Abstract
The Apicomplexa are a phylum of diverse obligate intracellular parasites including Plasmodium spp., the cause of malaria; Toxoplasma gondii and Cryptosporidium parvum, opportunistic pathogens of immunocompromised individuals; and Eimeria spp. and Theileria spp., parasites of considerable agricultural importance. These protozoan parasites share distinctive morphological features, cytoskeletal organization, and modes of replication, motility, and invasion. This review summarizes our current understanding of the cytoskeletal elements, the properties of cytoskeletal proteins, and the role of the cytoskeleton in polarity, motility, invasion, and replication. We discuss the unusual properties of actin and myosin in the Apicomplexa, the highly stereotyped microtubule populations in apicomplexans, and a network of recently discovered novel intermediate filament-like elements in these parasites.
THE APICOMPLEXA
Infection by parasitic protozoa of the phylum Apicomplexa causes incalculable morbidity and mortality to humans and agricultural animals (2, 3, 19, 26, 28, 39, 90). Apicomplexan parasites include Plasmodium spp., the agents of malaria; Toxoplasma gondii, a significant opportunistic pathogen in immunocompromised individuals; Eimeria spp., pathogens of chicken and cattle; Theileria spp., tick-borne parasites of cattle in Africa; and Cryptosporidium, an animal parasite as well as an opportunistic pathogen of humans. The phylum also includes gregarines, parasites of the guts of invertebrates including cockroaches and shrimp. This review is restricted to apicomplexan parasites of medical and agricultural importance since the bulk of the research has been done in this area.
All apicomplexans are obligate intracellular parasites. Most apicomplexan parasites grow and replicate within the parasitophorous vacuole, a nonphagosomal, membrane bound compartment that is segregated from most cellular trafficking pathways (78, 110, 143, 186). Proliferation of these organisms occurs by invasion of a host cell and is followed by parasite growth and cell division until the host cell is lysed by the replicating parasites. Parasites released by host cell lysis do not grow or undergo cell division extracellularly and must rapidly reinvade other host cells in order to survive. Repeated cycles of host cell invasion, parasite replication, host cell lysis, and parasite invasion of new cells account for much of the tissue damage associated with apicomplexan infections. Although apicomplexans are haploid for the bulk of their life cycles, they have complex life cycles, involving differentiation to forms that invade distinct tissues and hosts (Fig. 1). Differentiation can generate gametes that undergo fusion to generate a transient diploid zygote. The zygote immediately undergoes meiosis to reestablish haploid organisms. In some cases, differentiation also permits infection of organisms (such as mosquitoes or ticks) that serve as vectors to transmit parasites from host to host (2, 26, 39, 60, 67, 90, 157).
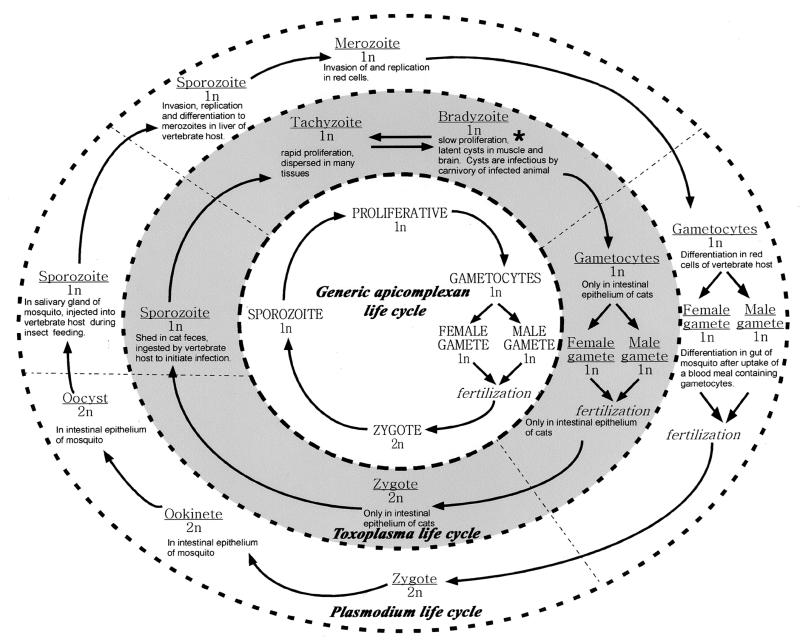
FIG. 1.
Life cycle of apicomplexan parasites. For the faint of heart or those not interested in details, the center circle illustrates a generic apicomplexan life cycle. The rapidly proliferating haploid form has the capacity to differentiate into gametes that fuse to produce a diploid zygote. Meiosis reestablishes the haploid state and leads to sporozoites, the form most widely associated with establishment of new infections after inoculation of a naive host by an infected vector. The outer two circles represent the specific life cycles for T. gondii and P. falciparum. The bradyzoite form of Toxoplasma (∗) is responsible for reactivation of latent infection and is an obligatory stage between tachyzoites and gametes. Plasmodium spp. do not have an analogous stage, although “hypnozoites” (latent sporozoites) are implicated in reactivation by P. vivax and P. ovale.
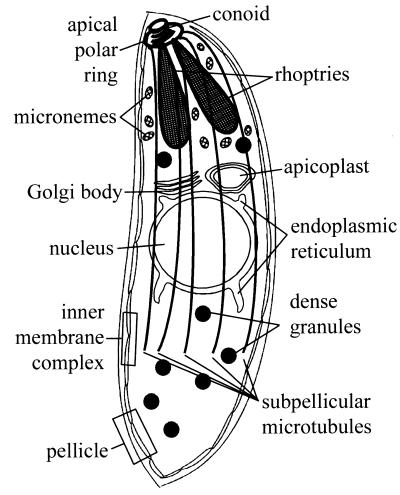
Apicomplexan parasites share a variety of morphological traits that are considered diagnostic for this phylum (Fig. 2). These protists have an elongated shape and a conspicuous specialization of the apical region (2, 3, 26, 157). Many of the distinct characteristics constitute a collection of unique organelles termed the apical complex. These organelles include the rhoptries, the micronemes, the apical polar ring, and the conoid. Rhoptries and micronemes are unique secretory organelles that contain products required for motility, adhesion to host cells, invasion of host cells, and establishment of the parasitophorous vacuole (2, 22, 24, 26, 41, 139). The conoid is a small cone-shaped structure composed of a spiral of unidentified filaments (120, 138). It is thought to play a mechanical role in invasion of host cells and is present in only some apicomplexans. The apical polar ring is a hallmark organelle of all members of the Apicomplexa (120, 134). It serves as one of the three microtubule-organizing centers (MTOCs) in these parasites; spindle pole plaques and centrioles/basal bodies are the other MTOCs (26, 157). In addition to the apical complex, the apicomplexans have other unique structural features, such as an essential chloroplast-like organelle called the apicoplast (87, 99, 169, 194). The parasites are bounded by the pellicle, a composite structure consisting of the plasma membrane and the closely apposed inner membrane complex (IMC) (2, 4, 26, 43, 44, 58, 103, 104, 177, 183). The pellicle is intimately associated with a number of cytoskeletal elements, including actin, myosin, microtubules, and a network of intermediate filament-like proteins (Fig. 3).
FIG. 2.
The morphology of apicomplexan parasites. Apicomplexans are highly polarized cells containing a collection of organelles that are specific to the phylum. Rhoptries, micronemes, and dense granules are secretory organelles that contain products required for motility, invasion, and establishment of the parasitophorous vacuole. The conoid is a small cone-shaped spiral of unidentified filaments. It is thought to play a mechanical role in invasion and can be protruded from or retracted into the apical polar ring. The apical polar ring serves as an MTOC for the subpellicular microtubules. The spirally arranged subpellicular microtubules closely follow the serpentine body shape of apicomplexans. The parasites are bounded by the pellicle, a composite structure consisting of the plasma membrane and the closely apposed IMC. The endoplasmic reticulum surrounds the nucleus, and the Golgi body is immediately above it. The apicoplast is immediately adjacent to the Golgi body.
FIG.3.
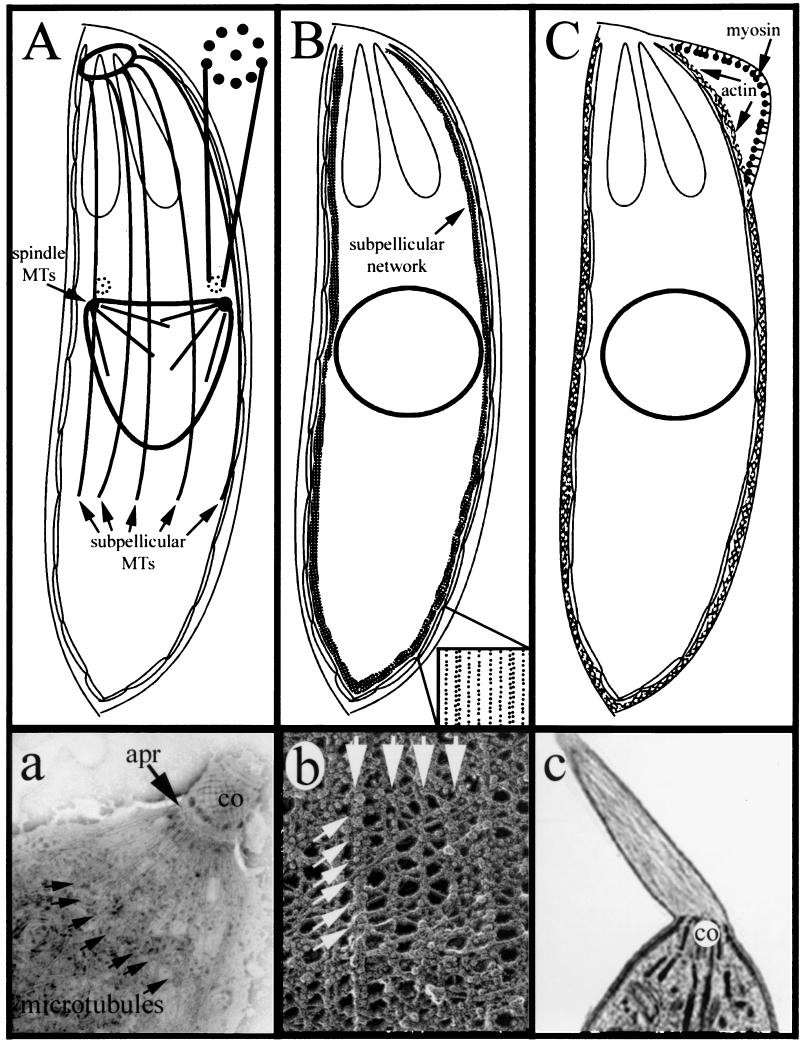
Cytoskeletal elements in the Apicomplexa. (A) The subpellicular microtubules radiate out of the apical polar ring and run down the cytosolic face of the IMC. The spindle microtubules are nucleated from spindle pole plaques within the nuclear membrane. Centrioles (consisting of nine singlet microtubules surrounding a central singlet microtubule) are adjacent to the spindle pole plaques. (a) Isolated, negatively strained conoid (co), apical polar ring (apr), and subpellicular microtubules from T. gondii (113). (B) A network of intermediate filaments, the subpellicular network, underlies the length of the IMC. The lower right inset illustrates the pattern of IMPs revealed by freeze fracture of the IMC. These particles may represent the transmembrane domains of receptors that link the subpellicular network to the cytoplasmic face of the inner membrane complex. (b) Glycerol- and detergent-extracted, freeze-dried replicas of Toxoplasma tachyzoites illustrate the regular array of the subpellicular network filaments. (Image of the Toxoplasma network kindly provided by J. Heuser.) (C) Actin is localized between the plasma membrane and the IMC. When the plasma membrane is separated from the IMC, actin remains associated with the IMC and myosin is associated with the plasma membrane. (c) Actin filaments protrude beyond the conoid (co) of Toxoplasma tachyzoites treated with jasplakinolide, a drug that induces actin polymerization. (Panel c reprinted from reference (151) with the permission of the publisher.)
Apicomplexan protozoa share a number of cytoskeletal elements (microtubules, actin, myosin, and intermediate filament-like proteins) with other “typical” eukaryotic systems used to study the cytoskeleton. Nonetheless, studies of the cytoskeletons of apicomplexans have revealed startling differences from model organisms that are worth mentioning at the outset. For example, the singlet subpellicular microtubules of apicomplexans are unusually stable and withstand the high pressure, cold, and detergents typically used to isolate them, conditions incompatible with survival for most microtubules (113). In contrast, the microfilaments of apicomplexans are thought to be exceedingly transient. Microfilaments are observed only after treatment with jasplakinolide (a drug that drives actin polymerization), and the bulk of actin (>98%) is sequestered in globular form (12, 36, 151). Apicomplexan myosins are unconventional, constituting a new class of unusually small “neckless” motors (68-70, 73, 123). In summary, apicomplexan protozoa constitute an ancient and diverse phylum with peculiar cell biological traits that make these parasites an intriguing topic for study. This review will survey our current understanding of the cytoskeleton of apicomplexan parasites.
MICROTUBULES IN THE APICOMPLEXA
Organization
The haploid forms of apicomplexan parasites have two discrete populations of microtubules: the subpellicular microtubules and the spindle microtubules (Fig. 3A and a). The subpellicular microtubules radiate from the apical polar ring and run down the cytosolic face of the pellicle, ending in the region below the nucleus (approximately two-thirds of the length of the parasite [1, 12, 13, 26, 31, 120, 131]). These spirally arranged microtubules closely follow the serpentine body shape of apicomplexans. Subpellicular microtubules confer both elongated shape and apical polarity to apicomplexan parasites. Replicative forms or drug-treated parasites that lack subpellicular microtubules are nonpolar, nonmotile, and noninvasive (72, 168). Subpellicular microtubules are organized by lateral association with the apical polar ring (APR), a circular MTOC (120, 134). Attachment of subpellicular microtubules to the APR is supported by blunt projections of the APR, which form a cogwheel pattern in transverse views. The plus end of the subpellicular microtubules is distal to this MTOC, and the ends of the subpellicular microtubules do not appear to be physically capped (134).
The arrangement of subpellicular microtubules varies among apicomplexan species, but the number, length, and organization are absolutely stereotyped within the life cycle stage of a species. The number of subpellicular microtubules ranges from a band of 3 or 4 in the tiny Plasmodium falciparum merozoites to ∼60 in the considerably larger Plasmodium ookinetes (2, 3, 12, 26, 136, 156, 157). In coccidian parasites (Toxoplasma and Eimeria), the subpellicular microtubules are evenly spaced beneath the periphery of the pellicle; however, in Plasmodium species, most of the microtubules occupy two-thirds of the circumference and one microtubule is centered within the latter one-third of the pellicle (3, 157, 180). P. falciparum merozoites are an exception to this spacing generalization; they have a reduced number (three or four) of subpellicular microtubules termed f-MAST (falciparum merozoite-associated assemblage of subellicular microtubules) that extend down one side of the merozoite membrane from the apex toward the posterior (12, 59). Other P. falciparum life cycle stages (sporozoites and ookinetes) have a full complement of subpellicular microtubules, as do merozoites of other Plasmodium species. Theileria sporozoites also deviate from this pattern. Although the tick-borne kinete stage of Theileria contains subpellicular microtubules and an IMC, Theileria sporozoites lack subpellicular microtubules and the IMC altogether and enter host cells in a distinct fashion (see below) (49, 50, 147, 148, 152).
Replicating parasites employ spindle microtubules during mitosis. Nuclear division proceeds without nuclear membrane breakdown (12, 26, 59, 79, 130, 161). Spindle microtubules are nucleated from electron-dense, amorphous plaques associated with nuclear invaginations and embedded in the nuclear membrane (12, 26, 82, 109, 140, 146, 157). This spindle-organizing structure has been variously referred to as the centrocone, the centriolar plaque, the spindle pole body, or the centriolar equivalent. We have chosen to characterize this structure as a “spindle pole plaque” to distinguish it from the adjacent centrioles that are sometimes present in members of the Apicomplexa. Centrioles are apparently not required for spindle assembly, since Plasmodium merozoites and Theileria sporozoites lack these structures and construct spindles by using only the spindle pole plaques (5, 12, 51, 140, 150, 157, 159). However, it is also possible that centrioles exist in these organisms and are obscured by inclusion in the electron-dense spindle pole plaque.
In many apicomplexans, centrioles are located in the cytoplasm close to but separate from the spindle pole plaques (26, 31, 42, 82, 100, 116, 157). Centrioles are highly ordered MTOCs typically consisting of a 9+0 structure of nine triplet microtubule blades organized in a turbine fashion. Apicomplexan centrioles have an unconventional form consisting of a central single microtubule surrounded by nine singlet microtubules, a deviation from the canonical structure (31, 40, 42, 82, 157, 165, 182). It is curious that apicomplexans apparently contain both spindle pole plaque structures and centrioles in addition to the apical polar ring to organize microtubules. It may be that the centrioles are maintained throughout the asexual life cycle in order to serve as a template for construction of basal bodies that nucleate flagellar axonemes in the male gametes. The centriole is also associated with inheritance of the apicoplast during replication in Toxoplasma (169). One intriguing possibility is that the centrioles function as a “super-organizing center” coordinating the apical polar ring MTOC and the spindle pole plaque MTOC.
Although most parasite replication occurs by asexual division, apicomplexans also differentiate to gametes that fuse to form a diploid zygote. The male gamete (microgamete) is flagellated and swims to the female gamete (macrogamete) to carry out fertilization. The anterior end of apicomplexan microgametes is pointed and contains three basal bodies in close proximity to the apical pole (116, 137, 158, 181). These basal bodies nucleate two or three flagella that extend past the nucleus and away from the apical end. Additional microtubules originate in the basal apparatus zone and extend to the posterior end of the microgamete. Two of the flagella are long and are free from association with the gamete body. The third flagellum is shorter and is attached to surface of gamete at its anterior end. In some species this third flagellum is present only in rudimentary form as a band of microtubules that extends along the length of the gamete. In contrast to the atypical centrioles observed in other stages, the basal body of male gametes has a typical triplet microtubule structure with ninefold symmetry and the flagellar axoneme contains a conventional 9+2 arrangement of doublet microtubules surrounding the central pair of microtubules (137, 158-160, 181). In Plasmodium, genesis of the basal bodies involves an intermediate 9+1 singlet form similar to the centrioles in other apicomplexans (157).
Conoid
Some apicomplexan parasites (Toxoplasma, Eimeria, and Sarcocystis) contain an additional cytoskeletal structure, the conoid, not found in other apicomplexans (Plasmodium and Theileria). It has been suggested that the conoid plays a mechanical role in invasion by parasites that must penetrate the robust barrier of the intestinal epithelium of vertebrates (26, 108, 121, 136, 138). The conoid consists of a set of counterclockwise spiraling filaments that create a pointed or cone-shaped structure at the extreme apex of these parasites (26, 64, 136, 138, 153). Extrusion of the conoid can be stimulated by ionomycin-triggered calcium influx, whereas pretreatment with cytochalasin D inhibits conoid extrusion (108). The filamentous subunits of the conoid resemble microtubules but are curled into an extremely tight coil, suggesting that if they are microtubules they are unusually constructed or deformed. (Recent work by K. Hu, D. Roos, and J. Murray [submitted for publication] suggests that the Toxoplasma conoid is constructed from tubulin organized into a novel polymer form consisting of a comma-shaped sheet of nine protofilaments.) The conoid is approximately 250 nm in diameter and can be extended beyond or retracted into the apical polar ring. There are two ∼400-nm-long, closely associated microtubules in the middle of the conoid. These microtubules are tightly bound to each other and are eccentric to the longitudinal axis of the conoid. This arrangement may be due to contact with the conoid and preconoidal rings via lateral projections (120). The course of the conoid subunits parallels the counterclockwise spiral of the subpellicular microtubules.
Role in apicomplexan replication
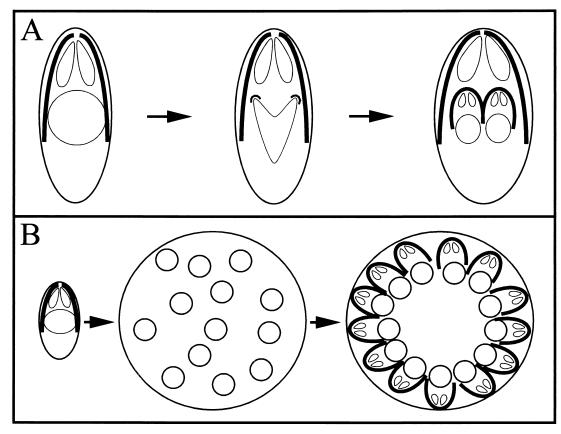
Apicomplexan parasites replicate by internal budding to create either two daughter cells or multiple progeny. Nuclear divisions within the Apicomplexa are cryptomitoses (the nuclear membrane remains intact throughout), and kariokinesis occurs without chromosomal condensation (26, 79, 130, 157). Replication in Toxoplasma and Neospora occurs by endodyogeny, which creates two daughter parasites (63, 71, 91, 154). Replication by Plasmodium, Theileria, Eimeria, and Babesia occurs by schizogeny, which can create 64 daughter parasites (3, 12, 72, 79, 150, 157). The processes of endodyogeny and schizogeny are quite similar, differing mainly in the preservation or loss of maternal cell specialization. In endodyogeny, two daughter parasites are formed within an intact, fully polarized mother parasite (Fig. 4A). This preserves the ability of replicating tachyzoites to invade throughout the cell cycle. The internal daughter cells are delimited by an IMC and associated subpellicular microtubules, and each contains (in addition to the nucleus, mitochondrion, Golgi, and plastid) a complete set of apical organelles (100, 154, 183). When the daughter cells are fully mature, the maternal apical complex is disassembled and the daughter parasites bud from the mother, adopting her plasma membrane. In schizogeny, after host cell invasion, the parasite subpellicular microtubules and apical complex are disassembled (Fig. 4B). After growth and multiple nuclear divisions, polarized parasites are regenerated when the nuclei move to the schizont periphery and associate with the assembling IMC, subpellicular microtubules, and apical organelles (3, 12, 72, 157). The newly repolarized parasites then bud out of the maternal cell as merozoites. Due to their large round shape and lack of apical specialization, replicating parasites are noninvasive during schizogeny.
FIG. 4.
Replication by the Apicomplexa. (A) Endodyogeny proceeds without loss of maternal cell shape and apical polarity. Formation of a curved nucleus is coupled to construction of two buds composed of nascent daughter IMC and subpellicular microtubules. The daughter cells develop surrounded by their own subpellicular microtubules and IMC in the fully polarized mother cell. Once the daughter cells are mature, they bud out of the remnants of the mother cell. (B) Schizogeny proceeds with loss of apical polarity. Invasion of a polarized elongated parasite is followed by disassembly of the subpellicular microtubules and IMC and by extensive cell growth and nuclear divisions. To reassemble polarized daughter cells, the multiple nuclei align with individual sets of apical organelles, subpellicular microtubules, and IMC at the periphery of the replicating cell.
P. falciparum replication has been studied by observing microtubule structures during replication in red cells (130). Premitotic intracellular parasites have a diffuse distribution of unpolymerized tubulin. The first visible structure is a spindle pole plaque that is localized within the nuclear membrane. This nucleates microtubules to give rise to a hemispindle, which partitions into two. Partitioning of the spindle pole plaque has also been observed by electron microscopy. A band of striated fibers termed the couche fibrillaire spans the separating plaques (42, 140, 161). This structure may represent centrin fibers, since similar structures are observed in algae and yeast (89, 164, 192). The spindle halves segregate in opposite directions by migration within the nuclear membrane to ultimately produce a full spindle. The multiple nuclear divisions of schizogeny are not completely synchronous; spindles in various stages of assembly are often present in a single late-stage schizont. Distinct postmitotic microtubule structures appear in late schizogeny. Each structure is associated with an individual nucleus and consists of extranuclear microtubules arranged in a regular radial array like the spokes on a cartwheel. These microtubules extend beyond the nucleus toward the center of the schizont. Remains of this microtubule organization persist as a rod-like structure in budding merozoites. This remnant may represent f-MAST, the reduced set of three or four subpellicular microtubules that occurs in P. falciparum merozoites (12, 59).
The microtubules of extracellular apicomplexans are not dynamic and are therefore impervious to microtubule-disrupting drugs (135). Assembly of microtubules occurs in the course of replication; therefore, intracellular parasites are susceptible to microtubule-depolymerizing drugs (Table 1) (168). In Toxoplasma and Plasmodium, the spindle and subpellicular microtubule populations are differentially stable to disruption by oryzalin or colchicine (14, 115). Lower concentrations (0.5 μM oryzalin or 1.0 mM colchicine) shorten microtubules. Under these conditions, Toxoplasma and Plasmodium continue to undergo nuclear division and budding but lack functional subpellicular microtubules and are incapable of invading new host cells. When removed from 0.5 μM oryzalin, Toxoplasma recovers normal morphology and is invasive (115). In contrast, higher concentrations of drug (2.5 μM oryzalin or 5.0 to 10.0 mM colchicine) disrupt both subpellicular and spindle microtubules (149, 168). Parasites under these conditions are incapable of nuclear division or budding, although cell growth, DNA synthesis, and centriole replication continue unchecked (115, 149, 168). When removed from 2.5 μM oryzalin, Toxoplasma tachyzoites attempt to bud as crescent-shaped parasites. Since the polyploid nuclear mass cannot be correctly segregated, daughter parasites are made that lack nuclei altogether (115).
TABLE 1.
Drug disruption of cytoskeletal elements in the Apicomplexa
| Drug and cytoskeletal element (drug mechanism) | Concn | Action | Comments |
|---|---|---|---|
| Actin microfilaments | |||
| Cytochalasin D (disrupter)a | 0.1-0.2 μM | Inhibits actin-based gliding motility (extracellular) and invasion | Inhibits gliding motility in extracellular parasites; use resistant host cells to avoid host cell effects. |
| Latrunculin (disrupter)b | 10 nM-5 μM | Inhibits motility and invasion | Will also show host cell actin effects. |
| Jasplakinolide (stabilizer)c | 50 nM-2 μM | Filament polymerization inhibits gliding and invasion | Acts on extracellular parasites; will also act on host cell actin in intracellular settings. |
| Microtubules | |||
| Oryzalin (disrupter)d | Also other dinitroanilines such as trifluralin, ethafluralin | ||
| Subpellicular microtubules | 0.5 μM | Parasites lack apical polarity | No effect on extracellular parasites; no effect on host cell microtubules, but intracellular parasites are affected. |
| Spindle microtubules | 2.5 μM | Parasites are incapable of replication | As for subpellicular microtubules. |
| Colchicine (disrupter)e | |||
| Subpellicular microtubules | 10 μM-1 mM | Parasites lack apical polarity | No drug effect on extracellular parasites; use resistant host cells to avoid host cell disruption (no microtubules in RBCs). |
| Spindle microtubules | 5-10 mM | Parasites are incapable of replication | As for subpellicular microtubules. |
| Colcemid (disrupter)f | 50 μM | Inhibits intracellular growth | No cell biological observations accompany study. |
| Vinblastine (disrupter)g | 15-100 nM | Inhibits intracellular growth | No cell biological observations accompany study. |
| Vincristine (disrupter)h | 7 nM | Inhibits intracellular growth | No cell biological observations accompany study. |
| Tubulozole (disrupter)i | 10 μM | Parasites are killed | May inhibit parasite protein synthesis rather than microtubules |
| Taxol (stabilizer)j | 0.1-0.5 μM | Parasites are incapable of budding | No drug effect on extracellular parasites; host cell microtubules will also be affected (no microtubules in RBCs). |
| Docetaxel/taxotere (stabilizer)k | 10 nm-50 μM | Parasites are incapable of replication | No drug effect on extracellular parasites; host cell microtubules will also be affected (no microtubules in RBCs). |
| Epithalone A (stabilizer)l | 0.1-0.5 μM | Parasites are incapable of budding | No drug effect on extracellular parasites; host cell microtubules will also be affected (no microtubules in RBCs). |
| Myosin | |||
| BDM (ATPase inhibitor)m | 20-40 mM | Inhibits actin-based gliding motility (extracellular) and invasion | Will also act on host cell myosins (not present in red cells). |
| KT5926 (LC kinase inhibitor)n | 1-5 μM | Inhibits actin-based gliding motility (extracellular) and invasion | Most probably inhibits secretion of TRAP family adhesins rather than myosin. |
Assessment of the activity of microtubule-destabilizing drugs on intracellular parasites is complicated by the activity of these drugs on host cells. In many cases, toxicity to host cells antedates any effects on parasite microtubules. In this situation, parasites fail to replicate, but this is due to adverse effects on the host cell rather than to direct inhibition of parasite functions. For these reasons, the most comprehensive examination of the effects of microtubule-disrupting drugs has been carried out with the P. falciparum erythrocytic stages in red cells (which lack microtubules). In vitro studies have established that replicating P. falciparum merozoites are susceptible to colchicine and colcemid, to vinblastine and vincristine, to the dinitroanilines trifluralin and pendimethalin, and to tubulozoles, including tubulozole-T, an isomer that is inactive in mammalian systems (15, 33, 34, 178). In contrast, merozoites show only low sensitivity to the benzimidazoles (albendazole, thiabendazole, mebendazole, and omeprazole) (33, 163). The results for benzimidazole are consistent with the deduced amino acid sequences of apicomplexan β-tubulins that lack Glu 198 and Phe 200, predictors of sensitivity to these compounds (44a, 81a). Plasmodium merozoites are also sensitive to microtubule-stabilizing drugs such as taxol, docetaxel, and epothilone A, which inhibit schizont nuclear division and budding (141, 162, 172). Colchicine, trifluralin, and taxol also perturb gametocyte and ookinete differentiation in Plasmodium (80, 88). Parallel experiments to examine the role of microtubules in other apicomplexans have been carried out with Eimeria, Toxoplasma, and Cryptosporidium by using dinitroaniline compounds such as oryzalin or trifluralin (10, 16, 168). These drugs specifically inhibit the microtubules of plants and protists and do not destabilize vertebrate microtubules. To assess the effects of other drugs such as colchicine and taxol, resistant host cells can be used. When colchicine- or taxol-resistant cells are used as host cells for Toxoplasma replication, the effects of colchicine and taxol are akin to what is observed in Plasmodium merozoites (115).
Tubulin and Microtubule-Associated Proteins
Apicomplexan tubulin genes have been cloned from T. gondii, Eimeria tenella, Babesia bovis, P. yoelii, P. berghei, C. parvum, and P. falciparum (8, 20, 21, 25, 29, 30, 74, 75, 118, 119, 145, 179, 197). In most of these apicomplexans, the α-tubulin and β-tubulin genes appear to be unlinked, single-copy genes containing up to three introns. The introns are in the same location and are similar in sequence in E. tenella, C. parvum, and T. gondii. The γ-tubulin gene has also been sequenced in P. falciparum. It is a single-copy gene and lacks introns (95). Curiously, P. falciparum and P. yoelii each have two α-tubulin genes, which are located on different chromosomes (8, 129). The α-tubulin-I gene is expressed throughout the parasite differentiation cycle, but the α-tubulin-II gene is specifically expressed in male gametes. The α-tubulin-II-specific monoclonal antibody 5E7 specifically labels stage III through mature male gametocytes and exflagellating and free male gametes (129). Immunoelectron microscopy using this antibody labels the axonemes of male gametes.
Microtubule-associated proteins (MAPs) are clearly critical to the highly organized structure of apicomplexan parasites. Bridges connecting the subpellicular microtubules to the inner membrane complex have been observed in thin sections of parasites (3, 131, 195). Isolated frozen-hydrated microtubules of T. gondii have a distinct 32-nm periodicity along their length as revealed by Fourier analysis (113). The periodicity most probably results from a MAP that heavily decorates these microtubules and that may account for their unusual stability after isolation. This MAP may coordinate the close interaction of the subpellicular microtubules with the IMC. The existence of a group of monoclonal antibodies that labels the subpellicular microtubules in Toxoplasma and cross-reacts with Plasmodium suggests that the MAPs may be conserved within the Apicomplexa (112, 114). The Plasmodium and Cryptosporidium genome databases and the Toxoplasma, Eimeria, and Neospora expressed sequence tag (EST) projects have sequences annotated as encoding putative kinesins and dyneins.
SUBPELLICULAR NETWORK OF THE APICOMPLEXA
Proteins of the Subpellicular Network
Deoxycholate extraction of Toxoplasma reveals a network of filamentous material that extends from the APR to the posterior of the parasite (98). The filaments have a diameter of 8 to 10 nm, and the network has the same general shape as the parasite, suggesting that these filaments may play a role in generating and maintaining cell shape. A polyclonal serum generated from the extracted pellicles has been used to identify two novel proteins that localize to the subpellicular network (98). These proteins, TgIMC-1 and TgIMC-2, are not homologous to any known proteins but are predicted to form regions of coiled coils and have weak similarity to the coiled-coil domains of cytoskeletal proteins such as myosin. TgIMC-1 is rich in valine and glutamic acid (together they constitute almost 30% of the protein). Interestingly, TgIMC-1 is similar to the articulins, a group of proteins that form a membrane skeleton in Euglena and other protists. Both the articulins and TgIMC-1 have a 12-amino-acid VPV repeat. Gold labeling with antisera to either TgIMC-1 or TgIMC-2 labels the cytoplasmic face of the pellicle between the subpellicular microtubules and the IMC (Fig. 3B). A homolog of TgIMC-1 is present in the P. falciparum genome. PfIMC-1 has an additional 220-amino-acid region near the C terminus, containing seven copies of a repeating sequence. The TgIMC-1 antiserum cross-reacts with Plasmodium, labeling late-stage schizonts during the formation of merozoites.
Organization of the Inner Membrane Complex and the Subpellicular Network
The IMC lies directly below the parasite plasma membrane and is closely associated with it, creating a three-layered pellicle characteristic of the Apicomplexa (26, 104, 183). In Plasmodium sporozoites, the IMC is constructed from a single large flattened vesicle joined by a single suture line traversing the long axis of the parasite (44). In other apicomplexans, it is made of many flattened vesicles aligned in longitudinal rows and joined in a patchwork fashion by sutures (43, 124). Glycerol-extracted, negatively stained pellicles from Sarcocystis ovifelis, Besnotia jellisoni, and Eimeria falciformis all have a mesh-like pattern that runs along the full length of the IMC (32). This mesh-like structure is similar to the network of IMC-associated filaments observed in Toxoplasma. Freeze fracture of Toxoplasma, Sarcocystis, Eimeria, and Plasmodium has shown that apicomplexans share a striking organization of the IMC (6, 43, 44, 104, 124). The membranes of the IMC are characterized by parallel alignment of intramembranous particles (IMPs). The lines of IMPs extend down the long axis of the parasite and are organized as single rows interspersed with double rows. The double rows correspond in number and arrangement to the underlying subpellicular microtubules (Fig. 3B, inset). The rows show continuity across the plates of the IMC, which is remarkable because each plate is a topologically distinct vesicle. Fourier analysis of the IMPs shows that they have a distinct 32-nm longitudinal repeat creating a two-dimensional lattice, with the second dimension at an angle of approximately 75° to the rows (113). The integrity of the particle lattice is not destroyed by disruption of actin or microtubules; this suggests the existence of additional cytoskeletal filaments. Glycerol- and detergent-extracted freeze-dried replicas of Toxoplasma tachyzoites reveal a filament network with striking similarity to the IMP lattice (Fig. 3b). The recent discovery of intermediate filament-like proteins that localize to the subpellicular network most probably identifies the lattice-generating network elements. We hypothesize that the IMP lattice may represent the transmembrane domains of receptors that anchor the subpellicular network to the IMC. This lattice may also anchor the subpellicular microtubules since they are decorated with a MAP that binds at 32-nm intervals (see above) (113).
ACTIN AND MYOSIN IN THE APICOMPLEXA
Properties and Localization of Actin
A large fraction of the actin in parasites of the Apicomplexa appears to be in monomeric rather than polymerized form. Experiments with Toxoplasma have established that tachyzoites have strikingly small amounts of assembled actin (36). Approximately 98% of Toxoplasma actin is globular, and this distribution is not shifted by the addition of agents that drive actin polymerization, such as phalloidin, MgCl2, exogenous actin, spermine, or phosphatidylinositol-4,5-bisphosphate (PIP2). In fact, although some researchers have observed phalloidin binding (127, 187) or filament stabilization with phalloidin (126), others have concluded that phalloidin does not bind to apicomplexan microfilaments (27, 36, 55, 56, 111, 151). Until quite recently, apicomplexan microfilaments had not even been observed by electron microscopy (12). However, in the presence of the microfilament-polymerizing drug jasplakinolide, Toxoplasma will form microfilaments (Fig. 3C and c) (126, 151). Jasplakinolide induces actin polymerization, most notably at the apical end of extracellular Toxoplasma tachyzoites (Table 1). The actin filaments in these membrane-enclosed apical projections are aligned parallel to the long axis of the parasite and can be up to 2 μM long (151). Jasplakinolide-induced filaments decorate with myosin subfragment 1, demonstrating that they are indeed actin; unfortunately, the filaments are too close together to determine polarity. Anti-actin antibodies label the apical projections in jasplakinolide treated parasites. In untreated parasites, heterologous actin antibodies label the apical region or appear as a diffuse distribution in the cytosol (47, 187, 196). A polyclonal antiserum directed against Toxoplasma actin labels a circumferential pattern in the tachyzoites, extending below the apical region (36). In hypotonically swelled parasites, a Toxoplasma-specific antiactin monoclonal antibody labels the region between the plasma membrane and the IMC (35).
Motility and Invasion
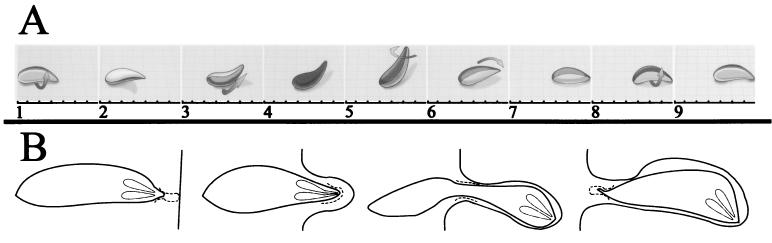
Most members of the Apicomplexa are motile. In these species, locomotion is intimately associated with host cell invasion and probably employs the same underlying cellular machinery. In fact, gliding locomotion and invasion often appear continuous and occur without a discernible change in speed. Apicomplexans (including gregarines) move by gliding motility (38, 52, 77, 84, 133). This movement does not exploit a discrete organelle (such as a flagellum) or result from amoeboid deformations of the cell. It is, however, substrate dependent. Motility has been analyzed in T. gondii and consists of three behaviors: circular gliding, upright twirling, and helical gliding (61, 66). When a crescent-shaped parasite lies on its right side, it moves in counterclockwise circles. Twirling occurs when the parasite is attached to the substrate by its posterior end, producing a clockwise spinning. Lastly, helical gliding is similar to twirling but occurs in horizontal parasites (Fig. 5A). This biphasic behavior consists of an 180° clockwise revolution (resulting in a corkscrewing forward movement) coupled to a parasite flip to return the parasite to its original face, so that it can initiate helical motion anew. Helical gliding is the only behavior that results in long-distance movement across a substrate. Actin-disrupting or -stabilizing drugs (cytochalasin D and jasplakinolide), as well as myosin inhibitors (butanedione monoxime [BDM]), disrupt Toxoplasma motility and invasion (35, 37, 126). This suggests that an actomyosin-based mechanism underlies these behaviors (Table 1). Cytochalasin inhibition of gliding motility and/or invasion has been demonstrated in C. parvum, T. gondii, E. tenella, E. acervulina, and Plasmodium species (37, 56, 77, 106, 133, 135). Motility and invasion are associated with translocation of secreted adhesive proteins from the apex to the parasite posterior and their shedding or deposition into a slime trail. Both adhesins and parasite surface antigens are deposited into a trail by gliding parasites (11, 22, 37, 48, 166, 167, 171). The presence of surface proteins in slime trails is likely to reflect the artificially sticky substrate used to capture adhesin trails.
FIG. 5.
Motility and invasion by the Apicomplexa. (A) Helical gliding is the only behavior associated with motility that leads to long-distance movement across a substrate. During this biphasic behavior, the parasite moves forward a body length and then repositions to repeat this cycle. This sequence illustrates the path taken by a gliding Toxoplasma tachyzoite to move forward one complete cycle. The parasite travels forward by moving a contact zone from its apex to its posterior in a helical path. Because the parasite is crescent shaped, it moves in a “corkscrew” fashion (frames 1 to 5). The crescent shape of the tachyzoite prevents the continuation of this motion because the parasite cannot contact the substrate with its concave face. To rectify this, the parasite rotates 180° without forward motion (frames 6 and 7) before initiating another cycle of ‘corkscrewing’ translational movement (frames 8 and 9). (Modified from reference (66) with the permission of the publisher.) (B) Apicomplexan invasion consists of attachment and apical orientation, induction of a parasitophorous vacuole, and translocation of the parasite into the vacuole. Apically secreted adhesins are capped along the moving junction to the posterior of the parasite. The moving junction is associated with a constriction of parasite shape that moves from the apex to the posterior.
Conserved adhesins have been found in Plasmodium, Toxoplasma, Eimeria, Neospora, and Cryptosporidium spp. (22, 24, 92, 132, 155, 171, 185). The thrombospondin-related anonymous protein (TRAP) family adhesins have common structural motifs and presumably an underlying mechanism of action. They contain a conserved adhesive domain consisting of a thrombospondin type 1 repeat that occurs in different numbers and locations within the individual apicomplexan adhesins. TRAP family proteins are localized to micronemes and are apically secreted during motility and invasion (22, 171). TRAP proteins are located on the surface of motile parasites in a transmembrane form and are capped from anterior to posterior in gliding parasites. The short cytoplasmic tail of these proteins is conserved and is thought to interact with the actomyosin cytoskeleton in order to translocate the protein backward. Adhesins are released as soluble protein by proteolytic cleavage at the posterior end of locomoting parasites (23). When the sporozoite-specific TRAP gene is knocked out in P. berghei merozoites, parasites behave normally until they differentiate to sporozoites within the mosquito. TRAP knockout sporozoites are immotile and noninvasive (171). Moreover, expression of a TRAP construct missing the last 14 amino acids of the cytoplasmic tail permits surface localization of the protein in knockout sporozoites (81). However, these sporozoites also display atypical behavior, repeatedly gliding one-third of a circle and snapping back to their original position. This suggests that the inability of TRAP to be appropriately translocated and/or released at the posterior end of locomoting parasites prevents their continued forward movement (81, 101). Menard has recently written a comprehensive review of gliding motility and the adhesins in the Apicomplexa (101).
The existence of an actomyosin-based mechanism for invasion was first implied by observations of the effect of cytochalasin B on Plasmodium invasion. Cytochalasin B blocks Plasmodium knowlesii merozoite invasion of red cells (106). Merozoites will attach irreversibly to red cells and form a vestigial parasitophorous vacuole but are inhibited from moving into the cell. Since red cells are unequivocally nonphagocytic, the effects of cytochalasin on Plasmodium invasion are likely to represent drug disruption of parasite (not host cell) microfilaments. However, for other apicomplexans, cytochalasin inhibition of invasion was sometimes attributed to inhibition of induced phagocytosis by the host cell. Active invasion of Toxoplasma tachyzoites can be distinguished from the phagocytic uptake of parasites because invasion of host cells (including macrophages) does not induce host cell membrane ruffling, actin microfilament reorganization, or tyrosine phosphorylation, which are all indicative of phagocytosis (111). Invasion is three to four times faster than phagocytosis (occurring within 25 to 40 s) and is characterized by parasite penetration into a tight-fitting vacuole formed by invagination of the plasma membrane. In contrast, phagocytosis of Toxoplasma involves membrane ruffling and the parasite is captured in a loose-fitting phagosome that forms over 2 to 4 min (78, 111, 121). Phagocytosis involves both reorganization of the host cytoskeleton and tyrosine phosphorylation of host proteins (111).
Experiments with cytochalasin D-resistant T. gondii have definitively established that invasion of Toxoplasma is critically dependent on actin filaments in the parasite but not in the host cell (37). Invasion of cytochalasin D-resistant host cells by wild-type (cytochalasin-sensitive) Toxoplasma tachyzoites is blocked by cytochalasin D. As observed with Plasmodium, attachment and apical orientation of Toxoplasma is normal in the presence of cytochalasin D. Cytochalasin D-resistant Toxoplasma mutants were isolated by chemical mutagenesis and selection for growth in cytochalasin D in resistant host cells. These resistant parasites have a point mutation in the single-copy actin gene ACT1 (A136G) and can invade wild-type host cells in the presence of cytochalasin D. Transformation of the mutant act1 allele into wild-type Toxoplasma confers cytochalasin D-resistant motility and invasion either as an allelic replacement or as a nonhomologous integration, generating a pseudodiploid parasite (37).
Apicomplexan invasion consists of three phases: (i) attachment with apical orientation, (ii) induction of a parasitophorous vacuole, and (iii) translocation of the parasite into the vacuole (Fig. 5B). Parasites attach to host cells and form an intimate connection through apical end contact (37, 53, 106). This results in sequential secretion from the parasite micronemes and rhoptries. Adhesins from the micronemes are translocated along the parasite length and are shed at the site of the moving junction; parasitophorous vacuole components from the rhoptries are secreted into this forming compartment (24). Both tight adhesion to the host cell and secretion into the host cell occur when parasites are immobilized early in invasion by cytochalasin treatment (37, 53, 65, 106).
The “moving junction” which forms during invasion is a circumferential zone of attachment at the orifice of the host cell invagination (7, 105). It is characterized by a markedly thickened host cell membrane with increased electron density and is frequently accompanied by a constriction in the parasite body. The parasite enters the nascent parasitophorous vacuole by capping the moving junction down its body. Ultimately, the parasite becomes enclosed within a cavity delimited by the invaginated host cell membrane. Formation of a moving junction that is capped to the posterior during invasion is likely to be a feature of invasion shared by many apicomplexans (Theileria is an exception [discussed below]). The moving junction is a highly specialized interface of the parasite with the host cell, presumably exploiting cytoskeletal proteins, signaling molecules, and receptors. Very little is known about this interface. P. falciparum MCP-1 (merozoite-capping protein 1) is a 60-kDa merozoite protein that moves from anterior to posterior with the moving junction during merozoite invasion of red cells (86). MCP-1 lacks both a signal sequence and transmembrane domain and is located in the parasite cytosol. The precise role of MCP-1 in invasion remains obscure. The protein has an amino-terminal domain that is conserved in bacterial and eukaryotic oxidoreductases (76). There are also a group of microneme proteins that may play a role in this junction that localize to the moving junction and the (exposed) posterior region during invasion (24, 57, 62).
Actin and Actin Binding Proteins
Genes encoding actin have been cloned in several members of the Apicomplexa (36, 83, 119, 189-191). In T. gondii and C. parvum, actin is encoded by a single-copy gene (36, 83, 119). In Cryptosporidium, the actin gene is intronless, and in Toxoplasma it has one intron. In P. falciparum, the situation is akin to that for α-tubulin. There are two genes encoding actin (189-191). One gene (actin I) is intronless and is expressed throughout the parasite life cycle. In contrast, the P. falciparum actin II gene has an intron and is transcribed only in the sexual stages (191). The amino acid sequence of actin II is divergent from that of previously characterized actins. Additionally, relative to the high degree of conservation shown by most actins, the 79% amino acid sequence similarity between Plasmodium actin I and actin II is quite low. Actin-related proteins (arps) have not been characterized in the Apicomplexa yet, but sequences in the Plasmodium genome are annotated as putative arps.
F-actin affinity chromatography has been used to isolate actin binding proteins from P. knowlesii and P. falciparum merozoites and from Toxoplasma tachyzoites (54, 125, 173). In P. knowlesii, five major proteins with molecular masses of 75, 70, 48, 40, and 32/34 kDa are eluted from F-actin columns (173). The 70-kDa protein has been identified as heat shock protein 70 (HSC70). The 32/34-kDa doublet coelutes with HSC70 from columns or in gel filtration chromatography; however, the identity of these proteins remains unknown. Highly enriched fractions of the Plasmodium HSC70-HSC32-HSC34 complex inhibit rabbit skeletal muscle actin polymerization in vitro. Biochemical experiments have established that this is due to a capping activity that is Ca2+ independent and is inhibited by PIP2.
Homologs of two widely conserved actin-associated proteins, coronin and actin-depolymerizing factor (ADF), have been characterized in the Apicomplexa. Coronin is a WD repeat containing actin binding protein that was first characterized in Dictyostelium discoideum, where it is essential for phagocytosis and motility. WD repeats (a tryptophan-aspartic acid motif) are found in diverse proteins; this motif is thought to mediate protein-protein interactions. Homologs of coronin are found in a large variety of eukaryotes, ranging from humans to C. elegans to yeast. A coronin homolog has been described in P. falciparum and is encoded by a single-copy gene (174). Compared to Dictyostelium coronin, the Plasmodium protein has conserved residues throughout the entire protein. A monoclonal antibody to D. discoideum coronin detects a 42-kDa protein in Triton X-100-insoluble extracts of P. falciparum schizonts. ADF/actophorin/cofilin is a widely conserved low-molecular-weight actin monomer-sequestering protein with filament-severing activity. An ADF homolog has been characterized in T. gondii (9). The single-copy gene encodes a 13.4-kDa protein. Toxoplasma ADF has a high degree of sequence similarity to other ADF homologs, particularly Acanthamoeba actophorin and plant ADFs. Toxoplasma ADF localizes to cytoplasm, especially under the plasma membrane. Recombinant Toxoplasma ADF purified from E. coli binds actin monomers and depolymerizes microfilaments in a pH-independent, concentration-dependent fashion.
One surprising finding is that a homolog of the actin monomer binding protein profilin has not been found in the Apicomplexa. In its place, Toxoplasma has apparently substituted a novel protein, toxofilin (125). Toxofilin is a 27-kDa actin monomer binding protein that was originally isolated from Toxoplasma extracts on G-actin affinity columns. In pyrene actin assays, toxofilin inhibits actin polymerization, acting as an actin-sequestering protein. It also slows microfilament disassembly through a filament end-capping activity. A single-copy gene encodes toxofilin. The protein has a pI of 9.63 and two coiled-coil domains and lacks consensus motifs or any similarity to known proteins. Overexpression of green fluorescent protein (GFP)-tagged toxofilin in vertebrate cells disrupts stress fibers and reduces microfilament levels by half. Toxofilin localizes to the apical cytoplasm in intracellular Toxoplasma but is found at the posterior of invading parasites. In motile parasites, toxofilin is localized throughout the entire cytoplasm.
Myosin
Evidence for myosins in the Apicomplexa was first suggested by observations that T. gondii gliding motility and host cell invasion are reversibly inhibited by the myosin inhibitors BDM (an ATPase inhibitor) and KT5926 (a myosin light-chain kinase inhibitor) (35). BDM also blocks motility and invasion in Plasmodium and in Cryptosporidium (56, 123). The effect of BDM is likely to be myosin specific; however, KT5926 blocks host cell attachment and motility by inhibiting the secretion of adhesins, proteins required for motility and cell attachment (Table 1). Immunofluorescence with heterologous myosin antibodies or antisera that recognizes the highly conserved myosin peptide LEAF localizes to the anterior pole or to a circumferential pattern that overlaps with the distribution of actin in T. gondii and P. falciparum (35, 144, 187). Immunoelectron microscopy of hypotonically swelled parasites (the plasma membrane is separated from the IMC) demonstrates that actin is associated with the IMC and that myosin is associated with the plasma membrane (Fig. 3C) (35). The LEAF peptide antiserum identifies a 90-kDa band in T. gondii lysates, and heterologous myosin antibodies label a Plasmodium protein of 86 kDa (187).
Apicomplexan myosins are highly atypical and were ultimately cloned in Toxoplasma by using degenerate PCR of conserved regions of the motor domain (70). A similar strategy has been used to identify myosins in Plasmodium, Neospora, Eimeria, Sarcocystis, Babesia, and Cryptosporidium (69). All apicomplexan myosins are extremely similar, suggesting that the diversity of myosins in these parasites is extremely limited (69). Phylogenetic analysis of the myosins places these motors in a novel, highly divergent class (XIV) in the myosin superfamily (69, 70). Apicomplexan myosins range from 91 to 125 kDa and include the smallest myosins characterized thus far (70, 73). There is a high degree of sequence conservation among all apicomplexan myosins throughout their whole length. All have short tails that do not have homology to any other myosin tails. However, these tails do contain a highly basic charge distribution similar to myosin I family members, suggesting that the apicomplexan myosins may interact with membranes. Generally, myosin motors have three domains; the amino terminus contains the motor domain, the central “neck” region binds light chains and acts as a lever arm, and the tail is diverse, carrying out different functions such as targeting to subcellular regions or binding to cargo (18, 102). The apicomplexan myosins do not contain the strictly conserved glycine residue at the fulcrum point of the lever arm and generally lack IQ motifs that bind calmodulin and calmodulin-related proteins (68, 70, 73). Additionally, the Toxoplasma myosins do not follow the TEDS rule, i.e., the presence of an acidic or phosphorylatable residue at a precise site close to the actin binding region (70). In lower eukaryotes, this residue is crucial for stimulation of the ATPase of class I myosins, but other exceptions to this rule have been described. Both the absence of an IQ motif and the nonadherence to the TEDS rule suggest that these motors may be regulated in a novel fashion.
T. gondii expresses five class XIV myosins: TgM-A, TgM-B, TgM-C, TgM-D, and TgM-E (68-70, 73). TgM-A is 93 kDa and lacks a discernible neck domain and IQ motifs (70). Epitope-tagged TgM-A localizes beneath the plasma membrane (73). Mutational analysis has established that a pair of arginine residues is essential to target TgM-A to the periphery (73). Since ectopically expressed TgM-A in HeLa cells does not target to the plasma membrane, peripheral localization in parasites may require a membrane-associated receptor. The P. falciparum homolog of TgM-A (PfM-A/Pf-myo1) is synthesized in mature schizonts and is present in merozoites but vanishes after the parasite enters the red cell (123). PfM-A is associated with the particulate parasite fraction, and immunofluorescence and immunogold analysis shows that PfM-A localizes to the periphery of mature schizonts and merozoites.
TgM-B and TgM-C are the products of differential RNA splicing and are 114 and 125 kDa respectively (70). They are identical throughout their head and neck domains and diverge in their distal tail structures. Both contain a single IQ motif. TgM-B has not been localized, but TgM-C localizes to a juxtanuclear region toward the apical pole of the parasite, consistent with an association with the Golgi apparatus (70, 73). TgM-D is a 91-kDa protein that has a punctate peripheral localization (73). TgM-E is the most recently discovered myosin and is currently being characterized (69, 73). Biochemical studies have established that the myosins bind actin in the absence but not the presence of ATP and that they are tightly associated with membranes (68, 73). The peripheral localization of TgM-A and of the GFP-TgM-A tail fusion is not dependent on an intact F-actin cytoskeleton (73). Truncation of the tail domains of TgM-A or TgM-D abolishes their peripheral localization and tight membrane association; fusion of the TgM-A or TgM-D tail to GFP is sufficient to confer plasma membrane localization (73).
MANIPULATION OF THE HOST CYTOSKELETON BY APICOMPLEXAN PARASITES
Reorganization of the Microvilli of Intestinal Epithelia by Cryptosporidium
Like other apicomplexans, C. parvum resides in a parasitophorous vacuole within the host cell, in this case the intestinal epithelium. However, the Cryptosporidium intracellular vacuole is extracytoplasmic, remaining at the apical surface of infected cells, in the region of the microvilli. The parasite induces host cell cytoskeletal rearrangement including the formation of branched microvilli clustered around the parasitophorous vacuole (55, 93). Moreover, Cryptosporidium induces the formation of a junctional complex that lies between it and the cytoplasm of the infected epithelial cell, keeping the parasite in the region of the microvilli. The junctional complex is associated with a plaque containing host cell actin. Accumulation of host cell actin, arp2, arp3, neural Wiskott-Aldrich syndrome protein (N-WASP), vasodilator-stimulated phosphoprotein (VASP), and α-actinin begins before entry is complete and these proteins localize beneath the invading parasite (45, 46). The plaque does not contain other actin binding proteins found in the intestinal epithelium, such as the catenins, zyxin, or plakoglobin (45). As parasites grow within the host cell, α-actinin is lost from the plaque, but the plaque size continues to increase to accommodate the increasing size of the replicating parasites. In addition to the above results, other have described localization of phosphotyrosine and villin at the site of parasite attachment (55). Expression of dominant negative constructs of Scar1 or N-WASP in host cells blocks Cryptosporidium invasion, suggesting that parasite-induced host cell actin reorganization is required for invasion (45, 46).
Plasmodium Modification and Mimicry of Erythrocyte Cytoskeletal Proteins
Infection with Plasmodium merozoites results in dramatic changes to the shape and biochemical properties of the parasitized red cell. Red cells infected with Plasmodium have increased phosphorylation of band 4.1, and a cysteine protease from the parasite cleaves red cell ankyrin (96, 128). P. falciparum induces knob formation of the surface of red cells, and the normal discoid shape of these cells becomes spherical (176). These structural alterations contribute to sequestration of infected red cells in organ capillaries, preventing their circulation and exposure to the spleen (107). The Plasmodium proteins RESA, MESA, and HRP-1 are anchored to the red cell membrane by association with spectrin, band 4.1, and the band 3 binding domain of ankyrin, respectively (17, 94, 97, 122). P. falciparum growth is decreased in human erythrocytes containing abnormal spectrin or band 4.1 (142). Parasites invade these cells normally, suggesting that an intact red cell membrane skeleton is required for parasite growth.
Plasmodium erythrocytic stages also synthesize proteins which are similar to ankyrin and spectrin and which are hypothesized to play a role in reorganization of the cytoskeleton of red cells. Plasmodium chabaudi ROPE (repetitive organellar protein) has a structure similar to that of spectrin (188). This 229-kDa protein is localized to the apical end of merozoites, possibly in the rhoptries. ROPE has characteristics of a cytoskeletal protein. A 364-amino-acid repetitive region based on 32 11-mer repeats suggests that the protein forms an helical coiled-coil triple helix containing a leucine-histidine zipper. Strikingly, this three-dimensional arrangement resembles the structure of spectrin. It has been postulated that ROPE may be involved in invasion, by interacting with the erythrocyte cytoskeleton via molecular mimicry of spectrin. P. falciparum expresses an 88-kDa phosphoprotein that is nearly identical to the amino-terminal region of ankyrin, a region of the protein that binds band 3 (170). This protein may also help the parasite reorganize the membrane skeleton via molecular mimicry.
Theileria Exploitation of Host Cell Microtubules
With respect to behavior and morphology, Theileria parva sporozoites are certainly the most distinct members of the Apicomplexa and do not conform to many of the general properties of the Apicomplexa described in this review. Theileria sporozoites are bounded by a simple plasma membrane structure and lack both an inner membrane complex and subpellicular microtubules. Host cell entry does not require apical orientation of the Theileria sporozoite and occurs by a zippering rather than a moving-junction mechanism (49, 50, 79, 147, 148, 152). Once inside the host cell, the parasite escapes from the parasitophorous vacuole and takes up residence in the cytoplasm. The parasite plasma membrane becomes coated with a number of host cell-derived microtubules organized in arrays tangential to the sporozoite surface (49, 79, 117, 147, 175). Sporozoite-associated microtubules are highly resistant to nocodazole disruption (147, 175). These parasites also activate NF-κB, specifically inducing clonal expansion of infected cells (193). Infected lymphocytes will proliferate indefinitely in culture until antiparasitic drugs halt unchecked replication (193). Sporozoites undergo nuclear divisions to form a multinucleate schizont. The host microtubules associated with the schizont are captured by the spindle of the proliferating host lymphocytes, pulling fragments of the schizont into each daughter lymphocyte (117, 175). The coupling of induction of host cell proliferation and association with host cell microtubules ensures that infected cells are specifically expanded and that the resulting progeny continue to harbor the parasite.
CONCLUSIONS
Although researchers studying apicomplexan parasites have amassed many data regarding the cytoskeleton, we still are missing explicit evidence linking cytoskeletal components to cellular properties. The following discussion suggests possible links between the cytoskeletal elements and behavioral traits that are common to the members of the Apicomplexa. One obvious generalization is that in these cells, the subpellicular network and the subpellicular microtubules are critical to cell shape while actin and myosin are essential for motility and invasion. This is not to say that future work will not uncover additional functions for the cytoskeleton.
Although apicomplexan parasites are profoundly deformed during host cell invasion, they retain membrane integrity during host cell entry. This may be ascribed to their robust arrangement of plasma membrane, IMC, and subpellicular network. The newly identified proteins IMC-1 and IMC-2 are implicated in subpellicular network formation (98). In addition to the obvious questions of how these proteins form filaments and how filament assembly is regulated is the issue of how this network associates with the IMC. The lattice of intramembranous particles observed after freeze fracture of the pellicle could reflect the transmembrane domains of receptors for the subpellicular network; however, the identity of these highly organized particles remains undetermined, as does any physical connection between the particles and the lattice proteins (43, 113, 124).
Apicomplexan parasites multiply by endodyogeny or schizogeny. As described above, these processes require independent regulation of spindle and subpellicular microtubules. Perhaps consequently, subpellicular microtubules and spindle microtubules are organized by different MTOCs: the apical polar ring and the spindle pole plaque/centrioles, respectively. In endodyogeny, parasites must discriminate between maternal and daughter apical polar rings and between maternal and daughter subpellicular microtubules. In schizogeny, daughter cell budding is induced after movement of multiple nuclei to the periphery of a maternal cell that lacks subpellicular microtubules. It is likely that the spindle pole plaques then induce or coordinate the formation of the apical polar rings, coupling each nucleus with a set of subpellicular microtubules. Daughter cell budding is distinct from vertebrate cytokinesis. In fact, inhibitor studies suggest that parasite scission may not utilize microfilaments such as are required at the vertebrate cleavage furrow (149). However, a recent study (28a) of the alternatively spliced myosins MyoB and MyoC in Toxoplasma demonstrates that overexpression of MyoB causes defects in cell division, and the parasites make extremely large residual bodies. Tagged MyoB localizes in a punctate cytosolic pattern and tagged MyoC localizes to the apical and posterior polar rings of tachyzoites. These latter observations suggest that MyoB and MyoC may play a role in parasite cell division, implicating an acto-myosin ring in parasite scission. Recent microtubule inhibitor studies show that subpellicular microtubule assembly can be disconnected from nuclear division, creating Toxoplasma tachyzoites that lack nuclei, although budding and scission from the maternal mass is completed (115). Multiple MTOCs permit apicomplexans to control nuclear division independently from cell polarity and cytokinesis. Although this grants greater cell cycle flexibility to these parasites, it abolishes the checks for coregulation of nuclear division and cytokinesis that are found in other eukaryotes.
Rigidity, cell shape, and apical polarity are provided by the subpellicular microtubules, and the apical polar ring organizes these microtubules (120, 134, 168). The apical polar ring represents a MTOC that is unique to the Apicomplexa. We know very little about its genesis and nothing about its component proteins. If the apical polar ring controls daughter cell budding, it must be replicated in a highly regulated fashion. It will also be informative to understand how it nucleates the subpellicular microtubules. The number of subpellicular microtubules and their organization are invariable within a life cycle stage of a particular species of apicomplexan parasite. The apical polar ring is a highly ordered structure and may contain signals that determine the number and placement of subpellicular microtubules.
Like the subpellicular network, the subpellicular microtubules also have intimate connections with the inner membrane complex (3, 113, 131, 195). To understand these interactions, it will be necessary to identify and characterize MAPs. MAPs may dictate subpellicular microtubule length and position under the pellicle. It is unclear why some apicomplexans distribute their microtubules uniformly beneath the pellicle while others center one microtubule beneath one-third of the circumference and evenly space the remainder below the other two-thirds of the pellicle. In studies of motility, it is clear that the convex and concave sides of the parasite are not equivalent. The asymmetry of microtubules in some apicomplexans may simply reflect areas that are more or less closely involved in force generation during motility or other essential functions. Subpellicular microtubules may contribute to motility by providing tracks that direct the acto-myosin-based capping activity. Toxoplasma tachyzoites and Plasmodium merozoites with shortened subpellicular microtubules (due to drug treatment) are noninvasive, supporting this notion (14, 115). However, it is not absolutely clear how microtubules could serve as tracks for the acto-myosin system, since actin and myosin are believed to act between the plasma membrane and the IMC while microtubules localize to the cytoplasmic face of the IMC (35, 120).
Many studies have implicated actin in apicomplexan motility, although apicomplexan microfilaments are apparently quite labile under most circumstances. Polymerized actin is observed only in the presence of jasplakinolide, and in untreated cells nearly all the actin is found as G-actin (36, 151). The apical actin filaments observed after treatment of Toxoplasma with jasplakinolide may reflect the location of actin regulators that nucleate or otherwise facilitate filament polymerization (151). Alternately, the apical localization of an F-actin projection after jasplakinolide treatment may represent the “path of least resistance” since the apical region is the only area of the pellicle not surrounded by three unit membranes and the subpellicular network. The short-lived nature of microfilaments suggests that actin assembly and disassembly are closely regulated. The Plasmodium HSC70 complex caps F-actin, limiting filament growth, and the apicomplexan homologs of ADF/cofilin are likely to sever filaments and sequester monomers, facilitating rapid disassembly of actin filaments (9, 173). Additionally, in Toxoplasma, actin may be kept monomeric by sequestration by toxofilin, a novel monomer binding protein (125). BLAST searches of the Cryptosporidium and Plasmodium genomes do not identify homologs of toxofilin, suggesting that distinct proteins may provide this function in other apicomplexans (unpublished data).
Myosin motors are also implicated in motility and invasion (35, 56, 66, 123). The apicomplexan myosins are quite divergent from myosins in other organisms, constituting a new class of motors in the myosin family (68-70, 73, 123). Apicomplexan myosins are all quite similar but have different subcellular localizations. Myosin-A is most likely to be involved in motility since it is found beneath the plasma membrane, whereas myosin-B is located to the Golgi and myosin-D is found on vesicles, consistent with roles for these latter motors in membrane traffic (68, 73, 123). Ectopic expression of myosin-A has shown that it does not localize to the plasma membrane in nonapicomplexans and therefore must be targeted to this region in parasites by additional proteins (73).
The precise mechanism by which parasites use an acto-myosin motor to generate motility is unclear. Since actin filaments are rare and since the apicomplexan myosins lack typical regulatory domains, it has been suggested that the movement of myosin is limited by filament generation. Consistent with this, jasplakinolide-treated parasites show increased motility, although drug treatment inhibits rather than enhances parasite invasiveness (151). For the myosin motors to have force-generating movement, the microfilaments must be tethered so that they remain in place. Actin filaments may be immobilized by interactions with the IMC, and the rigidity provided by the subpellicular network and the subpellicular microtubules could provide the extra stability required for myosin movement to transport adhesins to the posterior end of the parasite. Myosin movement along the actin filaments would lead to capping of adhesins down the length of the parasite and ultimately to gliding motility or invasion. Gliding motility is a trait shared with gregarines, apicomplexan parasites of invertebrates that are quite distinct from other members of the phylum described here (84, 85, 184).
This review has generalized the behavior of apicomplexans as a group. By doing so, we have undoubtedly glossed over differences, particularly with the more divergent members of this phylum. However, in many cases, atypical attributes are particular to the life cycle stage of the parasite rather than characteristic of the organism as a whole. This is most clearly illustrated with P. falciparum merozoites and Theileria sporozoites. Although most apicomplexans have many subpellicular microtubules and use an acto-myosin mechanism to glide and to invade cells, Plasmodium merozoites have reduced these traits and Theileria sporozoites have eliminated them. P. falciparum merozoites have a drastically scaled-back set of subpellicular microtubules that may reflect the greatly reduced size of merozoites relative to liver and insect stage parasites; both traits may be dictated by the smaller size of the host red cells. Merozoites do not display gliding motility, although they actively invade red cells in an actin-dependent fashion. Other Plasmodium stages are larger, have prominent subpellicular microtubules, and are clearly motile. Similarly, Theileria sporozoites lack subpellicular microtubules and infect lymphocytes after entering in a novel and nonmotile fashion. Tick-stage Theileria kinetes are larger and have subpellicular microtubules. Although very little work has been done on this stage, we assume that they are motile and actively invade host cells. Nonetheless, there are clearly dangers in overgeneralizing the behavior of apicomplexan parasites. Other exceptions to these generalizations will undoubtedly be found within this diverse group of parasites.
At present, the P. falciparum genome project is nearing completion and several genome projects are under way for other Plasmodium species. The Cryptosporidium genome is also nearing completion; genome projects for Toxoplasma, Theileria, and Babesia are under way; and there are ongoing EST projects for Toxoplasma, Neospora, Eimeria, Sarcocystis, and Plasmodium. In the past few years, researchers have developed techniques to transfect Toxoplasma and Plasmodium, to make targeted deletions, and to create gene replacements. With the amassed information about the cytoskeleton and these new resources and tools, we are truly poised to understand the mechanisms underlying cytoskeletal functions in the apicomplexan parasites. Disease caused by these protozoa has tremendous medical and economic impact worldwide. For the cell biologist, the unique biology of the Apicomplexa represents an intriguing departure from standard eukaryotic behaviors; for the clinician, these distinctions may represent unique drug targets.
Acknowledgments
We thank Antonio Barragan, Audra Charron, John Cooper, Susan Dutcher, Olivia Giddings, Dan Goldberg, Wallace Marshall, Alissa Weaver, and Dawn Wetzel for commenting on the manuscript.
N.S.M. is supported by Individual NRSA fellowship F32 GM20484-01A1 and was previously supported by an NIH training grant in Infectious Disease held by the Washington University School of Medicine. (T32; AI-0717221).
REFERENCES
- 1.Adams, J. H., and K. S. Todd, Jr. 1983. Transmission electron microscopy of intracellular sporozoites of Eimeria vermiformis (Apicomplexa, Eucoccidiida) in the mouse. J. Protozool. 30:114-118. [DOI] [PubMed] [Google Scholar]
- 2.Aikawa, M. 1988. Fine structure of malaria parasites in the various stages of development, p. 97-129. In W. H. Wernsdorf and I. McGregor (ed.), Malaria: principles and practice of malariology. Churchill Livingstone, Edinburgh, United Kingdom.
- 3.Aikawa, M. 1971. Parasitological review. Plasmodium: the fine structure of malarial parasites. Exp. Parasitol. 30:284-320. [DOI] [PubMed] [Google Scholar]
- 4.Aikawa, M. 1967. Ultrastructure of the pellicular complex of Plasmodium fallax. J. Cell Biol. 35:103-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aikawa, M., and R. L. Beaudoin. 1968. Studies on nuclear division of a malarial parasite under pyrimethamine treatment. J. Cell Biol. 39:749-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aikawa, M., A. H. Cochrane, R. S. Nussenzweig, and J. Rabbege. 1979. Freeze-fracture study of malaria sporozoites: antibody-induced changes of the pellicular membrane. J. Protozool. 26:273-279. [DOI] [PubMed] [Google Scholar]
- 7.Aikawa, M., L. H. Miller, J. Johnson, and J. Rabbege. 1978. Erythrocyte entry by malarial parasites. A moving junction between erythrocyte and parasite. J. Cell Biol. 77:72-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akella, R., P. Arasu, and A. B. Vaidya. 1988. Molecular clones of alpha-tubulin genes of Plasmodium yoelii reveal an unusual feature of the carboxy terminus. Mol. Biochem. Parasitol. 30:165-174. [DOI] [PubMed] [Google Scholar]
- 9.Allen, M. L., J. M. Dobrowolski, H. Muller, L. D. Sibley, and T. E. Mansour. 1997. Cloning and characterization of actin depolymerizing factor from Toxoplasma gondii. Mol. Biochem. Parasitol. 88:43-52. (Erratum, 90:399, 1997.) [DOI] [PubMed]
- 10.Arrowood, M. J., J. R. Mead, L. Xie, and X. You. 1996. In vitro anticryptosporidial activity of dinitroaniline herbicides. FEMS Microbiol. Lett. 136:245-249. [DOI] [PubMed] [Google Scholar]
- 11.Arrowood, M. J., C. R. Sterling, and M. C. Healey. 1991. Immunofluorescent microscopical visualization of trails left by gliding Cryptosporidium parvum sporozoites. J. Parasitol. 77:315-317. [PubMed] [Google Scholar]
- 12.Bannister, L. H., and G. H. Mitchell. 1995. The role of the cytoskeleton in Plasmodium falciparum merozoite biology: an electron-microscopic view. Ann. Trop. Med. Parasitol. 89:105-111. [DOI] [PubMed] [Google Scholar]
- 13.Beaudoin, R. L., and C. P. Strome. 1973. Plasmodium lophurae: the ultrastructure of the exoerythrocytic stages. Exp. Parasitol. 34:313-336. [DOI] [PubMed] [Google Scholar]
- 14.Bejon, P. A., L. H. Bannister, R. E. Fowler, R. E. Fookes, S. E. Webb, A. Wright, and G. H. Mitchell. 1997. A role for microtubules in Plasmodium falciparum merozoite invasion. Parasitology 114:1-6. [DOI] [PubMed] [Google Scholar]
- 15.Bell, A., B. Wernli, and R. M. Franklin. 1993. Effects of microtubule inhibitors on protein synthesis in Plasmodium falciparum. Parasitol. Res. 79:146-152. [DOI] [PubMed] [Google Scholar]
- 16.Benbow, J. W., E. L. Bernberg, A. Korda, and J. R. Mead. 1998. Synthesis and evaluation of dinitroanilines for treatment of cryptosporidiosis. Antimicrob. Agents Chemother. 42:339-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bennett, B. J., N. Mohandas, and R. L. Coppel. 1997. Defining the minimal domain of the Plasmodium falciparum protein MESA involved in the interaction with the red cell membrane skeletal protein 4.1. J. Biol. Chem. 272:15299-15306. [DOI] [PubMed] [Google Scholar]
- 18.Berg, J. S., B. C. Powell, and R. E. Cheney. 2001. A millennial myosin census. Mol. Biol. Cell 12:780-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Black, M. W., and J. C. Boothroyd. 2000. Lytic cycle of Toxoplasma gondii. Microbiol. Mol. Biol. Rev. 64:607-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonafonte, M. T., D. Garmon, and J. R. Mead. 1999. Characterization of an alpha-tubulin gene of Cryptosporidium parvum. J. Eukaryot. Microbiol. 46:545-547. [DOI] [PubMed] [Google Scholar]
- 21.Caccio, S., G. La Rosa, and E. Pozio. 1997. The beta-tubulin gene of Cryptosporidium parvum. Mol. Biochem. Parasitol. 89:307-311. [DOI] [PubMed] [Google Scholar]
- 22.Carruthers, V. B., O. K. Giddings, and L. D. Sibley. 1999. Secretion of micronemal proteins is associated with toxoplasma invasion of host cells. Cell. Microbiol. 1:225-235. [DOI] [PubMed] [Google Scholar]
- 23.Carruthers, V. B., G. D. Sherman, and L. D. Sibley. 2000. The Toxoplasma adhesive protein MIC2 is proteolytically processed at multiple sites by two parasite-derived proteases. J. Biol. Chem. 275:14346-14353. [DOI] [PubMed] [Google Scholar]
- 24.Carruthers, V. B., and L. D. Sibley. 1997. Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur. J. Cell Biol. 73:114-123. [PubMed] [Google Scholar]
- 25.Casu, R. E. 1993. Sequence of a cDNA encoding beta-tubulin from Babesia bovis. Mol. Biochem. Parasitol. 59:339-340. [DOI] [PubMed] [Google Scholar]
- 26.Chobotar, W., and E. Scholtyseck. 1982. Ultrastructure, p. 101-165. In P. L. Long (ed.), The biology of the coccidia. University Park Press, Baltimore, Md.
- 27.Cintra, W. M., and W. De Souza. 1985. Immunocytochemical localization of cytoskeletal proteins and electron microscopy of detergent extracted tachyzoites of Toxoplasma gondii. J. Submicrosc. Cytol. 17:503-508. [PubMed] [Google Scholar]
- 28.Clark, D. P. 1999. New insights into human cryptosporidiosis. Clin. Microbiol. Rev. 12:554-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28a.Delbac, F., A. Sanger, E. M. Neuhaus, R. Stratmann, J. W. Ajioka, C. Toursel, A. Herm-Gotz, S. Tomavo, T. Soldati, and D. Soldati. 2001. Toxoplasma gondii myosins B/C: one gene, two tails, two localizations, and a role in parasite division. J. Cell Biol. 155:613-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delves, C. J., P. Alano, R. G. Ridley, M. Goman, S. P. Holloway, J. E. Hyde, and J. G. Scaife. 1990. Expression of alpha and beta tubulin genes during the asexual and sexual blood stages of Plasmodium falciparum. Mol. Biochem. Parasitol. 43:271-278. [DOI] [PubMed] [Google Scholar]
- 30.Delves, C. J., R. G. Ridley, M. Goman, S. P. Holloway, J. E. Hyde, and J. G. Scaife. 1989. Cloning of a beta-tubulin gene from Plasmodium falciparum. Mol. Microbiol. 3:1511-1519. [DOI] [PubMed] [Google Scholar]
- 31.Desser, S. S. 1980. An ultrastructural study of the asexual development of a presumed Isospora sp. in mononuclear, phagocytic cells of the evening grosbeak (Hesperiphona vespertina). J. Parasitol. 66:601-612. [PubMed] [Google Scholar]
- 32.D'Haese, J., H. Mehlhorn, and W. Peters. 1977. Comparative electron microscope study of pellicular structures in coccidia (Sarcocystis. Besnoitia and Eimeria). Int. J. Parasitol. 7:505-518. [DOI] [PubMed] [Google Scholar]
- 33.Dieckmann-Schuppert, A., and R. M. Franklin. 1989. Compounds binding to cytoskeletal proteins are active against Plasmodium falciparum in vitro. Cell. Biol. Int. Rep. 13:207-214. [DOI] [PubMed] [Google Scholar]
- 34.Dieckmann-Schuppert, A., and R. M. Franklin. 1990. Mode of action of tubulozoles against Plasmodium falciparum in vitro. Antimicrob. Agents Chemother. 34:1529-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dobrowolski, J. M., V. B. Carruthers, and L. D. Sibley. 1997. Participation of myosin in gliding motility and host cell invasion by Toxoplasma gondii. Mol. Microbiol. 26:163-173. [DOI] [PubMed] [Google Scholar]
- 36.Dobrowolski, J. M., I. R. Niesman, and L. D. Sibley. 1997. Actin in the parasite Toxoplasma gondii is encoded by a single copy gene, ACT1 and exists primarily in a globular form. Cell Motil. Cytoskeleton 37:253-262. [DOI] [PubMed] [Google Scholar]
- 37.Dobrowolski, J. M., and L. D. Sibley. 1996. Toxoplasma invasion of mammalian cells is powered by the actin cytoskeleton of the parasite. Cell 84:933-939. [DOI] [PubMed] [Google Scholar]
- 38.Doran, D. J. 1982. Behavior of coccidia in vitro, p. 229-285. In P. L. Long (ed.), The biology of the coccidia. University Park Press, Baltimore, Md.
- 39.Dubey, J. P. 1977. Toxoplasma, Hammondia, Besnoitia, Sarcocystis, and other tissue cyst-forming coccidia of man and animals, p. 101-237. In J. P. Kreier (ed.), Parasitic protozoa, vol. III. Academic Press, Inc., New York, N.Y. [Google Scholar]
- 40.Dubremetz, J. F. 1975. Genesis of merozoites in the coccidia, Eimeria necatrix. Ultrastructural study. J. Protozool. 22:71-84. (In French.) [DOI] [PubMed] [Google Scholar]
- 41.Dubremetz, J. F., A. Achbarou, D. Bermudes, and K. A. Joiner. 1993. Kinetics and pattern of organelle exocytosis during Toxoplasma gondii/host-cell interaction. Parasitol. Res. 79:402-408. [DOI] [PubMed] [Google Scholar]
- 42.Dubremetz, J. F., and Y. Y. Elsner. 1979. Ultrastructural study of schizogony of Eimeria bovis in cell cultures. J. Protozool. 26:367-376. [DOI] [PubMed] [Google Scholar]
- 43.Dubremetz, J. F., and G. Torpier. 1978. Freeze fracture study of the pellicle of an eimerian sporozoite (Protozoa, Coccidia). J. Ultrastruct. Res. 62:94-109. [DOI] [PubMed] [Google Scholar]
- 44.Dubremetz, J. F., G. Torpier, P. Maurois, G. Prensier, and R. Sinden. 1979. Structure de la pellicule du sporozoite de Plasmodium yoelii etude par cryofracture. C. R. Acad. Sci. Paris 288:623-626. [Google Scholar]
- 44a.Edlind, T., G. Visvesvara, J. Li, and S. Katiyar. 1994. Cryptosporidium and microsporidial beta-tubulin sequences: predictions of benzimidazole sensitivity and phylogeny. J. Eukaryot. Microbiol. 41:385. [PubMed]
- 45.Elliott, D. A., and D. P. Clark. 2000. Cryptosporidium parvum induces host cell actin accumulation at the host-parasite interface. Infect. Immun. 68:2315-2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elliott, D. A., D. J. Coleman, M. A. Lane, R. C. May, L. M. Machesky, and D. P. Clark. 2001. Cryptosporidium parvum infection requires host cell actin polymerization. Infect. Immun. 69:5940-5942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Endo, T., K. Yagita, T. Yasuda, and T. Nakamura. 1988. Detection and localization of actin in Toxoplasma gondii. Parasitol. Res. 75:102-106. [DOI] [PubMed] [Google Scholar]
- 48.Entzeroth, R., G. Zgrzebski, and J. F. Dubremetz. 1989. Secretion of trials during gliding motility of Eimeria nieschulzi (Apicomplexa, Coccidia) sporozoites visualized by a monoclonal antibody and immuno-gold-silver enhancement. Parasitol. Res. 76:174-175. [DOI] [PubMed] [Google Scholar]
- 49.Fawcett, D., A. Musoke, and W. Voigt. 1984. Interaction of sporozoites of Theileria parva with bovine lymphocytes in vitro. I. Early events after invasion. Tissue Cell 16:873-884. [DOI] [PubMed] [Google Scholar]
- 50.Fawcett, D. W., S. Doxsey, D. A. Stagg, and A. S. Young. 1982. The entry of sporozoites of Theileria parva into bovine lymphocytes in vitro. Electron microscopic observations. Eur. J. Cell Biol. 27:10-21. [PubMed] [Google Scholar]
- 51.Fawcett, D. W., A. S. Young, and B. I. Leitch. 1985. Sporogeny in Theileria (Apicomplexa: Piroplasmida). A comparative ultrastructural study. J. Submicrosc. Cytol. 17:299-314. [Google Scholar]
- 52.Fayer, R. 1972. Penetration of cultured cells by Eimeria meleagrimitis and E. tenella sporozoites. J. Parasitol. 58:921-927. [PubMed] [Google Scholar]
- 53.Field, S. J., J. C. Pinder, B. Clough, A. R. Dluzewski, R. J. Wilson, and W. B. Gratzer. 1993. Actin in the merozoite of the malaria parasite, Plasmodium falciparum. Cell Motil. Cytoskeleton 25:43-48. [DOI] [PubMed] [Google Scholar]
- 54.Forero, C., and M. Wasserman. 2000. Isolation and identification of actin-binding proteins in Plasmodium falciparum by affinity chromatography. Mem. Inst. Oswaldo Cruz 95:329-337. [DOI] [PubMed] [Google Scholar]
- 55.Forney, J. R., D. B. DeWald, S. Yang, C. A. Speer, and M. C. Healey. 1999. A role for host phosphoinositide 3-kinase and cytoskeletal remodeling during Cryptosporidium parvum infection. Infect. Immun. 67:844-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Forney, J. R., D. K. Vaughan, S. Yang, and M. C. Healey. 1998. Actin-dependent motility in Cryptosporidium parvum sporozoites. J. Parasitol. 84:908-913. [PubMed] [Google Scholar]
- 57.Fourmaux, M. N., A. Achbarou, O. Mercereau-Puijalon, C. Biderre, I. Briche, A. Loyens, C. Odberg-Ferragut, D. Camus, and J. F. Dubremetz. 1996. The MIC1 microneme protein of Toxoplasma gondii contains a duplicated receptor-like domain and binds to host cell surface. Mol. Biochem. Parasitol. 83:201-210. [DOI] [PubMed] [Google Scholar]
- 58.Foussard, F., Y. Gallois, G. Tronchin, R. Robert, and G. Mauras. 1990. Isolation of the pellicle of Toxoplasma gondii (Protozoa, Coccidia): characterization by electron microscopy and protein composition. Parasitol. Res. 76:563-565. [DOI] [PubMed] [Google Scholar]
- 59.Fowler, R. E., R. E. Fookes, F. Lavin, L. H. Bannister, and G. H. Mitchell. 1998. Microtubules in Plasmodium falciparum merozoites and their importance for invasion of erythrocytes. Parasitology 117:425-433. [DOI] [PubMed] [Google Scholar]
- 60.Frenkel, J. K. 1973. Toxoplasmosis: parasite life cycle, pathology, and immunology, p. 343-410. In D. M. Hammond and P. L. Long (ed.), The coccidia: Eimeria, Isospora, Toxoplasma, and related genera. University Park Press, Baltimore, Md.
- 61.Frixione, E., R. Mondragon, and I. Meza. 1996. Kinematic analysis of Toxoplasma gondii motility. Cell Motil. Cytoskeleton 34:152-163. [DOI] [PubMed] [Google Scholar]
- 62.Garcia-Reguet, N., M. Lebrun, M. N. Fourmaux, O. Mercereau-Puijalon, T. Mann, C. J. Beckers, B. Samyn, J. Van Beeumen, D. Bout, and J. F. Dubremetz. 2000. The microneme protein MIC3 of Toxoplasma gondii is a secretory adhesin that binds to both the surface of the host cells and the surface of the parasite. Cell. Microbiol. 2:353-64. [DOI] [PubMed] [Google Scholar]
- 63.Gavin, M. A., T. Wanko, and L. Jacobs. 1962. Electron microscope studies of reproducing and interkinetic Toxoplasma. J. Protozool. 9:222-234. [DOI] [PubMed] [Google Scholar]
- 64.Gustafson, P. V., H. D. Agar, and D. I. Cramer. 1954. An electron microscope study of Toxoplasma. Am. J. Trop. Med. Hyg. 3:1008-1021. [PubMed] [Google Scholar]
- 65.Hakansson, S., A. J. Charron, and L. D. Sibley. 2001. Toxoplasma evacuoles: a two-step process of secretion and fusion forms the parasitophorous vacuole. EMBO J. 20:3132-3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hakansson, S., H. Morisaki, J. Heuser, and L. D. Sibley. 1999. Time-lapse video microscopy of gliding motility in Toxoplasma gondii reveals a novel, biphasic mechanism of cell locomotion. Mol. Biol. Cell 10:3539-3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hammond, D. M. 1973. Life cycles and development of coccidia, p. 45-79. In D. M. Hammond and P. L. Long (ed.), The coccidia: Eimeria, Isospora, Toxoplasma, and related genera. University Park Press, Baltimore, Md.
- 68.Heintzelman, M. B., and J. D. Schwartzman. 1999. Characterization of myosin-A and myosin-C: two class XIV unconventional myosins from Toxoplasma gondii. Cell Motil. Cytoskeleton 44:58-67. [DOI] [PubMed] [Google Scholar]
- 69.Heintzelman, M. B., and J. D. Schwartzman. 2001. Myosin diversity in Apicomplexa. J. Parasitol. 87:429-432. [DOI] [PubMed] [Google Scholar]
- 70.Heintzelman, M. B., and J. D. Schwartzman. 1997. A novel class of unconventional myosins from Toxoplasma gondii. J. Mol. Biol. 271:139-146. (Erratum, 297:829, 2000.) [DOI] [PubMed]
- 71.Hemphill, A., B. Gottstein, and H. Kaufmann. 1996. Adhesion and invasion of bovine endothelial cells by Neospora caninum. Parasitology 112:183-197. [DOI] [PubMed] [Google Scholar]
- 72.Hepler, P., C. Huff, and H. Sprinz. 1966. The fine structure of the exoerythrocytic stages of Plasmodium fallax. J. Cell Biol. 30:333-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hettmann, C., A. Herm, A. Geiter, B. Frank, E. Schwarz, T. Soldati, and D. Soldati. 2000. A dibasic motif in the tail of a class XIV apicomplexan myosin is an essential determinant of plasma membrane localization. Mol. Biol. Cell 11:1385-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Holloway, S. P., M. Gerousis, C. J. Delves, P. F. Sims, J. G. Scaife, and J. E. Hyde. 1990. The tubulin genes of the human malaria parasite Plasmodium falciparum, their chromosomal location and sequence analysis of the alpha-tubulin II gene. Mol. Biochem. Parasitol. 43:257-270. [DOI] [PubMed] [Google Scholar]
- 75.Holloway, S. P., P. F. Sims, C. J. Delves, J. G. Scaife, and J. E. Hyde. 1989. Isolation of alpha-tubulin genes from the human malaria parasite, Plasmodium falciparum: sequence analysis of alpha-tubulin. Mol. Microbiol. 3:1501-1510. [DOI] [PubMed] [Google Scholar]
- 76.Hudson-Taylor, D. E., S. A. Dolan, F. W. Klotz, H. Fujioka, M. Aikawa, E. V. Koonin, and L. H. Miller. 1995. Plasmodium falciparum protein associated with the invasion junction contains a conserved oxidoreductase domain. Mol. Microbiol. 15:463-471. [DOI] [PubMed] [Google Scholar]
- 77.Jensen, J. B., and S. A. Edgar. 1978. Fine structure of penetration of cultured cells by Isospora canis sporozoites. J. Protozool. 25:169-173. [Google Scholar]
- 78.Joiner, K. A., S. A. Fuhrman, H. M. Miettinen, L. H. Kasper, and I. Mellman. 1990. Toxoplasma gondii: fusion competence of parasitophorous vacuoles in Fc receptor-transfected fibroblasts. Science 249:641-646. [DOI] [PubMed] [Google Scholar]
- 79.Jura, W. G., C. G. Brown, and A. C. Rowland. 1983. Ultrastructural characteristics of in vitro parasite-lymphocyte behaviour in invasions with Theileria annulata and Theileria parva. Vet. Parasitol. 12:115-134. [DOI] [PubMed] [Google Scholar]
- 80.Kaidoh, T., J. Nath, H. Fujioka, V. Okoye, and M. Aikawa. 1995. Effect and localization of trifluralin in Plasmodium falciparum gametocytes: an electron microscopic study. J. Eukaryot. Microbiol. 42:61-64. [DOI] [PubMed] [Google Scholar]
- 81.Kappe, S., T. Bruderer, S. Gantt, H. Fujioka, V. Nussenzweig, and R. Menard. 1999. Conservation of a gliding motility and cell invasion machinery in Apicomplexan parasites. J. Cell Biol. 147:937-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81a.Katiyar, S. K., V. R. Gordon, G. L. McLaughlin, and T. D. Edlind. 1994. Antiprotozoal activities of benzimidazoles and correlations with beta-tubulin sequence. Antimicrob. Agents Chemother. 38:2086-2090. [DOI] [PMC free article] [PubMed]
- 82.Kelley, G. L., and D. M. Hammond. 1972. Fine structural aspects of early development of Eimeria ninakohlyakimovae in cultured cells. Z. Parasitenkd. 38:271-284. [DOI] [PubMed] [Google Scholar]
- 83.Kim, K., L. Gooze, C. Petersen, J. Gut, and R. G. Nelson. 1992. Isolation, sequence and molecular karyotype analysis of the actin gene of Cryptosporidium parvum. Mol. Biochem. Parasitol. 50:105-113. [DOI] [PubMed] [Google Scholar]
- 84.King, C. A. 1988. Cell motility of the sporozoan protozoa. Parasitol. Today 4:315-319. [DOI] [PubMed] [Google Scholar]
- 85.King, C. A. 1981. Cell surface interaction of the protozoan Gregarina with concanavalin A beads—implications for models of gregarine gliding. Cell. Biol. Int. Rep. 5:297-305. [DOI] [PubMed] [Google Scholar]
- 86.Klotz, F. W., T. J. Hadley, M. Aikawa, J. Leech, R. J. Howard, and L. H. Miller. 1989. A 60-kDa Plasmodium falciparum protein at the moving junction formed between merozoite and erythrocyte during invasion. Mol. Biochem. Parasitol. 36:177-185. [DOI] [PubMed] [Google Scholar]
- 87.Kohler, S., C. F. Delwiche, P. W. Denny, L. G. Tilney, P. Webster, R. J. Wilson, J. D. Palmer, and D. S. Roos. 1997. A plastid of probable green algal origin in Apicomplexan parasites. Science 275:1485-1489. [DOI] [PubMed] [Google Scholar]
- 88.Kumar, N., M. Aikawa, and C. Grotendorst. 1985. Plasmodium gallinaceum: critical role for microtubules in the transformation of zygotes into ookinetes. Exp. Parasitol. 59:239-247. [DOI] [PubMed] [Google Scholar]
- 89.Lechtreck, K. F., and A. Grunow. 1999. Evidence for a direct role of nascent basal bodies during spindle pole initiation in the green alga Spermatozopsis similis. Protist 150:163-181. [DOI] [PubMed] [Google Scholar]
- 90.Levine, N. D. 1973. Introduction, history, and taxonomy, p. 1-22. In D. M. Hammond and P. L. Long (ed.), The coccidia: Eimeria, Isospora, Toxoplasma, and related genera. University Park Press, Baltimore, Md.
- 91.Lindsay, D. S., C. A. Speer, M. A. Toivio-Kinnucan, J. P. Dubey, and B. L. Blagburn. 1993. Use of infected cultured cells to compare ultrastructural features of Neospora caninum from dogs and Toxoplasma gondii. Am. J. Vet. Res. 54:103-106. [PubMed] [Google Scholar]
- 92.Lovett, J. L., D. K. Howe, and L. D. Sibley. 2000. Molecular characterization of a thrombospondin-related anonymous protein homologue in Neospora caninum. Mol. Biochem. Parasitol. 107:33-43. [DOI] [PubMed] [Google Scholar]
- 93.Lumb, R., K. Smith, P. J. O'Donoghue, and J. A. Lanser. 1988. Ultrastructure of the attachment of Cryptosporidium sporozoites to tissue culture cells. Parasitol. Res. 74:531-536. [DOI] [PubMed] [Google Scholar]
- 94.Lustigman, S., R. F. Anders, G. V. Brown, and R. L. Coppel. 1990. The mature-parasite-infected erythrocyte surface antigen (MESA) of Plasmodium falciparum associates with the erythrocyte membrane skeletal protein, band 4.1. Mol. Biochem. Parasitol. 38:261-270. [DOI] [PubMed] [Google Scholar]
- 95.Maessen, S., J. G. Wesseling, M. A. Smits, R. N. Konings, and J. G. Schoenmakers. 1993. The gamma-tubulin gene of the malaria parasite Plasmodium falciparum. Mol. Biochem. Parasitol. 60:27-35. [DOI] [PubMed] [Google Scholar]
- 96.Magowan, C., J. Liang, J. Yeung, Y. Takakuwa, R. L. Coppel, and N. Mohandas. 1998. Plasmodium falciparum: influence of malarial and host erythrocyte skeletal protein interactions on phosphorylation in infected erythrocytes. Exp. Parasitol. 89:40-49. [DOI] [PubMed] [Google Scholar]
- 97.Magowan, C., W. Nunomura, K. L. Waller, J. Yeung, J. Liang, H. Van Dort, P. S. Low, R. L. Coppel, and N. Mohandas. 2000. Plasmodium falciparum histidine-rich protein I associates with the band 3 binding domain of ankyrin in the infected red cell membrane. Biochim. Biophys. Acta 1502:461-470. [DOI] [PubMed] [Google Scholar]
- 98.Mann, T., and C. Beckers. 2001. Characterization of the subpellicular network, a filamentous membrane skeletal component in the parasite Toxoplasma gondii. Mol. Biochem. Parasitol. 115:257-268. [DOI] [PubMed] [Google Scholar]
- 99.McFadden, G. I., and R. F. Waller. 1997. Plastids in parasites of humans. Bioessays 19:1033-1040. [DOI] [PubMed] [Google Scholar]
- 100.Mehlhorn, H., and A. O. Heydorn. 1978. The sarcosporidia (Protozoa, Sporozoa): life cycle and fine structure. Adv. Parasitol. 16:43-91. [DOI] [PubMed] [Google Scholar]
- 101.Menard, R. 2001. Gliding motility and cell invasion by Apicomplexa: insights from the Plasmodium sporozoite. Cell. Microbiol. 3:63-73. [DOI] [PubMed] [Google Scholar]
- 102.Mermall, V., P. L. Post, and M. S. Mooseker. 1998. Unconventional myosins in cell movement, membrane traffic, and signal transduction. Science 279:527-533. [DOI] [PubMed] [Google Scholar]
- 103.Meszoely, C. A., E. F. Erbe, L. M. Beaudoin, and R. L. Beaudoin. 1989. Freeze-fracture studies on the sporoblast and sporozoite development in the early oocyst. Am. J. Trop. Med. Hyg. 41:499-503. [DOI] [PubMed] [Google Scholar]
- 104.Meszoely, C. A., E. F. Erbe, R. L. Steere, N. D. Pacheco, and R. L. Beaudoin. 1982. Plasmodium berghei: architectural analysis by freeze-fracturing of the intraoocyst sporozoite's pellicular system. Exp. Parasitol. 53:229-241. [DOI] [PubMed] [Google Scholar]
- 105.Michel, R., K. Schupp, W. Raether, and F. W. Bierther. 1980. Formation of a close junction during invasion of erythrocytes by Toxoplasma gondii in vitro. Int. J. Parasitol. 10:309-313. [DOI] [PubMed] [Google Scholar]
- 106.Miller, L. H., M. Aikawa, J. G. Johnson, and T. Shiroishi. 1979. Interaction between cytochalasin B-treated malarial parasites and erythrocytes. Attachment and junction formation. J. Exp. Med. 149:172-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Miller, L. H., M. F. Good, and G. Milon. 1994. Malaria pathogenesis. Science 264:1878-1883. [DOI] [PubMed] [Google Scholar]
- 108.Mondragon, R., and E. Frixione. 1996. Ca(2+)-dependence of conoid extrusion in Toxoplasma gondii tachyzoites. J. Eukaryot. Microbiol. 43:120-127. [DOI] [PubMed] [Google Scholar]
- 109.Moore, J., and R. E. Sinden. 1974. Fine structure of Plasmodium mexicanum. J. Parasitol. 60:825-834. [PubMed] [Google Scholar]
- 110.Mordue, D. G., N. Desai, M. Dustin, and L. D. Sibley. 1999. Invasion by Toxoplasma gondii establishes a moving junction that selectively excludes host cell plasma membrane proteins on the basis of their membrane anchoring. J. Exp. Med. 190:1783-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Morisaki, J. H., J. E. Heuser, and L. D. Sibley. 1995. Invasion of Toxoplasma gondii occurs by active penetration of the host cell. J. Cell Sci. 108:2457-2464. [DOI] [PubMed] [Google Scholar]
- 112.Morrissette, N. S., V. Bedian, P. Webster, and D. S. Roos. 1994. Characterization of extreme apical antigens from Toxoplasma gondii. Exp. Parasitol. 79:445-459. [DOI] [PubMed] [Google Scholar]
- 113.Morrissette, N. S., J. M. Murray, and D. S. Roos. 1997. Subpellicular microtubules associate with an intramembranous particle lattice in the protozoan parasite Toxoplasma gondii. J. Cell Sci. 110:35-42. [DOI] [PubMed] [Google Scholar]
- 114.Morrissette, N. S., and D. S. Roos. 1998. Toxoplasma gondii: a family of apical antigens associated with the cytoskeleton. Exp. Parasitol. 89:296-303. [DOI] [PubMed] [Google Scholar]
- 115.Morrissette, N. S., and L. D. Sibley. Disruption of microtubules uncouples budding and nuclear division in Toxoplasma gondii. J. Cell Sci., in press. [DOI] [PubMed]
- 116.Mueller, B. E., S. S. Desser, and A. Haberkorn. 1981. Ultrastructure of developing gamonts of Eimeria contorta Haberkorn, 1971 (Protozoa, Sporozoa), with emphasis on the host-parasite interface. J. Parasitol. 67:487-495. [PubMed] [Google Scholar]
- 117.Musisi, F. L., R. G. Bird, C. G. Brown, and M. Smith. 1981. The fine structural relationship between Theileria schizonts and infected bovine lymphoblasts from cultures. Z. Parasitenkd. 65:31-41. [DOI] [PubMed] [Google Scholar]
- 118.Nagel, S. D., and J. C. Boothroyd. 1988. The alpha- and beta-tubulins of Toxoplasma gondii are encoded by single copy genes containing multiple introns. Mol. Biochem. Parasitol. 29:261-273. [DOI] [PubMed] [Google Scholar]
- 119.Nelson, R. G., K. Kim, L. Gooze, C. Petersen, and J. Gut. 1991. Identification and isolation of Cryptosporidium parvum genes encoding microtubule and microfilament proteins. J. Protozool. 38:52S-55S. [PubMed]
- 120.Nichols, B. A., and M. L. Chiappino. 1987. Cytoskeleton of Toxoplasma gondii. J. Protozool. 34:217-226. [DOI] [PubMed] [Google Scholar]
- 121.Nichols, B. A., and G. R. O'Connor. 1981. Penetration of mouse peritoneal macrophages by the protozoon Toxoplasma gondii. New evidence for active invasion and phagocytosis. Lab. Investig. 44:324-335. [PubMed] [Google Scholar]
- 122.Oh, S. S., S. Voigt, D. Fisher, S. J. Yi, P. J. LeRoy, L. H. Derick, S. Liu, and A. H. Chishti. 2000. Plasmodium falciparum erythrocyte membrane protein 1 is anchored to the actin-spectrin junction and knob-associated histidine-rich protein in the erythrocyte skeleton. Mol. Biochem. Parasitol. 108:237-247. [DOI] [PubMed] [Google Scholar]
- 123.Pinder, J. C., R. E. Fowler, A. R. Dluzewski, L. H. Bannister, F. M. Lavin, G. H. Mitchell, R. J. Wilson, and W. B. Gratzer. 1998. Actomyosin motor in the merozoite of the malaria parasite, Plasmodium falciparum: implications for red cell invasion. J. Cell Sci. 111:1831-1839. [DOI] [PubMed] [Google Scholar]
- 124.Porchet, E., and G. Torpier. 1977. Freeze fracture study of Toxoplasma and Sarcocystis infective stages. Z. Parasitenkd. 54:101-124. (In French.) [DOI] [PubMed]
- 125.Poupel, O., H. Boleti, S. Axisa, E. Couture-Tosi, and I. Tardieux. 2000. Toxofilin, a novel actin-binding protein from Toxoplasma gondii, sequesters actin monomers and caps actin filaments. Mol. Biol. Cell 11:355-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Poupel, O., and I. Tardieux. 1999. Toxoplasma gondii motility and host cell invasiveness are drastically impaired by jasplakinolide, a cyclic peptide stabilizing F-actin. Microbes Infect. 1:653-662. [DOI] [PubMed] [Google Scholar]
- 127.Preston, T. M., and C. A. King. 1992. Evidence for the expression of actomyosin in the infective stage of the sporozoan protist Eimeria. Cell Biol. Int. Rep. 16:377-381. [DOI] [PubMed] [Google Scholar]
- 128.Raphael, P., Y. Takakuwa, S. Manno, S. C. Liu, A. H. Chishti, and M. Hanspal. 2000. A cysteine protease activity from Plasmodium falciparum cleaves human erythrocyte ankyrin. Mol. Biochem. Parasitol. 110:259-272. [DOI] [PubMed] [Google Scholar]
- 129.Rawlings, D. J., H. Fujioka, M. Fried, D. B. Keister, M. Aikawa, and D. C. Kaslow. 1992. Alpha-tubulin II is a male-specific protein in Plasmodium falciparum. Mol. Biochem. Parasitol. 56:239-250. [DOI] [PubMed] [Google Scholar]
- 130.Read, M., T. Sherwin, S. P. Holloway, K. Gull, and J. E. Hyde. 1993. Microtubular organization visualized by immunofluorescence microscopy during erythrocytic schizogony in Plasmodium falciparum and investigation of post-translational modifications of parasite tubulin. Parasitology 106:223-232. [DOI] [PubMed] [Google Scholar]
- 131.Roberts, W. L., D. M. Hammond, L. C. Anderson, and C. A. Speer. 1970. Ultrastructural study of schizogony in Eimeria callospermophili. J. Protozool. 17:584-592. [DOI] [PubMed] [Google Scholar]
- 132.Robson, K. J., J. R. Hall, M. W. Jennings, T. J. Harris, K. Marsh, C. I. Newbold, V. E. Tate, and D. J. Weatherall. 1988. A highly conserved amino-acid sequence in thrombospondin, properdin and in proteins from sporozoites and blood stages of a human malaria parasite. Nature 335:79-82. [DOI] [PubMed] [Google Scholar]
- 133.Russell, D. G. 1983. Host cell invasion by Apicomplexa: an expression of the parasite's contractile system? Parasitology 87:199-209. [DOI] [PubMed] [Google Scholar]
- 134.Russell, D. G., and R. G. Burns. 1984. The polar ring of coccidian sporozoites: a unique microtubule-organizing centre. J. Cell Sci. 65:193-207. [DOI] [PubMed] [Google Scholar]
- 135.Russell, D. G., and R. E. Sinden. 1981. The role of the cytoskeleton in the motility of coccidian sporozoites. J. Cell Sci. 50:345-359. [DOI] [PubMed] [Google Scholar]
- 136.Scholtyseck, E. 1973. Ultrastructure, p. 81-144. In D. M. Hammond and P. L. Long (ed.), The coccidia: Eimeria, Isospora, Toxoplasma, and related genera. University Park Press, Baltimore, Md.
- 137.Scholtyseck, E., H. Mehlhorn, and D. M. Hammond. 1972. Electron microscope studies of microgametogenesis in coccidia and related groups. Z. Parasitenkd. 38:95-131. [DOI] [PubMed] [Google Scholar]
- 138.Scholtyseck, E., and H. Melhorn. 1970. The fine structure of the conoid of sporozoa and related organisms. Z. Parasitenkd. 34:68-94. [Google Scholar]
- 139.Scholtyseck, E., and H. Melhorn. 1970. Ultrastrucural study of characteristic organelles (paired organelles, micronemes, micropores) of sporozoa and related organisms. Z. Parasitenkd. 34:97-127. [DOI] [PubMed] [Google Scholar]
- 140.Schrevel, J., G. Asfaux-Foucher, and J. M. Bafort. 1977. Ultrastructural study of multiple mitoses during sporogony of Plasmodium b. berghei. J. Ultrastruct. Res. 59:332-350. (In French.) [DOI] [PubMed]
- 141.Schrevel, J., V. Sinou, P. Grellier, F. Frappier, D. Guenard, and P. Potier. 1994. Interactions between docetaxel (Taxotere) and Plasmodium falciparum-infected erythrocytes. Proc. Natl. Acad. Sci. USA 91:8472-8476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Schulman, S., E. F. Roth, Jr., B. Cheng, A. C. Rybicki, I. I.Sussman, M. Wong, W. Wang, H. M. Ranney, R. L. Nagel, and R. S. Schwartz. 1990. Growth of Plasmodium falciparum in human erythrocytes containing abnormal membrane proteins. Proc. Natl. Acad. Sci. USA 87:7339-7343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Schwab, J. C., C. J. Beckers, and K. A. Joiner. 1994. The parasitophorous vacuole membrane surrounding intracellular Toxoplasma gondii functions as a molecular sieve. Proc. Natl. Acad. Sci. USA 91:509-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schwartzman, J. D., and E. R. Pfefferkorn. 1983. Immunofluorescent localization of myosin at the anterior pole of the coccidian, Toxoplasma gondii. J. Protozool. 30:657-661. [DOI] [PubMed] [Google Scholar]
- 145.Sen, K., and G. N. Godson. 1990. Isolation of alpha- and beta-tubulin genes of Plasmodium falciparum using a single oligonucleotide probe. Mol. Biochem. Parasitol. 39:173-182. [DOI] [PubMed] [Google Scholar]
- 146.Senaud, J. 1967. Contribution a l'etude des Sarcosporidies et des Toxoplasmes (Toxoplasmea). Protistologica 3:167-232. [Google Scholar]
- 147.Shaw, M. K. 1997. The same but different: the biology of Theileria sporozoite entry into bovine cells. Int. J. Parasitol. 27:457-474. [DOI] [PubMed] [Google Scholar]
- 148.Shaw, M. K. 1999. Theileria parva: sporozoite entry into bovine lymphocytes is not dependent on the parasite cytoskeleton. Exp. Parasitol. 92:24-31. [DOI] [PubMed] [Google Scholar]
- 149.Shaw, M. K., H. L. Compton, D. S. Roos, and L. G. Tilney. 2000. Microtubules, but not actin filaments, drive daughter cell budding and cell division in Toxoplasma gondii. J. Cell Sci. 113:1241-1254. [DOI] [PubMed] [Google Scholar]
- 150.Shaw, M. K., and L. G. Tilney. 1992. How individual cells develop from a syncytium: merogony in Theileria parva (Apicomplexa). J. Cell Sci. 101:109-123. [DOI] [PubMed] [Google Scholar]
- 151.Shaw, M. K., and L. G. Tilney. 1999. Induction of an acrosomal process in Toxoplasma gondii: visualization of actin filaments in a protozoan parasite. Proc. Natl. Acad. Sci. USA 96:9095-9099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shaw, M. K., L. G. Tilney, and A. J. Musoke. 1991. The entry of Theileria parva sporozoites into bovine lymphocytes: evidence for MHC class I involvement. J. Cell Biol. 113:87-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sheffield, H. G., and D. M. Hammond. 1966. Fine structure of first-generation merozoites of Eimeria bovis. J. Parasitol. 52:595-606. [PubMed] [Google Scholar]
- 154.Sheffield, H. G., and M. L. Melton. 1968. The fine structure and reproduction of Toxoplasma gondii. J. Parasitol. 54:209-226. [PubMed] [Google Scholar]
- 155.Sibley, L. D., S. Hakansson, and V. B. Carruthers. 1998. Gliding motility: an efficient mechanism for cell penetration. Curr. Biol. 8:R12-R14 [DOI] [PubMed] [Google Scholar]
- 156.Sinden, R. E. 1985. A cell biologist's view of host cell recognition and invasion by malarial parasites. Trans. R. Soc. Trop. Med. Hyg. 79:598-605. [DOI] [PubMed] [Google Scholar]
- 157.Sinden, R. E. 1978. Cell biology, p. 85-168. In R. Killick-Kendrick and W. Jones (ed.), Rodent malaria. Academic Press, Inc., New York, N.Y.
- 158.Sinden, R. E. 1983. The cell biology of sexual development in plasmodium. Parasitology 86:7-28. [DOI] [PubMed] [Google Scholar]
- 159.Sinden, R. E. 1982. Gametocytogenesis of Plasmodium falciparum in vitro: an electron microscopic study. Parasitology 84:1-11. [DOI] [PubMed] [Google Scholar]
- 160.Sinden, R. E., and M. E. Smalley. 1979. Gametocytogenesis of Plasmodium falciparum in vitro: the cell-cycle. Parasitology 79:277-296. [DOI] [PubMed] [Google Scholar]
- 161.Sinden, R. E., and K. Strong. 1978. An ultrastructural study of the sporogonic development of Plasmodium falciparum in Anopheles gambiae. Trans. R. Soc. Trop. Med. Hyg. 72:477-491. [DOI] [PubMed] [Google Scholar]
- 162.Sinou, V., Y. Boulard, P. Grellier, and J. Schrevel. 1998. Host cell and malarial targets for docetaxel (Taxotere) during the erythrocytic development of Plasmodium falciparum. J. Eukaryot. Microbiol. 45:171-183. [DOI] [PubMed] [Google Scholar]
- 163.Skinner-Adams, T. S., T. M. Davis, L. S. Manning, and W. A. Johnston. 1997. The efficacy of benzimidazole drugs against Plasmodium falciparum in vitro. Trans. R. Soc. Trop. Med. Hyg. 91:580-584. [DOI] [PubMed] [Google Scholar]
- 164.Spang, A., I. Courtney, U. Fackler, M. Matzner, and E. Schiebel. 1993. The calcium-binding protein cell division cycle 31 of Saccharomyces cerevisiae is a component of the half bridge of the spindle pole body. J. Cell Biol. 123:405-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sterling, C. R., and M. Aikawa. 1973. A comparative study of gametocyte ultrastructure in avian haemosporidia. J. Protozool. 20:81-92. [DOI] [PubMed] [Google Scholar]
- 166.Stewart, M. J., and J. P. Vanderberg. 1992. Electron microscopic analysis of circumsporozoite protein trail formation by gliding malaria sporozoites. J. Protozool. 39:663-671. [DOI] [PubMed] [Google Scholar]
- 167.Stewart, M. J., and J. P. Vanderberg. 1991. Malaria sporozoites release circumsporozoite protein from their apical end and translocate it along their surface. J. Protozool. 38:411-421. [DOI] [PubMed] [Google Scholar]
- 168.Stokkermans, T. J., J. D. Schwartzman, K. Keenan, N. S. Morrissette, L. G. Tilney, and D. S. Roos. 1996. Inhibition of Toxoplasma gondii replication by dinitroaniline herbicides. Exp. Parasitol. 84:355-370. [DOI] [PubMed] [Google Scholar]
- 169.Striepen, B., M. J. Crawford, M. K. Shaw, L. G. Tilney, F. Seeber, and D. S. Roos. 2000. The plastid of Toxoplasma gondii is divided by association with the centrosomes. J. Cell Biol. 151:1423-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Suetterlin, B. W., B. Kappes, P. Jenoe, and R. M. Franklin. 1992. An 88-kDa protein of Plasmodium falciparum is related to the band-3-binding domain of human erythrocyte ankyrin. Eur. J. Biochem. 207:455-461. [DOI] [PubMed] [Google Scholar]
- 171.Sultan, A. A., V. Thathy, U. Frevert, K. J. Robson, A. Crisanti, V. Nussenzweig, R. S. Nussenzweig, and R. Menard. 1997. TRAP is necessary for gliding motility and infectivity of plasmodium sporozoites. Cell 90:511-522. [DOI] [PubMed] [Google Scholar]
- 172.Taraschi, T. F., D. Trelka, T. Schneider, and I. Matthews. 1998. Plasmodium falciparum: characterization of organelle migration during merozoite morphogenesis in asexual malaria infections. Exp. Parasitol. 88:184-193. [DOI] [PubMed] [Google Scholar]
- 173.Tardieux, I., I. Baines, M. Mossakowska, and G. E. Ward. 1998. Actin-binding proteins of invasive malaria parasites and the regulation of actin polymerization by a complex of 32/34-kDa proteins associated with heat shock protein 70kDa. Mol. Biochem. Parasitol. 93:295-308. [DOI] [PubMed] [Google Scholar]
- 174.Tardieux, I., X. Liu, O. Poupel, D. Parzy, P. Dehoux, and G. Langsley. 1998. A Plasmodium falciparum novel gene encoding a coronin-like protein which associates with actin filaments. FEBS Lett. 441:251-256. [DOI] [PubMed] [Google Scholar]
- 175.Tilney, L. G., M. S. Tilney, and M. K. Shaw. 1994. Theileria: strategy of infection and survival, p. 335-350. In D. Russell (ed.), Balliere's clinical infectious disease, vol. 1(2). Balliere-Tindall, London, United Kingdom. [Google Scholar]
- 176.Torii, M., and M. Aikawa. 1998. Ultrastructure of asexual stages, p. 123-134. In I. Sherman (ed.), Malaria: parasite biology, pathogenesis and protection. ASM Press, Washington, D.C.
- 177.Torpier, G., M. L. Darde, H. Caron, F. Darcy, and A. Capron. 1991. Toxoplasma gondii: membrane structure differences between zoites demonstrated by freeze fracture analysis. Exp. Parasitol. 72:99-102. [DOI] [PubMed] [Google Scholar]
- 178.Usanga, E. A., E. O' Brien, and L. Luzzato. 1986. Mitotic inhibitors arrest the growth of Plasmodium falciparum. FEBS Lett. 209:23-27. [DOI] [PubMed] [Google Scholar]
- 179.van Belkum, A., C. Janse, and B. Mons. 1991. Nucleotide sequence variation in the beta-tubulin genes from Plasmodium berghei and Plasmodium falciparum. Mol. Biochem. Parasitol. 47:251-254. [DOI] [PubMed] [Google Scholar]
- 180.Vanderberg, J., J. Rdodin, and M. Yoeli. 1967. Electron microscopic and histochemical studies of sporozoite formation in Plasmodium berghei. J. Protozool. 14:82-103. [Google Scholar]
- 181.Vetterling, J. M., N. D. Pacheco, and R. Fayer. 1973. Fine structure of gametogony and oocyst formation in Sarcocystis sp. in cell culture. J. Protozool. 20:613-621. [DOI] [PubMed] [Google Scholar]
- 182.Vivier, E., and A. Petitprez. 1972. Donees ultrastructurales complementaires, morphologiques et cytochimiques sur Toxoplasma gondii. Protistologica 8:199-221. [Google Scholar]
- 183.Vivier, E., and A. Petitprez. 1969. Le complexe membranaire superficiel et son evolution lors de l'elaboration des individus-fils chez Toxoplasma gondii. J. Cell Biol. 43:329-342. [PMC free article] [PubMed] [Google Scholar]
- 184.Walker, M., C. Mackenzie, S. Bainbridge, and C. Orme. 1979. A study of the structure and gliding movement of Gregarina garnhami. J. Protozool. 26:566-574. [Google Scholar]
- 185.Wan, K. L., V. B. Carruthers, L. D. Sibley, and J. W. Ajioka. 1997. Molecular characterisation of an expressed sequence tag locus of Toxoplasma gondii encoding the micronemal protein MIC2. Mol. Biochem. Parasitol. 84:203-214. [DOI] [PubMed] [Google Scholar]
- 186.Ward, G. E., L. H. Miller, and J. A. Dvorak. 1993. The origin of parasitophorous vacuole membrane lipids in malaria-infected erythrocytes. J. Cell Sci. 106:237-248. [DOI] [PubMed] [Google Scholar]
- 187.Webb, S. E., R. E. Fowler, C. O'Shaughnessy, J. C. Pinder, A. R. Dluzewski, W. B. Gratzer, L. H. Bannister, and G. H. Mitchell. 1996. Contractile protein system in the asexual stages of the malaria parasite Plasmodium falciparum. Parasitology 112:451-457. [DOI] [PubMed] [Google Scholar]
- 188.Werner, E. B., W. R. Taylor, and A. A. Holder. 1998. A Plasmodium chabaudi protein contains a repetitive region with a predicted spectrin-like structure. Mol. Biochem. Parasitol. 94:185-196. [DOI] [PubMed] [Google Scholar]
- 189.Wesseling, J. G., J. M. de Ree, T. Ponnudurai, M. A. Smits, and J. G. Schoenmakers. 1988. Nucleotide sequence and deduced amino acid sequence of a Plasmodium falciparum actin gene. Mol. Biochem. Parasitol. 27:313-320. [DOI] [PubMed] [Google Scholar]
- 190.Wesseling, J. G., M. A. Smits, and J. G. Schoenmakers. 1988. Extremely diverged actin proteins in Plasmodium falciparum. Mol. Biochem. Parasitol. 30:143-153. [DOI] [PubMed] [Google Scholar]
- 191.Wesseling, J. G., P. J. Snijders, P. van Someren, J. Jansen, M. A. Smits, and J. G. Schoenmakers. 1989. Stage-specific expression and genomic organization of the actin genes of the malaria parasite Plasmodium falciparum. Mol. Biochem. Parasitol. 35:167-176. [DOI] [PubMed] [Google Scholar]
- 192.Wiech, H., B. M. Geier, T. Paschke, A. Spang, K. Grein, J. Steinkotter, M. Melkonian, and E. Schiebel. 1996. Characterization of green alga, yeast, and human centrins. Specific subdomain features determine functional diversity. J. Biol. Chem. 271:22453-22461. [DOI] [PubMed] [Google Scholar]
- 193.Williams, R. O., and D. A. Dobbelaere. 1993. The molecular basis of transformation of lymphocytes by Theileria parva infection. Semin. Cell Biol. 4:363-371. [DOI] [PubMed] [Google Scholar]
- 194.Wilson, R. J., and D. H. Williamson. 1997. Extrachromosomal DNA in the Apicomplexa. Microbiol. Mol. Biol. Rev. 61:1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wong, T., and S. Desser. 1976. Fine structure of oocyst transformation and the sporozoites of Leukozoon dubreuili. J. Protozool. 23:115-126. [DOI] [PubMed] [Google Scholar]
- 196.Yasuda, T., K. Yagita, T. Nakamura, and T. Endo. 1988. Immunocytochemical localization of actin in Toxoplasma gondii. Parasitol. Res. 75:107-113. [DOI] [PubMed] [Google Scholar]
- 197.Zhu, G., and J. S. Keithly. 1996. The beta tubulin gene of Eimeria tenella. Mol. Biochem. Parasitol. 76:315-319. [DOI] [PubMed] [Google Scholar]