Abstract
The timing of dietary total fat intake influences glucose homeostasis, however, the impact of unsaturated fat (USFA) intake has yet to be explored. This 12-week, double-blind, randomized, controlled, 2 × 2 factorial-designed feeding trial investigated the effects of timing (lunch or dinner) and types of dietary USFA (high monounsaturated fat or polyunsaturated fat diet) intake on glucose metabolism in seventy prediabetes participants (mean age, 57 years). Sixty participants with completed fecal samples were included in the final analysis (n = 15 for each group). Postprandial serum glucose was first primary outcome, postprandial insulin levels and insulin sensitivity indices were co-primary outcomes Secondary outcomes were continuous glucose levels, serum fatty acid profile, gut microbiome (metagenomic sequencing) and fecal metabolites. Results showed no significant differences in postprandial glucose between groups. However, USFA intake at lunch (vs. dinner) improved insulin sensitivity and reduced postprandial insulin and serum free saturated fatty acid (Ptiming < 0.05, Ptype > 0.05, Pinteraction > 0.05), which was associated with alterations in gut microbiome and bile acid metabolism, regardless of USFA type. In summary, these results suggest that advancing timing of USFA intake improves insulin sensitivity through the gut microbiome and bile acid metabolism. Trial registration: ChiCTR2100045645.
Subject terms: Pre-diabetes, Nutrition
Here, Ying Li et al. present the results of a randomized controlled feeding trial in prediabetes participants, finding that advancing timing of unsaturated fat (USFA) intake improves insulin sensitivity through the gut microbiome and bile acid metabolism, regardless of USFA type.
Introduction
Lifestyle modification, particularly dietary intervention, is the most effective way to prevent the progression from prediabetes to type 2 diabetes (T2DM)1. Despite the well-recognized benefits of unsaturated fat (USFA) on insulin sensitivity and glycemic control, controversy remains regarding which of the two types of USFAs, namely monounsaturated fat (MUFA) versus polyunsaturated fat (PUFA), primarily contribute to these effects. Some isocaloric feeding trials evaluating the impact of dietary macronutrient composition on glucose-insulin homeostasis have reported that PUFA is superior to MUFA in reducing fasting glucose, HbA1C and serum lipids2. Conversely, another meta-analysis of patients with diabetes indicates that MUFA-rich diets offer advantages in improving glycemic control over PUFA-rich diets3. Yet neither the effects of different types of USFAs on glycemic control nor the possible mechanisms for their difference have been clarified.
Accumulating evidence suggests that the timing of fat intake play an important role in the progression of insulin resistance and T2DM. Fat intake could entrain the diurnal oscillation of genes and metabolites involved in glucose and lipid metabolism4. High-fat diets disrupt the circadian clock in the liver and diurnal fluctuation of gut microbiota, leading to obesity and insulin resistance5. While modulating the timing or daily distribution of fat intake produced metabolic benefits in both animal and human studies6,7. Epidemiological studies found that replacing 5% of USFAs consumed at dinner with an equivalent amount of USFAs at breakfast was associated with lower diabetes mortality8. In a 7-day trial, Froy and colleagues also found that consuming more calories at breakfast compared to dinner resulted in improved postprandial glucose and insulin sensitivity in participants with diabetes9. However, whether and how timing of different types of USFA influence the insulin sensitivity and glycemic control remain obscure.
The gut microbiota, which can be influenced by dietary fats10, has been verified to play an important role in the pathomechanism of T2DM11. Moreover, emerging studies have reported that the gut microbiota exhibits robust circadian rhythms12–16. However, a cohort study found that the circadian rhythms of gut microbial composition and function are disrupted in T2DM17. Recently, a modulatory effect of the chrononutrition on the gut microbiota has been observed in both humans and animals. Early calorie loading (before 15:00) enhances gut microbial richness in healthy volunteers18. Similarly, restricted calorie intake during the active phase increases the abundance of an obesity-protective microbiome in rodents19. Remodeling the gut microenvironment is considered a key intervention in the treatment of metabolic diseases, such as insulin resistance and T2DM. However, if the gut microbiome responds differently to the intake timing of the two types of USFA is not elucidated.
In this work, we performed a 12-week randomized, controlled, factorial-designed feeding trial in subjects with prediabetes, followed by metagenomics and metabolomics analyses and fecal transplantation to mice, to investigate the effect of timing and types of USFA intake on glucose metabolism, insulin sensitivity, and to elucidate the potential role of the gut microbiome in this process. Here we show the timing of USFA intake influences insulin sensitivity, and this is mediated by gut microbiome-mediated bile acid metabolism, while the type does not.
Results
Participants
Among 475 participants screened, 172 were identified as potentially qualified based on their serum glucose levels, and their serum fatty acids profile were measured to evaluate their eligibility for the trial. A total of 70 eligible participants agreed to participant in this trial, and were randomly assigned to one of four groups: MUFA-rich diet at lunch (HM-L), MUFA-rich diet at dinner (HM-D), PUFA-rich diet at lunch (HP-L), and PUFA-rich diet at dinner (HP-D). Finally, 60 participants (mean ± SD: age, 57.2 ± 8.1 years; weight, 67.5 ± 9.3 kg; BMI, 26.4 ± 2.6 kg/m2) with complete gut microbiome data were included in the final analysis (N = 15 for each group, Supplementary fig. 1). Baseline characteristics, including demographics, anthropometry, and biochemical indexes, were well-balanced across the four groups (Table 1). Habitual daily energy and macronutrient intake were also comparable among the groups (Supplementary fig. 2A). Additionally, baseline characteristics were similar between completers and non-completers (Supplementary Table 1).
Table 1.
Measurable indicators and biochemical parameters at baseline
| HP-L | HP-D | HM-L | HM-D | P Value | |
|---|---|---|---|---|---|
| Age (years) | 57.80 ± 10.20 | 57.60 ± 4.70 | 54.87 ± 9.48 | 58.47 ± 7.12 | 0.464 |
| Female, n (%) | 11 (73.3) | 11 (73.3) | 11 (73.3) | 11 (73.3) | |
| Body weight (kg) | 67.68 ± 11.30 | 66.00 ± 8.35 | 69.73 ± 10.65 | 66.48 ± 6.76 | 0.928 |
| BMI (kg/m2) | 25.84 ± 2.35 | 26.45 ± 2.51 | 26.83 ± 2.49 | 26.41 ± 3.17 | 0.746 |
| Fat mass% | 31.41 ± 3.94 | 32.79 ± 5.60 | 31.21 ± 5.08 | 32.22 ± 7.13 | 0.621 |
| Fasting glucose (mg/dL) | 101.82 ± 15.40 | 101.70 ± 11.43 | 102.29 ± 15.08 | 104.64 ± 17.50 | 0.944 |
| Postprandial glucose (mg/dL) | 135.62 ± 10.50 | 143.84 ± 18.76 | 143.39 ± 21.69 | 149.36 ± 25.07 | 0.092 |
| AUCglucose (mg/dL*h) | 307.78 ± 39.19 | 331.05 ± 45.10 | 332.05 ± 51.19 | 331.51 ± 74.57 | 0.437 |
| HbA1c (%) | 6.06 ± 0.27 | 6.23 ± 0.28 | 6.19 ± 0.20 | 6.30 ± 0.52 | 0.432 |
| HbA1c (mmol/mol) | 42.72 ± 2.91 | 44.53 ± 3.07 | 44.16 ± 2.13 | 45.29 ± 5.69 | 0.432 |
| Fasting insulin (mIU/mL) | 6.28 ± 2.90 | 5.84 ± 2.57 | 6.38 ± 3.47 | 8.23 ± 4.95 | 0.283 |
| Postprandial insulin (mIU/mL) | 21.04 ± 13.30 | 35.95 ± 29.58 | 32.57 ± 19.69 | 34.94 ± 25.08 | 0.382 |
| AUCinsulin (mIU/mL*h) | 66.02 ± 37.05 | 65.31 ± 27.03 | 78.52 ± 54.72 | 83.11 ± 36.17 | 0.513 |
| Gutt index | 91.74 ± 24.47 | 79.14 ± 22.40 | 80.50 ± 22.41 | 80.05 ± 37.10 | 0.264 |
| Stumvoll index | 0.101 ± 0.011 | 0.090 ± 0.019 | 0.092 ± 0.016 | 0.091 ± 0.019 | 0.366 |
| Triglycerides (mmol/L) | 1.25 ± 0.46 | 1.98 ± 3.02 | 1.26 ± 0.78 | 1.80 ± 1.31 | 0.178 |
| Total cholesterol (mmol/L) | 5.38 ± 1.34 | 5.58 ± 1.37 | 5.24 ± 1.19 | 5.56 ± 0.90 | 0.814 |
| HDL-c (mmol/L) | 1.46 ± 0.29 | 1.42 ± 0.39 | 1.43 ± 0.29 | 1.53 ± 0.23 | 0.517 |
| LDL-c (mmol/L) | 3.28 ± 1.20 | 3.29 ± 1.31 | 3.18 ± 1.02 | 3.26 ± 0.63 | 0.972 |
Values are presented as mean ± SD. HP-L high polyunsaturated fat diet at lunch, HP-D high polyunsaturated fat diet at dinner, HM-L high monounsaturated fat diet at lunch, HM-D high monounsaturated fat diet at dinner, BMI body mass index, AUCglucose area under the curve of glucose, HbA1c glycated hemoglobin A1c, AUCinsulin area under the curve of insulin, HDL-c high-density lipoprotein cholesterol, LDL-c low-density lipoprotein cholesterol. Analyses were performed using one-way ANOVA (two-sided) followed by post hoc Bonferroni test for normally distributed data and Kruskal-Waillis test (two-sided) followed by Dunn’s multiple comparison’s test for non-normally distributed data, χ2 test (two-sided) was for categorical data.
Changes in main and secondary outcomes
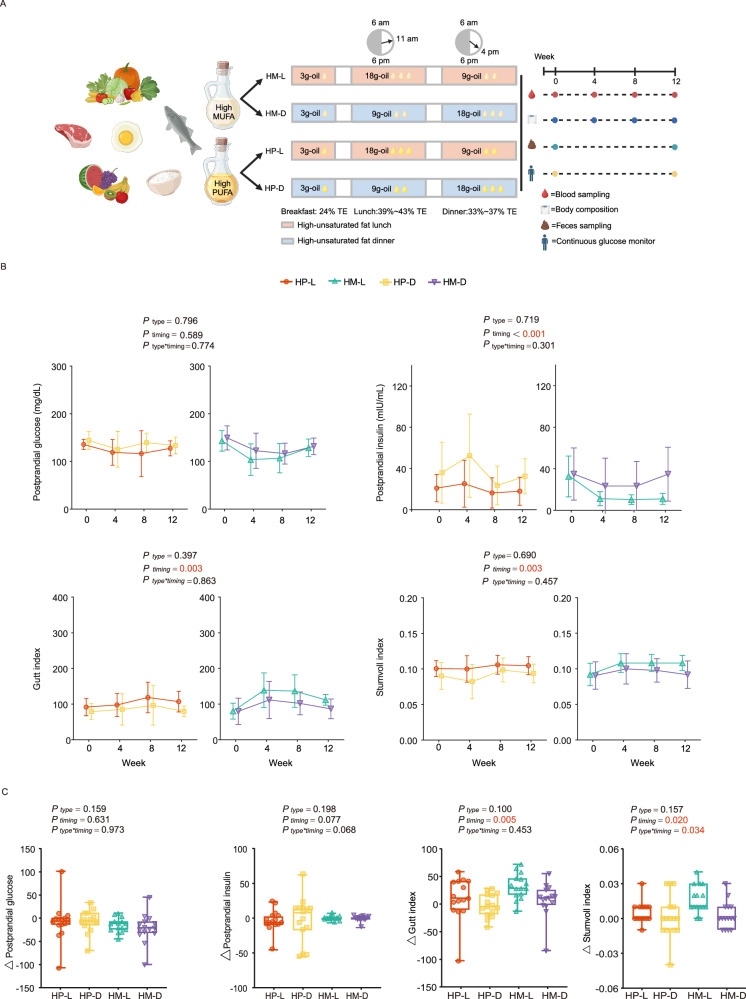
After a 12-week intervention, both the timing of USFA intake and the specific type of USFA did not influence postprandial glucose levels, Area under the curve (AUC) of glucose levels, and AUC of insulin levels (all På 0.05, Table 2). While USFA-rich diet at lunch (HU-L, USFA-rich diet at lunch, which included HP-L and HM-L) significantly decreased postprandial insulin levels (18.00 ± 13.59, 32.54 ± 17.06, 11.01 ± 5.23 and 34.80 ± 25.97 mIU/mL for HP-L, HP-D, HM-L and HM-D, respectively) and improved the insulin sensitivity indexes, such as Gutt index (107.12 ± 29.07, 79.86 ± 14.56, 112.15 ± 15.06 and 86.92 ± 27.68 for HP-L, HP-D, HM-L and HM-D, respectively) and the Stumvoll index (0.105 ± 0.013, 0.094 ± 0.013, 0.108 ± 0.011 and 0.092 ± 0.019 for HP-L, HP-D, HM-L and HM-D, respectively), compared with USFA-rich diet at dinner (HU-D, USFA-rich diet at dinner, including HP-D and HM-D) (Ptiming = <0.001, 0.003 and 0.003, respectively, Fig. 1B & Table 2). No difference was found in groups consuming diets rich in different types of USFA (all Ptype > 0.05, Fig. 2B). Furthermore, the increase in Gutt index and Stumvoll index from baseline to 12 weeks was also significantly greater in the HU-L groups than HU-D groups (Ptiming = 0.005 and Ptiming = 0.020, respectively, Fig. 1C). In terms of diets rich in MUFA or PUFA, there were no significant effects on insulin levels or insulin sensitivity (Fig. 1C).
Table 2.
Measurable indicators and biochemical parameters at the end of intervention
| HP-L | HP-D | HM-L | HM-D | Ptiming Value | Ptype Value | Pinteraction Value | |
|---|---|---|---|---|---|---|---|
| Body weight (kg) | 65.56 ± 10.26 | 64.90 ± 8.83 | 66.48 ± 10.60 | 65.68 ± 5.73 | 0.811 | 0.815 | 0.977 |
| BMI (kg/m2) | 25.07 ± 2.45 | 25.96 ± 2.37 | 25.59 ± 2.78 | 26.11 ± 2.96 | 0.591 | 0.882 | 0.789 |
| Fat mass% | 31.29 ± 4.42 | 32.24 ± 5.32 | 30.72 ± 4.61 | 33.41 ± 6.89 | 0.178 | 0.555 | 0.536 |
| Fasting glucose (mg/dL) | 94.48 ± 9.89 | 96.13 ± 10.98 | 92.03 ± 8.07 | 94.57 ± 13.14 | 0.517 | 0.691 | 0.873 |
| Postprandial glucose (mg/dL) | 127.42 ± 15.85 | 133.44 ± 17.48 | 128.35 ± 18.44 | 131.80 ± 17.55 | 0.589 | 0.796 | 0.774 |
| AUCglu (mg/dL*h) | 276.89 ± 26.99 | 315.65 ± 50.01 | 299.16 ± 38.24 | 291.59 ± 46.44 | 0.618 | 0.117 | 0.034 |
| HbA1c (%) | 6.05 ± 0.23 | 6.21 ± 0.22 | 6.10 ± 0.20 | 6.23 ± 0.45 | 0.237 | 0.820 | 0.869 |
| HbA1c (mmol/mol) | 42.61 ± 2.54 | 44.31 ± 2.44 | 43.16 ± 2.23 | 44.58 ± 4.96 | 0.235 | 0.818 | 0.871 |
| Fasting insulin (mIU/mL) | 6.39 ± 2.94 | 6.87 ± 2.75 | 6.92 ± 3.47 | 7.87 ± 4.23 | 0.447 | 0.422 | 0.787 |
| Postprandial insulin (mIU/mL) | 18.00 ± 13.59 | 32.54 ± 17.06 | 11.01 ± 5.23 | 34.80 ± 25.97 | <0.001* | 0.719 | 0.301 |
| AUCins (mIU/mL*h) | 64.19 ± 46.50 | 71.46 ± 38.14 | 60.02 ± 34. 26 | 74.18 ± 50.25 | 0.369 | 0.862 | 0.756 |
| Gutt index | 107.12 ± 29.07 | 79.86 ± 14.56 | 112.15 ± 15.06 | 86.92 ± 27.68 | 0.003* | 0.397 | 0.863 |
| Stumvoll index | 0.105 ± 0.013 | 0.094 ± 0.013 | 0.108 ± 0.011 | 0.092 ± 0.019 | 0.003* | 0.690 | 0.457 |
| Triglycerides (mmol/L) | 1.42 ± 0.60 | 1.56 ± 0.88 | 1.43 ± 0.85 | 1.54 ± 0.70 | 0.711 | 0.934 | 0.924 |
| Total cholesterol (mmol/L) | 5.18 ± 1.27 | 5.43 ± 1.31 | 5.03 ± 0.85 | 5.01 ± 0.72 | 0.966 | 0.292 | 0.638 |
| HDL-c (mmol/L) | 1.44 ± 0.25 | 1.46 ± 0.41 | 1.48 ± 0.36 | 1.42 ± 0.30 | 0.632 | 0.747 | 0.634 |
| LDL-c (mmol/L) | 3.15 ± 1.13 | 3.34 ± 1.09 | 3.02 ± 0.62 | 2.96 ± 0.63 | 0.862 | 0.249 | 0.599 |
Values are presented as mean ± SD. HP-L high polyunsaturated fat diet at lunch, HP-D high polyunsaturated fat diet at dinner, HM-L high monounsaturated fat diet at lunch, HM-D high monounsaturated fat diet at dinner, BMI body mass index, AUCglucose area under the curve of glucose, HbA1c glycated hemoglobin A1c, AUCinsulin area under the curve of insulin, HDL-c high-density lipoprotein cholesterol, LDL-c low-density lipoprotein cholesterol. Analysis was done using three-way repeated measures ANOVA (two-sided) followed by post hoc Bonferroni test. *P < 0.05.
Fig. 1. Study design and changes in glycemic control.
A Overall trial design (created in BioRender. Xu, X. (2025) https://BioRender.com/s17o93p). B Dynamic changes of postprandial glucose level, postprandial insulin level, Gutt index, Stumvoll index of each group at baseline and 4, 8, 12 weeks after intervention. Data are presented as mean ± SD. P values using three-way repeated measures ANOVA (two-sided) followed by post hoc Bonferroni test were displayed. The exact Ptiming value for the postprandial insulin levels was 0.000361. P values were colored red if significant. Number of participants: n = 15 for each group. C Changes of postprandial glucose level, postprandial insulin level, Gutt index, Stumvoll index at week 12 from baseline. Data are shown as box-and whisker plots, centre line, box boundaries, lower and upper whisker represent the median, quartile, minimum and maximum range respectively. P values using two-way ANOVA (two-sided) followed by post hoc Bonferroni test were displayed. Number of participants: n = 15 for each group. MUFA: monounsaturated fatty acid; PUFA: polyunsaturated fatty acid; HP-L: high polyunsaturated fat diet at lunch; HP-D: high polyunsaturated fat diet at dinner; HM-L: high monounsaturated fat diet at lunch; HM-D: high monounsaturated fat diet at dinner. TE: total energy.
Fig. 2. Daily glucose profile and serum fatty acid profile.
A Twenty-four-hour temporal profile of glucose levels measured by continuous glucose monitoring after a 12-week intervention. The solid lines represent the median glucose, the shaded area is the 25%-75% interval, and the lightly shaded area is the 5%-95% interval. B Calculated 24 h glucose metrics of each group at the end of the trial. Data are shown as box-and whisker plots, centre line, box boundaries, lower and upper whisker represent the median, quartile, minimum and maximum range respectively. P values using two-way ANOVA was displayed. HP-L: n = 14, HP-D: n = 13, HM-L: n = 14 for AUC_sleep, n = 15 for the other parameters, HM-D: n = 13 for AUC_sleep, n = 14 for the other parameters. C Serum free fatty acid (FFA) profile and proportion of different type of FFAs at baseline and after a 12-week intervention. Mixed models (two-sided) with post hoc Bonferroni correction was used. *Ptiming < 0.05. HP-L: high polyunsaturated fat diet at lunch; HP-D: high polyunsaturated fat diet at dinner; HM-L: high monounsaturated fat diet at lunch; HM-D: high monounsaturated fat diet at dinner; SD: standard deviation; eHbA1C: estimated glycated hemoglobin A1c; MAGE: mean amplitude of glycemic excursion; AUC: area under the curve; SFA: saturated fatty acid; MUFA: monounsaturated fatty acid; PUFA: polyunsaturated fatty acid.
No difference was found in calculated parameters from continuous glucose monitor (CGM) at baseline (Supplementary fig. 2B). At the end of intervention, mean glucose levels from CGM data tended to be lower in HU-L groups than in HU-D groups (Ptiming = 0.089, Fig. 2A). Moreover, mean amplitude of glycemic excursion (MAGE) and the AUC of glucose levels during sleep tended to be smaller in HU-L groups than in HU-D groups, although not significant (Ptiming = 0.125 and 0.072, respectively Fig. 2B). No such tendency was found in groups consuming diets rich in different types of USFA (Fig. 2B & Supplementary fig. 2C).
Although the serum triglycerides, total cholesterol, HDL-c, LDL-c did not differ among groups (Table 2), HU-L led to a significant decrease in serum saturated fatty acid (SFA) percentage, compared with HU-D (Ptiming < 0.001, Ptype = 0.667, Pinteraction = 0.432, Fig. 2C). HU-L groups showed decreased levels of myristic acid (C14:0), palmitic acid (C16:0) and stearic acid (C18:0) compared with HU-D (all Ptiming < 0.05, Fig. 2C).
Changes in diversity and composition of the gut microbiome
No significant differences in microbial richness (Chao1 index), evenness (Shannon index) or taxonomic composition (Bray-Curtis dissimilarity) were observed across the four groups at baseline (Fig. 3A). After the 12-weeks intervention, the HU-L groups exhibited a significant increase in microbial richness compared to HU-D groups, but no change in microbial evenness (Fig. 3A). A notable difference in taxonomic composition was observed between groups with different timing of USFA intake, but not between those with diets rich in MUFA or PUFA (Fig. 3B).
Fig. 3. Compositional changes of gut microbiome.
A Comparison of alpha diversity (Chao1 and Shannon indexes) at baseline and week 12 based on species levels. Data are shown as box-and whisker plots, centre line, box boundaries, lower whisker, upper whisker and light-colored circles represent the median, quartile, minimum range, maximum range and outliers respectively. Number of participants: n = 15 for each group. B PCoA based on bray-curtis dissimilarity showed different taxonomic compositions after 12-week intervention, and no significance was showed at baseline. P value was measured by PERMANOVA (two-sided) with 999 permutation tests, followed by Benjamini-Hochberg correction. P values were colored red if significant. C Barplots showed the percentage of the composition of fecal microbiome at the family level in each group. D Abundant of Prevotellaceae family and Lachnospiraceae family after the 12-week intervention (no difference at baseline). Data are shown as box-and whisker plots, centre line, box boundaries, lower and upper whisker represent the median, quartile, minimum and maximum range respectively. Analysis was done using MaAsLin2 (two-sided) followed by Benjamini-Hochberg correction.*P < 0.05. Number of participants: n = 15 for each group. E Abundance of significant species that were significantly correlated with clinical phenotypes. F Partial Spearman correlations of glucose- and lipid-related characteristics with significant species with baseline age, sex and body mass index adjusted. *P < 0.05. HP-L: high polyunsaturated fat diet at lunch; HP-D: high polyunsaturated fat diet at dinner; HM-L: high monounsaturated fat diet at lunch; HM-D: high monounsaturated fat diet at dinner. Source data are provided as a Source Data file.
To uncover taxa potentially associated with timing of USFA intake, we performed a Multivariate Analysis by Linear Models (MaAsLin2). After the 12-week intervention, we observed differential abundance patterns at the family levels, with no differences at baseline. Specifically, the HU-L groups exhibited a decrease in Lachnospiraceae (belonging to Firmicutes) and an increase in Prevotellaceae (belonging to Bacteroidetes) compared to the HU-D groups, after adjusting for baseline age, sex and BMI (Fig. 3C, D).
Within the Lachnospiraceae family, species such as Enterocloster bolteae and Ruminococcus gnavus increased in the HU-D groups after the 12-week intervention compared to baseline. Additionally, Ruminococcus gnavus Enterocloster hominis, Eubacterium rectale, were less abundant in HU-L groups than in HU-D groups at week 12 (Fig. 3E & Supplementary fig. 3). In contrast, Species in Prevotellaceae family, including Prevotella stercorea, Segatella sinensis and Segatella copri were more abundant in HU-L groups compared to HU-D groups at week 12 (Supplementary fig. 3 & Supplementary data file).
Other changes included an increase in Erysipelatoclostridium ramosum, a taxa often linked to obesity, infection, and Crohn’s disease20–22, following the HM-D intervention. This resulted in higher abundance in the HM-D group compared to the HM-L group by week 12 (Fig. 3E & Supplementary fig. 3). In contrast, species that contribute to anti-inflammatory effects and intestinal barrier function, such as Holdemanella biformis23, and Ruminococcus callidus24 were more abundant in the HU-L groups than in the HU-D groups (Supplementary fig. 3 & Supplementary data file).
Although the overall taxonomic composition remained largely similar between the HM-L and HP-L groups, significant species turnover was observed in response to types of USFA intake. Specifically, the 12-week HP-L intervention led to a decrease in species that ameliorate obesity and diabetes, such as Bifidobacterium longum25 and Blautia wexlerae26 (Supplementary fig. 3). In contrast, the 12-week HM-L intervention increased species with beneficial effects on insulin resistance, such as Alistipes indistinctus27 (Supplementary data file).
After adjusting for multiple comparisons, ten species within the Lachnospiraceae and Prevotellaceae family were identified affected by intake timing of USFA with FDR < 0.20. Among the differentially abundant species, Ruminococcus gnavus was also increased in the HP-D groups after the 12-week intervention (FDR = 0.087). No species affected by type of USFA passed the FDR correction. Furthermore, we analyzed results that combined groups with higher MUFA or PUFA consumption at the same mealtime since the effect of type of USFA on glycemic control and gut microbiome in this study was relatively minor. Ruminococcus gnavus was also identified as a key species (FDR = 0.027, Supplementary fig. 4 & Supplementary data file).
We subsequently aimed to enhance our understand of the bacterial-phenotype associations by conducting a spearman correlation analysis of all significant bacterial species with clinical phenotypes, with adjustment for age, sex and BMI. Our findings revealed several species within Lachnospiraceae family exhibited negative correlations with glycemic control parameters. Specifically, Enterocloster bolteae was negatively correlated with Stumvoll index and positively correlated with serum C14:0, C16:0 and C18:0 concentrations. Ruminococcus gnavus was negatively correlated with both Gutt index and Stumvoll index, while positively correlating with postprandial glucose levels. Additionally, Eubacterium rectale was found to positively correlated with fasting glucose levels and serum C14:0 concentrations. In contrast, Prevotella stercorea, a member of the Prevotellaceae family, was negatively correlated with postprandial insulin levels (Fig. 3F).
Furthermore, Bacteroides fragilis, a member of the Bacteroidaceae family, was observed to increase in the HP-D group by week 12 compared to baseline. This species, known to decrease following metformin treatment28, showed a positive association with fasting glucose levels. Additionally, the Clostridiaceae family, reported to play an important role in insulin resistance associated with statin use29, was presented by two species in this study: Clostridium sp.AF32-12BH, Clostridium sp.AF20-17LB. Both species decreased in the HP-D group by week 12 compared to baseline and exhibited positive correlations with the Gutt index.
Changes in gut microbiome functional pathways
To determine whether shifts in microbial composition translated into functional capacity, we profiled functional features of the gut microbiome utilizing the MetaCyc database. We compared the abundance of MetaCyc metabolic pathways after log transformation and adjusted for age, sex and BMI using -MaAsLin2. A total of 129 MetaCyc metabolic pathways involved in fatty acid and lipids degradation, carbohydrates biosynthesis, amino acids biosynthesis, secondary metabolites biosynthesis and degradation were differentially represented between HU-L and HU-D groups (119 pathways for HP-L vs HP-D and 11 pathways for HM-L vs HM-D). Pathways associated with carbohydrate metabolism, known to be enriched in diabetes30, were less abundant in the HU-L groups compared to the HU-D groups. For instance, we noted a reduced abundance of glycogen biosynthesis I (from ADP-D-Glucose, GLYCOGENSYNTH-PWY), 1,5-anhydrofructose degradation (PWY-6992), D-galactose degradation I (Leloir pathway, PWY-6317), pentose phosphate pathway (non-oxidative branch I, NONOXIPENT-PWY) in the HU-L groups (Supplementary data file).
Additionally, functional pathways associated with the biosynthesis of saturated fatty acids, which may potentially contribute to insulin resistance, were less abundant in the HU-L groups than in the HU-D groups. These pathways include the superpathway of fatty acid biosynthesis initiation (E.coli, FASYN-INITIAL-PWY), fatty acid salvage (PWY-7094), CDP-diacylglycerol biosynthesis I (PWY-5667), CDP-diacylglycerol biosynthesis II (PWY0-1319). Furthermore, functions related to the production of lipopolysaccharides (LPS)31, namely GDP-D-glycero-alpha-D-manno-heptose biosynthesis (PWY-6478) and superpathway of (Kdo)2-lipid A biosynthesis (KDO-NAGLIPASYN-PWY), were also downregulated in the HU-L groups compared to the HU-D groups (Supplementary data file).
We then examined the correlation between microbial functional pathways and clinical phenotypes to evaluate the role of these functions in glycemic control. As anticipated, Glycogen biosynthesis I (from ADP-D-Glucose, GLYCOGENSYNTH-PWY), D-galactose degradation I (Leloir pathway, PWY-6317), pentose phosphate pathway (non-oxidative branch I, NONOXIPENT-PWY) exhibited positive correlations with postprandial glucose and postprandial insulin levels, and negative associations with Gutt index and Stumvoll index (Fig. 4A). Additionally, CDP-diacylglycerol biosynthesis I (PWY-5667), CDP-diacylglycerol biosynthesis II (PWY0-1319) were positively associated with postprandial insulin levels and serum SFA concentrations, and negatively associated with Gutt index (Fig. 4A). Furthermore, the abundance of Enterocloster bolteae, Ruminococcus gnavus, Bacteroides fragilis, Erysipelatoclostridium ramosum showed positive associations with these pathways, whereas Clostridium sp.AF32-12BH displayed negative associations (Fig. 4B).
Fig. 4. Timing of unsaturated fatty acid intake altered gut microbiota functional profile.
A Partial Spearman correlations (two-sided) between gut microbiota energy metabolism and clinical phenotypes with baseline age, sex and BMI adjusted and Benjamini-Hochberg correction. *P < 0.05, **P < 0.01, ***P < 0.001. HDL-c: high-density lipoprotein cholesterol; LDL-c: low-density lipoprotein cholesterol; TCHO: total cholesterol; TG: triglyceride; SFA: saturated fatty acid; BMI: body mass index; AUCglucose: area under the curve of glucose; AUCinsulin: area under the curve of insulin. B Partial Spearman correlations (two-sided) between gut microbial energy metabolism and significant species with baseline age, sex and BMI adjusted and Benjamini-Hochberg correction. *P < 0.05, **P < 0.01, ***P < 0.001, #FDR < 0.20. Source data are provided as a Source Data file.
Intake timing and type of USFA alters fecal metabolites
For further insight into the effects of the timing and type of USFA intake on the metabolism of the human host and the commensal intestinal microbiome, we performed a targeted metabolomics analysis of fecal samples collected before and after the intervention. A total of 546 fecal metabolites were measured. The timing of USFA intake exhibited distinct influences on the overall metabolite changes (Fig. 5A). Concurrently, the type of dietary USFA intake significantly affected the changes in the overall metabolite profiles (Fig. 5A).
Fig. 5. Changes in fecal metabolites.
A Difference in overall changes of fecal metabolome after timing and type of unsaturated fatty acid intake, Log2-transformed fold-change profile for all individual metabolites were used in partial least-squares discriminant analysis (PLS-DA). B Partial Spearman correlations (two-sided) between significant differential metabolites and clinical phenotypes with baseline age, sex and BMI adjusted and Benjamini-Hochberg correction. *P < 0.05, **P < 0.01, ***P < 0.001, #FDR < 0.20. C Abundance and Log2-transformed fold-change of significant differential bile acids between HP-L and HP-D (upper), HM-L and HM-D (lower) as determined by wilcoxon test (two-sided). *P < 0.05, #FDR < 0.20. D Partial Spearman correlations (two-sided) between significant differential bile acids and significant differential species with baseline age, sex and BMI adjusted and Benjamini-Hochberg correction. *P < 0.05, **P < 0.01, ***P < 0.001, #FDR < 0.20. E BA ratios for each group at week 12. Data are shown as box-and whisker plots, centre line, box boundaries, lower and upper whisker represent the median, quartile, minimum and maximum range respectively. Analysis was done using General linear model (two-sided) with post hoc Bonferroni correction. Number of participants: n = 15 for each group. HP-L: high polyunsaturated fat diet at lunch; HP-D: high polyunsaturated fat diet at dinner; HM-L: high monounsaturated fat diet at lunch; HM-D: high monounsaturated fat diet at dinner. CAR:carnitine; LPC: Lysophosphatidyl choline; HDL-c: high-density lipoprotein cholesterol; LDL-c: low-density lipoprotein cholesterol; TCHO: total cholesterol; TG: triglyceride; SFA: saturated fatty acid; BMI: body mass index; AUCglucose: area under the curve of glucose; AUCinsulin: area under the curve of insulin; Log2FC (L/D): Log2 transformed fold change of HP-L/HP-D or HM-L/HM-D; BA: bile acid; CA:cholic acid; CDCA:chenodeoxycholic acid; LCA:lithocholic acid; GLCA:glycocholic acid. Source data are provided as a Source Data file.
At the individual metabolite level, the timing of MUFA intake (both HM-L and HM-D) altered 67 metabolites (P < 0.05, FDR > 0.20) by week 12 compared to baseline. Similarly, the timing of PUFA intake (both HP-L and HP-D) affected 109 metabolites (of which 79 metabolites were P < 0.05, FDR < 0.20) by week 12 compared to baseline. Furthermore, we identified 21 metabolites in the MUFA groups and 54 metabolites in the PUFA groups that were influenced by the timing of intake at the end of the intervention (P < 0.05, FDR > 0.20), despite showing no differences at baseline (P > 0.05, FDR > 0.20). These metabolites encompass amino acid and its derivatives, organic acid and its derivatives, and bile acids (Supplementary data file). In terms of the type of USFA intake, 14 metabolites differed between the HM-L and HP-L group. Beneficial metabolites such as 2-Aminoisobutyric Acid, 2-Aminooctanoic acid and Indole-3-glyoxylic acid were more abundant in HM-L group than in HP-L group (FDR > 0.20, Supplementary data file).
Spearman correlation analyses revealed multiple significant correlations between the identified metabolites and clinical parameters. Bile acids (BA), including glycochenodeoxycholic acid and taurocholic acid, Lysophosphatidylcholine (LPC), including 1-Myristoyl-sn-glycero-3-phosphocholine and 1-Palmitoyl-sn-glycero-3-phosphocholine, were positively correlated with postprandial insulin levels and negatively correlated with insulin sensitivity indices, such as the Gutt index and Stumvoll index (Fig. 5B). The amino acid beta-alanine, and the organic acid 2-hydroxyisovaleric acid showed similar correlations. Indoleacrylic acid, an antioxidant organic acid derived from tryptophan degradation, was negatively correlated with postprandial insulin levels and positively correlated with insulin sensitivity indices (Fig. 5B). Amino acids and its derivates (tyrosine, L-Homocitrulline) was negatively correlated with postprandial glucose levels and the organic acid Potassium Phenyl Sulfate was negatively correlated with fasting glucose levels, while indolelactic acid, 4-methoxyphenylacetic acid were positively correlated with postprandial glucose levels (Fig. 5B).
Additionally, several metabolites were significantly correlated with insulin resistance risk factors, including BMI and body fat percentage. Specifically, 7-ketodeoxycholic acid, chenodeoxycholic acid, cholic acid glycohyocholic acid, glycohyodeoxycholic acid, taurocholic acid, L-palmitoylcarnitine, and choline were positively correlated with BMI, whereas isoallolithocholic acid, isolithocholic acid were negatively correlated with BMI (Fig. 5B). Taurolithocholic acid was also found to be correlated with body fat percentage (Fig. 5B).
The differential species identified in this study (such as Enterocloster bolteae, Ruminococcus gnavus, and Bacteroides fragilis) are associated with bile acid (BA) metabolism. Consequently, we examined the changes in bile acids following timing of USFA intake, common changes included a decreased in taurolithocholic acid in the HU-L groups and an increased in 7-ketodeoxycholic acid in the HU-D groups at week 12 compared to baseline (Fig. 5C), and 7-ketodeoxycholic acid significantly increased in HP-D group after the 12-week intervention (FDR = 0.16). Additionally, specific changes related to the type of USFA intake were observed. For instance, chenodeoxycholic acid, lithocholic acid, and 7-ketolithocholic acid increased with the 12-week HP-D intervention (FDR = 0.06, 0.21 and 0.19, respectively), while glycocholic acid increased in the HM-L group, and 3β-deoxycholic acid increased in the HM-D group by week 12 compared to baseline (both FDR > 0.20, Fig. 5C). Furthermore, analyses after combination revealed that 9 of aforementioned BAs including taurolithocholic acid and 7-ketodeoxycholic acid were affected by intake timing of USFA (FDR < 0.20, Supplementary fig. 4). Spearman correlations were conducted to link differential metabolites and differential microbial species, revealing several significant correlations between bacterial species and bile acid abundance. Notably, taurolithocholic acid and 7-ketodeoxycholic acid were positively correlated with Ruminococcus gnavus and negatively correlated with Eubacteriaceae bacterium (Fig. 5D).
The ratio of secondary BA to primary BA serves as an indicator of gut microbiome activity. HU-L exhibited a trend towards increasing this ratio (Ptiming = 0.062, Ptype = 0.529, Pinteraction = 0.161) compared with HU-D (Fig. 5E). Moreover, HU-L significantly elevated the ratio of lithocholic acid (LCA) to chenodeoxycholic acid (CDCA) (LCA/CDCA, Ptiming = 0.019, Ptype = 0.640, Pinteraction = 0.237), and also tended to increase the ratio of cholic acid (CA) to CDCA (CA/CDCA, Ptiming = 0.057, Ptype = 0.507, Pinteraction = 0.743) and glycocholic acid (GLCA) to CDCA (GLCA/CDCA, Ptiming = 0.067, Ptype = 0.532, Pinteraction = 0.374) compared with HU-D. It is worth noting that types of dietary USFA did not have an impact on BA ratios (Fig. 5E).
Effect of fecal microbial transplantation on glucose homeostasis in mice
Finally, we conducted a fecal microbial transplantation (FMT) to examine the causal relationship between differentially shaped microbiome and changes in insulin sensitivity and serum fatty acids by timing of USFA intake. After a 4-week FMT, mice colonized with microbiomes from donors in the HP-L and HM-L groups displayed significantly improved blood glucose tolerance compared to those receiving microbiomes from the HP-D and HM-D groups respectively (all P < 0.05, Fig. 6B). Furthermore, mice receiving microbiomes from the HP-L and HM-L groups also showed decreased levels of serum saturated fatty acids (C14:0, C16:0 and C18:0, Fig. 6E). However, no significant differences in blood glucose tolerance or serum fatty acid levels were observed between recipients of the HP-L and HM-L groups (AUCOGTT P = 0.885, På 0.05 for serum fatty acids, Fig. 6B, E). There were no significant differences in body weight or the amounts of perinephric, peritesticular and omental fat among the four groups (Fig. 6C, D).
Fig. 6. Fecal microbiota transplantation experiment.
A Brief work flow of FMT experiment and the evidence of the high-fat modeling (Created in BioRender. Wei, C. (2025) https://BioRender.com/502ohcd). Comparisons between two groups were performed using Student’s t-test (two-sided) for normally distributed data or the Mann-Whitney U test (two-sided) for non-normally distributed data. Data are presented as mean ± SD. Number of mice: n = 7 in NC group and n = 28 in HF group in modeling period, n = 7 in each group in FMT period. *P < 0.05, **P < 0.01,***P < 0.001. B Changes in blood glucose tolerance after 4-week FMT experiment. Comparisons between two groups were performed using Student’s t-test (two-sided). Data are presented as mean ± SD. Number of mice: n = 6 in each group. *P < 0.05, **P < 0.01. The exact p values were AUC(RPD vs RPL): 0.004, AUC(RMD vs RML): 0.016, OGTT-120min(RMD vs RML):0.045. C Trends in weight change during the FMT period. Data are presented as mean ± SD. Number of mice: n = 7 in each group. D Fat weight of recipients in each group after FMT. Data are presented as mean ± SD. Number of mice: n = 7 in each group. E Serum fatty acids of recipients in each group. Two-way ANOVA (two-sided) followed by post hoc Bonferroni test was used. Number of mice: n = 7 in each group. *Ptiming < 0.05. HF: high fat group; NC: normal diet control group; HFD: high fat diet; FMT: fecal microbiota transplantation; Pre-FMT:pre-fecal microbiota transplantation; ABX: Antibiotics; RPL, recipient of high polyunsaturated fat diet at lunch; RPD, recipient of recipient of high polyunsaturated fat diet at dinner; RML, recipient of high monounsaturated fat diet at lunch; RMD, recipient of high monounsaturated fat diet at dinner; AUC: area under the curve. Source data are provided as a Source Data file.
Disscussion
This study demonstrates that the timing of dietary USFA intake significantly influenced insulin sensitivity and serum fatty acid profile in individuals with prediabetes. And alterations in the gut microbiome and fecal metabolites exhibited a correlation with improvements in clinical phenotypes. Subsequent FMT experiments in mice confirmed that these beneficial shifts can be transferred to the host by constructing a similar gut micro-environment. However, diets rich in MUFA or PUFA did not exhibit significant differences in these outcomes.
The metabolic benefits of increasing dietary USFA intake for maintaining glucose homeostasis have been widely accepted. However, the glucose-regulating effects between the two types of dietary USFA (MUFA and PUFA) remain controversial. In this isocaloric feeding trial, where all food ingredients were consistent, participants were administrated diets rich in either MUFA or PUFA, delivered through the use of designed cooking oils. The fatty acid ratios (SFA: MUFA: PUFA) was 13%: 58%: 29%) for the MUFA-rich oil and 16%: 26%:58% for the PUFA-rich oils respectively. This trial simulated the effects of different types of dietary USFA intake on glycemic control. After a 12-week intervention, no difference in glycemic control was observed between diets rich in MUFA or PUFA. This finding underscores the importance of considering not only the type of USFA, but also exploring novel dietary strategies for the prevention and treatment of diabetes.
Recent studies indicates that the timing of food intake affects glucose and lipid metabolism, with early calorie loading (before 15:00) shown to improve glycemic control32,33. However, for individuals with diabetes, current guidelines recommending increased USFA intake do not specify the optimal timing. In this study, we attempt to investigate the suitable timing for dietary USFA intake. The fat distribution for lunch and dinner was: 42% and 24% for HP-L, 28% and 37% for HP-D, 42% and 25% for HM-L, and 29% and 35% for HM-D, with approximately 80% of total fat intake coming from USFA. Our findings align with previous studies, demonstrating that early dietary USFA intake significantly enhances insulin sensitivity and improves serum fatty acid profile in participants with prediabetes.
The gut microbiome has long been regarded as the ‘sensitive additional organ’ in response to dietary factors, which also play important role in the etiology of diabetes34,35. To further explore how early dietary USFA intake improves insulin sensitivity, we investigated the impact of dietary USFA intake timing on the gut microbiome. In this study, HU-L increased richness of microbial community, which is associated with a healthier gut microbiome36. Our analysis suggested that the timing of dietary USFA intake, rather than the type, may play a role in shaping the structure and function of the gut microbial community. At the family level, we observed elevated levels of Lachnospiraceae in the HU-D groups, which are known to be involved in the conversion of secondary bile acids. Previous studies have reported that Lachnospiraceae is enriched in patients with diabetes37, and its increased abundance has been positively correlated with hyperglycemia, hyperlipidemia and ectopic fat accumulation in animal studies38,39. Here, we also observed species within Lachnospiraceae (such as Ruminococcus gnavus and Enterocloster bolteae) were elevated after the HU-D intervention compared to baseline (Fig. 3E). These species have been previously linked to obesity40, diabetes41 and prediabetes42 and in our study, they were negatively correlated with insulin sensitivity indices (Gutt and Stumvoll index) in this study. Additionally, we observed that HU-L increased Prevotellaceae compared to HU-D after a 12-week intervention. Prevotellaceae is known to improve glycemic control through the production of short-chain fatty acids43. In summary, our findings indicate that the composition and function of gut microbiome may be influenced by dietary USFA intake timing, and these changes could potentially be associated with insulin sensitivity.
Fecal metabolites play a key role in regulating host glucose and lipid metabolism. Consistent with bile acid metabolism potential of key species identified in this study, participants in the HU-L groups showed a decrease in taurolithocholic acid, whereas those in the HU-D groups exhibited an increase in 7-ketodeoxycholic acid after the 12-week intervention compared to baseline (Fig. 5C). Intestinal BA levels have been previously linked to obesity-related diseases, such as diabetes and dyslipidemia44. Additionally, elevated levels of fecal taurolithocholic acid have been associated with cholestasis, inflammation45 and hepatic fat accumulation46 in other studies. Based on these observations, we hypothesize that dietary USFA intake at dinner might contribute to increased lipid absorption and inflammatory responses, which could potentially impaire insulin sensitivity.
Primary bile acids are converted to secondary bile acids in the intestine and the ratio of these bile acids is critical for glucose and lipid metabolism. 7-ketodeoxycholic acid is an intermediate in the biotransformation of primary to secondary bile acid. Its accumulation indicates a decreased efficiency in this biotransformation process. In this study, the increased ratio of secondary to primary BAs in the HU-L groups could suggest a higher efficiency in BA transformation by the gut microbiome with early unsaturated fat intake. Additionally, the higher ratio of LCA/CDCA in HU-L groups might indicate an increased transformation from CDCA to LCA, which has been previously reported to enhance TGR5 activity47, and could potentially improve glucose metabolism. Taken together, these findings raise the possibility that a higher dietary USFA intake at lunch might enhance the conversion efficiency of bile acid and yield metabolic benefits.
Furthermore, we performed a 4-week FMT experiment to further validate the causal relationship between the microbiome shaped by timing of USFA intake and changes in glucose homeostasis. As anticipated, after the FMT, mice receiving microbiomes from donors in the HP-L and HM-L groups (aforementioned feeding trial groups) displayed significantly improved blood glucose tolerance compared to those receiving microbiomes from the HP-D and HM-D groups (all P < 0.05, Fig. 6B). Serum fatty acid analysis revealed a decrease in saturated fatty acids (C14:0, C16:0 and C18:0) in mice colonized with microbiomes from HP-L and HM-L groups, aligning with the outcomes of our feeding trial. Collectively, these results substantiate the hypothesis that favorable changes in glycemic control are transferrable by replicating a similar gut microenvironment.
To our knowledge, this is the first factorial-designed feeding trial to dissect the relationship between the timing of dietary USFA intake, glycemic homeostasis and the gut microbiome. A notable strength lies in our focus on prediabetic individuals who are not taking hypoglycemic agents, as many studies have demonstrated that hypoglycemic agents can significantly modulate the gut microbiome48. Additionally, in this feeding trial, we use cooking oils rich in MUFA or PUFA and maintained consistent food ingredients across all groups, thereby simulating the types of dietary USFA intake in real-life condition. However, there are still several limitations in our study. Firstly, the sample size is relatively small, and duration of our intervention is relatively short. The sample size calculation and power were based on postprandial glucose changes, whereas other co-primary outcomes were not considered. However, the type I error was controlled (α/4) in their analysis. These limitations may result in our study being particularly underpowered for secondary analyses; therefore, these results should be interpreted with caution as exploratory. Although the sample size is comparable to that of another well controlled feeding trial32, this does not mitigate the limitations imposed by a smaller sample size. Additionally, we did not conduct sex-stratified analyses due to concerns that further stratification would underpower the results given the relative small sample size. While previous studies have reported positive response to timing of calorie loading for both males and females. Further long-term, multi-center trials with larger sample sizes are needed to validate our findings. Secondly, fecal samples were collected at a fixed time at baseline and post-intervention, additional studies are required to assess the diurnal oscillation of the gut microbiome and its response to intake timing and types of USFA. Furthermore, future animal studies are pivotal in elucidating the precise mechanisms by which the timing of USFA intake affects the dynamic rhythmicity of the gut microbiome and fecal metabolites, thereby influencing metabolic health.
In conclusion, our study demonstrates that advancing the timing of USFA intake improves insulin sensitivity and reduces serum SFAs in participants with prediabetes. This beneficial effect is consistent across different types of USFA consumed. The gut microbiome and bile acid metabolism were identified as key factors contributing to the beneficial effects of USFA intake timing on insulin sensitivity.
Methods
Study design
This trial was registered at Chinese Clinical Trial Registry website (ChiCTR2100045645). The study protocol was approved by the Ethics Committee of Harbin Medical University. All participants who met eligibility criteria have signed written informed consent. This study was conducted in accordance with the tenets of the Declaration of Helsinki and Good Clinical Practice guidelines. The study was conducted according to the 2 × 2 factorial-design. For the investigation of intake timing, subjects consumed a USFA-rich diet either at lunch or at dinner. The amount of the cooking oil used in lunch and dinner was distributed as 18 g and 9 g for USFA-rich diet at lunch (HU-L) group, and 9 g and 18 g for USFA-rich diet at dinner (HU-D) group, respectively, with the amount of oil used in breakfast fixed at 3 g. Regarding the type of dietary USFA, a PUFA-rich diet (based on the ratios observed in the daily eating habits of Chinese population) and a MUFA-rich diet (according to the WHO recommendation 202349) were designed. Based on the above design, enrolled participants were randomly assigned to 4 groups: MUFA-rich diet at lunch (HM-L), MUFA-rich diet at dinner (HM-D), PUFA-rich diet at lunch (HP-L), and PUFA-rich diet at dinner (HP-D) after sex stratification. Sex was defined based on self-report. The habitual dietary intake of participants was collected to analyze their daily energy intake and macronutrient intake. All participants were fed an energy balanced diet for 7 days, and then an all-food-supplied diet for 12 weeks.
The ingredients used in each group were completely identical. MUFA-rich blended oil (SFA: MUFA: PUFA was 13%: 58%: 29%) and PUFA-rich soybean oil (SFA: MUFA: PUFA was 16%: 26%: 58%) were used. The proportions of energy provided by carbohydrate, fat and protein were 49%, 33% and 18%, respectively (according to dietary fat intake recommendation for adults with diabetes in China50). The energy distribution for USFA-rich diet at lunch (HU-L, including HM-L and HP-L) groups was 25% at breakfast, 40% at lunch and 35% at dinner. The energy distribution for USFA-rich diet at dinner (HU-D, including HM-D and HP-D) groups was 25% at breakfast, 36% at lunch and 39% at dinner. The total USFA (food and oil) counts for 4%: 11%: 7% versus 4%: 8%: 10% of TE at breakfast, lunch, and dinner, respectively. The ratio of saturated fat (SFA): MUFA: PUFA was 5%: 9%: 11% of total energy (TE) for high PUFA groups and 5%: 13%: 8% TE for high MUFA groups, respectively. Detailed information on nutrient distribution and the menu of provided diet were provided in Supplementary data file.
Participants were asked to finish their meals within 30 min. Participants were encouraged to eat all the dishes provided. In rare cases, if a meal was not fully consumed, the actual amount of food consumed was calculated by subtracting the remaining portion from the target meal. Throughout the study, participants were instructed to abstain from consuming alcohol and caffeinated energy drinks. In addition, they were instructed to maintain their regular physical activity levels during the trial.
Participant recruitment
This randomized, controlled, factorial-designed, feeding trial was conducted in Qiqihar, China between September 2021 and November 2021 (prospectively registered on ClinicalTrials.gov as ChiCTR2100045645, all the participants were recruited on 10 August 2021). A total of 475 participants were recruited from the same workplace for physical examination to evaluate their suitability to the trial. To be included, the participants should be at prediabetic status and altered serum fatty acid profile (elevated SFA percentage, decreased USFA percentage). To be qualified as at prediabetic status, participants should have elevated fasting glucose (100–126 mg/dL) or postprandial glucose (140–199 mg/dL). Participants were excluded if they met any of the following criteria: 1) belonged to the lowest quintile of serum saturated fatty acid percentage, or the highest quintile of serum monounsaturated fatty acid percentage or serum polyunsaturated fatty acid percentage; 2) having fever, infectious diseases, gastrointestinal diseases; 3) had comorbid hepatobiliary disease, malignant tumor, endocrine diseases or other autoimmune diseases, or severe organ damage such as heart, liver, brain and kidney; 4) had taken antibiotics, probiotics or prebiotics, or blood lipid-regulating drugs within 4 weeks prior to enrollment; 5) being unable to commit sufficient time to participate in this trial. Additionally, four participants who were diagnosed with diabetes during this physical examination were also enrolled. Following an evaluation by endocrinologists and considering the participants’ opinions, these newly diagnosed diabetes participants were also included in the randomized allocation process. The results were not changed if the four newly diagnosed diabetes participants were not included (Ptiming = 0.0001, Ptype = 0.5264 for postprandial insulin levels; Ptiming < 0.0001, Ptype = 0.1209 for Gutt index; Ptiming = 0.0003, Ptype = 0.3323 for Stumvoll index).
Experimental procedures
Participants were required to visit the laboratory for physical measurements and blood sampling after the 7-day balanced diet and every 4 weeks thereafter until the last intervention. Standardized procedures were followed to measure body weight, height, fat mass ratio. BMI was calculated by dividing weight (in kilograms) by the square of height (in meters). After fasting blood sampling, participants consumed a 200 mL solution containing 75 g glucose. Additional blood samples were taken at 30, 60 and 120 min after the first consumption to monitor the dynamic glucose and insulin levels. Fasting serum samples were used to analyze triglycerides, LDL cholesterol, HDL cholesterol, total cholesterol. Fasting whole blood samples were collected to measure HbA1c levels. Participants were also fitted with a continuous glucose monitor (CGM, Abbott Freestyle Libre) at the beginning and the last two weeks of the intervention to record their glycemic response. Fecal samples were collected before and at the end of the intervention. The detailed procedures of continuous glucose monitoring, blood and fecal sample collection are reported in Supplementary Material.
Main outcomes measurement
Postprandial serum glucose was the first primary outcome, which was used to calculate sample size and the power of the test. Insulin levels and insulin sensitivity indices (Gutt index and Stumvoll index) were second co-primary outcomes. Serum glucose was measured using an automatic biochemistry analyzer (Hitachi 7100, Japan). Serum insulin level was measured using an automatic immunoanalyzer (Beckman UniCel Dxl 800, USA). The Gutt index was calculated as [75000 + (fasting glucose-2-h glucose) ×0.19×weight] / (120×log [(fasting insulin+2-h insulin)/2] × [(fasting glucose+2-h glucose)/2]51. Stumvoll index was calculated as 0.222-(0.0033×BMI)-(0.0000779×7.175×2-h insulin)-(0.000422 × age)52.
Secondary outcomes measurement
Serum lipid profile (including triglycerides, LDL cholesterol, HDL cholesterol, total cholesterol levels) was measured using an automatic biochemistry analyzer. Whole-blood HbA1c levels were measured using an automatic glycosylated hemoglobin analyzer (HA-8380; ARKRAY, Tokyo, Japan). Area under the time concentration curve (AUC) of glucose and insulin in OGTT was calculated using the trapezoidal methods. Glucose parameters, such as mean glucose, standard deviation (SD), estimated HbA1c, mean amplitude of glycemic excursion (MAGE), and area under the curve (AUC) were calculated from the CGM data. The serum free fatty acid profile was determined using gas chromatography-mass spectrometry (GC-MS, Thermo Fisher Scientific, USA). Analysis of the gut microbiome was performed using metagenomic sequencing (Metware, Wuhan, China). Fecal targeted metabolites were quantified using platforms combining LC-MS/MS with GC-MS/MS (Metware, Wuhan, China). For more detailed information regarding CGM monitoring, fatty acid detection, metagenomic sequencing, fecal metabolites quantification, please refer to the Supplementary Material.
Mouse model design of the fecal microbial transplantation
The animal study was approved by Ethics Committee of Harbin Medical University. In the 8-week high-fat modeling period of the mouse study, 8-week-old male C57BL/6 N mice (Beijing Vital River Laboratory Animal Technology Co., Ltd, China) were randomly divided into 2 groups after one week of acclimatization: normal-control diet group (referred to as ‘NC group’, N = 7), high-fat diet group (‘HF group’, N = 28, 60% high-fat diet). The use of male mice was specifically chosen to eliminate the potential influence of estrogen on the gut microbiome. The NC group was fed with AIN93M diet, while the HF group received a modified high-fat diet based on the AIN93M formulation (Supplementary Table 4). All mice were provided with ad libitum access to food and water. The animals were housed in a controlled environment maintained at 22–24 °C with a 12 h light-dark cycle and relative humidity of 50–60%. An oral glucose tolerance test was performed at the end of the modelling period.
In the fecal microbial transplantation (FMT) period, 28 mice in the HF group were further randomly divided into 4 groups after 9 weeks of feeding: RA group (N = 7, received stool samples from feeding trial group HP-L), RB group (N = 7, received stool samples from feeding trial group HP-D), RC group (N = 7, received stool samples from feeding trial group HM-L) and RD group (N = 7, received stool samples from feeding trial group HM-D). All mice were maintained on a 60% HF diet during the FMT experiment. An oral glucose tolerance test was performed at the end of the 4-week FMT period. Details of fecal microbial transplantation are shown in Supplementary material.
Statistical analysis
Sixty participants with completed fecal samples were included in the final analysis (shown in Supplementary fig. 1).
Sample size estimation
The sample size was calculated using Power Analysis and Sample Size Software (PASS) 2021 software (UT, USA, https://www.ncss.com/software/pass/). Glucose (OGTT-2h) was considered as the first primary outcome. Seventeen subjects per group was determined for an 80% statistical power to detect a difference of 12 mg/dL in glucose levels during an OGTT (two-sided test, α = 0.05), assuming the standard deviation of 10 mg/dL and 15% dropout rate. Insulin, Gutt index, and Stumvoll index were considered as second co-primary outcomes, and p values of these co-primary outcomes less than 0.0125 (0.05/4) were defined as significant.
Measurable and biochemical parameters
For measurable and biochemical parameters, analyses were performed using SPSS version 26 (SPSS Inc., USA). P < 0.05 was considered statistically significant. Baseline data were tested for normality using Shapiro-Wilk test. Non-normally distributed data were analyzed using Kruskal-Waillis test followed by Dunn’s multiple comparison’s test. Normally distributed data were analyzed using one-way ANOVA for group comparisons followed by post hoc Bonferroni test. According to the factorial design of our study, a three-way repeated measures ANOVA followed by post hoc Bonferroni test was performed to analyze biochemical parameters post-intervention, with follow-up timepoint as a within subject factor, and timing group and type group as between-subject factors (Fig. 1B). Additionally, to adjust for the influence of baseline levels, main outcome parameters at week 12 were analyzed using General linear model (GLM) with Bonferroni correction, adjusting for baseline values (Supplementary Table 2). The changes of measurable and biochemical parameters at week 12 from baseline were analyzed using two-way ANOVA followed by post hoc Bonferroni test. Comparisons between completers and non-completers were performed using Student’s t-test or Mann-Whitney U test for continuous parameters after assessing for normality with the Shapiro-Wilk test. The χ2 test was used for categorical data.
Continuous glucose parameters and serum free fatty acids
Continuous glucose parameters were analyzed using R software53 version 4.4.0 and R package ‘iglu’54. The effects of intake timing and the type of USFA, along with their interaction, on continuous glucose and serum free fatty acids were analyzed using mixed models with post hoc Bonferroni correction. The models included random effect terms for participants and fixed effect terms for intake timing, the type of USFA, and their interaction, with baseline measurements adjusted as covariates (using the ‘lme4’ package)55.
Metagenomic analysis
Metagenomic sequencing analyses were conducted using the R package ‘vegan’56. Chao1 and Shannon indexes were calculated based on species level to represent the richness and evenness of alpha diversity, and Bray-Curtis dissimilarity was performed to represent beta diversity. Permutational multivariate analysis of variance (PERMANOVA) with 999 permutations was applied to assess group differences. Differential abundance analyses at the family, species and metagenomic pathways levels were performed using the Multivariate Analysis by Linear Models (‘MaAsLin2’ package)57, which included covariates such as baseline age, sex and BMI. The relative abundance of family and species were arcsine square root transformed, and Metacyc pathways abundance were analyzed using CPLM with total sum scaling normalization. Differential species were defined as those that:1) differed from baseline at week 12, or 2) showed no differences at baseline but differed at week 12. Significance was defined as P < 0.05. P values were further adjusted using the Benjamini–Hochberg correction in MaAsLin2, with an FDR cutoff at 0.20 to further filter key species.
Fecal metabolic analysis
Total sixty samples (N = 15 for each group) were measured. We conducted partial least-squares discriminant analysis (PLS-DA) on log2-transformed fold-change of all individual metabolites (‘mixOmics’ package). Differential metabolite analysis was performed using Wilcoxon signed-rank test to examine differences between week 12 and baseline, and Wilcoxon rank-sum test to assess differences between HU-L and HU-D groups at week 12 (‘dplyr’ package)58. Ratio of bile acids were log10-transformed and analyzed using General linear model with post hoc Bonferroni correction. P < 0.05 was considered significant. P values were corrected for multiple comparisons (Benjamini–Hochberg correction) using the ‘stats’ R package, with an FDR cutoff at 0.20 to further filter key species.
Correlation analysis
Partial Spearman correlations revealing the relationships between clinical parameters, species and metabolites at the end of the study were performed using ‘ppcor’ package59, adjusting for baseline age, sex and BMI. P < 0.05 was considered significant. P values were corrected for multiple comparisons (Benjamini–Hochberg correction) using ‘stats’ R package, with an FDR cutoff at 0.20 to further filter key species.
Animal experimental data
For animal experiments, all analyses were conducted using SPSS version 26 (SPSS Inc., USA). Comparisons between two groups were performed using Student’s t test for normally distributed data or the Mann-Whitney U test for non-normally distributed data. For multiple group comparisons, we employed one-way ANOVA followed by post hoc Bonferroni test for normally distributed data, and the Kruskal-Waillis test followed by Dunn’s test for non-normally distributed data.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Source data
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (Key Program 82030100 to Y.L.) and Joint Fund Project (U24A20768 to Y.L.). The authors thank the study participants and the staff at Department of Nutrition and Food Hygiene for helping to complete the trial.
Author contributions
Y.L., C.S., T.H. and C.W. contributed to the study design. C.W., X.X., X.W., F.L., Y.C., X.S., G.G., Y.P., X.Y. participated in the execution of the study. C.W., X.X., J.Z., Y.Z., H.X. performed the statistical analysis and wrote the first draft of the manuscript. T.H., X.W., and R.L. repeated and validated the statistical analysis. Z.M., Y.Z. measured serum biochemical parameters. K.D., S.P measured serum fatty acid profile. Q.L.and H.Q. performed the animal experiments. J.Z. revised the manuscript. All coauthors commented on the manuscript and agreed with the manuscript results and conclusions.
Peer review
Peer review information
Nature Communications thanks Susanne Brix, and the other, anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Data availability
The metagenomic sequencing data generated in this study have been deposited in the NCBI database under BioProject accession code PRJNA1238858 and the Genome Sequence Archive in National Genomics Data Center (BioProject:PRJCA033596). The metabolomics data are available in MetaboLights under the accession number MTBLS12353. All individual de-identified participant data supporting the findings of this study are available under restricted access due to compliance with data protection regulations and participant consent agreements. Requests for access can be made by contacting liying_helen@163.com, and should include a detailed research proposal and ethical approval documentation. Requests will be promptly reviewed by Harbin Medical University within 4 weeks to assess potential intellectual property or confidentiality obligations. Access will be granted only to qualified researchers for non-commercial academic purposes. Upon approval, access to the controlled data will be granted for a period of one year. Source data are provided with this paper. The processed data are available within the Source Data file. Source data are provided with this paper.
Code availability
The R codes for data analysis are available at zenodo 10.5281/zenodo.15078756.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Changhao Sun, Email: changhaosun2002@163.com.
Ying Li, Email: liying_helen@163.com.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-025-58937-6.
References
- 1.Knowler, W. C. et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med.346, 393–403 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Imamura, F. et al. Effects of Saturated Fat, Polyunsaturated Fat, Monounsaturated Fat, and Carbohydrate on Glucose-Insulin Homeostasis: A Systematic Review and Meta-analysis of Randomised Controlled Feeding Trials. PLoS Med.13, e1002087 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qian, F., Korat, A. A., Malik, V. & Hu, F. B. Metabolic Effects of Monounsaturated Fatty Acid-Enriched Diets Compared With Carbohydrate or Polyunsaturated Fatty Acid-Enriched Diets in Patients With Type 2 Diabetes: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Diabetes Care39, 1448–1457 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eckel-Mahan, K. L. et al. Reprogramming of the circadian clock by nutritional challenge. Cell155, 1464–1478 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leone, V. et al. Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism. Cell Host Microbe17, 681–689 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hatori, M. et al. Time-Restricted Feeding without Reducing Caloric Intake Prevents Metabolic Diseases in Mice Fed a High-Fat Diet. Cell Metab.15, 848–860 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kessler, K. et al. The effect of diurnal distribution of carbohydrates and fat on glycaemic control in humans: a randomized controlled trial. Sci. Rep. 7, 44170 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han, T. S. et al. The Association of Energy and Macronutrient Intake at Dinner Versus Breakfast With Disease-Specific and All-Cause Mortality Among People With Diabetes: The US National Health and Nutrition Examination Survey, 2003-2014. Diabetes Care43, 1442–1448 (2020). [DOI] [PubMed] [Google Scholar]
- 9.Jakubowicz, D. et al. High-energy breakfast with low-energy dinner decreases overall daily hyperglycaemia in type 2 diabetic patients: a randomised clinical trial. Diabetologia58, 912–919 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Schoeler, M. et al. The interplay between dietary fatty acids and gut microbiota influences host metabolism and hepatic steatosis. Nat. Commun. 14, 5329 (2023). [DOI] [PMC free article] [PubMed]
- 11.Crudele, L., Gadaleta, R. M., Cariello, M. & Moschetta, A. Gut microbiota in the pathogenesis and therapeutic approaches of diabetes. Ebiomedicine97, 104821 (2023). [DOI] [PMC free article] [PubMed]
- 12.Leone, V. et al. Effects of Diurnal Variation of Gut Microbes and High-Fat Feeding on Host Circadian Clock Function and Metabolism. Cell Host Microbe17, 681–689 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zarrinpar, A., Chaix, A., Yooseph, S. & Panda, S. Diet and Feeding Pattern Affect the Diurnal Dynamics of the Gut Microbiome. Cell Metab20, 1006–1017 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thaiss, C. A. et al. Transkingdom Control of Microbiota Diurnal Oscillations Promotes Metabolic Homeostasis. Cell159, 514–529 (2014). [DOI] [PubMed] [Google Scholar]
- 15.Liang, X., Bushman, F. D. & FitzGerald, G. A. Rhythmicity of the intestinal microbiota is regulated by gender and the host circadian clock. Proc. Natl. Acad. Sci. USA.112, 10479–10484 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi, H., Rao, M. C. & Chang, E. B. Gut microbiota as a transducer of dietary cues to regulate host circadian rhythms and metabolism. Nat. Rev. Gastroenterol. Hepatol.18, 679–689 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reitmeier, S. et al. Arrhythmic Gut Microbiome Signatures Predict Risk of Type 2 Diabetes. Cell Host Microbe28, 258–272.e256 (2020). [DOI] [PubMed] [Google Scholar]
- 18.Xie, Z. B. et al. Randomized controlled trial for time-restricted eating in healthy volunteers without obesity. Nat. Commun. 13, 1003 (2022). [DOI] [PMC free article] [PubMed]
- 19.Zarrinpar, A., Chaix, A., Yooseph, S. & Panda, S. Diet and feeding pattern affect the diurnal dynamics of the gut microbiome. Cell Metab.20, 1006–1017 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Chatelier, E. et al. Richness of human gut microbiome correlates with metabolic markers. Nature500, 541 (2013). [DOI] [PubMed] [Google Scholar]
- 21.Zuo, T. et al. Alterations in Gut Microbiota of Patients With COVID-19 During Time of Hospitalization. Gastroenterology159, 944–955.e948 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagayama, M. et al. TH1 cell-inducing Escherichia coli strain identified from the small intestinal mucosa of patients with Crohn’s disease. Gut Microbes12, 1788898 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pujo, J. et al. Bacteria-derived long chain fatty acid exhibits anti-inflammatory properties in colitis. Gut70, 1088–1097 (2021). [DOI] [PubMed] [Google Scholar]
- 24.Man, S. M., Kaakoush, N. O. & Mitchell, H. M. The role of bacteria and pattern-recognition receptors in Crohn’s disease. Nat. Rev. Gastroenterol. Hepatol.8, 152–168 (2011). [DOI] [PubMed] [Google Scholar]
- 25.Kim, G. et al. Bifidobacterial carbohydrate/nucleoside metabolism enhances oxidative phosphorylation in white adipose tissue to protect against diet-induced obesity. Microbiome10, 188 (2022). [DOI] [PMC free article] [PubMed]
- 26.Hosomi, K. et al. Oral administration of ameliorates obesity and type 2 diabetes via metabolic remodeling of the gut microbiota. Nat. Commun.13, 4477 (2022). [DOI] [PMC free article] [PubMed]
- 27.Semo, D., Reinecke, H. & Godfrey, R. Gut microbiome regulates inflammation and insulin resistance: a novel therapeutic target to improve insulin sensitivity. Signal Transduct. Tar.9, 35 (2024). [DOI] [PMC free article] [PubMed]
- 28.Sun, L. L. et al. Gut microbiota and intestinal FXR mediate the clinical benefits of metformin. Nat. Med. 24, 1919 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.She, J. Q. et al. Statins aggravate insulin resistance through reduced blood glucagon-like peptide-1 levels in a microbiota-dependent manner. Cell. Metab. 36, 408–421.e5 (2024). [DOI] [PubMed]
- 30.Takeuchi, T. et al. Gut microbial carbohydrate metabolism contributes to insulin resistance. Nature621, 389 (2023). -+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Si, J. et al. Gut microbiome signatures distinguish type 2 diabetes mellitus from non-alcoholic fatty liver disease. Comput Struct Biotec19, 5920–5930 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sutton, E. F. et al. Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metab.27, 1212–1221.e1213 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie, Z. et al. Randomized controlled trial for time-restricted eating in healthy volunteers without obesity. Nat. Commun.13, 1003 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Howard, E. J., Lam, T. K. T. & Duca, F. A. The Gut Microbiome: Connecting Diet, Glucose Homeostasis, and Disease. Annu. Rev. Med.73, 469–481 (2022). [DOI] [PubMed] [Google Scholar]
- 35.Lynch, S. V. & Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med.375, 2369–2379 (2016). [DOI] [PubMed] [Google Scholar]
- 36.Heiman, M. L. & Greenway, F. L. A healthy gastrointestinal microbiome is dependent on dietary diversity. Mol Metab5, 317–320 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qin, J. et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature490, 55–60 (2012). [DOI] [PubMed] [Google Scholar]
- 38.Xu, A. A. et al. Dietary Fatty Acid Intake and the Colonic Gut Microbiota in Humans. Nutrients14, 2722 (2022). [DOI] [PMC free article] [PubMed]
- 39.Lozano, C. P. et al. Associations of the Dietary Inflammatory Index with total adiposity and ectopic fat through the gut microbiota, LPS, and C-reactive protein in the Multiethnic Cohort-Adiposity Phenotype Study. Am. J. Clin. Nutr.115, 1344–1356 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palmas, V. et al. Gut microbiota markers associated with obesity and overweight in Italian adults. Sci. Rep.11, 5532 (2021). [DOI] [PMC free article] [PubMed]
- 41.Kulkarni, P., Devkumar, P. & Chattopadhyay, I. Could dysbiosis of inflammatory and anti-inflammatory gut bacteria have an implications in the development of type 2 diabetes? A pilot investigation. BMC Res. Notes14, 52 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Allin, K. H. et al. Aberrant intestinal microbiota in individuals with prediabetes. Diabetologia61, 810–820 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu, J. et al. Association Between Intestinal Prevotella copri Abundance and Glycemic Fluctuation in Patients with Brittle Diabetes. Diabetes Metabolic Syndrome Obesity16, 1613–1621 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chavez-Talavera, O., Tailleux, A., Lefebvre, P. & Staels, B. Bile Acid Control of Metabolism and Inflammation in Obesity, Type 2 Diabetes, Dyslipidemia, and Nonalcoholic Fatty Liver Disease. Gastroenterology152, 1679–1694.e1673 (2017). [DOI] [PubMed] [Google Scholar]
- 45.Li, S. W. et al. Cytoplasmic Tyrosine Phosphatase Shp2 Coordinates Hepatic Regulation of Bile Acid and FGF15/19 Signaling to Repress Bile Acid Synthesis. Cell Metab20, 320–332 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fling, R. R. & Zacharewski, T. R. Aryl Hydrocarbon Receptor (AhR) Activation by 2,3,7,8-Tetrachlorodibenzo--Dioxin (TCDD) Dose-Dependently Shifts the Gut Microbiome Consistent with the Progression of Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci.22, 12431 (2021). [DOI] [PMC free article] [PubMed]
- 47.Ladurner, A. et al. Allspice and Clove As Source of Triterpene Acids Activating the G Protein-Coupled Bile Acid Receptor TGR5. Front Pharmacol8, 468 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Montandon, S. A. & Jornayvaz, F. R. Effects of Antidiabetic Drugs on Gut Microbiota Composition. Genes (Basel)8 (2017). [DOI] [PMC free article] [PubMed]
- 49.Organization, W. H. in Saturated Fatty Acid and Trans-Fatty Acid Intake for Adults and Children: WHO Guideline WHO Guidelines Approved by the Guidelines Review Committee (2023). [PubMed]
- 50.China, N. H. C. o. t. P. s. R. o. Dietary Guidelines for d Adults with Diabetes (2023), <http://www.nhc.gov.cn/sps/s7887k/202301/0e55a01df50c47d9a4a43db026e3afc3/files/4fcbecd2c18e46baaf291bf46c2b79cd.pdf?xueqiu_status_id=264389131&xueqiu_status_from_source=htl&xueqiu_status_source=statusdetail&xueqiu_private_from_source=0105&xueqiu_link_type=status_detail_link_custom&xueqiu_link_text=%E7%BD%91%E9%A1%B5%E9%93%BE%E6%8E%A5> (2023). English version <https://en.chinacdc.cn/health_topics/nutrition_health/202303/t20230316_264324.html>.
- 51.Gutt, M. et al. Validation of the insulin sensitivity index (ISI(0,120)): comparison with other measures. Diabetes Res. Clin. Pract.47, 177–184 (2000). [DOI] [PubMed] [Google Scholar]
- 52.Stumvoll, M., Van Haeften, T., Fritsche, A. & Gerich, J. Oral glucose tolerance test indexes for insulin sensitivity and secretion based on various availabilities of sampling times. Diabetes Care24, 796–797 (2001). [DOI] [PubMed] [Google Scholar]
- 53.Team, R. C. R: A language and environment for statistical computing. MSOR Connections1 (2014) https://www.r-project.org/.
- 54.Broll, S. et al. Interpreting blood GLUcose data with R package iglu. Plos One16, e0248560 (2021). [DOI] [PMC free article] [PubMed]
- 55.Bates, D., Mächler, M., Bolker, B. M. & Walker, S. C. Fitting Linear Mixed-Effects Models Using lme4. J Stat Softw67, 1–48 (2015). [Google Scholar]
- 56.Oksanen, J. et al. Vegan: Community Ecology Package. R Package Version 2.2-12, 1–2 (2015). [Google Scholar]
- 57.Mallick, H. et al. Multivariable association discovery in population-scale meta-omics studies. Plos Comput. Biol.17, e1009442 (2021). [DOI] [PMC free article] [PubMed]
- 58.Wickham, H., François, R., Henry, L. & Müller, K. Welcome to the Tidyverse. J. Open Source Softw.4, 1686 (2019).
- 59.Kim, S. ppcor: An R Package for a Fast Calculation to Semi-partial Correlation Coefficients. Commun Stat Appl Methods22, 665–674 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The metagenomic sequencing data generated in this study have been deposited in the NCBI database under BioProject accession code PRJNA1238858 and the Genome Sequence Archive in National Genomics Data Center (BioProject:PRJCA033596). The metabolomics data are available in MetaboLights under the accession number MTBLS12353. All individual de-identified participant data supporting the findings of this study are available under restricted access due to compliance with data protection regulations and participant consent agreements. Requests for access can be made by contacting liying_helen@163.com, and should include a detailed research proposal and ethical approval documentation. Requests will be promptly reviewed by Harbin Medical University within 4 weeks to assess potential intellectual property or confidentiality obligations. Access will be granted only to qualified researchers for non-commercial academic purposes. Upon approval, access to the controlled data will be granted for a period of one year. Source data are provided with this paper. The processed data are available within the Source Data file. Source data are provided with this paper.
The R codes for data analysis are available at zenodo 10.5281/zenodo.15078756.