Abstract
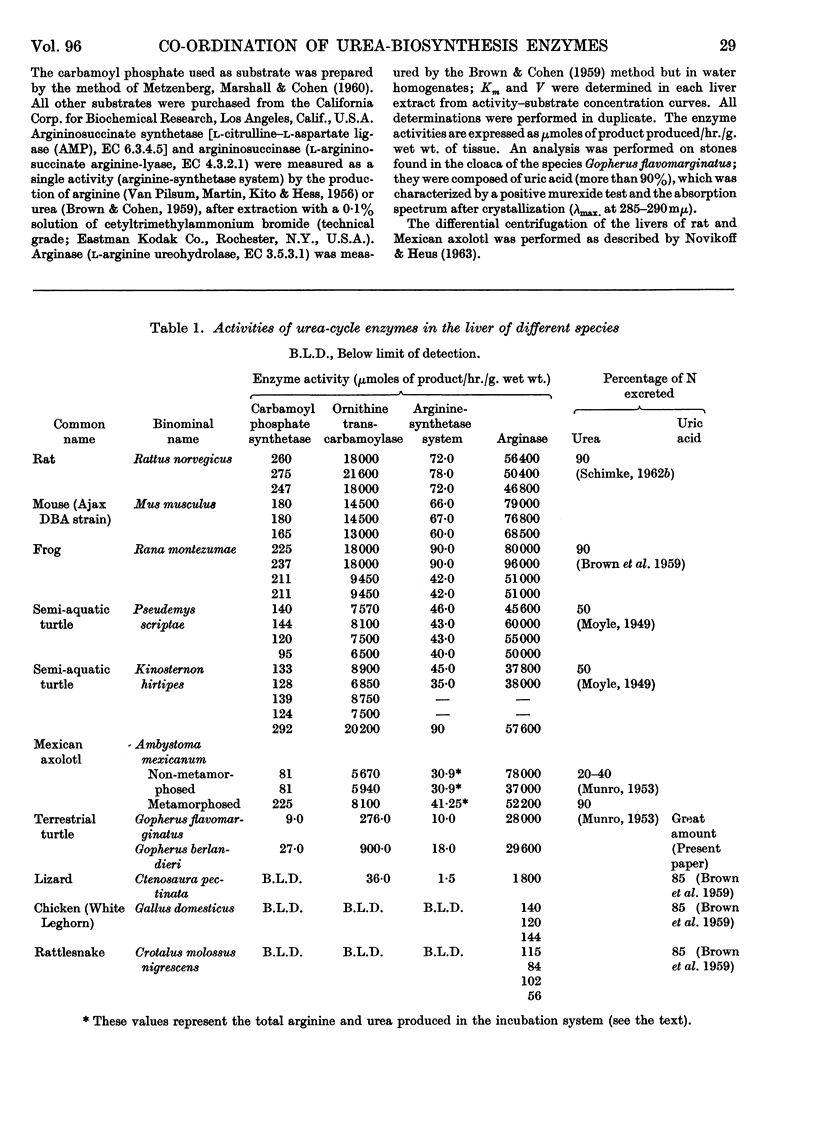
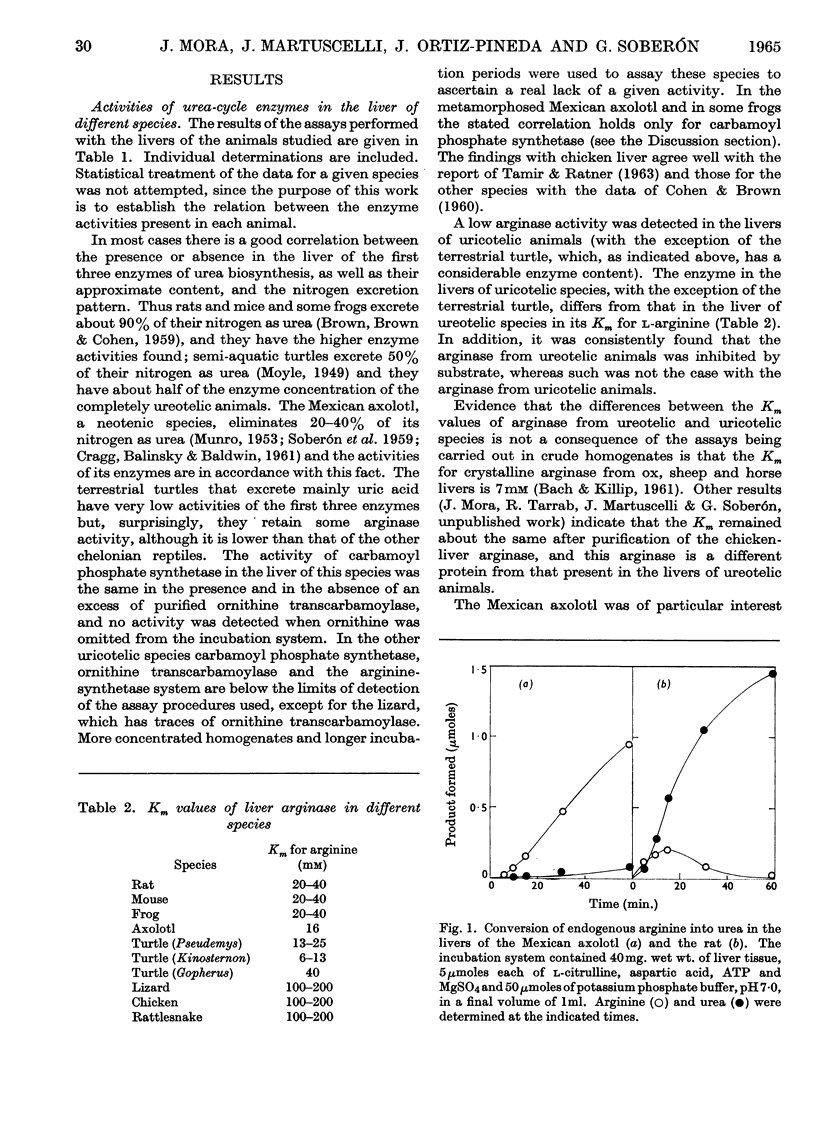
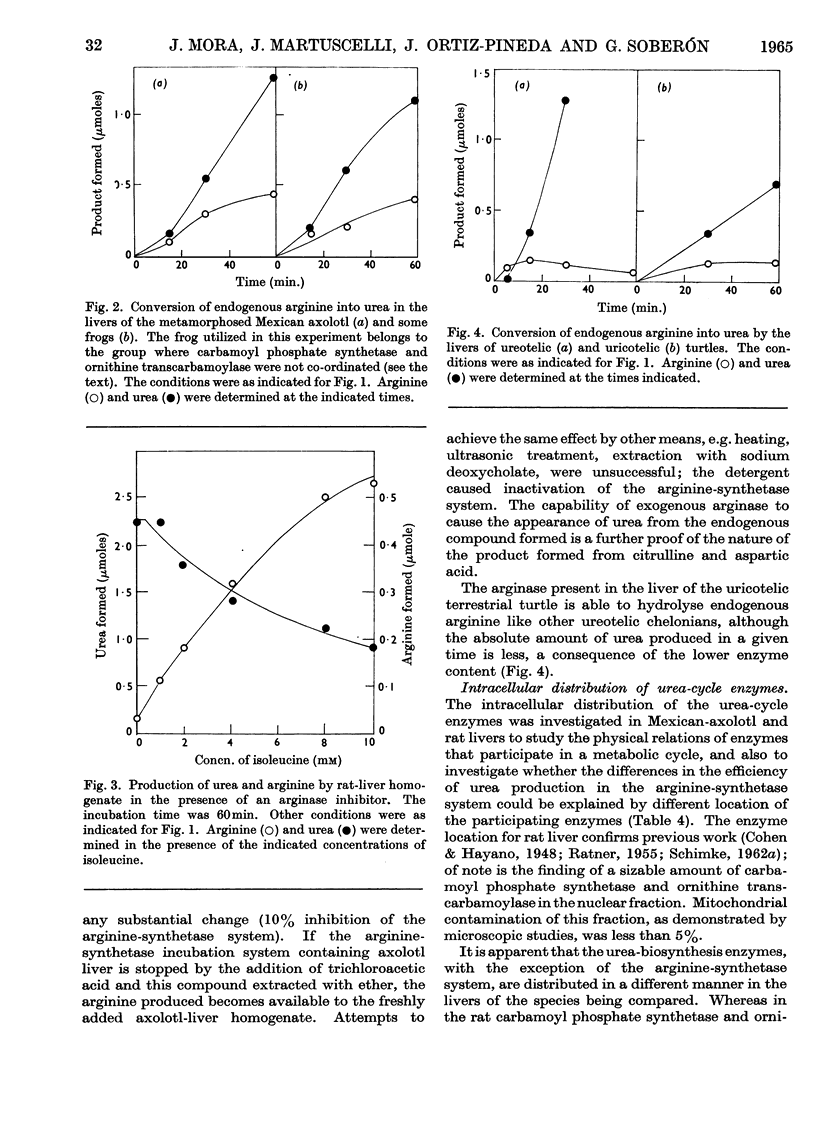
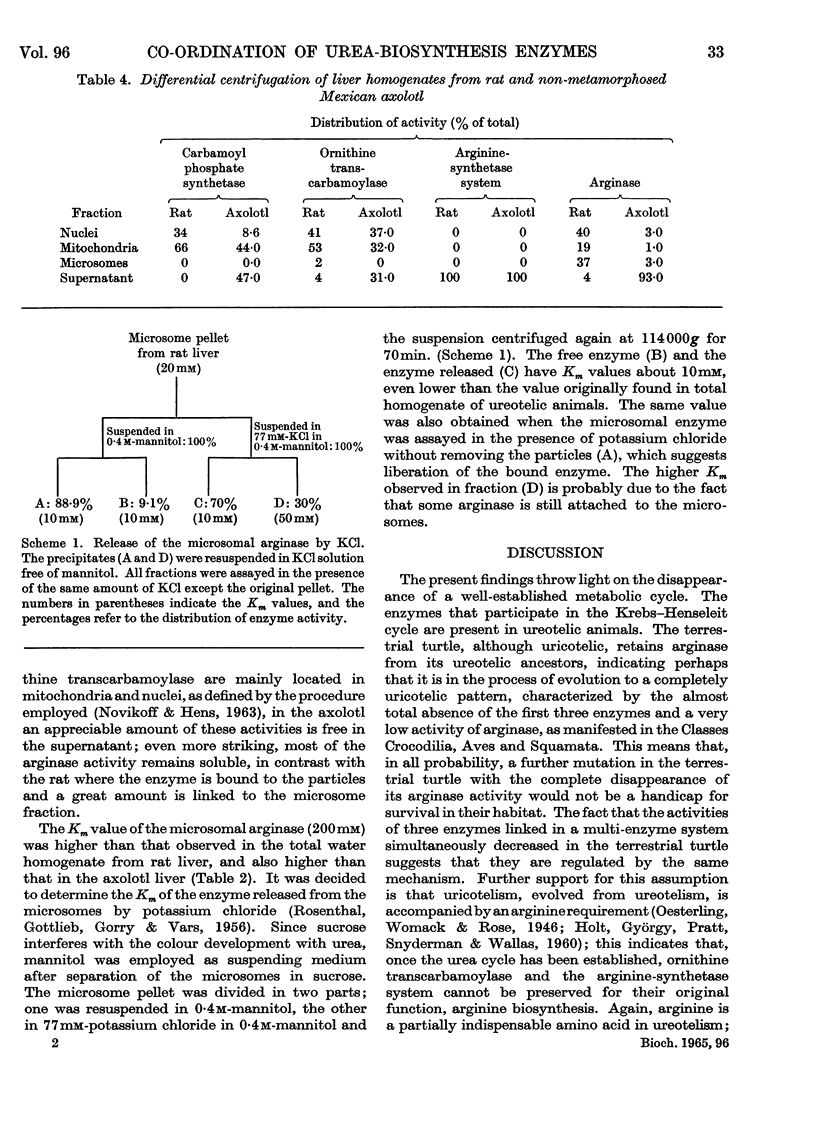
1. Carbamoyl phosphate synthetase, ornithine transcarbamoylase, the arginine-synthetase system and arginase were measured in the livers of ammoniotelic, ureotelic and uricotelic animals. The chelonian reptiles, whose nitrogen excretory patterns vary according to the habitat, and the Mexican axolotl, a neotenic species, were also studied. 2. The levels of the activities of the first three enzymes mentioned correlate with the amount of nitrogen excreted as urea. 3. The terrestrial turtle, which excretes mainly uric acid, maintains a high arginase activity but has very low levels of the activities of the other three enzymes. 4. The first three enzymes of the urea cycle vary in the phylogenic scale in a co-ordinated manner, which suggests that they are under the same regulatory mechanism. 5. Urea formation from endogenous arginine in vitro has a low efficiency in the Mexican axolotl. 6. The induction of metamorphosis in the Mexican axolotl by the administration of l-tri-iodothyronine, which causes a shift from ammonio-ureotelism to complete ureotelism, is accompanied by an increase mainly in carbamoyl phosphate synthetase and also by an improvement in the efficiency of hydrolysis of endogenous arginine in vitro to give urea. 7. The results obtained by differential centrifugation of the urea-cycle enzymes in rat and Mexican-axolotl livers are presented. The location requirements for the integration of a metabolic cycle are discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BACH S. J., KILLIP J. D. Studies on the purification and the kinetic properties of arginase from beef, sheep and horse liver. Biochim Biophys Acta. 1961 Feb 18;47:336–343. doi: 10.1016/0006-3002(61)90294-3. [DOI] [PubMed] [Google Scholar]
- BROWN G. W., Jr, BROWN W. R., COHEN P. P. Comparative biochemistry of urea synthesis. II. Levels of urea cycle enzymes in metamorphosing Rana catesbeiana tadpoles. J Biol Chem. 1959 Jul;234(7):1775–1780. [PubMed] [Google Scholar]
- CLEVER U. [Gene activity in the giant chromosomes of Chironomus tentans and its relation to development. I. Gene activation by ecdysone]. Chromosoma. 1961;12:607–675. doi: 10.1007/BF00328945. [DOI] [PubMed] [Google Scholar]
- CRAGG M. M., BALINSKY J. B., BALDWIN E. A comparative study of nitrogen excretion in some Amphibia and reptiles. Comp Biochem Physiol. 1961 Nov;3:227–235. doi: 10.1016/0010-406x(61)90008-1. [DOI] [PubMed] [Google Scholar]
- GORRY J. D., GOTTLIEB B., ROSENTHAL O., VARS H. M. Influence of cations on the intracellular distribution of rat liver arginase. J Biol Chem. 1956 Nov;223(1):469–478. [PubMed] [Google Scholar]
- HESS J., KITO E., MARTIN R. P., VAN PILSUM J. F. Determination of creatine, creatinine, arginine, guanidinoacetic acid, guanidine, and methylguanidine in biological fluids. J Biol Chem. 1956 Sep;222(1):225–235. [PubMed] [Google Scholar]
- JACOB F., MONOD J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961 Jun;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- KNIVETT V. A. Phosphorylation coupled with anaerobic breakdown of citrulline. Biochem J. 1954 Apr;56(4):602–606. [PMC free article] [PubMed] [Google Scholar]
- MCLEAN P., GURNEY M. W. Effect of adrenalectomy and of growth hormone on enzymes concerned with urea synthesis in rat liver. Biochem J. 1963 Apr;87:96–104. doi: 10.1042/bj0870096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUNRO A. F. The ammonia and urea excretion of different species of Amphibia during their development and metamorphosis. Biochem J. 1953 Apr;54(1):29–36. doi: 10.1042/bj0540029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyle V. Nitrogenous excretion in Chelonian reptiles. Biochem J. 1949;44(5):581–584. doi: 10.1042/bj0440581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOVIKOFF A. B., HEUS M. A microsomal nucleoside diphosphatase. J Biol Chem. 1963 Feb;238:710–716. [PubMed] [Google Scholar]
- ROSADO A., FLORES G., MORA J., SOBERON G. Distribution of an ammonia load in the normal rat. Am J Physiol. 1962 Jul;203:37–42. doi: 10.1152/ajplegacy.1962.203.1.37. [DOI] [PubMed] [Google Scholar]
- SCHIMKE R. T. Adaptive characteristics of urea cycle enzymes in the rat. J Biol Chem. 1962 Feb;237:459–468. [PubMed] [Google Scholar]
- SCHIMKE R. T., BARILE M. F. ARGININE METABOLISM IN PLEUROPNEUMONIA-LIKE ORGANISMS ISOLATED FROM MAMMALIAN CELL CULTURE. J Bacteriol. 1963 Aug;86:195–206. doi: 10.1128/jb.86.2.195-206.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHIMKE R. T. Differential effects of fasting and protein-free diets on levels of urea cycle enzymes in rat liver. J Biol Chem. 1962 Jun;237:1921–1924. [PubMed] [Google Scholar]
- SCHIMKE R. T. ENZYMES OF ARGININE METABOLISM IN MAMMALIAN CELL CULTURE. I. REPRESSION OF ARGININOSUCCINATE SYNTHETASE AND ARGININOSUCCINASE. J Biol Chem. 1964 Jan;239:136–145. [PubMed] [Google Scholar]
- SCHIMKE R. T. Studies on factors affecting the levels of urea cycle enzymes in rat liver. J Biol Chem. 1963 Mar;238:1012–1018. [PubMed] [Google Scholar]
- SOBERON G., GUSTAVO FLORES Q. F., MORA J., TORRES J. [Some aspects of the metabolism of urea and ammonia]. Gac Med Mex. 1959 Nov-Dec;89:955–980. [PubMed] [Google Scholar]
- TAMIR H., RATNER S. ENZYMES OF ARGININE METABOLISM IN CHICKS. Arch Biochem Biophys. 1963 Aug;102:249–258. doi: 10.1016/0003-9861(63)90178-4. [DOI] [PubMed] [Google Scholar]
- WAGNER R. P., BERGQUIST A. Synthesis of valine and isoleucine in the presence of a particulate cell fraction of Neurospora. Proc Natl Acad Sci U S A. 1963 Jun;49:892–897. doi: 10.1073/pnas.49.6.892. [DOI] [PMC free article] [PubMed] [Google Scholar]


