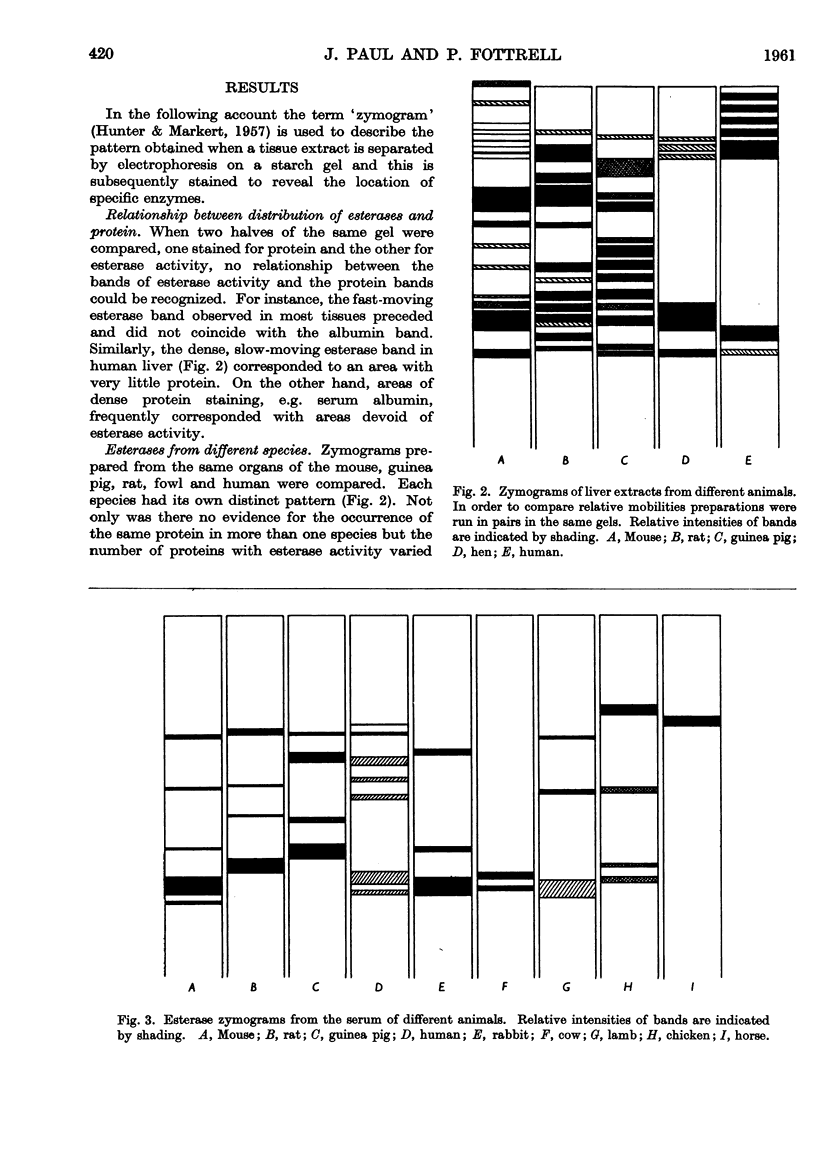
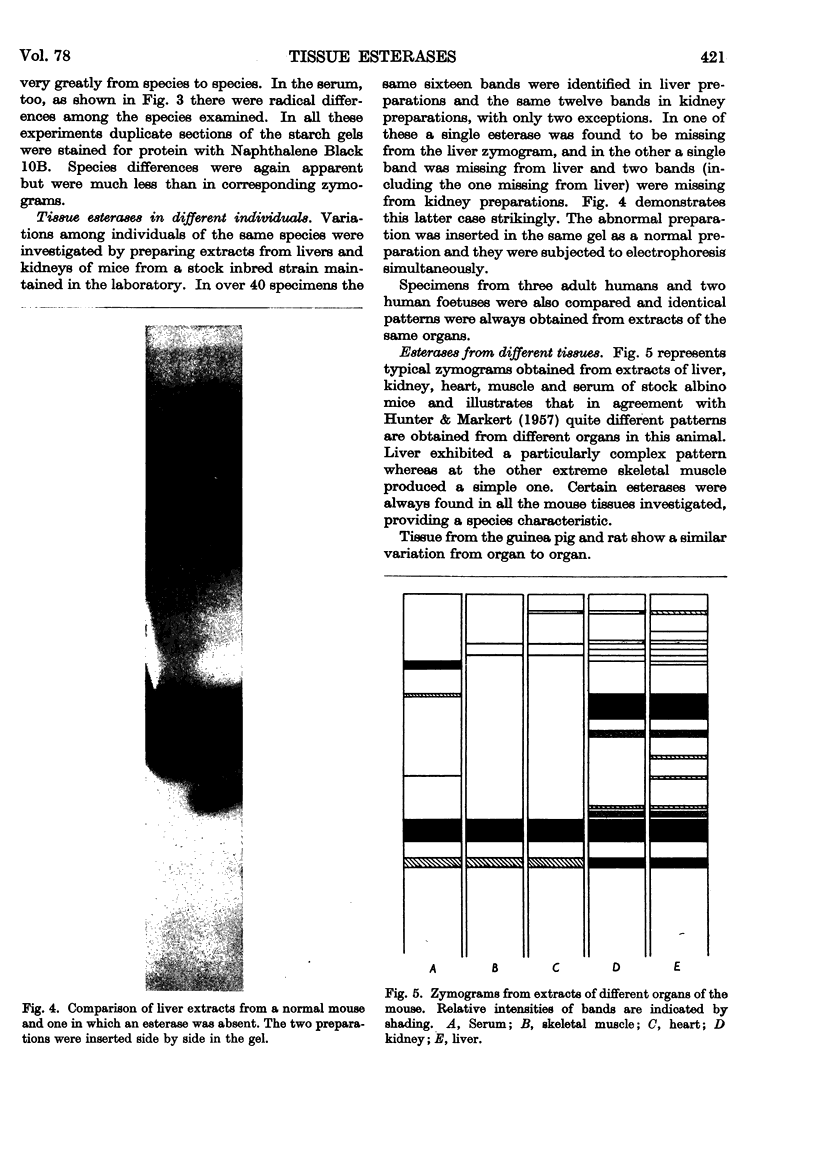
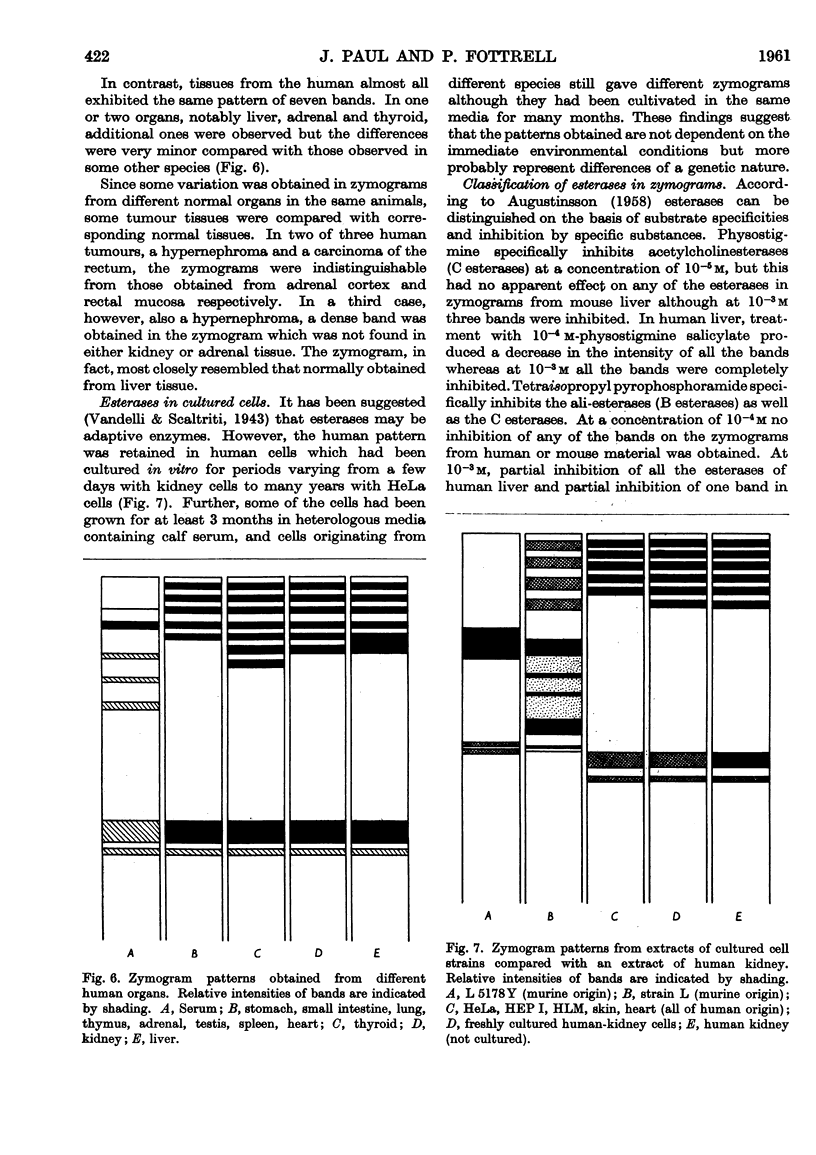
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AUGUSTINSSON K. B. Electrophoretic separation and classification of blood plasma esterases. Nature. 1958 Jun 28;181(4626):1786–1789. doi: 10.1038/1811786a0. [DOI] [PubMed] [Google Scholar]
- COHEN J. A., JANSZ H. S., OOSTERBAAN R. A. The chemical structure of the reactive group of esterases. Biochim Biophys Acta. 1956 May;20(2):402–403. doi: 10.1016/0006-3002(56)90310-9. [DOI] [PubMed] [Google Scholar]
- FULTON W. C., LESLIE I., SINCLAIR R. Biochemical tests for malignancy applied to a new strain of human cells. Nature. 1956 Nov 24;178(4543):1179–1180. doi: 10.1038/1781179a0. [DOI] [PubMed] [Google Scholar]
- HARRIS J. I., LI C. H. Corticotropins (ACTH). IV. The action of carboxypeptidase on alpha-corticotropin, and the C-terminal amino acid sequence. J Biol Chem. 1955 Apr;213(2):499–507. [PubMed] [Google Scholar]
- HENION W. F., SUTHERLAND E. W. Immunological differences of phosphorylases. J Biol Chem. 1957 Jan;224(1):477–488. [PubMed] [Google Scholar]
- HILL R. L., SMITH E. L. Hydrolysis of mercuripapain by leucine aminopeptidase without loss of enzymic activity. J Biol Chem. 1958 Mar;231(1):117–134. [PubMed] [Google Scholar]
- HIRS C. H. W., MOORE S., STEIN W. H. A chromatographic investigation of pancreatic ribonuclease. J Biol Chem. 1953 Feb;200(2):493–506. [PubMed] [Google Scholar]
- HUNTER R. L., MARKERT C. L. Histochemical demonstration of enzymes separated by zone electrophoresis in starch gels. Science. 1957 Jun 28;125(3261):1294–1295. doi: 10.1126/science.125.3261.1294-a. [DOI] [PubMed] [Google Scholar]
- KREBS E. G. Yeast Glyceraldehyde-3-phosphate dehydrogenase. I. Electrophoresis of fractions precipitated by nucleic acid. J Biol Chem. 1953 Feb;200(2):471–478. [PubMed] [Google Scholar]
- LATNER A. L., ZAKI A. H. Starch-gel electrophoresis of animal sera. Nature. 1957 Dec 14;180(4598):1366–1367. doi: 10.1038/1801366a0. [DOI] [PubMed] [Google Scholar]
- MALMSTROM B. G. The purification of yeast enolase by zone electrophoresis and ion-exchange chromatography, and the existence of several active forms of the enzyme. Arch Biochem Biophys. 1957 Jul;70(1):58–69. doi: 10.1016/0003-9861(57)90080-2. [DOI] [PubMed] [Google Scholar]
- MEISTER A. Reduction of alpha gamma-diketo and alpha-keto acids catalyzed by muscle preparations and by crystalline lactic dehydrogenase. J Biol Chem. 1950 May;184(1):117–129. [PubMed] [Google Scholar]
- MILLS G. T., PAUL J., SMITH E. E. B. Studies on beta-glucuronidase. II. The preparation and properties of three-ox-spleen beta-glucuronidase fractions. Biochem J. 1953 Jan;53(2):232–245. doi: 10.1042/bj0530232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markert C. L., Møller F. MULTIPLE FORMS OF ENZYMES: TISSUE, ONTOGENETIC, AND SPECIES SPECIFIC PATTERNS. Proc Natl Acad Sci U S A. 1959 May;45(5):753–763. doi: 10.1073/pnas.45.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEILANDS J. B. Studies on lactic dehydrogenase of heart. I. Purity, kinetics, and equilibria. J Biol Chem. 1952 Nov;199(1):373–381. [PubMed] [Google Scholar]
- NISSELBAUM J. S., BODANSKY O. Reactions of lactic dehydrogenase from various rabbit organs with antirabbit muscle lactic dehydrogenase. J Biol Chem. 1959 Dec;234:3276–3280. [PubMed] [Google Scholar]
- OOSTERBAAN R. A., KUNST P., VAN ROTTERDAM J., COHEN J. A. The reaction of chymotrypsin and diisopropylphosphorofluoridate. I. Isolation and analysis of diisopropylphosphoryl-peptides. Biochim Biophys Acta. 1958 Mar;27(3):549–555. doi: 10.1016/0006-3002(58)90385-8. [DOI] [PubMed] [Google Scholar]
- PEDERSEN K. O. Size relationship among similar proteins; association and dissociation reactions of protein units. Cold Spring Harb Symp Quant Biol. 1950;14:140–152. doi: 10.1101/sqb.1950.014.01.018. [DOI] [PubMed] [Google Scholar]
- PERLMANN G. E. Formation of enzymatically active, dialysable fragments during autodigestion of pepsin. Nature. 1954 Feb 27;173(4400):406–406. doi: 10.1038/173406a0. [DOI] [PubMed] [Google Scholar]
- PORTER G. R., RYDON H. N., SCHOFIELD J. A. Nature of the reactive serine residue in enzymes inhibited by organo-phosphorus compounds. Nature. 1958 Oct 4;182(4640):927–927. doi: 10.1038/182927a0. [DOI] [PubMed] [Google Scholar]
- POULIK M. D. Starch gel electrophoresis in a discontinous system of buffers. Nature. 1957 Dec 28;180(4600):1477–1479. doi: 10.1038/1801477a0. [DOI] [PubMed] [Google Scholar]
- RICHARDS F. M. On an active intermediate produced during the digestion of ribonuclease by subtilisin. C R Trav Lab Carlsberg Chim. 1955;29(17-19):329–346. [PubMed] [Google Scholar]
- ROGERS W. I., KALNITSKY G. Partially hydrolyzed ribonuclease with enzymic activity. Biochim Biophys Acta. 1957 Mar;23(3):525–532. doi: 10.1016/0006-3002(57)90372-4. [DOI] [PubMed] [Google Scholar]
- SCHLAMOWITZ M. Immunochemical studies on alkaline phosphatase. Ann N Y Acad Sci. 1958 Oct 13;75(1):373–379. doi: 10.1111/j.1749-6632.1958.tb36885.x. [DOI] [PubMed] [Google Scholar]
- SMITHIES O. An improved procedure for starch-gel electrophoresis: further variations in the serum proteins of normal individuals. Biochem J. 1959 Mar;71(3):585–587. doi: 10.1042/bj0710585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITHIES O. Zone electrophoresis in starch gels: group variations in the serum proteins of normal human adults. Biochem J. 1955 Dec;61(4):629–641. doi: 10.1042/bj0610629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TALLAN H. H., STEIN W. H. Chromatographic studies on lysozyme. J Biol Chem. 1953 Feb;200(2):507–514. [PubMed] [Google Scholar]
- TIMASHEFF S. N., STURTEVANT J. M., BIER M. On the electrophoretic heterogeneity of trypsin. Arch Biochem Biophys. 1956 Jul;63(1):243–246. doi: 10.1016/0003-9861(56)90027-3. [DOI] [PubMed] [Google Scholar]
- TOOLAN H. W. Transplantable human neoplasms maintained in cortisone-treated laboratory animals: H.S. No. 1; H.Ep. No. 1; H.Ep. No. 2; H.Ep. No. 3; and H.Emb.Rh. No. 1. Cancer Res. 1954 Oct;14(9):660–666. [PubMed] [Google Scholar]



