Abstract
Background:
Conventional palatoplasty involves operating in a narrow oral cavity space with surgical loupe visualization. Today, some surgeons resort to surgical microscopes to improve visualization and obtain more precise muscle dissection. The RoboticScope is a head movement–controlled system that moves the robotic arm loaded with 3-dimensional visualization technology, allowing surgeons complete freedom to use their hands during surgery.
Methods:
This was a retrospective pilot analysis of 8 pediatric patients, split into 2 cohorts of 4, comparing conventional palatoplasty to RoboticScope-assisted palatoplasty performed in 2023. Patient demographics collected included age, sex, and cleft palate classification (Veau I–IV). Variables measured included operative time, postoperative analgesia, oral intake, hospital stay, and complication rates. Ergonomic evaluations were performed through surgeons documenting their feedback on comfort and surgical efficiency, including dissection and repair.
Results:
RoboticScope-assisted palatoplasty averaged 28 minutes longer than the conventional method. Postoperative analgesic needs also differed: 100% of the patients who underwent conventional palatoplasty required morphine, whereas only 50% of the RoboticScope patients required morphine. Seventy-five percent of the RoboticScope patients demonstrated good oral intake on day 1, and 50% of the patients were discharged 1 day earlier than the conventional group.
Conclusions:
This study concludes that RoboticScope-assisted palatoplasty is safe and feasible, and is associated with fewer analgesic needs, better oral intake, and ergonomic benefits for surgeons than conventional palatoplasty. However, due to the limited nature of the study sample, future multicenter studies with a larger cohort group would be essential to validate these outcomes.
Takeaways
Question: Does the RoboticScope provide enhanced visualization and ergonomic benefits for surgeons during primary cleft palate surgery when compared with conventional surgical microscopes?
Findings: This pilot study demonstrated that the RoboticScope improves surgeon ergonomics, reduces physical strain, and offers high-quality visualization of the surgical field. The study included primary cleft palate procedures and highlighted significant benefits in surgical performance and workflow efficiency, and excellent patient outcomes.
Meaning: The RoboticScope poses significant benefits to patients and surgical staff, providing a promising tool for improving outcomes in primary cleft palate surgery.
INTRODUCTION
Cleft palate, either alone or accompanied by a cleft lip, is one of the most common congenital anomalies affecting the craniofacial region and presents a significant global health challenge. Infants with cleft deformities experience substantial difficulties in feeding due to the defective coordination of sucking, swallowing, and breathing required for effective nutrition.1 These challenges eventually lead to serious feeding and weight-related complications, including insufficient caloric consumption and poor nutritional status that negatively impact the infant’s growth.2 However, these challenges can be greatly improved with specialized cleft care, which includes anticipatory guidance, ongoing healthcare management, feeding support, and individualized preoperative preparation, followed by primary surgical intervention, known as palatoplasty (cleft palate repair).3
Traditional approaches to cleft palate repair have predominantly involved conventional surgical techniques, which, while effective, carry a risk of complications due to the requirement for wide exposure posing a significant risk of scarring and variable outcomes in terms of function and aesthetics. Robotic-assisted procedures have emerged as a promising alternative to conventional methods that reduce the complications associated with these intricate procedures. Procedures performed by robotic systems such as the da Vinci Robotic Surgical System (Intuitive Surgical, Inc., Sunnyvale, CA) have shown great reductions in complications such as infection, excessive blood loss, and damage to nearby vessels, and a significant improvement in postoperative care, therefore resulting in an optimized postoperative recovery period and greater patient satisfaction.4
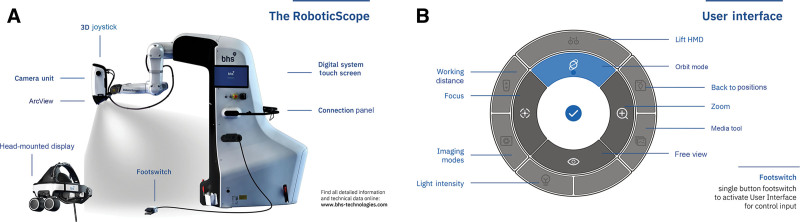
The RoboticScope (BHS Technologies, GmbH, Innsbruck, Austria) represents an advancement in surgical visualization, offering surgeons unparalleled precision and ergonomic flexibility. Unlike traditional surgical microscopes, the RoboticScope offers a head-mounted display (HMD) system that carries 2 digital microdisplays to bring real-time 3-dimensional (3D) images where they are needed, with the aid of a fully integrated 6-axis robotic arm with millimeter precision (Fig. 1A). The functions of the RoboticScope can be selected and controlled with intuitive head gestures in the user interface (Fig. 1B), which allows the surgeon to change the magnification and perspective of the surgical field and control the robotic arm at the same time without interruptions. This allows surgeons to easily navigate and maintain complex viewing angles while preserving an immersive 3D visualization of the surgical site, always maintaining a comfortable posture.
Fig. 1.
RoboticScope technology. A, Image of RoboticScope’s 6-axis robotic arm and HMD system. Image provided by BHS Technologies (GmbH, Innsbruck, Austria). Copyright 2024 BHS Technologies. B, Illustration showing the user interface of the RoboticScope, allowing intuitive control through head gestures. Image provided by BHS Technologies (GmbH, Innsbruck, Austria). Copyright 2024 BHS Technologies.
This retrospective pilot study evaluates the efficacy of the RoboticScope in cleft palate procedures, comparing its performance to traditional palatoplasty techniques. Through a detailed analysis of surgical outcomes, ergonomic advantages, and workflow efficiency, this study aims to highlight the potential of the RoboticScope in revolutionizing precision and surgeon comfort in common plastic surgery procedures.
METHODOLOGY
This retrospective pilot study was conducted in 2024 to evaluate the surgical outcomes of cleft palate repair at a tertiary pediatric hospital (Al Jalila Children’s Hospital) in Dubai, United Arab Emirates. This study aimed to compare conventional cleft palate repair with the RoboticScope manufactured by BHS Technologies (Innsbruck, Austria). A total of 8 pediatric patients were enrolled in the study and divided equally into 2 cohorts. The study adhered to ethical standards and was approved by the Mohammed Bin Rashid University of Medicine and Health Sciences Research Ethics Committee (Ref. No. MBRU IRB-2024-196), and Dubai Scientific Research Ethics Committee (Ref. No. DSREC-07/2024_34).
The inclusion criterion for this study was pediatric patients who presented with isolated, nonsyndromic cleft palate, without comorbidities or coexisting anomalies. All patients had been diagnosed with cleft palate, categorized by the standardized Veau classification system. The baseline study group characteristics recorded from the patient records included their age (months), sex (male or female), and cleft type (Veau I–IV) (Table 1).
Table 1.
Baseline Patient Demographics and Cleft Palate Severity Categorized by the Veau Classification System
| Study Group Characteristics | Overall Population (n = 8) | |
|---|---|---|
| RoboticScope Cohort (n = 4) | Conventional Palatoplasty Cohort (n = 4) | |
| Age, mo mean | 11 | 8.75 |
| Male | 2 | 1 |
| Female | 2 | 3 |
| Veau class I | 0 | 1 |
| Veau class II | 2 | 2 |
| Veau class III | 2 | 1 |
Each patient underwent surgery via the RoboticScope or conventional palatoplasty procedure under standardized conditions. Surgical simulation of a palatoplasty using the RoboticScope was undertaken at the BHS Technologies facility in the UAE before clinical utilization. Patients with Veau I and Veau II underwent palatoplasty through a medial-access incision, whereas patients with Veau III and Veau IV underwent a Bardach 2-flap palatoplasty technique.5 The following steps were implemented for each case: Local anesthesia was administered to both sides of the palate via lidocaine/epinephrine solution. Incisions were made along the edge of the cleft, with attention given to preserving enough mucosa for nasal lining closure, especially in wide clefts. Mucoperiosteal flaps were raised from the hard palate via an elevator, with flaps being based on the greater palatine vessels. Care was taken to avoid extensive traction or cutting near the greater palatine foramen. Enhanced visualization with the RoboticScope allowed for the complete release of all the retaining ligaments around the posterior edge of the hard palate, hamulus, and greater palatine pedicle foramen.
Although the focus of the article was not on the surgical technique, the authors emphasize the benefits of obtaining a magnified view of the oblique angulation around the pedicle, hamulus, and tensor tendon area, particularly when using the medial-access incision, whereas the ergonomic posture of the surgeon’s back and neck was maintained.6 The velar muscles (tensor and levator veli palatini) were freed from their abnormal attachments to the hard palate and divided accordingly.7 These muscles were then retropositioned to form a functional muscle sling, aiding in tension-free cleft closure. The nasal myomucosal edge was freed from the palatal shelves, and the vomer flaps were elevated for midline closure, continuing from the anterior cleft edge to the adenoid tissue. The nasal lining was sutured to the vomer with absorbable sutures. The repositioned muscles were approximated and sutured to close the muscle layer.7 Vertical mattress sutures were placed at the junction of the soft and hard palates to prevent fistulas. The oral mucosa was then closed at the midline, and the lateral edges of the flaps were tacked to the palate. Care was taken to ensure the oral flaps approximated in a tension-free manner, ensuring that all retaining tissues were released. The exposed bone laterally was covered with Surgicel, a powder absorbable hemostat (Ethicon, Johnson & Johnson, New Brunswick, NJ) and loosely tacked with absorbable sutures.
Variables were assessed across 2 main stages: the intraoperative stage and the postoperative period (from 24 h until discharge of the patients). Data from the intraoperative stage included the following: RoboticScope versus conventional palatoplasty, duration of surgery (in minutes), intraoperative blood loss (in milliliters), and surgeon ergonomics. Data from the postoperative period included the type of analgesia required (paracetamol, nonsteroidal anti-inflammatory drugs, morphine, and nalbuphine), quality of oral intake (classified as good or poor based on the child’s ability to feed, as recorded by the nutritionist), postoperative complications, and length of hospital stay (in days). All variables were analyzed using R version 4.4.0, statistical software utilized to evaluate outcomes between the RoboticScope-assisted procedures and conventional palatoplasty.
RESULTS
Baseline Study Group Characteristics
The mean age of the RoboticScope group was 11 months, whereas the conventional palatoplasty group had a mean age of 8.75 months. The RoboticScope group included 2 men (50%) and 2 women (50%), whereas the conventional palatoplasty group included 3 women (75%) and 1 man (25%). In accordance with the Veau classification system, the RoboticScope cohort included the following patients: Veau class I: none (0%), Veau class II: 2 patients (50%), and Veau class III: 2 patients (50%). In the conventional palatoplasty cohort, the following patients were included: Veau class I: 1 patient (25%), Veau class II: 2 patients (50%), and Veau class III: 1 patient (25%).
Operative Time and Blood Loss
The operative time for the RoboticScope procedures averaged 121 minutes, approximately 28 minutes longer than that of the conventional palatoplasty procedures, with an average of 93 minutes (Fig. 2). The differences observed in patients who underwent palatoplasty via the RoboticScope corresponded to the device’s setup, familiarity with the technology, staff training, and navigation during surgery. However, in the subsequent procedures, RoboticScope-assisted palatoplasty averaged 10%–35% shorter than the first procedure, indicating a promising linear learning curve.
Fig. 2.
Bar chart illustrating the duration of operations in the RoboticScope and conventional palatoplasty cohorts.
Surgeon’s Subjective Data
Based on the subjective data provided by the surgeon involved, it was observed that comfort during RoboticScope-assisted procedures was notably superior to that of the conventional surgical group. The RoboticScope facilitates a straight, ergonomic posture for the back and neck, combined with a hands-free advantage, while simultaneously providing enhanced surgical magnification. Furthermore, the ability to lock in certain oblique palatal views allows the surgeon to maintain a comfortable straight posture while focusing on the desired view.
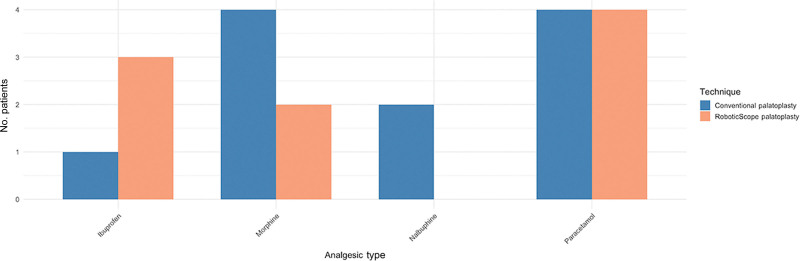
Postoperative Analgesic Requirements
Postoperatively, all patients were started on paracetamol (Fig. 3), with analgesia adjusted based on pain levels. In the RoboticScope group, 75% (n = 3) received ibuprofen initially, whereas 25% (n = 1) required only paracetamol throughout their stay. Opioid escalation was needed in 50% (n = 2) of patients, with morphine being the drug of choice, and none required nalbuphine. In the conventional palatoplasty group, only 25% (n = 1) received ibuprofen. Opioids were required in all patients in the conventional palatoplasty group, with 50% (n = 2) needing both morphine and nalbuphine and the remaining 50% (n = 2) only needing morphine. Overall, opioid use was lower in the RoboticScope group compared with the conventional palatoplasty group.
Fig. 3.
Bar chart comparing postoperative analgesic requirements between the RoboticScope and conventional palatoplasty cohorts.
Patient Recovery and Complications
Twenty-five percent (n = 1) of the conventional palatoplasty cohort experienced dehiscence of the uvula; however, no complications were observed in the RoboticScope cohort. The rates of oral feeding and speech in the short postoperative period remained comparable between both cohorts (Fig. 4).
Fig. 4.
Pie chart depicting postoperative oral intake outcomes in the RoboticScope and conventional palatoplasty cohorts.
With respect to the total duration of the patients’ hospital stay, in the conventional palatoplasty group, 75% (n = 3) of patients’ length of stay was 2 days, and 25% (n = 1) of patients were discharged after 1 day of hospitalization. In the RoboticScope group, 50% (n = 2) remained for 2 days, whereas the other 50% (n = 2) were discharged after 1 day of hospitalization. Patients traveled long distances within the country, often requiring 3–4 hours of travel. To ensure a safe and comfortable journey back home, the surgical team prioritized effective pain management and optimal oral intake before discharge.
DISCUSSION
The RoboticScope (BHS Technologies, GmbH, Innsbruck, Austria) is a novel head-mounted 3D virtual reality microscope that has 3 components: a 6-axis robotic arm, a camera unit, and an HMD. It was developed out of a need to advance from the current state-of-the-art microscopes, which have their equipment-related constraints and the need to manually control the settings every time the position is changed.8 Other alternatives to the microscope, such as endoscopes and exoscopes, are also limited by constant fog, with the need to always control the camera piece and rely on a large external screen kept at a safe working distance.8 The RoboticScope provides hands-free control of a head-mounted 3D camera while enhancing surgeon ergonomics for the entire length of the surgery. The use of the RoboticScope has been published in numerous surgical subspecialties, such as hand surgery, cochlear implant surgery, head and neck surgery, microvascular surgery, lymphovenous anastomosis, and plastic surgery.9–14 To the best of our knowledge, this is the first study to investigate its application in cleft palate surgery.
Sommerlad15 was the first to introduce a revolutionary operating microscope using the Zeiss Multidisciplinary Operating Microscope (Oberkochen, Germany) for cleft palate and pharyngeal surgery, enhancing surgical precision through improved visualization and ergonomics. His work highlighted the significant benefits of the operating microscope, which included a clear view of the operative field, allowing for more accurate tissue handling and suturing, and enhanced educational experience, allowing trainees and staff the ability to view the operation through a video monitor, replicating the surgeon’s perspective.15
Cleft surgeons are at a greater risk of developing back pain due to prolonged bending and neck strain in acute hyperflexion angles during prolonged surgery, increasing their likelihood of work-related musculoskeletal disorders. Demetz et al16 conducted a questionnaire study on 34 neurosurgeons using the RoboticScope. The advantage of the HMD ensured a neutral position during surgery, with a median head and body displacement of 0 degrees. Although there was no objective investigation of surgeon ergonomics during this study, the subjective findings concluded that neck and back posture was straight and in a relaxed position, enabling comfortable execution of all the steps of the surgery at all times.16
Compared with the RoboticScope, the traditional microscope needs to be manually handled for every position change, particularly when changing the view at an oblique angle. In contrast, the RoboticScope is hands-free with a head-controlled robotic arm that can move with 6 degrees of freedom and has a magnification of 4.3–34.4× (Table 2). The surgical workflow is uninterrupted when the surgeon needs to change the angulation view. Once an oblique view is locked in, the surgeon can get back to their original posture while still seeing the same oblique/deep-angle surgical field (Figs. 5, 6). This technology represents a significant advancement over current state-of-the-art microscopes with benefits to surgeon ergonomics and the promotion of long-term surgeon health.
Table 2.
Comparative Analysis of the RoboticScope and Traditional Microscopes
| Domains | RoboticScope | Surgical Microscope |
|---|---|---|
| Image quality | 3D magnification, up to 30.1× | 2D, latest versions have 3D |
| Brightness | Dual light source | Single light source |
| Ergonomics | Head and back straight | Manually needs to be adjusted for every position change |
| Easy hands-free movement of the camera unit with small head movements | Head and back are adjusted based on the microscope view and height | |
| Operating room integration | Base device positioned in front of the surgeon (straight or diagonal) | Main equipment comes at right angles to the surgeon |
| Assistant experience | Assistant wears HMD | Assistant has to coordinate position with every manual adjustment |
| Theater staff observation | Using HMD (if no assistant) or following on the RoboticScope-implemented monitor or connecting an external monitor (2D or 3D) | Staff can see the operative field on the monitor without special equipment |
| Approximate cost comparison | $400,000 (USD) | $125,000–$150,000 (USD) |
Fig. 5.
Visual representation of the intraoperative surgical field using the RoboticScope.
Fig. 6.
Illustration of intraoperative surgeon ergonomics during RoboticScope-assisted procedures.
This study has shown encouraging positive outcomes in those patients who underwent RoboticScope-assisted cleft palate surgery. The surgical setup time was prolonged in the RoboticScope group, with the initial learning curve and training of staff. It was observed that the setup time decreased as the staff became more familiar with the technology. The learning curve in robotic-assisted surgery is a widely studied topic, with substantial variability in its definition and measurement across surgical specialties.17–19 In our study, several factors determined the length of the procedure using the RoboticScope, such as familiarization with the technology, the complexity of the procedure as defined by the Veau classification system, and the surgeon’s previous experience with robotic-assisted procedures. This was evidenced by the first procedure conducted on a Veau class II patient, which averaged 180 minutes compared with 106 minutes in the conventional surgical group.
Soomro et al20 conducted a systematic review to investigate surgeon learning curves in robotic surgery, investigating factors associated with user performance. The study found that variables were mostly reported based on time-based metrics related to the duration of surgery; however, other variables such as length of hospital stay, morbidity, and mortality rates were underreported. Of note, the study suggested early performance variability was influenced by the user’s prior exposure to technology-rich environments, which underscores the promising potential of younger generations who have grown up with extensive use of digital tools and technology.20 This relates to the growing technology of the RoboticScope and achieving tailored training programs addressing these disparities between the different generations. Building on the systematic review of Soomro et al,20 we suggest that a multi-metric approach that includes task completion time, precision, and error rates be used to comprehensively evaluate proficiency with the use of the RoboticScope.
This study poses several limitations that warrant consideration. First, the investigation was conducted with a small sample size and involved a single surgical team, limiting the generalizability of the findings. Larger, multicenter studies are essential to objectively validate the observed benefits. Ergonomic advantages observed in this study were based on subjective feedback from the surgeon, highlighting the need for future research incorporating objective metrics to fully quantify these benefits. The disparity in analgesia requirements was due to anesthesia provider preference for each case.
Additionally, the RoboticScope technology presents a steep learning curve, with those experienced in gaming or digital tools adapting faster, as discussed previously. Furthermore, its high cost presents an economic limitation, with the RoboticScope priced up to US $400,000, compared with standard surgical microscopes ranging from $125,000–$150,000, potentially impacting accessibility and cost-effectiveness (Table 2). Finally, the system’s design introduces challenges, such as the HMD being bulky, posing potential discomfort for some surgeons with preexisting health conditions, limiting its widespread adoption. Future studies would be essential to thoroughly investigate these limitations, enabling a more comprehensive understanding of the RoboticScope’s capabilities and challenges.
CONCLUSIONS
This study concludes that the RoboticScope represents a highly advantageous tool for cleft palate surgery, particularly suited to addressing the inherent challenges of operating within the confined and complex anatomy of the small oral cavity. Additionally, the ergonomic design of the RoboticScope ensures that surgeons maintain a neutral posture throughout the procedure, significantly reducing physical strain on the neck and back—an essential factor in promoting long-term musculoskeletal health. These features, combined with the observed reduction in postoperative analgesic requirements, improved oral intake recovery, and comparable safety profile, establish the RoboticScope as a safe, feasible, and transformative advancement in cleft palate surgery when compared with conventional methods. However, these results are limited to a small sample size and would need to be replicated in a larger prospective cohort study.
DISCLOSURE
The authors have no financial interest to declare in relation to the content of this article.
ETHICAL APPROVAL
Ethical approval has been granted by the Al Jalila Children’s Specialty Hospital Research Ethics Committee, Mohammed Bin Rashid University of Medicine and Health Sciences Research Ethics Committee, and Dubai Scientific Research Ethics Committee.
Footnotes
Presented at the 93rd Annual Plastic Surgery the Meeting, September 26–29, 2024, San Diego Convention Center, San Diego, CA; and Dubai Health Research Conference, November 15–17, 2024, Mohammed Bin Rashid University of Medicine and Health Sciences, Dubai, UAE.
Limitations regarding long-term follow-up inherently exist in this article type.
Disclosure statements are at the end of this article, following the correspondence information.
REFERENCES
- 1.Denadai R, Lo LJ. Reducing delayed detection of isolated cleft palate-related deformity: a call for routine intraoral examination of newborns. J Pediatr (Rio J). 2024;100:350–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Penny C, McGuire C, Bezuhly M. A systematic review of feeding interventions for infants with cleft palate. Cleft Palate Craniofac J. 2022;59:1527–1536. [DOI] [PubMed] [Google Scholar]
- 4.Rivero-Moreno Y, Echevarria S, Vidal-Valderrama C, et al. Robotic surgery: a comprehensive review of the literature and current trends. Cureus. 2023;15:e42370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bardach J, Salyer KE. Surgical Techniques in Cleft Lip and Palate. Year Book Medical Publishers; 1987. [Google Scholar]
- 6.Sommerlad BC, Li S. Sommerlad’s technique of cleft palate repair. In: Shi B, Sommerlad BC, eds. Cleft Lip and Palate Primary Repair. Springer; 2013:265–276. [Google Scholar]
- 7.Cutting CB, Rosenbaum J, Rovati L. The technique of muscle repair in the cleft soft palate. Oper Tech Plast Reconstruct Surg. 1995;2:215–222. [Google Scholar]
- 8.Schär M, Röösli C, Huber A. Preliminary experience and feasibility test using a novel 3D virtual-reality microscope for otologic surgical procedures. Acta Otolaryngol. 2021;141:23–28. [DOI] [PubMed] [Google Scholar]
- 9.Battiston B, Artiaco S, Ciclamini D. The robotic scope can be a useful tool for hand and microsurgical procedures during the COVID-19 pandemic. J Hand Microsurg. 2021;13:255–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riepl R, Greve J, Schild LR, et al. Application of a new computer‐assisted robotic visualization system in cochlear implantation—proof of concept. Int J Med Robot. 2021;17:e2301. [DOI] [PubMed] [Google Scholar]
- 11.Boehm F, Graesslin R, Theodoraki MN, et al. Current advances in robotics for head and neck surgery—a systematic review. Cancers. 2021;13:1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boehm F, Schuler PJ, Riepl R, et al. Performance of microvascular anastomosis with a new robotic visualization system: proof of concept. J Robot Surg. 2022;16:705–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scaglioni MF, Meroni M, Fritsche E, et al. Use of the BHS robotic scope to perform lymphovenous anastomosis. Microsurgery. 2021;41:298–299. [DOI] [PubMed] [Google Scholar]
- 14.Dermietzel A, Aitzetmüller M, Klietz ML, et al. Free flap breast reconstruction using a novel robotic microscope. J Plast Reconstr Aesthet Surg. 2022;75:2387–2440. [DOI] [PubMed] [Google Scholar]
- 15.Sommerlad BC. A technique for cleft palate repair. Plast Reconstr Surg. 2003;112:1542–1548. [DOI] [PubMed] [Google Scholar]
- 16.Demetz M, Abramovic A, Krigers A, et al. Cadaveric study of ergonomics and performance using a robotic exoscope with a head-mounted display in spine surgery. J Robot Surg. 2024;18:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frieberg H, Nilsson A, Önefeldt D, et al. Novel robot-assisted microsurgery—what does the learning curve look like? J Plast Reconstr Aesthet Surg. 2023;87:S9. [Google Scholar]
- 18.Barbon C, Grünherz L, Uyulmaz S, et al. Exploring the learning curve of a new robotic microsurgical system for microsurgery. JPRAS Open. 2022;34:126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Von Reibnitz D, Weinzierl A, Grünherz L, et al. Learning curve of robotic assisted microsurgery in surgeons with different skill levels: a prospective preclinical study. J Robot Surg. 2024;18:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soomro NA, Hashimoto DA, Porteous AJ, et al. Systematic review of learning curves in robot-assisted surgery. BJS Open. 2020;4:27–44. [DOI] [PMC free article] [PubMed] [Google Scholar]