ABSTRACT
Background
The use of sex hormones in the clinic for the management of musculoskeletal conditions is increasingly common. Despite this, the role of sex hormones in various joint tissues such as the intervertebral disc (IVD), temporomandibular joint (TMJ), and articular cartilage remains poorly understood. Here, we employ a database search strategy to critically examine the available literature in this field through a scoping review.
Methods
Using a 4‐step protocol, primary research articles pertaining to sex hormones and the IVD, TMJ, or articular cartilage were identified and reviewed by two independent reviewers. ~3900 articles were identified in our initial search, and after review, ~140 were identified to be relevant to our tissues of interest and the effects of sex hormones.
Results
Within all joint tissues investigated here, there were limited investigations on the effects of testosterone. Studies reported here for these tissues indicate that sex hormones are likely beneficial in the context of age‐associated joint diseases, but there are important limitations to how this translates to the clinic given that various animal models can display distinct responses to sex hormone exposure. Direct comparisons of sex hormone therapies are limited between biological sexes, but evidence indicates that the molecular responses are likely similar. Current evidence indicates that sex hormone exposure likely has anti‐inflammatory effects within joint tissues at the level of gene and protein expression, but the mechanism is unknown.
Conclusion
Sex hormones such as testosterone and estrogen play an important role in inflammatory signaling within joint tissues, which could lead to novel interventions within the clinic for joint degeneration. However, understanding the biological mechanisms of hormones in these distinct tissues, between sexes, and with age is imperative for their proper implementation.
Keywords: aging, arthritis, estrogen, testosterone
The role of sex hormones within cartilaginous tissues remains poorly understood. To address this, we assembled and summarized the available literature regarding the effects of sex hormones in the intervertebral disc, temporomandibular joint, and articular cartilage. By summarizing the available literature, we were able to compare and contrast tissue specific responses, and next steps required within the field.

1. Introduction
1.1. Rationale
Musculoskeletal disorders are a tremendous burden on human health and health care systems around the world. Low back pain, for example, is the leading cause of years lived with disability worldwide [1]; and is often associated with degeneration of the intervertebral disc (IVD), the fibrocartilaginous joint located between joints of the spine. Osteoarthritis, also a leading cause of disability, is a heterogeneous disease affecting numerous joints, including the knee and hands. Although the cell types and overall tissue structure differ between articular and intervertebral joints, disc degeneration and osteoarthritis share numerous hallmarks and molecular pathways [2]. Despite their prevalence, both conditions lack disease‐modifying treatments. Of note, the incidence and severity of both IVD degeneration and osteoarthritis have been associated with sex differences [1, 3], and, specifically, the loss of endogenous sex hormones [4, 5]. Men display a higher prevalence of back pain until the fifth decade of life, after which women have a higher prevalence than men, a change likely corresponding to the onset of menopause [6, 7] and acute loss of sex hormone production in females. Menopause has also been linked to the progression of osteoarthritis, but there are mixed results on the direct effects of sex hormones on the health of the joint [8]. While the effects of sex hormones in bone are widely studied, their effects on joint cells and tissues are poorly understood, despite likely correlations between changes in sex hormone production and disease in cartilaginous tissues. The aim of this scoping review was to summarize the available literature on the effects of sex hormones on joint tissues to contextualize their effects and suggest future directions for the field. While sex hormones are important regulators of bone, this topic has been extensively reviewed [9, 10, 11, 12, 13, 14] and as such will not be addressed here, aside from subchondral bone as it relates to joint health and homeostasis.
1.2. Research Questions
(1) What are the roles of sex hormones within various joint tissues (intervertebral disc, temporomandibular joint, and articular cartilage)? (2) Are the roles of sex hormones dependent on biological sex? (3) Do these joints respond differently to hormone stimulation?
2. Methods
2.1. Search Strategy
A four‐step protocol using Covidence software to search multiple databases and identify studies of interest was applied. Then, two reviewers (JLH, AJH, or JF) independently screened titles and abstracts for inclusion eligibility. Discrepancies in agreement for inclusion were discussed individually and were then included or excluded based on mutual decision. Cohen's Kappa test for Title and Abstract screening was 0.745. Selected articles (221) were then retrieved for full‐text review and screened independently by two reviewers (JLH, AJH, or JF). Papers without English translations and papers that were unobtainable online were excluded from the study (N = 12). Details including study design, study subjects, and sample size, methodology, and results from each study were extracted and summarized.
2.2. Eligibility Criteria
This review included peer‐reviewed primary research articles that describe the effects of sex hormone supplementation or loss of endogenous production in articular cartilage, IVD, and the temporomandibular joint. No restrictions were set based on species, age, sex, ethnicity, or health status.
2.3. Information Sources
Only qualitative and quantitative articles from peer‐reviewed journals were considered. Narrative reviews, letters, and editorials were screened to ensure that original sources were included. No restrictions were set regarding the original publication language or year of publication. Four electronic databases were searched: PubMed, Web of Science, MEDLINE Ovid, and Scopus. These databases encompass a broad overview of literature pertaining to biomedicine, health, life, and physical sciences. Exclusion criteria included removal of secondary literature or opinion articles, papers that examined the effects of sex hormones on only bone (except for subchondral bone), and papers that did not test agonism or antagonism of sex hormones in the context of joint health.
2.4. Data Charting Process
A single reviewer charted data from each of the included full text articles. Data charted included citation information, the study design, cell and animal models used, major interventions or surgical models used, and their general outcomes.
2.5. Synthesis of Results
Data from the charting process was tabulated and organized by tissue or cell types (fibrocartilage or articular cartilage) and study design. For each tissue of interest, the sex hormones studied were tabulated to create an overview of their roles in each respective tissue.
3. Results
3.1. Selection of Sources of Evidence
We identified 3855 articles after the four‐database search (Web of Science, PubMed, Ovid Medline, Scopus) on April 16, 2024. After removal of duplicate articles, 3622 citations were evaluated by two independent reviewers in a screening process assessing the title and abstracts, followed by full‐text review. Inclusion criteria for this study required primary research articles addressing the loss of, supplementation, or treatment with sex hormones in vitro or in vivo and joint tissues. This included treatment of sex hormones on joint cells and tissues, in addition to models of ovariectomy/orchidectomy, and sex hormones in joint disease. Cohen's kappa reported a 0.78 agreement in Title and Abstract screening, and 0.48 for the full‐text review. At both stages, discrepancies in the agreement were discussed after the independent review to determine eligibility. After compiling the search results, there were 148 papers identified in this search (Figure 1).
FIGURE 1.
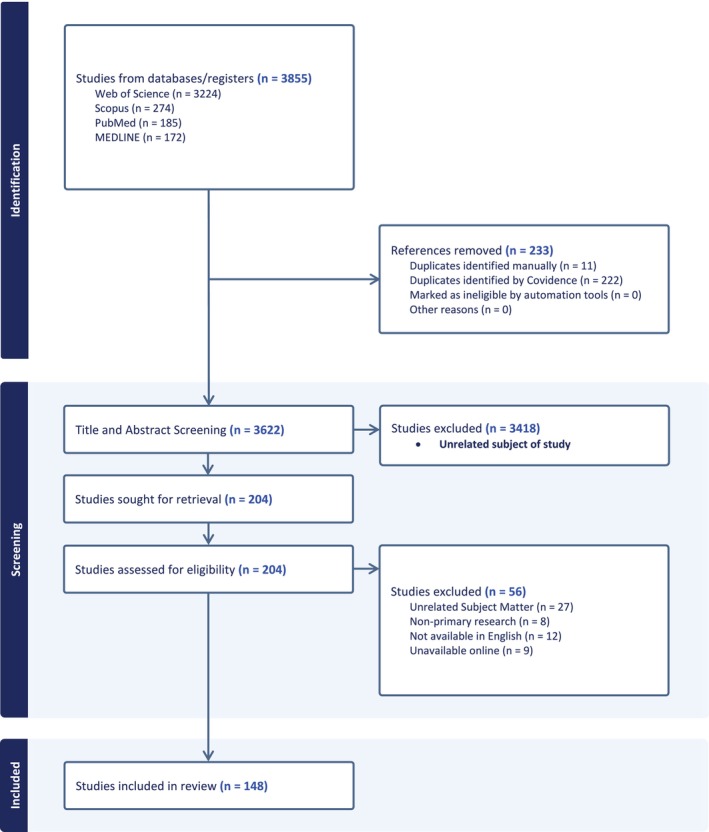
PRISMA flow diagram for article retrieval and selection for inclusion. Primary research articles related to the role of sex hormones and IVD, TMJ, and articular cartilage homeostasis were identified through multiple databases. Studies were screened by two independent reviewers, which identified 148 studies for this review.
We categorized the papers by joint of interest: the intervertebral disc (Table 1), the temporomandibular joint (Table 2), and the articular cartilage (Table 3).
TABLE 1.
Included articles for the intervertebral disc: information and study design.
| First author | Year | Study design | Species (strain) | Tissue/celltype | Anatomical region | Biological sex | Age of subject | Primary interventions or assessment |
|---|---|---|---|---|---|---|---|---|
| Paatsama [15] | 1969 | Non‐randomized experimental study | Canine | IVDs | Lumbar spine | Male and female | 1 week to 10 months | Testosterone and E2 |
| Brynhildsen [16] | 1998 | Questionnaire | Human | Low back pain | Lumbar spine | Female | 40–69 years | HRT |
| Gruber [17] | 2002 | Non‐randomized experimental study | Human | IVD cell culture | Lumbar spine | Male and female | 32–46 years | E2 |
| Wang [18] | 2004 | Non‐randomized experimental study | Rats (Sprague Dawley) | IVD | Lumbar IVDs | Female | 3 months | OVX |
| Baron [19] | 2005 | Cohort study | Human | IVD | T12‐L4 | Female | ~50 ± 8 years | HRT |
| Muscat Baron [20] | 2007 | Randomized controlled trial | Human | IVD | T12‐L3 | Female | Post‐menopausal | HRT |
| Gambacciani [21] | 2007 | Cross sectional study | Human | IVD | IVDs (T12‐L4) | Female | 53 ± 14 years | Pre‐, menopausal, and post‐menopausal |
| Li X [22] | 2008 | Non‐randomized experimental study | Bovine | Nucleus pulposus and annulus fibrosus cells | Caudal IVDs | NA | 15–18 months | Resveratrol |
| Zhang [23] | 2009 | Non‐randomized experimental study | Rats (Sprague Dawley) | Facet joint | Lumbar spine | Female | 3 months | E2 and OVX |
| Baron [24] | 2009 | Randomized controlled trial | Human | IVD | T2‐L3 | Female | Pre‐ and postmenopausal | HRT |
| Rowas [25] | 2012 | Non‐randomized experimental study | Mice (C57BL/6) | IVD | Lumbar spine | Male and Female | 3 months | Estrogen agonist (diethylbestrol) |
| Yang [26] | 2013 | Non‐randomized experimental study | Rats (Sprague Dawley) | Nucleus pulposus cells | Lumbar spine | Male | 2 months | E2 |
| Song [27] | 2014 | Non‐randomized experimental study | Human | IVD | Lumbar spine | Male and female | 65–77 years | Aging |
| Wang [28] | 2014 | Non‐randomized experimental study | Rats (Sprague Dawley) | Nucleus pulposus and annulus fibrosus cells | Lumbar spine | Male | 5–8 weeks | E2 |
| Lou [29] | 2014 | Randomized controlled trial | Human | IVDs | Lumbar spine | Female | 56.3 ± 12.9 years | Pre‐, menopausal, and post‐menopausal |
| Bertolo [30] | 2014 | Randomized controlled trial | Human | Nucleus pulposus cells | Cervical, thoracic and lumbar spine | Male and female | 25–57 years | Testosterone |
| Wang [31] | 2015 | Cohort study | Human | IVD | Lumbar spine | Male and female | > 64 years | Aging |
| Dubick [32] | 2015 | Case series | Human | Low back pain | Lumbar spine | Male and female | 58 ± 11 years | Testosterone and growth hormone |
| Ning [33] | 2016 | Non‐randomized experimental study | Rats (Sprague Dawley) | Nucleus pulposus cells | Lumbar spine | Male | ~5–8 weeks (~200‐220 g) | E2 |
| Wang [34] | 2016 | Non‐randomized experimental study | Human | Nucleus pulposus cells | NA | NA | NA | E2 |
| Yang [35] | 2016 | Non‐randomized experimental study | Rats (Sprague Dawley) | Nucleus Pulposus Cells | Lumbar spine | Male | 2 months | E2 |
| Zhao [36] | 2016 | Non‐randomized experimental study | Rats (Wistar) | Annulus fibrosus | Lumbar spine | Male | ~5–8 weeks (~200 g) | E2 |
| Jia [37] | 2016 | Non‐randomized experimental study | Rats (Sprague Dawley) | IVD | Lumbar spine | Female | 3 months | E2, PTH and OVX |
| Wei [38] | 2016 | Non‐randomized experimental study | Human | Nucleus pulposus cells | Lumbar spine | Male and female | 41 ± 15 years | ER agonists |
| Chatha [39] | 2016 | Randomized controlled trial | Rabbits (unidentified strain) | Annulus fibrosus injury | NA | Male | NA | Estradiol dipropionate |
| Lou [40] | 2017 | Randomized controlled trial | Human | IVD | Lumbar spine | Male and female | 68.4 ± 10.9 (female) and 66.8 ± 12.1 (male) | Aging |
| Li [41] | 2017 | Non‐randomized experimental study | Rats (Sprague Dawley) | IVD organ culture and nucleus pulposus cells | Lumbar spine | Male | 12 weeks | E2 |
| Song [42] | 2017 | Non‐randomized experimental study | Human | NP tissues | L4/5 and L5/S1 | Female | 69.3 ± 2.6 years | E2 and Substance P |
| Ao [43] | 2018 | Non‐randomized experimental study | Rats (Sprague Dawley) | Nucleus Pulposus cells | Lumbar Spine | Male | 6–8 weeks | E2 |
| Chen [44] | 2018 | Non‐randomized experimental study | Rats (Sprague Dawley) | IVD | Caudal IVDs | Female | 3 months of age | E2 |
| Sheng [45] | 2018 | Randomized controlled trial | Human | IVD and cartilage endplates | Lumbar spine | Male and Female | 39 ± 11 years | E2 |
| Jin [46] | 2018 | Non‐randomized experimental study | Rats (Sprague Dawley) | IVD | Lumbar spine | Female | 6 weeks | E2 and OVX |
| Liu [47] | 2018 | Non‐randomized experimental study | Rats (Sprague Dawley) | IVD | Caudal IVDs | Female | 3 months | E2 and OVX |
| Sheng [45] | 2018 | Non‐randomized experimental study | Rats (Sprague Dawley) | Cartilage endplate cells | NA | Female | 5–8 weeks | E2 and OVX |
| Wu [48] | 2018 | Non‐randomized experimental study | Mice (C57BL/6) | Facet Joint | Lumbar Spine | Female | 12 weeks | OVX |
| Xiao [49] | 2018 | Non‐randomized experimental study | Mice (C57BL/6) | IVD | Lumbar spine | Female | 8 weeks | OVX |
| Wen [50] | 2019 | Non‐randomized experimental study | Human | Nucleus pulposus cells | Lumbar spine | Male and Female | 59 ± 7.2 years | Beta‐ecdysterone |
| Guo [51] | 2019 | Non‐randomized experimental study | Rats (Sprague Dawley) | Nucleus pulposus cells | Lumbar IVDs | Male | 2 months | E2 and ER antagonist |
| Liu [52] | 2019 | Non‐randomized experimental study | Rats (Sprague Dawley) | IVD | Cranial | Female | 3 months | HRT and OVX |
| Zhang [53] | 2019 | Non‐randomized experimental study | Rats (Sprague Dawley) | IVD | Lumbar spine | Female | 3 months | OVX |
| Cai [54] | 2020 | Non‐randomized experimental study | Human | Nucleus Pulposus cells | Lumbar spine | Male and Female | 43 ± 6 years | E2 |
| Gao [55] | 2020 | Non‐randomized experimental study | Human | Nucleus Pulposus cells | NA | NA | NA | E2 |
| Bai [56] | 2021 | Non‐randomized experimental study | Human | Nucleus pulposus cells | NA | NA | NA | E2 |
| Song [57] | 2021 | Randomized controlled trial | Human | NP tissue | NA | NA | 28–45 years | E2 |
| Song [58] | 2021 | Non‐randomized experimental study | Mice (C57BL/6) | Nucleus pulposus | Lumbar spine | Female | 8 weeks | E2 and OVX |
| Tucci [59] | 2021 | Non‐randomized experimental study | Rats (Sprague Dawley) | IVD | Lumbar spine | Female | 3 months | E2 and OVX |
| Tian [60] | 2021 | Non‐randomized experimental study | Rats (Sprague Dawley) | IVD | Caudal IVDs | Female | 4, 8, and 12 weeks | E2 and OVX and IVD puncture |
| Sun [61] | 2021 | Non‐randomized experimental study | Rats (Sprague Dawley) | IVD | Lumbar spine | Female | 3 months | ER agonists and OVX |
| Zhao [62] | 2021 | Case control study | Human | IVD | Lumbar Spine | Female | 62.40 ± 10.64 years | OVX Patients |
| Bhadouria [63] | 2022 | Non‐randomized experimental study | Mice (C57BL/6) | IVD | Lumbar spine | Female | 4 and 22 months | Raloxifene |
| Li [64] | 2022 | Case control study | Human | IVD | Lumbar spine | Female | 47 ± 6 years | Tamoxifen |
| Heuch [65] | 2023 | Cohort study | Human | Low back pain | Lumbar spine | Female | 40–69 years | Aging |
| Widmayer [66] | 2023 | Non‐randomized experimental study | Bovine | IVD | Caudal IVDs | Male | 12–24 months | E2 and vibration |
| Stevenson [67] | 2023 | Non‐randomized experimental study | Human | IVD | Lumbar Spine | Female | 55.4 years | E2, postmenopausal |
| Elmounedi [68] | 2023 | Non‐randomized experimental study | Rats (Sprague Dawley) | IVD | Lumbar spine | Female | 3 months | OVX |
| Song [69] | 2023 | Non‐randomized experimental study | Mice (C57BL/6) | Spinal cord | Lumbar spine | Female | 18 weeks | OVX and diarylpropionitrile |
Abbreviations: E2, 17beta‐estradiol; ER, estrogen receptor; HRT, hormone replacement therapy; IVD, intervertebral disc; OCX, orchidectomy; OVX, ovariectomy; PTH, parathyroid hormone.
TABLE 2.
Included articles for the temporomandibular joint: information and study design.
| First author | Year | Study design | Species (strain) | Tissue or cell type a | Biological sex | Age of subject | Primary interventions or assessment |
|---|---|---|---|---|---|---|---|
| Aufdemorte [70] | 1986 | Non‐randomized experimental study | Baboon | Articular disc, condylar surface | Female | 19.1 years | OVX and tritiated HRT |
| Orajarvi [71] | 2011 | Non‐randomized experimental study | Rats (Sprague Dawley) | Condylar cartilage | Female | 2 months | OVX and diet hardness |
| Orajarvi [72] | 2012 | Non‐randomized experimental study | Rats (Sprague Dawley) | Condylar Cartilage | Female | 5 and 14 months | OVX and diet hardness |
| Robinson [73] | 2017 | Non‐randomized experimental study | Mice (C57BL/6) | Condylar cartilage | Male | 49 days | ERα KO |
| Abubaker [74] | 1996 | Non‐randomized experimental study | Rats (Wistar) | Articular disc | Male and female | 3 weeks | OVX or OC and HRT |
| Yamada [75] | 2003 | Non‐randomized experimental study | Rats (Wistar) | Synovium, articular disc, and mandibular condyle | Male | 4 weeks | ER localization |
| Min [76] | 2007 | Non‐randomized experimental study | Mice (ICR) | Condylar cartilage | Female | 4 months | OVX |
| Puri [77] | 2009 | Non‐randomized experimental study | Rats (Sprague Dawley) | Synovium, articular disc, and mandibular condyle | Female | 7 months | OVX and E2 |
| Wu [78] | 2010 | Non‐randomized experimental study | Rats (Sprague Dawley) | Whole joint | Female | Adult | OVX and E2 |
| Madani [79] | 2013 | Cross sectional study | Human | Blood from TMJD patients | Female | 20–40 years | TMJ disease |
| Chen [80] | 2014 | Non‐randomized experimental study | Rats (Sprague Dawley) | Condylar cartilage, subchondral bone | Female | 7 months | OVX |
| Chen [81] | 2014 | Non‐randomized experimental study | Mice (C57BL/6) | Condylar cartilage | Female | 21 days | OVX, ERβ KO, and HRT |
| Stemig [82] | 2015 | Non‐randomized experimental study | Human | N/A | Male and Female | N/A | Genotypic association with TMJD |
| Figueroba [83] | 2015 | Non‐randomized experimental study | Rats (Wistar) | Condylar cartilage, synovium | Male and Female | 3 months | OCX and OVX |
| Nicot [84] | 2016 | Cohort study | Human | N/A | Male and Female | 14–37 years | Genotypic association with TMJD |
| Fanton [85] | 2017 | Non‐randomized experimental study | Rats (Wistar) | Nervous system | Male and Female | 3 months | OVX or OCX |
| Ahmad [86] | 2018 | Randomized controlled trial | Mice (C57BL/6) | TMJ fibrocartilage | Female | 12 weeks | ERα and ERβ overexpression and knockdown |
| Flake [87] | 2006 | Non‐randomized experimental study | Rats (Sprague Dawley) | TMJ blood vessels | Male and Female | N/A | OVX and HRT |
| Wang [88] | 2013 | Non‐randomized experimental study | Rats (Sprague Dawley) | Condylar cartilage, subchondral bone | Female | N/A | OVX and HRT |
| Wu [89] | 2019 | Non‐randomized experimental study | Mice (C57BL/6) | Condylar cartilage, subchondral bone | Female | 8 weeks | OVX and mechanical stress |
| Zhang [90] | 2023 | Non‐randomized experimental study | Mice (C57BL/6) | Condylar cartilage | Female | 8 weeks | E2 deficiency and mechanical stress |
| Okuda [91] | 1996 | Non‐randomized experimental study | Rats (Wistar) | Condylar cartilage, subchondral bone | Female | 4 weeks | OVX |
| Yasuoka [92] | 2000 | Non‐randomized experimental study | Rats (Wistar) | Condylar cartilage, subchondral bone | Female | 4 weeks | OVX and hormone replacement |
| Hauru [93] | 2024 | Non‐randomized experimental study | Rats (Sprague Dawley) | Condylar cartilage | Female | 5 and 14 months | OVX, aging, HRT, dietary loading |
| Yu [52] | 2019 | Non‐randomized experimental study | Rats (Sprague Dawley) | Condylar cartilage | Female | 5 and 14 months | OVX, dietary loading |
| Sheridan [94] | 1985 | Non‐randomized experimental study | Baboon | Condylar cartilage | Female | 18–21 | 3H‐estradiol‐17 β |
Abbreviations: E2, 17beta‐estradiol; ER, estrogen receptor; HRT, hormone replacement therapy; IVD, intervertebral disc; KO, Knockout; OCX, orchidectomy; OVX, ovariectomy; PTH, parathyroid hormone.
Anatomical location was the TMJ for all studies listed above.
TABLE 3.
Included articles for articular cartilage: information and study design.
| First author | Year | Study design | Species (strain) | Tissue or cell type | Anatomical region | Biological sex | Age of subject | Primary interventions or assessment |
|---|---|---|---|---|---|---|---|---|
| DaSilva [95] | 1994 | Non‐randomized experimental study | Mice (BALB/C) and rats (Wistar) | Articular cartilage | Femoral head | Male and Female | 9–10 weeks (Mice), (160‐180 g male rats, 150‐160 g female rats) | OVX or OCX +/− HRT |
| Rasanen [96] | 1999 | Non‐randomized experimental study | Rabbits (New Zealand White) | Articular cartilage | Femoral head | Female | 18–23 weeks | OVX and HRT |
| Dai [97] | 2006 | Non‐randomized experimental study | Guinea pigs (Dunkin Hartley) | Articular cartilage | Femoral condyle, tibial plateau | Female | 32 months | OVX |
| Dayani [98] | 1988 | Non‐randomized experimental study | Rabbits (Fauve de Bourgogne) | Primary chondrocytes | Long bones | Male and Female | 15–25 and 40–60 days old | ER expression by articular cartilage cells |
| Osman [99] | 2019 | Non‐randomized experimental study | Rats (Sprague Dawley) | Articular cartilage | Knee | Female | 3–4 months | OVX and HRT |
| Tang [100] | 2020 | Non‐randomized experimental study | Human | Primary chondrocytes | Knee | Male and Female | 62–68 years | OA and arctigenin |
| Rosner [101] | 1982 | Non‐randomized experimental study | Rabbits (New Zealand White) | Articular cartilage | Knee | Female | N/A | OVX |
| Corvol [102] | 1987 | Non‐randomized experimental study | Rabbits (Fauve de Bourgogne) | Primary epiphyseal articular cells | Long bones | Male and Female | 2–80 days | Age, sex hormone (testosterone, DHT, E2) |
| Tsai [103] | 1993 | Non‐randomized experimental study | Rabbits (New Zealand White) | Articular cartilage | Lateral femoral condyle, patella | Female | N/A | OVX + E2 |
| Turner [104] | 1997 | Non‐randomized experimental study | Ewe | Articular cartilage | Femur and Tibia | Female | 4–5 years | OVX |
| Claassen [105] | 2002 | Non‐randomized experimental study | Pigs (gottingen miniature pigs) | Articular cartilage | Proximal wrist joint | Female | 33 months | OVX, dietary Ca2+ |
| Lee [106] | 2003 | Randomized controlled trial | Human | Primary chondrocytes | Knee | Female | 55–84 | E2 |
| Hoegh‐Andersen [107] | 2004 | Non‐randomized experimental study | Rats (Sprague Dawley) | Articular cartilage | Knee | Female | 5 and 7 months | OVX and SERM |
| Claassen [108] | 2005 | Non‐randomized experimental study | Bovine | Articular Chondrocytes | Intertarsal joint | Female | 2 and 7 years | E2 |
| Claassen [109] | 2006 | Non‐randomized experimental study | Bovine | Articular chondrocytes | Transverse tarsal joint | Female | N/A | E2, insulin, tamoxifen, ICI |
| Oshima [110] | 2007 | Non‐randomized experimental study | Rats (Wistar) | Articular cartilage, subchondral bone | Femur | Male and Female | 1 day, 1 week, 1 month, 3 month, 24 month | OVX, sex |
| Castaneda [111] | 2010 | Non‐randomized experimental study | Rabbits (New Zealand White) | Articular cartilage | Knee | Female | 8 months | OVX, glucocorticoid |
| Sniekers [112] | 2010 | Non‐randomized experimental study | Mice (C3H/HeJ) | Articular cartilage, subchondral bone | Knee | Female | 24 weeks | OVX, E2, bisphosphonate |
| Claassen [113] | 2010 | Non‐randomized experimental study | Human | Articular Chondrocytes | Hip, knee, or ankle | Male and Female | 28–85 years | E2 |
| Bay‐Jensen [114] | 2011 | Non‐randomized experimental study | Rats (Sprague Dawley) | Articular cartilage | Knee | Female | 6 months | OVX |
| Yang [115] | 2012 | Non‐randomized experimental study | Rats (Sprague Dawley) | Bone, articular cartilage | Knee | Female | 7 months | OVX, E2, progesterone |
| Fontinele [116] | 2013 | Non‐randomized experimental study | Rats (Wistar) | Articular cartilage | Tibial proximal epiphysis | Female | 6 months | OVX, physical activity |
| Yan [117] | 2014 | Non‐randomized experimental study | Guinea Pigs (Dunkin Hartley) | Articular cartilage, subchondral bone | Tibia | Female | 41 months | Age |
| Liang [118] | 2016 | Non‐randomized experimental study | Human | Primary chondrocytes | Knee | Female | > 53 years | E2, IL‐1β |
| Lou [119] | 2016 | Non‐randomized experimental study | Human | Articular cartilage | Knee | Female | 36–83 | Pain |
| Lee [120] | 2017 | Non‐randomized experimental study | Rats (Sprague Dawley) | Articular cartilage | Knee | Female | 8 weeks | OVX |
| Ge [121] | 2019 | Non‐randomized experimental study | Mice (C57BL/6) and human chondrocytes | Articular cartilage | Knee | Male and Female | Mice; 10 weeks, human, 53–70 years | OVX |
| Hughbanks [122] | 2021 | Case control study | Human | Articular cartilage | Knee | Female | N/A | Total knee arthroplasty and ACL reconstruction |
| Li [123] | 2022 | Non‐randomized experimental study | Mice (C57BL/6) | Articular cartilage, subchondral bone | Knee | Female | 3 months | OVX, diet, physical activity, metformin |
| Huang [124] | 2022 | Non‐randomized experimental study | Human and rabbit (New Zealand) | Primary chondrocytes | Knee | N/A | Adult | Psoralen |
| Polur [125] | 2015 | Non‐randomized experimental study | Mice (C57BL/6) | Articular cartilage, subchondral bone | TMJ | Male and Female | 21 days | ERβ KO |
| Richmond [126] | 2000 | Non‐randomized experimental study | Monkeys (cynomolgus) | Articular cartilage | Knee | N/A | Adult | OVX, E2 |
| Wluka [127] | 2001 | Non‐randomized experimental study | Human | Articular cartilage | Knee | Female | > 50 years | Postmenopausal |
| Ham [128] | 2002 | Non‐randomized experimental study | Monkeys (cynomolgus) | Articular cartilage, subchondral bone | Knee | Female | 9.6–15.8 years | OVX, E2, soy phytoestrogen |
| Mouritzen [129] | 2003 | Randomized controlled trial | Human | Articular cartilage | Urinary CTX‐II degradation products | Male and Female | 20–87 years | Age, sex, menopause, HRT, and BMI |
| Cicuttini [130] | 2003 | Randomized controlled trial | Human | Articular cartilage | Patella | Female | > 50 | ERT |
| Wluka [131] | 2004 | Non‐randomized experimental study | Human | Articular cartilage | Tibia | Female | > 50 | ERT |
| Ham [132] | 2004 | Non‐randomized experimental study | Monkeys (Cynomolgus) | Articular cartilage | Knee | Female | 10.7–13.6 | OVX |
| Cake [133] | 2005 | Non‐randomized experimental study | Ovine | Articular cartilage | Femoro‐tibial joint | Female | 7 years | OVX |
| Dai [134] | 2005 | Non‐randomized experimental study | Guinea Pigs (Dunkin Hartley) | Articular cartilage | Tibial plateau | Female | 2 months | OVX |
| Oestergaard [135] | 2006 | Non‐randomized experimental study | Rats (Sprague Dawley) | Articular cartilage | Serum CTX‐II degradatio product | Female | 6 months | OVX + early/delayed E2 |
| Kato [136] | 2010 | Non‐randomized experimental study | Mice (C57BL/6) | Articular cartilage, primary chondrocytes | Knee | Female | 4 and 15 months | ERα KO mice |
| Wei [137] | 2011 | Cross sectional study | Human | Articular cartilage | Knee | Female | 50–80 years | Parity |
| Kavas [138] | 2013 | Non‐randomized experimental study | Rats (Sprague Dawley) | Articular chondrocytes | Knee | N/A | N/A | Raloxifene |
| Hamdi [139] | 2022 | Non‐randomized experimental study | Human | Articular chondrocytes | Knee | Male and Female | 63–85 years | E2, raloxifene, enterolactone |
| Jiang [140] | 2023 | Non‐randomized experimental study | Rats (Sprague Dawley) | Articular chondrocytes and subchondral osteoblasts | N/A | Female | 6 months | OVX induced OA |
| Bei [141] | 2020 | Non‐randomized experimental study | Rats (Sprague Dawley) | Articular cartilage, subchondral bone | Patello‐femoral joint | Female | 3 months | OVX, raloxifene |
| Ziemian [142] | 2021 | Non‐randomized experimental study | Mice (C57BL/6) | Articular cartilage, subchondral bone, joint capsule | Knee | Female | 26 weeks | ERα KO mice |
| Wang [143] | 2016 | Non‐randomized experimental study | Rats (Sprague Dawley) | Articular cartilage | Knee | Female | 10 months | OVX |
| Xu [144] | 2019 | Non‐randomized experimental study | Rats (Sprague Dawley) | Articular cartilage, subchondral bone | Knee | Female | 6 months | OVX, E2, SERM, raloxifene |
| Jin [145] | 2017 | Cohort study | Human | Articular cartilage | Knee | Male and Female | 56.7–69.3 years | Symptomatic knee OA |
| Duan [146] | 2021 | Non‐randomized experimental study | Rats (unidentified strain) | Articular cartilage | Knee | Female | 6 months | ERα targeted knockdown (PROTAC) |
| Saeki Fernandes [147] | 2018 | Non‐randomized experimental study | Rats (Wistar) | Articular cartilage | Femur | Female | Adult | OVX, E2, diabetes |
| Qin [148] | 2013 | Non‐randomized experimental study | Rabbits (undefined strain) | Articular cartilage | Distal femur | Female | 18.5 months | OVX, E2, electro‐acupuncture |
| Luo [149] | 2009 | Non‐randomized experimental study | Rats (Wistar) | Articutlar cartilage | Medial condyle of femur, tibial plateau | Female | 3 months | OVX and HRT |
| Sniekers [150] | 2009 | Non‐randomized experimental study | Mice (C57BL/6) | Articular cartilage | Knee | Female | 6 months | ERα and ERβ KO |
| Silva [151] | 2024 | Non‐randomized experimental study | Mice (CD‐1) | Articular cartilage | Femoro‐tibial joint | Female | Adult | OVX, DV and DSG |
| Sondergaard [152] | 2007 | Non‐randomized experimental study | Rats (Sprague Dawley) | Articular cartilage | Knee | Female | 8 months | OVX, E2, calcitonin, 5CNAC |
| Zaychenko [153] | 2023 | Non‐randomized experimental study | Rats (Wistar) | Articular cartilage, subchondral bone, subchondral plate | Knee | Female | 6–8 months | OVX, resveratrol |
| daSilva [154] | 1993 | Non‐randomized experimental study | Mice (BALB/C) and Rats (Wistar) | Articular cartilage | Femoral head | Female | 9–10 weeks | OVX |
| Christgau [155] | 2004 | Non‐randomized experimental study | Rats (Sprague Dawley) | Articular cartilage | Knee | Female | 6 months | SERM or E2 exposure after OVX |
| Harris [156] | 2003 | Non‐randomized experimental study | Rats (Lewis) | Articular cartilage | Tarsal joint | Male and Female | 12 weeks | OA and ERβ agonist (ERB‐041) |
| Chang [157] | 2014 | Non‐randomized experimental study | Rabbits (Japenese White) | Articular chondrocytes | Knee | Male and Female | 12 weeks | E2 and testosterone |
| Hui [158] | 2021 | Non‐randomized experimental study | Rabbits (New Zealand White) | Articular chondrocytes | Knee | Male | 3 months | Androgen receptor overexpression |
| Kinney [159] | 2005 | Non‐randomized experimental study | Human | Articular chondrocytes | Knee | Male and Female | 16–39 years | 17β‐Estrogen |
| Rouge [160] | 2023 | Non‐randomized experimental study | Horse | Osteochondral sections | Metacarpel | Male | 11 and 18 months | OCX |
Abbreviations: DHT; 5α‐dihydrotestosterone, E2; 17β‐estradiol, ER; estrogen receptor, HRT; hormone replacement therapy, IVD; intervertebral disc, KO; Knockout, OA; osteoarthritis, OCX; orchidectomy, OVX; ovariectomy, PTH; parathyroid hormone. SERM; selective estrogen receptor modulator.
3.2. The Effects of Sex Hormones on the IVD
3.2.1. Study Design
Various study designs were employed, including non‐randomized experimental (39), cohort (3), case control (2), randomized controlled trial (8), case series (1), questionnaires (1), and cross‐sectional (1) studies. Publications by year are reported for all studies identified (Figure 2A). The research typically focused on the lumbar spine using cell or organ culture (23), or in vivo analyses (33). Animal models used varied based on study design. Experimental studies used mouse, rat, rabbit, canine, bovine, and human cells or tissues, while questionnaire or cohort studies assessed human patients attending clinics or human participants (Figure 2B).
FIGURE 2.
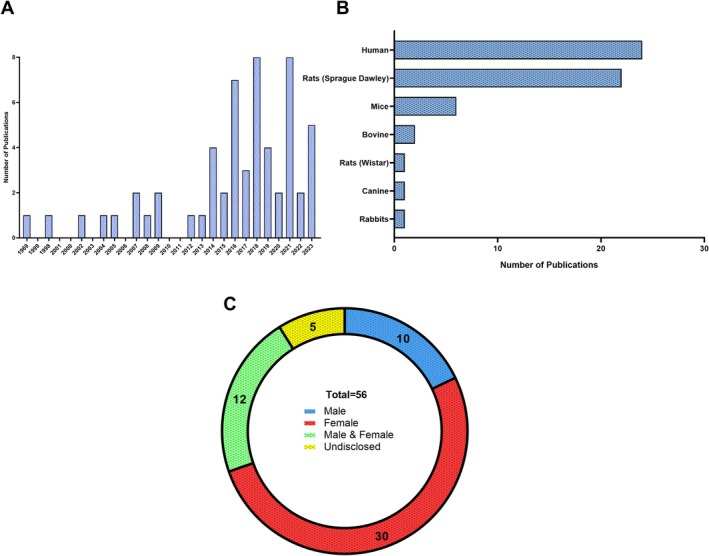
Primary characteristics in IVD research investigating the role of sex hormones. (A) Publications identified in our search stratified by year of publication. (B) Model species used in the laboratory and/or clinic in each study. (C) Distribution of biological sexes investigated in IVD research related to sex hormones.
3.2.2. Distribution of Biological Sex, Age, and Hormones Studied
Biological sex of the model systems was not evenly distributed, as females were more frequently studied (52%) compared to males (18%). Some studies used both biological sexes (21%) and a small proportion did not disclose biological sex (9%) (Figure 2C). Interventions used in these studies were primarily centered around estrogen removal, replacement, or use of estrogen agonists and antagonists (48). Three studies directly investigated testosterone exposure [15, 30, 32]. Some studies also investigated menopause‐associated changes (4). Age distribution varied from juvenile animal models to middle‐aged human (40–60 years old) individuals.
3.2.3. Key Findings: IVD
Literature examining the role of sex hormones on the IVD focused on each of its tissue constituents: the nucleus pulposus (NP), annulus fibrosus (AF), and cartilage endplates (CEP). Of these, studies investigated changes in tissue hydration, apoptosis, and matrix degrading enzyme expression and activity (41/55). Clinical assessments such as IVD height and low back pain in patients were also reported (14/55). Studies focused on the nucleus pulposus consistently reported that estrogen attenuates apoptotic and pro‐inflammatory signals induced by cytokines, ovariectomy (OVX), or disc injury [26, 36, 38, 41, 51, 57]. Additionally, estrogen exposure attenuated matrix degrading enzyme expression and increased proteoglycan and collagen content in cell cultures in a dose‐dependent fashion [47]. It is important to note that many of these studies focused solely on NP cell culture models (14), and few studies investigated the effects of estrogen on the AF (3) or CEP (2). Of those that investigated the AF, primary investigations reported that estrogen prevented apoptotic signaling [36], increased cell proliferation [17], and recruited neutrophil precursors [39]. Studies focused on the role of estrogen in the CEP also observed decreased endplate mineralization [44] and increased proteoglycan content [47].
In vivo, estrogen deprivation through OVX consistently induced IVD degeneration across multiple animal models [18, 23, 44, 46, 48, 49, 52, 57, 58, 68, 69, 161]. This phenomenon is also reported in postmenopausal women [16, 19, 20, 21, 24, 27, 29, 40, 62, 67, 162]. Estrogen replacement mitigated many degenerative changes in animal models, including maintaining the redox balance by reducing reactive oxygen species [46] and reducing histopathological changes within IVDs [59]. IVD injury and estrogen deprivation by OVX were partially ameliorated by estrogen supplementation [60]. In women, estrogen replacement following menopause correlated with increased disc height [19, 20, 67], but these patients also reported increased low back pain [16]. Vertebral subchondral bone of mice was decreased following ovariectomy [48, 49].
The effects of testosterone on IVD biology are poorly investigated, limited to two studies. In 1969, a group investigated the effects of testosterone, estrogen, and parathyroid hormone on AF lamellae structure in a canine model [15]. They reported structural changes to the lamellar structure, including fragmentation and “loosening,” with unknown biological consequences. The second study, in 2014, was a primary human cell culture experiment investigating the chondrogenic potential of testosterone in NP cells to facilitate the in vitro formation of tissue constructs [30]. Testosterone increased collagen type 2 and aggrecan gene expression in male IVD cells, and changes in gene expression were abolished by an aromatase inhibitor, preventing the conversion of testosterone into estrogen. A single case series study investigated the effects of testosterone in humans, where 60 patients (combination of male and female) with chronic lower back pain received testosterone and recombinant growth hormone injections locally to the site of pain [32]. The study reported a decrease in patients self‐reported low back pain after 12 months.
3.3. The Effect of Sex Hormones on the Temporal Mandibular Joint
3.3.1. Study Design
Most articles identified for the TMJ were non‐randomized experimental study designs (23); the remaining articles used randomized controlled trial (1), cohort (1), and cross‐sectional study (1) designs. Publications by year are reported for all studies identified (Figure 3A). The animal models used varied, with rats as the primary animal model used in studies (15), followed by mice (6), human (3), and baboon (2) models (Figure 3B).
FIGURE 3.
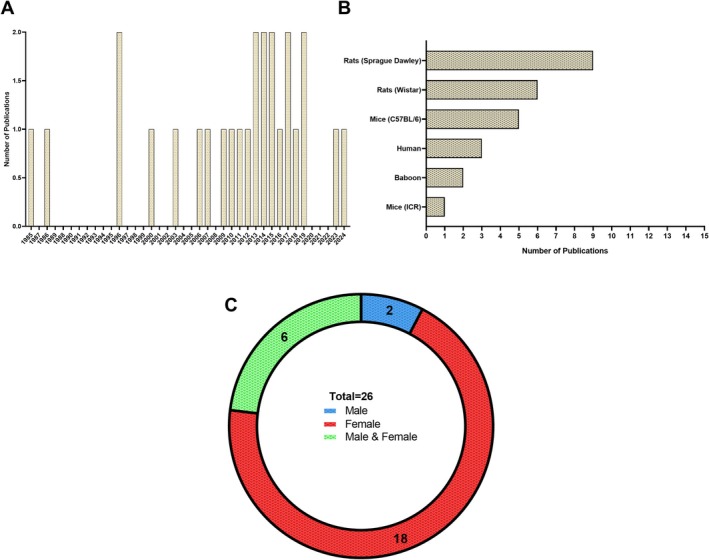
Primary characteristics in TMJ research investigating the role of sex hormones. (A) Publications identified in our search stratified by year of publication. (B) Model species used in laboratory and/or clinic in each study. (C) Distribution of biological sexes investigated in TMJ research related to sex hormones.
3.3.2. Distribution of Biological Sex, Age, and Hormones
Studies focused predominantly on female sex (69%), with two studies identified in our search examining males. Six studies used both male and female models (Figure 3C). Studies primarily focused on the effects of estrogen deprivation through ovariectomy (OVX) on the temporomandibular joint (54%) and estrogen receptor activation (35%). Three studies investigated the effects of testosterone on pain and collagen content following a gonadectomy. Human patients were examined in three studies ranging from adolescent to young adult (14–40 years of age) to identify associations between TMJ prevalence and genotypic variations.
3.3.3. Key Findings: Temporomandibular Joint
In the late 1980s and early 2000s, estrogen receptors were identified within TMJ cells of the baboon [94] and rat [75]. Following this discovery, various animal models were used to investigate the effects of estrogen on TMJ biology, including changes induced by estrogen deprivation, receptor agonism, and receptor knockout [86, 89]. The effects of OVX or orchidectomy (OCX) on the integrity of the TMJ are still unclear. Some groups report increased cartilage thickness after sex hormone deprivation [71, 81], while another group showed decreased cartilage thickness [90]. Importantly, these studies differed in their use of model systems to evaluate TMJ cartilage thickness, with mouse models ranging from 21 days to 2 months of age and 2‐month‐old rat models. At baseline, there are sex‐based differences in rat condylar cartilage within the TMJ. Male rats had higher collagen content than female animals, which was abrogated with castration in both sexes [74]. Estrogen receptor knockout has also been studied in rodent models of TMJ disease and suggests that loss of estrogen signaling has a detrimental effect on extracellular matrix production. For example, both male and female ERβ knockout mice had decreased collagen type X expression and subchondral bone integrity in the TMJ [125], similar to an ovariectomy study in rats which showed a reduction in collagen type II and X gene expression [72]. ERβ knockout mice were resistant to cartilage thickening observed in the ovariectomized mice [81] and ERα knockout negatively influenced TMJ maturation in mice, but not TMJ degeneration [163].
Interestingly, estradiol has pro‐inflammatory effects within the TMJ. Castration increased cytokine levels in both sexes [83], and while testosterone supplementation mitigated pain [85] and TMJ damage, estradiol exacerbated TMJ damage in the presence of inflammation [87], TRPV1‐mediated pain [78], and increased the expression of matrix metalloproteases [86].
3.4. The Effect of Sex Hormones on Articular Cartilage and Chondrocytes
3.4.1. Study Design
Study design for the assessment of articular cartilage was almost entirely non‐randomized experimental studies (60), with few randomized controlled trials (3) and a single cross‐sectional, case‐control, and cohort study. Publications by year are reported for all studies identified (Figure 4A). Model systems primarily consisted of rat (20), human (14), rabbit (7), and mouse (6) subjects (Figure 4B).
FIGURE 4.
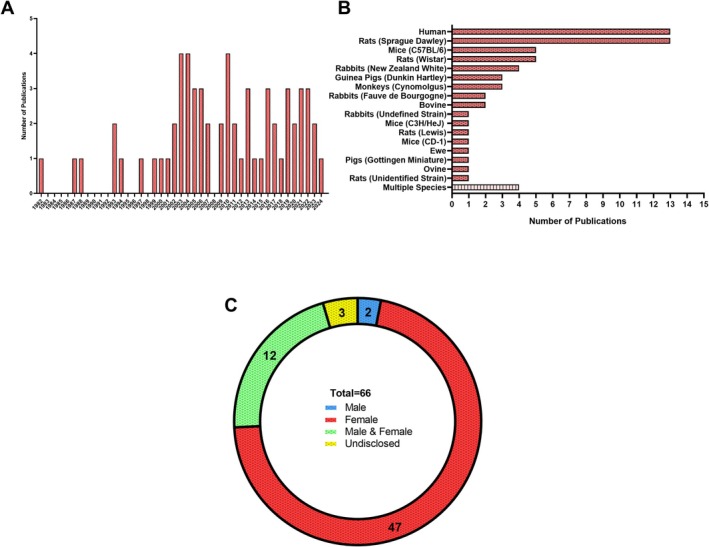
Primary characteristics in articular cartilage research investigating the role of sex hormones. (A) Publications identified in our search stratified by year of publication. (B) Model species used in the laboratory and/or clinic in each study. (C) Distribution of biological sexes investigated in articular cartilage research related to sex hormones.
3.4.2. Distribution of Biological Sex, Age, and Hormones
Most studies investigated female animals (71%), while only two investigated males (3%), and several used both sexes (21%). Three studies did not disclose the sex of individuals (1 human, 1 rabbit, 1 monkey, 5%) (Figure 4C). The most widely used interventions were OVX or OCX (37) to induce pathogenic features such as inflammation or osteoarthritis, or primary cell culture experimentation (16). Changes in osteoarthritis pathogenesis were then characterized following joint destabilization surgery alone or following treatment with various sex hormone agonists or hormone replacement therapy. Studies in humans examined age‐ and menopause‐associated changes in osteoarthritis (> 50 years of age) and used primary cells isolated from tissue at the time of surgery, which enabled the use of tissues from a large range of ages (16–87 years of age). In vitro animal‐based experiments used tissues and cells from neonatal to skeletally mature adult animals.
3.4.3. Key Findings: Articular Cartilage
How sex hormones regulate joint health and the progression of osteoarthritis is a multifaceted research question, given that both involve interactions between cartilage, bone, and synovium and that osteoarthritis studies often focus on the management of patient‐reported pain. Here we specifically focus on the effects of sex hormones on articular cartilage. Early cell culture studies showed estrogen receptor localization within rabbit and rat articular chondrocytes [98] and that rats expressed estrogen receptor α (ERα) in both biological sexes, which decreased after OVX [110]. This and subsequent studies characterized various effects of estrogen, including the attenuation of inflammatory gene expression in primary human chondrocytes isolated from the hip, knee, and ankle of OA patients [106, 113, 124], and increased miR‐140 expression, which suppressed MMP‐13 expression [118]. In primary chondrocyte cultures, estradiol reduced oxidative stress [108]. Estradiol and testosterone positively regulate chondrogenesis in male and female rabbit cells, with a larger effect from estrogen than testosterone in cells derived from both sexes [157].
Despite these positive findings in cell culture experiments, they contrast with findings on the effects of sex hormones on articular joints in vivo. Much of the variation in outcome is likely dependent on which animal model was used. In contrast to other reports suggesting negative effects of OVX on cartilage health, rabbits have increased cartilage thickness [96] and stiffness and decreased histopathological scores [111] with OVX, and high doses of estradiol resulted in cartilage defects and fibrillations [101]. However, this response was dose‐dependent; a low dose of estradiol did not result in changes to the knee cartilage [103]. In another study using primary rabbit chondrocytes, testosterone, dihydrotestosterone, and estradiol exposure all increased glycosaminoglycan production in an age‐dependent manner (increased response with increased age) in male and female rabbits [102]. Androgen receptor overexpression was investigated in one study using rabbit models and showed regeneration of cartilage defects [158].
There is a consensus regarding the effects of depletion, replacement, or supplementation of sex hormones based on studies in the rat model. OVX induces osteoarthritis [114, 143], and both OVX and OCX decreased glycosaminoglycan content in articular cartilage [95]. In female rats, OVX‐induced osteoarthritis was prevented with hormone replacement therapy [99]. Estrogen receptor beta agonist (ERβ‐041) reduced histopathological joint scores, inflammation, and synovitis in rats [156]. It is noteworthy that few studies used male animals (15/62). Reports in murine models are consistent with those in rats; OVX in mice increased OA progression [123] and estradiol prevented cartilage damage following OVX [112]. Female mice treated with DHT and estradiol had reduced GAG loss in cartilage explant cultures [154].
Human studies investigating the effects of sex hormones on cartilage volume and the progression of osteoarthritis produced conflicting reports. For instance, cartilage degradation correlates positively with years elapsed since menopause [119], and estrogen replacement decreased cartilage turnover in postmenopausal women [131]. Tibial cartilage volume was also increased in patients who received estrogen replacement [127], and a reduction in matrix degrading enzyme expression [106], implying that estrogen signaling was important for maintaining cartilage health. However, there are also reports that estradiol exposure does not influence cartilage volume [130] or the progression of knee OA [145]. Furthermore, there were also reports that ERα was increased with age in OA patients [122], and that treatment of articular chondrocytes with low doses of estrogen increased chondrogenic markers, but high doses of estrogen decreased these markers [139]. Childbearing also increased the number of cartilage defects observed in knee cartilage [137]. Taken together, these studies show that the relationship between sex hormones and articular cartilage is multifaceted and depends on biological sex and model system.
4. Discussion
4.1. Summary of Evidence
The objective of this scoping review was to summarize the body of literature pertaining to the effects of sex hormones on joint tissues, particularly the IVD, TMJ, and articular cartilage. Our search results suggest that while this research is still in its infancy, sex hormones are important regulators of joint homeostasis, and removal of endogenous sex hormone production can underlie joint disease and certainly exacerbates age‐ and injury‐associated joint pathologies [60, 92, 133, 155]. Importantly, the response of the animal models to sex hormone treatment, or removal, was conditional upon the sex, age, and the species being investigated. For instance, while there were positive implications within the IVD for disc height [67], and chondrogenesis [163], both indicators of IVD health, it should also be noted the incidence of low back pain was higher in groups receiving estrogen hormone replacement therapy [6]. Studies investigating the effects of testosterone and its derivatives on each of these joint tissues are extremely limited. Results from testosterone studies indicate positive molecular outcomes such as increased proteoglycan and collagen synthesis [30], and a reduction in patient‐reported pain after testosterone treatment [32]. Importantly, we identified that while there are apparent sex differences in joint pathologies following treatment, some outcomes of testosterone or estrogen exposure were irrespective of biological sex [74, 95, 102], including a clinical case series following testosterone injections in men and women [32]. These reported differences require further investigation so that laboratory models can better simulate clinical outcomes. Given the presence of sex hormone receptors on cells of multiple joint tissues in both sexes, it is also likely that there are independent and coactivation of androgen and estrogen receptors, which impact joint tissues.
A consistent finding across studies examining the various joint tissues investigated was the role of sex hormones in modulating the inflammatory response. Aberrant inflammatory signaling can promote disease pathology such as IVD degeneration [164, 165], TMJ disease, and osteoarthritis [166, 167]. Few studies investigated the effects of both testosterone and estrogen within their model systems, and this is a limitation to drawing strong conclusions, as the enzyme aromatase can actively convert testosterone to estrogen, and therefore the effects of testosterone could be confounded by those of estrogen. The relationship between the conversion of testosterone to estrogen could be addressed in these models using non‐aromatizable testosterone (DHT), or aromatase inhibitors, as previously described [30].
Important to note, IVD height, an indicator of IVD health, was assessed in women receiving hormone replacement therapy, as was the rate of height loss between men and women with aging. Disc height correlated negatively with the onset of menopause, whereas men had increased disc degeneration at a younger age, perhaps due to differences in injury rates between men and women [40, 168]. Importantly, disc height was increased in women receiving hormone replacement [19, 67]. There were no studies that assessed clinically the effects of sex hormones on the temporomandibular joint; however, similar to the IVD, articular cartilage volume is negatively correlated with menopause [119], and hormone replacement therapy increased articular cartilage volume in post‐menopausal women [131]. Of note, we did not identify any clinical studies investigating testosterone replacement therapy and age‐associated changes in disc height, temporomandibular joint disease, or articular cartilage volume. Although aging results in the loss of circulating testosterone at a much different rate in men than women, approximately 1% per year after 30 years of age [169], there still remains an important gap in our research knowledge regarding how sex hormones influence joint health in the aging male. Importantly, testosterone and estrogen use is not limited to male and female biological sex, respectively, and can be used by both sexes in the context of sport [170, 171, 172] or treatment of gender dysphoria [173, 174]. Given an increasing population of individuals who seek hormone‐based therapy for sexual transitioning each year [173], it is imperative that research explores the effects of these types of hormone regimes on joint homeostasis and long‐term health in these individuals.
4.2. Limitations Within the Field
There are some limitations impacting the data acquired throughout this search that we feel are important to bear in mind when interpreting the results of this review. First, the retrieval of human samples are often limited to those being excised during surgery or post‐mortem; and confounding factors such as surgery‐ or necrosis‐induced degeneration are a consideration that may impact findings. Due to the degenerative features or advanced age of these tissues, the effects of sex hormones on joint homeostasis are poorly understood, limiting analysis to their influence on the degenerative phenotype. Although randomized experimental trials were limited in human subjects, and cross‐sectional, cohort, or longitudinal studies are limited by sampling bias, they remained powerful assessments for determining sex‐based differences in aging and the effects of hormone replacement. Animal models are also limited, as almost all laboratory animals do not enter menopause with age [175, 176]. This necessitates researchers to use castration models to block sex hormone synthesis to mimic the effects of menopause, often requiring surgery or complex medication that could interfere with the progression of disease.
5. Conclusion
In summary, we found that reports show the effects of sex hormones on the IVD, TMJ, and articular cartilage are sex‐, species‐, age‐, and tissue‐dependent. Overall, the role of estrogen may not be entirely positive as predicted by pre‐clinical animal models, as commonly believed. While there are positive implications in clinical assessments such as IVD height and cartilage production, patients may also report increased pain. Many reports, particularly focused on the TMJ and articular cartilage, are inconsistent. Studies on the effects of testosterone on joint tissues are limited, and although there are beneficial implications for IVD, TMJ, and articular cartilage biology, further studies are warranted to understand the biology underlying joint health and to develop therapeutics for patients suffering from joint pathologies.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding: This work was funded by the Canadian Institutes of Health Research and the Arthritis Society (grants to C.A.S.). J.L.H. was supported by awards from the CONNECT! NSERC CREATE Training Program, a Transdisciplinary Training Award Western's Bone and Joint Institute, the Arthritis Society, and the Ontario Graduate Scholarship (OGS) Program. C.A.S. is supported by a Career Development award from the Arthritis Society.
References
- 1. Wu A., March L., Zheng X., et al., “Global Low Back Pain Prevalence and Years Lived With Disability From 1990 to 2017: Estimates From the Global Burden of Disease Study 2017,” Annals of Translational Medicine 8 (2020): 299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fine N., Lively S., Séguin C. A., Perruccio A. V., Kapoor M., and Rampersaud R., “Intervertebral Disc Degeneration and Osteoarthritis: A Common Molecular Disease Spectrum,” Nature Reviews Rheumatology 19 (2023): 136–152. [DOI] [PubMed] [Google Scholar]
- 3. Peshkova M., Lychagin A., Lipina M., et al., “Gender‐Related Aspects in Osteoarthritis Development and Progression: A Review,” International Journal of Molecular Sciences 23 (2022): 2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shelby T., Mills E. S., Ton A., et al., “The Role of Sex Hormones in Degenerative Disc Disease,” Global Spine Journal 13 (2023): 2096–2099, 10.1177/21925682231152826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Linn S., Murtaugh B., and Casey E., “Role of Sex Hormones in the Development of Osteoarthritis,” PM & R: The Journal of Injury, Function, and Rehabilitation 4 (2012): S169–S173. [DOI] [PubMed] [Google Scholar]
- 6. Wáng Y. X. J., Wáng J.‐Q., and Káplár Z., “Increased Low Back Pain Prevalence in Females Than in Males After Menopause Age: Evidences Based on Synthetic Literature Review,” Quantitative Imaging in Medicine and Surgery 6 (2016): 199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang Y.‐X. J. and Griffith J. F., “Effect of Menopause on Lumbar Disk Degeneration: Potential Etiology,” Radiology 257 (2010): 318–320. [DOI] [PubMed] [Google Scholar]
- 8. Mei Y., Williams J. S., Webb E. K., Shea A. K., MacDonald M. J., and al‐Khazraji B. K., “Roles of Hormone Replacement Therapy and Menopause on Osteoarthritis and Cardiovascular Disease Outcomes: A Narrative Review,” Frontiers in Rehabilitation Sciences 3 (2022): 825147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Compston J. E., “Sex Steroids and Bone,” Physiological Reviews 81 (2001): 419–447. [DOI] [PubMed] [Google Scholar]
- 10. Rizzoli R. and Bonjour J.‐P., “Hormones and Bones,” Lancet 349 (1997): S20–S23. [DOI] [PubMed] [Google Scholar]
- 11. Bachrach L. K., “Hormonal Contraception and Bone Health in Adolescents,” Frontiers in Endocrinology 11 (2020): 603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Khosla S. and Monroe D. G., “Regulation of Bone Metabolism by Sex Steroids,” Cold Spring Harbor Perspectives in Medicine 8 (2018): a031211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carnevale V., Romagnoli E., Cipriani C., et al., “Sex Hormones and Bone Health in Males,” Archives of Biochemistry and Biophysics 503 (2010): 110–117. [DOI] [PubMed] [Google Scholar]
- 14. Vico L. and Vanacker J.‐M., “Sex Hormones and Their Receptors in Bone Homeostasis: Insights From Genetically Modified Mouse Models,” Osteoporosis International 21 (2010): 365–372. [DOI] [PubMed] [Google Scholar]
- 15. Paatsama S., Rissanen P., and Rokkanen P., “Effect of Estradiol, Testosterone, Cortisone Acetate, Somatotropin, Thyrotropin and Parathyroid Hormone on the Lumbar Intervertebral Disc in Growing Dogs,” Journal of Small Animal Practice 10 (1969): 351–354. [DOI] [PubMed] [Google Scholar]
- 16. Brynhildsen J. O., Bjors E., Skarsgard C., and Hammar M. L., “Is Hormone Replacement Therapy a Risk Factor for Low Back Pain Among Postmenopausal Women?,” Spine 23 (1998): 809–813. [DOI] [PubMed] [Google Scholar]
- 17. Gruber H. E., Yamaguchi D., Ingram J., et al., “Expression and Localization of Estrogen Receptor‐Beta in Annulus Cells of the Human Intervertebral Disc and the Mitogenic Effect of 17‐Beta‐Estradiol In Vitro,” BMC Musculoskeletal Disorders 3 (2002): 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang T., Zhang L., Huang C., Cheng A. G., and Dang G. T., “Relationship Between Osteopenia and Lumbar Intervertebral Disc Degeneration in Ovariectomized Rats,” Calcified Tissue International 75 (2004): 205–213. [DOI] [PubMed] [Google Scholar]
- 19. Baron Y. M., Brincat M. P., Galea R., and Calleja N., “Intervertebral Disc Height in Treated and Untreated Overweight Post‐Menopausal Women,” Human Reproduction 20 (2005): 3566–3570. [DOI] [PubMed] [Google Scholar]
- 20. Muscat Baron Y., Brincat M. P., Galea R., and Calleja N., “Low Intervertebral Disc Height in Postmenopausal Women With Osteoporotic Vertebral Fractures Compared to Hormone‐Treated and Untreated Postmenopausal Women and Premenopausal Women Without Fractures,” Climacteric Journal ‐ International Menopause Society 10 (2007): 314–319. [DOI] [PubMed] [Google Scholar]
- 21. Gambacciani M., Pepe A., Cappagli B., Palmieri E., and Genazzani A. R., “The Relative Contributions of Menopause and Aging to Postmenopausal Reduction in Intervertebral Disk Height,” Climacteric 10 (2007): 298–305. [DOI] [PubMed] [Google Scholar]
- 22. Li X., Phillips F. M., An H. S., et al., “The Action of Resveratrol, a Phytoestrogen Found in Grapes, on the Intervertebral Disc,” Spine (Phila Pa 1976) 33 (2008): 2586–2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang L., Xu Z., Lin S., et al., “Effect of Estrogen on the Degeneration of Rat Cervical Intervertebral Disc,” Medical Journal of Wuhan University 30 (2009): 788–790. [Google Scholar]
- 24. Baron Y. M., Brincat M. P., Calleja‐Agius J., and Calleja N., “Intervertebral Disc Height Correlates With Vertebral Body T‐Scores in Premenopausal and Postmenopausal Women,” Menopause International 15 (2009): 58–62. [DOI] [PubMed] [Google Scholar]
- 25. Rowas S. A., Haddada R., Gawri R., et al., “Effect of In Utero Exposure to Diethylstilbestrol on Lumbar and Femoral Bone, Articular Cartilage, and the Intervertebral Disc in Male and Female Adult Mice Progeny With and Without Swimming Exercise,” Arthritis Research & Therapy 14 (2012): R17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yang S.‐D., Yang D.‐L., Sun Y.‐P., et al., “17β‐Estradiol Protects Against Apoptosis Induced by Interleukin‐1β in Rat Nucleus Pulposus Cells by Down‐Regulating MMP‐3 and MMP‐13,” Apoptosis 20 (2013): 348–357. [DOI] [PubMed] [Google Scholar]
- 27. Song X., Yu Y. J., Li X. F., Liu Z. D., Yu B. W., and Guo Z., “Estrogen Receptor Expression in Lumbar Intervertebral Disc of the Elderly: Gender‐ and Degeneration Degree‐Related Variations,” Joint, Bone, Spine 81 (2014): 250–253. [DOI] [PubMed] [Google Scholar]
- 28. Wang H., Ding W., Yang D., Gu T., Yang S., and Bai Z., “Different Concentrations of 17β‐Estradiol Modulates Apoptosis Induced by Interleukin‐1β in Rat Annulus Fibrosus Cells,” Molecular Medicine Reports 10 (2014): 2745–2751. [DOI] [PubMed] [Google Scholar]
- 29. Lou C., Chen H. L., Feng X. Z., et al., “Menopause Is Associated With Lumbar Disc Degeneration: A Review of 4230 Intervertebral Discs,” Climacteric 17 (2014): 700–704. [DOI] [PubMed] [Google Scholar]
- 30. Bertolo A., Baur M., Aebli N., Ferguson S. J., and Stoyanov J., “Physiological Testosterone Levels Enhance Chondrogenic Extracellular Matrix Synthesis by Male Intervertebral Disc Cells In Vitro, but Not by Mesenchymal Stem Cells,” Spine Journal 14 (2014): 455–468. [DOI] [PubMed] [Google Scholar]
- 31. Wang Y. X. J., “Postmenopausal Chinese Women Show Accelerated Lumbar Disc Degeneration Compared With Chinese Men,” Journal of Orthopaedic Translation 3, no. 4 (2015): 205–211, 10.1016/j.jot.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dubick M. N., Ravin T. H., Michel Y., and Morrisette D. C., “Use of Localized Human Growth Hormone and Testosterone Injections in Addition to Manual Therapy and Exercise for Lower Back Pain: A Case Series With 12‐Month Follow‐Up,” Journal of Pain Research 8 (2015): 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ning S.‐H., Yang S.‐D., Zhang X., et al., “Protective Effect of 17β‐Estradiol on Hydrogen Peroxide Induced Apoptosis of Rat Nucleus Pulposus Cells,” International Journal of Clinical and Experimental Medicine 9 (2016): 17281–17294. [Google Scholar]
- 34. Wang T., Yang S. D., Liu S., Wang H., Liu H., and Ding W. Y., “17β‐Estradiol Inhibites Tumor Necrosis Factor‐α Induced Apoptosis of Human Nucleus Pulposus Cells via the PI3K/Akt Pathway,” Medical Science Monitor 22 (2016): 4312–4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yang S. D., Ma L., Yang D. L., and Ding W. Y., “Combined Effect of 17β‐Estradiol and Resveratrol Against Apoptosis Induced by Interleukin‐1β in Rat Nucleus Pulposus Cells via PI3K/Akt/Caspase‐3 Pathway,” PeerJ 4 (2016): e1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhao C.‐M., Chen Q., Zhang W. J., et al., “17β‐Estradiol Protects Rat Annulus Fibrosus Cells Against Apoptosis via α1 Integrin‐Mediated Adhesion to Type I Collagen: An In‐Vitro Study,” Medical Science Monitor 22 (2016): 1375–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jia H., Ma J., Lv J., et al., “Oestrogen and Parathyroid Hormone Alleviate Lumbar Intervertebral Disc Degeneration in Ovariectomized Rats and Enhance Wnt/β‐Catenin Pathway Activity,” Scientific Reports 6 (2016): 27521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wei A., Shen B., Williams L. A., et al., “Expression and Functional Roles of Estrogen Receptor GPR30 in Human Intervertebral Disc,” Journal of Steroid Biochemistry and Molecular Biology 158 (2016): 46–55. [DOI] [PubMed] [Google Scholar]
- 39. Chatha W. A., “Response of Vertebral Bone Marrow to Female Sex Hormone Five Days After Injury to Annulus Fibrosus,” Pakistan Journal of Medical & Health Sciences 10 (2016): 28–31. [Google Scholar]
- 40. Lou C., Chen H., Mei L., et al., “Association Between Menopause and Lumbar Disc Degeneration: An MRI Study of 1,566 Women and 1,382 Men,” Menopause 24 (2017): 1136–1144. [DOI] [PubMed] [Google Scholar]
- 41. Li P., Gan Y., Xu Y., et al., “17beta‐Estradiol Attenuates TNF‐α‐Induced Premature Senescence of Nucleus Pulposus Cells Through Regulating the ROS/NF‐κB Pathway,” International Journal of Biological Sciences 13 (2017): 145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Song H., Luo Y., Wang W., et al., “Effects of Alendronate on Lumbar Intervertebral Disc Degeneration With Bone Loss in Ovariectomized Rats,” Spine (Phila Pa 1976) 17 (2017): 995–1003. [DOI] [PubMed] [Google Scholar]
- 43. Ao P., Huang W., Li J., et al., “17β‐Estradiol Protects Nucleus Pulposus Cells From Serum Deprivation‐Induced Apoptosis and Regulates Expression of MMP‐3 and MMP‐13 Through Promotion of Autophagy,” Biochemical and Biophysical Research Communications 503 (2018): 791–797. [DOI] [PubMed] [Google Scholar]
- 44. Chen C. H., Chen W. C., Lin C. Y., Tsuang Y. H., and Kuo Y. J., “Sintered Dicalcium Pyrophosphate Treatment Attenuates Estrogen Deficiency‐Associated Disc Degeneration in Ovariectomized Rats,” Drug Design, Development and Therapy 12 (2018): 3033–3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sheng B., Zhou J., Liu X., et al., “Protective Effect of Estrogen Against Calcification in the Cartilage Endplate,” International Journal of Clinical and Experimental Pathology 11 (2018): 1660–1666. [PMC free article] [PubMed] [Google Scholar]
- 46. Jin L.‐Y., Lv Z. D., Wang K., et al., “Estradiol Alleviates Intervertebral Disc Degeneration Through Modulating the Antioxidant Enzymes and Inhibiting Autophagy in the Model of Menopause Rats,” Oxidative Medicine and Cellular Longevity 2018 (2018): 7890291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Liu S., Yang S. D., Huo X. W., Yang D. L., Ma L., and Ding W. Y., “17β‐Estradiol Inhibits Intervertebral Disc Degeneration by Down‐Regulating MMP‐3 and MMP‐13 and Up‐Regulating Type II Collagen in a Rat Model,” Artificial Cells, Nanomedicine, and Biotechnology 46 (2018): 182–191. [DOI] [PubMed] [Google Scholar]
- 48. Wu T., Ni S., Cao Y., et al., “Three‐Dimensional Visualization and Pathologic Characteristics of Cartilage and Subchondral Bone Changes in the Lumbar Facet Joint of an Ovariectomized Mouse Model,” Spine (Phila Pa 1976) 18, no. 4 (2018): 663–673, 10.1016/j.spinee.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 49. Xiao Z.‐F., He J. B., Su G. Y., et al., “Osteoporosis of the Vertebra and Osteochondral Remodeling of the Endplate Causes Intervertebral Disc Degeneration in Ovariectomized Mice,” Arthritis Research & Therapy 20 (2018): 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wen F., Yu J., He C.‐J., Zhang Z.‐W., and Yang A.‐F., “Beta‐Ecdysterone Protects Against Apoptosis by Promoting Autophagy in Nucleus Pulposus Cells and Ameliorates Disc Degeneration,” Molecular Medicine Reports 19 (2019): 2440–2448. [DOI] [PubMed] [Google Scholar]
- 51. Guo H. T., Yang S. D., Zhang F., et al., “17β‐Estradiol Protects Against Interleukin‐1β‐Induced Apoptosis in Rat Nucleus Pulposus Cells via the mTOR/Caspase‐3 Pathway,” Molecular Medicine Reports 20 (2019): 1523–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Liu Q., Wang X., Hua Y., et al., “Estrogen Deficiency Exacerbates Intervertebral Disc Degeneration Induced by Spinal Instability in Rats,” Spine 44 (2019): E510–E519. [DOI] [PubMed] [Google Scholar]
- 53. Zhang N., Tian F., Gou Y., et al., “Protective Effect of Alendronate on Lumbar Facet Degeneration in Ovariectomized Rats,” Medical Science Monitor 25 (2019): 4907–4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cai Z., Li K., Yang K., Luo D., and Xu H., “Suppression of miR‐203‐3p Inhibits Lipopolysaccharide Induced Human Intervertebral Disc Inflammation and Degeneration Through Upregulating Estrogen Receptor Alpha,” Gene Therapy 27 (2020): 417–426. [DOI] [PubMed] [Google Scholar]
- 55. Gao X. W., Su X. T., Lu Z. H., and Ou J., “17β‐Estradiol Prevents Extracellular Matrix Degradation by Downregulating MMP3 Expression via PI3K/Akt/FOXO3 Pathway,” Spine (Phila Pa 1976) 45, no. 5 (2020): 292–299, 10.1097/BRS.0000000000003263. [DOI] [PubMed] [Google Scholar]
- 56. Bai X., Guo X., Zhang F., Zheng L., Ding W., and Yang S., “Resveratrol Combined With 17β‐Estradiol Prevents IL‐1β Induced Apoptosis in Human Nucleus Pulposus via the PI3K/AKT/Mtor and PI3K/AKT/GSK‐3β Pathway,” Journal of Investigative Surgery 34 (2021): 904–911. [DOI] [PubMed] [Google Scholar]
- 57. Song X.‐X., Jin L.‐Y., Li X.‐F., Luo Y., and Yu B.‐W., “Substance P Mediates Estrogen Modulation Proinflammatory Cytokines Release in Intervertebral Disc,” Inflammation 44 (2021): 506–517. [DOI] [PubMed] [Google Scholar]
- 58. Song M.‐X., Ma X. X., Wang C., et al., “Protective Effect of Estrogen Receptors (ERα/β) Against the Intervertebral Disc Degeneration Involves Activating CCN5 via the Promoter,” European Review for Medical and Pharmacological Sciences 25, no. 4 (2021): 1811–1820, 10.26355/eurrev_202102_25075. [DOI] [PubMed] [Google Scholar]
- 59. Tucci M., Wilson G. A., McGuire R., and Benghuzzi H. A., “The Effects of NPY1 Receptor Antagonism on Intervertebral Disc and Bone Changes in Ovariectomized Rats,” Global Spine Journal 11 (2021): 1166–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tian T., Wang H., Li Z., Yang S., and Ding W., “Intervertebral Disc Degeneration Induced by Needle Puncture and Ovariectomy: A Rat Coccygeal Model,” BioMed Research International 2021 (2021): 5510124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sun Q., Tian F. M., Liu F., et al., “Denosumab Alleviates Intervertebral Disc Degeneration Adjacent to Lumbar Fusion by Inhibiting Endplate Osteochondral Remodeling and Vertebral Osteoporosis in Ovariectomized Rats,” Arthritis Research & Therapy 23 (2021): 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhao Y., Wang H., Li Z., et al., “Lumbar Disk Degeneration in Female Patients With and Without Ovariectomy: A Case‐Control Study,” World Neurosurgery 156 (2021): 68–75. [DOI] [PubMed] [Google Scholar]
- 63. Bhadouria N., Berman A. G., Wallace J. M., and Holguin N., “Raloxifene Stimulates Estrogen Signaling to Protect Against Age‐ and Sex‐Related Intervertebral Disc Degeneration in Mice,” Frontiers in Bioengineering and Biotechnology 10 (2022): 924918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Li Y., Wei Y., Li H., et al., “Exogenous Parathyroid Hormone Alleviates Intervertebral Disc Degeneration Through the Sonic Hedgehog Signalling Pathway Mediated by CREB,” Oxidative Medicine and Cellular Longevity 2022 (2022): 9955677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Heuch I., Heuch I., Hagen K., Storheim K., and Zwart J.‐A., “Menopausal Hormone Therapy, Oral Contraceptives and Risk of Chronic Low Back Pain: The HUNT Study,” BMC Musculoskeletal Disorders 24 (2023): 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Widmayer F., Neidlinger‐Wilke C., Witz F., et al., “Oestrogen and Vibration Improve Intervertebral Disc Cell Viability and Decrease Catabolism in Bovine Organ Cultures,” International Journal of Molecular Sciences 24 (2023): 6143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Stevenson T. E. J., Brincat M. P., Pollacco J., and Stevenson J. C., “Effect of Hormone Replacement Therapy on Intervertebral Disc Height,” Climacteric Journal ‐ International Menopause Society 26 (2023): 110–113. [DOI] [PubMed] [Google Scholar]
- 68. Elmounedi N., Bahloul W., Aoui M., Sahnoun N., Ellouz Z., and Keskes H., “Original Animal Model of Lumbar Disc Degeneration,” Libyan Journal of Medicine 18 (2023): 2212481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Song X.‐X., Jin L.‐Y., Li Q., Li X.‐F., and Luo Y., “Estrogen Receptor β/Substance P Signaling in Spinal Cord Mediates Antinociceptive Effect in a Mouse Model of Discogenic Low Back Pain,” Frontiers in Cellular Neuroscience 16 (2023): 1071012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Aufdemorte T. B., van Sickels J. E., Dolwick M. F., et al., “Estrogen Receptors in the Temporomandibular Joint of the Baboon ( Papio cynocephalus ): An Autoradiographic Study,” Oral Surgery, Oral Medicine, and Oral Pathology 61 (1986): 307–314. [DOI] [PubMed] [Google Scholar]
- 71. Orajärvi M., Hirvonen O., Yu S.‐B., et al., “Effect of Estrogen and Altered Diet Hardness on the Expression of Estrogen Receptor Alpha and Matrix Metalloproteinase‐8 in Rat Condylar Cartilage,” Journal of Oral & Facial Pain and Headache 25 (2011): 261–268. [PubMed] [Google Scholar]
- 72. Orajärvi M., Puijola E., Yu S.‐B., et al., “Effect of Estrogen and Dietary Loading on Condylar Cartilage,” Journal of Oral & Facial Pain and Headache 26 (2012): 328–336. [PubMed] [Google Scholar]
- 73. Robinson J. L., Gupta V., Soria P., et al., “Estrogen Receptor Alpha Mediates Mandibular Condylar Cartilage Growth in Male Mice,” Orthodontics & Craniofacial Research 20, no. Suppl 1 (2017): 167–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Abubaker A. O., Hebda P. C., and Gunsolley J. N., “Effects of Sex Hormones on Protein and Collagen Content of the Temporomandibular Joint Disc of the Rat,” Journal of Oral and Maxillofacial Surgery 54 (1996): 721–727, discussion 727. [DOI] [PubMed] [Google Scholar]
- 75. Yamada K., Nozawa‐Inoue K., Kawano Y., et al., “Expression of Estrogen Receptor Alpha (ER Alpha) in the Rat Temporomandibular Joint,” Anatomical Record. Part A, Discoveries in Molecular, Cellular, and Evolutionary Biology 274 (2003): 934–941. [DOI] [PubMed] [Google Scholar]
- 76. Min H.‐J., Lee M. J., Kim J. Y., et al., “Alteration of BMP‐4 and Runx2 Expression Patterns in Mouse Temporomandibular Joint After Ovariectomy,” Oral Diseases 13 (2007): 220–227. [DOI] [PubMed] [Google Scholar]
- 77. Puri J., Hutchins B., Bellinger L. L., and Kramer P. R., “Estrogen and Inflammation Modulate Estrogen Receptor Alpha Expression in Specific Tissues of the Temporomandibular Joint,” Reproductive Biology and Endocrinology 7 (2009): 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wu Y.‐W., Bi Y. P., Kou X. X., et al., “17‐Beta‐Estradiol Enhanced Allodynia of Inflammatory Temporomandibular Joint Through Upregulation of Hippocampal TRPV1 in Ovariectomized Rats,” Journal of Neuroscience: The Official Journal of the Society for Neuroscience 30 (2010): 8710–8719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Madani A. S., Shamsian A. A., Hedayati‐Moghaddam M. R., et al., “A Cross‐Sectional Study of the Relationship Between Serum Sexual Hormone Levels and Internal Derangement of Temporomandibular Joint,” Journal of Oral Rehabilitation 40 (2013): 569–573. [DOI] [PubMed] [Google Scholar]
- 80. Chen K., Zhang N., Ding L., Zhang W., Hu J., and Zhu S., “Early Intra‐Articular Injection of Alendronate Reduces Cartilage Changes and Subchondral Bone Loss in Rat Temporomandibular Joints After Ovariectomy,” International Journal of Oral and Maxillofacial Surgery 43 (2014): 996–1004. [DOI] [PubMed] [Google Scholar]
- 81. Chen J., Kamiya Y., Polur I., et al., “Estrogen via Estrogen Receptor Beta Partially Inhibits Mandibular Condylar Cartilage Growth,” Osteoarthritis and Cartilage 22 (2014): 1861–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Stemig M., Myers S. L., Kaimal S., and Islam M. S., “Estrogen Receptor‐Alpha Polymorphism in Patients With and Without Degenerative Disease of the Temporomandibular Joint,” Cranio: Journal of Craniomandibular Practice 33 (2015): 129–133. [DOI] [PubMed] [Google Scholar]
- 83. Figueroba S. R., Franco G. C. N., Omar N. F., Groppo M. F., and Groppo F. C., “Dependence of Cytokine Levels on the Sex of Experimental Animals: A Pilot Study on the Effect of Oestrogen in the Temporomandibular Joint Synovial Tissues,” International Journal of Oral and Maxillofacial Surgery 44 (2015): 1368–1375. [DOI] [PubMed] [Google Scholar]
- 84. Nicot R., Vieira A. R., Raoul G., et al., “ENPP1 and ESR1 Genotypes Influence Temporomandibular Disorders Development and Surgical Treatment Response in Dentofacial Deformities,” Journal of Cranio‐Maxillo‐Facial Surgery 44, no. 9 (2016): 1226–1237, 10.1016/j.jcms.2016.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Fanton L. E., Macedo C. G., Torres‐Chavez K. E., Fischer L., and Tambeli C. H., “Activational Action of Testosterone on Androgen Receptors Protects Males Preventing Temporomandibular Joint Pain,” Pharmacology, Biochemistry, and Behavior 152 (2017): 30–35. [DOI] [PubMed] [Google Scholar]
- 86. Ahmad N., Chen S., Wang W., and Kapila S., “17beta‐Estradiol Induces MMP‐9 and MMP‐13 in TMJ Fibrochondrocytes via Estrogen Receptor Alpha,” Journal of Dental Research 97 (2018): 1023–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Flake N. M., Hermanstyne T. O., and Gold M. S., “Testosterone and Estrogen Have Opposing Actions on Inflammation‐Induced Plasma Extravasation in the Rat Temporomandibular Joint,” American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 291 (2006): R343–R348. [DOI] [PubMed] [Google Scholar]
- 88. Wang H., Tian Y., Wang J., et al., “Inflammatory Cytokines Induce NOTCH Signaling in Nucleus Pulposus Cells: Implications in Intervertebral Disc Degeneration,” Journal of Biological Chemistry 288 (2013): 16761–16774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wu Y., Kadota‐Watanabe C., Ogawa T., and Moriyama K., “Combination of Estrogen Deficiency and Excessive Mechanical Stress Aggravates Temporomandibular Joint Osteoarthritis In Vivo,” Archives of Oral Biology 102 (2019): 39–46. [DOI] [PubMed] [Google Scholar]
- 90. Zhang J., Zhang S., Qi W. J., et al., “Mechanism and Potential Contributing Factors to Temporomandibular Joint Osteoarthritis,” Oral Diseases 29 (2023): 1060–1069. [DOI] [PubMed] [Google Scholar]
- 91. Okuda T., Yasuoka T., Nakashima M., and Oka N., “The Effect of Ovariectomy on the Temporomandibular Joints of Growing Rats,” Journal of Oral and Maxillofacial Surgery. Official Journal of the American Association of Oral and Maxillofacial Surgeons 54 (1996): 1201. [DOI] [PubMed] [Google Scholar]
- 92. Yasuoka T., Nakashima M., Okuda T., and Tatematsu N., “Effect of Estrogen Replacement on Temporomandibular Joint Remodeling in Ovariectomized Rats,” Journal of Oral and Maxillofacial Surgery 58 (2000): 189–196. [DOI] [PubMed] [Google Scholar]
- 93. Hauru R., Rieppo L., Tuomisto T., et al., “Fourier‐Transform Infrared Study on Effects of Ageing, Oestrogen Level and Altered Dietary Loading on Rat Mandibular Condylar Cartilage,” Orthodontics & Craniofacial Research 27 (2024): 151–164. [DOI] [PubMed] [Google Scholar]
- 94. Sheridan P. J., Aufdemorte T. B., Holt G. R., and Gates G. A., “Cartilage of the Baboon Contains Estrogen Receptors,” Rheumatology International 5 (1985): 279–281. [DOI] [PubMed] [Google Scholar]
- 95. Da Silva J. A. P., Larbre J. P., Seed M. P., et al., “Sex Differences in Inflammation Induced Cartilage Damage in Rodents. The Influence of Sex Steroids,” Journal of Rheumatology 21 (1994): 330–337. [PubMed] [Google Scholar]
- 96. Räsänen T. and Messner K., “Articular Cartilage Compressive Stiffness Following Oophorectomy or Treatment With 17β‐Estradiol in Young Postpubertal Rabbits,” Acta Obstetricia et Gynecologica Scandinavica 78 (1999): 357–362. [PubMed] [Google Scholar]
- 97. Dai G., Wang S., Li J., Liu C., and Liu Q., “The Validity of Osteoarthritis Model Induced by Bilateral Ovariectomy in Guinea Pig,” Journal of Huazhong University of Science and Technology. Medical Sciences. Hua Zhong Ke Ji Xue Xue Bao Yi Xue Ying Wen Ban Huazhong Keji Daxue Xuebao Yixue Yingdewen Ban 26, no. 6 (2006): 716–719, 10.1007/s11596-006-0624-2. [DOI] [PubMed] [Google Scholar]
- 98. Dayani N., Corvol M. T., Robel P., et al., “Estrogen Receptors in Cultured Rabbit Articular Chondrocytes: Influence of Age,” Journal of Steroid Biochemistry 31 (1988): 351–356. [DOI] [PubMed] [Google Scholar]
- 99. Osman F. R. O., Yacoub M. F. Y., Mohammed S. H. A.‐K., and El Sayed O. E. M., “Comparative Histological Study Between the Effect of Calcitonin Versus Hormonal Replacement Therapy on Cartilage of Knee Joint of Ovariectomized Albino Rat,” Egyptian Journal of Histology 42 (2019): 515–525. [Google Scholar]
- 100. Tang S., Zhou W., Zhong X., et al., “Arctigenin Prevents the Progression of Osteoarthritis by Targeting PI3K/Akt/NF‐κB Axis: In Vitro and In Vivo Studies,” Journal of Cellular and Molecular Medicine 24 (2020): 4183–4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Rosner I. A., Malemud C. J., Goldberg V. M., Papay R. S., Getzy L., and Moskowitz R. W., “Pathologic and Metabolic Responses of Experimental Osteoarthritis to Estradiol and an Estradiol Antagonist,” Clinical Orthopaedics and Related Research 171 (1982): 280–286. [PubMed] [Google Scholar]
- 102. Corvol M. T., Carrascosa A., Tsagris L., Blanchard O., and Rappaport R., “Evidence for a Direct In Vitro Action of Sex Steroids on Rabbit Cartilage Cells During Skeletal Growth: Influence of Age and Sex,” Endocrinology 120 (1987): 1422–1429. [DOI] [PubMed] [Google Scholar]
- 103. Tsai C. L. and Liu T. K., “Estradiol‐Induced Knee Osteoarthrosis in Ovariectomized Rabbits,” Clinical Orthopaedics and Related Research 291 (1993): 295–302. [PubMed] [Google Scholar]
- 104. Turner A. S., Athanasiou K. A., Zhu C. F., Alvis M. R., and Bryant H. U., “Biochemical Effects of Estrogen on Articular Cartilage in Ovariectomized Sheep,” Osteoarthritis and Cartilage 5 (1997): 63–69. [DOI] [PubMed] [Google Scholar]
- 105. Claassen H., Hornberger F., Scholz‐Ahrens K., Schünke M., Schrezenmeir J., and Kurz B., “The Effect of Estrogens and Dietary Calcium Deficiency on the Extracellular Matrix of Articular Cartilage in Göttingen Miniature Pigs,” Annals of Anatomy 184 (2002): 141–148. [DOI] [PubMed] [Google Scholar]
- 106. Lee Y. J., Lee E. B., Kwon Y. E., et al., “Effect of Estrogen on the Expression of Matrix Metalloproteinase (MMP)‐1, MMP‐3, and MMP‐13 and Tissue Inhibitor of Metalloproternase‐1 in Osteoarthritis Chondrocytes,” Rheumatology International 23 (2003): 282–288. [DOI] [PubMed] [Google Scholar]
- 107. Høegh‐Andersen P., Tankó L. B., Andersen T. L., et al., “Ovariectomized Rats as a Model of Postmenopausal Osteoarthritis: Validation and Application,” Arthritis Research & Therapy 6 (2004): R169–R180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Claassen H., Schünke M., and Kurz B., “Estradiol Protects Cultured Articular Chondrocytes From Oxygen‐Radical‐Induced Damage,” Cell and Tissue Research 319, no. 3 (2005): 439–445, 10.1007/s00441-004-1029-9. [DOI] [PubMed] [Google Scholar]
- 109. Claassen H., Schlüter M., Schünke M., and Kurz B., “Influence of 17beta‐Estradiol and Insulin on Type II Collagen and Protein Synthesis of Articular Chondrocytes,” Bone 39 (2006): 310–317. [DOI] [PubMed] [Google Scholar]
- 110. Oshima Y., Matsuda K. I., Yoshida A., Watanabe N., Kawata M., and Kubo T., “Localization of Estrogen Receptors Alpha and Beta in the Articular Surface of the Rat Femur,” Acta Histochemica et Cytochemica 40 (2007): 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Castañeda S., Largo R., Calvo E., Bellido M., Gómez‐Vaquero C., and Herrero‐Beaumont G., “Effects of Estrogen Deficiency and Low Bone Mineral Density on Healthy Knee Cartilage in Rabbits,” Journal of Orthopaedic Research 28 (2010): 812–818. [DOI] [PubMed] [Google Scholar]
- 112. Sniekers Y. H., Weinans H., van Osch G. J., and Leeuwen J. P., “Oestrogen Is Important for Maintenance of Cartilage and Subchondral Bone in a Murine Model of Knee Osteoarthritis,” Arthritis Research & Therapy 12, no. 5 (2010): R182, 10.1186/ar3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Claassen H., Steffen R., Hassenpflug J., et al., “17β‐Estradiol Reduces Expression of MMP‐1, ‐3, and ‐13 in Human Primary Articular Chondrocytes From Female Patients Cultured in a Three Dimensional Alginate System,” Cell and Tissue Research 342 (2010): 283–293. [DOI] [PubMed] [Google Scholar]
- 114. Bay‐Jensen A. C., Nielsen R. H., Segovia‐Silvestre T., et al., “A Microarray Analysis of Full Depth Knee Cartilage of Ovariectomized Rats,” BMC Research Notes 4 (2011): 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Yang J. H., Kim J. H., Lim D. S., and Oh K. J., “Effect of Combined Sex Hormone Replacement on Bone/Cartilage Turnover in a Murine Model of Osteoarthritis,” Clinics in Orthopedic Surgery 4 (2012): 234–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Fontinele R. G., Mariotti V. B., Vazzoleré A. M., Ferrão J. S. P., Junior J. R. K., and de Souza R. R., “Menopause, Exercise, and Knee. What Happens?,” Microscopy Research and Technique 76 (2013): 381–387. [DOI] [PubMed] [Google Scholar]
- 117. Yan J. Y., Tian F. M., Wang W. Y., et al., “Age Dependent Changes in Cartilage Matrix, Subchondral Bone Mass, and Estradiol Levels in Blood Serum, in Naturally Occurring Osteoarthritis in Guinea Pigs,” International Journal of Molecular Sciences 15 (2014): 13578–13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Liang Y., Duan L., Xiong J., et al., “E2 Regulates MMP‐13 via Targeting miR‐140 in IL‐1β‐Induced Extracellular Matrix Degradation in Human Chondrocytes,” Arthritis Research & Therapy 18 (2016): 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Lou C., Xiang G., Weng Q., et al., “Menopause Is Associated With Articular Cartilage Degeneration: A Clinical Study of Knee Joint in 860 Women,” Menopause 23 (2016): 1239–1246. [DOI] [PubMed] [Google Scholar]
- 120. Lee H. W., Ko B.‐S., Kang S., Ryuk J. A., Kim M. J., and Park S., “Dangguijihwang‐Tang and Dangguijakyak‐San Prevent Menopausal Symptoms and Dangguijihwang‐Tang Prevents Articular Cartilage Deterioration in Ovariectomized Obese Rats With Monoiodoacetate‐Induced Osteoarthritis,” Evidence‐Based Complementary and Alternative Medicine 2017 (2017): 5658681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Ge Y., Zhou S., Li Y., et al., “Estrogen Prevents Articular Cartilage Destruction in a Mouse Model of AMPK Deficiency via ERK‐mTOR Pathway,” Annals of Translational Medicine 7 (2019): 336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Hughbanks M. L., Rodriguez‐Fontan F., Kleck C. J., and Van der Burger‐ Walt E., “Estrogen Receptor Alpha in Human Knee Articular Cartilage of Healthy and Osteoarthritic Females,” Journal of Orthopaedics 27 (2021): 1–8, 10.1016/j.jor.2021.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Li H., Gou Y., Tian F., et al., “Combination of Metformin and Exercise Alleviates Osteoarthritis in Ovariectomized Mice Fed a High‐Fat Diet,” Bone 157 (2022): 116323. [DOI] [PubMed] [Google Scholar]
- 124. Huang K., Wu B., Hou Z., et al., “Psoralen Downregulates Osteoarthritis Chondrocyte Inflammation via an Estrogen‐Like Effect and Attenuates Osteoarthritis,” Aging 14 (2022): 6716–6726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Polur I., Kamiya Y., Xu M., et al., “Oestrogen Receptor Beta Mediates Decreased Occlusal Loading Induced Inhibition of Chondrocyte Maturation in Female Mice,” Archives of Oral Biology 60 (2015): 818–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Richmond R. S., Carlson C. S., Register T. C., Shanker G., and Loeser R. F., “Functional Estrogen Receptors in Adult Articular Cartilage: Estrogen Replacement Therapy Increases Chondrocyte Synthesis of Proteoglycans and Insulin‐Like Growth Factor Binding Protein 2,” Arthritis and Rheumatism 43 (2000): 2081–2090. [DOI] [PubMed] [Google Scholar]
- 127. Wluka A. E., Davis S. R., Bailey M., Stuckey S. L., and Cicuttini F. M., “Users of Oestrogen Replacement Therapy Have More Knee Cartilage Than Non‐Users,” Annals of the Rheumatic Diseases 60 (2001): 332–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Ham K. D., Loeser R. F., Lindgren B. R., and Carlson C. S., “Effects of Long‐Term Estrogen Replacement Therapy on Osteoarthritis Severity in Cynomolgus Monkeys,” Arthritis and Rheumatism 46 (2002): 1956–1964. [DOI] [PubMed] [Google Scholar]
- 129. Mouritzen U., Christgau S., Lehmann H.‐J., Tanko L. B., and Christiansen C., “Cartilage Turnover Assessed With a Newly Developed Assay Measuring Collagen Type II Degradation Products: Influence of Age, Sex, Menopause, Hormone Replacement Therapy, and Body Mass Index,” Annals of the Rheumatic Diseases 62 (2003): 332–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Cicuttini F. M., Wluka A. E., Wang Y., Stuckey S. L., and Davis S. R., “Effect of Estrogen Replacement Therapy on Patella Cartilage in Healthy Women,” Clinical and Experimental Rheumatology 21 (2003): 79–82. [PubMed] [Google Scholar]
- 131. Wluka A. E., Wolfe R., Davis S. R., Stuckey S., and Cicuttini F. M., “Tibial Cartilage Volume Change in Healthy Postmenopausal Women: A Longitudinal Study,” Annals of the Rheumatic Diseases 63 (2004): 444–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Ham K. D., Oegema T. R., Loeser R. F., and Carlson C. S., “Effects of Long‐Term Estrogen Replacement Therapy on Articular Cartilage IGFBP‐2, IGFBP‐3, Collagen and Proteoglycan Levels in Ovariectomized Cynomolgus Monkeys,” Osteoarthritis and Cartilage 12 (2004): 160–168. [DOI] [PubMed] [Google Scholar]
- 133. Cake M. A., Appleyard R. C., Read R. A., Smith M. M., Murrell G. A. C., and Ghosh P., “Ovariectomy Alters the Structural and Biomechanical Properties of Ovine Femoro‐Tibial Articular Cartilage and Increases Cartilage iNOS,” Osteoarthritis and Cartilage 13 (2005): 1066–1075. [DOI] [PubMed] [Google Scholar]
- 134. Dai G., Li J., Liu X., Liu Q., and Liu C., “The Relationship of the Expression of Estrogen Receptor in Cartilage Cell and Osteoarthritis Induced by Bilateral Ovariectomy in Guinea Pig,” Journal of Huazhong University of Science and Technology. Medical Sciences 25 (2005): 683–686. [DOI] [PubMed] [Google Scholar]
- 135. Oestergaard S., Sondergaard B. C., Hoegh‐Andersen P., et al., “Effects of Ovariectomy and Estrogen Therapy on Type II Collagen Degradation and Structural Integrity of Articular Cartilage in Rats: Implications of the Time of Initiation,” Arthritis and Rheumatism 54 (2006): 2441–2451. [DOI] [PubMed] [Google Scholar]
- 136. Kato M., Takaishi H., Yoda M., et al., “GRIP1 Enhances Estrogen Receptor Alpha‐Dependent Extracellular Matrix Test in Chondrogenic Cells,” Osteoarthritis and Cartilage 18 (2010): 934–941. [DOI] [PubMed] [Google Scholar]
- 137. Wei S., Venn A., Ding C., et al., “The Associations Between Parity, Other Reproductive Factors and Cartilage in Women Aged 50‐80 Years,” Osteoarthritis and Cartilage 19 (2011): 1307–1313. [DOI] [PubMed] [Google Scholar]
- 138. Kavas A., Cagatay S. T., Banerjee S., Keskin D., and Tezcaner A., “Potential of Raloxifene in Reversing Osteoarthritis‐Like Alterations in Rat Chondrocytes: An In Vitro Model Study,” Journal of Biosciences 38 (2013): 135–147. [DOI] [PubMed] [Google Scholar]
- 139. Hamdi T. and Gouissem I., “Potential Effect of Enterolactone and Raloxifene in Reversing Osteoarthritis Markers in Cultured Human Articular Chondrocytes,” ARP Rheumatology 1 (2022): 30–41. [PubMed] [Google Scholar]
- 140. Jiang H., Tang Q., Zheng D., Gu Y., and Man C., “Parathyroid Hormone Enhances the Therapeutic Effect of Mesenchymal Stem Cells on Temporomandibular Joint Osteoarthritis in Rats,” American Journal of Stem Cells 12 (2023): 73–82. [PMC free article] [PubMed] [Google Scholar]
- 141. Bei M.‐J., Tian F. M., Xiao Y. P., et al., “Raloxifene Retards Cartilage Degradation and Improves Subchondral Bone Micro‐Architecture in Ovariectomized Rats With Patella Baja‐Induced—Patellofemoral Joint Osteoarthritis,” Osteoarthritis and Cartilage 28 (2020): 344–355. [DOI] [PubMed] [Google Scholar]
- 142. Ziemian S. N., Ayobami O. O., Rooney A. M., et al., “Low Bone Mass Resulting From Impaired Estrogen Signaling in Bone Increases Severity of Load‐Induced Osteoarthritis in Female Mice,” Bone 152 (2021): 116071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Wang Q., Liu Z., Wang Y., et al., “Quantitative Ultrasound Assessment of Cartilage Degeneration in Ovariectomized Rats With Low Estrogen Levels,” Ultrasound in Medicine & Biology 42 (2016): 290–298. [DOI] [PubMed] [Google Scholar]
- 144. Xu X., Li X., Liang Y., et al., “Estrogen Modulates Cartilage and Subchondral Bone Remodeling in an Ovariectomized Rat Model of Postmenopausal Osteoarthritis,” Medical Science Monitor: International Medical Journal of Experimental and Clinical Research 25 (2019): 3146–3153, 10.12659/msm.916254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Jin X., Wang B. H., Wang X., et al., “Associations Between Endogenous Sex Hormones and MRI Structural Changes in Patients With Symptomatic Knee Osteoarthritis,” Osteoarthritis and Cartilage 25 (2017): 1100–1106. [DOI] [PubMed] [Google Scholar]
- 146. Duan L., Xu X., Xu L., et al., “ERα‐Targeting PROTAC as a Chemical Knockdown Tool to Investigate the Estrogen Receptor Function in Rat Menopausal Arthritis,” Frontiers in Pharmacology 12 (2021): 764154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Saeki Fernandes A., Fonseca C. C. N., da Silva Sasso G. R., et al., “Combined Effects of Ovariectomy and Streptozotocin‐Induced Diabetes in the Articular Cartilage of Rats,” Climacteric: The Journal of the International Menopause Society 21 (2018): 75–81. [DOI] [PubMed] [Google Scholar]
- 148. Qin Y., He J., Xia L., Guo H., and He C., “Effects of Electro‐Acupuncture on Oestrogen Levels, Body Weight, Articular Cartilage Histology and MMP‐13 Expression in Ovariectomised Rabbits,” Acupuncture in Medicine 31 (2013): 214–221. [DOI] [PubMed] [Google Scholar]
- 149. Luo Q., Li S. S., He C. Q., He H. C., Yang L., and Deng L., “Pulse Electromagnetic Fields Effects on Serum E2 Levels, Chondrocyte Apoptosis, and Matrix Metalloproteinase‐13 Expression in Ovariectomized Rats,” Rheumatology International 29 (2009): 927–935. [DOI] [PubMed] [Google Scholar]
- 150. Sniekers Y. H., van Osch G. J. V. M., Ederveen A. G. H., et al., “Development of Osteoarthritic Features in Estrogen Receptor Knockout Mice,” Osteoarthritis and Cartilage 17 (2009): 1356–1361. [DOI] [PubMed] [Google Scholar]
- 151. Silva L. A. S., Araújo J. M. D., Almeida D. R., et al., “Effect of Dioscorea villosa Extract and the Phytoestrogen Diosgenin on Ovariectomized Mice With Zymosan‐Induced Arthritis,” Brazilian Journal of Pharmaceutical Sciences 60 (2024): 1–13. [Google Scholar]
- 152. Sondergaard B. C., Oestergaard S., Christiansen C., Tankó L. B., and Karsdal M. A., “The Effect of Oral Calcitonin on Cartilage Turnover and Surface Erosion in an Ovariectomized Rat Model,” Arthritis and Rheumatism 56 (2007): 2674–2678. [DOI] [PubMed] [Google Scholar]
- 153. Zaychenko G., Belenichev I., Hnatiuk V., et al., “Protective Effect of Vaginal Resveratrol Administration on Joint Tissues in Ovariectomized Rats: Targeting mTOR and Сaspase 3,” Biomedicine & Pharmacotherapy 165 (2023): 115176. [DOI] [PubMed] [Google Scholar]
- 154. da Silva J. A., Colville‐Nash P., Spector T. D., Scott D. L., and Willoughby D. A., “Inflammation‐Induced Cartilage Degradation in Female Rodents. Protective Role of Sex Hormones,” Arthritis & Rheumatism 36 (1993): 1007–1013. [DOI] [PubMed] [Google Scholar]
- 155. Christgau S., Tankó L. B., Cloos P. A. C., et al., “Suppression of Elevated Cartilage Turnover in Postmenopausal Women and in Ovariectomized Rats by Estrogen and a Selective Estrogen‐Receptor Modulator (SERM),” Menopause 11, no. 5 (2004): 508–518, 10.1097/01.wcb.0000121484.18437.98. [DOI] [PubMed] [Google Scholar]
- 156. Harris H. A., Albert L. M., Leathurby Y., et al., “Evaluation of an Estrogen Receptor‐β Agonist in Animal Models of Human Disease,” Endocrinology 144 (2003): 4241–4249. [DOI] [PubMed] [Google Scholar]
- 157. Chang S. J., Kuo S. M., Lin Y. T., and Yang S.‐W., “The Biological Effects of Sex Hormones on Rabbit Articular Chondrocytes From Different Genders,” BioMed Research International 2014 (2014): 932737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Hui L., Shoumei X., Zhoujing Z., et al., “Effects of Androgen Receptor Overexpression on Chondrogenic Ability of Rabbit Articular Chondrocytes,” Tissue Engineering and Regenerative Medicine 18 (2021): 641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Kinney R. C., Schwartz Z., Week K., Lotz M. K., and Boyan B. D., “Human Articular Chondrocytes Exhibit Sexual Dimorphism in Their Responses to 17beta‐Estradiol,” Osteoarthritis and Cartilage 13 (2005): 330–337. [DOI] [PubMed] [Google Scholar]
- 160. Rouge M., Legendre F., Elkhatib R., et al., “Early Castration in Horses Does Not Impact Osteoarticular Metabolism,” International Journal of Molecular Sciences 24 (2023): 16778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Jin L., Balian G., and Li X. J., “Animal Models for Disc Degeneration–An Update,” Histology and Histopathology 33 (2018): 543–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Li X., Zhao R., Rudd S., Ding W., and Yang S., “Correlation Analysis Between Tamoxifen and Lumbar Intervertebral Disc Degeneration: A Retrospective Case‐Control Study,” Pain Research & Management 2022 (2022): 3330260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Robinson J. L., Soria P., Xu M., et al., “Estrogen Promotes Mandibular Condylar Fibrocartilage Chondrogenesis and Inhibits Degeneration via Estrogen Receptor Alpha in Female Mice,” Scientific Reports 8 (2018): 8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Risbud M. V. and Shapiro I. M., “Role of Cytokines in Intervertebral Disc Degeneration: Pain and Disc‐Content,” Nature Reviews Rheumatology 10 (2014): 44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Johnson Z. I., Schoepflin Z. R., Choi H., Shapiro I. M., and Risbud M. V., “Disc in Flames: Roles of TNF‐α and IL‐1β in Intervertebral Disc Degeneration,” European Cells & Materials 30 (2015): 104–116, discussion 116–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Goldring M. B. and Otero M., “Inflammation in Osteoarthritis,” Current Opinion in Rheumatology 23 (2011): 471–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Houard X., Goldring M. B., and Berenbaum F., “Homeostatic Mechanisms in Articular Cartilage and Role of Inflammation in Osteoarthritis,” Current Rheumatology Reports 15 (2013): 375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Wang Y. X. J., “Menopause as a Potential Cause for Higher Prevalence of Low Back Pain in Women Than in Age‐Matched Men,” Journal of Orthopaedic Translation 8 (2017): 1–4, 10.1016/j.jot.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Stanworth R. D. and Jones T. H., “Testosterone for the Aging Male; Current Evidence and Recommended Practice,” Clinical Interventions in Aging 3 (2008): 25–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Handelsman D. J. and Bermon S., “Detection of Testosterone Doping in Female Athletes,” Drug Testing and Analysis 11 (2019): 1566–1571. [DOI] [PubMed] [Google Scholar]
- 171. Booth A., Shelley G., Mazur A., Tharp G., and Kittok R., “Testosterone, and Winning and Losing in Human Competition,” Hormones and Behavior 23 (1989): 556–571. [DOI] [PubMed] [Google Scholar]
- 172. Wood R. I. and Stanton S. J., “Testosterone and Sport: Current Perspectives,” Hormones and Behavior 61 (2012): 147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Leinung M. C. and Joseph J., “Changing Demographics in Transgender Individuals Seeking Hormonal Therapy: Are Trans Women More Common Than Trans Men?,” Transgender Health 5 (2020): 241–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Unger C. A., “Hormone Therapy for Transgender Patients,” Translational Andrology and Urology 5 (2016): 877–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Finch C. E., Felicio L. S., Mobbs C. V., and Nelson J. F., “Ovarian and Steroidal Influences on Neuroendocrine Aging Processes in Female Rodents,” Endocrine Reviews 5 (1984): 467–497. [DOI] [PubMed] [Google Scholar]
- 176. Diaz Brinton R., “Minireview: Translational Animal Models of Human Menopause: Challenges and Emerging Opportunities,” Endocrinology 153 (2012): 3571–3578. [DOI] [PMC free article] [PubMed] [Google Scholar]


