Abstract
Certain filamentous nitrogen-fixing cyanobacteria generate signals that direct their own multicellular development. They also respond to signals from plants that initiate or modulate differentiation, leading to the establishment of a symbiotic association. An objective of this review is to describe the mechanisms by which free-living cyanobacteria regulate their development and then to consider how plants may exploit cyanobacterial physiology to achieve stable symbioses. Cyanobacteria that are capable of forming plant symbioses can differentiate into motile filaments called hormogonia and into specialized nitrogen-fixing cells called heterocysts. Plant signals exert both positive and negative regulatory control on hormogonium differentiation. Heterocyst differentiation is a highly regulated process, resulting in a regularly spaced pattern of heterocysts in the filament. The evidence is most consistent with the pattern arising in two stages. First, nitrogen limitation triggers a nonrandomly spaced cluster of cells (perhaps at a critical stage of their cell cycle) to initiate differentiation. Interactions between an inhibitory peptide exported by the differentiating cells and an activator protein within them causes one cell within each cluster to fully differentiate, yielding a single mature heterocyst. In symbiosis with plants, heterocyst frequencies are increased 3- to 10-fold because, we propose, either differentation is initiated at an increased number of sites or resolution of differentiating clusters is incomplete. The physiology of symbiotically associated cyanobacteria raises the prospect that heterocyst differentiation proceeds independently of the nitrogen status of a cell and depends instead on signals produced by the plant partner.
INTRODUCTION
The word “bacteria” generally conjures up images of individual rods or spheres swimming around, every bug for itself. In fact, however, the solitary life is rare in the microbial world; bacteria interact with other microbes and macroorganisms in the environment and with their own siblings, and these interactions often result in complex social interactions. Many bacterial species exchange signals amongst themselves in order to assess cell density and to respond appropriately (62, 71). Myxobacteria take signaling a step further and use it to direct their differentiation into complex multicellular structures, enabling them to survive periods of starvation (47). Rhizobia communicate outside of their species. They respond to signals from leguminous plants (e.g., beans) to differentiate into nitrogen-fixing cells. At the same time, they produce signals that induce the plants to form specialized nodules, microoxic cavities required for the O2-sensitive process of N2 fixation (198).
Like myxobacteria, certain N2-fixing species of filamentous cyanobacteria generate signals to direct their own multicellular development, and, like rhizobia, they respond to signals from plants, initiating or altering the extent of their cellular differentiation. A main objective of this review is to consider the degree to which signals used by cyanobacteria to control their own differentiation are appropriated by plants to achieve stable symbioses. General reviews on the biology of symbioses between cyanobacteria and plants have recently appeared (3, 151).
Symbiosis of Cyanobacteria with Plants
What makes cyanobacteria valuable partners in symbioses?
Cyanobacteria are an ancient and diverse class of bacteria characterized by their ability to use light energy to split water into oxygen and reductant, which is subsequently consumed in photosynthesis. Some cyanobacteria are also able reduce atmospheric dinitrogen gas to ammonium (N2 fixation), giving them the simplest of nutritional requirements: air, water, a few inorganic nutrients, and light. Nitrogen fixation and oxygenic photosynthesis, however, are intrinsically incompatible, because nitrogenase, the enzyme responsible for reduction of N2, is inactivated by minute concentrations of oxygen (52). Certain multicellular cyanobacteria, e.g., the genera Anabaena and Nostoc in the order Nostocales, overcome this incompatibility by differentiating specialized, nearly anoxic cells at intervals within chains of other cells, like isolated pearls in a string of beads (Fig. 1). Nitrogen fixation takes place in these specialized terminally differentiated cells (called heterocysts), and photosynthesis takes place in the other cells (vegetative cells) (214).
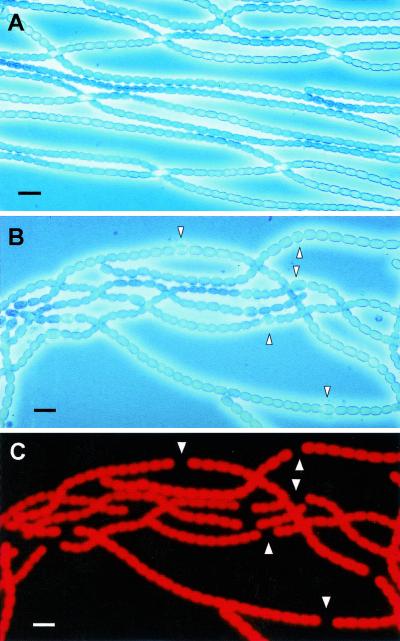
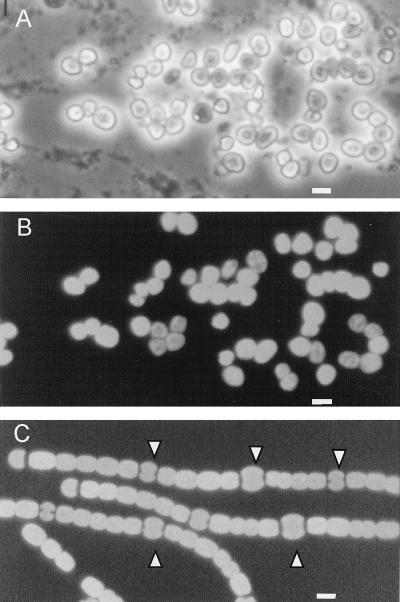
FIG. 1.
Photomicrographs of vegetative and heterocyst-containing filaments of the cyanobacterium N. punctiforme. (A) Phase-contrast image of vegetative filaments grown in the presence of ammonium. No heterocysts are visible. (B) Phase-contrast image of filaments grown in the absence of any combined nitrogen source in the culture medium. Heterocysts, identified by arrowheads, are present at well-spaced intervals. (C) Epifluorescence image of the same filaments as in panel B. Excitation was at 510 to 560 nm (green), exciting phycoerythrin, and emission was greater than 600 nm. Heterocysts have negligible fluorescence, while vegetative cells have intense combined fluorescence from phycobiliproteins and chlorophyll a. Bar, 10 μm.
The ability of these cyanobacteria to fix N2 is evidently prized by other organisms, because N2-fixing cyanobacteria, primarily in the genus Nostoc, form symbioses with a wide variety of fungi, plants (Table 1), and other organisms (148, 165). Cyanobacterial symbioses occur with representatives of all four of the major phylogenetic divisions of terrestrial plants: primitive spore-producing bryophytes (hornworts and liverworts) and ferns (Azolla) and the two classes of seed-producing plants, gymnosperms (cycads), and angiosperms (Gunnera). By contrast, rhizobia form associations only with angiosperm legumes (43) and one genus, Parasponia, of nonleguminous trees (14). Various N2-fixing and non-N2-fixing cyanobacteria also associate with fungi (to establish lichens) and with marine organisms (171). This review will focus on cyanobacterial associations with terrestrial plants.
TABLE 1.
Plants that form symbioses with nitrogen-fixing cyanobacteria
| Taxonomic division of plant partner (references)a | Plant genus | Tissue localization of cyanobacterial symbiont | Heterocyst frequency (%)b |
|---|---|---|---|
| Angiospermae (16, 85, 17416, 85, 174) | Gunnera (1 genus) | Intracellular, within cells of the stem gland | 1-60 |
| Gymnospermae (105, 135, 106105, 135, 106) | Cycads (11 genera) | Extracellular, within specialized coralloid roots | 5-45 |
| Pteridophyta (ferns) (101, 119, and 185, 78c | Azolla (1 genus) | Extracellular, within cavities of the dorsal leaves | 0-30 |
| Bryophyta (116, 49, 163 | Liverworts (2 genera), hornworts (4-6 genera) | Extracellular, within cavities of the gametophyte | 25-45 |
| Fungi (149, 8, 149)d | Lichens (perhaps 300-350 genera) | Extracellular, localized in cephadolia or evenly distributed in the thallus | 2-6 10-30 |
For each symbiosis, the first reference is to a general review, the second describes reconstitution of the symbiosis, and the third reports heterocyst frequency.
Fraction of the total cyanobacterial cells that are heterocysts, which may vary by position within the plant. For fungi, the first range is for bipartite lichens (cyanobacterium plus fungus) and the second range is for tripartite lichens (cyanobacterium plus green alga plus fungus).
Cyanobacterial symbiont has yet to be cultured according to references 119 and 185 (while historically identified as Anabaena, it is likely to be in the genus Nostoc; we refer to it as Nostoc/Anabaena).
Reconstitution has been attained but is rare (8).
In such associations, fixed nitrogen provided by heterocyst-forming cyanobacteria is able to support the nitrogen needs of the plant partner (148). Even free-living heterocyst-forming cyanobacteria can supply all of the nitrogen needed by nonsymbiotic plants under laboratory conditions (64, 134, 175). Plant partners do not, therefore, need to provide a specialized environment for cyanobacterial partners, as do legumes for rhizobia. A haven safe from competitors and predators may be sufficient.
What do plants bring to the symbiosis?
The self-sufficiency of heterocyst-forming cyanobacteria with respect to N2 fixation may account for the relatively simple plant structures that house symbiotic cyanobacteria, in contrast to those that house rhizobia. Infecting rhizobia induce plants to differentiate nodules, which have been likened in their complexity to new plant organs (201). Nodules differ from surrounding root tissue in several regards, many of them related to the control of O2 tension. One accommodation is the production of plant- and bacterium-encoded leghemoglobin, a protein that binds O2, keeping it at a concentration low enough to permit N2 fixation but high enough to permit rhizobial respiration.
In contrast, the plant structures inhabited by cyanobacteria exist even in the absence of the cyanobacterial symbiont, at sites that differ from plant to plant (Fig. 2). Gunnera possesses a unique gland on its stem that attracts cyanobacteria. Cycads have specialized lateral roots (coralloid roots) in which a layer has been prepared for infecting cyanobacteria. Bryophytes come with cavities in the gametophyte tissue that are infected by cyanobacteria. Cyanobacteria associated with Azolla in cavities of the dorsal leaves are unique in that they appear to be obligate symbionts (141), but Azolla cured of cyanobacteria by treatment with antibiotics continues to grow in the presence of combined nitrogen with empty leaf cavities (60). These different plant structures will be referred to collectively as symbiotic cavities, and the cyanobacterial populations within them will be referred to as symbiotic colonies.
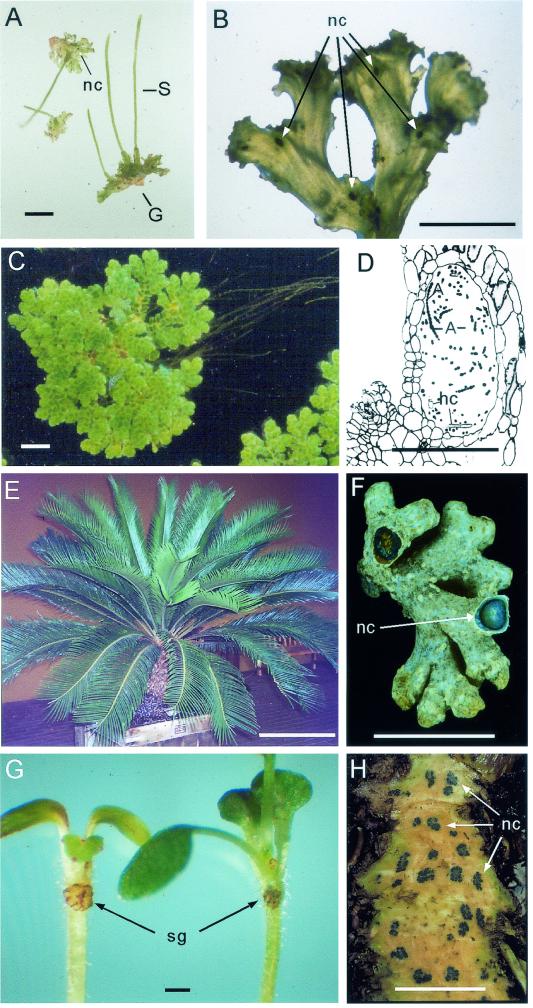
FIG. 2.
Photographs of plant partners showing the location of symbiotic cavities housing associated cyanobacteria. (A and B) Sporophyte (S) and gametophyte (G) generations of the bryophyte Anthoceros punctatus (A) and Nostoc colonies (nc) (B) within the gametophyte tissue. Bar, 1.0 cm. (C and D) Azolla caroliniana floating sporophyte leaves and trailing submerged roots (C). The cavity of a dorsal leaf housing symbiotic Nostoc/Anabaena is shown in a vertical position. A, Nostoc/Anabaena filaments; hc, Azolla hair cells (D). Photographs copyright Gerald Peters. Panel D reprinted from reference 142 with permission of the author. Bar, 0.25 cm and 0.25 mm in panels C and D, respectively. (E and F) Stem and leaves of the cycad Cycas taiwaniana (E) and ventral view and cross section of a coralloid root cluster from C. taiwaniana (F). Nostoc is present in a green annular ring (nc) within the root. Bar, 0.5 m and 0.5 cm in panels E and F, respectively. (G and H) Seedling of stoloniferous Gunnera manicata showing the location of the stem gland (sg) (G) and tangential stem section of a giant Gunnera chilensis showing location of the Nostoc colonies (nc) (H). Photographs copyright Warwick Sylvester. Panel G reprinted from reference 22 with permission of the publisher. Bar, 1.0 cm.
Although the symbiotic cavities exist without symbiosis, the environment within the cavities is clearly specialized for the symbiotic state. A feature that may be common to all symbiotic cavities is the presence of mucilage, a complex polysaccharide (133, 143, 155, 157, 168, 196). The cavities are also relatively axenic, except for the cyanobacterium itself (84). Cavities of Azolla are exceptional in supporting other bacteria (26) that may play an important role in the symbiosis (60). Finally, the symbiotic cavities respond to the presence of cyanobacteria by elaborating long, finger-like cells (103, 163) that may serve to increase the surface area for nutrient exchange.
It is a matter of controversy whether all symbiotic cavities, like the nodules of legumes, have O2 tensions much lower than ambient. Microelectrode measurements have indicated relatively high O2 tension within coralloid roots of cycads (35), slightly lower than ambient (83%) in symbiotic cavities of Azolla (68), and very low (near to anoxic) in the bryophyte hornwort Anthoceros (34). No plant-derived hemoglobin-like protein has been reported. Cyanoglobin, a heme protein with high affinity for oxygen, is found in many Nostoc strains, but some strains, including a few symbiotic strains, lack the protein (79). Free-living cyanobacteria can maintain high nitrogenase activities while growing under atmospheres ranging from 1 to 50% O2 (131).
How specific are associations between plants and cyanobacteria?
Strains or species of the genus Nostoc are the dominant but not exclusive symbiotic cyanobacteria in plant associations. The relatively low requirements of plant-cyanobacterium symbioses are illustrated by the lack of strain specificity between the partners in reconstitution experiments (22, 49, 85). Of 10 strains of Nostoc isolated from symbioses with Gunnera, 6 achieved an intracellular association with a different species of Gunnera. Of four strains of Nostoc isolated from symbioses with cycads, Anthoceros, or Peltigera (a lichenized fungus), three successfully infected Gunnera. Only one of nine Nostoc strains isolated from various plant or lichen symbioses failed to infect Anthoceros. Free-living isolates have generally not established symbioses, although exceptions have been noted (49, 135). DNA-fingerprinting techniques revealed a wide cluster of symbiotic and free-living isolates from a single field site associated with the hornwort Phaeoceros (202). The isolates included primarily 65 Nostoc strains, but three each of Calothrix and Chlorogloeopsis were also identified illustrating some flexibility in cyanobacterial partners. Except for one Nostoc isolate, all reconstituted a symbiotic association with Phaeoceros or the liverwort Blasia.
DEVELOPMENTAL REPERTOIRE OF FREE-LIVING CYANOBACTERIA
The relative lack of discrimination by plants with regard to their Nostoc partners indicates that the properties required for a wide variety of associations are found in most strains. It is difficult to imagine that cyanobacteria, unlike rhizobia, have evolved to exchange signals with multiple partners spanning nearly the entire plant kingdom. The alternative is that different plants have independently learned how to exploit the capabilities common to free-living Nostoc. In all resulting symbioses, plants influence two developmental responses of Nostoc—the formation of hormogonia and the differentiation of heterocysts. Vegetative cells of Nostoc have a third developmental alternative in the differentiation into perennating spore-like cells called akinetes (6). Akinetes are hypothesized to be evolutionary precursors of heterocysts (214). Heterocysts and akinetes may (99) or may not (217) have common developmental regulatory elements. Akinetes are present in large numbers in older Azolla leaves and in the developing sporocarp, where they presumably play a role in generational continuity (141). However, akinetes have not consistently been observed in all symbioses, and there have been no studies on their symbiotically associated differentiation or germination.
In this section, information from studies of free-living cyanobacterial strains on heterocyst and hormogonium developmental responses is considered. In subsequent sections, we will examine possible mechanisms by which plants may draw on the free-living signal transduction systems to assert symbiotic control.
Patterned Differentiation to Heterocysts, Well-Spaced Nitrogen-Fixing Cells
Filaments of Anabaena or Nostoc grown in the presence of a combined nitrogen source, such as NH4+ or NO3−, may consist solely of vegetative cells, morphologically indistinguishable from one another. When the source of nitrogen is removed (nitrogen step-down) or exhausted, heterocysts appear within one to two cell generations at nearly regular intervals along the filament (Fig. 1). Plants appear to alter both the number and the pattern of heterocyst spacing, perhaps to maximize the rate of N2 fixation at the expense of cyanobacterial growth.
What are heterocysts, and how do they work?
The major function of heterocysts is to provide a microoxic environment necessary for the production and proper functioning of nitrogenase and other proteins related to N2 fixation (214). In essentially all heterocyst-forming cyanobacteria, heterocysts appear to be the only sites for N2 fixation within the filament (48, 188). The exception is Anabaena variabilis strain ATCC 29413 (and a few closely related strains), which expresses a vegetative cell-specific nitrogenase under anoxic incubation conditions (188). The heterocyst achieves a near anoxic state by at least three means. First, photosystem II, the O2-producing end of the photosynthetic electron transport chain, is dismantled during heterocyst differentiation (186), so that the heterocyst need contend only against O2 produced by neighboring vegetative cells and that dissolved in the environment. Second, heterocysts are invested with a specialized envelope (214) that limits the influx of gases (199). Two layers within the envelope have been implicated in O2 protection (130): an inner layer composed of a hydroxylated glycolipid and an outer layer of polysaccharide. Neither layer is found in vegetative cells. Third, much of the O2 that overcomes these barriers is consumed by the high oxidase activity associated with heterocysts (144).
Abandoning complete photosynthesis allows the recycling of the amino acids contained in phycobiliproteins, light-harvesting pigments associated primarily with photosystem II that may account for up to 50% of the soluble protein within the cyanobacterial cell (21). Nitrogen deprivation elicits a general increase in proteolysis and degradation of phycobiliproteins (187, 218). At the onset of nitrogenase activity, phycobiliproteins return to their original levels in vegetative cells (23). The new heterocysts themselves, however, are grossly deficient in fluorescence associated with phycobiliproteins (197), as shown in Fig. 1C. After several days, heterocysts in some strains may regain this fluorescence (145, 190), but filaments tend to liberate aged heterocysts as single fluorescent cells. Loss of fluorescence in specific cells can thus be used as a diagnosis of differentiation; even in mutants that cannot complete differentiation, a pattern of well-spaced nonfluorescent cells is indicative of at least initiation of differentiation (48).
In the absence of complete photosynthesis, the heterocyst becomes dependent on adjacent vegetative cells for reduced carbon, just as the vegetative cells are dependent on heterocysts for reduced nitrogen. Nitrogen fixed within the heterocyst as ammonium is first converted to glutamine and then passes as amino acids to adjacent vegetative cells (191). In return, fixed carbon, probably sucrose (166), flows from vegetative cells to heterocysts (208). Sucrose synthase, or perhaps sucrose phosphate synthase (146), is present in high activity specifically in vegetative cells, while alkaline invertase, which converts sucrose to glucose and fructose, is confined largely to heterocysts (166). The resulting hexoses are catabolized in the heterocyst via the oxidative pentose phosphate cycle, providing reductant for nitrogenase and respiratory activities (181).
Heterocyst-forming cyanobacteria have made a number of adaptations in their nitrogen metabolism, which are important in our understanding of the basis of heterocyst differentiation and interactions with vegetative cells (Fig. 3). Like other cyanobacteria and, so far as we know, unlike any other organism on Earth, heterocyst-forming strains store amino nitrogen in a nonprotein polymeric form, as a branched polypeptide called cyanophycin (10, 111). Cyanophycin is a copolymer of aspartate and arginine and is present in both vegetative cells and mature heterocysts. Just as phycobiliproteins may serve as a source of amino nitrogen during differentiation, so may cyanophycin.
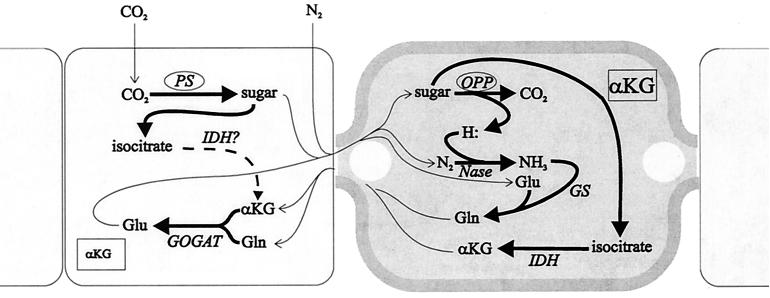
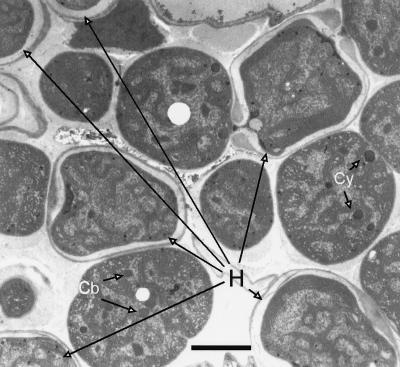
FIG. 3.
Metabolic interactions between heterocysts and vegetative cells. A lighter vegetative cell exchanges metabolites (thin lines) with a darker heterocyst bound by its characteristic thick envelope. The heterocyst has polar plugs at either end. Thick lines indicate metabolic pathways. The dotted line indicates a pathway whose existence is uncertain. Carbon dioxide is fixed in vegetative cells through the dark reactions of photosynthesis (PS), and the resulting triose is metabolized to pyruvate through the partial tricarboxylic acid cycle to isocitrate and then via IDH to α-ketoglutarate (αKG). The α-ketoglutarate combines with glutamine (Gln) via glutamate synthase (GOGAT) to form glutamate (Glu). In heterocysts, carbohydrate from vegetative cells enters the oxidative pentose phosphate (OPP) pathway to produce reductant (H:) used to support the activity of nitrogenase (Nase) to produce ammonium and concurrently yield α-ketoglutarate. Ammonium combines with glutamate, derived from the vegetative cell, through a reaction catalyzed by GS to form Gln. These reactions (if confirmed) should serve to reduce the level of α-ketoglutarate in the vegetative cell (small type) and increase it in heterocysts (large type). See the text for a discussion of the proposed cellular levels of α-ketoglutarate.
There is a partitioning in the enzymes for assimilation of N2-derived NH4+ between vegetative cells and heterocysts (113, 191). In cyanobacteria, the glutamine synthetase (GS)-glutamate synthase (GOGAT) cycle is the route of NH4+ assimilation for growth (56). GS, which incorporates NH4+ into glutamate to form glutamine, is present in both cell types, but most, if not all, of the assimilation of N2-derived NH4+ occurs in heterocysts. The bulk of the glutamate required by GS, however, must come from vegetative cells, because GOGAT is present at negligible levels in heterocysts (113, 191). GOGAT catalyzes the transfer of the amide nitrogen in glutamine to α-ketoglutarate.
Like glutamine, α-ketoglutarate may be made primarily in heterocysts; under N2-fixing conditions, in vitro activity of isocitrate dehydrogenase (IDH) appears to be largely absent from vegetative cell extracts (136), although, based on immunological assays, the IDH protein is present in equal amounts in vegetative cells and heterocysts (113). IDH is highly active in heterocyst extracts, which appears anomalous, since the primary metabolic role of α-ketoglutarate is to serve as precursor to glutamate (α-ketoglutarate dehydrogenase is absent in cyanobacteria, which thus lack a complete citric acid cycle [170]). However, IDH-generated NADPH may also contribute to the reductant pool for nitrogenase and respiration in heterocysts (109). In brief, glutamine is made in heterocysts, using glutamate transferred from vegetative cells, derived from α-ketoglutarate, which may arise in heterocysts. A possible rationale for this state of affairs in which α-ketoglutarate also serves as a signaling molecule will be offered in a subsequent section.
What are the regulatory circuits in heterocyst differentiation?
Much of what we know about heterocyst differentiation comes from the study of the nonsymbiotic laboratory strain Anabaena sp. strain PCC 7120. Based on DNA-DNA hybridization and sequence analysis of DNA encoding small-subunit rRNA, this strain is closer to those in the genus Nostoc (160), but since the name is entrenched in the literature we will continue to refer to it as Anabaena strain PCC 7120. Within one or two generations (about 18 to 36 h under standard conditions), well-spaced vegetative cells suddenly deprived of a source of combined nitrogen differentiate into N2-fixing heterocysts. The program of development begins with the sensation of nitrogen deprivation and culminates in the expression of the N2 fixation apparatus in the mature heterocyst. In between, there is an ordered sequence of events controlled to a great degree at the level of transcription.
Heterocyst differentiation is inhibited by the presence of a usable source of nitrogen, such as NH4+ or NO3−. Even 3 to 7 μM NH4+ is sufficient to repress the appearance of heterocysts in Anabaena strain PCC 7120 (122). Since heterocysts persist in Nostoc within symbiotic cavities despite a level of ammonium that is much higher than this (35, 36, 118, 120, 169), it is imperative to understand the basis of the repression. The following discussion will not detail all genes now known to influence heterocyst formation and function; a more thorough listing can be found in references 211 and 212.
(i) Earliest signals in heterocyst differentiation.
In Escherichia coli and other proteobacteria, nitrogen starvation is signaled in part by the glnB-encoded protein PII, which interacts with GlnE to modify GS activity by adenylylation and interacts with NtrB to modify the regulatory activity of NtrC by phosphorylation (124). NtrC-P enhances the transcription of several genes that respond to nitrogen conditions, including glnA, the structural gene of GS. The metabolic status of the cell is sensed directly by PII through its binding to α-ketoglutarate and ATP and through a uridylyltransferase (encoded by glnD) that modifies PII in response to the levels of glutamine.
PII is the only component of this regulatory web that has been found in cyanobacteria (65, 70, 108, 192). In unicellular cyanobacteria, the PII protein is modified by phosphorylation, and not uridylylation as in E. coli (58), in response to the level of α-ketoglutarate (83). Unmodified PII appears to regulate nitrate assimilation in unicellular Synechococcus sp. strain PCC 7942 (96). While a mutant strain of Synechococcus strain PCC 7942 in which glnB has been insertionally inactivated is minimally affected in nitrogen-related functions (59), it has proven impossible to obtain a similar mutant in N. punctiforme, implying that the glnB gene is essential in heterocyst-forming cyanobacteria (70). It should be noted that cyanobacteria, unlike many bacteria, possess only one GlnB-like protein (11) (see http://www.jgi.doe.gov/ and http://www.kuzusa.or.jp/cyano/).
The earliest documented step in the response of cyanobacteria to nitrogen deprivation is activation of the DNA binding protein NtcA (77, 110). Mutants lacking functional ntcA are unable to respond to nitrogen deprivation; they fail to increase levels of GS, to respond to alternate nitrogen sources (such as NO3−), to make heterocysts, or to induce nitrogenase expression (61, 200). Many genes whose transcription is altered by nitrogen deprivation (61) are preceded by a consensus sequence (GTAnCaannnnTAC in Anabaena strain PCC 7120 [77]) to which NtcA binds (153). One such gene is the NtcA-regulated gene glnB from the non-heterocyst-forming cyanobacterium Synechococcus strain PCC 7942 (97). Transcription of glnB from N. punctiforme is also induced by nitrogen limitation (70). NtcA binding activity is present in both vegetative cells and heterocysts (153). Although the transcription of ntcA is subject to variation over the course of heterocyst differentiation (200), perhaps owing to a NtcA binding site 5" to the gene (154), the means by which NtcA controls heterocyst differentiation remains unknown.
An unresolved question is the mechanism by which NtcA directly or indirectly senses nitrogen status or, even more generally, how nitrogen status is translated into the decision of a cell to differentiate. It would be convenient if the known ability of PII to sense levels of α-ketoglutarate could account for that decision, but if so, the role of the protein in heterocyst-forming cyanobacteria would be significantly different from that in unicellular strains. The regulation of transcription of nitrogen-regulated genes in unicellular Synechococcus strain PCC 7942 is little affected by the absence or modification of PII (59), although the state of the protein is critical in mediating the repression of nitrate/nitrite transport by ammonium (96). The nitrogen status in cyanobacteria thus appears to be sensed in two different ways: by PII, leading to the alteration of enzyme activity, and (perhaps indirectly) by NtcA, leading to the alteration of gene transcription.
The ability of ammonium to suppress heterocyst differentiation is widely believed to be indirect, requiring the activity of GS. When ammonium assimilation in A. cylindrica (164) and other cyanobacteria (210) is blocked by the presence of methionine-dl-sulfoximine, an inhibitor of GS, heterocysts differentiate in the typical spacing pattern in the presence of ammonium. This result implies that the suppressor signal must be a consequence of ammonium assimilation, such as increased glutamine or decreased α-ketoglutarate levels. This conclusion has been questioned as the result of experiments exploiting the ability of A. variabilis to express an alternative nitrogenase in vegetative cells under anoxic growth conditions (189). Under these conditions, this strain can grow on N2, even when the heterocyst-specific nitrogenase is inactivated by mutation. Despite the production of ammonia by the alternative nitrogenase, expressed uniformly in all vegetative cells, heterocysts persist and with the usual spacing. The normal appearance of heterocysts has been taken as evidence that exogenous ammonium is metabolized differently from that produced by endogenous nitrogenase and that only the former inhibits differentiation (189). Alternatively, the minimal level of fixed nitrogen required to suppress differentiation may be markedly higher than the minimal level required to support growth. This is a reasonable supposition, since it is consistent with the behavior of A. cylindrica in more natural circumstances, where the cyanobacterium responds to gradually diminishing supplies of fixed nitrogen well before it is exhausted (111).
Fragmentary evidence points to a crucial role of DNA replication early in heterocyst differentiation. The DNA-damaging agent mitomycin C (5) and the DNA gyrase inhibitor nalidixic acid (M. Gantar and J. Elhai, unpublished results) block heterocyst differentiation when individually applied during the first hour after nitrogen deprivation. Mitomycin C at the effective level has no discernible effect on new RNA synthesis. A mutant of Anabaena strain PCC 7120 defective in a gene, hanA, which encodes a histone-like protein (HU) is blocked in the earliest known stage of heterocyst differentiation (89). HU protein from Anabaena strain PCC 7120 is able to substitute for E. coli HU protein to promote the initiation of DNA synthesis in vitro (132). hanA is preceded by a NtcA binding site. HU protein is by far the most abundant DNA binding protein in vegetative cells, based on autoradiography of acrylamide gels, The vegetative cell band is replaced by a slightly larger DNA binding protein on gels of heterocyst proteins (132), but the protein has not yet been characterized.
Heterocyst differentiation is also blocked by ethionine (93), an analogue of methionine. At the micromolar levels that are effective, ethionine has no effect on bulk protein, DNA, or RNA synthesis or on growth, but it reportedly prevents the rise in hetR expression (P. S. Duggan and D. G. Adams, personal communication) that normally accompanies the earliest stage of differentiation. Ethionine may act by affecting S-adenosylmethionine-dependent methylation of DNA. Such a mechanism has been demonstrated to explain the induction by ethionine of spores of Bacillus subtilis (9).
(ii) Signals specific to early differentiating cells.
Several genes have been found that are required for nitrogen deprivation to evoke any visible sign of heterocyst differentiation. One of these, hetR, clearly plays a central role in the decision of a nitrogen-starved cell to differentiate. Mutants with mutations in hetR, unlike those with mutations in ntcA, are able to grow normally in NO3− (unaffected in a general response to nitrogen deprivation), but they do not differentiate. On the other hand, strains in which wild-type hetR is present on a multicopy plasmid (94) form heterocysts even in the presence of NO3− (27), and the fraction of cells that become heterocysts when hetR is overexpressed in the absence of combined nitrogen can reach 29% (28).
The expression of hetR also is consistent with a role for the protein as an important molecular switch. Transcription of hetR, which is low in vegetative cells, begins to increase as little as 0.5 h after nitrogen deprivation (20, 27, 28, 216). By 3.5 h after deprivation, expression (PhetR::luxAB) is confined largely to a small fraction of cells, with a spacing in the filaments similar to that of heterocysts, even though morphological differentiation is not evident until many hours later. Presuming that all the increase in hetR expression is confined to cells with high expression, the increase per cell can be estimated to be 20-fold, relative to expression under nitrogen-replete conditions (20). The starvation-dependent increase requires functional ntcA (61, 216) and hanA (89). The increase also requires the function of HetR, positively regulating its own expression (20). A positive autoregulatory switch, which magnifies small differences in expression and locks expression into one of two states, is known to stabilize many developmental decisions: lysogeny by coliphage lambda (147), cell type determination by Bacillus (92, 182), and commitment to different developmental fates by eukaryotic cells (7, 123).
The predicted amino acid sequence of HetR gives no clues to its function. For example, it possesses none of the sequence motifs typical of DNA binding proteins (27). Only one function of the protein, autoproteolytic activity, has been detected, evidently stimulated in vivo by the presence of a nitrogen source (223). The active site for proteolysis is required for heterocyst differentiation although not for the HetR-dependent induction of hetR (44). The protein appears to be modified in vivo, since HetR isolated under nitrogen-depleted conditions is considerably more acidic than the protein isolated from nitrogen-replete cells or than the recombinant protein isolated from E. coli (222).
Two genes, hetF and patA, appear to positively influence heterocyst differentiation by modulating the expression or activity of HetR. The hetF gene, best characterized in N. punctiforme (216), encodes a constitutively expressed protein that is essential for heterocyst differentiation; hetF mutants do not initiate even the earliest stages of heterocyst differentiation. The absence of HetF does not alter NtcA-dependent hetR transcription, but its absence eliminates HetR-dependent hetR transcription and the subsequent accumulation of HetR in differentiating cells. HetF bears no obvious similarity to any known protein. A second protein, PatA, has multiple domains, the carboxyl-terminal of which has similarity to the receiver domain of response regulators (100). Mutants defective in patA form heterocysts only at the ends of filaments and are consequently unable to grow well on N2 (100). The multiple contiguous heterocyst phenotype of strains overexpressing hetR is suppressed in patA mutants (28). These results imply that PatA is in the same regulatory circuit as HetR and may function downstream of hetR transcription to modulate HetR activity. Since HetR appears to be modified to a more acidic form in nitrogen-limited cultures, it is tempting to speculate that PatA is part of a phosphorelay signal transduction pathway that activates HetR by phosphorylation (28).
Another gene, hetC, encodes a product important in early heterocyst differentiation. Mutants insertionally inactivated in hetC fail to show any morphological signs of differentiation, but, as in wild-type filaments, mutant filaments lose fluorescent pigments in cells spaced as one would expect of differentiating heterocysts (90). Like hetR, hetC is induced early in the course of nitrogen starvation, is dependent on NtcA (128), and requires its own function for full expression.
HetC shows considerable sequence similarity to members of the superfamily of ATP binding cassette (ABC) proteins (90), which couple the cleavage of ATP to the transport of molecules across membranes (51). This similarity, the propensity of hetC mutants to exhibit pairs of nonfluorescent (partially differentiated) cells, and the confinement of expression of hetC to proheterocysts raise the possibility that HetC participates not in the differentiation of heterocysts but, rather, in the export of an inhibitor of differentiation (90).
Other heterocyst-specific genes have been identified that appear to play a role in later heterocyst differentiation (53, 211, 212). Most, like hepA (formerly hetA [82]) and devBCA (54, 112) are needed in the formation of the heterocyst-specific envelope, and so it is not surprising that they are expressed specifically in well-spaced and presumably differentiating cells (112, 213). The epistatic relationships between the genes required for heterocyst differentiation are just beginning to be worked out (28, 29).
The diversity in times after nitrogen step-down at which genes become active implies that there may be a hierarchy of transcriptional regulators, each controlling the expression of a set of genes at a characteristic time. Such ideas are inspired by the interlocking tiers of regulators and their modifiers that govern differentiation in B. subtilis (180) and other bacteria (161).
Much attention has been given to the role of sigma factors, modular subunits of RNA polymerase that determine promoter specificity in bacteria, in defining the course of differentiation. While alternative sigma factors have been identified in filamentous cyanobacteria (25, 32), none have so far been found to markedly influence the course of heterocyst differentiation. In fact, of the eight group 2 sigma factors that can be readily discerned from the sequence of Anabaena strain PCC 7120, all except the essential main sigma factor (24) are dispensable for heterocyst differentiation (88a; I. Khudyakov and J. Golden, personal communication).
Nonetheless, it is very likely that some such differentiation-specific sigma factors or other proteins regulating transcription initiation exist. Several genes are now known that are expressed in both vegetative cells and heterocysts. In such cases, transcriptional start sites have proven to be different under nitrogen-replete and N2 growth conditions (24, 55, 193). Sites active in nitrogen-replete cultures are preceded by sequences similar to the promoter sequence recognized by the principal E. coli sigma factor, while sites active under N2 growth conditions are preceded by quite different sequences (41). The principal sigma factor, attached to RNA polymerase from Anabaena strain PCC 7120, binds and transcribes only at sites presumptively active in vegetative cells (152, 167). Taken together, these results are consistent with a few possibilities: (i) combinations of redundant sigma factors are required for differentiation-specific transcription; (ii) Anabaena strain PCC 7120 possesses sigma factors essential for differentiation that cannot be recognized by their sequences; or (iii) proteins distinct from canonical sigma factors significantly alter the specificity of RNA polymerase during differentiation. Regardless of which possibility, if any, is true, an increased understanding of transcriptional regulation during heterocyst differentiation promises to teach us something new about how bacteria regulate their behavior.
(iii) Signals specific to mature differentiated cells.
A large suite of genes begins to be expressed at about the same time after the emergence of immature heterocysts (proheterocysts), an event that takes place at about 12 h after the nitrogen step-down. Some of the new gene expression presumably relates to the increase in respiratory capacity that occurs in proheterocysts at about this time. The resulting drop in the intracellular O2 concentration is required for expression of genes encoding nitrogenase (73), but low O2 content and nitrogen deprivation are not sufficient for nitrogenase expression (48). Late gene expression is accompanied by at least three DNA rearrangements (72), removing insertions within genes encoding nitrogenase (67); a heterocyst-specific electron donor, ferredoxin (66); and a hydrogenase (37, 114). While ammonium has been reported several times to inhibit nitrogenase activity in heterocysts, this effect appears to be indirect, at least in A. variabilis ATCC 29413, acting by alteration of oxygen levels (50). At present, it is not clear whether nitrogenase synthesis and activity within mature heterocysts in general is directly repressed by nitrogen in the environment.
How is the spacing of heterocysts determined?
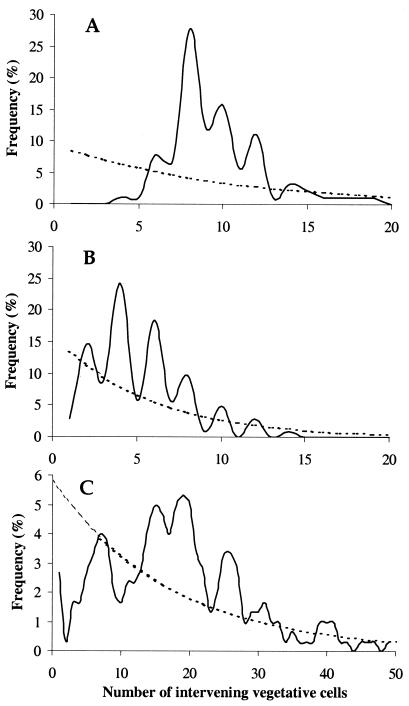
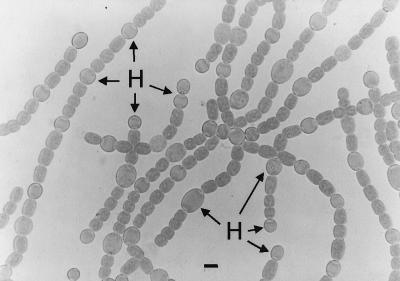
From the perspective of a developmental biologist, the most striking behavior of Anabaena and Nostoc is the elaboration of spaced heterocysts at nearly regular intervals along their filaments (Fig. 1B). The nonrandomness of heterocyst spacing in wild-type Nostoc is obvious by inspection, but for purposes of comparison, it is important to have a clear picture in mind of what a truly random distribution of heterocysts would look like. Figure 4A shows a random distribution of heterocysts, calculated on the assumption that each cell differentiates, or not, independently of other cells in the filament.
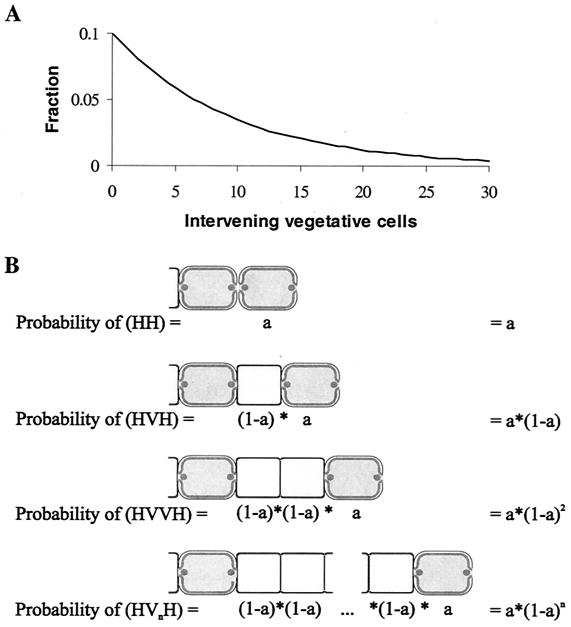
FIG. 4.
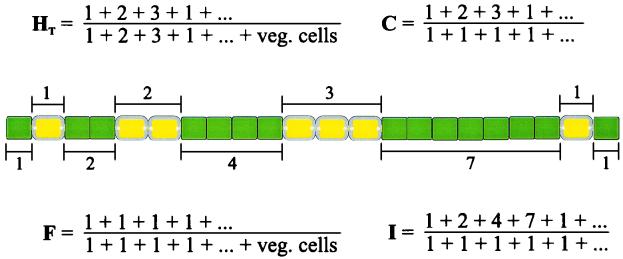
Distribution of randomly spaced heterocysts. (A) Calculated distribution of heterocysts if each cell differentiates with a probability of 0.1 (inspired by references 207 and 209). The graph shows the fraction of intervals with a given number of vegetative cells intervening between two heterocysts, according to the formula Pn = a(1 − a)n, where n represents the number of intervening vegetative cells and a is the probability that a specific cell differentiates. The average heterocyst spacing is (1 − a)/a, which in this case is one heterocyst every 9 vegetative cells. (B) Graphical derivation of the formula that predicts the distribution of heterocysts given random differentiation.
The monotonic decline in probability as the heterocyst distance increases may be surprising at first glance, since many feel intuitively that the peak probability should coincide with the average heterocyst spacing. This is not the case, for reasons illustrated in Fig. 4B. Suppose that each cell differentiates with a fixed probability, say 10% (given as a in Fig. 4B). If one wanted to find an interval of a specific length, say a given heterocyst separated from another heterocyst by 9 vegetative cells, then certainly the cell 9 vegetative cells away from the first heterocyst would need to differentiate. This, by hypothesis, would occur 10% of the time. However, this is not enough. Even if the cell differentiates, the interval would still be less than 9 vegetative cells any time an intermediate cell also differentiates. Therefore, the probability of an interval with of exactly 9 vegetative cells from a given heterocyst is reduced by the probability that each intervening cell does not differentiate. Seen this way, the probability of a shorter interval is always greater than the probability of a longer interval, because fewer intervening cells are required not to differentiate.
In fact, the peakless function predicted by random differentiation bears little resemblance to the actual distribution of intervals (Fig. 5A), and so heterocyst spacing is demonstrably nonrandom. The bias toward even-numbered intervals is expected if cells destined to vegetative cells status, but not those marked for differentiation, divide precisely once prior to the time of observation. A model that accounts for all spatial influences on heterocyst differentiation should be able to predict the distribution in Fig. 5A. Furthermore, if all the influences comprising the model can be removed (e.g. by mutation), the model predicts that heterocyst spacing should then be random, i.e., as shown in Fig. 4A.
FIG. 5.
Spacing of heterocysts. Distances between heterocysts, measured as the number of intervening vegetative cells, are given, and their relative frequencies by actual count (solid line) and by calculation presuming no influence of one cell on another (dashed line) (see Fig. 4 for the equation defining P, the probable heterocyst frequency). The value of a, the probability that a cell differentiates, was taken to be the heterocyst density (heterocysts per total cells) from the given data. Graphs inspired by reference 215, using data taken from reference 221. The frequencies of interheterocyst intervals of length zero are not shown in the first two panels. (A) Wild-type Anabaena strain PCC 7120 grown on nitrate and shifted to no fixed nitrogen for 24 h (P = 9.5%). (B) patS Anabaena strain PCC 7120 mutant grown on nitrate and shifted to no fixed nitrogen for 24 h (P = 16.1%). (C) patS Anabaena strain PCC 7120 mutant grown on ammonium and shifted to nitrate for 96 h (P = 5.6%). Owing to the dispersed nature of the data under the conditions in panel C, the frequencies have been displayed as a rolling average, averaging three intervals at a time centered about the given number.
We have found it useful to consider first a minimal set of assumptions (the one-stage model) that can explain the observed pattern of heterocyst differentiation. To accommodate inconsistencies in this model, as well as more recent observations and concepts, we shall then modify the first model into what we will call the two-stage model.
(i) Characteristics of the one-stage model.
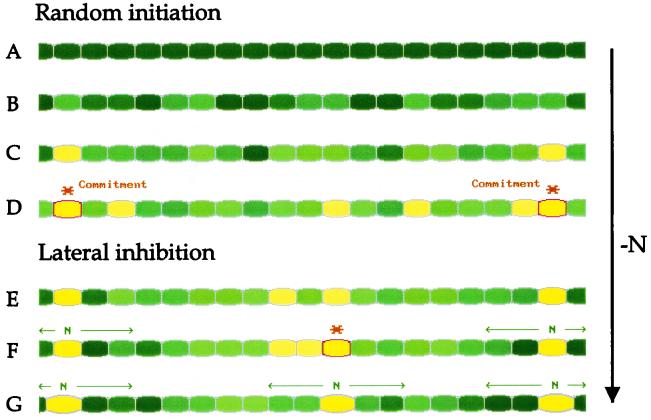
Wolk and Quine (215) showed by computer simulation that two assumptions were sufficient to reproduce a pattern of spaced heterocysts indistinguishable from that actually observed in filaments of A. cylindrica. First, they assumed that any cell is competent to differentiate at the moment when nitrogen is removed from the environment and that the choice of cells that initiate differentiation is random. Second, they postulated the existence of a diffusible inhibitor made by heterocysts and differentiating cells and consumed by nondifferentiating cells, as predicted by experimental data (127, 204, 205, 207, 210).
The model is attractive for its simplicity and because its elements may be interpreted in ways that are physically appealing. Figure 6 illustrates one interpretation of the one-stage model, in which differentiation is initiated in cells that are randomly distributed through the filament and have attained a critical level of starvation for nitrogen. These cells respond both by synthesizing the components particular to heterocysts and by releasing a compound that inhibits adjacent cells from differentiating. The compound may pass from cell to cell through junctions or through the periplasm, the region between the cytoplasmic membrane and the outer membrane of gram-negative bacteria that includes the peptidoglycan polymer. Because the outer membrane is contiguous in filamentous cyanobacteria and does not envelop individual cells (46), the periplasm is thought to be common to all cells in the filament, at least in the interval between adjacent heterocysts.
FIG. 6.
Physical interpretation of the one-stage model of heterocyst spacing: Each line represents a filament consisting of many cyanobacterial cells. The color within each cell represents its nitrogen status: the darker the color, the greater the amount of available nitrogen. (A to D) A filament is suddenly starved for nitrogen. Each cell draws on nitrogen reserves, postulated to be available in different cells to different degrees. When a cell has depleted its reserves to the extent that a critical level of starvation is reached (∗), it becomes committed to heterocyst differentiation. (E to G) Commitment is postulated to have two effects. The committed cell releases a signal (N) that diffuses to adjacent cells and prevents them from differentiating. In this interpretation, the signal is postulated to be a nitrogenous substance that feeds adjacent cells (symbolized by a darkening of their color). In addition, commitment prevents the committed cell from responding to its own inhibitor. Cells distant from the committed cells continue to starve, until one reaches the critical level. The position of the first cells that initiate differentiation is not critical to spacing. Spacing is determined by lateral inhibition.
Why is it that the inhibitor made by a differentiating cell, according to the model, does not inhibit the cell's own differentiation? First, the cell may have rendered itself oblivious to the metabolic effects of the signal. For example, if, early in differentiation, the cell turns off the synthesis of GOGAT and turns on the synthesis of IDH (129), then the concentration of α-ketoglutarate may be set at a high level. If α-ketoglutarate acts downstream from the target of the putative diffusible inhibitor, perhaps by blocking the modification of the PII protein, as it does in Synechococcus strain PCC 7942 (83), then the effect of the inhibitor may be diminished.
Alternatively, the cell may have committed itself genetically to differentiation through a positive-feedback switch. The model of Wilcox et al. (204), similar to the ideas of Turing (194), included not only a diffusible inhibitor, Y, but also a nondiffusible component, X, that acts to increase its own synthesis, to increase the synthesis of inhibitor, and to promote differentiation. Once X has reached a level sufficient to feedback on itself, the cell may be irreversibly locked into a state of differentiation. Reasons to equate HetR with X have already been discussed.
Finally, the inhibitor may be made only after its export from the cell. One can imagine that PatS protein (see below) may be inactive until cleaved outside the differentiating cell (2).
(ii) Multiple contiguous heterocysts.
The distinctive attribute of the one-stage model is that the pattern of heterocyst spacing in the filament is an outcome solely of lateral inhibition by differentiating cells. The pattern does not rely on the choice of cells initially selected to differentiate. In its basic form, the one-stage model postulates that initiation is random (215), but this need not be the case and evidently is not so. Wilcox et al. (204) reported seeing strings of adjacent differentiating cells in A. catenula that eventually resolved to single heterocysts, and they interpreted them as normal intermediates of pattern formation. Such intermediates would imply that initiation of differentiation is not random but, rather, takes place in clusters of contiguous cells along the filament.
Apart from the observations of Wilcox et al. (204), strings of differentiating cells have rarely been seen with wild-type strains of cyanobacteria, including Anabaena strain PCC 7120 (212); however, they are common results of chemical and genetic manipulations (Table 2) (for a more extensive discussion, see reference 214). Exceptionally high light intensity (4) and several chemicals often produce strings of two or more heterocysts where single heterocysts would otherwise be expected. Among the chemicals that produce strings of heterocysts are 7-azatryptophan (1, 38, 126), cyclic AMP (172), and rifampin (215), an inhibitor of RNA polymerase. Whether the strings produced chemically occur at random, suggestive of random initiation, or in a pattern, suggestive of a separate pattern-generating mechanism, has to our knowledge never been addressed. Mutant strains that show contiguous heterocysts have been reported (19, 206, 216, 220).
TABLE 2.
Conditions that markedly affect heterocyst differentiation and spacing
| Gene or treatment (reference) | No heterocysts | Contiguous heterocysts | Function and interpretation |
|---|---|---|---|
| Positively acting systems | |||
| ntcA (61, 200) | Mutant | Initiation: mediates sensing of nitrogen deprivation | |
| hanA (89) | Mutant | Initiation?: HU histone-like protein | |
| Ethioninea | 1 μM | Initiation?: blocks DNA methylation | |
| hetR (27) | Mutant | Multicopy | Resolution: master regulator of heterocyst differentiation |
| hetF (216) | Mutant | Multicopy | Resolution: regulates transcription of hetR |
| hetC (90) | Mutantb | Resolution: ABC transporter (of diffusible inhibitor?) | |
| Negatively acting systems | |||
| patS (220) | Multicopy | Mutant | Resolution: peptide signal |
| hetN (13, 19) | Multicopyc | Mutant | Resolution: unknown signal |
| 7-Azatryptophan (38) | 10 μMd | Resolution: unknown synthesis |
P. Duggan and D. Adams, personal communication.
Pattern of nonfluorescent cells still present.
Inhibits only secondary heterocysts; no effect on heterocyst differentiation within 24 h of nitrogen step-down.
Effect is strain dependent.
HetR is connected with two examples of contiguous differentiating cells. First, when hetR is present on a multicopy plasmid in Anabaena strain PCC 7120 (27) and N. punctiforme (216), removal of nitrogen from the medium leads to the appearance of contiguous heterocysts. Second, hours before the first sign of morphological differentiation, there is a moderate increase in hetR expression (judged by fusion of the hetR promoter to green fluorescent protein), sometimes seen in groups of two or four contiguous cells in Anabaena strain PCC 7120 28; B. Buikema, personal communication). Under these conditions, only single heterocysts are ultimately observed, so that the initial distribution of hetR expression may represent a normally invisible coarse pattern that resolves to well-spaced individual heterocysts. Only hours later is hetR fully induced, and then only in well-separated, differentiating cells. patS expression provides a better studied case in which gene expression in contiguous cells precedes expression confined to heterocysts (221).
(iii) Apparent inconsistencies with the one-stage model.
The one-stage model makes a few predictions that have sometimes not been fully borne out. Chief among these is the existence and behavior of an inhibitory signal emanating from differentiating cells. The one-stage model predicts that abolishing this signal would lead to random spacing of heterocysts while overproducing it might suppress heterocyst formation altogether.
Bauer et al. (13) found two regions of the chromosome that could suppress heterocyst formation when present in Anabaena strain PCC 7120 on a multicopy vector. One of the effective regions was narrowed to patS, a small open reading frame capable of encoding a 13- or perhaps 17-amino-acid polypeptide (220). Controlled overexpression of patS also led to suppression of heterocyst differentiation. Moreover, suppression was observed by exogenous application of a chemically synthesized peptide corresponding to the five C-terminal amino acids of PatS but not by other similar peptides or by the component amino acids. These results indicate that a secreted peptide encoded by patS might serve as the signal mediating lateral inhibition of differentiation.
Deletion of patS resulted in a mutant Anabaena strain PCC 7120 that made heterocysts even in the presence of nitrate and produced strings of heterocysts in the absence of a combined nitrogen source (220, 221). In the latter case, heterocyst clusters are spaced nonrandomly, as judged by the absence of a monotonic decline in frequency as the interheterocyst distance increases (Fig. 5B). Indeed, if one accounts for the fact that contiguous heterocysts diminish the distance between clusters by decreasing the number of dividing, nondifferentiating cells, then the distribution of interheterocyst distances of the patS mutant is much closer to that of the wild-type strain than is at first apparent. The PatS polypeptide, thus, cannot by itself be the signal called for by the one-stage model, since its loss does not lead to random differentiation. Rather, the polypeptide is evidently required to prevent contiguous differentiating cells from producing strings of heterocysts.
The absence of randomly distributed heterocysts in a patS mutant grown on nitrate (221) is equally informative. The presence of the nitrogen source should inhibit the production of the hetN-associated signal (see below), and one might not expect a gradient of nitrogenous compounds emanating from the nitrogenase-deficient heterocysts that appear in the patS mutant. Still, the distribution of interheterocyst distances is not random but, instead, is biased against short intervals; this can be seen by comparing Fig. 5C to the predicted random distribution of heterocysts in Fig. 4A. Thus, there is evidently another pattern-producing signal besides those associated with patS and hetN. The signal may be related to the position of cells in their cell cycles, as discussed below, or the result of heterocysts serving as a sink for glutamate or a source of α-ketoglutarate, glutamine, or some other amino acid produced from glutamine (121). The presence of an exogenous nitrogen source does not necessarily imply the absence of a gradient of nitrogenous compounds.
The second heterocyst-suppressing region (13, 19), hetN, encodes a protein with similarity to NAD(P)H-dependent oxidoreductases involved in fatty acid biosynthesis. Mutants with insertions hetN exhibit an unstable phenotype that is initially similar to that of patS mutants but then changes to a total inability to differentiate heterocysts (19), presumably the result of secondary mutations (19,30). HetN is expressed exclusively in heterocysts and only after hetR is induced during nitrogen step-down experiments (13, 30). It therefore plays no role in the initial pattern of heterocyst differentiation. However, conditional hetN mutants exhibit, under nonpermissive conditions, multiple contiguous heterocysts from secondary rounds of differentiation (30).
Mutants that produce nonrandomly placed strings of heterocysts are therefore readily obtained. In contrast, no mutant has been reported in which heterocysts are spaced at random, contrary to what one would expect if pattern were produced by a single mechanism. We take from this the lesson that there is a complicated and dispensable machinery that resolves strings of differentiating cells but that the fundamental mechanism that produces well-spaced heterocysts is embedded in basic cellular functions (and thus immutable) or is the result of multiple redundant processes.
Mitchison et al. (127) observed that differentiation is apparent only in cells where 6 to 8 h have elapsed since cell division in A. catenula. This raises the possibility that the position of a cell within the cell cycle may govern whether it is competent to differentiate (5). Consistent with this idea, synchronization of a mutant of Anabaena strain PCC 7120 that grows as single cells gives a culture that rises and falls twofold in competence to differentiate over the course of a generation time (Gantar and Elhai, unpublished). If competence were tied to position within the cell cycle, then any preexisting pattern in filaments with respect to the cycle would contribute to the ultimate pattern of heterocysts. Such a pattern exists within filaments of Anabaena strain PCC 7120 grown on nitrate (R. Bucheimer, A. Meng, and J. Elhai, unpublished results). On average, neighboring cells tend to divide at similar times while cells separated by six or seven positions along the filament tend to divide about 180° out of phase.
(iv) Two-stage model of pattern formation.
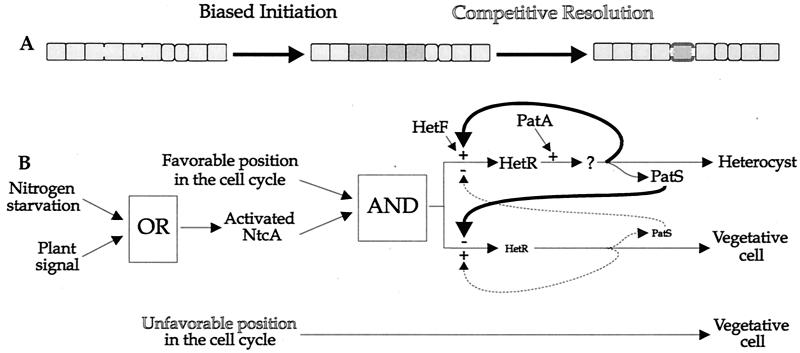
The one-stage model places the entire burden of pattern formation on lateral inhibition. The common appearance of contiguous differentiating cells in genetically or chemically perturbed strains raises the possibility that the pattern emerges in two stages: first, the development of a crude pattern consisting of regions of differentiating cells, and second, the resolution of these regions into single heterocysts. Influenced by the requirement of heterocyst differentiation for DNA replication (5, 6) and other results already discussed, we present a two-stage model in which one stage of pattern development is dependent on the position of a cell in the cell cycle (Fig. 7), but it is possible, of course, that the crude pattern may be governed by other influences.
FIG. 7.
Two-stage model of heterocyst spacing. (A) In response to nitrogen deprivation, four contiguous cells at similar stages in their cell cycles (shown here beginning cell division) initiate differentiation. The string of differentiating cells (dark) is resolved by competitive interaction to a single heterocyst (thick envelope). (B) Either nitrogen deprivation or the presence of a plant signal activates NtcA protein in all cells. The presence of activated NtcA protein and passage of the cell through a critical stage in the cell cycle induce hetR to a middle level of expression, mediated through HetF. Some initiate cells attain a PatA-dependent state (?; perhaps activation of HetR), characterized by a further induction of hetR expression. In these cells, PatS is highly expressed and diffuses to adjacent cells, where it is taken up and inhibits HetR expression. Cells where the positive intracellular HetR feedback loop (thick line) dominates become heterocysts. Cells where the negative intercellular PatS effect dominates revert to vegetative status. As they do, their own positive-feedback loop weakens as PatS inhibits HetR expression and those cells produce less PatS (dotted lines). Cells that are not passing through the critical stage of the cell cycle when nitrogen deprivation takes hold do not initiate differentiation and remain vegetative cells.
(a) Stage 1 (initiation).
All cells are postulated to experience the early effects of nitrogen starvation, presumably sensed directly or indirectly by NtcA. Only a fraction of the cells, possibly those in a critical stage of the cell cycle, are competent to initiate differentiation, and only in these cells is the transcription of hetR induced to the first, moderate level. The initiate cells are generally contiguous, since sibling cells are typically at the same stage in the cell cycle.
(b) Stage 2 (resolution).
Expression of hetR prompts the synthesis and release of an inhibitory signal, presumably PatS (and, after long periods of starvation, perhaps a HetN-related product). Each of the cells that have initated differentiation must choose one of two directions away from their unstable state. A relatively high intracellular level of HetR would, through positive feedback, tend to increase the expression of HetR, committing the cell to differentiation. Conversely, a high level of PatS transported from adjacent initiate cells would tend to decrease the level of hetR expression, leading to regression.
The existence of genes whose mutation leads to the failure to initiate heterocyst differentiation when mutated and whose overexpression leads to multiple contiguous heterocysts, or vice versa (Table 2), is explicable by their roles in resolution: an overactive resolution stage eliminates all heterocysts, while an underactive resolution stage leaves the intermediate stage intact. By the same token, the many treatments that lead to multiple contiguous heterocysts also can be seen as interfering with resolution. Mutations in some genes are proposed to act on initiation; these include ntcA, through the requirement for nitrogen deprivation, and perhaps hanA, through the requirement for DNA replication.
Some observations do not fit readily into this simple model. A patA mutant produces heterocysts only at the ends of filaments (100), similar to the behavior of cyanobacteria of the genus Cylindrospermum grown continuously on N2 (215). A patB mutant of Anabaena strain PCC 7120 (101) and a patN mutant of N. punctiforme (F. C. Wong and J. C Meeks, unpublished results) both exhibit a higher frequency of heterocysts than normal, but not as multiple contiguous heterocysts. However, an increased frequency is also seen in wild-type A. cylindrica exposed to high-intensity light (4). It is conceivable that a metabolic deficiency affecting cell division, such as that described in the patB mutant, might account for the phenotype. Mitchison and Wilcox (125) observed that cell division in A. catenula is asymmetric and that heterocysts arise only from the smaller of the two daughter cells. While asymmetric division has been reported in only one other heterocyst-forming strain (4), asymmetry not apparent by light microscopy is still possible. The rules found by Mitchison and Wilcox (125) make the strong prediction that if adjacent cells differentiate (as in a patS mutant), they will be no closer than three generations distant (i.e., they will share the same greatgrandparent cell). The two-stage model predicts instead that the adjacent cells are most likely to be siblings.
Within the context of the two-stage model, it is possible to see that plants could influence the number of heterocysts by increasing initiation, perhaps by altering the cell's perception of its nitrogen status or by modulating resolution, or both.
Global Differentiation to Motile Filaments: the Hormogonium Cycle
What are hormogonia, and what is their role in the cyanobacterial life cycle?
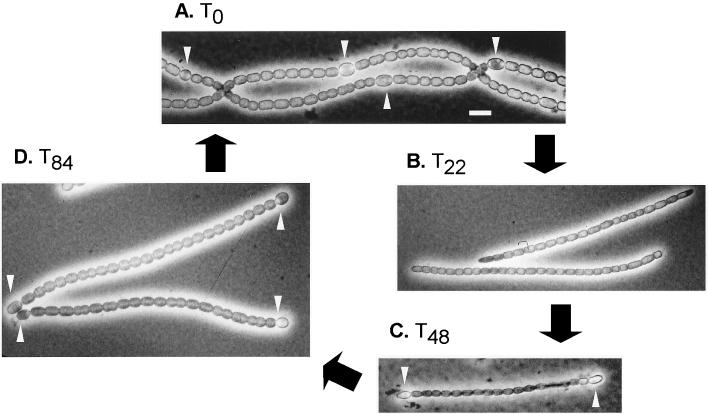
Hormogonia are short filaments that are released from parental filaments of a variety of heterocyst-forming and non-heterocyst-forming cyanobacteria (75, 184). They are distinguished from vegetative filaments primarily by their gliding motility, the small size of their cells (Fig. 8), and the absence of heterocysts. In some species, hormogonia cells contain gas vacuoles, which control buoyancy.
FIG. 8.
Photomicrograph of filaments of N. punctiforme in the N2-dependent vegetative growth state and at different stages of the hormogonium cycle. Phase-contrast images of vegetative filaments (A) and hormogonia (B to D) are shown. Note the process of heterocyst differentiation by the end cells of the hormogonium filaments starting at T48(C), with the appearance of mature heterocysts and an increase in size of vegetative cells by T84(D); this is followed by vegetative cell growth and division and eventually by differentiation of intercalary heterocysts. Bar, 10 μm.
Hormogonia play a major role in short-distance dispersal of filamentous cyanobacteria, through their gliding motility when in contact with a solid substratum or their buoyancy in the water column. Hormogonia of many strains display positive phototaxis (95), which is important in the colonization of illuminated portions of the habitat by these photoautotrophic organisms. While there is evidence of chemotaxis by a Nostoc species to extracellular products of a symbiotic plant partner (see below), there is none for chemotaxis by hormogonia of any species to inorganic or organic nutrients.
The mechanism by which hormogonia glide is unknown. A protein termed oscillin, which may be essential for gliding, has been identified in vegetative filaments of non-heterocyst-forming Phormidium uncinatum (81). Oscillin forms as fibrils external to the outer membrane of the cell wall and is proposed to passively direct the flow of mucilage between the filament and substratum. A similar mechanism may be involved in hormogonium motility.
The acquisition of motility comes at a price. Hormogonia are unable to fix nitrogen or grow. Phycobiliprotein synthesis ceases (42), leading through attrition to an accompanying decrease in fluorescence. Cells of hormogonia continue to photosynthesize and assimilate exogenous ammonium if it is available, although the rates of CO2 fixation and NH4+ incorporation are only 70 and 62%, respectively, of those of vegetative filaments (33). The metabolic fates of the photosynthate and newly synthesized or regenerated amino acids in the non-growth hormogonium state are not known. However, much of the metabolic output is probably devoted to the production of the proton motive force (80) and the synthesis and secretion of mucilage (81) thought to be required for gliding motility.
Since cells within hormogonia do not grow, the state by necessity is transient. One can therefore describe a hormogonium cycle, starting with the induction of hormogonia and ending with the return of hormogonia to the vegetative state within the equivalent of two or three generation times (Fig. 8). On induction, cell division begins at a nearly simultaneous rate in all cells of a filament, but not necessarily at the same time in all filaments. The result is that except for a very short period, filaments at a given moment consist of all newly divided cells (reflecting global differentiation) or virtually no newly divided cells (33, 42). There is no significant net synthesis of DNA or protein during hormogonium differentiation (75), although some protein synthesis is still required by the process (33); therefore, the smaller hormogonium cells result from multiple cell divisions in the absence of cell growth. Cell division without DNA synthesis is possible because cyanobacteria, in general, contain multiple copies of their genomes (74). Even in the absence of DNA replication, hormogonium cells are thus likely to receive at least one copy of the genome following multiple septations of the parental cell. Shortly after cell divisions, the filaments begin to fragment in a random manner. In filaments containing heterocysts, fragmentation takes place preferentially between heterocysts and their adjacent vegetative cells (33). The detachment of heterocysts from vegetative cells results in an interruption in the flow of reduced carbon from vegetative cells that supports both nitrogenase activity and respiration, and O2 protection is lost. Detached heterocysts therefore cannot fix N2, and nor can hormogonia.
The hormogonia remain in the gliding state for about 36 to 48 h, after which they cease to move. Only at this stage, in the absence of combined nitrogen, do heterocysts differentiate, first at the ends of the filaments and later, as the vegetative cells grow, with the typical spacing pattern within the filaments. Based on the resumption of pigment and total protein synthesis, the conversion of hormogonia back to vegetative filaments is complete within 96 h after their induction. Preliminary experiments indicate that cultures of Nostoc (E. L. Campbell and J. C. Meeks, unpublished data) and Calothrix (184) that have extensively differentiated hormogonia require a vegetative growth period before they are again able to differentiate a substantial number of hormogonia.
What induces the differentiation of hormogonia?
Combined nitrogen deprivation is sufficient to induce heterocyst differentiation in all capable free-living cyanobacteria. In contrast, no single environmental factor has been identified that will induce hormogonium differentiation in all capable strains. The one common factor in initiation of hormogonium differentiation appears to be a change in some environmental parameter: an increase or decrease of a nutrient or a change in the quantity or quality of light. The most frequently used inducer of hormogonium differentiation in the laboratory is the transfer of a dense culture, near stationary growth phase, to fresh medium (159). This procedure has the possibility of multiple environmental changes, including the dilution of an endogenous repressor of hormogonium differentiation (63, 75), increased light intensity, and replete nutrients. We consider these changes to signal either a positive or negative stress condition for growth. We attempt below to find some order in a complicated set of observations.
(i) Hormogonium differentiation in response to changes in the chemical environment.
When certain strains of either Calothrix or Nostoc (including symbiotic N. punctiforme), grown in the presence of combined nitrogen, are transferred to hormogonium-inducing conditions in the absence of combined nitrogen, the majority of filaments enter into the hormogonium cycle rather than differentiate heterocysts (42, 64, 75). The extent of hormogonium induction after such a shift is variable and may depend on the position of the culture in the growth curve. Under these conditions, hormogonium differentiation supersedes nitrogen-dependent differentiation of heterocysts. Heterocysts appear in these cultures only as the hormogonium filaments reenter the vegetative growth state. These observations also establish that hormogonium differentiation and heterocyst differentiation are mutually exclusive processes in any one filament.
In addition to nitrogen-limited stress signaling, supplementation with excess nutrients, such as iron (45) or phosphate (219), induces hormogonium differentiation in certain strains. Osmolarity may also serve as a stimulus, as illustrated by a symbiotic Nostoc strain that consistently forms hormogonia following an immediate decrease in osmolarity previously established by glucose or salt (91). With some other strains of Nostoc, a drop in ionic strength triggers hormogonium formation (12, 63). The mat-forming thermophilic cyanobacterium Mastigocladus laminosus (distantly related to Nostoc and Calothrix) produces hormogonia when liquid suspension cultures are transferred to agar-solidified plate medium, perhaps in response to water stress (76).
(ii) Hormogonium differentiation in response to changes in light quality.
Many Nostoc and Calothrix strains grown in green or white light differentiate hormogonia when the cultures are switched to growth in red light (184). This manipulation may seem at first no more than a laboratory curiosity, but it reflects the ambient conditions faced by a cyanobacterium when shielded by plant tissue or when buried deep in the water column. The induction of hormogonia by red light can be reversed by subsequent exposure to green light. This photoreversible response bears similarities to the process of complementary chromatic adaptation (CCA), in which certain cyanobacteria alter their pigmentation in order to use most efficiently the wavelengths of incident light (69). However, the two processes are not linked (184). CCA occurs in strains that do not form hormogonia, and many hormogonium-producing strains are incapable of CCA.
Photoreversible hormogonium induction in CCA-responsive Calothrix appears to be a consequence of an imbalance in electron flow through the photosynthetic electron transport chain rather than the direct action of a morphologically active pigment (31). In cyanobacteria, short-term exposure to red light tends to oxidize the electron transport components in the chain between photosystems II and I, while green light tends to reduce those components. Inhibitors that block the reduction of the quinone pool of the electron transport chain mimic red light and induce hormogonium formation while blocking heterocyst differentiation. Conversely, inhibitors that block oxidation of the quinone pool mimic green light and inhibit hormogonium formation while allowing heterocyst differentiation (31).
The mechanisms by which an imbalanced electron transport signal (i.e., the oxidation-reduction state of the quinone pool) could be integrated into the developmental response are unclear. It has been suggested that the PII protein, described earlier as a sensor of carbon and nitrogen status, also acts as a sensor of the oxidation state of the quinone pool (184). However, there is currently no direct evidence for an essential role of the PII protein in hormogonium differentiation.
(iii) Differential gene expression in the hormogonium cycle.
It is not clear how much specific gene expression occurs during the hormogonium cell cycle or if the major response is the activation or inactivation of constitutively expressed proteins. To date, only genes encoding gas vesicles in Calothrix (42) and an alternative sigma subunit in N. punctiforme (32) have been shown to be specifically transcribed in hormogonia. The transcription of other genes may be enhanced from a constitutive level in vegetative cells.
PLANT CONTROL OVER THE INFECTION PROCESS
All symbiotically competent filamentous cyanobacteria differentiate hormogonia, and these motile filaments function as the infective units in the formation of a symbiotic association with plants (16, 115, 116). For any individual hormogonium, the time window for infection is that of active gliding, or the period of 12 to 60 h after induction. Plants appear to send out chemical signals that influence at least three characteristics of the hormogonium cycle in relation to the infection process: (i) induction of hormogonium differentiation (a higher frequency of hormogonium differentiation in the cyanobacterial population will increase the probability of an infection event); (ii) control of hormogonium behavior (use of chemoattractants to influence the direction and perhaps speed of gliding in colonization of the symbiotic cavities); and (iii) regulation of the period between exit from and reentry into the hormogonium cycle (this response prevents continual entry into a nongrowth state in the presence of a stimulus and allows the differentiation of heterocysts).
Plant Induction of Hormogonium Differentiation
There is evidence for the extracellular production of one or more hormogonium-inducing factors (HIF) by four plant partners, the hornwort Anthoceros (33), the liverwort Blasia (91), the cycad Zamia (135), and the angiosperm Gunnera (155), as well as a plant not known to participate in symbioses, wheat (63). Anthoceros and Blasia produce the HIF activity in the suspension medium of liquid cultures (conditioned medium). In Zamia, the activity appears to be present in an aqueous extract of seeds and to be secreted from the specialized coralloid roots of the plant. In Gunnera, the HIF activity is present in the acidic mucilage that is excreted onto the surface of the stem by the stem glands that are colonized by the Nostoc organisms. The HIF activity is produced by the roots of wheat seedlings and exuded into the medium of hydroponic cultures.
The HIF has yet to be isolated and characterized from any plant. However, the activity from both Anthoceros and Gunnera exudates is more easily interpreted as a direct effect of the plant-derived substance on cyanobacteria rather than as a secondary effect acting through the chemical environment. For examples, low pH (5.0) and darkness tend to inhibit hormogonium differentiation, but the presence of exudate from Gunnera and Anthoceros overrides the inhibition inducing hormogonia without changing the bulk pH. The fact that seed rinses and aqueous extracts of leaves, stems (apart from the gland area), and roots of Gunnera do not induce hormogonia indicates that the activity in the gland mucilage is tissue specific and not a nonspecific plant metabolite. Moreover, in Anthoceros, the HIF activity is produced primarily by nitrogen-limited tissue (33). The activity therefore appears only when N2-fixing cyanobacteria would be useful to the plant.
It is not clear whether the HIF activities prepared from different plants represent similar or different substances. HIF activity from both Anthoceros (33) and Gunnera (155) is inactivated by heat (autoclaving) and, based on dialysis experiments, correlates with a small molecule of between 0.5 and 12 to 14 kDa. In contrast, HIF from wheat was retained by dialysis tubing with a 12- to 14-kDa cutoff (63). The HIF activity was lost in Gunnera mucilage treated with proteinase K, indicating that the HIF could be a small polypeptide or a ligand-modified polypeptide. On the other hand, HIF activity from Anthoceros was complexed by polyvinylpyrrolidone, which characteristically binds polyphenol-like molecules. Very different stabilities have been reported for HIF activities from different plants (39, 91, 155), but the conditions were too dissimilar to permit comparison.
Behavioral Characteristics of Hormogonia during Infection
There exist Nostoc strains that differentiate a high frequency of motile hormogonia in response to HIF yet either do not infect Gunnera (85) or Anthoceros (49) tissues or cause a delayed, low level of Anthoceros infection (116). This implies that an additional specific response, such as chemotaxis by the Nostoc strain to the plant tissue, is required for efficient infection. Indeed, since N2-fixing cyanobacteria are in low abundance in soils, it is difficult to imagine how new plant-cyanobacterium associations might arise without chemotaxis.
Hormogonia of Nostoc are positively phototactic (95), yet they migrate away from light in the colonization of interior gland cells in the Gunnera association. Based on this observation, Johansson and Bergman (84) suggested that chemotaxis must be involved in the infection of the gland cells. However, a chemotactic response could not be observed in specific plate assays using the gland mucilage (155). Knight and Adams (91) unequivocally demonstrated chemotaxis of a symbiotic Nostoc strain toward exudate from Blasia, using preinduced hormogonia to separate the phenomena of chemotaxis from hormogonium induction.
Evidence of specific cyanobacterial responses beyond hormogonium induction is emerging from studies of the infection of Anthoceros by genetically altered Nostoc mutants. Mutation of an alternative sigma factor (SigH) of RNA polymerase in N. punctiforme results in a sixfold-higher initial infection of Anthoceros compared to that by the parental strain (32). Relative to the parental culture, the mutant does not differentiate more hormogonia, nor do the hormogonia remain in a motile state for longer or reenter the hormogonium cycle more frequently. Therefore, it is the behavior of the hormogonia that appears to be altered in the mutant. sigH is not transcribed under various stress or vegetative growth conditions, with or without combined nitrogen, but it is specifically induced in a burst pattern between 1.5 and 6 h following exposure to Anthoceros conditioned medium containing HIF. The genes whose transcription is dependent on SigH are not known, but they clearly modulate the infection process in some manner.
Conversely, mutation of ntcA in N. punctiforme yields a noninfective phenotype in the Anthoceros association (217). While the ntcA mutant forms hormogonia following shifts in growth conditions, its response to HIF is markedly less strong than that of wild-type cultures and the hormogonia formed by either method fail to infect Anthoceros tissue. Since mutants unable to fix N2 infect Anthoceros at a rate similar to that of wild-type cultures, the symbiotic defect in the ntcA mutant is not related to the inability of the mutant to fix nitrogen. These observations indicate that NtcA may have a wider role than response to nitrogen status in cyanobacteria.
Plant Regulation of the Hormogonium Cycle
Hormogonia are a nongrowing developmental state; therefore, the continual entry into the hormogonium cycle is presumably lethal by extinction. When in prolonged coculture with Anthoceros, very few of the Nostoc filaments, both within and outside of the symbiotic cavities and in the suspension medium, are in the hormogonium state (49), yet HIF is continually present (33). Similarly, Nostoc appears to show a growth stimulation during prolonged incubation on plates with gland mucilage from Gunnera, which also contains HIF (155). These observations imply that Nostoc must have a mechanism to block either the sensing of or the response to the presence of HIF. Various Nostoc strains produce an unidentified autogenic repressor of hormogonium differentiation into the culture medium (63, 160, 162). It is conceivable that plants also produce an activator of the synthesis of this or a similar repressor.
The benefit of such a repressor to the plant would not be in blocking hormogonium formation in the immediate surrounding (i.e., the medium) but in the symbiotic cavities after infection has occurred. Hormogonium formation is clearly counterproductive to nitrogen fixation, and the provision of fixed nitrogen by the cyanobacterium for the plant is presumably the selective pressure in the establishment of these symbiotic associations; therefore, a repressor of hormogonium formation in the symbiotic cavities could also shift the developmental direction of associated Nostoc toward heterocyst differentiation and N2 fixation.
A plant-induced hormogonium-regulating locus (hrm) was identified in N. punctiforme by analysis of a transposition mutant that is 8- to 10-fold more infective of Anthoceros than is the wild-type parental strain (39, 117) and produces 2- to 3-fold more hormogonia when challenged with HIF. The mutant strain UCD 328 grows identically to the wild-type in the free-living state, in the presence or absence of combined nitrogen, but responds quite differently in coculture with Anthoceros. Within one week following inoculation, short hormogonium-like filaments and clusters of detached heterocysts are present in the cocultures with strain UCD 323, in contrast to the predominantly vegetative filaments in cocultures with wild-type Nostoc. Within 2 weeks the mutant had died, while the wild-type accumulated biomass in the medium and epiphytically on the gametophyte tissue. These observations indicate that the mutant may have emerged from the initially induced hormogonium state and differentiated heterocysts but that the filaments apparently immediately reentered the hormogonium cycle. The loss of the mutant Nostoc from the coculture medium is consistent with an extinction effect of continual entry into the hormogonium cycle.
The transposon had inserted into a gene termed hrmA (39). The sequence of the region around hrmA revealed a five-gene locus (117) that shows a remarkable similarity in gene sequence and genomic organization to the uxu and exu operons of E. coli, encoding enzymes responsible for glucuronic and galacturonic acid catabolism, as well as a transcriptional repressor (102). However, inducers of these operons, sodium glucuronate, sodium galacturonate, and sodium gluconate, failed to induce luxAB expression in a hrmA::luxAB mutant, nor did they alter infection by wild-type Nostoc (E. L. Campbell, F. C. Wong, and J. C. Meeks, unpublished data). The substrates metabolized by gene products of the hrm locus are currently unknown and need not be glucuronic or galacturonic acids. Transcription of genes in the locus is induced by exposure to an aqueous extract of the Anthoceoros gametophyte tissue that also blocks hormogonium formation in the wild-type strain (39). The factor in the extract was termed a hormogonium-repressing factor (HRF), since it appeared to prevent the entry of vegetative filaments containing an intact copy of hrmA into the HIF-induced hormogonium cycle, in part by upregulation of hrmA.
It is unlikely that enzymes of the hrm locus produce the autogenic repressor of hormogonium differentiation postulated to exist in dense cultures (75). The mixing of mutant and wild-type Nostoc cultures in reconstitution of the association with Anthoceros does not repress the high-frequency infection by the mutant (39). It would appear that if the hrm-encoded metabolic pathway does produce a repressor, it is not released into the medium at an effective level by wild-type cells.
Although sigH and hrmA null mutants yield a high infection phenotype, the phenotype is not the consequence of SigH-dependent transcription of the hrm locus. Two results lead to this conclusion: (i) sigH is induced by HIF while hrmA is not, and (ii) hrmA is induced by HRF in a sigH mutant background (31). The similarity in phenotype is probably a consequence of at least two unlinked metabolic systems under control by the plant that influence hormogonium differentiation (hrm) and behavior (sigH). One can conclude from these studies that plants clearly exert both positive and negative regulatory control over hormogonium differentiation in Nostoc. The mechanisms and signal molecules remain to be defined.
PLANT CONTROL OVER THE CYANOBACTERIAL SYMBIOTIC GROWTH STATE
The cyanobacteria are the passive partners in all associations with plants. The extent to which this is true might be appreciated by comparing plant-cyanobacterium associations with the carefully orchestrated dance of legumes and rhizobia. Interactions of rhizobia with roots elicits gross morphological transformations in the plant: the curling of young root hairs, the formation of the infection thread, and the differentiation of new nodules. Likewise, the plant induces rhizobia to differentiate into bacteroids, a form that does not exist outside the plant.
In contrast, when cyanobacteria enter into a symbiotic association with plants, only the cyanobacterial partner undergoes dramatic physiological and morphological changes, but no change represents a behavior outside of its normal repertoire of the free-living growth state. Although the plants may increase the size of the preexisting symbiotic cavities and elaborate transfer cells for metabolite exchange in response to the presence of the cyanobacteria, these changes are relatively minor. Plants have thus learned how to activate regulatory pathways that function normally in free-living cyanobacteria, but cyanobacteria scarcely affect the plant in return, except, of course, to supply it with combined nitrogen. In this section, we focus on the cyanobacterial behavior modified by plants in the symbiotic cavities.
Physiological Characteristics of Cyanobacteria in the Symbiotic Growth State
A decrease in the rates of three physiological processes have been documented when cyanobacteria enter the symbiotic state; these processes are growth, assimilation of CO2, and assimilation of NH4+. Conversely, there is an increase in the rate of N2 fixation that accompanies the increased frequency of heterocyst differentiation. During balanced growth in the free-living state, these processes are regulated such that photosynthetically reduced carbon is provided as required for growth and N2 fixation while the amount of nitrogen that is fixed and assimilated is proportional to the organic nitrogen needs for growth. The uncoupling of N2 fixation and NH4+ assimilation reflects an unbalanced physiological state in symbiosis, but it is similar to the physiology of rhizobial bacteroids and consistent with the cyanobacteria providing N2-derived NH4+ (or a derivative) for growth of the plant partner.
Do plants control the growth of symbiotically associated Nostoc?
If Nostoc grows too slowly within the plant, the cyanobacterial symbiont will gradually be lost. If it grows too fast, it will outgrow the plant and compete with it for light and nutrients. The growth rate of cyanobacteria in symbiosis has been shown to be proportional to that of the plant partner in the Anthoceros association (49), as it should, and a similar proportionality is evident in other associations. Whereas free-living Nostoc strains may have doubling times that range from 15 to 48 h in nitrogen-fixing culture, Anthoceros doubles in biomass under optimal laboratory conditions within 5 days when supplied with combined nitrogen or 10 days when dependent on N2-derived nitrogen from Nostoc (49). Thus, in the maintenance of a stable association, the growth of the cyanobacterium must be slowed in some manner.
It appears that Anthoceros regulates both the size of the symbiotic Nostoc colony and its nitrogenase specific activity. This assertion is based on the results of two experiments. First, under steady-state culture conditions of relatively low light intensity in medium supplemented with glucose (49), new symbiotic Nostoc colonies emerge at the margins of the growing gametophyte tissue and enlarge up to a relatively uniform size and density. The new infections are dependent on a low level of hormogonia produced from the existing Nostoc colonies. The immature and mature Nostoc colonies provide a constant rate of fixed nitrogen per colony and per unit of gametophyte tissue. New infections can be prevented by transient treatment of the culture with penicillin, which lyses Nostoc filaments in the medium. One might anticipate that this intervention would result in fewer colonies and lower nitrogen production. Instead, while new colonies are prevented, preexisting symbiotic colonies increase in size, with a proportional increase in the rate of N2 fixation per colony (49). The symbiosis maintains a constant rate of N2 fixation per unit of the growing gametophyte tissue through an increase in cyanobacterial growth without a change in nitrogenase specific activity.
Second, when the Anthoceros-Nostoc association is grown under high light intensity without glucose, the growth rate of the associated tissue and that of N2 fixation per unit of gametophyte tissue is identical to that of tissue grown under low light intensity in glucose-supplemented medium (177). However, both the number and the biomass of individual high-light-intensity-grown Nostoc colonies are approximately half those of low-light-intensity-grown colonies, and the specific activity of nitrogenase is fivefold higher. Here, the symbiosis maintains a constant rate of N2 fixation through a decrease in growth response and increase in nitrogenase specific activity. The mechanisms regulating these opposite responses have not been examined, but they clearly illustrate plant-mediated control over growth and specific protein synthesis of the associated Nostoc in order for the plant partner to maintain a constant growth rate.
What causes impaired photosynthesis in symbiotically associated Nostoc?
Complete photosynthesis, culminating in the reduction of CO2 to organic carbon, is highly depressed in cyanobacteria associated with a eukaroytic photosynthetic partner (116). The rate of light-dependent CO2 fixation by freshly separated symbiotic Nostoc is generally low relative to that in free-living cultures: undetectable from cycad (107), 1% from Gunnera (173), and 12% from Anthoceros (176) associations. An exception is the cyanobacterium associated with Azolla; when immediately separated from the fern, it has 85% of the rate of typical free-living Nostoc/Anabaena cultures (156). However, 14CO2 pulse-chase experiments indicate that the associated cyanobacterium fixes little or no CO2 in the intact Azolla association (88). Therefore, the cyanobacterial partner in all associations defers its independent photosynthetic potential and assumes a dependent heterotrophic role, exchanging fixed nitrogen for fixed carbon.
Heterocyst differentiation is accompanied by the loss of photosynthetic antenna biliproteins, but the depressed rates of light-dependent CO2 fixation by the symbiotic Nostoc cannot be explained by the loss of these pigments. Epifluorescence light microscopic and immunoelectron microscopic analyses have shown that the light-harvesting phycobiliproteins are present in vegetative cells of Nostoc in all symbiotic associations at concentrations similar to those in free-living cultures (Fig. 9) (116). Symbiotic Nostoc strains therefore retain phycobiliproteins even though they may be of little functional value, just as free-living cyanobacteria retain phycobiliproteins even during prolonged dark heterotrophic culture (158, 203). Even heterocysts retain phycobiliproteins and their attendant fluorescence in association with some plants (Fig. 9) (87, 115; but see also reference 150), in stark contrast to the loss of fluorescence within heterocysts of free-living Nostoc strains (Fig. 1C). The phycobiliproteins in heterocysts of the Azolla symbiont are functional, as judged by action spectra for nitrogenase activity (195).
FIG. 9.
Photomicrographs of symbiotically associated cyanobacteria. (A and B) Nostoc associated with Anthoceros; (C) Nostoc/Anabaena associated with Azolla. Panel A is a phase-contrast image and panels B and C are epifluorescence images with excitation light near 510 to 560 nm and emission greater than 600 nm. Arrowheads point to heterocysts in panel C. Bar, 10μm. Compare the cell sizes to those in Fig. 1 and 8. Panels A and B reprinted from reference 116 with permission of the publisher. Panel C reprodinted from reference 195 with permission of the publisher (kindly provided by Gerald Peters).
Another possibility to explain the loss of photosynthetic capacity is a decrease in the activity of ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco), the primary carboxylating enzyme of the carbon-assimilating Calvin-Benson-Bassham cycle (183), but here the situation is more complicated. Immunological titrations have demonstrated that the Rubisco protein accumulates to cellular levels that are similar to those in free-living cultures in the Nostoc strains of all associations examined (116). Rubisco activity is another matter. Nostoc cultures freshly separated from Anthoceros have only 12% of the in vitro Rubisco activity of corresponding free-living cultures, similar to the in vivo rate of symbiotic light-dependent CO2 assimilation (176). In contrast, the in vitro Rubisco activities in Nostoc from both the cycad (107) and Gunnera (173) associations are equivalent to those in free-living cultures. It may be that the low rate of in vivo CO2 fixation by Nostoc in association with cycads or Gunnera is attributable to a reversible inhibitor of Rubisco, which is lost by dilution in the in vitro assay.
Collectively, these results indicate that the mechanisms resulting in the common phenotype of impaired complete photosynthesis in Nostoc varies in the different associations, perhaps as a consequence of unique plant signals affecting different key reactions of the metabolic system.
What are the causes and consequences of uncoupled nitrogen fixation and ammonium assimilation in symbiotically associated Nostoc?
Nitrogen metabolism is profoundly different in symbiotically associated Nostoc strains from that in strains grown outside the plants. First, the specific activity of nitrogenase in Nostoc associated with Anthoceros (177), cycads (106), and Gunnera (174) is three- to fivefold higher than that in free-living cultures. Second, much of the nitrogen that is fixed is released and made available to the plant. The amounts of N2-derived NH4+ released by symbionts in associations grown under controlled conditions in the laboratory are 40% in Azolla (120), 80% in Anthoceros (118), and 90% in Gunnera (169). These values are in contrast to 6% (57) to 20% (178) of NH4+ and organic nitrogen combined by free-living N2-fixing cyanobacteria under variable conditions in the natural habitat. An undefined amount of cell lysis undoubtedly contributes to the measured nitrogen release in the habitat. These results point to an imbalance in cyanobacterial metabolism associated with the symbiotic state and raise several important questions addressed below. At the root is the question why Nostoc associated with plants adopts a developmental profile appropriate for extreme nitrogen deprivation when in fact the cyanobacterium is immersed in the products of N2 fixation.
(i) Sources of reductant for symbiotic nitrogen fixation.
Nostoc in the free-living state produces enough reductant through photosynthesis to provide exactly for N2 fixation and other metabolic needs. What is the case with symbiotically associated Nostoc? Steinberg and Meeks (177) addressed this question by performing experiments with a Nostoc mutant that is resistant to 3-(3,4-dichlorophenol)-1,1-dimethylurea (DCMU), an inhibitor of electron transport out of the photosystem II reaction center. The addition of DCMU thus serves to separate photosynthesis by resistant Nostoc from that by sensitive Anthoceros. Associations with the wild-type (DCMU-sensitive) Nostoc, when sufficiently depleted of internal stores of reduced carbon, showed no nitrogenase activity in the dark or in the light in the presence of DCMU, as expected. Associations with the DCMU-resistant Nostoc, incubated in the light and in the presence of DCMU to inhibit plant photosynthesis, had 30% of the uninhibited light-dependent rate of nitrogenase activity. This result verified that the Nostoc is capable of complete photosynthesis when in the Anthoceros association, albeit at a low rate, and that maximal nitrogenase activity is maintained primarily by photosynthate from the plant.
Full nitrogenase activity can be restored to light grown, DCMU inhibited associations by the addition of sucrose, fructose, or glucose but not the dicarboxylic acids favored by rhizobia in the legume associations (201). The few effective carbohydrates are the same as those able to support heterotrophic growth in free-living Nostoc cultures. Of these hexoses, sucrose appears to be the natural currency between the plant and the cyanobacterial symbiont, at least in the Azolla association (88).
One can conclude from these results that steady-state N2 fixation by symbiotic Nostoc probably relies on plant-derived hexoses. The symbiotic state does not change the types of exogenous organic compounds that Nostoc can metabolize but, rather, alters the usual connection between cyanobacterial photosynthesis and N2 fixation.
(ii) Routes of carbon catabolism by symbiotic Nostoc.
Carbohydrate translocated into heterocysts is catabolized by the initial dehydrogenases of oxidative pentose phosphate metabolism to generate the reductant required for both nitrogenase and oxidative respiration. Oxidative pentose phosphate metabolism in vegetative cells or heterocysts can proceed in either a cyclic or a linear manner. In the purely cyclic mode, glucose-6-phosphate is ultimately catabolized to CO2 while generating reductant, whereas in the linear mode the intermediates can be metabolized to pyruvate and ultimately to α-ketoglutarate (181). Free-living Nostoc growing on sugar must divert a substantial amount of carbon through the linear pathway to generate biosynthetic precursors, including the α-ketoglutarate essential for net assimilation of the N2-derived NH4+ into glutamate via the GS-GOGAT cycle. However, in the symbiotic growth state, where an elevated demand for reductant derived from glucose-6-phosphate is not paralleled by an elevated demand for carbon skeletons, Nostoc should favor flux through the cyclic oxidative pentose phosphate metabolic route to avoid the detrimental accumulation of tricarboxylic acid cycle intermediates, such as α-ketoglutarate. This predicted change in metabolism evoked by the transition from free-living to symbiotic states has not yet been identified, nor has any regulatory mechanism susceptible to exploitation by the plant.
(iii) Regulation of ammonium assimilation in symbiotic Nostoc.
Free-living cyanobacteria growing on N2 typically release very little fixed nitrogen (57). In contrast, Nostoc in the symbiotic state releases much or most of the nitrogen it fixes, in the form of NH4+ in its symbioses with Anthoceros (118), Azolla (120), and Gunnera (169) or organic nitrogen in its symbiosis with the cycad Zamia (137). To explain this behavior, studies have focused on the presence and activities of GS, the first enzyme in the pathway of NH4+ assimilation. It may be instructive to consider the associations with Anthoceros, cycads, and Gunnera, for which it has proven possible to compare the same strain of Nostoc in its symbiotic and free-living states. These comparisons are complicated by the fact that heterocysts in free-living cultures have twice as much GS activity (191) and GS antigen (17) as do vegetative cells. Since it is not technically possible to separate heterocysts from vegetative cells in Nostoc associated with plants, activity measurements are necessarily averaged over both cell types, and cell-type-specific effects brought on by the symbiotic state could be obscured.
GS is greatly affected by the transition of Nostoc from the free-living state to association with Anthoceros. While there is no significant difference in the overall concentration of GS protein, the in vitro GS catalytic activity of symbiotic Nostoc is only 15% that of free-living cultures (86). Immunoelectronmicroscopic analyses indicated that the accumulation of GS protein in the heterocysts of symbiotic Nostoc in the Anthoceros association was lower than that in the heterocysts in the free-living growth state and equal to that in the heterocysts in vegetative cells (150). In summary, the symbiotic state with Anthoceros may lead to small differences in the level of GS protein but large differences in activity, perhaps the result of modification of the enzyme.
The lower GS activity in Nostoc in symbiosis with Anthoceros does not, however, appear to be sufficient to account for the amount of N2-derived NH4+ released. To illustrate, an acetylene reduction rate of approximately 25 nmol per min per mg of protein, as reported for the Anthoceros, cycad, and Gunnera associations (summarized in reference 116), using a 4:1 ratio of acetylene reduced to N2 reduced (179), leads to a value of 12.5 nmol of NH4+ produced per min per mg of protein. The GS specific biosynthetic activity of 19.8 nmol of NH4+ assimilated per min per mg of protein for Nostoc in the Anthoceros association (98) would be sufficient to assimilate all of the N2-derived NH4+, and yet 80% of the ammonium is released.
The Nostoc in association with cycads has a GS specific activity similar to that of free-living cultures (104), but this activity is consistent with a report that the Nostoc in this association releases organic nitrogen for use by the plant partner (137).
The release by Nostoc in association with Gunnera of 90% of its N2-derived NH4+ (169) cannot be ascribed to low GS capacity, since the symbiotic Nostoc is reported to have as much as 70% of the in vitro GS activity of free-living cultures (18). It is possible, of course, that metabolic flux through GS in vivo is low owing to substrate concentration or allosteric inhibition.
Collectively, these observations indicate that the release of fixed nitrogen by symbiotic Nostoc, depending on the association, may or may not be due in part to the low activity of GS. The crucial question arises: is it the specific Nostoc strain or the plant partner that determines the response of GS to a particular association? The only information on the subject is the observation that when N. punctiforme ATCC 29133, isolated from the cycad Macrozamina (159), is reconstituted in the Anthoceros association, its GS is now subject to the irreversible inhibition that is characteristic of the Anthoceros association (K.-Y. Lee and J. C. Meeks, unpublished results). It is unknown if Nostoc in Macrozamina, as in Zamia, has normal GS activity, but perhaps it is the plant that controls the output of fixed nitrogen. Even in Anthoceros, the enzymatic capacity of GS is only part of the story; there must be other mechanisms that contribute to the release of fixed nitrogen by Nostoc to sustain the symbiotic relationship.
Morphological Characteristics of Cyanobacteria in the Symbiotic Growth State
Rhizobia within legume root nodules differentiate into a form, bacteroids, that lies beyond the repertoire of rhizobia in their growth environment outside the plant. The morphology of Nostoc cells is also affected by associations with plants, but, unlike the case with rhizobial associations, cyanobacterial associations are typified by differences in degree rather than kind. The morphology of vegetative cells within associations is also seen outside them on occasion. Heterocysts exist, of course, in free-living cyanobacteria, but to nowhere near the frequency at which they are found in cyanobacteria associated with plants.
Morphological alteration of vegetative cells.
Vegetative cells of Nostoc within mature symbiotic cavities of Azolla (15) and other plants (116) are up to fourfold larger than the vegetative cells of free-living cyanobacteria, but the heterocysts that differentiate from these vegetative cells are also proportionally larger (Fig. 9 and 10). Moreover, the cell-cell connections of Nostoc in associations with bryophytes, cycads, and Gunnera are very fragile. The characteristic of enlarged vegetative cells and weak cell-cell connections are seen also in the aseriate stage of the Nostoc life cycle (95). Filaments enter the aseriate stage through cell divisions that occur at various angles and that depart from the transverse plane of the filament. Confinement of the filament within the semirigid sheath typical of Nostoc results in the loss of obvious filamentous structure, hence the classical reference to cells not being in a series. The degree of communication among cells in this aseriate stage is unknown. The aseriate stage is one of slow growth. The regulatory mechanisms for entry into and exit from the aseriate stage have not been studied, because this morphological form is rapidly lost during laboratory culture. Based on the similarities in growth and filamentation, it is conceivable that the symbiotic growth state is equivalent to the aseriate growth stage, with the plant partners appropriating the regulatory signals that control entry into and maintenance of this state.
FIG. 10.
Photomicrograph of Nostoc/Anabaena in association with Azolla. The bright-field image shows intact filaments, heterocyst frequency, and vegetative cell intervals. Bar, 10 μm. Reprinted from reference 140 with permission of the publisher (kindly provided by Gerald Peters).
Nothing is known of the function, if any, of this morphological change. Nevertheless, it clearly reflects an alteration in peptidoglycan organization of the Nostoc strains growing within the plant.
What are the heterocyst frequencies of symbiotically associated Nostoc?
It has proven difficult to distinguish heterocysts from vegetative cells in most symbiotically associated Nostoc, whether by bright-field or fluorescence microscopy (Fig. 9). Heterocysts can clearly be distinguished from vegetative cells by electron microscopy (EM), based on the extra wall layers and in many cases by organization of the cytoplasm in heterocysts (Fig. 11). Heterocyst frequencies derived from EM studies range from 25 to 80% of the total cells, depending on the association and maturity of the symbiotic colony (Table 3) (148). The major limitation in EM studies is visualization of a continuous filament. The aseriate morphology of the cyanobacteria in all but the Azolla association yields a filament with cells that pass into and out of a sectional plane. This limitation makes it difficult to analyze the pattern of heterocyst spacing in the symbiotic growth state; doublet heterocysts can be recognized, but identification of higher orders of contiguous heterocysts and of the numbers of vegetative cells between heterocysts is equivocal in the absence of serial sectioning.
FIG. 11.
Electron micrograph of a portion of a Nostoc colony in association with Anthoceros. There are six heterocysts (H) distinguishable from nine vegetative cells in this field of view. Cells contain carboxysomes (Cb), where Rubisco is localized, and cyanophycin granules (Cy). Bar, 10 μm. Reprinted from reference 115 with permission of the publisher (kindly provided by Yvonne Weeden-Garcia).
TABLE 3.
Parameters of heterocyst spacing in free-living and plant-associated cyanobacteriaa
| Sample | Heterocyst frequency (HT)b | Single heterocysts (h1)c | Doublet heterocysts (h2)c | Contiguity (C)d | Foci (F)e | Average interval (I)f |
|---|---|---|---|---|---|---|
| Anabaena strain PCC 7120g | 0.098 | 1.0 | 0 | 1.0 | 0.098 | 9.2 |
| Anabaena strain PCC 7120 patSg | 0.29 | 0.39 | 0.55 | 1.79 | 0.20 | 3.6 |
| N. punctiformeh | 0.087 | 0.89 | 0.06 | 1.19 | 0.081 | 11.1 |
| N. punctiforme patNh | 0.26 | 0.97 | 0.04 | 1.03 | 0.255 | 2.90 |
| Nostoc in Anthocerosh | 0.25 | 0.86 | 0.13 | 1.17 | 0.23 | 3.2 |
| Nostoc/Anabaena in Azollai | 0.26 | 0.96 | 0.04 | 1.04 | 0.25 | 3.0 |
| Nostoc in Zamia (section 1)j | 0.167 | 0.856 | 0.14 | 1.14 | 0.15 | 5.4 |
| Nostoc in Zamia (section 2)j | 0.30 | 0.62 | 0.34 | 1.3 | 0.24 | 2.9 |
| Nostoc in Zamia (section 3)j | 0.38 | 0.49 | 0.37 | 1.59 | 0.27 | 2.3 |
| Nostoc in Zamia (section 7)j | 0.45 | 0.32 | 0.46 | 1.92 | 0.28 | 2.0 |
Numbers in normal font are derived from data presented in the indicated sources. Numbers in italics are calculated from the data. For a conceptual visualization, see Fig. 12.
HT = fraction of total cells that are heterocysts.
hn = fraction of total heterocysts that are part of a string of n = 1 or more than 1 contiguous heterocysts (e.g., h1 = H1/HT).
C = contiguity, i.e., the average number of heterocysts per string = Σ(n·hn), where n is the number of contiguous heterocysts.
F = foci, i.e., the fraction of cells that are heterocysts strings, counting each string as a single entity = Σ(Hn/n), where n is the number of contiguous heterocysts. When there are only single heterocysts, F = HT.
I = ratio of vegetative cells to foci of differentiating cells = V/F = (1 − HT)/F, where V is the fraction of total cells that are vegetative cells. As the length of the filament increases, I approaches the average interval of vegetative cells intervening between heterocyst strings.
From an analysis of at least 1,000 patS cells and a unstated number of wild-type cells (data from reference 220).
From an analysis of 1,258 cells of free-living N. punctiforme, 499 cells of the patN mutant, and 761 cells of Nostoc in association with Anthoceros (from light micrographs and collages of electron micrographs, [F. C. Wong, Y. Weeden-Garcia, and J. C. Meeks, unpublished data]).
From an analysis of 172 cells of Nostoc/Anabaena in Azolla (taken from Fig. 10, which is Fig. 3 of reference 140).
From an analysis of at least 4,750 cells taken from a coralloid root of the cycad Zamia. Section 1 was closest to the tip of the root and represented the youngest part. Sections are increasingly more distant from the tip (data from reference 106).
Heterocyst frequencies in the Azolla (15, 78), Zamia (103), and Gunnera (16, 174) associations are a function of the position of, or in, the cyanobacterial colony. A developmental gradient from low to high heterocyst frequencies is clearly seen in the stem axis of Azolla and Gunnera and in the tip to the base of coralloid roots of Zamia. In Anthoceros, new symbiotic colonies form near the marginal meristem of the growing gametophyte tissue, with mature and degenerating colonies at successive distances from the margin. No specific morphological or physiological comparisons have been reported between young marginal colonies and those deep in the Anthoceros gametophyte tissue. The stem-apical meristem of Azolla contains filaments of undifferentiated Nostoc/Anabaena (our taxonomic equivocation of the cyanobacterial symbiont is given in Table 1); heterocysts appear as the filaments are enclosed within the symbiotic cavity in leaf 1 and increase in frequency with successive leaves, reaching a maximum frequency of 25 to 45% by leaves 12 to 15 (15, 78). Absolute values vary with the Azolla species and growth conditions. Doublet and triplet or higher numbered contiguous heterocysts can be observed, but they are as rare in the Azolla associations as they are in free-living cultures. Heterocyst frequencies increase from 15% at the tip of the Zamia coralloid root to 45% within 7 to 10 mm back from the tip (106). In the association with a small stoloniferous Gunnera species, colonies in glands near the stem apex contain 19% heterocysts, which increases to an average of 55% by 11 to 20 mm from the apex (16). Doublet, triplet, and higher numbered contiguous heterocysts are routinely seen in the Zamia and Gunnera associations.
Increasing nitrogenase activity parallels increasing heterocyst frequency only during the early portions of the developmental gradient in associations with Azolla (15, 78, 87), Zamia (106), and Gunnera (16, 174). The peak of nitrogenase activity consistently correlates with heterocyst frequencies between 25 and 35% in these three associations and declines as a function of distance from the stem apex or root tip, while heterocyst frequencies increase in the Zamia and Gunnera associations. At the lower heterocyst frequencies, doublets and higher-order multiple contiguous heterocysts are rare, but they increase in frequency with distance from the tip or apex conversely with the decline in nitrogenase activity until they may comprise 60% or more of the total heterocysts (174). Ultrastructure analysis in the Anthoceros association indicates that approximately 40% of the heterocysts have a degenerative cytoplasmic organization and are likely to be nonfunctional (Y. Weeden-Garcia and J. C. Meeks, unpublished results); therefore, maximal nitrogenase activity may correlate with a functional heterocyst frequency of 25 to 30% in the Anthoceros association.
The correlation of maximal nitrogenase activity with a heterocyst frequency of approximately 30% in all of the associations may represent a physiological optimum. The unique bilayered polysaccharide and glycolipid wall of the heterocyst retards the translocation of chemical substrates as well as gases; the region of transport into and out of heterocysts is the relatively small junction with adjacent vegetative cells. In the free-living state, photosynthate generated in vegetative cells is transported into heterocysts via this junction and catabolized to provide reductant (214). If reductant for heterocyst function in symbiosis is ultimately derived from the photosynthate of the plant partner, vegetative cells would provide a greater surface area to accumulate that photosynthate and perhaps be required to convert it into a form that is translocated into heterocysts. Based on source-sink considerations, in both the free-living and symbiotic growth states a heterocyst that is attached to a vegetative cell on each pole has a probability of greater reductant supply than one attached to a single vegetative cell, as is the case for doublet heterocysts and heterocysts at the ends of a filament. On average, a 30% heterocyst frequency would allow both poles of each heterocyst to be associated with a vegetative cell (e.g., two vegetative cells specifically supplying each heterocyst). The activity of heterocysts located at the center of a cluster of multiple contiguous heterocysts would be considerably lower than that of a singlet heterocyst, because it would be dependent both on the length of the diffusion pathway and on the utilization of substrate by heterocysts between it and the nearest vegetative cell.
How Plants Overcome the Normal Controls over Heterocyst Differentiation
Does the high frequency of heterocysts in plant-associated cyanobacteria result from an increased frequency of initiation at discrete sites or a lack of resolution of multiple contiguous heterocysts?
In the context of the proposed two-stage model of heterocyst differentiation and pattern formation, the high heterocyst frequencies in symbiotic associations could be the consequence of increased sites of initiation of differentiation, a decrease in the resolution of multiple contiguous heterocysts differentiating at a single site, or both. If failure to resolve strings of heterocysts is responsible, one would expect to see an increase in the average number of heterocysts per string. If there is increased initiation, one would expect to see an increased number of sites or focal points of differentiation.
An analysis of heterocyst frequency and spacing for free-living Anabaena strain PCC 7120 and N. punctiforme, both wild-type and mutant strains, and Nostoc in symbiotic associations is summarized in Table 3 and modeled in Fig. 12. Wild-type Anabaena strain PCC 7120 produces only singlet heterocysts, and so the contiguity factor (C; heterocysts per string) is 1.0 and the frequency of focal points (F) is the same as the frequency of heterocysts, about 0.10 (Table 3). Mutation of patS results in much higher values of both contiguity of heterocysts (C = 1.79) and of frequency of foci (F = 0.20). N. punctiforme grown apart from the plant behaves similar to Anabaena strain PCC 7120, with a slightly higher contiguity (C = 1.19). The N. punctiforme patN mutant has a higher frequency of foci (F = 0.255) than the patS mutant, coupled with an essentially unaltered contiguity factor.
FIG. 12.
Model illustrating heterocyst strings (C), differentiation sites (F), and vegetative cell intervals (I). The filament is shown, where green cells represent vegetative cells and yellow cells represent heterocysts. A sample calculation for each of four parameters is explained further in the text. HT is the fraction of total cells that are heterocysts (7/22 in this example). C is the contiguity, i.e., the average number of heterocysts per string (7/4 in this example). F is the fraction of cells that are heterocyst strings or foci (4/19 in this example). I is the ratio of vegetative cells to foci of differentiating cells (15/4 in this example).
Analyses of the symbiotically associated Nostoc show that the F value increases in all associations, approximately in proportion to the general increase in heterocyst frequency, up to a certain limit. However, in the Anthoceros and Azolla associations and near the tip of the Zamia coralloid root, the contiguity factor resembles that of wild-type cells and the patN mutant, but not the patS mutant with its distinctive multiple contiguous heterocysts. The multiple singular heterocyst pattern in symbiosis with low C and high F, yielding a vegetative-cell average interval of 3.0, can be visualized in the photomicrograph of the Nostoc/Anabaena in association with Azolla in Fig. 10. In the more distal and mature portions of the Zamia coralloid root, C increases reflecting the presence of multiple contiguous heterocysts.
The high C value in more mature regions of the cycad coralloid root, as well as Gunnera stems, probably does not represent a failure to resolve strings of heterocysts. If resolution were completely defective, then the contiguous heterocysts would differentiate simultaneously. Instead, the frequency of multiple heterocysts increases with distance (time) from the growing tip. Since nitrogenase activity does not follow the increase in heterocyst number and ultimately decreases, it is likely that many of the heterocysts are nonfunctional, informationally separated from the filament. Heterocysts lose function after several generations of growth in free-living culture (214), but they pinch off and separate from the filament and therefore do not appear in the usual accounting. Heterocysts in symbiotic cavities may be prevented by the matrix from separating fully from their parent filaments. A mutant A. variabilis strain has been described that produces strings of heterocysts in a sequential manner, none of which are able to fix nitrogen (40, 214).
The N. punctiforme patN mutant with a high F and unaltered C is currently being characterized, but we know that it has no significant growth defect in medium containing combined nitrogen. Therefore, we provisionally conclude that the mutation is unlikely to be in an essential cell cycle gene and that the multiple singular heterocyst phenotype may be a consequence of aberrant resolution of an initial cluster, rather than increased sites of initiation. The current evidence therefore does not distinguish between an increase in initiation of heterocyst differentiation within symbiotic cavities, relative to that seen in free-living cultures, and an incomplete resolution of an initial cluster of differentiating cells similar to the situation, perhaps, in the patN mutant. It is of clear interest to determine how association with the plant modifies cyanobacterial behavior in either way.
What is the signal(s) initiating heterocyst differentiation in the symbiotic growth state?
We have argued that initiation of heterocyst differentiation in the free-living growth state occurs when NtcA receives a signal of nitrogen limitation and elicits a sequence of transcriptional activation events in clusters of cells able to respond to the signal. We have further noted that the nature of the nitrogen status signaling system is unresolved in filamentous cyanobacteria. Hence, the way such a signaling and response system could be modified in symbiosis to yield the markedly higher heterocyst frequencies cannot be modeled. However, three lines of evidence, largely drawn from the physiological characteristics presented above, imply that the signal initiating the developmental cascade in symbiosis is independent of the nitrogen status of the Nostoc cells and may involve a symbiosis-specific signaling and sensing system (Fig. 7).
First, vegetative cells of symbiotic Nostoc in all associations exhibit the characteristics of cells growing under nitrogen excess and not nitrogen-limited conditions. Electron micrographs show the presence of carboxysomes, cyanophycin granules (Fig. 11), and phycobilisomes (150), structures that in free-living cultures grown under nitrogen starvation are consumed to provide amino acids for the synthesis of new proteins (10). Cyanophycin granules re-form in heterocysts, but phycobiliproteins and Rubisco typically remain absent. Thus, the vegetative cells that differentiate into heterocysts in symbiosis appear not to reflect a nitrogen-limited physiology. This idea is supported by the observation that heterocysts in cyanobacteria associated with Azolla (Fig. 9) (195) and perhaps Anthoceros (Fig. 9) retain phycobiliproteins. The presence of phycobiliproteins in heterocysts implies that they are not degraded in the precursor vegetative cells as they differentiate into heterocysts, perhaps because the cells do not receive a signal of nitrogen limitation.
Second, N2 fixation is uncoupled from NH4+ assimilation, indicating that heterocysts in plant-associated cyanobacteria may result from a different developmental program. The heterocysts are physiologically distinguished from those of free-living cyanobacteria by their high demand for reductant in the form of carbohydrate and their low demand for carbon skeletons, such as α-ketoglutarate, for the assimilation of NH4+. Consistent with this, levels of GS may remain equivalent to those in vegetative cells in heterocysts of symbiotically associated cyanobacteria due to the lack of a nitrogen starvation signal.
Third, high concentrations of combined nitrogen do not appear to directly repress heterocyst differentiation in symbiosis. Ammonium is excreted from the Nostoc vegetative cells in the Azolla, Anthoceros, and Gunnera associations and accumulates in the symbiotic cavities. Due to the limited permeability of the heterocyst envelope, the excess N2-derived NH4+ must be translocated from heterocysts to vegetative cells and then extracellularly into the symbiotic cavity. Thus, the vegetative cells are exposed to relatively high concentrations of both internal and external NH4+, and yet they continue to differentiate into heterocysts. Why is it, then, that exogenously supplied NH4+ or NO3+ represses heterocyst differentiation and nitrogenase expression in cyanobacteria associated with Anthoceros (49) and, to a lesser extent, Azolla (138, 139)? Analysis of a N. punctiforme mutant unable to assimilate NO3− supports the idea that repression requires the mediation of the plant partner (34). Nitrate neither permitted growth of the mutant in the free-living state nor repressed heterocyst differentiation and nitrogenase expression. When the mutant was reconstituted into the Anthoceros symbiotic association, however, NO3− repressed nitrogenase activity in a kinetic pattern similar to associations with the wild-type N. punctiforme. Control experiments and analyses eliminated reversion of the mutant to NO3− prototrophy and accumulation of NO3−-derived NH4+ as contributors to the repression.
Taken together, these results are consistent with the existence of a signal produced by the host plant that regulates the differentiation of heterocysts. The nature of the plant-derived signal and where it might interact with the developmental cascade of heterocyst differentiation is unknown. Results of analysis of N. punctiforme mutants in the Anthoceros association have established that hetR is essential for symbiotic heterocyst differentiation; therefore, the signal must enter the developmental pathway prior to hetR (217). It is likely that the symbiotic signal enters the pathway prior to activation of NtcA (Fig. 7), because NtcA appears to be essential for the transcription of genes expressed late in heterocyst maturation and for nitrogenase function (e.g., excision of the nifD element [200]). Interestingly, mutants of N. punctiforme defective in ntcA fail to infect Anthoceros tissue; therefore, their ability to respond to plant signals influencing symbiotic heterocyst differentiation cannot readily be determined (217).
CONCLUSIONS AND FUTURE DIRECTIONS
Many societies have learned to domesticate the cow. This is because it is necessary only to learn the ways of the cow, what it needs, and how to control its behavior. It is not necessary for the cow to learn each society's idiosyncrasies. We have drawn a picture of certain filamentous cyanobacteria as a cow that has been domesticated repeatedly by diverse plants. The cyanobacteria that enter into symbiotic relationships are not highly specialized for the task. Rather, the plant exploits behaviors that the cyanobacteria otherwise exhibit by themselves.
Plants have learned how to induce motility in cyanobacteria, direct the motile cyanobacteria to symbiotic cavities, and shut off the response when the filaments have achieved their final destination. Plants have also gained control over cyanobacterial growth and metabolism within the symbiotic cavities in the process of converting the cyanobacteria into NH4+-producing factories.
We are only just beginning to figure out how plants achieve their control over cyanobacteria. If we understand how they do it, we may then understand the fundamental mechanisms governing cyanobacterial differentiation. We may also perceive how to extend the abilities possessed by plants capable of symbiosis to crop plants that are not capable of symbiosis. Identification of the regulatory circuits in Anabaena and Nostoc that are involved in cellular differentiation and those that are appropriated by the plant partners will soon be approachable by global genomic and proteomic analyses, following completion of the genomic sequences of N. punctiforme ATCC 29133 (accessible at http://www.jgi.doe.gov/) and Anabaena strain PCC 7120 (accessible at http://www.kazusa.or.jp/cyano).
Acknowledgments
We thank Francis Wong for his thorough and creative analysis of heterocyst frequencies and spacing in symbiosis, Yvonne Weeden-Garcia for electron microscopy, Gerald A. Peters and Warwick B. Silvester for generous contributions of photomicrographs, Jim Golden and Peter Wolk for key discussions and other interactions, and Elsie Campbell, Kari Hagen, John L. Ingraham, and Francis Wong for critical reviews of the manuscript.
Work in the laboratory of J.C.M. is supported by the NSF (grants IBN 95-14787, 00-80805, and MCB 96-04270) and the USDA (grant NRICGP 98-35305-6748), and work in the laboratory of J.E. is supported by the DOE (grant DE-FG02-97ER12211) and the NSF (grant IBN 99-75002).
REFERENCES
- 1.Adams, D. G. 1992. The effect of DL-7-azatryptophan on heterocyst development in the cyanobacterium Anabaena cylindrica. J. Gen. Microbiol. 138:355-362. [Google Scholar]
- 2.Adams, D. G. 2000. Heterocyst formation in cyanobacteria. Curr. Opin. Microbiol. 3:618-624. [DOI] [PubMed] [Google Scholar]
- 3.Adams, D. G. 2000. Symbiotic internactions, p. 523-561. In B. A. Whitton and M. Potts (ed.), The ecology of cyanobacteria. Their diversity in time and space. Kluwer Academic Publishers, Boston, Mass.
- 4.Adams, D. G., and N. G. Carr. 1981. Heterocyst differentiation and cell division in the cyanobacterium Anabaena cylindrica: effect of high light intensity. J. Cell Sci. 49:341-352. [DOI] [PubMed] [Google Scholar]
- 5.Adams, D. G., and N. G. Carr. 1989. Control of heterocyst development in the cyanobacterium Anabaena cylindrica. J. Gen. Microbiol. 135:839-849. [Google Scholar]
- 6.Adams, D. G., and P. S. Duggan. 1999. Heterocyst and akinete differentiation in cyanobacteria. New Phytol. 144:3-33. [Google Scholar]
- 7.Adams, T. H., M. T. Boylan, and W. E. Timberlake. 1988. brlA is necessary and sufficient to direct conidiophore development in Aspergillis nidulans. Cell 54:353-362. [DOI] [PubMed] [Google Scholar]
- 8.Ahmadjian, V. 1992. Basic mechanisms of signal exchange, recognition and regulation in lichens, p. 675-697. In W. Reisser (ed.), Algae and symbioses. Biopress Ltd., Bristol, United Kingdom.
- 9.Allen, E. R., C. Orrego, H. Wabiko, and E. Freese. 1986. An ethA mutation in Bacillus subtilis 168 permits induction of sporulation by ethionine and increases DNA modification of bacteriophage φ105. J. Bacteriol. 166:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allen, M. M. 1984. Cyanobacterial cell inclusions. Annu. Rev. Microbiol. 38:1-25. [DOI] [PubMed] [Google Scholar]
- 11.Arcondeguy, T., R. Jack, and M. Merrick. 2001. PII signal transduction proteins, pivotal players in microbial nitrogen control. Microbiol. Mol. Biol. Rev. 65:8-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Armstrong, R. E., P. K. Hayes, and A. E. Walsby. 1983. Gas vacuole formation in hormogonia of Nostoc muscorum. J. Gen. Microbiol. 128:263-270. [Google Scholar]
- 13.Bauer, C. C., K. S. Ramaswamy, S. Endley, L. A. Scappino, J. W. Golden, and R. Haselkorn. 1997. Suppression of heterocyst differentiation in Anabaena PCC 7120 by a cosmid carrying wild-type genes encoding enzymes for fatty acid synthesis. FEMS Microbiol. Lett. 151:23-30. [DOI] [PubMed] [Google Scholar]
- 14.Becking, T. H. 1992. The Rhizobium symbiosis of the nonlegume Parasponia, p. 497-559.In G. A. Stacey, R. H. Burris, and H. J. Evans (ed.), Biological nitrogen fixation. Chapman & Hall, New York, N.Y.
- 15.Becking, T. H., and M. Donze. 1981. Pigment distribution and nitrogen fixation in Anabaena azollae. Plant Soil 61:203-226. [Google Scholar]
- 16.Bergman, B., C. Johansson, and E. Soderback. 1992. The Nostoc-Gunnera symbiosis. New Phytol. 122:379-400. [DOI] [PubMed] [Google Scholar]
- 17.Bergman B., P. Lindblad, A. Pettersson, E. Renstrom, and E. Tiberg. 1985. Immunogold localization of glutamine synthetase in a nitrogen fixing cyanobacterium (Anabaena cylindrica). Planta 166:329-334. [DOI] [PubMed] [Google Scholar]
- 18.Bergman, B., A. N. Rai, C. Johansson, and E. Soderback. 1992. Cyanobacterial-plant symbioses. Symbiosis 14:61-81. [Google Scholar]
- 19.Black, T. A., and C. P. Wolk. 1994. Analysis of a Het− mutation in Anabaena sp. strain PCC 7120 implicates a secondary metabolite in the regulation of heterocyst spacing. J. Bacteriol. 176:2282-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Black, T. A., Y. Cai, and C. P. Wolk. 1993. Spatial expression and autoregulation of hetR, a gene involved in the control of heterocyst development in Anabaena. Mol. Microbiol. 9:77-84. [DOI] [PubMed] [Google Scholar]
- 21.Bogorad, L. 1975. Phycobiliproteins and complementary chromatic adaptation. Annu. Rev. Plant Physiol. 26:369-401. [Google Scholar]
- 22.Bonnett, H. T., and W. B. Silvester. 1981. Specificity in the Gunnera-Nostoc endosymbiosis. New Phytol. 89:121-128. [Google Scholar]
- 23.Bradley, S., and N. G. Carr. 1976. Heterocyst and nitrogenase development in Anabaena cylindrica. J. Gen. Microbiol. 96:175-184. [DOI] [PubMed] [Google Scholar]
- 24.Brahamsha, B., and R. Haselkorn. 1991. Isolation and characterization of the gene encoding the primary sigma factor of the vegetative cell RNA polymerase from the cyanobacterium Anabaena sp. strain PCC 7120. J. Bacteriol. 173:2442-2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brahamsha, B., and R. Haselkorn. 1992. Identification of multiple RNA polymerase sigma factor homologs in the cyanobacterium Anabaena sp. strain PCC 7120: cloning, expression, and inactivation of the sigB and sigC genes. J. Bacteriol. 174:7273-7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Braun-Howland E. B., and S. A. Nierzwicki-Bauer. 1990. Azolla-Anabaena symbiosis: biochemistry, physiology, ultrastructure, and molecular biology, p. 65-119. In A. N. Rai (ed.), Handbook of symbiotic cyanobacteria. CRC Press, Inc., Boca Raton, Fla.
- 27.Buikema, W. J., and R. Haselkorn. 1991. Characterization of a gene controlling heterocyst differentiation in the cyanobacterium Anabaena 7120. Genes Dev. 5:321-330. [DOI] [PubMed] [Google Scholar]
- 28.Buikema, W. J., and R. Haselkorn. 2001. Expression of the Anabaena hetR gene from a copper-regulated promoter leads to heterocyst differentiation under repressing conditions. Proc. Natl. Acad. Sci. USA 98:2729-2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cai, Y., and C. P. Wolk. 1997. Anabaena sp. strain PCC 7120 responds to nitrogen deprivation with a cascade-like sequence of transcriptional activations. J. Bacteriol. 179:267-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Callahan, S. M., and W. J. Buikema. 2001. The role of HetN in maintenance of the heterocyst pattern in Anabaena sp. PCC 7120. Mol. Microbiol. 40:941-950. [DOI] [PubMed] [Google Scholar]
- 31.Campbell, D., J. Houmard, and N. Tandeau de Marsac. 1993. Electron transport regulates cellular differentiation in the filamentous cyanobacterium Calothrix. Plant Cell 5:451-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campbell, E. L., B. Brahamsha, and J. C. Meeks. 1998. Mutation of an alternative sigma factor in the cyanobacterium Nostoc punctiforme results in increased infection of its symbiotic plant partner, Anthoceros punctatus. J. Bacteriol. 180:4938-4941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Campbell, E. L., and J. C. Meeks. 1989. Characteristics of hormogonia formation by symbiotic Nostoc spp. in response to the presence of Anthoceros punctatus or its extracellular products. Appl. Environ. Microbiol. 55:125-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Campbell, E. L., and J. C. Meeks. 1992. Evidence for plant-mediated regulation of nitrogenase expression in the Anthoceros-Nostoc symbiotic association. J. Gen. Microbiol. 138:473-480. [Google Scholar]
- 35.Canini, A., and M. Grilli Caiola. 1993. Characterization of gonidial zone of Cycas revoluta coralloid roots by means of microelectrodes. FEMS Microbiol. Lett. 109:75-80. [Google Scholar]
- 36.Canini, A., M. Grilli Caiola, and M. Mascini. 1990. Ammonium content, nitrogenase activity, and heterocyst frequency within Azolla filiculoides Lam. FEMS Microbiol. Lett. 71:205-210. [Google Scholar]
- 37.Carrasco, C. D., J. A. Buettner, and J. W. Golden. 1995. Programmed DNA rearrangement of a cyanobacterial hupL gene in heterocysts. Proc. Natl. Acad. Sci. USA 92:791-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen, C., C. van Baalen, and F. R. Tabita. 1987. Nitrogen starvation mediated by dl-7-azatryptophan in the cyanobacterium Anabaena sp. strain CA. J. Bacteriol. 169:1107-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cohen, M. F., and J. C. Meeks. 1997. A hormogonium regulating locus, hrmUA, of the cyanobacterium Nostoc punctiforme strain ATCC 29133 and its response to an extract of a symbiotic plant partner Anthoceros punctatus. Mol. Plant-Microbe Interact. 10:280-289. [DOI] [PubMed] [Google Scholar]
- 40.Currier, T. C., J. F. Haury, and C. P. Wolk. 1977. Isolation and preliminary characterization of auxotrophs of a filamentous cyanobacterium. J. Bacteriol. 129:1556-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Curtis, S. E., and J. A. Martin. 1994. The transcription apparatus and the regulation of transcription initiation, p. 613-639. In D. A. Bryant, (ed.), The molecular biology of cyanobacteria. Kluwer Academic Publishers, Boston, Mass.
- 42.Damerval, T., G. Guglielmi, J. Houmard, and N. Tandeau de Marsac. 1991. Hormogonium differentiation in the cyanobacterium Calothrix: a photoregulated developmental process. Plant Cell 3:191-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Denarie, J., F. Drebelle, and C. Rosenburg. 1992. Signaling and host range variation in nodulation. Annu. Rev. Microbiol. 46:497-531. [DOI] [PubMed] [Google Scholar]
- 44.Dong, Y., X. Huang, X.-Y. Wu, and J. Zhao. 2000. Identification of the active site of HetR protease and its requirement for heterocyst differentiation in the cyanobacterium Anabaena sp. strain PCC 7120. J. Bacteriol. 182:1575-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Douglas, D., A. Peat, B. A. Whitton, and P. Wood. 1986. Influence of iron status on structure of the cyanobacterium Calothrix 7601. Gene 54:83-92. [Google Scholar]
- 46.Drews, G., and J. Weckesser. 1982. Function, structure and composition of cell walls and external layers, p. 333-357. In N. G. Carr and B. A. Whitton (ed.), The biology of cyanobacteria. Blackwell Scientific Publications, Ltd., Oxford, United Kingdom.
- 47.Dworkin, M. 1996. Recent advances in the social and developmental biology of the myxobacteria. Microbiol. Rev. 60:70-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Elhai, J., and C. P. Wolk. 1990. Developmental regulation and spatial pattern of expression of the structural genes for nitrogenase in the cyanobacterium Anabaena. EMBO J. 9:3379-3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Enderlin, C. S., and J. C. Meeks. 1983. Pure culture and reconstitution of the Anthoceros-Nostoc symbiotic association. Planta 158:157-165. [DOI] [PubMed] [Google Scholar]
- 50.Ernst, A., S. Reich, and P. Boger. 1990. Modification of dinitrogenase reductase in the cyanobacterium Anabaena variabilis due to C starvation and ammonia. J. Bacteriol. 172:748-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fath, M. J., and R. Kolter. 1993. ABC transporters: bacterial exporters. Microbiol. Rev. 57:995-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fay, P. 1992. Oxygen relations of nitrogen fixation in cyanobacteria. Microbiol. Rev. 56:340-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fernandez-Pinas, F., F. Leganes, and C. P. Wolk. 1994. A third genetic locus required for the formation of heterocysts in Anabaena sp. strain PCC 7120. J. Bacteriol. 176:5277-5283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fiedler, G., M. Arnold, S. Hannus, and I. Maldener. 1998. The DevBCA exporter is essential for envelope formation in heterocysts of the cyanobacterium Anabaena sp. strain PCC 7120. Mol. Microbiol. 27:1193-1202. [DOI] [PubMed] [Google Scholar]
- 55.Floriano, B., A. Herrero, and E. Flores. 1994. Analysis of expression of the argC and argD genes in the cyanobacterium Anabaena sp. strain PCC 7120. J. Bacteriol. 176:6397-6401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Flores, E., and A. Herrero. 1994. Assimilatory nitrogen metabolism and its regulation, p.487-517. In D. A. Bryant (ed.). The molecular biology of cyanobacteria. Kluwer Academic Publishers, Boston, Mass.
- 57.Fogg, G. E. 1952. The production of extracellular nitrogenous substances by a blue-green alga. Proc. R. Soc. London Ser. B 139:372-397. [DOI] [PubMed] [Google Scholar]
- 58.Forchhammer, K., and N. Tandeau de Marsac. 1994. The PII protein in the cyanobacterium Synechococcus sp. strain PCC 7942 is modified by serine phosphorylation and signals the cellular N status. J. Bacteriol. 176:84-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Forchhammer, K., and N. Tandeau de Marsac. 1995. Functional analysis of the phosphoprotein PII (glnB gene product) in the cyanobacterium Synechococcus sp. strain PCC 7942. J. Bacteriol. 177:2033-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Forni, C., E. Tel-Or, E. Bar, and M. Grilli Caiola. 1991. Effects of antibiotic treatments on Azolla-Anabaena and Arthrobacter. Plant Soil 137:151-155. [Google Scholar]
- 61.Frias, J. E., E. Flores, and A. Herrero. 1994. Requirement of the regulatory protein NtcA for expression of nitrogen assimilation and heterocyst development genes in the cyanobacterium Anabaena sp. PCC 7120. Mol. Microbiol. 14:823-832. [DOI] [PubMed] [Google Scholar]
- 62.Fuqua, W. C., S. C. Winans, and E.P. Greenberg. 1994. Quorum sensing in bacteria: the LuxR-LuxI family of cell density-response transcriptional regulators. J. Bacteriol. 176:269-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gantar, M., N. W. Kerby, and P. Rowell. 1993. Colonization of wheat (Triticum vulgare L.) by N2-fixing cyanobacteria. III. The role of a hormogonia-promoting factor. New Phytol. 124:505-513. [DOI] [PubMed] [Google Scholar]
- 64.Gantar, M., N. W. Kerby, P. Rowell, Z. Obreht, and C. Scrimgeour. 1995. Colonization of wheat (Triticum vulgare L.) by N2-fixing cyanobacteria. IV. Dark nitrogenase activity and effect of cyanobacteria on natural 15N abundance in the plants. New Phytol. 129:337-343. [DOI] [PubMed] [Google Scholar]
- 65.Garcia-Dominquez, M., and F. Florencio. 1997. Nitrogen availability and electron transport control the expression of glnB genes (encoding PII protein) in the cyanobacterium Synechococystis sp. PCC 6803. Plant Mol. Biol. 35:723-734. [DOI] [PubMed] [Google Scholar]
- 66.Golden, J. W., C. D. Carrasco, M. E. Mulligan, G. J. Schneider, and R. Haselkorn. 1988. Deletion of a 55-kilobase-pair DNA element from the chromosome during heterocyst differentiation of Anabaena sp. strain PCC 7120. J. Bacteriol. 170:5034-5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Golden, J. W., S. J. Robinson, and R. Haselkorn. 1985. Rearrangement of nitrogen fixation genes during heterocyst differentiation in the cyanobacterium Anabaena. Nature 314:419-423. [DOI] [PubMed] [Google Scholar]
- 68.Grilli Caiola, M., A. Canini, and D. Moscone. 1989. Oxygen concentration, nitrogenase activity and heterocyst frequency in the leaf cavities of Azolla filiculoides Lam. FEMS Microbiol. Lett. 59:283-288. [Google Scholar]
- 69.Grossman, A. R., M. R. Schaefer, G. G. Chiang, and J. L. Collier. 1994. The responses of cyanobacteria to environmental conditions: light and nutrients, p. 641-675. In D. A. Bryant (ed.), The molecular biology of cyanobacteria. Kluwer Academic Publishers, Boston, Mass.
- 70.Hanson, T. E., K. Forchhammer, N. Tandeau de Marsac, and J. C. Meeks. 1998. Characterization of the glnB gene product of Nostoc punctiforme strain ATCC 29133:glnB or the PII protein may be essential. Microbiology 144:1537-1547. [DOI] [PubMed] [Google Scholar]
- 71.Hardman, A., G. S. Stewart, and P. Williams. 1998. Quorum sensing and the cell-cell communication-dependent regulation of gene expression in pathogenic and nonpathogenic bacteria. Antonie Leeuwenhoek 74:188-210. [DOI] [PubMed] [Google Scholar]
- 72.Haselkorn, R. 1992. Developmentally regulated gene rearrangements in prokaryotes. Annu. Rev. Genet. 26:113-130. [DOI] [PubMed] [Google Scholar]
- 73.Haselkorn, R., D. Rice, S. E. Curtis, and S. J. Robinson. 1983. Organization and transcription of genes important in Anabaena heterocyst differentiation. Ann. Microbiol. (Inst. Pasteur) 134B:181-193. [DOI] [PubMed]
- 74.Herdman, M., M. Janvier, R. Rippka, and R. Y. Stanier. 1977. Genome size of cyanobacteria. J. Gen. Microbiol. 111:73-85. [Google Scholar]
- 75.Herdman, M., and R. Rippka. 1988. Cellular differentiation: hormogonia and baeocytes. Methods Enzymol. 167:232-242. [Google Scholar]
- 76.Hernandez-Muniz, W., and S. E. Stevens, Jr. 1987. Characterization of the motile hormogonia of Mastigocladus laminosus. J. Bacteriol. 169:218-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Herrero, A., A. M. Muro-Pastor, and E. Flores. 2001. Nitrogen control in cyanobacteria. J. Bacteriol. 183:411-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hill, D. J. 1975. The pattern of development of Anabaena in the Azolla-Anabaena symbiosis. Planta 122:179-184. [DOI] [PubMed] [Google Scholar]
- 79.Hill, D. R., T. J. Belbin, M. V. Thorsteinsson, D. Bassam, S. Brass, S. Ernst, P. Boeger, H. Paerl, M. E. Mulligan, and M. Potts. 1996. GlbN (cyanoglobin) is a peripheral membrane protein that is restricted to certain Nostoc spp. J. Bacteriol. 178:6587-6598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hirose, M. 1987. Energy supply system for the gliding movement of hormogonia of the cyanobacterium Nostoc cycadae. Plant Cell Physiol. 28:587-597. [Google Scholar]
- 81.Hoiczyk, E. 2000. Gliding motility in cyanobacteria: observations and possible explanations. Arch Microbiol. 174:11-17. [DOI] [PubMed] [Google Scholar]
- 82.Holland, D., and C. P. Wolk. 1990. Identification and characterization of hetA, a gene that acts early in the process of morphological differentiation of heterocysats. J. Bacteriol. 172:3131-3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Irmler, A., S. Sanner, H. Dierks, and K. Forchhammer. 1997. Dephosphorylation of the phosphoprotein PII in Synechococcus PCC 7942: identification of an ATP and 2-oxoglutarate-regulated phosphatase activity. Mol. Microbiol. 26:81-90. [DOI] [PubMed] [Google Scholar]
- 84.Johansson, C., and B. Bergman. 1992. Early events during the establishment of the Gunnera/Nostoc symbiosis. Planta 188:403-413. [DOI] [PubMed] [Google Scholar]
- 85.Johansson, C., and B. Bergman. 1994. Reconstitution of the symbiosis of Gunnera manicata Linden: cyanobacterial specificity. New Phytol. 126:643-652. [Google Scholar]
- 86.Joseph, C. M., and J. C. Meeks. 1987. Regulation of expression of glutamine synthetase in a symbiotic Nostoc strain associated with Anthoceros punctatus. J. Bacteriol. 169:2471-2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kaplan, D., H. E. Calvert, and G. A. Peters. 1986. The Azolla-Anabaena azollae relationship. XII. Nitrogenase activity and phycobiliproteins of the endophyte as a function of leaf age and cell type. Plant Physiol. 80:884-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kaplan, D., and G. A. Peters. 1988. Interaction of carbon metabolism in the Azolla-Anabaena symbiosis. Symbiosis 6:53-68. [Google Scholar]
- 88a.Khudyakov, I. Y., and J. W. Golden. 2001. Identification and inactivation of three group 2 sigma factor genes in Anabaena sp. strain PCC 7120. J. Bacteriol. 183:6667-6675. [DOI] [PMC free article] [PubMed]
- 89.Khudyakov, I., and C. P. Wolk. 1996. Evidence that the hanA gene coding for HU protein is essential for heterocyst differentiation in, and cyanophage A-4(L) sensitivity of, Anabaena sp. strain PCC 7120. J. Bacteriol. 178:3572-3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Khudyakov, I., and C. P. Wolk. 1997. hetC, a gene coding for a protein similar to bacterial ABC protein exporters, is involved in early regulation of heterocyst differentiation in Anabaena sp. strain PCC 7120. J. Bacteriol. 179:6971-6978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Knight, C. D., and D. G. Adams. 1996. A method for studying chemotaxis in nitrogen fixing cyanobacterium-plant symbioses. Physiol. Mol. Plant Pathol. 49:73-77. [Google Scholar]
- 92.Kroos, L., B. Kunkel, and R. Losick. 1989. Switch protein alters specificity of RNA polymerase containing a compartment-specific sigma factor. Science 243:526-529. [DOI] [PubMed] [Google Scholar]
- 93.Kumar, A., and H. D. Kumar. 1980. Differential effects of amino acid analogs on growth and heterocyst differentiation in two nitrogen fixing blue-green algae. Curr. Microbiol. 3:213-218. [DOI] [PubMed] [Google Scholar]
- 94.Lang, J. D., and R. Haselkorn. 1991. A vector for analysis of promoters in the cyanobacterium Anabaena sp. strain PCC 7120. J. Bacteriol. 173:2729-2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lazaroff, N. 1973. Photomorphogenesis and Nostocacean development, p. 279-319. In N. G. Carr and B. A. Whitton (ed.), The biology of blue-green algae. Blackwell Scientific Publications, Ltd., Oxford, United Kingdom.
- 96.Lee, H. M., E. Flores, K. Forchhammer, A. Herrero, and N. Tandeau de Marsac. 2000. Phosphorylation of the signal transducer protein PII and an additional effector are required for the PII-mediated regulation of nitrate and nitrite uptake in the cyanobacterium Synechococcus sp. PCC 7942. Eur. J. Biochem. 267:591-600. [DOI] [PubMed] [Google Scholar]
- 97.Lee, H. M., M. F. Vasquez-Bermudez, and N. Tandeau de Marsac. 1999. The global nitogen regulator NtcA regulates transcription of the signal transducer PII (GlnB) and influences its phosphorylation level in response to nitrogen and carbon supplies in the cyanobacterium Synechococcus sp. strain PCC 7942. J. Bacteriol. 181:2697-2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee, K. Y., C. M. Joseph, and J. C. Meeks. 1988. Glutamine synthetase specific activity and protein concentration in symbiotic Anabaena associated with Azolla caroliniana. Antonie Leeuwenhoek 54:345-355. [DOI] [PubMed] [Google Scholar]
- 99.Leganes, F., F. Fernandez-Piñas, and C. P. Wolk. 1994. Two mutations that block heterocyst differentiation have different effects on akinete differentiation in Nostoc ellipsosporum. Mol. Microbiol. 12:679-684. [DOI] [PubMed] [Google Scholar]
- 100.Liang, J., L. Scappino, and R. Haselkorn. 1992. The patA gene product, which contains a region similar to cheY of Escherichia coli, controls heterocyst pattern formation in the cyanobacterium Anabaena 7120. Proc. Natl. Acad. Sci. USA 89:5655-5659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liang, J., L. Scappino, and R. Haselkorn. 1993. The patB gene product, required for growth of the cyanobacterium Anabaena sp. strain PCC 7120 under nitrogen-limiting conditions, contains ferredoxin and helix-turn-helix domains. J. Bacteriol. 175:1697-1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lin, C. C. 1996. Dissimilatory pathways for sugars, polyols, and carboxylates, p. 307-342. In F. C. Neidhardt et al. (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, D.C.
- 103.Lindblad, P., and B. Bergman. 1985. The cyanobacterium-Zamia symbiosis: an ultrastructural study. New Phytol. 101:707-716. [Google Scholar]
- 104.Lindblad, P., and B. Bergman. 1986. Glutamine synthetase: activity and localization in cyanobacteria of the cycads Cycas revoluta and Zamia skinneri. Planta 169:1-7. [DOI] [PubMed] [Google Scholar]
- 105.Lindblad, P., and B. Bergman. 1990. The cycad-cyanobacterial symbiosis, p. 136-159. In A. N. Rai (ed.), Handbook of symbiotic cyanobacteria. CRC Press, Inc., Boca Raton, Fla.
- 106.Lindblad, P., L. Hallbom, and B. Bergman. 1985. The cyanobacterium-Zamia symbiosis: C2H2-reduction and heterocyst frequency. Symbiosis 1:19-28. [Google Scholar]
- 107.Lindblad, P., A. N. Rai, and B. Bergman. 1987. The Cycas revoluta-Nostoc symbiosis: enzyme activities of nitrogen and carbon metabolism in the cyanobiont. J. Gen. Microbiol. 133:1695-1699. [Google Scholar]
- 108.Liotenberg, S., D. Campbell, A.-M. Castets, J. Houmard, and N. Tandeau de Marsac. 1996. Modification of the PII protein in response to carbon and nitrogen availability in filamentous cyanobacteria. FEMS Microbiol. Lett. 144:185-190. [Google Scholar]
- 109.Lockau, W., R. B. Peterson, C. P. Wolk, and R. H. Burris. 1978. Modes of reduction of nitrogenase in heterocysts isolated from Anabaena species. Biochim. Biophys. Acta 502:298-308. [DOI] [PubMed] [Google Scholar]
- 110.Luque, I., E. Flores, and A. Herrero. 1994. Molecular mechanisms of the operation of nitrogen control in cyanobacteria. EMBO J. 13:2862-2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mackerras, A. H., N. M. de Chazal, and G. D. Smith. 1990. Transient accumulations of cyanophycin in Anabaena cylindrica and Synechocystis 6803. J. Gen. Microbiol. 136:2057-2065. [Google Scholar]
- 112.Maldener, I., G. Fiedler, A. Ernst, F. Fernandez-Pinas and C. P. Wolk. 1994. Characterization of devA, a gene required for the maturation of proheterocysts in the cyanobacterium Anabaena sp. strain PCC 7120. J. Bacteriol. 176:7543-7549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Martin-Figueroa, E., F. Navarro, and F. J. Florencio. 2000. The GS-GOGAT pathway is not operative in the heterocyst. Cloning and expression of glsF gene from the cyanobacterium Anabaena sp. PCC 7120. FEBS Lett. 476:282-286. [DOI] [PubMed] [Google Scholar]
- 114.Matveyev, A. V., E. Rutgers, E. Soderback, and B. Bergman. 1994. A novel genome rearrangement involved in heterocyst differentiation of the cyanobacteirum Anabaena sp. PCC 7120. FEMS Microbiol. Lett. 116:210-208. [Google Scholar]
- 115.Meeks, J. C. 1990. Cyanobacterial-bryophyte associations, p. 43-63. In A. N. Rai (ed.), Handbook of symbiotic cyanobacteria. CRC Press, Inc., Boca Raton, Fla.
- 116.Meeks, J. C. 1998. Symbiosis between nitrogen-fixing cyanobacteria and plants. BioScience 48:266-276. [Google Scholar]
- 117.Meeks, J. C., E. L. Campbell, K. Hagen, T. Hanson, N. Hitzeman, and F. Wong. 1999. Developmental alternatives of symbiotic Nostoc punctiforme in response to its plant partner Anthoceros punctatus, p. 665-678. In G. A. Peschek, W. Loeffelhardt, and G. Schmettterer (ed.), The phototrophic prokaryotes. Plenum Publishing Corp., New York, N.Y.
- 118.Meeks, J. C., C. S. Enderlin, C. M. Joseph, J. S. Chapman, and M. W. L. Lollar. 1985. Fixation of [13N]N2 and transfer of fixed nitrogen in the Anthoceros-Nostoc symbiotic association. Planta 164:406-414. [DOI] [PubMed] [Google Scholar]
- 119.Meeks, J. C., C. M. Joseph, and R. Haselkorn. 1988. Organization of the nif genes in cyanobacteria in symbiotic association with Azolla and Anthoceros. Arch. Microbiol. 150:61-71. [DOI] [PubMed] [Google Scholar]
- 120.Meeks, J. C., N. A. Steinberg, C. S. Enderlin, C. M. Joseph, and G. A. Peters. 1987. Azolla-Anabaena relationship. XIII. Fixation of [13N]N2. Plant Physiol. 84:883-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Meeks, J. C., C. P. Wolk, J. Thomas, W. Lockau, P. W. Shaffer, S. M. Austin, W.-S. Chien, and A. Galonsky. 1977. The pathways of assimilation of 13NH4+ by the cyanobacterium Anabaena cylindrica. J. Biol. Chem. 252:7894-7900. [PubMed] [Google Scholar]
- 122.Meeks, J. C., K. L. Wycoff, J. S. Chapman, and C. S. Enderlin. 1983. Regulation of expression of nitrate and dinitrogen assimilation by Anabaena species. Appl. Environ. Microbiol. 45:1351-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Meinhardt, H. 1994. Biological pattern formation: New observations provide support for theoretical predictions. Bioessays 16:827-632. [DOI] [PubMed] [Google Scholar]
- 124.Merrick, M. J., and R. A. Edwards. 1995. Nitrogen control in bacteria. Microbiol. Rev. 59:604-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mitchison, G. J., and M. Wilcox. 1972. Rule governing cell division in Anabaena. Nature 239:110-111. [Google Scholar]
- 126.Mitchison, G. J., and M. Wilcox. 1973. Alteration in heterocyst pattern of Anabaena produced by 7-azatryptophan. Nat. New Biol. 246:229-233 [DOI] [PubMed] [Google Scholar]
- 127.Mitchison, G. J., M. Wilcox, and R. J. Smith. 1976. Measurement of an inhibitory zone. Science 191:866-868. [DOI] [PubMed] [Google Scholar]
- 128.Muro-Pastor, A. M., A. Valladares, E. Flores, and A. Herrero. 1999. The hetC gene is a direct target of the NtcA transcriptional regulator in cyanobacterial heterocyst development. J. Bacteriol. 181:6664-6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Muro-Pastor, M. I., and F. J. Florencio. 1994. NADP+-isocitrate dehydrogenase from the cyanobacterium Anabaena sp. strain PCC 7120: purification and characterization of the enzyme and cloning, sequencing, and disruption of the icd gene. J. Bacteriol. 176:2718-2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Murry, M. A., and C. P. Wolk. 1989. Evidence that the barrier to the penetration of oxygen into heterocysts depends upon two layers of the cell envelope. Arch. Microbiol. 151:469-474. [Google Scholar]
- 131.Murry, M. A., P. C. Hallenbeck, D. Esteva, and J. R. Benemann. 1983. Nitrogenase inactivation by oxygen and enzyme turnover in Anabaena cylindrica. Can. J. Microbiol. 29:1286-1294. [Google Scholar]
- 132.Nagaraja, R., and R. Haselkorn. 1994. Protein HU from the cyanobacterium Anabaena. Biochemie 76:1082-1089. [DOI] [PubMed] [Google Scholar]
- 133.Nathanielsz, C. O., and I. A. Staff. 1975. A mode of entry of blue-green algae into the apogeotrophic roots of Macrozamia communis. Am. J. Bot. 62:232-235. [Google Scholar]
- 134.Obrecht, Z., N. W. Kerby, M. Gantar, and P. Rowell. 1993. Effects of root-associated N2-fixing cyanobacteria on the growth and nitrogen content of wheat (Triticum vulgare L.) seedlings. Biol. Fertil. Soils 15:68-72. [Google Scholar]
- 135.Ow, M. C., M. Gantar, and J. Elhai. 1999. Reconstitution of a cycad-cyanobacterial association. Symbiosis 27:125-134. [Google Scholar]
- 136.Papen, H., G. Neuer, M. Refaian, and H. Bothe. 1983. The isocitrate dehydrogenase from cyanobacteria. Arch. Microbiol. 134:73-79. [DOI] [PubMed] [Google Scholar]
- 137.Pate, J. S., P. Lindblad, and C. A. Atkins. 1988. Pathways of assimilation and transfer of fixed nitrogen in coralloid roots of cycad-Nostoc symbioses. Planta 176:461-471. [DOI] [PubMed] [Google Scholar]
- 138.Peters, G. A., H. E. Calvert, D. Kaplan, O. Ito, and R. E. Toia, Jr. 1982. The Azolla-Anabaena symbiosis: morphology, physiology and use. Isr. J. Bot. 31:305-323. [Google Scholar]
- 139.Peters, G. A., O. Ito, V. V. S. Tyagi, and D. Kaplan. 1981. Physiological studies on N2-fixing Azolla, p. 343-362. In J. M. Lyons, R. C. Valentine, D. A. Phillips, D. W. Rains, and R. C. Huffaker (ed.), Genetic engineering of symbiotic nitrogen fixation and conservation of fixed nitrogen. Plenum Publishing Corp., New York, N.Y.
- 140.Peters, G. A., and B. C. Mayne. 1974. The Azolla-Anabaena azollae relationship. I. Initial characterization of the association. Plant Physiol. 53:813-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Peters, G. A., and J. C. Meeks. 1989. The Azolla-Anabaena symbiosis: basic biology. Annu. Rev. Plant Physiol. Plant Mol. Biol. 40:193-210. [Google Scholar]
- 142.Peters, G. A, T. B., Ray, B. C. Mayne, and R. E. Toia, Jr. 1980. Azolla-Anabaena association: morphological and physiological studies, p. 293-309. In W. E. Newton and W. H. Orme-Johnson (ed.), Nitrogen fixation, vol. II. University Park Press, Baltimore, Md. [Google Scholar]
- 143.Peters, G. A., R. E. Toia, Jr., D. Raveeed, and N. J. Levine. 1978. The Azolla-Anabaena azollae relationship. VI. Morphological aspects of the association. New Phytol. 80:583-593. [Google Scholar]
- 144.Peterson, R. B., and R. H. Burris. 1976. Properties of heterocysts isolated with colloidial silica. Arch. Microbiol. 108:35-40. [DOI] [PubMed] [Google Scholar]
- 145.Peterson, R. B., E. R. Shaw, E. Dolan, and B. Ke. 1981. A photochemically active heterocyst preparation from Anabaena variabilis. Photochem. Photobiophys. 2:79-84. [Google Scholar]
- 146.Porchia, A. C., and G. L. Salerno. 1996. Sucrose biosynthesis in a prokaryotic organism: presence of two sucrose-phosphate synthases in Anabaena with remarkable differences compared with the plant enzymes. Proc. Natl. Acad. Sci. USA 93:13600-13604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ptashne, M. 1992. A genetic switch, 2nd ed. Blackwell Scientific Publications, Ltd., Oxford, United Kingdom.
- 148.Rai, A. N. 1990. Handbook of symbiotic cyanobacteria. CRC Press, Inc., Boca Raton, Fla.
- 149.Rai, A. N. 1990. Cyanobacteria-fungal symbioses: the cyanolichens, p. 9-41. In A. N. Rai (ed.), Handbook of symbiotic cyanobacteria. CRC Press, Inc., Boca Raton, Fla.
- 150.Rai, A. N., M. Borthakur, S. Singh, and B. Bergman. 1989. Anthoceros-Nostoc symbiosis: immunoelectronmicroscopic localization of nitrogenase, glutamine synthetase, phycoerythrin and ribulose-1,5 bisphosphate carboxylase/oxygenase in the cyanobiont and the cultured (free-living) isolate Nostoc 7801. J. Gen. Microbiol. 135:385-395. [Google Scholar]
- 151.Rai, A. N., E. Söderbäck, and B. Bergman. 2000. Cyanobacterium-plant symbioses. New Phytol. 147:449-481. [DOI] [PubMed] [Google Scholar]
- 152.Ramasubramanian, T. S., F. Pu, and J. W. Golden. 1995. Isolation of the Anabaena sp. strain PCC 7120 sigA gene in a transciptional interference selection. J. Bacteriol. 177:6676-6678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ramasubramanian, T. S., T.-F. Wei, and J. W. Golden. 1994. Two Anabaena sp. strain PCC 7120 DNA-binding factors interact with vegetative cell- and heterocyst-specific genes. J. Bacteriol. 176:1214-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ramasubramanian, T. S., T.-F. Wei, A. K. Oldham, and J. W. Golden. 1996. Transcription of the Anabaena sp. strain PCC 7120 ntcA gene: multiple transcripts and NtcA binding. J. Bacteriol. 178:922-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rasmussen, U., C. Johansson, and B. Bergman. 1994. Early communication in the Gunnera-Nostoc symbiosis: plant induced cell differentiation and protein synthesis in the cyanobacterium. Mol. Plant-Microbe Interact. 7:696-702. [Google Scholar]
- 156.Ray, T. B., B. C. Mayne, R. E. Toia Jr., and G. A. Peters. 1979. Azolla-Anabaena azollae relationship. VIII. Photosynthetic characterization of the association and individual partners. Plant Physiol. 64:791-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ridgeway, J. E. 1967. The biotic relationship of Anthoceros and Phaeoceros to certain cyanophyta. Ann. Mo. Bot. Gard. 54:95-102. [Google Scholar]
- 158.Rippka, R. 1972. Photoheterotrophy and chemoheterotrophy among unicellular blue-green algae. Arch. Microbiol. 87:303-322. [DOI] [PubMed] [Google Scholar]
- 159.Rippka, R., J. Deruelles, J. B. Waterbury, M. Herdman, and R. Y. Stanier. 1979. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 111:1-61. [Google Scholar]
- 160.Rippka, R., and M. Herdman. 1992. Pasteur culture collection of cyanobacteria in axenic culture. Institut Pasteur, Paris, France.
- 161.Roberts, R. C., C. D. Mohr, and L. Shapiro. 1996. Developmental programs in bacteria. Curr. Topics Dev. Biol. 34:207-257. [DOI] [PubMed] [Google Scholar]
- 162.Robinson, B. L., and J. H. Miller. 1970. Photomorphogenesis in the blue-green alga Nostoc commune 584. Physiol. Plant. 23:461-472. [Google Scholar]
- 163.Rodgers, G. A., and W. D. P. Stewart. 1977. The cyanophyte-hepatic symbiosis. I. Morphology and physiology. New Phytol. 78:441-458. [Google Scholar]
- 164.Rowell, R., and W. D. P. Stewart. 1975. Effects of l-mthionine-dl-sulfoximine on the similation of newly fixed NH3, acetylene reduction and heterocyst production in Anabaena cylindrica. Biochem. Biophys. Res. Commun. 65:846-856. [DOI] [PubMed] [Google Scholar]
- 165.Schenk, H. E. A. 1992. Cyanobacterial symbioses, p. 3819-3854. In A. Balows, H. G. Trüper, M. Dworkin, W. Harder, and K.-H. Schleifer (ed.). The prokaryotes, 2nd ed. Springer-Verlag, New York, N.Y.
- 166.Schilling, N., and K. Ehrnsperger. 1985. Cellular differentiation of sucrose metabolism in Anabaena variabilis. Z. Naturforsch. 40c:776-779.
- 167.Schneider, G. J., J. D. Lang, and R. Haselkorn. 1991. Promoter recognition by the RNA polymerase from vegetative cells of the cyanobacterium Anabaena 7120. Gene 105:51-60. [DOI] [PubMed] [Google Scholar]
- 168.Schuster, R. M. 1966. The Hepaticae and Anthocerotae of North America east of the hundredth meridian, vol. I. Columbia University Press., New York, N.Y.
- 169.Silvester, W. B., R. Parsons, and P. W. Watt. 1996. Direct measurement of release and assimilation of ammonia in the Gunnera-Nostoc symbiosis. New Phytol. 132:617-625. [DOI] [PubMed] [Google Scholar]
- 170.Smith, A. J. 1982. Modes of cyanobacterial carbon metabolism, p. 47-85. In N. G. Carr and B. A. Whitton (ed.), The biology of cyanobacteria. Blackwell Scientific Publications, Ltd., Oxford, United Kingdom.
- 171.Smith, D. C., and A. E. Douglas. 1987. The biology of symbiosis. Edward Arnold, London, United Kingdom.
- 172.Smith, G., and J. D. Ownby. 1981. Cyclic AMP interfers with pattern formation in the cyanobacterium Anabaena variabilis. FEMS Microbiol. Lett. 11:175-180. [Google Scholar]
- 173.Soderback, E., and B. Bergman. 1992. The Nostoc-Gunnera magellanica symbiosis: phycobiliproteins, carboxysomes and rubisco in the cyanobiont. Physiol. Plant. 84:425-432. [Google Scholar]
- 174.Soderback, E., P. Lindblad, and B. Bergman. 1990. Developmental patterns related to nitrogen fixation in the Nostoc-Gunnera magellanica Lam. symbiosis. Planta 182:355-362. [DOI] [PubMed] [Google Scholar]
- 175.Spiller, H., W. Stallings, T. Woods, and M. Gunasekaran. 1993. Requirement for direct association of ammonia-excreting Anabaena variabilis mutant (SA-1) with roots for maximal growth and yield of wheat. Appl. Microbiol. Biotechnol. 40:557-566. [Google Scholar]
- 176.Steinberg, N. A., and J. C. Meeks. 1989. Photosynthetic CO2 fixation and ribulose bisphosphate carboxylase/oxygenase activity of Nostoc sp. strain UCD 7801 in symbiotic association with Anthoceros punctatus. J. Bacteriol. 171:6227-6233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Steinberg, N. A., and J. C. Meeks. 1991. Physiological sources of reductant for nitrogen fixation activity in Nostoc sp. strain UCD 7801 in symbiotic association with Anthoceros punctatus. J. Bacteriol. 173:7324-7329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Stewart, W. D. P. 1973. Nitrogen fixation by photosynthetic microorganisms. Annu. Rev. Microbiol. 27:283-316. [DOI] [PubMed] [Google Scholar]
- 179.Stewart, W. D. P. 1980. Systems involving blue-green algae (cyanobacteria), p. 583-680. In F. J. Bergersen (ed.), Methods for evaluating biological nitrogen fixation. John Wiley & Sons, Ltd., London, United Kingdom.
- 180.Stragier, P., and R. Losick. 1996. Molecular genetics of sporulation in Bacillus subtilis. Annu. Rev. Genet. 30:297-341. [DOI] [PubMed] [Google Scholar]
- 181.Summers, M. L., J. G. Wallis, E. L. Campbell, and J. C. Meeks. 1995. Genetic evidence of a major role for glucose-6-phosphate dehydrogenase in nitrogen fixation and dark heterotrophic growth of the cyanobacterium Nostoc sp. strain ATCC 29133. J. Bacteriol. 177:6184-6194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sun, D., R. M. Cabrera-Martinez, and P. Setlow. 1991. Control of transcription of the Bacillus subtilis spoIIIG gene, which codes for the forespore-specific transcription factor σG. J. Bacteriol. 173:2977-2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Tabita, F. R. 1988. Molecular and cellular regulation of autotrophic carbon dioxide fixation in microorganisms. Microbiol. Rev. 52:155-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Tandeau de Marsac, N. 1994. Differentiation of hormogonia and relationships with other biological processes, p. 825-842. In D. A. Bryant (ed.), The molecular biology of cyanobacteria. Kluwer Academic Publishers, Boston, Mass.
- 185.Tang, L. F., I. Watanabe, and C. C. Liu. 1990. Limited multiplication of symbiotic cyanobacteria of Azolla spp. on artifical media. Appl. Environ. Microbiol. 56:3623-3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Tel-Or, E., and W. D. P. Stewart. 1977. Photosynthetic components and activities of nitrogen-fixing heterocysts of Anabaena cylindrica. Proc. R. Soc. London Ser. B 198:61-86. [Google Scholar]
- 187.Thiel, T. 1990. Protein turnover and heterocyst differentiation in the cyanobacteirum Anabaena variabilis. J. Bacteriol. 26:50-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Thiel, T., E. M. Lyons, J. C. Erker, and A. Ernst. 1995. A second nitrogenase in vegetative cells of a heterocyst-forming cyanobacterium. Proc. Natl. Acad. Sci. USA 92:9358-9362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Thiel, T., and B. Pratte. 2001. Effect on heterocyst differentiation of nitrogen fixation in vegetative cells of the cyanobacterium Anabaena variabilis ATCC 29413. J. Bacteriol. 183:280-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Thomas, J. 1972. Relationship between age of culture and occurrence of the pigments of photosystem II of photosynthesis in heterocysts of a blue-green alga. J. Bacteriol. 110:92-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Thomas, J., J. C. Meeks, C. P. Wolk, P. W. Shaffer, S. M. Austin, and W-S. Chien. 1977. Formation of glutamine from [13N]ammonia, [13N]dinitrogen, and [14C]glutamate by heterocysts isolated from Anabaena cylindrica. J. Bacteriol. 129:15435-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Tsinoremas, N. F., A.-M. Castets, M. A. Harrison, J. F. Allen, and N. Tandeau de Marsac. 1991. Photosynthetic electron transport controls nitrogen assimilation in cyanobacteria by means of post-translational modification of the glnB gene product. Proc. Natl. Acad. Sci. USA 88:4565-4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Tumer, N. E., S. J. Robinson, and R. Haselkorn. 1983. Different promoters for the Anabaena glutamine synthetase gene during growth using molecular or fixed nitrogen. Nature 306:337-342. [Google Scholar]
- 194.Turing, A. M. 1952. The chemical basis of morphogenesis. Philos. Trans. R. Soc. London Ser. B 237:37-72. [Google Scholar]
- 195.Tyagi, V. V. S., T. B. Ray, B. C. Mayne, and G. A. Peters. 1981. The Azolla-Anabaena azollae relationship. XI. Phycobiliproteins in the action spectrum for nitrogenase-catalyzed acetylene reduction. Plant Physiol. 68:1479-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Uheda, E. 1986. Isolation of empty packets from Anabaena-free Azolla. Plant Cell Physiol. 27:1187-1190. [Google Scholar]
- 197.van Gorkom, H. J., and M. Donze. 1971. Localization of nitrogen fixation in Anabaena. Nature (London) 234:231-232. [DOI] [PubMed] [Google Scholar]
- 198.van Rhijn, P., and J. Vanderleyden. 1995. The Rhizobium-plant symbioses. Microbiol. Rev. 59:124-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Walsby, A. E. 1985. The premeability of heterocysts to the gases nitrogen and oxygen. Proc. R. Soc. London Ser. B 226:345-366. [Google Scholar]
- 200.Wei, T.-F., T. S. Ramasubramanian, and J. W. Golden. 1994. Anabaena sp. strain PCC 7120 ntcA gene required for growth on nitrate and heterocyst development. J. Bacteriol. 176:4473-4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Werner, D. 1992. Physiology of nitrogen-fixing legume nodules: compartments and functions, p. 399-431. In G. Stacey, R. H. Burris, and H. J. Evans (ed.), Biological nitrogen fixation. Chapman & Hall, New York, N.Y.
- 202.West, N. J., and D. G. Adams. 1997. Phenotypic and genotypic comparison of symbiotic and free-living cyanobacteria from a single field site. Appl. Environ. Microbiol. 63:4479-4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.White, A. W., and M. Shilo. 1975. Heterotrophic growth of the filamentous blue-green alga Plectonema boryanum. Arch. Microbiol. 102:123-127. [DOI] [PubMed] [Google Scholar]
- 204.Wilcox, M., G. J. Mitchison, and R. J. Smith. 1973. Pattern formation in the blue-green alga Anabaena. I. Basic mechanisms. J. Cell Sci. 12:707-725. [DOI] [PubMed] [Google Scholar]
- 205.Wilcox, M., G. J. Mitchison, and R. J. Smith. 1973. Pattern formation in the blue-green alga Anabaena. II. Controlled proheterocyst regression. J. Cell Sci. 13:637-649. [DOI] [PubMed] [Google Scholar]
- 206.Wilcox, M., G. J. Mitchison, and R. J. Smith. 1975. Mutants of Anabaena cylindrica altered in heterocyst spacing. Arch. Microbiol. 103:219-223. [DOI] [PubMed] [Google Scholar]
- 207.Wolk, C. P. 1967. Physiological basis of the pattern of vegetative growth of a blue-green alga. Proc. Natl. Acad. Sci. USA 57:1246-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Wolk, C. P. 1968. Movement of carbon from vegetative cells to heterocysts in Anabaena cylindrica. J. Bacteriol. 96:2138-2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wolk, C. P. 1979. Intercellular interactions and pattern formation in filamentous cyanobacteria, p. 247-266. In S. Subtelny and I. R. Konigsberg (ed.), Determinants of spatial organization. Academic Press, Inc., New York, N.Y.
- 210.Wolk, C. P. 1982. Heterocysts, p. 359-386. In N. G. Carr and B. A. Whitton (ed.), The biology of cyanobacteria. Blackwell Scientific Publications, Ltd., Oxford, United Kingdom.
- 211.Wolk, C. P. 1996. Heterocyst formation. Annu. Rev. Genet. 30:59-78. [DOI] [PubMed] [Google Scholar]
- 212.Wolk, C. P. 2000. Heterocyst formation in Anabaena, p. 83-104. In Y. V. Brun and L. J. Shimkets (ed.), Prokaryotic development. American Society for Microbiology, Washington, D.C.
- 213.Wolk, C. P., J. Elhai, T. Kuritz, and D. Holland. 1993. Amplified transcriptional pattern formed during development of Anabaena. Mol. Microbiol. 7:441-445. [DOI] [PubMed] [Google Scholar]
- 214.Wolk, C. P., A. Ernst, and J. Elhai. 1994. Heterocyst metabolism and development, p. 769-823. In D. A. Bryant (ed.), The molecular biology of cyanobacteria. Kluwer Academic Publishers, Boston, Mass.
- 215.Wolk, C. P., and M. P. Quine. 1975. Formation of one-dimensional patterns by stochastic processes and by filamentous blue-green algae. Dev. Biol. 46:370-382. [DOI] [PubMed] [Google Scholar]
- 216.Wong, F. C. Y., and J. C. Meeks. 2001. The hetF gene product is essential to heterocyst differentiation and affects HetR function in the cyanobacterium Nostoc punctiforme. J. Bacteriol. 183:2654-2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Wong, F. C. Y., and J. C. Meeks. 2002. Establishment of a functional symbiosis between the cyanobacterium Nostoc punctiforme and the bryophyte hornwort Anthocerospunctatus requires genes involved in nitrogen control and initiation of heterocyst differentiation. Microbiology 148:315-323. [DOI] [PubMed]
- 218.Wood, N. B., and R. Haselkorn. 1980. Control of phycobiliprotein proteolysis and heterocyst differentiation in Anabaena. J. Bacteriol. 141:1375-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Wood, P., A. Peat, and B. A. Whitton. 1986. Influence of phosphorus status on the fine structure of the cyanobacterium (blue-green alga) Calothrix parietina. Cytobiosis 47:89-99. [Google Scholar]
- 220.Yoon, H.-S., and J. S. Golden. 1998. Heterocyst pattern formation controlled by a diffusible peptide. Science 282:935-938. [DOI] [PubMed] [Google Scholar]
- 221.Yoon, H.-S., and J. S. Golden. 2001. PatS and products of nitrogen fixation control heterocyst pattern. J. Bacteriol. 183:2605-2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Zhou, R., Z. Cao, and J. Zhao. 1998. Characterization of HetR protein and turnover in Anabaena sp. PCC 7120. Arch. Microbiol. 169:417-423. [DOI] [PubMed] [Google Scholar]
- 223.Zhou, R., X. Wei, N. Jiang, H. Li, Y. Dong, K.-L. His, and J. Zhao. 1998. Evidence that HetR protein is an unusual serine-type protease. Proc. Natl. Acad. Sci. USA. 95:4959-4963. [DOI] [PMC free article] [PubMed] [Google Scholar]