Abstract
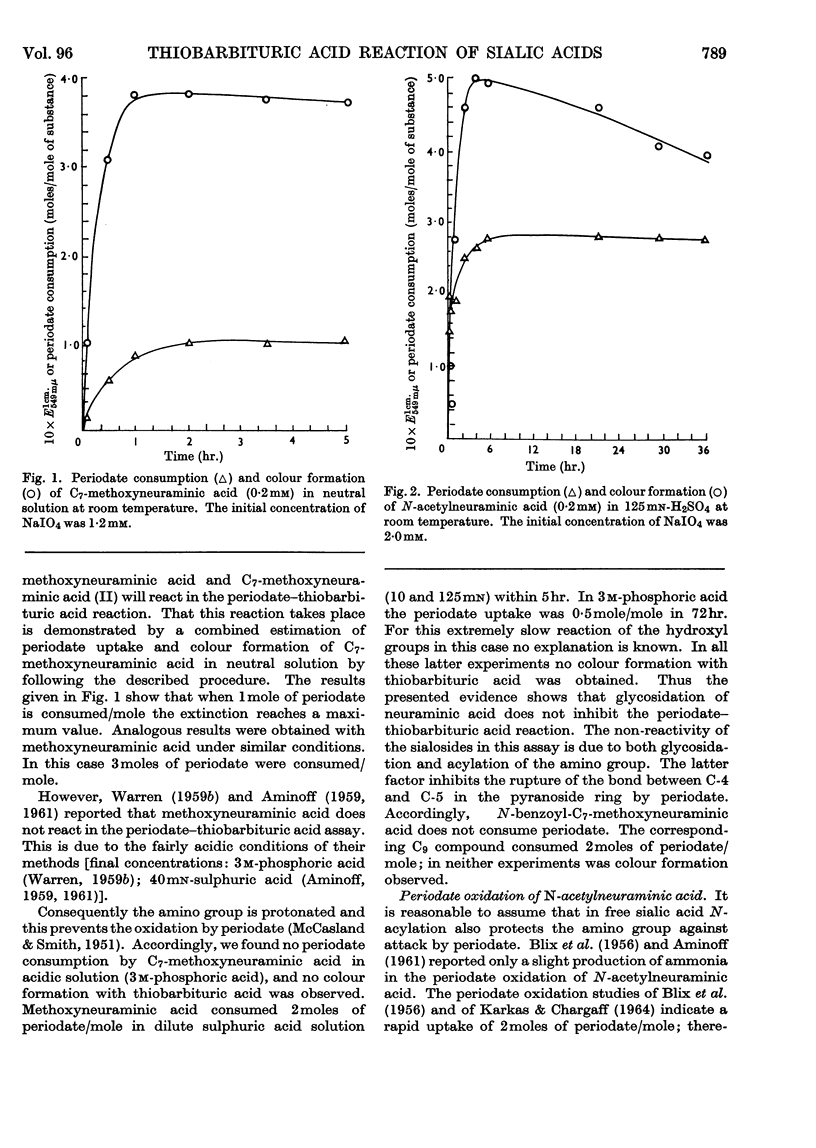
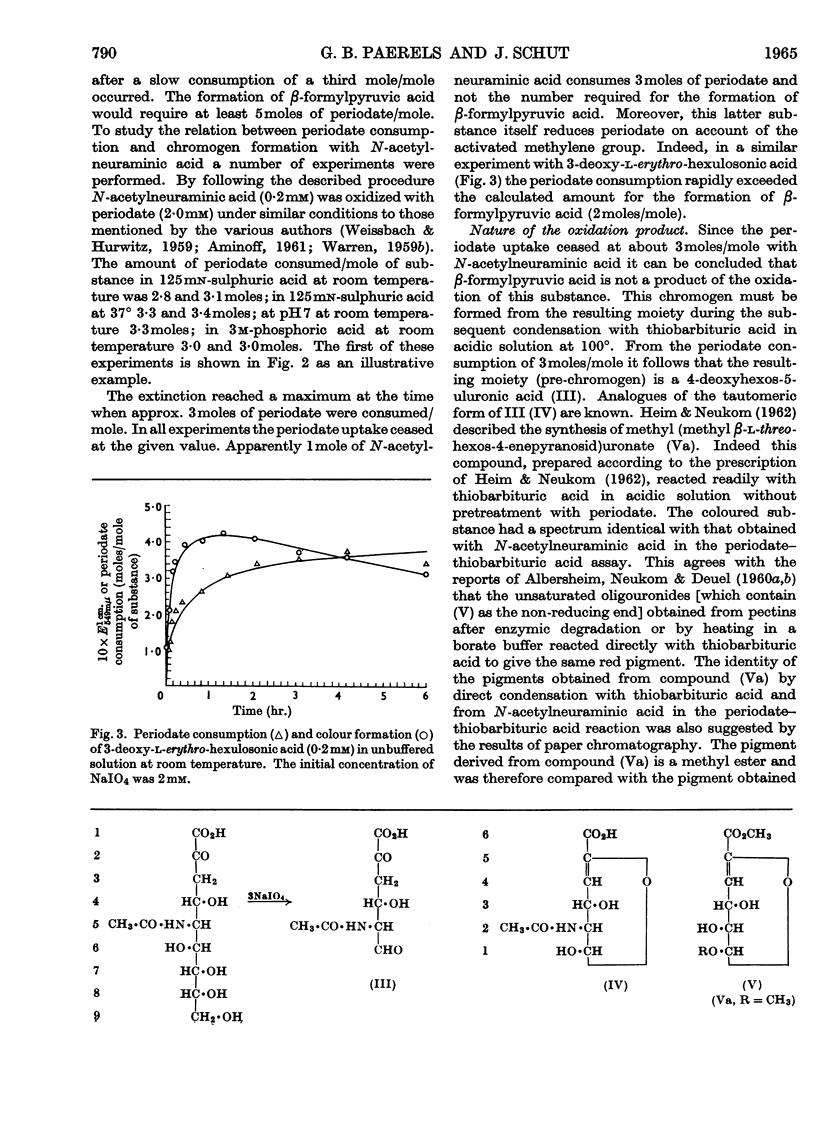
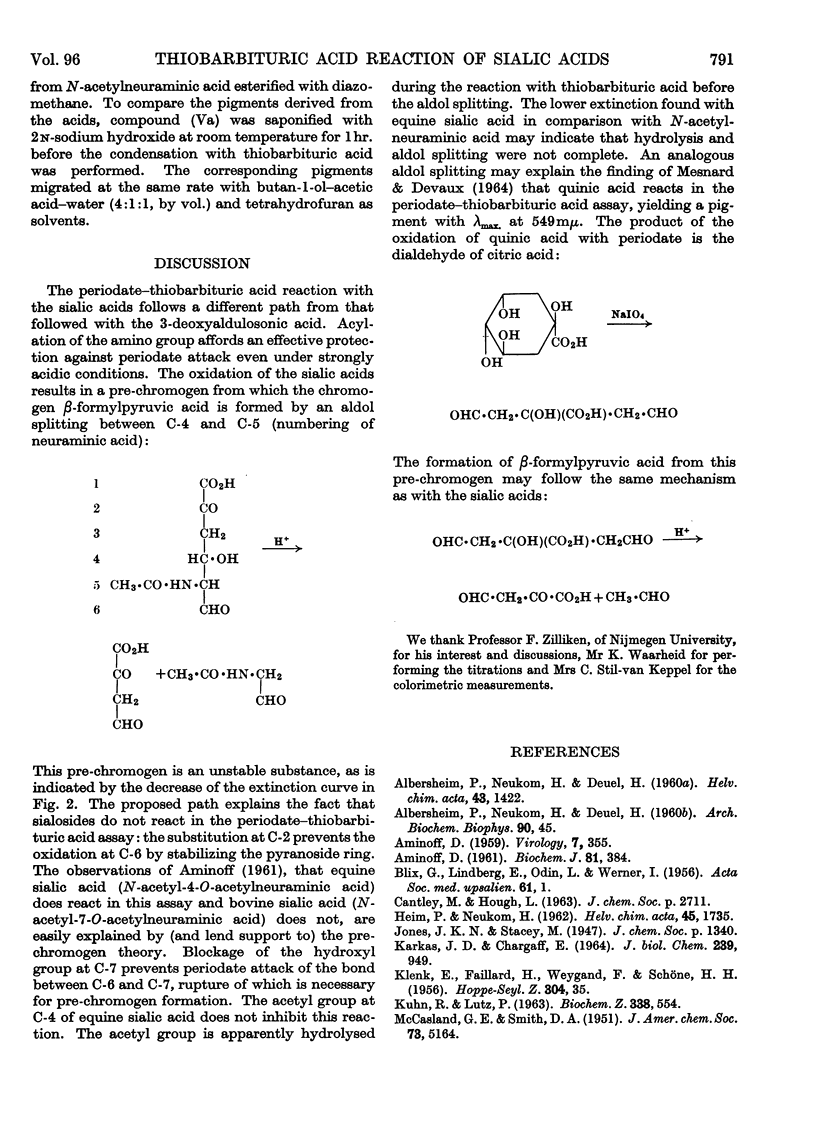
1. The chromogen formation from N-acetylneuraminic acid in the periodate–thiobarbituric acid reaction was investigated. Measurement of periodate consumption showed an uptake of approx. 3moles/mole of substrate in neutral as well as in strongly acidic solution. Therefore the chromogen β-formylpyruvic acid is not a direct product of the periodate oxidation; it is presumed to be formed from the true oxidation product, a hexos-5-uluronic acid, by aldol splitting during the reaction in hot acidic solution with thiobarbituric acid. 2. Methyl (methyl β-l-threo-hexos-4-enepyranosid)uronate, an analogue of the pre-chromogen, has been shown to yield with thiobarbituric acid in acidic solution a pigment exhibiting an identical absorption spectrum and showing the same behaviour on paper chromatography as the pigment obtained from N-acetylneuraminic acid in the periodate–thiobarbituric acid assay. 3. The substitution at C-2 of methoxyneuraminic acid does not inhibit the periodate–thiobarbituric acid reaction. In neutral solution methoxyneuraminic acid is oxidized by periodate to a substance that reacts readily with thiobarbituric acid in acidic solution. When periodate oxidation is attempted in acidic solution, protonation of the amino group protects this group against oxidation, rendering methoxyneuraminic acid negative in the assay systems of Warren (1959a,b) and Aminoff (1959, 1961).
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMINOFF D. Methods for the quantitative estimation of N-acetylneuraminic acid and their application to hydrolysates of sialomucoids. Biochem J. 1961 Nov;81:384–392. doi: 10.1042/bj0810384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AMINOFF D. The determination of free sialic acid in the presence of the bound compound. Virology. 1959 Mar;7(3):355–357. doi: 10.1016/0042-6822(59)90207-7. [DOI] [PubMed] [Google Scholar]
- KARKAS J. D., CHARGAFF E. STUDIES ON THE STABILITY OF SIMPLE DERIVATIVES OF SIALIC ACID. J Biol Chem. 1964 Apr;239:949–957. [PubMed] [Google Scholar]
- KUHN R., LUTZ P. UBER FORMYL-BRENZTRAUBENSAEURE UND DEN FARBSTOFF DER WARREN-REAKTION. Biochem Z. 1963;338:554–560. [PubMed] [Google Scholar]
- SRINIVASAN P. R., SPRINSON D. B. 2-Keto-3-deoxy-D-arabo-heptonic acid 7-phosphate synthetase. J Biol Chem. 1959 Apr;234(4):716–722. [PubMed] [Google Scholar]
- WARAVDEKAR V. S., SASLAW L. D. A method of estimation of 2-deoxyribose. Biochim Biophys Acta. 1957 May;24(2):439–439. doi: 10.1016/0006-3002(57)90224-x. [DOI] [PubMed] [Google Scholar]
- WARAVDEKAR V. S., SASLAW L. D. A sensitive colorimetric method for the estimation of 2-deoxy sugars with the use of the malonaldehyde-thiobarbituric acid reaction. J Biol Chem. 1959 Aug;234(8):1945–1950. [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- WEISSBACH A., HURWITZ J. The formation of 2-keto-3-deoxyheptonic acid in extracts of Escherichia coli B. I. Identification. J Biol Chem. 1959 Apr;234(4):705–709. [PubMed] [Google Scholar]
- WEYGAND F., RINNO H. Abbau der Methoxy-neuraminsäure um zwei CHOH-Gruppen. Hoppe Seylers Z Physiol Chem. 1957 Feb 5;306(4-6):177–179. [PubMed] [Google Scholar]


