Abstract
Aims
Patients surviving acute pulmonary embolism (PE) necessitate long-term treatment and follow-up. We estimated, the chronic economic impact of PE on the German healthcare system.
Methods and results
We calculated the direct cost of illness during the first year after discharge for the index PE, analysing data from a multicentre prospective cohort study in Germany. Main and accompanying readmission diagnoses were used to calculate DRG-based hospital reimbursements; anticoagulation costs were estimated from the exact treatment duration and each drug's unique national identifier; and outpatient post-PE care costs from guidelines-recommended algorithms and national reimbursement catalogues. Of 1017 patients enrolled at 17 centres, 958 (94%) completed ≥3-month follow-up; of those, 24% were rehospitalized (0.34 [95% CI 0.30–0.39] readmissions per PE survivor). Age, coronary artery, pulmonary and kidney disease, diabetes, and (in the sensitivity analysis of 837 patients with complete 12-month follow-up) cancer, but not recurrent PE, were independent cost predictors by hurdle gamma regression accounting for zero readmissions. The estimated rehospitalization cost was €1138 (95% CI 896–1420) per patient. Anticoagulation duration was 329 (IQR 142–365) days, with estimated average per-patient costs of €1050 (median 972; IQR 458–1197); costs of scheduled ambulatory follow-up visits amounted to €181. Total estimated direct per-patient costs during the first year after PE ranged from €2369 (primary analysis) to €2542 (sensitivity analysis).
Conclusion
By estimating per-patient costs and identifying cost drivers of post-PE care, our study may inform decisions concerning implementation and reimbursement of follow-up programmes aiming at improved cardiovascular prevention.
Keywords: Pulmonary embolism, Cost of illness, Economic burden, Readmission, Anticoagulation, Long-term care
Graphical Abstract
Graphical Abstract.
Key Learning Points.
What is already known on this topic
The annual incidence of pulmonary embolism is climbing and an increasing number of patients surviving an acute PE episode need chronic treatment and follow-up.
Data on the long-term impact of PE on European healthcare systems are sparse and still based on pooled analyses of older cohorts and adaptation of US cost models.
What this study adds
We calculated the direct cost of illness during the first year after acute PE, analysing data from a large multicentre cohort study in Germany with prospective follow-up of consecutive unselected PE survivors.
Estimated annual costs amounted to €2369–2542 per study patient, which would correspond to approximately €2.5 million per million population; they were mainly related to rehospitalizations and chronic anticoagulation.
ESC guidelines-recommended outpatient follow-up visits aiming at early detection of chronic complications and general cardiovascular prevention were only a minor contributor to the overall costs of post-PE care.
Introduction
Survival rates in the acute phase of pulmonary embolism (PE) are improving,1,2 paralleled by a decrease of PE-related mortality in the population.3–5 This is related, at least partly, to increased awareness and early diagnosis of the disease, combined with recent advances in antithrombotic treatment.6–8 On the other hand, the annual incidence of acute PE continues to climb as the population ages worldwide,1,2 meaning that an ever-increasing number of patients surviving an acute PE episode need chronic treatment and follow-up. The long-term risk of symptomatic recurrence of venous thromboembolism (VTE) is substantial,9 and extended anticoagulation beyond the first 3–6 months is often recommended.10,11 Moreover, the risk of any-cause rehospitalization after a first-time PE may be high as recently suggested by population data in Denmark.12 Finally, a broad spectrum of clinical and/or functional abnormalities may persist or appear de novo after PE,13,14 directly affecting morbidity and quality of life over the long term15–17 and supporting the recommendation for structured ambulatory follow-up programmes.8,18
In view of the above, patient care after an acute PE episode poses a substantial burden on healthcare systems globally, which, however, has not yet been systematically analysed. Existing models for estimating the annualized cost of illness of VTE in Europe date back to the past decade and were based on pooled data from older cohorts19 and adaptation of US cost models.20 This is in contrast to myocardial infarction and stroke, for which estimates at the European level were recently updated.21
Aiming to address this gap of knowledge, we analysed in the present study a large multicentre cohort of unselected patients with acute symptomatic PE who were prospectively followed after hospital discharge.15 We sought to determine the direct cost of illness of PE during the first year after hospital discharge, dissecting it into the costs related to (i) rehospitalizations from any cause, (ii) chronic anticoagulant medication, and (iii) scheduled outpatient follow-up and testing.
Methods
Study population
The FOllow-up after aCUte pulmonary emboliSm (FOCUS) study (German Clinical Trials registry number: DRKS00005939) was conducted at 17 large-volume centres across Germany and prospectively enrolled consecutive unselected patients (all comers) with confirmed diagnosis of acute symptomatic PE irrespective of clinical severity or thrombus load.15 Following hospital discharge, patients were followed with a standardized assessment plan; this comprised clinical, functional, imaging, and laboratory testing as well as patient-reported health status and outcomes, at pre-specified visits 3, 12, and 24 months after the index PE episode. Since FOCUS was an observational study, patient treatment was at the investigators’ discretion and in adherence to European Society of Cardiology (ESC) guidelines in effect at the time of patient recruitment.22,23 Detailed demographic and clinical data, diagnostic procedures, and outcomes were prospectively recorded in an electronic case report form (eCRF). FOCUS was approved by the ethics committees of the participating sites and written informed consent was obtained from all patients.
The present study analysed data from FOCUS related to the period between hospital discharge and the end of 1-year follow-up.
Prospectively collected and calculated variables, cost estimations
At the follow-up visits, the FOCUS investigators documented any rehospitalizations of the study patients since hospital discharge (for the 3-month visit), or since the previous outpatient visit (for the 12-month and 24-month visit). The information included the reason(s) for readmission to the hospital (main and accompanying diagnoses) as well as reimbursement-relevant procedures, and it was obtained from medical reports at hospital discharge; these were provided by the patient or (if not in their possession) by the patient's general practitioner. International Statistical Classification of Diseases (ICD) codes, specifically the 10th revision with German modification (ICD-10-GM), and the German procedure codes (Operationen- und Prozedurenschlüssel; OPS) were generated by two of the authors (K.M. and K.C.). Subsequently, the information was entered into an official online German Diagnosis Research Groups (G-DRG) calculator,24 and the calculated DRG was translated into the amount of hospital reimbursement for the year 2023, assuming the average length of hospital stay for the given indication.
The duration of oral anticoagulant treatment after hospital discharge and the exact anticoagulant drug(s) taken by the patient, including the dose, switch from one anticoagulant to another, date of discontinuation, and resumption of treatment after a discontinuation, all were documented in the eCRF at each follow-up visit. Based on the national central unique drug identifier, including the prices for the year 2023, we calculated the daily costs of treatment with each drug and multiplied them by the respective duration of treatment in days to obtain the total anticoagulation costs per patient.
Current guidelines recommend a structured schedule of outpatient follow-up visits after acute PE to ensure timely detection of treatment complications and late sequelae.8,18 This schedule corresponds to an algorithm, in which the diagnostic evaluation and the specific tests to be performed at each step depend on the results of the tests at the previous step.8 Since all the follow-up examinations included in the ESC guidelines algorithm had been implemented in the FOCUS study,15 we were able to calculate the probability of necessitating a given visit or test based on the actual results of the preceding examinations. Multiplying this probability by the costs of the visit/test based on the current (January 2024) ambulatory healthcare services reimbursement catalogue of the National Association of Statutory Health Insurance Physicians (Einheitlicher Bewertungsmaßstab der Kassenärztlichen Bundesvereinigung) in Germany25 allowed us to estimate the total current costs of the recommended outpatient post-PE evaluation per study patient.
Statistical analysis
The primary analysis was conducted in all patients with complete 3-month follow-up data. To assess to what extent the minority of patients with data limited to the first 3 months might have led to underestimation of the 12-month costs, a sensitivity analysis was performed, including only those patients with complete 12-month data. The cumulative incidence of hospital readmissions was plotted with Kaplan-Meier curves accounting for the competing risk of death. Differences between sexes and age groups were analysed with Gray's test.26
The number of rehospitalizations over the first year after PE was observed together with a set of baseline variables expected to provide possible risk factors for readmission. We anticipated a substantial proportion of study patients without readmissions, and that the number of rehospitalizations would exhibit a Poisson pattern in the remaining cases. Consequently, we applied a zero-inflated Poisson (ZIP) regression to assess the influence of the risk factors on the number of rehospitalizations; the model provides a parameter for excess zeros (zero process) on top of a Poisson regression model (count process).27 The zero process was modelled as a binomial with the logit link function, while the count process used the canonical log link. The ZIP regression was applied in a univariable fashion (separately) to each baseline variable; in addition, a multivariable regression was applied to all baseline variables simultaneously.
The number of readmissions per patient included in the analysis is shown as the marginal mean with bootstrapped 95% confidence intervals (CI). The effect estimates of univariable models for each predictor, and of the multivariable model, are displayed as average marginal effects with the corresponding Wald 95% CI. These indicate how strongly the expected value (here, number of readmissions) changes in the model (i) with the increase per age year; (ii) in women (with male sex serving as a reference); (iii) in the presence versus absence of a given categorical baseline variable (for the comorbidities tested); or (iv) in intermediate-risk or high-risk PE, each compared with low-risk PE (the latter being the reference severity category). Of note, the value of the marginal effect is influenced by two factors, a shift in the zero component and a shift in the distribution of readmission numbers. We also report (in the Supplementary material online) the results of the individual models as incidence rate ratios for the count processes, and as odds ratios for the estimation of excess zeros.
Total rehospitalization-related costs per patient during 1-year follow-up were analysed following a two-step (hurdle) process: In the first step, logistic regression was used to estimate whether costs were higher than zero. In the second, a gamma regression with a log link estimated the effects. The marginal means of the average costs per patient were calculated with the corresponding bootstrapped 95% CI. We additionally calculated these costs employing Bang and Tsiatis’ weighted complete case estimator as a control for the possible effect of censoring due to loss to follow-up.28 Furthermore, we sought to identify predictors of rehospitalization costs, entering the same baseline variables with the same categorization as for the model analysing the number of readmissions (see above). The effect estimates of univariable models, and of the multivariable model, are reported as average marginal effects with Wald 95% CI. In analogy to the approach presented in the previous paragraph, we also report (Supplementary material online) the results of the individual models as change in log costs on the condition of costs being greater than zero in the gamma regression with log link, and as odds ratios to predict that costs were zero in the logistic regression.
All analyses were performed using R version 4.3.2, package cmprsk for cumulative incidences, and packages pscl and glmmTMB for ZIP and hurdle regression models, respectively. Marginal effects were calculated with the package marginaleffects. Cost estimation accounting for censored data was performed using the ccostr package.
Results
Baseline characteristics and clinical outcomes
A total of 1017 patients were analysed in the FOCUS study; their baseline characteristics have been described previously.15 Of those, 958 (94.2%) completed at least the first (3-month) follow-up visit and were included in the primary analysis. Out of the latter patients, 837 (82.3% of the entire study population) also had complete 12-month follow-up data and were included in the sensitivity analysis. The key baseline characteristics of the population are shown in Table 1; the patients’ median age was 64 years (interquartile range [IQR] 51–74 years), 45% were women, and acute PE belonged to the intermediate-risk or high-risk class in 48% of the cases. A comparison of the patients included in the primary and sensitivity analyses is displayed in Supplementary material online, Table S1 of the Online Supplement; no differences in baseline characteristics were observed, with the exception of cancer which was more frequent in the primary analysis population. A total of 40 (4.2%) deaths occurred within the first 12 months after discharge. Of these, 16 occurred before the 3-month follow-up visit, and 24 between 3 and 12 months.
Table 1.
Baseline characteristics of the study patients (n = 958)
| Patient demographics | |
| Women, n | 435 (45%) |
| Age (years), median (IQR) | 64 (51–74) |
| Comorbidities and other risk factors, n | |
| Heart failure | 45 (4.7%) |
| Coronary artery disease | 85 (8.9%) |
| Chronic pulmonary disease | 149 (16%) |
| Cancer | 92 (9.6%) |
| Chronic kidney disease or eGFR <50 mL/min/1.73 m2 | 157 (16%) |
| Diabetes mellitus | 104 (11%) |
| History of VTE | 237 (25%) |
| Risk class of index PE | |
| Low | 497 (52%) |
| Intermediate | 433 (45%) |
| High | 28 (2.9%) |
eGFR, estimated glomerular filtration rate; IQR, interquartile range; PE, pulmonary embolism; VTE, venous thromboembolism.
Overall, 226 (23.6%) patients were readmitted to the hospital at least once, for an estimated average of 34 (95% CI 30–39) readmissions per 100 acute PE survivors. The total number of readmissions was 325, as 70/226 (31%) patients were rehospitalized more than once. As shown in Figure 1, the cumulative incidence of readmissions was independent from the patient's sex, whereas it differed between age groups. Leading causes of readmission were cardiovascular disease (mostly decompensated heart failure and coronary artery disease) in 54 cases (16.6%), cancer in 36 (11.1%), infectious or other acute inflammatory disease in 34 (10.5%), pulmonary disease in 28 (8.6%), bleeding in 14 (4.3%), and VTE recurrence in 12 (3.7%); in 147 cases (45.2%), the readmission was symptom-related.
Figure 1.
Kaplan-Meier estimator of the cumulative incidence of readmissions in the entire FOCUS population of survivors of acute pulmonary embolism, stratified by sex (left panel; same-colour shaded areas surrounding each curve denote the corresponding 95% confidence interval) and by age group (right panel). Differences between subgroups were analysed with Gray's test.26 FOCUS: FOllow-up after aCUte pulmonary emboliSm study.
Costs related to rehospitalizations
Table 2 (left columns) shows the marginal effects of the patients’ baseline clinical characteristics on the number of hospital readmissions over the first 12 months; the results of the individual models (Poisson regression for count and logistic regression for estimation of excess zeros) are shown in Supplementary material online, Table S2. In the simple (univariable) model, increasing age, coronary heart disease, chronic heart failure, chronic pulmonary disease, chronic kidney disease, diabetes, and cancer all were associated with a larger number of hospitalizations; in the adjusted (multivariable) model, only age, coronary artery disease, and chronic pulmonary disease remained independent predictors. The sensitivity analysis conducted in the patients with complete 12-month follow-up added cancer to the list of independent predictors of readmissions (Supplementary material online, Table S4; left columns).
Table 2.
Association of the patients’ baseline clinical characteristics with the number of hospital readmissions as well as with total rehospitalization costs over the first 12 months of follow-up after acute pulmonary embolism (n = 958 patients)
| Number of excess | Excess rehospitalization | |||
|---|---|---|---|---|
| readmissions (95% CI) | cost in euros (95% CI) | |||
| Zero-inflated Poisson regression | Hurdle gamma regression | |||
| Predicting variable | Univariable | Multivariable | Univariable | Multivariable |
| Age (per year increase) | 0.008 (0.004–0.011) | 0.005 (0.001–0.009) | 36 (23–49) | 15 (3–28) |
| Sex (women vs. men) | −0.05 (−0.14 to 0.04) | −0.03 (−0.12 to 0.06) | 99 (−257 to 455) | −50 (−373 to 274) |
| Coronary heart disease | 0.43 (0.23–0.63) | 0.20 (0.03–0.38) | 1939 (964–2914) | 684 (48–1321) |
| Chronic heart failure | 0.39 (0.08–0.70) | 0.12 (−0.10 to 0.35) | 2786 (873–4700) | 891 (−170 to 1953) |
| Chronic pulmonary disease | 0.38 (0.22–0.54) | 0.26 (0.12–0.41) | 1414 (745–2084) | 721 (222–1221) |
| Chronic kidney disease | 0.29 (0.14–0.44) | 0.13 (0.00–0.27) | 1782 (1020–2543) | 760 (206–1315) |
| Diabetes mellitus | 0.22 (0.04–0.41) | 0.08 (−0.07 to 0.23) | 1946 (864–3028) | 875 (120–1631) |
| Cancer | 0.23 (0.02–0.43) | 0.17 (−0.01 to 0.35) | 483 (−205–1170) | 558 (−113 to 1230) |
| Previous VTE | −0.06 (−0.16 to 0.04) | −0.04 (−0.14 to 0.06) | −106 (−511 to 298) | −171 (−528 to 185) |
| Risk class of index PE | ||||
| Low | Ref. | Ref. | Ref. | Ref. |
| Intermediate | 0.02 (−0.07 to 0.11) | −0.07 (−0.16 to 0.02) | 321 (−44 to 687) | −35 (−367 to 297) |
| High | 0.07 (−0.18 to 0.31) | 0.05 (−0.24 to 0.34) | 5 (−794 to 805) | 52 (−794 to 897) |
In both regression models (univariable and multivariable analysis of the number of readmissions [left columns], and of total rehospitalization costs [right columns] per patient), the effect estimates of are displayed as average marginal effects with the corresponding Wald 95% CI. Statistically significant effects (P < 0.05) appear in bold.
In the zero-inflated Poisson regression (left columns), the estimated number of readmissions per patient is always below 1 because the majority of patients in the FOCUS study had no readmissions at all during the first year of follow-up. For the same reason, the additive rehospitalization costs per patient appear ‘low’ in the hurdle gamma regression.
CI, confidence interval; PE, pulmonary embolism; Ref, reference; VTE, venous thromboembolism.
The estimated average total rehospitalization cost was €1138 (95% CI 896–1420) per patient included in the main analysis. Table 2 (right columns) shows the marginal effects of the patients’ baseline clinical characteristics on excess hospital readmissions over the first 12 months after acute PE; the results of the individual models (conditional gamma regression for cost count and logistic regression for the excess zeros) are shown in Supplementary material online, Table S3. The predictors of costs in both the univariable and the adjusted (multivariable) models largely overlapped with those of the number of readmissions. The sensitivity analysis of patients with complete 12-month follow-up, shown in Supplementary material online, Table S4, right columns, estimated an overall slightly higher total rehospitalization cost of €1171 (908–1479); analysis using the weighted complete case estimator was consistent with these results, yielding a total cost of €1172.
Costs related to anticoagulation and ambulatory care
After hospital discharge for the index PE, patients remained on anticoagulation for a median duration of 329 (IQR 142–365) days. The total days and the median duration (in days, with the corresponding IQR) of treatment on each anticoagulant are shown in Table 3; Supplementary material online, Table S5 shows the results of the sensitivity analysis in the patients with a complete 12-month follow-up. From these data and based on current daily costs as per each drug's central unique identifier, we calculated a total of anticoagulant treatment costs amounting to an average of €1050 per patient (median 972; IQR 458–1197) included in the primary analysis and €1190 (median 1168; IQR 931–1197) per patient in the sensitivity analysis.
Table 3.
Total patient-days after discharge and median duration of treatment with each anticoagulant drug during the study period (n = 958 patients)
| Anticoagulant | Total patient-days per anticoagulant in FOCUS during the first follow-up yeara | Median duration of treatment (IQR) |
|---|---|---|
| Low molecular weight heparin | 14 437 | 48 (42–181) |
| Fondaparinux | 135 | 135 (135–135) |
| Rivaroxaban | 141 772 | 365 (213–365) |
| Apixaban | 69 917 | 365 (220–365) |
| Edoxaban | 9866 | 324 (220–365) |
| Dabigatran | 10 597 | 316 (164–365) |
| VKA—Phenprocoumon | 33 566 | 322 (187–365) |
| VKA—Warfarin | 707 | 149 (136–154) |
| Other | 229 | 229 (229–229) |
| Any anticoagulant | 281 541 | 329 (142–365) |
IQR, interquartile range; VKA, vitamin K antagonist.
The FOllow-up after aCUte pulmonary emboliSm (FOCUS) study prospectively collected data on the type and duration of anticoagulant treatment in each patient, including switches from one anticoagulant to another, the exact date of discontinuation, and any resumption of anticoagulant treatment after discontinuation.
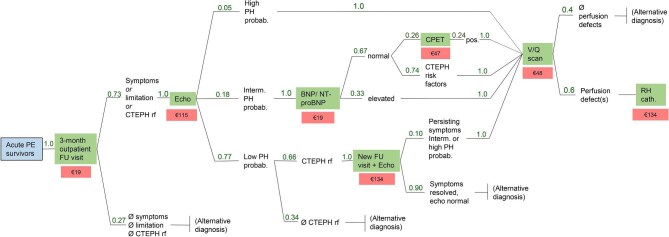
Costs of scheduled ambulatory visits and tests were calculated based on the decision tree recommended by current guidelines8 and the actual results of the tests in the FOCUS study population.15 The calculated probability and current reimbursement (in euros) of each visit and test are shown in Figure 2. Total costs amounted to €181 per patient.
Figure 2.
Decision tree-based outpatient follow-up visits and tests after acute pulmonary embolism (green boxes), with the respective reimbursement in euros (red boxes). The sequence of visits and tests correspond to the pulmonary embolism follow-up algorithm recommended by current European guidelines.8,18 The standard outpatient follow-up visit includes clinical examination, an electrocardiogram, and a 6-min walking test, followed by specific functional, imaging and/or laboratory tests as indicated. Numbers in green indicate the probability of needing a specific visit or test based on the findings at the previous visit or test as documented in the FOCUS study population. The amounts in euros below each visit/test are based on the current ambulatory healthcare services reimbursement catalogue of the National Association of Statutory Health Insurance Physicians (Einheitlicher Bewertungsmaßstab der Kassenärztlichen Bundesvereinigung) in Germany.25 The indicated echocardiogram reimbursement is for a test performed by a board-certified cardiologist. BNP, brain natriuretic peptide; CPET, cardiopulmonary exercise testing (spiroergometry); CTEPH, chronic thromboembolic pulmonary hypertension; Echo, outpatient transthoracic echocardiogram including Doppler examination; FOCUS: FOllow-up after aCUte pulmonary emboliSm study; FU, follow-up; interm. PH probab., intermediate echocardiographic probability for (chronic) pulmonary hypertension as defined by the 2022 European Guidelines on Pulmonary Hypertension;18 low PH probab., low echocardiographic probability for (chronic) pulmonary hypertension;18 NT-proBNP, N-terminal pro brain natriuretic peptide; PE, pulmonary embolism; PE survivors, all patients who survived the acute phase of the index PE event and were discharged alive; PH, pulmonary hypertension; pos, positive; rf, risk factor(s); RH cath., right heart catheterization; V/Q scan, ventilation-perfusion lung scintigraphy.
Taking into consideration all the above categories and calculations, the sum of direct per-patient costs during the first year after acute PE was estimated at €2369. The estimate based on the sensitivity analysis was slightly higher, €2542.
Discussion
We calculated the chronic cost of illness of PE during the first year after the index acute event, analysing prospectively collected data from a large prospective multicentre cohort study in Germany. Our results can be summarized as follows: (1) An average of 34 hospital readmissions per 100 survivors of acute PE occurred; (2) the estimated average total rehospitalization cost per patient was €1138–1172, with age, coronary artery disease, chronic pulmonary disease, and (in the sensitivity analysis of patients with complete 12-month follow-up) cancer being independent cost drivers; (3) average chronic anticoagulation costs over one year amounted to €1050–1190 per patient; and (4) the ambulatory post-PE care recommended by current guidelines (€181 per patient) was only a minor contributor to chronic costs. Accordingly, the total direct per-patient costs during the first year after acute PE were estimated at €2369–2542.
Data on the chronic cost of illness of PE are sparse. A study published in 2016 built decision trees populating them with data from a total of 25 studies of various designs conducted in different European countries.19 Estimated direct chronic costs concentrated on known complications of VTE and its treatment, notably recurrent events and bleedings. Medication and post-PE care costs were not included.19 A more recent study based on a European registry analysed prospectively collected data on cost drivers, also focusing, within the direct costs, (only) on those presumed to be ‘directly’ PE-related.29 A similar approach was followed in a cohort study which, in contrast to FOCUS, included only patients with low-risk PE.30 Importantly, however, we found in the present study that recurrent VTE or bleeding was the primary diagnosis in only a minority (3.7 and 4.3%, respectively) of hospital readmissions. Instead, we identified chronic heart, lung and kidney disease, diabetes, and (in the sensitivity analysis) cancer as key predictors of the number and cost of rehospitalizations. These findings are generally in agreement with those of a nationwide registry-based study, although our estimated annual readmission rate is below the 49% absolute risk reported for the Danish population.12 Importantly however, our study went beyond the description of readmissions and their predictors, calculating current DRG-based hospital reimbursement in the healthcare system of Germany, a country with a population of approximately 84 million. This allowed us to quantify the contribution of hospital readmissions to the direct annual costs after PE and dissect cost predictors among the patients’ demographic data and comorbidities. In doing so, we employed zero-inflated statistical models to take into account that these costs were generated by only a minority of the study patients and did not follow a normal distribution.
The results of the present study, conducted in unselected patients (all-comers) with PE, highlight changes across the broader spectrum of management and cost drivers after the acute phase. In the era of effective and safe direct oral anticoagulants,8,11 VTE recurrence or bleeding no longer appear to be major determinants of the direct chronic economic burden of PE. Of course, treatment with these drugs is still costly, affecting costs to a similar extent as rehospitalizations in our study. However, anticoagulant prices are expected to fall in the coming years as generic oral anticoagulants will become available.
Our findings further suggest that structured post-PE follow-up programmes as recommended by current guidelines8,18 have only a minor impact on the total annual costs after PE in Germany. In fact, such programmes may contribute to direct and indirect cost savings via early detection and treatment of a broad spectrum of persisting clinical and functional abnormalities comprising the so-called ‘post-PE syndrome’.15,16,31–33 Moreover, by identifying the most frequent causes of (co)morbidity in the general population as key drivers of direct costs after PE, our study underlines the need for integration of broader cardiovascular prevention and lifestyle modification in post-PE care.13 Outpatient cardiopulmonary rehabilitation programmes34,35 may, if successfully tested in future randomized trials, improve the patients’ well-being and quality of life, also offering the potential for reduction of the cost of illness of PE over the long term.
Based on our results and considering an annual incidence of 98 000 hospitalizations for acute PE in Germany along with a 13% in-hospital case fatality for the most recent years available,36 total annual direct costs after acute PE might be expected to range between €202 and €217 million in Germany, corresponding to €2.4–2.6 million per million population. Clearly, this estimate is to be interpreted with caution, since the analysis of a multicentre cohort such as that of the FOCUS study cannot be directly extrapolated to the population of an entire country. On the other hand, the per-patient costs reported in the present study were generated after the acute phase of PE in the ‘real world’ of the German healthcare system and based on European and national guideline recommendations. Therefore, they may provide at least a partial insight into the total estimated annual burden of this disease. Of note, these costs do not include the diagnosis and treatment of chronic thromboembolic pulmonary hypertension.
The present study has some limitations. First, we did not obtain actual costs after PE from administrative databases, nor did we record unscheduled outpatient healthcare resource utilization.29,30 However, we consider our estimates to be robust, since we relied on prospectively collected data, (i) on the date as well as the main and accompanying diagnoses of rehospitalizations; (ii) on the type, duration, and switches of anticoagulant medication over 1 year; and (iii) on the probability of needing follow-up tests in post-PE care based on established algorithms. Second, our estimates focused on direct costs after PE; costs due to loss of productivity may also be substantial.19,29 Third, our primary analysis included the vast majority (94%) of the patients enrolled in FOCUS, but ‘only’ for 82% of all patients was complete 1-year follow-up information available. Our sensitivity analysis of these latter patients indicates that some shifts in cost drivers, notably cancer, may occur. Finally, we consider the inclusion of all readmissions (instead of only those traditionally ascribed to PE, such as VTE recurrence or major bleeding) and their costs to be a strength of our analysis. However, it is likely that at least some of these rehospitalizations would have occurred in the study patients even if they had not suffered acute PE. To directly address the issue of excess PE-inflicted healthcare costs, as recently attempted in patients with atherosclerotic cardiovascular disease,37 future studies will need to compare the clinical course of PE survivors with that of appropriately matched individuals from large population cohorts.
In conclusion, the present study provides an estimate of the direct per-patient costs in the first year after acute PE, identifying and dissecting cost drivers. Our results may stimulate health economic research on post-PE care in further countries. They may further inform decisions by policymakers concerning the implementation and reimbursement of improved follow-up programmes aiming at broader cardiovascular prevention and lifestyle modification.
Supplementary Material
Acknowledgements
The FOCUS Investigators: Mainz: Stefano Barco, Dorothea Becker, Brunhilde Fischer, Lukas Hobohm, Anja Käberich, Karsten Keller, Frederikus A. Klok, Stavros V. Konstantinides, Nadine Martin, Anna C. Mavromanoli, Silke Otto, Kai-Helge Schmidt, Luca Valerio, and Philipp S. Wild; Berlin, Unfallkrankenhaus: Leonhard Bruch, Stefanie Geistert, and Katrin Schüler; Greifswald: Ralf Ewert, Claudia Pohl, and Jeannette Pieper; Esslingen: Martin Faehling, Birgit Blaich, and Annika Landmesser; Cologne: Stephan Rosenkranz, Felix Gerhardt, Jasmin Rudolph, and Sibel Gün; Giessen: Hossein-Ardeschir Ghofrani, and Ute George; Heidelberg: Ekkehard Grünig, Benjamin Egenlauf, Amina Salkić, and Eva-Maria Heier; Dresden: Michael Halank, Kristin Tausche, Tina Rink, and Diana Jäkel; Würzburg: Matthias Held, and Barbara Schröder; Hannover: Marius M. Hoeper, Julia Freise, and Susanne Tayler; Neuwittelsbach: Hanno H. Leuchte, and Annika Horn; Munich Bogenhausen: F. Joachim Meyer, Dagmar Emge-Rossa, and Karine Thabaret; Munich LMU: Claus Neurohr, and Juergen Barton; Berlin Westend: Christian Opitz, and Ines Bressem; Leipzig: Hans-Jürgen Seyfarth, Patricia Berger, and Angela Hennig; Göttingen: Rolf Wachter, Kristian Hellenkamp, Carmen Sentler, and Martina Schulte; Homburg/Saar: Heinrike Wilkens, Franziska Trudzinski, and Ines Holtz.
Independent Adjudication (Critical Events) Committee: Eckhard Mayer (Chair), David Fistera, and Aleksandar Grgic.
Trial registration number: DRKS00005939.
Contributor Information
Katharina Mohr, Center for Thrombosis and Hemostasis (CTH), University Medical Center of the Johannes Gutenberg University, 55131 Mainz, Germany; Institute of Medical Biometry and Statistics, Faculty of Medicine and Medical Center, University of Freiburg, 79104 Freiburg, Germany.
Philipp Mildenberger, Institute of Medical Biostatistics, Epidemiology and Informatics, University Medical Center of the Johannes Gutenberg University, 55131 Mainz, Germany.
Thomas Neusius, Wiesbaden Business School, RheinMain University of Applied Sciences, 65183 Wiesbaden, Germany.
Konstantinos C Christodoulou, Center for Thrombosis and Hemostasis (CTH), University Medical Center of the Johannes Gutenberg University, 55131 Mainz, Germany.
Ioannis T Farmakis, Center for Thrombosis and Hemostasis (CTH), University Medical Center of the Johannes Gutenberg University, 55131 Mainz, Germany; Department of Cardiology, University Medical Center of the Johannes Gutenberg University, 55131 Mainz, Germany.
Klaus Kaier, Institute of Medical Biometry and Statistics, Faculty of Medicine and Medical Center, University of Freiburg, 79104 Freiburg, Germany.
Stefano Barco, Center for Thrombosis and Hemostasis (CTH), University Medical Center of the Johannes Gutenberg University, 55131 Mainz, Germany; Department of Angiology, University Hospital Zurich, 8091 Zurich, Switzerland.
Frederikus A Klok, Center for Thrombosis and Hemostasis (CTH), University Medical Center of the Johannes Gutenberg University, 55131 Mainz, Germany; Department of Medicine - Thrombosis and Hemostasis, Leiden University Medical Center, 2300 RC Leiden, The Netherlands.
Lukas Hobohm, Center for Thrombosis and Hemostasis (CTH), University Medical Center of the Johannes Gutenberg University, 55131 Mainz, Germany; Department of Cardiology, University Medical Center of the Johannes Gutenberg University, 55131 Mainz, Germany.
Karsten Keller, Center for Thrombosis and Hemostasis (CTH), University Medical Center of the Johannes Gutenberg University, 55131 Mainz, Germany; Department of Cardiology, University Medical Center of the Johannes Gutenberg University, 55131 Mainz, Germany; Medical Clinic VII, Department of Sports Medicine, University Hospital Heidelberg, 69120 Heidelberg, Germany.
Dorothea Becker, Center for Thrombosis and Hemostasis (CTH), University Medical Center of the Johannes Gutenberg University, 55131 Mainz, Germany.
Christina Abele, Center for Thrombosis and Hemostasis (CTH), University Medical Center of the Johannes Gutenberg University, 55131 Mainz, Germany; School of Life Sciences, University of Siegen, 57068 Siegen, Germany.
Leonhard Bruch, Klinik für Innere Medizin und Kardiologie, Unfallkrankenhaus Berlin, 12683 Berlin, Germany.
Ralf Ewert, Clinic for Internal Medicine, Greifswald University Hospital, 17475 Greifswald, Germany.
Irene Schmidtmann, Institute of Medical Biostatistics, Epidemiology and Informatics, University Medical Center of the Johannes Gutenberg University, 55131 Mainz, Germany.
Philipp S Wild, Center for Thrombosis and Hemostasis (CTH), University Medical Center of the Johannes Gutenberg University, 55131 Mainz, Germany; Department of Preventive Cardiology and Preventive Medicine, Center for Cardiology, University Medical Center of the Johannes Gutenberg University, 55131 Mainz, Germany; German Center for Cardiovascular Research (DZHK), Partner Site RheinMain, Germany.
Stephan Rosenkranz, Department of Cardiology, Heart Center at the University Hospital Cologne, and Cologne Cardiovascular Research Center, 50937 Cologne, Germany.
Stavros V Konstantinides, Center for Thrombosis and Hemostasis (CTH), University Medical Center of the Johannes Gutenberg University, 55131 Mainz, Germany; Department of Cardiology, Democritus University of Thrace, 68100 Alexandroupolis, Greece.
Harald Binder, Institute of Medical Biometry and Statistics, Faculty of Medicine and Medical Center, University of Freiburg, 79104 Freiburg, Germany.
Luca Valerio, Center for Thrombosis and Hemostasis (CTH), University Medical Center of the Johannes Gutenberg University, 55131 Mainz, Germany; Department of Cardiology, University Medical Center of the Johannes Gutenberg University, 55131 Mainz, Germany.
The FOCUS Investigators:
Stefano Barco, Dorothea Becker, Brunhilde Fischer, Lukas Hobohm, Anja Käberich, Karsten Keller, Frederikus A Klok, Stavros V Konstantinides, Nadine Martin, Anna C Mavromanoli, Silke Otto, Kai-Helge Schmidt, Luca Valerio, Philipp S Wild, Leonhard Bruch, Stefanie Geistert, Katrin Schüler, Ralf Ewert, Claudia Pohl, Jeannette Pieper, Martin Faehling, Birgit Blaich, Annika Landmesser, Stephan Rosenkranz, Felix Gerhardt, Jasmin Rudolph, Sibel Gün, Hossein-Ardeschir Ghofrani, Ute George, Ekkehard Grünig, Benjamin Egenlauf, Amina Salkić, Eva-Maria Heier, Michael Halank, Kristin Tausche, Tina Rink, Diana Jäkel, Matthias Held, Barbara Schröder, Marius M Hoeper, Julia Freise, Susanne Tayler, Hanno H Leuchte, Annika Horn, F Joachim Meyer, Dagmar Emge-Rossa, Karine Thabaret, Claus Neurohr, Juergen Barton, Christian Opitz, Ines Bressem, Hans-Jürgen Seyfarth, Patricia Berger, Angela Hennig, Rolf Wachter, Kristian Hellenkamp, Carmen Sentler, Martina Schulte, Heinrike Wilkens, Franziska Trudzinski, Ines Holtz, Eckhard Mayer, David Fistera, and Aleksandar Grgic
Funding
FOCUS is an independent, investigator-initiated study with an academic sponsor, the University Medical Center of the Johannes Gutenberg University, Mainz, Germany. The work of Stavros V. Konstantinides and Philipp S. Wild was supported by the German Federal Ministry of Education and Research (BMBF 01EO1003 and 01EO1503). In addition, the sponsor received a grant from Bayer AG (ISS, 006_PH/Schau(32690), FOCUS17257). The funding bodies had no influence on the design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Conflict of interest:
K.M., P.M., T.N., K.C.C., I.T.F., K.K. (Kaier), K.K. (Keller), D.B., C.A., L.B., I.S., H.B., and L.V.: no disclosures; S.B.: Boston Scientific, Medtronic, Bayer, sanofi, institutional research support by Board; Boston Scientific, Penumbra, Viatris, personal fees/honoraria; R.E.: Janssen, OMT, Astra-Zeneca, Novartis, Boehringer-Ingelheim, AOP, LungPacer personal lecture/consultant fees; F.K.: research support from Bayer, BMS, BSCI, AstraZeneca, MSD, Leo Pharma, Actelion, Farm-X, The Netherlands Organization for Health Research and Development, The Dutch Thrombosis Foundation, The Dutch Heart Foundation and the Horizon Europe Program, all outside this manuscript and paid to his institution.; L.H.: MSD, Janssen, Inari Medical, personal lecture/consultant fees; P.S.W.: research funding from Bayer AG for the FOCUS BioSeq Study and outside the submitted work, consulting fees from Astra Zeneca, research funding from Bayer AG, research funding, consulting and lecturing fees from Bayer Health Care, lecturing fees from Bristol Myers Squibb, research funding and consulting fees from Boehringer Ingelheim, research funding and consulting fees from Daiichi Sankyo Europe, consulting fees and non-financial support from Diasorin, non-financial research support from I.E.M., research funding and consulting fees from Novartis Pharma, lecturing fees from Pfizer Pharma, non-financial grants from Philips Medical Systems, research funding and consulting fees from sanofi-aventis. He is principal investigator of the future cluster ‘curATime’ (BMBF 03ZU1202AA, 03ZU1202CD, 03ZU1202DB, 03ZU1202JC, 03ZU1202KB, 03ZU1202LB, 03ZU1202MB, and 03ZU1202OA) and principal investigator of the DIASyM research core (BMBF 161L0217A), and principal investigator of the DZHK (German Center for Cardiovascular Research), Partner Site Rhine-Main, Mainz, Germany; S.R.: remuneration for lectures and/or consultancy from Abbott, Acceleron, Actelion, Aerovate, Altavant, AOP, AstraZeneca, Bayer, Bristol-Myers Squibb, Boehringer-Ingelheim, Edwards, Ferrer, Gossamer, Inari, Janssen, Lilly, MSD, Novartis, Pfizer, and United Therapeutics; and research support to his institution from Actelion, AstraZeneca, Bayer, and Janssen; S.K.: Bayer AG, Boston Scientific, Daiichi-Sankyo, LumiraDx, Penumbra, Inari Medical, personal lecture/advisory fees and research grants to institution; MSD, Pfizer—Bristol-Myers Squibb, personal lecture/advisory fees.
Data Availability
Proposals for data access will be considered by the FOCUS Steering Committee in accordance with the data access policy of the academic study sponsor (University Medical Centre Mainz, Germany).
Authors’ contribution
K.M., P.M., I.S., S.V.K., H.B., and L.V. conceived and planned the study. K.M., P.M., and K.C.C. extracted the data and performed the statistical analysis with guidance and supervision by H.B. and L.V. T.N., K.K. (Kaier), C.A., and I.S. provided expert advice and support in statistics and health economic analysis, both of which were crucial for meeting the objectives of this study. S.B., F.A.K., L.H., I.F., and K.K. (Keller) provided expert input into the analysis of events documented during long-term follow-up after pulmonary embolism. D.B., L.B., R.E., S.R., P.S.W., and S.V.K. were responsible for the integrity of the data in the FOCUS study, representing the entire FOCUS investigator team. S.V.K., P.S.W., and S.R. obtained the funding for conducting the FOCUS study. K.M., P.M., and L.V. provided the first draft of the manuscript. All authors interpreted the data, made critical revisions of the manuscript for important intellectual content, and gave final approval for its submission.
References
- 1. Keller K, Hobohm L, Ebner M, Kresoja K-P, Münzel T, Konstantinides SV et al. Trends in thrombolytic treatment and outcomes of acute pulmonary embolism in Germany. Eur Heart J 2020;41:522–529. 10.1093/eurheartj/ehz236 [DOI] [PubMed] [Google Scholar]
- 2. Bikdeli B, Wang Y, Jimenez D, Parikh SA, Monreal M, Goldhaber SZ et al. Pulmonary embolism hospitalization, readmission, and mortality rates in US older adults, 1999-2015. JAMA 2019;322:574–576. 10.1001/jama.2019.8594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barco S, Mahmoudpour SH, Valerio L, Klok FA, Münzel T, Middeldorp S et al. Trends in mortality related to pulmonary embolism in the European Region, 2000-15: analysis of vital registration data from the WHO Mortality Database. Lancet Respir Med 2020;8:277–287. 10.1016/S2213-2600(19)30354-6 [DOI] [PubMed] [Google Scholar]
- 4. Martin KA, Molsberry R, Cuttica MJ, Desai KR, Schimmel DR, Khan SS. Time trends in pulmonary embolism mortality rates in the United States, 1999 to 2018. JAHA 2020;9:e016784. 10.1161/JAHA.120.016784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barco S, Valerio L, Ageno W, Cohen AT, Goldhaber SZ, Hunt BJ et al. Age-sex specific pulmonary embolism-related mortality in the USA and Canada, 2000-18: an analysis of the WHO Mortality Database and of the CDC Multiple Cause of Death database. Lancet Respir Med 2021;9:33–42. 10.1016/S2213-2600(20)30417-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Götzinger F, Lauder L, Sharp ASP, Lang IM, Rosenkranz S, Konstantinides S et al. Interventional therapies for pulmonary embolism. Nat Rev Cardiol 2023;20:670–684. 10.1038/s41569-023-00876-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pruszczyk P, Klok FK, Kucher N, Roik M, Meneveau N, Sharp AS et al. Percutaneous treatment options for acute pulmonary embolism: a clinical consensus statement by the ESC working group on pulmonary circulation and right ventricular function and the European Association of Percutaneous Cardiovascular Interventions. EuroIntervention 2022;18:e623–e638. 10.4244/EIJ-D-22-00246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing G-J, Harjola V-P et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J 2020;41:543–603. 10.1093/eurheartj/ehz405 [DOI] [PubMed] [Google Scholar]
- 9. Khan F, Rahman A, Carrier M, Kearon C, Weitz JI, Schulman S et al. Long term risk of symptomatic recurrent venous thromboembolism after discontinuation of anticoagulant treatment for first unprovoked venous thromboembolism event: systematic review and meta-analysis. BMJ 2019;366:l4363. 10.1136/bmj.l4363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ortel TL, Neumann I, Ageno W, Beyth R, Clark NP, Cuker A et al. American Society of Hematology 2020 guidelines for management of venous thromboembolism: treatment of deep vein thrombosis and pulmonary embolism. Blood Adv 2020;4:4693–4738. 10.1182/bloodadvances.2020001830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stevens SM, Woller SC, Kreuziger LB, Bounameaux H, Doerschug K, Geersing G-J et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. Chest 2021;160:e545–e608. 10.1016/j.chest.2021.07.055 [DOI] [PubMed] [Google Scholar]
- 12. Sindet-Pedersen C, El-Chouli M, Nouhravesh N, Lamberts M, Christensen DM, Kümler T et al. High risk of rehospitalization within 1 year following a pulmonary embolism-insights from the Danish nationwide registries from 2000-2020. Eur Heart J Qual Care Clin Outcomes 2024;10:256–264. 10.1093/ehjqcco/qcad046 [DOI] [PubMed] [Google Scholar]
- 13. Klok FA, Ageno W, Ay C, Bäck M, Barco S, Bertoletti L et al. Optimal follow-up after acute pulmonary embolism: a position paper of the European Society of Cardiology Working Group on Pulmonary Circulation and Right Ventricular Function, in collaboration with the European Society of Cardiology Working Group on Atherosclerosis and Vascular Biology, endorsed by the European Respiratory Society. Eur Heart J 2022;43:183–189. 10.1093/eurheartj/ehab816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Klok FA, Van Der Hulle T, Den Exter PL, Lankeit M, Huisman MV, Konstantinides S. The post-PE syndrome: a new concept for chronic complications of pulmonary embolism. Blood Rev 2014;28:221–226. 10.1016/j.blre.2014.07.003 [DOI] [PubMed] [Google Scholar]
- 15. Valerio L, Mavromanoli AC, Barco S, Abele C, Becker D, Bruch L et al. Chronic thromboembolic pulmonary hypertension and impairment after pulmonary embolism: the FOCUS study. Eur Heart J 2022;43:3387–3398. 10.1093/eurheartj/ehac206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Valerio L, Barco S, Jankowski M, Rosenkranz S, Lankeit M, Held M et al. Quality of life 3 and 12 months following acute pulmonary embolism: analysis from a prospective multicenter cohort study. Chest 2021;159:2428–2438. 10.1016/j.chest.2021.01.071 [DOI] [PubMed] [Google Scholar]
- 17. Kahn SR, Akaberi A, Granton JT, Anderson DR, Wells PS, Rodger MA et al. Quality of life, dyspnea, and functional exercise capacity following a first episode of pulmonary embolism: results of the ELOPE cohort study. Am J Med 2017;130:990.e9 e999-990 e921. 10.1016/j.amjmed.2017.03.033 [DOI] [PubMed] [Google Scholar]
- 18. Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M et al. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J 2022;43:3618–3731. 10.1093/eurheartj/ehac237 [DOI] [PubMed] [Google Scholar]
- 19. Barco S, Woersching AL, Spyropoulos AC, Piovella F, Mahan CE. European Union-28: an annualised cost-of-illness model for venous thromboembolism. Thromb Haemost 2016;115:800–808. 10.1160/TH15-08-0670 [DOI] [PubMed] [Google Scholar]
- 20. Mahan CE, Borrego ME, Woersching AL, Federici R, Downey R, Tiongson J et al. Venous thromboembolism: annualised United States models for total, hospital-acquired and preventable costs utilising long-term attack rates. Thromb Haemost 2012;108:291–302. 10.1160/TH12-03-0162 [DOI] [PubMed] [Google Scholar]
- 21. Luengo-Fernandez R, Walli-Attaei M, Gray A, Torbica A, Maggioni AP, Huculeci R et al. Economic burden of cardiovascular diseases in the European Union: a population-based cost study. Eur Heart J 2023;44:4752–4767. 10.1093/eurheartj/ehad583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galiè N et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC)Endorsed by the European Respiratory Society (ERS). Eur Heart J 2014;35:3033–3073. ehu283[pii]; 10.1093/eurheartj/ehu283 [doi] [DOI] [PubMed] [Google Scholar]
- 23. Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016;37:67–119. 10.1093/eurheartj/ehv317 [DOI] [PubMed] [Google Scholar]
- 24. DRG-Research-Group . Wegbrouper G-DRG 2023. https://www.drg-research-group.de/index.php?option=com_webgrouper&view=webgrouper&Itemid=112 (1 July 2024)
- 25. Kassenärztliche Bundesvereinigung . Enheitlicher Bewertungsmassstab (EBM). https://www.kbv.de/html/online-ebm.php (23 January 2024)
- 26. Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Statist 1988;16:1141–1154. 10.1214/aos/1176350951 [DOI] [Google Scholar]
- 27. Zeileis A, Kleiber C, Jackman S. Regression models for count data in R. J Stat Soft 2008;27:1–25. 10.18637/jss.v027.i08 [DOI] [Google Scholar]
- 28. Bang H. Estimating medical costs with censored data. Biometrika 2000;87:329–343. 10.1093/biomet/87.2.329 [DOI] [Google Scholar]
- 29. Farmakis IT, Barco S, Mavromanoli AC, Agnelli G, Cohen AT, Giannakoulas G et al. Cost-of-illness analysis of long-term health care resource use and disease burden in patients with pulmonary embolism: insights from the PREFER in VTE registry. JAHA 2022;11:e027514. 10.1161/JAHA.122.027514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Farmakis IT, Kaier K, Hobohm L, Mohr K, Valerio L, Barco S et al. Healthcare resource utilisation and associated costs after low-risk pulmonary embolism: pre-specified analysis of the Home Treatment of Pulmonary Embolism (HoT-PE) study. Clin Res Cardiol 2024; published online ahead of print. 10.1007/s00392-023-02355-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Farmakis IT, Valerio L, Barco S, Alsheimer E, Ewert R, Giannakoulas G et al. Cardiopulmonary exercise testing during follow-up after acute pulmonary embolism. Eur Respir J 2023;61:2300059. 10.1183/13993003.00059-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Barco S, Mavromanoli AC, Kreitner K-F, Bunck AC, Gertz RJ, Ley S et al. Preexisting chronic thromboembolic pulmonary hypertension in acute pulmonary embolism. Chest 2023;163:923–932. 10.1016/j.chest.2022.11.045 [DOI] [PubMed] [Google Scholar]
- 33. Farmakis IT, Valerio L, Barco S, Christodoulou KC, Ewert R, Giannakoulas G et al. Functional capacity and dyspnea during follow-up after acute pulmonary embolism. J Thromb Haemost 2024;22:163–171. 10.1016/j.jtha.2023.08.024 [DOI] [PubMed] [Google Scholar]
- 34. Jervan Ø, Haukeland-Parker S, Gleditsch J, Tavoly M, Klok FA, Steine K et al. The effects of exercise training in patients with persistent dyspnea following pulmonary embolism: a randomized controlled trial. Chest 2023;164:981–991. 10.1016/j.chest.2023.04.042 [DOI] [PubMed] [Google Scholar]
- 35. Boon GJAM, Janssen SMJ, Barco S, Bogaard HJ, Ghanima W, Kroft LJM et al. Efficacy and safety of a 12-week outpatient pulmonary rehabilitation program in post-PE syndrome. Thromb Res 2021;206:66–75. 10.1016/j.thromres.2021.08.012 [DOI] [PubMed] [Google Scholar]
- 36. Mohr K, Hobohm L, Kaier K, Farmakis IT, Valerio L, Barco S et al. Drivers and recent trends of hospitalisation costs related to acute pulmonary embolism. Clin Res Cardiol 2024; published online ahead of print. 10.1007/s00392-024-02437-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sidelnikov E, Dornstauder E, Jacob C, Maas C, Pinto L, Leidl R et al. Healthcare resource utilization and costs of cardiovascular events in patients with atherosclerotic cardiovascular disease in Germany—results of a claims database study. J Med Econ 2022;25:1199–1206. 10.1080/13696998.2022.2141964 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Proposals for data access will be considered by the FOCUS Steering Committee in accordance with the data access policy of the academic study sponsor (University Medical Centre Mainz, Germany).