Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROWN E. G. Evidence for the involvement of ferrous iron in the biosynthesis of delta-aminolaevulic acid by chicken erythrocyte preparations. Nature. 1958 Aug 2;182(4631):313–315. doi: 10.1038/182313b0. [DOI] [PubMed] [Google Scholar]
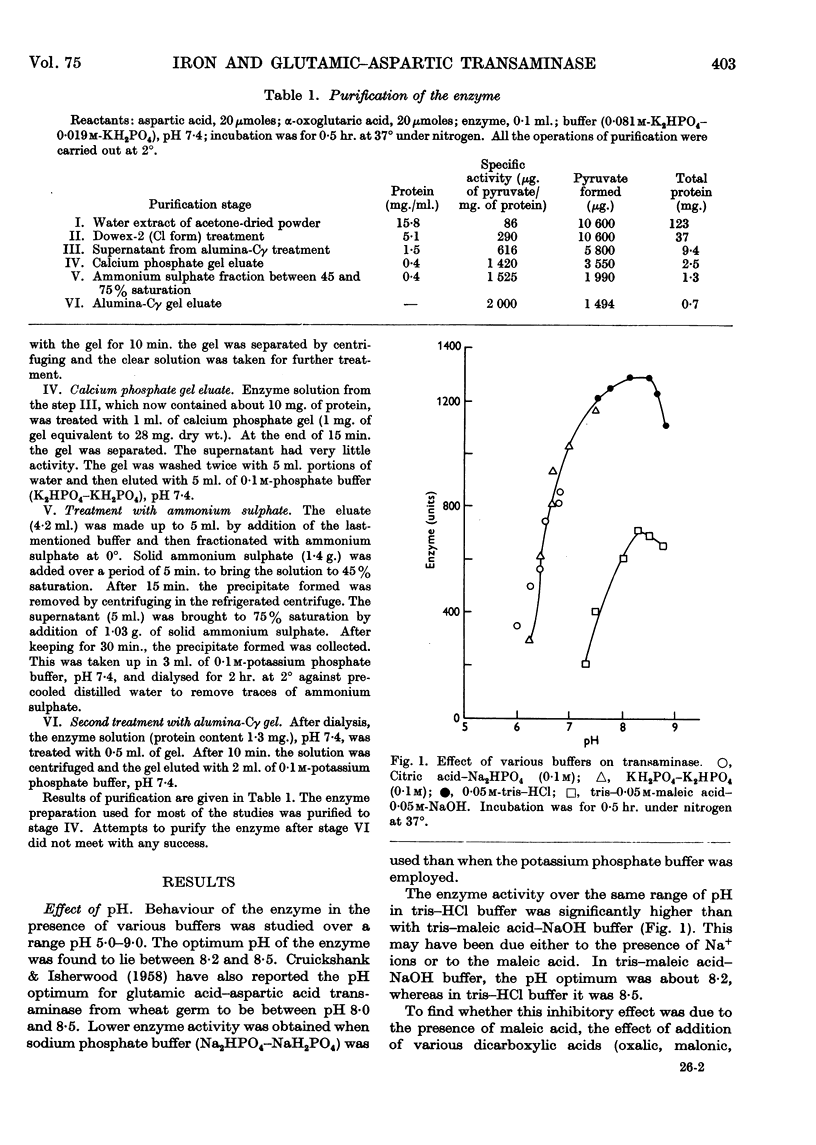
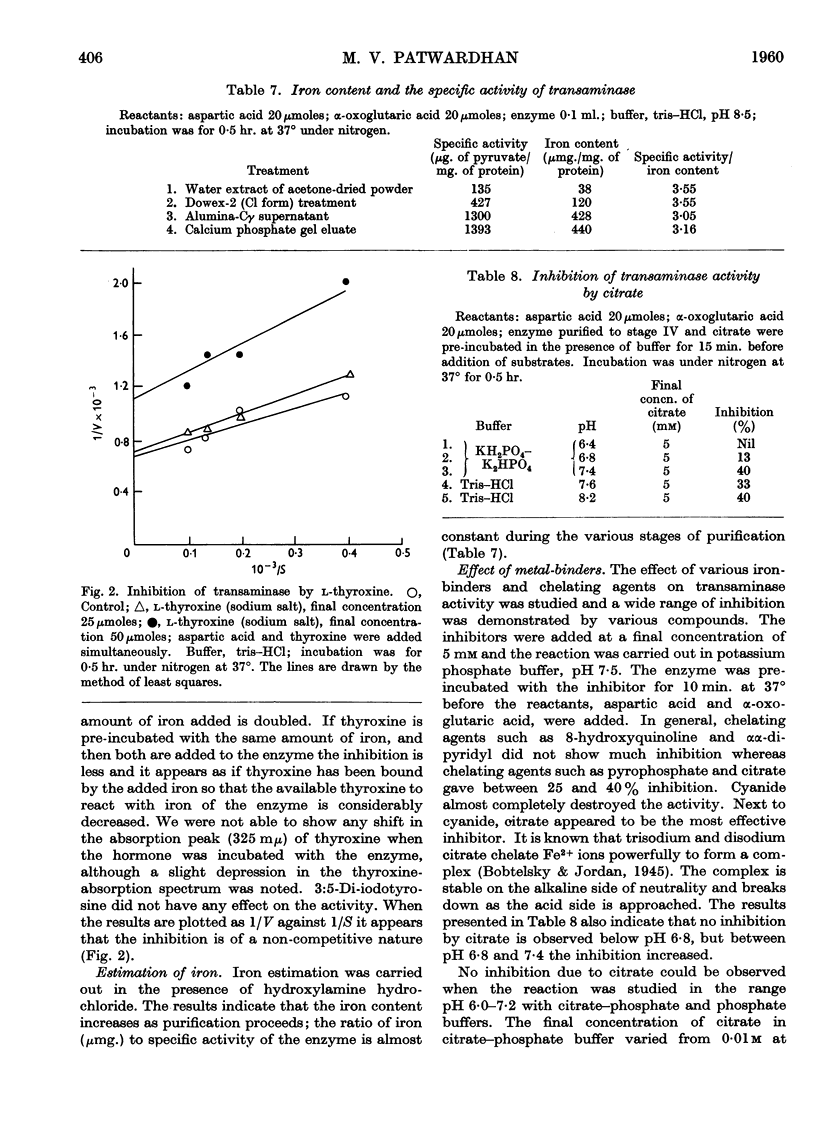
- CRUICKSHANK D. H., ISHERWOOD F. A. Glutamic-alanine and glutamic-aspartic transaminases of wheat germ. Biochem J. 1958 Jun;69(2):189–195. doi: 10.1042/bj0690189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EGGLESTON L. V. Effects of cations on amino acid decarboxylases. Biochem J. 1958 Mar;68(3):557–560. doi: 10.1042/bj0680557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAPPOLD F. C., STRUYVENBERG A. The activation of tryptophanase apo-enzyme by potassium, ammonium and rubidium ions. Biochem J. 1954 Nov;58(3):379–382. doi: 10.1042/bj0580379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAPPOLD F. C., TURNER J. M. Effect of magnesium ions on heart-muscle transaminase. Nature. 1957 Jan 19;179(4551):155–156. doi: 10.1038/179155a0. [DOI] [PubMed] [Google Scholar]
- ISHERWOOD F. A., CRUICKSHANK D. H. Chromatographic separation and analysis of mixtures of pyruvic, oxalacetic and alpha-ketoglutaric acids. Nature. 1954 Jan 16;173(4394):121–122. doi: 10.1038/173121a0. [DOI] [PubMed] [Google Scholar]
- JAKOBY W. B., BONNER D. M. Kynureninase from Neurospora: purification and properties. J Biol Chem. 1953 Dec;205(2):699–707. [PubMed] [Google Scholar]
- Krebs H. A. The effect of inorganic salts on the ketone decomposition of oxaloacetic acid. Biochem J. 1942 Apr;36(3-4):303–305. doi: 10.1042/bj0360303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MAHLER H. R., ELOWE D. G. Studies on metalloflavoproteins. II. The rôle of iron in diphosphopyridine nucleotide cytochrome c reductase. J Biol Chem. 1954 Sep;210(1):165–179. [PubMed] [Google Scholar]
- PATWARDHAN M. V. Role of ferrous iron in enzymatic transamination. Nature. 1958 Jan 18;181(4603):187–187. doi: 10.1038/181187a0. [DOI] [PubMed] [Google Scholar]
- SALL T., RICHARDS H. K., HARRISON E., MYERSON R. M. A colorimetric procedure for the determination of serum glutamic oxalacetic transaminase. J Lab Clin Med. 1957 Aug;50(2):297–303. [PubMed] [Google Scholar]
- SRINIVASAN M. Effects of certain protein foods on blood-sugar levels and glucose tolerance. Lancet. 1957 Aug 17;273(6990):317–320. doi: 10.1016/s0140-6736(57)92209-2. [DOI] [PubMed] [Google Scholar]
- TONHAZY N. E., WHITE N. G., UMBREIT W. W. A rapid method for the estimation of the glutamic-aspartic transaminase in tissues and its application to radiation sickness. Arch Biochem. 1950 Aug;28(1):36–42. [PubMed] [Google Scholar]
- TULPULE P. G., PATWARDHAN V. N. Application of paper chromatography to the study of the transminase system. Nature. 1952 Apr 19;169(4303):671–671. doi: 10.1038/169671a0. [DOI] [PubMed] [Google Scholar]
- WOLFF J., WOLFF E. C. The effect of thyroxine on isolated dehydrogenases. Biochim Biophys Acta. 1957 Nov;26(2):387–396. doi: 10.1016/0006-3002(57)90021-5. [DOI] [PubMed] [Google Scholar]
- WOLFF J., WOLFF E. C. The effect of thyroxine on isolated dehydrogenases. Biochim Biophys Acta. 1957 Nov;26(2):387–396. doi: 10.1016/0006-3002(57)90021-5. [DOI] [PubMed] [Google Scholar]
- YANOFSKY C. D-Serine dehydrase of Neurospora. J Biol Chem. 1952 Sep;198(1):343–352. [PubMed] [Google Scholar]


