Abstract
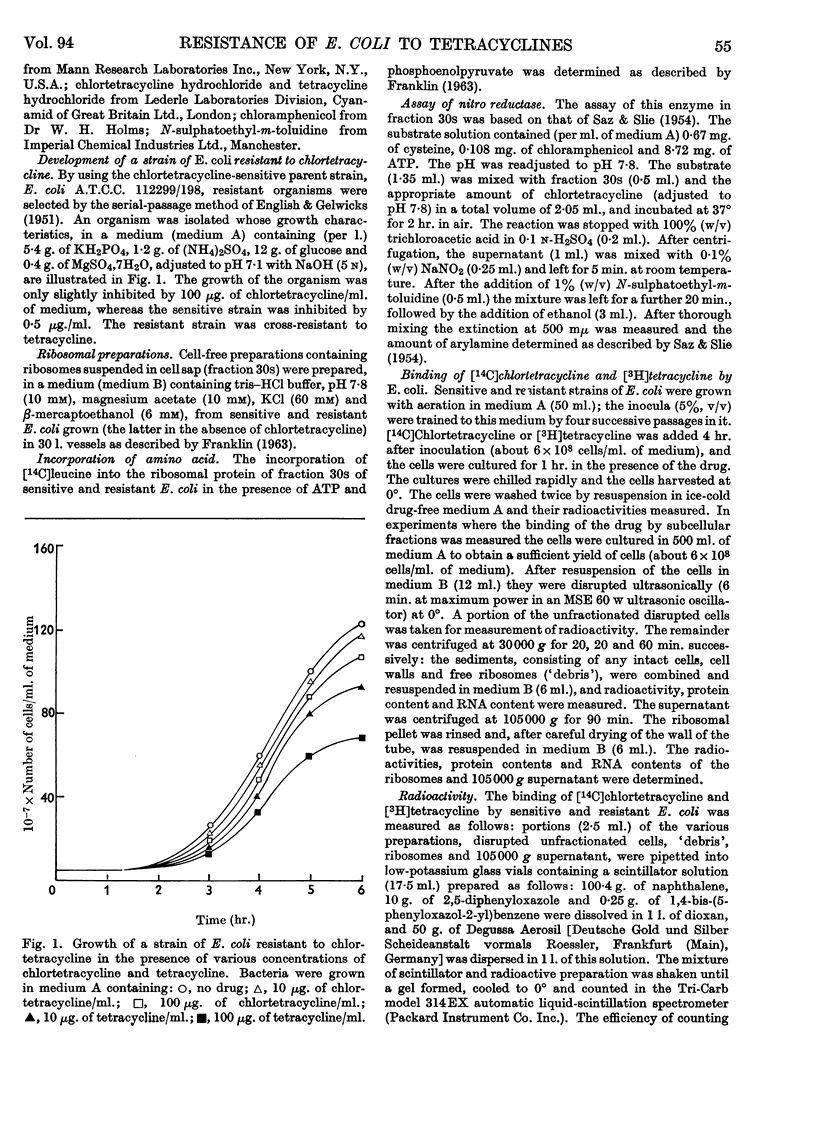
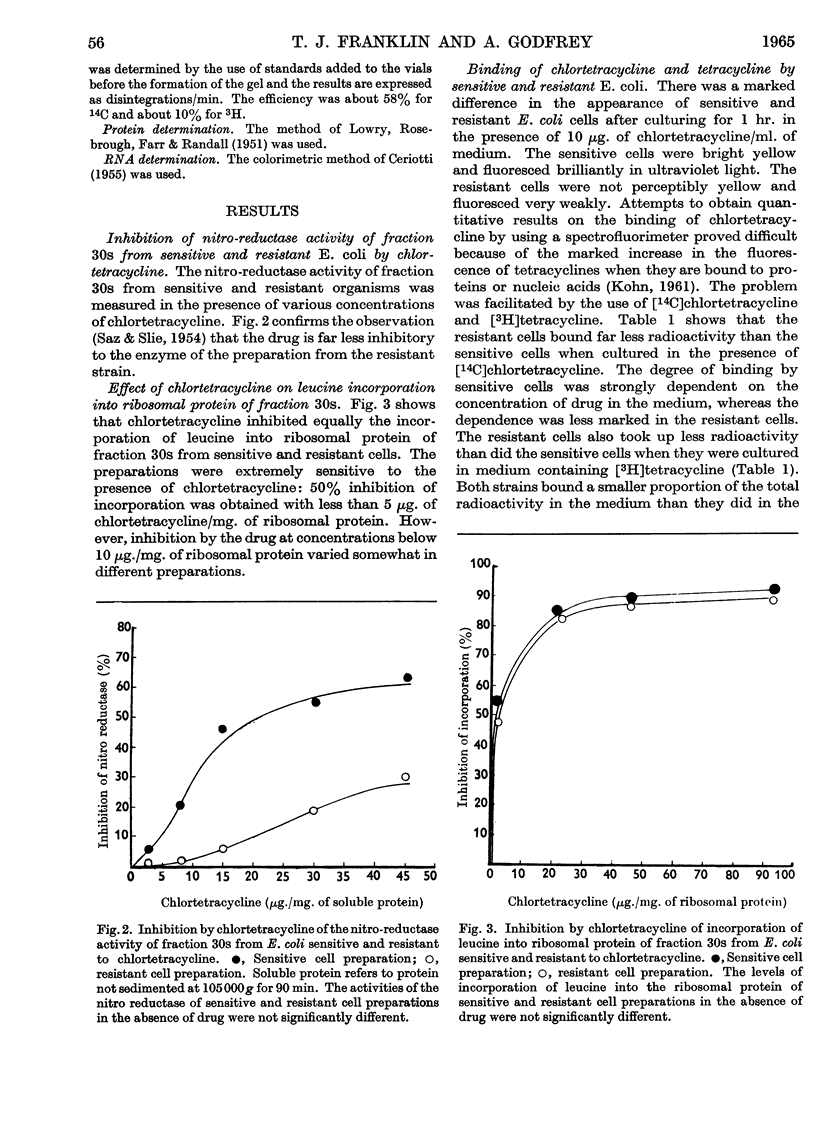
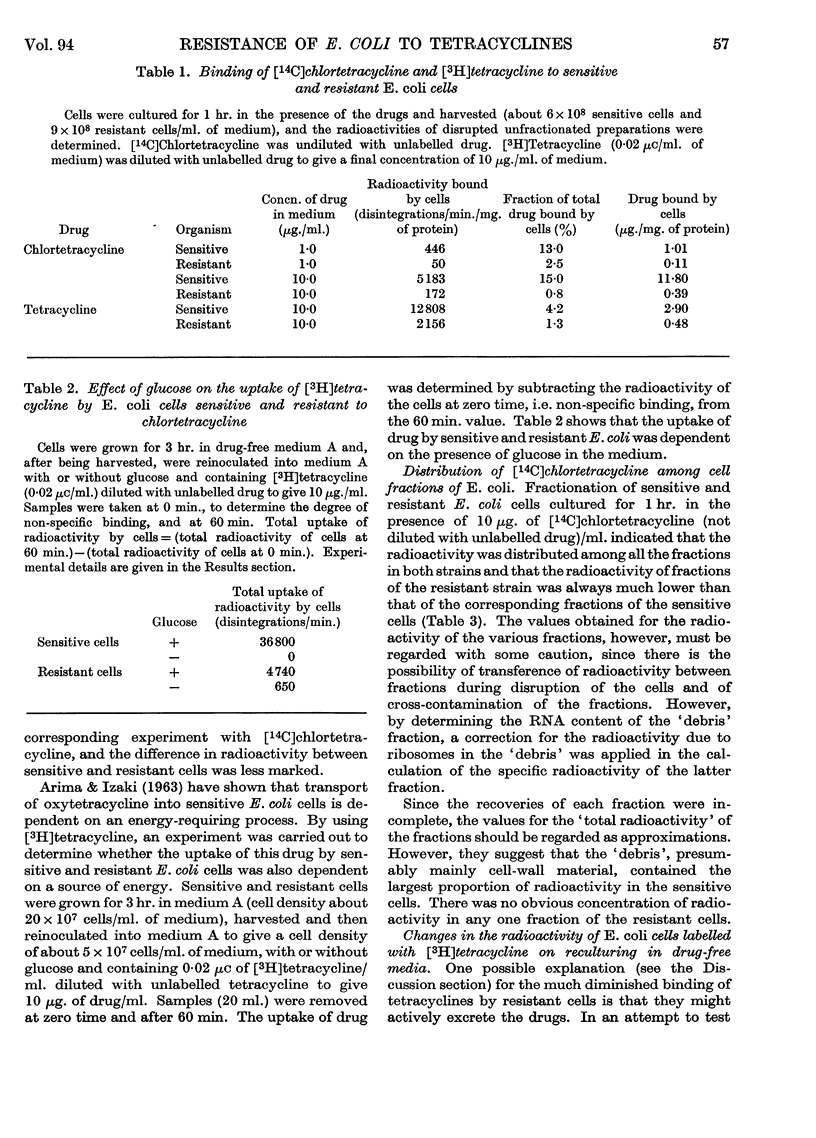
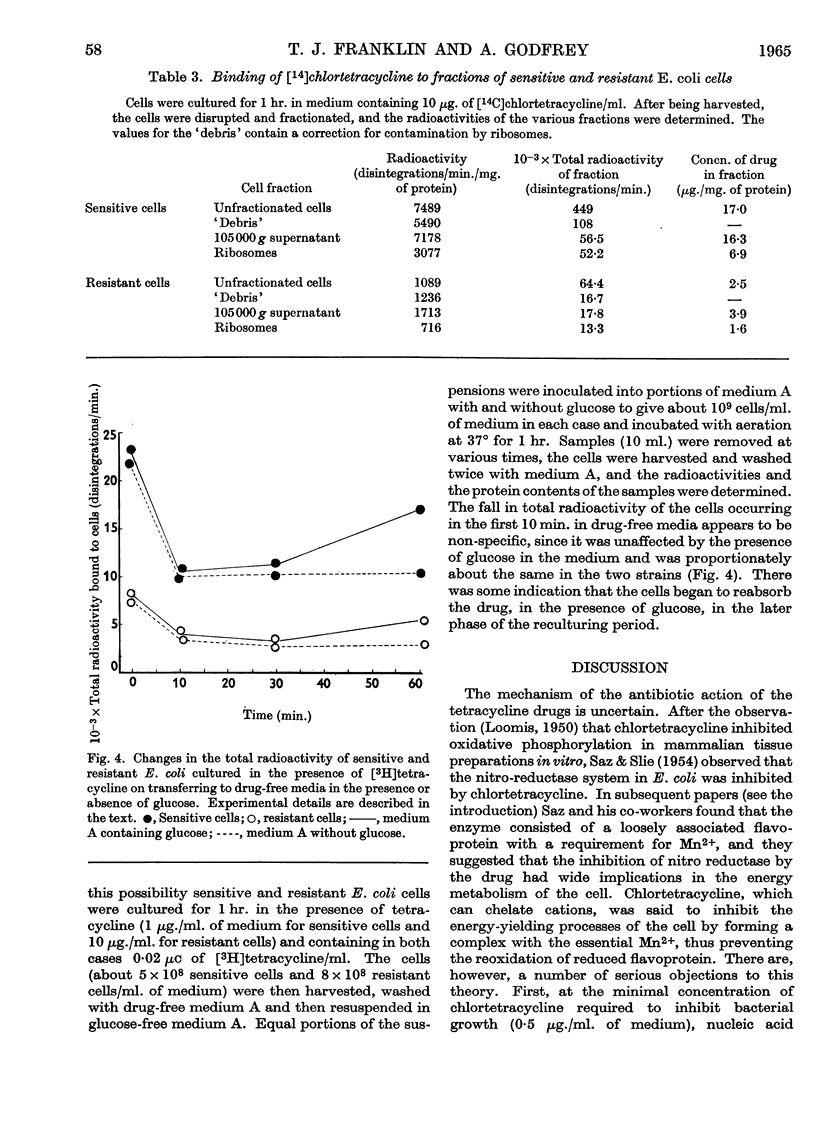
1. A strain of Escherichia coli highly resistant to chlortetracycline and partially cross-resistant to tetracycline has been isolated. 2. The nitro-reductase system of the resistant cells was inhibited to a smaller extent by chlortetracycline than was the corresponding enzyme of sensitive cells. 3. The incorporation of leucine in vitro into the ribosomal protein of cell-free preparations from sensitive and resistant cells was equally inhibited by chlortetracycline. 4. Resistant cells accumulated much less chlortetracycline and tetracycline than did sensitive cells when both were cultured in the presence of these drugs. 5. The uptake of tetracycline by both sensitive and resistant E. coli was dependent on the presence of glucose in the medium. 6. Fractionation of cells cultured in medium containing [14C]chlortetracycline indicated that the largest proportion of radioactivity in sensitive cells was in the fraction consisting mainly of cell-wall material. There was no concentration of radioactivity in any one fraction of the resistant cells. 7. No evidence could be obtained for a specific tetracycline-excretion system in the resistant cells. 8. The significance of these results in relation to current theories of the antibiotic action of and resistance to the tetracycline drugs is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARIMA K., IZAKI K. ACCUMULATION OF OXYTETRACYCLINE RELEVANT TO ITS BACTERICIDAL ACTION IN THE CELLS OF ESCHERICHIA COLI. Nature. 1963 Oct 12;200:192–193. doi: 10.1038/200192a0. [DOI] [PubMed] [Google Scholar]
- CERIOTTI G. Determination of nucleic acids in animal tissues. J Biol Chem. 1955 May;214(1):59–70. [PubMed] [Google Scholar]
- FLAKS J. G., COX E. C., WITTING M. L., WHITE J. R. Polypeptide synthesis with ribosomes from streptomycin-resistant and dependent E. coli. Biochem Biophys Res Commun. 1962 May 11;7:390–393. doi: 10.1016/0006-291x(62)90321-2. [DOI] [PubMed] [Google Scholar]
- FRANKLIN T. J. The inhibition of incorporation of leucine into protein of cell-free systems from rat liver and Escherichia coli by chlortetracycline. Biochem J. 1963 Jun;87:449–453. doi: 10.1042/bj0870449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin T. J. The effect of chlortetracycline on the transfer of leucine and 'transfer' ribonucleic acid to rat-liver ribosomes in vitro. Biochem J. 1964 Mar;90(3):624–628. doi: 10.1042/bj0900624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALE E. F., FOLKES J. P. The assimilation of amino-acids by bacteria. XV. Actions of antibiotics on nucleic acid and protein synthesis in Staphylococcus aureus. Biochem J. 1953 Feb;53(3):493–498. doi: 10.1042/bj0530493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IZAKI K., ARIMA K. DISAPPEARANCE OF OXYTETRACYCLINE ACCUMULATION IN THE CELLS OF MULTIPLE DRUG-RESISTANT ESCHERICHIA COLI. Nature. 1963 Oct 26;200:384–385. doi: 10.1038/200384a0. [DOI] [PubMed] [Google Scholar]
- KOHN K. W. Mediation of divalent metal ions in the binding of tetracycline to macromolecules. Nature. 1961 Sep 16;191:1156–1158. doi: 10.1038/1911156a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laskin A. I., May Chan W. Inhibition by tetracyclines of polyuridylic acid directed phenylalanine incorporation in Escherichia coli cell-free systems. Biochem Biophys Res Commun. 1964;14:137–142. doi: 10.1016/0006-291x(64)90243-8. [DOI] [PubMed] [Google Scholar]
- Loomis W. F. On the Mechanism of Action of Aureomycin. Science. 1950 Apr 28;111(2887):474–474. doi: 10.1126/science.111.2887.474. [DOI] [PubMed] [Google Scholar]
- MARTINEZ L. M., SAZ A. K. Enzymatic basis of resistance to aureomycin. I. Differences between flavoprotein nitro reductases of sensitive and resistant Escherichia coli. J Biol Chem. 1956 Nov;223(1):285–292. [PubMed] [Google Scholar]
- RAMSEY C. H., EDWARDS P. R. Resistance of Salmonellae isolated in 1959 and 1960 to tetracyclines and chloramphenicol. Appl Microbiol. 1961 Sep;9:389–391. doi: 10.1128/am.9.5.389-391.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAZ A. K., BROWNELL L. W., SLIE R. B. Aureomycin-resistant cell-free nitro reductase from aureomycin-resistant Escherichia coli. J Bacteriol. 1956 Apr;71(4):421–424. doi: 10.1128/jb.71.4.421-424.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAZ A. K., MARTINEZ L. M. Enzymiatic basis of resistance to aureomycin. III. Inhibition by aureomycin of protein-stimulated electron transport in Eschericia coli. J Bacteriol. 1960 Apr;79:527–531. doi: 10.1128/jb.79.4.527-531.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAZ A. K., SLIE R. B. The inhibition of organiz nitro reductase by aureomycin in cell-free extracts. II. Cofactor requirements for the nitro reductase enzyme complex. Arch Biochem Biophys. 1954 Jul;51(1):5–16. doi: 10.1016/0003-9861(54)90447-6. [DOI] [PubMed] [Google Scholar]


