Abstract
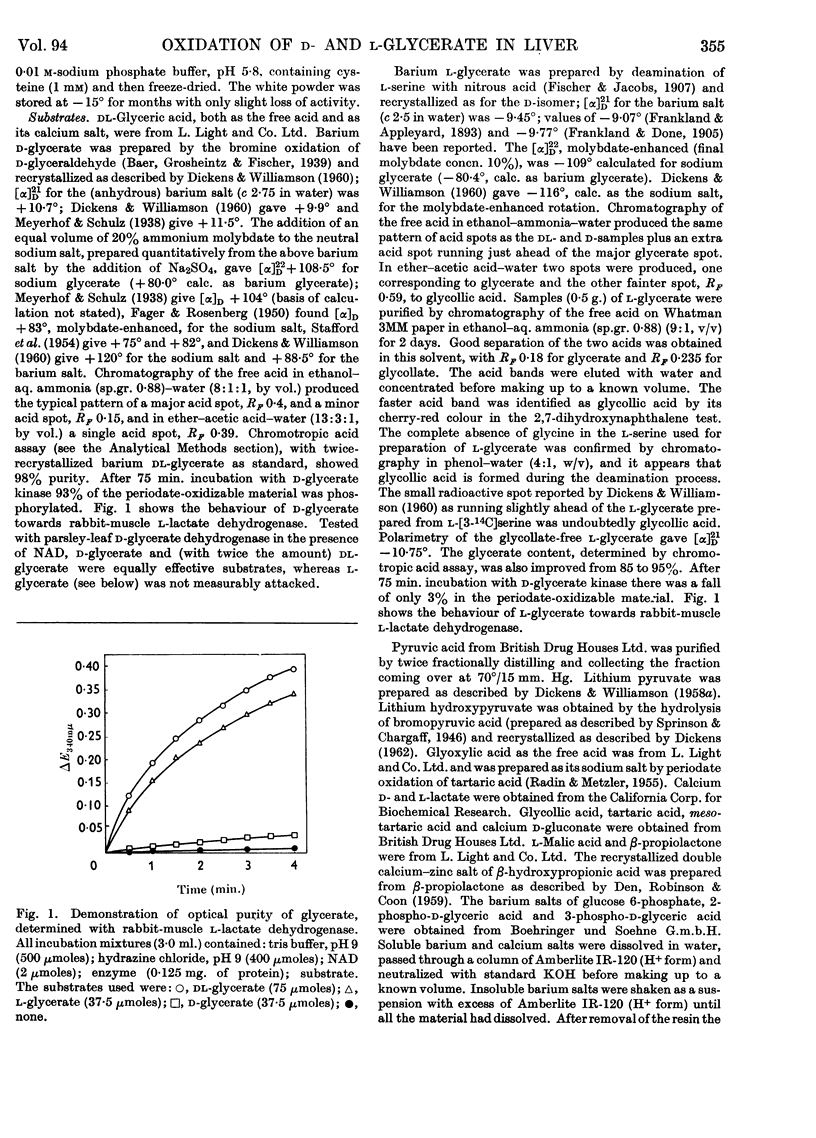
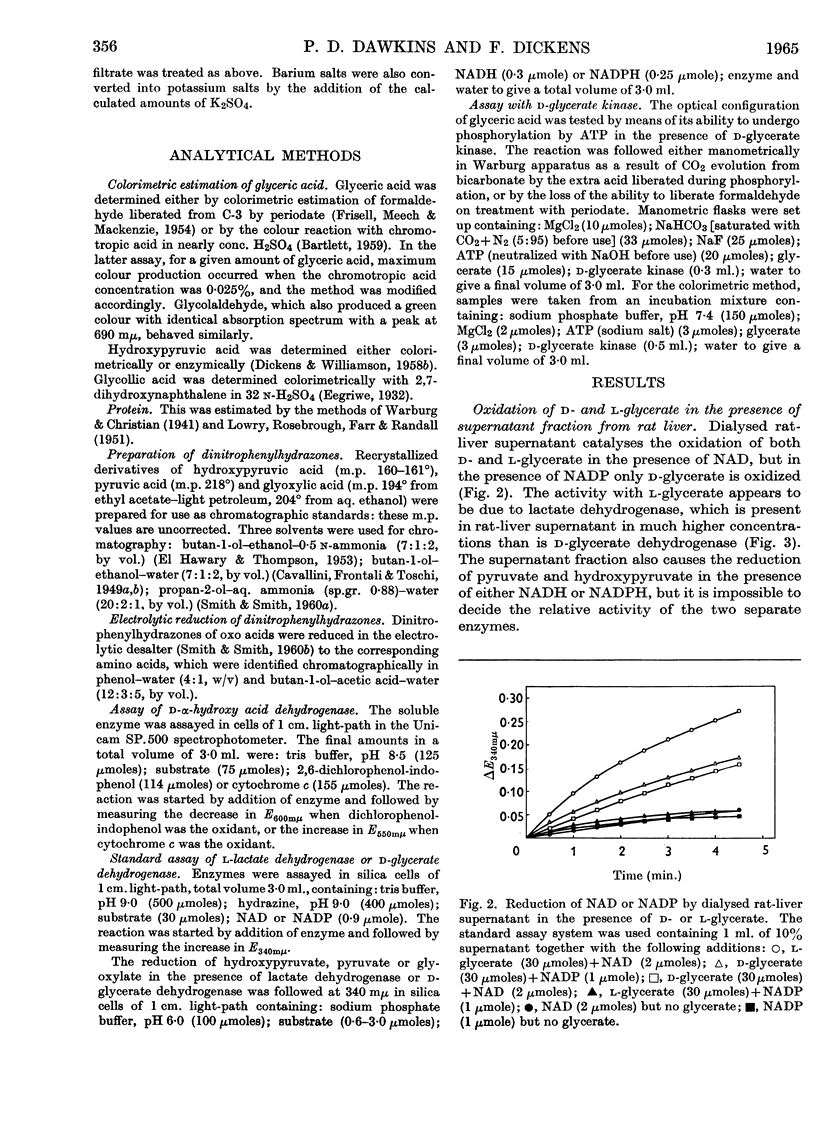
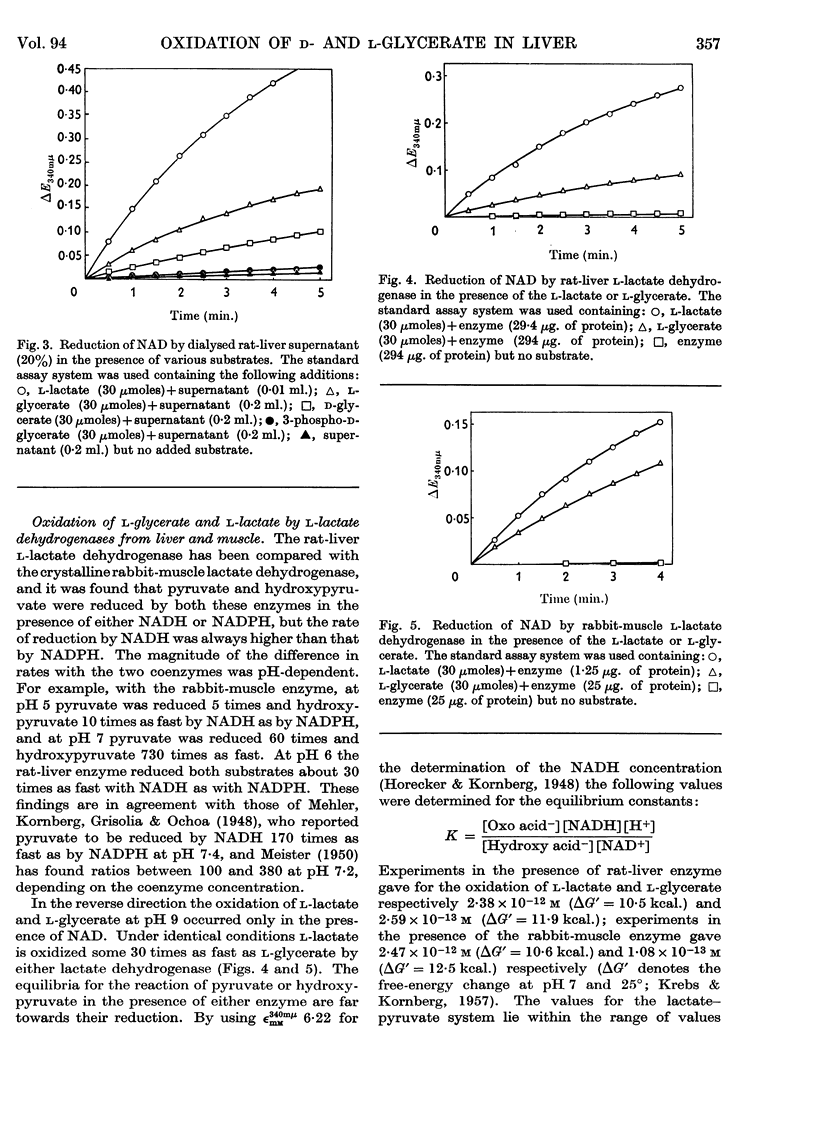
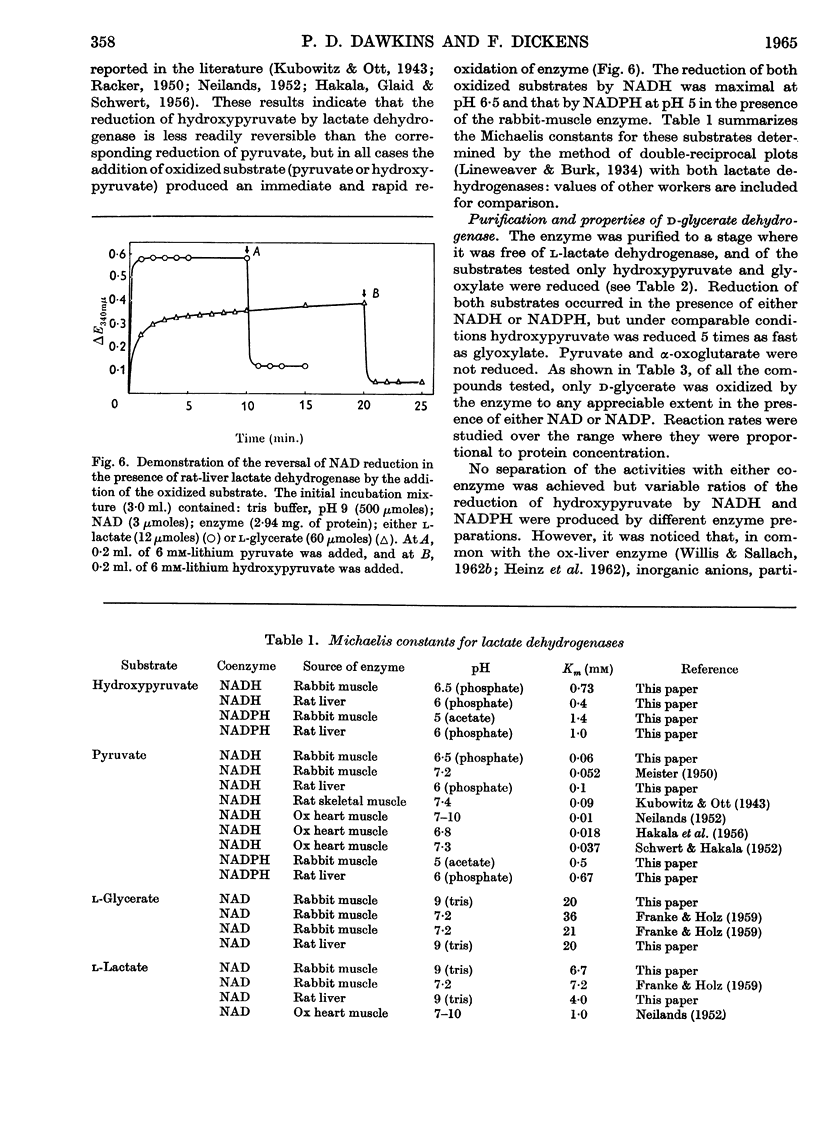
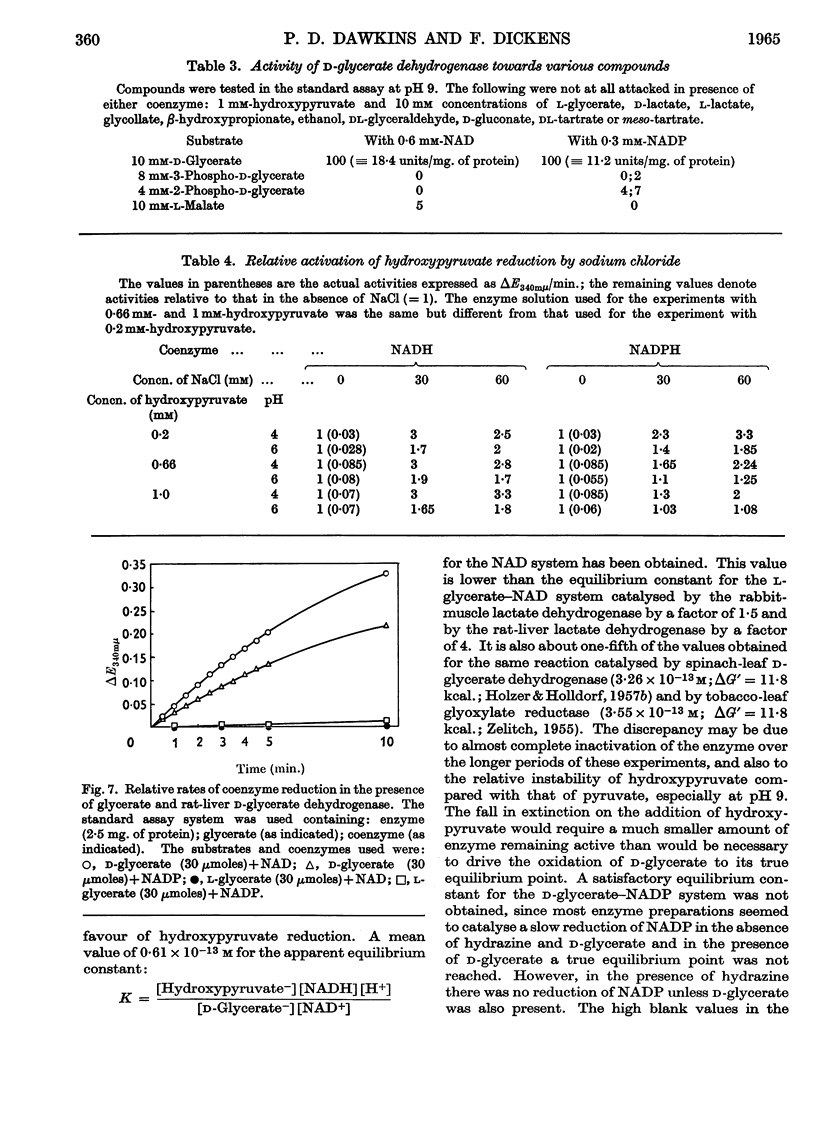
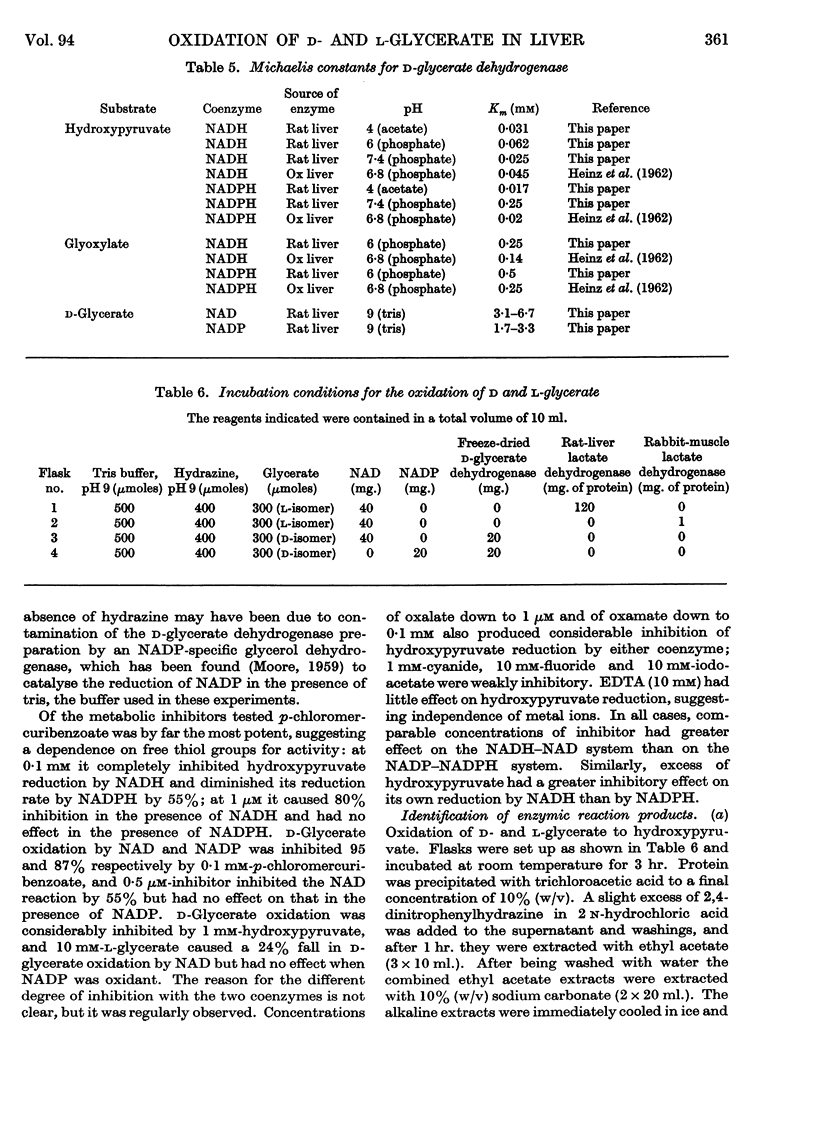
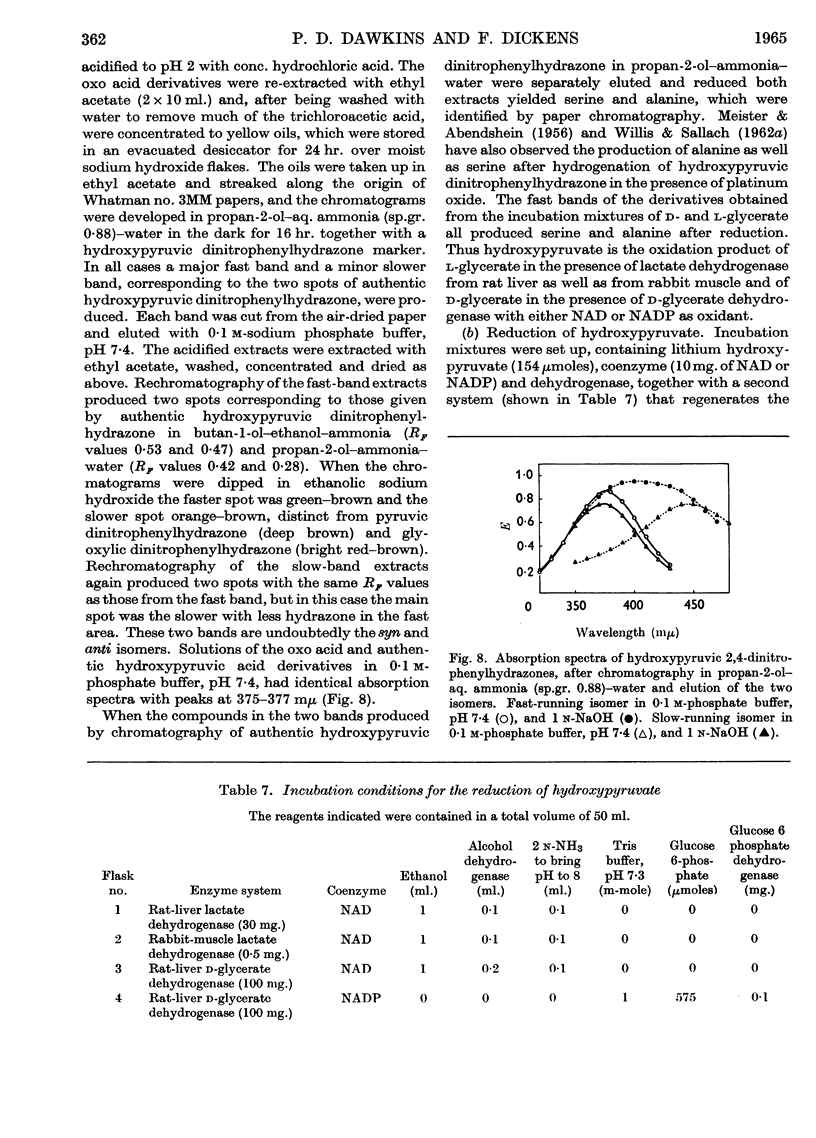
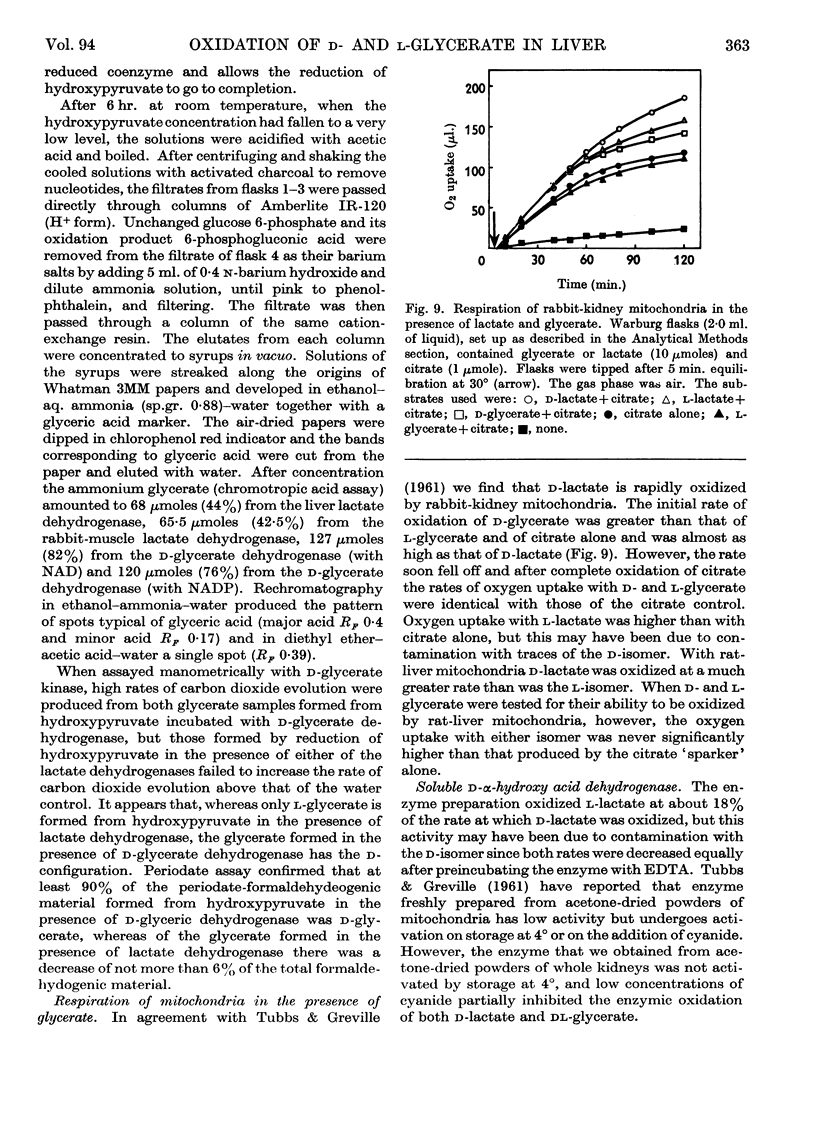
1. The interconversion of hydroxypyruvate and l-glycerate in the presence of NAD and rat-liver l-lactate dehydrogenase has been demonstrated. Michaelis constants for these substrates together with an equilibrium constant have been determined and compared with those for pyruvate and l-lactate. 2. The presence of d-glycerate dehydrogenase in rat liver has been confirmed and the enzyme has been purified 16–20-fold from the supernatant fraction of a homogenate, when it is free of l-lactate dehydrogenase, with a 23–29% recovery. The enzyme catalyses the interconversion of hydroxypyruvate and d-glycerate in the presence of either NAD or NADP with almost equal efficiency. d-Glycerate dehydrogenase also catalyses the reduction of glyoxylate, but is distinct from l-lactate dehydrogenase in that it fails to act on pyruvate, d-lactate or l-lactate. The enzyme is strongly dependent on free thiol groups, as shown by inhibition with p-chloromercuribenzoate, and in the presence of sodium chloride the reduction of hydroxypyruvate is activated. Michaelis constants for these substrates of d-glycerate dehydrogenase and an equilibrium constant for the NAD-catalysed reaction have been calculated. 3. An explanation for the lowered Vmax. with d-glycerate as compared with dl-glycerate for the rabbit-kidney d-α-hydroxy acid dehydrogenase has been proposed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Colorimetric assay methods for free and phosphorylated glyceric acids. J Biol Chem. 1959 Mar;234(3):469–471. [PubMed] [Google Scholar]
- CAVALLINI D., FRONTALI N., TOSCHI G. Keto-acid content of human blood and urine. Nature. 1949 Nov 5;164(4175):792–792. doi: 10.1038/164792b0. [DOI] [PubMed] [Google Scholar]
- COWGILL R. W. Paper chromatographic separation of 2-phosphoglyceric acid and 3-phosphoglyceric acid. Biochim Biophys Acta. 1955 Apr;16(4):614–614. doi: 10.1016/0006-3002(55)90300-0. [DOI] [PubMed] [Google Scholar]
- DE LA HABA G., LEDER I. G., RACKER E. Crystalline transketolase from bakers' yeast: isolation and properties. J Biol Chem. 1955 May;214(1):409–426. [PubMed] [Google Scholar]
- DEN H., ROBINSON W. G., COON M. J. Enzymatic conversion of beta-hydroxypropionate to malonic semialdehyde. J Biol Chem. 1959 Jul;234(7):1666–1671. [PubMed] [Google Scholar]
- DICKENS F., WILLIAMSON D. H. Metabolism of D- and L-[3-14C]glycerate by liver tissue of the rat. Biochem J. 1960 Nov;77:356–363. doi: 10.1042/bj0770356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DICKENS F., WILLIAMSON D. H. Studies on the metabolism of hydroxypyruvate in animal tissues. Biochem J. 1959 Jul;72:496–508. doi: 10.1042/bj0720496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DICKENS F., WILLIAMSON D. H. The determination of hydroxypyruvate and glycolaldehyde. Biochem J. 1958 Jan;68(1):84–88. doi: 10.1042/bj0680084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DICKENS F., WILLIAMSON D. H. The preparation and properties of lithium hydroxypyruvate and hydroxypyruvic acid. Biochem J. 1958 Jan;68(1):74–81. doi: 10.1042/bj0680074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EL HAWARY M. F. S., THOMPSON R. H. S. Separation and estimation of blood keto acids by paper chromatography. Biochem J. 1953 Feb;53(3):340–347. doi: 10.1042/bj0530340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAGER E. W., ROSENBERG J. L. Phosphoglyceric acid in photosynthesis. Science. 1950 Nov 24;112(2917):617–618. doi: 10.1126/science.112.2917.617. [DOI] [PubMed] [Google Scholar]
- FRISELL W. R., MEECH L. A., MACKENZIE C. G. A simplified photometric analysis for serine and formaldehyde. J Biol Chem. 1954 Apr;207(2):709–716. [PubMed] [Google Scholar]
- GIBSON D. M., DAVISSON E. O., BACHHAWAT B. K., RAY B. R., VESTLING C. S. Rat liver lactic dehydrogenase. I. Isolation and chemical properties of the crystalline enzyme. J Biol Chem. 1953 Jul;203(1):397–409. [PubMed] [Google Scholar]
- GLOCK G. E., MCLEAN P. Levels of oxidized and reduced diphosphopyridine nucleotide and triphosphopyridine nucleotide in animal tissues. Biochem J. 1955 Nov;61(3):388–390. doi: 10.1042/bj0610388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENBERG D. M., ICHIHARA A. Further studies on the pathway of serine formation from carbohydrate. J Biol Chem. 1957 Jan;224(1):331–340. [PubMed] [Google Scholar]
- HAKALA M. T., GLAID A. J., SCHWERT G. W. Lactic dehydrogenase. II. Variation of kinetic and equilibrium constants with temperature. J Biol Chem. 1956 Jul;221(1):191–209. [PubMed] [Google Scholar]
- HEDRICK J. L., SALLACH H. J. The metabolism of hydroxypyruvate. II. The enzymatic oxidation and decarboxylation of hydroxypyruvate. J Biol Chem. 1961 Jul;236:1872–1875. [PubMed] [Google Scholar]
- HEINZ F., BARTELSEN K., LAMPRECHT W. [D-glycerate dehydrogenase from liver. Contribution to serine metabolism]. Hoppe Seylers Z Physiol Chem. 1962 Nov 15;329:222–240. doi: 10.1515/bchm2.1962.329.1.222. [DOI] [PubMed] [Google Scholar]
- HEINZ F., LAMPRECHT W. [D-Glycerate dehydrogenase in the liver. Preliminary report]. Hoppe Seylers Z Physiol Chem. 1961 Sep 20;325:280–282. doi: 10.1515/bchm2.1961.325.1.280. [DOI] [PubMed] [Google Scholar]
- HOLZER H., HOLLDORF A. Isolierung von D-Glycerat-dehydrogenase, einige Eigenschaften des Enzyms und seine Verwendung zur enzymatisch-optischen Bestimmung von Hydroxypyruvat neben Pyruvat. Biochem Z. 1957;329(4):292–312. [PubMed] [Google Scholar]
- HORECKER B. L., SMYRNIOTIS P. Z., KLENOW H. The formation of sedoheptulose phosphate. J Biol Chem. 1953 Dec;205(2):661–682. [PubMed] [Google Scholar]
- ICHIHARA A., GREENBERG D. M. Studies on the purification and properties of D-glyceric acid kinase of liver. J Biol Chem. 1957 Apr;225(2):949–958. [PubMed] [Google Scholar]
- JAGANNATHAN V., SCHWEET R. S. Pyruvic oxidase of pigeon breast muscle. I. Purification and properties of the enzyme. J Biol Chem. 1952 May;196(2):551–562. [PubMed] [Google Scholar]
- KATTERMANN R., DOLD U., HOLZER H. [D-Glycerate in fructose degradation in the liver]. Biochem Z. 1961;334:218–226. [PubMed] [Google Scholar]
- KREBS H. A., KORNBERG H. L., BURTON K. A survey of the energy transformations in living matter. Ergeb Physiol. 1957;49:212–298. [PubMed] [Google Scholar]
- LABEYRIE F., NASLIN L., CURDEL A., WURMSER R. [A new determination of the redox potential of the lactate-pyruvate system]. Biochim Biophys Acta. 1960 Jul 15;41:509–515. doi: 10.1016/0006-3002(60)90049-4. [DOI] [PubMed] [Google Scholar]
- LAMPRECHT W., HEINZ F., DIAMANTSTEINT [Phosphorylation of D-glyceric acid to 2-phospho-D-glyceric acid with glycerate kinase in the liver. II. Identification of the reaction products by paper chromatography]. Hoppe Seylers Z Physiol Chem. 1962 Aug 6;328:204–206. doi: 10.1515/bchm2.1962.328.1.204. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MEISTER A. Enzymatic preparation of alpha-keto acids. J Biol Chem. 1952 May;197(1):309–317. [PubMed] [Google Scholar]
- MEISTER A., FRASER P. E., TICE S. V. Enzymatic desulfuration of beta-mercaptopyruvate to pyruvate. J Biol Chem. 1954 Feb;206(2):561–575. [PubMed] [Google Scholar]
- MEISTER A. Reduction of alpha gamma-diketo and alpha-keto acids catalyzed by muscle preparations and by crystalline lactic dehydrogenase. J Biol Chem. 1950 May;184(1):117–129. [PubMed] [Google Scholar]
- NEILANDS J. B. Studies on lactic dehydrogenase of heart. I. Purity, kinetics, and equilibria. J Biol Chem. 1952 Nov;199(1):373–381. [PubMed] [Google Scholar]
- SALLACH H. J. Formation of serine hydroxypryuvate and L-alanine. J Biol Chem. 1956 Dec;223(2):1101–1108. [PubMed] [Google Scholar]
- SAVAGE N. Preparation and properties of highly purified diaphorase. Biochem J. 1957 Sep;67(1):146–155. doi: 10.1042/bj0670146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWERT G. W., HAKALA M. T. Lactic dehydrogenase. I. Kinetics. Arch Biochem Biophys. 1952 Jul;38:55–65. doi: 10.1016/0003-9861(52)90009-x. [DOI] [PubMed] [Google Scholar]
- SMITH V. H. Removal of internally deposited plutonium. Nature. 1958 Jun 28;181(4626):1792–1793. doi: 10.1038/1811792a0. [DOI] [PubMed] [Google Scholar]
- STAFFORD H. A., MAGALDI A., VENNESLAND B. The enzymatic reduction of hydroxypyruvic acid to D-glyceric acid in higher plants. J Biol Chem. 1954 Apr;207(2):621–629. [PubMed] [Google Scholar]
- Straub F. B. Crystalline lactic dehydrogenase from heart muscle. Biochem J. 1940 Apr;34(4):483–486. doi: 10.1042/bj0340483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TUBBS P. K., GREVILLE G. D. The oxidation of D-alpha-hydroxy acids in animal tissues. Biochem J. 1961 Oct;81:104–114. doi: 10.1042/bj0810104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIS J. E., SALLACH H. J. Activation of mammalian glycerate dehydrogenase by inorganic salts. Biochim Biophys Acta. 1962 Aug 13;62:443–444. doi: 10.1016/0006-3002(62)90282-2. [DOI] [PubMed] [Google Scholar]
- WILLIS J. E., SALLACH H. J. Evidence for a mammalian D-glyceric dehydrogenase. J Biol Chem. 1962 Mar;237:910–915. [PubMed] [Google Scholar]
- ZELITCH I. The isolation and action of crystalline glyoxylic acid reductase from tobacco leaves. J Biol Chem. 1955 Oct;216(2):553–575. [PubMed] [Google Scholar]


