Abstract
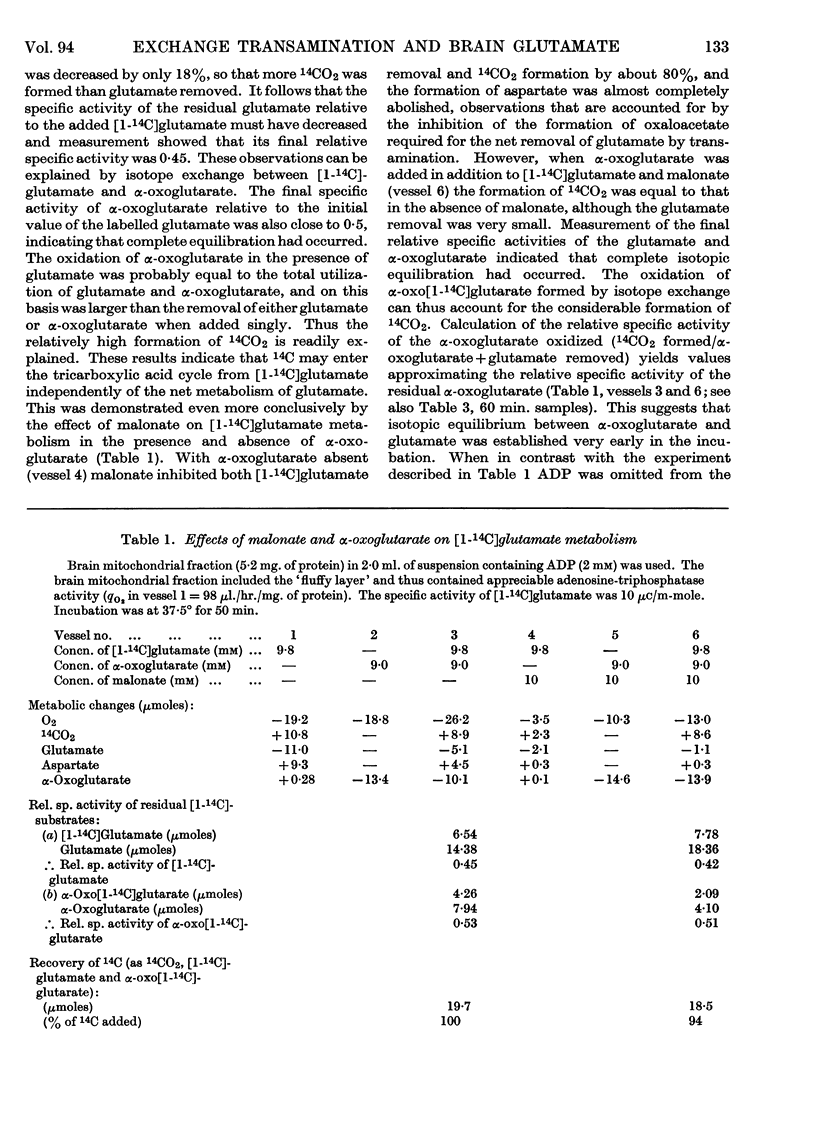
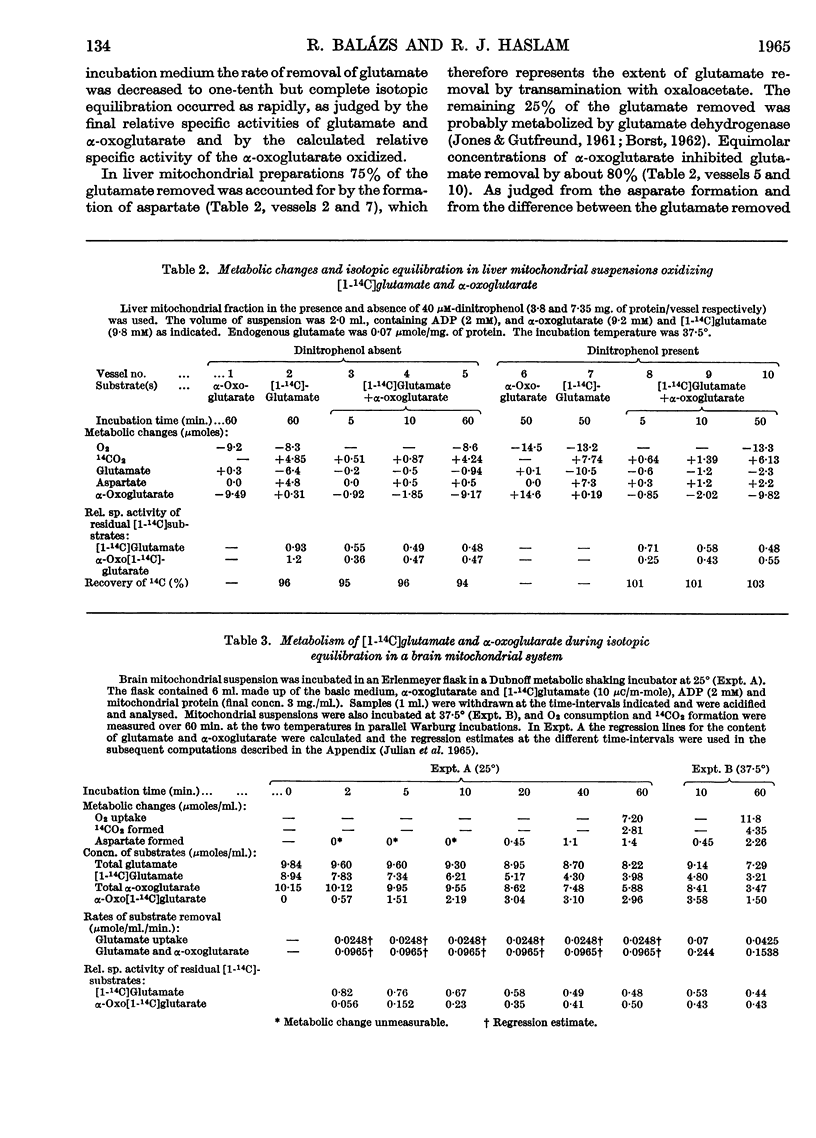
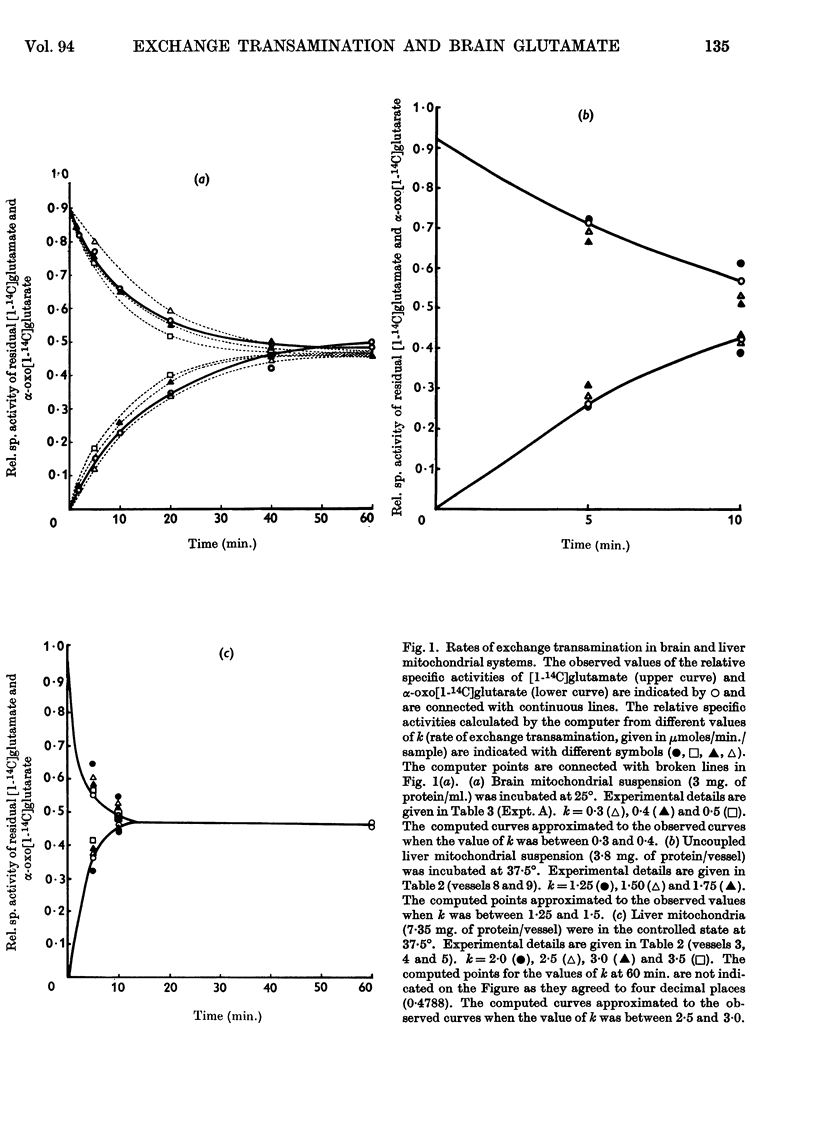
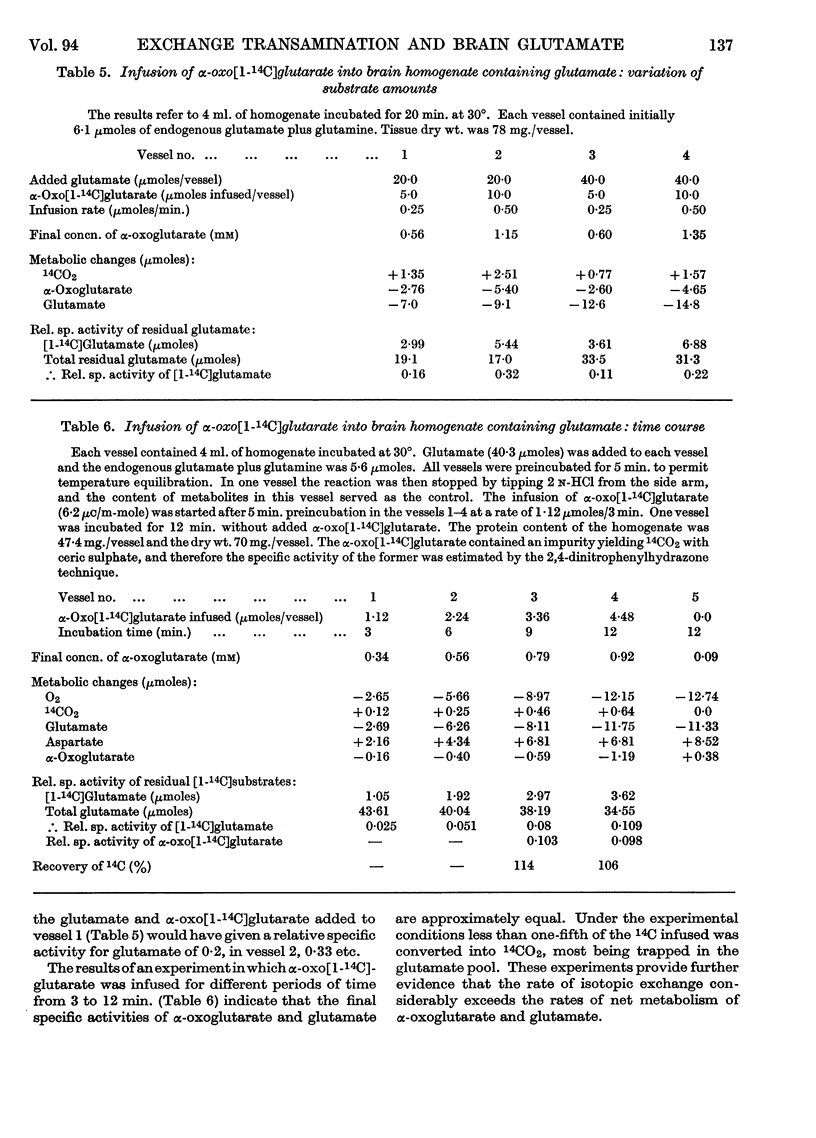
1. Experiments were performed to throw light on why the incorporation of 14C from labelled carbohydrate precursors into glutamate has been found to be more marked in brain than in other tissues. 2. Rapid isotope exchange between labelled glutamate and unlabelled α-oxoglutarate was demonstrated in brain and liver mitochondrial preparations. In the presence but not in the absence of α-oxoglutarate the yield of 14CO2 from [1-14C]glutamate exceeded the net glutamate removal, and the final relative specific activities of the two substrates indicated that complete isotopic equilibration had occurred. Also, when in a brain preparation net glutamate removal was inhibited by malonate, isotope exchange between [1-14C]glutamate and α-oxoglutarate and the formation of 14CO2 were unaffected. 3. The time-course of isotope exchange between labelled glutamate and unlabelled α-oxoglutarate was followed in uncoupled brain and liver mitochondrial fractions, and the rate of exchange calculated by a computer was found to be 3–8 times more rapid than the maximal rate of utilization of the two substrates. 4. The physiological situation was imitated by the continuous infusion of small amounts of α-oxo[1-14C]glutarate into brain homogenate containing added glutamate. The fraction of 14C infused that was retained in the glutamate pool depended on the size of the latter, and the final relative specific activities of the two substrates indicated almost complete isotope exchange. Isotopic equilibration also occurred when α-oxoglutarate was generated from pyruvate through the tricarboxylic acid cycle in a brain mitochondrial preparation containing [1-14C]glutamate. 5. The differences in the incorporation of 14C from labelled glucose into the glutamate of brain and liver are discussed in terms of the rates of isotope exchange, the glutamate pool sizes and the rates of formation of labelled α-oxoglutarate in the two tissues. It is concluded that the differences between tissues in the incorporation of glucose carbon into glutamate reflect features of their metabolism largely unrelated to that of glutamate.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALDRIDGE W. N. Liver and brain mitochondria. Biochem J. 1957 Nov;67(3):423–431. doi: 10.1042/bj0670423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASHMORE J., CAHILL G. F., Jr, HASTINGS A. B., ZOTTU S. Studies on carbohydrate metabolism in rat liver slices. VIII. Effect of ions and hormones on pathways of glucose-6-phosphate metabolism. J Biol Chem. 1957 Jan;224(1):225–235. [PubMed] [Google Scholar]
- BALAZS R., BIESOLD D., MAGYAR K. SOME PROPERTIES OF RAT BRAIN MITOCHONDRIAL PREPARATIONS: RESPIRATORY CONTROL. J Neurochem. 1963 Oct;10:685–708. doi: 10.1111/j.1471-4159.1963.tb08926.x. [DOI] [PubMed] [Google Scholar]
- BARKULIS S. S., GEIGER A., KAWAKITA Y., AGUILAR V. A study on the incorporation of 14C derived from glucose into the free amino acids of the brain cortex. J Neurochem. 1960 Jun;5:339–348. doi: 10.1111/j.1471-4159.1960.tb13372.x. [DOI] [PubMed] [Google Scholar]
- BELOFF-CHAIN A., CATANZARO R., CHAIN E. B., MASI I., POCCHIARA F. Fate of uniformly labelled 14C glucose in brain slices. Proc R Soc Lond B Biol Sci. 1955 Aug 16;144(914):22–28. doi: 10.1098/rspb.1955.0031. [DOI] [PubMed] [Google Scholar]
- BORST P. The pathway of glutamate oxidation by mitochondria isolated from different tissues. Biochim Biophys Acta. 1962 Feb 26;57:256–269. doi: 10.1016/0006-3002(62)91119-8. [DOI] [PubMed] [Google Scholar]
- BOYD J. W. The intracellular distribution, latency and electrophoretic mobility of L-glutamate-oxaloacetate transaminase from rat liver. Biochem J. 1961 Nov;81:434–441. doi: 10.1042/bj0810434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUSCH H., GOLDBERG M. H., ANDERSON D. C. Substrate effects on metabolic patterns of pyruvate-2-C14 in tissue slices. Cancer Res. 1956 Feb;16(2):175–181. [PubMed] [Google Scholar]
- CREMER J. E. AMINO ACID METABOLISM IN RAT BRAIN STUDIED WITH 14C-LABELLED GLUCOSE. J Neurochem. 1964 Mar;11:165–185. doi: 10.1111/j.1471-4159.1964.tb06127.x. [DOI] [PubMed] [Google Scholar]
- GAITONDE M. K., MARCHI S. A., RICHTER D. THE UTILIZATION OF GLUCOSE IN THE BRAIN AND OTHER ORGANS OF THE CAT. Proc R Soc Lond B Biol Sci. 1964 Apr 14;160:124–136. doi: 10.1098/rspb.1964.0031. [DOI] [PubMed] [Google Scholar]
- GEY K. F. The concentration of glucose in rat tissues. Biochem J. 1956 Sep;64(1):145–150. doi: 10.1042/bj0640145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUTFREUND H., EBNER K. E., MENDIOLA L. Transamination and the control of mitochondrial pathways. Nature. 1961 Dec 2;192:820–823. doi: 10.1038/192820a0. [DOI] [PubMed] [Google Scholar]
- HASLAM R. J., KREBS H. A. Substrate competition in the respiration of animal tissues. The metabolic interactions of pyruvate and alpha-oxoglutarate in rat-liver homogenates. Biochem J. 1963 Mar;86:432–446. doi: 10.1042/bj0860432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASLAM R. J., KREBS H. A. THE METABOLISM OF GLUTAMATE IN HOMOGENATES AND SLICES OF BRAIN CORTEX. Biochem J. 1963 Sep;88:566–578. doi: 10.1042/bj0880566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENKINS W. T., SIZER I. W. Glutamic aspartic transaminase. II. The influence of pH on absorption spectrum and enzymatic activity. J Biol Chem. 1959 May;234(5):1179–1181. [PubMed] [Google Scholar]
- JONES E. A., GUTFREUND H. The control of some oxidative pathways in guinea-pig mammary-gland mitochondria. Biochem J. 1961 Jun;79:608–614. doi: 10.1042/bj0790608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian T., Balázs R., Haslam R. J. Appendix-Calculation of rate of exchange transamination. Biochem J. 1965 Jan;94(1):142–142. doi: 10.1042/bj0940142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARMEN A. A note on the spectrometric assay of glutamic-oxalacetic transaminase in human blood serum. J Clin Invest. 1955 Jan;34(1):131–133. [PubMed] [Google Scholar]
- KINI M. M., QUASTEL J. H. Carbohydrate--amino-acid inter-relations in brain cortex in vitro. Nature. 1959 Jul 25;184:252–256. doi: 10.1038/184252a0. [DOI] [PubMed] [Google Scholar]
- KREBS H. A., BELLAMY D. The interconversion of glutamic acid and aspartic acid in respiring tissues. Biochem J. 1960 Jun;75:523–529. doi: 10.1042/bj0750523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- NISONOFF A., BARNES F. W., Jr, ENNS T., VON SCHUCHING S. Mechanisms in enzymatic transamination reaction between carbon chains of same length. Bull Johns Hopkins Hosp. 1954 Mar;94(3):117–127. [PubMed] [Google Scholar]
- OLSON J. A., ANFINSEN C. B. The crystallization and characterization of L-glutamic acid dehydrogenase. J Biol Chem. 1952 May;197(1):67–79. [PubMed] [Google Scholar]
- ROBERTS E., BREGOFF H. M. Transamination of gamma-aminobutyric acid and beta-alanine in brain and liver. J Biol Chem. 1953 Mar;201(1):393–398. [PubMed] [Google Scholar]
- ROBERTS R. B., FLEXNER J. B., FLEXNER L. B. Biochemical and physiological differentiation during morphogenesis. XXIII. Further observations relating to the synthesis of amino acids and proteins by the cerebral cortex and liver of the mouse. J Neurochem. 1959 Apr;4(1):78–90. doi: 10.1111/j.1471-4159.1959.tb13176.x. [DOI] [PubMed] [Google Scholar]
- SCHWERIN P., BESSMAN S. P., WAELSCH H. The uptake of glutamic acid and glutamine by brain and other tissues of the rat and mouse. J Biol Chem. 1950 May;184(1):37–44. [PubMed] [Google Scholar]
- SWAIMAN K. F., MILSTEIN J. M., COHEN M. M. INTERRELATIONSHIPS OF GLUCOSE AND GLUTAMIC ACID METABOLISM IN DEVELOPING RABBIT BRAIN. J Neurochem. 1963 Sep;10:635–639. doi: 10.1111/j.1471-4159.1963.tb08935.x. [DOI] [PubMed] [Google Scholar]
- Smith M. J., Moses V. Uncoupling reagents and metabolism. 1. Effects of salicylate and 2:4-dinitrophenol on the incorporation of C from labelled glucose and acetate into the soluble intermediates of isolated rat tissues. Biochem J. 1960 Sep;76(3):579–585. doi: 10.1042/bj0760579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VELICK S. F., VAVRA J. A kinetic and equilibrium analysis of the glutamic oxaloacetate transaminase mechanism. J Biol Chem. 1962 Jul;237:2109–2122. [PubMed] [Google Scholar]
- VRBA R., GAITONDE M. K., RICHTER D. The conversion of glucose carbon into protein in the brain and other organs of the rat. J Neurochem. 1962 Sep-Oct;9:465–475. doi: 10.1111/j.1471-4159.1962.tb04199.x. [DOI] [PubMed] [Google Scholar]


