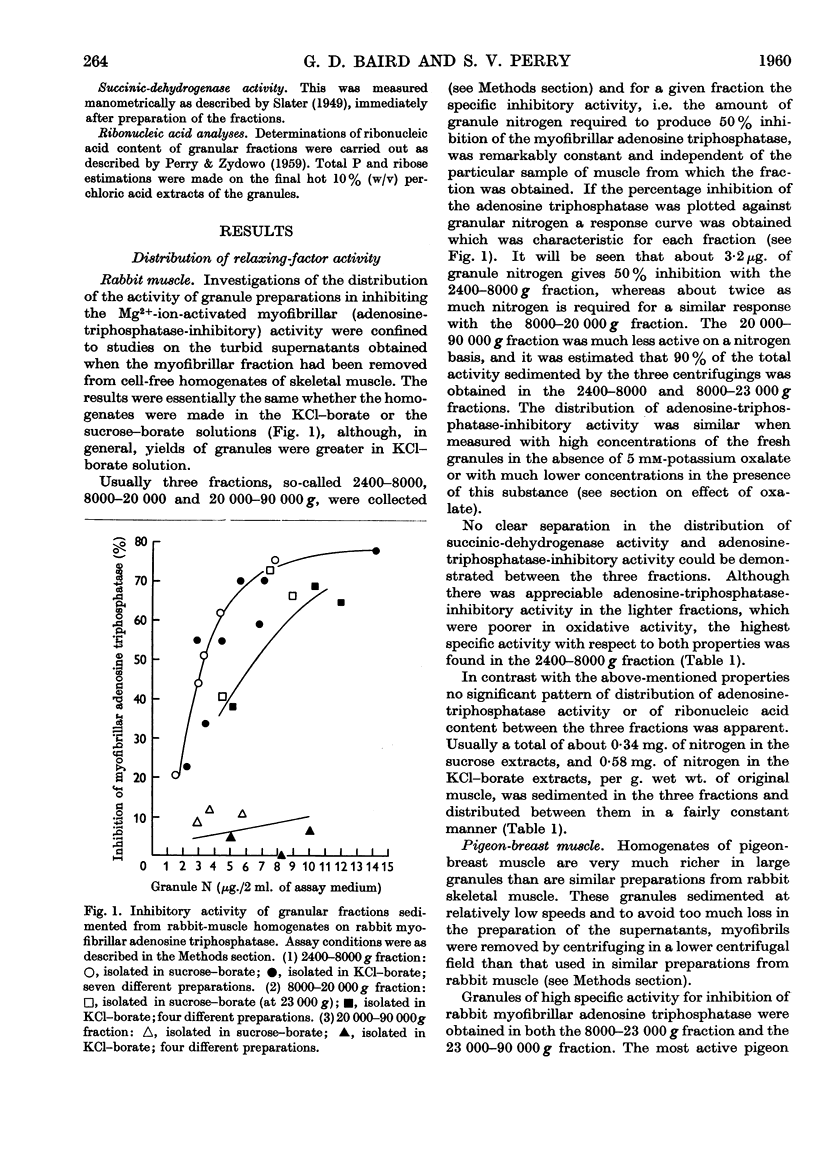
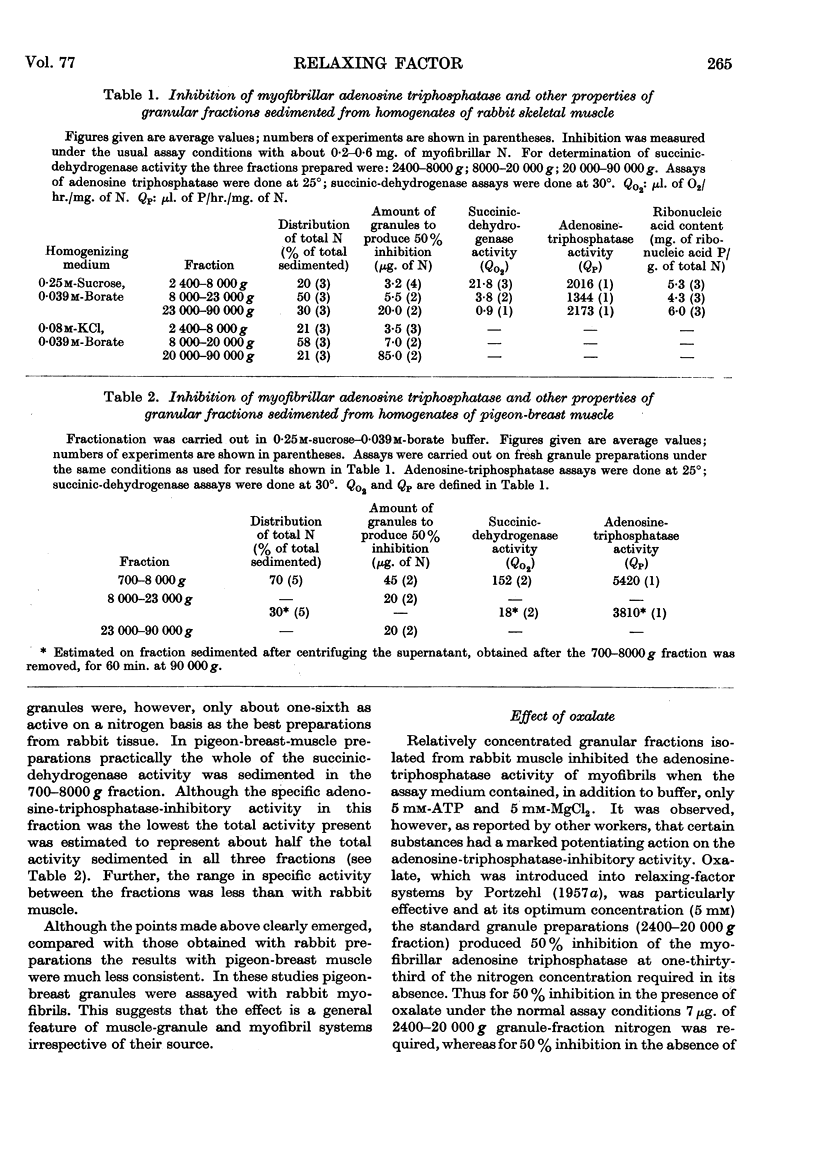
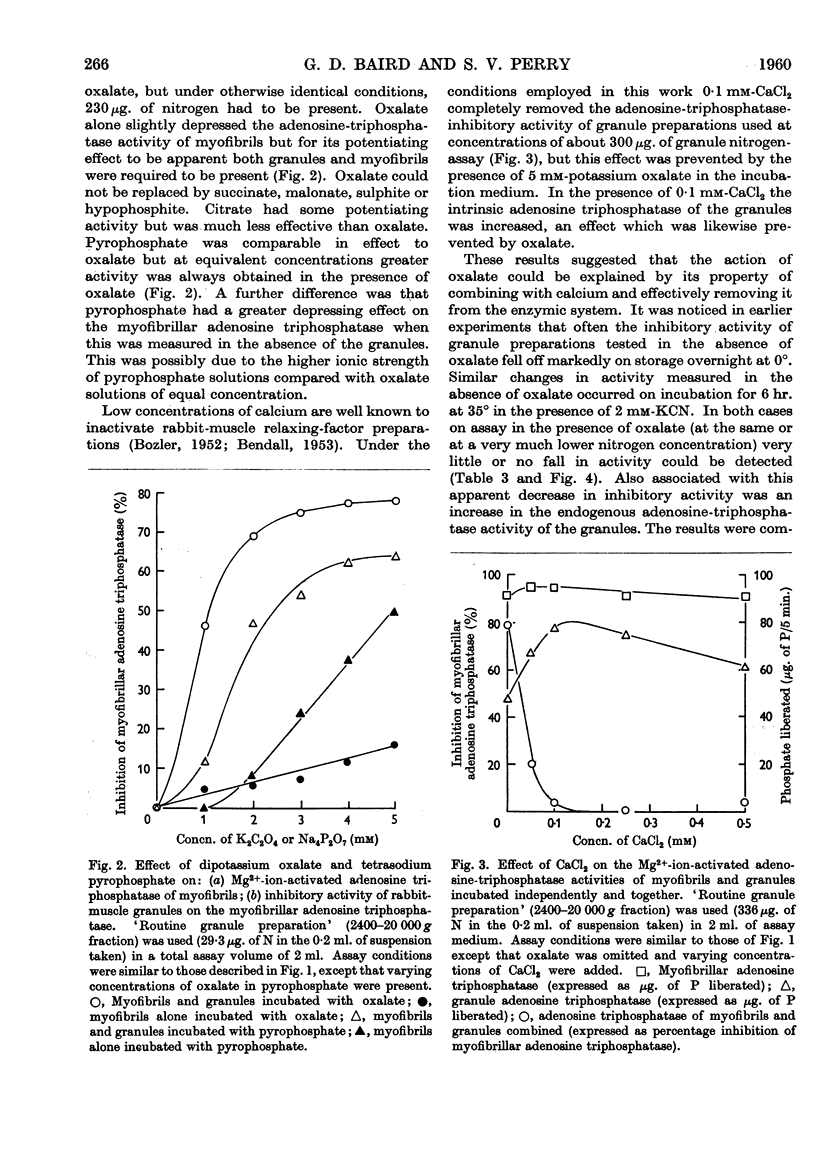
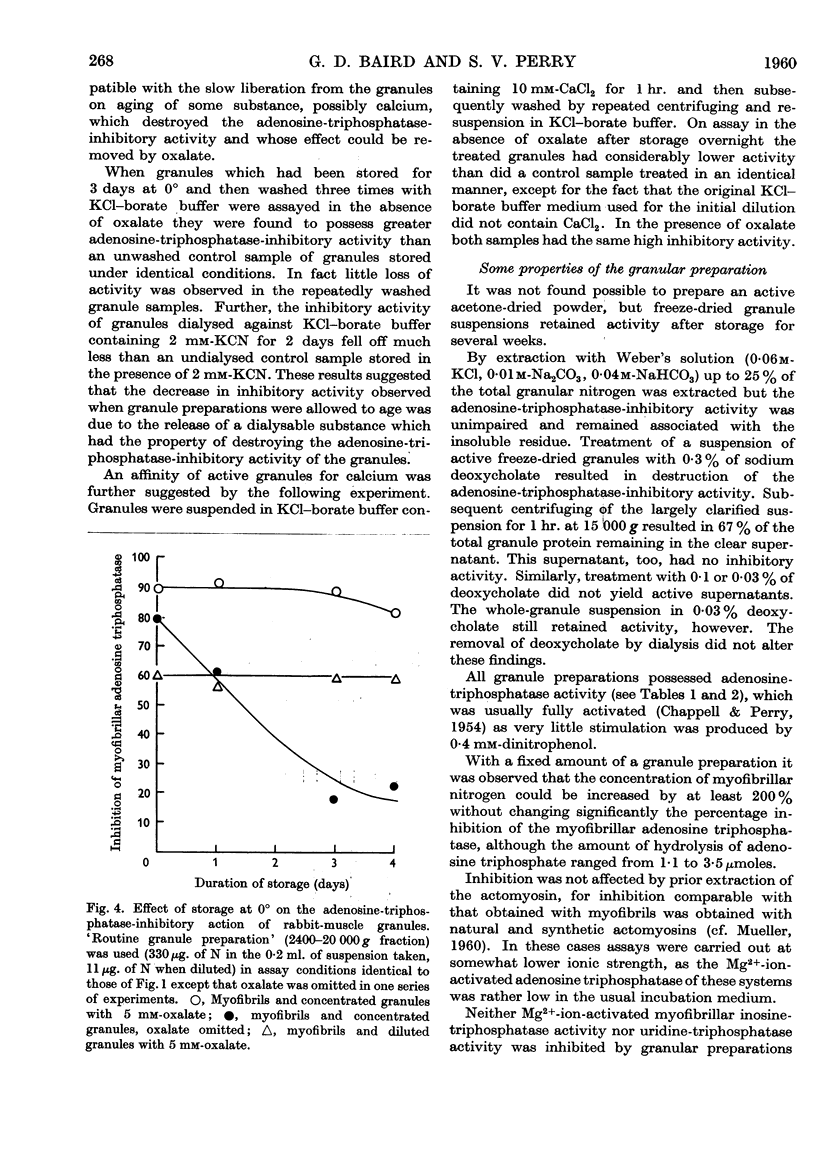
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENDALL J. R. Further observations on a factor (The `Marsh' factor) effecting relaxation of ATP-shortened muscle-fibre models and the effect of Ca and Mg ions upon it. J Physiol. 1953 Aug;121(2):232–254. doi: 10.1113/jphysiol.1953.sp004944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENDALL J. R. Muscle-relaxing factors. Nature. 1958 Apr 26;181(4617):1188–1190. doi: 10.1038/1811188a0. [DOI] [PubMed] [Google Scholar]
- BENDALL J. R. The relaxing effect of myokinase on muscle fibres; its identity with the Marsh factor. Proc R Soc Lond B Biol Sci. 1954 May 27;142(908):409–426. doi: 10.1098/rspb.1954.0033. [DOI] [PubMed] [Google Scholar]
- BOZLER E. Evidence of an ATP-actomyosin complex in relaxed muscle and its response to calcium ions. Am J Physiol. 1952 Mar;168(3):760–765. doi: 10.1152/ajplegacy.1952.168.3.760. [DOI] [PubMed] [Google Scholar]
- BOZLER E., PRINCE J. T. The control of energy release in extracted muscle fibers. J Gen Physiol. 1953 Sep;37(1):53–61. doi: 10.1085/jgp.37.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRIGGS F. N., KALDOR G., GERGELY J. Participation of a dialyzable cofactor in the relaxing factor system of muscle. I. Studies with single glycerinated fibres. Biochim Biophys Acta. 1959 Jul;34:211–218. doi: 10.1016/0006-3002(59)90249-5. [DOI] [PubMed] [Google Scholar]
- CHAPPELL J. B., PERRY S. V. Biochemical and osmotic properties of skeletal muscle mitochondria. Nature. 1954 Jun 5;173(4414):1094–1095. doi: 10.1038/1731094a0. [DOI] [PubMed] [Google Scholar]
- GOODALL M. C., SZENT-GYORGYI A. G. Relaxing factors in muscle. Nature. 1953 Jul 11;172(4367):84–85. doi: 10.1038/172084a0. [DOI] [PubMed] [Google Scholar]
- GREY T. C., PERRY S. V. A study of the effects of substrate concentration and certain relaxing factors on the magnesium-activated myofibrillar adenosine triphosphatase. Biochem J. 1956 Sep;64(1):184–192. doi: 10.1042/bj0640184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASSELBACH W. Die Wechselwirkung verschiedener Nukleosidtriphosphate mit Aktomyosin in Gelzustand. Biochim Biophys Acta. 1956 May;20(2):355–368. doi: 10.1016/0006-3002(56)90297-9. [DOI] [PubMed] [Google Scholar]
- HASSELBACH W., WEBER H. H. Der Einfluss des MB-Faktors auf die Kontraktion des Fasermodells. Biochim Biophys Acta. 1953 May;11(1):160–161. doi: 10.1016/0006-3002(53)90021-3. [DOI] [PubMed] [Google Scholar]
- LORAND L. Adenosine triphosphate-creatine transphosphorylase as relaxing factor of muscle. Nature. 1953 Dec 26;172(4391):1181–1183. doi: 10.1038/1721181a0. [DOI] [PubMed] [Google Scholar]
- MARSH B. B. The effects of adenosine triphosphate on the fibre volume of a muscle homogenate. Biochim Biophys Acta. 1952 Sep;9(3):247–260. doi: 10.1016/0006-3002(52)90159-5. [DOI] [PubMed] [Google Scholar]
- MOLNAR J., LORAND L. Transphosphorylating enzymes and muscle relaxation. Nature. 1959 Apr 11;183(4667):1032–1034. doi: 10.1038/1831032a0. [DOI] [PubMed] [Google Scholar]
- PERRY S. V., CORSI A. Extraction of proteins other than myosin from the isolated rabbit myofibril. Biochem J. 1958 Jan;68(1):5–12. doi: 10.1042/bj0680005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERRY S. V. Relation between chemical and contractile function and structure of the skeletal muscle cell. Physiol Rev. 1956 Jan;36(1):1–76. doi: 10.1152/physrev.1956.36.1.1. [DOI] [PubMed] [Google Scholar]
- PERRY S. V. The protein components of the isolated myofibril. Biochem J. 1953 Aug;55(1):114–122. doi: 10.1042/bj0550114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERRY S. V., ZYDOWO M. A ribonucleoprotein of skeletal muscle and its relation to the myofibril. Biochem J. 1959 Aug;72:682–690. doi: 10.1042/bj0720682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORTER K. R., PALADE G. E. Studies on the endoplasmic reticulum. III. Its form and distribution in striated muscle cells. J Biophys Biochem Cytol. 1957 Mar 25;3(2):269–300. doi: 10.1083/jcb.3.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORTZEHL H. Bewirkt das System Phosphokreatin-Phosphokinase die Erschlaffung des lebenden Muskels? Biochim Biophys Acta. 1957 Jun;24(3):474–482. doi: 10.1016/0006-3002(57)90235-4. [DOI] [PubMed] [Google Scholar]
- PORTZEHL H. Die Bindung des Erschlaffungsfaktors von Marsh an die Muskelgrana. Biochim Biophys Acta. 1957 Nov;26(2):373–377. doi: 10.1016/0006-3002(57)90019-7. [DOI] [PubMed] [Google Scholar]
- SLATER E. C., CLELAND K. W. The effect of calcium on the respiratory and phosphorylative activities of heart-muscle sarcosomes. Biochem J. 1953 Nov;55(4):566–590. doi: 10.1042/bj0550566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater E. C. A comparative study of the succinic dehydrogenase-cytochrome system in heart muscle and in kidney. Biochem J. 1949;45(1):1–8. doi: 10.1042/bj0450001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEBER A. On the role of calcium in the activity of adenosine 5'-triphosphate hydrolysis by actomyosin. J Biol Chem. 1959 Oct;234:2764–2769. [PubMed] [Google Scholar]


