Abstract
Symbiotic nitrogen fixation is carried out in specialized organs, the nodules, whose formation is induced on leguminous host plants by bacteria belonging to the family Rhizobiaceae. Nodule development is a complex multistep process, which requires continued interaction between the two partners and thus the exchange of different signals and metabolites. NH4+ is not only the primary product but also the main regulator of the symbiosis: either as ammonium and after conversion into organic compounds, it regulates most stages of the interaction, from the production of nodule inducers to the growth, function, and maintenance of nodules. This review examines the adaptation of bacterial NH4+ metabolism to the variable environment generated by the plant, which actively controls and restricts bacterial growth by affecting oxygen and nutrient availability, thereby allowing a proficient interaction and at the same time preventing parasitic invasion. We describe the regulatory circuitry responsible for the downregulation of bacterial genes involved in NH4+ assimilation occurring early during nodule invasion. This is a key and necessary step for the differentiation of N2-fixing bacteroids (the endocellular symbiotic form of rhizobia) and for the development of efficient nodules.
INTRODUCTION
Rhizobium-Plant Interaction
Soil bacteria of the family Rhizobiaceae are able to colonize the roots of compatible legume plants and to induce specialized organs therein, the N2-fixing nodules, thus making the plant autotrophic for external N. Most of the literature deals with the properties of bacteria belonging to the genera Rhizobium and Sinorhizobium and, to a lesser extent, Azorhizobium, Bradyrhizobium, and Mesorhizobium. In this article we use the term rhizobia to include these five genera. From the point of view of the bacterial partner, nodule formation is a multistage process involving bacterial multiplication in the rhizosphere, recognition of the host plant, infection of the root hairs, and bacterial growth inside a network of tube-like structures made of plant cell wall-like material, called infection threads (ITs). When the ITs reach the center of a nodule primordium (formed by successive rounds of mitotic events), bacteria are released from the tip of ITs into the plant cells by endocytosis. Bacteria, at this stage called bacteroids (BTs), become enclosed in membrane vesicles arising from the plasma membrane, which act as a physical barrier between the symbiotic partners.
Once in the cytoplasm of plant cells, BTs divide a few times, arrest division, and differentiate morphologically and physiologically into a form able to reduce atmospheric N2 and release the fixed N to the plant (for reviews, see references 19, 124, and 197). The organelle-like structure formed by the BT, together with the peribacteroid space and the surrounding plant membrane, is called the symbiosome (SB). After the onset of N2 fixation, SBs continue to differentiate and eventually proceed towards lysis. It was recently shown that, in the symbiosis between Sinorhizobium meliloti and Medicago sativa, undifferentiated bacteria remaining in intracellular ramifications of the ITs infect the cytoplasm of senescent cells growing as free-living bacteria (186). This process occurs after the SBs are degraded. This observation may explain the benefit of the bacterial partner in the symbiosis.
Root Nodule
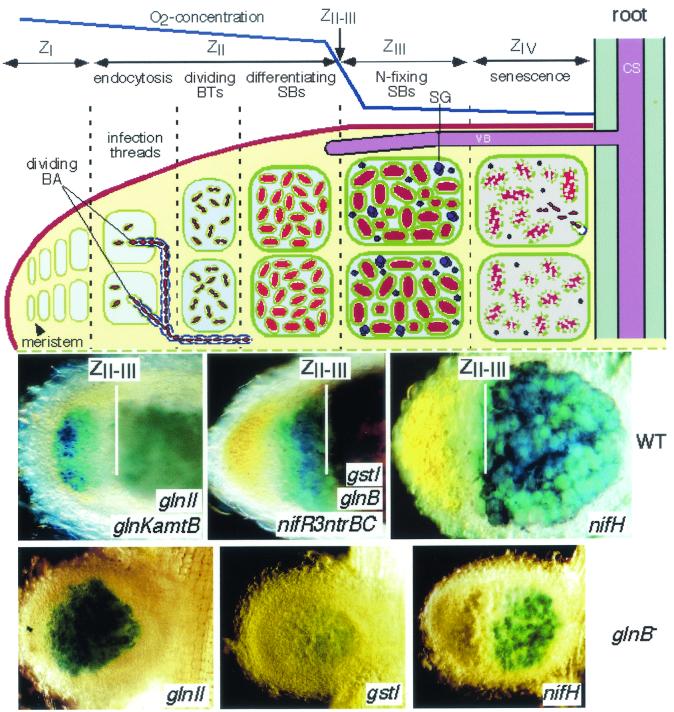
Fully developed nodules are vascularized plant organs formed by two main tissues, peripheral and central. The central tissue (CT) includes thousands of SB-containing cells, which in the literature are called either infected or invaded. Since SBs do not behave as pathogenic bacteria, we use the term invaded cells (ICs) for SB-containing cells. SBs are designed to accommodate the requirements for bacteroidal N2 fixation and the metabolic exchanges occurring between the two symbiotic partners. Both partners genetically regulate nodule organogenesis and nitrogen fixation. The most studied nodules are of two types, indeterminate and elongated (e.g., from Medicago sativa, Pisum sativum, and Vicia hirsuta) and determinate and globose (e.g., from Glycine max and Phaseolus vulgaris). Indeterminate nodules are elongated because of the presence of a persistent meristem located at their apex (Fig. 1). As a consequence, in a longitudinal section they show different developmental zones containing ICs at different stages of differentiation, in which SBs also show a progressive differentiation (186, 199).
FIG. 1.
(Top) Scheme of the indeterminate elongated nodule. BA, bacteria; BTs, bacteroids; SBs, symbiosomes; SG, starch grain; CS, central stele; VB, vascular bundle. The nodule zones (Z) are indicated. (Bottom) Histochemical localization of β-galactosidase activity in nodules of V. hirsuta induced by the wild-type (WT) and the glnB (PII−) strain of R. leguminosarum carrying lacZ transcriptional fusions to the genes indicated.
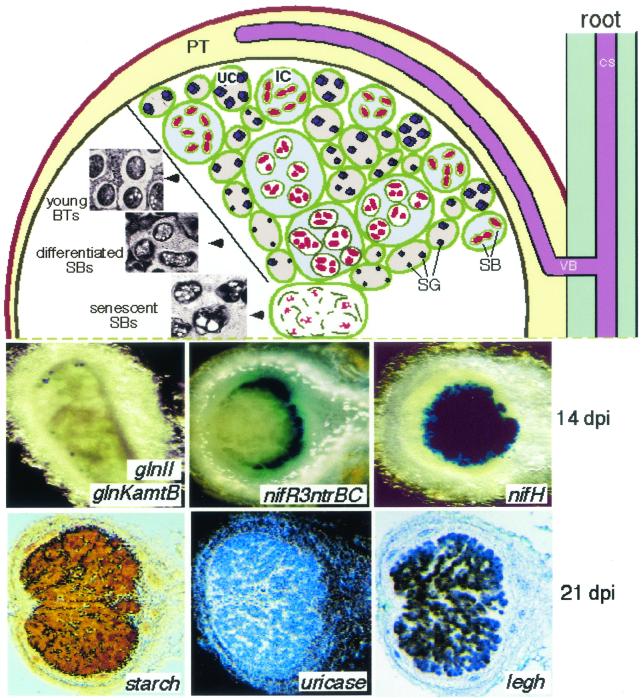
Determinate nodules are globose because they develop from a spherical meristem that is not evident in mature nodules (Fig. 2). They show a peripheral and a central tissue and, on the basis of histological observations (124, 125), it has been concluded that at each step of development all the ICs and the SBs of the CT are at a similar stage of differentiation. However, more recently it was shown that in developing determinate nodules of P. vulgaris, there is also a spatial component of development. In fact, during nodule organogenesis, it is possible to observe differentiating ICs containing only a few undifferentiated SBs and mature ICs filled with differentiated SBs formed by more than one BT (25). Moreover, some plant and bacterial genes are not expressed simultaneously in all cells of the CT (144, 176) (see below and Fig. 2) and signs of nodule senescence, such as the lysis of ICs, initiate their appearance specifically at its center (25).
FIG. 2.
(Top) Scheme of the determinate globose nodule. CS, central stele; VB, vascular bundle; PT, peripheral tissue; BTs, bacteroids; SBs, symbiosomes; SG, starch grain; IC, invaded cell; UC, uninvaded cell. (Middle) Histochemical localization of β-galactosidase activity in 14-day-postinoculation (dpi) nodules of P. vulgaris induced by R. etli carrying lacZ transcriptional fusions to the genes indicated. (Bottom) Sections of P. vulgaris nodules taken at 21 days postinoculation, showing amyloplast (starch) accumulation, and the in situ localization of uricase and leghemoglobin (legh) transcripts.
Finally, the heterogeneity of the ICs is confirmed by the observation that in nodules elicited by bacterial mutants having delayed invasion ability, not all the meristematic cells are invaded simultaneously. This suggests either a difference in their susceptibility to invasion (181, 182) or that the first invaded meristematic cells divide several times before differentiation (149). Plant molecular markers are differentially expressed through the CT also in G. max nodules (83).
Nodule organogenesis, such as the formation of other plant organs (flowers, leaves, roots), proceeds through many temporally and spatially regulated developmental steps. It is induced by rhizobia, but the development of a mature and functional nodule requires the exchange of several signal molecules and metabolites between the prokaryotic and eukaryotic partners. Nodule organogenesis is studied with the help of bacterial and plant mutants, which reveal specific patterns of differentiation, and with the in situ study of molecular markers, which in this case are not only eukaryotic, but also prokaryotic. The combined efforts of physiologists, embryologists, molecular biologists, and cytologists are needed to make progress in this field.
Purpose of This Review
In the rhizobium-legume symbiosis, the bacterial partner differentiates into nondividing endocellular symbionts that, through the induction of the nitrogenase complex, convert atmospheric N2 to NH3/NH4+, which is eventually used by the plant. In contrast, most NH4+ produced through N2 fixation in free-living diazotrophs is assimilated and used for bacterial growth. Null mutants of rhizobia (e.g., Tn5 induced) (37, 174) affected in different genes involved in NH4+ assimilation were found to be able to elicit the formation of efficient nodules (Nod+/Fix+ phenotype). Moreover, it has been shown that in isolated N2-fixing BTs, NH4+ assimilation activities are either absent or expressed at very low levels (22, 37, 77, 162). These studies led to the conclusion that the bacterial ability to assimilate NH4+ is not needed for an efficient symbiosis (80a, 192a). However, they do not deal with the mechanism(s) of gene regulation acting during SB differentiation to uncouple N2 fixation and NH4+ assimilation activities and do not exclude the possibility that the switching off of this pathway is essential for an efficient symbiosis.
The purpose of this review is to show that more detailed studies have revealed a very fine regulation of bacterial NH4+ metabolism during the establishment of the symbiosis. Downregulation of structural and regulatory genes involved in NH4+ assimilation is a key and necessary step for the differentiation of efficient SBs and thus for the development of effective nodules. During SB differentiation, a conserved molecular mechanism acts selectively to switch off gene transcription and to remove the corresponding proteins, either before or when N2 fixation is turned on.
NH4+ AS A REGULATORY SIGNAL IN SYMBIOSIS
Small supplements of combined N, such as NO3−, NH4+, and urea, added at sowing may benefit symbiosis by increasing seedling growth rate and the number, size, and efficiency of the resulting nodules. Excessive amounts of combined N depress nodulation by reducing the number of infective sites and/or the number of successful infections on the primary roots and by inhibiting nodule growth and functionality (for a review, see reference 213). NH4+ is at the same time the primary product and the main regulator of the symbiosis: either as such, or after conversion into organic compounds such as glutamate and glutamine, it regulates most stages of the symbiotic interaction. Table 1 lists some of the genes and phenotypes described below.
TABLE 1.
Genes for ammonium metabolism and their expression during symbiosis
| Gene(s) | Function(s) of the product(s) | Mutant phenotype, free-living//symbiosisa (reference[s]) | Promoter activity
|
|
|---|---|---|---|---|
| Elongated/indeterminate | Globose/determinate | |||
| aapM | General amino acid permease | Rl unable to use glutamine-glutamate as C source//Nod+ Fix+ (204, 204a) | Unknown | Unknown |
| aatA | Aspartate aminotransferase | Sm unable to use aspartate//Nod+ Fix− partially empty nodules (207) | High levels of enzyme activity in BTs (207) | Unknown |
| cysG | Syroheme synthetase | Re unable to grow on NO3−//Nod+ Fix+ (178) | Unknown | Unknown |
| glnA/glnII/glnT | Glutamine synthetase I, II, or III | Single mutants show no phenotype (54)//single mutants are Nod+ Fix+ (37); Bj glnA glnII only nodule primordia (22) | pglnII ON in ITs, OFF in BTs of ZI and later on (183) | pglnII ON in ITs, OFF in BTs after endocytosis (179) |
| glnB | PII regulatory protein | Rl NtrC-activated promoters ON//Nod+ Fix+/− (3, 183); Sm NtrC-activated promoters OFF//Nod+/− Ara+ Fix−, abnormal ITs (5) | ON in ITs and in BTs of ZI and ZII; OFF in BTs of ZIII and later on (52) | ON in younger BTs; OFF in differentiated BTs (R. Tatè, unpublished data) |
| glnD | Uridylyl removing/transferase of PII | Rl constitutively deuridylylated PII, NtrC-activated promoters OFF//Nod+ Fix+ (159) | ON at all stages of BTs differentiation (159) | Unknown |
| glnK | AmtB (NH4+ uptake) regulator | No mutants described | Same pattern as that of pglnII (179) | As pglnII (179) |
| gltB/gltD | Glutamate synthase subunits | Glutamate auxotrophs//Sm or Re Nod+ Fix+ (24, 95); Bj and As Nod+ Fix− (71, 133) | High levels of enzyme activity in BTs (136) | High levels of enzyme activity in BTs (24) |
| gstI | glnII translation inhibitor | No mutants described | Same pattern as that of pnifR3-ntrBC (183) | As pnifR3-ntrBC (R. Tatè, unpublished) |
| nifA | Transcriptional activator | Early degeneration and lysis of BTs in both types of nodules (25, 58, 73, 209) | Same pattern as that of pnifHDK (212) | Unknown |
| nifHDK | Nitrogenase subunits | Sm Nod+ Fix−, ZIII not formed, early senescence (72, 73, 199); Bj and Re Nod+ Fix−, no early histological alterations, abnormal BT differentiation (8, 25, 58) | OFF in ITs and in BTs of ZI and ZII; ON in BTs of interzone II-III and in distal ZIII (144, 179, 180, 212) | OFF in ITs; ON in younger differentiating BTs (12, 180) |
| nifR3-ntrBC | Transcriptional regulator | Unable to use low concentration of NO3− or amino acids as N source//Nod+ Fix+ (108, 123, 174) | ON in BTs of ZI and ZII, OFF in N2-fixing BTs of ZIII and ZIV (144) | ON in ITs and in younger BTs; OFF in differentiated BTs (144) |
| putA | Proline dehydrogenase | Unable to use proline//Nod+/− Fix+ (80) | Same pattern as that of pnifR3-ntrBC (80) | Unknown |
Rl, Rhizobium leguminosarum; Re, Rhizobium etli; Bj, Bradyrhizobium japonicum; Sm, Sinorhizobium meliloti; As, Azorhizobium sesbaniae; Z, zone; ON, active; OFF, inactive.
Action on Early Steps of Symbiosis
The production of flavonoids, such as luteolin and naringenin (146, 163), that are secreted by the root cells is induced by stress of many types, including N starvation (28, 33). Flavonoids induce transcription of the bacterial nod genes and thus the production of lipochitooligosaccharides called Nod factors. The Nod factors are in turn able to elicit the induction of a nodule primordium in the specific host plant and to stimulate the plant to produce flavonoids (16, 39, 97, 171). Except for the case of Rhizobium leguminosarum (9), the flavonoid-mediated induction of nod gene transcription is abolished in the presence of NH4+, which appears to act through its assimilation products (48, 114, 205).
NH4NO3 is known to be a potent inhibitor of root hair deformation, initial cortical cell division, and IT formation, but its effect on the Nod factor perception has been excluded (70). The presence of NH4+ in the medium reduces the ability of Sinorhizobium meliloti to produce exopolysaccharides, which play a critical role in the process of nodule invasion (44). Although bacterial lipopolysaccharides are necessary for competitiveness (93) and nodule invasion (23, 203), no reports on the regulation of their structure and synthesis by NH4+ have been reported. Their structure is modified in response to the pH of the medium (175), and the latter is strongly influenced by the N source. Thus, differences in N metabolism may indirectly provoke the structural alterations of the lipopolysaccharides observed in bacteria growing inside the ITs (164). The addition of N compounds, including NH4+, lowers the expression and/or the activity of some nodulins, plant gene products that are either specifically expressed or highly induced in the nodule (27; for a review, see reference 156).
Positive and Negative Regulator of Nodule Development
NH4+ acts as a negative signal when added from without. This effect has been mostly studied on the elongated nodules of M. sativa and P. sativum. When combined N is added to nodulated roots, nodule growth is arrested and nodule endodermis grows to progressively surround the apical meristem, which becomes inactive. When the N source is removed, the apical meristem is reactivated, and thus nodule growth resumes (189; G. Truchet, personal communication). Addition of NH4+ (and NO3−) also inhibits nitrogenase activity, possibly not directly but after conversion to glutamine, or through the regulation of a plant function (173), e.g., the reduction of the O2 or C supply by the plant (15). In fact, it has been shown that isolated SBs do not take up NH4+ and that SBs in the nodule do not express the amtB gene, which is required for high-affinity NH4+ uptake activity (92, 179). Moreover, externally added NH4+ may never reach the SBs, since the NH4+-assimilating enzymes are highly expressed in the cytoplasm of ICs.
NH4+ produced through the action of the nitrogenase complex appears to be required as a positive signal necessary for normal development of indeterminate nodules (5, 199). Nodules induced by S. meliloti mutants unable to reduce N2 because of a mutation in one of the structural genes for nitrogenase (nifHDK), and thus unable to produce NH4+, show normal early organogenesis, but later on the N2-fixing zone (zone III) of the nodule is not formed, the apical meristem is inactivated, and early nodule senescence is observed (72, 73, 199). Therefore, completion of nodule development and sustained action of the meristem require nitrogenase activity. The regulatory signal for completion of nodule development may be either the NH4+ released by SBs or some other metabolic changes resulting from nitrogenase activity, such as enhanced consumption of O2 and/or reducing power.
The presence of normal Fix+ nodules inhibits the induction of further nodules on the root. In fact, plants inoculated with Fix− rhizobia show a greater number of nodules than in normal nodulation due to reinfections taking place on secondary roots (72). In conclusion, NH4+ produced in the nodule appears to act locally as a positive regulatory signal for nodule and SB maintenance, whereas it acts systemically as a negative signal inhibiting the reinfection process. Moreover, NH4+ produced in the nodule may repress flavonoid and isoflavonoid production (the latter compounds being involved in the defense reaction [for a review, see reference 16]). In fact, the chs (chalcone synthase) gene, involved in flavonoids and isoflavonoid biosynthesis, is induced in mature and invaded (18 days postinoculation) nodules elicited by a Fix− mutant of S. meliloti and not in nodules elicited by the wild-type strain (67). In nodules elicited by an S. meliloti strain mutated in the nifA regulatory gene, the SBs degenerate at an earlier stage of development than in nodules formed by a nifH mutant (73), indicating that NifA is involved in the expression of bacterial functions that are required for SB viability, in addition to those involving N2 fixation.
A different behavior is observed in the case of determinate nodules. When G. max and P. vulgaris are inoculated with a nifH mutant strain and Vigna unguiculata is nodulated in the absence of N2 (replaced by argon), the resulting nodules are similar (in shape, dimension, and histology) to normal nodules (8, 25, 58). This suggests that NH4+ is not a signal for nodule organogenesis and that younger BTs release NH4+ by a mechanism not involving nitrogenase action, such as the utilization of amino acids as a C source (see below). Instead, if these plants are infected with a nifA mutant strain, SBs are degraded at an early stage (25, 58, 209). This indicates that the nifA regulatory gene, as in the case of elongated nodules, is required for SB maintenance, while nifH, and thus nitrogenase activity, is not (60, 129).
Action on Symbiosomes
During development of both types of nodules, SBs undergo profound physiological and morphological changes. One of the most relevant is that, even though reached in different ways, their surface/volume ratio decreases during differentiation, reducing the capacity of SBs to exchange metabolites (and/or signals) with the plant.
SBs of elongated nodules during nodule development maintain a single BT, which increases up to 30-fold in size and shows polar swellings and Y shapes (199). Moreover, BTs undergo significant changes in DNA content and in the ratio of chromosomal to symbiotic plasmid DNA (14, 202, 210). The morphological changes of SBs become evident after the arrest of BT divisions occurring in the middle of zone II (Fig. 1) and thus before the onset of nitrogenase activity. The same shape modifications are observed in nodules elicited by Fix− strains and when free-living bacteria are treated with cell cycle inhibitors (94) and C4-dicarboxylic acids (193), excluding the involvement of NH4+ in this process. However, the appearance of C4-dicarboxylic acids may provoke, at the corresponding stage of differentiation, severe changes in N metabolism, such as the inhibition of the utilization of amino acids as a C source and thus of NH4+ excretion (see below). Furthermore, the completion of SB differentiation takes place after nitrogenase activation (199).
Globose nodules develop SBs formed by multiple rod-shaped BTs, which do not increase in size (25, 34). The number of BTs per SB appears to increase more by fusion of younger SBs than by BT division inside a single membrane vesicle (25, 55, 127). Moreover, the merging of SBs proceeds through the fusion of their membranes. It increases during nodule senescence, when BT division stops, and appears to be related to the N status of the cell, since it is also accelerated when the plant is infected with Fix− mutants (25). Lack of nitrogenase activity, and therefore of NH4+ production, might cause a change in pH at the cytoplasmic surface of SBs, thus activating a homotypic mechanism of membrane fusion that may involve SNARE proteins (49).
NH4+ METABOLISM
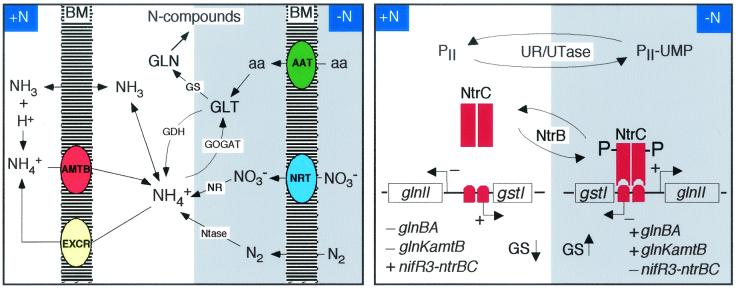
Rhizobia are able to take up and assimilate NH4+ from the external medium, to produce it intracellularly from N2, nitrate, and amino acids, and to excrete it when in excess (Fig. 3).
FIG. 3.
NH4+ metabolism (left) and regulation of NH4+ assimilation (right), at high (left side of each panel) and low (right side) N concentrations. (Left panel) NH3 is supposed to diffuse freely through the bacterial membrane (BM), while NH4+ requires a transporter (AmtB) for uptake and is supposed to need a different one for excretion (EXCR). N sources alternative to NH4+ are amino acids (aa), which require specific transport systems (AAT), and NO3−, requiring a transport system (NRT) (for reviews, see references 64, 82, and 121), and N2. Ntase, nitrogenase; NR, nitrate reductase; GOGAT, glutamate synthase; GDH, catabolic glutamate dehydrogenase; GS, glutamine synthetase isozymes; GLT, glutamate; GLN, glutamine. (Right panel) Gene symbols are described in the text. A switch from high N (left side of the panel) to low N (right side) causes inactivation (uridylylation) of the PII protein (PII-UMP), phosphorylation of the NtrC protein (NtrC-P), and its binding to a sequence located at the glnII-gstI intergenic region, thus activating glnII and repressing gstI expression. The effect of NtrC-P on the transcription of other genes, such as glnBA, glnK-amtB, and nifR3-ntrBC, is indicated. PII-UMP also mediates the posttranslational deadenylylation of GSI and thus its activation.
NH4+ Uptake
NH4+ is in equilibrium with NH3, the pKa being 9.25, and it is assumed that NH3 crosses most but not all (86) biological membranes by unmediated diffusion and thus supports bacterial growth under certain conditions (see references 87 and 169 for discussion). Conversely, NH4+ crosses the membranes only through specific transporters. Evidence for a low-affinity NH4+ uptake system has been recently reported for Corynebacterium glutamicum (112). A high-affinity, energy-dependent transport system for NH4+, detected by uptake assays with radioactive methylammonium (an NH4+ analogue), has been described in many bacterial species, including rhizobia (77, 87, 134, 179). In rhizobia this system is activated under poor N conditions, such as the utilization of nitrate and glutamate as the sole N source, either to retrieve the NH3/NH4+ lost to the outside or to sense their extracellular concentration (179, 191).
The amtB gene from Azorhizobium caulinodans and Rhizobium etli has been shown to be required for high-affinity NH4+ transport (117, 179). This gene codes for an integral membrane protein highly conserved in many organisms (184, 185) and it is cotranscribed with the glnK gene (coding for a PII-like protein), forming a glnK-amtB operon. This operon is expressed from a promoter located upstream of glnK, whereas no promoter activity was detected in the intergenic region (179). The glnK promoter becomes inactive very early during symbiosis, and in correlation isolated BTs do not show high-affinity NH4+ uptake activity (92, 134, 179, 180). Downregulation of amtB expression is essential for the normal function of BTs (see below).
Nitrogen Fixation
The organization and regulation of the rhizobial genes coding for the nitrogenase complex have been reviewed (59). Their transcription is dependent on the NifA protein, which is expressed and/or activated under specific environmental conditions, including low O2 tension (11). In S. meliloti the expression of the nifA gene under microaerobic free-living conditions requires the FixLJ two-component system and is negatively regulated by NH4+ through a process mediated by the FixL protein (35, 131). The posttranslational inactivation of nitrogenase mediated by the draGT genes and induced by NH4+, reported for other members of the diazotrophic α-proteobacteria (for a review, see reference 6), has not been identified in rhizobia.
In free-living diazotrophs such as Klebsiella pneumoniae, the NH4+ produced through the action of nitrogenase is assimilated for bacterial growth. The nitrogenase complex may be induced in free-living rhizobia but, with the exception of A. caulinodans and Rhizobium trifolii strain 0403 (40, 194), most of the NH4+ produced is excreted (132, 194). Therefore, in rhizobia, N2 fixation and assimilation of the NH4+ produced are uncoupled, and this is essential for an effective symbiotic interaction. In fact, plants nodulated by strains unable to express an active nitrogenase complex and unable to release the NH4+ produced show signs of N deprivation, which are relieved by exogenously added combined N (5, 72, 73). However, it has recently been reported that an ntrR mutant strain of S. meliloti induces nodules with an enhanced N2 fixation capacity due to the higher expression of nif genes (135). Moreover, this phenotype is not abolished by the exogenous addition of N, suggesting that in S. meliloti the expression of the nif genes is also N regulated during the symbiosis.
The symbiosis may be viewed as an exchange of photosynthate (in the form of C4-dicarboxylic acids) for fixed N, produced by the SBs (80a, 147, 192a). During SB differentiation, BTs of globose nodules accumulate polyhydroxybutyrate and glycogen granules, perhaps because their capacity to fix N2 decreases while C supply is still plentiful. In fact, ineffective nodules of P. vulgaris induced by a Fix− R. etli mutant strain show an earlier and higher accumulation of polyhydroxybutyrate compared to effective nodules (25). On the other hand, nodules induced by a mutant strain blocked in polyhydroxybutyrate biosynthesis show a higher and prolonged nitrogenase activity compared to nodules induced by the wild-type strain, indicating that nitrogenase and polyhydroxybutyrate biosyntheses compete for reducing power (26). Rhizobium tropici mutants unable to synthesize glycogen also show a better symbiotic performance (107a).
Internal Production from Nitrate and Amino Acids
NH4+ is produced inside rhizobia from nitrate (Fig. 3), which in the soil is formed by oxidation of NH4+ from residual fertilizer and mineralization of organic N and may reach concentrations up to 20 mM (for a review, see reference 213). Most species grow on NO3− as a sole N source and possess an assimilatory NO3− reductase, although some of them, including R. etli, do not express it in isolated SBs (104). A mutant strain of R. etli unable to reduce NO2− because of a mutation in the cysG gene and consequent lack of the siroheme cofactor does not grow on NO3−, but it induces effective nodules (178). Moreover, mutants of Bradyrhizobium japonicum lacking assimilatory NO3− reductase elicit the formation of normal nodules with somewhat better N2 fixation characteristics compared to the parental strain (41). An R. leguminosarum glnB mutant strain lacking the regulatory PII protein does not grow on NO3−, but it is Nod+ and invades developing nodules (3). In conclusion, the rhizobial ability to assimilate NO3− is dispensable either for its growth during nodule invasion or for SB activity, even though NO3− may be the most abundant soil N source and at low levels stimulates nodulation (154, 213).
NH4+ is also produced from amino acids, which, like NO3−, behave as poor N sources in the presence of a rich C source. Unlike enteric bacteria, free-living rhizobia (and Pseudomonas aeruginosa) can use a single amino acid (such as glutamine, asparagine, or histidine) as the sole source of C and energy (99). Some mammalian cell lines also use glutamine as the principal energy source in the presence of hexoses (for a review, see reference 151). Amino acid utilization requires active uptake, deamination/deamidation, common metabolic pathways, such as the citric acid cycle and gluconeogenesis, and NH4+ excretion.
R. etli mutants showing very low levels of glutaminase, asparaginase, and aspartase activity are Nod+/Fix+ (46, 47, 50, 79). Enzymes involved in gluconeogenesis are expressed in BTs of R. leguminosarum, S. meliloti, and Rhizobium sp. strain NGR234 (57, 111, 137), and R. etli mutants impaired in this pathway induce only a few nodule primordia (R. Tatè, unpublished). Mutant strains altered in sugar catabolism are Nod+/Fix+ (for a review, see reference 204a). In the utilization of glutamine and histidine as a C source, glutamate is produced, which, for further catabolism, requires a dissimilatory glutamate dehydrogenase (GDH), distinct from the assimilatory one (99). Rhizobial mutants lacking this enzyme have not been described, but some mutants altered in glutamate catabolism show an altered symbiotic phenotype (see below). However, since aap (general amino acid permease) mutant strains of R. leguminosarum are unable to grow with glutamate as a carbon source but are Nod+/Fix+, it has been concluded that glutamate is not an important carbon and energy source in BTs of R. leguminosarum (204).
Assimilation
Assimilation of NH4+ in enteric bacteria takes place through the GS/GOGAT (glutamine synthetase/glutamate synthase) pathway and, at higher NH4+ concentrations, through GDH (for a review, see reference 152). As shown below, assimilatory GDH appears to be absent in rhizobia, while there are two GS isozymes, GSI and GSII. In some species a third GS has been identified (30, 53, 54, 162), which in free-living rhizobia is expressed only under special conditions. Why rhizobia express more than one GS isozyme remains an unresolved question. The present evidence is in agreement with the hypothesis that GSI is preferentially used for the assimilation of external NH4+, while GSII appears to be preferred for the assimilation of internal NH4+ when it is produced at low concentrations.
GSI, encoded by the glnA gene, is a dodecameric enzyme similar to the GS present in enteric bacteria. The intracellular concentration of GSI increases two- to fourfold when R. leguminosarum is grown on a poor N source compared to a rich one, while its activity is regulated by reversible adenylylation (153). When R. leguminosarum is switched from N-rich to N-poor conditions, GSI activity is essential to allow an efficient rate of GSII derepression (183). This suggests that under these conditions, GSI is required for a prompt synthesis of glutamine to be used for protein synthesis. Regulation of GSI concentration and activity (adenylylation) is similar in other rhizobial species (for a review, see reference 116).
GSII, encoded by the glnII gene, is a heat-sensitive (relative to GSI) octameric enzyme (103). The expression of the glnII gene is activated under N deficiency (for a review, see reference 54) and requires an upstream activating sequence (140). When R. leguminosarum is switched to an NH4+-containing medium, glnII transcription is 10-fold repressed, but GSII activity and the GSII protein become undetectable (103, 140). That this is due to a posttranscriptional control mechanism of glnII expression has recently been demonstrated (172, 183). The inhibition of glnII mRNA translation is mediated by the product of the gstI gene, located upstream and transcribed divergently of glnII. Under N-excess conditions, glnII transcription is low and gstI transcription is high, while the opposite is found under N-limiting conditions (Fig. 3, right panel).
The gstI gene codes for a small protein (63 amino acids) that inhibits translation of glnII mRNA, probably through direct binding to the 5′ untranslated region. In fact, in silico remote modeling predicts that the GstI protein may fold to match a domain of the Escherichia coli glutaminyl-tRNA synthetase. On the other hand, the 5′ untranslated region of glnII mRNA may adopt a secondary structure involving two loops, both of which contain the UUG anticodon sequence for glutamine (172, 183). The reason for the presence of such a sophisticated regulation of glnII expression is unknown. It is possible that GstI not only inhibits translation but at the same time stabilizes glnII mRNA for prompt expression when the intracellular concentration of glutamine becomes limiting. Under microaerobic free-living conditions as well as in isolated BTs of B. japonicum, glnII mRNA but neither GSII activity nor GSII protein was detected, and thus a posttranscriptional mechanism of regulation of glnII expression has been hypothesized (108). However, no evidence for the presence of the gstI gene in this bacterium has been reported. GSI but not GSII activity was detected in isolated BTs of R. etli (122).
Very low levels of GS activity are found in isolated N2-fixing SBs, and genetic manipulation indicates that none of the GS isozymes is necessary for effective symbiosis (37, 54, 116, 192). However, a glnA glnII double mutant of S. meliloti induces normal invaded nodules (37), and GSIII activity was detected (162). A glnA glnII mutant of B. japonicum is severely affected in nodulation (22), and GSIII is absent in this species. These results apparently lead to contradictory conclusions that may be clarified if the symbiotic interaction is considered as a multistage process. In fact, while the ability of rhizobia to invade developing nodules may be dependent on the capacity to synthesize glutamine, GS activity appears to be dispensable for BT function.
Assimilatory GDH activity (Fig. 3, left panel) is absent in R. leguminosarum, and consequently this bacterium grows better on glutamine than on NH4+ as the sole N source (153). It is also absent in other rhizobial species (18, 76, 100, 114). This suggests that assimilatory GDH activity is not needed for efficient nodulation and function. In contrast, it has been shown that the expression of the E. coli gdhA gene, coding for an assimilatory GDH, reduces nodulation ability when expressed in R. etli, whereas if expressed in N2-fixing BTs, it has detrimental effects on nodule efficiency (see below).
GOGAT activity is present in both free-living rhizobia and isolated SBs (20), and the structural genes, gltB and gltD, coding for GOGAT in R. etli have been isolated (24). Due to the absence of assimilatory GDH activity, mutants lacking GOGAT activity are glutamate auxotrophs (24, 45, 88, 95, 133, 136) (Fig. 3). S. meliloti and R. etli mutants lacking GOGAT are not altered in their symbiotic behavior (24, 95). Thus, during symbiosis, glutamate is provided in the nodule by the plant and is synthesized through a different pathway, such as amino acid degradation. In the case of R. etli, lack of GOGAT activity causes an enhancement of nodulation efficiency, probably correlated to an increased ability of BTs to release fixed N (24). In contrast, strains of B. japonicum and A. caulinodans lacking GOGAT are defective in symbiotic N2 fixation (71, 133).
NH4+ Efflux
When rhizobia use either histidine or glutamine as the sole C source, due to the stoichiometry of the C and N requirements for bacterial growth, NH4+ is excreted and the extracellular concentration of NH4+ may become as high as 90 mM (42, 46). Since high intracellular concentrations of NH4+ are toxic, the extracellular levels are probably reached by means of an active mechanism of efflux (Fig. 3, left panel), but neither bacterial genes nor mutations involved in NH4+ efflux in rhizobia and other bacteria have been described. It has recently been proposed that the human Rhesus-associated RhAG proteins are involved in NH4+ efflux of mammalian cells. In fact, these proteins, showing similarity to members of the AMT/MEP NH4+ transporter family (105), facilitate the NH4+ efflux when expressed in Saccharomyces cerevisiae (106). Other members of the Rh superfamily are predominantly expressed in organs (liver, kidney, and skin) involved in ammonia genesis, excretion, and secretion (96). Glutamine (the most abundant amino acid in plasma) is the preferred oxidation substrate of erythrocytes (150) and of other mammalian cell lines (for a review, see reference 151), suggesting the necessity for NH4+ efflux.
In the rhizobium-legume symbiosis, most of the N2 fixed in BTs is liberated to the host plant to satisfy its demand for N, and consequently the growth of nodulated plants becomes almost independent of alternative N sources (69). The form (e.g., NH3/NH4+ and organic compounds, such as amino acids) and the mechanism (passive diffusion and transport) by which fixed N is exported are unclear. B. japonicum SBs isolated from crushed nodules have been shown to excrete alanine (206). R. leguminosarum SBs excrete different ratios of alanine and NH4+ according to the experimental conditions used (open or closed systems), but NH4+ excretion prevails in an open system where the supernatant is continuously removed (2, 147). It should be pointed out that in an intact nodule the N compounds excreted by SBs are promptly utilized by the plant, which is known to derepress NH4+ assimilation activities, as for example a nodule-specific GS (for a review, see reference 156). Therefore, the primary form excreted in the symbiosis by SBs is most likely NH4+, whereas other compounds like alanine are synthesized when NH4+ accumulates either in vitro (closed chamber) or in vivo when the NH4+ assimilation capacity of the plant is reduced (2, 13).
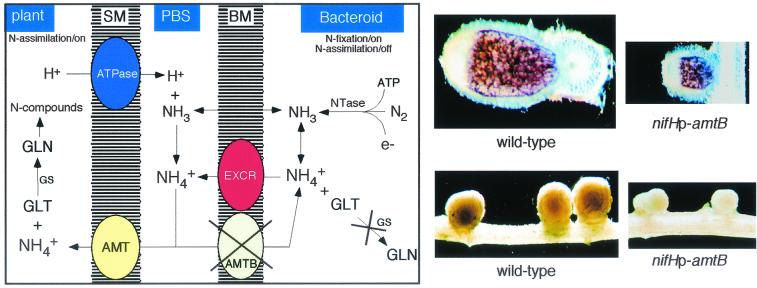
It has been proposed (36, 192) that NH3 might passively diffuse across the BT membrane into the relatively acidic peribacteroid space, where it is converted into NH4+. However, the present evidence does not exclude the existence of a channel for NH3 or NH4+ efflux in free-living bacteria and in BTs during symbiosis (Fig. 4, left panel). The NH3/NH4+ diffused or excreted outside the BTs reaches the plant cytosol through a channel located on the SB membrane and capable of transporting NH4+ (190) or through a channel mediating NH3 flux (36). However, the plant gene(s) involved in this process remains unrecognized (81, 107). By complementation of a triple MEP mutant strain of Saccharomyces cerevisiae, genes coding for NH4+ transport systems have been identified in different plants (for a review, see reference 78) and recently in the legume Lotus japonicus (155). One of the MEP genes, coding for the high-affinity NH4+ uptake system of S. cerevisiae, may act as a sensor for extracellular NH4+ (98).
FIG. 4.
NH4+ flux and assimilation inside invaded cells (left panel). NH3 produced through nitrogenase (Ntase) action diffuses freely through the BT membrane (BM) and is converted into NH4+, which cannot be assimilated into glutamate (GLT) and glutamine (GLN) through glutamine synthetase (GS) action and is excreted, probably by an active mechanism (EXCR) into the peribacteroid space (PBS), where an ATPase present on the symbiosome membrane (SM) pumping protons keeps a high NH4+/NH3 ratio, and a transport system for NH4+ uptake (AMT) moves NH4+ to the plant cytoplasm, where is assimilated. Note that, as described in the text, in BTs the NH4+ transporter AmtB is not expressed. (Right panel) Top: Longitudinal sections of V. hirsuta nodules elicited by the wild-type strain of R. leguminosarum and by the same strain carrying the amtB gene under the control of the nifH promoter. Bottom: Nodules of Phaseolus vulgaris induced by the wild-type strain of R. etli and by the same strain expressing amtB from the nifH promoter.
REGULATION OF NH4+ ASSIMILATION UNDER FREE-LIVING CONDITIONS
ntr Regulatory System
The regulation of N metabolism is best known in enteric bacteria, where the global N regulatory (ntr) system has been described (for reviews, see references 116 and 152). The ntr system senses the ratio of the intracellular concentration of glutamine to α-ketoglutarate and, through a cascade of regulatory proteins, regulates the activity of different promoters by means of a two-component system, comprising the protein kinase NtrB and the transcriptional regulator NtrC (Fig. 3, right panel). Most studies on the rhizobial ntr system have used the knowledge of the enteric one as a paradigm, even though differences in gene organization and regulation were found.
The R. etli ntrBC genes are not cotranscribed with glnA as in enteric bacteria, but with an open reading frame (ORF) that shows significant homology at the amino acid level with an ORF located upstream of the fis gene of E. coli and named yhdG (141). The YhdG protein belongs to the NifR3-SMM1 protein family of unknown function (Prosite PS01136). The same gene organization has been described for Azospirillum brasilense, Mesorhizobium loti, and Rhodobacter capsulatus (62, 82, 102). In the following description, the ORF1-ntrBC operon has been renamed nifR3 ntrBC. The nifR3 ntrBC operon of R. etli is transcribed from a promoter containing a sequence homologous to the −35/−10 consensus, identified by deletion analysis and runoff transcription experiments using specific oligonucleotides as DNA competitors (110). The switch from N-rich to N-poor conditions causes a 14-fold increase in NtrC concentration in enteric bacteria, but no increase (actually a 2-fold decrease) in R. etli. Under N deficiency, the DNA-binding activity of NtrC increases, and as a consequence some promoters (such as glnII) are activated, whereas others (such as gstI) are repressed (Fig. 3) (140-143, 183). This shows that, while in enteric bacteria N availability regulates both the intracellular concentration of NtrC and its activity, in R. etli only NtrC activity is regulated (110, 142). On the other hand, a mutant strain expressing a truncated form of the NtrC protein shows an intracellular concentration of nifR3 ntrBC mRNA at least 20-fold higher than that of the wild-type strain (141). This demonstrates the presence of a mechanism of autogenous regulation acting on either promoter activity or mRNA stability.
In R. etli the ntrC gene does not affect the utilization of either glutamine or histidine as the sole N source when added at 1 g/liter (123). Moreover, an ntrC mutant strain grows on 10 mM nitrate, but not on 3 mM (123). The same phenotype was observed in ntrC mutant strains of B. japonicum and S. meliloti (108, 174). It is possible that lack of growth at low nitrate concentrations is a consequence of a reduced bacterial capacity to maintain NH4+ inside the cells and/or to assimilate it. In fact, the genes involved in NH4+ uptake and assimilation (like amtB and glnII) are highly expressed in an NtrC-dependent mode in bacteria grown on nitrate (140, 143, 179). Moreover, it has been reported that the majority of the N limitation-induced genes are not regulated by the ntr system (119). It seems, therefore, that in rhizobia the main function of NtrC is the transcriptional regulation of genes involved in NH4+ metabolism.
In free-living diazotrophs, NtrC is necessary for expression of the nifA gene, which in turn activates expression of the other nif promoters (43), and as a consequence ntrC mutants are unable to grow when N2 is the sole N source. By contrast, different rhizobia mutated in the ntrC gene are Nod+ Fix+ (108, 123, 174), showing that NtrC is not essential either for activation of nif and fix genes or for an efficient symbiosis. However, P. vulgaris plants nodulated with an R. etli ntrC mutant are invaded faster (R. Tatè, unpublished) and accumulate more N than plants nodulated with the wild-type strain (165). This suggests that NtrC exerts a negative control on other functions related to the symbiotic interaction. In fact, in S. meliloti, NtrC regulates nod gene expression as a function of the NH4+ concentration (48). In R. leguminosarum, NtrC represses the expression of the aap (general amino acid permease) operon (204), and in R. etli NtrC prevents the expression of the symbiotic terminal oxidase (167) and affects the degree of exopolysaccharide polymerization (C. Giordano and E. J. Patriarca, unpublished data). Moreover, NtrC is necessary for the alteration of nodulation exerted by the ectopic expression of the amtB and gdhA genes (114, 180).
PII Regulatory Protein
In bacteria, the PII protein, encoded by the glnB gene, has a key role in the regulation of NH4+ metabolism. In fact, PII is involved in the covalent (and reversible) modification of both NtrC (through phosphorylation) and GSI (adenylylation), and thus in the regulation of their activity (5, 31, 32, 74, 75). In S. meliloti, B. japonicum, and R. leguminosarum, glnB is cotranscribed with glnA (GSI), forming a glnBA operon. The same gene organization is present in the genome of M. loti (82). An N-regulated promoter, activated by NtrC and located upstream of glnB, drives the generation of glnBA transcripts (29, 52, 109). Hence, glnB expression in R. leguminosarum is N regulated (fourfold lower in NH4+ compared to NO3−), while it is constitutive in enteric bacteria (for a review, see reference 84). The PII proteins of R. leguminosarum and S. meliloti, as in enteric bacteria, are subject to covalent uridylylation, which modulates their activity (6, 32, 159). The degree of uridylylation is high under N limitation and undetectable under N excess (32). In bacteria growing in an NH4+-rich medium, unmodified PII stimulates the adenylylation of GSI, inactivating the enzyme (4, 65). However, the influence of the different forms of PII on the activity of NtrC varies among different rhizobia.
In R. leguminosarum the PII protein is essential to inactivate (dephosphorylate) NtrC under conditions of N excess, but it is dispensable for the activation (phosphorylation) of NtrC, which, in the absence of PII, becomes active independently of the N conditions (Fig. 3, right panel). In fact, transcription of the glnII gene (requiring NtrC for expression) is constitutive in a glnB mutant (3). In a glnD mutant lacking uridylyl removing/uridylyltransferase (UR/UTase), the enzyme catalyzing uridylylation/deuridylylation of PII, the latter is constitutively deuridylylated, and therefore NtrC cannot be activated. As a consequence, the glnII promoter also remains silent under N-limiting conditions (159). An R. leguminosarum glnB mutant induces normal early organogenesis (3), but nitrogenase is only weakly expressed compared to the level in nodules induced by the wild-type strain (183). In contrast, a glnD mutant strain elicits the formation of normal, N2-fixing nodules (159). Altogether, these data indicate that PII, in its deuridylylated form, is essential for normal levels of nitrogenase expression, most probably due to the inactivation exerted by PII on NtrC and/or GSI, ensuring a reduced NH4+ assimilation capacity of BTs (see below). This is supported by the observation that NtrC-activated promoters are switched off, in a PII-dependent manner, very early during symbiosis (183).
In S. meliloti PII is instead essential to activate (phosphorylate) NtrC under conditions of N starvation, and as a consequence, NtrC-dependent promoters are inactive in a glnB mutant (5). When M. sativa is infected with this mutant strain, most ITs abort early. Since strains lacking NtrC are able to induce efficient nodules, the abnormal phenotype of the glnB mutant cannot be explained by a requirement for NtrC phosphorylation. Therefore, PII should be involved in the regulation of another bacterial function(s) essential at the onset of nodulation (growth in the ITs), such as the activation of genes needed for the synthesis of Nod factors, exopolysaccharides, and lipopolysaccharides. Otherwise, a constitutively dephosphorylated NtrC might repress and alter the expression of the above-mentioned genes. The phenotype of the S. meliloti glnB mutant strain cannot be explained by an action of the PII protein on GSI adenylylation, since a glnA mutant strain impaired in adenylylation of GSI shows a normal symbiotic phenotype (4).
In the R. leguminosarum glnB mutant, the NtrC-activated genes (such as glnII and glnK-amtB) are highly expressed independently of the N source, but this strain does not grow on nitrate (3). It appears therefore that PII activates another function, as yet unknown, required for nitrate utilization.
As mentioned above, a second glnB-like gene, glnK, located upstream of the amtB gene is present in different rhizobia (6, 82, 179). In R. etli the glnK-amtB operon is transcribed from a σ54-dependent promoter requiring NtrC for activity (179). This promoter does not show a clear-cut sequence for NtrC binding, and therefore its activation by NtrC may be indirect. In some bacterial species, glnB and glnK are functionally equivalent (for a review, see reference 6). In rhizobia the function of GlnK is unknown, although it has recently been shown that in E. coli and Azotobacter vinelandii, GlnK binds to the membrane in an AmtB-dependent manner and that GlnK acts as a negative regulator of the transport activity of AmtB (33a). This interaction represents a novel form of signal transduction pathway sensing extracellular NH4+.
In A. caulinodans, a second two-component system (NtrY/NtrX) showing considerable homology to NtrBC has been identified (145) and found to regulate the expression of NtrC and NifA. A. caulinodans is a diazotrophic bacterium in which NtrC mediates the induction of nifA transcription under N limitation. Homologous genes are also present in M. loti, R. etli, and S. meliloti (64, 82; E. J. Patriarca and M. Merrick, unpublished data), but no evidence for their presence has been reported in the case of enteric bacteria. Sequence analysis predicts that NtrY (the homologue of the protein kinase NtrB) may be membrane associated through a transmembrane element. Therefore, NtrY may modulate the activity of NtrX (the NtrC equivalent) as a function of the extracellular N. If so, its function might be linked to that of the glnK-amtB operon.
REGULATION OF NH4+ ASSIMILATION IN DIFFERENTIATING SYMBIOSOMES
In Situ Detection
Most studies describing the presence of NH4+ assimilation enzymes in nodules use an in vitro biochemical approach. This means crushing nodules, purifying BTs and intact SBs, and assaying a specific gene product. Although direct and quantitative, it assumes reproducibility in the yield of isolation of SBs at different stages of development, the absence of contaminating plant products, and stability during the isolation procedure. The results obtained are the average values of a heterogeneous population of SBs and therefore are of limited usefulness for the understanding of in situ NH4+ metabolism in the nodule. Moreover, SBs analyzed in vitro are out of their natural environment, namely the cytoplasm of the plant cell, which may explain some of the contradictions that are found in the literature when comparing results obtained through biochemical and genetic methods.
Studies on the pattern of gene expression in developing nodules clarify the above contradictions. Although in situ localization of bacterial mRNA has been reported (158, 212), the histochemical localization of the product of a reporter gene (gfp, gusA, and lacZ) is more generally used because of the simplicity and efficacy of the method (7, 63, 161). Most studies make use of lacZ fusions because the protocol used to lower the background of β-galactosidase activity (7) and the use of stable plasmids (170, 208) permit clear establishment of when and where during nodulation a bacterial promoter is active.
As shown below, the in situ analysis of the activity of different promoters in the elongated nodule confirms and extends the conclusions reached by histological analysis (186, 199). In fact, with respect to the regulation of NH4+ metabolism in V. hirsuta nodules, this kind of analysis confirms the activation of nif gene expression in BTs of SBs located at the II-III interzone (144, 179) and allows the description of two previously unrecognized metabolic events (Fig. 1). The first one is the downregulation of genes required for NH4+ uptake and assimilation, observed when bacteria are released, by endocytosis, from the ITs into the meristematic host cells. The second one takes place later on, a few cell layers before the II-III interzone, where promoters of several N-regulatory genes (including gstI, ntrC, and glnB) are switched off.
In the globose nodule of Proteus vulgaris, the in situ analysis of bacterial promoters, together with earlier evidence of gradients of expression of eukaryotic gene products in this nodule (176), has been instrumental in revealing a spatial component of development. In particular, downregulation of the nifR3-ntrBC promoter clearly defines a previously unrecognized developmental zone (144). Furthermore, the analysis of the nifHc promoter indicates that nif genes are activated much earlier in the globose nodule than in the elongated nodule (180). Table 1 lists the topological expression of bacterial genes in nodules.
Expression of Bacterial Genes
The promoters of the glnK-amtB operon of R. etli and of the glnII gene of R. leguminosarum are N regulated and strictly dependent on NtrC for activation (see above) (140, 179). Their activity is lower in bacteria growing on glutamine as a sole C source (thus causing NH4+ efflux) than when growing on NH4+ (R. Tatè, unpublished). In the symbiosis, these promoters are switched off very early during nodule invasion (179, 183). In both elongated and globose nodules (Fig. 1 and 2), both promoters are active in bacteria growing inside the ITs but become inactive just when bacteria begin to invade nodule cells and thus are the earliest negatively regulated promoters so far recognized. In fact, transcription of nod genes, which mediates the nodule primordium induction, is switched off later during SB differentiation (161).
The gstI promoter in free-living R. leguminosarum is induced in an N-rich medium, while it is repressed under N-poor conditions (183). In developing nodules of V. hirsuta, the gstI promoter is active in SBs released in the first ICs of the inner cortex during primordium formation. In 1-week-old nodules, a decreasing distal-proximal gradient (with respect to the root) is observed, which becomes more evident later on, when the activity is restricted to the SBs present in the most distal cells of the invasion zone (zone II according to reference 199) (Fig. 1). The gstI promoter becomes inactive before transcription of the nif genes is activated (183). The pattern of gstI expression suggests that dividing BTs located in the distal part of zone II of elongated nodule sense a signal of N excess, whereas the most proximal ones are under severe N limitation and are unable to activate the gstI promoter. In the presence of GstI, glnII expression in BTs may be inhibited not only at the transcriptional level but also at the posttranscriptional level, as occurs under free-living conditions (183).
The R. etli nifR3-ntrBC promoter in V. hirsuta nodules is active in bacteria growing in ITs and in dividing BTs located in differentiating host cells of the distal zone II. It becomes inactive in the SBs of the last two or three cell layers before nif gene expression is switched on (Fig. 1) (144). The same pattern of expression is observed in Fix− nodules, confirming that downregulation of the nifR3-ntrBC promoter is not due to the synthesis of NH3 produced through nitrogenase activity. In globose nodules of P. vulgaris, the nifR3-ntrBC promoter is active in bacteria growing in the ITs, in the BTs released in the first ICs of the primordium, and in all ICs of early emergent nodules. In developed nodules, expression is limited to the BTs located in the two and three most peripheral layers of ICs (Fig. 2) (144). Immunolocalization of NtrC confirms this pattern of expression and shows that not only is transcription of the nifR3-ntrBC operon switched off but also that, since BTs at this stage do not divide, the NtrC protein is removed.
The R. leguminosarum glnBA promoter in V. hirsuta nodules shows a pattern of activity quite similar to that of the nifR3-ntrBC and gstI promoters, since it is active in bacteria growing inside the ITs and in BTs released in the first ICs of the inner cortex during primordium formation. In more mature nodules it is switched off, in correlation with the disappearance of NtrC, a few cell layers before nif gene expression (52).
In nodules of P. vulgaris, the R. etli nifHc promoter is induced at a very early stage of nodule invasion, namely, in BTs just released in the plant cells (180). This promoter is induced in a NifA-dependent manner both under free-living microaerobic conditions and during symbiosis (195). Instead, in elongated nodules the R. etli nifHc promoter is switched on abruptly in SBs located at interzone II-III (144), exactly where predicted by an in situ hybridization analysis revealing the endogenous nifH mRNA (212). In interzone II-III, the ICs are filled with nondividing, almost completely differentiated SBs (199), and a sharp drop in O2 tension takes place (168) (Fig. 1). The earlier timing of NifA activation, and therefore of nifHc expression, in globose versus elongated nodules may be related to differences either in the organizational structure of the parenchyma (211) or in plant and/or bacterial metabolism, which generate a low O2 tension environment (91, 120).
That the invasion of globose nodules takes place under microaerobic conditions is supported by the following data. First, in the youngest ICs of developing nodules of P. vulgaris, harboring only a few SBs formed by dividing BTs, a large number of condensed (crista-rich) mitochondria, which cluster, aggregate, and divide, are observed (25). Second, a terminal oxidase complex, which is expressed under microaerobic conditions in free-living bacteria, is required for the invasion process, since a cytochrome c-deficient mutant of R. etli induces empty nodules (166). Third, nitrogenase activity (requiring microaerobiosis) is detected in young soybean nodules, in which most of the BTs in the host cells are still actively dividing (12). These data indicate that the nitrogenase complex is induced in young dividing BTs during the invasion of globose nodules, even though the analysis of nif mutants indicates that nitrogenase activity is not essential for invasion.
ntr System
The glnK-amtB and glnII promoters become inactive in younger BTs when the nifR3-ntrC promoter is still active and the NtrC protein is present (see above). This suggests the inactivation of NtrC, because of a signal of N excess transmitted by PII, and the activation of a different regulatory circuit involving a specific repressor(s) operating on both promoters. The availability of an R. leguminosarum glnB mutant and the in situ analysis of the glnII (NtrC-activated) and gstI (NtrC-repressed) promoters allowed us to test whether the ntr system senses the N status of SBs. In dividing BTs located in the ICs of the distal zone II of nodules induced by the wild-type strain, the NtrC-repressed gstI promoter is active, whereas the NtrC-activated glnII promoter is inactive (Fig. 1 and 2). In contrast, in nodules induced by the glnB mutant, the gstI promoter is switched off during endocytosis, whereas the glnII promoter remains active (Fig. 1) (183). The same reciprocal pattern of regulation occurs in wild-type and free-living glnB bacteria. This indicates that PII is necessary for regulation of NtrC activity not only in free-living bacteria but also in BTs. The PII-mediated inactivation of NtrC is the first recognized step in the molecular mechanism leading to the uncoupling of NH4+ assimilation and N2 fixation in differentiated SBs. This event occurs early during host cell invasion, just when bacteria are released into the host cell, and therefore much earlier than when N2 fixation begins to take place.
During symbiosis, the N status of rhizobia is determined by the composition of the medium in which they are immersed. The analysis of ntr-regulated promoters indicates that during endocytosis, the N metabolism of BTs changes from a condition of N deficiency to one of N richness (and C deficiency, which would result in a relative abundance of available N). The movement of the bacterium from a C-rich environment, the cell wall-like matrix of the ITs, into the intracellular environment of the BTs, which are surrounded only by the SB membrane, may reduce C availability. A condition of C limitation and N excess may also be generated by the utilization of amino acids as a C source (see below). A second factor causing a condition of C deficiency is the permeability of the SB membrane. The latter arises from an invagination of the plasma membrane, so that if it does not invert the polarity of its transport systems, the peribacteroid space is, at least initially, equivalent to the extracellular space. This implies that the IC may take up solutes, such as sugars, not only from the extracellular space but also from the peribacteroid space of the youngest BTs. However, later on during differentiation, the structure and function of the SB membrane as well as the composition of the peribacteroid space gradually change (200, 201). For instance, the induction of a C4-dicarboxylate carrier on the SB membrane (138) may drastically change the physiology of the SBs.
N Sources
A successful symbiosis may take place in the absence of added N, and therefore nodule (and root) growth uses N sources provided by the seed. Bacteria grow extensively in the ITs, and BTs grow inside the ICs of the nodule (at least in elongated nodules) before nitrogenase begins to be expressed. An effective symbiosis is induced by a few bacteria, which multiply up to many millions. Sustained bacterial growth implies that the plant provides the necessary N compounds in return for a more abundant supply later on. The N source needed for growth is not NH4+ and NO3− (see above) and therefore should be a single or several N-containing organic compounds. Determining the symbiotic phenotypes (Nod and Fix) of auxotrophic mutants is a way to test the availability to rhizobia of a given substance in the developing nodule.
N-containing compounds such as purines and pyrimidines are not provided by the corresponding plant partners to B. japonicum, R. etli, Rhizobium fredii, and R. leguminosarum, even in the ITs, since auxotrophic strains induce only empty pseudonodules (128, 130). Mutants of S. meliloti induce Fix− nodules, but a histological analysis was not performed (85).
R. etli strains defective in the biosynthesis of amino acids (such as aromatics, aspartate, cysteine, glutamate, glycine, histidine, isoleucine-valine, leucine, lysine, phenylalanine, and tryptophan) with the exception of an arginine and a methionine auxotroph, are able to induce nodules (24, 56, 178, 181, 182; S. Ferraioli, unpublished data). Analogous observations have been reported in the case of S. meliloti. In fact, except for an isoleucine-valine auxotroph (1), all other mutants analyzed (such as those requiring asparagine, leucine, methionine, ornithine, tryptophan, and tyrosine) are able to induce nodules on M. sativa (10, 85, 148, 188). With minor differences, the same conclusion was reached by analyzing amino acid-requiring mutants of R. leguminosarum (139). In most cases, however, the resulting nodules are partially or totally ineffective, but without histological observations, the nature of these alterations in most cases remains unrecognized.
Auxotrophic strains of R. etli incubated in minimal medium in the absence of their nutritional requirements are unable to produce Nod factors, but when inoculated on the roots of P. vulgaris without exogenously added amino acids, they are able to induce nodules (56, 178, 181, 182). Since Nod factors are essential for nodule induction, the plant must provide the necessary amino acids to the bacterium. The presence of amino acids in the root exudates of some legumes has been demonstrated (196), and it has been proposed that they are produced by lysis of the root cells detached from its cortex due to the outgrowth of developing lateral roots. However, it is not possible to exclude that root cells even under N starvation (a condition necessary for symbiosis) release amino acids to stimulate rhizobia both to divide and to produce Nod factors. Chemotaxis towards root exudates has been demonstrated (21).
The ability of most of the amino acid-auxotrophic strains to invade developing nodules indicates that the plant provides the amino acids necessary for bacterial growth occurring inside the ITs. The origin of the amino acids in the ITs is unknown, but, similar to what happens in the case of pollen tubes, membrane vesicles may fuse with the tip of developing ITs, allowing their elongation (16, 68). In this way they would provide nutrients in the form of amino acids and proteins. This may explain why bacteria grow only at the tip of ITs (63). After endocytosis, the composition of the peribacteroid space, as well as the composition of the SB membrane, is modified by fusion of the SB with secretory vesicles formed by budding of the Golgi cisternae and/or originated from the endoplasmic reticulum (201). A variety of hydrolytic enzymes, including proteases and protease inhibitors, have been identified in the peribacteroid space (113). Since BTs divide several times before differentiation and the SB membrane is relatively impermeable to amino acids (192), proteolytic activity may generate a mixture of free amino acids from proteins released through vesicle fusion.
Different lines of evidence indicate that undifferentiated BTs may use amino acids as a source of C and energy and that C4-dicarboxylic acids are the C source utilized after SBs begin to differentiate (51, 198). An S. meliloti mutant strain altered in proline catabolism shows reduced nodulation efficiency and competitiveness, and the promoter for the putA (proline utilization) gene is active during root invasion and nodule formation, but not in N2-fixing BTs (80). An S. meliloti mutant strain missing succinic semialdehyde dehydrogenase (involved in the γ-aminobutyrate bypass), and therefore impaired in glutamate catabolism, shows reduced nitrogenase activity and thus biomass-accumulating ability (61). The γ-aminobutyrate bypass is responsible, even though partly, for the catabolism of glutamate in BTs of B. japonicum (90). A mutant of S. meliloti missing aspartate aminotransferase (aatA gene) induces Fix− nodules in which only a few BTs are present in the ICs (207). Since proline catabolism and aspartate aminotransferase activity generate glutamate, together these data suggest that glutamate catabolism plays an important role in the dividing BTs.
A very strong inactivation of the glnII promoter (NtrC activated) and derepression of the gstI promoter (NtrC repressed) are observed in free-living R. leguminosarum when an amino acid, such as glutamine and glutamate, is used as the sole C source (see above) (183). Therefore, the almost complete inactivation of NtrC taking place during endocytosis supports the notion that BTs use amino acids as a C source. Moreover, when rhizobia utilize an amino acid as a C source, they excrete NH4+, and if under these conditions amtB expression is forced by means of an inducible promoter, bacterial growth is inhibited (E. J. Patriarca, unpublished data). NH4+ excretion by young BTs may prepare the SBs and the cytoplasm of ICs, through the induction of specific plant genes, for the transport and assimilation of the NH4+ produced later by the nitrogenase.
Global Regulation
During SB differentiation, the transcription of genes coding for N-regulatory proteins (such as NtrC, GstI, and PII) is switched off. It is clear, however, that transcriptional downregulation of these and other genes is not a consequence of the nitrogenase activity and of the NH4+ produced. In fact, many promoters are silenced at the same stage of differentiation in nodules induced by a Fix− mutant (52, 144, 177). Moreover, some of these promoters, such as gstI and nifR3-ntrBC, are induced in free-living bacteria growing with NH4+ as an N source (183).
With almost the same pattern, the lipA (lipoic acid synthetase), nod (Nod factors), putA (proline utilization), and ropA (outer membrane protein III) genes are also downregulated (38, 80, 158, 161, 177). Over 350 proteins, including several proteins involved in N metabolism and the cell cycle, were found to be expressed at lower levels or undetectable in isolated SBs of S. meliloti compared to free-living bacteria (126). These include proteins encoded by genes that are either never expressed or inactivated very early (in the ITs) during symbiosis. This proteome analysis, however, reflects the pattern of expression obtained from a mixture of BTs at different stages of differentiation. Therefore, proteins encoded by genes that are downregulated (even abruptly) later on during BT differentiation, such as in interzone II-III (Fig. 1) could be considered as not altered. Therefore, the number of genes downregulated may be higher. Downregulation is extensive, involving genes related to different metabolic pathways, uncorrelated with respect to regulation and function. It seems, therefore, that in differentiating SBs there is a global mechanism for inactivation of gene expression.
It is known that in bacteria one of the mechanisms used to exert a global control of gene expression is the modification of the chromosome structure. DNA supercoiling varies as a function of the cellular energy status and plays an essential role in bacterial adaptation and survival to suboptimal growth conditions, such as stationary-phase growth, osmotic stress, and aerobic to anaerobic transition (for a review, see reference 157). It has been shown that before the sharp drop in O2 concentration taking place at interzone II-III of nodules, a shallow gradient of decrease is observed in zone II (168) and, in addition, that the DNA superhelicity increases in BTs (66). The energy status (the ADP/ATP ratio) of BTs may change as a function of the decrease in the O2 tension that is encountered inside the nodule, thus altering the superhelical density of the chromosome.
Another event taking place at this stage, in the middle of zone II, is the transition from dividing BTs (distal zone II) to nondividing differentiating BTs (proximal zone II). In bacteria sigma factors are considered global regulators of gene expression. The sigA gene, coding for the putative vegetative sigma factor of R. etli, has been identified (101). The intracellular levels of sigA, lipA, and nifR3-ntrBC transcripts are very high just after bacterial dilution in complete medium, decrease during bacterial divisions, and become very low, almost undetectable, as bacterial growth continues (101, 144, 177). In a similar manner, sigA may be downregulated during BT differentiation (144, 177) (Fig. 2), and as a consequence, a large set of genes necessary for bacterial growth and invasion but dispensable for the function of N2-fixing BTs would be silenced.
A combination of immunolocalization and promoter analysis (and in situ mRNA hybridization) shows, in all cases analyzed, the colocalization of transcripts and proteins. Different proteins, including β-galactosidase, expressed from lacZ transcriptional fusions become undetectable in BTs located in proximal zone II. Since BTs in proximal zone II change their shape and enlarge but do not divide (199), protein disappearance is better explained by a mechanism of proteolysis than by dilution. Activation of proteolysis is observed in rhizobia incubated in minimal medium with glucose and mannitol but without added N (E. J. Patriarca, unpublished data). Changes in C and N supply, namely a switch from amino acids to C4-dicarboxylic acids, which may result in severe N starvation, appear to take place in correlation with the beginning of BT differentiation. The removal of a large set of proteins, including the vegetative sigma factor, may explain the irreversibility of the process of BT differentiation. A parallel with the very early steps of the sporulation of Bacillus subtilis has been suggested (144). In fact, during the initial stage of sporulation induced by bacterial dilution in N-free medium, transcription of a set of genes is downregulated by inactivation of the vegetative sigma factor (187) and protein degradation is activated in the mother cell (89).
DOWNREGULATION OF NH4+ ASSIMILATION IS REQUIRED FOR EFFICIENT SYMBIOSIS
As described above, the promoters of genes for NH4+ uptake (glnK-amtB) and for its conversion into glutamine (glnII) are switched off very early during invasion of host cells, whereas those of genes involved in the regulation of NH4+ metabolism such as ntrBC, glnBA, and gstI are downregulated later on during SB differentiation. Accordingly, N2-fixing BTs show very low levels of NH4+ assimilation activities, and mutant strains lacking genes required for NH4+ assimilation, such as glnA, glnII, and gltB, are able to induce normal N2-fixing nodules. A key question is if this downregulation is necessary for the efficient functioning of the symbiosis. Of course, it is not possible to give an answer by using null mutants for these genes, and in fact, our present understanding of the necessity for downregulation comes from bacteria in which a specific function, NH4+ uptake and assimilation, is forced to be expressed in the symbiosis.
The expression of the E. coli gdhA gene (coding for an assimilatory GDH) was used to enhance the NH4+ assimilation capacity of R. etli, which does not possess an assimilatory GDH. Bacteria expressing gdhA show up to sixfold lower GOGAT activity, a sixfold-higher glutamate pool, and excrete some amino acids into the medium (114). The same alterations are observed when gdhA is expressed in an ntrC derivative. The ability of the wild-type strain of R. etli to induce nodules was completely inhibited when this strain carried gdhA (17, 18, 114). In contrast, the symbiotic behavior of the ntrC mutant strain was unaffected by GDH activity. Consistent with these results, gdhA expression reduced the capacity of the wild-type strain but not of the ntrC mutant to induce nod genes and thus to produce Nod factors in the presence of the appropriate flavonoid inducer (naringenin). These results indicate that bacteria must be N deprived to be able to nodulate and that NtrC is an essential component of the N-dependent regulatory circuit, which modulates the expression of nod genes as a function of the N status of the cells (114).
By driving the expression of gdhA from the nifHc promoter (which is under NifA control), it has been demonstrated that a high level of NH4+ assimilation in BTs is also detrimental at later steps of the symbiosis (115). The expression of GDH, and thus the enhancement of the NH4+ assimilation capacity of BTs, has a negative consequence on the ability for N translocation to the plant (115). In fact, GDH mediates an increase in the amino acid pool of BTs (including glutamate), whereas it reduces the ureide (the form of N transported to bean leaves) content in the xylem sap (115). Moreover, nitrogenase activity but not nifHDK expression is reduced in BTs expressing GDH, and premature senescence of some nodules is observed.
The effect of ectopic expression of the amtB gene allows investigation of whether BTs release NH4+. In the current model of NH4+ flux in the symbiosis (36, 192), the NH3 produced in the BTs by the nitrogenase complex would diffuse to the peribacteroid space, where it is converted to NH4+. These NH4+ ions would reach the cytoplasm of the plant cell through an NH4+ channel located in the SB membrane and would then be fixed into amino acids. The requirements for this process are (i) a low level of GS activity (NH4+ assimilation) in BTs; (ii) the presence of NH3/NH4+ in the peribacteroid space; (iii) the absence of an NH4+ uptake system in the bacteroid membrane; (iv) the presence of an NH4+ carrier in the SB membrane; and (v) high levels of assimilatory GS and GDH activities in the cytoplasm of the ICs.
The release of NH3/NH4+ into the peribacteroid space was tested by forcing the expression of amtB in N2-fixing BTs, in which this gene is normally not expressed (179, 180). It was predicted that the presence in BTs of an active mechanism for the uptake of NH4+ should retrieve the NH4+ present in the peribacteroid space, thus generating an energy-consuming futile cycle with negative consequence for the symbiotic interaction. The results obtained confirmed this prediction. In fact, driving the expression of amtB from the nifHc promoter (specifically activated by NifA under microaerobic conditions), the symbiosis is profoundly affected. Nodules (12 days old) are small, show abnormal pigmentation, a reduced number of ICs containing only a few undifferentiated SBs, and, in correlation, almost undetectable nitrogenase activity (measured as acetylene reduction). By forcing the expression of amtB in different genetic backgrounds, it was shown that the detrimental effect of amtB expression depends on the capacity of BTs to fix N and to efficiently assimilate NH4+ (180).
Surprisingly, the earliest effect of the expression of amtB was the arrest of bacterial division inside the host cells. Bacterial division, as well as nitrogenase activity, requires energy in the form of ATP and reducing equivalents. In Azotobacter vinelandii, it was observed that the addition of NH4+ to N2-fixing cells rapidly inhibits nitrogenase activity by decreasing the flow of reducing equivalents, whereas at high concentrations it also reduces the intracellular ATP/ADP ratio (92). In contrast, addition of NH4+ does not affect nitrogenase activity of isolated SBs of R. leguminosarum, which are instead unable to take up NH4+ actively, and therefore it was concluded that NH4+ could affect the energized state of the membrane only when it is taken up (92). The amtB-mediated negative effect on BT growth supports the notion that, unlike free-living diazotrophs, BTs are programmed not only to fix N2 but also to release most of the N fixed and that the N2 fixation process in BTs is regulated to satisfy the plant requirement for N.
The ectopic expression of the R. etli amtB gene was also studied in V. hirsuta (R. Tatè, unpublished data) (Fig. 4, right panel). Nodules induced by an R. leguminosarum strain expressing amtB ectopically from the nifH promoter, and thus expressing it specifically in N2-fixing BTs of zone III, are smaller than the control ones and their nitrogenase activity is strongly reduced. However, as predicted by the timing of NifA activation (Fig. 1), the detrimental effects mediated by amtB are evident after interzone II-III, similar to what was observed in nodules induced by Fix− mutant strains.
In summary, the evidence obtained by driving the expression of gdhA and amtB in BTs permits us to conclude that the symbiotic association is based on the ability of rhizobia to uncouple N2 fixation and NH4+ assimilation and that at least part of the NH3/NH4+ produced by nitrogenase activity is directly released by the BTs.
CONCLUDING REMARKS
In the rhizobium-legume symbiosis, most of the fixed N is released to the plant and used by it (69). Nitrogenase expression is switched on, bacterial assimilation of the NH4+ produced is prevented, and fixed N is exported. The uncoupling of NH4+ production and assimilation is crucial in the symbiosis, but it has received relatively little attention (192a). Results obtained when analyzing free-living diazotrophs cannot be extrapolated to rhizobia. In fact, in K. pneumoniae, the NH3 produced by N2 fixation is assimilated for growth, whereas in free-living rhizobia it is not assimilated for growth but excreted.
Until now, the transition of rhizobial N metabolism that takes place when free-living bacteria differentiate into endosymbionts has been considered in terms of a single-step mechanism. Instead, it should be regarded as a multistep process requiring recognition of the plant, invasion of specific cortical cells, growth in the ITs, endocytosis, differentiation in the ICs into N2-fixing BTs, and finally senescence. This implies several check points effected through the exchange of signal and metabolites, inside a plant that maintains a controlled internal environment through regulation and adaptation of its own N metabolism (for a review, see reference 118). During nodule development, the plant provides the necessary N compounds for bacterial growth and for BT differentiation in the ICs, while it simultaneously prepares itself to assimilate and transport the fixed N as glutamine in temperate legumes and as ureides in tropical legumes (see reference 160 for a review).
As emphasized in this review, the use of modern histochemical techniques (including in situ RNA hybridization, immunolocalization of proteins, and in situ promoter activity), combined with classical cytological and ultrastructural analyses, has made it possible to observe developmentally regulated changes (temporal and spatial) in bacterial and plant gene expression during the symbiotic interaction. The main conclusions reported in this review follow.
(i) For the growth of bacteria in the rhizosphere and inside the ITs, a mixture of amino acids is available, whereas for the differentiation of BTs inside the ICs, a smaller number of amino acids are available. This change in amino acid availability coincides with the internalization of BTs in the ICs and thus with the formation of the SB membrane.
(ii) Bacteria in the ITs are N starved, indicating that amino acids are used as an N source, whereas at the onset of endocytosis BTs are under conditions of N excess, as if from this stage amino acids are also used as a C source (resulting in an excess of NH4+).
(iii) Endocytosis is a key step in the symbiotic regulation of bacterial N metabolism in both elongated and globose nodules, since NtrC-activated promoters are switched off at this stage. The N-regulatory PII protein plays a central role in the inactivation of NtrC, indicating that an intracellular signal of N excess occurs in BTs at endocytosis.
(iv) Downregulation of NH4+ assimilation begins with the early inactivation of the NtrC protein followed by downregulation of the nifR3-ntrBC promoter and is completed with the removal of NtrC. The absence of NtrC in BTs explains why nitrogenase expression and regulation of N metabolism are uncoupled. In fact, in free-living diazotrophs, NtrC activity is essential for the coordinated expression of genes involved in N2 fixation and NH4+ assimilation.
(v) Nitrogenase expression takes place when gene products for NH4+ sensing (PII), regulation (NtrC), and assimilation (AmtB and GS) are either inactivated or absent. As a consequence, most of the NH3/NH4+ produced must be released to the plant to avoid detrimental effects on the SBs.
Symbiotic N2 fixation provides N to cultivated plants, thus avoiding the negative environmental effects caused by chemical fertilizers. In the last two decades, the considerable interest in the rhizobium-legume symbiosis has produced scant results in the improvement of this process due to underestimation of its complexity and ignorance about the regulatory mechanisms involved. Knowledge of the regulation of N metabolism constitutes an important step towards correcting this situation. In fact, as described in this review, the efficiency of symbiotic N2 fixation is improved by (i) increasing nitrogenase activity (overexpression of the nif genes and enhanced respiration); (ii) reducing the NH4+ assimilation capacity of the bacterial partner (NtrC- and GOGAT-deficient mutants); (iii) enhancing the NH4+ assimilation capacity of the plant (high levels of GS); and (iv) decreasing the sensitivity of the symbiotic interaction to the presence of inhibiting concentrations of NH4+ (NtrR). Vice versa, genetic manipulations that modify these processes in the opposite direction result in reduced N2 fixation efficiency.
Acknowledgments
Work in our laboratory described in this review was supported by EEC (BIOTEC94-2310), MURST-CNR Programme Legge 488/92 (cluster 02), MIPAF (Ministero delle Politiche Agricole e Forestali), and Fondazione Viamarconidieci.
We acknowledge the constant experimental help of A. Lamberti and A. Riccio. We thank A. Secondulfo for help with the manuscript and M. Merrick and M. Nuti for reviewing the manuscript.
REFERENCES
- 1.Aguilar, O. M., and D. H. Grasso. 1991. The product of the Rhizobium meliloti ilvC gene is required for isoleucine and valine synthesis and nodulation of alfalfa. J. Bacteriol. 173:7756-7764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allaway, D., E. M. Lodwig, L. A. Crompton, M. Wood, R. Parsons, T. R. Wheeler, and P. S. Poole. 2000. Identification of alanine dehydrogenase and its role in mixed secretion of ammonium and alanine by pea bacteroids. Mol. Microbiol. 36:508-515. [DOI] [PubMed] [Google Scholar]
- 3.Amar, M., E. J. Patriarca, G. Manco, P. Bernard, A. Riccio, A. Lamberti, R. Defez, and M. Iaccarino. 1994. Regulation of nitrogen metabolism is altered in a glnB mutant strain of Rhizobium leguminosarum. Mol. Microbiol. 11:685-693. [DOI] [PubMed] [Google Scholar]
- 4.Arcondéguy, T., I. Huez, J. Fourment, and D. Kahn. 1996. Symbiotic nitrogen fixation does not require adenylylation of glutamine synthetase I in Rhizobium meliloti. FEMS Microbiol. Lett. 145:33-40. [DOI] [PubMed] [Google Scholar]
- 5.Arcondéguy, T., I. Huez, P. Tillard, C. Gangneux, F. de Billy, A. Gojon, G. Truchet, and D. Kahn. 1997. The Rhizobium meliloti PII protein, which controls bacterial nitrogen metabolism, affects alfalfa nodule development. Genes Dev. 11:1194-1206. [DOI] [PubMed] [Google Scholar]
- 6.Arcondéguy, T., R. Jack, and M. Merrick. 2001. PII signal transduction proteins, pivotal players in microbial nitrogen control. Microbiol. Mol. Biol. Rev. 65:80-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arsène, F., S. Katupitiya, I. R. Kennedy, and C. Elmerich. 1994. Use of lacZ fusions to study the expression of nif genes of Azospirillum brasilense in association with plants. Mol. Plant-Microbe Interact. 7:748-757. [Google Scholar]
- 8.Atkins, C. A., B. J. Shelp, P. J. Storer, and J. S. Pate. 1984. Nitrogen nutrition and the development of biochemical functions associated with nitrogen fixation and ammonia assimilation of nodules of cowpea seedlings. Planta 162:327-333. [DOI] [PubMed] [Google Scholar]
- 9.Baev, N., M. Amar, R. Defez, and M. Iaccarino. 1992. The expression of the nodD and nodABC genes of Rhizobium leguminosarum is not regulated in response to combined nitrogen. FEMS Microbiol. Lett. 97:205-208. [Google Scholar]
- 10.Barsomian, G. D., A. Urzainqui, K. Lohman, and G. C. Walker. 1992. Rhizobium meliloti mutants unable to synthesize anthranilate display a novel symbiotic phenotype. J. Bacteriol. 174:4416-4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Batut, J., and P. Boistard. 1994. Oxygen control in Rhizobium. Antonie Leeuwenhoek 66:129-150. [DOI] [PubMed] [Google Scholar]
- 12.Bergersen, F. J., and D. J. Goodchild. 1973. Aeration pathways in soybean root nodules. Aust. J. Biol. Sci. 26:729-740. [Google Scholar]
- 13.Bergersen, F. J., and G. L. Turner. 1990. Bacteroids from soybean root nodules: respiration and N2 fixation in flow chamber reactions with oxyleghemoglobin. Proc. R. Soc. Lond. B 238:295-320. [Google Scholar]
- 14.Bisseling, T., R. C. van den Bos, A. van Kammen, M. van der Ploeg, P. van Duijn, and A. Houwers. 1977. Cytofluorometrical determination of the DNA content of bacteroids and corresponding broth cultured Rhizobium bacteria. J. Gen. Microbiol. 101:79-84. [Google Scholar]
- 15.Bisseling, T., R. C. van den Bos, and A. van Kammen. 1978. The effect of ammonium nitrate on the synthesis of nitrogenase and the concentration of leghemoglobin in pea root nodules induced by Rhizobium leguminosarum. Biochim. Biophys. Acta 539:1-11. [DOI] [PubMed] [Google Scholar]
- 16.Bladergroen, M. R., and H. P. Spaink. 1998. Genes and signal molecules involved in the rhizobia-Leguminosae symbiosis. Curr. Opin. Plant Biol. 1:353-359. [DOI] [PubMed] [Google Scholar]
- 17.Bravo, A., B. Becerril, and J. Mora. 1988. Introduction of the Escherichia coli gdhA gene into Rhizobium phaseoli: effect on nitrogen fixation. J. Bacteriol. 170:985-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bravo, A., and J. Mora. 1988. Ammonium assimilation in Rhizobium phaseoli by the glutamine synthetase-glutamate synthase pathway. J. Bacteriol. 170:980-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brewin, N. J. 1991. Development of the legume root nodule. Annu. Rev. Cell Biol. 7:191-226. [DOI] [PubMed] [Google Scholar]
- 20.Brown, C. M., and J. M. Dilworth. 1975. Ammonia assimilation by Rhizobium cultures and bacteroids. J. Gen. Microbiol. 86:39-48. [DOI] [PubMed] [Google Scholar]
- 21.Caetano-Anolles, G., D. K. Crist-Estes, and W. D. Bauer. 1988. Chemotaxis of Rhizobium meliloti to the plant flavone luteolin requires functional nodulation genes. J. Bacteriol. 170:3164-3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carlson, T. A., G. B. Martin, and B. K. Chelm. 1987. Differential transcription of the two glutamine synthetase genes of Bradyrhizobium japonicum. J. Bacteriol. 169:5861-5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carlson, R. W., B. Reuhs, T. B. Chen, U. R. Bhat, and K. D. Noel. 1995. Lipopolysaccharide core structures in Rhizobium etli and mutants deficient in O-antigen. J. Biol. Chem. 270:11783-11788. [DOI] [PubMed] [Google Scholar]
- 24.Castillo, A., H. Taboada, A. Mendoza, B. Valderrama, S. Encarnacion, and J. Mora. 2000. Role of GOGAT in carbon and nitrogen partitioning in Rhizobium etli. Microbiology 146:1627-1637. [DOI] [PubMed] [Google Scholar]
- 25.Cermola, M., E. Fedorova, R. Tatè, A. Riccio, R. Favre, and E. J. Patriarca. 2000. Nodule invasion and symbiosome differentiation during Rhizobium etli-Phaseolus vulgaris symbiosis. Mol. Plant-Microbe Interact. 13:733-741. [DOI] [PubMed] [Google Scholar]
- 26.Cevallos, M. A., S. Encarnacion, A. Leija, Y. Mora, and J. Mora. 1996. Genetic and physiological characterization of a Rhizobium etli mutant strain unable to synthesize poly-β-hydroxybutyrate. J. Bacteriol. 178:1646-1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Charon, C., C. Johansson, E. Kondorosi, A. Kondorosi, and M. Crespi. 1997. enod40 induces dedifferentiation and division of root cortical cells in legumes. Proc. Natl. Acad. Sci. USA 94:8901-8906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Charrier, B., C. Coronado, A. Kondorosi, and P. Ratet. 1995. Molecular characterization and expression of alfalfa (Medicago sativa L.) flavanone-3-hydroxylase and dihydroflavonol-4-reductase encoding genes. Plant Mol. Biol. 29:773-786. [DOI] [PubMed] [Google Scholar]
- 29.Chiurazzi, M., and M. Iaccarino. 1990. Transcriptional analysis of the glnB-glnA region of Rhizobium leguminosarum biovar viciae. Mol. Microbiol. 4:1727-1735. [DOI] [PubMed] [Google Scholar]
- 30.Chiurazzi, M., R. Meza, M. Lara, A. Lahm, R. Defez, M. Iaccarino, and G. Espin. 1992. The Rhizobium leguminosarum biovar phaseoli glnT gene, encoding glutamine synthetase III. Gene 119:1-8. [DOI] [PubMed] [Google Scholar]
- 31.Colonna-Romano, S., A. Riccio, M. Guida, R. Defez, A. Lamberti, M. Iaccarino, W. Arnold, U. Priefer, and A. Puhler. 1987. Tight linkage of glnA and a putative regulatory gene in Rhizobium leguminosarum. Nucleic Acids Res. 15:1951-1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colonna-Romano, S., E. J. Patriarca, M. Amar, P. Bernard, G. Manco, A. Lamberti, M. Iaccarino, and R. Defez. 1993. Uridylylation of the PII protein in Rhizobium leguminosarum. FEBS Lett. 330:95-98. [DOI] [PubMed] [Google Scholar]
- 33.Coronado, C., J. Zuanazzi, S. Poirier, B. Charrier, P. Ratet, and A. Kondorosi. 1992. Regulation of flavonoid production in Medicago sativa-Rhizobium meliloti symbiosis, p. 334. In R. Palacios, J. Mora, and W. E. Newton (ed.), New horizons in nitrogen fixation. Kluwer Academic Publishers, London, United Kingdom.
- 33a.Coutts, G., G. Thomas, D. Blakey, and M. Merrick. 2002. Membrane sequestration of the signal transduction protein GlnK by the ammonium transporter AmtB. EMBO J. 21:536-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dart, P. J. 1975. Legume root nodule initiation and development. p. 467-506. In J. G. Torrey and D. T. Clarkson (ed.), The development and function of roots. Academic Press, London, United Kingdom.
- 35.David, M., M. L. Daveran, J. Batut, A. Dedieu, O. Domergue, J. Ghai, C. Hertig, P. Boistard, and D. Kahn. 1988. Cascade regulation of nif gene expression in Rhizobium meliloti. Cell 54:671-683. [DOI] [PubMed] [Google Scholar]
- 36.Day, D. A., P. S. Poole, S. D. Tyerman, and L. Rosendahl. 2001. Ammonia and amino acid transport across symbiotic membranes in nitrogen-fixing legume nodules. Cell. Mol. Life Sci. 58:61-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Bruijn, F. J., S. Rossbach, M. Schneider, P. Ratet, S. Messmer, W. W. Szeto, F. M. Ausubel, and J. Schell. 1989. Rhizobium meliloti 1021 has three differentially regulated loci involved in glutamine biosynthesis, none of which is essential for symbiotic nitrogen fixation. J. Bacteriol. 171:1673-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Maagd, R. A., W. C. Yang, L. Goosen-de Roo, I. H. M. Mulders, H. P. Roest, H. P. Spaink, T. Bisseling, and B. J. J. Lugtenberg. 1994. Downregulation of expression of the Rhizobium leguminosarum outer membrane protein gene ropA occurs abruptly in interzone II-III of pea nodules and can be uncoupled from nif gene activation. Mol. Plant-Microbe Interact. 7:276-281. [Google Scholar]
- 39.Denarié, J., F. Debellé, and J. C. Promé. 1996. Rhizobium lipo-chitooligosaccharide nodulation factors: signaling molecules mediating recognition and morphogenesis. Annu. Rev. Biochem. 65:503-535. [DOI] [PubMed] [Google Scholar]
- 40.Denèfle, P., A. Kush, F. Norel, A. Paquelin, and C. Elmerich. 1987. Biochemical and genetic analysis of the nifHDKE region of Rhizobium ORS571. Mol. Gen. Genet. 207:280-287. [Google Scholar]
- 41.de Vasconcelos, L., L. Miller, and C. A. Neyra. 1980. Free-living and symbiotic characteristics of chlorate resistant mutants of Rhizobium. Can. J. Microbiol. 26:338-342. [DOI] [PubMed] [Google Scholar]
- 42.Dilworth, M. J., and A. R. Glenn. 1982. Movements of ammonia in Rhizobium leguminosarum. J. Gen. Microbiol. 128:29-37. [Google Scholar]
- 43.Dixon, R., R. R. Eady, G. Espin, S. Hill, M. Iaccarino, D. Kahn, and M. Merrick. 1980. Analysis of regulation of Klebsiella pneumoniae nitrogen fixation (nif) gene cluster with gene fusions. Nature 286:128-132. [DOI] [PubMed] [Google Scholar]
- 44.Doherty, D., J. A. Leigh, J. Glazebrook, and G. C. Walker. 1988. Rhizobium meliloti mutants that overproduce the R. meliloti acidic calcofluor-binding exopolysaccharide. J. Bacteriol. 170:4249-4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Donald, R. G., and R. A. Ludwig. 1984. Rhizobium sp. strain ORS571 ammonium assimilation and nitrogen fixation. J. Bacteriol. 158:1144-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duran, S., G. Du Pont, A. Huerta-Zepeda, and J. Calderon. 1995. The role of glutaminase in Rhizobium etli: studies with a new mutant. Microbiology 141:2883-2889. [DOI] [PubMed] [Google Scholar]
- 47.Duran, S., L. Sanchez-Linares, A. Huerta-Saquero, G. Du Pont, A. Huerta-Zepeda, and J. Calderon. 1996. Identification of two glutaminases in Rhizobium etli. Biochem. Genet. 34:453-465. [DOI] [PubMed] [Google Scholar]
- 48.Dusha, I., and A. Kondorosi. 1993. Genes at different regulatory levels are required for the ammonia control of nodulation in Rhizobium meliloti. Mol. Gen. Genet. 240:435-444. [DOI] [PubMed] [Google Scholar]
- 49.Edwardson, J. M. 1998. Membrane fusion: all done with SNAREpins? Curr. Biol. 8:R390-393. [DOI] [PubMed] [Google Scholar]
- 50.Encarnacion, S., J. Calderon, A. S. Gelbard, A. J. Cooper, and J. Mora. 1998. Glutamine biosynthesis and the utilization of succinate and glutamine by Rhizobium etli and Sinorhizobium meliloti. Microbiology 144:2629-2638. [DOI] [PubMed] [Google Scholar]
- 51.Engelke, T., D. Jording, D. Kapp, and A. Puhler. 1989. Identification and sequence analysis of the Rhizobium meliloti dctA gene encoding the C4-dicarboxylate carrier. J. Bacteriol. 171:5551-5560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ercolano, E., R. Mirabella, M. Merrick, and M. Chiurazzi. 2001. The Rhizobium leguminosarum glnB gene is downregulated during symbiosis. Mol. Gen. Genet. 264:555-564. [DOI] [PubMed] [Google Scholar]
- 53.Espin, G., S. Moreno, M. Wild, R. Meza, and M. Iaccarino. 1990. A previously unrecognized glutamine synthetase expressed in Klebsiella pneumoniae from the glnT locus of Rhizobium leguminosarum. Mol. Gen. Genet. 223:513-516. [DOI] [PubMed] [Google Scholar]
- 54.Espin, G., S. Moreno, and J. Guzman. 1994. Molecular genetics of the glutamine synthetases in Rhizobium species. Crit. Rev. Microbiol. 20:117-123. [DOI] [PubMed] [Google Scholar]
- 55.Fedorova, E., R. Thomson, L. F. Whitehead, O. Maudoux, M. K. Udvardi, and D. A. Day. 1999. Localization of H(+)-ATPases in soybean root nodules. Planta 209:25-32. [DOI] [PubMed] [Google Scholar]
- 56.Ferraioli, S., R. Tatè, E. Caputo, A. Lamberti, A. Riccio, and E. J. Patriarca. 2001. The Rhizobium etli argC gene is essential for arginine biosynthesis and nodulation of Phaseolus vulgaris. Mol. Plant-Microbe Interact. 14:250-254. [DOI] [PubMed] [Google Scholar]
- 57.Finan, T. M., E. McWhinnie, B. Driscoll, and R. J. Watson. 1991. Complex symbiotic phenotypes result from gluconeogenic mutations in Rhizobium meliloti. Mol. Plant-Microbe Interact. 4:386-392. [Google Scholar]
- 58.Fischer, H.-M., A. Alvarez-Morales, and H. Hennecke. 1986. The pleiotropic nature of symbiotic regulatory mutants: Bradyrhizobium japonicum nifA gene is involved in control of nif gene expression and formation of determinate symbiosis. EMBO J. 5:1165-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fischer, H.-M. 1994. Genetic regulation of nitrogen fixation in rhizobia. Microbiol. Rev. 58:352-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fischer, H.-M., L. Velasco, M. J. Delgado, E. J. Bedmar, S. Scharen, D. Zingg, M. Gottfert, and H. Hennecke. 2001. One of two hemN genes in Bradyrhizobium japonicum is functional during anaerobic growth and in symbiosis. J. Bacteriol. 183:1300-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fitzmaurice, A. M., and F. O'Gara. 1993. A Rhizobium meliloti mutant, lacking a functional γ-aminobutyrate (GABA) bypass, is defective in glutamate catabolism and symbiotic nitrogen fixation. FEMS Microbiol. Lett. 109:195-202. [Google Scholar]
- 62.Foster-Hartnett, D., P. J. Cullen, K. K. Gabbert, and R. G. Kranz. 1993. Sequence, genetic, and lacZ fusion analysis of a nifR3-ntrB-ntrC operon in Rhodobacter capsulatus. Mol. Microbiol. 8:903-914. [DOI] [PubMed] [Google Scholar]
- 63.Gage, D. J., T. Bobo, and S. R. Long. 1996. Use of green fluorescent protein to visualize the early events of symbiosis between Rhizobium meliloti and alfalfa (Medicago sativa). J. Bacteriol. 178:7159-7166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Galibert, F., T. M. Finan, S. R. Long, et al. 2001. The composite genome of the legume symbiont Sinorhizobium meliloti. Science 293:668-672. [DOI] [PubMed] [Google Scholar]
- 65.Ginsburg, A., and E. R. Stadtman. 1973. Regulation of glutamine synthetase in Escherichia coli, p. 9-94. In S. Prusiner and E. R. Stadtman (ed.), The enzymes of glutamine metabolism. Academic Press, New York, N.Y.
- 66.Gober, J. W., and E. R. Kashket. 1989. Role of DNA superhelicity in regulation of bacteroid-associated functions of Bradyrhizobium sp. strain 32H1. Appl. Environ. Microbiol. 55:1420-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grosskopf, E., D. T. Cam Ha, R. Wingender, H. Rohrig, J. Szecsi, E. Kondorosi, J. Schell, and A. Kondorosi. 1993. Enhanced levels of chalcone synthase in alfalfa nodules induced by a Fix− mutant of Rhizobium meliloti. Mol. Plant-Microbe Interact. 6:173-181. [Google Scholar]
- 68.Gualtieri, G., and T. Bisseling. 2000. The evolution of nodulation. Plant Mol. Biol. 42:181-194. [PubMed] [Google Scholar]
- 69.Gutschick, V. P. 1980. Energy flows in the nitrogen cycle, especially in fixation, p. 17-27. In W. E. Newton and W. H. Orme-Johnson (ed.), Nitrogen fixation. Kettering International Symposium on Nitrogen Fixation, Madison, Wis. Baltimore University Park Press, Baltimore, Md.
- 70.Heidstra, R., G. Nilsen, F. Martinez-Abarca, A. van Kammen, and T. Bisseling. 1997. Nod factor-induced expression of leghemoglobin to study the mechanism of NH4NO3 inhibition on root hair deformation. Mol. Plant-Microbe Interact. 10:215-220. [DOI] [PubMed] [Google Scholar]
- 71.Hilgert, U., J. Schell, and F. J. de Bruijn. 1987. Isolation and characterization of Tn5-induced NADPH-glutamate synthase (GOGAT−) mutants of Azorhizobium sesbaniae ORS571 and cloning of the corresponding glt locus. Mol. Gen. Genet. 210:195-202. [Google Scholar]
- 72.Hirsch, A. M., M. Bang, and F. M. Ausubel. 1983. Ultrastructural analysis of ineffective alfalfa nodules formed by nif::Tn5 mutants of Rhizobium meliloti. J. Bacteriol. 155:367-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hirsch, A. M., and C. A. Smith. 1987. Effects of Rhizobium meliloti nif and fix mutants on alfalfa root nodule development. J. Bacteriol. 169:1137-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Holtel, A., and M. Merrick. 1988. Identification of the Klebsiella pneumoniae glnB gene: nucleotide sequence of wild-type and mutant alleles. Mol. Gen. Genet. 215:134-138. [DOI] [PubMed] [Google Scholar]
- 75.Holtel, A., S. Colonna-Romano, M. Guida, A. Riccio, M. J. Merrick, and M. Iaccarino. 1989. The glnB gene of Rhizobium leguminosarum biovar viceae. FEMS Microbiol. Lett. 58:203-208. [Google Scholar]
- 76.Howitt, S. M., and P. M. Gresshoff. 1985. Ammonia regulation of glutamine synthetase in Rhizobium sp. ANU289. J. Gen. Microbiol. 131:1433-1440. [Google Scholar]
- 77.Howitt, S. M., K. Udvardi, D. Day, and P. M. Gresshoff. 1986. Ammonia transport in free-living and symbiotic Rhizobium sp. ANU289. J. Gen. Microbiol. 132:257-261. [Google Scholar]
- 78.Howitt, S. M., and M. K. Udvardi. 2000. Structure, function and regulation of ammonium transporters in plants. Biochim. Biophys. Acta 1465:152-170. [DOI] [PubMed] [Google Scholar]
- 79.Huerta-Zepeda, A., L. Ortuno, G. Du Pont, S. Duran, A. Lloret, H. Merchant-Larios, and J. Calderon. 1997. Isolation and characterization of Rhizobium etli mutants altered in degradation of asparagine. J. Bacteriol. 179:2068-2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jimenez-Zurdo, J. L., F. M. Garcia-Rodriguez, and N. Toro. 1997. The Rhizobium meliloti putA gene: its role in the establishment of the symbiotic interaction with alfalfa. Mol. Microbiol. 23:85-93. [DOI] [PubMed] [Google Scholar]
- 80a.Kahn, M. L., J. Kraus, and J. E. Somerville. 1985. A model for nutrient exchange in the Rhizobium-legume symbiosis, p. 193-199. In H. J. Evans (ed.), Nitrogen fixation research progress. M. N. Nijhoff Publisher, Dordrecht, The Netherlands.
- 81.Kaiser, B. N., P. M. Finnegan, S. D. Tyerman, L. F. Whitehead, F. J. Bergersen, D. A. Day, and M. K. Udvardi. 1998. Characterization of an ammonium transport protein from the peribacteroid membrane of soybean nodules. Science 281:1202-1206. [DOI] [PubMed] [Google Scholar]
- 82.Kaneko, T., Y. Nakamura, S. Sato, E. Asamizu, T. Kato, S. Sasamoto, A. Watanabe, K. Idesawa, A. Ishikawa, K. Kawashima, T. Kimura, Y. Kishida, C. Kiyokawa, M. Kohara, M. Matsumoto, A. Matsuno, Y. Mochizuki, S. Nakayama, N. Nakazaki, S. Shimpo, M. Sugimoto, C. Takeuchi, M. Yamada, and S. Tabata. 2000. Complete genome structure of the nitrogen-fixing symbiotic bacterium Mesorhizobium loti (supplement). DNA Res. 7:381-406. [DOI] [PubMed] [Google Scholar]
- 83.Kavroulakis, N., E. Flemetakis, G. Aivalakis, and P. Katinakis. 2000. Carbon metabolism in developing soybean root nodules: the role of carbonic anhydrase. Mol. Plant-Microbe Interact. 13:14-22. [DOI] [PubMed] [Google Scholar]
- 84.Kennedy, C., N. Doetsch, D. Meletzus, E. J. Patriarca, M. Amar, and M. Iaccarino. 1994. Ammonium sensing in nitrogen fixing bacteria: functions of the glnB and glnD gene products. Plant Soil 161:43-57. [Google Scholar]
- 85.Kerppola, T. K., and M. L. Kahn. 1988. Symbiotic phenotypes of auxotrophic mutants of Rhizobium meliloti 104A14. J. Gen. Microbiol. 134:913-919. [DOI] [PubMed] [Google Scholar]
- 86.Kikeri, D., A. Sun, M. L. Zeidel, and S. C. Hebert. 1989. Cell membranes impermeable to NH3. Nature 339:478-480. [DOI] [PubMed] [Google Scholar]
- 87.Kleiner, D. 1985. Bacterial ammonium transport. FEMS Microbiol. Rev. 32:87-100. [Google Scholar]
- 88.Kondorosi, A., Z. Svab, G. B. Kiss, and R. A. Dixon. 1977. Ammonia assimilation and nitrogen fixation in Rhizobium meliloti. Mol. Gen. Genet. 151:221-226. [Google Scholar]
- 89.Kornberg, A., J. A. Spudich, D. L. Nelson, and M. P. Deutscher. 1968. Origin of proteins in sporulation. Annu. Rev. Biochem. 37:51-78. [DOI] [PubMed] [Google Scholar]
- 90.Kouchi, H., K. Fukai, and A. Kihara. 1991. Metabolism of glutamate and aspartate in bacteroids isolated from soybean root nodules. J. Gen. Microbiol. 137:2901-2910. [Google Scholar]
- 91.Kuzma, M. M., H. Winter, P. Storer, I. I. Oresnik, C. A. Atkins, and D. B. Layzell. 1999. The site of oxygen limitation in soybean nodules. Plant Physiol. 119:399-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Laane, C., W. Krone, W. Konings, H. Haaker, and C. Veeger. 1980. Short-term effect of ammonium chloride on nitrogen fixation by Azotobacter vinelandii and by bacteroids of Rhizobium leguminosarum. Eur. J. Biochem. 103:39-46. [DOI] [PubMed] [Google Scholar]
- 93.Lagares, A., G. Caetano-Anolles, K. Niehaus, J. Lorenzen, H. D. Ljunggren, A. Puhler, and G. Favelukes. 1992. A Rhizobium meliloti lipopolysaccharide mutant altered in competitiveness for nodulation of alfalfa. J. Bacteriol. 174:5941-5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Latch, J. N., and W. Margolin. 1997. Generation of buds, swellings, and branches instead of filaments after blocking the cell cycle of Rhizobium meliloti. J. Bacteriol. 179:2373-2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lewis, T. A., R. Gonzalez, and J. L. Botsford. 1990. Rhizobium meliloti glutamate synthase: cloning and initial characterization of the glt locus. J. Bacteriol. 172:2413-2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu, Z., J. Pen, R. Mo, C. Hui, and C. H. Huang. 2001. Rh type B glycoprotein is a member of the Rh superfamily and a putative ammonia transporter in mammals. J. Biol. Chem. 276:1424-1433. [DOI] [PubMed] [Google Scholar]
- 97.Long, S. R. 1996. Rhizobium symbiosis: nod factors in perspective. Plant Cell 8:1885-1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lorenz, M. C., and J. Heitman. 1998. The MEP2 ammonium permease regulates pseudohyphal differentiation in Saccharomyces cerevisiae. EMBO J. 17:1236-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lu, C. D., and A. T. Abdelal. 2001. The gdhB gene of Pseudomonas aeruginosa encodes an arginine-inducible NAD+-dependent glutamate dehydrogenase which is subject to allosteric regulation. J. Bacteriol. 183:490-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ludwig, R. A. 1978. Control of ammonium assimilation in Rhizobium 32H1. J. Bacteriol. 135:114-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Luka, S., E. J. Patriarca, A. Riccio, M. Iaccarino, and R. Defez. 1996. Cloning of the rpoD Analog from Rhizobium etli: sigA of R. etli is growth phase regulated. J. Bacteriol. 178:7138-7143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Machado, H. B., M. G. Yates, S. Funayama, L. U. Rigo, M. B. Steffens, E. M. Souza, and F. O. Pedrosa. 1995. The ntrBC genes of Azospirillum brasilense are part of a nifR3-like-ntrB-ntrC operon and are negatively regulated. Can. J. Microbiol. 41:674-684. [DOI] [PubMed] [Google Scholar]
- 103.Manco, G., M. Rossi, R. Defez, A. Lamberti, G. Percuoco, and M. Iaccarino. 1992. Dissociation by NH4Cl treatment of the enzymatic activities of glutamine synthetase II from Rhizobium leguminosarum biovar viciae. J. Gen. Microbiol. 138:1453-1460. [DOI] [PubMed] [Google Scholar]
- 104.Manhart, J. R., and P. P. Wong. 1979. Nitrate reductase activities of rhizobia and the correlation between nitrate reduction and nitrogen fixation. Can. J. Microbiol. 25:1169-1174. [DOI] [PubMed] [Google Scholar]
- 105.Marini, A. M., A. Urrestarazu, R. Beauwens, and B. André. 1997. The Rh (rhesus) blood group polypeptides are related to NH4+ transporters. Trends Biochem. Sci. 22:460-461. [DOI] [PubMed] [Google Scholar]
- 106.Marini, A. M., G. Matassi, V. Raynal, B. André, J. P. Cartron, and B. Cherif-Zahar. 2000. The human Rhesus-associated RhAG protein and a kidney homologue promote ammonium transport in yeast. Nat. Genet. 26:341-344. [DOI] [PubMed] [Google Scholar]
- 107.Marini, A. M., J. Y. Springael, W. B. Frommer, and B. André. 2000. Cross-talk between ammonium transporters in yeast and interference by the soybean SAT1 protein. Mol. Microbiol. 35:378-385. [DOI] [PubMed] [Google Scholar]
- 107a.Marroqui, S., A. Zorreguieta, C. Santamaria, F. Temprano, M. Soberon, M. Megias, and J. A. Downie. 2001. Enhanced symbiotic performance by Rhizobium tropici glycogen synthase mutants. J. Bacteriol. 183:854-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Martin, G. B., K. A. Chapman, and B. K. Chelm. 1988. Role of the Bradyrhizobium japonicum ntrC gene product in differential regulation of the glutamine synthetase II gene (glnII). J. Bacteriol. 170:5452-5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Martin, G. B., M. F. Thomashow, and B. K. Chelm. 1989. Bradyrhizobium japonicum glnB, a putative nitrogen-regulatory gene, is regulated by NtrC at tandem promoters. J. Bacteriol. 171:5638-5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Martino, M., A. Riccio, R. Defez, M. Iaccarino, and E. J. Patriarca. 1996. In vitro characterization of the ORF1-ntrBC promoter of R. etli. FEBS Lett. 388:53-58. [DOI] [PubMed] [Google Scholar]
- 111.McKay, I. A., A. R. Glenn, and M. J. Dilworth. 1985. Gluconeogenesis in Rhizobium leguminosarum MNF3841. J. Gen. Microbiol. 131:2067-2073. [Google Scholar]
- 112.Meier-Wagner, J., L. Nolden, M. Jakoby, R. Siewe, R. Kramer, and A. Burkovski. 2001. Multiplicity of ammonium uptake systems in Corynebacterium glutamicum: role of Amt and AmtB. Microbiology 147:135-143. [DOI] [PubMed] [Google Scholar]
- 113.Mellor, R. B. 1989. Bacteroids in the Rhizobium-legume symbiosis inhabit a plant lytic compartment: implications for other microbial endosymbioses. J. Exp. Bot. 40:831-839. [Google Scholar]
- 114.Mendoza, A., A. Leija, E. Martinez-Romero, G. Hernandez, and J. Mora. 1995. The enhancement of ammonium assimilation in Rhizobium etli prevents nodulation of Phaseolus vulgaris. Mol. Plant-Microbe Interact. 8:584-592. [DOI] [PubMed] [Google Scholar]
- 115.Mendoza, A., B. Valderrama, A. Leija, and J. Mora. 1998. NifA-dependent expression of glutamate dehydrogenase in Rhizobium etli modifies nitrogen partitioning during symbiosis. Mol. Plant-Microbe Interact. 11:83-90. [DOI] [PubMed] [Google Scholar]
- 116.Merrick, M., and R. A. Edwards. 1995. Nitrogen control in bacteria. Microbiol. Rev. 59:604-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Michel-Reydellet, N., N. Desnoues, M. de Zamaroczy, C. Elmerich, and P. A. Kaminski. 1998. Characterization of the glnK-amtB operon and the involvement of AmtB in methylammonium uptake in Azorhizobium caulinodans. Mol. Gen. Genet. 258:671-677. [DOI] [PubMed] [Google Scholar]
- 118.Miflin, B. J., and J. V. Cullimore. 1984. Nitrogen assimilation in the legume-Rhizobium symbiosis: a joint endeavor, p. 129-178. In D. P. S. Verma and T. Hohn (ed.), Genes involved in microbe-plant interaction. Springer-Verlag, Berlin, Germany.
- 119.Milcamps, A., D. M. Ragatz, P. Lim, K. A. Berger, and F. J. de Bruijn. 1998. Isolation of carbon- and nitrogen-deprivation-induced loci of Sinorhizobium meliloti 1021 by Tn5-luxAB mutagenesis. Microbiology 144:3205-3218. [DOI] [PubMed] [Google Scholar]
- 120.Minchin, F. R. 1997. Regulation of oxygen diffusion in legume nodules. Soil Biol. Biochem. 29:881-888. [Google Scholar]
- 121.Moir, J. W., and N. J. Wood. 2001. Nitrate and nitrite transport in bacteria. Cell. Mol. Life Sci. 58:215-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Moreno, S., R. Meza, J. Guzman, A. Carabez, and G. Espin. 1991. The glnA gene of Rhizobium leguminosarum bv. phaseoli and its role in symbiosis. Mol. Plant-Microbe Interact. 4:619-622. [Google Scholar]
- 123.Moreno, S., E. J. Patriarca, M. Chiurazzi, R. Meza, R. Defez, A. Lamberti, A. Riccio, M. Iaccarino, and G. Espin. 1992. Phenotype of a Rhizobium leguminosarum ntrC mutant. Res. Microbiol. 143:161-171. [DOI] [PubMed] [Google Scholar]
- 124.Mylona, P., K. Pawlowski, and T. Bisseling. 1995. Symbiotic nitrogen fixation. Plant Cell 7:869-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nap, J.-P., and T. Bisseling. 1990. Developmental biology of a plant-prokaryote symbiosis: the legume root nodule. Science 250:948-954. [DOI] [PubMed] [Google Scholar]
- 126.Natera, S. H., N. Guerreiro, and M. A. Djordjevic. 2000. Proteome analysis of differentially displayed proteins as a tool for the investigation of symbiosis. Mol. Plant-Microbe Interact. 13:995-1009. [DOI] [PubMed] [Google Scholar]
- 127.Newcomb, W., and L. McIntyre. 1981. Development of root nodules of mung bean (Vigna radiata): a reinvestigation of endocytosis. Can. J. Bot. 59:2478-2499. [Google Scholar]
- 128.Newman, J. D., R. J. Diebold, B. W. Schultz, and K. D. Noel. 1994. Infection of soybean and pea nodules by Rhizobium spp. purine auxotrophs in the presence of 5-aminoimidazole-4-carboxamide riboside. J. Bacteriol. 176:3286-3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nienaber, A., A. Huber, M. Gottfert, H. Hennecke, and H. M. Fischer. 2000. Three new NifA-regulated genes in the Bradyrhizobium japonicum symbiotic gene region discovered by competitive DNA-RNA hybridization. J. Bacteriol. 182:1472-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Noel, K. D., R. J. Diebold, J. R. Cava, and B. A. Brink. 1988. Rhizobial purine and pyrimidine auxotrophs: nutrient supplememtation, genetic analysis, and the symbiotic requirements for de novo purine biosynthesis. Arch. Microbiol. 149:499-506. [Google Scholar]
- 131.Noonan, B., M. Motherway, and F. O'Gara. 1992. Ammonia regulation of the Rhizobium meliloti nitrogenase structural and regulatory genes under free-living conditions: involvement of the fixL gene product? Mol. Gen. Genet. 234:423-428. [DOI] [PubMed] [Google Scholar]
- 132.O'Gara, F., and K. T. Shanmugam. 1976. Regulation of nitrogen fixation by Rhizobia. Export of fixed N2 as NH+4. Biochim. Biophys. Acta 437:313-321. [DOI] [PubMed] [Google Scholar]
- 133.O'Gara, F., S. Manian, and J. Meade. 1984. Isolation of an Asm-mutant of Rhizobium japonicum defective in symbiotic N2 [nitrogen]-fixation. FEMS Microbiol. Lett. 24:241-245. [Google Scholar]
- 134.O'Hara, G. W., I. T. Riley, A. R. Glenn, and M. J. Dilworth. 1985. The ammonium permease of Rhizobium leguminosarum MNF3841. J. Gen. Microbiol. 131:757-764. [Google Scholar]
- 135.Olah, B., E. Kiss, Z. Gyorgypal, J. Borzi, G. Cinege, G. Csanadi, J. Batut, A. Kondorosi, and I. Dusha. 2001. Mutation in the ntrR gene, a member of the vap gene family, increases the symbiotic efficiency of Sinorhizobium meliloti. Mol. Plant-Microbe Interact. 14:887-894. [DOI] [PubMed] [Google Scholar]
- 136.Osburne, M. S., and E. R. Signer. 1980. Ammonium assimilation in Rhizobium meliloti. J. Bacteriol. 143:1234-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Osteras, M., T. M. Finan, and J. Stanley. 1991. Site-directed mutagenesis and DNA sequence of pckA of Rhizobium NGR234, encoding phosphoenolpyruvate carboxykinase: gluconeogenesis and host-dependent symbiotic phenotype. Mol. Gen. Genet. 230:257-269. [DOI] [PubMed] [Google Scholar]
- 138.Ou Yang, L., M. K. Udvardi, and D. A. Day. 1990. Specificity and regulation of the dicarboxylate carrier on the peribacteroid membrane of soybean nodules. Planta 182:437-444. [DOI] [PubMed] [Google Scholar]
- 139.Pain, A. N. 1979. Symbiotic properties of antibiotic-resistant and auxotrophic mutants of Rhizobium leguminosarum. J. Appl. Bacteriol. 47:53-64. [Google Scholar]
- 140.Patriarca, E. J., M. Chiurazzi, G. Manco, A. Riccio, A. Lamberti, A. De Paolis, M. Rossi, R. Defez, and M. Iaccarino. 1992. Activation of the Rhizobium leguminosarum glnII gene by NtrC is dependent on upstream DNA sequences. Mol. Gen. Genet. 234:337-345. [DOI] [PubMed] [Google Scholar]
- 141.Patriarca, E. J., A. Riccio, R. Tatè, S. Colonna-Romano, M. Iaccarino, and R. Defez. 1993. The ntrBC genes of Rhizobium leguminosarum are part of a complex operon subject to negative regulation. Mol. Microbiol. 9:569-577. [DOI] [PubMed] [Google Scholar]
- 142.Patriarca, E. J., A. Riccio, S. Colonna-Romano, R. Defez, and M. Iaccarino. 1994. DNA binding activity of NtrC from Rhizobium grown on different nitrogen sources. FEBS Lett. 354:89-92. [DOI] [PubMed] [Google Scholar]
- 143.Patriarca, E. J., R. Tatè, R. Defez, and M. Iaccarino. 1994. Nitrogen assimilation in free-living Rhizobium and in the symbiosis, p. 38-44. In G. B. Kiss and G. Endre (ed.), Proceedings of the 1st European Nitrogen Fixation Conference. Officina Press, Szeged, Hungary.
- 144.Patriarca, E. J., R. Tatè, E. Fedorova, A. Riccio, R. Defez, and M. Iaccarino. 1996. Downregulation of the Rhizobium ntr system in the determinate nodule of Phaseolus vulgaris identifies a specific developmental zone. Mol. Plant-Microbe Interact. 9:243-251. [Google Scholar]
- 145.Pawlowski, K., U. Klosse, and F. J. de Bruijn. 1991. Characterization of a novel Azorhizobium caulinodans ORS571 two-component regulatory system, NtrY/NtrX, involved in nitrogen fixation and metabolism. Mol. Gen. Genet. 231:124-138. [DOI] [PubMed] [Google Scholar]
- 146.Peters, N. K., J. W. Frost, and S. R. Long. 1986. A plant flavone, luteolin, induces expression of Rhizobium meliloti nodulation genes. Science 233:977-980. [DOI] [PubMed] [Google Scholar]
- 147.Poole, P., and D. Allaway. 2000. Carbon and nitrogen metabolism in Rhizobium. Adv. Microb. Physiol. 43:117-163. [DOI] [PubMed] [Google Scholar]
- 148.Prasad, C. K., K. E. Vineetha, H. R. Hassani, R. Gupta, and G. S. Randhava. 2000. Isolation and symbiotic characterization of aromatic amino acid auxotrophs of Sinorhizobium meliloti. Indian J. Exp. Bot. 38:1041-1049. [PubMed] [Google Scholar]
- 149.Rae, A. L., P. Bonfante-Fasolo, and N. J. Brewin. 1992. Structure and growth of infection threads in the legume symbiosis with Rhizobium leguminosarum. Plant J. 2:385-395. [Google Scholar]
- 150.Rapoport, S., J. Rost, and M. Schultze. 1971. Glutamine and glutamate as respiratory substrates of rabbit reticulocytes. Eur. J. Biochem. 23:166-170. [DOI] [PubMed] [Google Scholar]
- 151.Reitzer, L. J., B. M. Wice, and D. Kennell. 1979. Evidence that glutamine, not sugar, is the major energy source for cultured HeLa cells. J. Biol. Chem. 254:2669-2676. [PubMed] [Google Scholar]
- 152.Reitzer, L. J. 1996. Ammonia assimilation and the biosynthesis of glutamine, glutamate, aspartate, asparagine, l-alanine, and d-alanine, p. 391-407. In F. C. Neidhardt et al. (ed.), Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, D.C.
- 153.Rossi, M., R. Defez, M. Chiurazzi, A. Lamberti, A. Fuggi, and M. Iaccarino. 1989. Regulation of glutamine synthetase isoenzymes in Rhizobium leguminosarum biovar viciae. J. Gen. Microbiol. 135:629-637. [Google Scholar]
- 154.Rys, G. J., and T. Phung. 1984. Effect of nitrogen form and counterion on establishment of the Rhizobium trifolii-Trifolium repens symbiosis. J. Exp. Bot. 35:1811-1820. [Google Scholar]
- 155.Salvemini, F., A. M. Marini, A. Riccio, E. J. Patriarca, and M. Chiurazzi. 2001. Functional characterization of an ammonium transporter gene from Lotus japonicus. Gene 270:237-243. [DOI] [PubMed] [Google Scholar]
- 156.Sanchez, F., J. E. Padilla, J., H. Perez, and M. Lara. 1991. Control of nodulin genes in root-nodule development and metabolism. Annu. Rev. Plant Physiol. Plant Mol. Biol. 42:507-528. [Google Scholar]
- 157.Schaechter, M. 2001. Escherichia coli and Salmonella 2000: the view from here. Microbiol. Mol. Biol. Rev. 65:119-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Schlaman, H. R., B. Horvath, E. Vijgenboom, R. J. Okker, and B. J. Lugtenberg. 1991. Suppression of nodulation gene expression in bacteroids of Rhizobium leguminosarum biovar viciae. J. Bacteriol. 173:4277-4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Schluter, A., M. Nohlen, M. Kramer, R. Defez, and U. B. Priefer. 2000. The Rhizobium leguminosarum bv. viciae GlnD gene, encoding a uridylyltransferase/uridylyl-removing enzyme, is expressed in the root nodule but is not essential for nitrogen fixation. Microbiology 146:2987-2996. [DOI] [PubMed] [Google Scholar]
- 160.Schubert, K. R. 1986. Products of biological nitrogen fixation in higher plants: synthesis transport and metabolism. Annu. Rev. Plant Physiol. 37:539-574. [Google Scholar]
- 161.Sharma, S. B., and E. R. Signer. 1990. Temporal and spatial regulation of the symbiotic genes of Rhizobium meliloti in planta revealed by transposon Tn5-gusA. Genes Dev. 4:344-356. [DOI] [PubMed] [Google Scholar]
- 162.Shatters, R. G., Y. Liu, and M. L. Kahn. 1993. Isolation and characterization of a novel glutamine synthetase from Rhizobium meliloti. J. Biol. Chem. 268:469-475. [PubMed] [Google Scholar]
- 163.Shirley, B. W. 1996. Flavonoid biosynthesis: new functions for an “old” pathway. Trends Plant Sci. Bacteriol. 1:377-381. [Google Scholar]
- 164.Sindhu, S. S., N. J. Brewin, and E. L. Kannenberg. 1990. Immunochemical analysis of lipopolysaccharides from free-living and endosymbiotic forms of Rhizobium leguminosarum. J. Bacteriol. 172:1804-1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Soberon, M., H. D. Williams, R. K. Poole, and E. Escamilla. 1989. Isolation of a Rhizobium phaseoli cytochrome mutant with enhanced respiration and symbiotic nitrogen fixation. J. Bacteriol. 171:465-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Soberon, M., G. R. Aguilar, and F. Sanchez. 1993. Rhizobium phaseoli cytochrome-c-deficient mutant induces empty nodules on Phaseolus vulgaris L. Mol. Microbiol. 8:159-166. [DOI] [PubMed] [Google Scholar]
- 167.Soberon, M., O. Lopez, C. Morera, M. L. Girard, M. L. Tabche, and J. Miranda. 1999. Enhanced nitrogen fixation in a Rhizobium etli ntrC mutant that overproduces the Bradyrhizobium japonicum symbiotic terminal oxidase cbb3. Appl. Environ. Microbiol. 65:2015-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Soupène, E., M. Foussard, P. Boistard, G. Truchet, and J. Batut. 1995. Oxygen as a key developmental regulator of Rhizobium meliloti N2 fixation gene expression within the alfalfa root nodule. Proc. Natl. Acad. Sci. USA 92:3759-3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Soupène, E., L. He, D. Yan, and S. Kustu. 1998. Ammonia acquisition in enteric bacteria: physiological role of the ammonium/methylammonium transport B (AmtB) protein. Proc. Natl. Acad. Sci. USA 95:7030-7034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Spaink, H. P., R. J. H. Okker, C. A. Wijffelman, E. Pees, and B. J. Lugtenberg. 1987. Promoters in the nodulation region of the Rhizobium leguminosarum Sym plasmid pRL1JI. Plant Mol. Biol. 9:27-39. [DOI] [PubMed] [Google Scholar]
- 171.Spaink, H. P., and B. J. Lugtenberg. 1994. Role of rhizobial lipo-chitin oligosaccharide signal molecules in root nodule organogenesis. Plant Mol. Biol. 26:1413-1422. [DOI] [PubMed] [Google Scholar]
- 172.Spinosa, M., A. Riccio, L. Mandrich, G. Manco, A. Lamberti, M. Iaccarino, M. Merrick, and E. J. Patriarca. 2000. Inhibition of glutamine synthetase II expression by the product of the gstI gene. Mol. Microbiol. 37:443-452. [DOI] [PubMed] [Google Scholar]
- 173.Streeter, J. 1988. Inhibition of legume formation and N2 fixation by nitrate. Crit. Rev. Plant Sci. 7:1-23. [Google Scholar]
- 174.Szeto, W. W., B. T. Nixon, C. W. Ronson, and F. M. Ausubel. 1987. Identification and characterization of the Rhizobium meliloti ntrC gene: R. meliloti has separate regulatory pathways for activation of nitrogen fixation genes in free-living and symbiotic cells. J. Bacteriol. 169:1423-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Tao, H., N. J. Brewin, and K. D. Noel. 1992. Rhizobium leguminosarum CFN42 lipopolysaccharide antigenic changes induced by environmental conditions. J. Bacteriol. 174:2222-2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tatè, R., E. J. Patriarca, A. Riccio, R. Defez, and M. Iaccarino. 1994. Development of Phaseolus vulgaris root nodules. Mol. Plant-Microbe Interact. 7:582-589. [Google Scholar]
- 177.Tatè, R., A. Riccio, M. Iaccarino, and E. J. Patriarca. 1997. Cloning and transcriptional analysis of the lipA (lipoic acid synthetase) gene from Rhizobium etli. FEMS Lett. 149:165-172. [DOI] [PubMed] [Google Scholar]
- 178.Tatè, R., A. Riccio, M. Iaccarino, and E. J. Patriarca. 1997. A cysG mutant strain of Rhizobium etli pleiotropically defective in sulfate and nitrate assimilation. J. Bacteriol. 179:7343-7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Tatè, R., A. Riccio, M. Merrick, and E. J. Patriarca. 1998. The Rhizobium etli amtB gene coding for an NH4+ transporter is downregulated early during bacteroid differentiation. Mol. Plant-Microbe Interact. 11:188-198. [DOI] [PubMed] [Google Scholar]
- 180.Tatè, R., M. Cermola, A. Riccio, M. Iaccarino, M. Merrick, R. Favre, and E. J. Patriarca. 1999. Ectopic expression of the Rhizobium etli amtB gene affects the symbiosome differentiation process and nodule development. Mol. Plant-Microbe Interact. 12:515-525. [Google Scholar]
- 181.Tatè, R., A. Riccio, E. Caputo, M. Cermola, R. Favre, and E. J. Patriarca. 1999. The Rhizobium etli trpB gene is essential for an effective symbiotic interaction with Phaseolus vulgaris. Mol. Plant-Microbe Interact. 12:926-933. [DOI] [PubMed] [Google Scholar]
- 182.Tatè, R., A. Riccio, E. Caputo, M. Iaccarino, and E. J. Patriarca. 1999. The Rhizobium etli metZ gene is essential for methionine biosynthesis and nodulation of Phaseolus vulgaris. Mol. Plant-Microbe Interact. 12:24-34. [DOI] [PubMed] [Google Scholar]
- 183.Tatè, R., L. Mandrich, M. R. Spinosa, A. Riccio, A. Lamberti, M. Iaccarino, and E. J. Patriarca. 2001. The GstI protein reduces the NH4+ assimilation capacity of Rhizobium leguminosarum. The endocytosis as a key step for gene regulation in developing nodules. Mol. Plant-Microbe Interact. 14:823-831. [DOI] [PubMed] [Google Scholar]
- 184.Thomas, G., G. Coutts, and M. Merrick. 2000. The glnKamtB operon. A conserved gene pair in prokaryotes. Trends Genet. 16:11-14. [DOI] [PubMed] [Google Scholar]
- 185.Thomas, G. H., J. G. Mullins, and M. Merrick. 2000. Membrane topology of the Mep/Amt family of ammonium transporters. Mol. Microbiol. 37:331-344. [DOI] [PubMed] [Google Scholar]
- 186.Timmers, A. C., E. Soupène, M. C. Auriac, F. de Billy, J. Vasse, P., Boistard, and G. Truchet. 2000. Saprophytic intracellular rhizobia in alfalfa nodules. Mol. Plant-Microbe Interact. 13:1204-1213. [DOI] [PubMed] [Google Scholar]
- 187.Tjian, R., and R. Losick. 1974. An immunological assay for the sigma subunit of RNA polymerase in extracts of vegetative and sporulating Bacillus subtilis. Proc. Natl. Acad. Sci. USA 71:2872-2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Truchet, G., M. Michel, and J. Dénarié. 1980. Sequential analysis of the organogenesis of lucerne (Medicago sativa) root nodules using symbiotically defective mutants of Rhizobium meliloti. Differentiation 16:163-169. [Google Scholar]
- 189.Truchet, G. L., and F. B. Dazzo. 1982. Morphogenesis of lucerne root nodules in the presence of combined nitrogen. Medicago sativa, histology and ultrastructure. Planta 154:352-360. [DOI] [PubMed] [Google Scholar]
- 190.Tyerman, S. D., L. F. Whitehead, and D. A. Day. 1995. A channel-like transporter for NH4+ on the symbiotic interface of N2-fixing plants. Nature 378:629-632. [Google Scholar]
- 191.Udvardi, M. K., D. L. Lister, and D. Day. 1992. Isolation and characterization of a ntrC mutant of Bradyrhizobium (Parasponia) sp. ANU289. J. Gen. Microbiol. 138:1019-1025. [DOI] [PubMed] [Google Scholar]
- 192.Udvardi, M. K., and D. Day. 1997. Metabolite transport across symbiotic membranes of legume nodules. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48:493-523. [DOI] [PubMed] [Google Scholar]
- 192a.Udvardi, M. K., and M. L. Kahn. 1993. Evolution of the (Brady) Rhizobium-legume symbiosis: why do bacteroids fix nitrogen? Symbiosis 14:87-101. [Google Scholar]
- 193.Urban, J. E., and F. B. Dazzo. 1982. Succinate-induced morphology of Rhizobium trifolii 0403 resembles that of bacteroids in clover nodules. Appl. Environ. Microbiol. 44:219-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Urban, J. E., L. C. Davis, and S. J. Brown. 1986. Rhizobium trifolii 0403 is capable of growth in the absence of combined nitrogen. Appl. Environ. Microbiol. 52:1060-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Valderrama, B., A. Davalos, L. Girard, E. Morett, and J. Mora. 1996. Regulatory proteins and cis-acting elements involved in the transcriptional control of Rhizobium etli reiterated nifH genes. J. Bacteriol. 178:3119-3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Van Egeraat, A. W. S. M. 1975. Ninhydrin-positive compounds present in seedling roots of leguminous plants. Plant Soil 43:503-507. [Google Scholar]
- 197.Van Rhijn, P., and J. Vanderleyden. 1995. The Rhizobium-plant symbiosis. Microbiol. Rev. 59:124-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.van Slooten, J. C., T. V. Bhuvanasvari, S. Bardin, and J. Stanley. 1992. Two C4-dicarboxylate transport systems in Rhizobium sp. NGR234: rhizobial dicarboxylate transport is essential for nitrogen fixation in tropical legume symbioses. Mol. Plant-Microbe Interact. 5:179-186. [DOI] [PubMed] [Google Scholar]
- 199.Vasse, J., F. de Billy, S. Camut, and G. Truchet. 1990. Correlation between ultrastructural differentiation of bacteroids and nitrogen fixation in alfalfa nodules. J. Bacteriol. 172:4295-4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Verma, D. P. S. 1992. Signals in root nodule organogenesis and endocytosis of Rhizobium. Plant Cell 4:373-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Verma, D. P., and Z. Hong. 1996. Biogenesis of the peribacteroid membrane in root nodules. Trends Microbiol. 4:364-368. [DOI] [PubMed] [Google Scholar]
- 202.Vierny, C., and M. Iaccarino. 1989. Comparative study of the symbiotic plasmid DNA in free-living bacteria and bacteroids of Rhizobium leguminosarum. FEMS Microbiol. Lett. 60:15-20. [DOI] [PubMed] [Google Scholar]
- 203.Vinuesa, P., B. L. Reuhs, C. Breton, and D. Werner. 1999. Identification of a plasmid-borne locus in Rhizobium etli KIM5s involved in lipopolysaccharide O-chain biosynthesis and nodulation of Phaseolus vulgaris. J. Bacteriol. 181:5606-5614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Walshaw, D. L., C. J. Reid, and P. S. Poole. 1997. The general amino acid permease of Rhizobium leguminosarum strain 3841 is negatively regulated by the Ntr system. FEMS Microbiol. Lett. 152:57-64. [DOI] [PubMed] [Google Scholar]
- 204a.Walshaw, D. L., A. Wilkinson, M. Mundy, M. Smith, and P. S. Poole. 1997. Regulation of the TCA cycle and the general amino acid permease by overflow metabolism in Rhizobium leguminosarum. Microbiology 143:2209-2221. [DOI] [PubMed] [Google Scholar]
- 205.Wang, S. P., and G. Stacey. 1990. Ammonia regulation of nod genes in Bradyrhizobium japonicum. Mol. Gen. Genet. 223:329-331. [DOI] [PubMed] [Google Scholar]
- 206.Waters, J. K., B. L. Hughes, L. C. Purcell, K. O. Gerhardt, T. P. Mawhinney, and D. W. Emerich. 1998. Alanine, not ammonia, is excreted from N2-fixing soybean nodule bacteroids. Proc. Natl. Acad. Sci. USA 95:12038-12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Watson, R. J., and V. K. Rastogi. 1993. Cloning and nucleotide sequencing of Rhizobium meliloti aminotransferase genes: an aspartate aminotransferase required for symbiotic nitrogen fixation is atypical. J. Bacteriol. 175:1919-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Weinstein, M., R. C. Roberts, and D. R. Helinski. 1992. A region of the broad-host-range plasmid RK2 causes stable in planta inheritance of plasmids in Rhizobium meliloti cells isolated from alfalfa root nodules. J. Bacteriol. 174:7486-7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Werner, D., E. Morschel, R. Kort, R. B. Mellor, and S. Bassarab. 1984. Lysis of bacteroids in the vicinity of the host cell nucleus in an ineffective (fix-) root nodule of soybean (Glycine max). Planta 162:8-16. [DOI] [PubMed] [Google Scholar]
- 210.Wheatcroft, R., D. G. McRae, and R. W. Miller. 1990. Changes in the Rhizobium meliloti genome and the ability to detect supercoiled plasmid during bacteroid development. Mol. Plant-Microbe Interact. 3:9-17. [Google Scholar]
- 211.Witty, J. F., L. Skot, and N. P. Revsbech. 1987. Direct evidence for changes in the resistance of legume root nodules to O2 diffusion. J. Exp. Bot. 38:1129-1140. [Google Scholar]
- 212.Yang, W. C., B. Horvath, J. Hontelez, A. Van Kammen, and T. Bisseling. 1991. In situ localization of Rhizobium mRNAs in pea root nodules: nifA and nifH localization. Mol. Plant-Microbe Interact. 4:464-468. [Google Scholar]
- 213.Zahran, H. H. 1999. Rhizobium-legume symbiosis and nitrogen fixation under severe conditions and in an arid climate. Microbiol. Mol. Biol. Rev. 63:968-989. [DOI] [PMC free article] [PubMed] [Google Scholar]