Abstract
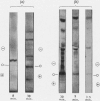
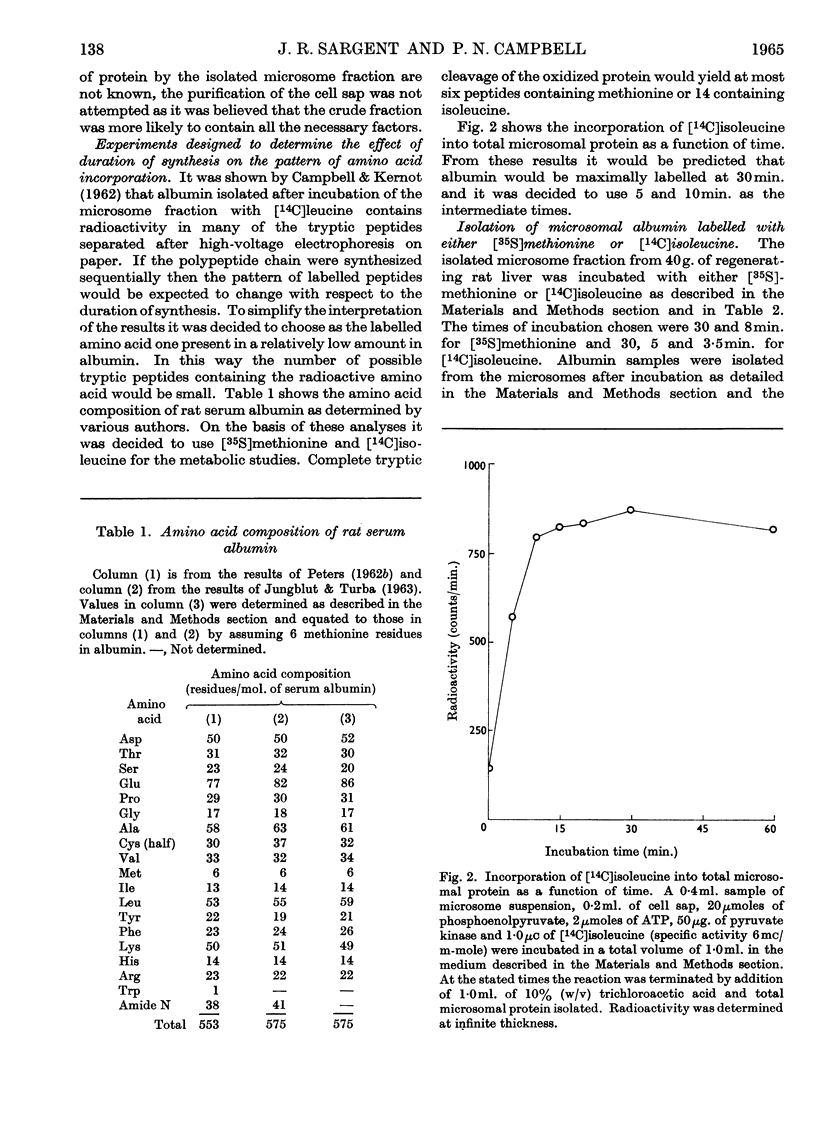
1. The isolated microsome fraction of regenerating rat liver was incubated with cell sap, a source of energy and [35S]methionine, [14C]isoleucine or [14C]leucine for different periods of time, and microsomal albumin isolated. 2. The distribution of these isotopes in albumin was determined by separation of tryptic peptides from the protein. Radioactivity was measured in peptides either qualitatively by radioautography or quantitatively by labelling with both 3H and 14C. 3. A gradient of radioactivity existed at all times in albumin isolated after incubating microsomes. 4. The shorter the incubation time the fewer the peptides labelled in albumin, but the peptides with highest specific activity after short incubation times corresponded to those with highest specific activities after long incubation times. 5. Leucine released from the C-terminus of albumin had a higher specific activity than the mean specific activity of the remaining leucine residues in albumin. 6. The peptide with the highest specific activity in albumin is probably derived from the C-terminus of the protein. 7. [14C]Glutamic acid is incorporated into the N-terminus of albumin after incubating the microsome fraction with this isotopically labelled amino acid, cell sap and a source of energy. The specific activity of the N-terminal glutamic acid under these conditions is less than the mean specific activity of the remaining glutamic acid and glutamine residues in albumin. 8. The results are interpreted as reflecting a sequential synthesis of serum albumin in the isolated microsome fraction of rat liver. The direction of synthesis of albumin is from the N-terminus towards the C-terminus. 9. The bulk of incorporation of radioactive amino acid into albumin in the isolated microsome fraction is due to completion of partially completed, pre-existing peptide and polypeptide chains. A limited synthesis of new chains of albumin does, however, occur.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUSCH H., FUJIWARA E., FIRSZT D. C. Studies on the metabolism of radioactive albumin in tumor-bearing rats. Cancer Res. 1961 Apr;21:371–377. [PubMed] [Google Scholar]
- Bishop J., Leahy J., Schweet R. FORMATION OF THE PEPTIDE CHAIN OF HEMOGLOBIN. Proc Natl Acad Sci U S A. 1960 Aug;46(8):1030–1038. doi: 10.1073/pnas.46.8.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPBELL P. N., GREENGARD O., KERNOT B. A. Studies on the synthesis o serum albumin by the isolated microsome fraction from rat liver. Biochem J. 1960 Jan;74:107–117. doi: 10.1042/bj0740107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CANFIELD R. E., ANFINSEN C. B. NONUNIFORM LABELING OF EGG WHITE LYSOZYME. Biochemistry. 1963 Sep-Oct;2:1073–1078. doi: 10.1021/bi00905a028. [DOI] [PubMed] [Google Scholar]
- Campbell P. N., Kernot B. A. The incorporation of [C]leucine into serum albumin by the isolated microsome fraction from rat liver. Biochem J. 1962 Feb;82(2):262–266. doi: 10.1042/bj0820262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALGLIESH C. E. The template theory and the role of transpeptidation in protein biosynthesis. Nature. 1953 Jun 6;171(4362):1027–1028. doi: 10.1038/1711027a0. [DOI] [PubMed] [Google Scholar]
- DEBRO J. R., TARVER H., KORNER A. The determination of serum albumin and globulin by a new method. J Lab Clin Med. 1957 Nov;50(5):728–732. [PubMed] [Google Scholar]
- GOLDSTEIN A., BROWN B. J. Addition of amino acid to C-terminal ends of growing protein chains in Escherichia coli. Biochim Biophys Acta. 1961 Oct 28;53:438–439. doi: 10.1016/0006-3002(61)90467-x. [DOI] [PubMed] [Google Scholar]
- GROSS E., WITKOP B. Nonenzymatic cleavage of peptide bonds: the methionine residues in bovine pancreatic ribonuclease. J Biol Chem. 1962 Jun;237:1856–1860. [PubMed] [Google Scholar]
- HIRS C. H. The oxidation of ribonuclease with performic acid. J Biol Chem. 1956 Apr;219(2):611–621. [PubMed] [Google Scholar]
- HUNT J. A., INGRAM V. M. Abnormal human haemoglobins. II. The chymotryptic digestion of the trypsin-resistant core of haemoglobins A and S. Biochim Biophys Acta. 1958 Jun;28(3):546–549. doi: 10.1016/0006-3002(58)90517-1. [DOI] [PubMed] [Google Scholar]
- LEDER P., NIRENBERG M. RNA CODEWORDS AND PROTEIN SYNTHESIS. II. NUCLEOTIDE SEQUENCE OF A VALINE RNA CODEWORD. Proc Natl Acad Sci U S A. 1964 Aug;52:420–427. doi: 10.1073/pnas.52.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOFTFIELD R. B. THE FREQUENCY OF ERRORS IN PROTEIN BIOSYNTHESIS. Biochem J. 1963 Oct;89:82–92. doi: 10.1042/bj0890082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUCK D. N., BARRY J. M. THE ORDER IN TIME IN WHICH AMINO ACIDS ARE INCORPORATED INTO PANCREATIC RIBONUCLEASE. J Mol Biol. 1964 Jul;9:186–192. doi: 10.1016/s0022-2836(64)80099-1. [DOI] [PubMed] [Google Scholar]
- NAUGHTON M. A., DINTZIS H. M. Sequential biosynthesis of the peptide chains of hemoglobin. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1822–1830. doi: 10.1073/pnas.48.10.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETERS T., Jr The biosynthesis of rat serum albumin. III. Amino acid composition of rat albumin. J Biol Chem. 1962 Jul;237:2182–2183. [PubMed] [Google Scholar]
- RENDI R., HULTIN T. Preparation and amino acid incorporating ability of ribonucleoprotein-particles from different tissues of the rat. Exp Cell Res. 1960 Mar;19:253–266. doi: 10.1016/0014-4827(60)90006-9. [DOI] [PubMed] [Google Scholar]
- RYLE A. P., SANGER F., SMITH L. F., KITAI R. The disulphide bonds of insulin. Biochem J. 1955 Aug;60(4):541–556. doi: 10.1042/bj0600541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANGER F. The terminal peptides of insulin. Biochem J. 1949;45(5):563–574. doi: 10.1042/bj0450563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHLESSINGER D. PROTEIN SYNTHESIS BY POLYRIBOSOMES ON PROTOPLAST MEMBRANES OF B. MEGATERIUM. J Mol Biol. 1963 Nov;7:569–582. doi: 10.1016/s0022-2836(63)80103-5. [DOI] [PubMed] [Google Scholar]
- SLAYTER H. S., WARNER J. R., RICH A., HALL C. E. THE VISUALIZATION OF POLYRIBOSOMAL STRUCTURE. J Mol Biol. 1963 Dec;7:652–657. doi: 10.1016/s0022-2836(63)80112-6. [DOI] [PubMed] [Google Scholar]
- Von Der Decken A., Campbell P. N. Studies on the synthesis of serum albumin by ribonucleoprotein particles isolated from rat liver. Biochem J. 1962 Sep;84(3):449–455. doi: 10.1042/bj0840449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARNER J. R., KNOPF P. M., RICH A. A multiple ribosomal structure in protein synthesis. Proc Natl Acad Sci U S A. 1963 Jan 15;49:122–129. doi: 10.1073/pnas.49.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEBER G., YOUNG L. B. FRAGMENTATION OF BOVINE SERUM ALBUMIN BY PEPSIN. II. ISOLATION, AMINO ACID COMPOSITION, AND PHYSICAL PROPERTIES OF THE FRAGMENTS. J Biol Chem. 1964 May;239:1424–1431. [PubMed] [Google Scholar]
- WILLIAMS R. B., DAWSON R. M. C. The biosynthesis of L-cystine and L-methionine labelled with radioactive sulphur (35S). Biochem J. 1952 Oct;52(2):314–317. doi: 10.1042/bj0520314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOSHIDA A., TOBITA T. Studies on the mechanism of protein synthesis. Nouniform incorporation of [14C]leucine into alpha-amylase and the presence of alpha-amylase precursos. Biochim Biophys Acta. 1960 Jan 29;37:513–520. doi: 10.1016/0006-3002(60)90508-4. [DOI] [PubMed] [Google Scholar]
- ZAMECNIK P. C., LOFTFIELD R. B., STEPHENSON M. L., STEELE J. M. Studies on the carbohydrate and protein metabolism of the rat hepatoma. Cancer Res. 1951 Aug;11(8):592–602. [PubMed] [Google Scholar]