Abstract
Alzheimer's disease (AD) is characterized by progressive neurodegeneration, marked by the accumulation of amyloid-β (Aβ) plaques and tau tangles. Emerging evidence suggests that mitochondrial dysfunction plays a pivotal role in AD pathogenesis, driven by impairments in mitochondrial quality control (MQC) mechanisms. MQC is crucial for maintaining mitochondrial integrity through processes such as proteostasis, mitochondrial dynamics, mitophagy, and precise communication with other subcellular organelles. In AD, disruptions in these processes lead to bioenergetic failure, gene dysregulation, the accumulation of damaged mitochondria, neuroinflammation, and lipid homeostasis impairment, further exacerbating neurodegeneration. This review elucidates the molecular pathways involved in MQC and their pathological relevance in AD, highlighting recent discoveries related to mitochondrial mechanisms underlying neurodegeneration. Furthermore, we explore potential therapeutic strategies targeting mitochondrial dysfunction, including gene therapy and pharmacological interventions, offering new avenues for slowing AD progression. The complex interplay between mitochondrial health and neurodegeneration underscores the need for innovative approaches to restore mitochondrial function and mitigate the onset and progression of AD.
Keywords: Alzheimer's disease, Mitochondrial quality control, Amyloid beta, Tauopathy, Gene therapy, Pharmacotherapy
Introduction
Alzheimer's disease (AD) is the most common form of neurodegeneration, affecting an estimated 6.9 million Americans aged 65 and older, with a projected increase to 13.8 million by 2060 [1]. The initial clinical symptoms of AD vary among individuals but often include a decline in non-memory cognitive functions such as word-finding, visuospatial issues, and impaired reasoning or judgment [2]. As the disease progresses to its later stages, symptoms include significant cognitive decline, difficulty communicating, and the need for full-time assistance with daily personal care, which places a heavy burden on caregivers and family members [2]. In 2024, the projected cost of AD and other neurodegenerative diseases in the United States is estimated to be $360 billion, potentially rising to nearly $1 trillion by 2050 [1]. The substantial human and economic toll underscores the urgent need for advancements in the understanding, prevention, and treatment of AD.
In recent decades, extensive efforts have been made to elucidate the mechanisms underlying AD pathogenesis. Beyond the well-known Amyloid and Tau hypotheses [3,4], increasing attention is directed toward the mitochondrial hypothesis. Mitochondrial integrity and function are compromised early in the disease, both in the context of amyloidogenesis and tauopathy, highlighting a potentially convergent pathway in AD development [5]. This early mitochondrial dysfunction is now recognized as a critical factor in the progression of AD, contributing to synaptic failure, neuroinflammation, lipid disturbance, oxidative stress, and neuronal loss [[5], [6], [7], [8]]. Therefore, understanding mitochondrial abnormalities in AD could provide crucial insights into the disease's pathogenesis, offering new therapeutic targets for the development of effective treatments.
Mitochondria are dynamic organelles essential for cellular energy production via oxidative phosphorylation (OXPHOS) and ATP generation, particularly crucial for the high energy demands of neuronal cells [9,10]. Normal mitochondrial function encompasses energy production, calcium homeostasis, metabolism, and redox regulation, all vital for cellular growth, proliferation, and survival [11,12]. During OXPHOS, mitochondria also produce and eliminate reactive oxygen species (ROS). Excessive ROS can impair protein function and trigger inflammatory responses, leading to neuronal death [13]. Therefore, under stress or disease conditions, mitochondrial quality control (MQC) mechanisms are necessary to maintain mitochondrial function. MQC includes the mitochondrial unfolded protein response (UPRmt), which rescues protein homeostasis [14]; mitochondrial dynamics and transportation, which maintain mitochondrial morphology [15,16]; and mitophagy pathways, which eliminate damaged mitochondria [17]. Collectively, these mechanisms ensure the preservation of mitochondrial integrity and functionality, thereby supporting overall cellular health and resilience in the face of physiological and pathological challenges.
In AD, these MQC mechanisms become compromised, leading to mitochondrial dysfunction. Impairments such as defective mitochondrial import [18], excessive activation of UPRmt [19,20], overproduction of ROS [21], disruptions in mitochondrial dynamics like fragmentation [22], and impaired mitophagy result in the accumulation of damaged mitochondria [23]. These abnormalities destabilize the mitochondrial genome, causing bioenergetic failure and the release of mitochondrial DNA (mtDNA), which further exacerbates AD progression [24,25]. Given the key role of mitochondria in neuronal health, these MQC impairments highlight the importance of targeting mitochondrial function in AD. Recent therapeutic strategies have focused on restoring MQC, showing promise in mitigating mitochondrial dysfunction and improving overall cellular health. This review summarizes the molecular basis of MQC, encompassing its mechanistic pathways, physiological functions, and pathological relevance in AD, and discuss potential MQC-targeted therapeutic strategies for AD.
Mitochondrial proteostasis and AD
The mitochondrial proteostasis system refers to the maintenance of protein homeostasis within the mitochondria. Mitochondria are unique in their separation of proteome and genome from the rest of the cell by inner and outer membranes. They contain 1100–1300 proteins, but only ∼37 of these proteins are encoded by the mtDNA [26,27]. This indicates that the majority of mitochondrial proteins are translated in the cytoplasm and imported into the mitochondria. Both mitochondrial and nuclear-encoded proteins work together to sustain mitochondrial functions, including energy homeostasis and metabolism [28,29]. The import of mitochondrial precursor proteins, surveillance, and regulation of mitochondrial proteins are crucial for maintaining mitochondrial protein homeostasis. Nuclear-encoded mitochondrial precursor proteins are synthesized by cytosolic ribosomes and have mitochondrial targeting sequences, with 70 % residing within the N-terminus [[30], [31], [32]]. The mitochondrial protein import system includes several complexes which include TOMM, MIM, SAM, MIA, TIMM23, TIMM22, and PAM [33]. TOMM is the most critical as it first contacts the majority of nuclear-encoded mitochondrial proteins, facilitating their entry into the intermembrane space [33]. The precursor proteins are recognized by TOMM20, transferred to TOMM22, and then translocated through the TOMM40 channel into the mitochondrial matrix (Fig. 1a). Once inside, the targeting sequence is proteolytically removed by mitochondrial processing protease (MPP) [33]. Chaperones such as mitochondrial heat shock protein 70 (mtHsp70) and mtHsp60 (chaperonin) then fold the proteins [34]. The mature proteins are recognized by specific machinery like the TIMM or MIA complexes, which escort them to their destinations. This intricate import system is essential for maintaining mitochondrial function and cellular energy homeostasis, underscoring the complexity and precision of mitochondrial protein import.
Fig. 1.
Mitochondrial protein import and the mitochondrial unfolded protein response. (a) Nuclear-encoded mitochondrial precursor proteins are imported into the mitochondria via the TOM complex. Precursor proteins with matrix-targeting presequences are further translocated by the TIM23 complex, where mitochondrial processing peptidase (MPP) removes the presequences, and chaperones like mtHsp70 fold the proteins into their mature forms (b) Mitochondrial proteostasis involves chaperones (e.g., mtHsp). and proteases (e.g., Lon peptidase 1, LONP1) to manage misfolded and damaged proteins. When overwhelmed, the mitochondrial unfolded protein response (UPRmt) is activated, triggering stress signals. (c) Kinases JNK and PKR activate transcription factor c-Jun, which binds to AP-1 elements and induces CHOP and C/EBPβ transcription. The CHOP–C/EBPβ complex binds to CHOP elements flanked by MURE1 and MURE2, promoting UPRmt gene expression. (d) The integrated stress response is activated through UPRmt. Kinases GCN2 and PERK phosphorylate eIF2α, leading to the nuclear translocation of eIF2α, which upregulates the translation of CHOP, ATF4, and ATF5, thereby promoting stress response genes. (e) In AD, amyloid-β (Aβ) peptides inhibit the import of nuclear-encoded mitochondrial precursor proteins via the TOM complex. f. Aβ is imported into the mitochondria via the TOM complex. Aβ accumulates in the OMM or be imported into the IMM via the TIM complex. Although Aβ can be degraded by mitochondrial presequence peptidase (PreP), it may inhibit PreP function. The accumulation of Aβ peptides aggregates triggers UPRmt activation.
The mitochondrial proteostasis system includes chaperones and proteases to manage misfolded and damaged proteins. Chaperones such as mtHsp10 [35], mtHsp40 (DNAJ) [36], mtHsp60 [37], mtHsp70 [38,39], mtHsp90 [40], mtHsp100 [41], and small heat shock proteins (sHsp) [42] recognize and refold misfolded proteins. Proteases such as Lon peptidase 1 (LONP1) [43], ClpP [44], OMA1 [45], and HtrA serine peptidase 2 (HTRA2) [46] located in the inner mitochondrial space (IMS), along with m-AAA [47], and i-AAA [48] in the IMM, recognize and degrade misfolded and damaged proteins. This refolding and degrading mechanism is essential for a healthy proteome. However, when overwhelmed, chaperones and proteases activate the UPRmt to alleviate proteotoxicity and re-establish proteostasis [49]. The UPRmt is a retrograde signal between the mitochondria and nucleus that induces the production of mitochondrial chaperones, proteases, and antioxidant enzymes [50]. It is triggered by accumulation of unfolded proteins, excessive ROS, depletion of mtDNA, and or disruptions in OXPHOS complexes [51] (Fig. 1b). In mammalian cells, UPRmt activation involves multiple pathways (Fig. 1c). Under mitochondrial stress, the kinases JNK and PKR phosphorylate and activate the transcription factor c-Jun, which binds to AP-1 elements, activating CHOP and C/EBP-β transcription [52]. The CHOP–C/EBP-β complex binds to CHOP elements flanked by mitochondrial unfolded protein response elements MURE1 and MURE2, promoting UPRmt response genes such as HSP60, ClpP, and mitochondrial import proteins. Additionally, CHOP, ATF4, and ATF5 activate UPRmt [52]. The integrated stress response (ISR) is activated, leading to eIF2α phosphorylation by GCN2 and PERK kinases, triggered by amino acid depletion, excessive ROS, and ER stress [52] (Fig. 1d). Phosphorylated eIF2α reduces protein synthesis and preferentially translates mRNAs encoding CHOP, ATF4, and ATF5, which induce mitochondrial proteases and chaperones, including HSP60, mtHSP70, and LONP1 [52]. These mitochondrial proteases and chaperones aid in alleviating accumulated misfolded and damaged proteins, helping restore balance to the mitochondrial proteostasis system. Dysfunction in IMS proteostasis can stimulate a protective response mediated by SIRT3 [53,54]. SIRT3 deacetylates and activates FOXO3a, which translocates to the nucleus, acting as a transcription factor to induce antioxidant enzymes such as SOD2, PGC-1α, and CAT [55,56]. In the mitochondria, SIRT3-activated SOD2 converts O2˙ into H2O2, which CAT breaks down into water and oxygen O2 [55]. This protective mechanism is crucial for mitigating oxidative damage and maintaining cellular redox balance, thereby ensuring mitochondrial and overall cellular health.
The mitochondrial proteome is altered in AD, affecting mitochondrial protein import, mitochondrial proteases, and the UPRmt. Aβ peptides from the cytosol coaggregate with mitochondrial precursor proteins [57]. These Aβ-coaggregates have their mitochondrial targeting sequence exposed, which is recognized by the TOM complex [58]. These Aβ-coaggregates can either clog or inhibit the TOM complex, leading to dysfunction in mitochondrial protein import, or enter the mitochondria [[18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58]] (Fig. 1e). Once inside, the Aβ-coaggregates are translocated to the IMM by the TIM23 complex [[18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59]].
In addition to Aβ-coaggregates clogging or inhibiting import proteins, Aβ-coaggregates can prevent essential mitochondrial proteins from reaching their destination resulting in mitochondrial dysfunction [18]. Additionally, Aβ peptides with positively charged amino acid residues at the N-terminal cation-binding domain can be recognized and imported into the mitochondria by the TOM complex, leading to the accumulation of Aβ within the IMS (Fig. 1f) [61]. Aβ is also recognized and imported into the IMM by the TIM23 complex, causing further accumulation within both the IMS and IMM [18]. Within the IMM, mitochondrial presequence peptidase (PreP) degrades Aβ peptides [62]. However, Aβ can inhibit PreP's function in degrading presequences and processing protein intermediates after translocation into the IMM [63]. This inhibition results in the accumulation of non-folded mitochondrial preproteins and processing intermediates, decreasing mitochondrial function [63]. Dysfunctional preprotein maturation leads to rapid protein degradation and an imbalanced organellar proteome. Consequently, these disruptions contribute significantly to the pathophysiology of AD.
The relationship between the UPRmt and AD has only recently been explored. The UPRmt is activated in both 3- and 9-month-old APP/PS1 mice brains and within SH-SY5Y cells exposed to Aβ treatment [19]. UPRmt-related genes are upregulated in AD post-mortem prefrontal cortex samples [64]. Additionally, a deficiency in the mitochondrial peptidase PITRM1 induces UPRmt and increases Aβ accumulation within cerebral organoids generated from human induced pluripotent stem cells (iPSCs) [65]. Pharmacological inhibition of UPRmt results in a drastic accumulation of Aβ [65]. Therefore, future studies should aim to clarify whether both inhibition and persistent activation of UPRmt contribute to AD.
Mitochondrial dynamics & transport and AD
Mitochondria are highly dynamic organelles involved in ATP production, cellular metabolism, stress responses, and the maintenance of homeostasis. Mitochondria continuously undergo fission and fusion processes. Mitochondrial dynamics regulate the length, size, and connectivity of mitochondria, contributing to maintain the mitochondrial morphology [66]. Maintain the regular morphology of cristae and mitochondria is crucial to support the cell daily activity. Mitochondrial contact site and cristae organizing system (MICOS) in the IMM is involves in the formation and maintenance of cristae structure [67]. MICOS complex contains Mic60 (Mitofilin), Mic10 (MINOS1), Mic19 (CHCHD3), Mic25 (CHCHD6), Mic23, Mic27, Mic13, and Mic14 (CHCHD10) which form MIB complex with SAM and DnaJC11 across the IMM and OMM [68]. During mitosis, mitochondrial fission generates two daughter mitochondria that inherit the same mtDNA and proteins, ensuring proper function after cell division. Selective removal of damaged or dysfunctional mitochondria during mitosis also supports normal cellular function [69]. During mouse embryogenesis and cell differentiation, mitochondrial morphology and localization change. For example, in neuronal development, mitochondria transition from a fragmented to an elongated morphology, indicating that fusion occurs to shift energy production from glycolysis to OXPHOS. As synapses form, mitochondria translocate to areas requiring more energy, such as presynaptic terminals [70,71]. Under cellular stress, such as bacterial or viral infection, upregulation of mitochondrial fission promotes apoptosis to eliminate damaged cells [72,73]. Mitochondrial dynamics also regulate metabolism under different nutrient conditions: starvation induces mitochondrial fusion to enhance ATP production via OXPHOS, while in nutrient-rich environments, cells undergo mitochondrial fission to reduce ATP production. These processes are tightly regulated by specific proteins to support cellular activity [74].
Mitochondrial fission involves the division of a mitochondrion into two, separating both the outer mitochondrial membrane (OMM) and inner mitochondrial membrane (IMM) without losing soluble proteins from the matrix. The GTPase dynamin-related protein 1 (DRP1) translocates to the OMM, where it interacts with other proteins, such as mitochondrial fission protein 1 (FIS1), mitochondrial dynamics proteins 49 and 51 kDa (MiD49 and MiD51), and mitochondrial fission factor (MFF), to mediate OMM separation [[75], [76], [77], [78]]. Phosphorylation of MFF triggers DRP1 recruitment and activates mitochondrial fission. DRP1's function is regulated by various post-translational modifications, including phosphorylation, which controls its GTPase activity. Several proteins regulate mitochondrial fission through the phosphorylation and dephosphorylation of DRP1 at specific sites, such as Ser-616 and Ser-637 [79]. For instance, mitochondrial serine/threonine-protein phosphatase PGAM5 dephosphorylates DRP1 at Ser-637 to induce GTPase activity and promote fission, while cyclin-dependent kinase (CDK) 1/Cyclin B or CDK5 phosphorylates DRP1 at Ser-616 during mitosis to activate fission [80,81]. PTEN-induced kinase 1 (PINK1) and mitogen-activated protein kinase (MAPK1) also phosphorylate DRP1 at Ser-616, promoting mitochondrial fission [82,83].
Mitochondrial fusion involves the merging of the OMM and IMM. The dynamin GTPases mitofusin 1 (MFN1) and mitofusin 2 (MFN2) form heterodimers or homodimers to mediate OMM fusion [71]. Following OMM fusion, IMM and matrix fusion occurs, a critical step during mitochondrial fusion since the matrix contains essential functional components such as mtDNA, metabolites, and proteins. Optic atrophy 1 (OPA1), a dynamin-related GTPase, interacts with cardiolipin to maintain cristae structure. OPA1 is regulated through post-transcriptional modifications and alternative splicing, producing long and short forms of the protein [[74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84]]. The long form promotes mitochondrial fusion and supports cellular homeostasis, while the short form leads to loss of fusion, bioenergetics, and cristae disruption. The balance between mitochondrial fusion and fission is crucial for maintaining cellular homeostasis and activity. An imbalance can cause mitochondrial dysfunction, leading to cell cycle arrest and apoptosis. Mitochondrial dynamics, including fission, fusion, and transport, are involved in processes like mitophagy and distribution, ensuring normal mitochondrial function in healthy cells (Fig. 2a). Accumulation of damaged mitochondria contributes to cell loss and senescence, playing a role in the development of neurodegenerative diseases.
Fig. 2.
Mitochondria dynamic and transport. (a) Mitochondrial Dynamics in Healthy Cells: Mitochondrial dynamics in healthy cells is characterized by a balance between fission and fusion, essential for maintaining cellular homeostasis. During mitochondrial fusion, MFN1 and MFN2, located on opposing OMMs, form homo- or hetero-oligomeric complexes, facilitating the tethering of two mitochondria. Fusion of the inner mitochondrial membrane is mediated by OPA1, resulting in the formation of an enlarged mitochondrion. Conversely, during mitochondrial fission, DRP1 is recruited to the OMM by fission receptors such as FIS1. DRP1 oligomerizes, wrapping around and constricting the mitochondrion, ultimately leading to its division into two daughter mitochondria. (b) In neurons, mitochondria are transported along microtubules from the soma to the synapse through anterograde transport. The outer mitochondrial membrane protein Miro-1 forms a complex with MTX1, MTX2, and KLC-1, facilitating kinesin-1-mediated anterograde transport. Retrograde transport, moving mitochondria back toward the soma, is mediated by a complex of Miro-1, MTX2, and TRAK-1, which interacts with dynein to enable this process. (c) In AD, mitochondrial dynamics are disrupted. The downregulation of MFN1, MFN2, and OPA1 reduces mitochondrial fusion, while the upregulation of DRP1 leads to excessive mitochondrial fission. (d) Mitochondrial transport dysfunction in AD. The hyperphosphorylation of tau leads to tau's disassociation from the microtubule leading to microtubule instability. Microtubule instability leads to damage and disintegration of axonal microtubules impair mitochondrial transport. Accumulation of mitochondria in the soma leads to perinuclear clustering, increasing ROS production. Reduced mitochondrial transport to the synapse contributes to synaptic dysfunction.
Neuronal cells are polarized with long axons and dendrites, making proper mitochondrial distribution essential. Mitochondrial transport along microtubules ensures redistribution to provide sufficient ATP for neurotransmission at synaptic terminals and along axons [85]. The complex responsible for mitochondrial transport includes motor and adaptor proteins (Fig. 2b). Kinesin-related protein (KIF5), dynein, and myosin are motor proteins that facilitate mitochondrial movement along microtubules [85]. The heavy chain of KIF5 has a head domain with ATPase activity that binds to microtubules and a tail domain that binds to cargo. KIF5 transports mitochondria to distal axons and synaptic terminals, while dynein moves them toward the soma [86]. Dynein consists of catalytic heavy chains, intermediate chains, light intermediate chains, and light chains. Like KIF5, dynein's heavy chains serve as motor domains that bind to microtubules [87]. Myosin, in contrast, is responsible for short-distance mitochondrial transport [88]. Dynein directly binds to both mitochondria and microtubules, whereas KIF5 requires adaptor proteins to attach to mitochondria [86]. Several mitochondrial outer membrane (MOM) proteins serve as adaptors. Mitochondrial Rho (Miro), a GTPase, binds to Milton, also known as trafficking of kinesin-binding protein (TRAK), forming a complex that attaches to KIF5's tail domain [89,90]. Miro contains calcium-binding sites regulated by calcium signaling and synaptic activity. Upregulation of Miro enhances complex formation. When mitochondria reach energy-deficient regions, calcium binds to Miro, causing KIF5 to dissociate from microtubules, and mitochondria stabilize in axons via anchoring protein syntaphilin [[89], [90], [91]]. Metaxin 2 (MTX2) bind to the Miro1 and function together with Metaxin 1 (MTX1) to contribute kinesin-mediated mitochondrial movement with KLC1. MTX2/Miro complex also bind to TRAK1 and support dynein-mediated mitochondrial trafficking [92]. Mitochondrial dynamics-related proteins have been shown to interact with Miro proteins, supporting mitochondrial transport. Mitochondrial dynamics are tightly regulated by proteins to maintain regular cellular activity under varying conditions. Thus, an imbalance in mitochondrial dynamics during disease development can lead to abnormal cellular functions.
In the progression of AD, the accumulation of Aβ and hyperphosphorylated Tau alters mitochondrial mass by influencing mitochondrial dynamics, transport and morphology. Imbalances in mitochondrial fission and fusion have been observed in various brain regions associated with AD [[21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87], [88], [89], [90], [91], [92], [93]]. In both AD mouse models and human patients, reduced levels of MFN1 and MFN2 and overexpression of DRP1 have been detected in the hippocampus and cerebral cortex. In the APPSWE/PS1 transgenic mice model, mitochondrial fission is upregulated while mitochondrial fusion is downregulated [93]. The 5xFAD mice model, which overexpresses APP695 with the Swedish (K670 N, M671L), Florida (I716V), and London (V717I) familial AD mutations, along with human PS with two familial AD mutations, also shows disturbed mitochondrial dynamics in an age- and gender-dependent manner. For instance, the mitochondrial fusion protein MFN2 decreases in 2- and 8-month-old female mice, while the mitochondrial fission protein MFF is phosphorylated in 6- and 8-month-old 5xFAD mice. FIS1 levels decrease in both male and female mice at 4 months old. DRP1, another key fission protein, decreases in female mice at 2 months old and in males at 4 months old [94]. Tau, a microtubule-associated protein, is a hallmark of neurological disorders known as tauopathies. During aging, elevated Tau levels in mitochondria downregulate mitochondrial fusion proteins like OPA1 [95]. In the Tau P301S transgenic mice model, which is commonly used to study tauopathies, mitochondria become fragmented, and mitochondrial length decreases in neuronal cells. Overexpression of MFN2 specifically in neuronal cells of P301S mice has been shown to attenuate mitochondrial fragmentation, neurodegeneration, synaptic loss, cognitive deficits, and neuronal inflammation [96].
In AD patients and mouse models, mitochondrial fission is significantly influenced, with DRP1 being the most studied protein. DRP1 translocates from the cytosol to the mitochondria, binds to FIS1, and forms a complex that splits the mitochondria [76]. Phosphorylation at Ser-616 and dephosphorylation at Ser-637 activate DRP1, leading to increased mitochondrial fission [79]. Overexpression of DRP1 has been observed in the brain tissue of AD patients [97]. In neuronal cell cultures, phosphorylated Tau and toxic Aβ phosphorylate DRP1 and facilitate its translocation to the mitochondria, disrupting mitochondrial dynamics [98]. Excessive mitochondrial fission in neuronal cells can lead to mitochondrial fragmentation, ultimately inducing cell death and neurodegeneration (Fig. 2c). Impairment of morphology can not only be induced by imbalance of mitochondrial fission and fusion, MICOS impairment also place a role in it. In APP knock in mice, deficiency of CHCHD6 caused MICOS loss, promote APP processing, mitochondrial damage, neuronal cholesterol accumulation, and cognitive deficits [99]. In addition to the imbalance between mitochondrial fission and fusion, dysfunction in mitochondrial transport also affects cellular activity. During AD development, the aggregation of toxic Aβ and hyperphosphorylated Tau promotes disease-like cytoskeletal abnormalities, disrupting the axonal transport of organelles, including mitochondria (Fig. 2d) [85]. Peri-nuclear mitochondrial clustering increased ROS level in the soma which damage the neuronal cell [100]. Also, lack of mitochondria at synaptic terminals and axons leads to synaptic degeneration, contributing to neuronal disorders [101]. Although the exact mechanisms behind mitochondrial transport disorders remain unknown, evidence from both in vitro and in vivo models suggests that stabilizing axonal transport can ameliorate cognitive deficits. In APP/PS1 double transgenic mice, stabilizing axonal transport with drugs can mitigate cognitive deficits [102,103]. In the 5xFAD mice model, decreased tubulin acetylation destabilizes the cytoskeleton, making it more susceptible to damage [104]. Tau is a protein with six isoforms and several phosphorylated species that normally associate with microtubules in mature neurons to maintain cytoskeletal stability. Abnormal Tau leads to the formation of neurofibrillary tangles, disrupting cytoskeletal stability. In various cell and animal models, reduced mitochondrial motility accompanies the aggregation of neurofibrillary tangles [[105], [106], [107], [108], [109], [110]]. Abnormal mitochondrial dynamics can lead not only to cellular loss but also to cellular senescence. In mouse models, downregulation of PGAM5 increases DRP1 phosphorylation at Ser-637, inducing mitochondrial fusion and cellular senescence. Overexpression of mitochondrial fusion prevents the removal of damaged mitochondria within the cell, and the accumulation of damaged mitochondria contributes to cellular senescence in the context of AD [111].
Mitophagy and AD
Mitophagy is the selective degradation of dysfunctional mitochondria via lysosomes, crucial for maintaining mitochondrial health, regulating numbers, and preventing ROS and cellular stress [112,113]. It balances functional mitochondria with autophagic degradation, ensuring homeostasis. During this process, damaged mitochondria are enclosed by a phagophore, forming a mitophagosome, which fuses with a lysosome for enzymatic breakdown into recyclable components [112]. Neurons rely heavily on mitochondria, making mitophagy vital for cellular health. As lysosomes are mainly in the soma, damaged mitochondria must be transported back for degradation via specialized mechanisms [114].
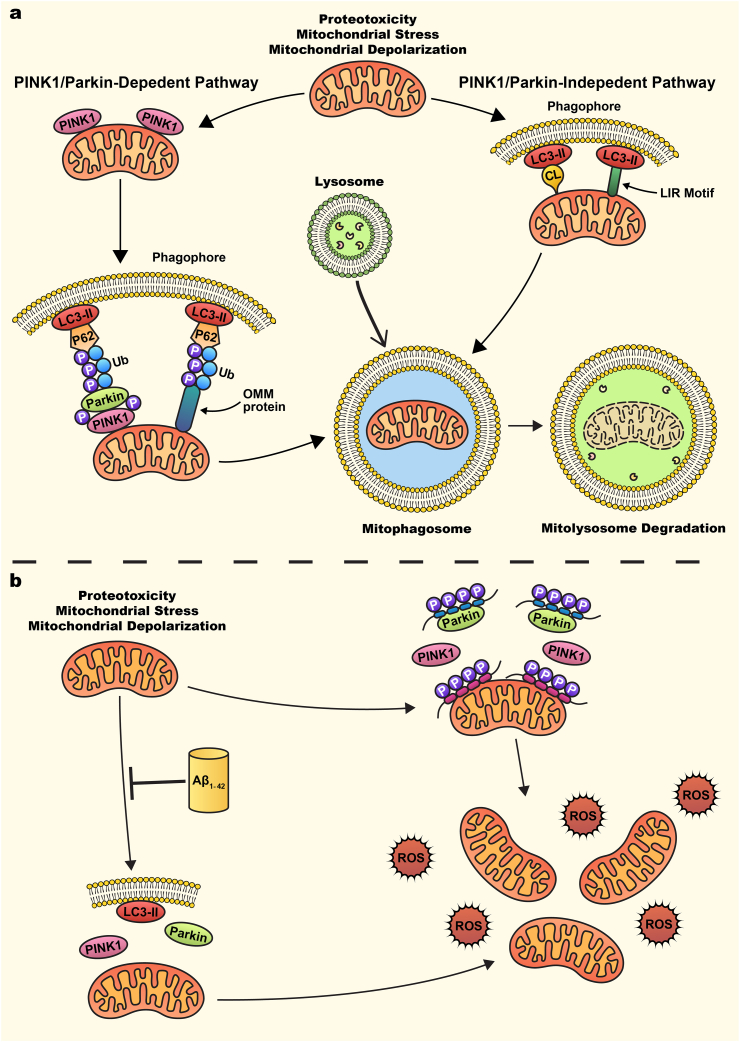
Fig. 3a shows both the PINK1/Parkin dependent and independent pathways. The PINK1/Parkin pathway is a prominent mechanism in mitophagy regulation. PINK1 and Parkin function as the first steps of a signaling pathway that activates mitochondrial quality control pathways in response to mitochondrial damage [115,116]. Under basal conditions, PINK1 is recruited and imported into mitochondria through the TOM-TIM complex, then cleaved by IMM-bound proteases such as matrix processing peptidase (MPP) and prescenilins-associated rhomboid-like protein (PARL) and subsequently degraded by the proteasome, leading to undetectable basal levels of PINK1 [[117], [118], [119], [120], [121]]. However, mitochondrial stressors such as membrane depolarization, mitochondrial complex dysfunction, and proteotoxicity, leading to PINK1 accumulation on the OMM [[112], [113], [114], [115], [116], [117], [118], [119], [120], [121], [122]]. The accumulation of OMM PINK1 leads to subsequent homodimerization and then autophosphorylation thus promoting kinase activation and facilitates the recruitment and binding of Parkin [[17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87], [88], [89], [90], [91], [92], [93], [94], [95], [96], [97], [98], [99], [100], [101], [102], [103], [104], [105], [106], [107], [108], [109], [110], [111], [112], [113], [114], [115], [116], [117], [118]]. Parkin is an E3 ubiquitin ligase that has minimal basal activity under normal conditions due to intramolecular interactions that blocks the active site and compete with E2 ligase binding [123]. Once mitochondrial damage occurs and PINK1 accumulation on the OMM occurs, PINK1 activates Parkin through two mechanisms. The first mechanism occurs through PINK1 phosphorylation of ubiquitin at S65, which competes with an autoinhibitory domain within Parkin and stabilizes it in an active conformation [115]. The second mechanism occurs by PINK1 direct phosphorylation of Parkin on S65 in Parkin's ubiquitin-like domain, which induces conformational changes allowing for the binding of the charged E2 ligase [115]. These two mechanisms increase Parkin's E3 ubiquitin ligase activity. Within the first 2 hours of Parkin recruitment and activation, Parkin ubiquitinates many OMM and mitochondrial matrix proteins then targets IMM proteins [115,[124], [125], [126]]. These include cytosolic proteins that can act as mitophagy receptors including p62, OPTEN, NDP52, NBR1, and TAX1BP1 that will recognize and bind to p-S65-Ub proteins [[127], [128], [129]]. The formation of the ubiquitin chains on the mitochondria then leads to the recruitment and binding of autophagy receptors. In PINK1/Parkin-independent mitophagy, phagosomes are recruited directly around mitochondria either via OMM receptors containing LIR motifs (such as NIX/BNIP3L, BNIP3, FUNDC1, BCL2L13, FKBP8, DISC1, AMBRA1, or MCL1) or through recognition of exposed cardiolipin (CL) on the OMM [[130], [131], [132], [133], [134], [135], [136], [137]]. These autophagy proteins lead to the assembly of microtubule-associated protein 1A/1Blight chain 3 (LC3)-positive phagophores. Phagophores sequester damaged mitochondria and deliver them to the lysosomes for degradation via acidic hydrolases [138,139]. This orchestrated process ensures mitochondrial quality control, vital for cellular function and neuronal health.
Fig. 3.
PINK1/Parkin dependent and independent mitophagy (a) Mitochondrial stress, such as mitochondrial membrane depolarization, triggers mitophagy, a degradation process mediated by either PINK1/Parkin-dependent or PINK1/Parkin-independent pathways. In the PINK1/Parkin-dependent pathway, mitochondrial stress leads to the accumulation and stabilization of PTEN-induced putative kinase 1 (PINK1) on the OMM. Stabilized PINK1 recruits the E3 ubiquitin ligase Parkin, which is phosphorylated by PINK1 at the ubiquitin-like domain (S65). This phosphorylation enhances Parkin's E3 ligase activity, leading to the ubiquitination of OMM proteins. Cytosolic mitophagy receptors, such as p62, recognize these polyubiquitinated proteins via their LC3-interacting region (LIR) motifs, facilitating the recruitment of autophagosomes through interaction with LC3-II on the autophagosome membrane. In the PINK1/Parkin-independent pathway, autophagosomes are recruited either through the exposure of cardiolipin (CL) on the OMM or via LIR motif-containing OMM receptors, such as FUNDC1, NIX/BNIP3L, and BNIP3. Following autophagosome engulfment of the damaged mitochondria, mitophagosomes fuse with lysosomes to form mitolysosomes, where damaged mitochondria are degraded by lysosomal acidic hydrolases. (b) Mitophagy impairments in AD. Aβ peptides inhibit mitophagy, characterized by upregulation of PINK1, Parkin, and LC3-II, along with the accumulation of damaged mitochondria. Human phosphorylated tau binds to the OMM, preventing PINK1 stabilization and subsequent recruitment of Parkin, thereby inhibiting mitophagy and leading to the buildup of damaged mitochondria. Additionally, expression of the tau P301L mutation leads to its interaction with Parkin, blocking its translocation to mitochondria. This impaired mitophagy results in the accumulation of damaged mitochondria, increased ROS production, and neuronal death.
Impaired mitophagy in AD has been linked to increased production of ROS, accumulation of mtDNA, proteins, and autophagic vacuoles with oxidative damage, and the presence of damaged mitochondria [23]. Fig. 3b shows both the PINK1/Parkin dependent and independent pathways in AD. In the hippocampus of early-stage AD patients, elevated PINK1 levels have been observed, while late-stage AD is marked by increased Parkin levels, suggesting a reduction in mitophagy flux due to a defect in the initiation of the PINK1/Parkin-dependent pathway [[23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87], [88], [89], [90], [91], [92], [93], [94], [95], [96], [97], [98], [99], [100], [101], [102], [103], [104], [105], [106], [107], [108], [109], [110], [111], [112], [113], [114], [115], [116], [117], [118], [119], [120], [121], [122], [123], [124], [125], [126], [127], [128], [129], [130], [131], [132], [133], [134], [135], [136], [137], [138], [139], [140]]. Additionally, PINK1 mRNA levels are reduced in the hippocampus of late-stage AD patients [141]. Basal mitophagy levels in the hippocampus of postmortem AD patients are reduced by 30–50 %, with these patients displaying an accumulation of damaged mitochondria characterized by reduced size, disorganized cristae, and diminished ATP production [142]. There are also defects in the initiation of mitophagy, such as reduced recruitment of activated LC3 to mitochondria, dysfunction in the AMPK signaling cascade, and impaired fusion of mitophagosomes with lysosomes [142]. Several mitophagy-related proteins, including AMBRA1, ATG5, ATG12, BCL-1, BNIP3, BNIP3L, FUNDC1, OPTEN, PI3K class III, ULK1, and VCP/P97, are downregulated in the brains of AD patients [143]. Mitochondrial fractions from late-stage AD brain samples reveal increased p62 levels, an elevated LC3II/I ratio, and decreased PINK1 and Parkin levels, indicating a failure in mitophagy recruitment [144]. In AD patient iPSC-derived neuronal cells, reduced expression levels of LC3 and TFEB lead to a decrease in mitophagy-related proteins [145].
The effects of Aβ peptide on mitophagy depend on the duration of exposure. In rat pheochromocytoma (PC12) cells, a 12 hour treatment with Aβ peptide impairs mitophagy, as indicated by reductions in PINK1, Parkin, BCL-1, and LC3II/I ratios, along with the accumulation of p62 [146]. Conversely, a 24 hour exposure to Aβ peptide in PC12 cells results in increased levels of Parkin, BCL-1, and the LC3II/I ratio [147]. Similarly, a 72 hour treatment with Aβ peptide in murine embryonic hippocampal neurons upregulates the expression of PINK1, Parkin, LC3II, and BCL-1, alongside a decrease in mitochondrial membrane potential, reduced ATP production, and increased mitochondrial ROS production [148]. Additionally, treatment of HEK293T cells with oligomeric Aβ initially induces mitophagy, but this is followed by a blockade of the process, characterized by increased levels of Parkin, an elevated LC3II/I ratio, and the accumulation of p62 within the mitochondrial fraction, along with the buildup of autophagosomes and mitochondria [149]. Rats subjected to intracerebroventricular injections of Aβ peptide exhibit reduced levels of PINK1, Parkin, and BCL-1, along with the accumulation of p62 in the hippocampus [150].
Tau has also been shown to interfere with mitophagy. Overexpression of human tau (hTau) induces mitophagy deficits in the hippocampus of hTau transgenic mice, primary hippocampal neurons, and HEK293T cells [151,152]. These deficits are characterized by the accumulation of total and phosphorylated tau on the OMM and an increase in mitochondrial membrane potential, which hinders PINK1 stabilization on the OMM, thereby preventing the recruitment of Parkin. However, overexpression of Parkin can rescue the mitophagy deficits induced by hTau [151]. The tau P301L mutant also inhibits mitophagy in C. Elegans and neuroblastoma cell models by interacting with Parkin's projection domain, preventing its translocation to mitochondria [153]. Contrarily, NH2-Tau fragments in mature hippocampal primary neurons trigger alterations in mitochondrial structure and neuronal synapses while enhancing mitophagy flux by recruiting Parkin to the mitochondria [154]. In both in vitro (overexpression of 2N4R, 1N4R, and 2N3R tau in SH-SY5Y cells) and in vivo (transgenic nematodes expressing human tau, 3xTg-AD mice) models, stimulation of mitophagy reduces tau hyperphosphorylation [[142], [143], [144], [145], [146], [147], [148], [149], [150], [151], [152]]. Collectively, these findings suggest that impaired mitophagy contributes to tau pathogenesis and underscore the distinct pathological features between different models of tau phosphorylation.
Genome instability and AD
Mitochondrial genome stability is crucial for maintaining regular mitochondrial function in healthy cells. Replication of mtDNA is required to maintain stable copy numbers supporting cellular energy demands while transcription of mtDNA into mitochondrial RNA (mtRNA), is required to translate OXPHOS proteins essential for electron transport chain (ETC) and overall mitochondrial bioenergetics (Fig. 4a) [155]. When this stability is compromised, mitochondrial dysfunction can occur, particularly in the context of AD (Fig. 4b). Unlike nuclear DNA, mtDNA lacks extensive non-coding regions and histones, making it more susceptible to mutations [156]. Additionally, mtDNA is located near sites of ROS production, increasing the likelihood of oxidative damage and mutations. Elevated oxidative stress and Aβ accumulation in AD exacerbate mtDNA mutations and damage, impairing the BER mechanism and other repair pathways. This impairment leads to the accumulation of mtDNA mutations, disrupting OXPHOS and resulting in energy failure [157]. Mutation in the mtDNA transcription and translation related protein has also been detected in AD. TFAM is the key protein regulate mtDNA transcription and translation. TFAM will transcript from nucleus, translate in the cytosol, and translocate to the mitochondria. Mutation in TFAM could cause decrease in mtDNA copy number [158]. Mitochondrial transcription factor 4 (MTERF4) is another protein has been altered in APP/PS1 mice [159]. In AD patients, increased oxidative mtDNA mutations and decreased mtDNA copy numbers are observed, contributing to metabolic comorbidities.
Fig. 4.
Mitochondrial genome stability. (a) Mitochondrial DNA (mtDNA) replicates to maintain stable copy numbers, supporting cellular energy demands. mtDNA is transcribed into mitochondrial RNA (mtRNA), which is translated into OXPHOS proteins essential for the electron transport chain (ETC) and overall mitochondrial bioenergetics. (b) In AD, mtDNA copy numbers decrease, and mtDNA becomes more susceptible to oxidative damage. This leads to impaired OXPHOS protein production, resulting in mitochondrial bioenergetic deficits. (c) Increased release of mtDNA through VDAC1 and mPTP into the cytoplasm activates the cGAS-STING pathway. cGAS detects mtDNA, catalyzing the formation of cGAMP, which activates STING. This triggers TANK-binding kinase 1 (TBK1) to phosphorylate interferon regulatory factor 3 (IRF3), promoting the transcription of NF-κB, proinflammatory cytokines, and interferon response proteins.
The release of mtDNA serves as a messenger that facilitates communication between neurons and innate immune cells, such as microglia, during AD progression [160]. In the APP/PS1 mouse model, mtDNA copy numbers decrease, while mtDNA methylation increases [161,162]. The oxidative stress in APP/PS1 mice leads to mtDNA mutations, disrupting transcription and replication [157]. Unlike nuclear DNA, mtDNA lacks modifications, leading cells to recognize it as a foreign molecular, similar to viral or bacterial DNA, which activates the cGAS-STING pathway (Fig. 4c) [163,164]. In AD patients and various mouse models, the accumulation of Aβ plaques and hyperphosphorylated Tau triggers mitochondrial dysfunction, resulting in the release of significant amounts of mtDNA [161,162]. This release activates the cGAS-STING pathway, causing neuronal inflammation primarily within microglia, leading to microgliosis and neuronal loss. Overexpression of the cGAS-STING pathway promotes cell death and further DNA release into the extracellular space. These foreign DNA fragments induce cellular stress in healthy cells, perpetuating the activation of the cGAS-STING pathway [168,169]. Beyond inducing cell death, mtDNA release also contributes to cellular senescence. Senescent cells release a senescence-associated secretory phenotype (SASP), which can convert healthy cells into senescent cells. The accumulation of senescent cells in the brain exacerbates neurodegeneration in AD [170]. Additionally, mtDNA damage directly leads to mitochondrial bioenergetic deficiency, with glucose hypometabolism consistently recognized as an early indicator of disease onset. Since bioenergetic failure in AD has been extensively reviewed elsewhere [[171], [172], [173], [174]], it will not be the focus of this review.
These studies demonstrate that MQC is disrupted in AD in both in vitro and in vivo models. Key processes, including mitochondrial protein import and the UPRmt are impaired. Aβ peptides disrupt precursor protein import, accumulate in mitochondrial membranes, and overactivate UPRmt. Altered mitochondrial dynamics, characterized by decreased fusion proteins (OPA1, MFN1, MFN2) and increased fission protein (DRP1), lead to mitochondrial fragmentation. Axonal damage disrupts mitochondrial transport, causing perinuclear clustering and increased ROS in the soma. Reduced mitophagy flux hampers the removal of damaged mitochondria, while mitochondrial genome instability, marked by reduced mtDNA copy numbers, diminishes ETC proteins and activates the pro-inflammatory cGAS-STING pathway. These findings highlight the interconnected nature of mitochondrial dysfunction and its critical importance of therapeutic potential in AD.
Therapeutics Development Enhancing Mitochondrial Function
Gene therapy targeting mitochondrial pathways
Adeno-associated virus (AAV)-based therapies have revolutionized the field of gene therapy, offering a versatile platform for the targeted delivery of therapeutic genes to treat a wide range of genetic and acquired diseases. The ability of AAV vectors to achieve long-term expression with minimal immunogenicity has made them a cornerstone for addressing disorders. Furthermore, their tissue-specific tropism and capacity for modification enhance their therapeutic precision. Despite challenges such as scaling up production, immune responses, and limited payload capacity, ongoing advancements in AAV vector engineering continue to expand their clinical utility, highlighting their transformative impact on modern medicine [183]. In the context of AD, this technique can potentially address mitochondrial dysfunction by enhancing mtDNA stability, bioenergetics, dynamics, and mitophagy. By stabilizing mitochondrial function, reducing oxidative stress, and improving neuronal health, AAV gene therapy holds potential for mitigating neurodegeneration in AD.
Table 1 displays multiple mitochondrial targeted AAV gene therapies within AD models. NDI1, an NADH dehydrogenase located in yeast mitochondria, is integral to the electron transport chain and apoptosis, functioning analogously to mammalian complex I. The introduction of NDH1 can effectively compensate for deficiencies in mammalian mitochondrial complex I [184]. Stereotaxic injection of AAV9-NDI1 into the hippocampus of an AD mouse model (created by Aβ1-42 injection) improved mitochondrial function, reduced histopathological abnormalities, and alleviated neurological deficits without causing adverse effects in healthy mice [175]. DJ-1 is a multifunctional protein involved in numerous cellular processes, including the regulation of apoptosis and pro-survival signaling, PINK1/parkin-mediated mitophagy, inflammatory responses, protection against oxidative stress through NRF2 activation, mitochondrial dynamics, mitochondrial-ER communication, and chaperone activity [185]. Delivery of AAV9-DJ1 to the hippocampus of 8-month-old APP/PS1 mice improved cognitive function and increased levels of NRF2, LC3, phosphorylated AMPK, and BCL-1 [176]. DJ-1 also enhanced antioxidant defenses, including total superoxide dismutase activity, total antioxidant capacity, and glutathione peroxidase activity [176]. Thioredoxin-1 (Trx-1) is a critical regulator of cellular functions, including redox homeostasis, cell proliferation, DNA synthesis, transcription factor modulation, and apoptosis control [186]. Acting as an antioxidant, Trx-1 directly scavenges ROS. Overexpression of Trx-1 via AAV-Trx-1 delivery to the hippocampus of APP/PS1 mice enhanced learning and memory, reduced hippocampal Aβ deposition, and restored mitochondrial biogenesis by upregulating key proteins such as AMPK, Sirt1, and PGC-1α [177]. PGC-1α is a transcriptional coactivator that plays a pivotal role in cellular energy metabolism, regulating metabolic gene expression, oxidative phosphorylation, and mitochondrial biogenesis [187]. In APP23 transgenic mice, overexpression of PGC-1α via a lentiviral vector delivered to the hippocampus and cortex during the preclinical stage of AD [178]. Four months post-injection treated mice exhibited improved spatial and recognition memory, reduced Aβ deposition, and decreased BACE1 expression. PGC-1α overexpression also attenuated neuroinflammation, lowered proinflammatory cytokine levels, and suppressed microglial activation, preserving pyramidal neurons in the CA3 region and enhancing neurotrophic factor expression [178]. In APP/PS1 mice, AAV-mediated PGC-1α overexpression alleviated deficits in spatial memory, working memory, and sensorimotor gating. Additionally, it restored mitochondrial homeostasis by balancing fission and fusion dynamics, increasing mitochondrial fusion proteins (OPA1, MFN2) while reducing fission proteins (DRP1, FIS1) [179].
Table 1.
Mitochondria AAV based therapeutics in Alzheimer's disease.
| AAV-Protein | Protein function | Model | Effects | Reference |
|---|---|---|---|---|
| AAV9-NDI1 | Yeast mitochondrial NADH dehydrogenase; electron transport chain; apoptosis | SH-SY5Y Aβ1-42 cell model, Aβ1-42 hippocampal injected mice. |
Restored oxidative phosphorylation; reduces ROS production | Li et al., 2024 |
| AAV9-DJ-1 | Regulation of apoptosis; PINK1/parkin-mediated mitophagy; Antioxidant response through NRF2 activation |
APP/PS1 | Enhanced cognitive function; increased levels of antioxidant genes (NRF2, SOD, GPx) | Peng et al., 2023 |
| AAV-Trx-1 | Maintains redox homeostasis; Antioxidant ROS scavenger |
APP/PS1 | Improved learning and memory; Reduced hippocampal Aβ deposition; increased expression of APRK, Sirt1, PGC-1α |
Jia et al., 2023 |
| AAV-PGC-1α | Regulator of oxidative phosphorylation; Regulator of mitochondrial biogenesis |
APP/PS1 | Improved learning and memory; Increase mitochondrial fusion protein (OPA1, MFN2); reduced mitochondrial fission protein (DRP1, FIS1) |
Wang et al., 2022 |
| AAV2-CEND1 | Neuronal lineage-specific modulator; Regulates mitochondrial dynamics via DRP1 |
5xFAD | Improved learning and memory; Increase mitochondrial fusion protein (OPA1, MFN2); reduced mitochondrial fission protein (DRP1) |
Xie et al., 2022 |
| AAV5-CHCHD6 | MICO complex component; Key component for mitochondrial crista formation |
APPNL−F-G KI AD | Reduced APP accumulation in the MAM; lowered C99 fragment; improved spatial working memory; restored synaptic function | Shang et al., 2022 |
| AAV9-MICU3 | Regulates mitochondrial calcium uptake | Tg-SwDI AD | Improved behavioral performance; enhance cerebral blood flow; reduce Aβ deposition | Zhou et al., 2023 |
| AAV2-PINK1 | Mitochondrial protein kinase; Mitophagy |
APPSwInd (J20) AD | Alleviated mitochondrial dysfunction and oxidative stress; increased ATP; Increased mitophagy; reduced Aβ accumulation |
Du et al., 2017 |
| AAV-MCL1 | OMM LIR motif receptor; Mitophagy; Regulates apoptosis |
APP/PS1 | Improved learning and memory; reduced hippocampal Aβ deposition; | Cen et al., 2020 |
| DISC1 | OMM LIR motif receptor; Mitophagy; Mitochondrial dynamics; |
APP/PS1 | Reduced expression of BACE1; reduced hippocampal Aβ deposition; improved learning and memory | Deng et al., 2016 |
CEND1 is a neuronal lineage-specific modulator that synchronizes cell cycle exit and differentiation of neuronal progenitors during nervous system development [188]. It is also localized in presynaptic mitochondria, where it regulates mitochondrial dynamics via DRP1 modulation. CEND1 depletion increases mitochondrial fission through DRP1 upregulation [188]. In 5xFAD mice, AAV-mediated overexpression of CEND1 in the hippocampus restored mitochondrial function, alleviated synaptic deficits, and improved learning and memory [180]. This overexpression promoted mitochondrial fusion by upregulating MFN2 and OPA1 levels while downregulating DRP1 expression [180]. Similarly, CHCHD6, a core component of the MICO complex, is critical for mitochondrial crista formation. AAV5-eGFP-CHCHD6 delivery to the hippocampus of APPNL-F-G knock-in Alzheimer's disease mouse models reduced APP accumulation in the MAM and lowered C99 fragment levels, suggesting reduced APP processing [99]. CHCHD6 overexpression also improved spatial working memory and restored hippocampal levels of synaptophysin and PSD95, proteins essential for synaptic function [99].
Mitochondrial calcium uptake 3 (MICU3) regulates mitochondrial calcium uptake by forming heterodimers with MICU1, enhancing MCU-dependent mitochondrial Ca2⁺ uptake [189]. The MICU1-MICU3 heterodimer promotes synaptic flexibility in neuronal cells by increasing mitochondrial calcium uptake at presynapses [189]. In Tg-SwDI transgenic AD mouse models, AAV9-mediated overexpression of MICU3 improved behavioral performance, enhanced cerebral blood flow, and significantly reduced Aβ deposition by modulating Aβ metabolism [181]. MICU3 overexpression also mitigated oxidative stress, mitochondrial dysfunction, ATP depletion, and mtDNA reduction in these models [181]. Targeting mitophagy-related proteins, such as PINK1, MCL1, and DISC1, has demonstrated improvements in cognitive and pathological features of AD. Overexpression of the mitochondrial protein kinase PINK1 via AAV2 delivery to the hippocampus of APPSwInd (J20) mice reduced cerebral and mitochondrial Aβ accumulation [141]. PINK1 overexpression alleviated mitochondrial dysfunction and oxidative stress, evidenced by increased ATP levels and decreased mitochondrial ROS. It also activated autophagy signaling by enhancing the recruitment of autophagy receptors NDP52 and OPTN to damaged mitochondria and increasing levels of the autophagy marker LC3-II [141]. PINK1-mediated autophagy reduced Aβ accumulation, improved synaptic function, and enhanced learning and memory in APPSwInd mice [141]. Similarly, AAV-mediated overexpression of OMM LIR motif receptors MCL1 or DISC1 in APP/PS1 mice produced comparable effects, including improved cognitive function and reduced extracellular Aβ plaque burden [[136], [137], [138], [139], [140], [141], [142], [143], [144], [145], [146], [147], [148], [149], [150], [151], [152], [153], [154], [155], [156], [157], [158], [159], [160], [161], [162], [163], [164], [165], [166], [167], [168], [169], [170], [171], [172], [173], [174], [175], [176], [177], [178], [179], [180], [181], [182]]. Collectively, these findings underscore the potential of gene therapy targeting mitochondrial pathways in AD treatment.
Mitochondria-targeted pharmacological strategy
Pharmacological strategies targeting mitochondrial function offer promising therapeutic avenues for AD. Various reagents have been reported to influence mitochondrial activities, particularly oxidative stress and energy metabolism, as summarized in Refs. [[190], [191], [192]]. However, many of these reagents may only indirectly affect mitochondrial function. This review focuses on pharmacological agents that selectively target mitochondrial proteins to develop AD therapies shown in Table 2.
Table 2.
Mitochondrial focused therapeutic agents in Alzheimer's disease.
| Agent | Molecular action | Model | Effects | Reference |
|---|---|---|---|---|
| MitoQ | Antioxidant | 3xTg AD | Reduces ROS levels; reduces Aβ levels; Improves synaptic function |
McManus et al., 2011; Young et al., 2019 |
| Szeto-Schiller tetrapeptides 31 (SS-31) | Binds to cardiolipin | APP/PS1 | Rescues mitochondrial morphology; reduces ROS levels; Reduces Aβ levels |
Mitchell et al., 2020; Jia et al., 2023 |
| Nicotinamide riboside (NR) | NAD+ precursor | APP/PS1 | Suppresses cGAS-STING pathway; reduces Aβ levels; Enhances mitophagy |
Hou et al., 2021 |
| DA1 | Binds to ATAD3A and suppresses its oligomerization | 5xFAD | Improves mitochondrial dynamics; reduces Aβ levels | Zhoa et al., 2022 |
| Mdivi-1 | Inhibits DRP1 | APP/PS1 | Improves mitochondrial dynamics; reduces Aβ levels; reduces ROS levels | Baek et al., 2017 |
| P110 | Blocks the interaction between DRP1 and FIS1 | 5xFAD | Improves mitochondrial dynamics; reduces Aβ levels; reduces ROS levels | Joshi et al., 2018 |
| UMI-77 | Mitophagy activator; BH3-mimetic for MCL-1 | APP/PS1 | Enhances mitophagy; reduces Aβ levels; reduces neuroinflammation | Chen et al., 2020 |
| Loganin | Promotes OPTN-mediated mitophagy | 3xTg AD | Enhances mitophagy; Improves synaptic plasticity; improves mitochondrial dynamics |
Zhou et al., 2023 |
Mitochondria-targeted antioxidant therapies represent a promising approach to restoring mitochondrial function in AD. Compounds such as MitoQ (ubiquinone) [193], MitoVitE (mitochondrially-targeted vitamin E) [194,195], and SS-31 (a tetrapeptide) [196,197] selectively accumulate within mitochondria, providing greater protection against oxidative damage compared to non-targeted antioxidants. MitoQ, for example, is a modified ubiquinone conjugated to triphenylphosphonium (TPP), allowing it to easily penetrate mitochondrial membranes and accumulate within the IMM. Once localized, MitoQ protects neurons from oxidative damage by converting H2O2 into H2O and O2, reducing ROS levels, and mimicking the activity of endogenous CoQ [198]. In AD models, MitoQ treatment has been shown to improve memory retention, reduce Aβ accumulation, decrease Tau hyperphosphorylation, and enhance neurite growth [199]. Long-term treatment with the antioxidant peptide SS-31 in APP/PS1 mice reduced mitochondrial ROS, improved synaptic integrity, decreased expression of mitochondrial fission proteins DRP1 and FIS1, and lowered Aβ levels [200]. These findings underscore the potential of mitochondria-targeted antioxidants in mitigating AD pathology and improving cognitive function. Nicotinamide adenine dinucleotide (NAD+), a vital metabolite in human cells, plays a key role in processes such as DNA repair and mitophagy. Hou et al. [201] demonstrated that treatment of APP/PS1 mutant mice with the NAD + precursor nicotinamide riboside (NR) for 5 months increased brain NAD + levels, reduced proinflammatory cytokine expression, and decreased microglia and astrocyte activation. Elevated cGAS-STING signaling observed in AD mice was normalized by NR treatment, which also induced mitophagy and improved cognitive and synaptic functions in the APP/PS1 mutant mice [201].
ATAD3A is a nuclear-encoded mitochondrial membrane protein that plays a critical role in maintaining MAMs, regulating mitochondrial dynamics, and ensuring mitochondrial genome stability. In various neurodegenerative diseases, including AD, oligomerization and accumulation of ATAD3A have been observed in hippocampal neurons in both patients and mouse models [202,203]. In 5xFAD mouse models and cell cultures, ATAD3A oligomers are enriched in the MAM fraction, leading to impaired MAM integrity and resulting in synaptic loss [203]. Genetic downregulation of ATAD3A in AD mouse models has been shown to restore cognitive function, reduce neuronal inflammation, and attenuate AD pathology [203]. Furthermore, inhibition of ATAD3A oligomerization using the inhibitor DA1 was found to suppress neuronal inflammation, reduce AD pathology, and protect against neurodegeneration. In these models, ATAD3A oligomerization also inhibits CYP46A1-mediated cholesterol metabolism, which promotes the processing of APP, contributing to AD pathology [203]. Thus, targeting ATAD3A represents a potential therapeutic approach to mitigate neurodegeneration and AD progression.
In AD, abnormal mitochondrial dynamics are characterized by increased mitochondrial fission and decreased fusion. Targeting mitochondrial fission proteins, such as DRP1, has shown potential in restoring balance to these dynamics. Mitochondrial division inhibitor-1 (Mdivi-1), for example, inhibits DRP1 to improve mitochondrial function [204,205]. In APP/PS1 mutant mice, treatment with Mdivi-1 restores mitochondrial length, decreased ROS production, and reduced hippocampal and cortex Aβ accumulation [206]. P110 is a seven-amino acid peptide that specifically blocks the interaction between DRP1 and FIS1, inhibits DRP1 enzymatic activity, and prevents DRP1 translocation to the mitochondria [207,208]. In 5xFAD mutant mice, P110 treatment reduces excessive mitochondrial fission, Aβ accumulation, energetic failure, and oxidative stress by inhibiting DRP1 activity [209].
Enhancing mitophagy can alleviate neuronal stress by clearing damaged mitochondria, a crucial factor in neurodegenerative diseases like Alzheimer's. In APP/PS1 mice, treatment with the BH3-mimetic UMI-77, which targets MCL1, has been shown to promote mitophagy [136]. This increase in mitophagy leads to a reduction in damaged mitochondria, decreased proinflammatory cytokines, and lower Aβ accumulation. Loganin, an iridoid glycoside derived from Corni Fructus, promotes mitophagy and improves learning and memory deficits, reduces Aβ accumulation, and restores mitochondrial dynamics in 3xTg AD mouse models [210]. Notably, loganin decreased the levels of LC3-II, p62, PINK1, and Parkin proteins, yet enhanced the localization of the mitophagy receptor OPTN to mitochondria, promoting mitophagy through a PINK1-Parkin independent pathway. Molecular docking analysis revealed that loganin has a strong binding affinity with OPTN [210]. These findings suggest that targeting mitophagy pathways may be a promising therapeutic strategy for alleviating mitochondrial dysfunction and neurodegeneration in AD.
Perspectives
The complex and multifactorial nature of AD presents significant challenges for both researchers and clinicians. The emerging mitochondrial hypothesis offers a promising new perspective on understanding AD beyond traditional frameworks. Mitochondria, far from being mere energy generators, are dynamic organelles crucial for maintaining cellular homeostasis, especially in neurons with high energy demands. Disruptions in MQC processes highlight a convergent pathway where mitochondrial dysfunction drives AD progression, positioning mitochondria at the heart of disease pathology. Given their critical role in AD, targeting mitochondrial dysfunction represents a novel and potentially transformative therapeutic strategy. Recent advances in gene therapy and pharmacological interventions aimed at restoring mitochondrial function have shown considerable promise in preclinical models. Therapies that enhance mitochondrial biogenesis, stabilize mitochondrial dynamics, or promote mitophagy could potentially mitigate the cascade of neurodegenerative events triggered by mitochondrial impairment.
Looking forward, several critical areas of research should be prioritized to advance the field. A key challenge is to elucidate the precise mechanisms by which mitochondrial dysfunction interacts with other pathological features of AD, such as Aβ deposition, tau hyperphosphorylation, and chronic neuroinflammation. Understanding these interactions will clarify whether mitochondrial dysfunction acts as a primary driver or a secondary amplifier of AD pathology, guiding the development of more targeted therapies. Additionally, while preclinical studies have demonstrated the feasibility of mitochondrial-targeted approaches, their translation to clinical settings requires careful evaluation of long-term safety and efficacy. Future studies should address the potential risks of mitochondrial interventions, such as unintended effects on mitophagy or mitochondrial dynamics, and optimize therapeutic windows and dosing regimens.
Another important direction is the development of biomarkers to monitor mitochondrial dysfunction in vivo. Such biomarkers would enable patient stratification, allowing for personalized treatment approaches based on specific mitochondrial impairments. Advances in biomarker development could also support the real-time assessment of treatment efficacy, facilitating the rapid translation of preclinical findings to clinical trials. Furthermore, the role of mitochondrial dysfunction in non-neuronal cells, particularly microglia and astrocytes, warrants greater attention. These glial cells contribute significantly to the inflammatory and metabolic landscape of the brain in AD, and their mitochondrial health may directly influence disease progression. Investigating mitochondrial dysfunction across cell types could open new therapeutic avenues targeting the brain's immune-metabolic axis. Finally, integrating mitochondrial-targeted therapies with established approaches that address Aβ and tau pathology may provide a more comprehensive strategy for managing AD. Combination therapies have the potential to address the multifactorial nature of the disease more effectively, reducing the burden of neurodegeneration and improving clinical outcomes.
In summary, understanding mitochondrial dysfunction in AD not only deepens our knowledge of disease mechanisms but also expands the scope of therapeutic possibilities. By addressing the outlined research priorities, we can advance innovative, targeted treatments that have the potential to slow or halt the progression of this devastating disease. The integration of mitochondrial-focused strategies with existing therapeutic approaches holds promise for developing more effective and personalized treatments, offering hope to millions of patients and their families.
Author Contributions
K.M.P., and Z.L. wrote the manuscript and X.Q. revised the manuscript. All authors reviewed and approved the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was supported by grants from the US National Institutes of Health (R01AG065240, R01NS115903, R01AG076051 and RF1AG074346 to X.Q.); Vinney Scholar Award for Alzheimer's disease to X.Q.
The authors thank Taylor Thor for his contributions to the figures.
Footnotes
This article is part of a special issue on Alzheimer's Disease published in Neurotherapeutics.
References
- 1.2024 Alzheimer's disease facts and figures. Alzheimers Dement. 2024;20(5):3708–3821. doi: 10.1002/alz.13809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Breijyeh Z., Karaman R. Comprehensive review on Alzheimer's disease: causes and treatment. Molecules. 2020;25(24) doi: 10.3390/molecules25245789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hardy J., Selkoe D.J. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 4.Alquezar C., Arya S., Kao A.W. Tau post-translational modifications: dynamic transformers of tau function, degradation, and aggregation. Front Neurol. 2020;11 doi: 10.3389/fneur.2020.595532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang W., Zhao F., Ma X., Perry G., Zhu X. Mitochondria dysfunction in the pathogenesis of Alzheimer's disease: recent advances. Mol Neurodegener. 2020;15(1):30. doi: 10.1186/s13024-020-00376-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Du H., Guo L., Yan S., Sosunov A.A., McKhann G.M., Yan S.S. Early deficits in synaptic mitochondria in an Alzheimer's disease mouse model. Proc Natl Acad Sci USA. 2010;107(43):18670–18675. doi: 10.1073/pnas.1006586107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yao J., Irwin R.W., Zhao L., Nilsen J., Hamilton R.T., Brinton R.D. Mitochondrial bioenergetic deficit precedes Alzheimer's pathology in female mouse model of Alzheimer's disease. Proc Natl Acad Sci USA. 2009;106(34):14670–14675. doi: 10.1073/pnas.0903563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peggion C., Calì T., Brini M. Mitochondria dysfunction and neuroinflammation in neurodegeneration: who comes first? Antioxidants. 2024;13(2) doi: 10.3390/antiox13020240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mink J.W., Blumenschine R.J., Adams D.B. Ratio of central nervous system to body metabolism in vertebrates: its constancy and functional basis. Am J Physiol. 1981;241(3):R203–R212. doi: 10.1152/ajpregu.1981.241.3.R203. [DOI] [PubMed] [Google Scholar]
- 10.Attwell D., Laughlin S.B. An energy budget for signaling in the grey matter of the brain. J Cerebr Blood Flow Metabol. 2001;21(10):1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Antico Arciuch V.G., Elguero M.E., Poderoso J.J., Carreras M.C. Mitochondrial regulation of cell cycle and proliferation. Antioxidants Redox Signal. 2012;16(10):1150–1180. doi: 10.1089/ars.2011.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giorgi C., Marchi S., Pinton P. The machineries, regulation and cellular functions of mitochondrial calcium. Nat Rev Mol Cell Biol. 2018;19(11):713–730. doi: 10.1038/s41580-018-0052-8. [DOI] [PubMed] [Google Scholar]
- 13.Johri A., Beal M.F. Mitochondrial dysfunction in neurodegenerative diseases. J Pharmacol Exp Therapeut. 2012;342(3):619–630. doi: 10.1124/jpet.112.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nargund A.M., Fiorese C.J., Pellegrino M.W., Deng P., Haynes C.M. Mitochondrial and nuclear accumulation of the transcription factor ATFS-1 promotes OXPHOS recovery during the UPR(mt) Mol Cell. 2015;58(1):123–133. doi: 10.1016/j.molcel.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan D.C. Mitochondrial dynamics and its involvement in disease. Annu Rev Pathol. 2020;15:235–259. doi: 10.1146/annurev-pathmechdis-012419-032711. [DOI] [PubMed] [Google Scholar]
- 16.Giacomello M., Pyakurel A., Glytsou C., Scorrano L. The cell biology of mitochondrial membrane dynamics. Nat Rev Mol Cell Biol. 2020;21(4):204–224. doi: 10.1038/s41580-020-0210-7. [DOI] [PubMed] [Google Scholar]
- 17.Pickles S., Vigié P., Youle R.J. Mitophagy and quality control mechanisms in mitochondrial maintenance. Curr Biol. 2018;28(4):R170. doi: 10.1016/j.cub.2018.01.004. -r85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bogorodskiy A., Okhrimenko I., Burkatovskii D., Jakobs P., Maslov I., Gordeliy V., et al. Role of mitochondrial protein import in age-related neurodegenerative and cardiovascular diseases. Cells. 2021;10(12) doi: 10.3390/cells10123528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen Y., Ding M., Xie Z., Liu X., Yang H., Jin S., et al. Activation of mitochondrial unfolded protein response in SHSY5Y expressing APP cells and APP/PS1 mice. Front Cell Neurosci. 2019;13:568. doi: 10.3389/fncel.2019.00568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muñoz-Carvajal F., Sanhueza M. The mitochondrial unfolded protein response: a hinge between healthy and pathological aging. Front Aging Neurosci. 2020;12 doi: 10.3389/fnagi.2020.581849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Misrani A., Tabassum S., Yang L. Mitochondrial dysfunction and oxidative stress in Alzheimer's disease. Front Aging Neurosci. 2021;13 doi: 10.3389/fnagi.2021.617588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blagov A.V., Grechko A.V., Nikiforov N.G., Borisov E.E., Sadykhov N.K., Orekhov A.N. Role of impaired mitochondrial dynamics processes in the pathogenesis of Alzheimer's disease. Int J Mol Sci. 2022;23(13) doi: 10.3390/ijms23136954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mary A., Eysert F., Checler F., Chami M. Mitophagy in Alzheimer's disease: molecular defects and therapeutic approaches. Mol Psychiatr. 2023;28(1):202–216. doi: 10.1038/s41380-022-01631-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yao J., Brinton R.D. Targeting mitochondrial bioenergetics for Alzheimer's prevention and treatment. Curr Pharmaceut Des. 2011;17(31):3474–3479. doi: 10.2174/138161211798072517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klein H.U., Trumpff C., Yang H.S., Lee A.J., Picard M., Bennett D.A., et al. Characterization of mitochondrial DNA quantity and quality in the human aged and Alzheimer's disease brain. Mol Neurodegener. 2021;16(1):75. doi: 10.1186/s13024-021-00495-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calvo S.E., Mootha V.K. The mitochondrial proteome and human disease. Annu Rev Genom Hum Genet. 2010;11:25–44. doi: 10.1146/annurev-genom-082509-141720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kotrys A.V., Szczesny R.J. Mitochondrial gene expression and beyond-novel aspects of cellular physiology. Cells. 2019;9(1) doi: 10.3390/cells9010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Couvillion M.T., Soto I.C., Shipkovenska G., Churchman L.S. Synchronized mitochondrial and cytosolic translation programs. Nature. 2016;533(7604):499–503. doi: 10.1038/nature18015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soto I., Couvillion M., Hansen K.G., McShane E., Moran J.C., Barrientos A., et al. Balanced mitochondrial and cytosolic translatomes underlie the biogenesis of human respiratory complexes. Genome Biol. 2022;23(1):170. doi: 10.1186/s13059-022-02732-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neupert W. Protein import into mitochondria. Annu Rev Biochem. 1997;66:863–917. doi: 10.1146/annurev.biochem.66.1.863. [DOI] [PubMed] [Google Scholar]
- 31.Pfanner N., Warscheid B., Wiedemann N. Mitochondrial proteins: from biogenesis to functional networks. Nat Rev Mol Cell Biol. 2019;20(5):267–284. doi: 10.1038/s41580-018-0092-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harbauer A.B., Zahedi R.P., Sickmann A., Pfanner N., Meisinger C. The protein import machinery of mitochondria-a regulatory hub in metabolism, stress, and disease. Cell Metabol. 2014;19(3):357–372. doi: 10.1016/j.cmet.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 33.Haastrup M.O., Vikramdeo K.S., Singh S., Singh A.P., Dasgupta S. The journey of mitochondrial protein import and the roadmap to follow. Int J Mol Sci. 2023;24(3) doi: 10.3390/ijms24032479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bahr T., Katuri J., Liang T., Bai Y. Mitochondrial chaperones in human health and disease. Free Radic Biol Med. 2022;179:363–374. doi: 10.1016/j.freeradbiomed.2021.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Höhfeld J., Hartl F.U. Role of the chaperonin cofactor Hsp10 in protein folding and sorting in yeast mitochondria. J Cell Biol. 1994;126(2):305–315. doi: 10.1083/jcb.126.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li J., Qian X., Sha B. Heat shock protein 40: structural studies and their functional implications. Protein Pept Lett. 2009;16(6):606–612. doi: 10.2174/092986609788490159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng M.Y., Hartl F.U., Horwich A.L. The mitochondrial chaperonin hsp60 is required for its own assembly. Nature. 1990;348(6300):455–458. doi: 10.1038/348455a0. [DOI] [PubMed] [Google Scholar]
- 38.Mayer M.P., Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci. 2005;62(6):670–684. doi: 10.1007/s00018-004-4464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saibil H. Chaperone machines for protein folding, unfolding and disaggregation. Nat Rev Mol Cell Biol. 2013;14(10):630–642. doi: 10.1038/nrm3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoter A., El-Sabban M.E., Naim H.Y. The HSP90 family: structure, regulation, function, and implications in health and disease. Int J Mol Sci. 2018;19(9) doi: 10.3390/ijms19092560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Voos W., Röttgers K. Molecular chaperones as essential mediators of mitochondrial biogenesis. Biochim Biophys Acta. 2002;1592(1):51–62. doi: 10.1016/s0167-4889(02)00264-1. [DOI] [PubMed] [Google Scholar]
- 42.Adriaenssens E., Asselbergh B., Rivera-Mejías P., Bervoets S., Vendredy L., De Winter V., et al. Small heat shock proteins operate as molecular chaperones in the mitochondrial intermembrane space. Nat Cell Biol. 2023;25(3):467–480. doi: 10.1038/s41556-022-01074-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matsushima Y., Takahashi K., Yue S., Fujiyoshi Y., Yoshioka H., Aihara M., et al. Mitochondrial Lon protease is a gatekeeper for proteins newly imported into the matrix. Commun Biol. 2021;4(1):974. doi: 10.1038/s42003-021-02498-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luo B., Ma Y., Zhou Y., Zhang N., Luo Y. Human ClpP protease, a promising therapy target for diseases of mitochondrial dysfunction. Drug Discov Today. 2021;26(4):968–981. doi: 10.1016/j.drudis.2021.01.007. [DOI] [PubMed] [Google Scholar]
- 45.Viana M.P., Levytskyy R.M., Anand R., Reichert A.S., Khalimonchuk O. Protease OMA1 modulates mitochondrial bioenergetics and ultrastructure through dynamic association with MICOS complex. iScience. 2021;24(2) doi: 10.1016/j.isci.2021.102119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Radke S., Chander H., Schäfer P., Meiss G., Krüger R., Schulz J.B., et al. Mitochondrial protein quality control by the proteasome involves ubiquitination and the protease Omi. J Biol Chem. 2008;283(19):12681–12685. doi: 10.1074/jbc.C800036200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patron M., Sprenger H.G., Langer T. m-AAA proteases, mitochondrial calcium homeostasis and neurodegeneration. Cell Res. 2018;28(3):296–306. doi: 10.1038/cr.2018.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Opalińska M., Jańska H. AAA proteases: guardians of mitochondrial function and homeostasis. Cells. 2018;7(10) doi: 10.3390/cells7100163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shpilka T., Haynes C.M. The mitochondrial UPR: mechanisms, physiological functions and implications in ageing. Nat Rev Mol Cell Biol. 2018;19(2):109–120. doi: 10.1038/nrm.2017.110. [DOI] [PubMed] [Google Scholar]
- 50.Zhao Q., Wang J., Levichkin I.V., Stasinopoulos S., Ryan M.T., Hoogenraad N.J. A mitochondrial specific stress response in mammalian cells. EMBO J. 2002;21(17):4411–4419. doi: 10.1093/emboj/cdf445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arnould T., Michel S., Renard P. Mitochondria retrograde signaling and the UPR mt: where are we in mammals? Int J Mol Sci. 2015;16(8):18224–18251. doi: 10.3390/ijms160818224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jovaisaite V., Mouchiroud L., Auwerx J. The mitochondrial unfolded protein response, a conserved stress response pathway with implications in health and disease. J Exp Biol. 2014;217(Pt 1):137–143. doi: 10.1242/jeb.090738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tseng A.H., Shieh S.S., Wang D.L. SIRT3 deacetylates FOXO3 to protect mitochondria against oxidative damage. Free Radic Biol Med. 2013;63:222–234. doi: 10.1016/j.freeradbiomed.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 54.Qureshi M.A., Haynes C.M., Pellegrino M.W. The mitochondrial unfolded protein response: signaling from the powerhouse. J Biol Chem. 2017;292(33):13500–13506. doi: 10.1074/jbc.R117.791061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weng H., Ma Y., Chen L., Cai G., Chen Z., Zhang S., et al. A new vision of mitochondrial unfolded protein response to the sirtuin family. Curr Neuropharmacol. 2020;18(7):613–623. doi: 10.2174/1570159X18666200123165002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kenny T.C., Hart P., Ragazzi M., Sersinghe M., Chipuk J., Sagar M.A.K., et al. Selected mitochondrial DNA landscapes activate the SIRT3 axis of the UPR(mt) to promote metastasis. Oncogene. 2017;36(31):4393–4404. doi: 10.1038/onc.2017.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cenini G., Rüb C., Bruderek M., Voos W. Amyloid β-peptides interfere with mitochondrial preprotein import competence by a coaggregation process. Mol Biol Cell. 2016;27(21):3257–3272. doi: 10.1091/mbc.E16-05-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hansson Petersen C.A., Alikhani N., Behbahani H., Wiehager B., Pavlov P.F., Alafuzoff I., et al. The amyloid beta-peptide is imported into mitochondria via the TOM import machinery and localized to mitochondrial cristae. Proc Natl Acad Sci USA. 2008;105(35):13145–13150. doi: 10.1073/pnas.0806192105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Anandatheerthavarada H.K., Biswas G., Robin M.A., Avadhani N.G. Mitochondrial targeting and a novel transmembrane arrest of Alzheimer's amyloid precursor protein impairs mitochondrial function in neuronal cells. J Cell Biol. 2003;161(1):41–54. doi: 10.1083/jcb.200207030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Anandatheerthavarada H.K., Devi L. Mitochondrial translocation of amyloid precursor protein and its cleaved products: relevance to mitochondrial dysfunction in Alzheimer's disease. Rev Neurosci. 2007;18(5):343–354. doi: 10.1515/revneuro.2007.18.5.343. [DOI] [PubMed] [Google Scholar]
- 61.Istrate A.N., Kozin S.A., Zhokhov S.S., Mantsyzov A.B., Kechko O.I., Pastore A., et al. Interplay of histidine residues of the Alzheimer's disease Aβ peptide governs its Zn-induced oligomerization. Sci Rep. 2016;6 doi: 10.1038/srep21734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Falkevall A., Alikhani N., Bhushan S., Pavlov P.F., Busch K., Johnson K.A., et al. Degradation of the amyloid beta-protein by the novel mitochondrial peptidasome. PreP. J Biol Chem. 2006;281(39):29096–29104. doi: 10.1074/jbc.M602532200. [DOI] [PubMed] [Google Scholar]
- 63.Mossmann D., Vögtle F.N., Taskin A.A., Teixeira P.F., Ring J., Burkhart J.M., et al. Amyloid-β peptide induces mitochondrial dysfunction by inhibition of preprotein maturation. Cell Metabol. 2014;20(4):662–669. doi: 10.1016/j.cmet.2014.07.024. [DOI] [PubMed] [Google Scholar]
- 64.Beck J.S., Mufson E.J., Counts S.E. Evidence for mitochondrial UPR gene activation in familial and sporadic Alzheimer's disease. Curr Alzheimer Res. 2016;13(6):610–614. doi: 10.2174/1567205013666151221145445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pérez M.J., Ivanyuk D., Panagiotakopoulou V., Di Napoli G., Kalb S., Brunetti D., et al. Loss of function of the mitochondrial peptidase PITRM1 induces proteotoxic stress and Alzheimer's disease-like pathology in human cerebral organoids. Mol Psychiatr. 2021;26(10):5733–5750. doi: 10.1038/s41380-020-0807-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang K., Petr J. Mitochondrial dynamics: updates and perspectives. Sci Rep. 2024;14(1):9936. doi: 10.1038/s41598-024-59998-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Khosravi S., Harner M.E. The MICOS complex, a structural element of mitochondria with versatile functions. Biol Chem. 2020;401(6-7):765–778. doi: 10.1515/hsz-2020-0103. [DOI] [PubMed] [Google Scholar]
- 68.Kozjak-Pavlovic V. The MICOS complex of human mitochondria. Cell Tissue Res. 2017;367(1):83–93. doi: 10.1007/s00441-016-2433-7. [DOI] [PubMed] [Google Scholar]
- 69.Horbay R., Bilyy R. Mitochondrial dynamics during cell cycling. Apoptosis. 2016;21(12):1327–1335. doi: 10.1007/s10495-016-1295-5. [DOI] [PubMed] [Google Scholar]
- 70.Noguchi M., Kasahara A. Mitochondrial dynamics coordinate cell differentiation. Biochem Biophys Res Commun. 2018;500(1):59–64. doi: 10.1016/j.bbrc.2017.06.094. [DOI] [PubMed] [Google Scholar]
- 71.Chen H., Detmer S.A., Ewald A.J., Griffin E.E., Fraser S.E., Chan D.C. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol. 2003;160(2):189–200. doi: 10.1083/jcb.200211046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lum M., Morona R. Dynamin-related protein Drp1 and mitochondria are important for Shigella flexneri infection. International journal of medical microbiology : IJMM. 2014;304(5-6):530–541. doi: 10.1016/j.ijmm.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 73.Kim S.J., Khan M., Quan J., Till A., Subramani S., Siddiqui A. Hepatitis B virus disrupts mitochondrial dynamics: induces fission and mitophagy to attenuate apoptosis. PLoS Pathog. 2013;9(12) doi: 10.1371/journal.ppat.1003722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Adebayo M., Singh S., Singh A.P., Dasgupta S. Mitochondrial fusion and fission: the fine-tune balance for cellular homeostasis. Faseb J. 2021;35(6) doi: 10.1096/fj.202100067R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Smirnova E., Shurland D.L., Ryazantsev S.N., van der Bliek A.M. A human dynamin-related protein controls the distribution of mitochondria. J Cell Biol. 1998;143(2):351–358. doi: 10.1083/jcb.143.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mozdy A.D., McCaffery J.M., Shaw J.M. Dnm1p GTPase-mediated mitochondrial fission is a multi-step process requiring the novel integral membrane component Fis1p. J Cell Biol. 2000;151(2):367–380. doi: 10.1083/jcb.151.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Palmer C.S., Osellame L.D., Laine D., Koutsopoulos O.S., Frazier A.E., Ryan M.T. MiD49 and MiD51, new components of the mitochondrial fission machinery. EMBO Rep. 2011;12(6):565–573. doi: 10.1038/embor.2011.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gandre-Babbe S., van der Bliek A.M. The novel tail-anchored membrane protein Mff controls mitochondrial and peroxisomal fission in mammalian cells. Mol Biol Cell. 2008;19(6):2402–2412. doi: 10.1091/mbc.E07-12-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cribbs J.T., Strack S. Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep. 2007;8(10):939–944. doi: 10.1038/sj.embor.7401062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang Z., Jiang H., Chen S., Du F., Wang X. The mitochondrial phosphatase PGAM5 functions at the convergence point of multiple necrotic death pathways. Cell. 2012;148(1-2):228–243. doi: 10.1016/j.cell.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 81.Taguchi N., Ishihara N., Jofuku A., Oka T., Mihara K. Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. J Biol Chem. 2007;282(15):11521–11529. doi: 10.1074/jbc.M607279200. [DOI] [PubMed] [Google Scholar]
- 82.Han H., Tan J., Wang R., Wan H., He Y., Yan X., et al. PINK1 phosphorylates Drp1(S616) to regulate mitophagy-independent mitochondrial dynamics. EMBO Rep. 2020;21(8) doi: 10.15252/embr.201948686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gui C., Ren Y., Chen J., Wu X., Mao K., Li H., et al. p38 MAPK-DRP1 signaling is involved in mitochondrial dysfunction and cell death in mutant A53T α-synuclein model of Parkinson's disease. Toxicol Appl Pharmacol. 2020;388 doi: 10.1016/j.taap.2019.114874. [DOI] [PubMed] [Google Scholar]
- 84.Alexander C., Votruba M., Pesch U.E., Thiselton D.L., Mayer S., Moore A., et al. OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat Genet. 2000;26(2):211–215. doi: 10.1038/79944. [DOI] [PubMed] [Google Scholar]
- 85.Lu D., Feng Y., Liu G., Yang Y., Ren Y., Chen Z., et al. Mitochondrial transport in neurons and evidence for its involvement in acute neurological disorders. Front Neurosci. 2023;17 doi: 10.3389/fnins.2023.1268883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pilling A.D., Horiuchi D., Lively C.M., Saxton W.M. Kinesin-1 and Dynein are the primary motors for fast transport of mitochondria in Drosophila motor axons. Mol Biol Cell. 2006;17(4):2057–2068. doi: 10.1091/mbc.E05-06-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Martin M., Iyadurai S.J., Gassman A., Gindhart J.G., Jr., Hays T.S., Saxton W.M. Cytoplasmic dynein, the dynactin complex, and kinesin are interdependent and essential for fast axonal transport. Mol Biol Cell. 1999;10(11):3717–3728. doi: 10.1091/mbc.10.11.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sokac A.M., Bement W.M. Regulation and expression of metazoan unconventional myosins. Int Rev Cytol. 2000;200:197–304. doi: 10.1016/s0074-7696(00)00005-x. [DOI] [PubMed] [Google Scholar]
- 89.Frederick R.L., McCaffery J.M., Cunningham K.W., Okamoto K., Shaw J.M. Yeast Miro GTPase, Gem1p, regulates mitochondrial morphology via a novel pathway. J Cell Biol. 2004;167(1):87–98. doi: 10.1083/jcb.200405100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.van Spronsen M., Mikhaylova M., Lipka J., Schlager M.A., van den Heuvel D.J., Kuijpers M., et al. TRAK/Milton motor-adaptor proteins steer mitochondrial trafficking to axons and dendrites. Neuron. 2013;77(3):485–502. doi: 10.1016/j.neuron.2012.11.027. [DOI] [PubMed] [Google Scholar]
- 91.Kang J.S., Tian J.H., Pan P.Y., Zald P., Li C., Deng C., et al. Docking of axonal mitochondria by syntaphilin controls their mobility and affects short-term facilitation. Cell. 2008;132(1):137–148. doi: 10.1016/j.cell.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhao Y., Song E., Wang W., Hsieh C.H., Wang X., Feng W., et al. Metaxins are core components of mitochondrial transport adaptor complexes. Nat Commun. 2021;12(1):83. doi: 10.1038/s41467-020-20346-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.de la Cueva M., Antequera D., Ordoñez-Gutierrez L., Wandosell F., Camins A., Carro E., et al. Author Correction: amyloid-β impairs mitochondrial dynamics and autophagy in Alzheimer's disease experimental models. Sci Rep. 2023;13(1) doi: 10.1038/s41598-023-46106-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sharma N., Banerjee R., Davis R.L. Early mitochondrial defects in the 5xFAD mouse model of Alzheimer's disease. J Alzheimers Dis. 2023;91(4):1323–1338. doi: 10.3233/JAD-220884. [DOI] [PubMed] [Google Scholar]
- 95.Pérez M.J., Vergara-Pulgar K., Jara C., Cabezas-Opazo F., Quintanilla R.A. Caspase-cleaved tau impairs mitochondrial dynamics in Alzheimer's disease. Mol Neurobiol. 2018;55(2):1004–1018. doi: 10.1007/s12035-017-0385-x. [DOI] [PubMed] [Google Scholar]
- 96.Wang L., Liu M., Gao J., Smith A.M., Fujioka H., Liang J., et al. Mitochondrial fusion suppresses tau pathology-induced neurodegeneration and cognitive decline. J Alzheimers Dis. 2021;84(3):1057–1069. doi: 10.3233/JAD-215175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Manczak M., Calkins M.J., Reddy P.H. Impaired mitochondrial dynamics and abnormal interaction of amyloid beta with mitochondrial protein Drp1 in neurons from patients with Alzheimer's disease: implications for neuronal damage. Hum Mol Genet. 2011;20(13):2495–2509. doi: 10.1093/hmg/ddr139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yan J., Liu X.H., Han M.Z., Wang Y.M., Sun X.L., Yu N., et al. Blockage of GSK3β-mediated Drp1 phosphorylation provides neuroprotection in neuronal and mouse models of Alzheimer's disease. Neurobiol Aging. 2015;36(1):211–227. doi: 10.1016/j.neurobiolaging.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 99.Shang Y., Sun X., Chen X., Wang Q., Wang E.J., Miller E., et al. A CHCHD6-APP axis connects amyloid and mitochondrial pathology in Alzheimer's disease. Acta Neuropathol. 2022;144(5):911–938. doi: 10.1007/s00401-022-02499-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dewitt D.A., Hurd J.A., Fox N., Townsend B.E., Griffioen K.J., Ghribi O., et al. Peri-nuclear clustering of mitochondria is triggered during aluminum maltolate induced apoptosis. J Alzheimers Dis. 2006;9(2):195–205. doi: 10.3233/jad-2006-9211. [DOI] [PubMed] [Google Scholar]
- 101.Calkins M.J., Reddy P.H. Amyloid beta impairs mitochondrial anterograde transport and degenerates synapses in Alzheimer's disease neurons. Biochim Biophys Acta. 2011;1812(4):507–513. doi: 10.1016/j.bbadis.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Guo B., Huang Y., Gao Q., Zhou Q. Stabilization of microtubules improves cognitive functions and axonal transport of mitochondria in Alzheimer's disease model mice. Neurobiol Aging. 2020;96:223–232. doi: 10.1016/j.neurobiolaging.2020.09.011. [DOI] [PubMed] [Google Scholar]
- 103.Xu M., Feng J., Tang M., Guo Q., Zhan J., Zhu F., et al. Blocking retrograde axonal transport of autophagosomes contributes to sevoflurane-induced neuron apoptosis in APP/PS1 mice. Acta Neurol Belg. 2021;121(5):1207–1215. doi: 10.1007/s13760-020-01359-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Choi H., Kim H.J., Kim J., Kim S., Yang J., Lee W., et al. Increased acetylation of Peroxiredoxin1 by HDAC6 inhibition leads to recovery of Aβ-induced impaired axonal transport. Mol Neurodegener. 2017;12(1):23. doi: 10.1186/s13024-017-0164-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sabui A., Biswas M., Somvanshi P.R., Kandagiri P., Gorla M., Mohammed F., et al. Decreased anterograde transport coupled with sustained retrograde transport contributes to reduced axonal mitochondrial density in tauopathy neurons. Front Mol Neurosci. 2022;15 doi: 10.3389/fnmol.2022.927195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Conze C., Rierola M., Trushina N.I., Peters M., Janning D., Holzer M., et al. Caspase-cleaved tau is senescence-associated and induces a toxic gain of function by putting a brake on axonal transport. Mol Psychiatr. 2022;27(7):3010–3023. doi: 10.1038/s41380-022-01538-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vossel K.A., Zhang K., Brodbeck J., Daub A.C., Sharma P., Finkbeiner S., et al. Tau reduction prevents Abeta-induced defects in axonal transport. Science. 2010;330(6001):198. doi: 10.1126/science.1194653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kopeikina K.J., Carlson G.A., Pitstick R., Ludvigson A.E., Peters A., Luebke J.I., et al. Tau accumulation causes mitochondrial distribution deficits in neurons in a mouse model of tauopathy and in human Alzheimer's disease brain. Am J Pathol. 2011;179(4):2071–2082. doi: 10.1016/j.ajpath.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shahpasand K., Uemura I., Saito T., Asano T., Hata K., Shibata K., et al. Regulation of mitochondrial transport and inter-microtubule spacing by tau phosphorylation at the sites hyperphosphorylated in Alzheimer's disease. J Neurosci. 2012;32(7):2430–2441. doi: 10.1523/JNEUROSCI.5927-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Trease A.J., George J.W., Roland N.J., Lichter E.Z., Emanuel K., Totusek S., et al. Hyperphosphorylated human tau accumulates at the synapse, localizing on synaptic mitochondrial outer membranes and disrupting respiration in a mouse model of tauopathy. Front Mol Neurosci. 2022;15 doi: 10.3389/fnmol.2022.852368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liang M.Z., Ke T.L., Chen L. Mitochondrial protein PGAM5 emerges as a new regulator in neurological diseases. Front Mol Neurosci. 2021;14 doi: 10.3389/fnmol.2021.730604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang S., Long H., Hou L., Feng B., Ma Z., Wu Y., et al. The mitophagy pathway and its implications in human diseases. Signal Transduct Targeted Ther. 2023;8(1):304. doi: 10.1038/s41392-023-01503-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hu D., Liu Z., Qi X. Mitochondrial quality control strategies: potential therapeutic targets for neurodegenerative diseases? Front Neurosci. 2021;15 doi: 10.3389/fnins.2021.746873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Han S., Zhang M., Jeong Y.Y., Margolis D.J., Cai Q. The role of mitophagy in the regulation of mitochondrial energetic status in neurons. Autophagy. 2021;17(12):4182–4201. doi: 10.1080/15548627.2021.1907167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ge P., Dawson V.L., Dawson T.M. PINK1 and Parkin mitochondrial quality control: a source of regional vulnerability in Parkinson's disease. Mol Neurodegener. 2020;15(1):20. doi: 10.1186/s13024-020-00367-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Quinn P.M.J., Moreira P.I., Ambrósio A.F., Alves C.H. PINK1/PARKIN signalling in neurodegeneration and neuroinflammation. Acta Neuropathologica Communications. 2020;8(1):189. doi: 10.1186/s40478-020-01062-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Eldeeb M.A., Bayne A.N., Fallahi A., Goiran T., MacDougall E.J., Soumbasis A., et al. Tom20 gates PINK1 activity and mediates its tethering of the TOM and TIM23 translocases upon mitochondrial stress. Proc Natl Acad Sci USA. 2024;121(10) doi: 10.1073/pnas.2313540121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pickrell A.M., Youle R.J. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson's disease. Neuron. 2015;85(2):257–273. doi: 10.1016/j.neuron.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kazlauskaite A., Muqit M.M. PINK1 and Parkin – mitochondrial interplay between phosphorylation and ubiquitylation in Parkinson's disease. FEBS J. 2015;282(2):215–223. doi: 10.1111/febs.13127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Thomas R.E., Andrews L.A., Burman J.L., Lin W.Y., Pallanck L.J. PINK1-Parkin pathway activity is regulated by degradation of PINK1 in the mitochondrial matrix. PLoS Genet. 2014;10(5) doi: 10.1371/journal.pgen.1004279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lazarou M., Jin S.M., Kane L.A., Youle R.J. Role of PINK1 binding to the TOM complex and alternate intracellular membranes in recruitment and activation of the E3 ligase Parkin. Dev Cell. 2012;22(2):320–333. doi: 10.1016/j.devcel.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gao F., Zhang Y., Hou X., Tao Z., Ren H., Wang G. Dependence of PINK1 accumulation on mitochondrial redox system. Aging Cell. 2020;19(9) doi: 10.1111/acel.13211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Seirafi M., Kozlov G., Gehring K. Parkin structure and function. FEBS J. 2015;282(11):2076–2088. doi: 10.1111/febs.13249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rüb C., Wilkening A., Voos W. Mitochondrial quality control by the Pink1/Parkin system. Cell Tissue Res. 2017;367(1):111–123. doi: 10.1007/s00441-016-2485-8. [DOI] [PubMed] [Google Scholar]
- 125.Sarraf S.A., Raman M., Guarani-Pereira V., Sowa M.E., Huttlin E.L., Gygi S.P., et al. Landscape of the PARKIN-dependent ubiquitylome in response to mitochondrial depolarization. Nature. 2013;496(7445):372–376. doi: 10.1038/nature12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rose C.M., Isasa M., Ordureau A., Prado M.A., Beausoleil S.A., Jedrychowski M.P., et al. Highly multiplexed quantitative mass spectrometry analysis of ubiquitylomes. Cell Syst. 2016;3(4):395–403.e4. doi: 10.1016/j.cels.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Geisler S., Holmström K.M., Skujat D., Fiesel F.C., Rothfuss O.C., Kahle P.J., et al. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol. 2010;12(2):119–131. doi: 10.1038/ncb2012. [DOI] [PubMed] [Google Scholar]
- 128.Richter B., Sliter D.A., Herhaus L., Stolz A., Wang C., Beli P., et al. Phosphorylation of OPTN by TBK1 enhances its binding to Ub chains and promotes selective autophagy of damaged mitochondria. Proc Natl Acad Sci USA. 2016;113(15):4039–4044. doi: 10.1073/pnas.1523926113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Whang M.I., Tavares R.M., Benjamin D.I., Kattah M.G., Advincula R., Nomura D.K., et al. The ubiquitin binding protein TAX1BP1 mediates autophagasome induction and the metabolic transition of activated T cells. Immunity. 2017;46(3):405–420. doi: 10.1016/j.immuni.2017.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li Y., Zheng W., Lu Y., Zheng Y., Pan L., Wu X., et al. BNIP3L/NIX-mediated mitophagy: molecular mechanisms and implications for human disease. Cell Death Dis. 2021;13(1):14. doi: 10.1038/s41419-021-04469-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li G., Li J., Shao R., Zhao J., Chen M. FUNDC1: a promising mitophagy regulator at the mitochondria-associated membrane for cardiovascular diseases. Front Cell Dev Biol. 2021;9 doi: 10.3389/fcell.2021.788634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Murakawa T., Yamaguchi O., Hashimoto A., Hikoso S., Takeda T., Oka T., et al. Bcl-2-like protein 13 is a mammalian Atg32 homologue that mediates mitophagy and mitochondrial fragmentation. Nat Commun. 2015;6(1):7527. doi: 10.1038/ncomms8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yoo S.M., Yamashita S.I., Kim H., Na D., Lee H., Kim S.J., et al. FKBP8 LIRL-dependent mitochondrial fragmentation facilitates mitophagy under stress conditions. Faseb J. 2020;34(2):2944–2957. doi: 10.1096/fj.201901735R. [DOI] [PubMed] [Google Scholar]
- 134.Wang Z.T., Lu M.H., Zhang Y., Ji W.L., Lei L., Wang W., et al. Disrupted-in-schizophrenia-1 protects synaptic plasticity in a transgenic mouse model of Alzheimer's disease as a mitophagy receptor. Aging Cell. 2019;18(1) doi: 10.1111/acel.12860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Strappazzon F., Nazio F., Corrado M., Cianfanelli V., Romagnoli A., Fimia G.M., et al. AMBRA1 is able to induce mitophagy via LC3 binding, regardless of PARKIN and p62/SQSTM1. Cell Death Differ. 2015;22(3):419–432. doi: 10.1038/cdd.2014.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cen X., Chen Y., Xu X., Wu R., He F., Zhao Q., et al. Pharmacological targeting of MCL-1 promotes mitophagy and improves disease pathologies in an Alzheimer's disease mouse model. Nat Commun. 2020;11(1):5731. doi: 10.1038/s41467-020-19547-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chu C.T., Ji J., Dagda R.K., Jiang J.F., Tyurina Y.Y., Kapralov A.A., et al. Cardiolipin externalization to the outer mitochondrial membrane acts as an elimination signal for mitophagy in neuronal cells. Nat Cell Biol. 2013;15(10):1197–1205. doi: 10.1038/ncb2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Li A., Gao M., Liu B., Qin Y., Chen L., Liu H., et al. Mitochondrial autophagy: molecular mechanisms and implications for cardiovascular disease. Cell Death Dis. 2022;13(5):444. doi: 10.1038/s41419-022-04906-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Onishi M., Yamano K., Sato M., Matsuda N., Okamoto K. Molecular mechanisms and physiological functions of mitophagy. EMBO J. 2021;40(3) doi: 10.15252/embj.2020104705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Martín-Maestro P., Gargini R., Perry G., Avila J., García-Escudero V. PARK2 enhancement is able to compensate mitophagy alterations found in sporadic Alzheimer's disease. Hum Mol Genet. 2016;25(4):792–806. doi: 10.1093/hmg/ddv616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Du F., Yu Q., Yan S., Hu G., Lue L.F., Walker D.G., et al. PINK1 signalling rescues amyloid pathology and mitochondrial dysfunction in Alzheimer's disease. Brain. 2017;140(12):3233–3251. doi: 10.1093/brain/awx258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fang E.F., Hou Y., Palikaras K., Adriaanse B.A., Kerr J.S., Yang B., et al. Mitophagy inhibits amyloid-β and tau pathology and reverses cognitive deficits in models of Alzheimer's disease. Nat Neurosci. 2019;22(3):401–412. doi: 10.1038/s41593-018-0332-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Martín-Maestro P., Gargini R., García E., Perry G., Avila J., García-Escudero V. Slower dynamics and aged mitochondria in sporadic Alzheimer's disease. Oxid Med Cell Longev. 2017;2017 doi: 10.1155/2017/9302761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Vaillant-Beuchot L., Mary A., Pardossi-Piquard R., Bourgeois A., Lauritzen I., Eysert F., et al. Accumulation of amyloid precursor protein C-terminal fragments triggers mitochondrial structure, function, and mitophagy defects in Alzheimer's disease models and human brains. Acta Neuropathol. 2021;141(1):39–65. doi: 10.1007/s00401-020-02234-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Martín-Maestro P., Sproul A., Martinez H., Paquet D., Gerges M., Noggle S., et al. Autophagy induction by bexarotene promotes mitophagy in presenilin 1 familial Alzheimer's disease iPSC-derived neural stem cells. Mol Neurobiol. 2019;56(12):8220–8236. doi: 10.1007/s12035-019-01665-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wang N., Wang H., Li L., Li Y., Zhang R. β-Asarone inhibits amyloid-β by promoting autophagy in a cell model of Alzheimer's disease. Front Pharmacol. 2019;10:1529. doi: 10.3389/fphar.2019.01529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang H., Jiang T., Li W., Gao N., Zhang T. Resveratrol attenuates oxidative damage through activating mitophagy in an in vitro model of Alzheimer's disease. Toxicol Lett. 2018;282:100–108. doi: 10.1016/j.toxlet.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 148.Zhang L., Fang Y., Zhao X., Zheng Y., Ma Y., Li S., et al. miR-204 silencing reduces mitochondrial autophagy and ROS production in a murine AD model via the TRPML1-activated STAT3 pathway. Mol Ther Nucleic Acids. 2021;24:822–831. doi: 10.1016/j.omtn.2021.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang H., Zhang T., Ge X., Chen J., Zhao Y., Fu J. Parkin overexpression attenuates Aβ-induced mitochondrial dysfunction in HEK293 cells by restoring impaired mitophagy. Life Sci. 2020;244 doi: 10.1016/j.lfs.2020.117322. [DOI] [PubMed] [Google Scholar]
- 150.Han Y., Wang N., Kang J., Fang Y. β-Asarone improves learning and memory in Aβ(1-42)-induced Alzheimer's disease rats by regulating PINK1-Parkin-mediated mitophagy. Metab Brain Dis. 2020;35(7):1109–1117. doi: 10.1007/s11011-020-00587-2. [DOI] [PubMed] [Google Scholar]
- 151.Hu Y., Li X.C., Wang Z.H., Luo Y., Zhang X., Liu X.P., et al. Tau accumulation impairs mitophagy via increasing mitochondrial membrane potential and reducing mitochondrial Parkin. Oncotarget. 2016;7(14):17356–17368. doi: 10.18632/oncotarget.7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Szabo L., Eckert A., Grimm A. Insights into disease-associated tau impact on mitochondria. Int J Mol Sci. 2020;21(17) doi: 10.3390/ijms21176344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Guha S., Fischer S., Johnson G.V.W., Nehrke K. Tauopathy-associated tau modifications selectively impact neurodegeneration and mitophagy in a novel C. elegans single-copy transgenic model. Mol Neurodegener. 2020;15(1):65. doi: 10.1186/s13024-020-00410-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Amadoro G., Corsetti V., Florenzano F., Atlante A., Ciotti M.T., Mongiardi M.P., et al. AD-linked, toxic NH2 human tau affects the quality control of mitochondria in neurons. Neurobiol Dis. 2014;62:489–507. doi: 10.1016/j.nbd.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 155.Gupta R., Kanai M., Durham T.J., Tsuo K., McCoy J.G., Kotrys A.V., et al. Nuclear genetic control of mtDNA copy number and heteroplasmy in humans. Nature. 2023;620(7975):839–848. doi: 10.1038/s41586-023-06426-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Stocker H., Gentiluomo M., Trares K., Beyer L., Stevenson-Hoare J., Rujescu D., et al. Mitochondrial DNA abundance in blood is associated with Alzheimer's disease- and dementia-risk. Mol Psychiatr. 2024 doi: 10.1038/s41380-024-02670-x. [DOI] [PubMed] [Google Scholar]
- 157.Liou C.W., Chen S.H., Lin T.K., Tsai M.H., Chang C.C. Oxidative stress biomarkers and mitochondrial DNA copy number associated with APOE4 allele and cholinesterase inhibitor therapy in patients with Alzheimer’s disease. Antioxidants. 2021;10(12):1971. doi: 10.3390/antiox10121971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Alvarez V., Corao A.I., Alonso-Montes C., Sánchez-Ferrero E., De Mena L., Morales B., et al. Mitochondrial transcription factor A (TFAM) gene variation and risk of late-onset Alzheimer's disease. J Alzheimers Dis. 2008;13(3):275–280. doi: 10.3233/jad-2008-13305. [DOI] [PubMed] [Google Scholar]
- 159.Wang X.L., Liu Q., Chen G.J., Li M.L., Ding Y.H. Overexpression of MTERF4 promotes the amyloidogenic processing of APP by inhibiting ADAM10. Biochem Biophys Res Commun. 2017;482(4):928–934. doi: 10.1016/j.bbrc.2016.11.135. [DOI] [PubMed] [Google Scholar]
- 160.Zhao Y., Liu B., Xu L., Yu S., Fu J., Wang J., et al. ROS-induced mtDNA release: the emerging messenger for communication between neurons and innate immune cells during neurodegenerative disorder progression. Antioxidants. 2021;10(12) doi: 10.3390/antiox10121917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Xu Y., Cheng L., Sun J., Li F., Liu X., Wei Y., et al. Hypermethylation of mitochondrial cytochrome b and cytochrome c oxidase II genes with decreased mitochondrial DNA copy numbers in the APP/PS1 transgenic mouse model of Alzheimer's disease. Neurochem Res. 2021;46(3):564–572. doi: 10.1007/s11064-020-03192-y. [DOI] [PubMed] [Google Scholar]
- 162.Xu Y., Xu L., Han M., Liu X., Li F., Zhou X., et al. Altered mitochondrial DNA methylation and mitochondrial DNA copy number in an APP/PS1 transgenic mouse model of Alzheimer disease. Biochem Biophys Res Commun. 2019;520(1):41–46. doi: 10.1016/j.bbrc.2019.09.094. [DOI] [PubMed] [Google Scholar]
- 163.White M.J., McArthur K., Metcalf D., Lane R.M., Cambier J.C., Herold M.J., et al. Apoptotic caspases suppress mtDNA-induced STING-mediated type I IFN production. Cell. 2014;159(7):1549–1562. doi: 10.1016/j.cell.2014.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Rongvaux A., Jackson R., Harman C.C., Li T., West A.P., de Zoete M.R., et al. Apoptotic caspases prevent the induction of type I interferons by mitochondrial DNA. Cell. 2014;159(7):1563–1577. doi: 10.1016/j.cell.2014.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bo H., Kang W., Jiang N., Wang X., Zhang Y., Ji L.L. Exercise-induced neuroprotection of hippocampus in APP/PS1 transgenic mice via upregulation of mitochondrial 8-oxoguanine DNA glycosylase. Oxid Med Cell Longev. 2014;2014 doi: 10.1155/2014/834502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhang L., Qian Y., Li J., Zhou X., Xu H., Yan J., et al. BAD-mediated neuronal apoptosis and neuroinflammation contribute to Alzheimer's disease pathology. iScience. 2021;24(9) doi: 10.1016/j.isci.2021.102942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Cervera-Carles L., Alcolea D., Estanga A., Ecay-Torres M., Izagirre A., Clerigué M., et al. Cerebrospinal fluid mitochondrial DNA in the Alzheimer's disease continuum. Neurobiol Aging. 2017;53:192.e1. doi: 10.1016/j.neurobiolaging.2016.12.009. -e4. [DOI] [PubMed] [Google Scholar]
- 168.Gulen M.F., Samson N., Keller A., Schwabenland M., Liu C., Glück S., et al. cGAS-STING drives ageing-related inflammation and neurodegeneration. Nature. 2023;620(7973):374–380. doi: 10.1038/s41586-023-06373-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wu Z., Tang W., Ibrahim F., Chen X., Yan H., Tao C., et al. Aβ induces neuroinflammation and microglial M1 polarization via cGAS-STING-IFITM3 signaling pathway in BV-2 cells. Neurochem Res. 2023;48(9):2881–2894. doi: 10.1007/s11064-023-03945-5. [DOI] [PubMed] [Google Scholar]
- 170.Yang H., Wang H., Ren J., Chen Q., Chen Z.J. cGAS is essential for cellular senescence. Proc Natl Acad Sci USA. 2017;114(23):E4612. doi: 10.1073/pnas.1705499114. -e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Dewanjee S., Chakraborty P., Bhattacharya H., Chacko L., Singh B., Chaudhary A., et al. Altered glucose metabolism in Alzheimer's disease: role of mitochondrial dysfunction and oxidative stress. Free Radic Biol Med. 2022;193(Pt 1):134–157. doi: 10.1016/j.freeradbiomed.2022.09.032. [DOI] [PubMed] [Google Scholar]
- 172.Patro S., Ratna S., Yamamoto H.A., Ebenezer A.T., Ferguson D.S., Kaur A., et al. ATP synthase and mitochondrial bioenergetics dysfunction in Alzheimer's disease. Int J Mol Sci. 2021;22(20) doi: 10.3390/ijms222011185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Strope T.A., Birky C.J., Wilkins H.M. The role of bioenergetics in neurodegeneration. Int J Mol Sci. 2022;23(16) doi: 10.3390/ijms23169212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Preeti K., Sood A., Fernandes V. Metabolic regulation of glia and their neuroinflammatory role in Alzheimer's disease. Cell Mol Neurobiol. 2022;42(8):2527–2551. doi: 10.1007/s10571-021-01147-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Li H., Chen Z., Shen Y., Xiong T., Chen A., Chen L., et al. Gene therapy in Aβ-induced cell and mouse models of Alzheimer's disease through compensating defective mitochondrial complex I function. J Transl Med. 2024;22(1):760. doi: 10.1186/s12967-024-05571-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Peng Y.Y., Li M.X., Li W.J., Xue Y., Miao Y.F., Wang Y.L., et al. DJ1 ameliorates AD-like pathology in the Hippocampus of APP/PS1 mice. Biomed Environ Sci. 2023;36(11):1028–1044. doi: 10.3967/bes2023.133. [DOI] [PubMed] [Google Scholar]
- 177.Jia J., Yin J., Zhang Y., Xu G., Wang M., Jiang H., et al. Thioredoxin-1 promotes mitochondrial biogenesis through regulating AMPK/Sirt1/PGC1α pathway in Alzheimer's disease. ASN Neuro. 2023;15 doi: 10.1177/17590914231159226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Katsouri L., Lim Y.M., Blondrath K., Eleftheriadou I., Lombardero L., Birch A.M., et al. PPARγ-coactivator-1α gene transfer reduces neuronal loss and amyloid-β generation by reducing β-secretase in an Alzheimer's disease model. Proc Natl Acad Sci USA. 2016;113(43):12292–12297. doi: 10.1073/pnas.1606171113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wang J., Liu W.J., Shi H.Z., Zhai H.R., Qian J.J., Zhang W.N. A role for PGC-1a in the control of abnormal mitochondrial dynamics in Alzheimer's disease. Cells. 2022;11(18) doi: 10.3390/cells11182849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Xie W., Guo D., Li J., Yue L., Kang Q., Chen G., et al. CEND1 deficiency induces mitochondrial dysfunction and cognitive impairment in Alzheimer's disease. Cell Death Differ. 2022;29(12):2417–2428. doi: 10.1038/s41418-022-01027-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zhou G., Ye Q., Xu Y., He B., Wu L., Zhu G., et al. Mitochondrial calcium uptake 3 mitigates cerebral amyloid angiopathy-related neuronal death and glial inflammation by reducing mitochondrial dysfunction. Int Immunopharm. 2023;117 doi: 10.1016/j.intimp.2022.109614. [DOI] [PubMed] [Google Scholar]
- 182.Deng Q.S., Dong X.Y., Wu H., Wang W., Wang Z.T., Zhu J.W., et al. Disrupted-in-Schizophrenia-1 attenuates amyloid-β generation and cognitive deficits in APP/PS1 transgenic mice by reduction of β-site APP-cleaving enzyme 1 levels. Neuropsychopharmacology. 2016;41(2):440–453. doi: 10.1038/npp.2015.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wang J.-H., Gessler D.J., Zhan W., Gallagher T.L., Gao G. Adeno-associated virus as a delivery vector for gene therapy of human diseases. Signal Transduct Targeted Ther. 2024;9(1):78. doi: 10.1038/s41392-024-01780-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Li H., Sun B., Huang Y., Zhang J., Xu X., Shen Y., et al. Gene therapy of yeast NDI1 on mitochondrial complex I dysfunction in rotenone-induced Parkinson's disease models in vitro and vivo. Mol Med. 2022;28(1):29. doi: 10.1186/s10020-022-00456-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mencke P., Boussaad I., Romano C.D., Kitami T., Linster C.L., Krüger R. The role of DJ-1 in cellular metabolism and pathophysiological implications for Parkinson's disease. Cells. 2021;10(2) doi: 10.3390/cells10020347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Oberacker T., Kraft L., Schanz M., Latus J., Schricker S. The importance of thioredoxin-1 in health and disease. Antioxidants. 2023;12(5) doi: 10.3390/antiox12051078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Mihaylov S.R., Castelli L.M., Lin Y.-H., Gül A., Soni N., Hastings C., et al. The master energy homeostasis regulator PGC-1α exhibits an mRNA nuclear export function. Nat Commun. 2023;14(1):5496. doi: 10.1038/s41467-023-41304-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gaitanou M., Segklia K., Matsas R. Cend1, a story with many tales: from regulation of cell cycle progression/exit of neural stem cells to brain structure and function. Stem Cell Int. 2019;2019 doi: 10.1155/2019/2054783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Patron M., Granatiero V., Espino J., Rizzuto R., De Stefani D. MICU3 is a tissue-specific enhancer of mitochondrial calcium uptake. Cell Death Differ. 2019;26(1):179–195. doi: 10.1038/s41418-018-0113-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Atlante A., Amadoro G., Latina V., Valenti D. Therapeutic potential of targeting mitochondria for Alzheimer's disease treatment. J Clin Med. 2022;11(22) doi: 10.3390/jcm11226742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Austad S.N., Ballinger S., Buford T.W., Carter C.S., Smith D.L., Jr., Darley-Usmar V., et al. Targeting whole body metabolism and mitochondrial bioenergetics in the drug development for Alzheimer's disease. Acta Pharm Sin B. 2022;12(2):511–531. doi: 10.1016/j.apsb.2021.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Bhatti G.K., Gupta A., Pahwa P., Khullar N., Singh S., Navik U., et al. Targeting mitochondrial bioenergetics as a promising therapeutic strategy in metabolic and neurodegenerative diseases. Biomed J. 2022;45(5):733–748. doi: 10.1016/j.bj.2022.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Bao X., Liu X., Wu Q., Ye F., Shi Z., Xu D., et al. Mitochondrial-targeted antioxidant MitoQ-mediated autophagy: a novel strategy for precise radiation protection. Antioxidants. 2023;12(2) doi: 10.3390/antiox12020453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Johri A. Disentangling mitochondria in Alzheimer's disease. Int J Mol Sci. 2021;22(21) doi: 10.3390/ijms222111520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Oliver D.M.A., Reddy P.H. Small molecules as therapeutic drugs for Alzheimer's disease. Mol Cell Neurosci. 2019;96:47–62. doi: 10.1016/j.mcn.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Jia Y.L., Sun S.J., Chen J.H., Jia Q., Huo T.T., Chu L.F., et al. SS31, a small molecule antioxidant peptide, attenuates β-amyloid elevation, mitochondrial/synaptic deterioration and cognitive deficit in SAMP8 mice. Curr Alzheimer Res. 2016;13(3):297–306. doi: 10.2174/1567205013666151218150004. [DOI] [PubMed] [Google Scholar]
- 197.Reddy P.H., Manczak M., Kandimalla R. Mitochondria-targeted small molecule SS31: a potential candidate for the treatment of Alzheimer's disease. Hum Mol Genet. 2017;26(8):1597. doi: 10.1093/hmg/ddx129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Sulaimon L.A., Afolabi L.O., Adisa R.A., Ayankojo A.G., Afolabi M.O., Adewolu A.M., et al. Pharmacological significance of MitoQ in ameliorating mitochondria-related diseases. Advances in Redox Research. 2022;5 [Google Scholar]
- 199.Young M.L., Franklin J.L. The mitochondria-targeted antioxidant MitoQ inhibits memory loss, neuropathology, and extends lifespan in aged 3xTg-AD mice. Mol Cell Neurosci. 2019;101 doi: 10.1016/j.mcn.2019.103409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Jia Y.-L., Wang W., Han N., Sun H.-L., Dong F.-M., Song Y.-X., et al. The mitochondria-targeted small molecule SS31 delays progression of behavioral deficits by attenuating β-amyloid plaque formation and mitochondrial/synaptic deterioration in APP/PS1 mice. Biochem Biophys Res Commun. 2023;658:36–43. doi: 10.1016/j.bbrc.2023.02.076. [DOI] [PubMed] [Google Scholar]
- 201.Hou Y., Wei Y., Lautrup S., Yang B., Wang Y., Cordonnier S., et al. NAD(+) supplementation reduces neuroinflammation and cell senescence in a transgenic mouse model of Alzheimer's disease via cGAS-STING. Proc Natl Acad Sci USA. 2021;118(37) doi: 10.1073/pnas.2011226118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zhao Y., Sun X., Hu D., Prosdocimo D.A., Hoppel C., Jain M.K., et al. ATAD3A oligomerization causes neurodegeneration by coupling mitochondrial fragmentation and bioenergetics defects. Nat Commun. 2019;10(1):1371. doi: 10.1038/s41467-019-09291-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zhao Y., Hu D., Wang R., Sun X., Ropelewski P., Hubler Z., et al. ATAD3A oligomerization promotes neuropathology and cognitive deficits in Alzheimer's disease models. Nat Commun. 2022;13(1):1121. doi: 10.1038/s41467-022-28769-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Yuan Y., Chen J., Ge X., Deng J., Xu X., Zhao Y., et al. Activation of ERK-Drp1 signaling promotes hypoxia-induced Aβ accumulation by upregulating mitochondrial fission and BACE1 activity. FEBS Open Bio. 2021;11(10):2740–2755. doi: 10.1002/2211-5463.13273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Reddy P.H., Manczak M., Yin X., Reddy A.P. Synergistic protective effects of mitochondrial division inhibitor 1 and mitochondria-targeted small peptide SS31 in Alzheimer's disease. J Alzheimers Dis. 2018;62(4):1549–1565. doi: 10.3233/JAD-170988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Baek S.H., Park S.J., Jeong J.I., Kim S.H., Han J., Kyung J.W., et al. Inhibition of Drp1 ameliorates synaptic depression, Aβ deposition, and cognitive impairment in an Alzheimer's disease model. J Neurosci. 2017;37(20):5099–5110. doi: 10.1523/JNEUROSCI.2385-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Qi X., Qvit N., Su Y.C., Mochly-Rosen D. A novel Drp1 inhibitor diminishes aberrant mitochondrial fission and neurotoxicity. J Cell Sci. 2013;126(Pt 3):789–802. doi: 10.1242/jcs.114439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Srivastava A., Johnson M., Renna H.A., Sheehan K.M., Ahmed S., Palaia T., et al. Therapeutic potential of P110 peptide: new insights into treatment of Alzheimer's disease. Life. 2023;13(11) doi: 10.3390/life13112156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Joshi A.U., Saw N.L., Shamloo M., Mochly-Rosen D. Drp1/Fis1 interaction mediates mitochondrial dysfunction, bioenergetic failure and cognitive decline in Alzheimer's disease. Oncotarget. 2018;9(5):6128–6143. doi: 10.18632/oncotarget.23640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Zhou Y., Luo D., Shi J., Yang X., Xu W., Gao W., et al. Loganin alleviated cognitive impairment in 3×Tg-AD mice through promoting mitophagy mediated by optineurin. J Ethnopharmacol. 2023;312 doi: 10.1016/j.jep.2023.116455. [DOI] [PubMed] [Google Scholar]