Abstract
Brain atrophy may precede symptoms in Alzheimer’s disease (AD), but it remains unclear whether atrophy in this preclinical stage falls within a distinct brain network or is associated with transitional cognitive changes. We investigated cortical thickness in cognitively unimpaired older adults with varying amyloid-β accumulation and estimated the connectivity of each individual’s atrophy pattern using a large normative connectome (n = 1000). A distinct network was connected to atrophy patterns in amyloid-β-positive (n = 1242) versus negative (n = 536) participants. This preclinical AD network was similar to a previously published atrophy network associated with AD dementia (r = 0.8284, p = 0.016). In leave-one-out cross-validation, atrophy patterns connected to the preclinical AD network were associated with lower cognitive performance (p = 0.0018), greater subjective cognitive decline (p < 0.001), and amyloid-β levels (p < 0.001). Atrophy in preclinical AD maps to a network similar to AD dementia that is associated with amyloid-β and cognition, demonstrating an atrophy-related network across the continuum of AD.
Introduction
Alzheimer’s disease (AD) pathologic changes, including abnormal accumulation of amyloid-β (Aβ), begin many years before individuals develop cognitive and functional impairment. It remains unclear whether Aβ accumulation is associated with a distinct pattern of network-level atrophy during this preclinical stage of the disease. Defining atrophy-related brain networks and clinical manifestations in preclinical AD is critical to understanding initial AD pathophysiology and could lead to better prediction of disease trajectories and potential novel treatment targets in preclinical AD.
Studies of atrophy in preclinical AD have yielded mixed results. In the preclinical stage, it may be difficult to distinguish Aβ-associated atrophy from age-related volume loss or early atrophy from other neurodegenerative conditions, and it emerges later than Aβ and tau pathologies.1 Some studies support AD-specific cortical thinning occurs in preclinical AD,2,3 while others found no cross-sectional difference4 or even increased cortical thickness.5 These mixed findings could be due to methodological and technical differences across studies, heterogeneity in the preclinical phase, or inadequate sample size.6,7
Mapping lesion-induced symptoms onto the functional brain networks instead of individual brain locations has allowed the localization of various neurologic/psychiatric symptoms.8 Recent work suggests that this lesion network mapping technique might be applied to neurodegenerative conditions using atrophy as the “lesion”. Tetreault et al. demonstrated that atrophy locations in individual patients map to brain networks associated with clinical, cognitive, and neuropsychiatric symptoms of AD dementia.9 However, this atrophy network mapping (ANM) has never been implemented in preclinical AD, and it is unclear if this method can detect subtle changes in cortical thickness at this stage.
This study uses ANM (Fig. 1) to compare network-level neurodegeneration to pathological and clinical manifestations in preclinical AD using a large, well-characterized cohort from an AD prevention clinical trial.10,11 We hypothesized that (1) atrophy in the preclinical stage of AD would localize to a distinct brain network, (2) this preclinical AD network would be similar to atrophy patterns previously implicated in AD dementia, and (3) this preclinical AD network would be associated with cognitive manifestations of preclinical AD.
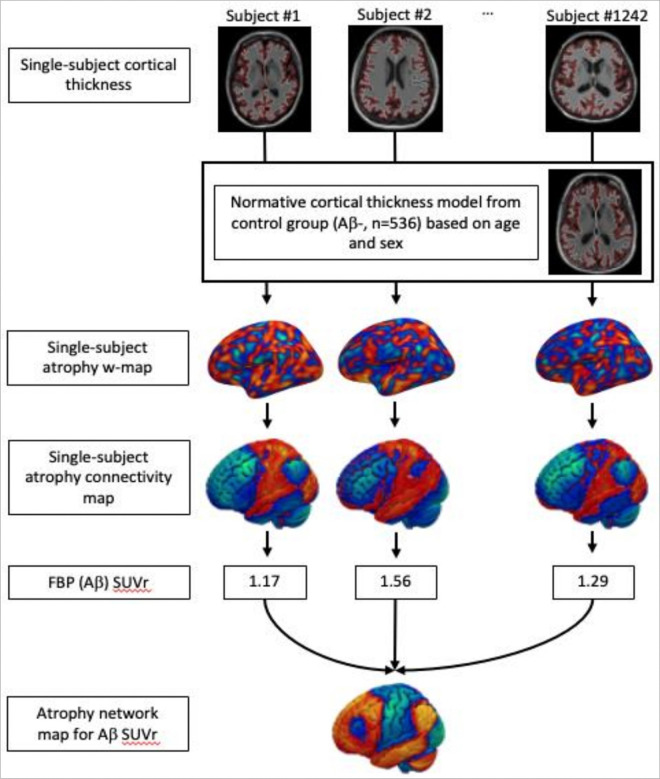
Figure 1.
Atrophy Network Mapping. A normative cortical thickness general linear model using Aβ negative subjects as a control group adjusting for age and sex. Comparing to the normative cortical thickness, atrophy w-maps were created for each subject. Then, using the normative functional connectome of the brain, single-subject atrophy connectivity maps were created with each voxel representing the connectivity to atrophy. Finally, comparing atrophy connectivity in each voxel with Aβ SUVr, an atrophy network map for Aβ was created.
Results
Characteristics of the sample
The sample included 1778 older adults from the Anti-Amyloid Treatment in Asymptomatic Alzheimer’s Disease (A4) (ClinicalTrials.gov identifier: NCT02008357) and Longitudinal Evaluation of Amyloid Risk and Neurodegeneration (LEARN) (ClinicalTrials.gov identifier: NCT02488720) studies. A4 was a double-blind, placebo-controlled Alzheimer’s disease prevention trial with anti-amyloid-β (Aβ) therapy in subjects with elevated Aβ accumulation (Aβ+) as determined by 18F-florbetapir (FBP) PET scan. LEARN was a companion study of A4 comprised of subjects without high Aβ accumulation (Aβ−).10,11 All participants were living independently and determined to be cognitively unimpaired based on a Clinical Dementia Rating global score of 0, a Mini-Mental State Examination (MMSE) score of 25–30, and a Wechsler Memory Scale Logical Memory Delayed Recall score of 6–18. Participants underwent brain imaging, including structural brain MRI and FBP PET.10,11 The Aβ status (Aβ + vs. Aβ−) was determined using a dual quantitative and qualitative algorithm, using global FBP-specific binding calculated from six regions (anterior cingulate, posterior cingulate, frontal cortex, temporal cortex, parietal cortex, and precuneus) and visual read. 10,11
Demographics and other covariates between the Aβ− and Aβ + groups are summarized in Table 1. The mean (SD) age was 71.5 (4.7) years, 1060 (59.6%) were female, and the mean (SD) education was for 16.6 (2.8) years. Cognitive performance was measured using the Preclinical Alzheimer Cognitive Composite (PACC).12,13 Subjective cognitive decline was assessed with the Cognitive Function Index (CFI),14,15 which consists of subjective cognitive concerns reported by study partners (CFI-SP) and study participants (CFI-PT).
Table 1.
Characteristics of A4/LEARN Baseline Cohort by Amyloid Status
| Total (N = 1778) | Aβ negative (N = 536) | Aβ positive (N = 1242) | p | |
|---|---|---|---|---|
| Age (Mean [SD]) | 71.54 [4.74] | 70.46 [4.29] | 72.00 [4.84] | < 0.001 |
| Sex (N of Female [%]) | 1060 [59.6%] | 328 [61.2%] | 732 [58.9%] | 0.37 |
| Education | 16.62 [2.76] | 16.76 [2.62] | 16.56 [2.81] | 0.16 |
| PACC | −0.20 [2.63] | 0.28 [2.41] | −0.41 [2.69] | 0.003 |
| Digit Symbol | 43.61 [8.93] | 44.15 [8.82] | 42.59 [8.93] | 0.18 |
| FCSRT | 75.88 [6.04] | 76.72 [5.71] | 75.52 [6.14] | 0.02 |
| Logical Memory Delay | 11.56 [3.34] | 11.89 [3.35] | 11.42 [3.32] | 0.1 |
| MMSE | 28.80 [1.24] | 28.94 [1.13] | 28.75 [1.28] | 0.05 |
| CFI | 7.48 [6.31] | 6.12 [5.77] | 7.94 [6.42] | < 0.001 |
| CFI-SP | 3.11 [3.87] | 2.53 [3.38] | 3.31 [4.00] | < 0.001 |
| CFI-PT | 4.31 [3.83] | 3.58 [3.55] | 4.63 [3.91] | < 0.001 |
| Average Cortical Thickness | 2.18 [0.08] | 2.19 [0.08] | 2.18 [0.08] | 0.23 |
| Hippocampal Occupancy * | 0.73 [0.08] | 0.76 [0.07] | 0.73 [0.08] | 0.51 |
| PET_SUVr | 1.23 [0.22] | 0.99 [0.07] | 1.33 [0.18] | < 0.001 |
SD = Standard Deviation, PACC = Preclinical Alzheimer Cognitive Composite, Digit Symbol = Digit Symbol Substitution Test score from the Wechsler Adult Intelligence Scale-Revised, Logical Memory Delay = Logical Memory IIa sub-test from the Wechsler Memory Scale, FCSRT = Free and Cued Selective Reminding Test, MMSE = Mini-Mental Status Exam, CFI = Cognitive Function Inventory, SP = Study Partner, PT = Participant.
Hippocampal Occupancy was calculated as the ratio of hippocampal volume to the sum of the hippocampal and inferior lateral ventricle volumes in each hemisphere, left and right hippocampal occupancy scores were averaged, and then normalized for age and sex.
p values for PACC, Digit Symbol, FCSRT, Logical Memory Delay, MMSE, CFI-PT, Average Cortical Thickness, Hippocampal Occupancy, and PET_SUVr are adjusted for age, sex, and education.
In comparison to the Aβ− group, the Aβ + group was older (p-value < 0.001, t statistic = −6.704), had lower PACC score (p-value < 0.001, t-value = 5.294), and reported greater CFI score (p-value < 0.001, t-value = −5.129), as reported in prior analyses of the same dataset.11 These differences remained significant when adjusting for age, sex, and education. (Table 1)
Cortical thinning and medial temporal lobe atrophy in Aβ + vs. Aβ−
First, we compared traditional metrics of atrophy between Aβ + and Aβ− groups. There was no significant difference between groups in terms of global cortical thickness or adjusted hippocampal volume. These results were unchanged when adjusting for age, sex, and education (Table 1). In a vertex-wise cortical thickness comparison between Aβ + and Aβ− groups, no specific regions remained statistically significant after family-wise error (FWE) correction using the permutation-based max t-stat method as implemented by Winkler et al.16 Thus, atrophy location alone was not significantly different between Aβ + and Aβ− groups. (Fig. 2.a)
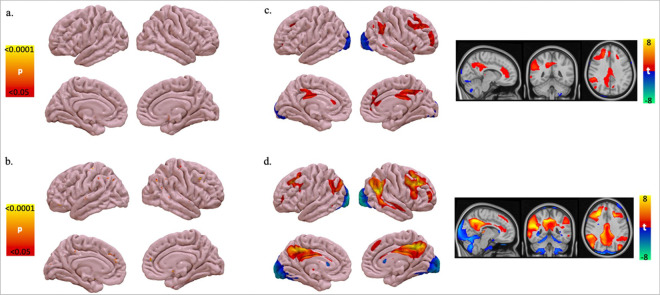
Figure 2.
Localizing atrophy in preclinical Alzheimer’s disease. a. Vertex-wise ANOVA of cortical thickness between Aβ+ Vs. Aβ-controlling for age, sex, and education. FWE-corrected p<0.05. b. Vertex-wise regression of cortical thickness with continuous FBP (Aβ)SUVr using PALM controlling for age, sex, and education. FWE-corrected p<0.05. c. Atrophy network map for preclinical Alzheimer’s disease: voxel-wise ANOVA of functional connectivity to atrophy between Aβ+ Vs. Aβ-controlling for age, sex, and education. FWE-corrected p<0.05. d. Atrophy network map for preclinical Alzheimer’s disease: voxel-wise linear regression of functional connectivity to atrophy and continuous FBP (Aβ) SUVr controlling for age, sex, and education. FWE-corrected p<0.05.
Next, we compared cortical thickness to continuous Aβ SUVr using voxel-wise Pearson correlation, as continuous scores may be more sensitive than binary classification,17 adjusting for age, sex, and education. After FWE correction, higher Aβ SUVr values were correlated with small, scattered clusters of cortical thinning in the superior frontal gyrus, postcentral gyrus, posterior cingulate/precuneus, superior temporal gyrus, parahippocampal gyrus, and cuneus. (Fig. 2.b)
Atrophy Network Maps for Preclinical AD: Aβ + vs. Aβ− and continuous Aβ SUVr
To test for network-level effects, which may be more sensitive than looking at individual regions of atrophy, we performed ANM according to the previously published method.9 We created a general linear model (GLM) for expected cortical thickness controlling for age and sex using 546 Aβ− subjects’ structural MRI. Next, atrophy w-maps were created for each subject, with each vertex representing an atrophy w-score in comparison to the normative cortical thickness from the GLM. Then, we estimated the whole-brain connectivity of each participant’s atrophy pattern using a normative connectome database (n = 1000),18,19 as in prior work on lesion and ANM. (Fig. 1)
To map the connectivity of atrophy patterns associated with AD pathology, we used a voxel-wise ANCOVA to compare the whole-brain atrophy connectivity between the Aβ + and Aβ− groups, adjusting for age, sex, and education. The Aβ + group had atrophy patterns with greater connectivity to the right prefrontal lobe, right precuneus, right temporoparietal junction (Fig. 2.c), which remained statistically significant after FWE correction.16 Similar results were seen when using continuous Aβ SUVr instead of binarizing Aβ + vs. Aβ−, but more brain regions survived multiple comparisons correction, including right dorsolateral and medial prefrontal lobes, left and right precuneus, left and right lateral parietal lobes, and the right temporoparietal junction and middle temporal gyrus (Fig. 2.d).
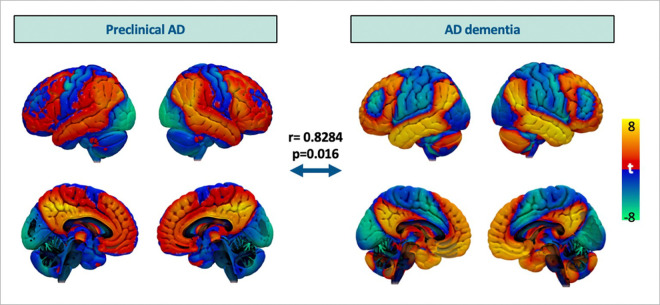
This atrophy network map using Aβ SUVr was compared to the previously published atrophy network map for AD dementia.9 This map was created using the data from Alzheimer’s Disease Neuroimaging Initiative II (ADNI-2) following the same procedure that was described above, but comparing patients with clinically adjudicated AD dementia with high Aβ to those who were deemed cognitively normal with low Aβ. The two maps were more similar to each other than by chance (r = 0.8284, p = 0.016) using permutation testing in which a spatial correlation was recomputed after each patient’s clinical measure was randomly permuted against a different patient’s neuroimaging, as in prior work.20 Thus, atrophy associated with Aβ positivity in preclinical AD localized to a similar network as atrophy associated with a clinical diagnosis of AD dementia. (Fig. 3)
Figure 3.
Comparison between atrophy network map for preclinical AD versus pre-established atrophy network map for AD dementia derived from ADNI-2 dataset. Preclinical AD map is based on continuous Aβ SUVr from the A4/LEARN dataset following the atrophy network mapping procedures described in Figure 1. Atrophy network map for AD dementia is created, comparing Aβ + subjects with AD dementia and Aβ−, cognitive normal subjects.
Atrophy Network Maps for Preclinical AD is associated with cognition.
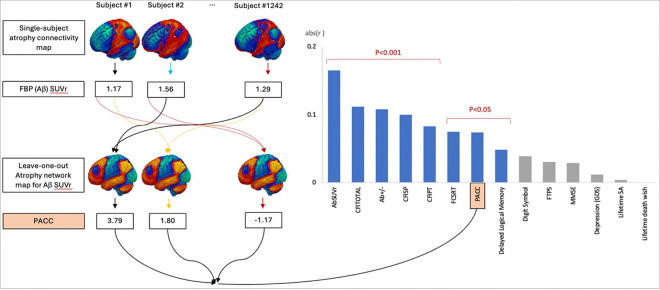
Next, we tested whether the Aβ SUVr atrophy network map could explain variance in clinical factors in an out-of-sample analysis, using a leave-one-out cross-validation. First, the Aβ SUVr atrophy network map was regenerated after excluding one participant. The resulting map was compared to the left-out map using spatial Pearson correlation. This leave-one-out spatial correlation was computed for each participant. (Fig. 4) As expected, atrophy to the preclinical AD network was associated with A β status in this out-of-sample analysis (r = 0.11, p < 0.001). Similarly, atrophy to the preclinical AD network was associated with Aβ SUVr (r = 0.17, p<0.001).
Figure 4.
Leave-one-out cross-validation: spatial correlation between individual atrophy connectivity map and leave-one-out atrophy network map for preclinical AD was associated with individual subjects’ Aβ SUVr, dichotomous Aβ+/− group, cognition measured by PACC and CFI, but not with some of PACC subdomains and behavioral variables including depression.
PACC=Preclinical Alzheimer Cognitive Composite, Digit Symbol= Digit Symbol Substitution Test score from the Wechsler Adult Intelligence Scale–Revised, Logical Memory Delay= Logical Memory IIa sub-test from the Wechsler Memory Scale, FCSRT= Free and Cued Selective Reminding Test, MMSE=Mini-Mental Status Exam, CFI=Cognitive Function Inventory, SP=Study Partner, PT=Participant. FTPS= Future Time Perspective Scale, GDS= Geriatric Depression Scale, SA= suicidal attempt.
The resulting spatial correlations were also compared with clinical manifestations, including objective cognitive performance measured by PACC and subjective cognitive decline measured by CFI total score, using Pearson correlations. (Fig. 4). The leave-one-out correlation was negatively associated with PACC scores (r= −0.0740, p = 0.0018) and positively associated with CFI total score (r = 0.1119, p < 0.001). The leave-one-out correlation was also associated with specific subdomains of the PACC, including the Digit Symbol Substitution Test (Digit Symbol), Free and Cued Selective Reminding Test (FCSRT), and Mini-Mental Status Exam (MMSE), but not Logical Memory IIa sub-test from the Wechsler Memory Scale (Delayed Logical Memory). The leave-one-out correlation was also significantly associated subjective cognitive decline as detected by both the CFI participant report and the CFI study partner report. In contrast, the leave-one-out correlation was not associated with other nonspecific behavioral variables, including depression measured by the Geriatric Depression Scale, optimism measured by the Future Time Perspective Scale, history of lifetime suicidal attempt, and history of lifetime death wishes.
Discussion
Atrophy network mapping revealed a discrete and localizable pattern of atrophy in preclinical AD, using a well-characterized, large cohort of cognitively unimpaired older adults in AD prevention studies.10,11 This pattern was tested against the gold standard of Aβ PET neuroimaging showing a significant association. This network was similar to the previously established atrophy network for AD dementia.9 Moreover, similarity of given subjects’ atrophy connectivity to this preclinical AD network was associated with transitional objective and subjective cognitive manifestations in the unimpaired subjects. These results add to the validity of the atrophy network map in preclinical AD. Furthermore, they indicate that network-level atrophy is already detectable during this initial biological stage of AD and is associated with early cognitive manifestations.
Understanding AD neuropathology, which begins to accrue years before symptoms arise, has paved the way for developing disease-modifying and potential disease-preventing therapies.10,21–23 This endeavor resulted in a biological definition of AD based on elevated neuropathological biomarkers even in individuals without overt cognitive and functional decline: preclinical AD.24 However, some have questioned whether this unimpaired stage should be considered a distinct disease entity.25,26 Here, we demonstrated that AD-specific neurodegeneration is detectable in preclinical AD by localizing atrophy connectivity in cognitively unimpaired older adults with Aβ. This confirms that the preclinical stage is associated not only with AD proteinopathies, but also with structural neurodegeneration. This supports that preclinical AD, defined by Aβ accumulation, is not a risk factor but rather an early part of the AD disease process.
Investigating atrophy in AD has faced challenges, partly due to its high individual variability. By applying network mapping to atrophy in Alzheimer’s disease, Tetreault et al. elucidated the localization of clinical, cognitive, and psychotic symptoms of AD dementia. These network-level patterns explained more variance than any individual regions of atrophy. However, the relevance of atrophy to preclinical AD, the stage of the AD continuum prior to impairment, has remained unclear.1 In this study, we demonstrated that atrophy network mapping could be used in preclinical AD to localize the atrophy connectivity patterns at this stage, which was similar to Tetreault’s AD dementia network. This validates the use of atrophy network mapping in preclinical AD, potentially broadening the use of this method to study other symptoms in preclinical AD datasets.
To our knowledge, this is the first study demonstrating a network-specific pattern of atrophy connectivity and its associations with cognition in preclinical AD. Leave-one-out atrophy network maps showed that the similarity of a given subject’s atrophy connectivity to the preclinical AD network is significantly associated with poor objective cognition and greater subjective cognitive decline. This was specific to cognitive manifestations relative to other behaviors associated with AD such as depression, suggesting a relevance of the preclinical AD network to cognitive changes characteristic of AD.
The atrophy network for preclinical AD included the right dorsolateral and medial prefrontal lobe, left and right precuneus, left and right lateral parietal lobes, and right middle temporal gyrus, key areas of the default mode network that are affected in Alzheimer’s disease.27,28 Interestingly, the medial temporal lobe did not survive correction for multiple comparisons. This could be explained by selection bias, that subjects with atrophy connected to the medial temporal lobe may more likely be symptomatic, and thus would have been excluded from the study.29 The asymmetry of this network could be due to a similar selection bias, as subjects with left cortical involvement may be more likely to progress to a symptomatic stage due to specific cognitive deficits related to language or verbal memory.30
Of note, the effect sizes detected in this study are relatively small. There are several sources of variability that may contribute to this. One key factor is that this was a cross-sectional study rather than a longitudinal study, so patients are being compared to one another rather than to their own baseline, which limits the explainable variance. Atrophy and cognition can be quantified more precisely when comparing a subject to their own prior scores. Also, this study analyzed subjects in preclinical AD solely based on Aβ without consideration of heterogeneity due to other factors, e.g. tau, that play a role in AD pathogenesis in this stage. While this could introduce variability, this study design represents a real-world population and is consistent with the latest revised criteria for diagnosis and staging of Alzheimer’s disease.24 There are also additional sources of noise that limit explainable variance; for instance, even though Aβ PET is now considered a gold standard, it only explains 50–60% of the variance in pathological Aβ levels.31 These factors are offset by the large sample size, which enabled us to detect a significant effect despite multiple sources of variance. For these reasons, the present analysis is informative to better understand the disease process, but future longitudinal studies would be necessary before considering its use for clinical diagnostic purposes. Other limitations include the use of a normative connectome database derived from healthy, young controls, which may not reflect the brain connectivity changes in AD or older adults. However, prior work suggests that the signal-to-noise benefit of a large connectome exceeds minor differences in patient- or disorder-specific connectivity.32
Overall, using ANM, we identified a distinct atrophy connectivity pattern in preclinical AD, supporting the notion that preclinical AD is part of the AD disease process with ongoing neurodegeneration.
Online Methods
Subjects
The cross-sectional clinical and neuroimaging data from cognitively unimpaired (CU) older adults were obtained from the publicly available Anti-Amyloid Treatment in Asymptomatic Alzheimer’s Disease (A4, ClinicalTrials.gov identifier: NCT02008357) and Longitudinal Evaluation of Amyloid Risk and Neurodegeneration (LEARN, ClinicalTrials.gov identifier: NCT02488720) dataset (https://ida.loni.usc.edu/login.jsp). The A4 study was a double-blind, placebo-controlled, secondary prevention trial testing whether the anti-amyloid antibody solanezumab could slow cognitive decline in CU older adults with elevated brain Amyloid-β (Aβ). The LEARN study, an observational companion study, followed individuals without elevated brain Aβ who were excluded from the A4 study. Screening procedures have been previously described.1,2 Eligible participants were ages 65–85, living independently, and cognitively unimpaired based on a Clinical Dementia Rating global score of 0, a Mini-Mental State Examination (MMSE) score of 25–30, and a Wechsler Memory Scale Logical Memory Delayed Recall score of 6–18. Exclusion criteria included a diagnosis of cognitive impairment or dementia, the use of AD medications, unstable medical conditions, or significant anxiety or depression that could pose a risk for disclosing a participant’s Aβ imaging results. The local Institutional Review Board approved the A4/LEARN studies at each of the clinical trial sites. All participants provided written informed consent prior to screening.
Demographic Information
The data included demographic information including age, sex, and education.
Objective Cognition
Cognitive performance in the A4/LEARN studies was measured using the Preclinical Alzheimer Cognitive Composite (PACC).3,4 The PACC score was computed as the mean z score from the Logical Memory Delayed Recall (Logical Memory), the MMSE, the Weschler Adult Intelligence Scale-Revised Digit Symbol Coding (Digit Symbol), and the Free and Cued Selective Reminding Test (FCSRT). A higher PACC z score denotes better cognitive functioning.
Subjective Cognitive Decline
Subjective cognitive decline was assessed with the Cognitive Function Index, a 15-item survey administered individually to the participant and their study partner that asks about changes in the participant’s cognitive function over the last year.5,6 Respondents chose “yes”, “no”, or “maybe” for each question. A total score was derived by summing the participant’s and study partner’s item scores, with a higher score indicating greater subjective cognitive decline.
Other Covariates
Depressive symptoms were measured by the 15-item version of the Geriatric Depression Scale,7 and perception of one’s future was measured by the 10-item Future Time Perspective Scale.8 The data also included a history of lifetime suicidal attempts and a history of lifetime passive death wishes.
Structural MRI Acquisition and Freesurfer Processing
3T brain structural MRI was obtained on study-qualified MRI scanners. T1 images were processed using Freesurfer 6.0.9
Global Cortical Thickness
Global cortical thickness was calculated by averaging the cortical thickness across all cortical vertices from cortical thickness output from Freesurfer 6.09’s recon-all process.
Hippocampal Occupancy Score
The hippocampal occupancy was calculated as the ratio of hippocampal volume to the sum of the hippocampal and inferior lateral ventricle volumes in each hemisphere, left and right hippocampal occupancy scores were averaged, and then normalized for age and sex.10
18F-florbetapir PET Imaging
All participants underwent Aβ PET using 18F-florbetapir (FBP).1 Aβ PET was acquired from 50 to 70 minutes after a 10mCi bolus injection and was reconstructed in 4x-5minute frames. Global FBP specific binding was calculated from six regions (anterior cingulate, posterior cingulate, frontal cortex, temporal cortex, parietal cortex, and precuneus) as a mean standardized uptake value ratio (SUVr) normalized to a whole-cerebellum reference region.11 Aβ status (Aβ+ vs. Aβ−) was determined using a dual quantitative and qualitative method. An SUVr of 1.15 or greater was the primary criterion for Aβ+ status.12 An SUVr between 1.10 and 1.15 was considered Aβ+ if a visual read confirmed positivity.
Statistical Analyses
Statistical analyses were completed using Matlab R2022b. The global cortical thickness and hippocampal occupancy score of Aβ+ group was compared against Aβ− group using ANCOVA with age, sex, and education as covariates. The vertex-wise cortical thickness was compared between Aβ+ and Aβ− groups with age, sex, and education as covariates, then evaluated using regression models by treating Aβ SUVr as a continuous variable adjusting for the same covariates. For the vertex-wise analysis, the p-value was corrected for multiple comparisons family-wise error correction using the permutation-based max t-stat method implemented by Winkler et al.13,14
Atrophy Network Mapping (ANM)
ANM was performed following the previously published procedure by Tetreault et al.15 (Figure 1) We created a vertex-wise general linear model (GLM) for cortical thickness for the cognitively unimpaired subjects without Aβ accumulation (Aβ−) using age and gender as covariates. Next, we calculated a vertex-wise w-score for cortical thickness in each patient (a w-score is a z-score adjusted for covariates, in this study, age and sex). Next, we derived an ‘atrophy connectivity map’ for each subject, defined as the brain regions functionally connected to each subject’s atrophy w-map. First, single-subject atrophy w-maps in surface space from each hemisphere were combined and converted to MNI volume space. Using a publicly available normative functional connectivity dataset of 1000 healthy subjects from the Genome Superstruct Project (GSP),16,17 we computed the average blood oxygen level-dependent (BOLD) time course for all voxels within each patient’s distributed single-subject atrophy w-map. Next, we correlated this mean time course within each single-subject atrophy w-map with the BOLD time course at every other brain voxel.
1) ANM of Preclinical AD Comparing Aβ− vs. Aβ+
We compared atrophy connectivity maps between Aβ− vs. Aβ+ groups at every voxel using ANCOVA, including age, sex, and education as covariates. The resulting atrophy network map was corrected for multiple comparisons by permutation-based family-wise error correction (p < 0.05).14
2) ANM of Preclinical AD Using Continuous Global Aβ SUVr
We performed voxel-wise analyses comparing atrophy connectivity maps to each subject’s global Aβ SUVr using a linear regression model adjusting for age, sex, and education. Again, the resulting atrophy network map was corrected for multiple comparisons by permutation-based family-wise error correction (p < 0.05).14
3) ANM of AD dementia using Alzheimer’s Disease Neuroimaging Initiative II (ADNI-2)
We replicated ANM of AD dementia in comparison to cognitively normal subjects from ADNI-2 dataset following the published ANM procedure by Tetreault et al.15 We defined the control group with cognitive normal subjects with low Aβ and AD dementia group with subjects with AD diagnosis and high Aβ, based on the same Aβ SUVr cut-off as the A4/LEARN studies.
Random Permutation Testing
To examine similarity of two brain maps, the atrophy network map for preclinical AD from A4/LEARN versus the atrophy network map for AD dementia from ADNI-2, a random permutation testing was performed as in prior work.18 Briefly, a spatial correlation was recomputed after each patient’s clinical measure was randomly permuted against a different patient’s neuroimaging. After repeating this permutation 1000 times, the chances of random spatial correlation values being greater than the true spatial correlation between the two maps were calculated.
Leave-one-out cross-validation
Leave-one-out atrophy network maps for preclinical AD using continuous Aβ SUVr were generated for each subject using the atrophy connectivity maps from the whole sample except for the particular subject. Each subject’s atrophy connectivity map was compared to its leave-one-out atrophy network map by spatial correlation. To examine if the similarity of each subject’s atrophy connectivity to each subject’s leave-one-out atrophy network map for preclinical AD is associated with the given subject’s true Aβ status, t test was performed between the r-value from the spatial correlation and the dichotomous Aβ group. Then, the same leave-one-out analysis was performed for Aβ SUVr by linear regression between the spatial r-value the continuous Aβ SUVr. To examine if the similarity of each subject’s atrophy connectivity to each subject’s leave-one-out atrophy network map for preclinical AD is associated with given subject’s cognition, linear regression was performed for the correlation coefficient as a predictor with objective cognition measured by PACC and subjective cognitive decline measured by CFI. The same analyses were performed for subdomains for PACC, Digit Symbol Substitution Test (Digit Symbol), Free and Cued Selective Reminding Test (FCSRT), and Mini-Mental Status Exam (MMSE), Logical Memory IIa sub-test from the Wechsler Memory Scale (Delayed Logical Memory), subjective cognitive decline reported by study partner (CFI-SP), subjective cognitive decline reported by participant (CFI-PT), depression measured by GDS, future-time perspective scale, history of life-time suicidal attempt, and history of life-time death wish.
Acknowledgments
The A4 Study is a secondary prevention trial in preclinical Alzheimer’s disease, aiming to slow cognitive decline associated with brain amyloid accumulation in clinically normal older individuals. The A4 Study is funded by a public-private-philanthropic partnership, including funding from the National Institutes of Health-National Institute on Aging, Eli Lilly and Company, Alzheimer’s Association, Accelerating Medicines Partnership, GHR Foundation, an anonymous foundation and additional private donors, with in-kind support from Avid and Cogstate. The companion observational Longitudinal Evaluation of Amyloid Risk and Neurodegeneration (LEARN) Study is funded by the Alzheimer’s Association and GHR Foundation. The A4 and LEARN Studies are led by Dr. Reisa Sperling at Brigham and Women’s Hospital, Harvard Medical School and Dr. Paul Aisen at the Alzheimer’s Therapeutic Research Institute (ATRI), University of Southern California. The A4 and LEARN Studies are coordinated by ATRI at the University of Southern California, and the data are made available through the Laboratory for Neuro Imaging at the University of Southern California. The participants screening for the A4 Study provided permission to share their de-identified data in order to advance the quest to find a successful treatment for Alzheimer’s disease. We would like to acknowledge the dedication of all the participants, the site personnel, and all of the partnership team members who continue to make the A4 and LEARN Studies possible. The complete A4 Study Team list is available at: a4study.org/a4-study-team. In addition, the investigators were funded by the following sources: S. Lee was supported by the Stuart T. Hauser Research Training Program in Biological and Social Psychiatry at Judge Baker Children’s Center, National Institute of Mental Health, National Institutes of Health, under Grant Number 2T32MH016259–34, philanthropic gifts (the Neuropsychiatry of Aging Research Fund and the Geriatric Psychiatry Fund) to the Division of Geriatric Psychiatry, and the Center for Brain Circuit Therapeutics, Brigham and Women’s Hospital. S.H.S. was supported by the NIH (grant nos. K23MH121657 and R21MH126271), the Brain and Behavior Research Foundation Young Investigator Grant, the Baszucki Brain Research Fund and the Department of Veterans Affairs (grant no. I01CX002293).
Footnotes
Declarations
Conflicts of Interests
There are no personal financial conflicts of interest related to the present results. S.Lee, S.R.B., G.T.B., S.T.P., W.J.D., B.S.Z., N.M.C., S.Lariviere report no conflicts with any concept discussed in this article. M.D.F. reports consulting fees from Magnus Medical, Sorterix, Abott, and Boston Scientific. M.D.F. also owns independent intellectual property involving the use of functional connectivity to target TMS for depression, which was filed in 2013, has yielded no royalties and does not cover the present work. R.A.S. has served as a paid consultant for AbbVie, AC Immune, Acumen, Alector, Apellis, Biohaven, Bristol Myers Squibb, Genentech, Ionis, Janssen, Oligomerix, Prothena, Roche, and Vaxxinity. She has received research funding from Eisai and Eli Lilly for public-private partnership clinical trials and receives research grant funding from the National Institute on Aging/National Institutes of Health, GHR Foundation, and the Alzheimer’s Association. Her spouse, K. Johnson, reports consulting fees from Novartis, Merck, and Janssen. N.J.D. reports receiving royalties from the American Psychiatric Association Press, and honoraria and travel support for Harvard University, Harvard Medical School, and NIH-sponsored events. S.H.S. is an owner of intellectual property involving the use of individualized resting-state network mapping to target TMS, which was filed in 2016, has yielded no royalties and does not cover the present work. S.H.S. is also a scientific consultant for Magnus Medical, received investigator-initiated research funding from Neuronetics (2019) and Brainsway (2022), received speaking fees from Brainsway (2021) and Otsuka (for PsychU.org, 2021), and is a shareholder in Brainsway (publicly traded) and Magnus Medical (not publicly traded).
Research Data Share Statement
The A4/LEARN data are publicly available online at the Image and Data Archive (https://ida.loni.usc.edu).
Contributor Information
Soyoung Lee, Brigham and Women’s Hospital.
Sheena Baratono, Brigham and Women’s Hospital.
Grace Burt, Brigham and Women’s Hospital.
Stephan Palm, Brigham and Women’s Hospital.
William Drew, Brigham and Women’s Hospital.
Benjamin Zide, Brigham and Women’s Hospital.
Nicole Chiulli, Brigham and Women’s Hospital.
Sara Lariviere, Brigham and Women’s Hospital.
Michael Fox, Brigham and Women’s Hospital.
Reisa Sperling, Harvard Aging Brain Study, Department of Neurology, Massachusetts General Hospital, Boston, MA 02114;Center for Alzheimer Research and Treatment, Department of Neurology, Brigham and Women’s.
Nancy Donovan, Brigham and Women’s Hospital, Massachusetts General Hospital.
Shan Siddiqi, Brigham and Women’s Hospital, Massachusetts General Hospital.
References
- 1.Sperling RA, Donohue MC, Raman R, et al. Association of Factors With Elevated Amyloid Burden in Clinically Normal Older Individuals. JAMA Neurology. 2020;77(6):735–745. doi: 10.1001/jamaneurol.2020.0387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sperling RA, Mormino EC, Schultz AP, et al. The impact of amyloid-beta and tau on prospective cognitive decline in older individuals. Ann Neurol. Feb 2019;85(2):181–193. doi: 10.1002/ana.25395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mormino EC, Papp KV, Rentz DM, et al. Early and late change on the preclinical Alzheimer’s cognitive composite in clinically normal older individuals with elevated amyloid β. Alzheimers Dement. Sep 2017;13(9):1004–1012. doi: 10.1016/j.jalz.2017.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donohue MC, Sperling RA, Salmon DP, et al. The preclinical Alzheimer cognitive composite: measuring amyloid-related decline. JAMA Neurol. Aug 2014;71(8):961–70. doi: 10.1001/jamaneurol.2014.803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amariglio RE, Donohue MC, Marshall GA, et al. Tracking early decline in cognitive function in older individuals at risk for Alzheimer disease dementia: the Alzheimer’s Disease Cooperative Study Cognitive Function Instrument. JAMA Neurol. Apr 2015;72(4):446–54. doi: 10.1001/jamaneurol.2014.3375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amariglio RE, Sikkes SAM, Marshall GA, et al. Item-Level Investigation of Participant and Study Partner Report on the Cognitive Function Index from the A4 Study Screening Data. J Prev Alzheimers Dis. 2021;8(3):257–262. doi: 10.14283/jpad.2021.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yesavage JA, Sheikh JI. 9/Geriatric Depression Scale (GDS). Clinical gerontologist. 1986/11/18 1986;5(1–2):165–173. doi: 10.1300/J018v05n01_09 [DOI] [Google Scholar]
- 8.Zimbardo PG, Boyd JN. Putting time in perspective: A valid, reliable individual-differences metric. Journal of Personality and Social Psychology. 1999;77(6):1271–1288. doi: 10.1037/0022-3514.77.6.1271 [DOI] [Google Scholar]
- 9.Fischl B. FreeSurfer. Neuroimage. Aug 15 2012;62(2):774–81. doi: 10.1016/j.neuroimage.2012.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heister D, Brewer JB, Magda S, Blennow K, McEvoy LK. Predicting MCI outcome with clinically available MRI and CSF biomarkers. Neurology. Oct 25 2011;77(17):1619–28. doi: 10.1212/WNL.0b013e3182343314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pontecorvo MJ, Arora AK, Devine M, et al. Quantitation of PET signal as an adjunct to visual interpretation of florbetapir imaging. European Journal of Nuclear Medicine and Molecular Imaging. 2017/05/01 2017;44(5):825–837. doi: 10.1007/s00259-016-3601-4 [DOI] [PubMed] [Google Scholar]
- 12.Johnson KA, Sperling RA, Gidicsin CM, et al. Florbetapir (F18-AV-45) PET to assess amyloid burden in Alzheimer’s disease dementia, mild cognitive impairment, and normal aging. Alzheimers Dement. Oct 2013;9(5 Suppl):S72–83. doi: 10.1016/j.jalz.2012.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Westfall PH, Young SS. Resampling-based multiple testing: Examples and methods for p-value adjustment. vol 279. John Wiley & Sons; 1993. [Google Scholar]
- 14.Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. Neuroimage. May 15 2014;92(100):381–97. doi: 10.1016/j.neuroimage.2014.01.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tetreault AM, Phan T, Orlando D, et al. Network localization of clinical, cognitive, and neuropsychiatric symptoms in Alzheimer’s disease. Brain. 2020;143(4):1249–1260. doi: 10.1093/brain/awaa058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yeo BTT, Krienen FM, Sepulcre J, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology. 2011;106(3):1125–1165. doi: 10.1152/jn.00338.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holmes AJ, Hollinshead MO, O’Keefe TM, et al. Brain Genomics Superstruct Project initial data release with structural, functional, and behavioral measures. Scientific Data. 2015/07/07 2015;2(1):150031. doi: 10.1038/sdata.2015.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siddiqi SH, Schaper F, Horn A, et al. Brain stimulation and brain lesions converge on common causal circuits in neuropsychiatric disease. Nat Hum Behav. Dec 2021;5(12):1707–1716. doi: 10.1038/s41562-021-01161-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
References
- 1.Dubois B, Hampel H, Feldman HH, et al. Preclinical Alzheimer’s disease: Definition, natural history, and diagnostic criteria. Alzheimer’s & Dementia. 2016;12(3):292–323. doi: 10.1016/j.jalz.2016.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dickerson BC, Bakkour A, Salat DH, et al. The cortical signature of Alzheimer’s disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cerebral cortex (New York, NY : 1991). Mar 2009;19(3):497–510. doi: 10.1093/cercor/bhn113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becker JA, Hedden T, Carmasin J, et al. Amyloid-β associated cortical thinning in clinically normal elderly. Ann Neurol. Jun 2011;69(6):1032–42. doi: 10.1002/ana.22333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Josephs KA, Whitwell JL, Ahmed Z, et al. Beta-amyloid burden is not associated with rates of brain atrophy. Ann Neurol. Feb 2008;63(2):204–12. doi: 10.1002/ana.21223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chételat G, Villemagne VL, Pike KE, et al. Larger temporal volume in elderly with high versus low beta-amyloid deposition. Brain. Nov 2010;133(11):3349–58. doi: 10.1093/brain/awq187 [DOI] [PubMed] [Google Scholar]
- 6.Pegueroles J, Vilaplana E, Montal V, et al. Longitudinal brain structural changes in preclinical Alzheimer’s disease. Alzheimer’s & Dementia. 2017/05/01/ 2017;13(5):499–509. doi: 10.1016/j.jalz.2016.08.010 [DOI] [PubMed] [Google Scholar]
- 7.Xie L, Wisse LEM, Das SR, et al. Longitudinal atrophy in early Braak regions in preclinical Alzheimer’s disease. Hum Brain Mapp. Nov 2020;41(16):4704–4717. doi: 10.1002/hbm.25151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fox MD. Mapping Symptoms to Brain Networks with the Human Connectome. New England Journal of Medicine. 2018;379(23):2237–2245. doi: 10.1056/NEJMra1706158 [DOI] [PubMed] [Google Scholar]
- 9.Tetreault AM, Phan T, Orlando D, et al. Network localization of clinical, cognitive, and neuropsychiatric symptoms in Alzheimer’s disease. Brain. 2020;143(4):1249–1260. doi: 10.1093/brain/awaa058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sperling RA, Donohue MC, Raman R, et al. Trial of Solanezumab in Preclinical Alzheimer’s Disease. N Engl J Med. Sep 21 2023;389(12):1096–1107. doi: 10.1056/NEJMoa2305032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sperling RA, Donohue MC, Raman R, et al. Association of Factors With Elevated Amyloid Burden in Clinically Normal Older Individuals. JAMA Neurology. 2020;77(6):735–745. doi: 10.1001/jamaneurol.2020.0387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mormino EC, Papp KV, Rentz DM, et al. Early and late change on the preclinical Alzheimer’s cognitive composite in clinically normal older individuals with elevated amyloid β. Alzheimers Dement. Sep 2017;13(9):1004–1012. doi: 10.1016/j.jalz.2017.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donohue MC, Sperling RA, Salmon DP, et al. The preclinical Alzheimer cognitive composite: measuring amyloid-related decline. JAMA Neurol. Aug 2014;71(8):961–70. doi: 10.1001/jamaneurol.2014.803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amariglio RE, Donohue MC, Marshall GA, et al. Tracking early decline in cognitive function in older individuals at risk for Alzheimer disease dementia: the Alzheimer’s Disease Cooperative Study Cognitive Function Instrument. JAMA Neurol. Apr 2015;72(4):446–54. doi: 10.1001/jamaneurol.2014.3375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amariglio RE, Sikkes SAM, Marshall GA, et al. Item-Level Investigation of Participant and Study Partner Report on the Cognitive Function Index from the A4 Study Screening Data. J Prev Alzheimers Dis. 2021;8(3):257–262. doi: 10.14283/jpad.2021.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. Neuroimage. May 15 2014;92(100):381–97. doi: 10.1016/j.neuroimage.2014.01.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Altman DG, Royston P. The cost of dichotomising continuous variables. Bmj. May 6 2006;332(7549):1080. doi: 10.1136/bmj.332.7549.1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yeo BTT, Krienen FM, Sepulcre J, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology. 2011;106(3):1125–1165. doi: 10.1152/jn.00338.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holmes AJ, Hollinshead MO, O’Keefe TM, et al. Brain Genomics Superstruct Project initial data release with structural, functional, and behavioral measures. Scientific Data. 2015/07/07 2015;2(1):150031. doi: 10.1038/sdata.2015.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siddiqi SH, Schaper F, Horn A, et al. Brain stimulation and brain lesions converge on common causal circuits in neuropsychiatric disease. Nat Hum Behav. Dec 2021;5(12):1707–1716. doi: 10.1038/s41562-021-01161-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. May 2011;7(3):280–92. doi: 10.1016/j.jalz.2011.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jack CR Jr., Bennett DA, Blennow K, et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. Apr 2018;14(4):535–562. doi: 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rafii MS, Sperling RA, Donohue MC, et al. The AHEAD 3–45 Study: Design of a prevention trial for Alzheimer’s disease. Alzheimers Dement. Apr 2023;19(4):1227–1233. doi: 10.1002/alz.12748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jack CR Jr, Andrews JS, Beach TG, et al. Revised criteria for diagnosis and staging of Alzheimer’s disease: Alzheimer’s Association Workgroup. Alzheimer’s & Dementia. 2024;20(8):5143–5169. doi: 10.1002/alz.13859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kozlov M. Should Alzheimer’s be diagnosed with a blood test? Proposal sparks controversy. Nature. Nov 2024;635(8039):530–531. doi: 10.1038/d41586-024-03629-2 [DOI] [PubMed] [Google Scholar]
- 26.Dubois B, Villain N, Schneider L, et al. Alzheimer Disease as a Clinical-Biological Construct—An International Working Group Recommendation. JAMA Neurology. 2024;81(12):1304–1311. doi: 10.1001/jamaneurol.2024.3770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sperling RA, Laviolette PS, O’Keefe K, et al. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. Jul 30 2009;63(2):178–88. doi: 10.1016/j.neuron.2009.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. Mar 30 2004;101(13):4637–42. doi: 10.1073/pnas.0308627101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jack CR Jr., Petersen RC, Xu YC, et al. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer’s disease. Neurology. Sep 1997;49(3):786–94. doi: 10.1212/wnl.49.3.786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Derflinger S, Sorg C, Gaser C, et al. Grey-matter atrophy in Alzheimer’s disease is asymmetric but not lateralized. J Alzheimers Dis. 2011;25(2):347–57. doi: 10.3233/jad-2011-110041 [DOI] [PubMed] [Google Scholar]
- 31.Clark CM, Schneider JA, Bedell BJ, et al. Use of Florbetapir-PET for Imaging β-Amyloid Pathology. Jama. 2011;305(3):275–283. doi: 10.1001/jama.2010.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Q, Akram H, Muthuraman M, et al. Normative vs. patient-specific brain connectivity in deep brain stimulation. Neuroimage. Jan 1 2021;224:117307. doi: 10.1016/j.neuroimage.2020.117307 [DOI] [PubMed] [Google Scholar]