Abstract
Traumatic brain injury (TBI) triggers a series of pathophysiological events, contributing significantly to secondary injury and long-term functional deficits. While exosome therapy is beginning to emerge as a promising avenue for various injuries, its efficacy in TBI, using preclinical models that mimic the biomechanics of human acceleration/deceleration TBI, remains largely unexplored. This study investigated the capacity of human Schwann cell-derived exosomes (hSC-Exo) to improve outcomes in a model of moderate fluid percussion injury (FPI). We found that jugular infusion of hSC-Exo 30 min after trauma attenuated acute proinflammatory responses in the ipsilateral cortex and hippocampus 24 h post-TBI, as demonstrated by a reduction in levels of key inflammasome components, and decreased activation of the STAT3/pSTAT3/SOCS3 pathway. Furthermore, exosome treatment mitigated subacute histopathological changes, including a significant decrease in cerebral edema and contusion volumes at 72 h post-injury. Immunohistochemical analysis revealed a decrease in microglial activation, characterized by a shift toward a more ramified morphology. Importantly, hSC-exosome therapy led to the preservation of both sensorimotor function subacutely and cognitive performance at chronic time points. Flow cytometry analysis of peripheral blood at 21 days post-TBI demonstrated a reduction in circulating neutrophils, indicating an attenuation of chronic systemic inflammation. These findings highlight the multifaceted therapeutic benefits of hSC-Exo in a clinically-relevant FPI model, targeting both acute and chronic neuroinflammatory processes to promote functional recovery. This study provides new evidence to support hSC-exosomes as a therapeutic strategy for TBI, and emphasizes the translational potential of human exosomes for treating acute and progressive neurological injury.
Keywords: Exosomes, Traumatic brain injury, Schwann cells, STAT3, Neuroinflammation, Functional recovery
Introduction
Traumatic brain injury (TBI) is a global epidemic that affects more than 50 million individuals each year [1]. TBI results in a combination of extensive neurological, emotional, and functional consequences, ranging from mild headache, sleep disturbances, and cognitive fog, to those much more severe, including amnesia, aphasia, emotional disorders, motor dysfunction, coma, or brain death. Furthermore, symptoms may be transient or permanent, and often exacerbate due to the progressive nature of injurious mechanisms [2,3]. Injury-induced alterations result from the primary mechanical injury that occurs at the time of trauma, which is characterized by tissue deformation, shearing and sliding of the parenchyma, as well as vascular rupture [4]. At the immediate onset of the injury, an extensive array of secondary injury mechanisms commences and can persist for decades [5,6]. Detrimental secondary mechanisms after TBI are multifold and complex. While prevention, such as continued safety education on the importance of wearing seat belts and helmets are strategies for proactively addressing the potential occurrence of primary injuries, secondary injury mechanisms still lack defined treatment strategies.
The acute phase following TBI is characterized by a series of detrimental events, including excitotoxicity, oxidative stress, mitochondrial dysfunction, and a considerable neuroinflammatory response [7]. TBI-induced neuroinflammation is comprised of a complex series of signaling cascades and molecular players that can present as the activation of resident microglia, infiltration of peripheral leukocytes, and the release of proinflammatory cytokines [8,9]. One of the key molecular players implicated in TBI-induced neuroinflammation is the inflammasome, a multiprotein complex that is crucial to the innate immune response [10]. The nod-like receptor protein 3 (NLRP3) is one of the most widely studied inflammasomes and heavily implicated in posttraumatic neuroinflammatory responses after TBI [11]. The NLRP3 inflammasome assembles in the cytosol of primarily astrocytes and microglia after sensing specific priming signals, including pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs), which are endogenous molecules released by injured and dying cells [12]. Undergoing a tightly regulated process of activation and assembly, the oligomerization of NLRP3 recruits the adaptor protein, apoptosis-associated speck-like protein containing a CARD (ASC), and procaspase-1. This assembled complex forms the active NLRP3 inflammasome, which enables the autocatalytic cleavage and activation of caspase-1, subsequent cleavage of gasdermin D (GSDMD) and pyroptotic pore formation, as well as the maturation of proinflammatory cytokines interleukin-1β (IL-1β) and interleukin-18 (IL-18). These cytokines are released into the extracellular space, where they exacerbate and amplify neuroinflammatory responses and contribute to the pathogenesis of TBI [[13], [14], [15], [16]].
Among TBI-induced neuroinflammatory signaling cascades is the Janus kinase (JAK)/Signal Transducer and Activator of Transcription 3 (STAT3) pathway. Upon receptor activation by a signaling molecule, such as a cytokine or growth factor, JAKs are activated and phosphorylate STAT3, which then translocates to the nucleus and initiates DNA transcription of specific genes that control various critical cellular processes, such as proliferation, differentiation, survival, death, development, immunity, and inflammation, among others [17,18]. STAT dysregulation can occur under pathological conditions and contributes to various disease pathology. In the context of TBI, STAT3 is aberrantly upregulated and positively correlates to neuroinflammation and a greater degree of pyroptopsis-mediated neuronal death [19]. It promotes the expression of proinflammatory cytokines, chemokines, and other mediators that exacerbate neuronal damage [20]. After FPI in particular, Oliva and colleagues showed a significant, acute upregulation of phosphorylated STAT3 (pSTAT3) in the ipsilateral cortex and hippocampus that was localized primarily to astrocytic nuclei [21]. In other models of CNS injury, there is significant activation of STAT3 in reactive microglia/macrophages, and attenuation of STAT3 activation reduced infarct volumes, decreased apoptosis, and rescued functional deficits [22]. However, the role of STAT3 signaling is complex and can be bidirectional as this pathway is also involved in processes such as neuronal survival, regeneration, and tissue repair [23]. The overall net effect of STAT3 is highly context-dependent, influenced by factors such as timing, mechanism of injury, cell type, and the specific kinases responsible for its activation. While further studies are needed to fully understand its role in post-TBI inflammation and repair, targeting STAT3 may be a viable therapeutic strategy.
Recent findings have implicated STAT3 activation in NLRP3 inflammasome regulation [24,25]. Luo et al. showed that STAT3 enabled the translocation of NLRP3 to mitochondria, which is an essential step for inflammasome assembly [25]. Furthermore, the disruption of the interaction between STAT3 and NLRP3 specifically suppressed NLRP3 inflammasome activation and improved histopathological outcomes both in vitro and in vivo [25]. Disrupting the recruitment and association of inflammasome components remains a viable target for therapeutic strategies in pathological neuroinflammation. The interplay of STAT3 signaling and NLRP3 inflammasome activation after TBI has yet to be delineated.
Schwann cells are the myelinating glial cells of the peripheral nervous system. They are vital for efficient nerve conduction and actively participate in nerve regeneration and repair by contributing to axonal regrowth and recovery [26]. While traditionally investigated as a treatment option in peripheral nerve injury, more recent studies have demonstrated the immunomodulatory and neuroprotective properties of Schwann cells in central nervous system (CNS) injuries, including TBI [[27], [28], [29], [30]]. Schwann cells have been shown to exert their therapeutic effects through various mechanisms, including the secretion of neurotrophic factors, modulation of the inflammatory response, and promotion of axonal regeneration, making them a promising candidate for cell-based transplantation therapies in nervous system injury, as evidenced by previous and ongoing clinical investigations evaluating their safety and efficacy in promoting sciatic nerve repair and after spinal cord injury [30,31]. While Schwann cell-based therapies hold promise, there are significant limitations, including post-transplant cell survival, inability to migrate within astrocytic parenchyma, difficulties in targeted delivery, and restricted axonal regeneration [30,32].
Exosomes are nanosized, extracellular vesicles that are secreted by various cell types and play critical roles in intercellular communication. They have garnered significant attention as a therapeutic approach given their ability to cross biological barriers, their low immunogenicity, and cell-free compatibility with host tissues [33]. Furthermore, exosomes can contain a variety of bioactive molecules which can be transported to targeted recipient cells, and in turn modulate cellular function [34]. Recent studies have highlighted the potential of exosomes derived from stem cells and other sources in modulating neuroinflammation and promoting tissue repair in various models of CNS injury and neurological disorders [14,[35], [36], [37], [38], [39]].
The use of human Schwann cell-derived exosomes (hSC-Exo) for TBI is a compelling strategy, as it combines the therapeutic potential of Schwann cells with the advantages of exosome-based therapy. Furthermore, investigating human exosomes has significant translational implications. In preclinical studies, hSC-exosomes have been shown to promote neuronal survival, enhance axonal regeneration, and attenuate neuroinflammation in various CNS injury models [40,41]. Most recently, Nishimura and colleagues showed that in a model of severe TBI, systemic administration of hSC-Exos was neuroprotective, reduced secondary inflammatory mechanisms, and decreased cavity and perilesional contusion volumes [40]. The therapeutic efficacy of hSC-Exo in the context of fluid percussion TBI however has not yet been previously explored.
The present study sought to investigate the neuroprotective effects of hSC-Exo in a moderate FPI model of TBI, focusing on the modulation of neuroinflammatory responses, histopathological alterations, and functional outcomes. We hypothesized that jugular infusion of hSC-Exosomes 30 min after TBI will attenuate neuroinflammation, reduce brain edema, and improve sensorimotor and cognitive function after TBI.
Material and methods
Human Schwann cell culture
Human peripheral nerves were obtained from organ donors by the transplant procurement team of the University of Miami School of Medicine. Primary human Schwann Cell (SC) cultures were prepared and expanded by using methods analogous to those used in clinical trials [30,32,39]. Banked cell cultures were obtained from non-pathological nerve tissues isolated from males and females, ages 10 to 66, that tested negative for blood borne viruses. The donor used in the present study was a 38-year-old female. Cryopreservation of cell stocks was done in medium consisting of dimethyl sulfoxide (DMSO) and fetal bovine serum (FBS) at a ratio of 1:9.
Cryovials of human SCs were thawed at 37 °C and re-suspended in 10–15 mL of DMEM (Life Technologies) containing 10 % FBS (heat-inactivated; Hyclone) prior to collection by centrifugation and plating directly onto (1μg/cm2) laminin-coated T-75 or T-150 flask at a density of 1–2 × 106 cells/flask. Cells were cultured in mitogen-supplemented growth media consisting of high-glucose DMEM, 10 % FBS, 4 mM l-Glutamine (Sigma-Aldrich), 50 μg/mL of gentamicin, (APP Pharmaceutical/Fresenius Kabi), 10 nM heregulin (Genentech) and 2 μM forskolin (Sigma-Aldrich). The cells were maintained in a 37 °C incubator set to 8–9% CO2 for optimal growth. The cultures were observed under a phase contrast microscope to confirm cell adhesion to the substrate within 2–3 h of seeding. Regular media changes were performed until the cells reached confluency (70–80 % confluence).
Exosome isolation and characterization
Regular media was removed from SCs, cells were washed two times with PBS to remove any remaining FBS and replaced by FBS-free medium and cultured for 48 h. Exosomes were then isolated from the supernatant of SCs. Briefly, the supernatant was collected, centrifugated at 3.000 g for 10 min to remove cells debris and filtered through 0.2 μm filter and then subjected to centrifugation under 100,000 g for 130 min at 4 °C. The supernatant was then aspirated, the pellet was resuspended into 500 μL of cold PBS, and then aliquoted and stored at −80 °C.
Characterization of SC-exosomes was performed using a multi-faceted approach as previously described [40]. Briefly, visualization and quantification were conducted employing Nanoparticle Tracking Analysis (NTA) with a NanoSight NS300 instrument (Malvern Instruments Ltd.) and NTA 3.3 software. Morphological analysis was achieved through transmission electron microscopy (TEM), confirming the characteristic size and structure of these vesicles. Biochemical analysis included determination of total protein content using a DC Protein Assay with a microplate assay protocol. Flow cytometry was utilized to assess the presence of exosomal surface markers CD63, CD81, and CD9, with positive expression observed for each (95.9 %, 95.9 %, and 94.8 %, respectively). TEM imaging revealed a homogenous population of round or elliptical vesicles with diameters ranging from approximately 30 to 150 nm.
Animals
All experimental procedures were approved by the University of Miami's Animal Care and Use Committee and were conducted in compliance with the ARRIVE guidelines and those established by the National Institute of Health Guide for the Care and Use of Laboratory Animals. Adult male Sprague-Dawley rats (310–340 g) were housed in a temperature-controlled room (22 °C) and exposed to a 12 h light/dark cycle. They were acclimated for at least seven days before surgery. Rats were designated into the following groups: Sham PBS-Vehicle (Veh), Sham hSC-exosomes (hSC-exo), TBI Veh, or TBI hSC-exo. We did not observe any differences in any parameter between Sham Veh and Sham hSC-exo animals, thus they were collapsed into a single Sham group.
Fluid percussion injury surgery
Day 1
Animals were anesthetized with 3.0 % isoflurane and a mixture of 70 % nitrous oxide (N2O) and 30 % oxygen (O2). Once an adequate level of anesthesia was reached, the animals were placed in a stereotaxic frame and the scalp surgically incised. A 4.8 mm parasagittal craniotomy was made overlying the right parietal cortex (3.8 mm posterior to bregma and 2.5 mm lateral to the midline), and a plastic injury tube (3.5 mm inner diameter) was adhered to the skull over the intact dura. The scalp was then closed, and animals were allowed to recover before being returned to their home cage. At this point, food was removed, and animals were fasted overnight to maintain standardization, including consistent glucose levels.
Day 2
Twenty-four hours later, animals were reanesthetized with 3.0 % isoflurane in a mixture of 70 % N2O and 30 % O2. The tail artery was cannulated and blood samples were collected at regular intervals to monitor blood gases (pO2 and pCO2), blood pH, glucose, and mean arterial blood pressure (MABP). The right jugular vein was also cannulated as the intravenous route for infusion of hSC exosomes. Rats were intubated, immobilized with pancuronium bromide, mechanically ventilated, and maintained on 0.5–1.5 % isoflurane in a mixture of 70 % N2O, and 30 % O2. The fluid percussion device consists of a plexiglass cylindrical reservoir bounded at one end by a rubber-covered plexiglass piston with the opposite end fitted with a transducer housing and a central injury screw adapted for the rat skull. The entire system is filled with isotonic saline. The aseptic metal injury screw was next firmly connected to the plastic injury tube of the intubated and anesthetized rat. The injury is induced by the descent of a metal pendulum striking the piston, thereby injecting a small volume of saline epidurally into the closed cranial cavity and producing a brief displacement (18 msec) of neural tissue. The amplitude of the resulting pressure pulse was measured in atmospheres by a pressure transducer and recorded on a PowerLab chart recording system. Sham animals underwent identical surgical procedures but were not subjected to the fluid percussion pulse. In the present study, a moderate (1.8–2.2 atm) injury was generated. Physiological variables were maintained in normal ranges 15 min prior to TBI and for up to 1 h post-injury (pO2, 105–140 mmHg; pCO2, 35–45 mmHg; pH, 7.35–7.45), and brain and body temperatures were maintained at 37 °C using a feedback heat lamp system. After either the TBI or sham injury, the injury cap was removed, and the scalp was closed using staples. Appropriate pain management measures were taken to alleviate discomfort.
Exosome infusion
30 min after the fluid percussion insult, or identical time point in the sham surgery procedure, 1 × 1011 hSC-exosomes suspended in 300 μL of sterile PBS, or sterile PBS alone for vehicle groups, were slowly injected via the right jugular vein, followed by 200 μL of PBS to flush. This dosage was used based on preliminary findings and previous data [40].
Behavioral testing
Spontaneous forelimb task: Sensorimotor capacity was evaluated using the spontaneous forelimb task as previously described [42]. A transparent Plexiglas cylinder (20 cm diameter, 30 cm high) encourages use of forelimbs for vertical wall exploration. Animals were placed in the cylinder for 5 min per test and filmed. The video was played in slow motion (one-fifth real-time speed), and blinded observers quantified the number of times the right forelimb, left forelimb, or both forelimbs simultaneously made contact with the cylinder wall during a rearing movement. Asymmetry index was calculated by dividing the number of contralateral placements by the total placements of both paws. Behavior cohort groups were tested 1 day before surgery (baseline) and 3 days after TBI.
Morris water maze (MWM)
To assess cognitive function, a cohort of animals designated for behavioral testing underwent spatial memory evaluation using the MWM, which was comprised of a round pool (122 cm diameter; 60 cm deep) filled with water at ∼25 °C. The maze was located in a quiet, windowless room, with a variety of distinct, prominent extramaze cues. Four points on the rim, designated as north (N), east (E), south (S), and west (W), served as starting positions and divided the maze into four quadrants. A round platform (10 cm in diameter) was placed 1.5 cm beneath the surface of the water at a location that varied according to the requirements of the task (see below). Animal movements were digitally acquired with a CCD video that recorded the swim path. This animal's swim path was then analyzed with the Ethovision (Noldus) software program, which determined path length, latency to reach the platform (in seconds), time spent in each quadrant of the water maze, and swim speed.
Hidden Platform Task: At 14 d post TBI, animals were tested on the hidden platform task of the MWM to assess memory acquisition. The submerged, nonvisible platform was placed in the northeast quadrant of the maze. Animals were released into the pool from various quadrants, with release-point order identical for all animal groups. Each animal received four 60-s trials per day for four consecutive days. If the animal successfully located the platform, it was allowed to remain for 10 s. If it did not locate the platform within 60 s, the animal was gently guided to the platform and allowed to remain for a period of 10 s for memory acquisition training. Inter-trial intervals were 5–6 min, during which rats were placed back in their cage and under a heat lamp.
Working Memory Task: For the working memory task, the animal was given 60 s to find the submerged (non-cued) platform. If the animal failed to find the platform within 60 s, the animal was placed on the platform for 10 s. Five seconds following Trial 1 for the same rat, a second identical trial was conducted with the same release point and platform location. Rats were placed under a heat lamp for 5–6 min between each paired trial. The platform was moved to a new location within the water maze and the procedure was repeated. Five paired trials were administered per animal over a period of two consecutive days, 19–20 days post TBI.
Western blot analysis
At 24 h post surgery, animals were euthanized, brains removed, and ipsilateral cortices and hippocampi were freshly dissected and snap frozen in liquid nitrogen prior to storage at −80 °C. Prior to immunoblot use, tissue was homogenized and lysed in RIPA buffer with the cellular debris removed via centrifugation. Aliquots were mixed with Laemmli sample buffer with β-mercaptoethanol and stored at −80 °C until use.
Western blotting was performed as previously described [16]. Briefly, tissue samples were boiled then separated on 4 %−15%TGX Stain-free Precast gels (Bio-Rad). Gels were then transferred onto polyvinylidene difluoride (PVDF) membranes utilizing Turbo Transfer mixed molecular weight protocol (Bio-Rad). Membranes were blocked in blocking buffer (Biorad) and then incubated in antibodies to inflammatory-associated proteins: caspase-1 (Protein Tech, 1:1000), ASC (Santa Cruz, 1:500), IL-18, (R&D Systems, 1:250), GSDMD (Abcam 1:1000) or STAT3-associated proteins: STAT3 (Cell Signaling, 1:1000), pSTAT3 (Cell Signaling 1:2000), and SOCS3 (Abcam, 1:500). Membranes were washed in TBS-T prior to exposure to secondary antibodies and prior to exposure to imaging reagents. After primary antibody exposure, membranes were incubated in secondary antibodies containing horseradish peroxidase: IgG antirabbit (Cell Signaling, 1:2000) and IgG antimouse (Cell Signaling, 1:2000). Membranes were imaged using a Chemidoc GO touch imaging system (Bio-Rad) with results analyzed utilizing Image-Lab (Bio-Rad) software. The amount of total density of the proteins of interest was determined by measuring specific band intensity and dividing it by the respective band intensity of a beta actin (Thermo Fisher Scientific) loading control.
IL-1β cytokine analysis
IL-1β levels in cortical cell lysates (as prepared in Western blot methods) were measured using a V-PLEX Rat IL-1β assay kit (Meso Scale Discovery, Cat #K153QPD-1). Briefly, 50 μl of lysate from the ipsilateral cortex was diluted with 150 μl of diluent and analyzed according to the manufacturer's instructions. Analyte concentrations were determined using a MESO Quickplex SQ 120 MM plate reader and Discovery Workbench 4.0.12 software. A standard curve was generated by plotting the known concentrations of standard samples against corresponding measurements, and a four-parameter logistic model was used to fit the curve. This model was applied to estimate the concentration of IL-1β in the samples.
Volumetric analysis and immunohistochemistry
In a separate cohort of animals at 72 h post TBI or sham surgery, animals were transcardially perfused with isotonic saline following by 4 % paraformaldehyde in 0.1 M phosphate buffer. Brains were removed at completion and switched over to formalin for subsequent paraffin-embedded tissue processing. Brains were sectioned on a microtome into 10 μm thick sections and adhered to slides. Sections were deparaffinized and either immunostained with hematoxylin and eosin (H&E) or washed in PBS (0.4 % Triton X100) and antigen retrieval buffer (citrate buffer, 20 min in steamer). Tissue sections were then blocked with 3 % normal animal serum in PBS + for 2 h at room temperature, and then incubated in antibodies for ionized calcium-binding adapter molecule 1 (Iba1) (Santa Cruz, 1:1000) overnight at 4 °C. Tissue sections were washed in PBS and incubated in respective fluorescent secondary antibodies (Abcam) for 2 h at room temperature. Sections were then washed in PBS, mounted on slides, and cover slipped with DAPI Vector shield (Vector Labs). Slides were stored in darkness at 4 °C until imaging using a confocal microscope with a 60× oil objective and analyzed utilizing Leica or Olympus software.
Volumetric analyses
Antibody penetration was verified in all sections using 60× magnification. Cortical contusion volumes were determined by tracing the contused areas in serial H&E sections with a 5× objective on an Axiophot 200 M microscope using Neurolucida software as previously described [43]. Briefly, cortical contusion boundaries were well demarcated by hemorrhage and shearing at the gray/white matter interface between the cortex and external capsule in the ipsilateral hemisphere. Serial bregma levels were observed beginning at −1.0 mm posterior to bregma. At first indication of contusion, all subsequent bregma levels were included for volumetric analysis up until −6.8 mm posterior to bregma.
Iba1-positive phenotype analysis
The degree of Iba1-positive cell activation was quantified via Imaris software similar to previously published methodology [44]. Imaris was used to trace the microglia processes and quantify the number of intersections at each sholl. An Olympus spinning disk dragonfly confocal microscope at 63× magnification was used to obtain the Imaris file type (IMS) images. The version of Imaris used to access the saved IMS files was Imaris 10.1.0. The images contained DAPI and Iba1 channels. In Imaris, the Filament Tracer Module enabled the three-dimensional reconstruction of the microglia cells. The Iba1 channel was selected as the primary channel for Iba1 microglia activation analysis. The DAPI channel was deselected prior to filament reconstruction. A background subtraction was performed on each image to amplify the main Iba1 signals and reduce background fluorescence. Additionally, the orthogonal slicer feature on the XY plane was used to ensure inclusion of all microglia cells in the imaged section. The green filament icon helped illustrate the projecting filaments from the soma. The slice feature was utilized to quantify the smallest and largest soma diameter. For this study, the smallest microglia recorded had a 1 μm diameter soma, while the largest microglia had a 6 μm soma. Therefore, the diameter range was standardized across all images (1–6 μm). The spherical blue starting point marked the soma, while the white seed points indicated the filaments projecting from the soma. Imaris automatically identified the microglia's somas and filaments. Manual adjustments to the starting and seed points ensured the inclusion of all microglia cells for a given image. All the microglia present in an image were included and no somas were excluded. Imaris connected the starting and seed points, ultimately rendering three-dimensional microglia. Each Imaris image was analyzed in its entirety via this filament reconstruction procedure.
Quantitative data analysis: The data type of interest included the “Filament Number of Sholl Intersections.” Through the statistics module, Imaris generated an Excel data sheet with the raw data. The data analysis included sorting the Radii from lowest to highest values. The Radii represented the radial distance from the soma in microns (μm). The Imaris Excel data sheet consisted of the number of intersections at various radii with the radius value capturing the microglia into different levels of microglia activation. Three brain sections were analyzed per animal. The data acquisition was performed at approximately −3 mm to −4.3 mm bregma for the cortex near the injury site. The distribution curve of microglial process length (x-axis = radial distance from soma, 0–12 μm) and the number of such processes intersecting the radii (y-axis = number of intersections) served as a measure of de-ramification/activation [45]. Larger values for both radii and number of intersections corresponded to lower levels of microglia activation, while smaller values for both radii and number of intersections corresponded to higher levels of microglia activation. Sholl analysis was conducted by averaging the number of intersections (measured at distances up to 23 μm from the soma) across the three analyzed sections obtained from each animal.
Edema
A separate cohort of animals was generated to assess injury-induced edema. At 72 h post sham or FPI surgery, animals were reanethesized with 3 % isoflurane, 70 % N2O, and 30 % O2 for 5 min. Animals were decapitated, the brain carefully removed, and the right ipsilateral cortex and hippocampus freshly dissected and placed onto pre-weighed aluminum foil squares for immediate weighing using a precision analytical balance. Samples and foil were stationed on a Petri dish and then placed in a 100 °C vacuum oven for 72 h until completely dry. Samples were then reweighed and percent brain water content was calculated as the difference in wet and dry weights, divided by the wet weight, x 100 to acquire a percentage.
Flow cytometry
To analyze the phenotypic profiles of white blood cells (WBCs), flow cytometry was employed 21 days after FPI or sham surgery. Animals were anesthetized with 3.0 % isoflurane in 70/30 % mixture N2O and O2, respectively. 2 mL of peripheral whole blood was collected via cardiac puncture into heparinzed syringes. 100 μL of blood in 50 μL of flow cytometry staining buffer (eBioscience) were incubated with 13 fluorophore-conjugated cell surface marker antibodies (1:150) to determine WBC phenotype. The antibodies used were: CD45, CD86, RP-1, CD11b, CD8b, RT-1D, CD44, CD4, CD25, CD49b, CD3, CD62L, and CD43. Cell viability was determined using LIVE/DEAD Fixable Dead Cell Stain Kit (Invitrogen). Cells were then fixed with BD Cytofix (BD Biosciences). Flow cytometry data analysis was restricted to live cells, gated using standard forward and side scatter parameters. Gating boundaries were established by utilizing isotype controls and by performing single-color controls for each marker. This allowed for precise identification and analysis of distinct cell populations based on their unique scatter properties and marker expression. To acquire the samples, we used Beckman Coulter CytoFLEX S with CytExpert 2.0 as acquisition software. The resulting FCS files were analyzed using Kaluza 1.5A software provided by Beckman Coulter.
Statistics
Statistical analysis was conducted utilizing GraphPad Prism 10. Outliers were removed using the Robust regression and Outlier removal (ROUT) method. Western blot, edema, IHC, and flow cytometry data analysis included ordinary one-way or two-way ANOVAs followed by Tukey or Bonferroni post hoc multiple comparisons. Contusion volumes data were analyzed using unpair two-tailed t-test and two-way ANOVA with Bonferroni multiple comparison. Behavioral analysis was conducted with two-way ANOVA and two-way repeated measures ANOVA followed by Tukey's multiple comparison test. F values, p-values, means, mean differences, SE of differences, q statistics, degrees of freedom (DF), R squared, and 95 % CI for significant findings are reported in entirety in Supplemental Table 1.
To ensure the scientific rigor and reproducibility of our study, we adhered to the following principles: animal group assignment was randomized to minimize selection bias and ensure group comparability at baseline. A power analysis was conducted indicating a sample size of 5–10 per group to achieve a power of 0.8 with an alpha level of 0.05, ensuring sufficient statistical power to detect effects. Furthermore, all observers involved in data analyses were blinded to the experimental conditions to prevent observer bias.
Results
TBI causes a robust inflammatory response, characterized in part by the upregulation of proinflammatory cytokines. At 24 h after moderate fluid percussion TBI or sham surgery, we probed for alterations in the levels of several key proteins associated with inflammation and cell death in the ipsilateral cortex and hippocampus across animal groups. Western blot analyses revealed that TBI Vehicle-treated animals had significantly greater staining intensity of proinflammatory inflammasome proteins, including ASC, caspase-1, GSDMD, and IL-18, in the ipsilateral cortex (Fig. 1a–e) and hippocampus (Fig. 1f– j) relative to sham, uninjured controls, consistent with this TBI model. We found that treatment with hSC-exo after TBI however effectively mitigated this neuroinflammatory response compared to the vehicle-treated TBI group as evidenced by a significant reduction of proinflammatory protein expression levels in both the cortex and hippocampus. For full statistical details of each analysis conducted, please see Supplemental Table 1.
Fig. 1.
hSC-exosome treated animals had a significant reduction of proinflammatory inflammasome proteins relative to TBI control animals. Twenty-four hours after TBI, a subset of animals was sacrificed for detection of neuroinflammatory protein expression in the ipsilateral cortex (a–e) and ipsilateral hippocampus (f–j) via Western blot. Densitometric analyses revealed that TBI significantly upregulated levels of inflammasome markers, ASC, Caspase-1, GSDMD, and IL-18. Treating TBI animals with hSC exosomes however reduced the degree of inflammatory responses with levels resembling that of Sham animals. e, j) Representative immunoblot images from the ipsilateral cerebral cortex and hippocampus 24 h post FPI. Data are presented as mean ± SEM. One-way ANOVA followed by Tukey's post hoc test; ∗p-value <0.05; ∗∗p-values <0.01; ∗∗∗p-values <0.001; ∗∗∗∗p-values <0.0001.
We also probed for STAT3 and pSTAT3 proteins given that this pathway is activated after injury and highly involved in neuroinflammatory responses, including NLRP3 inflammasome assembly. We found a significant upregulation of both STAT3 and pSTAT3 in the cortex (Fig. 2a, b, d) and pSTAT3 in the hippocampus (Fig. 2f,h) after moderate FPI. hSC-exosome therapy however decreased the expression levels of STAT3 and pSTAT3, which was reflective of a suppression of this inflammatory cascade. Interestingly, we also observed an attenuation of TBI-induced SOCS3 protein upregulation after hSC-exosome treatment in the ipsilateral cortex (Fig. 2c and d) and trending in the hippocampus (Fig. 2g and h).
Fig. 2.
hSC-exosome treated animals had a significant reduction of STAT3 pathway components relative to TBI control animals. Twenty-four hours after TBI, a subset of animals was sacrificed for detection of STAT3 protein expression in the ipsilateral cortex (a–d) and ipsilateral hippocampus (e-h) via Western blot. Densitometric analyses revealed that TBI significantly upregulated levels of the expression of STAT3, pSTAT3, and SOCS3 proteins. Treating TBI animals with hSC exosomes however reduced the degree of inflammatory responses with levels resembling that of Sham animals. d, h) Representative immunoblot images from the ipsilateral cerebral cortex and hippocampus 24 h post FPI. Data are presented as mean ± SEM. One-way ANOVA followed by Tukey's post hoc test; ∗p-value <0.05; ∗∗p-values <0.01; ∗∗∗p-values <0.001; ∗∗∗∗p-values <0.0001.
Taken together, Western blot protein expression quantification assays at 24 h post TBI indicated that TBI leads to early activation of inflammasome signaling as well as the STAT3 pathway in vulnerable brain regions. In contrast, administration of hSC-exosomes exerted a potent anti-inflammatory effect by suppressing inflammatory cascades and modulating key STAT3 signaling pathways in both the cortex and hippocampus after TBI.
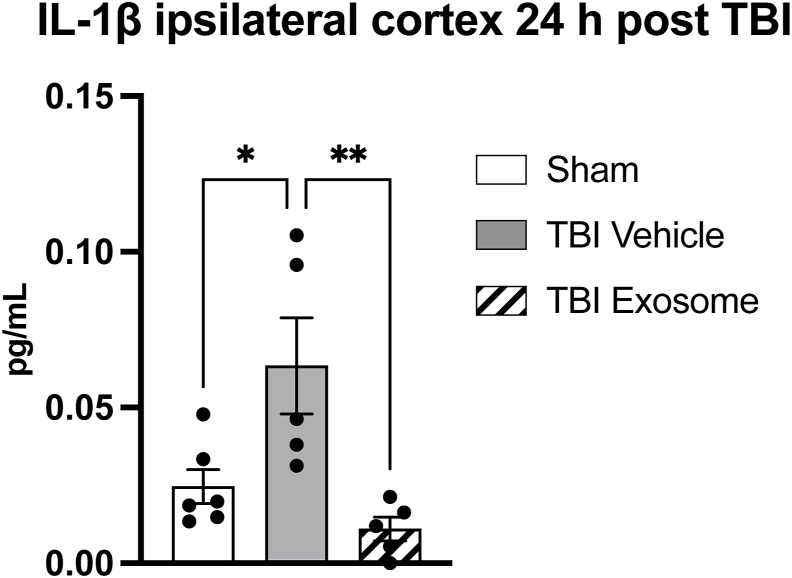
Additionally, using a Meso Scale Discovery (MSD) immunoassay, a highly sensitive, quantitative platform that also utilizes electrochemiluminescence detection for protein analysis, we observed a significant increase in interleukin 1 beta (IL-1β) levels in the ipsilateral cortex 24 h post TBI in the vehicle-treated group consistent with cerebral injury. Administration of hSC-exosomes however significantly attenuated this TBI-induced IL-1β upregulation demonstrating a neuroinflammatory profile comparable to sham uninjured animals (Fig. 3).
Fig. 3.
hSC-Exo administration mitigated IL-1β neuroinflammatory responses 24 h post TBI. Bar graph depicting IL-1β levels (in pg/mL) in the ipsilateral cortex 24 h post-TBI or sham surgery as quantified by a MSD multiplex immunoassay. Consistent with this injury model, IL-1β levels are upregulated in the acute stage after moderate fluid percussion impact. TBI-hSC exosome treated animals however showed significantly reduced protein levels and resembled sham, uninjured controls. One-way ANOVA followed by Tukey's post hoc test; ∗p-value <0.05; ∗∗p-value <0.01.
A separate cohort of animals was evaluated at a subacute 72 h post TBI time point to investigate histopathological alterations with and without hSC-exosome infusion. After parasagittal moderate FPI, our laboratory and others observe extensive edema in the injured brain resulting from the accumulation of excess fluid in the interstitial spaces and within cells [46]. Swelling of the brain parenchyma leads to increased intracranial pressure and secondary brain damage. We sought to assess whether exosome treatment would decrease edematous alterations after injury by measuring brain water content to provide a quantitative assessment of edema severity. 72 h after moderate FPI or sham surgery, animal groups were sacrificed and the ipsilateral cortex and hippocampus were dissected and weighed on a high-precision analytical balance to acquire wet-dry weights. Determining the percent brain water content across groups, we found that TBI-Vehicle control animals had a highly significant, elevated degree of edematous tissue in the ipsilateral cortex relative to the sham uninjured group. Administration of hSC-exosomes after TBI caused a significant reduction in edema (Fig. 4). We did not observe any alterations in the ipsilateral hippocampus.
Fig. 4.
Systemic hSC-exosome infusion reduced posttraumatic edema in the ipsilateral cortex 72 h post TBI. TBI resulted in a significant increase in percent brain water content compared to sham controls, indicating the development of edema consistent with this model. Treatment with hSC-exos significantly attenuated this increase with brain water content levels more closely resembling sham animals. These edematous alterations were not observed in the ipsilateral hippocampus. One-way ANOVA followed by Tukey's post hoc test; ∗∗p < 0.01, ∗∗∗∗p < 0.0001.
In addition to assessing the development of FPI-induced edema with and without exosome treatment, we also sought to compare the degree of contusion severity between injury groups at 72 h post TBI. Moderate FPI generates a distinct pattern of histopathology [47]. Adjacent to the impact site, a cortical contusion forms characterized by tissue disruption, hemorrhaging, and cell death [48]. Ventral to this area, at the gray/white matter interface, there exists a zone of architectural disruption, indicative of axonal injury. Using non-biased stereological methods and microscopy, we quantified the extent of these lesions and compared TBI-Vehicle and TBI-hSC exosome animals to determine if histological changes were present. We found that jugular infusion of hSC-exosomes 30 min post TBI significantly reduced contusion volumes compared to vehicle infusion, most notably at the epicenter of the injury (bregma level −4.3 mm) when comparing across the brain (Fig. 5).
Fig. 5.
hSC-exosome treatment reduced cortical contusion volumes at multiple bregma levels 72 h post TBI. Representative H&E sections showing the ipsilateral cortex of a TBI Vehicle (a) and TBI hSC-exosome (b) animal. Volumetric analyses revealed the TBI Veh group had a significantly greater cortical contusion volume relative to the hSC-exosome-treated TBI animals (c). This effect was observed across several bregma levels surrounding the injury epicenter (d). ∗p-value <0.05; ∗∗p-values <0.01. Unpaired two-tailed t-test; Two-way ANOVA with Bonferroni multiple comparison.
Given the robust anti-inflammatory effects we observed in hSC-exosome treated animals at 24 h post TBI, we further sought to determine the degree of activated microglia and macrophages at a 72-h post TBI time point as shown in Fig. 6. After FPI, resident microglia and infiltrating macrophages, identified by their Iba1-positive immunoreactivity, undergo a transformation in morphology. In the uninjured brain, as observed in the sham group, these cells exhibited a ramified quiescent state with small cell bodies and thin branching processes (Fig. 6a, left panel). However, in response to FPI, Iba1+ cells rapidly activated, transforming to an ameboid shape, with enlarged cell bodies and retraction of processes. Furthermore, activated Iba1+ cells clustered around the lesion core and were observed with greater fluorescence staining intensity relative to resting Iba1+ cells, as observed in TBI-Vehicle treated animals (Fig. 6a, middle panel). Using fluorescent immunohistochemical techniques and Sholl analyses [49], we quantitatively assessed the degree of Iba1+ cell activation across experimental groups (Fig. 6c and d). We found that the Sham uninjured group exhibited the highest number of intersections per Sholl, particularly at distances closer to the soma. This indicated that microglia in the Sham group had extensive branching and complex processes, characteristic of a resting or ramified state. The TBI Vehicle group however exhibited a marked decrease in the number of Sholl intersections, suggesting that microglia had undergone proinflammatory morphological changes in response to FPI with retracted processes and less ramification. This observation was indicative of proinflammatory microglial activation in response to injury. The TBI-hSC exosome group however demonstrated a partial recovery in the number of Sholl intersections compared to the TBI Vehicle group exhibiting a significantly greater ramified state and attenuated activation.
Fig. 6.
hSC-Exo treatment reduced the degree of activated microglia in the perilesional cortex 72 h after TBI. Fluorescent Iba1+ labeling in the penumbra of the cortical contusion showed varying degrees of microglia activation in three representative images from experimental groups (a and b). White lines demarcate area of cortical contusion (a) and yellow boxes areas of high magnification (b). C) Schematic depicting Sholl analysis approach on microglia with diverse morphologies. A series of concentric circles are overlaid onto images of Iba1+ cells, and the number of intersections between these circles and the cell's processes is quantified. A greater number of intersections at each Sholl are indicative of increased ramification, characterizing anti-inflammatory resting versus proinflammatory activated states. D) Sholl analyses of perilesional Iba1+ cells at 72 h post-TBI revealed a significantly higher proportion of ramified cells with greater process complexity (as indicated by intersections per Sholl) in both sham and exosome-treated animals compared to TBI-Vehicle animals. Two-way ANOVA followed by Bonferroni post hoc: at 0 μm, TV vs TE, TV vs S, TE vs S = ∗∗∗∗p-value <0.0001.
Whether improvement in histopathological outcomes and reduction in inflammatory responses had any effect on functional outcomes was next investigated. To this end, we generated an additional set of animals to assess sensorimotor function and cognitive capacity at a subacute and more chronic time point after FPI, respectively. The spontaneous forelimb placement task was employed to evaluate forelimb use asymmetry as a measure of sensorimotor capability before and after FPI [42]. As shown in Fig. 7, baseline asymmetry indices were comparable across all groups, with a value of 0.5 indicating symmetrical forelimb usage and intact sensorimotor function. Three days post-FPI, a significant increase in asymmetry (index value < 0.5), indicative of impaired sensorimotor function and a favoring of the injured left forelimb, was observed in the TBI Vehicle group, consistent with our moderate FPI model. Notably, hSC-exosome-treated TBI animals maintained a pre-injury asymmetry index near 0.5, demonstrating preserved sensorimotor function, which was comparable to sham uninjured animals.
Fig. 7.
hSC-exosome administration rescued sensorimotor deficits at 3 d post TBI. Animals underwent a sensorimotor task evaluating spontaneous forelimb placement. Left and right forelimbs placements were quantified and the degree of usage across groups was compared. Animals with an asymmetry index of less than 0.5 is indicative of a forelimb favor. Baseline pre-surgery assessment demonstrated groups utilized each left and right forelimbs equally. At 3 days-post surgery, TBI Veh animals demonstrated a left unilateral forelimb deficit consistent with our injury model. TBI animals treated with hSC-Exo however showed significantly equal forelimb usage comparable to sham groups. Two-way ANOVA followed by Tukey post hoc; ∗p-values <0.05.
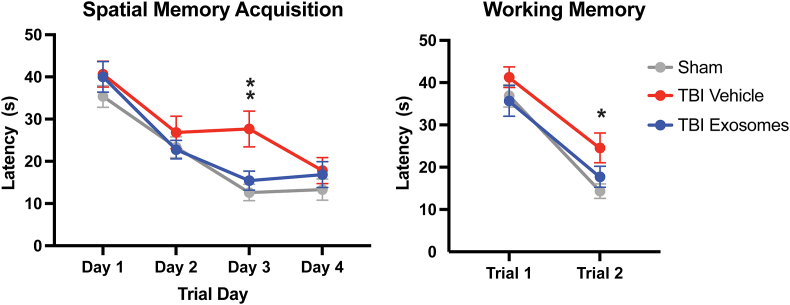
Our moderate FPI model results in impairments in hippocampal-dependent spatial learning and memory, evidenced by increased latencies to find a hidden platform in the Morris water maze [50]. Additionally, it causes short-term, working memory deficits, which can be observed in tasks requiring animals to remember a platform location across back-to-back trials. Using these well-established behavioral paradigms, we found that across 4 trial days, the sham group exhibited a steady decrease in latency, indicative of successful spatial learning acquisition (Fig. 8, left panel). In contrast, the TBI Vehicle group showed higher latencies, most notably on Day 3, demonstrating significantly impaired spatial learning at this chronic time point after injury. Injured animals receiving hSC-exosomes, however, maintained learning acquisition curves resembling sham animals, performing significantly better than their vehicle control counterparts.
Fig. 8.
Hippocampal-dependent spatial memory was preserved with hSC exosome treatment at chronic time points after TBI. Cognitive capacity of experimental groups was tested using the Morris water maze. A) In order to assess spatial memory acquisition, we utilized the hidden platform task. Sham animals (gray) exhibited a smooth, steadily decreasing trajectory of latencies as trial days progressed, which is indicative of an intact spatial memory learning curve. TBI-Vehicle-treated animas (red) however did not exhibit this same improvement pattern, instead revealed significantly impaired learning on Trial Day 3. TBI animals administered hSC-exosomes (blue) on the other hand did not show a TBI-induced learning impairment and maintained learning acquisition curves resembling sham animals. Two-way repeated measures ANOVA with Tukey post hoc: S vs TV ∗p-value = 0.013; TV vs TE, ∗p-value = 0.0491. B) In a short-term working memory assessment, we found that both Sham and TBI hSC-Exo groups were able to effectively recall the platform location as shown by a decreased latency between Trials 1 and 2. TBI Vehicle animals however were not able to recall location, demonstrating a significant short-term memory impairment compared to the Sham group. Two-way repeated measures ANOVA with Tukey post hoc: S vs TV ∗p-value = 0.0305.
In the second phase of cognitive testing, animals were assessed on short-term working memory, which is disrupted in this model of FPI. As expected, we observed that TBI Vehicle animals had greater difficulty in locating the platform on the second trial as evidenced by significantly increased latencies relative to the sham group (Fig. 8, right panel). Exosome treatment, however, attenuated this deficit, with TBI hSC-exosome animals demonstrating an intact ability to recall the platform location comparable to that of non-injured controls.
Our findings showed that hSC-exosome therapy significantly mitigated certain acute and subacute neuroinflammatory responses in cortical and subcortical structures after FPI (Fig. 1, Fig. 2, Fig. 3, Fig. 6). Given the reversal of sensorimotor deficits and preservation of cognitive function at later time points, we sought to determine whether chronic TBI-induced systemic inflammation might also be a viable target of systemically infused hSC-exosomes, with attenuation of its upregulation potentially contributing to improved functional outcomes. To this end, we collected peripheral whole blood from animals at 21 d post TBI to assess the degree of systemic inflammatory responses and investigate whether exosome treatment exerted therapeutic benefits in the context of leukocyte mobilization in response to injury. Using flow cytometry to identify and enumerate distinct leukocyte populations, we found that TBI significantly increased the percentage of RP-1+ neutrophils 21 d post TBI, indicative of a persistent systemic inflammatory response (Fig. 9). However, hSC-exosome therapy effectively attenuated this neutrophil recruitment, with RP-1+ cell percentages returning to levels comparable to sham controls. We did not observe any additional TBI- or TBI-exosome induced alterations in the other cell surface leukocyte markers assessed.
Fig. 9.
Flow cytometry analysis on peripheral whole blood showed a significantly reduced prevalence of infiltrating neutrophils in TBI hSC-Exo treated animals 21 d post injury. (a–c) Representative flow cytometry density plots showing RP-1+ leukocytes in whole blood from each experimental group. d) Gating quantification showed that hSC-Exo treatment after TBI normalized neutrophil levels to those comparable to sham uninjured animals. One-way ANOVA with Tukey multiple comparison.∗p-value <0.05.
Discussion
Traumatic brain injury triggers a complex cascade of pathophysiological events, including neuroinflammation, vascular disruption, and cellular dysfunction and death, which contribute to both acute and chronic neurological deficits. Despite dedicated and substantial research, effective therapeutic interventions for TBI are lacking. The present study explores the potential of human SC-derived exosomes as a novel therapeutic strategy for TBI.
The current findings demonstrate that hSC-exo therapy effectively mitigated the acute and subacute neuroinflammatory responses following TBI, as shown by a significant reduction in proinflammatory inflammasome proteins (ASC, caspase-1, GSDMD, IL-18, and IL-1β) 24 h post-injury as well as a decrease in microglial activation at a 72 h time point. The current results further revealed that hSC-exosome treatment significantly attenuated the TBI-induced upregulation of key components of the STAT3 pathway, including STAT3, pSTAT3, and SOCS3. As previously discussed, the STAT3 pathway is known to be acutely activated after brain injury, contributing to a detrimental pro-inflammatory environment characterized by excessive inflammation, cytokine expression, bias toward M1 microglia/macrophage polarization, and cell death and dysfunction [[19], [20], [21],51]. We sought to investigate this pathway given recent reports of STAT3 acting as a key player in NLRP3 inflammasome assembly as well as a recent study showing that Schwann cell-derived exosomes ameliorated STAT3-mediated activation of M1 macrophage polarization and promoted M2 phenotype [25,52].
In addition to a reduction of STAT3 and pSTAT3 activation after exosome administration, we also observed an exosome-mediated reduction of SOCS3 in the ipsilateral cortex. SOCS3 acts as a negative feedback regulator of the JAK/STAT3 pathway so as to prevent excessive signaling. We propose that hSC-exosomes were acting to restore balance to overly-activated detrimental inflammatory responses through a nuanced regulatory mechanism rather than indiscriminate pathway suppression. This hypothesis is supported by the findings of Ji et al. who reported that knockdown of SOCS3 expression in a model of intracerebral hemorrhage promoted M2 macrophage polarization and ameliorated the inflammatory microenvironment, resulting in neuroprotection and functional recovery, which they showed was a direct result of suppression of NF-κB activation [51]. Further investigation is warranted to delineate the precise mechanisms by which hSC-exos modulate these intricate and complex signaling pathways.
While the precise molecular mechanisms underlying the interplay between hSC-exosomes, the NLRP3 inflammasome, and STAT3 signaling in attenuating the pro-inflammatory environment remain to be fully elucidated, it is well-established that hSC-exos carry a diverse cargo of anti-inflammatory molecules capable of directly or indirectly suppressing the inflammatory cascade [40,53,54]. Furthermore, they have been shown to mediate microglia/macrophage polarization toward an M2 phenotype, thereby reducing the production of proinflammatory cytokines and chemokines [52]. In recent studies, a comprehensive profiling of human Schwann cell extracellular vesicles (SCEV) was performed, which identified 732 microRNAs (miRNAs), with the top 30 contributing to axon growth, neuroprotection, and the attenuation of neuroinflammation [59]. Importantly, these human SCEV molecular profiles aligned with the activation of key signaling pathways, such as STAT3 and phosphoinositide 3-kinase/protein kinase B (PI3K-Akt), known to promote nervous system repair.
The observed decrease in brain edema and reduction in contusion volume at 72 h post-TBI further supports the anti-inflammatory and neuroprotective effects of hSC-exos, as well as their innate ability to assuage the injured milieu and allow for a more permissive environment for cell survival. hSC-exosomes after FPI may also promote neuro- and cytoprotection, potentially by modulating additional signaling pathways involved in cell death and survival, beyond STAT3.
The significant improvement in sensorimotor function and preservation of cognitive abilities in hSC-exo-treated animals highlights the translational therapeutic potential of this approach. This model of FPI results in diffuse and focal injuries that affect cortical and subcortical structures, leading to deficits that can include incoordination and difficulty initiating movements, as well as sensory pathway disruption, impairing an animal's ability to perceive touch, proper proprioception, and other sensory information essential for guiding movement. Thus, injured rats exhibit asymmetrical limb use, favoring the non-impaired limb and neglecting the other as a compensatory strategy. hSC-exosomes may influence spontaneous forelimb task outcomes through multiple mechanisms, including the amelioration of inflammation and dampening of secondary brain damage within these vulnerable brain regions, the promotion of neuronal survival and repair within sensorimotor pathways, and the support of myelination and nerve transmission, ultimately improving function and coordination. Further investigation is needed to elucidate the specific mechanisms contributing to sensorimotor outcomes that we observed in the present study.
The attenuation of TBI-induced deficits in spatial learning and working memory suggests that hSC-exos may exert long-term beneficial effects on hippocampal function, possibly by promoting neurogenesis, synaptic plasticity, neurotrophic support, or neuronal repair, however the direct mechanisms responsible for the preservation of neurological function in the present study remain to be investigated. The hidden platform task is a means of assessing acquisition memory with intact spatial learning indicated by consistent improvement and a smooth downward trajectory of decreasing latency from Trial Day 1 through Trial Day 4. This pattern was observed in Sham animals as expected as well as in TBI-hSC Exo animals, demonstrating intact spatial memory after TBI (Fig. 7a). While TBI-Vehicle-treated animals had similar latencies to sham and hSC-Exo-treated animals on Trial Day 4, they showed statistically significant impairment on Trial Day 3. Thus, by not exhibiting the proper steady learning curve that is the benchmark of this behavioral paradigm, we can conclude that their spatial learning is deficient [55].
Our findings indicated that hSC-exo therapy also modulates the chronic, systemic inflammatory response observed after TBI [56], as evidenced by the reduced percentage of RP-1+ neutrophils in peripheral blood at 21 days post-injury relative to the TBI-vehicle control group, which demonstrated sustained upregulated levels. This suggests that hSC-exos may exert their therapeutic effects through both central and peripheral mechanisms, potentially by regulating the activity of immune cells to modulate systemic inflammation, and resulting in significantly improved functional outcomes.
It is yet to be fully understood how exosome treatment mechanistically exerts its therapeutic effects after fluid percussion brain trauma as FPI is characterized by a multifaceted series of complex pathophysiology. However, based on our findings, we propose that hSC-exosomes may primarily mitigate pathological neuroinflammation via STAT3 modulation and disruption of NLRP3 inflammasome assembly, promoting cell survival and function, and ultimately leading to improved functional and cognitive outcomes. Future investigations are needed to explore whether additional exosome-mediated actions, such as enhanced neurogenesis, neurotrophic support, or synaptic plasticity, also contribute to the observed benefits. Further research is needed to fully elucidate the STAT3 pathway, including the identification of its upstream and downstream regulators and the precise role of SOCS3 in modulating its activity. Highlighting the intricacies of STAT3 signaling in the context of our findings, Wen et al. showed that miRNA from exosomes derived from mesenchymal stem cells reversed TBI-induced neuroinflammation through the STAT3 pathway via IL-10 mediated activation, while a separate study demonstrated that SOCS3 reduction, which was observed in the present study, permitted anti-inflammatory IL-10/STAT3 signaling to predominate and persist [57,58]. Therefore, a comprehensive understanding of the intricate interplay between hSC-exosomes, the NLRP3 inflammasome, and the STAT3 signaling pathway, including the precise roles of upstream and downstream regulators and the nuanced influence of SOCS3 is crucial to harnessing the full therapeutic potential of hSC-exos in promoting neuroprotection and functional recovery after TBI.
The use of human Schwann cell-derived exosomes may offer several potential advantages over exosomes derived from other cell types or non-human species. First, human exosomes may be less likely to elicit an immune response, reducing the risk of rejection or adverse reactions in the clinical setting. Secondly, the use of hSC-exos aligns with the growing trend toward personalized medicine, as they can potentially be derived from the patient's own cells, further minimizing the risk of immune rejection and maximizing therapeutic efficacy. Third, hSC-exos may possess unique bioactive molecules or signaling pathways that are specific for human neuroregeneration and repair, such as a distinct isoform or variant of a molecule that may be particularly effective in humans. While research is ongoing to fully characterize the unique molecular cargo of human exosomes, some have already been identified and hold particular relevance for axon growth, neuroprotection, and the attenuation of neuroinflammation. In a recent paper, Khan et al. conducted a comprehensive profile assessing the bioactivity and molecular cargo of human SC-exosomes [59] Using omics characterization, they identified 136 proteins, over 700 miRNAs, and unique lipid profiles associated with key processes related to nervous system repair. Furthermore, these molecular profiles align closely with essential signaling pathways for human nervous system regeneration and neuroprotection, suggesting a high-therapeutic potential of hSC-exosomes in neurological applications.
Our study further adds support for the therapeutic potential of hSC-exo therapy in TBI. It is important that future research focus on further elucidating the mechanisms of action of hSC-exos, such as identifying the specific bioactive molecules and precise signaling pathways involved in the observed therapeutic effects. In this regard, our laboratory is currently pursuing novel approaches to fully characterize exosomes from multiple sample types using fluorescent imaging and single particle interferometry. Additionally, it is vital to optimize the production, purification, and delivery of hSC-exos to ensure their safety and efficacy in clinical settings. Advanced imaging techniques, such as magnetic resonance imaging and positron emission tomography scans, could be used to track the biodistribution of hSC-exos systemically and in the brain, providing valuable insight into therapeutic targets and activity. Furthermore, longitudinal studies are needed to assess the long-term effects of hSC-exo therapy on functional outcomes, cognitive function, and quality of life in TBI patients. It is critical to evaluate the safety and efficacy of hSC-exo therapy in clinical trials, comparing it to existing treatment options. Additionally, exploring its potential in combination with other neuroprotective or neuroregenerative therapies, such as hypothermia, could further enhance its efficacy, establishing exosomes as a compelling approach for developing targeted and effective TBI treatments.
There are several limitations to the present study that are worth noting. Firstly, the focus on a single time point (24 h) for inflammatory marker and STAT3 pathway assessment only provides a snapshot of the complex inflammatory process in response to hSC-exosome therapy. Future studies should incorporate multiple time points to fully understand the temporal dynamics and the specific targets of hSC-exosome action. This limitation also applies to assessment of functional outcomes. We evaluated sensorimotor function and cognitive capacity at only two specific time points. However, a more thorough assessment of functional recovery and evaluation of other behavioral paradigms, would provide a more comprehensive view of the therapeutic potential of hSC-exosomes. Furthermore, this study does not address potential confounding biological variables such as age or sex, which may significantly influence outcomes [[56], [60]]. Infusing hSC-exosomes intravenously into the jugular also presents with some limitations, including suboptimal biodistribution as well as possible off-target systemic effects that may be undesirable. Additionally, we infused a single dose of exosomes at 30 min post-TBI, which may not be as clinically relevant as administration at a later time point or as efficacious as additional dosing. Future research should investigate various treatment windows, doses, and routes of administration to maximize therapeutic effects for optimal clinical relevancy.
hSC-exo therapy represents a promising approach for the treatment of TBI, harnessing the regenerative and immunomodulatory potential of human Schwann cells. Our findings demonstrate that hSC-exos exert potent anti-inflammatory, neuroprotective, and immunomodulatory effects, leading to improved functional outcomes after TBI. Further research is warranted to continue to work toward possible clinical studies and to develop hSC-exo-based therapies for the treatment of TBI and other neurological disorders.
Author disclosure
HMB and WDD are cofounders and managing members of InflammaCORE, LLC and have licensed patents on inflammasome proteins as markers of injury and disease, as well as targeting inflammasome proteins for therapeutic purposes. HMB and WDD are Scientific Advisory Board Members of ZyVersa Therapeutics.
Author contributions
MOB, HMB, and WDD made substantial contributions to the conception and design of experiments, data acquisition, and data analysis and interpretation. All authors have also been involved in drafting and revising the manuscript. All authors have approved the final manuscript.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:W. Dalton Dietrich reports financial support was provided by University of Miami Miller School of Medicine. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors wish to acknowledge Dr. Fabiola Placeres Uray, Mr. Roey Hadad, Dr. Nathan Johnson, Dr. Coleen Atkins, and Ms. Yan Shi for their technical expertise.
This work was supported by NIH/NINDS R37NS133195 and RF1NS125578.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.neurot.2025.e00555.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Dams-O'Connor K., Juengst S.B., Bogner J., Chiaravalloti N.D., Corrigan J.D., Giacino J.T., et al. Traumatic brain injury as a chronic disease: insights from the United States traumatic brain injury model systems research program. Lancet Neurol. 2023;22(6):517–528. doi: 10.1016/S1474-4422(23)00065-0. [DOI] [PubMed] [Google Scholar]
- 2.Bramlett H.M., Dietrich W.D. Quantitative structural changes in white and gray matter 1 year following traumatic brain injury in rats. Acta Neuropathol. 2002;103(6):607–614. doi: 10.1007/s00401-001-0510-8. [DOI] [PubMed] [Google Scholar]
- 3.Bramlett H.M., Dietrich W.D. Long-term consequences of traumatic brain injury: current status of potential mechanisms of injury and neurological outcomes. J Neurotrauma. 2015;32(23):1834–1848. doi: 10.1089/neu.2014.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dietrich W.D., Alonso O., Busto R., Prado R., Zhao W., Dewanjee M.K., et al. Posttraumatic cerebral ischemia after fluid percussion brain injury: an autoradiographic and histopathological study in rats. Neurosurgery. 1998;43(3):585–593. doi: 10.1097/00006123-199809000-00105. ; discussion 93-4. [DOI] [PubMed] [Google Scholar]
- 5.Dietrich W.D., Alonso O., Busto R., Ginsberg M.D. Widespread metabolic depression and reduced somatosensory circuit activation following traumatic brain injury in rats. J Neurotrauma. 1994;11(6):629–640. doi: 10.1089/neu.1994.11.629. [DOI] [PubMed] [Google Scholar]
- 6.Dietrich W.D., Alonso O., Busto R., Prado R., Dewanjee S., Dewanjee M.K., et al. Widespread hemodynamic depression and focal platelet accumulation after fluid percussion brain injury: a double-label autoradiographic study in rats. J Cerebr Blood Flow Metabol. 1996;16(3):481–489. doi: 10.1097/00004647-199605000-00015. [DOI] [PubMed] [Google Scholar]
- 7.Aghili-Mehrizi S., Williams E., Yan S., Willman M., Willman J., Lucke-Wold B. Secondary mechanisms of neurotrauma: a closer look at the evidence. Diseases. 2022;10(2):30. doi: 10.3390/diseases10020030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simon D.W., McGeachy M.J., Bayır H., Clark R.S., Loane D.J., Kochanek P.M. The far-reaching scope of neuroinflammation after traumatic brain injury. Nat Rev Neurol. 2017;13(3):171–191. doi: 10.1038/nrneurol.2017.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corps K.N., Roth T.L., McGavern D.B. Inflammation and neuroprotection in traumatic brain injury. JAMA Neurol. 2015;72(3):355–362. doi: 10.1001/jamaneurol.2014.3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo H., Callaway J.B., Ting J.P. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21(7):677–687. doi: 10.1038/nm.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chakraborty R., Tabassum H., Parvez S. NLRP3 inflammasome in traumatic brain injury: its implication in the disease pathophysiology and potential as a therapeutic target. Life Sci. 2023;314 doi: 10.1016/j.lfs.2022.121352. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Q., Raoof M., Chen Y., Sumi Y., Sursal T., Junger W., et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464(7285):104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Rivero Vaccari J.P., Lotocki G., Marcillo A.E., Dietrich W.D., Keane R.W. A molecular platform in neurons regulates inflammation after spinal cord injury. J Neurosci. 2008;28(13):3404–3414. doi: 10.1523/JNEUROSCI.0157-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Rivero Vaccari J.P., Brand F., 3rd, Adamczak S., Lee S.W., Perez-Barcena J., Wang M.Y., et al. Exosome-mediated inflammasome signaling after central nervous system injury. J Neurochem. 2016;136(Suppl 1):39–48. doi: 10.1111/jnc.13036. (0 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson N.H., de Rivero Vaccari J.P., Bramlett H.M., Keane R.W., Dietrich W.D. Inflammasome activation in traumatic brain injury and Alzheimer's disease. Transl Res. 2023;254:1–12. doi: 10.1016/j.trsl.2022.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson N.H., Kerr N.A., de Rivero Vaccari J.P., Bramlett H.M., Keane R.W., Dietrich W.D. Genetic predisposition to Alzheimer's disease alters inflammasome activity after traumatic brain injury. Transl Res. 2023;257:66–77. doi: 10.1016/j.trsl.2023.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu X., Li J., Fu M., Zhao X., Wang W. The JAK/STAT signaling pathway: from bench to clinic. Signal Transduct Targeted Ther. 2021;6(1):402. doi: 10.1038/s41392-021-00791-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo Q., Jin Y., Chen X., Ye X., Shen X., Lin M., et al. NF-κB in biology and targeted therapy: new insights and translational implications. Signal Transduct Targeted Ther. 2024;9(1):53. doi: 10.1038/s41392-024-01757-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang S., Lai N., Xu L. Neuronal pyroptosis mediated by STAT3 in early brain injury after subarachnoid hemorrhage. Brain Res. 2024;1822 doi: 10.1016/j.brainres.2023.148666. [DOI] [PubMed] [Google Scholar]
- 20.Chen E., Xu D., Lan X., Jia B., Sun L., Zheng J.C., et al. A novel role of the STAT3 pathway in brain inflammation-induced human neural progenitor cell differentiation. Curr Mol Med. 2013;13(9):1474–1484. doi: 10.2174/15665240113139990076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oliva A.A., Jr., Kang Y., Sanchez-Molano J., Furones C., Atkins C.M. STAT3 signaling after traumatic brain injury. J Neurochem. 2012;120(5):710–720. doi: 10.1111/j.1471-4159.2011.07610.x. [DOI] [PubMed] [Google Scholar]
- 22.Akira S. Roles of STAT3 defined by tissue-specific gene targeting. Oncogene. 2000;19(21):2607–2611. doi: 10.1038/sj.onc.1203478. [DOI] [PubMed] [Google Scholar]
- 23.Murray P.J. STAT3-mediated anti-inflammatory signalling. Biochem Soc Trans. 2006;34(Pt 6):1028–1031. doi: 10.1042/BST0341028. [DOI] [PubMed] [Google Scholar]
- 24.Zhu L., Wang Z., Sun X., Yu J., Li T., Zhao H., et al. STAT3/Mitophagy Axis coordinates macrophage NLRP3 inflammasome activation and inflammatory bone loss. J Bone Miner Res. 2023;38(2):335–353. doi: 10.1002/jbmr.4756. [DOI] [PubMed] [Google Scholar]
- 25.Luo L., Wang F., Xu X., Ma M., Kuang G., Zhang Y., et al. STAT3 promotes NLRP3 inflammasome activation by mediating NLRP3 mitochondrial translocation. Exp Mol Med. 2024;56(9):1980–1990. doi: 10.1038/s12276-024-01298-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jessen K.R., Mirsky R. The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci. 2005;6(9):671–682. doi: 10.1038/nrn1746. [DOI] [PubMed] [Google Scholar]
- 27.Yang L., Fang J.S., Wang W., Chen R.K., Shen C.F. Transplantation of Schwann cells differentiated from adipose-derived stem cells modifies reactive gliosis after contusion brain injury in rats. J Int Med Res. 2011;39(4):1344–1357. doi: 10.1177/147323001103900421. [DOI] [PubMed] [Google Scholar]
- 28.Pearse D.D., Bastidas J., Izabel S.S., Ghosh M. Schwann cell transplantation subdues the pro-inflammatory innate immune cell response after spinal cord injury. Int J Mol Sci. 2018;19(9) doi: 10.3390/ijms19092550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lavdas A.A., Papastefanaki F., Thomaidou D., Matsas R. Schwann cell transplantation for CNS repair. Curr Med Chem. 2008;15(2):151–160. doi: 10.2174/092986708783330593. [DOI] [PubMed] [Google Scholar]
- 30.Guest J.D., Santamaria A.J., Solano J.P., de Rivero Vaccari J.P., Dietrich W.D., Pearse D.D., et al. Challenges in advancing Schwann cell transplantation for spinal cord injury repair. Cytotherapy. 2025;27(1):36–50. doi: 10.1016/j.jcyt.2024.08.005. [DOI] [PubMed] [Google Scholar]
- 31.Levi A.D., Burks S.S., Anderson K.D., Dididze M., Khan A., Dietrich W.D. The use of autologous Schwann cells to supplement sciatic nerve repair with a large gap: first in human experience. Cell Transplant. 2016;25(7):1395–1403. doi: 10.3727/096368915X690198. [DOI] [PubMed] [Google Scholar]
- 32.Anderson K.D., Guest J.D., Dietrich W.D., Bartlett Bunge M., Curiel R., Dididze M., et al. Safety of autologous human Schwann cell transplantation in subacute thoracic spinal cord injury. J Neurotrauma. 2017;34(21):2950–2963. doi: 10.1089/neu.2016.4895. [DOI] [PubMed] [Google Scholar]
- 33.Kalluri R., LeBleu V.S. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478) doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yáñez-Mó M., Siljander P.R., Andreu Z., Zavec A.B., Borràs F.E., Buzas E.I., et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4 doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reed S.L., Escayg A. Extracellular vesicles in the treatment of neurological disorders. Neurobiol Dis. 2021;157 doi: 10.1016/j.nbd.2021.105445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dong X., Dong J.F., Zhang J. Roles and therapeutic potential of different extracellular vesicle subtypes on traumatic brain injury. Cell Commun Signal. 2023;21(1):211. doi: 10.1186/s12964-023-01165-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Putthanbut N., Lee J.Y., Borlongan C.V. Extracellular vesicle therapy in neurological disorders. J Biomed Sci. 2024;31(1):85. doi: 10.1186/s12929-024-01075-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y., Chopp M., Meng Y., Katakowski M., Xin H., Mahmood A., et al. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J Neurosurg. 2015;122(4):856–867. doi: 10.3171/2014.11.JNS14770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldschmidt-Clermont P.J., Khan A., Jimsheleishvili G., Graham P., Brooks A., Silvera R., et al. Treating amyotrophic lateral sclerosis with allogeneic Schwann cell-derived exosomal vesicles: a case report. Neural Regen Res. 2025;20(4):1207–1216. doi: 10.4103/NRR.NRR-D-23-01815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nishimura K., Sanchez-Molano J., Kerr N., Pressman Y., Silvera R., Khan A., et al. Beneficial effects of human Schwann cell-derived exosomes in mitigating secondary damage after penetrating ballistic-like brain injury. J Neurotrauma. 2024;41(21–22):2395–2412. doi: 10.1089/neu.2023.0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lopez-Leal R., Court F.A. Schwann cell exosomes mediate neuron-glia communication and enhance axonal regeneration. Cell Mol Neurobiol. 2016;36(3):429–436. doi: 10.1007/s10571-015-0314-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blaya M.O., Bramlett H.M., Naidoo J., Pieper A.A., Dietrich W.D. Neuroprotective efficacy of a proneurogenic compound after traumatic brain injury. J Neurotrauma. 2014;31(5):476–486. doi: 10.1089/neu.2013.3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blaya M.O., Tsoulfas P., Bramlett H.M., Dietrich W.D. Neural progenitor cell transplantation promotes neuroprotection, enhances hippocampal neurogenesis, and improves cognitive outcomes after traumatic brain injury. Exp Neurol. 2015;264:67–81. doi: 10.1016/j.expneurol.2014.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andreu M., Matti N., Bramlett H.M., Shi Y., Gajavelli S., Dietrich W.D. Dose-dependent modulation of microglia activation in rats after penetrating traumatic brain injury (pTBI) by transplanted human neural stem cells. PLoS One. 2023;18(5) doi: 10.1371/journal.pone.0285633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morrison H.W., Filosa J.A. A quantitative spatiotemporal analysis of microglia morphology during ischemic stroke and reperfusion. J Neuroinflammation. 2013;10:4. doi: 10.1186/1742-2094-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Balleste A.F., Alvarez J.C., Placeres-Uray F., Mastromatteo-Alberga P., Torres M.D., Dallera C.A., et al. Improvement in edema and cognitive recovery after moderate traumatic brain injury with the neurosteroid prodrug NTS-104. Neurotherapeutics. 2024 doi: 10.1016/j.neurot.2024.e00456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thompson H.J., Lifshitz J., Marklund N., Grady M.S., Graham D.I., Hovda D.A., et al. Lateral fluid percussion brain injury: a 15-year review and evaluation. J Neurotrauma. 2005;22(1):42–75. doi: 10.1089/neu.2005.22.42. [DOI] [PubMed] [Google Scholar]
- 48.Dietrich W.D., Alonso O., Halley M. Early microvascular and neuronal consequences of traumatic brain injury: a light and electron microscopic study in rats. J Neurotrauma. 1994;11(3):289–301. doi: 10.1089/neu.1994.11.289. [DOI] [PubMed] [Google Scholar]
- 49.Green T.R.F., Murphy S.M., Rowe R.K. Comparisons of quantitative approaches for assessing microglial morphology reveal inconsistencies, ecological fallacy, and a need for standardization. Sci Rep. 2022;12(1) doi: 10.1038/s41598-022-23091-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bramlett H.M., Green E.J., Dietrich W.D. Hippocampally dependent and independent chronic spatial navigational deficits following parasagittal fluid percussion brain injury in the rat. Brain Res. 1997;762(1-2):195–202. doi: 10.1016/s0006-8993(97)00387-9. [DOI] [PubMed] [Google Scholar]
- 51.Ji X.C., Shi Y.J., Zhang Y., Chang M.Z., Zhao G. Reducing suppressors of cytokine signaling-3 (SOCS3) expression promotes M2 macrophage polarization and functional recovery after intracerebral hemorrhage. Front Neurol. 2020;11 doi: 10.3389/fneur.2020.586905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ren J., Zhu B., Gu G., Zhang W., Li J., Wang H., et al. Schwann cell-derived exosomes containing MFG-E8 modify macrophage/microglial polarization for attenuating inflammation via the SOCS3/STAT3 pathway after spinal cord injury. Cell Death Dis. 2023;14(1):70. doi: 10.1038/s41419-023-05607-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ghosh M., Pearse D.D. Schwann cell-derived exosomal vesicles: a promising therapy for the injured spinal cord. Int J Mol Sci. 2023;24(24):17317. doi: 10.3390/ijms242417317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu B., Kong Y., Shi W., Kuss M., Liao K., Hu G., et al. Exosomes derived from differentiated human ADMSC with the Schwann cell phenotype modulate peripheral nerve-related cellular functions. Bioact Mater. 2022;14:61–75. doi: 10.1016/j.bioactmat.2021.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Amelchenko E.M., Bezriadnov D.V., Chekhov O.A., Ivanova A.A., Kedrov A.V., Anokhin K.V., et al. Cognitive flexibility is selectively impaired by radiation and is associated with differential recruitment of adult-born neurons. J Neurosci. 2023;43(34):6061–6083. doi: 10.1523/JNEUROSCI.0161-22.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Suzuki T., Bramlett H.M., Dietrich W.D. The importance of gender on the beneficial effects of posttraumatic hypothermia. Exp Neurol. 2003;184(2):1017–1026. doi: 10.1016/S0014-4886(03)00389-3. [DOI] [PubMed] [Google Scholar]
- 57.Wen L., Wang Y.D., Shen D.F., Zheng P.D., Tu M.D., You W.D., et al. Exosomes derived from bone marrow mesenchymal stem cells inhibit neuroinflammation after traumatic brain injury. Neural Regen Res. 2022;17(12):2717–2724. doi: 10.4103/1673-5374.339489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johnston J.A., O'Shea J.J. Matching SOCS with function. Nat Immunol. 2003;4(6):507–509. doi: 10.1038/ni0603-507. [DOI] [PubMed] [Google Scholar]
- 59.Khan A., Oliveira J., Lee Y., Guest J., Silvera R., Pressman Y., et al. Human Schwann cell-derived extracellular vesicle isolation, bioactivity assessment, and omics characterization. Int J Nanomed. 2025;20:4123–4144. doi: 10.2147/IJN.S500159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Blaya M.O., Raval A.P., Bramlett H.M. Traumatic brain injury in women across lifespan. Neurobiol Dis. 2022;164:105613. doi: 10.1016/j.nbd.2022.105613. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.