Abstract
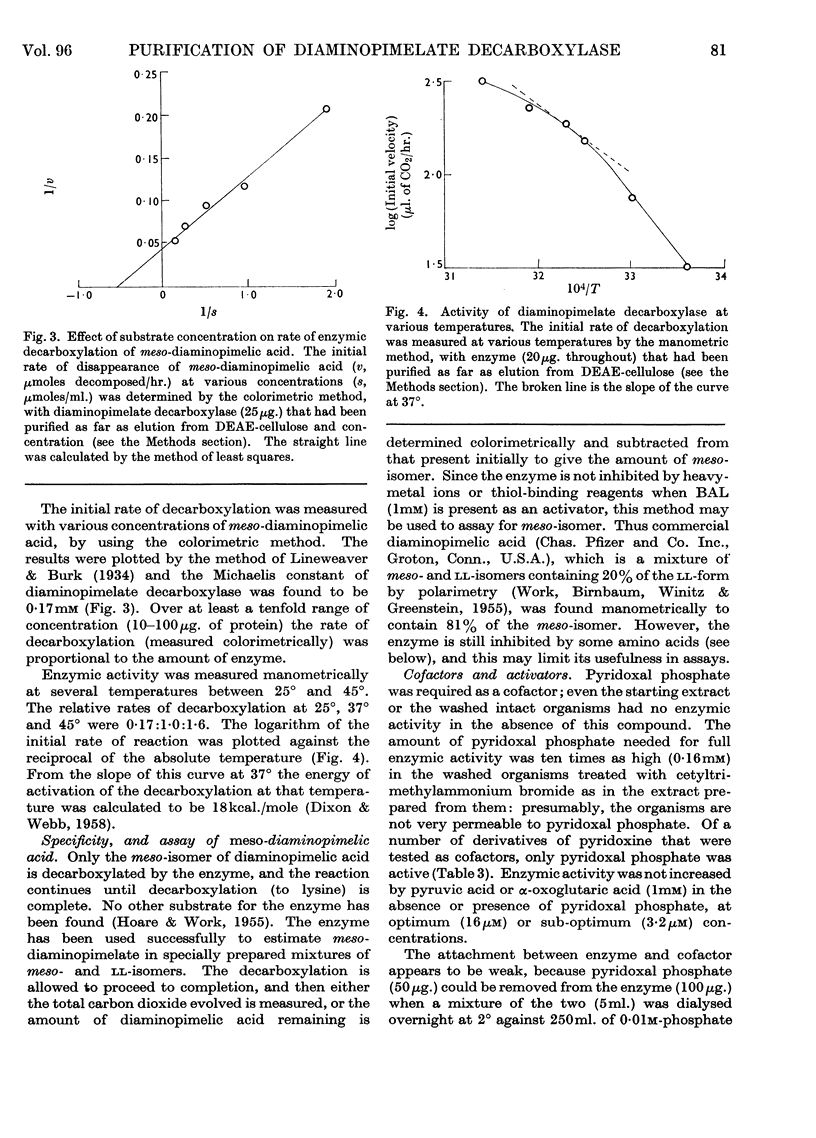
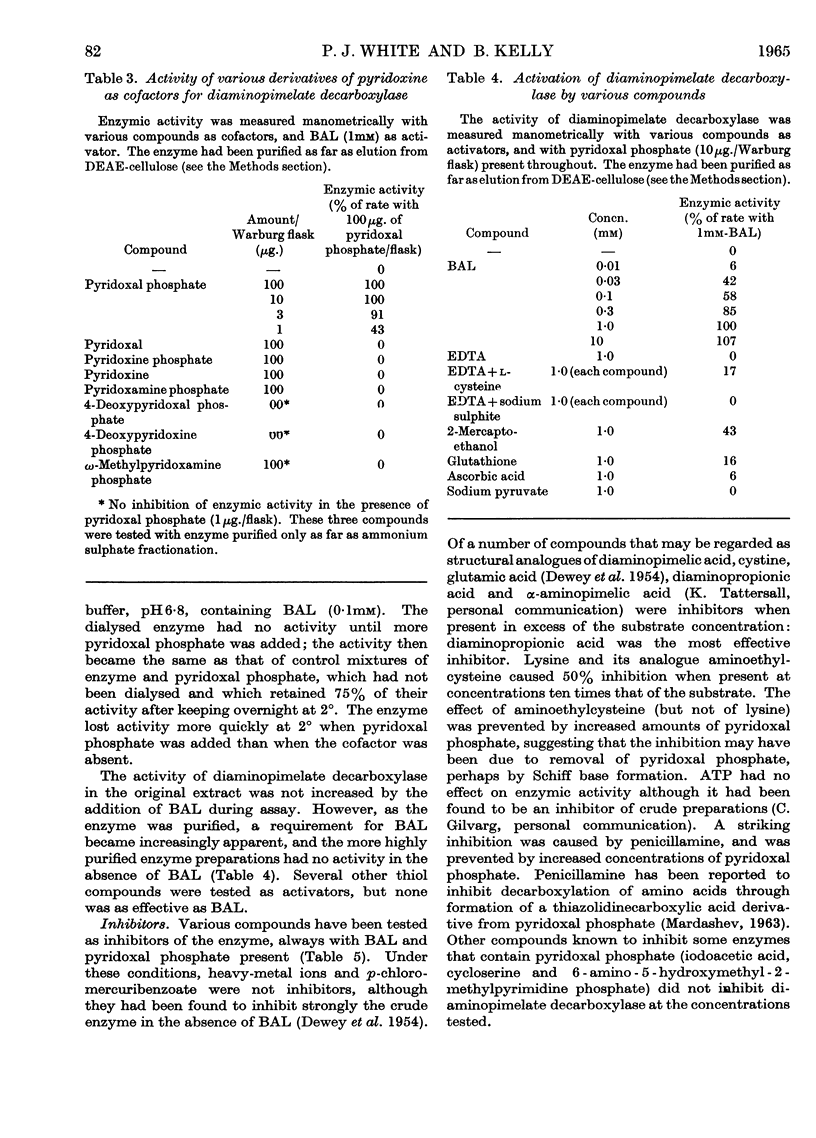
1. Diaminopimelate decarboxylase from a soluble extract of Escherichia coli A.T.C.C. 9637 was purified 200-fold by precipitation of nucleic acids, fractionation with acetone and then with ammonium sulphate, adsorption on calcium phosphate gel and chromatography on DEAE-cellulose or DEAE-Sephadex. 2. The purified enzyme showed only one component in the ultracentrifuge, with a sedimentation coefficient of 5·4s. One major peak and three much smaller peaks were observed on electrophoresis of the enzyme at pH8·9. 3. The mol.wt. of the enzyme was approx. 200000. The catalytic constant was 2000mol. of meso-diaminopimelic acid decomposed/min./mol. of enzyme, at 37°. The relative rates of decarboxylation at 25°, 37° and 45° were 0·17:1·0:1·6. At 37° the Michaelis constant was 1·7mm and the optimum pH was 6·7–6·8. 4. There was an excess of acidic amino acids over basic amino acids in the enzyme, which was bound only on basic cellulose derivatives at pH6·8. 5. The enzyme had an absolute requirement for pyridoxal phosphate as a cofactor; no other derivative of pyridoxine had activity. A thiol compound (of which 2,3-dimercaptopropan-1-ol was the most effective) was also needed as an activator. 6. In the presence of 2,3-dimercaptopropan-1-ol (1mm), heavy-metal ions (Cu2+, Hg2+) did not inhibit the enzyme, but there was inhibition by several amino acids with analogous structures to diaminopimelate, generally at high concentrations relative to the substrate. Penicillamine was inhibitory at relatively low concentrations; its action was prevented by pyridoxal phosphate.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANTIA M., HOARE D. S., WORK E. The stereoisomers of alpha epsilon-diaminopimelic acid. III. Properties and distribution of diaminopimelic acid racemase, an enzyme causing interconversion of the LL and meso isomers. Biochem J. 1957 Mar;65(3):448–459. doi: 10.1042/bj0650448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAVALLINI D., DE MARCO C., MONDOVI B., AZZONE G. F. A new synthetic sulfur-containing amino acid: S-aminoethylcysteine. Experientia. 1955 Feb 15;11(2):61–62. doi: 10.1007/BF02179026. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEWEY D. L., HOARE D. S., WORK E. Diaminopimelic acid decarboxylase in cells and extracts of Escherichia coli and Aerobacter aerogenes. Biochem J. 1954 Dec;58(4):523–531. doi: 10.1042/bj0580523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOARE D. S., WORK E. The stereoisomers of alpha epsilon-diaminopimelic acid. II. Their distribution in the bacterial order Actinomycetales and in certain Eubacteriales. Biochem J. 1957 Mar;65(3):441–447. doi: 10.1042/bj0650441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOARE D. S., WORK E. The stereoisomers of alpha epsilon-diaminopimelic acid: their distribution in nature and behaviour towards certain enzyme preparations. Biochem J. 1955 Dec;61(4):562–568. doi: 10.1042/bj0610562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLY C. D., LAYNE S. Bacteria found in the air over Canada and the American Arctic. Can J Microbiol. 1957 Apr;3(3):447–455. doi: 10.1139/m57-047. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MEADOW P., WORK E. The effects of vitamin B6 and its derivatives on diaminopimelic acid decarboxylase in Bacillus sphaericus asporogenous. Biochim Biophys Acta. 1958 Jul;29(1):180–187. doi: 10.1016/0006-3002(58)90159-8. [DOI] [PubMed] [Google Scholar]
- MILNER H. W., LAWRENCE N. S., FRENCH C. S. Colloidal dispersion of chloroplast material. Science. 1950 Jun 9;111(2893):633–634. doi: 10.1126/science.111.2893.633. [DOI] [PubMed] [Google Scholar]
- POGELL B. M. Enzyme purification by selective elution with substrate from substituted cellulose columns. Biochem Biophys Res Commun. 1962 Apr 20;7:225–230. doi: 10.1016/0006-291x(62)90179-1. [DOI] [PubMed] [Google Scholar]
- SUTTON C. R., KING H. K. Inhibition of leucine decarboxylase by thiol-binding reagents. Arch Biochem Biophys. 1962 Feb;96:360–370. doi: 10.1016/0003-9861(62)90421-6. [DOI] [PubMed] [Google Scholar]
- TSUBOI K. K., HUDSON P. B. Acid phosphatase. III. Specific kinetic properties of highly purified human prostatic phosphomonoesterase. Arch Biochem Biophys. 1955 Mar;55(1):191–205. doi: 10.1016/0003-9861(55)90557-9. [DOI] [PubMed] [Google Scholar]
- WORK E. Reaction of ninhydrin in acid solution with straight-chain amino acids containing two amino groups and its application to the estimation of alpha epsilon-diaminopimelic acid. Biochem J. 1957 Nov;67(3):416–423. doi: 10.1042/bj0670416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White P. J., Kelly B., Suffling A., Work E. Variation of activity of bacterial diaminopimelate decarboxylase under different conditions of growth. Biochem J. 1964 Jun;91(3):600–610. doi: 10.1042/bj0910600. [DOI] [PMC free article] [PubMed] [Google Scholar]


