Abstract
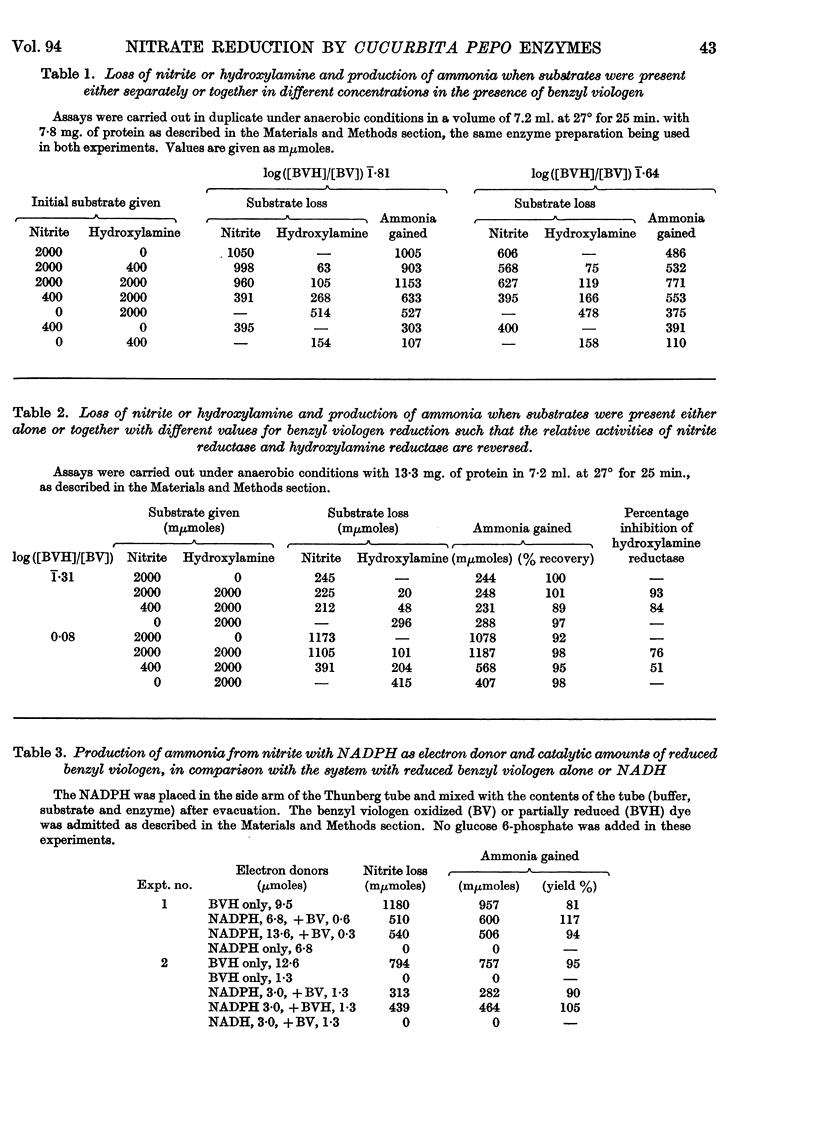
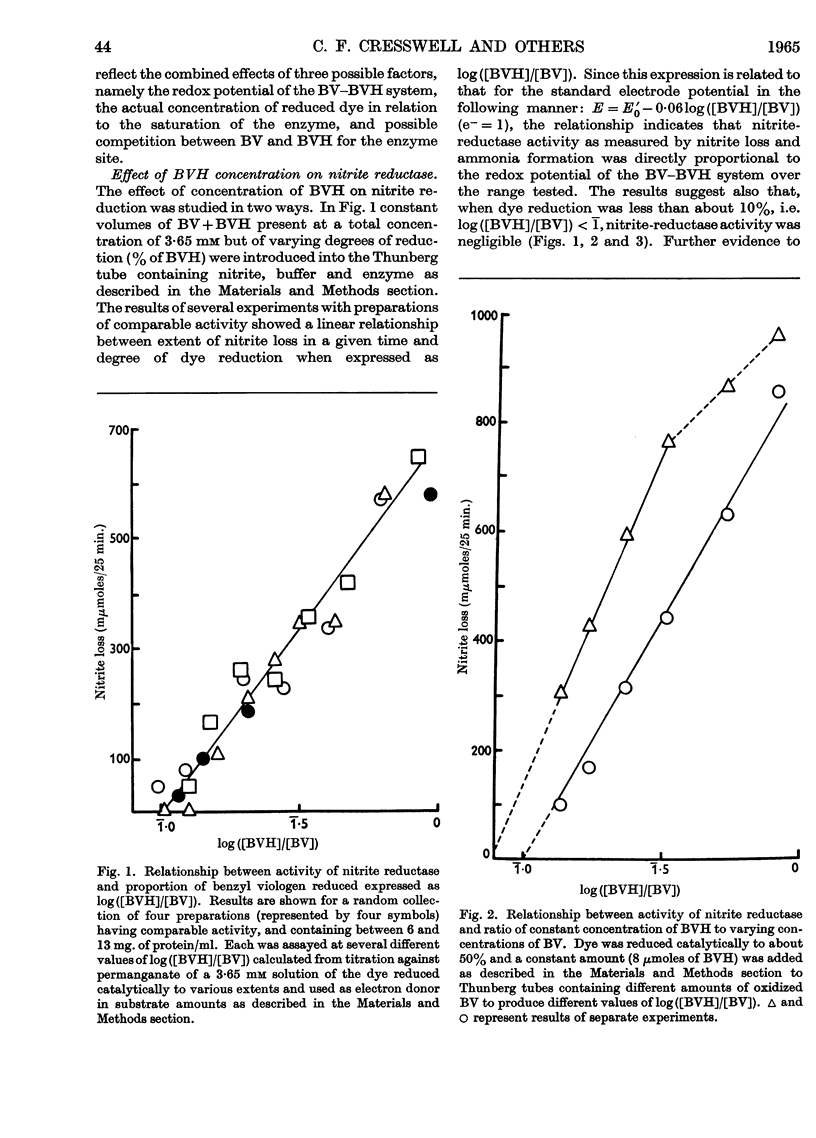
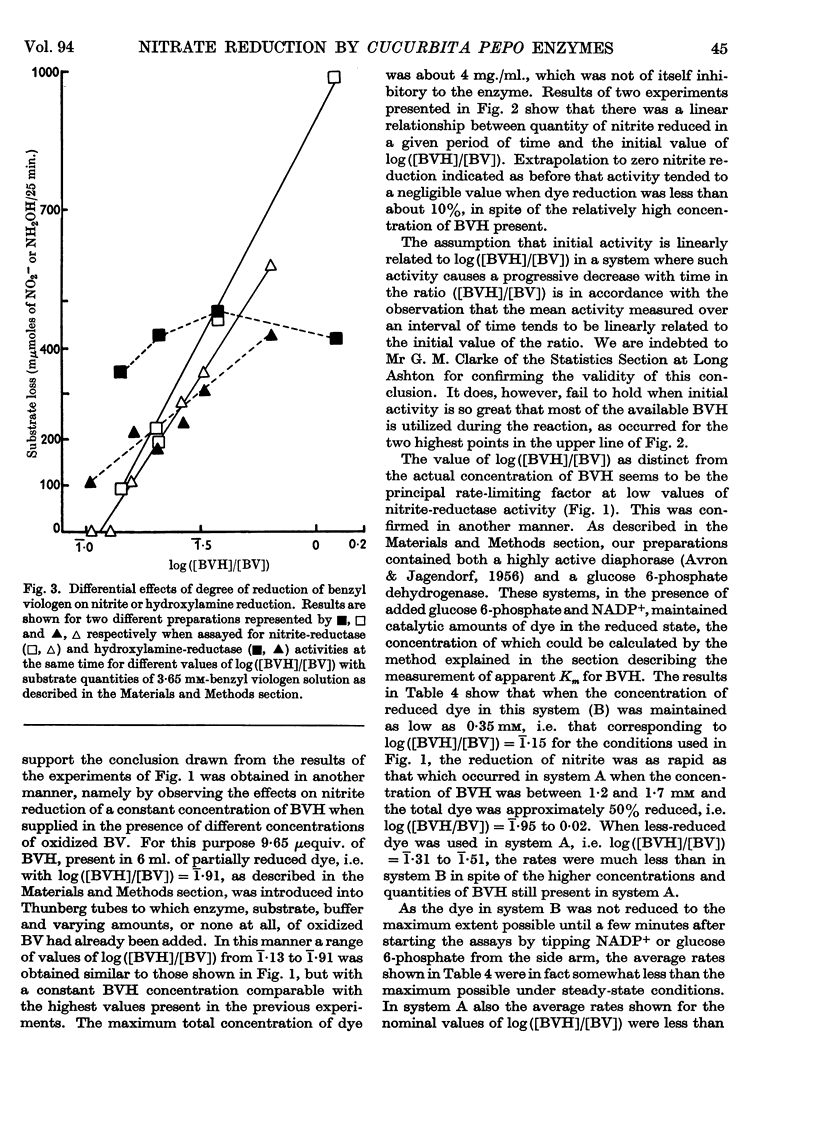
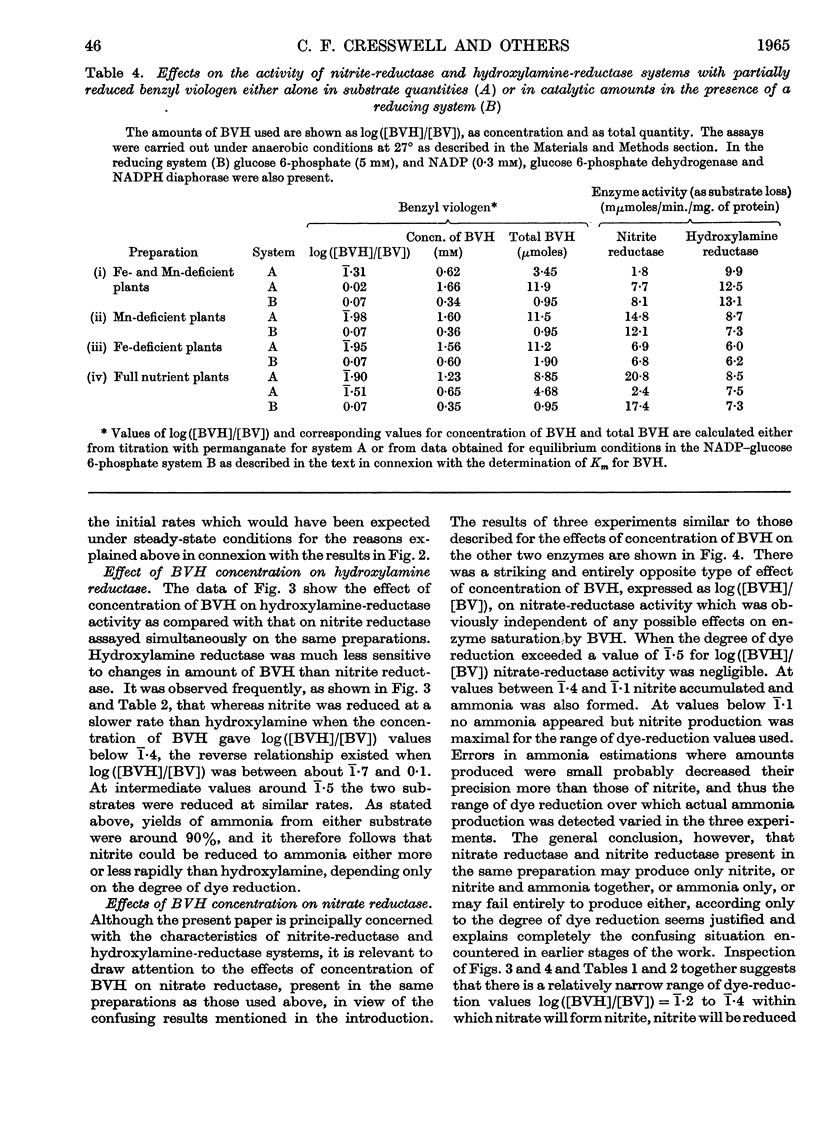
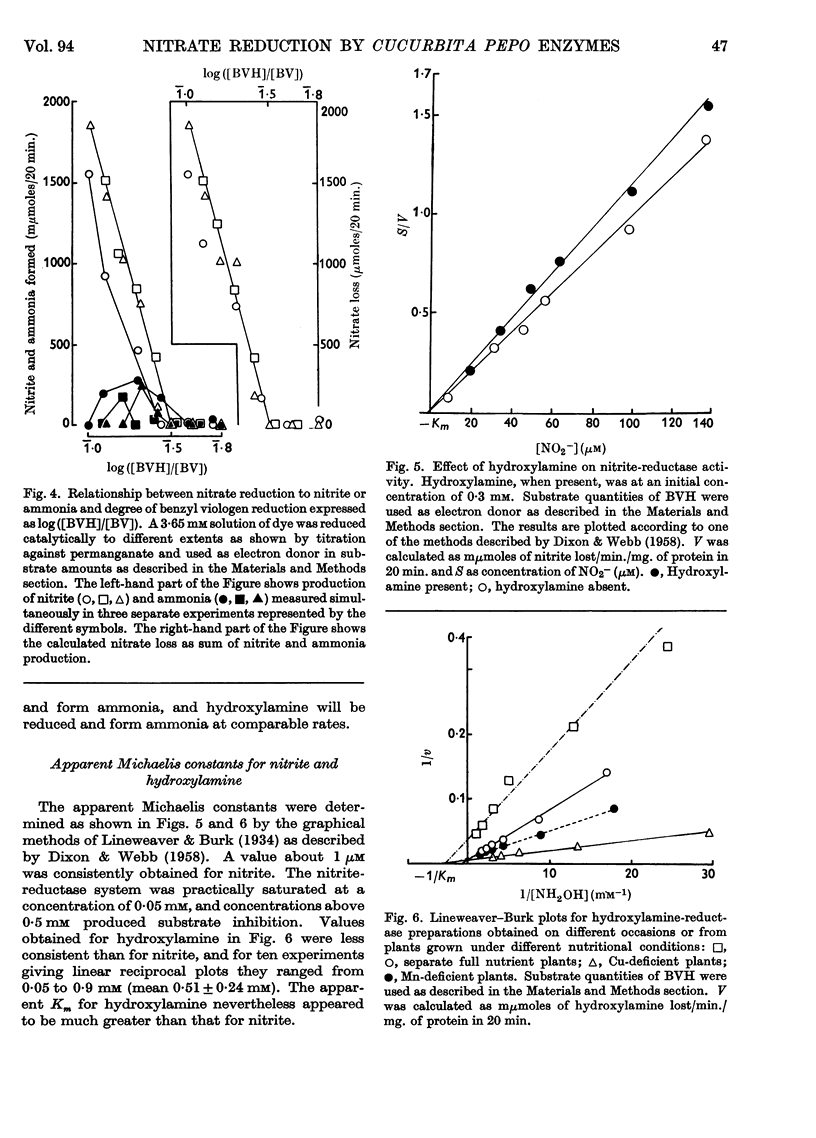
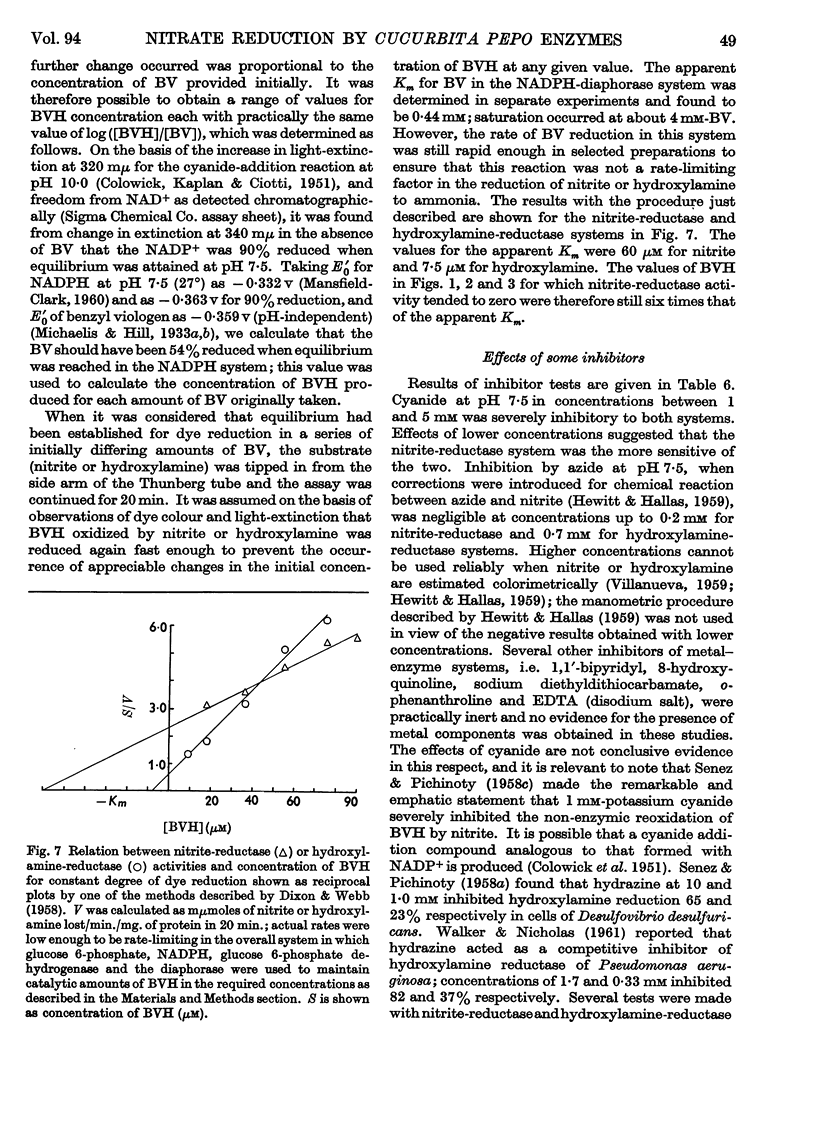
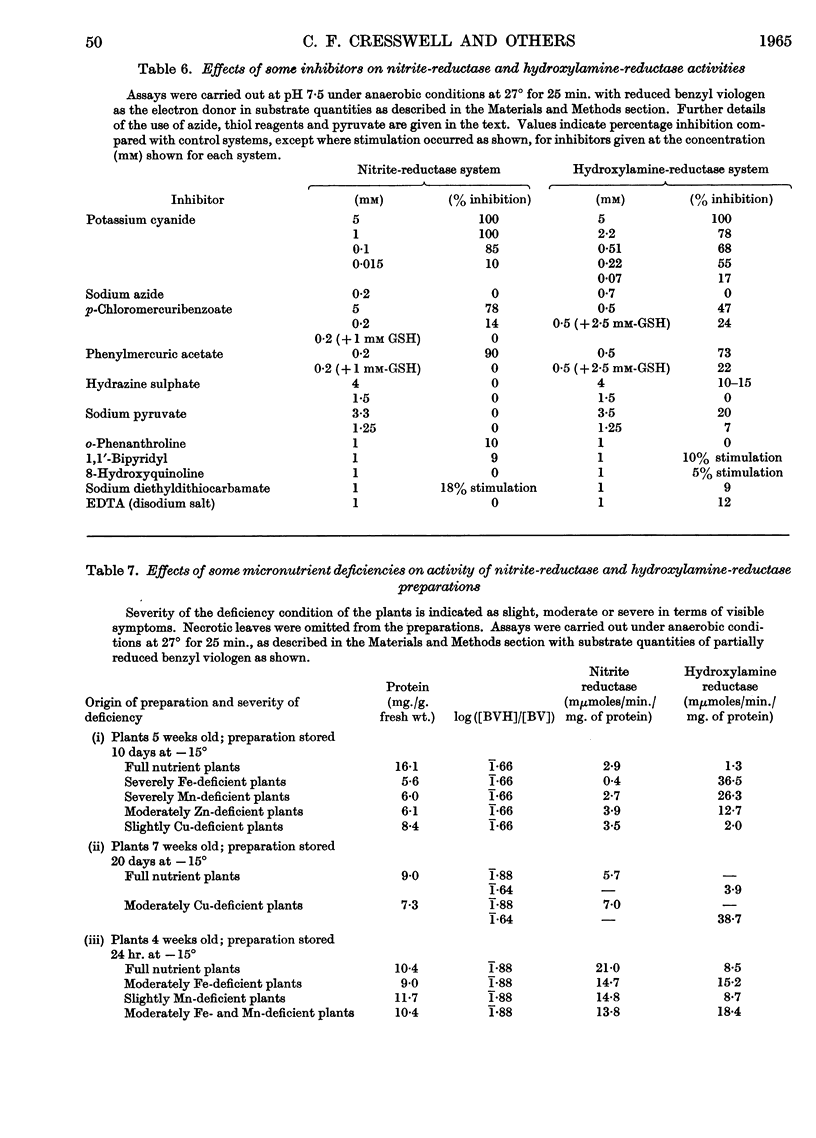
1. Enzyme systems from Cucurbita pepo have been shown to catalyse the reduction of nitrite and hydroxylamine to ammonia in yields about 90–100%. 2. Reduced benzyl viologen serves as an efficient electron donor for both systems. Activity of the nitrite-reductase system is directly related to degree of dye reduction when expressed in terms of the function for oxidation–reduction potentials, but appears to decrease to negligible activity below about 9% dye reduction. 3. NADH and NADPH alone produce negligible nitrite loss, but NADPH can be linked to an endogenous diaphorase system to reduce nitrite to ammonia in the presence of catalytic amounts of benzyl viologen. 4. The NADH– or NADPH–nitrate-reductase system that is also present can accept electrons from reduced benzyl viologen, but shows relationships opposite to that for the nitrite-reductase system with regard to effect of degree of dye reduction on activity. The product of nitrate reduction may be nitrite alone, or nitrite and ammonia, or ammonia alone, according only to the degree of dye reduction. 5. The relative activities of nitrite-reductase and hydroxylamine-reductase systems show different relationships with degree of dye reduction and may become reversed in magnitude when effects of degree of dye reduction are tested over a suitable range. 6. Nitrite severely inhibits the rate of reduction of hydroxylamine without affecting the yield of ammonia as a percentage of total substrate loss, but hydroxylamine has a negligible effect on the activity of the nitrite-reductase system. 7. The apparent Km for nitrite (1 μm) is substantially less than that for hydroxylamine, for which variable values between 0·05 and 0·9mm (mean 0·51 mm) have been observed. 8. The apparent Km values for reduced benzyl viologen differ for the nitrite-reductase and hydroxylamine-reductase systems: 60 and 7·5 μm respectively. 9. It is concluded that free hydroxylamine may not be an intermediate in the reduction of nitrite to ammonia by plants, and a possible mechanism for reduction of both compounds by the same enzyme system is discussed in the light of current ideas relating to other organisms.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVRON M., JAGENDORF A. T. A TPNH diaphorase from chloroplasts. Arch Biochem Biophys. 1956 Dec;65(2):475–490. doi: 10.1016/0003-9861(56)90207-7. [DOI] [PubMed] [Google Scholar]
- COLOWICK S. P., KAPLAN N. O., CIOTTI M. M. The reaction of pyridine nucleotide with cyanide and its analytical use. J Biol Chem. 1951 Aug;191(2):447–459. [PubMed] [Google Scholar]
- Candela M. I., Fisher E. G., Hewitt E. J. Molybdenum as a Plant Nutrient. X. Some Factors Affecting the Activity of Nitrate Reductase in Cauliflower Plants Grown with Different Nitrogen Sources and Molybdenum Levels in Sand Culture. Plant Physiol. 1957 Jul;32(4):280–288. doi: 10.1104/pp.32.4.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans H. J., Nason A. Pyridine Nucleotide-Nitrate Reductase from Extracts of Higher Plants. Plant Physiol. 1953 Apr;28(2):233–254. doi: 10.1104/pp.28.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FEWSON C. A., NICHOLAS D. J. Utilization of nitric oxide by micro-organisms and higher plants. Nature. 1960 Dec 3;188:794–796. doi: 10.1038/188794a0. [DOI] [PubMed] [Google Scholar]
- GIBBS M. B., BECKER E. L. Quantitation of haemagglutination by enumeration of free cells by an electronic counter. Nature. 1963 Apr 6;198:90–90. doi: 10.1038/198090a0. [DOI] [PubMed] [Google Scholar]
- HAGEMAN R. H., CRESSWELL C. F., HEWITT E. J. Reduction of nitrate, nitrite and hydroxylamine to ammonia by enzymes extracted from higher plants. Nature. 1962 Jan 20;193:247–250. doi: 10.1038/193247a0. [DOI] [PubMed] [Google Scholar]
- HEWITT E. J., HALLAS D. G. Measurement of inhibition by azide in biochemical assay systems involving nitrite estimation by diazotization. Nature. 1959 Nov 7;184(Suppl 19):1485–1485. doi: 10.1038/1841485a0. [DOI] [PubMed] [Google Scholar]
- KEMP J. D., ATKINSON D. E., EHRET A., LAZZARINI R. A. EVIDENCE FOR THE IDENTITY OF THE NICOTINAMIDE ADENINE DINUCLEOTIDE PHOSPHATE-SPECIFIC SULFITE AND NITRITE REDUCTASES OF ESCHERICHIA COLI. J Biol Chem. 1963 Oct;238:3466–3471. [PubMed] [Google Scholar]
- KESSLER E. Stoffwechselphysiologische Untersuchungen an Hydrogenase enthaltenden Grünalgen. II. Dunkel-Reduktion von Nitrat und Nitrit mit molekularem Wasserstoff. Arch Mikrobiol. 1957;27(2):166–181. [PubMed] [Google Scholar]
- LAZZARINI R. A., ATKINSON D. E. A triphosphopyridine nucleotide-specific nitrite reductase from Escherichia coli. J Biol Chem. 1961 Dec;236:3330–3335. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MAGER J. A TPNH-linked reductase and its relation to hydroxylamine reductase in Enterobacteriaceae. Biochim Biophys Acta. 1960 Jul 15;41:553–555. doi: 10.1016/0006-3002(60)90065-2. [DOI] [PubMed] [Google Scholar]
- MORTENSON L. E., VALENTINE R. C., CARNAHAN J. E. An electron transport factor from Clostridium pasteurianum. Biochem Biophys Res Commun. 1962 Jun 4;7:448–452. doi: 10.1016/0006-291x(62)90333-9. [DOI] [PubMed] [Google Scholar]
- NASON A. Symposium on metabolism of inorganic compounds. II. Enzymatic pathways of nitrate, nitrite, and hydroxylamine metabolisms. Bacteriol Rev. 1962 Mar;26:16–41. doi: 10.1128/br.26.1.16-41.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NASON A., TAKAHASHI H. Inorganic nitrogen metabolism. Annu Rev Microbiol. 1958;12:203–246. doi: 10.1146/annurev.mi.12.100158.001223. [DOI] [PubMed] [Google Scholar]
- SENEZ J. C., PICHINOTY F. Reduction de l'hydroxylamine liée à l'activité de l'hydrogénase de Desulfovibrio desulfuricans. I. Activité des cellules et des extraits. Biochim Biophys Acta. 1958 Mar;27(3):569–580. doi: 10.1016/0006-3002(58)90388-3. [DOI] [PubMed] [Google Scholar]
- SENEZ J. C., PICHINOTY F. Reduction de l'hydroxylamine liée à l'activité de l'hydrogénase de Desulfovibrio desulfuricans. Il. Nature du système enzymatique et du transporteur d'électrons intervenant dans la réaction. Biochim Biophys Acta. 1958 May;28(2):355–369. doi: 10.1016/0006-3002(58)90483-9. [DOI] [PubMed] [Google Scholar]
- STAHMANN M. A., BUCHANAN-DAVIDSON D. J., LAPRESEL C., GRABAR P. Immunochemical studies of polypeptidyl proteins and synthetic polypeptides. Nature. 1959 Aug 15;184(Suppl 8):549–550. doi: 10.1038/184549b0. [DOI] [PubMed] [Google Scholar]
- TAGAWA K., ARNON D. I. Ferredoxins as electron carriers in photosynthesis and in the biological production and consumption of hydrogen gas. Nature. 1962 Aug 11;195:537–543. doi: 10.1038/195537a0. [DOI] [PubMed] [Google Scholar]
- VALENTINE R. C., JACKSON R. L., WOLFE R. S. Role of ferredoxin in hydrogen metabolism of Micrococcus lactilyticus. Biochem Biophys Res Commun. 1962 Jun 4;7:453–456. doi: 10.1016/0006-291x(62)90334-0. [DOI] [PubMed] [Google Scholar]
- WALKER G. C., NICHOLAS D. J. Hydroxylamine reductase from Pseudomonas aeruginosa. Biochim Biophys Acta. 1961 May 13;49:361–368. doi: 10.1016/0006-3002(61)90135-4. [DOI] [PubMed] [Google Scholar]
- WHITELEY H. R., WOOLFOLK C. A. Ferredoxin-dependent reactions in Micrococcus lactilyticus. Biochem Biophys Res Commun. 1962 Dec 19;9:517–522. doi: 10.1016/0006-291x(62)90118-3. [DOI] [PubMed] [Google Scholar]
- YAMAFUJI K., AKITA T. On transoximation. Enzymologia. 1952 Sep 1;15(5):313–318. [PubMed] [Google Scholar]
- YAMAFUJI K., OSAJIMA Y. Ammonia dehydrogenase, hydroxylamine dehydrogenase, hyponitrite dehydrogenase and nitrite dehydrogenase. Nature. 1961 May 6;190:534–535. doi: 10.1038/190534a0. [DOI] [PubMed] [Google Scholar]


