Abstract
Background and aims:
Crohn’s disease is a chronic inflammatory disorder with rising global prevalence, marked by abdominal pain, diarrhea, and fatigue. Interleukin (IL)-23 plays a pivotal role in Crohn’s disease pathogenesis, making it a therapeutic target. Risankizumab, a monoclonal antibody targeting the IL-23 p19 subunit, has shown potential in clinical trials.
Objectives:
This meta-analysis evaluates the efficacy and safety of Risankizumab in achieving clinical remission, clinical response, and endoscopic remission in patients with moderate-to-severe Crohn’s disease.
Design:
A systematic review and meta-analysis were conducted following PRISMA 2020 guidelines.
Data sources and methods:
A comprehensive search of PubMed, Embase, Cochrane CENTRAL, Web of Science, and ClinicalTrials.gov was performed to identify randomized controlled trials (RCTs) assessing Risankizumab in Crohn’s disease. Primary outcomes were clinical remission, clinical response, and endoscopic remission, with secondary outcomes focusing on treatment-related adverse events. A random-effects model estimated odds ratios (ORs) with 95% confidence intervals. Meta-regression analyzed dose- and duration-dependent effects.
Results:
Four RCTs involving 1774 participants showed that Risankizumab significantly improved clinical remission (OR = 2.223), clinical response (OR = 2.483), and endoscopic remission (OR = 4.112). Dose-dependent improvements were observed, with treatment duration affecting clinical remission (p = 0.0158) but not clinical response or endoscopic remission. Adverse event rates were comparable between Risankizumab and placebo groups (OR = 0.872, p = 0.592).
Conclusion:
Risankizumab is effective in achieving clinical and endoscopic outcomes in moderate-to-severe Crohn’s disease, demonstrating dose-dependent benefits and a favorable safety profile, supporting its use as a therapeutic option. However, the limited number of studies may affect the robustness of these findings. Further large-scale RCTs are needed to validate its long-term efficacy, safety in elderly populations, and effectiveness in biologic-naïve patients.
Trial registration:
This systematic review and meta-analysis were registered with the INPLASY database under registration number INPLASY202530014. The full protocol is accessible at DOI: 10.37766/inplasy2025.3.0014.
Keywords: clinical remission, Crohn’s disease, endoscopic remission, IL-23 inhibitors, meta-analysis, Risankizumab
Plain language summary
How Risankizumab helps treat Crohn’s disease: a review of clinical trial results
Crohn’s disease is a long-term condition that causes inflammation in the digestive system. People with Crohn’s disease often experience symptoms like stomach pain, diarrhea, and tiredness, which can significantly affect their daily lives. Researchers have found that a protein called interleukin-23 (IL-23) plays a key role in this disease. Targeting IL-23 may help control the inflammation. Risankizumab is a medicine designed to block a part of IL-23, and it has been tested in clinical trials to see if it can help people with moderate-to-severe Crohn’s disease. To understand how well it works and whether it is safe, we combined the results of multiple high-quality studies in a process called a metaanalysis. Our analysis included four studies with a total of 1,774 patients. We found that Risankizumab significantly improved three key outcomes: Clinical remission: Fewer or no symptoms of Crohn’s disease. Clinical response: Noticeable improvement in symptoms. Endoscopic remission: Healing of the digestive tract, confirmed by a camera test. Patients who received Risankizumab were more likely to achieve these outcomes compared to those who received a placebo. The benefits were stronger with higher doses of the medication and with longer treatment durations for clinical remission. Importantly, the medicine was found to be safe. The rates of side effects were similar between patients taking Risankizumab and those taking a placebo. In conclusion, Risankizumab is an effective and safe treatment for people with moderate-to-severe Crohn’s disease. It helps reduce symptoms, promotes healing in the digestive tract, and works better at higher doses. This makes it a promising option for managing this challenging condition.
Introduction
Crohn’s disease is a chronic inflammatory condition of the gastrointestinal tract with rising global prevalence, affecting approximately 1 million individuals in the United States alone. 1 Common symptoms include abdominal pain, chronic diarrhea, weight loss, and fatigue. Diagnosis is typically established through clinical evaluation, imaging studies, endoscopic procedures, and histological analysis. 2 Patients with Crohn’s disease often experience significantly lower health-related quality of life compared to healthy individuals, driven by factors such as disease activity, work disability, and frequent hospitalizations. 3 Despite advances in treatment, many patients experience inadequate responses or intolerance to current therapies, highlighting the need for more effective and targeted treatment options.
Current treatment options for Crohn’s disease include advanced biological therapies. Anti-TNF agents (e.g., infliximab, adalimumab, and golimumab) are widely used for moderate-to-severe cases. Anti-integrin therapies—such as vedolizumab—block the migration of inflammatory cells and are associated with favorable safety profiles. Recently, interleukin (IL)-23 inhibitors have emerged as promising alternatives. IL-23, part of the IL-12 cytokine family, plays a central role in the pathogenesis of Crohn’s disease by promoting T helper 17 cell differentiation and survival. These cells produce pro-inflammatory cytokines, including IL-17A and IL-22, which drive intestinal inflammation. Structurally, IL-23 comprises a unique p19 subunit and a shared p40 subunit (with IL-12).4,5
Several IL-23 inhibitors—Risankizumab, Guselkumab, Brazikumab, and Mirikizumab—are currently in clinical use or under investigation for Crohn’s disease treatment. Guselkumab has shown promise in inducing clinical and endoscopic remission, although the study did not demonstrate a dose-dependent effect. 6 Brazikumab has demonstrated clinical benefits in patients with prior anti-TNF failure and has sustained long-term efficacy for over 100 weeks without significant safety concerns. 7 Mirikizumab has demonstrated superior endoscopic response compared to Ustekinumab, further supporting the therapeutic potential of IL-23 inhibition. 8
Risankizumab, a monoclonal antibody that selectively targets the IL-23 p19 subunit, prevents its interaction with the IL-23 receptor complex. This therapeutic agent is already approved for treating psoriatic arthritis. 9 Compared to other IL-23 inhibitors, risankizumab has been evaluated in more randomized controlled trials (RCTs), providing a more extensive clinical evidence base. While all IL-23 inhibitors share a common mechanism of action, risankizumab has demonstrated significant efficacy in achieving clinical remission, clinical response, and endoscopic remission, with emerging data suggesting potential advantages in dose optimization and long-term safety.
This systematic review and meta-analysis aim to comprehensively assess the efficacy and safety of risankizumab in Crohn’s disease by integrating data from multiple RCTs. Additionally, we aim to explore dose- and duration-dependent effects to provide insights into optimal treatment strategies. By synthesizing the available evidence, this study seeks to inform clinical decision-making and support the role of risankizumab as a targeted therapy for Crohn’s disease.
Materials and methods
Protocol and registration
This meta-analysis was conducted in accordance with the PRISMA 2020 guidelines (see Supplemental Table S1) and registered with INPLASY (http://INPLASY.com) under registration number INPLASY202530014. 10
Search strategy and data collection
The authors independently conducted electronic searches in the PubMed, Embase, Cochrane CENTRAL, Web of Science, and ClinicalTrials.gov databases, (“Risankizumab”) AND (“Crohn’s Disease” OR “Inflammatory Bowel Disease” OR “IBD”) AND (“efficacy” OR “treatment outcome” OR “clinical trial”) AND (“safety” OR “adverse effects” OR “side effects”) AND (“quality of life” OR “QOL”) AND (“endoscopic remission” OR “mucosal healing”) AND (“biologics” OR “Interleukin-23 inhibitor”). The search covered all available records from each database’s inception up to the search date (December 31, 2024). The full search strategy for this systematic review and meta-analysis is provided in the Supplemental Table S2.
Author conducted an initial screening of titles and abstracts to determine eligibility through a consensus-based approach. The PubMed and EMBASE databases were reviewed meticulously for any potentially relevant studies. In cases of disagreement, consensus was sought. No language restrictions were applied to this search.
Eligibility criteria and outcomes
This meta-analysis followed the P (Population): Participants with Crohn’s disease. I (Intervention): Risankizumab treatment. C (Comparison): Placebo or standard treatment (e.g., TNF inhibitors, corticosteroids). O (Outcome): Changes in disease activity (e.g., Crohn’s Disease Activity Index (CDAI)), endoscopic remission, or adverse events. Inclusion criteria included: (1) RCTs involving human participants, (2) RCTs with quantitative assessments of outcome before and after Risankizumab, and (3) trials providing data on pre- and post-intervention changes in disease activity. Exclusion criteria were: (1) non-RCT studies, (2) studies lacking quantitative disease activity assessments, and (3) studies with participant overlap with previously published trials.
Risk of bias and evidence quality
To evaluate the methodological quality of the included studies, we used the Cochrane Risk of Bias tool for randomized trials (version 2; RoB 2, London, UK). This tool assesses six key domains: randomization process, adherence to intervention, missing outcome data, outcome measurement, selective reporting, and overall risk of bias. For the intervention adherence aspect within RoB 2, two assessment approaches are available: intention-to-treat (based on intervention assignment) and per-protocol (based on adherence). We chose the per-protocol approach for this meta-analysis, as it better aligns with the study designs of our included trials. 11
Primary outcome (clinical remission, clinical response, and endoscopic remission)
This study primarily assessed changes in clinical remission, clinical response, and endoscopic remission following treatment with Risankizumab or placebo. Additionally, the validity and appropriateness of the scales used in each trial were evaluated,12 –14 or trials employing multiple scoring systems, the score included in the meta-analysis was determined through author consensus.
Secondary outcome (treatment-associated adverse event rates)
As a secondary outcome, this investigation also examined the rate of treatment-related adverse events. The outcomes were measured using odds ratios (ORs).
Data extraction and management
Data were extracted by the author from each study included in this meta-analysis, capturing demographic details, study design elements, information on Risankizumab and placebo treatments, and both primary and secondary outcome values. To maintain accuracy, evaluators carefully verified the direction of effect for each scale used. When data were unavailable in published reports, corresponding authors were contacted for original data. Data extraction, transformation, and consolidation across study arms with differing Risankizumab strategies followed the guidelines of the Cochrane Handbook for Systematic Reviews of Interventions and related medical literature. 15 When multiple post-treatment data points were available, we used the outcome reported at the intervention’s conclusion for statistical analysis. In crossover studies, only data from the initial study period were included to avoid carry-over effects. 16
Statistical analysis
Given the variability of the target populations in the studies, we conducted this meta-analysis using a random-effects model with Comprehensive Meta-Analysis software (version 4; Biostat, Englewood, NJ, USA). 17 Primary outcomes, such as changes in clinical remission, were measured using ORs with 95% confidence intervals (CIs). For secondary outcomes, including treatment-related adverse event rates, ORs with 95% CIs were calculated. Study heterogeneity was assessed using I2 and Cochran’s Q statistics, with I2 values of 25%, 50%, and 75% indicating low, moderate, and high heterogeneity, respectively. 18 Meta-regression analyses explored the relationship between Risankizumab’s effects and treatment dose or duration. 19 Sensitivity analyses using the one-study removal method were conducted to confirm the robustness of the meta-analysis. 20 Publication bias was evaluated following Cochrane Handbook guidelines, with funnel plots visually inspected for asymmetry. 21 These methodological approaches ensured a comprehensive assessment of Risankizumab’s efficacy and safety across diverse study populations.
Results
Study selection and characteristics
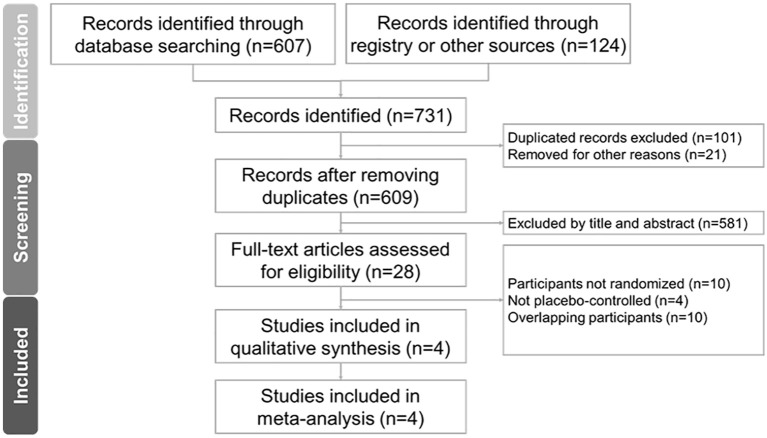
Figure 1 illustrates the PRISMA flowchart detailing the literature search process. After removing duplicates and excluding non-relevant articles based on title and abstract screening, we included four RCTs (one study included two RCTs) in the final analysis.12–14 Supplemental Table S3 lists excluded articles from the final stage, along with exclusion reasons. The 4 eligible RCTs comprised 1774 participants with a mean age of 38.3 ± 13.4 years, 48.2% (n = 964) of whom were male. Study durations ranged from 4 to 52 weeks, and all participants had moderate-to-severe Crohn’s disease. A summary of the trials is provided in Table 1.12 –14
Figure 1.
The PRISMA flowchart.
Table 1.
Summary of the retrieved trials investigating the effect of Risankizumab on Crohn’s disease in the enrolled participants.
| First author and year | Country | Population | Participants (female/male) | Age a | Study design | Allocation concealment | Randomization | Funding/grants/support |
|---|---|---|---|---|---|---|---|---|
| Feagan 2017 | North America, Europe, and Southeast Asia | • Moderate-to-severe Crohn’s disease • diagnosed for at least 3 months |
200 mg Risankizumab: 26/15
b
600 mg Risankizumab: 25/16 b Placebo: 23/16 |
39 ± 13
b
40 ± 13 36 ± 14 |
RCT, double-blind | Independent statistician | Stratified by prior TNF antagonist exposure | N/A |
| Ferrante 2022 | North and South America, Europe, Oceania, Africa, and the Asia-Pacific region | Moderately to severely active Crohn’s disease | 180 mg Risankizumab: 89/68
b
360 mg Risankizumab: 60/81 Placebo: 75/89 |
39.1 ± 14.8
b
37 ± 12.8 38 ± 13 |
RCT, double-blind | Independent statistician | Stratified | AbbVie |
| D’Haens 2022 (ADVANCE) |
39 countries | • Moderate-to-severe Crohn’s disease • Intolerance to one or more previous treatments |
Risankizumab 600 mg: 147/189 Risankizumab 1200 mg: 156/183 Placebo: 87/88 |
38.3 ± 13.3
b
37 ± 13.2 37.1 ± 13.4 |
RCT, double-blind | Not mentioned | Stratified the number of previous biologics that had failed, corticosteroid use at baseline, and the Simple Endoscopic Score for Crohn’s disease | AbbVie |
| D’Haens 2022 (MOTIVATE) |
40 countries | • Moderate-to-severe Crohn’s disease • Diagnosed for at least 3 months • Inadequate response or intolerance to at least one approved biologic therapy |
Risankizumab 600 mg: 99/92 Risankizumab 1200 mg: 89/102 Placebo: 88/99 |
40.2 ± 13.6
b
39.3 ± 12.9 39.3 ± 13.5 |
RCT, double-blind | Not mentioned | Stratified the number of previous biologics that had failed, corticosteroid use at baseline, and the Simple Endoscopic Score for Crohn’s disease | AbbVie |
Age is presented as means ± standard deviations or as medians (ranges).
Allocated participants.
RCT, randomized controlled trial.
Risk of bias and quality of evidence
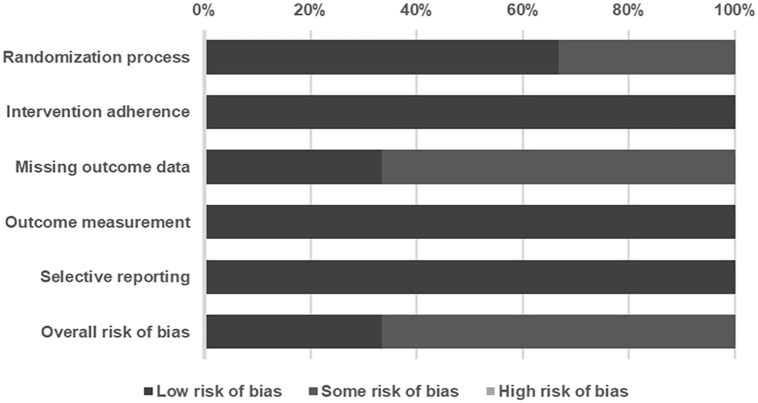
Among the included studies, 33.3% were rated as low risk of bias, 66.6% as having some risk of bias, and none as high risk (Figure 2). Two studies were classified as having “some” risk of bias due to insufficient information on allocation concealment and loss of follow-up participants. Details of the risk of bias assessment are summarized in Table 2.12 –14
Figure 2.
Summary of quality assessment of studies included in the meta-analysis using Cochrane risk of bias 2 tool.
Table 2.
Detailed quality assessment of included studies using Cochrane RoB 2 tool.
| First author | Year | Randomization process | Intervention adherence | Missing outcome data | Outcome measurement | Selective reporting | Overall RoB |
|---|---|---|---|---|---|---|---|
| Ferrante | 2022 | L | L | S a | L | L | S |
| Feagan | 2017 | L | L | L | L | L | L |
| D’Haens | 2022 | S b | L | S c | L | L | S |
The study excluded two subjects from risankizumab group because of lost follow-up.
The studies didn’t provide allocation concealment details.
The study excluded one subject from risankizumab group and two from placebo group because of lost follow-up.
H, high risk of bias; L, low risk of bias; RoB, risk of bias; S, risk of bias.
Primary outcome: effects of Risankizumab on clinical remission, clinical response, and endoscopic remission
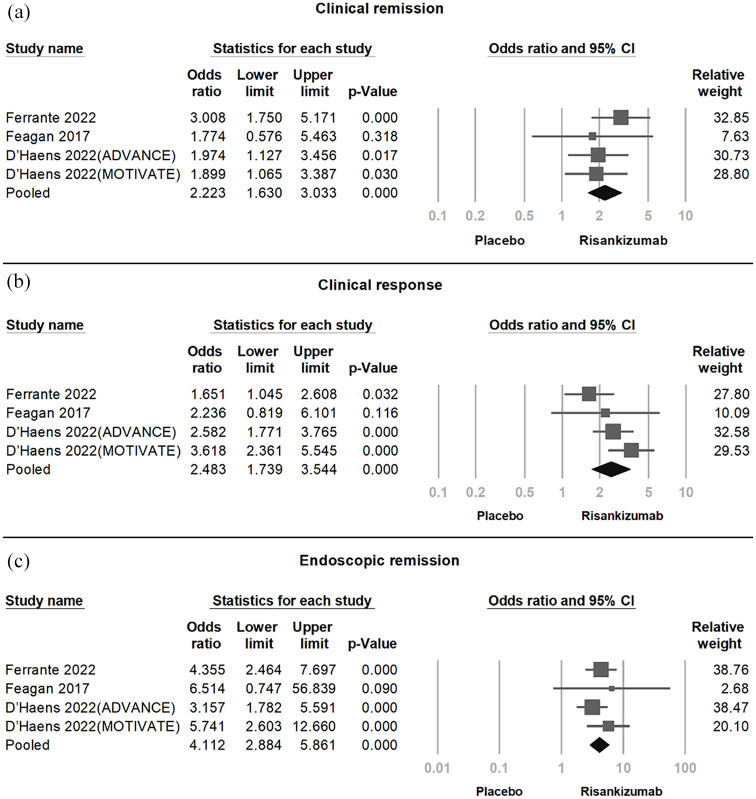
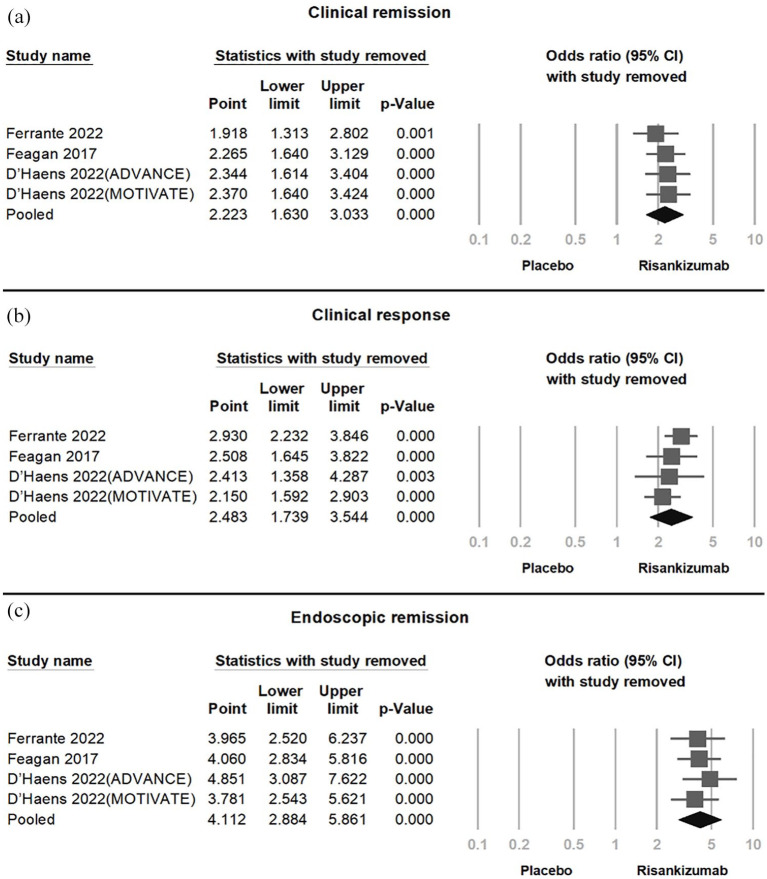
In the pooled analysis of four RCTs (Figure 3), Risankizumab demonstrated significant efficacy in achieving clinical remission (OR = 2.223, 95% CI = 1.630–3.033, p < 0.001, I2 < 0.01%), clinical response (OR = 3.618, 95% CI = 2.361–5.545, p < 0.001, I2 = 50.95%), and endoscopic remission (OR = 5.741, 95% CI = 2.603–12.66, p < 0.001, I2 < 0.01%). Moderate-to-high heterogeneity was observed in clinical response. Sensitivity analysis using the one-study removal method confirmed the robustness of these findings, with statistical significance remaining stable upon exclusion of any single study (Figure 4).
Figure 3.
Forest plot showing the significant efficacy of Crohn’s disease with Risankizumab across four trials. (a) Clinical remission (OR = 2.223, 95% CI = 1.630–3.033, p < 0.001, I2 < 0.01%). (b) Clinical response (OR = 2.483, 95% CI = 1.739–3.544, p < 0.001, I2 = 50.95%). (c) Endoscopic remission (OR = 4.112, 95% CI = 2.884–5.861, p < 0.001, I2 < 0.01%).
CI, confidence interval; OR, odds ratio.
Figure 4.
Sensitivity analysis confirming consistent Risankizumab effects on (a) clinical remission, (b) clinical response, and (c) endoscopic remission, with significance maintained across all study removals.
Meta-regression revealed that higher treatment dosage significantly correlated with improvements in clinical remission (coefficient = 0.0008 per mg, p = 0.0491), clinical response (coefficient = 0.0018 per mg, p = 0.0004), and endoscopic remission (coefficient = 0.0012 per mg, p = 0.0001; Figure 5). Treatment duration was significantly associated with clinical remission (coefficient = 0.0034 per day, p = 0.0158), but not with clinical response (coefficient = 0.0055 per day, p = 0.0670) or endoscopic remission (coefficient = 0.0026 per day, p = 0.3171; Figure 6).
Figure 5.
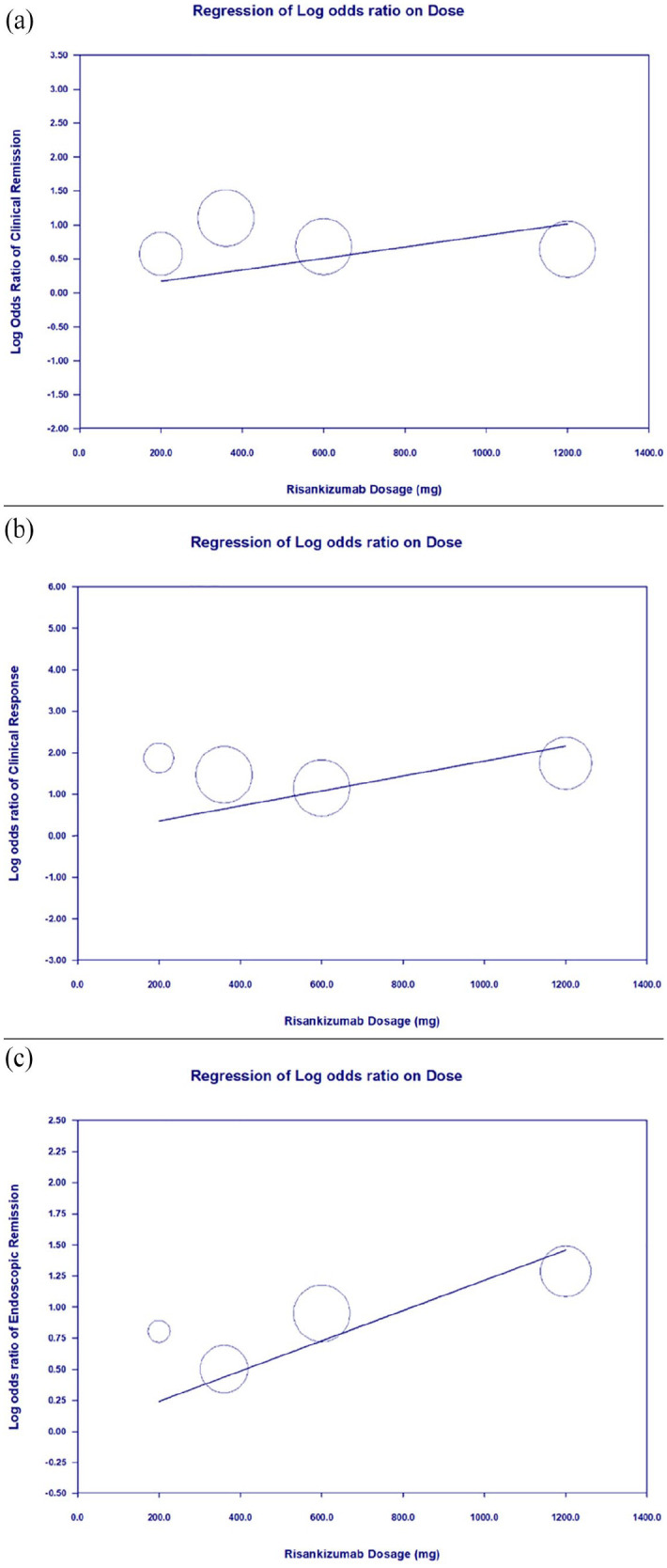
Meta-regression showing the correlation between treatment dosage and outcome. (a) Clinical remission (coefficient = 0.0008 per mg, p = 0.0491). (b) Clinical response (coefficient = 0.0018 per mg, p = 0.0004). (c) Endoscopic remission (coefficient = 0.0012 per mg, p = 0.0001).
Figure 6.
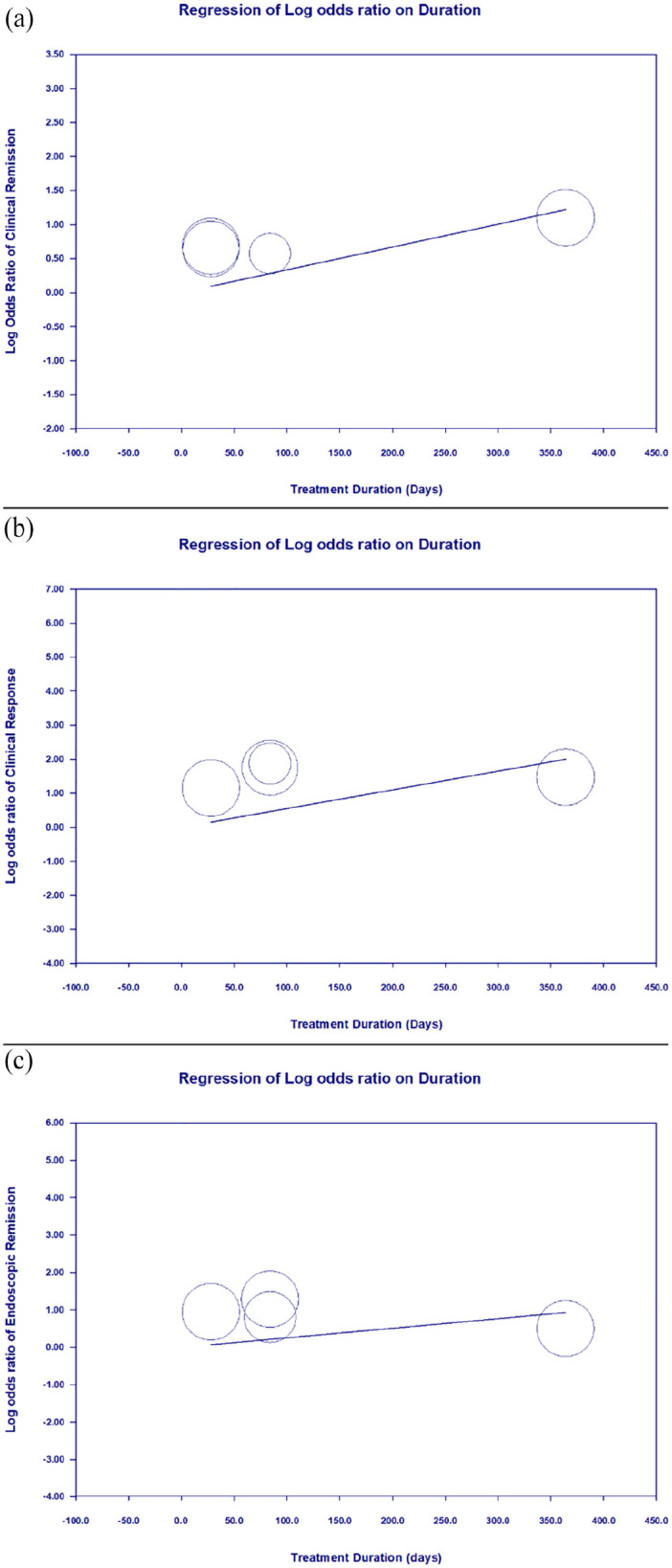
Meta-regression showing the correlation between treatment duration and outcome. (a) Clinical remission (coefficient = 0.0034 per day, p = 0.0158), achieved statistical significance. (b) Clinical response without statistical significance (coefficient = 0.0055 per day, p = 0.0670). (c) Endoscopic remission without statistical significance (coefficient = 0.0026 per day, p = 0.3171).
We conducted subgroup analyses to account for variations in disease duration and prior treatments, both of which can influence remission rates. For disease duration, we categorized studies based on a 10-year threshold and performed subgroup analyses accordingly. The association between Risankizumab and placebo remained consistent across both subgroups. In patients with a disease duration of less than 10 years, the ORs were as follows: clinical remission (OR = 2.454, 95% CI = 1.662–3.622, p < 0.001), clinical response (OR = 2.116, 95% CI = 1.438–3.115, p < 0.001), and endoscopic remission (OR = 3.710, 95% CI = 2.478–5.554, p < 0.001). Similarly, in patients with a disease duration exceeding 10 years, the ORs were: clinical remission (OR = 1.872, 95% CI = 1.119–3.132, p = 0.017), clinical response (OR = 1.872, 95% CI = 1.119–3.132, p < 0.001), and endoscopic remission (OR = 5.827, 95% CI = 2.772–12.248, p < 0.001; Supplemental Figure S1).
Regarding prior treatments, all patients had received conventional therapy, while 42% of participants in the D’Haens 2022 (ADVANCE) study were bio-naïve. 12 We conducted a subgroup analysis to compare outcomes between patients previously treated with biologic agents and bio-naïve patients. Among those with prior biologic exposure, the ORs were: clinical remission (OR = 2.344, 95% CI = 1.614–3.404, p < 0.001), clinical response (OR = 2.413, 95% CI = 1.358–4.287, p = 0.003), and endoscopic remission (OR = 4.851, 95% CI = 3.087–7.622, p < 0.001). In contrast, the ORs for bio-naïve patients were: clinical remission (OR = 1.974, 95% CI = 1.127–3.456, p = 0.017), clinical response (OR = 2.582, 95% CI = 1.071–6.226, p = 0.035), and endoscopic remission (OR = 3.157, 95% CI = 1.782–5.591, p < 0.001; Supplemental Figure S2).
The funnel plot showed slight asymmetry in effect size distributions (Supplemental Figure S3), and Egger’s regression test (p = 0.73410) indicated no evidence of publication bias.
Secondary outcome: treatment-associated adverse event rates
Of the 1437 participants receiving Risankizumab, 892 (62.07%) reported adverse events, including nasopharyngitis, arthralgia, headache, nausea, and diarrhea.12 –14 Similarly, adverse events were reported in placebo groups (385 of 616 participants, 62.5%). Meta-analysis of treatment-related adverse events (Supplemental Figure S4) revealed no significant difference between Risankizumab and placebo groups (OR = 0.872, 95% CI: 0.695–1.093, p = 0.592, I2 < 0.01%).
Discussion
Principal findings
In this systematic review and meta-analysis, we synthesized data from four RCTs to evaluate the efficacy of Risankizumab in Crohn’s disease. Our findings demonstrate that Risankizumab significantly improves clinical remission, clinical response, and endoscopic remission outcomes. Clinical remission was defined as a CDAI score of <150, while clinical response was defined as a reduction in CDAI of ⩾100 points from baseline. Endoscopic remission was characterized by a Simple Endoscopic Score for Crohn’s Disease ⩽4, with at least a 2-point reduction from baseline and no individual subscore >1, as assessed by central reviewers. These metrics are established benchmarks for assessing therapeutic outcomes in Crohn’s disease. 22 Unlike anti-TNF agents, the role of therapeutic drug monitoring for small-molecule agents is more complex. Drug clearance is influenced by individual factors such as low albumin levels, high fecal calprotectin (FCP) levels, and corticosteroid use at the time of induction. 23 Regarding the efficacy of IL-12/23 antagonists as induction therapy, the UNISTAR pediatric RCT study demonstrated that the high-dose arm of Ustekinumab (9 mg/kg or 390 mg) at 16 weeks resulted in better clinical remission compared to the low-dose arm (3 mg/kg or 130 mg). 24 Similarly, Risankizumab exhibits a linear dose–response with repeated administration. It requires three intravenous induction doses (600 mg at weeks 0, 4, and 8), followed by subcutaneous maintenance dosing every 8 weeks, with doses of either 180 or 360 mg starting from week 12. Steady-state concentrations are achieved by week 16. 25 Our regression analysis revealed a dose-dependent relationship, with higher doses of Risankizumab correlating with greater efficacy. This aligns with findings from prior reviews exploring the dose-dependent effects of Risankizumab. 26 When treatment duration was considered, only clinical remission showed significant improvement, likely because it reflects sustained and comprehensive patient recovery. In contrast, clinical response captures initial disease activity reduction without indicating long-term stability, and endoscopic remission depends on factors such as baseline mucosal damage and local tissue responses.
Risankizumab demonstrates significant efficacy in Crohn’s disease, regardless of disease duration or prior biologic exposure. The subgroup analysis shows that patients with shorter disease duration (<10 years) had slightly higher ORs for clinical remission and response, suggesting better outcomes with earlier intervention. However, patients with longer disease duration (>10 years) experienced more pronounced endoscopic remission, indicating Risankizumab’s ability to induce mucosal healing even in refractory cases. Both bio-naïve and biologic-exposed patients benefited from the treatment, with strong improvements in clinical and endoscopic outcomes. These findings support Risankizumab’s use as an effective option for both early and long-standing disease, as well as for patients with prior biologic failure.
The baseline conditions of participants, all of whom had moderate-to-severe Crohn’s disease, did not significantly affect Risankizumab’s clinical response. Some trials included patients intolerant to previous therapies, highlighting its potential role in refractory cases. 12 However, moderate heterogeneity in clinical response may be influenced by baseline disease severity (mean disease duration: 8.8 years in ADVANCE vs 11.7 years in MOTIVATE), previous biologic failure (ranging from 47% to 73% across trials), and trial duration (12-week induction vs 52-week maintenance). Sensitivity analyses confirmed the robustness of these findings. Previous meta-analyses have established Risankizumab’s efficacy in conditions like psoriatic arthritis. 9 Network meta-analyses also suggest Risankizumab offers the highest probability of early remission induction and superior clinical responses in biologic-exposed patients compared to other Crohn’s disease treatments. 27 Our findings are consistent, showing that Risankizumab produces mild-to-large improvements in clinical outcomes, depending on the assessment used.
The included RCTs showed no significant differences in adverse event rates between Risankizumab and placebo groups. Common adverse events included headache and nasopharyngitis. Serious infections (e.g., opportunistic infections, herpes zoster, and COVID-19) were rare and observed across all groups. Hepatic events were infrequent, occurring in less than 4% of participants, primarily as mild liver enzyme elevations. 28 Given the relatively short duration of the included trials (4–52 weeks), long-term safety remains a concern. However, no serious adverse effects, such as opportunistic infections, active tuberculosis, or herpes zoster, were observed. Nonetheless, long-term rates of infection and malignancy are still under investigation. While adverse events associated with Risankizumab are manageable, its significant efficacy in inducing clinical remission and response, particularly in biologic-exposed or refractory cases, underlines its potential as a promising therapeutic option for moderate-to-severe Crohn’s disease.
Insights for Clinical Practice Risankizumab, a humanized monoclonal antibody targeting the p19 subunit of IL-23, is approved for moderate-to-severe Crohn’s disease in adults. By inhibiting the IL-23/Th17 axis, Risankizumab reduces gene expression related to inflammation, biomarkers like FCP and high-sensitivity C-reactive protein (hs-CRP), and Th17 cells in the gut.5,25 Our study highlights Risankizumab as a promising therapeutic option. However, treatment outcomes can be influenced by several factors, including disease duration, prior bowel resection, extraintestinal manifestations (EIMs), previous biologic exposure, number of prior therapies, and patient-specific variables such as age, gender, and smoking status.
Patient-reported outcomes, including AP and stool frequency (SF), were assessed at baseline and weeks 24 and 52. Clinical remission was defined as an average daily SF ⩽2.8 and an AP score ⩽1, while clinical response was defined as a ⩾30% reduction in average daily AP and/or SF. In the two induction RCTs, ADVANCE (42% bio-naïve) and MOTIVATE (47% with only one prior biologic exposure), 37.6% of patients achieved steroid-free clinical remission at week 12. In the FORTIFY trial, subcutaneous risankizumab maintenance therapy, following induction with ADVANCE and MOTIVATE, led to approximately 60% of patients achieving steroid-free clinical remission at week 52 (51.8% in the 360 mg arm and 46.5% in the 180 mg arm). These findings suggest that durable clinical remission is correlated with subcutaneous risankizumab maintenance therapy.12,29 In a Belgian multicentric cohort study focusing on multirefractory Crohn’s disease (85.5% of patients exposed to at least four advanced therapies and 98.6% to ustekinumab, with 14 having an ostomy), clinical outcomes were assessed at weeks 24 and 52. Among patients without an ostomy, 61.8% (34/55) achieved a steroid-free clinical response, while 18.2% (10/55) attained remission at week 24. By week 52, these rates were 58.2% (32/55) and 27.3% (15/55), respectively. Outcomes for patients with an ostomy were comparable, with 42.9% achieving a steroid-free clinical response and 14.3% achieving remission. 29 These findings support the efficacy of risankizumab in treating multirefractory Crohn’s disease patients.
The STRIDE-II and SPIRIT initiatives outline treatment goals for Crohn’s disease, starting with clinical response, followed by clinical remission to ensure symptom control and biomarker reduction. Long-term objectives include endoscopic healing and improved quality of life.30,31 An open-label extension study by Ferrante et al. 14 demonstrated that median reductions were noted at week 0 (FCP ⩽250 mg/kg; hs-CRP ⩽5 mg/L) and sustained through week 52, reinforcing Risankizumab’s efficacy.
The Inflammatory Bowel Disease Questionnaire (IBDQ) is a validated, widely used disease-specific tool that assesses IBD across four dimensions: bowel symptoms, systemic symptoms, social functioning, and emotional well-being. 32 Among patients treated with Risankizumab, the percentage who achieved an IBDQ response and remission following 12 weeks of induction treatment was sustained through 52 weeks of maintenance therapy. Furthermore, a greater proportion of patients achieved IBDQ response and remission after 52 weeks of maintenance treatment with risankizumab. 33
EIMs, including skin, joint, and systemic organ involvement, are common challenges in Crohn’s disease. IL-23 plays a critical role in Th17-mediated inflammation, contributing to these manifestations. Meta-analyses have shown significant associations between psoriasis and IBD, with higher risk in Crohn’s disease (risk ratio, 2.53, 95% CI: 1.65–3.89).34,35 Risankizumab, a humanized monoclonal antibody targeting IL-23, has shown significant efficacy in treating moderate-to-severe plaque psoriasis, as demonstrated in head-to-head efficacy comparisons. 36 The 2024 ECCO guidelines on EIMs in Crohn’s disease recommend humanized monoclonal antibodies, such as Risankizumab, for refractory or severe skin conditions like pyoderma gangrenosum. However, TNF-α antagonists, commonly used in IBD, are associated with complications such as paradoxical psoriasis, psoriasiform eczema, and palmoplantar pustulosis. 37 These complications may result from a shift from Th1 to Th2 responses, inhibiting macrophage apoptosis and promoting abnormal T-cell activation. A case report highlighted the efficacy of Risankizumab in a Crohn’s disease patient with concurrent IgA nephropathy and guttate psoriasis. IgA nephropathy is marked by elevated Th2 and Th17 cell levels and reduced Th1 activity. IL-23 inhibition effectively downregulated Th17-mediated pathways, achieving promising disease control. 38 Spondyloarthritis (SpA) is another common EIM in IBD. Shared genetic loci between SpA and IBD, particularly within the IL-12/23 pathway, underscore their interconnected pathophysiology. The 2024 ECCO guidelines also highlight the effectiveness of ustekinumab in treating non-axial spondyloarthropathy, including psoriatic arthropathy and arthralgia, in Crohn’s disease patients. 39
Crohn’s disease is influenced by various factors, with smoking behavior serving as a significant genetic and environmental contributor. The interleukin-23 receptor (IL23R) has been identified as a critical determinant of disease susceptibility in independent patient cohorts. Database analyses suggest a potential mechanism linking IL23R variants, smoking behavior, and Crohn’s disease-associated genes, such as NOD2 and ATG16L1. IL-23, a key cytokine, plays a central role in orchestrating innate and T-cell-mediated immune responses during intestinal inflammation.40,41 Clinically, smoking exacerbates disease severity, increasing the frequency of flare-ups, penetrating complications, and the likelihood of requiring surgical intervention compared to nonsmokers. Moreover, therapeutic responses may differ across subgroups, underscoring the importance of personalized treatment approaches. Notably, a strong additive interaction exists between IL23R single nucleotide polymorphisms and smoking behavior, significantly elevating disease risk in specific genetic backgrounds. 42
The analyzed RCTs primarily included relatively young participants, with limited subgroup analyses addressing elderly patients. The efficacy and safety of biological agents, including Risankizumab, can vary with age. While some studies report similar outcomes between elderly and younger individuals, others indicate reduced efficacy or an increased risk of adverse events in older populations. 40 However, evidence on the use of Risankizumab in elderly patients remains scarce. 43 Future research should focus on biologic-naïve populations, age-specific effects, long-term follow-up, and large-scale RCTs to comprehensively evaluate the efficacy and safety of Risankizumab in Crohn’s disease.
Strengths and limitations
The strengths of this review include a robust and comprehensive search strategy, the inclusion of studies with diverse populations and Risankizumab treatment protocols, and the utilization of standardized outcome measures to ensure consistency and reliability. However, limitations include the relatively small number of studies analyzed and the heterogeneity in reporting corticosteroid use across trials, which precluded a formal subgroup analysis based on this variable. Furthermore, the overlap in patient cohorts across some included studies may introduce a degree of dependency, potentially influencing effect estimates. Additional research is needed to confirm these findings and further explore the long-term efficacy of risankizumab across different patient subgroups.
Relation to prior work
A prior systematic review assessed the efficacy and safety of selective IL-23p19 and IL-12/23p40 inhibitors in patients with moderate-to-severe Crohn’s disease, establishing the role of IL-23 antagonists in clinical care. However, registry-based data for IL-23p19 antagonists in Crohn’s disease are still needed. 44 Our study extends this body of work by identifying dose-dependent efficacy trends and emphasizing the potential benefits of prolonged treatment for achieving clinical remission. By employing a one-study-removed sensitivity analysis and ORs for statistical evaluation, we present evidence of moderate efficacy in improving clinical outcomes for patients with Crohn’s disease.
Conclusion
This systematic review and meta-analysis assessed the effects of Risankizumab on clinical outcomes in Crohn’s disease, synthesizing data from four RCTs. The results demonstrate that Risankizumab significantly enhances clinical remission, clinical response, and endoscopic remission, with efficacy showing a clear dose-dependent trend. The safety profile of Risankizumab is favorable, with manageable adverse events and no significant differences observed compared to placebo. Mechanistically, Risankizumab targets the IL-23/Th17 pathway, effectively mitigating inflammation associated with Crohn’s disease. However, the small number of studies may impact the reliability of these findings. Additional large-scale RCTs are required to confirm its long-term effectiveness, safety in elderly populations, and efficacy in biologic-naïve patients.
Supplemental Material
Supplemental material, sj-docx-1-tag-10.1177_17562848251338743 for Clinical insights into IL-23 inhibition: risankizumab for Crohn’s disease through a systematic review and meta-analysis of randomized controlled trials by Po-Feng Huang, Tien-Yu Huang, Yi-Chiao Cheng, Peng-Jen Chen, Wei-Kuo Chang and Chao-Feng Chang in Therapeutic Advances in Gastroenterology
Supplemental material, sj-tif-2-tag-10.1177_17562848251338743 for Clinical insights into IL-23 inhibition: risankizumab for Crohn’s disease through a systematic review and meta-analysis of randomized controlled trials by Po-Feng Huang, Tien-Yu Huang, Yi-Chiao Cheng, Peng-Jen Chen, Wei-Kuo Chang and Chao-Feng Chang in Therapeutic Advances in Gastroenterology
Supplemental material, sj-tif-3-tag-10.1177_17562848251338743 for Clinical insights into IL-23 inhibition: risankizumab for Crohn’s disease through a systematic review and meta-analysis of randomized controlled trials by Po-Feng Huang, Tien-Yu Huang, Yi-Chiao Cheng, Peng-Jen Chen, Wei-Kuo Chang and Chao-Feng Chang in Therapeutic Advances in Gastroenterology
Supplemental material, sj-tif-4-tag-10.1177_17562848251338743 for Clinical insights into IL-23 inhibition: risankizumab for Crohn’s disease through a systematic review and meta-analysis of randomized controlled trials by Po-Feng Huang, Tien-Yu Huang, Yi-Chiao Cheng, Peng-Jen Chen, Wei-Kuo Chang and Chao-Feng Chang in Therapeutic Advances in Gastroenterology
Supplemental material, sj-tif-5-tag-10.1177_17562848251338743 for Clinical insights into IL-23 inhibition: risankizumab for Crohn’s disease through a systematic review and meta-analysis of randomized controlled trials by Po-Feng Huang, Tien-Yu Huang, Yi-Chiao Cheng, Peng-Jen Chen, Wei-Kuo Chang and Chao-Feng Chang in Therapeutic Advances in Gastroenterology
Acknowledgments
None.
Footnotes
ORCID iD: Po-Feng Huang  https://orcid.org/0000-0003-3918-4540
https://orcid.org/0000-0003-3918-4540
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Po-Feng Huang, Division of Gastroenterology, Department of Internal Medicine, Kaohsiung Armed Forces General Hospital, Kaohsiung, Taiwan; Division of Gastroenterology, Department of Internal Medicine, Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan.
Tien-Yu Huang, Division of Gastroenterology, Department of Internal Medicine, Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan.
Yi-Chiao Cheng, Division of Colon and Rectal Surgery, Department of Surgery, Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan.
Peng-Jen Chen, Division of Gastroenterology, Department of Internal Medicine, Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan.
Wei-Kuo Chang, Division of Gastroenterology, Department of Internal Medicine, Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan.
Chao-Feng Chang, Division of Gastroenterology, Department of Internal Medicine, Tri-Service General Hospital, National Defense Medical Center, No. 325, Sec. 2, Chenggong Road, Neihu District, Taipei City 114202, Taiwan.
Declarations
Ethics approval and consent to participate: This study is a meta-analysis that exclusively utilizes data extracted from previously published trials, all of which have obtained ethical approval and informed consent from participants as per their respective study protocols. No new patient data were collected, and no direct patient interaction was involved. Therefore, additional informed consent was not required for this study. Regarding Institutional Review Board (IRB) approval, we applied to the Tri-Service General Hospital IRB under application number 62689, and it was classified as an exempt study.
Consent for publication: Not applicable.
Author contributions: Po-Feng Huang: Conceptualization; Investigation; Methodology; Writing – original draft.
Tien-Yu Huang: Conceptualization; Resources.
Yi-Chiao Cheng: Formal analysis; Software.
Peng-Jen Chen: Investigation; Writing – review & editing.
Wei-Kuo Chang: Funding acquisition; Validation; Visualization; Writing – review & editing.
Chao-Feng Chang: Formal analysis; Methodology; Project administration; Writing – original draft; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declare that there is no conflict of interest.
Availability of data and materials: Data are publicly available since this is a systematic review of published literature.
References
- 1. Lewis JD, Parlett LE, Funk MLJ, et al. Incidence, prevalence, and racial and ethnic distribution of inflammatory bowel disease in the United States. Gastroenterology 2023; 165(5): 1197–1205.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Torres J, Mehandru S, Colombel J-F, et al. Crohn’s disease. Lancet 2017; 389(10080): 1741–1755. [DOI] [PubMed] [Google Scholar]
- 3. van der Have M, van der Aalst KS, Kaptein AA, et al. Determinants of health-related quality of life in Crohn’s disease: a systematic review and meta-analysis. J Crohns Colitis 2014; 8(2): 93–106. [DOI] [PubMed] [Google Scholar]
- 4. Verstockt B, Salas A, Sands BE, et al. IL-12 and IL-23 pathway inhibition in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol 2023; 20(7): 433–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sarra M, Pallone F, Macdonald TT, et al. IL-23/IL-17 axis in IBD. Inflamm Bowel Dis 2010; 16(10): 1808–1813. [DOI] [PubMed] [Google Scholar]
- 6. Danese S, Panaccione R, Feagan BG, et al. Efficacy and safety of 48 weeks of guselkumab for patients with Crohn’s disease: maintenance results from the phase 2, randomised, double-blind GALAXI-1 trial. Lancet Gastroenterol Hepatol 2024; 9(2): 133–146. [DOI] [PubMed] [Google Scholar]
- 7. Danese S, Beaton A, Duncan EA, et al. Long-term safety of brazikumab in the open-label period of a randomized phase 2a study of patients with Crohn’s disease. BMC Gastroenterol 2023; 23(1): 451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ferrante M, D’Haens G, Jairath V, et al. Efficacy and safety of mirikizumab in patients with moderately-to-severely active Crohn’s disease: a phase 3, multicentre, randomised, double-blind, placebo-controlled and active-controlled, treat-through study. Lancet 2024; 404(10470): 2423–2436. [DOI] [PubMed] [Google Scholar]
- 9. Huang X, Shentu H, He Y, et al. Efficacy and safety of IL-23 inhibitors in the treatment of psoriatic arthritis: a meta-analysis based on randomized controlled trials. Immunol Res 2023; 71(4): 505–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLoS Med 2021; 18(3): e1003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019; 366: l4898. [DOI] [PubMed] [Google Scholar]
- 12. D’Haens G, Panaccione R, Baert F, et al. Risankizumab as induction therapy for Crohn’s disease: results from the phase 3 ADVANCE and MOTIVATE induction trials. Lancet 2022; 399(10340): 2015–2030. [DOI] [PubMed] [Google Scholar]
- 13. Feagan BG, Sandborn WJ, D’Haens G, et al. Induction therapy with the selective interleukin-23 inhibitor risankizumab in patients with moderate-to-severe Crohn’s disease: a randomised, double-blind, placebo-controlled phase 2 study. Lancet 2017; 389(10080): 1699–1709. [DOI] [PubMed] [Google Scholar]
- 14. Ferrante M, Panaccione R, Baert F, et al. Risankizumab as maintenance therapy for moderately to severely active Crohn’s disease: results from the multicentre, randomised, double-blind, placebo-controlled, withdrawal phase 3 FORTIFY maintenance trial. Lancet 2022; 399(10340): 2031–2046. [DOI] [PubMed] [Google Scholar]
- 15. Higgins JPT, Li T, Deeks JJ. Chapter 6: choosing effect measures and computing estimates of effect. In: Higgins JPT, Thomas J, Chandler J, et al. (eds) Cochrane handbook for systematic reviews of interventions. Version 6.5 (updated August 2024). Cochrane, UK, https://www.training.cochrane.org/handbook (2024). [Google Scholar]
- 16. Higgins JPT, Eldridge S, Li T. Chapter 23: Including variants on randomized trials. In: Higgins JPT, Thomas J, Chandler J, et al. (eds) Cochrane handbook for systematic reviews of interventions. Version 6.5 (updated August 2024). Cochrane, UK, https://www.training.cochrane.org/handbook (2024). [Google Scholar]
- 17. Borenstein M, Hedges LV, Higgins JPT, et al. Fixed-effect versus random-effects models. In: Borenstein M. (ed.) Introduction to meta-analysis. Hoboken, NJ: Wiley, 2009, pp. 77–86. [Google Scholar]
- 18. Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Higgins JPT, Li T, Deeks JJ. Chapter 9: Analysing data and undertaking meta-analyses. In: Cochrane handbook for systematic reviews of interventions. Version 6.5 (updated August 2024). Cochrane, UK, https://www.training.cochrane.org/handbook (2024). [Google Scholar]
- 20. Deeks JJ, Higgins JPT, Altman DG, et al.; Cochrane Statistical Methods Group. Analysing data and undertaking meta-analyses. 2019; In: Higgins JPT, Thomas J, Chandler J, et al. (eds) Cochrane handbook for systematic reviews of interventions. Version 6.5 (updated August 2024). Cochrane, UK, https://www.training.cochrane.org/handbook (2024). [Google Scholar]
- 21. Page MJ, Higgins JPT, Sterne JAC. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JPT, Thomas J, Chandler J, et al. (eds) Cochrane handbook for systematic reviews of interventions. Version 6.5 (updated August 2024). Cochrane, UK, https://www.training.cochrane.org/handbook (2024). [Google Scholar]
- 22. Moreira PL, Dignass A, Estevinho MM, et al.; International Organization for the Study of Inflammatory Bowel Diseases (IOIBD). Assessment of outcomes in Crohn’s disease: a systematic review of randomized clinical trials to inform a multiple outcome framework. United European Gastroenterol J 2024; 12(9): 1280–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Suleiman AA, Goebel A, Bhatnagar S, et al. Population pharmacokinetic and exposure–response analyses for efficacy and safety of risankizumab in patients with active Crohn’s disease. Clin Pharmacol Ther 2023; 113(4): 839–850. [DOI] [PubMed] [Google Scholar]
- 24. Rosh JR, Turner D, Griffiths A, et al. Ustekinumab in paediatric patients with moderately to severely active Crohn’s disease: pharmacokinetics, safety, and efficacy results from UniStar, a phase 1 study. J Crohns Colitis 2021; 15(11): 1931–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pang Y, D’Cunha R, Winzenborg I, et al. Risankizumab: mechanism of action, clinical and translational science. Clin Transl Sci 2024; 17(1): e13706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dutt K, Vasudevan A. Therapeutic drug monitoring for biologic and small-molecule therapies for inflammatory bowel disease. Medicina (Kaunas) 2024; 60(2): 250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Attauabi M, Steenholdt C, Poulsen A, et al. Network meta-analysis: comparative onset of early effect of biologics and small molecules in moderately to severely active luminal Crohn’s disease. Aliment Pharmacol Ther 2024; 60(2): 124–143. [DOI] [PubMed] [Google Scholar]
- 28. Choi D, Sheridan H, Bhat S. Risankizumab-rzaa: a new therapeutic option for the treatment of Crohn’s disease. Ann Pharmacother 2023; 57(5): 579–584. [DOI] [PubMed] [Google Scholar]
- 29. Alsoud D, Sabino J, Franchimont D, et al. Real-world effectiveness and safety of risankizumab in patients with moderate to severe multirefractory Crohn’s disease: a Belgian multicentric cohort study. Inflamm Bowel Dis 2024; 30(12): 2289–2296. [DOI] [PubMed] [Google Scholar]
- 30. Le Berre C, Peyrin-Biroulet L; SPIRIT-IOIBD Study Group. Selecting end points for disease-modification trials in inflammatory bowel disease: the SPIRIT consensus from the IOIBD. Gastroenterology 2021; 160(5): 1452–1460.e21. [DOI] [PubMed] [Google Scholar]
- 31. Turner D, Ricciuto A, Lewis A, et al.; International Organization for the Study of IBD. STRIDE-II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the international organization for the study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology 2021; 160(5): 1570–1583. [DOI] [PubMed] [Google Scholar]
- 32. Alrubaiy L, Rikaby I, Dodds P, et al. Systematic review of health-related quality of life measures for inflammatory bowel disease. J Crohns Colitis 2015; 9(3): 284–292. [DOI] [PubMed] [Google Scholar]
- 33. Peyrin-Biroulet L, Ghosh S, Lee SD, et al. Effect of risankizumab on health-related quality of life in patients with Crohn’s disease: results from phase 3 MOTIVATE, ADVANCE and FORTIFY clinical trials. Aliment Pharmacol Ther 2023; 57(5): 496–508. [DOI] [PubMed] [Google Scholar]
- 34. Ribaldone DG, Pellicano R, Actis GC. The gut and the inflammatory bowel diseases inside-out: extra-intestinal manifestations. Minerva Gastroenterol Dietol 2019; 65(4): 309–318. [DOI] [PubMed] [Google Scholar]
- 35. Fu Y, Lee C-H, Chi C-C. Association of psoriasis with inflammatory bowel disease: a systematic review and meta-analysis. JAMA Dermatol 2018; 154(12): 1417–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet 2018; 392(10148): 650–661. [DOI] [PubMed] [Google Scholar]
- 37. Gordon H, Burisch J, Ellul P, et al. ECCO guidelines on extraintestinal manifestations in inflammatory bowel disease. J Crohns Colitis 2024; 18(1): 1–37. [DOI] [PubMed] [Google Scholar]
- 38. Larson C, Munir N, Rao P, et al. Crohn’s disease associated with iga nephropathy effectively treated with the interleukin-23 inhibitor Risankizumab. ACG Case Rep J 2024; 11(7): e01437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Guillo L, D’Amico F, Danese S, et al. Ustekinumab for extra-intestinal manifestations of inflammatory bowel disease: a systematic literature review. J Crohns Colitis 2021; 15(7): 1236–1243. [DOI] [PubMed] [Google Scholar]
- 40. Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, et al. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol 2007; 8(9): 942–949. [DOI] [PubMed] [Google Scholar]
- 41. Wilson NJ, Boniface K, Chan JR, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol 2007; 8(9): 950–957. [DOI] [PubMed] [Google Scholar]
- 42. Doecke JD, Simms LA, Zhao ZZ, et al. Smoking behaviour modifies IL23r-associated disease risk in patients with Crohn’s disease. J Gastroenterol Hepatol 2015; 30(2): 299–307. [DOI] [PubMed] [Google Scholar]
- 43. Ruggiero A, Fabbrocini G, Cinelli E, et al. Anti-interleukin-23 for psoriasis in elderly patients: guselkumab, risankizumab and tildrakizumab in real-world practice. Clin Exp Dermatol 2022; 47(3): 561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vuyyuru SK, Solitano V, Hogan M, et al. Efficacy and safety of IL-12/23 and IL-23 inhibitors for Crohn’s disease: systematic review and meta-analysis. Dig Dis Sci 2023; 68(9): 3702–3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tag-10.1177_17562848251338743 for Clinical insights into IL-23 inhibition: risankizumab for Crohn’s disease through a systematic review and meta-analysis of randomized controlled trials by Po-Feng Huang, Tien-Yu Huang, Yi-Chiao Cheng, Peng-Jen Chen, Wei-Kuo Chang and Chao-Feng Chang in Therapeutic Advances in Gastroenterology
Supplemental material, sj-tif-2-tag-10.1177_17562848251338743 for Clinical insights into IL-23 inhibition: risankizumab for Crohn’s disease through a systematic review and meta-analysis of randomized controlled trials by Po-Feng Huang, Tien-Yu Huang, Yi-Chiao Cheng, Peng-Jen Chen, Wei-Kuo Chang and Chao-Feng Chang in Therapeutic Advances in Gastroenterology
Supplemental material, sj-tif-3-tag-10.1177_17562848251338743 for Clinical insights into IL-23 inhibition: risankizumab for Crohn’s disease through a systematic review and meta-analysis of randomized controlled trials by Po-Feng Huang, Tien-Yu Huang, Yi-Chiao Cheng, Peng-Jen Chen, Wei-Kuo Chang and Chao-Feng Chang in Therapeutic Advances in Gastroenterology
Supplemental material, sj-tif-4-tag-10.1177_17562848251338743 for Clinical insights into IL-23 inhibition: risankizumab for Crohn’s disease through a systematic review and meta-analysis of randomized controlled trials by Po-Feng Huang, Tien-Yu Huang, Yi-Chiao Cheng, Peng-Jen Chen, Wei-Kuo Chang and Chao-Feng Chang in Therapeutic Advances in Gastroenterology
Supplemental material, sj-tif-5-tag-10.1177_17562848251338743 for Clinical insights into IL-23 inhibition: risankizumab for Crohn’s disease through a systematic review and meta-analysis of randomized controlled trials by Po-Feng Huang, Tien-Yu Huang, Yi-Chiao Cheng, Peng-Jen Chen, Wei-Kuo Chang and Chao-Feng Chang in Therapeutic Advances in Gastroenterology