Abstract
Copper, an essential micronutrient, plays significant roles in numerous biological functions. Recent studies have identified imbalances in copper homeostasis across various cancers, along with the emergence of cuproptosis, a novel copper-dependent form of cell death that is crucial for tumor suppression and therapeutic resistance. As a result, manipulating copper levels has garnered increasing interest as an innovative approach to cancer therapy. In this review, we first delineate copper homeostasis at both cellular and systemic levels, clarifying copper’s protumorigenic and antitumorigenic functions in cancer. We then outline the key milestones and molecular mechanisms of cuproptosis, including both mitochondria-dependent and independent pathways. Next, we explore the roles of cuproptosis in cancer biology, as well as the interactions mediated by cuproptosis between cancer cells and the immune system. We also summarize emerging therapeutic opportunities targeting copper and discuss the clinical associations of cuproptosis-related genes. Finally, we examine potential biomarkers for cuproptosis and put forward the existing challenges and future prospects for leveraging cuproptosis in cancer therapy. Overall, this review enhances our understanding of the molecular mechanisms and therapeutic landscape of copper and cuproptosis in cancer, highlighting the potential of copper- or cuproptosis-based therapies for cancer treatment.
Subject terms: Cancer therapy, Cancer
Introduction
Copper, an essential trace element crucial for various physiological processes, has garnered increasing attention for its intricate role in cancer biology.1–4 Intracellular copper levels are maintained within a strict range, with even slight elevations potentially causing cytotoxic effects and cell death, emphasizing the need for precise regulation of copper absorption, distribution, and excretion.5–8 In the broader physiological context, copper homeostasis in the human body is primarily maintained through several key processes, including intestinal absorption, vascular transport, hepatic storage, biliary excretion, and utilization and excretion by other organs.9–11 At the cellular level, copper homeostasis involves its uptake and subsequent distribution across various cellular compartments, such as the cytoplasm, mitochondria, Golgi apparatus, and nucleus.12–14 This intricate process encompasses the storage and flux of copper within these organelles, as well as its expulsion from the cell.7,9,15 The entire sequence of events is coordinated through copper acting as a catalytic cofactor in its redox chemistry, which entails complex interactions with various intracellular enzymes and proteins.16
In the context of cancer, dysregulation of copper metabolism exerts a dual effect on tumor progression. On one hand, cuproplasia, the term that refers to copper-driven cellular growth and proliferation, involves multiple cancer pathways and regulatory mechanisms to promote tumor progression,17 which could be further accelerated by copper-dependent metastasis, angiogenesis, and immune escape.6,18–21 On the other hand, copper inhibits tumor growth by participating in the regulation of cell death processes, including apoptosis, pyroptosis, necroptosis, ferroptosis, and autophagy, as well as by activating immune responses.16,22–25 In 2022, Tsvetkov et al. introduced the concept of cuproptosis, marking a new milestone in the study of copper-induced cell death mechanisms.26 Cuproptosis involves the binding of copper to lipoylated enzymes within the tricarboxylic acid cycle, which triggers protein aggregation, and proteotoxic stress, and ultimately leads to cell death.26
Given the burgeoning interest in copper metabolism and cuproptosis, it is crucial to develop a comprehensive understanding of the molecular mechanism and therapeutic landscape of copper and cuproptosis in cancer. In this review, we first delineate copper homeostasis and clarify both its protumorigenic and antitumorigenic functions in cancer. We then outline key milestones and molecular mechanisms of cuproptosis and explore its roles in cancer biology. Additionally, we summarize emerging therapeutic opportunities targeting copper and discuss the clinical associations of cuproptosis-related genes. Finally, we examine potential biomarkers for cuproptosis and discuss existing challenges and future prospects for leveraging cuproptosis in cancer therapy.
Copper homeostasis in physiology
Copper overload or deficiency in the human body has been linked with a variety of diseases.9,27 Genetic mutations that disrupt copper homeostasis can lead to specific conditions such as Wilson’s disease (WD)28,29 and Menkes disease (MD).30,31 Copper imbalances are also associated with neurodegenerative diseases,32 cardiovascular diseases,33,34 and cancers,2,3,35 underscoring the critical importance of copper homeostasis in sustaining body health.36 Copper typically exists in biological systems in both copper (II) and copper (I) oxidation states.37 Copper (I) is predominantly found within the reducing environment of the cytoplasm, whereas copper (II) is more commonly in the oxidative conditions of the extracellular space.4 The highly oxidative and reductive nature imparts copper a dual role within physiological processes. On one hand, copper acts as a co-factor for numerous enzymes by facilitating electron transfer.4,38 On the other hand, excessive copper accumulation can disrupt cellular metabolism, potentially causing cellular damage or death.39 Thus, a strictly regulated copper homeostasis system is essential to ensure adequate copper levels for enzymatic functions while preventing toxic accumulation.40,41 Copper homeostasis encompasses the mechanisms of copper absorption, distribution, utilization, and excretion. This balance is maintained through a network of transporter proteins, chaperones, and storage molecules that orchestrate copper’s cellular entry, trafficking, enzyme incorporation, and elimination.42 We will outline the mechanisms of copper homeostasis in both systemic and cellular contexts.
Systemic copper homeostasis
Copper is widely present throughout the human body, with a total amount of 100–200 mg.43 The concentrations of copper in organs and tissues vary, ranging from 3 mg (kidneys) to 46 mg (bone) in an adult weighing 70 kg.44 To maintain systemic copper homeostasis, a daily copper intake between 0.8 and 2.4 mg is recommended.45
Copper is mostly absorbed from dietary sources, including animal offal, seafood, and nuts,46 and it initially exists in the digestive system as copper (II)10 (Fig. 1). The primary site of dietary copper absorption is the small intestine, predominantly in the duodenum and jejunum regions,47 where copper (II) can permeate the cytoplasm via the nonspecific divalent metal transporter 1 (DMT1, also known as solute carrier family 11 member 2, SLC11A2).48 Additionally, members of the six-transmembrane epithelial antigen of the prostate (STEAP) family (including STEAP2, STEAP3, and STEAP4) and duodenal cytochrome b (DCYTB) function as copper reductases, ensuring that copper (II) is maintained in its reduced state copper (I) to facilitating cellular uptake.9,49 The entry of copper (I) into cells is primarily mediated by copper transporter 1 (CTR1, also known as SLC31A1) and CTR2 (SLC31A2), which are located at the apical membrane of intestinal epithelial cells, representing a major pathway for copper absorption.50,51 Notably, DMT1 has also been implicated in the uptake of copper (I), potentially serving as a compensatory mechanism in the absence of CTR1.32,52
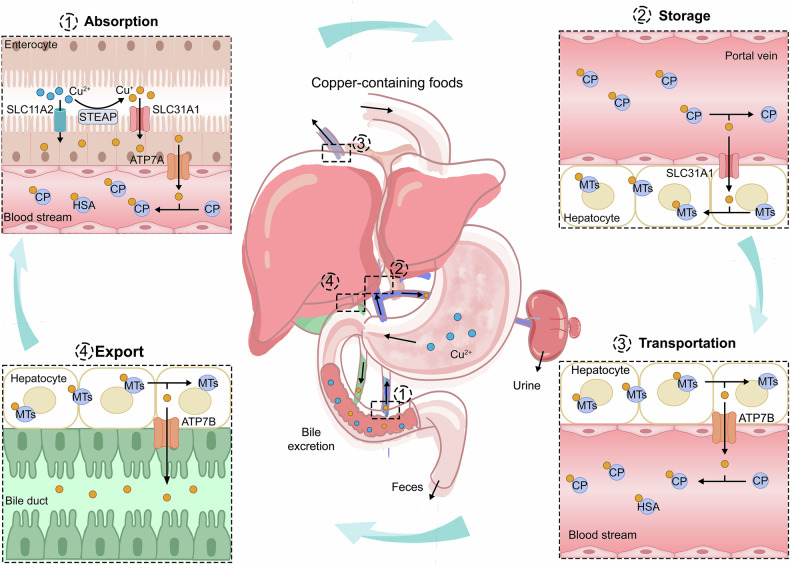
Fig. 1.
Systemic copper homeostasis. Systemic copper homeostasis involves intestinal absorption, hepatic storage, systemic transport, and biliary excretion. Dietary copper is predominantly absorbed in the small intestine, where copper (II) is reduced to copper (I) by members of the STEAP family. Copper (I) is then transported into enterocytes via SLC31A1, with a small amount entering through SLC11A2. Inside the intestinal epithelial cells, copper (I) binds to the copper chaperone and is transported to the basolateral side, where it is exported into the bloodstream via ATP7A. In the bloodstream, copper (I) binds to soluble copper chaperone proteins, primarily CP. Copper is transported to the liver through the portal vein, where hepatocytes uptake copper (I) from the bloodstream via SLC31A1. Within the hepatocytes, copper (I) can either be stored in MTs or re-enter the bloodstream through the ATP7B for distribution to other tissues. Excess copper is processed in the liver and excreted via bile, which is the primary route for copper elimination. Abbreviations: STEAP six-transmembrane epithelial antigen of the prostate, SLC31A1 solute carrier family 1 member 1, SLC11A2 solute carrier family 11 member 2, ATP7A/B ATPase copper transporter 7A and 7B, CP ceruloplasmin, MT metallothionein
The distribution of copper within the body can be divided into two phases. Dietary copper entering the bloodstream is initially transported to the liver and kidneys through the portal vein. Copper in the liver is then distributed to other tissues or organs via the blood circulatory system with the aid of circulating chaperone proteins.53 Specifically, after entering the intestinal epithelial cells, copper (I) binds to the copper chaperone antioxidant protein 1 (ATOX1) and is mainly transported to the opposite side of the epithelial cell, where it is exported into the bloodstream via copper-transporting P-type ATPases α (ATP7A).54 In the bloodstream, copper (II) predominantly binds to several copper chaperone proteins and is transported to the liver and kidneys. Approximately 75% of copper (II) binds to plasma ceruloplasmin (CP), 25% to human serum albumin, and about 0.2% to histidine and macroglobulin.55,56 The liver is the principal organ for copper storage and excretion, serving as the central regulatory mechanism for copper homeostasis. Within hepatocytes, copper is chelated by metallothionein isoforms 1 and 2 (MT1/2), facilitating its storage.57 MTs are thiol-rich reducing molecules that exhibit a high affinity for copper and are pivotal in maintaining copper equilibrium through the storage and timely release of excess copper.58 Excess copper is released into the bloodstream through the mediation of ATP7B, where it subsequently binds to the soluble chaperone proteins and is transported to specific tissues and organs.59 ATP7A and ATP7B, two subtypes of the P-type ATPase family, play a crucial role in copper export.60 ATP7A is predominantly expressed in the small intestine, whereas ATP7B is mainly found in the liver. Consequently, ATP7A is responsible for exporting copper from the intestines, while ATP7B facilitates copper export from the liver.
Copper, once released into the bloodstream from the liver, is transported to specific tissues and organs such as the brain, heart, muscles, and bones for utilization.37,61 In these organs, copper catalyzes a range of essential physiological processes, such as maintenance of redox homeostasis, mitochondrial energy production, remodeling of the extracellular matrix (ECM), and metabolism of tyrosine and neurotransmitters.9,62–64 For instance, copper participates in myelination and interacts with synaptic proteins and neurotransmitter receptors, highlighting its significant function at synapses.32,65 Excretion of excess copper occurs primarily through the following pathways: (I) metabolized copper in the liver is incorporated into bile and subsequently excreted from the body in the form of secretory vesicles, which represents the primary mechanism for endogenous copper elimination;66 (II) unabsorbed copper in the intestinal tract is excreted through feces;67 (III) a small amount of copper (10–50 µg/day) is also eliminated through urine via the kidneys;8 and (IV) sweat and menstruation also contribute to copper excretion.5 In instances of high copper intake, the human body mainly regulates copper homeostasis by increasing bile excretion or reducing absorption, and vice versa.67
Intracellular copper homeostasis
Once entering the cell, copper is intricately coordinated by a fine-tuning network, involving the intricmate crosstalk between cytoplasm and different organelles (Fig. 2). The intracellular copper form includes two forms: a tightly bound protein pool at the micromolar level, and a bioavailable, labile pool at the femtomolar level.68 Although the concentration of free copper in cells is almost negligible, it still has a potentially detrimental effect on cell membranes, proteins, and nucleic acids. Therefore, cellular copper homeostasis is rigorously regulated to maintain copper levels within a specific and narrow range.41
Fig. 2.
Intracellular copper homeostasis. Within the cell, copper (I) can be sequestered by MTs and GSH, forming a dynamic copper pool, or it can bind to copper chaperone proteins, including CCS, COX17, CuL, and ATOX1, for transport to various organelles. CCS delivers copper (I) to the SOD1, facilitating the conversion of superoxide radicals into oxygen and thereby protecting the cell from oxidative stress. COX17 transports copper (I) to mitochondrial SCO1 and COX11, essential for COX assembly. CuL binds to copper in the cytosol and triggers copper transport into the mitochondria via SLC25A3. ATOX1 directs copper (I) to ATP7A/B in the TGN. When there is an excess of copper, ATP7A/B translocates to vesicular compartments and fuses with the plasma membrane to expel the excess copper. Also, CCS and ATOX1 are involved in transporting copper to the cell nucleus, which is crucial for activating various transcription factors. Abbreviations: MT metallothionein, GSH glutathione, CCS copper chaperone for superoxide dismutase, COX cytochrome oxidase, SCO1 synthesis of cytochrome c oxidase 1, CuL copper ligands, ATOX1 antioxidant protein 1, SOD1 superoxide dismutase 1, ATP7A/B ATPase copper transporter 7A and 7B, TGN trans-Golgi network
Within the cytoplasm, superoxide dismutase (CCS), a soluble copper chaperone protein, immediately delivers copper (I) to the copper-binding site of superoxide dismutase 1 (SOD1).69,70 SOD1 is a principal antioxidant enzyme located in the cytosol and the mitochondrial intermembrane space (IMS), where it catalyzes the conversion of superoxide radicals to hydrogen peroxide and oxygen, thereby protecting cells from oxidative stress damage.71 The distribution of SOD1 between the cytosol and IMS is regulated by CCS, which mediates the formation of disulfide bonds in SOD1.72,73 This is crucial for its proper spatial conformation and enzymatic activity.74 Intracellular copper typically binds to MTs and non-proteinaceous ligands such as glutathione (GSH), which provide storage and detoxifying functions.75 The regulation of copper ion concentration is also mediated by the expression of MTs, which is increased in response to elevated copper levels.76,77 This mechanism ensures that the cell can adapt to fluctuating copper availability while maintaining essential biological processes.
Mitochondria are the major organelle for the storage and utilization of copper, playing a crucial role in cellular copper homeostasis.49,78 The copper chaperone COX17 facilitates the transport of copper to the mitochondria by shuttling between the cytoplasm and the mitochondria. Copper ligands (CuL), a non-proteinaceous and low molecular weight complex, are also involved in copper transport.17 Specifically, CuL binds to copper in the cytosol and triggers copper transport into the mitochondria through transporters such as SLC25A3.79,80 Copper is a fundamental element in mitochondria function, particularly through its role with cytochrome oxidase (COX), the enzyme complex that drives oxidative phosphorylation (OXPHOS).81 The COX complex, also known as mitochondrial respiratory chain complex IV and cytochrome C oxidase (CCO), requires copper and heme as essential cofactors to facilitate the process of ATP production by transferring electrons through the respiratory chain to molecular oxygen. Specifically, copper is involved in the assembly of the COX complex, which consists of the two core subunits COX1 and COX2, through two distinct pathways.70,82 On one hand, COX17 binds to and delivers Cu to the synthesis of cytochrome c oxidase 1 (SCO1) or SCO2, which then transfers the Cu to the CuA site in the core subunit of COX2. SCO1 particularly helps in the copper metallation of COX2 by binding copper on the intermembrane space side and inserting it into COX2. On the other hand, COX17 binds to and transfers copper to COX11, which conveys copper to the CuB site of COX1. As COX17 serves as a primary copper donor in the IMS,83 mutations in COX17 can reduce CCO activity and cause mitochondrial dysfunction and oxidative stress,84 further supporting copper’s significance in mitochondria.
The Golgi apparatus works as the central compartment for copper homeostasis.85–87 ATP7A and ATP7B are the primary transport proteins for exporting cellular copper, and their localization and function are vital for regulating copper homeostasis.88 Under physiological copper levels, ATP7A/B are situated in the TGN, where they pump copper into the lumen of the TGN by the copper chaperone ATOX1.89 When there is an excess of copper within the cell, ATP7A and ATP7B can relocate to vesicular compartments and fuse with the plasma membrane to expel excess copper, thus preventing copper toxicity.90 This relocation is essential for modulating copper efflux. Once copper levels return to physiological norms, ATP7A and ATP7B are recycled back to the TGN,60 where copper facilitates the synthesis of copper enzymes including tyrosinase, lysyl oxidase (LOX), CP, and SOD3.91 These enzymes are integral to various biological processes such as connective tissue development, iron metabolism, and melanin production.92–94
Copper also plays a pivotal role in the nucleus.32 Specifically, CCS and ATOX1 are both involved in transporting copper to the cell nucleus, where it is essential for activating different transcription factors.95–97 In human hepatocellular carcinoma (HCC) cells, the expression of genes induced under low oxygen conditions relies on the presence of CCS and copper. These components are critical for enabling hypoxia-inducible factor 1α (HIF1α) to bind to both the transcriptional co-activator protein p300 and to elements in target genes that respond to hypoxia.98 Moreover, ATOX1 carries copper into the nucleus and functions as a novel transcription factor, thereby contributing to cell proliferation.96
Copper functions in cancer
Extensive research has revealed unique metabolic patterns of copper in different cancers.17,27,99 Compared to normal tissues, tumor tissues exhibit a higher demand for copper.2,100 For instance, elevated levels of copper in the tumor tissue and serum have been observed in patients with oral,101 thyroid,102 breast,103,104 lung,105,106 pancreatic,107 gallbladder,108 colorectal,109 prostate,110 and gynecological cancers,111 and are significantly associated with poor prognostic outcomes. This increased copper demand is primarily because copper is required as a cofactor for multiple enzymes involved in cellular energy metabolism (such as CCO) and antioxidant defenses (such as SOD), thereby meeting the substantial energy needs of rapidly dividing tumor cells.112 Additionally, copper can also negatively affect tumors due to its redox activity and improper binding with functional macromolecules.26,113 Therefore, an imbalance in copper homeostasis could play a dual role, in promoting or suppressing tumors in various contexts.
Tumor-promoting functions of copper
Copper and cuproplasia
Copper, as a transition metal element and essential nutrient, plays diverse and critical roles in oncology.114 To better elucidate the association between copper and cancer, researchers coined the term “cuproplasia” in 2022, which is described as copper-dependent cellular growth and proliferation and exemplifies “metalloplasias”.17 The process encompasses the interactions of copper with various cellular mechanisms, including kinase signaling pathways, autophagy, the ubiquitin-proteasome system (UPS), epigenetic regulation, and metabolic pathways (Fig. 3a).
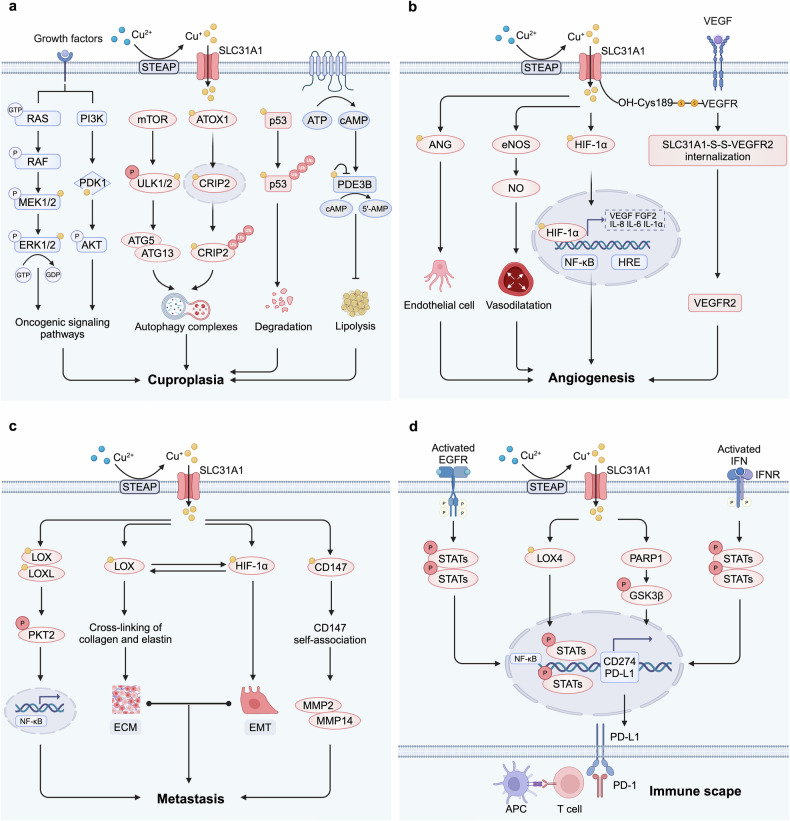
Fig. 3.
Mechanisms of copper in promoting tumor. a Copper binds to MEK1/2 and PDK1, activating oncogenic signaling pathways. It also activates ULK1/2 or enters the nucleus to induce the degradation of CRIP2, promoting autophagy. Additionally, copper interacts with the p53 protein, leading to its degradation. Copper also inhibits PDE3B, promoting lipolysis, which collectively contributes to cuproplasia. b Copper directly binds or activates angiogenic factors such as ANG and NO and interacts with HIF-1 to enhance NF-κB activity, promoting the expression of angiogenic mediators. Disulfide bonds formed between CTR1 and VEGFR2 activate VEGFR2 signaling, facilitating angiogenesis. c Copper promotes the expression of LOX/LOXL and HIF-1α, synergistically enhancing tumor metastasis through a positive feedback mechanism. Copper also binds to CD147 to promote its self-association, further enhancing metastasis. d Copper upregulates the expression of PD-L1 in cancer cells through multiple pathways, inhibiting T lymphocytes and inducing exhaustion, thereby facilitating immune escape. Created by BioRender. Abbreviations: ULK1/2 unc-51-like autophagy activating kinases 1 and 2, CRIP2 copper-binding protein cysteine-rich protein 2, PDE3B phosphodiesterase 3B, ANG angiogenin, NO nitric oxide, HIF-1 hypoxia-Inducible Factor-1, CTR1 copper transporter 1, VEGFR vascular endothelial growth factor receptor, LOX lysyl oxidase, LOXL lysyl oxidase-like protein, HIF-1α hypoxia-inducible factor-1α, PD-L1 programmed death-ligand 1
Copper activates a series of cancer-related kinase-signaling pathways, including the RAS-RAF-MEK-ERK1/2 and PI3K-PDK1-AKT pathways.115,116 Specifically, copper acts as a cofactor of MEK1/2 can allosterically enhance its capability to phosphorylate ERK1/2 in a dose-dependent manner, which further promotes the expression of c-myc, c-fos, and c-jun in the nucleus to regulate tumor growth.117–119 This copper-dependent vulnerability has been demonstrated in tumor models with BRAF or KRAS mutations. Mutations in BRAF, most commonly BRAFV600E, drive the development of various cancers, including melanoma, lung cancer, and thyroid cancer, and its carcinogenic signal transmission requires copper to bind to MEK and facilitate the activation of ERK1/2.120,121 Disruption of copper availability, either through the genetic ablation of the CTR1 or by introducing mutations that impair copper binding in the surface-accessible regions of MEK1, can decrease the signal transduction and tumorigenesis driven by BRAF.121 In the KRASG12V-mutated colorectal cancer (CRC) model, tumor cells can obtain copper to promote cell growth through an atypical mechanism involving micropinocytosis. Upregulation of ATP7A or exposure to the copper chelator tetrathiomolybdate (TTM) results in reduced phosphorylation of ERK1/2, indicating that copper bioavailability represents a KRAS-selective vulnerability.122 Additionally, copper can also bind to the H117 and H203 sites on PDK1, enhancing its interaction with AKT and activating AKT-mediated oncogenic signaling. By depleting CTR1 or using copper chelators to inhibit the copper axis, AKT signaling can be reduced, thereby decreasing tumorigenesis and cell proliferation.123
Copper stimulates autophagy to regulate cuproplasia in response to various stressors such as nutritional starvation, metabolic imbalance, hypoxia, oxidative stress, and oncogene activation. Cancer cells generate nutrients and energy through a cellular process known as autophagy, supporting the survival and proliferation of the tumor cells.124–126 Unc-51-like autophagy activating kinases 1 and 2 (ULK1/2), downstream targets of the major nutrient-sensing kinase mechanistic target of rapamycin complex 1 (mTORC1), serve as pivotal regulators within the autophagy pathway.126 The direct binding of copper to ULK1/2 is necessary for kinase activity and serves as a regulatory factor that promotes the phosphorylation and activation of the autophagy proteins ATG7 and ATG13, leading to the formation of the autophagy complexes and ultimately enhancing tumor growth.127 Fluctuations in intracellular copper concentrations can accordingly regulate ULK1/2 kinase activity.128 The absence of the CTR1 gene or mutations in ULK1/2 can impair its copper-binding capacity, thereby inhibiting copper-ULK1/2 dependent downstream signaling and the formation of autophagy complexes, reducing cancer cell proliferation and increasing sensitivity to “nutrient starvation”.128 Furthermore, copper can activate autophagy by binding to the nuclear copper-binding protein cysteine-rich protein 2 (CRIP2).129 Mechanistically, copper is transferred to the nucleus via ATOX1, binds to CRIP2, inducing changes in the secondary structure of the CRIP2 protein, and promotes its ubiquitin-mediated proteasome degradation. This process leads to ROS-mediated activation of autophagy.129
Copper activates the UPS to regulate the degradation of certain proteins, such as p53 and XIAP, thereby promoting the proliferation and growth of tumor cells. The tumor suppressor protein p53, encoded by the critical tumor suppressor gene TP53, plays a pivotal role in regulating cell growth. Mutations in TP53 can lead to functional impairments that promote uncontrolled cell proliferation and the development of cancer.130,131 Intracellular free zinc binds to p53 to ensure its activity and stability,132 which could be displaced by copper, leading to aberrant folding of the protein and subsequent degradation via the UPS.133 Specifically, copper promotes p53 degradation through positive allosteric activation of the E2 conjugating enzyme branch UBE2D1-UBE2D4.134 The X-linked inhibitor of apoptosis protein (XIAP) is a potent anti-apoptotic factor belonging to the inhibitor of apoptosis (IAP) family, capable of directly inhibiting caspases and regulating cell apoptosis.135 XIAP exhibits strong copper affinity, and reversible binding with copper leads to its rapid ubiquitination and subsequent proteasomal degradation, thereby reducing its anti-apoptotic inhibitory capacity.136
Copper inhibits several key mitochondrial enzymes, disrupting the production of metabolites involved in epigenetic regulation.137–139 For example, copper inhibits pyruvate dehydrogenase, which in turn suppresses the conversion of pyruvate to acetyl-CoA, a metabolite required for histone acetylation by histone acetyltransferases (HATs) and global transcription.140 The acetylation levels of histones are jointly regulated by HATs and histone deacetylases.141 Decreased acetylation can lead to increased proliferation and differentiation of tumor cells. In human hepatoma Hep3B cells, exposure to copper (II) leads to a reduction in histone H3 and H4 acetylation by inhibiting the activity of HATs.142 Additionally, inhibition of COX17 leads to elevated mitochondrial copper levels in leukemic stem cells, which results in reduced cellular levels of S-adenosylmethionine, subsequently decreasing global DNA methylation and increasing chromatin accessibility, which contributes to promoting the differentiation of acute myeloid leukemia cells while reducing stem cell-like characteristics.143
Copper modulates tumor metabolism by interacting with molecules involved in lipid metabolic pathways. Cancer cells facilitate their proliferation and metastasis by harnessing energy from lipid catabolism.144 The classic 3’,5’-cyclic AMP (cAMP) pathway, which is essential for the breakdown of triglycerides into fatty acids and glycerol, plays a crucial role in lipid metabolism within the body.145 Copper serves as an endogenous regulator by binding to a key conserved cysteine residue within the active site of phosphodiesterase 3B and inhibiting its activity. This inhibition prevents the degradation of cAMP, thereby promoting cAMP-dependent lipolysis.146
Copper and angiogenesis
Angiogenesis is a crucial process for the transport of oxygen and nutrients to tumors, facilitating tumor progression.147,148 Copper acts as a pivotal “switch” in angiogenic signaling by activating a multitude of pro-angiogenic and inflammatory factors100,149–151 (Fig. 3b). For example, copper binds directly to angiogenin (ANG), modulating its affinity towards endothelial cells and vascular smooth muscle cells, thereby promoting angiogenesis.152,153 Moreover, intracellular copper can stabilize the biochemical structure of transcription factor HIF-1 and enhance the activity of the nuclear factor NF-κB, thereby ensuring their transcriptional activity on angiogenesis genes such as VEGF and ceruloplasmin and promoting the expression of angiogenic mediators including FGF2, VEGF, IL-8, IL-6, and IL-1α.154–156 Copper also elevates endothelial nitric oxide synthase levels, thereby increasing the production of nitric oxide (NO). The increase in NO not only promotes vasodilation but also activates pro-tumor angiogenic signaling pathways.157
Copper homeostasis-associated proteins are also involved in copper-mediated angiogenesis. For example, CTR1 promotes angiogenesis by regulating the entry of copper into endothelial cells, activating a multitude of pro-angiogenic and inflammatory factors.158 CTR1 also transmits ROS signals induced by VEGF through the sulfenylation of cysteine 189 (Cys189) located at its C-terminal and promotes the formation of disulfide bonds between CTR1 and VEGFR2. The CTR1-VEGFR2 complex drives their co-endocytosis, activating sustained VEGFR2 signaling within endosomes, which is crucial for angiogenesis.19 Moreover, the copper-dependent transcription factor ATOX1 contributes to tumor angiogenesis and vascular remodeling by modulating the platelet-derived growth factor signaling pathway and activating the ATP7A-mediated LOX signaling pathway.159,160 Concurrently, ATP7A plays a critical role by inhibiting the autophagy-mediated degradation of VEGFR2, thus enhancing VEGFR2 signaling and further promoting angiogenesis.160 Additionally, the enzyme Amine oxidase copper-containing 3 (AOC3) has been shown to induce angiogenesis through a mechanism involving IL-1β-driven M2 macrophage infiltration.161 These interconnected mechanisms highlight the potential of targeting copper homeostasis as an anti-angiogenic therapeutic strategy for cancer.
Copper and metastasis
Tumor metastasis is a dynamic and complex process in which copper plays a significant role162,163 (Fig. 3c). On one hand, copper-mediated cell proliferation and angiogenesis serve as fundamental components of this process.164 On the other hand, copper activates enzymes and signaling cascades related to metastasis, regulating the remodeling of the ECM and epithelial-mesenchymal transition (EMT), both of which are key pathways facilitating the metastasis of cancer.164–166
LOX and LOX-like (LOXL) isoforms are copper-dependent metalloenzymes that have been established as contributors to cancer cell metastasis.167,168 Intracellular copper accumulation can activate LOX/LOXL, promoting the cross-linking of collagen and elastin in the ECM, thereby creating a microenvironment conducive to tumor cell metastasis.169–171 The activation of LOX/LOXL stimulates signaling pathways involving protein kinase C α and protein tyrosine kinase 2, further driving the activation of transcription factors associated with cancer cell proliferation and metastasis.172,173 Furthermore, LOX promotes the cross-linking of ECM components such as elastin and collagen through the copper-dependent oxidoreductase cell motility mediator 1, which influences cellular activities and leads to alterations in the cytoskeletal proteins, enhancing extracellular migration and metastasis.174,175 Notably, Copper ions can induce the secretion of LOX by activating HIF-1α,176 while LOX enhances the synthesis of HIF-1α protein through a positive feedback mechanism, with both factors working synergistically to regulate and promote tumor progression.177,178 Copper also activates the interaction between HIF-1α and hypoxia-responsive elements as well as the HIF1α-Snail/Twist signaling pathway, promoting the development of EMT.179,180 In triple-negative breast cancer (TNBC), cells expressing SOX2+/OCT4+ exhibit significant metastatic potential due to copper-mediated activation of the AMPK/mTORC1 signaling pathway.181
The interaction between certain proteins and copper also plays a role in tumor metastasis. CD147 acts as a receptor for extracellular copper ions.182 In HCC cells, copper (II) binds to the proximal extramembrane domain of CD147 and mediates its self-association. This process activates the PI3K/AKT signaling pathway, leading to the upregulation of MMP-2 and MMP-14, which enhances the invasiveness of HCC cells.182 Copper chaperone proteins also play a key role in linking copper homeostasis with cancer metastasis. ATOX1 mediates the metastasis of breast cancer cells through coordinated copper transport along the ATP7A-LOX axis,183 making the levels of ATOX1 in tumor cells a potential predictive marker of metastatic potential and a biomarker for copper depletion therapy. Furthermore, the Secreted protein acidic and rich in cysteine, also known as osteonectin, contains a copper-binding domain.184 The binding of this domain to copper ions has been demonstrated to regulate cell-matrix interactions and enhance the metastasis of tumor cells.185
Copper and immune escape
The interaction between programmed cell death protein 1 (PD-1) and its ligand PD-L1 is a core mechanism for tumor immune escape.186–188 Copper can upregulate the expression of PD-L1 in cancer cells through multiple pathways, inhibiting T lymphocytes and inducing exhaustion to facilitate immune escape (Fig. 3d). (1) Copper increases PD-L1 levels by inhibiting UPS-mediated degradation, facilitating the phosphorylation of STAT3 and EGFR, which are crucial proteins for tumor growth and immune escape.20,189 Copper chelators have also been shown to enhance the effectiveness of immune cells such as CD8+ T cells and natural killer (NK) cells in the tumor microenvironment (TME), thereby slowing tumor growth and improving survival rates in mice.20 (2) Copper promotes the secretion of LOXL4 by tumor cells. Exposure to LOXL4 results in macrophages within the TME adopting an immunosuppressive phenotype primarily mediated by interferon (IFN)-dependent signal transduction, which leads to transcription-dependent PD-L1 activation. The increased PD-L1 expression further impairs CD8+ T-cell function, promoting tumor immune escape and supporting tumor growth.190 (3) Copper ions contribute to tumor immune escape by upregulating the expression of CD274/PD-L1 (CD274 molecule), which serves as an immune checkpoint in cancer cells.191 (4) The disulfiram (DSF) and copper (DSF/Cu) complex upregulates PD-L1 expression by inhibiting poly (ADP-ribose) polymerase 1 (PARP1) activity and enhancing phosphorylation of glycogen synthase kinase-3β (GSK-3β) at Ser9 site, ultimately suppressing T cell infiltration.192
Tumor-suppressing functions of copper
Copper and apoptosis
Apoptosis is a strictly regulated form of PCD, characterized by distinct morphological changes in cells and the activation of specific caspase and mitochondrial regulatory pathways.193–195 The major apoptotic pathways are categorized into the mitochondrial pathway and death receptor pathways. Intracellular apoptotic signals typically activate the mitochondrial pathway,which stimulates the binding of BH3-only proteins with Bcl-2, leading to the aggregation of Bax/Bak on the mitochondrial membrane.196 This process triggers the release of pro-apoptotic proteins from the mitochondria, including cytochrome c and apoptosis-inducing factor (AIF), which subsequently activate caspases to initiate apoptosis. The extrinsic death receptor pathway begins with the binding of specific death receptors to ligands such as tumor necrosis factor-related TRAIL, TNF-α, and FASL, forming a death-inducing signaling complex that activates caspase-8, leading to apoptosis through a caspase cascade.197
Excessive copper can induce apoptosis through multiple molecular mechanisms. Firstly, copper can catalyze the production of ROS when present in excess, leading to oxidative stress198–200 (Fig. 4a). The oxidative damage to cellular components such as lipids, proteins, and DNA can trigger the apoptotic pathways. Additionally, the accumulation of ROS can disrupt mitochondrial function, resulting in a loss of mitochondrial membrane potential and the release of pro-apoptotic factors like cytochrome c and AIF into the cytosol, which activates the caspase cascade and causes DNA fragmentation, leading to apoptosis.201 Secondly, copper can directly or indirectly influence the activity of various proteins involved in apoptotic pathways. For instance, copper binds to and activates proteins like p53, a key regulatory protein in apoptosis, thereby enhancing its ability to promote cell death in response to DNA damage. In human breast cancer MCF7 cells, copper-induced transactivation of P53 enhances the expression of BAX, a Bcl-2 family member, and the p53-induced gene 3 product, leading to the opening of mitochondrial permeability transition pores and the subsequent production of ROS.202 This research highlights the mechanism by which copper amplifies P53 transcriptional activity to promote apoptosis. Thirdly, endoplasmic reticulum (ER) stress and nucleolar stress are also significant contributing factors to cellular death under copper exposure. Copper sulfate-induced ER stress promotes apoptosis in mouse hepatocytes by activating the CHOP, JNK, and caspase-12 signaling pathways.203 Furthermore, copper-induced nucleolar stress impedes ribosomal synthesis and triggers a p53-independent apoptotic pathway.204 Notably, DSF also robustly inhibits the activity of the 26S proteasome in various cancer cell lines in a copper-dependent manner, ultimately leading to cancer cell apoptosis.205 Additionally, the activation of the TNF receptor-1 (TNF-R1) signaling pathway is involved in the copper-induced extrinsic apoptosis pathway, marked by substantial increases in the mRNA and protein levels of TNF-R1, Fas-associated death domain, TNFR-associated death domain, and cleaved caspase-8.206 These findings provide compelling evidence that copper can induce apoptosis via multiple molecular pathways, underscoring its potential utility as a targeted strategy in cancer treatment. It is also worth mentioning that research has demonstrated copper can induce apoptosis through a mechanism that does not involve caspases, although the exact processes remain unclear.207
Fig. 4.
Mechanisms of copper-induced cell death. a Copper induces apoptosis primarily through the induction of ROS, DNA damage, and proteasome inhibition. Additionally, Copper induces TP53-dependent apoptosis by activating the transcription of TP53 target genes, while also triggering TP53-independent apoptosis through the inhibition of ribosomal synthesis and the induction of nucleolar stress. b Copper promotes pyroptosis by inducing ROS production and ER stress, resulting in NLRP3 inflammasome formation and membrane pore creation via GSDMD activation. c Copper toxicity activates the TLR4/NF-κB signaling pathway through oxidative stress, resulting in the phosphorylation and oligomerization of RIPK3 and MLKL, thereby triggering necroptosis. d Copper induces intracellular ROS through Fenton-like reactions and mitochondrial damage, leading to lipid peroxidation. It also binds to and induces the oligomerization of GPX4, promoting its autophagic degradation via the receptor TAXIBP1. e Copper initiates autophagy by activating AMPK, inhibiting mTOR, or directly binding to ULK1/2 kinases. Copper-mediated upregulation of autophagic genes and activation of the transcription factor TFEB contribute to the formation of autophagosomes and autolysosomes, which further promoting autophagy-dependent cell death. Created by BioRender. Abbreviations: ROS reactive oxygen species, ER endoplasmic reticulum, NLRP3 nod-like receptor family pyrin domain containing 3, GFDMD gasdermin D, TLR4 toll-like receptor 4, RIPK3 receptor-interacting protein kinase 3, MLKL mixed lineage kinase domain-like protein, GPX4 glutathione peroxidase 4, TAXIBP1 Tax1-binding protein 1, AMPK adenosine monophosphate-activated protein kinase, mTOR mammalian target of rapamycin, TFEB transcription factor EB
Copper and pyroptosis
Pyroptosis is a lytic form of PCD triggered by inflammasomes, primarily driven by the activation of caspase family proteins, including classical caspase-1 and non-classical caspase-4/11 or Caspase-5.208–210 This process is characterized by the cleavage of gasdermins (GSDMD) and the release of IL-1β and IL-18.211 The NLRP3 protein acts as a sensor for the mature inflammasome and is responsible for assembling the classical inflammasome during inflammatory stimulation.212
Copper-induced ROS generation and ER stress may contribute to pyroptosis (Fig. 4b). The accumulation of copper in hepatocytes leads to the expression of pyroptosis-related genes, including caspase-1, IL-18, IL-1β, and NLRP3.213 Likewise, exposing murine microglia to copper initiates an inflammatory response, activating the NLRP3/caspase-1/GSDMD axis and inducing cell pyroptosis.214 These effects are mediated by the activation of ROS/NF-κB pathway and subsequent disruption of mitophagy.214 Comparable outcomes have been observed in murine macrophages exposed to copper oxide nanoparticles, which induce oxidative stress and activate the NLRP3 inflammasome, leading to the expression of pro-IL-1β through the myeloid differentiation factor 88 (MyD88)-dependent TLR4 signaling pathway, subsequently activating NF-κB in macrophages.215 Besides, excessive copper increases the expression of pyroptosis-related genes in jejunal epithelial cells, primarily through the activation of the ER stress pathway.216 ER stress inhibitors, such as 4-phenylbutyric acid, can reduce copper-induced pyroptosis.216 These findings demonstrate there is crosstalk between copper and pyroptosis, necessitating further research to elucidate the precise underlying mechanisms and explore the implications of their interaction.
Copper and necroptosis
Necroptosis is a form of programmed necrosis, mediated by the interaction of two receptor-interacting protein kinases (RIPK1 and RIPK3) and the mixed lineage kinase domain-like pseudokinase (MLKL).217–219 RIPK3 regulates the phosphorylation of MLKL, inducing its oligomerization and translocation to the plasma membrane, where it forms pore complexes and results in the release of DAMPs, cellular swelling, and membrane rupture.220–222
Emerging evidence indicates that copper and copper-based compounds play a role in the activation of necroptosis (Fig. 4c). Excess copper can activate genes associated with necroptosis, including RIPK1, RIPK3, and MLKL. Moreover, DNA damage caused by copper (II) can be alleviated by necroptosis inhibitors.223,224 Copper toxicity activates the Toll-like receptor 4 (TLR4)/NF-κB signaling pathway through oxidative stress, leading to the phosphorylation and oligomerization of RIPK3 and MLKL, thereby triggering necroptosis.25 These studies suggest a close relationship between copper and necroptosis; however, the direct mechanisms underlying this interaction remain to be elucidated.
Copper and ferroptosis
Ferroptosis is an RCD driven by iron-dependent lipid peroxidation, primarily triggered by both intrinsic and extrinsic pathways.225–227 The extrinsic pathway inhibits the cystine/glutamate antiporter system XC-, which is essential for maintaining intracellular cystine and preventing lipid peroxide accumulation.228 The intrinsic pathway induces ferroptosis by inhibiting glutathione peroxidase 4 (GPX4), a critical enzyme that protects cells from oxidative stress.229
Both copper and iron possess potent redox potentials, enabling them to induce the production of hydroxyl radicals via Fenton or Fenton-like reactions230,231 (Fig. 4d). While previous research suggested that iron was the sole metal ion triggering ferroptosis,232 emerging evidence indicates that copper can also facilitate this form of cell death under certain conditions. Gao et al. have unveiled the potential mechanisms of anti-tumor mediated by the copper ionophore Elesclomol (ES)/Cu.233 Distinguished from other copper ionophores, ES exhibits the distinctive property of facilitating the degradation of ATP7A. Combining ES with copper leads to copper retention in mitochondria due to the degradation of ATP7A, which causes an accumulation of ROS. This promotes the degradation of SLC7A11, further intensifying oxidative stress and subsequent ferroptosis in CRC cells.233 Additionally, copper facilitates ferroptosis by inducing the autophagy of GPX4. Exogenous copper increases the ubiquitination of GPX4 through direct binding to the cysteine residues C107 and C148 of the GPX4 protein, promoting the formation of GPX4 aggregates. Tax1-binding protein 1 (TAX1BP1) serves as an autophagic receptor, orchestrating the degradation of GPX4, which collectively leads to ferroptosis in response to copper stress. The utilization of copper chelators can attenuate ferroptosis susceptibility without inhibiting other types of cell death.22 DSF/Cu is a promising anticancer drug with potential clinical applications. One mechanism by which it exerts its anticancer effects involves the mediation of ferroptosis through the activation of the ROS/MAPK and p53 signaling pathways.234 DSF/Cu treatment also profoundly impairs mitochondrial homeostasis, increasing the free iron pool and exacerbating lipid peroxidation, ultimately culminating in ferroptosis triggered by p62 phosphorylation-mediated NRF2 accumulation.235 Notably, a few studies have reported that copper may possess anti-ferroptotic properties, facilitated by a positive feedback loop mechanism between HIF1α and ceruloplasmin.236 Increased copper can also enhance the expression of GPX4, thereby impeding ferroptosis.237 These findings suggest that the complex interactions between copper and ferroptosis merit further investigation.
Copper and autophagy
Autophagy, a universal cellular catabolic route, is orchestrated by autophagy-related proteins (ATGs) and associated factors.238,239 This dynamic recycling system facilitates the formation of membranous structures such as phagophores, autophagosomes, and autolysosomes.240 The initiation of autophagy is triggered by the modulation of kinase activity, primarily through the activation of AMPK or the inhibition of the mTOR pathway.241,242 As a pivotal energy monitor, AMPK facilitates autophagic processes by phosphorylating several targets including mTOR complex 1 (mTORC1), ULK1, and the BECN1 (beclin 1) component within the phosphatidylinositol 3-kinase catalytic subunit type 3 (PIK3C3/VPS34) complex.243,244 Conversely, active mTORC1 suppresses autophagy by engaging and phosphorylating the ULK ensemble, which comprises ULK1, ULK2, and ATG13.245 Autophagy plays a dual role in cellular processes, acting as both a suppressor and a promoter of cell death. Here, we will focus on the regulation of autophagy by copper and its facilitative role in cell death.
Copper can promote autophagy through multiple mechanisms (Fig. 4e). Firstly, copper activates the autophagy process by upregulating the expression of autophagy-related genes such as Beclin1, ATG5, LC3, mTOR, and Dynein,246,247 while also activating autophagic kinases ULK1 and ULK2.128 Secondly, intracellular copper overload induces autophagy via a ROS-dependent AMPK-mTOR pathway.248 Additionally, excessive copper increases cellular autophagy levels through the Cu-mTORC1-TFEB signaling pathway, where TFEB acts as a regulator of lysosomal biogenesis.127
Copper-induced autophagy can also facilitate cell death in cases of overactivation, particularly through ferroptosis, a form of autophagy-dependent cell death.249 Specifically, copper-induced production of ROS can activate autophagy pathways leading to the degradation of anti-ferroptotic factors such as SLC7A11, ferritin, lipid droplets, GPX4, and Cadherin 2.250–253 Additionally, copper has been shown to promote ferroptosis in pancreatic cancer cells by triggering TAX1BP1-mediated autophagic degradation of GPX4 protein.22 Moreover, various copper compounds, including copper oxide nanoparticles254 and copper (II) complexes,255 have been demonstrated to induce autophagic cell death in cancer cells, an effect that can be enhanced by autophagy inhibitors.
Copper and the immune activation
Copper exerts tumor-suppressing functions by activating the immune cells. The cell membrane protein CD44, which serves as the primary receptor for hyaluronic acid and a regulator of cellular plasticity, can mediate an increase in intracellular copper. Within mitochondria, copper ions catalyze the oxidation of NADH molecules by hydrogen peroxide, resulting in the production of NAD+ ions. This biochemical process drives the basal metabolic activation of immune cells, specifically macrophages.256 DSF/Cu has demonstrated anti-tumor effects by provoking immunogenic cell death (ICD). ICD refers to a form of cell death that elicits an immune response against the antigens of dying cells. Cancer cells dying through ICD can release damage-associated molecular patterns (DAMPs) that are recognized by the immune system, thereby helping to prime the immune system against the tumor.257 Treatment with DSF/Cu enhanced the activation and maturation of dendritic cells (DCs). Furthermore, the additional blockade of CD47 further boosts DC maturation and enhances the cytotoxicity of CD8+ T cells. Mechanically, DSF/Cu facilitated the nuclear accumulation of Npl4, which disrupts the UPS and induces ER stress, leading to immune activation in HCC and improving the efficacy of CD 47 blockage.258 Moreover, macrophages can adopt different phenotypes within the tumor microenvironment, with M1 macrophages generally having pro-inflammatory and anticancer properties. DSF/Cu has been shown to promote the polarization of macrophages toward the M1 phenotype and to rewire glucose metabolism via the mTOR pathway. This shift supports antitumor immunity as M1 macrophages are efficient in phagocytosing tumor cells and presenting tumor antigens.259
Cuproptosis in cancer
Based on the aforementioned studies, copper has been proven to induce various forms of cell death. However, emerging research indicates that copper-induced cell death represents a distinct form of cellular demise, which has been termed cuproptosis.
Major milestone of cuproptosis
Over the past few decades, the evolution from understanding copper-induced cell death to the discovery of cuproptosis has reflected researchers’ growing focus on this field (Fig. 5). The term “cuproptosis” was introduced in 2022 to describe a unique form of cell death characterized by copper accumulation and its distinct mechanisms.26 However, the journey leading to this discovery has roots in earlier observations regarding the critical biological roles of copper. As early as 1928, Hart et al. identified copper as an essential element for the production of red blood cells in rats fed a milk-based diet, thereby highlighting the necessity of copper for human health.260 In 1965, de Jorge and colleagues provided the first evidence linking tumors to accumulated copper by demonstrating an 11-fold increase in copper concentrations in brain cancer.261 From 1980 onward, researchers began to understand that excess copper could be toxic, potentially leading to cellular damage and even cell death.262,263 A further study in 1988 corroborated that copper could accelerate the death of tumor cells.264 Consequently, the cytotoxic properties of copper are increasingly being harnessed in anticancer therapies.265,266
Fig. 5.
Major milestone of cuproptosis. The significance of copper ions has been recognized in 1928. Since 2022, research on cuproptosis and its regulatory mechanisms has surged. This timeline illustrates the evolution from the initial understanding of copper-induced cell death to the establishment of cuproptosis over the past few decades, providing insights into the major milestone surrounding cuproptosis and advancements in oncological research related to copper-associated cell death
During the 1990s, research increasingly focused on how copper induces cell damage through oxidative stress mechanisms. In 1991, copper was demonstrated to cause DNA damage by localizing on DNA and enhancing the production of oxygen radicals, particularly hydrogen peroxide (H2O2).267 However, whether there is an increased incidence of cancer in diseases associated with copper accumulation, such as WD and MD, remains to be determined.267 Then in 1993, the copper-transporting ATP7A was first isolated and identified among the candidate genes for MD.268 By 1994, research indicated that copper-induced ROS damage leads to DNA mutations, potentially serving as a mechanism for cancer development.269 Subsequent studies have also confirmed that superoxide anions and ROS, generated by copper within cells, can damage cellular lipids, proteins, and DNA.270–272 In 2000, Zhai et al. investigated the cytotoxic effects of copper and its underlying molecular mechanisms, revealing that copper (II) enhances the expression of Bax and ROS, subsequently inactivating NFκB and inducing apoptosis in the murine pre-B cell line BA/F3b.273 Until 2006, studies indicated that copper-induced human cell death could signify a unique form of non-apoptotic PCD, overturning prior constraints in our understanding and marking a milestone in the identification of “cuproptosis” as a novel mode of cell death.274 Additionally, it was reported that cell death induced by intracellular glutathione depletion depends on trace amounts of extracellular copper in 2007.275 In models related to WD, the disruption of copper balance through the suppression of ATP7B was also observed to induce cell death in 2014.276
Concurrently, the discovery and development of cuproptosis have been largely driven by explorations into the anticancer effects of copper-based compounds. By 1953, the discovery of copper chelators such as Dipyridyl and O-Phenanthroline, which could inhibit tumor growth, initiated the exploration of copper’s potential therapeutic applications.277 DSF, traditionally used as an alcohol abuse deterrent, was found to possess anticancer activity in 1974.278 However, it was not recognized as a copper ionophore until 2004 and is believed to cause cell death through mechanisms involving copper.279 The anticancer effects of TTM, a copper-chelating agent, were first described in 1999.280 A study conducted in 2000 revealed that copper-containing drugs could induce tumor cell death.281 Another copper ionophore, ES, demonstrated the capacity to induce cancer cell apoptosis in 2008.282 Copper ionophores are lipophilic molecules that reversibly bind copper and facilitate their transport across cellular membranes, including the plasma and mitochondrial membranes.283 While the exact mechanism of cell death induced by copper ionophores remains incompletely understood, research has suggested that it may involve the generation of ROS. Mitochondria, known for their role in cellular metabolism, are implicated in this process, as ROS are primarily derived from intracellular redox reactions, with mitochondria playing a significant role.284 Studies on cell death induced by ES indicated that elevated ROS levels, resulting from mitochondrial dysfunction contribute to cell death.285–288 In 2015, copper was reported to enhance the antitumor activity of DSF.289 Research in 2016 elucidated the anticancer mechanism of the DSF-copper complex, demonstrating that the DSF-dependent upregulation of intracellular copper concentration suppressed tumor growth through elevated ROS levels.290 In 2017, Npl4, an adaptor of the p97 segregase (also known as VCP), was identified as a molecular target for the tumor-suppressive effects of the DSF/Cu complex.291 Further research in 2021 revealed that copper induces the aggregation of p97-Npl4 by inhibiting ubiquitinated protein degradation, or directly binding to Npl4 and impeding its conformational changes, leading to cell death.292
The pioneering work of the team led by Peter Tsvetkov and Todd R. Golub established the foundation of cuproptosis. They discovered that ES can promote a distinct form of copper-dependent cell death and initially proposed the concept of cuproptosis.293 Notably, ferredoxin1 (FDX1) was identified as the direct target protein of ES, which interacts directly with ES-Copper and inhibits the formation of iron-sulfur clusters (Fe-S clusters).293 Additionally, subsequent research in 2021 on glioblastoma stem-like cells (GSCs) showed that ES/Cu directly targets the mitochondrial membrane, inducing a significant increase in mitochondrial ROS, ultimately leading to non-apoptotic, copper-dependent cell death.294 Until March 2022, Tsvetkov et al. formally defined this form of cell death as cuproptosis, which is distinct from known death mechanisms and reliant on copper and mitochondrial respiration, marking a significant advancement in the field.26 In the subsequent years, research primarily focused on further elucidating the mechanisms of cuproptosis and exploring anticancer drugs based on cuproptosis. In 2023, Sun et al. identified the atypical methyltransferase METTL16 as a key mediator of cuproptosis through its m6A modification of FDX1 mRNA.295 They established the copper-lactylated METTL16-FDX1-cuproptosis axis as a crucial regulatory mechanism in copper-related metabolism, filling a significant gap in our understanding of cuproptosis regulation. Liu et al. uncovered the abnormal activation of the Wnt/β-catenin signaling pathway imparts resistance to cuproptosis in tumor cells, proposing a precision medicine strategy for cancer treatment through the selective induction of cuproptosis.296 In the same year, Li et al. demonstrated that zinc transporter 1 (ZnT1) is a novel copper transport protein capable of mediating copper (II) uptake and inducing cuproptosis.297 Collectively, these studies contribute to a deeper understanding of the mechanisms and regulation of cuproptosis, highlighting its significance for therapeutic interventions.
Molecular mechanism of cuproptosis
Cuproptosis is a novel type of regulated cell death that depends on mitochondrial metabolism.26,293 However, recent research has unveiled the existence of mitochondrial-independent cuproptosis. Here, we delineate its core mechanisms, specifically emphasizing the mitochondrial-dependent and mitochondrial-independent pathways (Fig. 6).
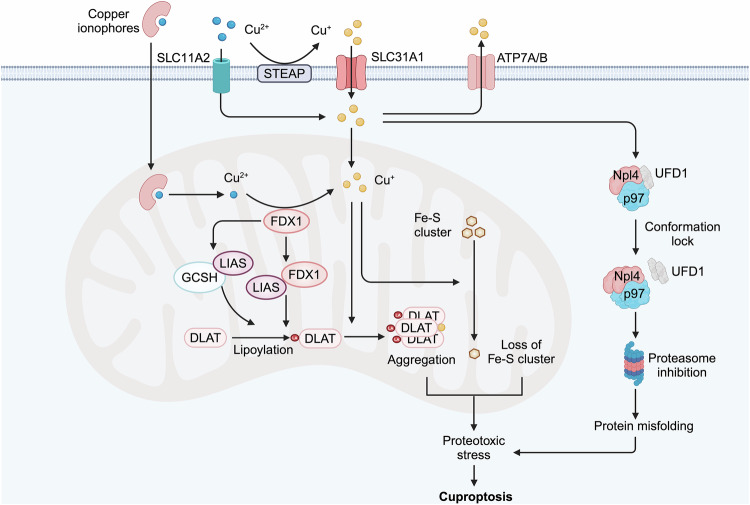
Fig. 6.
Mechanism of cuproptosis. The mechanism of cuproptosis includes both mitochondrial-dependent and mitochondrial-independent pathways. Excess copper (II) enters the mitochondria, where it is reduced to the more toxic copper (I) by the mitochondrial protein FDX1. FDX1 also promotes protein lipoylation by directly binding to the LIAS and enhancing its interaction with the GCSH. Copper (I) binding induces the aggregation of DLAT and destabilizes Fe-S cluster proteins, triggering cellular stress responses that result in cuproptosis. Additionally, DSF/Cu mediates the aggregation and conformation lock of the Npl4-p97 protein in the cytoplasm, inhibiting the ubiquitin-proteasome degradation pathway, which contributes to proteotoxic stress and cuproptosis. Created by BioRender. Abbreviations: FDX1 ferredoxin 1, LIAS lipoic acid synthase, GCSH glycine cleavage system protein H, DLAT Dihydrolipoamide S-Acetyltransferase, Fe-S cluster iron-sulfur cluster, DSF Disulfiram, Npl4 an adaptor of the p97 segregase (also known as VCP)
Mitochondria-dependent cuproptosis
Cuproptosis is different from the current well-known cell death, because using the inhibitors or knockouting these pathway genes failed to rescue cuproptic cells. However, cancer cells with mitochondrial respiration are significantly more sensitive to ES than those with glycolysis, suggesting the pivotal role of mitochondria in cuproptosis. Further studies using whole-genome CRISPR-Cas9 knockout screening identified FDX1, LIAS, and DLAT, which are components of the lipoic acid (LA) pathway or the pyruvate dehydrogenase complex, as essential genes required for cuproptosis.26,293 Excess copper (II) enters the mitochondria, where it is reduced to the more toxic copper (I) by the mitochondrial protein FDX1. FDX1 also promotes protein lipoylation by directly binding to lipoic acid synthase (LIAS) and enhancing its interaction with the glycine cleavage system protein H (GCSH).298 Notably, mitochondrial copper can directly bind to proteins following their lipoylation, a post-translational modification involving the covalent attachment of an eight-carbon organosulfur lipoic acid moiety to specific lysine residues, leading to the aggregation of lipoylated proteins.299,300 For example, copper binding induces the aggregation of dihydrolipoamide S-acetyltransferase (DLAT).301 Moreover, mitochondrial copper destabilizes Fe-S cluster proteins, which are crucial for protein lipoylation and electron transfer reactions in mitochondria. Consequently, copper toxicity results in improper folding of DLAT and the loss of proteins containing Fe-S clusters, leading to a cascade of cellular stress responses characterized by the increase of heat shock proteins, that ultimately culminate in cuproptosis.
Mitochondria-independent cuproptosis
FDX1 is a core regulator of mitochondria-dependent cuproptosis, as mentioned above. However, Attar et al. identified histone H3-H4 tetramer as a novel copper (II) reductase in the eukaryotic cells, indicating the fungibility of FDX1 in cuproptosis.302 Gale et al. demonstrated that ES/Cu induces FDX1-independent astrocyte toxicity mediated by oxidative stress, as FDX1 knockdown did not block ES/Cu toxicity to astrocytes.303 Additionally, inhibition of mitochondrial respiration failed to rescue the ES/Cu toxicity,303 and intracellular copper can be released from the ES/Cu complex and become bioavailable outside the mitochondria, suggesting a great likelihood of mitochondrial-independent cuproptosis.304 Similarly, DSF/Cu can mediate the aggregation and lock the conformational transition of cytoplasmic p97 complex that plays a central role in cellular protein homeostasis, inhibiting cellular ubiquitin-proteasome degradation pathways and further leads to increased proteotoxic stress and cell death.291,292,305 These findings highlight the existence of mitochondria-independent cuproptosis, although the molecular mechanisms remain inadequately understood. Hence, further exploration of mitochondrial-independent cuproptosis could trigger a novel breakthrough in the field of cuproptosis.
Functions of cuproptosis in cancer biology
Cuproptosis has emerged as a significant player in cancer biology. On one hand, it appears to function as an innate mechanism for tumor suppression. On the other hand, cancer cells evade cuproptosis, thereby promoting tumor progression and treatment resistance.
Cuproptosis induction in tumor suppression
The tumor suppressor protein p53 inhibits tumor development partly by inducing cuproptosis (Fig. 7a). P53 serves as a crucial metabolic regulator in the modulation of glycolysis and oxidative phosphorylation, two tightly coupled metabolic processes closely linked to cellular sensitivity to cuproptosis.306–308 Specifically, p53 inhibits glucose uptake and glycolysis, promoting a metabolic shift towards the tricarboxylic acid (TCA) cycle and oxidative phosphorylation, thereby increasing sensitivity to cuproptosis.309 Additionally, p53 regulates the biosynthesis of GSH to facilitate cuproptosis. Mechanistically, p53 suppresses the production of NADPH by inhibiting malic enzymes and G6PD, as well as the associated pentose phosphate pathway.310,311 NADPH is a crucial reductant for the regeneration of GSH, and its reduction leads to decreased GSH levels. Consequently, p53-mediated metabolic remodeling and cuproptosis may represent an effective strategy for eradicating cancer cells.
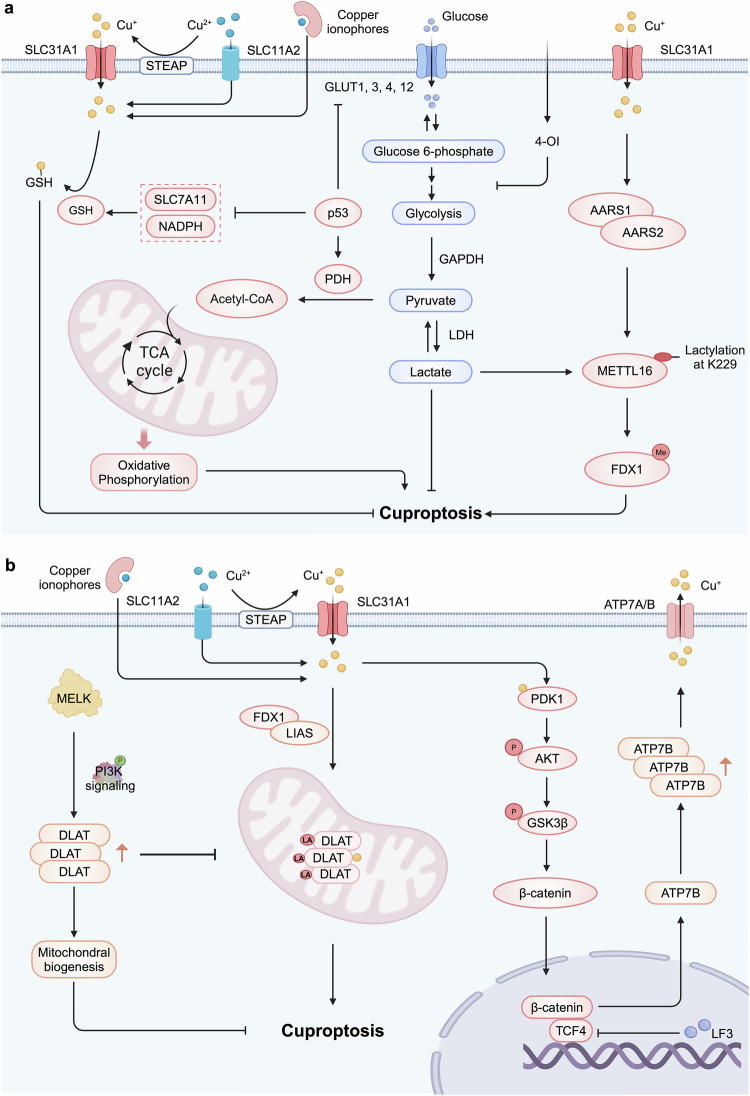
Fig. 7.
Cancer-related pathways in cuproptosis. a Cuproptosis induction in tumor suppression. The tumor suppressor p53 inhibits glucose uptake and glycolysis, shifting metabolism towards the TCA cycle and oxidative phosphorylation, which sensitizes cells to cuproptosis. It also suppresses NADPH production by inhibiting G6PD and the pentose phosphate pathway, resulting in decreased GSH levels. Intratumoral copper enhances METTL16-K229 lactylation and activity through its interaction with AARS1 or AARS2, leading to increased FDX1 expression and cuproptosis induction. Additionally, the metabolite 4-OI alkylates cysteine residues in GAPDH, inhibiting its activity and suppressing aerobic glycolysis, thereby further promoting cuproptosis. b Cuproptosis evasion in tumor progression and therapeutic resistance. Increased MELK expression in tumors activates the PI3K/mTOR signaling pathway, leading to elevated DLAT expression and stabilized mitochondrial function, which enhances resistance to cuproptosis. Additionally, copper binds to PDK1, activating the downstream AKT-GSK3β-β-catenin pathway and promoting CSC characteristics. CSCs demonstrate heightened resistance to cuproptosis, as the β-catenin/TCF4 transcription complex binds to the ATP7B promoter, facilitating copper expulsion. Created by BioRender. Abbreviations: TCA tricarboxylic acid cycle, NADPH nicotinamide adenine dinucleotide phosphate, G6PD glucose-6-phosphate dehydrogenase, GSH glutathione, METTL16 methyltransferase-like 16, 4-OI 4-octyl itaconate, GAPDH glyceraldehyde-3-phosphate dehydrogenase, MELK maternal embryonic leucine zipper kinase. CSC cancer stem cell
The epigenetic regulator METTL16 plays a vital role in tumor suppression by promoting cuproptosis. In mechanism, intratumoral copper ions promote the lactylation and activity of METTL16-K229 by increasing its interaction with AARS1 or AARS2. Lactylation of METTL16 induces methylation modifications at the FDX1-602 site, thus enhancing the expression of FDX1 and leading to cuproptosis. In contrast, SIRT2, a classical deacetylase, significantly inhibits the lactylation of METTL16 and the m6A modification of FDX1, thereby impeding cuproptosis.295 Consistently, AGK2, a SIRT2-specific inhibitor, promotes the therapeutic effects of ES by inducing the lactylation of METTL16-mediated cuproptosis in gastric tumors in vitro and in vivo. These findings suggested that the lactylation modification of METTL16 inhibits the development of gastric cancer partly through the induction of cuproptosis.
Tumor metabolites also inhibit tumor progression by inducing cuproptosis. For example, 4-octyl itaconate (4-OI), a derivative of the TCA cycle metabolite produced by aconitate decarboxylase 1, has been shown to promote cuproptosis by inhibiting aerobic glycolysis.312 Specifically, 4-OI alkylates the cysteine residues of GAPDH, inhibiting the enzymatic activity and thereby leading to the suppression of aerobic glycolysis. Meanwhile, another metabolite derived from the TCA cycle intermediate fumarate, dimethyl fumarate, can also inhibit aerobic glycolysis by targeting GAPDH, potentially promoting cuproptosis.313
Cuproptosis evasion in tumor progression and therapeutic resistance
Despite the presence of a cuproptosis-mediated tumor suppression mechanism, tumors can still arise and progress uncontrollably, suggesting the existence of evasion mechanisms against cuproptosis in cancer cells (Fig. 7b). The oncogene maternal embryonic leucine zipper kinase (MELK) functions as a suppressor of cuproptosis to contribute to tumorigenesis and tumor progression. Elevated expression of MELK in tumors enhances the activity of the PI3K/mTOR signaling pathway, which subsequently boosts the expression of DLAT and stabilizes mitochondrial functions.314 The increase in DLAT (not the oligomer, possibly lipoic acid-modified DLAT) helps improve mitochondrial respiration, eliminates excessive intracellular ROS, and also promotes resistance to ES-induced cuproptosis, thus contributing to the tumorigenesis and progression of HCC.
Additionally, aberrant activation of the Wnt/β-catenin signaling pathway endows tumor cells with the ability of therapeutic resistance by evading cuproptosis. Liu et al. indicated that the process of cuproptosis is accompanied by robust activation of the Wnt/β-catenin pathway.296 Mechanistically, copper ions in tumors directly bind to PDK1, activating the downstream AKT-GSK3β-β-catenin pathway and enhancing cancer stem cells (CSCs) traits. Interestingly, CSCs exhibit greater resistance to cuproptosis because the β-catenin/TCF4 transcriptional complex can directly bind to the ATP7B promoter and induce its expression, which is responsible for reducing intracellular copper levels. CSCs are often characterized by multi-drug resistance within tumors.315,316 Therefore, aberrant activation of the Wnt/β-catenin pathway aids CSCs in adapting to therapeutic interventions by evading cuproptosis.
Cuproptosis-mediated crosstalk within the tumor microenvironment (TME)
The TME refers to the multifaceted ecosystem surrounding tumor cells, encompassing various cellular and non-cellular components.317,318 These components interact intricately, significantly influencing tumor growth and progression.319 Tumor cells undergoing cuproptosis exhibit immunostimulatory effects in both direct and indirect manners. On one hand, cuproptotic tumor cells can directly activate their internal cyclic GMP-AMP synthase (cGAS)/stimulator of interferon genes (STING) signaling pathway, triggering the release of inflammatory factors to initiate immune responses within the TME. Specifically, cuproptosis-induced mitochondrial proteotoxic stress promotes the release of mitochondrial DNA (mtDNA), which acts as an intracellular DAMP,320 activating the tumor-immune-related mtDNA-cGAS-STING signaling pathway and leading to the secretion IFN-β and CXCL10.321 Subsequently, these cytokines facilitate DC maturation, cytotoxic CD8+ T cell infiltration, and natural killer (NK) cell recruitment, collectively promoting tumor regression in preclinical models. On the other hand, tumor cells undergoing curptoptosis release certain “eat me” signals, indirectly initiating a sustained anti-tumor immune response. The membranes of tumor cells damaged by cuproptosis release various DAMPs, such as ATP, HMGB1, and calreticulin (CRT), which enhance the maturation of DCs and the activation of CD8+ effector T cells, thereby triggering classical ICD.322–328 Intriguingly, the cGAS-STING signaling pathway is also activated in DCs by cuproptotic cancer cells, induced by ES and CuCl2, which subsequently enhances the release of inflammatory mediators including IL-2, TNF-α, IFN-γ, CXCL10, and CXCL11.329 These cuproptosis-mediated immunostimulatory effects were also observed in copper-based nanomedicine.325,330–338 For instance, TPP-CuET, a mitochondria-targeted copper complex modified with triphenylphosphine, effectively inhibits the mitochondrial tricarboxylic acid cycle, ATP synthesis, and the electron transport chain.330 Simultaneously, it activates the immune response of CD8+ T cells and NK cells via the MHC I pathway, enhancing antigen processing and presentation in cancer cells.330 A novel nucleic acid nanoplatforms can inhibit HIF-1 expression, thereby alleviating the immunosuppressive TME, and enhancing antigen presentation through the activation of Toll-like receptor 9 (TLR9) via the immune adjuvant polyCpG.331
However, cuproptosis also leads to the upregulation of PD-L1 protein in tumor cells, inhibiting the cytotoxic CD8+ T cell response.339 Consistently, elevated copper levels can promote PD-L1 expression by upregulating the JAK/STAT signaling pathway, thereby enhancing the negative regulatory effect on T cells that facilitates tumor immune escape.20,189 Moreover, certain nanomaterials such as CAT-ecSNA-Cu and NP@ESCu induce cuproptosis in tumor cells and increase PD-L1 expression, though creating favorable conditions for combined anti-tumor therapy with immunotherapy.326,331 These findings support the immunosuppressive effects of cuproptotic tumor cells, suggesting the complex immunoregulatory nature of cuproptosis.
Clinical associations of cuproptosis-related genes (CRGs)
Tsvetkov et al. identified ten genes through whole genome knockout screening that may modify susceptibility to cuproptosis, classifying FDX1, LIAS, LIPT1, DLD, DLAT, PDHA1and PDHB as positive regulators, and MTF1, GLS and CDKN2A as negative regulators.26 Additional analysis on copper homeostasis indicated that SLC31A1, ATP7A, and ATP7B could also influence cuproptosis by regulating intracellular copper concentrations. Among these 13 CRGs, FDX1, DLAT, LIAS, SLC31A1, ATP7A, and ATP7B are well-studied, and therefore, we will explore their expression levels and clinical relevance across different cancers.
FDX1 is predominantly downregulated in various types of cancer tissues, particularly in solid tumors, including clear cell renal cell carcinoma (ccRCC), breast invasive carcinoma, colon adenocarcinoma, lung adenocarcinoma, thyroid carcinoma, and HCC. whereas, it is upregulated in certain female reproductive tumors such as ovarian serous cystadenocarcinoma (OV) and uterine corpus endometrial carcinosarcoma, as well as in glioblastoma (GBM).340,341 High levels of FDX1 correlate with poor prognosis in brain lower-grade glioma, while better outcomes are observed in ccRCC and HCC.342–344 Particularly in ccRCC, low expression of FDX1 is significantly associated with advanced TNM staging, lymph node metastasis, and poorer prognosis.342 Single-cell RNA sequencing analysis also indicates that FDX1 is expressed in immune cells, with notable variations in expression levels among monocytes or macrophages.343 The effect of FDX1-dependent cuproptosis in these immune cells needs further clarification.
DLAT, the E2 subunit of the PDCs, is critical for TCA cycle.345,346 In gastric cancer cells, DLAT expression is notably upregulated, enhancing oxidative phosphorylation by catalyzing the conversion of pyruvate to acetyl-CoA, thereby supplying energy to tumor cells.347 Studies in non-small cell lung cancer (NSCLC) have shown that PM2.5 upregulates DLAT expression through a dual-regulatory mechanism involving the Sp1-DLAT and eIF4E-DLAT axes, promoting glycolysis and enhancing tumor cell proliferation.348 Based on multi-database and experimental verification, DLAT is significantly upregulated in ccRCC349 and HCC,350 revealing its role as a tumor suppressor gene.351 Guo et al. first explored the correlation between disease-free survival in bladder cancer patients and the expression of lipoylated DLAT protein targets using Gene Expression Profiling Interactive Analysis from the TCGA database.326 The findings indicate that patients with low DLAT expression have longer disease-free survival than those with high DLAT expression. Furthermore, DLAT expression positively correlates with the expression of FDX1 and LIAS. In bladder tumor tissues, DLAT expression is significantly positively correlated with PD-L1 expression. Additionally, the level of DLAT expression is positively associated with the immune infiltration levels of B cells, macrophages, neutrophils, and DCs.351
LIAS is linked to mitochondrial energy metabolism and antioxidant defense, functioning in the electron transport chain and oxidative phosphorylation within the TCA cycle. Therefore, tumors characterized by high levels of oxidative phosphorylation, such as breast cancer (BRCA), melanoma, and cholangiocarcinoma, are predominantly dependent on LIAS.352 Recent pan-cancer bioinformatics analyses have shown that high expression of LIAS correlates with favorable prognosis in patients with ccRCC, rectal adenocarcinoma, BRCA, and ovarian cancer. In contrast, high expression of LIAS is associated with poor prognosis in lung cancer patients.353 Furthermore, LIAS shows differential expression between recurrent and non-recurrent colorectal cancer samples.354
Dysregulation of copper homeostasis, mediated by factors such as SLC31A1, ATP7A, and ATP7B, can lead to cellular dysfunction.4,26 As previously mentioned, SLC31A1 mediates copper entry into cells, while ATP7A and ATP7B facilitate copper efflux, collectively functioning as copper carriers intimately involved in copper shuttling.355–358 In research focused on gliomas, SLC31A1 was considered a risk factor, whereas ATP7B was deemed a protective factor, further suggesting a potential connection between copper homeostasis and cancer.359 Furthermore, studies have established that microsatellites of SLC31A1 and ATP7B are linked to an increased risk of lung cancer, implying that the expression levels of copper homeostasis-related genes could influence cancer progression.360 Two independent studies suggested that high expression of SLC31A1 correlates with adverse clinical outcomes in BRCA patients.361,362 Additionally, elevated levels of ATP7A are associated with poor overall survival (OS) in BRCA and liver cancer patients.363,364 Notably, SLC31A1 demonstrates a strong correlation with the TME. Upregulation of SLC31A1 is associated with poor OS in cervical squamous cell carcinoma, esophageal cancer, BRCA, or head and neck squamous cell carcinoma patients.365 The analysis of tumor immune infiltration revealed associations between the expression of SLC31A1 and the presence of T cells, and macrophages within the TME. Interestingly, the expression of SLC31A1 is negatively correlated with OS and tumor infiltration of plasmacytoid DCs, NK cells, and CD8+ T cells, but it is positively correlated with the high abundance of characteristic immunosuppressive immune cell types in tumors of glioma patients.366 In BRCA patients, tumor expression of SLC31A1 is positively correlated with the infiltration of immune cells including CD4+ T cells, macrophages, neutrophils, and DCs, as well as with the expression of immune checkpoint genes such as CD274 and CTLA4, despite its association with poorer prognosis.366 Finally, a study employing TCGA and tissue microarray data revealed a significant positive correlation between the expression of SLC31A1 and CD274 across multiple cancer types.20
DLD, PDHA1, and PDHB, as crucial subunits of the PDC, play significant roles in the process of cuproptosis.345,367 According to a study by Ma et al., the expression levels of DLD, PDHA1, and PDHB1 are notably higher than those of other CRGs in almost all tumors according to the TCGA database.368 Furthermore, the expression levels of these genes are also considered to be associated with tumor prognosis. For instance, high levels of PDHA1 are linked to better prognosis in lung cancer patients and may serve as a biomarker for immunotherapy response.369 Additionally, CDKN2A, GLS, and MTF1 have been shown to correlate with cellular sensitivity to cuproptosis.26 CDKN2A is often overexpressed in most cancer patients and is associated with adverse prognostic outcomes.370 In HCC, CDKN2A promotes tumor cell proliferation and migration capabilities as well as attenuating cuproptosis.371 Furthermore, CDKN2A serves as a target for puromycin, which may contribute to preventing cancer progression.372 GLS and MTF1 potentially influence cellular sensitivity to cuproptosis by regulating the levels of intracellular copper-binding substances, such as MTs and GSH.373,374 Notably, the mitochondrial copper (I) transporter PiC2 is a target of MTF1.375 The LIPT1 enzyme facilitates the activation of 2-oxoglutarate dehydrogenases within the TCA cycle.376 Inhibition of LIPT1 expression has been demonstrated to hinder the growth, invasion, and metastasis of HCC cells.377 Interestingly, LIPT1 also exhibits a positive correlation with PD-L1 in a variety of cancer types, further emphasizing its potential relevance in cancer biology.378
Copper and cuproptosis targeting strategies for therapy
Copper and cuproptosis are intricately linked to cancer. Cellular copper levels are meticulously regulated, and its dyshomeostasis significantly inhibits tumor progression. Therefore, depleting copper and inducing cuproptosis through copper introduction presents new therapeutic strategies for cancer treatment. Notably, certain cancers characterized by enhanced mitochondrial respiration, stem cell-like traits, or drug resistance demonstrate greater responsiveness to cuproptosis-based therapies,80 indicating its potential as a valuable supplement to current chemotherapy, radiotherapy, and immunotherapy. Furthermore, cuproptosis-based therapies can enhance the sensitivity of cancer cells to these traditional treatments,293,296,324,327,334,379–388 making cuproptosis-based combination therapies a promising avenue for cancer treatment. Given these points, it is essential to deepen our understanding of copper chelators (Table 1) and ionophores (Table 2).
Table 1.
Copper chelators in clinical trials for cancer therapy
| Agent | Mechanism involved | Preclinical cancer type | Clinical cancer type | Combination | NCT | Phase |
|---|---|---|---|---|---|---|
| Tetrathiomolybdate (TTM) | Inhibits angiogenesis and metastasis | Ovarian carcinoma,508 Cervical cancer,509 Prostate cancer,510 Melanoma,392 Lung cancer,407 Pancreatic cancer,356 and Papillary thyroid cancer394 | Hormone-refractory prostate cancer408 | N/A | NCT00150995 | II |
| Hepatocellular carcinoma | N/A | NCT00006332 | II | |||
| Esophageal carcinoma511 | N/A | NCT00176800 | II | |||
| Breast cancer512 | N/A | NCT00195091 | II | |||
| Advanced kidney cancer406 | N/A | N/A | II | |||
| Metastatic non-small-cell lung cancer | Carboplatin and Pemetrexed | NCT01837329 | I | |||
| High risk for relapse of triple-negative breast cancer | Capecitabine and pembrolizumab | NCT06134375 | Ib and II | |||
| ATN-224 | Inhibits SOD1 activity |
Metastatic head and neck squamous cell carcinoma,410 lung cancer,513 leukemia,514 diffuse large B cell lymphoma,515 and breast cancer434 |
Advanced solid tumors(breast, melanoma, colon,renal and others)411 | N/A | N/A | I |
| Advanced melanoma | Temozolomide | NCT00383851 | II | |||
| Prostate cancer | N/A | NCT00405574 | II | |||
| Multiple myeloma | Bortezomib | NCT00352742 | I and II | |||
| Biochemically-recurrent hormone-naive prostate cancer412 | N/A | N/A | II | |||
| D-penicillamine (D-pen or PCA), | Inhibits angiogenesis and metastasis | Gliosarcoma,516 lung cancer,417 breast cancer,417 cervical cancer,415 and leukemia517 | Recurrent head and neck cancer | N/A | NCT06103617 | II |
| Glioblastoma418 | N/A | NCT00003751 | II | |||
| Triethylenetetramine (TETA/trientine) | Inhibits angiogenesis and enhances apoptosis | Hepatocellular carcinoma,422,423 and mesothelioma518 | Advanced malignancies426 | Carboplatin | NCT01178112 | I |
| BRAF mutated metastatic melanoma | Vemurafenib | NCT02068079 | I | |||
| Epithelial ovarian cancer425 | Pegylated liposomal doxorubicin and carboplatin | NCT03480750 | I and II | |||
| Tetraethylenepentamine pentahydrochloride (TEPA) | Inhibits PD-L1 expression | Neuroblastoma20 | N/A | N/A | N/A | N/A |
Table 2.
Copper ionophores in clinical trials for cancer therapy
| Agent | Mechanism involved | Preclinical cancer type | Clinical cancer type | Combination | NCT | Phase |
|---|---|---|---|---|---|---|
| Elesclomol (ES) | Induces apoptosis, cuproptosis and ferroptosis | Breast cancer,519 colorectal cancer,233,312 thyroid cancer,520 uveal melanoma,521 gastric cancer,295 and prostate cancer522 | Metastatic melanoma441 | Paclitaxel | NCT00084214 | I and II |
| Chemotherapy-naïve with advanced melanoma446 | Paclitaxel | NCT00522834 | III | |||
| Relapsed or refractory acute myeloid leukemia445 | N/A | NCT01280786 | I | |||
| Solid tumors | N/A | NCT00827203 | I | |||
| Refractory solid tumors444 | Paclitaxel | NCT00088114 | I | |||
| Metastatic prostate cancer | Docetaxel and prednisone | NCT00808418 | I | |||
| Recurrent ovarian epithelial cancer443 | Paclitaxel | NCT00888615 | II | |||
| Soft-tissue sarcomas | Paclitaxel | NCT00087997 | II | |||
| Non-small-cell lung cancer | Paclitaxel and Carboplatin | NCT00088088 | I and II | |||
| Disulfiram (DSF) | Induces apoptosis, cuproptosis and ferroptosis | Breast cancer,291,523–525 cervical cancer,526 chondrosarcoma,452 thyroid cancer,527,528 lung cancer,524 pancreatic cancer,529 hepatocellular carcinoma,192,235,530 and colon cancer531 | Advance gastric cancer | Cisplatin | NCT05667415 | N/A |
| Recurrent glioblastoma459 | Dietary copper and alkylating agents | NCT02678975 | II and III | |||
| Recurrent glioblastoma532 | Copper gluconate and temozolomide | NCT03034135 | II | |||
| Newly diagnosed glioblastoma multiform | Temozolomide | NCT01777919 | II | |||
| Newly diagnosed glioblastoma533 | Standard radiation therapy and temozolomide | NCT02715609 | I and II | |||
| Refractory germ cell tumors457 | Cisplatin | NCT03950830 | II | |||
| Metastatic melanoma | N/A | NCT00256230 | I and II | |||
| Metastatic pancreatic cancer | Chemotherapy | NCT02671890 | I | |||
| Metastatic breast cancer | N/A | NCT03323346 | II | |||
| Treatment-refractofy sarcomas | Copper gluconate and liposomal doxorubicin | NCT05210374 | I | |||
|
Refractory solid tumors involving the liver458 |
Copper gluconate | NCT00742911 | I | |||
| Treatment-refractory multiple myeloma | Copper gluconate | NCT04521335 | I | |||
| Metastatic castration-resistant prostate cancer534 | Copper gluconate | NCT02963051 | I | |||
| Non-small-cell-lung cancer456 | Chemotherapy | NCT00312819 | II and III | |||
| Clioquinol (CQ) | Induces apoptosis and inhibits proteasome | Prostate cancer,207,461,535,536 breast cancer,537,538 ovarian cancer,539 and cervical cancer540 | Advanced hematologic malignancies541 | N/A | NCT00963495 | I |
| 8-hydroxyquinoline (8-OHQ) | Induces apoptosis and paraptosis, as well as inhibits proteasome | Breast cancer,460 lung cancer,542 | N/A | N/A | N/A | N/A |
| NSC319726 | Induces oxidative stress and cell-cycle arrest | Glioblastomas,113 chromophobe renal cell carcinoma543 | N/A | N/A | N/A | N/A |
| Pyrithione | Induces ROS production and apoptosis | Breast cancer, hepatocellular carcinoma, and myeloma544 | N/A | N/A | N/A | N/A |
Copper chelators
Copper chelators, which bind to copper and reduce its bioavailability, are a critical strategy for targeting copper homeostasis in cancer therapy. Various copper chelators have proven potential in preclinical animal research and clinical trials aimed at cancer treatment, primarily by inhibiting angiogenesis and impairing tumor cell proliferation and metastasis.389,390 Representative agents include TTM, choline tetrathiomolybdate (ATN-224), D-penicillamine (D-pen or PCA), Triethylenetetramine (TETA, commonly known as trientine), and Tetraethylenepentamine pentahydrochloride (TEPA).390
TTM is an oral copper chelator that has been shown to inhibit tumor angiogenesis and metastasis.155,166,181,391 Recent studies indicate that TTM further inhibits the activity of MEK1/2 kinases by lowering copper levels, contributing to the suppression of papillary thyroid cancer and colon cancer, as well as BRAFV600E-driven tumorigenesis in melanoma.120,122,392–395 Importantly, TTM enhances the antitumor efficacy of MEK1/2 and BRAFV600E inhibitors, including sorafenib and vemurafenib.394 In clinical trials with TTM, a Phase I study with advanced cancer patients found that most achieved copper deficiency after 6–8 weeks of treatment.280 The notable toxicity associated with TTM was anemia, defined as a hematocrit below 80% of baseline, occurring in about one-third of patients. Rapidly reversible neutropenia without infection was also observed. Anemia was considered directly related to the degree of copper deficiency rather than TTM dosage, and all patients recovered without the need for transfusions within 5–7 days after discontinuing TTM.280 In a study involving high-risk breast cancer patients in stages II-IV, 75 individuals received TTM as adjuvant treatment across two cycles (induction and maintenance).396 With a median follow-up of 6.3 years, the event-free survival rate for the entire cohort was 72%, and the OS rate was 84%. Among patients with stage II-III disease, the 2-year OS rate was 96%, while it was 93% for those with stage IV. TTM was well tolerated, with only 5.7% of patients experiencing grade 3 or 4 adverse events, primarily neutropenia, all of which were reversible. Furthermore, lower levels of ceruloplasmin were associated with a reduction in circulating angiogenic progenitor cells (VEGFR2+) and decreased serum LOXL-2 levels, indicating that TTM treatment may influence the premetastatic niche.396 Another study involving 30 patients with malignant pleural mesothelioma revealed that postoperative TTM treatment delayed disease progression in stage I and II patients, extending the progression-free survival (PFS) from 10 to 20 months.397 Compared to traditional multimodal therapies, TTM showed anti-angiogenic effects after tumor resection, with minimal toxicity and comparable efficacy.397 TTM also exhibits radiosensitizing and chemosensitizing effects,398,399 enhancing the efficacy of chemotherapeutic agents like doxorubicin, mitomycin C, fenretinide, 5-fluorouracil, and cisplatin in preclinical models without increasing side effects.400–403 A pilot trial of TTM in combination with irinotecan, 5-fluorouracil, and leucovorin for metastatic colorectal cancer also demonstrates that TTM can be safely incorporated into combination chemotherapy regimens.404 Overall, TTM is a low-toxicity, safe, and well-tolerated copper-chelating agent that has shown promising antitumor effects, particularly as an adjuvant treatment and in combination treatments.155,166,280,396,397,404–407 Additionally, serum ceruloplasmin levels serve as a biomarker to monitor and assess copper depletion, facilitating adjustments to drug dosages and treatment durations.396,405 However, in a study with 19 patients diagnosed with Hormone-Refractory Prostate Cancer, TTM monotherapy failed to delay disease progression.408 A possible reason for this could be that the anti-angiogenic effects of copper depletion by TTM may require a longer lead-in time to effectively inhibit tumor progression.408 Therefore, extending drug exposure time and designing studies to assess disease progression over time may contribute to optimizing treatment strategies.
ATN-224, a second-generation TTM analog, serves as an inhibitor of SOD1 in anticancer therapy.409,410 A phase I clinical trial involving 80 patients investigated the pharmacokinetics and pharmacodynamics of ATN-224.411 Results indicated that oral ATN-224 (330 mg/day) was well-tolerated in patients with advanced solid tumors. Compared to TTM, ATN-224 exhibited improved stability and a longer half-life, achieving a reduction in serum copper levels in 80% of patients within an average of 21 days (versus approximately 35 days for TTM). However, in addition to copper depletion-related anemia and neutropenia, ATN-224 also led to grade 3 fatigue after 14 days of treatment, which was dose-limiting and correlated with the dosage of ATN-224 and the rate of cp reduction.411 A phase II clinical trial on biochemically recurrent prostate cancer patients found that low-dose ATN-224 (30 mg/day) may exhibit biological activity.412 However, the clinical significance of prostate-specific antigen (PSA) dynamics in this population remains uncertain. Due to the lack of a clear dose-response effect, there are currently no plans for further development of ATN-224 for prostate cancer treatment.
D-pen is the first copper-chelating agent used for the treatment of WD and has been demonstrated to inhibit tumor proliferation and angiogenesis in preclinical models.413 For instance, D-pen inhibits LOX enzymatic activity by depleting copper levels, thereby suppressing angiogenesis and tumor progression in glioblastoma multiforme.414 Furthermore, D-pen enhances the cytotoxic effects of both radiotherapy and chemotherapeutic agents. Combining D-pen with oxaliplatin or cisplatin exhibits a synergistic cytotoxic effect on oxaliplatin-resistant cancer cells.415 The potential mechanism underlying this synergy is that D-pen upregulates the copper transport proteins hCtr1 and ATP7A, which share a transport system with platinum,416 thereby increasing intracellular platinum levels.415 Additionally, co-administration of D-pen with hydrogen peroxide metabolism inhibitors boosts the responsiveness of lung and breast cancer cells to radiation and carboplatin via H2O2-mediated oxidative stress.417 A Phase II clinical trial involving 40 patients with newly diagnosed glioblastoma treated with D-pen in conjunction with radiation therapy indicated that D-pen-induced copper deficiency was well tolerated. Drug-related myelosuppression, elevated liver function tests, and rashes were rapidly reversed with copper supplementation. Nevertheless, this anti-angiogenic strategy did not improve survival rates for glioblastoma patients compared to historical controls, with a median OS of 11.3 months and a median PFS of 7.1 months.418 Currently, a new clinical trial is underway to investigate the role of D-pen in enhancing radiosensitivity in recurrent head and neck cancer.
Trientine is an alternative copper-chelating agent clinically utilized for WD patients with D-pen intolerance.419–421 In preclinical tumor models, both Tientine and D-pen inhibited tumor progression; however, Tientine demonstrated a more significant inhibitory effect, primarily by suppressing angiogenesis and enhancing apoptosis within the tumors.422,423 Clinical trials of Trientine mainly focus on its application in platinum-resistant tumors,424–426 which re-sensitizes cancer cells to carboplatin by enhancing hCtr1-mediated platinum uptake.427 A preliminary clinical study involving five patients with platinum-resistant high-grade epithelial ovarian cancer receiving a combination of carboplatin and Trientine indicated that Trientine can partially reverse the resistance of cancer cells to platinum therapy, warranting further evaluation in larger studies.424 Another Phase I clinical trial that included 55 patients with advanced malignancies who had failed platinum treatment demonstrated that trientine, administered as a maximum daily divided dose of 3000 mg, can be safely combined with carboplatin FDA-approved doses (AUC 6), showing good tolerance.426 The longest duration of treatment recorded was 17 months, with no dose-limiting toxicities or treatment-related deaths observed. Compared to historical experiences with single-agent platinum therapy, no more severe adverse events were noted. Furthermore, patients with relatively low levels of hCtr1 expression may derive the greatest benefit from the combination treatment of carboplatin and trientine, suggesting a potential clinical prognostic implication.
TEPA has demonstrated tumor-suppressive effects in preclinical models of neuroblastoma.20 Specifically, TEPA inhibits the phosphorylation of STAT3 and EGFR, promotes the ubiquitin-mediated degradation of PD-L1, and significantly increases the infiltration of CD8 + T cells and natural killer cells, thereby enhancing antitumor immunity and slowing tumor growth.20 Additionally, TEPA has the potential to reduce radioresistance in HCC by chelating copper, functioning as a copper-dependent selective radiosensitizer.236 However, there are notable gaps in the clinical trial of TEPA for cancer therapy.
Nanomedicine delivery systems are designed to enhance cancer treatment by improving drug targeting and delivery efficiency while ensuring biocompatibility and biodegradability.428 These systems often utilize nanocarriers, such as liposomes, dendrimers, or polymeric nanoparticles, which can preferentially accumulate in tumor tissues via the enhanced permeability and retention (EPR) effect or by conjugating targeting ligands to specific tumor markers.429,430 Their biocompatibility minimizes toxicity to healthy tissues, while biodegradability allows for bodily clearance after therapeutic delivery. Advanced strategies, such as pH-responsive or enzyme-sensitive release mechanisms, optimize drug delivery by releasing therapeutic agents in response to the tumor’s unique microenvironment, thus maximizing efficacy and minimizing side effects.431 The clinical application of copper chelation in cancer treatment is limited by poor tumor-targeting capabilities, leading to associated toxicity or ineffectiveness. Therefore, nanoparticle-based delivery systems have been developed to optimize the therapeutic effects of small-molecule copper chelators. For instance, prostate-specific membrane antigen and glucose transporter GLUT1 targeting agents have been designed to deliver copper chelators specifically to prostate cancer and pancreatic cancer, respectively.432 Ismail M et al. successfully developed an actively targeted biomimetic nanoparticle (Ang-MNPs@(Dp44mT/Reg)) that can target and deliver the copper chelator Dp44mT to GBM lesions.433 These nanoparticles exhibit significantly enhanced active targeting capabilities and prolonged circulation time in the bloodstream, while markedly reducing drug-related side effects. This strategy not only effectively inhibits tumor growth and extends the survival of GBM model mice but also demonstrates negligible systemic toxicity, further validating its safety and potential for treating other central nervous system disorders.433 A mitochondria-targeted copper-depleting nanoparticle (CDN) demonstrates the ability to inhibit tumor growth and significantly extend survival in mouse models of TNBC.434 Compared to existing copper chelators, CDNs exhibit lower toxicity, as they preferentially deplete copper from the mitochondria of cancer cells rather than causing systemic copper depletion. Moreover, co-delivering copper chelators with other antitumor agents within the same nanoparticle carrier can harness the synergistic effects of different drugs to achieve multiple antitumor effects. For example, PTDH/R848 nanoparticles, which combine copper chelators with TLR7 and TLR8 agonists, can serve as a therapeutic agent for metastatic breast cancer by integrating anti-angiogenic and immune-activating mechanisms.435 Currently, various copper chelation-based nanomaterials have demonstrated antitumor effects and exhibit good biocompatibility. For instance, the novel adhesive injectable thermosensitive hydrogel loaded with small molecule copper chelator (SO-N)436 and the Imi-OSi nanocomplex with high selectivity and efficient copper-chelating capacity have shown promising results.437 However, no related drugs have yet entered clinical trials.
Copper ionophores
Copper ionophores also referred to as cuproptosis-related drugs, typically form neutral, lipophilic complexes with copper, thereby increasing intracellular copper concentrations.438 A variety of copper ionophores have been developed as anticancer agents to promote cuproptosis, with ES and DSF being among the most extensively researched. In addition, several antimicrobial agents, such as clioquinol (CQ), 8-hydroxyquinoline (8-OHQ), NSC319726, and pyrithione, also function as copper ionophores.439 These compounds are instrumental in advancing the application of cuproptosis as a therapeutic strategy against cancer.
ES is a mitochondrion-targeting copper ionophore for cancer therapy.283,440,441 Previous studies have demonstrated that ES can induce copper-dependent apoptosis and ferroptosis by promoting the generation of ROS and facilitating the degradation of ATP7A, respectively.22,282,442 The discovery of cuproptosis has refined the specific cancer suppressor mechanism of ES, while enhanced mitochondrial metabolism increases the sensitivity of cancer cells to this agent.26 ES was initially developed as an adjunct chemotherapy agent for treating metastatic melanoma.440 Subsequently, sodium salt formulations of ES were developed for clinical trials, which could be used in combination with paclitaxel or as a monotherapy for various solid tumors and acute myeloid leukemia.441,443–445 Published data indicate that ES has a favorable safety profile but failed to show a favorable clinical response. A Phase I clinical trial involving patients with refractory solid tumors showed that the combination of ES and paclitaxel was well tolerated without increasing toxicity.444 In a Phase II trial conducted in patients with stage IV melanoma, the results indicated that the combination of paclitaxel and ES doubled the median PFS from 56 days to 112 days compared to paclitaxel alone, and reduced the risk of disease progression or death by 41.7%, extending the median OS from 7.8 months to 11.9 months.441 However, a large randomized double-blind Phase III trial involving 651 patients with advanced melanoma found that the combination of paclitaxel and ES did not achieve its primary endpoint of PFS.446 Interestingly, a post-hoc analysis indicated that baseline serum lactate dehydrogenase (LDH) levels could be a potential predictive factor for treatment response. In patients with low serum LDH levels, combination therapy improved median PFS by 1.6 months.446 Additionally, another Phase II clinical evaluation of the combination of ES and weekly paclitaxel for the treatment of recurrent or persistent platinum-resistant ovarian cancer, tubal cancer, or primary peritoneal cancer demonstrated good tolerability.443 However, the response rate was insufficient to support further research into this combination therapy.443
DSF, an FDA-approved aldehyde dehydrogenase (ALDH) inhibitor, exhibits multi-targeted anti-tumor activity across various cancer cell lines, making it a widely used anticancer agent.447 The toxicity of DSF is closely linked to its ability to promote intracellular accumulation of copper, significantly enhancing its anticancer efficacy when administered in conjunction with copper. The DSF/Cu complex targets multiple pathways, including inhibition of NF-κB, elevation of ROS levels, modulation of Npl4 aggregation, as well as induction of apoptosis, ferroptosis, and cuproptosis.305,447–449 Additionally, DSF possesses cytotoxic effects on ALDH-positive cancer stem cells which possess rapid self-renewal capabilities and strong tumorigenic potential and exhibit resistance to chemotherapy and radiotherapy.450–452 In preclinical models, the combination of DSF/Cu demonstrates potential synergistic effects when used in conjunction with established chemotherapy agents such as cisplatin, temozolomide (TMZ), gemcitabine, and doxorubicin.453–455 A Phase IIb multicenter, randomized, double-blind study involving 40 newly diagnosed NSCLC patients found that the addition of DSF to the combination therapy of cisplatin and vinorelbine was well-tolerated and prolonged median survival time from 7.1 to 10 months.456 Notably, two long-term survivors emerged in the DSF group.456 However, a Phase II clinical study involving 12 patients with recurrent and/or refractory germ cell tumors failed to achieve its primary endpoint.457 DSF showed limited activity in restoring cisplatin sensitivity, with only 2 patients achieving disease stabilization. The median PFS and OS were 1.4 months and 2.9 months, respectively. Current clinical trials suggest that DSF and DSF/Cu-based therapy have limited efficacy in cancer treatment. In a Phase I trial involving advanced solid tumor patients, participants received 250 mg of DSF with escalating doses of copper gluconate (2, 4, 6, or 8 mg of elemental copper), showing good overall tolerability.458 While five cases of grade 3 toxicity were observed (including anorexia, increased serum aspartate aminotransferase, increased serum alkaline phosphatase, fever, and fatigue), no dose-limiting toxicities were reported.458 However, a Phase II-III study with 88 patients suffering from recurrent glioblastoma, receiving 400 mg of DSF and 2.5 mg of copper daily in combination with alkylating chemotherapy, did not significantly improve the 6-month survival rate or median PFS.459 Additionally, the incidence of adverse events was notably higher in patients receiving the combination therapy compared to those on alkylating chemotherapy alone.459
Antimicrobial drugs inhibit microbial growth by increasing intracellular copper ion concentrations and can also function as copper ionophores to suppress cancer. CQ is an analog of 8-OHQ, both of which can induce apoptosis in tumor cells.460,461 Treatment with CQ in human tumor xenografts with high copper content also leads to cancer suppression, linked to proteasome inhibition in vivo. Further studies indicate that the proteasome inhibitory and growth suppressive effects of CQ and 8-OHQ on tumor cells require their ability to bind copper and facilitate its transport into cells.462 Additionally, CQ (at a concentration of 50 μM) induces the oxidation of the copper chaperone ATOX1, indicating its inactivation and subsequent impairment of copper transport. However, the inactivation of ATOX1, leading to disrupted cellular copper transport, is one of the mechanisms underlying the subacute bone marrow optic neuropathy neurotoxicity associated with CQ.463 Currently, the use of antimicrobial agents in the clinical treatment of cancer remains highly limited.
Cuproptosis, a novel copper-dependent mechanism of cell death, has garnered significant attention for its potent tumor-suppressive properties. However, its clinical application is limited by the low sensitivity of tumor cells, which can be attributed to several factors:326,333 insufficient copper ion concentration within mitochondria, high levels of GSH that chelate copper ions and inhibit copper-protein interactions, and the rapid clearance of copper ionophores like ES and DSF, which restrict copper uptake by tumor cells. Furthermore, relying solely on cuproptosis may not effectively control tumor growth. To overcome these challenges, nanomedicine delivery systems have emerged as a promising strategy to enhance copper accumulation and release at tumor sites, optimizing the therapeutic effects of cuproptosis.464 Research is focusing on synergistic cancer therapies that combine cuproptosis with various anti-tumor modalities, including chemotherapy,465 photodynamic therapy (PDT),466 photothermal therapy,467 immunotherapy,326 and gene therapy.468 For example, a study by Zhou et al. engineered a nanocarrier system (Au@MSN-Cu/PEG/DSF) for photothermally activated drug delivery, which utilizes the EPR effect to concentrate at tumor sites.467 Upon near-infrared laser exposure, localized heat triggers the release of copper (II), forming a cytotoxic complex that induces cuproptosis in tumors. Similarly, Xu et al. developed a nanoporous copper(I) 1,2,4-triazolate coordination polymer nanocarrier (GOx@[Cu(tz)]) that synchronizes therapies via cuproptosis, PDT, and starvation treatments by depleting glucose and GSH, thus enhancing tumor cell sensitivity.466 Additionally, Guo et al. created a ROS-sensitive polymer (PHPM) for co-encapsulation of ES and copper into nanoparticles (NP@ESCu), which, upon internalization, release these agents in response to elevated intracellular ROS. This approach not only induces cuproptosis but also increases PD-L1 expression, transforming “cold” tumors into “hot” tumors that respond to immunotherapy, significantly inhibiting tumor growth and activating a systemic anti-tumor immune response.326 Related therapeutic agents include T-HCN@CuMS,469 Cel-Cu NP,379 ES@CuO,327 Ce6@Cu NPs,470 ES-Cu-MOF,471 Cu@CDCN,472 mCGYL-LOx,337 AuTPyP,333 PDA-DTC/Cu,473 Cu-THBQ/AX,383 MACuS,474 CuO NPs,475 ZCA NSs,476 Cu2-xSe@cMOF,477 CSTD-Cu(II)@DSF,465and CuPEs@PApt,478 among others. Despite the lack of clinical trials, these findings highlight the promising clinical prospects of combining cuproptosis with various treatment modalities in cancer therapy.
Detection of cuproptosis
Cuproptosis is a newly identified form of regulated cell death, yet biomarkers for its detection remain incompletely defined. In this context, we outline four key aspects for detecting cuproptosis based on existing literature and identify several biomarkers associated with this process. Currently, these biomarkers are primarily utilized to detect cuproptosis in cell lines or animal models, but they hold significant potential for advancing research in the field.
Assessment of key cuproptosis-related genes or proteins
Cuproptosis is characterized by the oligomerization of DLAT, the decrease of Fe-S cluster proteins, and an increase in heat shock proteins, which can serve as indicators of cuproptosis. Two commonly employed methods for detecting DLAT oligomerization include non-reducing immunoblotting and confocal immunofluorescence imaging.26,327,331,466,479–481 Immunoblotting, immunofluorescence, and immunohistochemistry are typically used to analyze the expression of Fe-S cluster proteins (such as FDX1, LIAS, ACO2, ETFDH, NDUFV1, and NDUFS8) and heat shock proteins (HSP70).326,482 Notably, FDX1 is known to regulate the lipoylation of cellular proteins through direct interactions with LIAS.298 Consequently, the loss of FDX1 during cuproptosis is often accompanied by a reduction in the lipoylation of four mitochondrial enzymes: DBT, GCSH, DLST, and DLAT,300,301 among which lipoylated DLST and DLAT are also commonly detected via immunoblotting during cuproptosis. It is crucial to acknowledge that changes in the expression of these cuproptosis-related proteins are not specific to cuproptosis, as they are subject to complex transcriptional regulation and can be influenced by various factors, including cellular stress, signaling pathways, and genetic background. Therefore, while assessing these key proteins is essential, it is insufficient for researchers to definitively evaluate the occurrence of cuproptosis.
Examination of the ultrastructure of subcellular organelles
Morphologically, cuproptotic cells exhibit mitochondrial shrinkage, cell membrane rupture, endoplasmic reticulum damage, and chromatin fragmentation.483,484 These alterations are critical for identifying cuproptosis within cellular contexts. Transmission electron microscopy (TEM) plays a vital role in elucidating changes in these subcellular organelles. For instance, Zhao et al. utilized TEM to demonstrate that the treatment of zebrafish embryos with copper nanoparticles and CuSO4 resulted in a reduction of mitochondrial inner membranes and a loosening of the endoplasmic reticulum structure.200 Similarly, Liao et al. observed that excess copper led to mitochondrial vacuolization, membrane destruction, and chromatin rupture in chicken liver cells.213 Further investigations using Bio-TEM revealed shrunken mitochondria with reduced or absent cristae and increased membrane density, confirming copper-induced mitochondrial toxicity.338 These findings suggest that cuproptotic cells are characterized by distinct ultrastructural changes in subcellular organelles, including mitochondria, endoplasmic reticulum, and nucleus. However, it is important to recognize that these morphological features may also overlap with other forms of cellular stress or death, particularly apoptosis.200,213,215 Therefore, while examining the ultrastructure of subcellular organelles can provide preliminary evidence for cuproptosis, additional confirmatory assays are necessary to specifically attribute these changes to cuproptosis.
Copper levels assessment
Excess copper in the cytoplasm and subcellular organelles serves as a key inducer of cuproptosis. Thus, precise quantification and distribution mapping of copper in mitochondria, endoplasmic reticulum, nuclei, and cytoplasm are essential for researchers evaluating cuproptosis. Various techniques, including copper-specific fluorescent probes, colorimetric tests, and inductively coupled plasma mass spectrometry (ICP-MS), can be employed to measure copper levels.485–488 ICP-MS offers high sensitivity and accuracy in detecting trace amounts of copper ions, enabling researchers to identify subtle fluctuations in copper levels that may impact cellular metabolism and function.489 In colorimetric assays, copper ions in the sample react with a complexing agent to form a purple complex, allowing for indirect calculation of copper ion content.490 Given the well-established role of copper in mitochondria, copper-specific fluorescent probes and mito-tracker (a mitochondrial fluorescent probe) are commonly used to explore the relationship between copper and mitochondria.338,385,491 By employing mito-tracker alongside copper-specific fluorescent probes, researchers can effectively visualize the colocalization of copper ions within the mitochondrial matrix using fluorescence microscopy. In certain instances, researchers may also estimate copper levels indirectly by assessing the expression of copper importers (SLC31A1, also known as CTR1) and copper exporters (ATP7A and ATP7B),114 or by utilizing copper chelators such as tetrathiomolybdate or bathocuproine disulfonic acid to mitigate cuproptosis.
Metabolic biomarker assessment
During cuproptosis, copper does not directly target the electron transport chain but rather affects components of the TCA cycle, making its metabolites critical indicators of this cell death process.26 Tsvetkov et al. demonstrated changes in various metabolites, including elevated levels of citrate, cis-aconitate, GDP, and sedoheptulose-7-P, alongside decreased levels of glutamate, α-ketoglutarate, succinate, fumarate, malate, and C5-Carnitine.26 The reduction in α-ketoglutarate and succinate partially contributes to the decreased expression of FDX1, whereas the implications of other metabolite changes and their predictive value for cuproptosis warrant further investigation. These metabolites can be quantitatively assessed using techniques such as liquid chromatography coupled with mass spectrometry or NMR spectroscopy for comprehensive metabolite profiling.
Conclusion and perspectives
Copper functions as a double-edged sword within cells: it is an essential cofactor for numerous enzymes that facilitate tumor progression, yet its excess can lead to cuproptosis, resulting in cellular demise. In recent years, pharmacologically targeting copper and cuproptosis has emerged as a promising anticancer strategy. Therefore, exploring the foundational mechanistic aspects related to copper and cuproptosis could provide valuable treatment avenues for cancer. However, several challenges must be addressed in future research.
Firstly, monitoring copper levels within tumors and identifying biomarkers of cuproptosis in patients present significant challenges. Despite advancements in analytical techniques,492 including copper isotope measurement493 and chemical fluorescent probes,494 their application in cuproptosis-related research remains largely confined to cell lines or in vitro tissues. Progress in imaging technologies, such as copper-specific imaging agents,495,496 PET scans,497–499 and nanoprobes,465 could facilitate non-invasive monitoring of copper distribution in vivo. However, their sensitivity warrants improvement, and their safety in patients needs further clarification. Moreover, although cuproptotic cells are characterized by mitochondrial shrinkage, cell membrane rupture, endoplasmic reticulum damage, and chromatin fragmentation, these morphological changes overlap with those seen in apoptosis,200,213,215 complicating the issue. Thus, developing robust assays and biomarkers, along with standardized experimental protocols and methodologies, to accurately measure copper levels and assess cuproptosis in patients is imperative.
Secondly, the precise molecular mechanisms underlying cuproptosis remain poorly understood, despite its strong association with mitochondrial metabolic states. For instance, the mechanism by which mitochondrial respiration accelerates cuproptosis is unclear. One hypothesis posits that cells with active mitochondrial respiration may express higher levels of lipoylated enzymes, potentially leading to increased aggregation.500 However, there is currently no evidence indicating that tumor stem-like cells or certain drug-resistant cells with enhanced mitochondrial respiration exhibit elevated levels of lipoylated enzymes. Additionally, it remains uncertain why cell death caused by excess copper results in cuproptosis rather than established forms of cell death such as apoptosis or necroptosis. Further research is necessary to determine whether excess copper inhibits enzymes critical for these well-known cell death pathways. Furthermore, while mitochondria are known to play a crucial role in cuproptosis, it is uncertain if they are strictly required for the process or if cuproptosis can occur in mitochondria-depleted cells.
Thirdly, investigating the occurrence of cuproptosis under normal physiological conditions presents another challenge. Cuproptosis has been documented in several pathological conditions beyond Wilson’s disease, Menkes disease, and cancer. For example, Chen et al. demonstrated that cuproptosis occurs following myocardial ischemia-reperfusion injury, exacerbated by sleep fragmentation.480 Yang et al. reported that high glucose levels induce cuproptosis in human lens epithelial cells, which can be reversed by copper chelation.501 Additionally, SARS-CoV-2 infection has been found to reduce glutamine levels, leading to decreased glutathione levels, copper overload, and increased cuproptosis in immune cells, potentially exacerbating rapid tumor development.502 These findings underscore the prevalence of cuproptosis in various pathological conditions. Similarly, ferroptosis has been initially identified as contributing to the development of numerous diseases, including cancer, neurodegeneration, sepsis, ischemia-reperfusion injury, autoimmune disorders, and metabolic disorders.503 With the deepening of research, ferroptosis is identified to play a significant role under normal physiological conditions such as embryonic development and aging.504 Thus, it is speculated that cuproptosis must also occur under several normal physiological conditions, requiring further investigations.
Fourthly, despite numerous studies analyzing the connections between CRGs and various tumor characteristics, the lack of biological evidence and experimental validation presents an additional challenge. Many studies only indirectly demonstrate a link between cuproptosis and cancer,363,447,505–507 leaving unclear whether these genes play a direct role in this relationship or if they are influenced indirectly. Another unresolved question is whether other metabolic pathways, in addition to mitochondrial respiration, participate in cuproptosis. Additionally, while CTR1 and DMT1 are known receptors for copper uptake, recent work by Li et al. suggests that ZnT1, which exports zinc from cells, may also be involved in copper uptake and cuproptosis.297 This raises the possibility that other copper transporters mediating copper uptake or efflux warrant further investigation.
Lastly, it is crucial to identify patient groups likely to benefit from cuproptosis-based therapies, as certain cancers characterized by higher mitochondrial respiration, stem cell-like traits, or drug resistance have demonstrated better responsiveness to such treatments.80 A previous phase III clinical trial of ES in melanoma patients showed no clinical benefit;446 however, a post-hoc analysis indicated that patients with low plasma LDH levels may benefit from ES treatment, suggesting that LDH could serve as an important biomarker for screening patients who may respond favorably to copper-based therapy. A possible explanation for this is that lower LDH levels reflect a higher cellular dependency on mitochondrial respiration. Nevertheless, there is an urgent need to identify more reliable biomarkers to effectively screen potential beneficiaries. Furthermore, inhibitors of the proteasome or Wnt signaling pathways might synergize with copper ionophores in tumor therapy.293,296 Numerous studies suggest that cuproptosis-based therapies have the potential to enhance the efficacy of immunotherapies.324,327,334,379–388 Therefore, identifying effective combinational strategies for cuproptosis-based interventions is essential for future applications.
In summary, this review enhances our understanding of the molecular mechanisms and therapeutic landscape of copper and cuproptosis in cancer. Addressing the aforementioned challenges will not only deepen our comprehension of its role in cancer biology but also pave the way for the development of copper and cuproptosis-based therapies. We anticipate that intensive research efforts will facilitate the translation of the concept of cuproptosis from basic science into therapeutic reality.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Nos. 82272849 to G.D., 82403139 to L.Y.), Huxiang Youth Talent Program (Grant Nos. 2023RC3072 to G.D., 2024RC3043 to F.Z.), Natural Science Fund for Outstanding Youths in Hunan Province (Grant No. 2023JJ20093 to G.D.), Postdoctoral Fellowship Program of CPSF (Grant No. GZC20242053 to L.Y.), Changsha Municipal Natural Science Foundation (Grant No. kq243010 to L.Y.), and Xiangya Hospital Youth Fund (Grant No. 2023Q17 to L.Y.). We thank Biorender (https://www.biorender.com/) for the assistance for the illustration.
Author contributions
G.D., X.C., and F.Z. designed the review; Z.G., D.C., and L.Y. searched for literature and wrote the manuscript; Z.G., L.Y., and J.L., drew the figures; Y.S., D.L., and Y.D., helped edit and revise the manuscript. G.D., and F.Z., provided funding support. All authors have read and approved the article and agree with publication in this journal.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Ziyu Guo, Danyao Chen, Lei Yao.
Contributor Information
Furong Zeng, Email: zengfurong@csu.edu.cn.
Xiang Chen, Email: chenxiangck@126.com.
Guangtong Deng, Email: dengguangtong@csu.edu.cn.
References
- 1.Guan, D. et al. Copper in cancer: from pathogenesis to therapy. Biomed. Pharmacother.163, 114791 (2023). [DOI] [PubMed] [Google Scholar]
- 2.Garber, K. Cancer’s copper connections. Science349, 129 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Wang, Y. et al. Copper in colorectal cancer: from copper-related mechanisms to clinical cancer therapies. Clin. Transl. Med.14, e1724 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Festa, R. A. & Thiele, D. J. Copper: an essential metal in biology. Curr. Biol.21, R877–R883 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Linder, M. C. Copper homeostasis in mammals, with emphasis on secretion and excretion. a review. Int. J. Mol. Sci. 21, (2020). [DOI] [PMC free article] [PubMed]
- 6.Wang, X., Zhou, M., Liu, Y. & Si, Z. Cope with copper: from copper linked mechanisms to copper-based clinical cancer therapies. Cancer Lett.561, 216157 (2023). [DOI] [PubMed] [Google Scholar]
- 7.Tian, Z., Jiang, S., Zhou, J. & Zhang, W. Copper homeostasis and cuproptosis in mitochondria. Life Sci.334, 122223 (2023). [DOI] [PubMed] [Google Scholar]
- 8.An, Y. et al. The role of copper homeostasis in brain disease. Int. J. Mol. Sci. 23, (2022). [DOI] [PMC free article] [PubMed]
- 9.Chen, L., Min, J. & Wang, F. Copper homeostasis and cuproptosis in health and disease. Signal Transduct. Target Ther.7, 378 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim, B. E., Nevitt, T. & Thiele, D. J. Mechanisms for copper acquisition, distribution and regulation. Nat. Chem. Biol.4, 176–185 (2008). [DOI] [PubMed] [Google Scholar]
- 11.Li, Y. Copper homeostasis: emerging target for cancer treatment. IUBMB Life72, 1900–1908 (2020). [DOI] [PubMed] [Google Scholar]
- 12.Vulpe, C. D. & Packman, S. Cellular copper transport. Annu. Rev. Nutr.15, 293–322 (1995). [DOI] [PubMed] [Google Scholar]
- 13.Harris, E. D. Cellular copper transport and metabolism. Annu. Rev. Nutr.20, 291–310 (2000). [DOI] [PubMed] [Google Scholar]
- 14.Lutsenko, S. Dynamic and cell-specific transport networks for intracellular copper ions. J. Cell Sci. 134, (2021). [DOI] [PMC free article] [PubMed]
- 15.Wu, Z. et al. Copper in hepatocellular carcinoma: a double-edged sword with therapeutic potentials. Cancer Lett.571, 216348 (2023). [DOI] [PubMed] [Google Scholar]
- 16.Xue, Q. et al. Copper metabolism in cell death and autophagy. Autophagy19, 2175–2195 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ge, E. J. et al. Connecting copper and cancer: from transition metal signalling to metalloplasia. Nat. Rev. Cancer22, 102–113 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kong, R. & Sun, G. Targeting copper metabolism: a promising strategy for cancer treatment. Front. Pharm.14, 1203447 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Das, A. et al. Cysteine oxidation of copper transporter CTR1 drives VEGFR2 signalling and angiogenesis. Nat. Cell Biol.24, 35–50 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Voli, F. et al. Intratumoral copper modulates PD-L1 expression and influences tumor immune evasion. Cancer Res.80, 4129–4144 (2020). [DOI] [PubMed] [Google Scholar]
- 21.Zhou, Q. et al. Upregulation of postsynaptic cAMP/PKA/CREB signaling alleviates copper(II)-induced oxidative stress and pyroptosis in MN9D cells. Toxicology494, 153582 (2023). [DOI] [PubMed] [Google Scholar]
- 22.Xue, Q. et al. Copper-dependent autophagic degradation of GPX4 drives ferroptosis. Autophagy19, 1982–1996 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang, Y. et al. Copper-induced tumor cell death mechanisms and antitumor theragnostic applications of copper complexes. Nanomedicine17, 303–324 (2022). [DOI] [PubMed] [Google Scholar]
- 24.Tsui, K. H. et al. The cross-communication of cuproptosis and regulated cell death in human pathophysiology. Int. J. Biol. Sci.20, 218–230 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao, D. et al. Copper exposure induces inflammation and PANoptosis through the TLR4/NF-κB signaling pathway, leading to testicular damage and impaired spermatogenesis in Wilson disease. Chem. Biol. Interact.396, 111060 (2024). [DOI] [PubMed] [Google Scholar]
- 26.Tsvetkov, P. et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science375, 1254–1261 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turski, M. L. & Thiele, D. J. New roles for copper metabolism in cell proliferation, signaling, and disease. J. Biol. Chem.284, 717–721 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zischka, H. & Einer, C. Mitochondrial copper homeostasis and its derailment in Wilson disease. Int. J. Biochem. Cell Biol.102, 71–75 (2018). [DOI] [PubMed] [Google Scholar]
- 29.Bandmann, O., Weiss, K. H. & Kaler, S. G. Wilson’s disease and other neurological copper disorders. Lancet Neurol.14, 103–113 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bertini, I. & Rosato, A. Menkes disease. Cell Mol. Life Sci.65, 89–91 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guthrie, L. M. et al. Elesclomol alleviates Menkes pathology and mortality by escorting Cu to cuproenzymes in mice. Science368, 620–625 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gromadzka, G., Tarnacka, B., Flaga, A. & Adamczyk, A. Copper dyshomeostasis in neurodegenerative diseases-therapeutic implications. Int. J. Mol. Sci. 21 (2020). [DOI] [PMC free article] [PubMed]
- 33.Yang, L., Yang, P., Lip, G. Y. H. & Ren, J. Copper homeostasis and cuproptosis in cardiovascular disease therapeutics. Trends Pharm. Sci.44, 573–585 (2023). [DOI] [PubMed] [Google Scholar]
- 34.Parsanathan, R. Copper’s dual role: unravelling the link between copper homeostasis, cuproptosis, and cardiovascular diseases. Hypertens. Res.47, 1440–1442 (2024). [DOI] [PubMed] [Google Scholar]
- 35.da Silva, D. A. et al. Copper in tumors and the use of copper-based compounds in cancer treatment. J. Inorg. Biochem.226, 111634 (2022). [DOI] [PubMed] [Google Scholar]
- 36.Chen, J. et al. The molecular mechanisms of copper metabolism and its roles in human diseases. Pflug. Arch.472, 1415–1429 (2020). [DOI] [PubMed] [Google Scholar]
- 37.Vetchý, M. Biological role of copper as an essential trace element in the human organism. Ceska Slov. Farm67, 143–153 (2018). [PubMed] [Google Scholar]
- 38.Tsang, T., Davis, C. I. & Brady, D. C. Copper biology. Curr. Biol.31, R421–r427 (2021). [DOI] [PubMed] [Google Scholar]
- 39.Shi, H. et al. Copper metabolism in Saccharomyces cerevisiae: an update. Biometals34, 3–14 (2021). [DOI] [PubMed] [Google Scholar]
- 40.Harris, E. D. Copper homeostasis: the role of cellular transporters. Nutr. Rev.59, 281–285 (2001). [DOI] [PubMed] [Google Scholar]
- 41.Chambers, A. et al. An exposure-response curve for copper excess and deficiency. J. Toxicol. Environ. Health B Crit. Rev.13, 546–578 (2010). [DOI] [PubMed] [Google Scholar]
- 42.Burkhead, J. L. et al. Copper homeostasis. N. Phytol.182, 799–816 (2009). [DOI] [PubMed] [Google Scholar]
- 43.Focarelli, F., Giachino, A. & Waldron, K. J. Copper microenvironments in the human body define patterns of copper adaptation in pathogenic bacteria. PLoS Pathog.18, e1010617 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen, X. et al. Copper homeostasis and copper-induced cell death in the pathogenesis of cardiovascular disease and therapeutic strategies. Cell Death Dis.14, 105 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bost, M. et al. Dietary copper and human health: current evidence and unresolved issues. J. Trace Elem. Med. Biol.35, 107–115 (2016). [DOI] [PubMed] [Google Scholar]
- 46.Institute of Medicine Panel on, M. in Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc (National Academies Press (US) Copyright 2001 by the National Academy of Sciences. All rights reserved, 2001). [PubMed]
- 47.Mason, K. E. A conspectus of research on copper metabolism and requirements of man. J. Nutr.109, 1979–2066 (1979). [DOI] [PubMed] [Google Scholar]
- 48.Pezacki, A. T. et al. Oxidation state-specific fluorescent copper sensors reveal oncogene-driven redox changes that regulate labile copper(II) pools. Proc. Natl. Acad. Sci. USA119, e2202736119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garza, N. M. et al. Mitochondrial copper in human genetic disorders. Trends Endocrinol. Metab.34, 21–33 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wee, N. K., Weinstein, D. C., Fraser, S. T. & Assinder, S. J. The mammalian copper transporters CTR1 and CTR2 and their roles in development and disease. Int. J. Biochem. Cell Biol.45, 960–963 (2013). [DOI] [PubMed] [Google Scholar]
- 51.Rees, E. M., Lee, J. & Thiele, D. J. Mobilization of intracellular copper stores by the ctr2 vacuolar copper transporter. J. Biol. Chem.279, 54221–54229 (2004). [DOI] [PubMed] [Google Scholar]
- 52.Arredondo, M., Muñoz, P., Mura, C. V. & Nùñez, M. T. DMT1, a physiologically relevant apical Cu1+ transporter of intestinal cells. Am. J. Physiol. Cell Physiol.284, C1525–C1530 (2003). [DOI] [PubMed] [Google Scholar]
- 53.Tang, D., Kroemer, G. & Kang, R. Targeting cuproplasia and cuproptosis in cancer. Nat. Rev. Clin. Oncol.21, 370–388 (2024). [DOI] [PubMed] [Google Scholar]
- 54.Lönnerdal, B. Intestinal regulation of copper homeostasis: a developmental perspective. Am. J. Clin. Nutr.88, 846s–850s (2008). [DOI] [PubMed] [Google Scholar]
- 55.Cabrera, A. et al. Copper binding components of blood plasma and organs, and their responses to influx of large doses of (65)Cu, in the mouse. Biometals21, 525–543 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kirsipuu, T. et al. Copper(II)-binding equilibria in human blood. Sci. Rep.10, 5686 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xie, J., Yang, Y., Gao, Y. & He, J. Cuproptosis: mechanisms and links with cancers. Mol. Cancer22, 46 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Calvo, J., Jung, H. & Meloni, G. Copper metallothioneins. IUBMB Life69, 236–245 (2017). [DOI] [PubMed] [Google Scholar]
- 59.La Fontaine, S., Ackland, M. L. & Mercer, J. F. Mammalian copper-transporting P-type ATPases, ATP7A and ATP7B: emerging roles. Int. J. Biochem. Cell Biol.42, 206–209 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.La Fontaine, S. & Mercer, J. F. Trafficking of the copper-ATPases, ATP7A and ATP7B: role in copper homeostasis. Arch. Biochem. Biophys.463, 149–167 (2007). [DOI] [PubMed] [Google Scholar]
- 61.Rondanelli, M. et al. Copper as dietary supplement for bone metabolism: a review. Nutrients. 13, (2021). [DOI] [PMC free article] [PubMed]
- 62.Rees, E. M. & Thiele, D. J. From aging to virulence: forging connections through the study of copper homeostasis in eukaryotic microorganisms. Curr. Opin. Microbiol.7, 175–184 (2004). [DOI] [PubMed] [Google Scholar]
- 63.Kaplan, J. H. & Maryon, E. B. How mammalian cells acquire copper: an essential but potentially toxic metal. Biophys. J.110, 7–13 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rodríguez, C. & Martínez-González, J. The role of lysyl oxidase enzymes in cardiac function and remodeling. Cells. 8, (2019). [DOI] [PMC free article] [PubMed]
- 65.Gaier, E. D., Eipper, B. A. & Mains, R. E. Copper signaling in the mammalian nervous system: synaptic effects. J. Neurosci. Res91, 2–19 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aggett, P. J. An overview of the metabolism of copper. Eur. J. Med. Res.4, 214–216 (1999). [PubMed] [Google Scholar]
- 67.Turnlund, J. R., Keyes, W. R., Anderson, H. L. & Acord, L. L. Copper absorption and retention in young men at three levels of dietary copper by use of the stable isotope 65Cu. Am. J. Clin. Nutr.49, 870–878 (1989). [DOI] [PubMed] [Google Scholar]
- 68.Ackerman, C. M., Lee, S. & Chang, C. J. Analytical methods for imaging metals in biology: from transition metal metabolism to transition metal signaling. Anal. Chem.89, 22–41 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wong, P. C. et al. Copper chaperone for superoxide dismutase is essential to activate mammalian Cu/Zn superoxide dismutase. Proc. Natl. Acad. Sci. USA97, 2886–2891 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Robinson, N. J. & Winge, D. R. Copper metallochaperones. Annu. Rev. Biochem79, 537–562 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miao, L. & St Clair, D. K. Regulation of superoxide dismutase genes: implications in disease. Free Radic. Biol. Med.47, 344–356 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sturtz, L. A. et al. A fraction of yeast Cu,Zn-superoxide dismutase and its metallochaperone, CCS, localize to the intermembrane space of mitochondria. A physiological role for SOD1 in guarding against mitochondrial oxidative damage. J. Biol. Chem.276, 38084–38089 (2001). [DOI] [PubMed] [Google Scholar]
- 73.Okado-Matsumoto, A. & Fridovich, I. Subcellular distribution of superoxide dismutases (SOD) in rat liver: Cu, Zn-SOD in mitochondria. J. Biol. Chem.276, 38388–38393 (2001). [DOI] [PubMed] [Google Scholar]
- 74.Furukawa, Y., Torres, A. S. & O’Halloran, T. V. Oxygen-induced maturation of SOD1: a key role for disulfide formation by the copper chaperone CCS. Embo J.23, 2872–2881 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lorenzo-Gutiérrez, D. et al. Role of the Fusarium oxysporum metallothionein Mt1 in resistance to metal toxicity and virulence. Metallomics11, 1230–1240 (2019). [DOI] [PubMed] [Google Scholar]
- 76.McArdle, H. J., Mercer, J. F., Sargeson, A. M. & Danks, D. M. Effects of cellular copper content on copper uptake and metallothionein and ceruloplasmin mRNA levels in mouse hepatocytes. J. Nutr.120, 1370–1375 (1990). [DOI] [PubMed] [Google Scholar]
- 77.Fürst, P., Hu, S., Hackett, R. & Hamer, D. Copper activates metallothionein gene transcription by altering the conformation of a specific DNA binding protein. Cell55, 705–717 (1988). [DOI] [PubMed] [Google Scholar]
- 78.Cobine, P. A., Moore, S. A. & Leary, S. C. Getting out what you put in: copper in mitochondria and its impacts on human disease. Biochim. Biophys. Acta Mol. Cell Res.1868, 118867 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Baker, Z. N., Cobine, P. A. & Leary, S. C. The mitochondrion: a central architect of copper homeostasis. Metallomics9, 1501–1512 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang, Y. et al. Cuproptosis: a novel therapeutic target for overcoming cancer drug resistance. Drug Resist. Updat.72, 101018 (2024). [DOI] [PubMed] [Google Scholar]
- 81.Li, L. et al. Cuproptosis/OXPHOS tendency prediction of prognosis and immune microenvironment of esophageal squamous cell carcinoma: bioinformatics analysis and experimental validation. Gene902, 148156 (2024). [DOI] [PubMed] [Google Scholar]
- 82.Chojnacka, M. et al. Cox17 protein is an auxiliary factor involved in the control of the mitochondrial contact site and cristae organizing system. J. Biol. Chem.290, 15304–15312 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Horng, Y. C. et al. Specific copper transfer from the Cox17 metallochaperone to both Sco1 and Cox11 in the assembly of yeast cytochrome C oxidase. J. Biol. Chem.279, 35334–35340 (2004). [DOI] [PubMed] [Google Scholar]
- 84.Takahashi, Y. et al. Mammalian copper chaperone Cox17p has an essential role in activation of cytochrome C oxidase and embryonic development. Mol. Cell Biol.22, 7614–7621 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li, J. & Wang, Y. Golgi metal ion homeostasis in human health and diseases. Cells. 11, (2022). [DOI] [PMC free article] [PubMed]
- 86.Hartwig, C. et al. Golgi-dependent copper homeostasis sustains synaptic development and mitochondrial content. J. Neurosci.41, 215–233 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim, H. W. et al. Human macrophage ATP7A is localized in the trans-Golgi apparatus, controls intracellular copper levels, and mediates macrophage responses to dermal wounds. Inflammation35, 167–175 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Palmgren, M. G. & Nissen, P. P-type ATPases. Annu Rev. Biophys.40, 243–266 (2011). [DOI] [PubMed] [Google Scholar]
- 89.Hasan, N. M. et al. Molecular events initiating exit of a copper-transporting ATPase ATP7B from the trans-Golgi network. J. Biol. Chem.287, 36041–36050 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Petris, M. J. et al. Ligand-regulated transport of the Menkes copper P-type ATPase efflux pump from the Golgi apparatus to the plasma membrane: a novel mechanism of regulated trafficking. EMBO J.15, 6084–6095 (1996). [PMC free article] [PubMed] [Google Scholar]
- 91.Polishchuk, R. & Lutsenko, S. Golgi in copper homeostasis: a view from the membrane trafficking field. Histochem. Cell Biol.140, 285–295 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Petris, M. J., Strausak, D. & Mercer, J. F. The Menkes copper transporter is required for the activation of tyrosinase. Hum. Mol. Genet.9, 2845–2851 (2000). [DOI] [PubMed] [Google Scholar]
- 93.Sudhahar, V. et al. Caveolin-1 stabilizes ATP7A, a copper transporter for extracellular SOD, in vascular tissue to maintain endothelial function. Am. J. Physiol. Cell Physiol.319, C933–c944 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shanbhag, V. et al. ATP7A delivers copper to the lysyl oxidase family of enzymes and promotes tumorigenesis and metastasis. Proc. Natl. Acad. Sci. USA116, 6836–6841 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang, L., Ge, Y. & Kang, Y. J. Featured article: effect of copper on nuclear translocation of copper chaperone for superoxide dismutase-1. Exp. Biol. Med.241, 1483–1488 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Itoh, S. et al. Novel role of antioxidant-1 (Atox1) as a copper-dependent transcription factor involved in cell proliferation. J. Biol. Chem.283, 9157–9167 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Muller, P. A. & Klomp, L. W. ATOX1: a novel copper-responsive transcription factor in mammals? Int. J. Biochem. Cell Biol.41, 1233–1236 (2009). [DOI] [PubMed] [Google Scholar]
- 98.Feng, W. et al. Copper regulation of hypoxia-inducible factor-1 activity. Mol. Pharm.75, 174–182 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shanbhag, V. C. et al. Copper metabolism as a unique vulnerability in cancer. Biochim. Biophys. Acta Mol. Cell Res.1868, 118893 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Denoyer, D., Masaldan, S., La Fontaine, S. & Cater, M. A. Targeting copper in cancer therapy: ‘Copper That Cancer’. Metallomics7, 1459–1476 (2015). [DOI] [PubMed] [Google Scholar]
- 101.Khanna, S. S. & Karjodkar, F. R. Circulating immune complexes and trace elements (copper, iron and selenium) as markers in oral precancer and cancer : a randomised, controlled clinical trial. Head. Face Med2, 33 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Baltaci, A. K., Dundar, T. K., Aksoy, F. & Mogulkoc, R. Changes in the serum levels of trace elements before and after the operation in thyroid cancer patients. Biol. Trace Elem. Res.175, 57–64 (2017). [DOI] [PubMed] [Google Scholar]
- 103.Feng, J. F. et al. Serum total oxidant/antioxidant status and trace element levels in breast cancer patients. Int. J. Clin. Oncol.17, 575–583 (2012). [DOI] [PubMed] [Google Scholar]
- 104.Kuo, H. W. et al. Serum and tissue trace elements in patients with breast cancer in Taiwan. Biol. Trace Elem. Res.89, 1–11 (2002). [DOI] [PubMed] [Google Scholar]
- 105.Jin, Y. et al. Combined effects of serum trace metals and polymorphisms of CYP1A1 or GSTM1 on non-small cell lung cancer: a hospital-based case-control study in China. Cancer Epidemiol.35, 182–187 (2011). [DOI] [PubMed] [Google Scholar]
- 106.Lossow, K., Schwarz, M. & Kipp, A. P. Are trace element concentrations suitable biomarkers for the diagnosis of cancer? Redox Biol.42, 101900 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lener, M. R. et al. Serum concentrations of selenium and copper in patients diagnosed with pancreatic cancer. Cancer Res. Treat.48, 1056–1064 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Basu, S. et al. Heavy and trace metals in carcinoma of the gallbladder. World J. Surg.37, 2641–2646 (2013). [DOI] [PubMed] [Google Scholar]
- 109.Gupta, S. K. et al. Serum and tissue trace elements in colorectal cancer. J. Surg. Oncol.52, 172–175 (1993). [DOI] [PubMed] [Google Scholar]
- 110.Saleh, S. A. K., Adly, H. M., Abdelkhaliq, A. A. & Nassir, A. M. Serum levels of selenium, zinc, copper, manganese, and iron in prostate cancer patients. Curr. Urol.14, 44–49 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yaman, M., Kaya, G. & Simsek, M. Comparison of trace element concentrations in cancerous and noncancerous human endometrial and ovary tissues. Int. J. Gynecol. Cancer17, 220–228 (2007). [DOI] [PubMed] [Google Scholar]
- 112.Chen, Z. X. & Pervaiz, S. Involvement of cytochrome c oxidase subunits Va and Vb in the regulation of cancer cell metabolism by Bcl-2. Cell Death Differ.17, 408–420 (2010). [DOI] [PubMed] [Google Scholar]
- 113.Shimada, K. et al. Copper-binding small molecule induces oxidative stress and cell-cycle arrest in glioblastoma-patient-derived cells. Cell Chem. Biol.25, 585–594.e587 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang, W. et al. Cuproptosis: harnessing transition metal for cancer therapy. ACS Nano17, 19581–19599 (2023). [DOI] [PubMed] [Google Scholar]
- 115.Ullah, R., Yin, Q., Snell, A. H. & Wan, L. RAF-MEK-ERK pathway in cancer evolution and treatment. Semin. Cancer Biol.85, 123–154 (2022). [DOI] [PubMed] [Google Scholar]
- 116.Bahar, M. E., Kim, H. J. & Kim, D. R. Targeting the RAS/RAF/MAPK pathway for cancer therapy: from mechanism to clinical studies. Signal Transduct. Target Ther.8, 455 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Turski, M. L. et al. A novel role for copper in Ras/mitogen-activated protein kinase signaling. Mol. Cell Biol.32, 1284–1295 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mattie, M. D., McElwee, M. K. & Freedman, J. H. Mechanism of copper-activated transcription: activation of AP-1, and the JNK/SAPK and p38 signal transduction pathways. J. Mol. Biol.383, 1008–1018 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Grasso, M. et al. The copper chaperone CCS facilitates copper binding to MEK1/2 to promote kinase activation. J. Biol. Chem.297, 101314 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tsang, T. et al. BRAFV600E-driven lung adenocarcinoma requires copper to sustain autophagic signaling and processing. Mol. Cancer Res.20, 1096–1107 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Brady, D. C. et al. Copper is required for oncogenic BRAF signalling and tumorigenesis. Nature509, 492–496 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Aubert, L. et al. Copper bioavailability is a KRAS-specific vulnerability in colorectal cancer. Nat. Commun.11, 3701 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Guo, J. et al. Copper promotes tumorigenesis by activating the PDK1-AKT oncogenic pathway in a copper transporter 1-dependent manner. Adv. Sci.8, e2004303 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kroemer, G., Mariño, G. & Levine, B. Autophagy and the integrated stress response. Mol. Cell40, 280–293 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liu, G. et al. Role of autophagy and apoptosis in non-small-cell lung cancer. Int. J. Mol. Sci. 18, (2017). [DOI] [PMC free article] [PubMed]
- 126.Martin, K. R. et al. A potent and selective ULK1 inhibitor suppresses autophagy and sensitizes cancer cells to nutrient stress. iScience8, 74–84 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Polishchuk, E. V. et al. Activation of autophagy, observed in liver tissues from patients with Wilson disease and from atp7b-deficient animals, protects hepatocytes from copper-induced apoptosis. Gastroenterology156, 1173–1189.e1175 (2019). [DOI] [PubMed] [Google Scholar]
- 128.Tsang, T. et al. Copper is an essential regulator of the autophagic kinases ULK1/2 to drive lung adenocarcinoma. Nat. Cell Biol.22, 412–424 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chen, L. et al. APEX2-based proximity labeling of Atox1 identifies CRIP2 as a nuclear copper-binding protein that regulates autophagy activation. Angew. Chem. Int. Ed. Engl.60, 25346–25355 (2021). [DOI] [PubMed] [Google Scholar]
- 130.Hu, J. et al. Targeting mutant p53 for cancer therapy: direct and indirect strategies. J. Hematol. Oncol.14, 157 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Peuget, S., Zhou, X. & Selivanova, G. Translating p53-based therapies for cancer into the clinic. Nat. Rev. Cancer24, 192–215 (2024). [DOI] [PubMed] [Google Scholar]
- 132.Blanden, A. R. et al. Reactivating mutant p53 using small molecules as zinc metallochaperones: awakening a sleeping giant in cancer. Drug Discov. Today20, 1391–1397 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Alfadul, S. M., Matnurov, E. M., Varakutin, A. E. & Babak, M. V. Metal-based anticancer complexes and P53: how much do we know? Cancers15, (2023). [DOI] [PMC free article] [PubMed]
- 134.Arnesano, F. et al. Copper-triggered aggregation of ubiquitin. PLoS One4, e7052 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Galbán, S. & Duckett, C. S. XIAP as a ubiquitin ligase in cellular signaling. Cell Death Differ.17, 54–60 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Liao, Y. et al. Inflammation mobilizes copper metabolism to promote colon tumorigenesis via an IL-17-STEAP4-XIAP axis. Nat. Commun.11, 900 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kieffer, D. A. & Medici, V. Wilson disease: at the crossroads between genetics and epigenetics-a review of the evidence. Liver Res.1, 121–130 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jin, X. et al. Copper ions impair zebrafish skeletal myofibrillogenesis via epigenetic regulation. FASEB J.35, e21686 (2021). [DOI] [PubMed] [Google Scholar]
- 139.Tai, Z., Li, L., Zhao, G. & Liu, J. X. Copper stress impairs angiogenesis and lymphangiogenesis during zebrafish embryogenesis by down-regulating pERK1/2-foxm1-MMP2/9 axis and epigenetically regulating ccbe1 expression. Angiogenesis25, 241–257 (2022). [DOI] [PubMed] [Google Scholar]
- 140.Takahashi, H., McCaffery, J. M., Irizarry, R. A. & Boeke, J. D. Nucleocytosolic acetyl-coenzyme a synthetase is required for histone acetylation and global transcription. Mol. Cell23, 207–217 (2006). [DOI] [PubMed] [Google Scholar]
- 141.Audia, J. E. & Campbell, R. M. Histone modifications and cancer. Cold Spring Harb. Perspect. Biol.8, a019521 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kang, J., Lin, C., Chen, J. & Liu, Q. Copper induces histone hypoacetylation through directly inhibiting histone acetyltransferase activity. Chem. Biol. Interact.148, 115–123 (2004). [DOI] [PubMed] [Google Scholar]
- 143.Singh, R. P. et al. Disrupting mitochondrial copper distribution inhibits leukemic stem cell self-renewal. Cell Stem Cell26, 926–937.e910 (2020). [DOI] [PubMed] [Google Scholar]
- 144.Ma, K. & Zhang, L. Overview: lipid metabolism in the tumor microenvironment. Adv. Exp. Med. Biol.1316, 41–47 (2021). [DOI] [PubMed] [Google Scholar]
- 145.Carmen, G. Y. & Víctor, S. M. Signalling mechanisms regulating lipolysis. Cell Signal18, 401–408 (2006). [DOI] [PubMed] [Google Scholar]
- 146.Krishnamoorthy, L. et al. Copper regulates cyclic-AMP-dependent lipolysis. Nat. Chem. Biol.12, 586–592 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Liu, Z. L. et al. Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduct. Target Ther.8, 198 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Li, X. et al. New advances in the research of clinical treatment and novel anticancer agents in tumor angiogenesis. Biomed. Pharmacother.163, 114806 (2023). [DOI] [PubMed] [Google Scholar]
- 149.McAuslan, B. R. & Reilly, W. Endothelial cell phagokinesis in response to specific metal ions. Exp. Cell Res.130, 147–157 (1980). [DOI] [PubMed] [Google Scholar]
- 150.Hu, G. F. Copper stimulates proliferation of human endothelial cells under culture. J. Cell Biochem.69, 326–335 (1998). [DOI] [PubMed] [Google Scholar]
- 151.Jiang, X. et al. The role of microenvironment in tumor angiogenesis. J. Exp. Clin. Cancer Res.39, 204 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Soncin, F., Guitton, J. D., Cartwright, T. & Badet, J. Interaction of human angiogenin with copper modulates angiogenin binding to endothelial cells. Biochem. Biophys. Res. Commun.236, 604–610 (1997). [DOI] [PubMed] [Google Scholar]
- 153.Giacomelli, C. et al. ♦Copper (II) ions modulate Angiogenin activity in human endothelial cells. Int. J. Biochem. Cell Biol.60, 185–196 (2015). [DOI] [PubMed] [Google Scholar]
- 154.Liu, X. et al. Copper levels affect targeting of hypoxia-inducible factor 1α to the promoters of hypoxia-regulated genes. J. Biol. Chem.293, 14669–14677 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pan, Q. et al. Copper deficiency induced by tetrathiomolybdate suppresses tumor growth and angiogenesis. Cancer Res.62, 4854–4859 (2002). [PubMed] [Google Scholar]
- 156.Rigiracciolo, D. C. et al. Copper activates HIF-1α/GPER/VEGF signalling in cancer cells. Oncotarget6, 34158–34177 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Urso, E. & Maffia, M. Behind the link between copper and angiogenesis: established mechanisms and an overview on the role of vascular copper transport systems. J. Vasc. Res.52, 172–196 (2015). [DOI] [PubMed] [Google Scholar]
- 158.Narayanan, G. et al. CTR1 silencing inhibits angiogenesis by limiting copper entry into endothelial cells. PLoS One8, e71982 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kohno, T. et al. Novel role of copper transport protein antioxidant-1 in neointimal formation after vascular injury. Arterioscler. Thromb. Vasc. Biol.33, 805–813 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ash, D. et al. The P-type ATPase transporter ATP7A promotes angiogenesis by limiting autophagic degradation of VEGFR2. Nat. Commun.12, 3091 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nakao, S. et al. VAP-1-mediated M2 macrophage infiltration underlies IL-1β- but not VEGF-A-induced lymph- and angiogenesis. Am. J. Pathol.178, 1913–1921 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kamiya, T. Copper in the tumor microenvironment and tumor metastasis. J. Clin. Biochem. Nutr.71, 22–28 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhou, C. et al. Copper metabolism and hepatocellular carcinoma: current insights. Front. Oncol.13, 1186659 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Castaneda, M. et al. Mechanisms of cancer metastasis. Semin. Cancer Biol.87, 17–31 (2022). [DOI] [PubMed] [Google Scholar]
- 165.Blockhuys, S. et al. Defining the human copper proteome and analysis of its expression variation in cancers. Metallomics9, 112–123 (2017). [DOI] [PubMed] [Google Scholar]
- 166.Hassouneh, B. et al. Tetrathiomolybdate promotes tumor necrosis and prevents distant metastases by suppressing angiogenesis in head and neck cancer. Mol. Cancer Ther.6, 1039–1045 (2007). [DOI] [PubMed] [Google Scholar]
- 167.Barker, H. E., Cox, T. R. & Erler, J. T. The rationale for targeting the LOX family in cancer. Nat. Rev. Cancer12, 540–552 (2012). [DOI] [PubMed] [Google Scholar]
- 168.Laczko, R. & Csiszar, K. Lysyl Oxidase (LOX): functional contributions to signaling pathways. Biomolecules10, (2020). [DOI] [PMC free article] [PubMed]
- 169.Xiao, Q. & Ge, G. Lysyl oxidase, extracellular matrix remodeling and cancer metastasis. Cancer Microenviron.5, 261–273 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Erler, J. T. et al. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell15, 35–44 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Al-U’datt, D., Allen, B. G. & Nattel, S. Role of the lysyl oxidase enzyme family in cardiac function and disease. Cardiovasc. Res.115, 1820–1837 (2019). [DOI] [PubMed] [Google Scholar]
- 172.Zhan, X. H. et al. LOXL2 upregulates phosphorylation of ezrin to promote cytoskeletal reorganization and tumor cell invasion. Cancer Res.79, 4951–4964 (2019). [DOI] [PubMed] [Google Scholar]
- 173.Chen, X. et al. LOX upregulates FAK phosphorylation to promote metastasis in osteosarcoma. Genes Dis.10, 254–266 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.MacDonald, G. et al. Memo is a copper-dependent redox protein with an essential role in migration and metastasis. Sci. Signal7, ra56 (2014). [DOI] [PubMed] [Google Scholar]
- 175.Zhang, X. et al. Memo1 binds reduced copper ions, interacts with copper chaperone Atox1, and protects against copper-mediated redox activity in vitro. Proc. Natl. Acad. Sci. USA119, e2206905119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Murdocca, M. et al. LOX-1 and cancer: an indissoluble liaison. Cancer Gene Ther.28, 1088–1098 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wong, C. C. et al. Hypoxia-inducible factor 1 is a master regulator of breast cancer metastatic niche formation. Proc. Natl. Acad. Sci. USA108, 16369–16374 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Pez, F. et al. The HIF-1-inducible lysyl oxidase activates HIF-1 via the Akt pathway in a positive regulation loop and synergizes with HIF-1 in promoting tumor cell growth. Cancer Res.71, 1647–1657 (2011). [DOI] [PubMed] [Google Scholar]
- 179.Martin, F. et al. Copper-dependent activation of hypoxia-inducible factor (HIF)-1: implications for ceruloplasmin regulation. Blood105, 4613–4619 (2005). [DOI] [PubMed] [Google Scholar]
- 180.Li, S. et al. Copper depletion inhibits CoCl2-induced aggressive phenotype of MCF-7 cells via downregulation of HIF-1 and inhibition of Snail/Twist-mediated epithelial-mesenchymal transition. Sci. Rep.5, 12410 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ramchandani, D. et al. Copper depletion modulates mitochondrial oxidative phosphorylation to impair triple negative breast cancer metastasis. Nat. Commun.12, 7311 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ding, P. et al. CD147 functions as the signaling receptor for extracellular divalent copper in hepatocellular carcinoma cells. Oncotarget8, 51151–51163 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Blockhuys, S., Zhang, X. & Wittung-Stafshede, P. Single-cell tracking demonstrates copper chaperone Atox1 to be required for breast cancer cell migration. Proc. Natl. Acad. Sci. USA117, 2014–2019 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Weaver, M. S., Workman, G. & Sage, E. H. The copper binding domain of SPARC mediates cell survival in vitro via interaction with integrin beta1 and activation of integrin-linked kinase. J. Biol. Chem.283, 22826–22837 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Nagaraju, G. P., Dontula, R., El-Rayes, B. F. & Lakka, S. S. Molecular mechanisms underlying the divergent roles of SPARC in human carcinogenesis. Carcinogenesis35, 967–973 (2014). [DOI] [PubMed] [Google Scholar]
- 186.Yi, M. et al. Combination strategies with PD-1/PD-L1 blockade: current advances and future directions. Mol. Cancer21, 28 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sun, C., Mezzadra, R. & Schumacher, T. N. Regulation and function of the PD-L1 checkpoint. Immunity48, 434–452 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yi, M. et al. Regulation of PD-L1 expression in the tumor microenvironment. J. Hematol. Oncol.14, 10 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Cheng, F. et al. Relationship between copper and immunity: the potential role of copper in tumor immunity. Front. Oncol.12, 1019153 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Tan, H. Y. et al. Lysyl oxidase-like 4 fosters an immunosuppressive microenvironment during hepatocarcinogenesis. Hepatology73, 2326–2341 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Jiang, X. et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol. Cancer18, 10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zhou, B. et al. Disulfiram combined with copper induces immunosuppression via PD-L1 stabilization in hepatocellular carcinoma. Am. J. Cancer Res.9, 2442–2455 (2019). [PMC free article] [PubMed] [Google Scholar]
- 193.Carneiro, B. A. & El-Deiry, W. S. Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol.17, 395–417 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Newton, K., Strasser, A., Kayagaki, N. & Dixit, V. M. Cell death. Cell187, 235–256 (2024). [DOI] [PubMed] [Google Scholar]
- 195.Kumar, S., Dorstyn, L. & Lim, Y. The role of caspases as executioners of apoptosis. Biochem. Soc. Trans.50, 33–45 (2022). [DOI] [PubMed] [Google Scholar]
- 196.Hutt, K. J. The role of BH3-only proteins in apoptosis within the ovary. Reproduction149, R81–89 (2015). [DOI] [PubMed] [Google Scholar]
- 197.Bertheloot, D., Latz, E. & Franklin, B. S. Necroptosis, pyroptosis and apoptosis: an intricate game of cell death. Cell Mol. Immunol.18, 1106–1121 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ozcelik, D. et al. Copper-mediated oxidative stress in rat liver. Biol. Trace Elem. Res.96, 209–215 (2003). [DOI] [PubMed] [Google Scholar]
- 199.Yang, F. et al. Copper induces oxidative stress and apoptosis through mitochondria-mediated pathway in chicken hepatocytes. Toxicol. Vitr.54, 310–316 (2019). [DOI] [PubMed] [Google Scholar]
- 200.Zhao, G., Sun, H., Zhang, T. & Liu, J. X. Copper induce zebrafish retinal developmental defects via triggering stresses and apoptosis. Cell Commun. Signal18, 45 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kawakami, M. et al. Mechanism of apoptosis induced by copper in PC12 cells. Food Chem. Toxicol.46, 2157–2164 (2008). [DOI] [PubMed] [Google Scholar]
- 202.Ostrakhovitch, E. A. & Cherian, M. G. Role of p53 and reactive oxygen species in apoptotic response to copper and zinc in epithelial breast cancer cells. Apoptosis10, 111–121 (2005). [DOI] [PubMed] [Google Scholar]
- 203.Wu, H. et al. Copper sulfate-induced endoplasmic reticulum stress promotes hepatic apoptosis by activating CHOP, JNK and caspase-12 signaling pathways. Ecotoxicol. Environ. Saf.191, 110236 (2020). [DOI] [PubMed] [Google Scholar]
- 204.Chen, C. H., Chou, Y. T., Yang, Y. W. & Lo, K. Y. High-dose copper activates p53-independent apoptosis through the induction of nucleolar stress in human cell lines. Apoptosis26, 612–627 (2021). [DOI] [PubMed] [Google Scholar]
- 205.Chen, D., Cui, Q. C., Yang, H. & Dou, Q. P. Disulfiram, a clinically used anti-alcoholism drug and copper-binding agent, induces apoptotic cell death in breast cancer cultures and xenografts via inhibition of the proteasome activity. Cancer Res.66, 10425–10433 (2006). [DOI] [PubMed] [Google Scholar]
- 206.Liu, H. et al. Copper induces oxidative stress and apoptosis in the mouse liver. Oxid. Med. Cell Longev.2020, 1359164 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Tardito, S. et al. Copper-dependent cytotoxicity of 8-hydroxyquinoline derivatives correlates with their hydrophobicity and does not require caspase activation. J. Med. Chem.55, 10448–10459 (2012). [DOI] [PubMed] [Google Scholar]
- 208.Rao, Z. et al. Pyroptosis in inflammatory diseases and cancer. Theranostics12, 4310–4329 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Kovacs, S. B. & Miao, E. A. Gasdermins: effectors of pyroptosis. Trends Cell Biol.27, 673–684 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Liu, Z., Wang, C. & Lin, C. Pyroptosis as a double-edged sword: the pathogenic and therapeutic roles in inflammatory diseases and cancers. Life Sci.318, 121498 (2023). [DOI] [PubMed] [Google Scholar]
- 211.Elias, E. E., Lyons, B. & Muruve, D. A. Gasdermins and pyroptosis in the kidney. Nat. Rev. Nephrol.19, 337–350 (2023). [DOI] [PubMed] [Google Scholar]
- 212.Coll, R. C., Schroder, K. & Pelegrín, P. NLRP3 and pyroptosis blockers for treating inflammatory diseases. Trends Pharm. Sci.43, 653–668 (2022). [DOI] [PubMed] [Google Scholar]
- 213.Liao, J. et al. Inhibition of Caspase-1-dependent pyroptosis attenuates copper-induced apoptosis in chicken hepatocytes. Ecotoxicol. Environ. Saf.174, 110–119 (2019). [DOI] [PubMed] [Google Scholar]
- 214.Zhou, Q. et al. Copper induces microglia-mediated neuroinflammation through ROS/NF-κB pathway and mitophagy disorder. Food Chem. Toxicol.168, 113369 (2022). [DOI] [PubMed] [Google Scholar]
- 215.Tao, X. et al. A tandem activation of NLRP3 inflammasome induced by copper oxide nanoparticles and dissolved copper ion in J774A.1 macrophage. J. Hazard Mater.411, 125134 (2021). [DOI] [PubMed] [Google Scholar]
- 216.Liao, J. et al. Endoplasmic reticulum stress contributes to copper-induced pyroptosis via regulating the IRE1α-XBP1 pathway in pig jejunal epithelial cells. J. Agric. Food Chem.70, 1293–1303 (2022). [DOI] [PubMed] [Google Scholar]
- 217.Galluzzi, L., Kepp, O., Chan, F. K. & Kroemer, G. Necroptosis: mechanisms and relevance to disease. Annu. Rev. Pathol.12, 103–130 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Wegner, K. W., Saleh, D. & Degterev, A. Complex pathologic roles of RIPK1 and RIPK3: moving beyond necroptosis. Trends Pharm. Sci.38, 202–225 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Yan, J., Wan, P., Choksi, S. & Liu, Z. G. Necroptosis and tumor progression. Trends Cancer8, 21–27 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Morgan, M. J. & Kim, Y. S. Roles of RIPK3 in necroptosis, cell signaling, and disease. Exp. Mol. Med.54, 1695–1704 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Zhan, C., Huang, M., Yang, X. & Hou, J. MLKL: functions beyond serving as the executioner of necroptosis. Theranostics11, 4759–4769 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Zhang, T., Wang, Y., Inuzuka, H. & Wei, W. Necroptosis pathways in tumorigenesis. Semin. Cancer Biol.86, 32–40 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Tang, S. et al. ATP7B R778L mutant hepatocytes resist copper toxicity by activating autophagy and inhibiting necroptosis. Cell Death Discov.9, 344 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Chen, J. et al. CuS-NiS(2) nanomaterials for MRI-guided phototherapy of gastric carcinoma via triggering mitochondria-mediated apoptosis and MLKL/CAPG-mediated necroptosis. Nanotoxicology14, 774–787 (2020). [DOI] [PubMed] [Google Scholar]
- 225.Zhou, Q. et al. Ferroptosis in cancer: from molecular mechanisms to therapeutic strategies. Signal Transduct. Target Ther.9, 55 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Jiang, X., Stockwell, B. R. & Conrad, M. Ferroptosis: mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol.22, 266–282 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Dixon, S. J. & Olzmann, J. A. The cell biology of ferroptosis. Nat. Rev. Mol. Cell Biol.25, 424–442 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Liu, J., Kang, R. & Tang, D. Signaling pathways and defense mechanisms of ferroptosis. FEBS J.289, 7038–7050 (2022). [DOI] [PubMed] [Google Scholar]
- 229.Ursini, F. & Maiorino, M. Lipid peroxidation and ferroptosis: the role of GSH and GPx4. Free Radic. Biol. Med.152, 175–185 (2020). [DOI] [PubMed] [Google Scholar]
- 230.Sutton, H. C. & Winterbourn, C. C. On the participation of higher oxidation states of iron and copper in Fenton reactions. Free Radic. Biol. Med.6, 53–60 (1989). [DOI] [PubMed] [Google Scholar]
- 231.Qiao, L., Lu, Y., Liu, B. & Girault, H. H. Copper-catalyzed tyrosine nitration. J. Am. Chem. Soc.133, 19823–19831 (2011). [DOI] [PubMed] [Google Scholar]
- 232.Dixon, S. J. et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell149, 1060–1072 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Gao, W. et al. Elesclomol induces copper-dependent ferroptosis in colorectal cancer cells via degradation of ATP7A. Mol. Oncol.15, 3527–3544 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Li, Y. et al. Disulfiram/copper induces antitumor activity against both nasopharyngeal cancer cells and cancer-associated fibroblasts through ROS/MAPK and ferroptosis pathways. Cancers.12, (2020). [DOI] [PMC free article] [PubMed]
- 235.Ren, X. et al. Overcoming the compensatory elevation of NRF2 renders hepatocellular carcinoma cells more vulnerable to disulfiram/copper-induced ferroptosis. Redox Biol.46, 102122 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Yang, M. et al. COMMD10 inhibits HIF1α/CP loop to enhance ferroptosis and radiosensitivity by disrupting Cu-Fe balance in hepatocellular carcinoma. J. Hepatol.76, 1138–1150 (2022). [DOI] [PubMed] [Google Scholar]
- 237.Li, F. et al. Copper Depletion strongly enhances ferroptosis via mitochondrial perturbation and reduction in antioxidative mechanisms. Antioxidants11, (2022). [DOI] [PMC free article] [PubMed]
- 238.Mizushima, N. & Komatsu, M. Autophagy: renovation of cells and tissues. Cell147, 728–741 (2011). [DOI] [PubMed] [Google Scholar]
- 239.Levine, B. & Kroemer, G. Biological functions of autophagy genes: a disease perspective. Cell176, 11–42 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Miller, D. R. & Thorburn, A. Autophagy and organelle homeostasis in cancer. Dev. Cell56, 906–918 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Park, J. M., Lee, D. H. & Kim, D. H. Redefining the role of AMPK in autophagy and the energy stress response. Nat. Commun.14, 2994 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Kim, Y. C. & Guan, K. L. mTOR: a pharmacologic target for autophagy regulation. J. Clin. Investig.125, 25–32 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Kim, J., Kundu, M., Viollet, B. & Guan, K. L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol.13, 132–141 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Wirth, M., Joachim, J. & Tooze, S. A. Autophagosome formation-the role of ULK1 and Beclin1-PI3KC3 complexes in setting the stage. Semin Cancer Biol.23, 301–309 (2013). [DOI] [PubMed] [Google Scholar]
- 245.Dalle Pezze, P. et al. ATG13 dynamics in nonselective autophagy and mitophagy: insights from live imaging studies and mathematical modeling. Autophagy17, 1131–1141 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Yang, F. et al. Autophagy attenuates copper-induced mitochondrial dysfunction by regulating oxidative stress in chicken hepatocytes. Chemosphere204, 36–43 (2018). [DOI] [PubMed] [Google Scholar]
- 247.Hazari, Y. et al. Autophagy in hepatic adaptation to stress. J. Hepatol.72, 183–196 (2020). [DOI] [PubMed] [Google Scholar]
- 248.Guo, H. et al. Induction of autophagy via the ROS-dependent AMPK-mTOR pathway protects copper-induced spermatogenesis disorder. Redox Biol.49, 102227 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Chen, X. et al. International consensus guidelines for the definition, detection, and interpretation of autophagy-dependent ferroptosis. Autophagy20, 1213–1246 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Liu, J. et al. Autophagy-dependent ferroptosis: machinery and regulation. Cell Chem. Biol.27, 420–435 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Chen, X. et al. Cellular degradation systems in ferroptosis. Cell Death Differ.28, 1135–1148 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Wu, Z. et al. Chaperone-mediated autophagy is involved in the execution of ferroptosis. Proc. Natl. Acad. Sci. USA116, 2996–3005 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Chen, X. et al. Identification of HPCAL1 as a specific autophagy receptor involved in ferroptosis. Autophagy19, 54–74 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Sun, T. et al. Copper oxide nanoparticles induce autophagic cell death in A549 cells. PLoS One7, e43442 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Guo, W. J. et al. ROS-mediated autophagy was involved in cancer cell death induced by novel copper(II) complex. Exp. Toxicol. Pathol.62, 577–582 (2010). [DOI] [PubMed] [Google Scholar]
- 256.Solier, S. et al. A druggable copper-signalling pathway that drives inflammation. Nature617, 386–394 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Krysko, D. V. et al. Immunogenic cell death and DAMPs in cancer therapy. Nat. Rev. Cancer12, 860–875 (2012). [DOI] [PubMed] [Google Scholar]
- 258.Gao, X. et al. Disulfiram/copper induces immunogenic cell death and enhances CD47 blockade in hepatocellular carcinoma. Cancers14, (2022). [DOI] [PMC free article] [PubMed]
- 259.Zheng, Z. et al. Remodeling tumor immune microenvironment (TIME) for glioma therapy using multi-targeting liposomal codelivery. J. Immunother Cancer. 8, (2020). [DOI] [PMC free article] [PubMed]
- 260.Hart, E. B., Steenbock, H., Waddell, J. & Elvehjem, C. A. Iron in nutrition. VII. Copper as a supplement to iron for hemoglobin building in the rat. 1928. J. Biol. Chem.277, e22 (2002). [PubMed] [Google Scholar]
- 261.de Jorge, F. B., Canato, C. & Delascio, D. Biochemical studies on fibroleiomyoma. Matern Infanc.24, 649–654 (1965). [PubMed] [Google Scholar]
- 262.Beratis, N. G., Yee, M., LaBadie, G. U. & Hirschhorn, K. Effect of copper on Menkes’ and normal cultured skin fibroblasts. Dev. Pharm. Ther.1, 305–317 (1980). [PubMed] [Google Scholar]
- 263.Halliwell, B. & Gutteridge, J. M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem. J.219, 1–14 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Bégin, M. E., Ells, G. & Horrobin, D. F. Polyunsaturated fatty acid-induced cytotoxicity against tumor cells and its relationship to lipid peroxidation. J. Natl. Cancer Inst.80, 188–194 (1988). [DOI] [PubMed] [Google Scholar]
- 265.Haiduc, I. & Silvestru, C. Rhodium, iridium, copper and gold antitumor organometallic compounds (review). In Vivo3, 285–293 (1989). [PubMed] [Google Scholar]
- 266.Monti, E. et al. Cardiotoxicity and antitumor activity of a copper(II)-doxorubicin chelate. Cancer Chemother. Pharm.25, 333–336 (1990). [DOI] [PubMed] [Google Scholar]
- 267.Tkeshelashvili, L. K., McBride, T., Spence, K. & Loeb, L. A. Mutation spectrum of copper-induced DNA damage. J. Biol. Chem.266, 6401–6406 (1991). [PubMed] [Google Scholar]
- 268.Mercer, J. F. et al. Isolation of a partial candidate gene for Menkes disease by positional cloning. Nat. Genet.3, 20–25 (1993). [DOI] [PubMed] [Google Scholar]
- 269.Reid, T. M., Feig, D. I. & Loeb, L. A. Mutagenesis by metal-induced oxygen radicals. Environ. Health Perspect.102, 57–61 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Calderaro, M., Martins, E. A. & Meneghini, R. Oxidative stress by menadione affects cellular copper and iron homeostasis. Mol. Cell Biochem.126, 17–23 (1993). [DOI] [PubMed] [Google Scholar]
- 271.Schnitzer, E. et al. Copper-induced lipid oxidation in unfractionated plasma: the lag preceding oxidation as a measure of oxidation-resistance. Biochem. Biophys. Res. Commun.216, 854–861 (1995). [DOI] [PubMed] [Google Scholar]
- 272.Quinlan, G. J., Coudray, C., Hubbard, A. & Gutteridge, J. M. Vanadium and copper in clinical solutions of albumin and their potential to damage protein structure. J. Pharm. Sci.81, 611–614 (1992). [DOI] [PubMed] [Google Scholar]
- 273.Zhai, Q. et al. Copper induces apoptosis in BA/F3beta cells: Bax, reactive oxygen species, and NFkappaB are involved. J. Cell Physiol.184, 161–170 (2000). [DOI] [PubMed] [Google Scholar]
- 274.Tardito, S. et al. Non-apoptotic programmed cell death induced by a copper(II) complex in human fibrosarcoma cells. Histochem. Cell Biol.126, 473–482 (2006). [DOI] [PubMed] [Google Scholar]
- 275.Hultberg, M., Isaksson, A., Andersson, A. & Hultberg, B. Traces of copper ions deplete glutathione in human hepatoma cell cultures with low cysteine content. Chem. Biol. Interact.167, 56–62 (2007). [DOI] [PubMed] [Google Scholar]
- 276.Polishchuk, E. V. et al. Wilson disease protein ATP7B utilizes lysosomal exocytosis to maintain copper homeostasis. Dev. Cell29, 686–700 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Leiter, J. et al. Damage induced in sarcoma 37 with chemical agents. VI. Biphenyl, fluorene, phenanthrene, and tropolone derivatives. J. Natl. Cancer Inst.14, 365–374 (1953). [DOI] [PubMed] [Google Scholar]
- 278.Wattenberg, L. W. Inhibition of carcinogenic and toxic effects of polycyclic hydrocarbons by several sulfur-containing compounds. J. Natl. Cancer Inst.52, 1583–1587 (1974). [DOI] [PubMed] [Google Scholar]
- 279.Cen, D. et al. Disulfiram facilitates intracellular Cu uptake and induces apoptosis in human melanoma cells. J. Med. Chem.47, 6914–6920 (2004). [DOI] [PubMed] [Google Scholar]
- 280.Brewer, G. J. et al. Treatment of metastatic cancer with tetrathiomolybdate, an anticopper, antiangiogenic agent: phase I study. Clin. Cancer Res.6, 1–10 (2000). [PubMed] [Google Scholar]
- 281.De Vizcaya-Ruiz, A. et al. Induction of apoptosis by a novel copper-based anticancer compound, Cassiopeia II, in L1210 murine leukaemia and CH1 human ovarian carcinoma cells. Toxicol. Vitr.14, 1–5 (2000). [DOI] [PubMed] [Google Scholar]
- 282.Kirshner, J. R. et al. Elesclomol induces cancer cell apoptosis through oxidative stress. Mol. Cancer Ther.7, 2319–2327 (2008). [DOI] [PubMed] [Google Scholar]
- 283.Zheng, P. et al. Elesclomol: a copper ionophore targeting mitochondrial metabolism for cancer therapy. J. Exp. Clin. Cancer Res.41, 271 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Yang, Y. et al. Mitochondria and mitochondrial ROS in cancer: novel targets for anticancer therapy. J. Cell Physiol.231, 2570–2581 (2016). [DOI] [PubMed] [Google Scholar]
- 285.Nagai, M. et al. The oncology drug elesclomol selectively transports copper to the mitochondria to induce oxidative stress in cancer cells. Free Radic. Biol. Med.52, 2142–2150 (2012). [DOI] [PubMed] [Google Scholar]
- 286.Yadav, A. A., Patel, D., Wu, X. & Hasinoff, B. B. Molecular mechanisms of the biological activity of the anticancer drug elesclomol and its complexes with Cu(II), Ni(II) and Pt(II). J. Inorg. Biochem.126, 1–6 (2013). [DOI] [PubMed] [Google Scholar]
- 287.Hasinoff, B. B. et al. Cellular mechanisms of the cytotoxicity of the anticancer drug elesclomol and its complex with Cu(II). Biochem. Pharm.93, 266–276 (2015). [DOI] [PubMed] [Google Scholar]
- 288.Kwan, S. Y. et al. Loss of ARID1A expression leads to sensitivity to ROS-inducing agent elesclomol in gynecologic cancer cells. Oncotarget7, 56933–56943 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Li, Y. et al. Copper improves the anti-angiogenic activity of disulfiram through the EGFR/Src/VEGF pathway in gliomas. Cancer Lett.369, 86–96 (2015). [DOI] [PubMed] [Google Scholar]
- 290.Yang, Y. et al. Disulfiram chelated with copper promotes apoptosis in human breast cancer cells by impairing the mitochondria functions. Scanning38, 825–836 (2016). [DOI] [PubMed] [Google Scholar]
- 291.Skrott, Z. et al. Alcohol-abuse drug disulfiram targets cancer via p97 segregase adaptor NPL4. Nature552, 194–199 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Pan, M. et al. Seesaw conformations of Npl4 in the human p97 complex and the inhibitory mechanism of a disulfiram derivative. Nat. Commun.12, 121 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Tsvetkov, P. et al. Mitochondrial metabolism promotes adaptation to proteotoxic stress. Nat. Chem. Biol.15, 681–689 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Buccarelli, M. et al. Elesclomol-induced increase of mitochondrial reactive oxygen species impairs glioblastoma stem-like cell survival and tumor growth. J. Exp. Clin. Cancer Res.40, 228 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Sun, L. et al. Lactylation of METTL16 promotes cuproptosis via m(6)A-modification on FDX1 mRNA in gastric cancer. Nat. Commun.14, 6523 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Liu, Y. T. et al. Dysregulated Wnt/β-catenin signaling confers resistance to cuproptosis in cancer cells. Cell Death Differ. 31, 1452–1466 (2024). [DOI] [PMC free article] [PubMed]
- 297.Li, Y. et al. Zinc transporter 1 functions in copper uptake and cuproptosis. Cell Metab. 36, 2118–2129.e6 (2024). [DOI] [PubMed]
- 298.Dreishpoon, M. B. et al. FDX1 regulates cellular protein lipoylation through direct binding to LIAS. J. Biol. Chem.299, 105046 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Lin, C. H. et al. Protein lipoylation: mitochondria, cuproptosis, and beyond. Trends Biochem. Sci.49, 729–744 (2024). [DOI] [PubMed] [Google Scholar]
- 300.Rowland, E. A., Snowden, C. K. & Cristea, I. M. Protein lipoylation: an evolutionarily conserved metabolic regulator of health and disease. Curr. Opin. Chem. Biol.42, 76–85 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Solmonson, A. & DeBerardinis, R. J. Lipoic acid metabolism and mitochondrial redox regulation. J. Biol. Chem.293, 7522–7530 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Attar, N. et al. The histone H3-H4 tetramer is a copper reductase enzyme. Science369, 59–64 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Gale, J. R. et al. Copper induces neuron-sparing, ferredoxin 1-independent astrocyte toxicity mediated by oxidative stress. J. Neurochem167, 277–295 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Zulkifli, M. et al. FDX1-dependent and independent mechanisms of elesclomol-mediated intracellular copper delivery. Proc. Natl. Acad. Sci. USA120, e2216722120 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Skrott, Z. et al. Disulfiram’s anti-cancer activity reflects targeting NPL4, not inhibition of aldehyde dehydrogenase. Oncogene38, 6711–6722 (2019). [DOI] [PubMed] [Google Scholar]
- 306.Liu, Y. & Gu, W. The complexity of p53-mediated metabolic regulation in tumor suppression. Semin. Cancer Biol.85, 4–32 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Zhou, X. et al. Icaritin activates p53 and inhibits aerobic glycolysis in liver cancer cells. Chem. Biol. Interact.392, 110926 (2024). [DOI] [PubMed] [Google Scholar]
- 308.Gomes, A. S., Ramos, H., Soares, J. & Saraiva, L. p53 and glucose metabolism: an orchestra to be directed in cancer therapy. Pharm. Res131, 75–86 (2018). [DOI] [PubMed] [Google Scholar]
- 309.Xiong, C., Ling, H., Hao, Q. & Zhou, X. Cuproptosis: p53-regulated metabolic cell death? Cell Death Differ.30, 876–884 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Jiang, P. et al. p53 regulates biosynthesis through direct inactivation of glucose-6-phosphate dehydrogenase. Nat. Cell Biol.13, 310–316 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Jiang, P. et al. Reciprocal regulation of p53 and malic enzymes modulates metabolism and senescence. Nature493, 689–693 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Yang, W. et al. 4-Octyl itaconate inhibits aerobic glycolysis by targeting GAPDH to promote cuproptosis in colorectal cancer. Biomed. Pharmacother.159, 114301 (2023). [DOI] [PubMed] [Google Scholar]
- 313.Kornberg, M. D. et al. Dimethyl fumarate targets GAPDH and aerobic glycolysis to modulate immunity. Science360, 449–453 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Li, Z. et al. MELK promotes HCC carcinogenesis through modulating cuproptosis-related gene DLAT-mediated mitochondrial function. Cell Death Dis.14, 733 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Huang, T. et al. Stem cell programs in cancer initiation, progression, and therapy resistance. Theranostics10, 8721–8743 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Steinbichler, T. B. et al. Therapy resistance mediated by cancer stem cells. Semin. Cancer Biol.53, 156–167 (2018). [DOI] [PubMed] [Google Scholar]
- 317.Peng, C. et al. TME-related biomimetic strategies against cancer. Int. J. Nanomed.19, 109–135 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Chen, D., Zhang, X., Li, Z. & Zhu, B. Metabolic regulatory crosstalk between tumor microenvironment and tumor-associated macrophages. Theranostics11, 1016–1030 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Eggert, T. & Greten, T. F. Tumor regulation of the tissue environment in the liver. Pharm. Ther.173, 47–57 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Hu, M. M. & Shu, H. B. Mitochondrial DNA-triggered innate immune response: mechanisms and diseases. Cell Mol. Immunol.20, 1403–1412 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Yu, X. et al. Cuproptotic nanoinducer-driven proteotoxic stress potentiates cancer immunotherapy by activating the mtDNA-cGAS-STING signaling. Biomaterials307, 122512 (2024). [DOI] [PubMed] [Google Scholar]
- 322.Jin, X.-K. et al. Orchestrated copper-based nanoreactor for remodeling tumor microenvironment to amplify cuproptosis-mediated anti-tumor immunity in colorectal cancer. Mater. Today68, 108–124 (2023). [Google Scholar]
- 323.Chen, R., Zou, J., Kang, R. & Tang, D. The redox protein HMGB1 in cell death and cancer. Antioxid Redox Signal, (2023). [DOI] [PubMed]
- 324.Qiao, L. et al. Self-destructive copper carriers induce pyroptosis and cuproptosis for efficient tumor immunotherapy against dormant and recurrent tumors. Adv. Mater.36, e2308241 (2024). [DOI] [PubMed] [Google Scholar]
- 325.Huang, Q.-X. et al. Metal-organic framework nanoagent induces cuproptosis for effective immunotherapy of malignant glioblastoma. Nano Today51, 101911 (2023). [Google Scholar]
- 326.Guo, B. et al. Cuproptosis Induced by ROS-responsive nanoparticles with elesclomol and copper combined with αPD-L1 for enhanced cancer immunotherapy. Adv. Mater.35, e2212267 (2023). [DOI] [PubMed] [Google Scholar]
- 327.Lu, X. et al. Elesclomol-loaded copper oxide nanoplatform triggers cuproptosis to enhance antitumor immunotherapy. Adv. Sci.11, e2309984 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Liu, J. et al. HMGB1 is a mediator of cuproptosis-related sterile inflammation. Front. Cell Dev. Biol.10, 996307 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Jiang, A. et al. A new thinking: deciphering the aberrance and clinical implication of copper-death signatures in clear cell renal cell carcinoma. Cell Biosci.12, 209 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Lu, Y. et al. A mitochondria-targeted anticancer copper dithiocarbamate amplifies immunogenic cuproptosis and macrophage polarization. J. Mater. Chem. B12, 2006–2014 (2024). [DOI] [PubMed] [Google Scholar]
- 331.Huang, Y. et al. Enzyme core spherical nucleic acid that enables enhanced cuproptosis and antitumor immune response through alleviating tumor hypoxia. J. Am. Chem. Soc.146, 13805–13816 (2024). [DOI] [PubMed] [Google Scholar]
- 332.Chen, W. et al. Mild-photothermal effect induced high efficiency ferroptosis-boosted-cuproptosis based on Cu(2)O@Mn(3)Cu(3)O(8) Nanozyme. Adv. Sci.10, e2303694 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Bao, J. et al. Coordination self-assembled AuTPyP-Cu metal-organic framework nanosheets with pH/ultrasound dual-responsiveness for synergistically triggering cuproptosis-augmented chemotherapy. ACS Nano18, 9100–9113 (2024). [DOI] [PubMed] [Google Scholar]
- 334.Liu, Y. et al. Single-site nanozymes with a highly conjugated coordination structure for antitumor immunotherapy via cuproptosis and cascade-enhanced T lymphocyte activity. J. Am. Chem. Soc.146, 3675–3688 (2024). [DOI] [PubMed] [Google Scholar]
- 335.Li, R. et al. Self-cascade nanozyme reactor as a cuproptosis inducer synergistic inhibition of cellular respiration boosting radioimmunotherapy. Small20, e2306263 (2024). [DOI] [PubMed] [Google Scholar]
- 336.Yang, Z. et al. In-situ fabrication of novel Au nanoclusters-Cu(2+)@sodium alginate/hyaluronic acid nanohybrid gels for cuproptosis enhanced photothermal/photodynamic/chemodynamic therapy via tumor microenvironment regulation. J. Colloid Interface Sci.641, 215–228 (2023). [DOI] [PubMed] [Google Scholar]
- 337.Xu, X. et al. Biomimetic nano-regulator that induces cuproptosis and lactate-depletion mediated ROS storm for metalloimmunotherapy of clear cell renal cell carcinoma. Adv. Healthc. Mater., e2400204, (2024). [DOI] [PubMed]
- 338.Hu, F. et al. Stimulus-responsive copper complex nanoparticles induce cuproptosis for augmented cancer immunotherapy. Adv. Sci.11, e2309388 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Qin, Y. et al. Cuproptosis correlates with immunosuppressive tumor microenvironment based on pan-cancer multiomics and single-cell sequencing analysis. Mol. Cancer22, 59 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Xu, J. et al. Multi-omics pan-cancer study of cuproptosis core gene FDX1 and its role in kidney renal clear cell carcinoma. Front. Immunol.13, 981764 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Zhang, C. et al. Pan-cancer analyses confirmed the cuproptosis-related gene FDX1 as an immunotherapy predictor and prognostic biomarker. Front. Genet.13, 923737 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Wang, T. et al. Cuproptosis-related gene FDX1 expression correlates with the prognosis and tumor immune microenvironment in clear cell renal cell carcinoma. Front. Immunol.13, 999823 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Wang, Y. et al. Integrated analyses reveal the prognostic, immunological features and mechanisms of cuproptosis critical mediator gene FDX1 in KIRC. Genes Immun.24, 171–182 (2023). [DOI] [PubMed] [Google Scholar]
- 344.Huang, W. et al. Pan-cancer integrated bioinformatics analysis reveals cuproptosis-related gene FDX1 is a potential prognostic and immunotherapeutic biomarker for lower-grade gliomas. Front. Mol. Biosci.10, 963639 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Patel, M. S., Nemeria, N. S., Furey, W. & Jordan, F. The pyruvate dehydrogenase complexes: structure-based function and regulation. J. Biol. Chem.289, 16615–16623 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Casteel, J., Miernyk, J. A. & Thelen, J. J. Mapping the lipoylation site of Arabidopsis thaliana plastidial dihydrolipoamide S-acetyltransferase using mass spectrometry and site-directed mutagenesis. Plant Physiol. Biochem.49, 1355–1361 (2011). [DOI] [PubMed] [Google Scholar]
- 347.Goh, W. Q. et al. DLAT subunit of the pyruvate dehydrogenase complex is upregulated in gastric cancer-implications in cancer therapy. Am. J. Transl. Res.7, 1140–1151 (2015). [PMC free article] [PubMed] [Google Scholar]
- 348.Chen, Q. et al. PM2.5 promotes NSCLC carcinogenesis through translationally and transcriptionally activating DLAT-mediated glycolysis reprograming. J. Exp. Clin. Cancer Res.41, 229 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Huang, S. et al. Cuproptosis-related gene DLAT serves as a prognostic biomarker for immunotherapy in clear cell renal cell carcinoma: multi-database and experimental verification. Aging15, 12314–12329 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Zhang, P. et al. DLAT is a promising prognostic marker and therapeutic target for hepatocellular carcinoma: a comprehensive study based on public databases. Sci. Rep.13, 17295 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Bian, Z., Fan, R. & Xie, L. A novel cuproptosis-related prognostic gene signature and validation of differential expression in clear cell renal cell carcinoma. Genes13, (2022). [DOI] [PMC free article] [PubMed]
- 352.Zhao, Q. & Qi, T. The implications and prospect of cuproptosis-related genes and copper transporters in cancer progression. Front. Oncol.13, 1117164 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Cai, Y. et al. Comprehensive analysis of the potential cuproptosis-related biomarker LIAS that regulates prognosis and immunotherapy of pan-cancers. Front. Oncol.12, 952129 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Wu, W., Dong, J., Lv, Y. & Chang, D. Cuproptosis-Related genes in the prognosis of colorectal cancer and their correlation with the tumor microenvironment. Front. Genet.13, 984158 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Samimi, G. et al. Increased expression of the copper efflux transporter ATP7A mediates resistance to cisplatin, carboplatin, and oxaliplatin in ovarian cancer cells. Clin. Cancer Res.10, 4661–4669 (2004). [DOI] [PubMed] [Google Scholar]
- 356.Yu, Z. et al. Blockage of SLC31A1-dependent copper absorption increases pancreatic cancer cell autophagy to resist cell death. Cell Prolif.52, e12568 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Qiu, Z. et al. The copper transporter, SLC31A1, transcriptionally activated by ELF3, imbalances copper homeostasis to exacerbate cisplatin-induced acute kidney injury through mitochondrial dysfunction. Chem. Biol. Interact.393, 110943 (2024). [DOI] [PubMed] [Google Scholar]
- 358.Bartee, M. Y. & Lutsenko, S. Hepatic copper-transporting ATPase ATP7B: function and inactivation at the molecular and cellular level. Biometals20, 627–637 (2007). [DOI] [PubMed] [Google Scholar]
- 359.Chen, B. et al. A Cuproptosis Activation Scoring model predicts neoplasm-immunity interactions and personalized treatments in glioma. Comput. Biol. Med148, 105924 (2022). [DOI] [PubMed] [Google Scholar]
- 360.Yun, Y., Wang, Y., Yang, E. & Jing, X. Cuproptosis-related gene - SLC31A1, FDX1 and ATP7B - polymorphisms are associated with risk of lung cancer. Pharmgenom. Pers. Med.15, 733–742 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Li, X., Ma, Z. & Mei, L. Cuproptosis-related gene SLC31A1 is a potential predictor for diagnosis, prognosis and therapeutic response of breast cancer. Am. J. Cancer Res.12, 3561–3580 (2022). [PMC free article] [PubMed] [Google Scholar]
- 362.Li, L., Li, L. & Sun, Q. High expression of cuproptosis-related SLC31A1 gene in relation to unfavorable outcome and deregulated immune cell infiltration in breast cancer: an analysis based on public databases. BMC Bioinforma.23, 350 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Sha, S. et al. Prognostic analysis of cuproptosis-related gene in triple-negative breast cancer. Front. Immunol.13, 922780 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Liu, Y. et al. Development and validation of cuproptosis-related gene signature in the prognostic prediction of liver cancer. Front. Oncol.12, 985484 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Mi, J. et al. Elucidating cuproptosis-related gene SLC31A1 diagnostic and prognostic values in cancer. Am. J. Transl. Res.15, 6026–6041 (2023). [PMC free article] [PubMed] [Google Scholar]
- 366.Wang, J. et al. Cuproptosis-related gene SLC31A1 expression correlates with the prognosis and tumor immune microenvironment in glioma. Funct. Integr. Genom.23, 279 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Sheu, K. F., Hu, C. W. & Utter, M. F. Pyruvate dehydrogenase complex activity in normal and deficient fibroblasts. J. Clin. Investig.67, 1463–1471 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Ma, J., Gong, B. & Zhao, Q. Pan-cancer analysis of cuproptosis-promoting gene signature from multiple perspectives. Clin. Exp. Med.23, 4997–5014 (2023). [DOI] [PubMed] [Google Scholar]
- 369.Deng, L. et al. Comprehensive analyses of PDHA1 that serves as a predictive biomarker for immunotherapy response in cancer. Front. Pharm.13, 947372 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Zhang, D., Wang, T., Zhou, Y. & Zhang, X. Comprehensive analyses of cuproptosis-related gene CDKN2A on prognosis and immunologic therapy in human tumors. Medicines102, e33468 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Zhao, S. et al. Integrated machine learning and bioinformatic analyses used to construct a copper-induced cell death-related classifier for prognosis and immunotherapeutic response of hepatocellular carcinoma patients. Front. Pharm.14, 1188725 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Fan, K., Dong, Y., Li, T. & Li, Y. Cuproptosis-associated CDKN2A is targeted by plicamycin to regulate the microenvironment in patients with head and neck squamous cell carcinoma. Front. Genet.13, 1036408 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Yu, C. et al. A photodynamic-mediated glutamine metabolic intervention nanodrug for triple negative breast cancer therapy. Mater. Today Biol.19, 100577 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Gu, S. et al. Expression and functional analysis of the metallothionein and metal-responsive transcription factor 1 in Phascolosoma esculenta under Zn Stress. Int. J. Mol. Sci. 25, (2024). [DOI] [PMC free article] [PubMed]
- 375.McCann, C. et al. The mitochondrial Cu(+) transporter PiC2 (SLC25A3) is a target of MTF1 and contributes to the development of skeletal muscle in vitro. Front. Mol. Biosci.9, 1037941 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Solmonson, A. et al. Compartmentalized metabolism supports midgestation mammalian development. Nature604, 349–353 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Yan, C. et al. System analysis based on the cuproptosis-related genes identifies LIPT1 as a novel therapy target for liver hepatocellular carcinoma. J. Transl. Med.20, 452 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Lv, H. et al. Comprehensive analysis of cuproptosis-related genes in immune infiltration and prognosis in melanoma. Front Pharm.13, 930041 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Lu, S., Li, Y. & Yu, Y. Glutathione-scavenging celastrol-Cu nanoparticles induce self-amplified cuproptosis for augmented cancer immunotherapy. Adv. Mater.36, e2404971 (2024). [DOI] [PubMed] [Google Scholar]
- 380.Du, Y. et al. A vacancy-engineering ferroelectric nanomedicine for cuproptosis/apoptosis co-activated immunotherapy. Adv. Mater.36, e2403253 (2024). [DOI] [PubMed] [Google Scholar]
- 381.Yu, N. et al. Sono-triggered cascade lactate depletion by semiconducting polymer nanoreactors for cuproptosis-immunotherapy of pancreatic cancer. Angew. Chem. Int Ed. Engl.63, e202405639 (2024). [DOI] [PubMed] [Google Scholar]
- 382.Wu, L. et al. Bioorthogonal Cu single-atom nanozyme for synergistic nanocatalytic therapy, photothermal therapy, cuproptosis and immunotherapy. Angew. Chem. Int Ed. Engl.63, e202405937 (2024). [DOI] [PubMed] [Google Scholar]
- 383.Liu, Y. et al. Metal-organic framework-based nanovaccine for relieving immunosuppressive tumors via hindering efferocytosis of macrophages and promoting pyroptosis and cuproptosis of cancer cells. ACS Nano18, 12386–12400 (2024). [DOI] [PubMed] [Google Scholar]
- 384.Yan, C. et al. Inhalable metal-organic framework-mediated cuproptosis combined with PD-L1 checkpoint blockade for lung metastasis synergistic immunotherapy. Acta Pharm. Sin. B14, 2281–2297 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Li, Y., Liu, J., Weichselbaum, R. R. & Lin, W. Mitochondria-targeted multifunctional nanoparticles combine cuproptosis and programmed cell death-1 downregulation for cancer immunotherapy. Adv. Sci., e2403520, (2024). [DOI] [PMC free article] [PubMed]
- 386.Wu, H. et al. A self-amplifying ROS-responsive nanoplatform for simultaneous cuproptosis and cancer immunotherapy. Adv. Sci.11, e2401047 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Li, Y. et al. Nanoparticles synergize ferroptosis and cuproptosis to potentiate cancer immunotherapy. Adv. Sci.11, e2310309 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Dai, Y. et al. A biomimetic cuproptosis amplifier for targeted NIR-II fluorescence/photoacoustic imaging-guided synergistic NIR-II photothermal immunotherapy. Biomaterials305, 122455 (2024). [DOI] [PubMed] [Google Scholar]
- 389.Tisato, F. et al. Copper in diseases and treatments, and copper-based anticancer strategies. Med. Res. Rev.30, 708–749 (2010). [DOI] [PubMed] [Google Scholar]
- 390.Baldari, S., Di Rocco, G. & Toietta, G. Current biomedical use of copper chelation therapy. Int. J. Mol. Sci. 21, (2020). [DOI] [PMC free article] [PubMed]
- 391.Kim, K. K. et al. Tetrathiomolybdate inhibits mitochondrial complex IV and mediates degradation of hypoxia-inducible factor-1α in cancer cells. Sci. Rep.5, 14296 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Brady, D. C., Crowe, M. S., Greenberg, D. N. & Counter, C. M. Copper chelation inhibits BRAF(V600E)-driven melanomagenesis and counters resistance to BRAF(V600E) and MEK1/2 Inhibitors. Cancer Res.77, 6240–6252 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Kim, Y. J. et al. Inhibition of BCL2 family members increases the efficacy of copper chelation in BRAF(V600E)-driven melanoma. Cancer Res.80, 1387–1400 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Xu, M. et al. Copper chelation as targeted therapy in a mouse model of oncogenic BRAF-driven papillary thyroid cancer. Clin. Cancer Res.24, 4271–4281 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Sammons, S., Brady, D., Vahdat, L. & Salama, A. K. Copper suppression as cancer therapy: the rationale for copper chelating agents in BRAF(V600) mutated melanoma. Melanoma Manag.3, 207–216 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Chan, N. et al. Correction: influencing the tumor microenvironment: a phase II study of copper depletion using tetrathiomolybdate in patients with breast cancer at high risk for recurrence and in preclinical models of lung metastases. Clin. Cancer Res.26, 5051 (2020). [DOI] [PubMed] [Google Scholar]
- 397.Pass, H. I. et al. A phase II trial of tetrathiomolybdate after surgery for malignant mesothelioma: final results. Ann. Thorac. Surg.86, 383–389 (2008). [DOI] [PubMed] [Google Scholar]
- 398.Pan, Q. et al. Antiangiogenic tetrathiomolybdate enhances the efficacy of doxorubicin against breast carcinoma. Mol. Cancer Ther.2, 617–622 (2003). [PubMed] [Google Scholar]
- 399.Khan, M. K. et al. Radiotherapy and antiangiogenic TM in lung cancer. Neoplasia4, 164–170 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Kim, K. K. et al. Tetrathiomolybdate induces doxorubicin sensitivity in resistant tumor cell lines. Gynecol. Oncol.122, 183–189 (2011). [DOI] [PubMed] [Google Scholar]
- 401.Kim, K. K. et al. Tetrathiomolybdate sensitizes ovarian cancer cells to anticancer drugs doxorubicin, fenretinide, 5-fluorouracil and mitomycin C. BMC Cancer12, 147 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Morisawa, A. et al. Ammonium tetrathiomolybdate enhances the antitumor effects of cetuximab via the suppression of osteoclastogenesis in head and neck squamous carcinoma. Int. J. Oncol.52, 989–999 (2018). [DOI] [PubMed] [Google Scholar]
- 403.Ryumon, S. et al. Ammonium tetrathiomolybdate enhances the antitumor effect of cisplatin via the suppression of ATPase copper transporting beta in head and neck squamous cell carcinoma. Oncol. Rep.42, 2611–2621 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Gartner, E. M. et al. A pilot trial of the anti-angiogenic copper lowering agent tetrathiomolybdate in combination with irinotecan, 5-flurouracil, and leucovorin for metastatic colorectal cancer. Investig. New. Drugs27, 159–165 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Jain, S. et al. Tetrathiomolybdate-associated copper depletion decreases circulating endothelial progenitor cells in women with breast cancer at high risk of relapse. Ann. Oncol.24, 1491–1498 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Redman, B. G. et al. Phase II trial of tetrathiomolybdate in patients with advanced kidney cancer. Clin. Cancer Res9, 1666–1672 (2003). [PubMed] [Google Scholar]
- 407.Li, Y., Fang, M., Xu, Z. & Li, X. Tetrathiomolybdate as an old drug in a new use: as a chemotherapeutic sensitizer for non-small cell lung cancer. J. Inorg. Biochem.233, 111865 (2022). [DOI] [PubMed] [Google Scholar]
- 408.Henry, N. L. et al. Phase II trial of copper depletion with tetrathiomolybdate as an antiangiogenesis strategy in patients with hormone-refractory prostate cancer. Oncology71, 168–175 (2006). [DOI] [PubMed] [Google Scholar]
- 409.Juarez, J. C. et al. Copper binding by tetrathiomolybdate attenuates angiogenesis and tumor cell proliferation through the inhibition of superoxide dismutase 1. Clin. Cancer Res.12, 4974–4982 (2006). [DOI] [PubMed] [Google Scholar]
- 410.Yoo, J. Y. et al. ATN-224 enhances antitumor efficacy of oncolytic herpes virus against both local and metastatic head and neck squamous cell carcinoma. Mol. Ther. Oncol.2, 15008 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Lowndes, S. A. et al. Phase I study of copper-binding agent ATN-224 in patients with advanced solid tumors. Clin. Cancer Res.14, 7526–7534 (2008). [DOI] [PubMed] [Google Scholar]
- 412.Lin, J. et al. A non-comparative randomized phase II study of 2 doses of ATN-224, a copper/zinc superoxide dismutase inhibitor, in patients with biochemically recurrent hormone-naïve prostate cancer. Urol. Oncol.31, 581–588 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Danks, D. M. Penicillamine in Wilson’s disease. Lancet2, 435–436 (1982). [DOI] [PubMed] [Google Scholar]
- 414.Mammoto, T. et al. Role of collagen matrix in tumor angiogenesis and glioblastoma multiforme progression. Am. J. Pathol.183, 1293–1305 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Chen, S. J. et al. Mechanistic basis of a combination D-penicillamine and platinum drugs synergistically inhibits tumor growth in oxaliplatin-resistant human cervical cancer cells in vitro and in vivo. Biochem Pharm.95, 28–37 (2015). [DOI] [PubMed] [Google Scholar]
- 416.Lai, Y. H., Kuo, C., Kuo, M. T. & Chen, H. H. W. Modulating Chemosensitivity of Tumors to Platinum-Based Antitumor Drugs by Transcriptional Regulation of Copper Homeostasis. Int J Mol Sci. 19, (2018). [DOI] [PMC free article] [PubMed]
- 417.Sciegienka, S. J. et al. D-penicillamine combined with inhibitors of hydroperoxide metabolism enhances lung and breast cancer cell responses to radiation and carboplatin via H(2)O(2)-mediated oxidative stress. Free Radic. Biol. Med.108, 354–361 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Brem, S. et al. Phase 2 trial of copper depletion and penicillamine as antiangiogenesis therapy of glioblastoma. Neuro Oncol.7, 246–253 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Brewer, G. J., Fink, J. K. & Hedera, P. Diagnosis and treatment of Wilson’s disease. Semin. Neurol.19, 261–270 (1999). [DOI] [PubMed] [Google Scholar]
- 420.Lopez, J., Ramchandani, D. & Vahdat, L. Copper depletion as a therapeutic strategy in cancer. Met. Ions Life Sci. 19, (2019). [DOI] [PubMed]
- 421.Schilsky, M. L. et al. Trientine tetrahydrochloride versus penicillamine for maintenance therapy in Wilson disease (CHELATE): a randomised, open-label, non-inferiority, phase 3 trial. Lancet Gastroenterol. Hepatol.7, 1092–1102 (2022). [DOI] [PubMed] [Google Scholar]
- 422.Yoshii, J. et al. The copper-chelating agent, trientine, suppresses tumor development and angiogenesis in the murine hepatocellular carcinoma cells. Int. J. Cancer94, 768–773 (2001). [DOI] [PubMed] [Google Scholar]
- 423.Moriguchi, M. et al. The copper chelator trientine has an antiangiogenic effect against hepatocellular carcinoma, possibly through inhibition of interleukin-8 production. Int. J. Cancer102, 445–452 (2002). [DOI] [PubMed] [Google Scholar]
- 424.Fu, S. et al. Overcoming platinum resistance through the use of a copper-lowering agent. Mol. Cancer Ther.11, 1221–1225 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Huang, Y. F. et al. A dose escalation study of trientine plus carboplatin and pegylated liposomal doxorubicin in women with a first relapse of epithelial ovarian, tubal, and peritoneal cancer within 12 months after platinum-based chemotherapy. Front. Oncol.9, 437 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Fu, S. et al. Exploratory study of carboplatin plus the copper-lowering agent trientine in patients with advanced malignancies. Investig. New Drugs32, 465–472 (2014). [DOI] [PubMed] [Google Scholar]
- 427.Yellepeddi, V. K., Vangara, K. K., Kumar, A. & Palakurthi, S. Comparative evaluation of small-molecule chemosensitizers in reversal of cisplatin resistance in ovarian cancer cells. Anticancer Res.32, 3651–3658 (2012). [PubMed] [Google Scholar]
- 428.Fan, D. et al. Nanomedicine in cancer therapy. Signal Transduct. Target Ther.8, 293 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Matsumura, Y. & Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res.46, 6387–6392 (1986). [PubMed] [Google Scholar]
- 430.Peer, D. et al. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol.2, 751–760 (2007). [DOI] [PubMed] [Google Scholar]
- 431.Torchilin, V. P. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat. Rev. Drug Discov.13, 813–827 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Zhao, Z., Ukidve, A., Kim, J. & Mitragotri, S. Targeting strategies for tissue-specific drug delivery. Cell181, 151–167 (2020). [DOI] [PubMed] [Google Scholar]
- 433.Ismail, M. et al. Biomimetic Dp44mT-nanoparticles selectively induce apoptosis in Cu-loaded glioblastoma resulting in potent growth inhibition. Biomaterials289, 121760 (2022). [DOI] [PubMed] [Google Scholar]
- 434.Cui, L. et al. Mitochondrial copper depletion suppresses triple-negative breast cancer in mice. Nat. Biotechnol.39, 357–367 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Zhou, P. et al. Multifunctional nanoparticles based on a polymeric copper chelator for combination treatment of metastatic breast cancer. Biomaterials195, 86–99 (2019). [DOI] [PubMed] [Google Scholar]
- 436.Ren, Y. et al. Responsively aggregatable nanochelator-mediated antiangiogenesis to enhance interventional therapies for liver cancer. Chem. Eng. J. Adv.500, 156925 (2024). [Google Scholar]
- 437.Yang, Y. et al. Responsively aggregatable Sub-6 nm nanochelators induce simultaneous antiangiogenesis and vascular obstruction for enhanced tumor vasculature targeted therapy. Nano Lett.19, 7750–7759 (2019). [DOI] [PubMed] [Google Scholar]
- 438.Oliveri, V. Biomedical applications of copper ionophores. Chem. Commun.422, 213474 (2020). [Google Scholar]
- 439.Oliveri, V. Selective targeting of cancer cells by copper ionophores: an overview. Front. Mol. Biosci.9, 841814 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Chen, S. et al. Syntheses and antitumor activities of N'1,N'3-dialkyl-N'1,N'3-di-(alkylcarbonothioyl) malonohydrazide: the discovery of elesclomol. Bioorg. Med. Chem. Lett.23, 5070–5076 (2013). [DOI] [PubMed] [Google Scholar]
- 441.O’Day, S. et al. Phase II, randomized, controlled, double-blinded trial of weekly elesclomol plus paclitaxel versus paclitaxel alone for stage IV metastatic melanoma. J. Clin. Oncol.27, 5452–5458 (2009). [DOI] [PubMed] [Google Scholar]
- 442.Wang, W. et al. Ferroptosis inducers enhanced cuproptosis induced by copper ionophores in primary liver cancer. J. Exp. Clin. Cancer Res.42, 142 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Monk, B. J. et al. A phase II evaluation of elesclomol sodium and weekly paclitaxel in the treatment of recurrent or persistent platinum-resistant ovarian, fallopian tube or primary peritoneal cancer: an NRG oncology/gynecologic oncology group study. Gynecol. Oncol.151, 422–427 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Berkenblit, A. et al. Phase I clinical trial of STA-4783 in combination with paclitaxel in patients with refractory solid tumors. Clin. Cancer Res.13, 584–590 (2007). [DOI] [PubMed] [Google Scholar]
- 445.Hedley, D. et al. A phase I study of elesclomol sodium in patients with acute myeloid leukemia. Leuk. Lymphoma57, 2437–2440 (2016). [DOI] [PubMed] [Google Scholar]
- 446.O’Day, S. J. et al. Final results of phase III SYMMETRY study: randomized, double-blind trial of elesclomol plus paclitaxel versus paclitaxel alone as treatment for chemotherapy-naive patients with advanced melanoma. J. Clin. Oncol.31, 1211–1218 (2013). [DOI] [PubMed] [Google Scholar]
- 447.Lu, Y. et al. Leveraging disulfiram to treat cancer: Mechanisms of action, delivery strategies, and treatment regimens. Biomaterials281, 121335 (2022). [DOI] [PubMed] [Google Scholar]
- 448.Li, H. et al. The combination of disulfiram and copper for cancer treatment. Drug Discov. Today25, 1099–1108 (2020). [DOI] [PubMed] [Google Scholar]
- 449.Chu, M. et al. Disulfiram/copper induce ferroptosis in triple-negative breast cancer cell line MDA-MB-231.Front. Biosci. (Landmark Ed.)28, 186 (2023). [DOI] [PubMed] [Google Scholar]
- 450.Wu, L. et al. Disulfiram and BKM120 in combination with chemotherapy impede tumor progression and delay tumor recurrence in tumor initiating cell-rich TNBC. Sci. Rep.9, 236 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Guo, F. et al. Inhibitory effect on ovarian cancer ALDH+ stem-like cells by disulfiram and copper treatment through ALDH and ROS modulation. Biomed. Pharmacother.118, 109371 (2019). [DOI] [PubMed] [Google Scholar]
- 452.Wang, K. et al. Targeting cancer stem cells by disulfiram and copper sensitizes radioresistant chondrosarcoma to radiation. Cancer Lett.505, 37–48 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Viola-Rhenals, M. et al. Recent advances in antabuse (Disulfiram): the importance of its metal-binding ability to its anticancer activity. Curr. Med. Chem.25, 506–524 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Hothi, P. et al. High-throughput chemical screens identify disulfiram as an inhibitor of human glioblastoma stem cells. Oncotarget3, 1124–1136 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Triscott, J. et al. Disulfiram, a drug widely used to control alcoholism, suppresses the self-renewal of glioblastoma and over-rides resistance to temozolomide. Oncotarget3, 1112–1123 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Nechushtan, H. et al. A phase IIb trial assessing the addition of disulfiram to chemotherapy for the treatment of metastatic non-small cell lung cancer. Oncologist20, 366–367 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Mego, M. et al. Phase II study of disulfiram and cisplatin in refractory germ cell tumors. The GCT-SK-006 phase II trial. Investig. New Drugs40, 1080–1086 (2022). [DOI] [PubMed] [Google Scholar]
- 458.Kelley, K. C. et al. A Phase 1 dose-escalation study of disulfiram and copper gluconate in patients with advanced solid tumors involving the liver using S-glutathionylation as a biomarker. BMC Cancer21, 510 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Werlenius, K. et al. Effect of disulfiram and copper plus chemotherapy vs chemotherapy alone on survival in patients with recurrent glioblastoma: a randomized clinical trial. JAMA Netw. Open6, e234149 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Ma, L. et al. A novel 8-hydroxyquinoline derivative induces breast cancer cell death through paraptosis and apoptosis. Apoptosis27, 577–589 (2022). [DOI] [PubMed] [Google Scholar]
- 461.Yu, H., Zhou, Y., Lind, S. E. & Ding, W. Q. Clioquinol targets zinc to lysosomes in human cancer cells. Biochem J.417, 133–139 (2009). [DOI] [PubMed] [Google Scholar]
- 462.Zhai, S. et al. Tumor cellular proteasome inhibition and growth suppression by 8-hydroxyquinoline and clioquinol requires their capabilities to bind copper and transport copper into cells. J. Biol. Inorg. Chem.15, 259–269 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Katsuyama, M. et al. Clioquinol inhibits dopamine-β-hydroxylase secretion and noradrenaline synthesis by affecting the redox status of ATOX1 and copper transport in human neuroblastoma SH-SY5Y cells. Arch. Toxicol.95, 135–148 (2021). [DOI] [PubMed] [Google Scholar]
- 464.Lu, J., Miao, Y. & Li, Y. Cuproptosis: advances in stimulus-responsive nanomaterials for cancer therapy. Adv. Health. Mater.13, e2400652 (2024). [DOI] [PubMed] [Google Scholar]
- 465.Ni, C. et al. A tumor microenvironment-responsive core-shell tecto dendrimer nanoplatform for magnetic resonance imaging-guided and cuproptosis-promoted chemo-chemodynamic therapy. Acta Biomater.164, 474–486 (2023). [DOI] [PubMed] [Google Scholar]
- 466.Xu, Y. et al. An enzyme-engineered nonporous copper(I) coordination polymer nanoplatform for cuproptosis-based synergistic cancer therapy. Adv. Mater.34, e2204733 (2022). [DOI] [PubMed] [Google Scholar]
- 467.Zhou, J. et al. Photothermally triggered copper payload release for cuproptosis-promoted cancer synergistic therapy. Angew. Chem. Int. Ed. Engl.62, e202213922 (2023). [DOI] [PubMed] [Google Scholar]
- 468.Zhang, J. et al. Syphilis mimetic nanoparticles for cuproptosis-based synergistic cancer therapy via reprogramming copper metabolism. Int. J. Pharm.640, 123025 (2023). [DOI] [PubMed] [Google Scholar]
- 469.Xia, J. et al. Copper-loaded nanoheterojunction enables superb orthotopic osteosarcoma therapy via oxidative stress and cell cuproptosis. ACS Nano17, 21134–21152 (2023). [DOI] [PubMed] [Google Scholar]
- 470.Zhu, Y. et al. Carrier-free self-assembly nano-sonosensitizers for sonodynamic-amplified cuproptosis-ferroptosis in glioblastoma therapy. Adv. Sci.11, e2402516 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Luo, Y. et al. Copper(II)-based nano-regulator correlates cuproptosis burst and sequential immunogenic cell death for synergistic cancer immunotherapy. Biomater. Res.28, 0039 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Ding, H. et al. Cu(2+) -anchored carbon nano-photocatalysts for visible water splitting to boost hydrogen cuproptosis. Angew. Chem. Int. Ed. Engl.62, e202311549 (2023). [DOI] [PubMed] [Google Scholar]
- 473.Chang, J. et al. Copper deposition in polydopamine nanostructure to promote cuproptosis by catalytically inhibiting copper exporters of tumor cells for cancer immunotherapy. Small20, e2308565 (2024). [DOI] [PubMed] [Google Scholar]
- 474.Zu, H. et al. Tumor metabolism aiming Cu(2-x)S nanoagents mediate photothermal-derived cuproptosis and immune activation. ACS Nano18, 23941–23957 (2024). [DOI] [PubMed] [Google Scholar]
- 475.Su, Z. et al. Nanostrategy of targeting at embryonic trophoblast cells using CuO nanoparticles for female contraception. ACS Nano17, 25185–25204 (2023). [DOI] [PubMed] [Google Scholar]
- 476.Tang, W. et al. Bioactive layered double hydroxides for synergistic sonodynamic/cuproptosis anticancer therapy with elicitation of the immune response. ACS Nano18, 10495–10508 (2024). [DOI] [PubMed] [Google Scholar]
- 477.Zhao, C., Tang, X., Chen, X. & Jiang, Z. Multifaceted carbonized metal-organic frameworks synergize with immune checkpoint inhibitors for precision and augmented cuproptosis cancer therapy. ACS Nano18, 17852–17868 (2024). [DOI] [PubMed] [Google Scholar]
- 478.Wang, S. et al. Polyvalent aptamer nanodrug conjugates enable efficient tumor cuproptosis therapy through copper overload and glutathione depletion. J. Am. Chem. Soc.146, 30033–30045 (2024). [DOI] [PubMed] [Google Scholar]
- 479.Yang, X. et al. Targeting cuproptosis by zinc pyrithione in triple-negative breast cancer. iScience26, 108218 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Chen, N. et al. Sleep fragmentation exacerbates myocardial ischemia‒reperfusion injury by promoting copper overload in cardiomyocytes. Nat. Commun.15, 3834 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Ning, S. et al. Type-I AIE photosensitizer-loaded biomimetic system boosting cuproptosis to inhibit breast cancer metastasis and rechallenge. ACS Nano17, 10206–10217 (2023). [DOI] [PubMed] [Google Scholar]
- 482.Huo, S. et al. ATF3/SPI1/SLC31A1 signaling promotes cuproptosis induced by advanced glycosylation end products in diabetic myocardial injury. Int. J. Mol. Sci. 24 (2023). [DOI] [PMC free article] [PubMed]
- 483.Wang, Y., Zhang, L. & Zhou, F. Cuproptosis: a new form of programmed cell death. Cell Mol. Immunol.19, 867–868 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Wang, D. et al. The molecular mechanisms of cuproptosis and its relevance to cardiovascular disease. Biomed. Pharmacother.163, 114830 (2023). [DOI] [PubMed] [Google Scholar]
- 485.Lu, Y., Wei, X., Chen, M. & Wang, J. Non-ceruloplasmin-bound copper and copper speciation in serum with extraction using functionalized dendritic silica spheres followed by ICP-MS detection. Anal. Chim. Acta1251, 340993 (2023). [DOI] [PubMed] [Google Scholar]
- 486.Luo, Y. et al. Determination of interactions between antibody biotherapeutics and copper by size exclusion chromatography (SEC) coupled with inductively coupled plasma mass spectrometry (ICP/MS). Anal. Chim. Acta1079, 252–259 (2019). [DOI] [PubMed] [Google Scholar]
- 487.Duroux, C. & Hagège, A. Interactions between copper (II) and β-amyloid peptide using capillary electrophoresis-ICP-MS: Kd measurements at the nanogram scale. Anal. Bioanal. Chem.414, 5347–5355 (2022). [DOI] [PubMed] [Google Scholar]
- 488.Hayder, M. et al. Analysis of cerium oxide and copper oxide nanoparticles bioaccessibility from radish using SP-ICP-MS. J. Sci. Food Agric.100, 4950–4958 (2020). [DOI] [PubMed] [Google Scholar]
- 489.Cheng, X. et al. Associations of essential trace elements with epigenetic aging indicators and the potential mediating role of inflammation. Redox Biol.67, 102910 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Fernandes, G., Renné, W. G., Visser, M. B. & Sabatini, C. Esterase inhibition and copper release from copper iodide dental adhesives—an in vitro study. J. Adhes. Dent.22, 265–274 (2020). [DOI] [PubMed] [Google Scholar]
- 491.Deng, J. et al. Mitochondrial-targeted copper delivery for cuproptosis-based synergistic cancer therapy. Adv. Health. Mater.13, e2304522 (2024). [DOI] [PubMed] [Google Scholar]
- 492.Chang, C. J. Searching for harmony in transition-metal signaling. Nat. Chem. Biol.11, 744–747 (2015). [DOI] [PubMed] [Google Scholar]
- 493.Wang, W. et al. Identification of two-dimensional copper signatures in human blood for bladder cancer with machine learning. Chem. Sci.13, 1648–1656 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Pham, V. N. & Chang, C. J. Metalloallostery and transition metal signaling: bioinorganic copper chemistry beyond active sites. Angew. Chem. Int. Ed. Engl.62, e202213644 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Hu, K. et al. Marriage of black phosphorus and Cu(2+) as effective photothermal agents for PET-guided combination cancer therapy. Nat. Commun.11, 2778 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Que, E. L. et al. Copper-responsive magnetic resonance imaging contrast agents. J. Am. Chem. Soc.131, 8527–8536 (2009). [DOI] [PubMed] [Google Scholar]
- 497.Bartnicka, J. J. & Blower, P. J. Insights into trace metal metabolism in health and disease from PET: “PET metallomics”. J. Nucl. Med.59, 1355–1359 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Panichelli, P. et al. Imaging of brain tumors with copper-64 chloride: early experience and results. Cancer Biother. Radiopharm.31, 159–167 (2016). [DOI] [PubMed] [Google Scholar]
- 499.Piccardo, A. et al. (64)CuCl(2) PET/CT in prostate cancer relapse. J. Nucl. Med.59, 444–451 (2018). [DOI] [PubMed]
- 500.Kahlson, M. A. & Dixon, S. J. Copper-induced cell death. Science375, 1231–1232 (2022). [DOI] [PubMed] [Google Scholar]
- 501.Yang, J. et al. Qiju Dihuang Pill protects the lens epithelial cells via alleviating cuproptosis in diabetic cataract. J. Ethnopharmacol.333, 118444 (2024). [DOI] [PubMed] [Google Scholar]
- 502.Wang, D., Deng, X., Li, S. & Sana, S. Impact of SARS-CoV-2 infection on immune cell cuproptosis in patients with lung adenocarcinoma via glutamine regulation. Int. Immunopharmacol.140, 112912 (2024). [DOI] [PubMed] [Google Scholar]
- 503.Chen, F., Kang, R., Tang, D. & Liu, J. Ferroptosis: principles and significance in health and disease. J. Hematol. Oncol.17, 41 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Stockwell, B. R. Ferroptosis turns 10: Emerging mechanisms, physiological functions, and therapeutic applications. Cell185, 2401–2421 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Liu, J. Y. et al. The role of cuproptosis-related gene in the classification and prognosis of melanoma. Front. Immunol.13, 986214 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Chen, Y. et al. Identification and validation of a novel cuproptosis-related signature as a prognostic model for lung adenocarcinoma. Front Endocrinol.13, 963220 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Ran, X. M. et al. The effect of cuproptosis-relevant genes on the immune infiltration and metabolism of gynecological oncology by multiply analysis and experiments validation. Sci. Rep.13, 19474 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Ishida, S. et al. Bioavailable copper modulates oxidative phosphorylation and growth of tumors. Proc. Natl. Acad. Sci. USA110, 19507–19512 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Ishida, S., McCormick, F., Smith-McCune, K. & Hanahan, D. Enhancing tumor-specific uptake of the anticancer drug cisplatin with a copper chelator. Cancer Cell17, 574–583 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Gao, X. et al. Enzalutamide sensitizes castration-resistant prostate cancer to copper-mediated cell death. Adv. Sci.11, e2401396 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Schneider, B. J. et al. Pre-operative chemoradiation followed by post-operative adjuvant therapy with tetrathiomolybdate, a novel copper chelator, for patients with resectable esophageal cancer. Investig. New Drugs31, 435–442 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Chan, N. et al. Influencing the tumor microenvironment: a phase II study of copper depletion using tetrathiomolybdate in patients with breast cancer at high risk for recurrence and in preclinical models of lung metastases. Clin. Cancer Res.23, 666–676 (2017). [DOI] [PubMed] [Google Scholar]
- 513.Glasauer, A. et al. Targeting SOD1 reduces experimental non–small-cell lung cancer. J. Clin. Investig.124, 117–128 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Lee, K. et al. The copper chelator ATN-224 induces peroxynitrite-dependent cell death in hematological malignancies. Free Radic. Biol. Med.60, 157–167 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.Lee, K. et al. The copper chelator ATN-224 induces caspase-independent cell death in diffuse large B cell lymphoma. Int. J. Oncol.45, 439–447 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Yoshida, D., Ikeda, Y. & Nakazawa, S. Copper chelation inhibits tumor angiogenesis in the experimental 9L gliosarcoma model. Neurosurgery37, 287–292 (1995). discussion 292-283. [DOI] [PubMed] [Google Scholar]
- 517.Gupte, A. & Mumper, R. J. Copper chelation by D-penicillamine generates reactive oxygen species that are cytotoxic to human leukemia and breast cancer cells. Free Radic. Biol. Med.43, 1271–1278 (2007). [DOI] [PubMed] [Google Scholar]
- 518.Crowe, A. et al. Rapid copper acquisition by developing murine mesothelioma: decreasing bioavailable copper slows tumor growth, normalizes vessels and promotes T cell infiltration. PLoS One8, e73684 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Qu, Y. et al. Elesclomol, counteracted by Akt survival signaling, enhances the apoptotic effect of chemotherapy drugs in breast cancer cells. Breast Cancer Res. Treat.121, 311–321 (2010). [DOI] [PubMed] [Google Scholar]
- 520.Chen, G. et al. FDX1 inhibits thyroid cancer malignant progression by inducing cuprotosis. Heliyon9, e18655 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Li, Y. et al. Copper ionophore elesclomol selectively targets GNAQ/11-mutant uveal melanoma. Oncogene41, 3539–3553 (2022). [DOI] [PubMed] [Google Scholar]
- 522.Wen, H. et al. Cuproptosis enhances docetaxel chemosensitivity by inhibiting autophagy via the DLAT/mTOR pathway in prostate cancer. FASEB J.37, e23145 (2023). [DOI] [PubMed] [Google Scholar]
- 523.Zheng, X. et al. Disulfiram improves the Anti-PD-1 therapy efficacy by regulating PD-L1 expression via epigenetically reactivation of IRF7 in triple negative breast cancer. Front Oncol.11, 734853 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Kanellis, D. C. et al. Actionable cancer vulnerability due to translational arrest, p53 aggregation and ribosome biogenesis stress evoked by the disulfiram metabolite CuET. Cell Death Differ.30, 1666–1678 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Kim, J. Y. et al. Disulfiram targets cancer stem-like properties and the HER2/Akt signaling pathway in HER2-positive breast cancer. Cancer Lett.379, 39–48 (2016). [DOI] [PubMed] [Google Scholar]
- 526.Cao, H. Z., Yang, W. T. & Zheng, P. S. Cytotoxic effect of disulfiram/copper on human cervical cancer cell lines and LGR5-positive cancer stem-like cells. BMC Cancer22, 521 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527.Xie, J. et al. Disulfiram/Cu kills and sensitizes BRAF-mutant thyroid cancer cells to BRAF kinase inhibitor by ROS-dependently relieving feedback activation of MAPK/ERK and PI3K/AKT pathways. Int. J. Mol. Sci. 24, (2023). [DOI] [PMC free article] [PubMed]
- 528.Ni, Y. L. et al. Disulfiram/copper suppresses cancer stem cell activity in differentiated thyroid cancer cells by inhibiting BMI1 expression. Int. J. Mol. Sci. 23, (2022). [DOI] [PMC free article] [PubMed]
- 529.Huang, S. et al. Disulfiram combined with chemoimmunotherapy potentiates pancreatic cancer treatment efficacy through the activation of cGAS-STING signaling pathway via suppressing PARP1 expression. Am. J. Cancer Res.13, 2055–2065 (2023). [PMC free article] [PubMed] [Google Scholar]
- 530.Read, E. et al. The interaction of disulfiram and H(2)S metabolism in inhibition of aldehyde dehydrogenase activity and liver cancer cell growth. Toxicol. Appl. Pharm.426, 115642 (2021). [DOI] [PubMed] [Google Scholar]
- 531.Navrátilová, J., Hankeová, T., Beneš, P. & Šmarda, J. Acidic pH of tumor microenvironment enhances cytotoxicity of the disulfiram/Cu2+ complex to breast and colon cancer cells. Chemotherapy59, 112–120 (2013). [DOI] [PubMed] [Google Scholar]
- 532.Huang, J. et al. A multicenter phase II study of temozolomide plus disulfiram and copper for recurrent temozolomide-resistant glioblastoma. J. Neurooncol.142, 537–544 (2019). [DOI] [PubMed] [Google Scholar]
- 533.Huang, J. et al. A phase I study to repurpose disulfiram in combination with temozolomide to treat newly diagnosed glioblastoma after chemoradiotherapy. J. Neurooncol.128, 259–266 (2016). [DOI] [PubMed] [Google Scholar]
- 534.Zhang, T. et al. Prospective clinical trial of disulfiram plus copper in men with metastatic castration-resistant prostate cancer. Prostate82, 858–866 (2022). [DOI] [PubMed] [Google Scholar]
- 535.Yu, H., Lou, J. R. & Ding, W. Q. Clioquinol independently targets NF-kappaB and lysosome pathways in human cancer cells. Anticancer Res.30, 2087–2092 (2010). [PubMed] [Google Scholar]
- 536.Chen, D. et al. Clioquinol, a therapeutic agent for Alzheimer’s disease, has proteasome-inhibitory, androgen receptor-suppressing, apoptosis-inducing, and antitumor activities in human prostate cancer cells and xenografts. Cancer Res.67, 1636–1644 (2007). [DOI] [PubMed] [Google Scholar]
- 537.Lu, S. et al. Radiosensitization of clioquinol and zinc in human cancer cell lines. BMC Cancer18, 448 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.Yap, C., Short, J. L. & Nicolazzo, J. A. A combination of clioquinol, zinc and copper increases the abundance and function of breast cancer resistance protein in human brain microvascular endothelial cells. J. Pharm. Sci.110, 338–346 (2021). [DOI] [PubMed] [Google Scholar]
- 539.Tuller, E. R. et al. PPARalpha signaling mediates the synergistic cytotoxicity of clioquinol and docosahexaenoic acid in human cancer cells. Biochem. Pharm.77, 1480–1486 (2009). [DOI] [PubMed] [Google Scholar]
- 540.Du, T. et al. Clioquinol promotes cancer cell toxicity through tumor necrosis factor alpha release from macrophages. J. Pharm. Exp. Ther.324, 360–367 (2008). [DOI] [PubMed] [Google Scholar]
- 541.Schimmer, A. D. et al. A phase I study of the metal ionophore clioquinol in patients with advanced hematologic malignancies. Clin. Lymphoma Myeloma Leuk.12, 330–336 (2012). [DOI] [PubMed] [Google Scholar]
- 542.Balthazar, J. D. et al. 8-Hydroxyquinoline a natural chelating agent from Streptomyces spp. inhibits A549 lung cancer cell lines via BCL2/STAT3 regulating pathways. World J. Microbiol. Biotechnol.38, 182 (2022). [DOI] [PubMed] [Google Scholar]
- 543.Yang, Y. et al. Genomic and metabolic characterization of a chromophobe renal cell carcinoma cell line model (UOK276). Genes Chromosomes Cancer56, 719–729 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544.Liu, N. et al. A novel proteasome inhibitor suppresses tumor growth via targeting both 19S proteasome deubiquitinases and 20S proteolytic peptidases. Sci. Rep.4, 5240 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]