Significance
Understanding the divergence and evolution of novel genes and expression is an essential question in evolutionary biology. However, rapid transcriptomic divergence in tissues such as the testis has significantly hindered comparative single-cell RNA-Sequencing (scRNA-Seq) studies. Here, we provide a strategy—independent of preexisting cell type marker genes—to overcome this barrier. In finding how genome organization influences the evolution of transcriptional bursting dynamics via de novo transcript origination, our results support the cultivator model of de novo gene origination—a model emphasizing the genomic environment in shaping novel gene origination.
Keywords: single-cell RNA-seq, transcriptional divergence, de novo genes, Drosophila, testis
Abstract
Spermatogenesis is a key developmental process underlying the origination of newly evolved genes. However, rapid cell type–specific transcriptomic divergence of the Drosophila germline has posed a significant technical barrier for comparative single-cell RNA-sequencing studies. By quantifying a surprisingly strong correlation between species- and cell type–specific divergence in three closely related Drosophila species, we apply a statistical procedure to identify a core set of 198 genes that are highly predictive of cell type identity while remaining robust to species-specific differences that span over 25 to 30 My of evolution. We then utilize cell type classifications based on the 198-gene set to show how transcriptional divergence in cell type increases throughout spermatogenic developmental time. After validating these cross-species cell type classifications using RNA fluorescence in situ hybridization and imaging, we then investigate the influence of genome organization on the molecular evolution of spermatogenesis vis-a-vis transcriptional bursting. We first show altering transcriptional burst size contributes to premeiotic transcription and altering bursting frequency contributes to postmeiotic expression. We then report global differences in autosomal vs. X chromosomal transcription may arise in a developmental stage preceding full testis organogenesis by showing evolutionarily conserved decreases in X-linked transcription bursting kinetics in all examined somatic and germline cell types. Finally, we provide evidence supporting the cultivator model of de novo gene origination by demonstrating how the appearance of newly evolved testis-specific transcripts potentially provides short-range regulation of neighboring genes’ transcriptional bursting properties during key stages of spermatogenesis.
Gametogenesis is an essential process in all sexually reproducing species in nature, ensuring genetic material is successfully transferred between generations. Male gametogenesis, spermatogenesis, is a specialized developmental process that exhibits several key features. First, cell differentiation from stem cell to fully formed gamete is remarkably conserved across animals. Second, despite the necessary maintenance of gametic function, testis-expressed genes tend to evolve very rapidly, with some genes being newly originated. Third, although cell types in the testis exhibit conserved features, expression patterns of genes in each cell type vary drastically between species. Because of this, the expression dynamics of testis-expressed genes are an important issue for both evolutionary biology and developmental biology.
While the transcriptomes of highly conserved cell types in the testis are likely to remain stable over large evolutionary timescales, the rapid evolution of spermatogenesis in Drosophila presents significant challenges for comparative analyses of single-cell transcriptomic data, even among closely related species. For instance, many seemingly conserved marker genes from Drosophila melanogaster (1, 2) are lost or not annotated in other Drosophila species, challenging the assumption that these genes are evolutionarily conserved. Additionally, the rate of gene expression alteration drastically varies in Drosophila (3, 4), making it difficult to transfer cell types across different species. Our interest lies not only in the conserved transcriptional programs of cell types across species but also in complete gene expression patterns, including species-specific genes. The application of standard techniques (5–8) often fails to reproduce previous cell-type assignments in D. melanogaster samples, generating a large number of species-specific cell-type clusters that likely do not reflect true phenotypic differences.
Within approximately 30 My of evolution in the melanogaster species group, the process of germline differentiation has undergone many phenotypic changes (9–11). Alternatively, a large degree of spermatogenesis (Fig. 1A) has remained unaltered through similar evolutionary time scales (12, 13), including gross anatomical phenotypes such as organ structure or the developmental progression of various cell-type specific changes (14, 15). We therefore hypothesized that a high degree of species-specific gene expression evolution within each cell type is the primary factor driving technical difficulties in clustering and identifying cell type differences within comparative studies of Drosophila spermatogenesis (SI Appendix, Fig. S1).
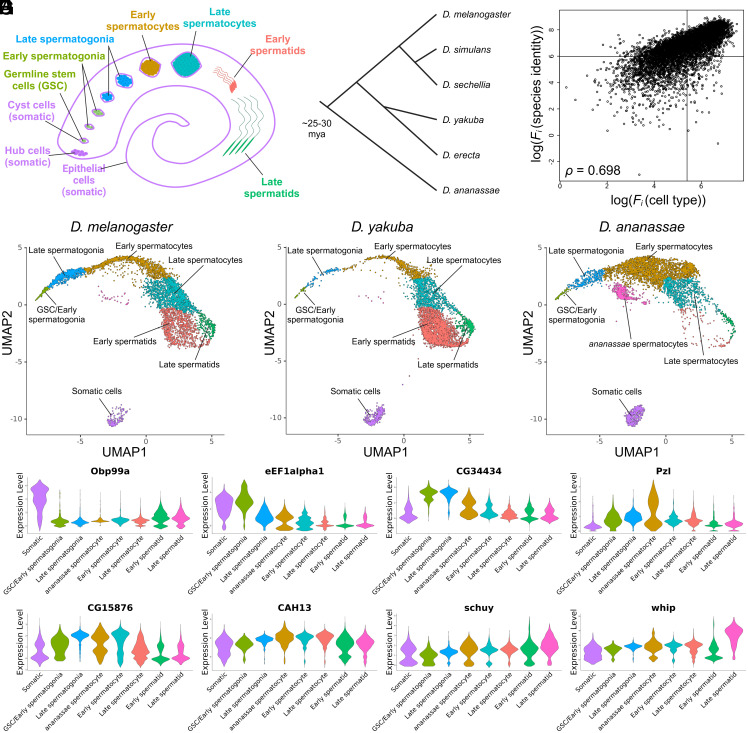
Fig. 1.
Identification of 198-gene list allows for cell type classification across species in a rapidly diverging process. (A) Illustration of spermatogenesis from germline stem cells to late spermatids. (B) Phylogeny of species used in this study with estimated divergence times. (C) ANOVA log(F-statistic) values from scRNA-Seq data using previously identified D. melanogaster cell types and cross-species labels for the three species show high correlation resulting in a large degree of species-specific cell type divergence. Drawn lines correspond to median F-statistic values for each (independent) marginal distribution. (D–F) Uniform Manifold Approximation and Projections (UMAPs) resulting from the 198-gene list reveal a progression of intermediate cell types spanning germline stem cells to late spermatids for the three species. (G) Violin plots from the most differentially expressed gene within each cell type.
Transcription is an intrinsically random process that may be modeled stochastically (16, 17). Single-molecule observations of transcriptional activity are intrinsically “bursty,” consisting of short bouts of transcription that produce discrete quantities of mRNAs (“on” state) followed by periods of inactivity (“off” state) (18–20). Two key parameters controlling the transcriptional kinetics of different genes are burst size (or burst amplitude), which reflects the number of transcripts produced per burst, and burst frequency, which reflects the number of bursts that occur per unit time (18, 21, 22). Importantly, the analysis of transcriptional bursting kinetics offers unique insight into understanding how certain genes are regulated. The kinetics underlying burst size is a property of core promoter elements, driven by the stoichiometry of RNA polymerase II (pol II) availability, while the kinetics of burst frequency is influenced primarily by enhancer elements, modulated by the probability of transcriptional initiation (23–26). Transcriptional bursting properties have provided a novel perspective into the molecular underpinnings of sex-biased transcription burst amplitude in male and female embryos of D. melanogaster (27). Recent work has shown that the upregulation of male X chromosome genes is primarily mediated by a higher pol II initiation rate and burst amplitude (27), paving the way for further studies of dosage compensation in spermatogenesis.
Despite this, the molecular mechanisms underlying downregulation of the X chromosome remain understudied (28). While prior work has suggested that expression of genes on the X chromosome is globally down-regulated during spermatogenesis in Drosophila (29), it is not possible to determine anything beyond the general reduction of expression for X-linked genes (28, 30). Alternatively, previous single-cell RNA-sequencing (scRNA-Seq) studies have proposed that there is some degree of noncanonical dosage compensation (31, 32). A decrease in X chromosome expression beyond that which would be expected by dosage has also led others to suggest that the entire Drosophila X chromosome is inactivated (33).
Beyond the X chromosome, our interest in the testis is also related to the observation that the testis is a hotspot for tissue-specific expression of newly evolved genes (34, 35), including novel genes and transcripts (e.g., noncoding RNAs) arising de novo from nongenic, transcriptionally inactive sequences. While the origination of de novo protein-coding genes has been relatively well-studied (36–38), the study of the evolutionary mechanisms underlying the origination of de novo transcripts, e.g., long noncoding RNA (lncRNA), in fruit flies has been lacking (36, 39, 40). This gap highlights the importance of comprehensively identifying these RNA genes in multiple Drosophila species (41). Such de novo transcripts are often expressed in a tissue-specific manner, typically in the adult testis of Drosophila (34, 36, 39). Intriguingly, scRNA-Seq data have shown that de novo originated genes, unlike recently evolved duplicate genes, are biased toward expression in mid-spermatogenesis (35). It is not yet clear whether this pattern is common across multiple Drosophila species and what function these transcripts may hold.
In our study, we undertook a comprehensive analysis of spermatogenesis in three Drosophila species, aiming to unravel the evolution of complex transcriptional dynamics and novel gene expression. We first developed an approach to study scRNA-Seq data from rapidly diverging species. We then quantified a strong correlation between species- and cell-type-specific transcriptomic divergence across these species, identifying a core set of genes crucial for predicting cell type identity over approximately 30 My of evolutionary divergence. After classifying cell types across these species, we analyzed how transcriptional divergence progresses throughout spermatogenic development. We further investigated the effects of genome organization on the molecular evolution of spermatogenesis, focusing on transcriptional bursting. We demonstrated variations in transcriptional burst size and frequency before and after meiosis, examining differences in autosomal versus X chromosomal transcription. Our findings support the cultivator model of de novo gene origination (42), showing that newly evolved testis-specific transcripts might play a regulatory role in the transcriptional bursting properties of neighboring genes.
Results
A Simple ANOVA-Based Statistical Procedure Identifies Gene Lists Predictive of Cell Type but Not Species Identity.
We used several commonly available software suites to analyze scRNA-Seq data from testis tissue derived from three species, D. melanogaster, Drosophila yakuba, and Drosophila ananassae, but were unable to obtain high-quality cell type assignments to correlate similar cell types across different species (SI Appendix, Fig. S1). For example, widely used markers in D. melanogaster did not robustly classify cell type identities in species such as D. ananassae. We thus hypothesized that species-specific cell type transcriptomic divergence significantly hinders cell type classification. Clear morphological and functional conservation of spermatogenesis across various Drosophila species, e.g., mitotic staging or spermatid differentiation, suggests some degree of evolutionary conservation of key genetic elements. In this case, challenges in cross-species clustering could potentially be overcome by focusing on key conserved elements, not the entire transcriptome. We thus developed a simple methodology to identify genes that are both evolutionarily conserved and important in determining cell identity. We hypothesized that these genes could be identified by calculating ANOVA F-statistic scores for cell type (where available) and species. To test this strategy, we generated a simulated dataset consisting of nine unique cell types evolving in three different species (SI Appendix, Fig. S2A), to simulate a scenario in which cell type differences are masked by species-level differences. (SI Appendix, Fig. S2A). A good cell type marker gene, indexed by variable i, for multiple species should exhibit a large Fi for cell type and a small Fi for species identity (SI Appendix, Fig. S2 B, Bottom-Right quadrant). We selected the top 200 genes showing high Fi (cell type) and low Fi (species identity). The genes identified through our methodology effectively captured the most informative simulated genes, preferentially containing cell type information over species identity information (SI Appendix, Fig. S2C).
Uncorrected simulated data resulted in 27 nonoverlapping cell types, as they were clustered based on both cell type and species identity (SI Appendix, Fig. S2D). When standard batch correction techniques, such as Monocle3’s implementation of batchelor via “align_cds()” (6, 43), are applied to remove species-specific effects, Uniform Manifold Approximation and Projection (UMAP) and Principle Component Analysis (PCA) projections show that these effects have been removed with entirely overlapping cell type assignments despite species-specific differences in cell type (SI Appendix, Fig. S2E). This stands in contrast to the removal of species-specific changes using these same batch correction techniques in real scRNA-Seq data derived from testis tissue (SI Appendix, Fig. S1), as remaining species-specific differences hinder the identification of cell types across species in these datasets. This is likely due to the excessively simplistic and additive nature of the simulations in contrast to the more complex, nonlinear nature of cell-type specific evolution. Regardless, the application of standard batch correction techniques remained insufficient for cell type identification in real data.
Projection and clustering using the top 200 gene list in the simulated data showed nine distinct cell type clusters that still maintained species-specific differences (SI Appendix, Fig. S2 F and G). Cell type identities are readily recovered, while species-specific divergence within these cell types is also clearly visualized. These show that our method identifies gene lists that are predictive of cell type but not species identity, which is important for clustering cell types across species. The methodology is also effective across phylogenies of varying degrees of asymmetry (SI Appendix, Fig. S3 and Performance of HSQ Criteria in Asymmetric Phylogenies).
Application of the ANOVA-Based Statistical Procedure Identifies 198 Gene List in Related Drosophila Species.
We then applied our nonparametric statistical procedure on our scRNA-Seq dataset derived from testis tissue in D. melanogaster, D. yakuba, and D. ananassae. The divergence time between D. melanogaster and D. yakuba has been estimated to be about 7 Mya while the divergence time between D. melanogaster and D. ananassae has been estimated to be about 25 to 30 Mya (44) (Fig. 1B). Prior data and cell type labels from D. melanogaster were obtained from ref. 35, while 5,000 cells derived from D. yakuba and 5,000 cells derived from D. ananassae were sequenced (SI Appendix). Raw data were aligned in “cellranger” to reference genomes and annotations obtained from FlyBase to generate read counts. Further orthology information between the three species’ annotations was obtained using FlyBase (dmel-r6.44) and used to generate “melanogasterized” D. yakuba (dyak-r1.05) and D. ananassae (dana-r1.06) genomes. Specifically, each genome was reduced to a core set of single-copy genes that possessed strict one-to-one orthology between the three species. After processing in “Monocle3” (43), data from one-to-one orthologs were combined together and retained. A summary of key processing parameters is presented in SI Appendix, Table S1.
The distributions of Fi (cell type) and Fi (species identity) were calculated using our experimentally derived data (Fig. 1C and Dataset S1). Interestingly, the joint distribution of log(Fi (cell type)) and log(Fi (species identity)) showed an exceedingly large correlation (Pearson ρ = 0.698, P < 2 × 10−16, Fig. 1C). This result is on par with the correlation found in simulated data (SI Appendix, Fig. S2B, Pearson ρ = 0.74, P < 2 × 10−16), suggesting a high degree of species-specific cell type evolution in Drosophila testis tissue. The medians of the independent distributions Fi (cell type) and Fi (species identity) were chosen as threshold pcell-type and pspecies-identity, respectively (Fig. 1C, solid lines). Note that the independent distributions of Fi (cell type) and Fi (species identity) are not fully represented in the joint distribution of Fi (cell type) and Fi (species identity), because some genes were missing Fi (cell type) or Fi (species identity) values. This definition of pcell-type and pspecies-identity produced a new 198-gene (SI Appendix, Table S2).
To support the biological significance of the 198-gene set, we performed a gene ontology analysis using the PANTHER database (45, 46) (SI Appendix, Table S3). The 11 statistically significant major categories (FDR < 0.05) were highly concordant with known function during spermatogenesis, including “cilium mobility,” “dynein-associated pathways,” “insulin signaling,” “centrosome cycle,” “protein maturation,” “proteolysis,” and “general reproduction.” Notably, all of these major categories showed overrepresentation in comparison to what is expected for all genes in D. melanogaster, demonstrating that this statistical procedure effectively identifies transcriptionally important, functionally conserved genes in the rapidly evolving Drosophila testis transcriptome.
Cell Type Clustering of Testis scRNA-Seq Data Reveals Increasing Transcriptomic Divergence Throughout Spermatogenesis.
To validate the expression of the 198-gene set we identified, we visualized both UMAP and PCA projections of all analyzed cells with an overlay of previously identified cell type assignments (35) (SI Appendix, Fig. S4A). Importantly, cells from all species can be projected onto the same visualization (subsetted by the 198-gene list) without extraneous processing beyond standard single-cell transcriptomics pipelines (Fig. 1 D–F and SI Appendix, Fig. S4B). We note that batch correction for species was applied to the simulated 200- and this 198-gene list as part of Monocle3’s standard pipeline. A visual examination of these projections shows striking features concordant with known spermatogenesis function. 1) While the sequence of spermatogenesis was not explicitly encoded into our statistical procedure or in the visualization process, the developmental progression of cell types in the projection follows a known developmental progression from germline stem cells to late spermatids. Despite being a continuous developmental process where each stage does not produce spatially segregated clusters, the stages of previous cell type assignments form relatively distinct boundaries. This eliminates the need for more advanced pseudotime projection techniques. 2) All somatic cell types cluster separately from germ cell types. 3) We observe a gradual increase in cell-to-cell gene expression variance as development progresses. For example, germline stem cells appear to have low expression variance across all species, while spermatids show much larger variance (Fig. 1 D–F). Note that all these features are readily observed in PCA projections of the same dataset (SI Appendix, Fig. S4B).
Importantly, the application of this methodology allows for recapitulation of previously identified cell type clusters using unsupervised clustering on a unified, panspecies UMAP with the clustering resolution set to produce the same number of clusters reported in previous studies (35) (Leiden clustering, resolution = 3 × 10−4, nine clusters). While it is likely to be the result of residual species-specific differences, these cell type assignments show that fewer cells are represented in the earlier germline stem cell, spermatogonia, and spermatocyte stages of D. yakuba when compared to D. melanogaster. We also observe a correlated accumulation of cells in later spermatid developmental stages. These cell type assignments also reveal the presence of an additional cell cluster/expression present in D. ananassae that appears to be absent from D. melanogaster and D. yakuba. We believe that this cluster is likely a result of transcriptomic divergence (over evolutionary timescales) that persists even after batch correction. Specifically, these cells likely represent a set of spermatocyte cells that demonstrate a stereotypical expression pattern that exists only in D. ananassae (New Spermatocyte Cell Cluster Reveals a Unique Expression Pattern and SI Appendix: ‘ananassae’ spermatocyte cluster). We also observe a low abundance of early and late spermatid stages in D. ananassae (Fig. 1 D–F and SI Appendix, Fig. S4B). These changes in cell type assignment and visualized patterns among species reflect changes in the expression patterns of the 198-gene list, possible technical biases during data generation, and fundamental processes driving species-specific differences in reproduction (New Spermatocyte Cell Cluster Reveals a Unique Expression Pattern and SI Appendix: ‘ananassae’ spermatocyte cluster). These cell clusters were further investigated using RNA fluorescence in situ hybridization (FISH) (RNA In Situ Hybridization Chain Reaction Validates Cross-species Cell Type Assignments.)
High Differential Expression across Cell Types in D. melanogaster Spermatogenesis Does Not Necessarily Indicate Differential Expression across Cell Types in Closely Related Species.
A cross-species analysis of the five most significantly differentially expressed genes for each cell type/cluster was performed. Unless specifically noted, the phrase “differential expression” indicates analyses of differential expression across cell types that do not take species identity into account. For example, if a gene is said to be differentially expressed in Germline stem cell (GSC)/Early spermatogonia, we mean that the expression of this gene is altered when comparing GSC/Early spermatogonia for all species to all other cell types for all species. This is achieved by combining GSC/Early spermatogonia from D. melanogaster, D. yakuba, and D. ananassae into one category and comparing these cells to all other cells detected in D. melanogaster, D. yakuba, and D. ananassae.
Interestingly, only one (MtnA) of 13 previously reported markers (aly, zfh1, Fas1, Hsp23, MtnA, His2Av, aub, bam, fzo, twe, soti, Dpy-30L2, p-cup) (35) remains as one of the top-five differentially expressed genes by cell type (all spp., e.g. all GSC from all spp. considered together, SI Appendix, Fig. S5). These results suggest that while the 13 marker genes identified in previous D. melanogaster studies may be highly differentially expressed across cell types in D. melanogaster, these expression patterns are likely to have evolved in a species-biased manner. A lack of one-to-one orthology may also be excluded as a driving factor in these discrepancies, as only one of these 13 markers (zfh1) was removed due to this reason.
We then analyzed all significantly differentially expressed genes for each cell type, testing for both over- and underexpression (SI Appendix, Table S4 and Dataset S2). This analysis was performed by comparing cell types without regard to species. Among the 12 previously used markers present in the dataset, Fas1, fzo, and Dpy-30L2 were not found to be differentially expressed in any of these cell types (all spp.). In the somatic cells, Hsp23 and MtnA remained highly differentially expressed (all spp.) with the correct cell type specificity. Similarly, early spermatogenesis markers did appear to be differentially expressed in GSC/Early spermatogonia cell types, but with highly attenuated signals. For example, of all 1,326 significantly differentially expressed genes in GSC/Early spermatogonia, the most differentially expressed classic marker gene was His2Av, which was ranked 375 when sorted by log(fold change), while the next closest marker was aub, ranked 600. This attenuation of signal appears to continue through later stages of spermatogenesis but reverses at the late spermatid stage. There, p-cup is ranked 18/639 and soti is ranked 24/639 respectively. Interestingly, cells demonstrating the “ananassae-specific” expression pattern showed significant overexpression of both early and late spermatogenesis markers: His2Av (ranked 245/366) and p-cup (ranked 73/366), respectively. The attenuation of cell-type information of previously utilized marker genes highlights how cell type–specific divergence (over evolutionary time) contributed to the masking of proper cell type assignment between species.
Among the 12 classic marker genes in our dataset, only aly appears in our 198-gene list, indicating that the remaining D. melanogaster marker genes’ ability to predict cell type likely occurs in a lineage-specific manner. Eight of the 12 marker genes did not appear in the 198-gene list as they showed high levels of species information, while the remaining four marker genes remained relatively similar across species. Of those, two genes (aub, bam) were excluded for having undefined Fi (cell type) values resulting from little to no variance in certain cell types. This low/no variance occurs due to having uniformly few to no counts within different cell types. Interestingly, the remaining two genes (Hsp23, MtnA) were not excluded for having evolutionarily diverged expression patterns, but they were excluded for having poor predictive ability for cell type. This appears to contradict the appearance of MtnA in an analysis of the most highly differentially expressed genes across cell types (regardless of species; see Dataset S2). This discrepancy arises from differences in statistical methodologies. MtnA expression is found only in a very small number of cells, primarily somatic cells. Differential expression analysis, which compares expression within one cell type vs. expression across all other cell types (“one-vs.-all”), revealed elevated MtnA expression in somatic cells (all spp.) despite its low detection in many other cell types (all spp.). In contrast, ANOVA compares the between-cell type variance across all cell types, where MtnA’s limited detection results in a low F-statistic value due to minimal variance in most cell types. This highlights the value of performing cell type assignment on genes that show highly specific yet robust expression across cell types (Fig. 1G). This effect is particularly important in the context of scRNA-Seq technology, where the absence of detection may be as much the result of low read count as it is of biologically low expression. Focusing on quantitative differences within widely expressed genes like those in our 198-gene list, rather than a reliance on nondetection events of relatively lowly expressed genes, may lead to more consistent cell type classification in future comparative scRNA-Seq studies.
RNA In Situ Hybridization Chain Reaction Validates Cross-Species Cell Type Assignments.
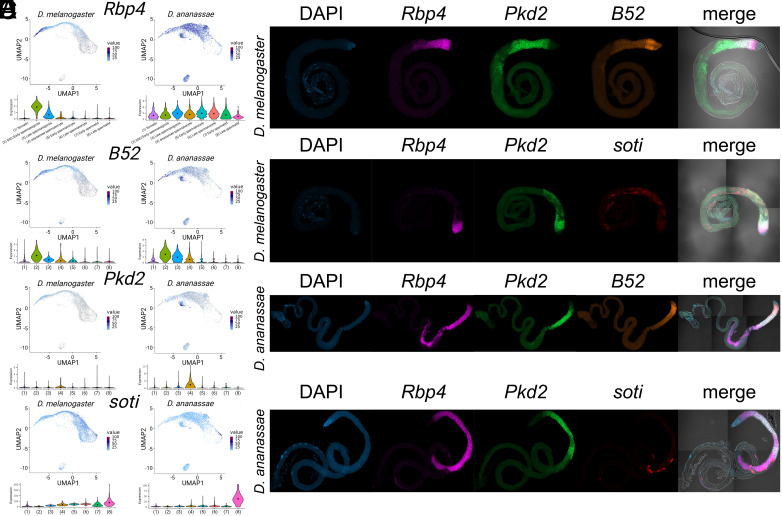
While we were able to transfer a reference species’ cell type labels across species both in our simulated datasets and in our three-species dataset, it was unclear whether the cell type assignments reflected biological reality. To assess the accuracy of the cell type assignments, we performed in situ Hybridization Chain Reactions (HCR) RNA FISH (47–49) in whole-mount testis tissue from D. melanogaster and D. ananassae. Four genes were chosen: Rbp4, B52, Pkd2, and soti (Fig. 2) to fulfill multiple criteria: 1) highlighting predicted expression differences between species in common marker genes, 2) demonstrating high cell-type specificity to aid interpretation of subsequent in situ imaging, and 3) providing maximal information regarding our newly detected “ananassae spermatocyte” cluster. We note that during imaging, there was a large degree of crosstalk between the fluorophores representing B52 and soti, so images were not amplified using both fluorophores in the same sample.
Fig. 2.
Validation of expression pattern divergence and cell type classification using HCR RNA FISH. (A) scRNA-Seq UMAP shows predicted expression patterns in D. melanogaster and D. ananassae for four genes: Rbp4, B52, Pkd2, and soti. Key for cell type labels is found under Rbp4: Somatic, Germline stem cell (GSC)/Early spermatogonia, Late spermatogonia, ananassae spermatocyte, Early spermatocyte, Late spermatocyte, Early spermatid, Late spermatid. (B–E) RNA FISH images in D. melanogaster (B and C) and D. ananassae (D and E) using these same genes, with DAPI nuclear staining (B–E, Left column) and overlay of these channels over bright-field microscopy (B–E, Right column). Rbp4 is a marker gene used in D. melanogaster to indicate germline stem cells/spermatogonia. Our scRNA-Seq cell type classification predicts that Rbp4 has a broad expression pattern in D. ananassae. B52 is a gene with a restricted expression pattern in D. melanogaster germline stem cells/spermatogonia. This expression pattern is predicted to be slightly broader in D. ananassae, extending into the new ananassae spermatocyte cluster. Pkd2 is predicted to be primarily restricted to the newly identified D. ananassae spermatocyte cluster in both D. melanogaster and D. ananassae. soti is a marker gene used in D. melanogaster to indicate spermatid cell types, and our classification predicts that soti has both higher overall expression levels and lower cell type specificity in D. melanogaster than in D. ananassae.
Rpb4 was chosen as it was a previously employed cell-type marker for D. melanogaster GSC/Early spermatogonia cell types in prior publications (1). Our cross-species cell type classification predicted that this marker gene had highly differing expression patterns between D. melanogaster and D. ananassae (Fig. 2A). This prediction was validated, as Rbp4 showed an unexpectedly wide expression pattern in D. ananassae beginning with early spermatogonia and culminating in expression overlap with late spermatids (soti expression and DAPI staining) (Fig. 2 B–E). This is a surprising finding, as Rpb4, an RNA-binding protein, was found to be a regulator of mitosis. Rbp4 interacts with Fest to regulate the translation of Cyclin B (50). Similarly, the genes Lut and Syp interact with Fest, regulating posttranscriptional control of Cyclin B (51). We then investigated the cell type–specific expression of the genes Cyclin B, Fest, and Syp in our dataset (SI Appendix, Fig. S6). Lut was excluded as it was not present as a one-to-one ortholog in our dataset. The expression pattern of Cyclin B agrees with previously reported expression patterns in D. melanogaster (50), while also adopting a similarly specific pattern in D. yakuba. Interestingly, the expression pattern of Cyclin B appears to be nonspecific in D. ananassae, showing a broad expression across all of spermatogenesis like Rbp4. While the D. melanogaster expression patterns of Fest and Syp were less specific than for Rbp4 and Cyclin B, this low specificity decreased even further in D. ananassae (SI Appendix, Fig. S6). This result indicates that changes in cell type–biased patterns of genes potentially reflect underlying functional and molecular changes.
Finally, soti was chosen as it was previously employed as a cell-type marker for D. melanogaster late spermatid cell types (1, 2, 31, 35). Our cross-species cell type classification predicted that transcript levels in D. melanogaster would both be higher and less specific than in D. ananassae. This prediction was validated using our in situ imaging. For example, D. melanogaster soti expression appears to be broader than in D. ananassae, forming a broader, “smearier” expression pattern when compared to the tighter, more individualized puncta seen in D. ananassae (Fig. 2). Note that these figures show maximal intensity projections on z-stacks.
New Spermatocyte Cell Cluster Reveals a Unique Expression Pattern.
A striking result of the previous sections is the identification of a D. ananassae-specific cluster that appears in our cross-species UMAP clustering. We thus use our scRNA-Seq and RNA FISH data to further investigate this newly identified cluster. As mentioned in the previous section, this “ananassae-specific” cluster appears in the UMAP of D. ananassae cells but does not seem well-represented in D. melanogaster and D. yakuba projections. While the appearance of this cluster seems correlated with the inferred disappearance of spermatid cell types in D. ananassae, the new cluster also appears in close proximity with other known spermatocyte cell types in our unified cross-species projections (Fig. 1 D–F).
To resolve ambiguity and to further investigate the specificity of this new cluster, we identified genes that are differentially expressed (all spp.) in this cluster in comparison to all other clusters (High Differential Expression across Cell Types in D. melanogaster Spermatogenesis Does Not Necessarily Indicate Differential Expression across Cell Types in Closely Related Species). The 20 most highly differentially expressed genes in the ananassae spermatocyte cluster are reported in SI Appendix, Table S5.
While many of these differentially expressed genes are predicted to have expression in more than one tissue type, Pkd2, the 6th most differentially expressed gene in this cluster, was also found to have relatively specific expression. In particular, Pkd2 demonstrated elevated cluster-specific expression in D. ananassae. While specific, we note that Pkd2 is still predicted to have residual expression from the GSC/Early spermatogenesis stage to the Early spermatid stages in both D. melanogaster as well as D. ananassae (Fig. 2A).
A detailed examination of the RNA FISH data for Pkd2 reveals surprisingly broad expression throughout spermatogenesis (Fig. 2 B–E), particularly in contrast to B52 expression. B52 was chosen because it is predicted to express in early spermatogonia as well as in our newly identified ananassae-specific cluster. As predicted, B52 showed high specificity in germline stem cells and spermatogonia, with a residual expression detected in our ananassae spermatocyte cell cluster. These results confirm that cells with the ananassae spermatocyte expression pattern are a type of spermatocyte.
Expression of Pkd2 in D. melanogaster and D. ananassae was found to overlap with GSC/Early spermatogonia as indicated by the expression pattern of B52 (both species) and Rbp4 (D. melanogaster only, “Rbp4-mel”). The expression of Pkd2 subsequently decreases in concert with B52 and Rbp4-mel as we continue along the germline differentiation process. Interestingly, Pkd2 remains relatively highly expressed even in late-stage spermatocytes and onion-stage spermatids preceding spermatid elongation. This late-stage expression is most interestingly highlighted by the occasional presence of round cells that express Pkd2 well into the spermatid stages of differentiation as represented by soti expression (Fig. 2 B and E). This combination of scRNA-Seq and RNA FISH data suggests that the ananassae spermatocyte cell cluster has a biological component and is not the sole result of technical artifacts. However, we stress that this cluster does not likely represent a cell type that is functionally different from previously identified spermatocytes, but rather a shift in the expression pattern of a set of these cells. This is further discussed in SI Appendix, “ananassae” Spermatocyte Cluster.
Transcriptional Bursting in Spermatogenesis Is Controlled via Opposing Mechanisms during Meiotic Transition.
We used our validated cell type assignments to study the evolution of transcriptional control by examining how the transcriptional bursting properties of different genes change during development. General transcriptional activity first increases and then dramatically decreases during the stage of spermatogenesis between the 16-spermatocyte stage and the second round of meiosis. These divisions subsequently result in 64 round spermatids in all three species (11). Importantly, the total pool of remaining transcripts is slowly degraded as they are translated in subsequent stages, supplemented only by a small number of genes that are transcribed postmeiotically. The increased resolution of scRNA-Seq analysis (26, 52) and MS2-MS2 coat protein system imaging (24, 25) has brought significant advances in understanding the fundamental molecular mechanisms underlying transcription, shedding light on the chemical kinetics that drive stochastic gene expression. However, global control of the pre- to postmeiotic transition during Drosophila spermatogenesis has remained understudied.
Aside from higher-order eukaryotic regulation via nucleosomes and histones, sequence-based transcriptional control is exerted by genomic segments that are broadly classified as promoter and enhancer sequences. Prior results have mechanistically demonstrated how this transcriptional control of promoters alters burst size via pol II availability, while control of enhancers mechanistically alters burst frequency via BRD4 and the Mediator complex (20, 26, 53–55). To investigate how transcriptional control of germline differentiation is controlled in Drosophila, we begin by calculating burst size and frequency for all annotated genes by cell type using “txburst,” using our three-species data (Fig. 3) (26). After applying default txburst quality control criteria, the number of genes that were analyzed per cell type varied by cell type and species (SI Appendix, Fig. S7 and Table S6). Interestingly, we find that in the early, premeiotic stages of spermatogenesis (i.e., germline stem cells, early spermatogonia, late spermatogonia, and early spermatocytes), transcriptional bursting is primarily controlled via alterations in burst size. While burst frequency also increases during these stages, overall burst size appears to reach a maximum during the late spermatogonia stage before declining during spermatocyte stages (Fig. 3). Interestingly, in D. melanogaster, burst size drops sharply between the early and late spermatocyte stages, while in D. yakuba and D. ananassae, burst size decreases between the spermatogonia and spermatocyte stages, remaining relatively stable from early to late spermatocyte stages (Fig. 3 A–F). This genome-wide transition from burst size-dominant control (reflecting promoter-based regulation) to burst frequency-dominated control (reflecting enhancer-based regulation) suggests that phenotypic divergence during spermatogenesis in Drosophila may be evolving via global, genome-level alterations of transcriptional control via pol II initiation (“kini”) in alignment with prior observations (20, 53, 54, 56), rather than through alterations in a smaller subset of genes.
Fig. 3.
Pre- and postmeiotic cell types in spermatogenesis are controlled by opposing mechanisms. The burst size and burst frequency distributions for (A and B) D. melanogaster, (C and D) D. yakuba, and (E and F) D. ananassae reveal that transcription in the earlier stages of spermatogenesis are primarily controlled via burst size, while transcription in later stages of spermatogenesis is primarily controlled via burst frequency. This transition between control mechanisms correlates with the transition between mitosis and meiosis.
The control of bursting frequency appears to become the more dominant mechanism during postmeiotic stages of spermatogenesis (i.e., late spermatocyte, and spermatid stages, Fig. 3B). An increase in bursting frequency in D. melanogaster is observed in the late spermatocyte stage simultaneously with a correlated decrease in burst size. A similar change is observed between late spermatogonia and early spermatocyte in D. yakuba, where a decrease in burst size occurs simultaneously with an increase in bursting frequency (Fig. 3 C and D). In D. ananassae, an increase in burst frequency is observed in the early spermatocyte stage followed by an even larger increase in the late spermatocyte stage (Fig. 3 E and F). While the increase in burst frequency during the transition between late spermatogonia and early spermatocyte stages is correlated with a decrease in burst size in the same stages, the second increase of burst frequency during the late spermatocyte stage does not show a correlated change in burst size. This increase in burst frequency is potentially an outlier, as not only did a very low number of annotated genes pass quality control measures (202 genes, SI Appendix, Fig. S7C), but also the variance in the observed distribution is also quite high. These observations could also reflect a deeper evolutionary divergence in transcriptomic control or cell type, resulting from a weaker cell type assignment as revealed by its sparse representation in UMAP and PCA projections (Fig. 1E). If the inferred distribution is closer in value to distributions found for the early spermatocytes and D. ananassae spermatocyte cell types, it would appear that D. melanogaster may have undergone a species-specific alteration where the size-to-frequency control transition has been delayed from the early spermatocyte stage to the late spermatocyte stage.
Overall, these results are consistent with a model of transcriptional control where an abundance of pol II is present (20, 26, 53, 54, 56) during earlier stages of spermatogenesis and becomes depleted during the meiotic transition. During the period of high overall pol II availability and activity, general levels of transcription remain high (20, 26, 53, 54, 56), and bursting kinetics are dominated by large burst size. Subsequent to this depletion, transcription then decreases significantly, shifting global transcriptional control into a more burst frequency-dominated mode of activity. It is likely that this modulation of burst frequency is achieved by enhancer-related changes in transcription factor stoichiometry (23, 25, 57).
Bursting Kinetics of X-Linked vs. Autosomal Genes Reveals That Partial Dosage Compensation Likely Occurs in Somatic and Germline Tissue.
To help elucidate the timing of dosage compensation during spermatogenesis, we examined the transcriptional bursting kinetics for both X-linked (N = 2,198) and autosomal (N = 11,749) protein-coding genes. Interestingly, our dataset shows a highly consistent pattern where transcriptional burst size and frequency are significantly lower for X-linked genes than for autosomal genes across cell types and species (Mann–Whitney U test, Benjamini–Hochberg correction, FDR < 0.05) (Fig. 4). Significant differences between X-linked and autosomal bursting kinetics remain consistent throughout spermatogenesis. Perhaps most surprisingly, this difference is detected in D. melanogaster somatic tissue. While X-linked genes typically show lower activity than autosomal genes (even in statistically insignificant comparisons), the transcriptional bursting frequency for X-linked genes is higher than for autosomal genes in D. yakuba early spermatocytes. Additionally, while nearly every cell type in D. melanogaster and D. yakuba produces a significant difference between X- and autosomal-linked genes’ transcriptional kinetics, the observed differences in D. ananassae are typically not significant after multiple hypothesis correction. This is likely because the number of genes that pass quality control in txburst is much lower for D. ananassae than for D. melanogaster and D. yakuba, resulting from the relatively poor reference genome quality and read depth for D. ananassae vs. D. melanogaster and D. yakuba (SI Appendix, Table S1).
Fig. 4.
X-linked genes show lower bursting kinetics than autosomal genes prior to spermatogenesis. The burst size and burst frequency distributions for (A and B) D. melanogaster, (C and D) D. yakuba, and (E and F) D. ananassae reveal a conserved pattern of smaller average burst sizes and lower bursting frequencies for X-linked than autosomal genes across cell types (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Note these are p-values before Benjamini–Hochberg correction, FDR < 0.05). No stage of spermatogenesis shows lower burst kinetics for X-linked genes than autosomal genes. The significant repression of X-linked genes in somatic cells suggests that decreased meiotic X chromosome expression occurs in a developmental stage preceding testis maturation.
Despite these differences, the ratio between X-linked and autosomal genes passing quality-control remains relatively constant across all stages and species (26) (Fig. 4 and SI Appendix, Fig. S7), reflecting both the number of genes that are expressed in each stage as well as read depth. The consistent ratio of X to autosomal genes expressed does not reveal a particular stage of spermatogenesis during which the entire X chromosome is entirely inactivated. After either the early spermatocyte stage in D. melanogaster or the late spermatogonia stage in D. yakuba and D. ananassae, there is a drop-off in the number of genes that pass QC (SI Appendix, Fig. S7). Despite this change, the number of genes expressed on the X vs. autosomal chromosomes remains at a relatively constant ratio. As this ratio remains constant even when comparing somatic cell types to germline cell types (Fig. 4), it is possible that the process of X-downregulation occurs prior to the establishment of somatic vs. germline identity during the differentiation of testis tissue (58). This pattern could potentially be further tested by comparing the transcriptional bursting characteristics of X-linked to autosomal genes in earlier stages of testis organogenesis, e.g., in larval testis tissue.
Origination of New Transcripts Alters Regulation of Neighboring Genes by Increasing Burst Size during Meiotic Transition.
The cultivator model of de novo gene origination hypothesizes that the most evolvable method of altering the expression of preexisting genes is the appearance of a new proximal promoter element (42). Unlike changes to existing transcription-factor-binding sites, as enhancer sequences typically act on longer range distances (≥~1.5 kb), the cultivator model posits that the appearance of a promoter would act on relatively short ranges (~100 to 500 bp), likely through enhancer-mediated promoter interference (24) or supercoiling-mediated transcriptional coupling (59). One key prediction of this model would be that ncRNAs are found to be closer to their protein-coding neighbors than expected by chance—this is indeed the case (42). Another key prediction of this model is that the origination of a de novo transcript would alter the regulation of its immediate neighboring genes, but not more distant neighbors as might be expected by enhancers. To test this prediction, we examined the transcriptional bursting characteristics of protein-coding genes that are neighboring newly activated de novo transcripts.
We begin by identifying four neighboring protein-coding genes for all 170 transcripts identified through bulk RNA-Seq analysis (Dataset S3), two upstream and two downstream (“L2,” “L1,” “R1,” and “R2” respectively, Fig. 5). Note that the L2 and L1 genes (or “5’-2” and “5’-1”) are defined as being in the 5’ direction of the de novo transcript, relative to its strand orientation—that is, upstream with respect to the transcript’s own direction. Similarly, R2 and R1 genes (or “3’-1” and “3’-2”) are defined to be to the 3’ direction. Using these neighboring genes’ orthologous genes in D. yakuba and D. ananassae, we compared the burst size and frequency for each class of neighbors to genome-wide distributions. Unlike the 170 evolutionarily young de novo transcripts identified through our comparative analysis of bulk RNA-Seq data, the neighboring protein-coding genes are not expected to be biased in an evolutionary age.
Fig. 5.
Higher burst sizes in L1 neighboring genes of de novo transcripts support a cultivator model of de novo gene origination. (A) The initial step of the model suggests that a new promoter element near a preexisting gene may serve to regulate nearby genes in cis. We tested this by comparing the bursting kinetics of genes immediately neighboring new de novo transcripts to further neighboring genes. Note that in our study, de novo transcripts include those unique to D. melanogaster and those shared across the melanogaster-simulans or the melanogaster-yakuba clades. (B–D) L1 neighboring genes just upstream of de novo transcripts show significantly higher burst sizes than the distribution of all genes during the early and late spermatocyte stages, aligning with the meiosis onsite. These differences remain significant after Benjamini–Hochberg correction (FDR < 0.05). No other significant differences were observed in further neighboring genes or within spermatocytes preceding the de novo transcript origination (SI Appendix, Fig. S8).
Consistent with the cultivator model, the origination of these de novo transcripts is associated with a significant increase in transcriptional burst size in their 1st upstream neighboring genes, but not further neighboring genes. These differences are also found concurrently with the meiotic transition, suggesting that these genes may be functionally important in later stages of spermatogenesis. More specifically, significant differences are found either in the early spermatocyte (D. melanogaster, P = 5.17 × 10−4 and D. yakuba, P = 4.56 × 10−4, Mann–Whitney U test) and late spermatocyte (D. yakuba, P = 8.25 × 10−4) stages. These results remain significant after correction for multiple hypothesis testing using a Benjamini–Hochberg procedure (4 neighbors × 8 clusters × 3 species = 96 hypotheses, FDR < 0.05; Fig. 5 and SI Appendix, Figs. S8 and S9). Importantly, the cultivator model also predicts that there is no significant difference in transcriptional bursting kinetics prior to the origination of these de novo transcripts as these neighboring genes predate the appearance of the new de novo transcripts in the outgroup species, D. ananassae. Consistent with this hypothesis, no significant differences in burst size are detected in any neighboring genes. Furthermore, no significant differences are detected in burst frequency in any neighbors at any stage in any species. Given an increase in burst size but no alteration of burst frequency, these results suggest that a promoter-based effect, rather than an enhancer-based mechanism, drives the cis-regulatory effect of new promoter birth. We note that while it is possible that de novo transcripts appear next to older genes that already have large burst size kinetics, this does not explain why these neighboring genes show significantly elevated burst size after, but not before, de novo transcript origination.
To further validate this finding, we compared the genomic distribution of mean read count per cell (i.e. expression) with the same distributions calculated for L2, L1, R1, and R2 neighboring gene groups (SI Appendix, Fig. S10 and Table S7). While many significant differences in mean read count per cell (cell type–specific expression) were found, most of these significant differences persisted between in-group (D. melanogaster and D. yakuba) and outgroup (D. ananassae) comparisons or had opposing directions between species (e.g., significantly increased in D. melanogaster and decreased in D. yakuba). Only three neighboring group/cell type combinations showed significant differences within in-group species (D. melanogaster and D. yakuba) while also being directionally consistent: L1 neighbors in early spermatocytes (increase), R1 neighbors in early spermatocytes (decrease), and R1 neighbors in late spermatids (decrease).
In alignment with our previous finding of increased burst size, we find significantly elevated expression of L1 neighboring genes in early spermatocytes in D. melanogaster and D. yakuba but not D. ananassae (SI Appendix, Fig. S10). Interestingly, we also find significantly decreased expression of R1 neighboring genes in both early spermatocytes and early spermatids when compared to genomic expression. The combination of increased L1 neighboring gene expression as well as decreased R1 neighboring gene expression is particularly striking as it is consistent with supercoiling-mediated coupling (59). The appearance of a new promoter element in the tandem upstream/downstream conformations as shown in Fig. 5A would be expected to generate underwinding just upstream of and overwinding just downstream of the new promoter. This would result in both increased transcription in the upstream gene (the L1 neighbor), and decreased transcription of the downstream gene (the R1 neighbor).
Discussion
Feature Selection through ANOVA Allows for the Identification of Conserved Cell Type–Specific Genes.
Our work demonstrates how the identification of the evolutionarily conserved 198-gene list allowed for cell type classifications from D. melanogaster to be extended to a panel of closely related Drosophila species. While it was utilized in this study to overcome cell type–specific evolution, the methodology used here should be generalizable to a large range of problems where conditions and transcriptome expression vary in a correlated manner. For example, strong cell type–specific transcriptome-wide responses to external treatment (e.g., heat shock) could obscure cell type assignments. The application of an ANOVA-based methodology could be utilized to identify genes that differ strongly between cell types but remain robust to treatment.
Interestingly, the 198-gene list that we identified did not show a large overlap with more traditionally utilized cell type markers in D. melanogaster while still being significantly enriched for previously identified spermatogenesis-related function. One potential interpretation of this observation is that while the markers in the 198-gene list do have known cell type–specific function, it is possible that they do not perform as well as other, more classic markers within D. melanogaster. Alternatively, that the list of classic marker genes might reflect the historical discovery process regarding D. melanogaster spermatogenesis alone rather than underlying biological functions within the larger melanogaster species group. Regardless of the reason, our work has shown that the utilization of these marker genes in D. melanogaster does not necessarily imply that they are the most optimal markers when examining multiple species’ genomes.
Biological Differences in Cell Type Classification.
When we examined the ananassae spermatocyte cluster, we found biological evidence that there may be meaningful transcriptional differences in these cells compared to other cell types. For example, while these cells are poorly represented in D. melanogaster and D. yakuba, we found unusual patterns in our differential expression analysis in our full dataset. Recall that we found significant differential expression of His2Av, an early spermatogenesis marker in D. melanogaster, as well as p-cup, a late spermatogenesis marker in D. melanogaster. Consistent with this observation, we also note the early expression of Pkd2 in GSC/Early spermatogonia, as well as the occasional presence of cells expressing Pkd2 well into spermatid differentiation (Fig. 2 C and E). Notably, neither His2Av nor p-cup are found in our 198-gene list used for classification. Such transcriptional differences during the spermatocyte stage, combined with the detection of the “ananassae-specific spermatocyte” cluster, and unique D. ananassae reproductive biology during meiosis (60), suggests that the rapid evolution of the testis may be dominated by changes during this stage, reminiscent of earlier observations that de novo genes tend to express most highly in spermatocytes (35).
We also found that Rpb4 has a surprisingly broad expression pattern in D. ananassae. This provokes the interesting question of whether this broad expression pattern is reflective of the ancestral state or evolved in an ancestor of D. ananassae subsequent to D. melanogaster speciation. It is likely that investigation into the upstream transcriptional differences that drive the alteration of Rpb4 expression would lead to more general insight into the evolution of transcriptional programming in the testis. Similarly, investigation into the downstream effects of broad Rpb4 expression would likely be fruitful. Rpb4, in conjunction with fest and Syp, has been shown to regulate Cyclin B during meiosis in D. melanogaster (50, 51). However, Rbp4 and Cyclin B have much higher cell type specificity in D. melanogaster than D. ananassae, but in slightly different tissue types. How did these expression patterns evolve? Is the evolutionary appearance of this specificity coupled between the two genes? Alternatively, did this loss of specificity occur only in the D. ananassae lineage? These questions require further investigation in the future, particularly with the utilization of additional outgroup species.
We also note that our cross-species categorization detected an absence of spermatids in our scRNA-Seq data. However, our in situ results showed highly specific expression of soti, while our bright-field microscopy revealed cells that can clearly be identified as late spermatids based on morphology alone. Interestingly, the gross morphology of these D. ananassae spermatids appeared to diverge from D. melanogaster as well, as they appeared to be “stringier” and “fuzzier” in addition to downstream morphological differences observed in the accessory gland, where tissue appeared to be larger and whiter. As the total amount of transcripts becomes increasingly depleted as spermatids fully mature, the transcriptional programming controlling these phenotypic differences likely occurs in earlier stages, e.g. “ananassae spermatocyte.” Consequently, the downstream biological effects of this altered transcriptional programming will likely be opaque to transcription-based techniques, highlighting the need for proteomic analysis or the application of traditional genetic tools beyond D. melanogaster.
Transcriptional Bursting Kinetics Offers Insight into Male Sex Chromosome Regulation.
Many unanswered questions remain regarding the mechanisms that drive decreased X chromosome expression during spermatogenesis, leading to a large variety of proposed models. For example, differential polymerase elongation rates have been suggested as a mechanism underlying dosage compensation in males (61). Alternatively, a more canonical pathway involving the lncRNAs roX1, roX2, and the male-specific lethal complex (MSL) is possible (62, 63). One attractive aspect of the canonical MSL pathway hypothesis is that recent results have shown that the intrinsically disordered C-terminal region of MSL2 and the roX lncRNAs induce X chromosome compartmentalization that functions similarly to phase separation (64). This has led to the suggestion that this X chromosome compartment could be enriched for pol II, resulting in increased initiation of transcription and an increase in transcriptional burst size (27). However, it has also been suggested that elements of the MSL complex, such as maleless, do not associate with the X chromosome during spermatogenesis (65).
Our results demonstrate a clear transition from a burst size-dominated mode of regulation to a burst frequency-dominated mode during the meiotic transition, suggesting that this transition may be driven by a global decrease in polymerase availability. However, in alignment with a prior analysis of previous results (31), our analysis of transcriptional bursting kinetics demonstrates that significantly decreased burst size and burst frequency for X-linked genes precedes not only the meiotic transition, but possibly also the establishment of germline stem cells. This decreased burst size is consistent with a study demonstrating decreased pol II binding on X chromosomes derived from both isolated spermatocytes and dissociated testis in comparison to wing imaginal discs (66). While these results are consistent with some manner of incomplete dosage compensation (31, 67), both decreased burst size and burst frequency suggest that a depletion of pol II localized to the X chromosome alone is not a complete explanation for the observed X-linked decrease in expression. More specifically, a local depletion of pol II would decrease transcriptional burst size without altering burst frequency as observed in the developed embryo (27). Instead, the as-yet-unknown mechanism for decreased X chromosome expression should affect both the binding rate and the initiation rate of pol II to X chromosome–linked promoter sequences.
Another interesting result from our analyses is species-specific differences in kinetic bursting parameter values. The txburst software performs inference over raw read counts, and so it is likely that these differences are related to differences in read depth (SI Appendix, Fig. S10). Differences in transcript decay rates across species may also be a contributing factor (53). These differences are unlikely to reflect true biological differences in transcriptional burst kinetics, as these differences appear to be unbiased in regard to transcript identity (e.g., housekeeping genes). Importantly, we do not compare kinetic parameters across species, so such differences do not affect our conclusions; further experimental evidence, e.g., single-molecule studies, would be needed for proper cross-species comparisons.
Methods and Materials
Information on Methods and Materials used is available in SI Appendix. Briefly, we generated scRNA-Seq data from the testis of D. melanogaster, D. yakuba, and D. ananassae. ANOVA was used to transfer cell type classifications across species and applicability was validated by simulation and HCR RNA FISH (SI Appendix, Table S8). De novo transcripts were computationally identified, and bursting kinetics were inferred using maximum likelihood (26).
Supplementary Material
Appendix 01 (PDF)
Dataset S01 (CSV)
Dataset S02 (CSV)
Dataset S03 (CSV)
Acknowledgments
We thank Helen Duan and Connie Zhao from The Rockefeller University Genomics Resource Center for the help with scRNA-Seq library preparation, RRID:SCR_020986. Confocal microscopy was performed in the Rockefeller University’s Bio-Imaging Resource Center, RRID:SCR_017791. We thank the Zhao lab members for helpful discussion and Sasha Mills for editing help. U.L. would like to thank Huang Suqi for helpful discussions regarding statistics. This work was supported by NIH MIRA R35GM133780, the Robertson Foundation, and an Allen Distinguished Investigator Award from Paul G. Allen Family Foundation to L.Z., and NSF postdoctoral fellowship 2410289 to U.L. The content of this study is solely the responsibility of the authors and does not necessarily represent the official views of the funders.
Author contributions
U.L. and L.Z. designed research; U.L., C.L., C.B.L., N.S., and L.Z. performed research; U.L., C.L., C.B.L., and N.S. contributed new reagents/analytic tools; U.L. analyzed data; and U.L. and L.Z. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
scRNA-Seq reads were uploaded to NCBI under the project number PRJNA995212 (68). Scripts for analyses may be found at https://github.com/LiZhaoLab/denovo_bursting_2025 (69). An R package for HSQ implementation may be found at https://github.com/ulee-sciscripts/scHSQ (70).
Supporting Information
References
- 1.Raz A. A., et al. , Emergent dynamics of adult stem cell lineages from single nucleus and single cell RNA-Seq of Drosophila testes. Elife 12, e82201 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Witt E., Langer C. B., Svetec N., Zhao L., Transcriptional and mutational signatures of the Drosophila ageing germline. Nat. Ecol. Evol. 7, 440–449 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nuzhdin S. V., Wayne M. L., Harmon K. L., McIntyre L. M., Common pattern of evolution of gene expression level and protein sequence in Drosophila. Mol. Biol. Evol. 21, 1308–1317 (2004). [DOI] [PubMed] [Google Scholar]
- 4.Khodursky S., Svetec N., Durkin S. M., Zhao L., The evolution of sex-biased gene expression in the Drosophila brain. Genome Res. 30, 874–884 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hao Y., et al. , Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587.e29 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haghverdi L., Lun A. T. L., Morgan M. D., Marioni J. C., Batch effects in single-cell RNA-sequencing data are corrected by matching mutual nearest neighbors. Nat. Biotechnol. 36, 421–427 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Korsunsky I., et al. , Fast, sensitive and accurate integration of single-cell data with Harmony. Nat. Methods 16, 1289–1296 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tarashansky A. J., et al. , Mapping single-cell atlases throughout Metazoa unravels cell type evolution. Elife 10, e66747 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Snook R. R., Markow T. A., Karr T. L., Functional nonequivalence of sperm in Drosophila pseudoobscura. Proc. Natl. Acad. Sci. U.S.A. 91, 11222–11226 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joly D., Korol A., Nevo E., Sperm size evolution in Drosophila: Inter- and intraspecific analysis. Genetica 120, 233–244 (2004). [DOI] [PubMed] [Google Scholar]
- 11.Schärer L., Da Lage J.-L., Joly D., Evolution of testicular architecture in the Drosophilidae: A role for sperm length. BMC Evol. Biol. 8, 143 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamashita Y. M., Fuller M. T., Jones D. L., Signaling in stem cell niches: Lessons from the Drosophila germline. J. Cell Sci. 118, 665–672 (2005). [DOI] [PubMed] [Google Scholar]
- 13.Lim C., Tarayrah L., Chen X., Transcriptional regulation during Drosophila spermatogenesis. Spermatogenesis 2, 158–166 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alvi Z. A., Chu T.-C., Schawaroch V., Klaus A. V., Protamine-like proteins in 12 sequenced species of Drosophila. Protein Pept. Lett. 20, 17–35 (2013). [PubMed] [Google Scholar]
- 15.Chang C.-H., Mejia Natividad I., Malik H. S., Expansion and loss of sperm nuclear basic protein genes in Drosophila correspond with genetic conflicts between sex chromosomes. Elife 12, e85249 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McAdams H. H., Arkin A., Stochastic mechanisms in gene expression. Proc. Natl. Acad. Sci. U.S.A. 94, 814–819 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raj A., van Oudenaarden A., Nature, nurture, or chance: Stochastic gene expression and its consequences. Cell 135, 216–226 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Golding I., Paulsson J., Zawilski S. M., Cox E. C., Real-time kinetics of gene activity in individual bacteria. Cell 123, 1025–1036 (2005). [DOI] [PubMed] [Google Scholar]
- 19.Cai L., Friedman N., Xie X. S., Stochastic protein expression in individual cells at the single molecule level. Nature 440, 358–362 (2006). [DOI] [PubMed] [Google Scholar]
- 20.Bothma J. P., et al. , Dynamic regulation of eve stripe 2 expression reveals transcriptional bursts in living Drosophila embryos. Proc. Natl. Acad. Sci. U.S.A. 111, 10598–10603 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raj A., Peskin C. S., Tranchina D., Vargas D. Y., Tyagi S., Stochastic mRNA synthesis in mammalian cells. PLoS Biol. 4, e309 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suter D. M., et al. , Mammalian genes are transcribed with widely different bursting kinetics. Science 332, 472–474 (2011). [DOI] [PubMed] [Google Scholar]
- 23.Walters M. C., et al. , Enhancers increase the probability but not the level of gene expression. Proc. Natl. Acad. Sci. U.S.A. 92, 7125–7129 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fukaya T., Lim B., Levine M., Enhancer control of transcriptional bursting. Cell 166, 358–368 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zoller B., Little S. C., Gregor T., Diverse spatial expression patterns emerge from unified kinetics of transcriptional bursting. Cell 175, 835–847.e25 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larsson A. J. M., et al. , Genomic encoding of transcriptional burst kinetics. Nature 565, 251–254 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Forbes Beadle L., Zhou H., Rattray M., Ashe H. L., Modulation of transcription burst amplitude underpins dosage compensation in the Drosophila embryo. Cell Rep. 42, 112382 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vibranovski M. D., Meiotic sex chromosome inactivation in Drosophila. J. Genomics 2, 104–117 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hense W., Baines J. F., Parsch J., X chromosome inactivation during Drosophila spermatogenesis. PLoS Biol. 5, e273 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meiklejohn C. D., Landeen E. L., Cook J. M., Kingan S. B., Presgraves D. C., Sex chromosome-specific regulation in the Drosophila male germline but little evidence for chromosomal dosage compensation or meiotic inactivation. PLoS Biol. 9, e1001126 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Witt E., Svetec N., Benjamin S., Zhao L., Transcription factors drive opposite relationships between gene age and tissue specificity in male and female Drosophila gonads. Mol. Biol. Evol. 38, 2104–2115 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Witt E., Shao Z., Hu C., Krause H. M., Zhao L., Single-cell RNA-sequencing reveals pre-meiotic X-chromosome dosage compensation in Drosophila testis. PLoS Genet. 17, e1009728 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kemkemer C., Hense W., Parsch J., Fine-scale analysis of X chromosome inactivation in the male germ line of Drosophila melanogaster. Mol. Biol. Evol. 28, 1561–1563 (2011). [DOI] [PubMed] [Google Scholar]
- 34.Long M., VanKuren N. W., Chen S., Vibranovski M. D., New gene evolution: Little did we know. Annu. Rev. Genet. 47, 307–333 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Witt E., Benjamin S., Svetec N., Zhao L., Testis single-cell RNA-seq reveals the dynamics of de novo gene transcription and germline mutational bias in Drosophila. Elife 8, e47138 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levine M. T., Jones C. D., Kern A. D., Lindfors H. A., Begun D. J., Novel genes derived from noncoding DNA in Drosophila melanogaster are frequently X-linked and exhibit testis-biased expression. Proc. Natl. Acad. Sci. U.S.A. 103, 9935–9939 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen S., Zhang Y. E., Long M., New genes in Drosophila quickly become essential. Science 330, 1682–1685 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gubala A. M., et al. , The Goddard and Saturn genes are essential for Drosophila male fertility and may have arisen de novo. Mol. Biol. Evol. 34, 1066–1082 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao L., Saelao P., Jones C. D., Begun D. J., Origin and spread of de novo genes in Drosophila melanogaster populations. Science 343, 769–772 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cridland J. M., Majane A. C., Sheehy H. K., Begun D. J., Polymorphism and divergence of novel gene expression patterns in Drosophila melanogaster. Genetics 216, 79–93 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao L., Svetec N., Begun D. J., De novo genes. Annu. Rev. Genet. 58, 211–232 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee U., Mozeika S., Zhao L., A synergistic, cultivator model of de novo gene origination. Genome Biol. and Evol. 16, evae103 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qiu X., et al. , Reversed graph embedding resolves complex single-cell trajectories. Nat. Methods 14, 979–982 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suvorov A., et al. , Widespread introgression across a phylogeny of 155 Drosophila genomes. Curr. Biol. 32, 111–123.e5 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mi H., Muruganujan A., Casagrande J. T., Thomas P. D., Large-scale gene function analysis with the PANTHER classification system. Nat. Protoc. 8, 1551–1566 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thomas P. D., et al. , PANTHER: Making genome-scale phylogenetics accessible to all. Protein Sci. 31, 8–22 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choi H. M. T., et al. , Third-generation in situ hybridization chain reaction: Multiplexed, quantitative, sensitive, versatile, robust. Development 145, dev165753 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bruce H. S., et al. , Hybridization Chain Reaction (HCR) in situ protocol (2021). 10.17504/protocols.io.bunznvf6. Accessed 11 April 2025. [DOI]
- 49.Thayer R. C., Polston E. S., Xu J., Begun D. J., Regional specialization, polyploidy, and seminal fluid transcripts in the Drosophila female reproductive tract. Proc. Natl. Acad. Sci. U.S.A. 121, e2409850121 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baker C. C., Gim B. S., Fuller M. T., Cell type-specific translational repression of Cyclin B during meiosis in males. Development 142, 3394 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baker C. C., et al. , Cell-type-specific interacting proteins collaborate to regulate the timing of Cyclin B protein expression in male meiotic prophase. Development 150, dev201709 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hunt G., et al. , Tissue-specific RNA Polymerase II promoter-proximal pause release and burst kinetics in a Drosophila embryonic patterning network. Genome Biol. 25, 2 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trzaskoma P., et al. , 3D chromatin architecture, BRD4, and Mediator have distinct roles in regulating genome-wide transcriptional bursting and gene network. Sci. Adv. 10, eadl4893 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gupta S., Cai J. J., Gene function revealed at the moment of stochastic gene silencing. Commun. Biol. 8, 1–14 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Palacio M., Taatjes D. J., Real-time visualization of reconstituted transcription reveals RNA polymerase II activation mechanisms at single promoters. bioXriv [Preprint] (2025). https://www.biorxiv.org/content/10.1101/2025.01.06.631569v1 (Accessed 9 February 2025).
- 56.Ohishi H., et al. , Transcription-coupled changes in genomic region proximities during transcriptional bursting. Sci. Adv. 10, eadn0020 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alamos S., et al. , Minimal synthetic enhancers reveal control of the probability of transcriptional engagement and its timing by a morphogen gradient. Cell Syst. 14, 220–236.e3 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mahadevaraju S., et al. , Dynamic sex chromosome expression in Drosophila male germ cells. Nat. Commun. 12, 892 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Johnstone C. P., Galloway K. E., Supercoiling-mediated feedback rapidly couples and tunes transcription. Cell Rep 41, 111492 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Singh B. N., Drosophila ananassae: A species characterized by spontaneous male recombination in appreciable frequency. J. Genet. 99, 12 (2020). [PubMed] [Google Scholar]
- 61.Larschan E., et al. , X chromosome dosage compensation via enhanced transcriptional elongation in Drosophila. Nature 471, 115–118 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Franke A., Baker B. S., The rox1 and rox2 RNAs are essential components of the compensasome, which mediates dosage compensation in Drosophila. Mol. Cell 4, 117–122 (1999). [DOI] [PubMed] [Google Scholar]
- 63.Conrad T., Akhtar A., Dosage compensation in Drosophila melanogaster: Epigenetic fine-tuning of chromosome-wide transcription. Nat. Rev. Genet. 13, 123–134 (2012). [DOI] [PubMed] [Google Scholar]
- 64.Valsecchi C. I. K., et al. , RNA nucleation by MSL2 induces selective X chromosome compartmentalization. Nature 589, 137–142 (2021). [DOI] [PubMed] [Google Scholar]
- 65.Rastelli L., Kuroda M. I., An analysis of maleless and histone H4 acetylation in Drosophila melanogaster spermatogenesis. Mech. Dev. 71, 107–117 (1998). [DOI] [PubMed] [Google Scholar]
- 66.Anderson J. T., Henikoff S., Ahmad K., Chromosome-specific maturation of the epigenome in the Drosophila male germline. Elife 12, RP89373 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wei K. H.-C., Chatla K., Bachtrog D., Single-cell RNA-seq of Drosophila miranda testis reveals the evolution and trajectory of germline sex chromosome regulation. PLoS Biol. 22, e3002605 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee U., et al. , BioProject PRJNA995212 Single cell RNA sequencing of Drosophila testis tissue. NCBI Sequence Read Archive. https://www.ncbi.nlm.nih.gov/bioproject/PRJNA995212. Deposited 15 July 2023.
- 69.Lee U., et al. , Code for Comparative single cell analysis of transcriptional bursting reveals the role of genome organization in de novo transcript origination. GitHub. https://github.com/LiZhaoLab/denovo_bursting_2025. Deposited 27 February 2025. [DOI] [PMC free article] [PubMed]
- 70.Lee U., scHSQ. GitHub. https://github.com/ulee-sciscripts/scHSQ. Deposited 4 February 2025.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Dataset S01 (CSV)
Dataset S02 (CSV)
Dataset S03 (CSV)
Data Availability Statement
scRNA-Seq reads were uploaded to NCBI under the project number PRJNA995212 (68). Scripts for analyses may be found at https://github.com/LiZhaoLab/denovo_bursting_2025 (69). An R package for HSQ implementation may be found at https://github.com/ulee-sciscripts/scHSQ (70).