Abstract
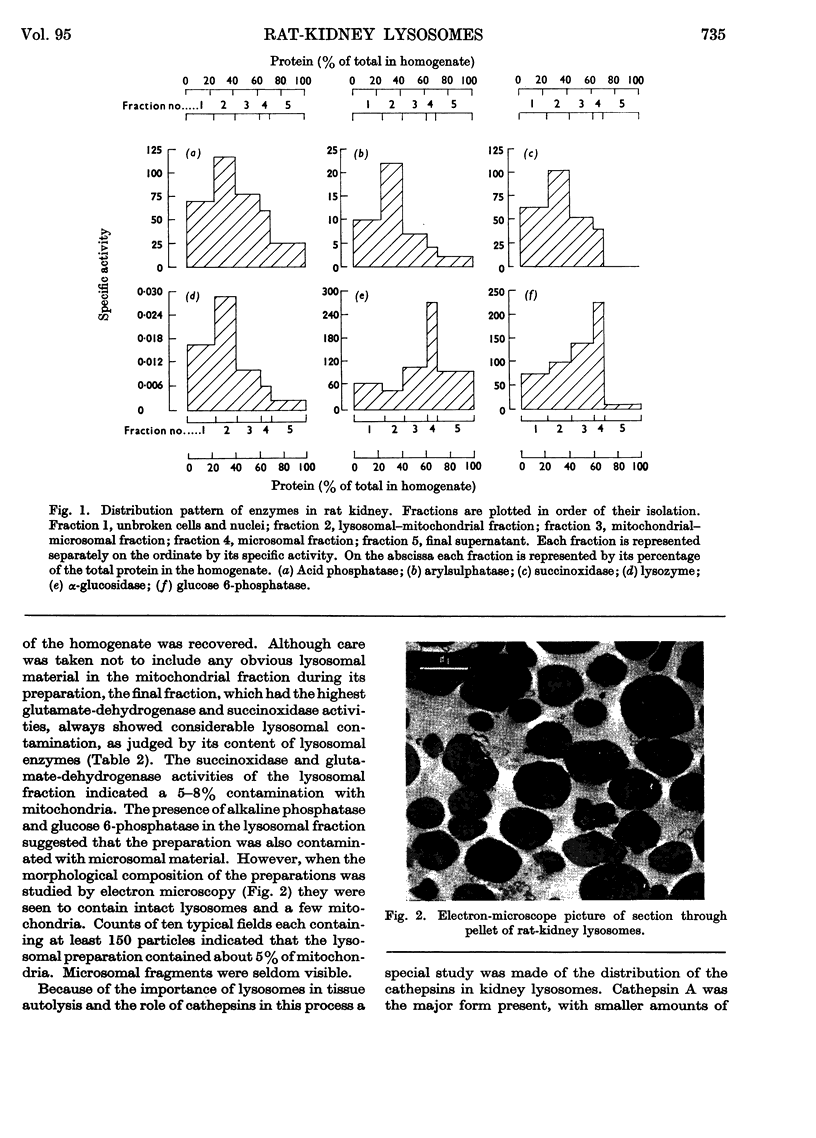
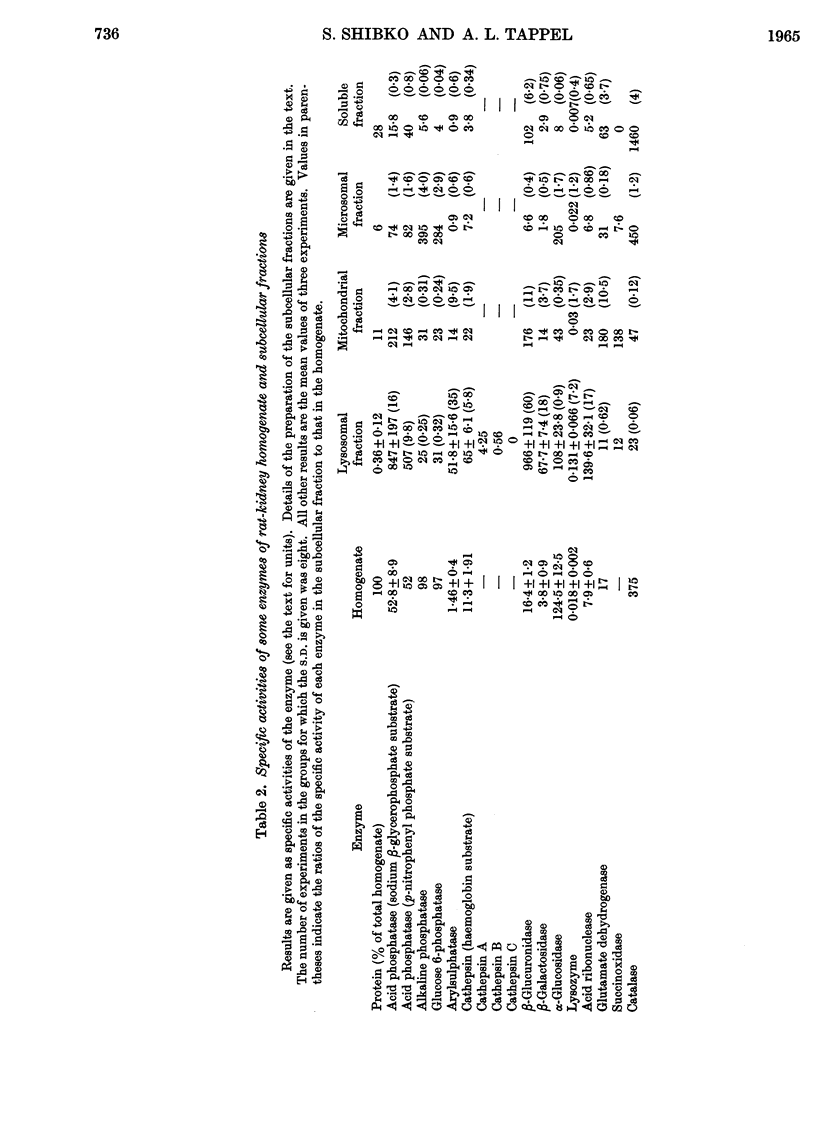
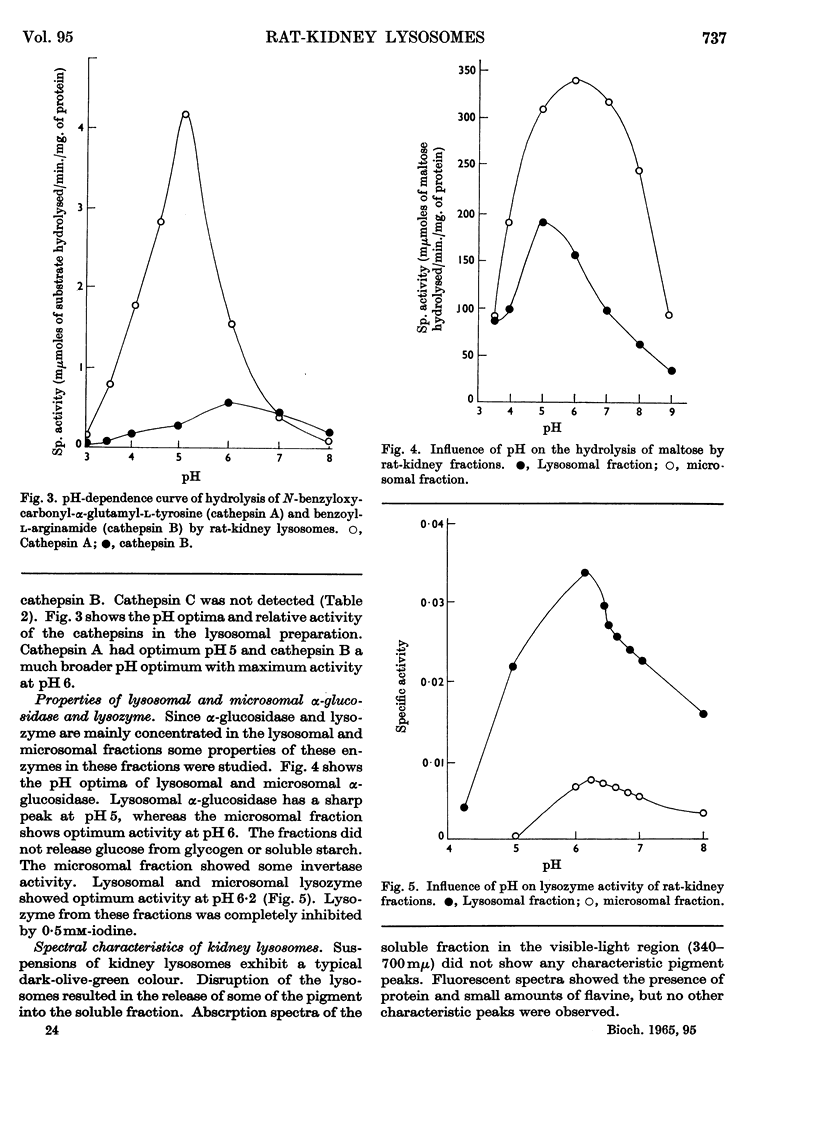
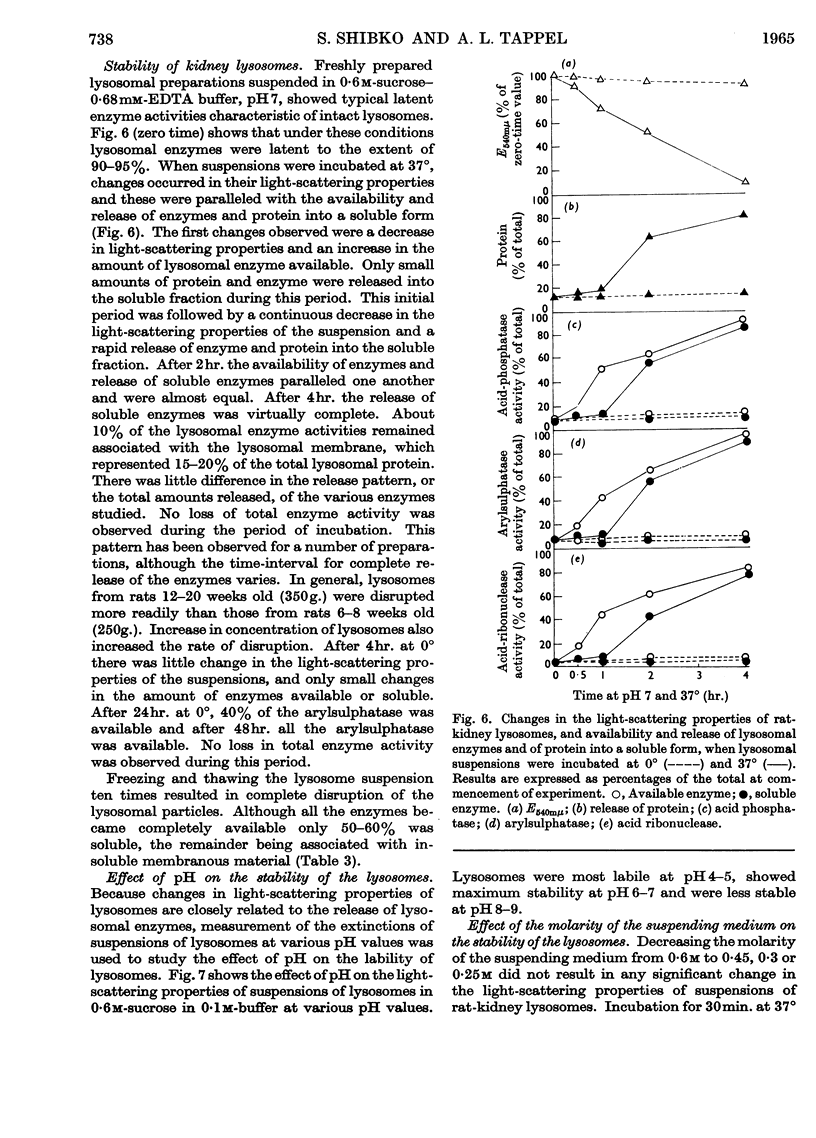
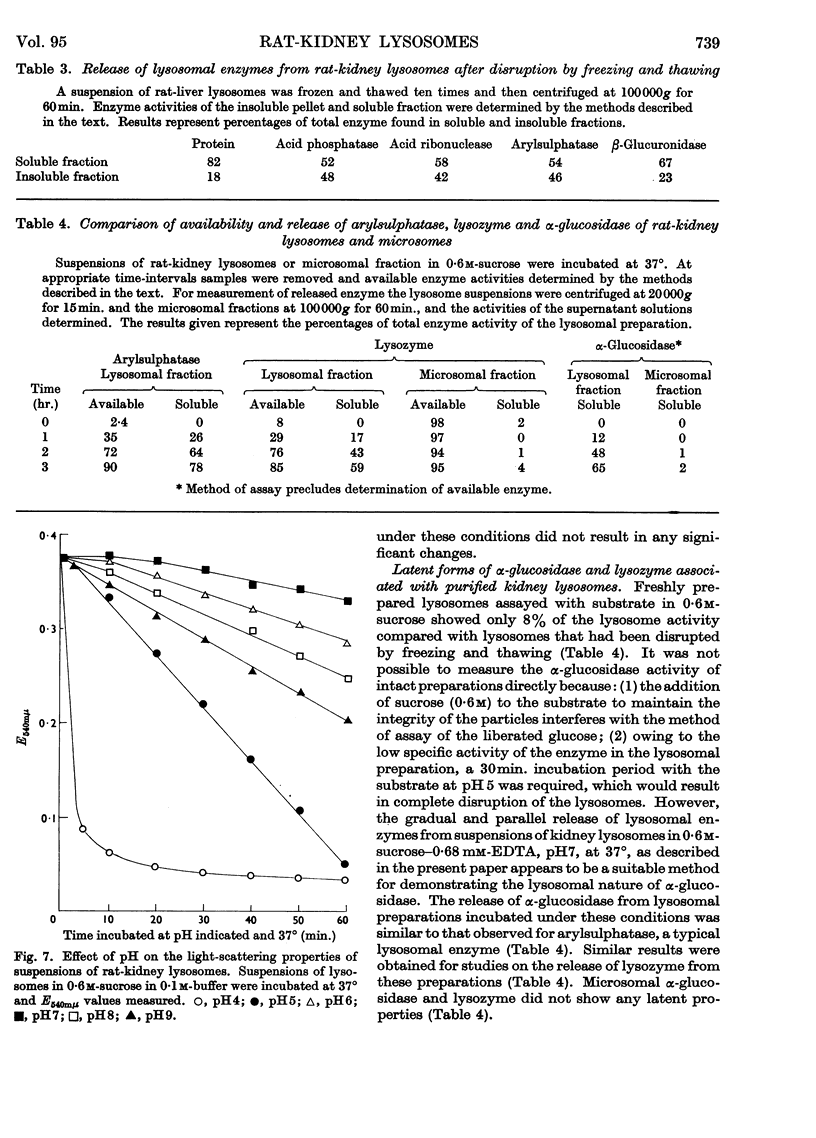
1. The activities of lysosomal enzymes in the cortexes and medullas and the principal subcellular fractions of rat kidney were measured. 2. A method is described for the isolation of rat-kidney lysosomes and a detailed analysis of the enzymic composition of the lysosomes is reported. Enzyme analysis of the other principal subcellular fractions is included for comparison. 3. Studies of the distribution of α-glucosidase showed that the lysosomal fraction contained only 10% of the total enzyme activity. The microsomal fraction contained most of the particulate α-glucosidase. Lysozyme was concentrated mainly in the lysosomal fraction with only small amounts present in the microsomal fraction. Lysosomal α-glucosidase had optimum pH5 whereas the microsomal form had optimum pH6. Both lysosomal and microsomal lysozyme had optimum pH6·2. 4. The stability of lysosomal suspensions was studied. Incubation at 37° and pH7 resulted in first an increased availability of enzymes without parallel release of enzyme. This was followed by a second stage during which the availability of enzymes was closely related to the release of enzymes. These changes were closely paralleled by changes in light-scattering properties of lysosomes. 5. The latent nature of the α-glucosidase and lysozyme of intact kidney lysosomes was demonstrated by their graded and parallel release with other typical lysosomal enzymes. 6. Isolated lysosomes were unstable at pH values lower than 5, most stable at pH6–7 and less stable at pH 8–9. Lysosomes were not disrupted when the osmolarity of the suspending medium was decreased from 0·6m to 0·25m. 7. The discussion compares the properties and composition of kidney lysosomes, liver lysosomes and the granules of macrophages. 8. The possible origin of the lysozyme in kidney lysosomes by reabsorption of the lysozyme in blood is discussed.
Full text
PDF





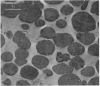
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALBERTI K. G., BARTLEY W. The production of amino acids by cell fractions, particularly rat-liver mitochondria. Biochem J. 1963 Apr;87:104–114. doi: 10.1042/bj0870104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A., HIRSCH J. G. The isolation and properties of the specific cytoplasmic granules of rabbit polymorphonuclear leucocytes. J Exp Med. 1960 Dec 1;112:983–1004. doi: 10.1084/jem.112.6.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIANETTO R., DE DUVE C. Tissue fractionation studies. 4. Comparative study of the binding of acid phosphatase, beta-glucuronidase and cathepsin by rat-liver particles. Biochem J. 1955 Mar;59(3):433–438. doi: 10.1042/bj0590433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDBARG J. A., TSOU K. C., RUTENBURG S. H., RUTENBURG A. M., SELIGMAN A. M. A method for the colorimetric determination of alpha-D-glucosidase with a chromogenic substrate. Arch Biochem Biophys. 1958 Jun;75(2):435–442. doi: 10.1016/0003-9861(58)90443-0. [DOI] [PubMed] [Google Scholar]
- HERS H. G. alpha-Glucosidase deficiency in generalized glycogenstorage disease (Pompe's disease). Biochem J. 1963 Jan;86:11–16. doi: 10.1042/bj0860011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOGEBOOM G. H., SCHNEIDER W. C. Intracellular distribution of enzymes. XI. Glutamic dehydrogenase. J Biol Chem. 1953 Sep;204(1):233–238. [PubMed] [Google Scholar]
- KORNER A., TARVER H. Studies on protein synthesis in vitro. VI. Incorporation and release of amino acids in particulate preparations from livers of rats. J Gen Physiol. 1957 Sep 20;41(1):219–231. doi: 10.1085/jgp.41.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KREBS H. A., BENNETT D. A., DE GASQUET P., GASQUET P., GASCOYNE T., YOSHIDA T. Renal gluconeogenesis. The effect of diet on the gluconeogenic capacity of rat-kidney-cortex slices. Biochem J. 1963 Jan;86:22–27. doi: 10.1042/bj0860022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEJEUNE N., THINES-SEMPOUX D., HERS H. G. Tissue fractionation studies. 16. Intracellular distribution and properties of alpha-glucosidases in rat liver. Biochem J. 1963 Jan;86:16–21. doi: 10.1042/bj0860016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LITWACK G. Effects of induced states on tissue lysozyme activity. Proc Soc Exp Biol Med. 1957 Apr;94(4):764–767. doi: 10.3181/00379727-94-23079. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROBERTS N. R., WU M. L., HIXON W. S., CRAWFORD E. J. The quantitative histochemistry of brain. II. Enzyme measurements. J Biol Chem. 1954 Mar;207(1):19–37. [PubMed] [Google Scholar]
- PERRI G. C., ANIGSTEIN R. Ehrlich-reacting protein in kidneys of tumor-bearing rats. Cancer Res. 1960 Aug;20:1054–1058. [PubMed] [Google Scholar]
- PERRI G. C., FAULK M., MELLORS J., STOCK C. C. Crystallization of a basic protein (lysozyme) from kidneys of tumour-bearing rats (Jensen sarcoma). Nature. 1962 Feb 17;193:649–651. doi: 10.1038/193649a0. [DOI] [PubMed] [Google Scholar]
- PERRI G. C., FAULK M., MONEY W. L. THYROID FUNCTION AND KIDNEY MURAMIDASE. Proc Soc Exp Biol Med. 1964 Jan;115:185–188. doi: 10.3181/00379727-115-28865. [DOI] [PubMed] [Google Scholar]
- ROY A. B. The sulphatase of ox liver. I. The complex nature of the enzyme. Biochem J. 1953 Jan;53(1):12–15. doi: 10.1042/bj0530012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SELLINGER O. Z., BEAUFAY H., JACQUES P., DOYEN A., DE DUVE C. Tissue fractionation studies. 15. Intracellular distribution and properties of beta-N-acetylglucosaminidase and beta-galactosidase in rat liver. Biochem J. 1960 Mar;74:450–456. doi: 10.1042/bj0740450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRAUS W. CYTOCHEMICAL OBSERVATIONS ON THE RELATIONSHIP BETWEEN LYSOSOMES AND PHAGOSOMES IN KIDNEY AND LIVER BY COMBINED STAINING FOR ACID PHOSPHATASE AND INTRAVENOUSLY INJECTED HORSERADISH PEROXIDASE. J Cell Biol. 1964 Mar;20:497–507. doi: 10.1083/jcb.20.3.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRAUS W. Colorimetric investigation of the uptake of an intravenously injected protein (horseradish peroxidase) by rat kidney and effects of competition by egg white. J Cell Biol. 1962 Feb;12:231–246. doi: 10.1083/jcb.12.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRAUS W. Isolation and biochemical properties of droplets from the cells of rat kidney. J Biol Chem. 1954 Apr;207(2):745–755. [PubMed] [Google Scholar]
- TAPPEL A. L., ZALKIN H., CALDWELL K. A., DESAI I. D., SHIBKO S. Increased lysosomal enzymes in genetic muscular dystrophy. Arch Biochem Biophys. 1962 Feb;96:340–346. doi: 10.1016/0003-9861(62)90418-6. [DOI] [PubMed] [Google Scholar]
- WHITAKER J. R. Ninhydrin assay in the presence of thiol compounds. Nature. 1961 Feb 25;189:662–663. doi: 10.1038/189662a0. [DOI] [PubMed] [Google Scholar]