Abstract
Human metapneumovirus (hMPV) is a significant respiratory pathogen, primarily impacting young, elderly, and immunocompromised populations. While the clinical presentations are similar to those of other respiratory viruses such as respiratory syncytial virus (RSV), influenza, and SARS-CoV-2, they can still lead to serious complications. The virus primarily transmits via respiratory droplets, with outbreaks peaking during winter and spring. In resource-limited settings, administration of multiplex PCR assays is essential for precise diagnosis, yet it presents significant challenges. Recent studies indicate a 6.24% infection rate in hospitalized patients presenting with acute respiratory infections (ARIs). Enhanced surveillance and prevention are essential given the morbidity and mortality rates of hMPV, which are comparable to those of influenza and RSV. Effective management requires enhanced diagnostic tools, improved public health strategies, and continuous research into antiviral therapies and vaccines. This study highlighted the growing importance of hMPV as a respiratory pathogen, focusing on its seasonal patterns, clinical manifestations in at-risk populations, transmission dynamics, and diagnostic challenges compared to other respiratory viruses.
Keywords: Human metapneumovirus (hMPV), Respiratory infections, RSV, Influenza, SARS-CoV-2
Background
Human metapneumovirus (hMPV) is a significant respiratory pathogen, first identified in 2001, and known for a broad spectrum of respiratory infections, varying from mild upper respiratory infections to severe lower respiratory tract diseases, including bronchiolitis and pneumonia. The condition especially concerns children, the senior population, and those with compromised immune systems [1, 2]. It is worth noting that acute respiratory infections (ARIs) continue to be a significant contributor to global mortality, responsible for an estimated 4.25 million deaths, particularly affecting low- or middle-income countries disproportionately [3]. Numerous viral pathogens are responsible for acute respiratory infections (ARIs). However, recent investigations indicate that hMPV, when combined with respiratory syncytial virus (RSV) and influenza, can result in serious and sometimes fatal complications, especially among vulnerable populations [4, 5]. Recent global burden analyses have highlighted the significant impact of respiratory infections, revealing an estimated 33 million episodes of RSV-associated acute lower respiratory infection and 3.6 million RSV-related hospital admissions worldwide in 2019, particularly affecting children under five, with approximately 101,400 deaths annually [6]. Lower respiratory infections (LRTIs) impose a significant burden on both morbidity and mortality, with a staggering 344 million new cases and 2.18 million deaths reported globally in 2021 [7].
The recent alerts from the World Health Organization regarding hMPV outbreaks, including the 2024 outbreak in China, have underscored the increasing significance of this virus in public health discussions. Considering its significant role in ARI-related morbidity and mortality, a more profound insight into hMPV’s epidemiology, transmission dynamics, and clinical implications is crucial [8].
The Pneumoviridae family comprises two genera: Metapneumovirus, which encompasses hMPV, and Orthopneumovirus, which includes RSV [9]. The hMPV can lead to respiratory tract infections across all age groups, with symptomatic cases predominantly observed in young children and older adults [10]. The burden of hMPV includes classical and non-classical acute respiratory infections, which involve exacerbations of asthma or chronic obstructive pulmonary disease (COPD) symptoms during the disease [11, 12]. Previous data indicate that hMPV infection is seasonal and frequently occurs in co-infection with other respiratory pathogens [13]. A study showed an hMPV infection rate of 0.2% in 2007, 4.3% in 2008, and 0.3% in 2009 [14]. According to another study, the estimated rate of hMPV infections among hospitalized cases with ARI was 6.24% [15].
This review represented hMPV epidemiology, pathophysiology, clinical manifestations in both community and hospital settings, unique transmission dynamics, and challenges in diagnosis and therapy. It also compared it to other respiratory viruses to contextualize its impact and facilitate future investigation and public health efforts.
Transmission dynamics
hMPV is distributed worldwide and is among the most common viruses responsible for ARIs, alongside influenza, coronaviruses, and RSV [16]. hMPV can circulate within community and healthcare settings, each with specific pathways. Centers for Disease Control and Prevention (CDC) considers respiratory secretions (from coughing or sneezing), close contact (such as handshakes), and contaminated objects and surfaces as the primary modes of hMPV transmission [17].
In temperate regions, hMPV outbreaks follow a seasonal pattern, typically peaking from February to April (Table 1) [18]. Environmental factors, such as temperature and humidity, influence transmission rates through various mechanisms, including changes in viral behavior, host susceptibility, environmental conditions, and human behaviors [19]. Wang et al. showed that the number of cases of hMPV was negatively related to both temperature and rainfall levels. This indicates that climatic conditions can affect the spread of viruses [13].
Table 1.
Comparison of seasonal trends of hMPV with other respiratory viruses
| Virus | Season onset | Peak activity | Season offset | Outbreak duration | Seasonal peak |
|---|---|---|---|---|---|
| hMPV | Early January | January-March | Early June | 12–16 weeks | Winter-spring peak |
| RSV | Late October | Late December | April | 16–22 weeks | Winter-spring peak |
| Influenza | December | January-February | March-April | 6–8 weeks | Winter peak; earlier than hMPV |
| HPIV-3 | April-June | May-June | Late summer | Variable | Summer |
| HPIV-4 | September | October | Autumn | Variable | Autumn |
Like other respiratory viruses, hMPV transmission risk factors include crowded settings such as schools and nursing homes, close contact with infected individuals or contaminated surfaces, lower socioeconomic status, and travel to epidemic regions [17, 20]. Close contact with hospitalized infected individuals, even in asymptomatic cases, can help spread the infection, especially in settings where aerosol-generating procedures (AGPs) are administered and among immunocompromised patients [21, 22]. Asymptomatic carriers are especially important in crowded settings, including schools and households, as viral shedding occurs 7–14 days post-infection [23]. Studies have proven that both symptomatic and asymptomatic carriers, as observed among healthcare workers (HCWs), contribute to spreading infections in daycare and households [22]. The COVID-19 pandemic confirmed these risk factors, as hMPV incidence decreased during the implementation of restrictions and subsequently increased after removing these measures [24].
Few studies have specifically addressed hMPV transmission rates in community settings. Bell et al. used an RT-PCR respiratory pathogen panel to study school-aged children and found that this age group is a major source of hMPV transmission. The study found that higher household density correlated with increased transmission rates, as high as 12.2%, while index-case age negatively correlated with transmission rates. Notably, this study might not be representative of other age groups or regions [25]. On the contrary, a study in a daycare center following the death of a 33-month-old girl from hMPV pneumonia found that 36% of the remaining 22 children tested positive for hMPV using an RT-PCR panel [26]. Such discrepancy in transmission rates could be due to selection bias, differences in study populations, and the large number of undetected cases, as asymptomatic and milder cases do not usually seek further testing [27].
Similarly, Matsuzaki et al. reported higher transmission rates from older index cases, consistent with Bell et al.’s findings. However, it may not apply universally due to study design or sample limitations [28]. It was thought by both studies that the time between the first symptoms in index cases and secondary household cases was about four to five days. This is in line with the idea that most viruses are shed during the first week of illness [29]. The overall transmission rate for hMPV varies among studies.
As noted, hMPV outbreaks have also been reported in healthcare settings [26, 30]. Respiratory viruses, like hMPV, are more likely to spread in healthcare facilities because of close contact between patients and higher viral loads in patients with severe symptoms [31]. Additionally, the extended shedding period in young and immunocompromised individuals underscores the difficulties of preventing hMPV propagation in these conditions.
Since no precise information is available on hMPV virus transmission rates, such variations complicate direct comparisons and hinder the generalization of findings across all settings.
Additionally, the World Health Organization (WHO) alerted that China’s hMPV outbreak is overwhelming hospitals, raising worries of a worldwide pandemic. High population density, significant global connection, and difficulty in providing accurate risk assessments and conducting swift responses in the COVID-19 era raise concerns about rapid international transmission from any Chinese outbreak [32].
Mechanisms of transmission
Community transmission
hMPV is mainly transmitted through respiratory droplets that are released when one speaks, sneezes, or coughs. Schools, daycare centers, and family settings serve as major transmission hubs due to frequent and close encounters among individuals [33]. According to lab study findings, respiratory droplets carrying hMPV are more permanent and transmissible at lower humidity and colder temperatures. In contrast, higher temperatures and humidity limit viral stability, restricting its transmission during the warmer months. These seasonal patterns support epidemiological findings and highlight how environmental factors contribute to community spread [33].
The risk of transmission increases with prolonged shedding, especially in close-contact settings like schools and childcare centers, and the shedding duration is dependent on the host’s immune status, age, and viral load [34]. According to studies, children usually continue to shed the virus for 7–14 days post-infection. Moreover, it can occur for up to 28 days or longer in certain situations, especially in immunocompromised individuals, as the viral shedding in these individuals can last for a long time—up to 182 days [35]. Moreover, asymptomatic carriers have been reported to shed the virus for an average of 5–7 days [36].
Hospital transmission
AGPs, defined as any procedure that generates aerosols that eventually are suspended within the air, such as suctioning, bronchoscopy, and intubation, greatly increase the risk of virus transmission in hospital settings. Aerosols containing infectious virus particles may stay in the air for an extended time, increasing the risk of inhalation [37]. Improved personal protective equipment (PPE) rules, high-efficiency particulate air (HEPA) filtering systems, and getting rid of unnecessary AGPs have all been used to lower this risk, which has resulted in less nosocomial hMPV transmission [38]. Depending on environmental factors such as temperature, humidity, and surface type, hMPV can survive on inanimate surfaces for extended periods. According to laboratory research, hMPV can survive for up to 24 to 48 h at room temperature with moderate humidity on nonporous surfaces such as plastic and stainless steel. On the other hand, the virus only lasts a much shorter time on permeable substances consisting of paper or cloth [39]. Although there is little direct proof of fomite-based hMPV transmission in clinical settings, there is substantial indirect evidence of its role. Recent research has shown that viable hMPV exists on contaminated surfaces in hospitals, such as medical instruments, doorknobs, and bed railings, which presents a substantial risk of indirect transmission [40].
Although, all ages are at risk of hMPV infection, immunocompromised, and older patients are most at risk. Despite advances in understanding hMPV, the epidemiology of the virus in immunocompromised patients, as well as the possible role of HCWs in transmission, remain unexplored [37, 41]. Immunocompromised individuals are more vulnerable to hMPV, including those undergoing hematopoietic stem cell transplantation (HSCT), cancer therapy, or living with primary or secondary immunodeficiencies [4, 42]. HCWs play an important role in preventing and potentially exacerbating the transmission of hMPV in healthcare settings. HCWs are more vulnerable to infection and virus transmission from frequent contact with symptomatic and asymptomatic carriers [43]. During the 2003 SARS epidemic, evidence from a nosocomial outbreak underscores this risk, as HCWs were substantially associated with hMPV infection. hMPV-RNA was found in 20% of SARS patients’ nasopharyngeal aspirates during the pandemic, demonstrating how prevalent it is in healthcare settings [44].
Pathogenesis and virology
Genomic structure and replication cycle
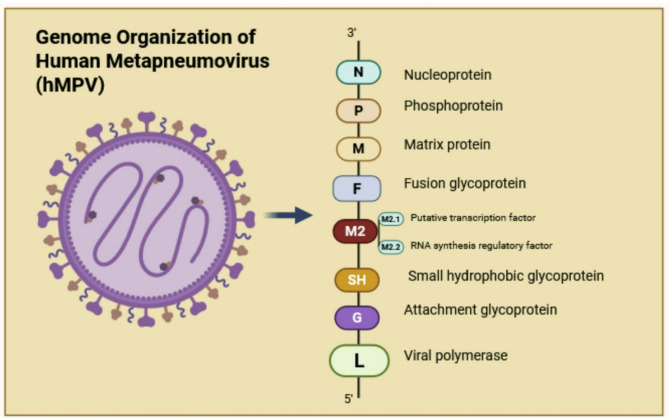
hMPV is a single-stranded, negative-sense, non-segmented RNA virus that belongs to the Paramyxoviridae family and the Pneumovirinae subfamily. The 13.3 kb genome encodes nine structural proteins in this order: nucleoprotein (N), phosphoprotein (P), matrix protein (M), fusion protein (F), M2-1/2 protein, small hydrophobic protein (SH), glycoprotein (G), and large polymerase protein [45]. The F protein is first generated in an inactive form known as F0, which is subsequently broken into two components, F2 and F1, to form a functional prefusion trimer, a protein complex that mediates viral entry into host cells and is the primary target for vaccine development [46, 47]. The activation process is initiated by serine proteases, including transmembrane serine protease 2 (TMPRSS2), which cleave F0 at a particular site. Upon activation, the F protein proceeds through a permanent transformation from its initial metastable trimeric form. The fusion peptide is released during this process, which enables it to enter into the host cell membrane and facilitate the fusion of the viral and host membranes [48]. The hMPV F protein’s main role in viral entry, coupled with its propensity to stimulate effective neutralizing antibody responses in its prefusion structure, makes it a promising target for vaccine development [49]. Figure 1 depicts the genomic organization of hMPV and highlights the unique roles of each protein encoded by the genome.
Fig. 1.
The genomic structure of human metapneumovirus (hMPV) resembles that of respiratory syncytial virus (RSV). The antisense RNA genome of hMPV has eight open reading frames, organized in 3’N-P-M-F-M2-SH-G-L-5’. The N protein is essential for RNA encapsidation. The P protein functions as a cofactor for the polymerase complex. The M protein participates in viral budding and assembly. The F protein is a membrane fusion protein that facilitates the binding of a virus to a host cell. The M2 protein participates in the transcription and replication of viral RNA via its subunits 1 and 2. The SH protein may operate as a viroporin, facilitating viral immune evasion. The G protein facilitates the virus’s adhesion to host cells, whereas the L protein serves a catalytic function in viral replication. This figure was created by BioRender
Attachment, fusion, transcription, replication, assembly, and release are the steps in the hMPV replication process. The replication of hMPV occurs when its G protein attaches itself to glycosaminoglycans on respiratory epithelial cells. Following the F protein binding, the membranes fuse, letting the viral RNA genome enter the host cell’s cytoplasm [50]. Several steps need to occur for viral replication. The G protein first attaches to certain receptors on the surface of the host cell, thus beginning attachment. Subsequently, the F protein enables the virus to enter the host cell by promoting membrane fusion. RNA-dependent RNA polymerase then translates the viral RNA genome into messenger RNAs and reproduces it. The following step is assembly, during which viral components, including proteins and genomes, assemble in the host cell membrane. The host cell ultimately releases the newly produced virus particles through a process known as budding [51]. The ciliated epithelial cells in the respiratory system are primarily affected by hMPV, leading to a variety of symptoms. These symptoms can range in intensity from mild upper respiratory conditions to serious illnesses like bronchiolitis and pneumonia [52]. Mucociliary clearance relies on ciliated epithelial cells to protect the respiratory system against inhaled pathogens. These cells’ usual activity is altered by hMPV infection, which reduces ciliary motion. The primary cause of the typical symptoms of hMPV infection, including coughing, wheezing, and airway blockage, is this disturbance [53].
Molecular variability
The hMPV indicates high genetic variability, leading to its classification into certain subtypes. According to genomic characteristics, hMPV is classified into two primary types, A and B, which are further divided into four primary subtypes: A1, A2, B1, and B2 [54]. More specifically, A2a and A2b are subtypes of A2. Since 2015, new mutations have been found in this subtype, such as 111 and 180 nucleotides in the G gene. These findings highlight hMPV’s continual evolution and emphasize the importance of constant genomic surveillance to identify the clinical significance of new variations [55, 56].
Classifying the virus into subtypes has required analyzing the N, M, F, G, and L genes, which are the primary HMPV genes. The highly conserved F protein, which is essential for the virus to infect cells, has 95% of its amino acid sequence shared between groups A and B. However, there is significant variance in the G gene, with only 53% commonality between the two groups. The G and SH genes show a lot of variation across subtypes, according to studies, whereas the F and N genes show fewer variations within each subgroup [57].
F and G gene variants have a major impact on hMPV pathogenicity. Although the F protein is typically conserved, certain mutations may modify its structure, thus possibly improving the virus’s ability to infect host cells. For instance, a study has demonstrated that specific mutations in the F protein can increase its fusion activity, resulting in increased viral replication and, as a result, increased virulence [58]. The G protein shows notable genetic variability. This variation may impact the virus’s pathogenicity by influencing its capacity to attach to host cells and evade the immune system. The hMPV G protein is constantly changing, according to studies, and antigenic variability is a result of genetic variation. Such alterations may enable the virus to evade previous immunity, thereby worsening illness severity [55]. Genetic variability in hMPV influences its transmissibility, particularly in its fusion (F) and attachment (G) proteins. The F protein is extensively conserved, with research demonstrating no genetic change over decades [59]. The F protein’s ability to be conserved through lineages allows efficient host cell entrance, which is essential for viral transmission. On the other hand, transmissibility may be impacted by lineage-specific differences that affect fusion kinetics and receptor binding efficiency [60]. Conversely, the G protein demonstrates substantial genetic and antigenic diversity. In addition to possibly influencing its transmission dynamics, this variability allows hMPV to adjust according to different host populations and environmental pressures [61]. Epidemiological studies have documented seasonal shifts in the prevalent hMPV genotypes, indicating that genetic variation plays a role in the virus’s capacity to survive in communities. To illustrate the ever-changing nature of hMPV transmission patterns, studies have shown that genotype prevalence shifts with the seasons [62]. The genetic variety of the G gene is the main cause of immune evasion, which is an indication of hMPV infection. With its antigenic diversity in the G protein, hMPV can escape neutralizing antibodies, which weakens the host immune response [63]. Furthermore, the G protein interacts with host immune modulators, such as Toll-like receptor 4 (TLR4), to inhibit innate immune signaling pathways [64]. In recent genomic studies, mutations or duplications in the G gene have been identified as having the potential to enhance these immune evasion mechanisms, thereby allowing the virus to survive in the host for extended periods [65]. Through the prefusion and post fusion conformations of the F protein, which can boost neutralizing antibody responses, the F protein indirectly contributes to immune evasion by changing the immunogenicity of the system [48].
Population-specific immune variability
Children have distinct immune response patterns to hMPV due to their underdeveloped immune systems, making them highly vulnerable to severe infections (Fig. 2). According to studies, hMPV infections in children frequently result in severe respiratory consequences such as bronchiolitis and pneumonia, as well as longer disease courses than in adults. For instance, studies have reported longer periods of viral shedding (averaging 10–14 days) and higher viral loads in respiratory secretions in children with hMPV [23]. This leads to more severe clinical symptoms, such as hypoxemia and respiratory distress, and higher hospitalization rates, especially in children under two [16]. Pediatric patients frequently have lower T-cell-mediated immunity, which causes delayed viral clearance, in contrast to adults who depend on stronger cytotoxic T-cell responses [66, 67]. This delay causes respiratory disorders by facilitating prolonged viral replication and raising the chance of co-infections. According to Pelletier et al. [68], 20% of children with hMPV infection needed intensive care unit (ICU) admission, highlighting the virus’s clinical burden in this susceptible population. hMPV virus loads in children have been measured in recent research using reverse transcription-polymerase chain reaction (RT-PCR), providing important new information. The hMPV viral loads were not directly associated with the duration of illness in 18 children hospitalized with lower respiratory tract infections; however, peak viral shedding occurred between 6- and 11-days post-infection [69]. The result implies that viral load influences the dynamics of virus transmission, but it could fail to be an accurate prediction of clinical outcomes.
Fig. 2.
Age-stratified risk factors and prevalence of human metapneumovirus (hMPV) infection. Three distinct life stages are shown: Early Life (infants and young children), characterized by immature immunity and prevalence rates of 5–20%; Middle Life (adolescents and adults), showing generally lower risk (5–10% prevalence) with increased susceptibility in chronic disease patients; and Late Life (elderly), demonstrating elevated risk (10–15% prevalence) due to immunosenescence and comorbidities. Co-infection patterns are indicated, with viral co-infections (RSV, influenza, rhinovirus, SARS-CoV-2) occurring in 10–30% of early life cases, and bacterial superinfections (Streptococcus pneumoniae, Haemophilus influenzae, Staphylococcus aureus) in 15–20% of cases, predominantly in older populations. The figure was created using BioRender
In the elderly, hMPV-induced respiratory infections are prevalent and more severe due to immunosenescence and pre-existing comorbidities (Fig. 2) [70]. Infection susceptibility, vaccine efficacy, and infectious illness mortality increase with age due to immunosenescence. Studies have shown that both innate and adaptive immune responses are functionally compromised in older adults. One example is the modulation of pro-inflammatory cytokine production, which frequently results in inflammation or chronic inflammation [71]. This condition of immunological failure has been associated with serious outcomes in viral infections, including hMPV, which is particularly concerning given its frequency in older people during respiratory virus seasons [72]. Multiple chronic diseases, including diabetes, hypertension, and COPD, are common among the elderly and can exacerbate immune dysfunction. Once these comorbidities are present, infections frequently provoke a hyper-inflammatory response, which may lead to severe clinical consequences [73]. Interferons (IFNs), especially type I IFNs (IFN-α and IFN-β), activate antiviral defenses. In older adults, type I IFN production and signaling are severely compromised, which results in inadequate and delayed viral clearance [74]. Improving outcomes in hMPV infections requires addressing weaknesses in IFN responses and antibody production in aged people. Administering recombinant IFN-λ or IFN-ε can improve viral clearance, reduce illness severity, and prevent excessive inflammation [75].
Immunocompromised patients have longer viral shedding, more severe illness, and higher mortality rates, in contrast to healthy individuals who typically experience self-limiting infections [76, 77]. Despite developments in understanding hMPV pathophysiology, a lack of targeted antiviral treatments and vaccines adds to the illness burden in vulnerable groups [78]. Impaired innate and adaptive immune responses are the cause of prolonged hMPV infections in immunocompromised patients. Viral loads persisted for up to nine days post-infection in studies employing immunosuppressed cotton rats (Sigmodon hispidus), which showed substantially higher pulmonary and nasal virus titers than immunocompetent animals [79]. Delayed pulmonary chemokine expression and histological alterations were found, indicating an alternative inflammatory response. Clinical research has also shown that immunocompromised patients frequently experience acute and organizing lung injury, which frequently necessitates extended hospital stays [78]. In hMPV-induced pulmonary disease, neutrophil infiltration and cytokine dysregulation play a crucial role. In immunosuppressed animals, higher levels of macrophage inflammatory protein-1 alpha (MIP-1α) and IFN-gamma-inducible protein-10 (IP-10) have been connected with delayed pathogenesis [79]. This dysregulated inflammatory response contributes to disease severity, highlighting the importance of immunomodulatory therapy. RSV, hMPV, and parainfluenza virus are major causes of severe respiratory illness that can lead to mortality or need extensive respiration support. A large retrospective study of hospitalized people found that hMPV infection increased the chance of getting serious illnesses (incidence rate ratio [IRR]: 1.82; 95% CI: 1.71–1.93; p < 0.001). Additionally, compared to patients without respiratory viral infections, hospital admissions were longer, averaging an additional 0.88 ± 0.28 days [80]. The use of corticosteroid medications and cytotoxic treatments increases vulnerability to acute viral respiratory infections. In children with cancer, these components lead to extended viral shedding and a rapid progression from upper to lower respiratory tract infections [77].
Clinical manifestations
In pediatric and adult populations, hMPV infections typically manifest as mild and self-limiting respiratory illnesses [81]. A detailed comparison of clinical manifestations across different age groups and risk categories is presented in Tables 2 and 3. These infections are often characterized by symptoms such as coryza, cough, and low-grade fever, which usually resolve spontaneously within a relatively short period [16]. However, the precise relationship between viral shedding dynamics and clinical disease severity remains incomplete due to limitations in current research methodologies, particularly those involving human participants. The challenges in monitoring viral load excretion and symptom intensity concurrently in human subjects lead to ongoing uncertainty in establishing a definitive correlation. Bronchiolitis, bronchitis, and pneumonia are the most common diagnoses for hMPV patients [82]. The common symptoms are almost nonspecific, including fever, cough, hypoxia, upper respiratory tract infection, lower respiratory tract infection, rhinorrhea, sore throat, and wheezing [83]. Fever usually lasts 10 days, with a peak during the illness. In hospitalized cases, the main manifestations are bronchiolitis and pneumonia [84].
Table 2.
Comparative outcomes and prognosis of hMPV with other respiratory viruses: At-glance
| Viruses | HMPV | RSV | Influenza A/B | SARS-CoV-2 | Parainfluenza | Rhinovirus/Enterovirus | Adenovirus |
|---|---|---|---|---|---|---|---|
| Outcome | |||||||
| Common symptoms | Cough, fever, nasal congestion, wheezing | Cough, fever, nasal congestion, wheezing | Fever, cough, sore throat, muscle aches, headache, fatigue | Fever, cough, shortness of breath, loss of taste/smell, fatigue | Cough, fever, nasal congestion, wheezing | Runny nose, sore throat, cough, sneezing, mild fever | Fever, sore throat, cough, red eyes, diarrhea |
| Average duration of fever (days) | 3–5 | 3–8 | 3–7 | 2–10 | 3–7 | 1–3 | 3–5 |
| Mortality rate | ~ 8–10% in hospitalized patients | ~ 8–10% in hospitalized patients | 0.1–2% (seasonal); up to 10% (pandemic) | 1–3% (original strain); varies by variant | < 5% in hospitalized patients | < 1% in hospitalized patients | < 5% in hospitalized patients |
| Morbidity (Severity) | Mild to moderate; severe in children and elderly | Mild to moderate; severe in children and elderly | Mild to severe; can lead to pneumonia | Mild to severe; can lead to ARDS | Mild; severe in immunocompromised | Mild; can lead to bronchitis in rare cases | Mild; can lead to pneumonia in rare cases |
| Hospitalization rate | Hospitalization in severe cases, especially in children and elderly | Hospitalization in severe cases, especially in children and elderly | Hospitalization in severe cases or in those with comorbidities | Hospitalization in severe cases or in those with comorbidities | Hospitalization in severe cases or in immunocompromised patients | Rarely requires hospitalization | Rarely requires hospitalization |
| Length of hospital stay (depends on severity) | Typically, 5–7 days | Typically, 5–7 days | Typically, 3–7 days | Typically, 7–14 days | Typically, 3–5 days | Typically, 2–4 days | Typically, 5–7 days |
| Co-infection risks | High risk of co-infection with other respiratory viruses | High risk of co-infection with other respiratory viruses | High risk of co-infection with bacterial pathogens or other viruses | High risk of co-infection with bacterial pathogens or other viruses | High risk of co-infection with other respiratory viruses | High risk of co-infection with other respiratory viruses | High risk of co-infection with bacterial pathogens or other viruses |
| Economic burden | Significant cost from hospitalization | Significant cost from hospitalization | Substantial cost associated with hospitalizations | Substantial cost associated with hospitalizations | Cost associated with treatment | Low cost, mostly outpatient treatment | Low cost, mostly outpatient treatment |
| Prognosis | |||||||
| Population most affected | Young children, elderly, and immunocompromised individuals | Young children, elderly, and immunocompromised individuals | All ages; elderly and those with comorbidities at higher risk | All ages; elderly and those with comorbidities at higher risk | Young children and immunocompromised individuals | Young children; generally mild in adults | Young children and immunocompromised individuals |
| Reinfection rate | Moderate; immunity wanes after initial infection | High; immunity wanes over time | Moderate; varies by strain and vaccination status | Moderate; immunity wanes over time | High; immunity wanes over time | Moderate; frequent in children | Moderate; immunity wanes over time |
| Complications | Pneumonia, bronchiolitis, secondary bacterial infections | Pneumonia, bronchiolitis, secondary bacterial infections | Pneumonia, myocarditis, exacerbation of chronic conditions | ARDS, organ failure, long-COVID syndrome | Pneumonia, bronchiolitis | Bronchitis, exacerbation of asthma | Pneumonia, hepatitis, or myocarditis |
| Preventive measures | No licensed vaccine; hygiene practices | Palivizumab (for high-risk infants), hygiene practices | Annual vaccination, hygiene practices | Vaccination, hygiene practices, masks, social distancing | No vaccine; hygiene practices | Hygiene practices; no specific vaccine | Hygiene practices; no specific vaccine |
| Treatment availability | Supportive care; no specific antiviral available | Supportive care; no specific antiviral available | Antivirals (oseltamivir, zanamivir); supportive care | Antivirals (e.g., remdesivir); supportive care | Supportive care; no specific antiviral available | Supportive care; no specific antiviral available | Supportive care; no specific antiviral available |
| Seasonal patterns | Peaks in late winter and spring | Peaks in late fall, winter, and early spring | Peaks in winter; varies with geographic location | Peaks in winter; pandemic waves vary seasonally | Peaks in late fall and early winter | Year-round; peaks in spring and fall | Year-round; peaks in spring and summer |
Table 3.
Age and risk group-based comparison of clinical manifestations for hMPV
| Age group | Typical presentations | Risk of delayed diagnosis | Severe presentations | ICU admissions rate | Complications |
|---|---|---|---|---|---|
| Community-Level Cases | |||||
| Children | Runny nose, cough, wheezing, fever | Moderate (symptoms resemble common cold) | Rare severe bronchiolitis | Low | Secondary bacterial infections1 |
| Adolescents | Sore throat, cough, mild fever | High (symptoms are often mild) | Rare severe respiratory distress | Very low | Asthma exacerbation |
| Adults | Persistent cough, fatigue, mild fever | High (non-specific symptoms) | Rare severe pneumonia | Very low | Chronic respiratory disease |
| Hospital-Based Cases | |||||
| Children | Severe cough, hypoxia, wheezing, retractions | Moderate (often misdiagnosed as RSV) | Bronchiolitis, respiratory failure | Moderate | Secondary bacterial infections |
| Adolescents | Persistent cough, fever, mild dyspnea | High (atypical presentations) | Severe pneumonia | Low | Reactive airway disease |
| Adults | High fever, dyspnea, chest pain | Moderate | Severe pneumonia | Moderate | ARDS2, sepsis |
| Elderly | Severe dyspnea, confusion, chest discomfort | High (non-specific symptoms) | Severe respiratory failure | High | Multi-organ dysfunction |
| High-Risk Groups | |||||
| Immunocompromised | Persistent fever, severe dyspnea, hypoxia | Very high (symptoms overlap with baseline disease) | Severe pneumonia, respiratory failure | High | Disseminated infection, sepsis |
| Elderly | Weakness, confusion, shortness of breath | High | Severe respiratory distress, ARDS | High | Exacerbation of chronic conditions |
| Specific groups | Depends on underlying condition (e.g., asthma, COPD3, heart disease) | Very high | Severe respiratory or cardiac decompensation | High | Cardiac arrest, secondary bacterial infections |
1. Secondary infections refer to infections that occur after the primary infection
2. Acute Respiratory Distress Syndrome
3. Chronic Obstructive Pulmonary Disease
Community-level cases
Children and adolescents
The hMPV exhibits a broad age distribution, indicating its presence across diverse demographic groups without restriction to any particular age cohorts. Nevertheless, the incidence of hMPV infection is notably heightened in pediatric and geriatric populations, signifying that both young children and older adults are disproportionately impacted by this virus relative to other age segments [85]. hMPV ranks as the second most prevalent etiological agent of LRTIs in young children, surpassed only by RSV in terms of frequency [86]. The heightened prevalence of hMPV as a causative factor in LRTIs within this vulnerable cohort underscores its critical implications for pediatric respiratory health. Therefore, it is imperative to consider hMPV as a prominent pathogen in the differential diagnoses of respiratory infections. The relatively frequent occurrences of hMPV-induced LRTIs illustrate the substantial burden this virus imposes on the health of young children and the broader healthcare systems.
A cohort study conducted in Finland examined 1,338 children under the age of 13 for viral detection upon presentation of respiratory infection symptoms. The findings indicated that the age-specific incidence of human metapneumovirus (hMPV) infection was notably highest, at 7.6%, in children younger than 2 years. Among those diagnosed with hMPV, 97% exhibited a cough, 90% presented with rhinitis, and 72% experienced fever, with a median duration of symptomatic illness recorded at 8 days. Wheezing was noted in 10% of the hMPV-infected cohort, while laryngitis was observed in 8%. The most prevalent complication associated with hMPV infection was acute otitis media (AOM), diagnosed via pneumatic otoscopy, which occurred in 61% of individuals under 3 years of age. Notably, none of these patients required referral to a hospital [87].
Additionally, a separate investigation aimed to elucidate data collected from children under the age of 14 diagnosed with community-acquired pneumonia (CAP). All participants presented with fever, characterized by an axillary temperature surpassing 38 °C, and exhibited signs and symptoms consistent with CAP. The results of the viral testing indicated that respiratory syncytial virus (RSV) was the most commonly detected pathogen in 188 children (31.7%), followed by rhinovirus (24.3%), bocavirus (10.1%), influenza viruses (9.6%), hMPV (8.2%), coronaviruses (5.6%), enterovirus (3.5%), adenovirus (1.8%), and parainfluenza viruses (1.8%). High-grade fever (≥ 39 °C) was diagnosed more frequently in hMPV-positive children rather than rhinovirus‐positive [88].
Among the various infection types in pediatric populations, the median temperature (IQR) observed in mild and severe cases is typically 36.8 (36.5, 37.2) degrees Celsius. Besides fever, additional clinical manifestations, such as cough and wheezing, are prevalent in mild and severe cases. Notably, patients with mild infections tend to exhibit a higher prevalence of concurrent cough and fever, whereas wheezing is reported with greater frequency among the severe cases [89]. The concurrence of fever, cough, and wheezing is particularly characteristic of patients with more severe presentations [90]. In pediatric patients under 18 years of age, CAP manifests with symptoms analogous to those found in adult populations, primarily cough and fever. However, younger patients frequently present with supplementary symptoms, including anorexia and dyspnea, which may signify a more protracted and complex clinical course. Furthermore, the study elucidated that respiratory virus constitute the predominant etiological agents of pneumonia within this demographic, thereby underscoring the significance of considering viral infections in the diagnostic and therapeutic approaches to pneumonia in pediatric patients [91].
A comprehensive study evaluating human metapneumovirus (hMPV) infection in both outpatient and inpatient populations established that the average age of affected patients was 11.7 years, with a standard deviation of 14.6 years. The research consistently identified specific symptoms—namely fever, cough, rhinorrhea, and dyspnea—across various healthcare settings, underscoring the prevalence of these manifestations as common indicators of hMPV infection, irrespective of whether individuals received outpatient care or required hospitalization [92]. The hMPV, like many other respiratory virus infections, is marked by a considerable number of asymptomatic cases, wherein infected individuals do not show any clinical symptoms despite harboring the virus. This characteristic of hMPV infection closely resembles other well-known respiratory pathogens, highlighting the challenges in implementing effective disease control and containment strategies. It emphasizes that the absence of symptoms in an individual does not necessarily indicate that they are not infected or potentially contagious [93].
Adults
Fever is identified as one of the most frequently occurring clinical symptoms in adult patients diagnosed with hMPV, as demonstrated in a study [94]. A broad spectrum of other symptoms often accompanies this finding, including myalgia, rhinorrhea, sneezing, cough, sputum production, sore throat, chills, headache, and muscle pain, all of which are commonly reported in the outpatient setting among those with hMPV [95].
The similarities with other respiratory viruses become evident when comparing these symptoms to those of RSV infection. Patients infected with RSV often present with a similar profile, including fever, cough, wheezing, dyspnea, rhinorrhea, and sputum production [96]. Therefore, clinicians must be attentive to the overlapping symptomatic presentations of hMPV and RSV infections, especially in adults seeking outpatient care.
Asymptomatic hMPV infection also occurs in adults. The percentage of asymptomatic infection is most significant in the young group compared to the healthy elderly group and the high-risk group [97].
Inpatient level cases
Children and adolescents
The primary reasons for hospitalization among children with hMPV infections are the development of acute lower respiratory tract illnesses, specifically bronchiolitis, and pneumonia [98]. Additionally, patients who have a coinfection with RSV tend to experience more severe illness, particularly children less than two years of age, resulting in a greater likelihood of admission to the intensive care unit and a 10-fold increase in the need for mechanical ventilation [9, 99]. However, additional evidence is required to substantiate this hypothesis. The findings underscore the considerable clinical implications of hMPV on pediatric respiratory health, as the virus frequently precipitates conditions that necessitate intensive medical intervention and inpatient care [100]. Age under six months and household crowding have been identified as risk factors for hospitalizations due to hMPV. Furthermore, in hospitalized patients, factors such as female sex, prematurity, and infection with genotype B are recognized as significant predictors of severe hMPV disease (Fig. 2) [101].
Some interventions in specific populations, such as immunization of HIV-infected children with pneumococcal vaccine, could decrease the prevalence of hMPV-associated lower respiratory tract illnesses [102]. Among hMPV-hospitalized children, fever was more frequently reported. Cough, wheezing, increased work of breathing, loss of appetite, and rhinorrhea have been reported in almost 80% of cases [101]. Other clinical manifestations are vomiting, diarrhea, otalgia, and cyanosis. Otitis, pharyngitis, and conjunctivitis are also reported [103]. Clinical characterizations of hMPV in a population-based prospective surveillance study revealed that fever was less common among children with HMPV infection than children with influenza. In contrast, symptoms like difficulty breathing and wheezing were more common in children with HMPV than influenza [104].
A recent study investigating the clinical manifestations of hMPV and RSV infections found that fever is more commonly observed in individuals infected with hMPV. Other mild symptoms, such as gastrointestinal (GI) issues and upper respiratory manifestations, were comparable between both hMPV and RSV cases. However, more severe complications, including seizures, chest recessions, and hypoxemia, were reported in both hMPV and RSV infections. Among children hospitalized with hMPV, the clinical phenotype indicating lower respiratory tract involvement was noted in 90.2% of cases, while this figure was slightly higher at 95% for RSV cases [96]. Rare presentations, including encephalitis, have been reported in children with upper respiratory tract hMPV disease [105]. The causal link between hMPV and these cases is unclear, as viral replication is understood to be mainly limited to the respiratory tract. Sepsis syndrome occurs less than in hMPV-infected children in comparison with other related viruses occurring in < 2% of cases [106].
Adults
Like other viral infections, hMPV can lead to serious complications that need admission to the hospital and, in severe cases, can lead to ICU admission due to requiring mechanical ventilation, hemodynamic management, and treatment of possible coinfection. In adults with acute respiratory tract infections (ARTIs), RSV and hMPV are the main suspicious [107].
In adults, hMPV primarily manifests as an influenza-like illness, often accompanied by signs of upper and/or acute lower respiratory tract infections, with a study revealing that the two most prevalent symptoms were dyspnea and cough; notably, the combination of fever, cough, and dyspnea was observed in 42% of patients, while additional symptoms included nasal congestion, sputum production, fatigue, wheezing, crackles, and reports of gastrointestinal issues [108]. The comparative clinical presentations and outcomes of hMPV versus other respiratory viruses are summarized in Table 2. In another study, symptoms of hospitalized adults included shortness of breath, cough, fever, and chest pain [109]. Moreover, the rates of ICU admission, mechanical ventilation, hospital length of stay, and ICU duration for adults requiring hospitalization due to hMPV were found to be comparable to those associated with RSV and influenza infections [108]. The clinical manifestations of influenza and hMPV in hospitalized adults are generally similar, and the numbers of patients with symptoms like fever, cough, and myalgia are comparable. Weakness, headache, and sore throat are primarily presented in influenza infection [108]. The average duration of symptoms prior to hospitalization was longer for infections caused by RSV and hMPV compared to those caused by influenza. Patients with hMPV tended to be older than those with influenza and were more likely to present with acute heart failure during their hospital stay; however, the rates of respiratory and cardiovascular complications were comparable in both groups [110, 111]. During the SARS-CoV-2 pandemic in China, a case was reported involving an adult with hMPV infection whose respiratory symptoms closely mimicked those of COVID-19 [112]. Furthermore, evidence indicates that smoking may not be a significant risk factor for hMPV infection among hospitalized adults [109].
Special population
The hMPV infections in some groups of patients with underlying conditions could be more complicated. Here, we summarize data that indicates considerable significance in these populations. Table 3 provides a comprehensive overview of clinical manifestations across different age groups and high-risk populations.
Adults with chronic cardiopulmonary disease
Respiratory virus infections are a serious trigger for COPD exacerbations. These patients have more symptomatic disease in contrast with healthy populations and are also more likely to use medical care services. Studies also showed that hMPV in hospitalized patients due to COPD or asthma exacerbation was frequent [16, 113, 114].
Immunocompromised patients
The incidence of morbidity and mortality is elevated among immunocompromised patients, whose clinical trajectories are often prolonged, with respiratory failure occurring more frequently and presenting commonly with symptoms such as fever, cough, and rhinorrhea, especially in immunocompromised children [4, 5, 115]. A major complication in this population is severe lymphopenia (defined as < 1000 lymphocytes/mL), alongside elevated liver enzyme levels, and respiratory failure also significantly contributes to mortality, with bacterial or fungal co-infections identified as the primary risk factor for death [5]. In a cohort study, hMPV was detected in six patients. Four of them were immunocompromised, primarily due to lung transplantation. However, the rate of hMPV detection was comparable with the RSV detection [116]. Regarding children with HIV infection, a study in South Africa indicated that the incidence of hMPV-associated hospitalization was higher in HIV-infected subjects than in HIV-uninfected [117]. In a survey of immunocompromised hMPV-infected children, 23% of patients required intensive care unit admission and/or supplemental oxygen and were more likely to be neutropenic [66].
Patients with malignancy were also more likely to be admitted to ICU level care and had a mortality rate of hMPV infection [118]. The burden of hMPV infection was higher in children and adults with cancer, especially patients with hematologic malignancies [119]. The hMPV infection involving the upper and lower respiratory tract was common in adults with hematologic malignancies. Respiratory coinfections included five cases of pseudomonal pneumonia and six cases of proven invasive fungal infection. Other complications associated with oxygen requirement without any significant effect on mortality [120]. Following hematopoietic stem cell transplantation (HCT), severe hMPV infections can also occur, especially with the pattern of progression from upper to lower respiratory tract disease with the risk factors including systemic glucocorticoid use and low lymphocyte counts [35]. In a study that evaluated adults following HCT, nasopharyngeal hMPV infection was persistent in most participants for months, although they were asymptomatic [121]. Lung transplant patients are also more susceptible to the hMPV complex form of infection based on a study that a third of hMPV-infected lung transplant cases presented chronic lung allograft dysfunction (CLAD) progression in the first year, with lack of early lung function recovery predicting long-term CLAD progression [122].
Role of co-infections and comorbidities
Co-infection of hMPV with other respiratory pathogens, such as RSV, bocavirus, rhinovirus or enterovirus, parainfluenza virus, coronavirus, influenza A, influenza B, and influenza has been shown in several investigations (Fig. 2) [83, 123]. Studies have also revealed the superinfection of hMPV with bacterial pathogens such as Streptococcus pneumoniae, Mycoplasma pneumoniae, and Chlamydia pneumoniae [83]. Co-infections with bacteria, including Streptococcus pneumoniae or Staphylococcus aureus, complicate outcomes by leading to secondary bacterial pneumonia or sepsis. It is noteworthy that more than 50% of secondary infections involve the lower respiratory tract, often occurring concurrently with primary infections [124, 125]. While several studies have indicated an association between co-infections and an elevated rate of ICU admissions, as well as prolonged hospital stays, other investigations have demonstrated no significant correlation between co-infection and the severity of the disease [83, 126].
Co-infections significantly worsen the clinical progression of hMPV infections, particularly when they occur alongside RSV, influenza, or bacterial pathogens, as co-infection with RSV can lead to serious complications in the lower respiratory tract, often resulting in ICU admissions due to increased inflammation and airway injury, especially in children under the age of one who are most likely to contract these viruses. According to Nair et al. [127], RSV can cause serious lower respiratory tract diseases in children, such as bronchiolitis, bronchospasm, pneumonia, and acute respiratory failure. Children, particularly those presenting with upper or lower respiratory tract infections as well as asymptomatic cases, are primarily affected by human metapneumovirus (hMPV), with acute bronchiolitis and pneumonia being the most prevalent conditions leading to hospitalization in this population [128]. However, immunocompromised persons and the elderly are also at risk. 5 to 10% of hospitalizations for children with ARIs are due to hMPV [129, 130]. In children under two years old infected with RSV, co-infection with hMPV is associated with severe RSV bronchiolitis and a heightened probability of necessitating ICU hospitalization [83]. The clinical progression of hMPV closely resembles that of RSV infection, with bronchiolitis, pneumonia, and asthma exacerbations being the principal symptoms [131]. The incidence of severe hMPV infection in children requiring hospitalization in the ICU or respiratory support is relatively low. Infants with co-infection of both RSV and hMPV face a tenfold increased risk of needing mechanical ventilation compared to those with isolated RSV infections [83].
Co-infection with influenza, although less common, can exacerbate respiratory symptoms and increase the risk of complications, such as Acute Respiratory Distress Syndrome (ARDS) [132]. Several factors heighten the risk of severe human metapneumovirus (hMPV) infection, including premature birth, young age, a history of healthcare-associated infections, and pre-existing lung, cardiac, or neurological conditions. A recent investigation into an hMPV outbreak in two skilled nursing facilities reported an 11% fatality rate. Hospitalization rates for hMPV infection are comparable to the combined rates of influenza and parainfluenza viruses 1, 2, and 3. This underscores the critical need for advancing our understanding of hMPV pathogenesis and ongoing vaccine research [133, 134].
Comorbidities significantly influence the severity of hMPV infections; conditions such as asthma and COPD can exacerbate symptoms and lead to repeated hospitalizations, while cardiovascular issues like heart failure may further increase respiratory discomfort [135]. Moreover, immunocompromised patients, including those with HIV or cancer, are more susceptible to severe and prolonged infections, often complicated by secondary bacterial or fungal infections [78]. Additionally, metabolic disorders such as diabetes and obesity compromise immune function, hindering viral clearance and exacerbating clinical outcomes, whereas neurological abnormalities increase the risk of respiratory complications and aspiration pneumonia [16, 136].
Underlying conditions such as COPD, asthma, and cardiovascular diseases have a profound impact on immune responses, often increasing the susceptibility to and severity of respiratory infections like hMPV [137]. COPD impairs pathogen protection by disrupting mucociliary clearance, altering the balance of cytokines, and leading to chronic airway inflammation and structural damage [138]. In patients with asthma, heightened Th2-driven immune responses result in hyperresponsive airways and increased inflammation, which can delay the clearance of viruses and exacerbate symptoms [139]. Furthermore, heart failure and other cardiovascular diseases elevate the risk of severe outcomes by inducing systemic inflammation and diminishing immune cell activation due to poor blood perfusion and endothelial dysfunction [137]. Together, these altered immune conditions contribute to slower healing, accelerated disease progression, and a greater likelihood of recurrent infections.
Diagnostic strategies
ARIs, which affect both the upper and lower respiratory systems, are one of the leading causes of mortality and morbidity globally, responsible for approximately 4.25 million deaths each year. Although infection rates declined during 2021–2022, a rising trend was noted in 2022–2023. These infections set a considerable burden on healthcare resources [3]. A range of viral pathogens is responsible for ARIs across various age groups, with notable contributors including hMPV, rhinovirus, adenovirus, RSV, influenza, parainfluenza virus, and SARS-CoV-2 [80, 124, 140–143].
In recent years, advancements in diagnostic measurements, including molecular assays, cell cultures, and antigen-based tests, have reached their pinnacle in accuracy and rapidity [144]. Currently, nucleic-acid-based techniques (NAAT), including polymerase chain reactions (PCRs), are one of the major diagnostic approaches, replacing traditional methods, including cell cultures and immunofluorescence assay (IFA) [145, 146]. Molecular assay techniques encompass a range of PCR-based methods, including nested PCR, multiplex PCR, real-time PCR (rtPCR), reverse transcriptase PCR (RT-PCR), loop-mediated isothermal amplification (LAMP), and DNA microarrays [147]. Notably, multiplex RT-PCR is favored for its high sensitivity, specificity, and convenience, making it the standard tool for detecting multiple viral pathogens. This process involves the reverse transcription of RNA to DNA, followed by amplification through PCR after the extraction and purification of nucleic acids [148].
Influenza virus diagnosis includes a combination of clinical judgment and laboratory diagnostic tools. These techniques include viral culture, enzyme-linked immunosorbent (ELISA)‐based, and molecular-based tests [149]. Although culture is the gold standard diagnostic measurement, its time-consuming nature and the need for diagnostic tools with higher accuracy have shifted the use of molecular assay tests in clinical practice [150]. These methods, including rapid tests (RIDT) and NAATs, have shown promising results compared to RIDTs and comprise various methods, including RT-PCR, LAMP, and DNA-microarray and sequencing tests [149, 151], commonly used for the detection of HMPV, HPIV, RSV, SARSCoV-2, adenovirus, and rhinovirus. Despite the development of NAATs, traditional approaches, including cell culture, are still in practice as recently a novel ELISA method has been developed that detects RSV in a short period, with a sensitivity and specificity of 94% and 96.8%, which has attracted the attention of resource-limited countries due to its cost-effectiveness [152]. RSV detection tests include viral culture, RADT, direct fluorescent antibody (DFA), and RT-PCR [152–154]. Similarly, NAATs are widely used for HMPV detection, viral culture, and IFA [83]. Although shell viral culture is currently the gold standard diagnostic approach, RT-PCR and multiplex RT-PCR, two novel NAATs, are the most common approaches for HMPV detection [143]. Additionally, IFA, including DFA and ELISAs, are used alongside shell viral culture to detect viral antigens, enhancing specificity and sensitivity. Moreover, previous methods include serological assays, which are now less frequent due to the better performance of other methods [143]. SARSCoV-2 is detected mainly via NAATs, including RT-PCR, reverse transcription LAMP (RT-LAMP), real-time RT-LAMP (rRT-LAMP), and real-time RT-PCR (rRT-PCR) [155–158]. Adenovirus diagnostic methods are similar to those previously discussed. Traditional measurements include viral culture, neutralization test (NT), which is based on the detection of the hexon protein’s antigenic determinant, indirect ELISA, latex agglutination test (LAT), and enzyme immunoassay (EIA) [159]. However, the robust reliability of molecular-based assays, including rtPCR, has outperformed other methods [160]. IFA, ELISA, complement fixation test (CFT), hemagglutination inhibition test (HIT), and rRT-PCR are standard diagnostic methods for rhinovirus, with the latter accounting for the majority due to its reliability, rapidness, high specificity, and sensitivity [161, 162]. Parainfluenza virus diagnostic tests in clinical follow the same pattern, including traditional viral culture and IFA, as well as more advanced and novel molecular assays (PCR) with superior sensitivity and specificity [140, 163].
When comparing the diagnostic precision of various methods for hMPV and other viral pathogens, RT-PCR for hMPV demonstrates a sensitivity of 95–100% and a specificity of 100%, similar to the results in RSV and influenza RT-PCR sensitivity and specificities [164]. Moreover, one study reported ELISA superiority regarding sensitivity for RSV compared to hMPV (88% vs. 83%). Moreover, ELISA specificity was the highest for adenovirus (100%) compared to hMPV (92%) and RSV (88%) [165].
NAATs are the preferred tests in clinical practice with increasing accessibility. However, their complexity, limited equipment, poor infrastructure, high costs, and the need for professional laboratory technicians impede their accessibility in resource-limited countries [166, 167]. This limitation was well observed during the COVID-19 pandemic, where the costliness of RT-PCR due to the need for thermocyclers impeded adequate and timely testing in these countries, leading to substantial mortality and morbidity. Moreover, the absence of primary resources for developing accurate diagnostic tools complicates this problem in resource-challenged settings. These include the lack of centrifugation machines required for serum tests and trained professionals for viral genome detection [168, 169]. Additionally, technical complexity and the need for multiple primer pairs optimization to avoid cross-reactivity and -contamination, which requires trained personnel, the required multiple stages of amplification and detection, lack of a proper supply chain infrastructure, and technical troubleshooting support often unavailable in limited-resources settings, and limited available equipment including thermocyclers and fluorescence detectors have hindered their broad administration in many low- and middle-income countries [170, 171].
Thus, resource-limited countries rely heavily on traditional measurements, including ELISA and IFA [172]. The implications of these limitations are significant, as they underscore the urgent need for more affordable and accessible diagnostic tools in these regions.
Management and therapeutic approaches
Supportive care
Oxygen therapy remains a cornerstone treatment for patients with severe hMPV infection, particularly when hypoxemia is present. This intervention is especially critical for high-risk groups, including children under five, older adults, and those with underlying comorbidities [120, 173, 174]. Proper fluid management is essential to prevent dehydration, especially in pediatric and elderly patients who are susceptible to fluid imbalances due to fever, tachypnea, and reduced oral intake [120, 174].
The balance of electrolyte replacement and careful monitoring of fluid overload is necessary across all age groups, given that viral infections can trigger systemic inflammation. Patients exhibiting significant respiratory distress may demonstrate elevated work of breathing, necessitating an individualized approach to fluid therapy. Clinical judgment regarding fluid volume, rate, and type (oral vs. IV) is paramount [174].
Symptomatic management forms another cornerstone of supportive care. Antipyretics such as acetaminophen (paracetamol) and occasionally ibuprofen help alleviate fever, reduce discomfort, and improve overall well-being. For individuals who exhibit signs of respiratory failure or significant work of breathing, both non-invasive ventilation (CPAP, BiPAP) and invasive mechanical ventilation may be necessary [173, 174].
Emerging antiviral options
Currently, there are no hMPV-specific antivirals licensed for routine clinical use [174]. The primary treatments remain supportive, particularly for hospitalized infants and children, focusing on oxygen supplementation and intravenous hydration. While bronchodilators and corticosteroids are used empirically, no data substantiate their efficacy [100].
Ribavirin, a nucleoside analogue, has been extensively explored for hMPV treatment. It functions by disrupting viral purine metabolism and inhibiting viral RNA polymerase, while also demonstrating immunomodulatory effects through up-regulation of CD4 and CD8 T lymphocyte-derived cytokines and down-regulation of Th2 cytokines like IL-10 [175]. However, evidence of its clinical benefit remains inconclusive [120]. The various administration routes present distinct challenges: aerosolized delivery raises concerns about high costs, teratogenicity risks for healthcare workers, and potential respiratory function deterioration; oral administration faces bioavailability issues; and intravenous administration shows limited and conflicting outcomes. Notable success has been reported when combining ribavirin with IVIG in immunocompromised patients, particularly lung transplant recipients and those with hematological conditions [100, 176].
Research into monoclonal antibodies (mAbs) offers promising avenues for treatment. Several candidates target the fusion (F) protein of hMPV, aiming to block viral entry and replication within respiratory epithelial cells [174, 177]. Significant developments include MAb 338, which demonstrates effectiveness against all four hMPV subtypes, and MPE8, which shows potential for cross-neutralization with RSV. The 54G10 antibody has also demonstrated substantial neutralizing capacity in experimental models [175, 176]. Investigations into palivizumab—originally designed for RSV—have suggested some cross-reactivity, although its efficacy against hMPV remains limited [174, 177]. In immunocompromised populations, monoclonal antibodies may be deployed prophylactically to prevent severe complications, echoing strategies used for RSV in high-risk neonates. However, larger-scale clinical trials are needed to confirm their safety and efficacy for hMPV [120, 174].
Novel therapeutic approaches under investigation include fusion inhibitors, which impede viral entry into host cells, and RNA-based technologies. The HRA2 peptide, derived from heptad repeat domains of the hMPV F protein, has shown promising results in reducing viral load and inflammation in animal models. Small interfering RNAs, particularly siRNA45 targeting the nucleoprotein and siRNA60 targeting the phosphoprotein, have demonstrated potent antiviral activity without inducing cytokines or off-target effects [176]. These developments align with broader investigations focusing on inhibiting viral polymerases and preventing the fusion process by stabilizing prefusion F protein structures [178–180].
The integration of advanced structural biology techniques, including cryo-electron microscopy, continues to refine drug design by revealing key vulnerability sites in hMPV’s protein structures. Accelerating this pipeline from bench to bedside requires collaborations between academia, pharmaceutical companies, and public health agencies. While funding remains a challenge due to smaller market incentives compared to established pathogens like influenza, the burden of hMPV hospitalizations underscores the urgent need for targeted therapies [178–180].
Public health implications and prevention strategies
Role of hygiene, masking, and public education
Infection prevention relies on comprehensive public health measures, with consistent hand hygiene—via soap and water or alcohol-based sanitizers—being essential for reducing transmission. Proper respiratory etiquette, such as covering the mouth and nose or coughing/sneezing into the elbow, further limits droplet dissemination. Despite their apparent simplicity, these preventive measures are both cost-effective and profoundly impactful in hospital and community environments [181].
Lessons from recent respiratory virus outbreaks, particularly the COVID-19 pandemic, highlight that masking and physical distancing can significantly reduce transmission. Universal masking in healthcare settings, nursing homes, and schools has proven particularly effective during seasonal outbreaks. Mask usage, especially by symptomatic individuals, reduces the release of infectious droplets, while physical distancing decreases person-to-person contact [182].
The success of community-level interventions heavily depends on public education campaigns. By promoting early symptom recognition, hand hygiene, and appropriate mask-wearing, these programs encourage timely self-isolation, reducing chain transmission. Behavioral interventions, such as staying home when ill, further limit virus spread [183]. These strategies can be optimized for different demographics by adapting successful approaches from influenza and RSV campaigns.
Potential for vaccination and diagnostic development
An effective vaccine would be a significant step toward controlling hMPV, with multiple vaccine platforms under active investigation. Traditional formalin-inactivated vaccine approaches have proven problematic, showing enhanced disease severity upon challenge. However, novel approaches using nano emulsion-based inactivation have demonstrated better safety profiles. Live attenuated vaccines, generated through gene deletion or temperature-sensitive mutations, have shown encouraging results in animal models, with particular success seen in deletions of the G, SH, and M2-2 genes [175, 176].
Many candidates target the prefusion conformation of the F protein, leveraging improvements in immunogenicity—a strategy that proved successful in RSV vaccine research. Subunit vaccines, a category of vaccines including specific viral pathogens rather than the entire viral pathogen, focusing on the F protein have demonstrated potential, utilizing various delivery systems including viral vectors and bacterial expression systems. Virus-like particles represent another promising approach, demonstrating strong humoral immune responses against both homologous and heterologous strains. These particles effectively mimic viral structure while avoiding the risks associated with live virus vaccines [176, 184].
The genetic diversity of hMPV (genotypes A and B) poses a challenge, making cross-protection critical. Some investigators explore multivalent approaches that combine hMPV and RSV antigens to broaden prophylactic coverage. Though phase 1 results from certain candidates are promising, more expansive clinical trials are needed for validation, particularly in infants, the elderly, and immunocompromised individuals [177, 180, 185].
Better diagnostic tools have markedly improved hMPV surveillance. Multiplex PCR and next-generation sequencing (NGS) allow simultaneous identification of hMPV and co-circulating respiratory pathogens (e.g., RSV, influenza, parainfluenza, rhinovirus). Rapid, accurate testing guides patient triage and isolation protocols, reducing hospital-acquired infections. These advances also support real-time epidemiological assessments, as health authorities track which pathogens dominate in local outbreaks and tailor interventions accordingly [176, 186].
Strengthening surveillance systems with comprehensive panels
Advanced multiplex PCR panels and NGS platforms enable simultaneous detection of hMPV alongside other respiratory pathogens. These technologies support real-time epidemiological assessments and allow health authorities to track pathogen prevalence patterns in local outbreaks. Investing in high-throughput laboratories capable of multiplex respiratory panels is integral to robust hMPV surveillance. Automated platforms shorten turnaround times, allowing alerts about hMPV surges to be broadcast in near real-time [186–188].
Integration of laboratory networks and data-sharing platforms enables rapid detection of outbreak patterns and facilitates timely implementation of control measures. This systematic approach supports better resource allocation, from antiviral stockpiles to intensive care unit capacity management. Implementing such capacity in areas with historically limited diagnostics expands outbreak detection and ensures early interventions, particularly for vulnerable populations [181, 189].
Effective communication among hospitals, clinics, and public health bodies enables real-time data sharing of laboratory results and clinical outcomes. Healthcare systems can rapidly detect anomalies—such as spikes in hMPV-positive tests—and implement prompt control measures through this coordinated approach [181, 185].
Ultimately, hMPV management hinges on supportive policies and targeted resources. Governments should prioritize funding for research on hMPV vaccines, drawing from RSV and influenza modeling. Expanding diagnostic capabilities—especially in under-resourced areas—ensures equitable access to rapid, accurate testing. Sustained public health campaigns can bolster preventive measures like hygiene practices. Incorporating hMPV preparedness into broader pandemic frameworks acknowledges its significant contribution to pediatric and geriatric morbidity. Guiding principles for infection control, antibiotic stewardship, and immunomodulatory use will further refine clinical standards of care [120, 174, 181].
Conclusion
Human metapneumovirus (hMPV) is a significant respiratory pathogen with profound global health implications. Its complex transmission dynamics, influenced by environmental and host factors, particularly affect vulnerable populations such as young children, the elderly, and immunocompromised individuals. The overlap of hMPV’s clinical manifestations with those of other respiratory viruses, notably respiratory syncytial virus (RSV) and influenza, alongside a lack of specific antiviral treatments and licensed vaccines, poses substantial challenges for healthcare providers. The resurgence of hMPV transmission following the easing of COVID-19 restrictions further emphasizes the need for robust surveillance and effective containment strategies.
Advancing hMPV management necessitates focused efforts in several key areas. These include developing antiviral therapeutics targeting viral proteins, especially the fusion (F) protein, and creating effective vaccines that offer broad protection against diverse genotypes. Thus, future research should focus on identifying immune responses involved in the hMPV infectious process, developing antiviral treatments that target viral proteins, and inventing vaccines that provide cross-protection given the diverse hMPV strains, with special attention given to immunocompromised individuals and younger patients due to both the longer viral shedding period and disease severity.
Additionally, implementing rapid and cost-effective diagnostic tools for resource-limited settings and establishing comprehensive surveillance networks to monitor viral evolution and outbreak patterns are essential. Given the evolving landscape of respiratory viral infections, international collaboration is critical in addressing these challenges, thereby reducing the global burden of hMPV and enhancing preparedness for future emerging infectious diseases.
Acknowledgements
N/A.
Abbreviations
- ALRI
Acute lower respiratory infection
- ARDS
Acute respiratory distress syndrome
- ARIs
Acute respiratory infections
- AGPs
Aerosol-generating medical procedures
- AOM
Acute otitis media
- CAP
Community-acquired pneumonia
- CDC
Centers for disease control and prevention
- CFT
Complement fixation test
- CLAD
Chronic lung allograft dysfunction
- COPD
Chronic obstructive pulmonary disease
- CPAP
Continuous positive airway pressure
- DFA
Direct fluorescent antibody
- EIA
Enzyme immunoassay
- ELISA
Enzyme-linked immunosorbent assay
- GI
Gastrointestinal
- HCT
Hematopoietic cell transplantation
- HCWs
Healthcare workers
- HEPA
High-efficiency particulate air
- HIT
Hemagglutination inhibition test
- hMPV
Human metapneumovirus
- ICU
Intensive care unit
- IFA
Immunofluorescence assay
- IFN
Interferon
- IP-10
Interferon-gamma-inducible Protein-10
- IQR
Interquartile range
- IRR
Incidence rate ratio
- LAMP
Loop-mediated isothermal amplification
- LAT
Latex agglutination test
- LRTIs
Lower respiratory tract infections
- mAbs
Monoclonal antibodies
- MIP-1α
Macrophage inflammatory protein-1 alpha
- NAAT
Nucleic-acid-based techniques
- NGS
Next-generation sequencing
- NT
Neutralization test
- PCR
Polymerase chain reaction
- PPE
Personal protective equipment
- RADT
Rapid antigen detection test
- RIDT
Rapid influenza diagnostic test
- RNA
Ribonucleic acid
- rRT-LAMP
Real-time reverse transcription LAMP
- rRT-PCR
Real-time reverse transcription PCR
- RSV
Respiratory syncytial virus
- RT-LAMP
Reverse transcription LAMP
- RT-PCR
Reverse transcription polymerase chain reaction
- rtPCR
Real-time PCR
- SARS
Severe acute respiratory syndrome
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- SH
Small hydrophobic (protein)
- TLR4
Toll-like receptor 4
- TMPRSS2
Transmembrane serine protease 2
- VLP
Virus-like particle
- WHO
World health organization
Author contributions
M.Sh.: Conceptualization, Data curation, Investigation, Methodology, Supervision, Validation, Writing - Original draft, Writing - Review & editing, K.M., S.F.: These authors contributed equally (†), K.M.: Data curation, Investigation, Methodology, Writing - Original draft, Writing - Review & editing, S.F.: Data curation, Investigation, Methodology, Writing - Original draft, Writing - Review & editing, Sh.Y.: Data curation, Investigation, Methodology, Writing - Original draft, Writing - Review & editing, Z.V.: Data curation, Investigation, Methodology, Writing - Original draft, Writing - Review & editing, E.R.: Data curation, Investigation, Methodology, Writing - Original draft, Writing - Review & editing, M.G.: Data curation, Investigation, Methodology, Writing - Original draft, Writing - Review & editing, R.S.: Data curation, Investigation, Methodology, Writing - Original draft, Writing - Review & editing, B.K.: Data curation, Investigation, Methodology, Writing - Original draft, Writing - Review & editing, M.R.S. and A.S.: Data curation, Investigation, Methodology, Writing - Original draft, Writing - Review & editing. All authors critically reviewed and approved the final version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Ethical approval was not required for this review.
Consent for publication
Informed consent was not required for this study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Keyhan Mohammadi and Samireh Faramarzi Contributed Equally as Co-first Authors.
References
- 1.Zhou F, Wang Y, Liu Y, Liu X, Gu L, Zhang X, Pu Z, Yang G, Liu B, Nie Q. Disease severity and clinical outcomes of community-acquired pneumonia caused by non-influenza respiratory viruses in adults: a multicentre prospective registry study from the CAP-China network. Eur Respir J 2019;54. [DOI] [PubMed]
- 2.Satapathy T, Satapathy A, Satapathy A, Yadav N, Chandrakar M, Chandrakar K. Recent outbreaks of human metapneumovirus (HMPV): prevention, diagnosis and therapeutic insights. J Drug Delivery Ther 2025;15.
- 3.Cui C, Timbrook TT, Polacek C, Heins Z, Rosenthal NA. Disease burden and high-risk populations for complications in patients with acute respiratory infections: a scoping review. Front Med 2024;11. [DOI] [PMC free article] [PubMed]
- 4.Martinez-Rodriguez C, Banos-Lara MDR. HMPV in immunocompromised patients: frequency and severity in pediatric oncology patients. Pathogens 2020;9. [DOI] [PMC free article] [PubMed]
- 5.Scheuerman O, Barkai G, Mandelboim M, Mishali H, Chodick G, Levy I. Human metapneumovirus (hMPV) infection in immunocompromised children. J Clin Virol. 2016;83:12–6. [DOI] [PubMed] [Google Scholar]
- 6.Wang X, Li Y, Shi T, Bont LJ, Chu HY, Zar HJ, Wahi-Singh B, Ma Y, Cong B, Sharland E. Global disease burden of and risk factors for acute lower respiratory infections caused by respiratory syncytial virus in preterm infants and young children in 2019: a systematic review and meta-analysis of aggregated and individual participant data. Lancet 2024.
- 7.Bender RG, Sirota SB, Swetschinski LR, Dominguez R-MV, Novotney A, Wool EE, Ikuta KS, Vongpradith A, Rogowski ELB, Doxey M. Global, regional, and national incidence and mortality burden of non-COVID-19 lower respiratory infections and aetiologies, 1990–2021: a systematic analysis from the Global Burden of Disease Study 2021. The Lancet Infectious Diseases 2024. [DOI] [PMC free article] [PubMed]
- 8.Trends of Acute Respiratory Infection. Including human metapneumovirus (hMPV). Disease outbreak news (DON), 6 January 2025. World Health Organization, [https://www.who.int/emergencies/disease-outbreak-news/item/2025-DON550.].
- 9.Kuang L, Xu T, Wang C, Xie J, Zhang Y, Guo M, Liang Z, Zhu B. Changes in the epidemiological patterns of respiratory syncytial virus and human metapneumovirus infection among pediatric patients and their correlation with severe cases: a long-term retrospective study. Front Cell Infect Microbiol. 2024;14:1435294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barrera-Badillo G, Olivares-Flores B, Ruiz-López A, Fierro-Valdez M, Gutiérrez-Vargas RI, López-Martínez I. Human metapneumovirus: etiological agent of severe acute respiratory infections in hospitalized and deceased patients with a negative diagnosis of influenza. Pathogens 2020;9. [DOI] [PMC free article] [PubMed]
- 11.Zwaans WA, Mallia P, van Winden ME, Rohde GG. The relevance of respiratory viral infections in the exacerbations of chronic obstructive pulmonary disease—a systematic review. J Clin Virol. 2014;61:181–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rudd PA, Thomas BJ, Zaid A, MacDonald M, Kan OK, Rolph MS, Soorneedi AR, Bardin PG, Mahalingam S. Role of human metapneumovirus and respiratory syncytial virus in asthma exacerbations: where are we now? Clin Sci (Lond). 2017;131:1713–21. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Chen Z, Yan YD, Guo H, Chu C, Liu J, Ding Y, Shao X, Xu J, Ji W. Seasonal distribution and epidemiological characteristics of human metapneumovirus infections in pediatric inpatients in Southeast China. Arch Virol. 2013;158:417–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnott A, Vong S, Sek M, Naughtin M, Beauté J, Rith S, Guillard B, Deubel V, Buchy P. Genetic variability of human metapneumovirus amongst an all ages population in Cambodia between 2007 and 2009. Infect Genet Evol. 2013;15:43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lefebvre A, Manoha C, Bour JB, Abbas R, Fournel I, Tiv M, Pothier P, Astruc K. Aho-Glélé LS: human metapneumovirus in patients hospitalized with acute respiratory infections: A meta-analysis. J Clin Virol. 2016;81:68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uddin S, Thomas M. Human metapneumovirus. StatPearls. Treasure Island (FL): StatPearls publishing copyright © 2025. StatPearls Publishing LLC.; 2025. [PubMed]
- 17.Centers for Disease Control and Prevention (CDC). (2023, December 19). About Human Metapneumovirus. https://www.cdc.gov/human-metapneumovirus/about/index.html
- 18.Aberle JH, Aberle SW, Redlberger-Fritz M, Sandhofer MJ, Popow-Kraupp T. Human metapneumovirus subgroup changes and seasonality during epidemics. Pediatr Infect Dis J. 2010;29:1016–8. [DOI] [PubMed] [Google Scholar]
- 19.He Y, Liu WJ, Jia N, Richardson S, Huang C. Viral respiratory infections in a rapidly changing climate: the need to prepare for the next pandemic. EBioMedicine. 2023;93:104593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papenburg J, Hamelin ME, Ouhoummane N, Carbonneau J, Ouakki M, Raymond F, Robitaille L, Corbeil J, Caouette G, Frenette L, De Serres G, Boivin G. Comparison of risk factors for human metapneumovirus and respiratory syncytial virus disease severity in young children. J Infect Dis. 2012;206:178–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lucar J, Navalkele B, Becker BP, Reed CD, Parham J. Health care personnel exposure to a patient with asymptomatic SARS-CoV2 infection during a prolonged surgical intervention. Am J Infect Control. 2020;48:955–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akingbola A, Adegbesan A, TundeAlao S, Adewole O, Ayikoru C, Benson AE, Shekoni M, Chuku J. Human metapneumovirus: an emerging respiratory pathogen and the urgent need for improved diagnostics, surveillance, and vaccine development. Infect Dis. 2025;57:304–10. [DOI] [PubMed] [Google Scholar]
- 23.Schuster JE, Williams JV. Human metapneumovirus. Pediatr Rev. 2013;34:558–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schüz ML, Dallmeyer L, Fragkou PC, Omony J, Krumbein H, Hünerbein BL, Skevaki C. Global prevalence of respiratory virus infections in adults and adolescents during the COVID-19 pandemic: A systematic review and meta-analysis. Int J Infect Dis 2023. [DOI] [PubMed]
- 25.Bell C, He C, Norton D, Goss M, Chen G, Temte J. Household transmission of human metapneumovirus and seasonal coronavirus. Epidemiol Infect. 2024;152:e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lormeau B, Foulongne V, Baccino E, Adriansen A, Pidoux O, Prodhomme O, Haquet A, Guyon G, Jeziorski E. Epidemiological survey in a day care center following toddler sudden death due to human metapneumovirus infection. Arch Pediatr. 2019;26:479–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sarkar J. To test or not to test? A new behavioral epidemiology framework for COVID-19. PLoS ONE. 2024;19:e0309423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsuzaki Y, Itagaki T, Ikeda T, Aoki Y, Abiko C, Mizuta K. Human metapneumovirus infection among family members. Epidemiol Infect. 2013;141:827–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Human Metapneumovirus, Red Book: 2021–2024 Report of the Committee on Infectious Diseases, Committee on Infectious Diseases, American Academy of Pediatrics, David W. Kimberlin, MD, FAAP, Elizabeth D. Barnett, MD, FAAP, Ruth Lynfield, MD, FAAP, Mark H. Sawyer, MD, FAAP.
- 30.Liao RS, Appelgate DM, Pelz RK. An outbreak of severe respiratory tract infection due to human metapneumovirus in a long-term care facility for the elderly in Oregon. J Clin Virol. 2012;53:171–3. [DOI] [PubMed] [Google Scholar]
- 31.Haas LE, Thijsen SF, van Elden L, Heemstra KA. Human metapneumovirus in adults. Viruses. 2013;5:87–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trends of Acute Respiratory Infection. Including Human Metapneumovirus (hMPV). Disease Outbreak News (DON), 6 January 2025. World Health Organization. https://www.who.int/emergencies/disease-outbreak-news/item/2025-DON550. Accessed 8 January 2025.
- 33.Otomaru H, Sornillo JBT, Kamigaki T, Bado SLP, Okamoto M, Saito-Obata M, Inobaya MT, Segubre-Mercado E, Alday PP, Saito M. Risk of transmission and viral shedding from the time of infection for respiratory syncytial virus in households. Am J Epidemiol. 2021;190:2536–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Birger R, Morita H, Comito D, Filip I, Galanti M, Lane B, Ligon C, Rosenbloom D, Shittu A, Ud-Dean M. Asymptomatic shedding of respiratory virus among an ambulatory population across seasons. Msphere. 2018;3. 10.1128/msphere. 00249– 00218. [DOI] [PMC free article] [PubMed]
- 35.Seo S, Gooley TA, Kuypers JM, Stednick Z, Jerome KR, Englund JA, Boeckh M. Human metapneumovirus infections following hematopoietic cell transplantation: factors associated with disease progression. Clin Infect Dis. 2016;63:178–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murata T, Sakurai A, Suzuki M, Komoto S, Ide T, Ishihara T, Doi Y. Shedding of Viable Virus in Asymptomatic SARS-CoV-2 Carriers. mSphere 2021, 6. [DOI] [PMC free article] [PubMed]
- 37.Klompas M, Milton DK, Rhee C, Baker MA, Leekha S. Current insights into respiratory virus transmission and potential implications for infection control programs: a narrative review. Ann Intern Med. 2021;174:1710–8. [DOI] [PubMed] [Google Scholar]
- 38.Grant K, Andruchow JE, Conly J, Lee DD, Mazurik L, Atkinson P, Lang E. Personal protective equipment preservation strategies in the covid-19 era: A narrative review. Infect Prev Pract. 2021;3:100146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wißmann JE, Kirchhoff L, Brüggemann Y, Todt D, Steinmann J, Steinmann E. Persistence of pathogens on inanimate surfaces: a narrative review. Microorganisms. 2021;9:343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Foley DA, Sikazwe CT, Minney-Smith CA, Ernst T, Moore HC, Nicol MP, Smith DW, Levy A, Blyth CC. An unusual resurgence of human metapneumovirus in Western Australia following the reduction of non-pharmaceutical interventions to prevent SARS-CoV-2 transmission. Viruses. 2022;14:2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sundell N, Andersson L-M, Brittain-Long R, Lindh M, Westin J. A four year seasonal survey of the relationship between outdoor climate and epidemiology of viral respiratory tract infections in a temperate climate. J Clin Virol. 2016;84:59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martinez-Rodriguez C, Banos-Lara MR. HMPV in immunocompromised patients: frequency and severity in pediatric oncology patients. Pathogens. 2020;9:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Asanati K, Majeed A, Shemtob L, Cresswell F. Healthcare workers potentially exposed to HIV: an update. J R Soc Med. 2022;115:286–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee N, Chan P, Yu I, Tsoi K, Lui G, Sung J, Cockram C. Co-circulation of human metapneumovirus and SARS-associated coronavirus during a major nosocomial SARS outbreak in Hong Kong. J Clin Virol. 2007;40:333–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gálvez NM, Andrade CA, Pacheco GA, Soto JA, Stranger V, Rivera T, Vásquez AE, Kalergis AM. Host components that modulate the disease caused by hMPV. Viruses. 2021;13:519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rappazzo CG, Hsieh CL, Rush SA, Esterman ES, Delgado T, Geoghegan JC, Wec AZ, Sakharkar M, Más V, McLellan JS, Walker LM. Potently neutralizing and protective anti-human metapneumovirus antibodies target diverse sites on the fusion glycoprotein. Immunity. 2022;55:1710–e17241718. [DOI] [PubMed] [Google Scholar]
- 47.Lee Y-Z, Han J, Zhang Y-N, Ward G, Braz Gomes K, Auclair S, Stanfield RL, He L, Wilson IA, Zhu J. Rational design of uncleaved prefusion-closed trimer vaccines for human respiratory syncytial virus and metapneumovirus. Nat Commun. 2024;15:9939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Battles MB, Más V, Olmedillas E, Cano O, Vázquez M, Rodríguez L, Melero JA, McLellan JS. Structure and immunogenicity of pre-fusion-stabilized human metapneumovirus F glycoprotein. Nat Commun. 2017;8:1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hsieh C-L, Rush SA, Palomo C, Chou C-W, Pickens W, Más V, McLellan JS. Structure-based design of prefusion-stabilized human metapneumovirus fusion proteins. Nat Commun. 2022;13:1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adamson P, Thammawat S, Muchondo G, Sadlon T, Gordon D. Diversity in glycosaminoglycan binding amongst hMPV G protein lineages. Viruses. 2012;4:3785–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cifuentes-Muñoz N, Branttie J, Slaughter KB, Dutch RE. Human metapneumovirus induces formation of inclusion bodies for efficient genome replication and transcription. J Virol 2017, 91:10.1128/jvi. 01282– 01217. [DOI] [PMC free article] [PubMed]
- 52.Feuillet F, Lina B, Rosa-Calatrava M, Boivin G. Ten years of human metapneumovirus research. J Clin Virol. 2012;53:97–105. [DOI] [PubMed] [Google Scholar]
- 53.Soto Ramírez JA, Gálvez Arriagada NMS, Benavente F, Pizarro Ortega MS, Lay MK, Riedel C. Bueno Ramírez S, González Muñoz PA: Human metapneumovirus: mechanisms and molecular targets used by the virus to avoid the immune system. 2018. [DOI] [PMC free article] [PubMed]
- 54.Wei T, Wang C, Ma F, Guo J, Chen A, Huang Y, Xie Z, Zheng L. Whole genome sequencing and evolution analyses of human metapneumovirus. Virus Genes. 2023;59:524–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chow WZ, Chan YF, Oong XY, Ng LJ, Nor’E SS, Ng KT, Chan KG, Hanafi NS, Pang YK, Kamarulzaman A. Genetic diversity, seasonality and transmission network of human metapneumovirus: identification of a unique sub-lineage of the fusion and attachment genes. Sci Rep. 2016;6:27730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saikusa M, Nao N, Kawakami C, Usuku S, Sasao T, Toyozawa T, Takeda M, Okubo I. A novel 111-nucleotide duplication in the G gene of human metapneumovirus. Microbiol Immunol. 2017;61:507–12. [DOI] [PubMed] [Google Scholar]
- 57.Al-Turab M, Chehadeh W, Al-Nakib W. Phylogenetic analysis of human metapneumovirus detected in hospitalized patients in Kuwait during the years 2009–2011. J Infect Public Health. 2015;8:448–57. [DOI] [PubMed] [Google Scholar]
- 58.Mas V, Herfst S, Osterhaus AD, Fouchier RA, Melero JA. Residues of the human metapneumovirus fusion (F) protein critical for its strain-related fusion phenotype: implications for the virus replication cycle. J Virol. 2011;85:12650–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shetty PP, Parida U, Varamballi P, Mukhopadhyay P. Molecular detection and genotyping of HMPV in patients with severe acute respiratory infection in India. Ann Med. 2024;56:2398719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schuster JE, Williams JV. Human metapneumovirus. Antibodies Infect Dis 2015:237–47.
- 61.Agrawal AS, Roy T, Ghosh S, Chawla-Sarkar M. Genetic variability of attachment (G) and fusion (F) protein genes of human metapneumovirus strains Circulating during 2006–2009 in Kolkata, Eastern India. Virol J. 2011;8:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou Z, Zhang P, Cui Y, Zhang Y, Qin X, Li R, Liu P, Dou Y, Wang L, Zhao Y. Experiments investigating the competitive growth advantage of two different genotypes of human metapneumovirus: implications for the alternation of genotype prevalence. Sci Rep. 2020;10:2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen Q, Zhang J, Wang P, Zhang Z. The mechanisms of immune response and evasion by the main SARS-CoV-2 variants. Iscience 2022;25. [DOI] [PMC free article] [PubMed]
- 64.Ren J, Wang Q, Kolli D, Prusak DJ, Tseng C-TK, Chen ZJ, Li K, Wood TG, Bao X. Human metapneumovirus M2-2 protein inhibits innate cellular signaling by targeting MAVS. J Virol. 2012;86:13049–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Piñana M, González-Sánchez A, Andrés C, Abanto M, Vila J, Esperalba J, Moral N, Espartosa E, Saubi N, Creus A. The emergence, impact, and evolution of human metapneumovirus variants from 2014 to 2021 in Spain. J Infect. 2023;87:103–10. [DOI] [PubMed] [Google Scholar]
- 66.Chu HY, Renaud C, Ficken E, Thomson B, Kuypers J, Englund JA. Respiratory tract infections due to human metapneumovirus in immunocompromised children. J Pediatr Infect Dis Soc. 2014;3:286–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kloc M, Ghobrial RM, Kuchar E, Lewicki S, Kubiak JZ. Development of child immunity in the context of COVID-19 pandemic. Clin Immunol. 2020;217:108510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pelletier G, Déry P, Abed Y, Boivin G. Respiratory tract reinfections by the new human metapneumovirus in an immunocompromised child. Emerg Infect Dis. 2002;8:976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peng D, Zhao X, Liu E, Huang Y, Yang X, Zhao Y, Chen X, Zhang Z. Analysis of viral load in children infected with human metapneumovirus. Iran J Pediatr. 2010;20:393. [PMC free article] [PubMed] [Google Scholar]
- 70.Seynaeve D, Augusseau-Rivière B, Couturier P, Morel-Baccard C, Landelle C, Bosson JL, Gavazzi G, Mallaret MR. Outbreak of human metapneumovirus in a nursing home: A clinical perspective. J Am Med Dir Assoc. 2020;21:104–e109101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A. Inflammaging: a new immune–metabolic viewpoint for age-related diseases. Nat Reviews Endocrinol. 2018;14:576–90. [DOI] [PubMed] [Google Scholar]
- 72.Oh SJ, Lee JK, Shin OS. Aging and the immune system: the impact of Immunosenescence on viral infection, immunity and vaccine immunogenicity. Immune Netw. 2019;19:e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shi T, Vennard S, Jasiewicz F, Brogden R, Nair H. Disease burden estimates of respiratory syncytial virus related acute respiratory infections in adults with comorbidity: a systematic review and meta-analysis. J Infect Dis. 2022;226:S17–21. [DOI] [PubMed] [Google Scholar]
- 74.Feng E, Balint E, Poznanski SM, Ashkar AA, Loeb M. Aging and interferons: impacts on inflammation and viral disease outcomes. Cells. 2021;10:708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sojati J, Parks OB, Zhang Y, Walters S, Lan J, Eddens T, Lou D, Fan L, Chen K, Oury TD. IFN-λ drives distinct lung immune landscape changes and antiviral responses in human metapneumovirus infection. Mbio. 2024;15:e00550–00524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Choi SH, Hong SB, Huh JW, Jung J, Kim MJ, Chong YP, Kim SH, Sung H, Koo HJ, Do KH, et al. Outcomes of severe human metapneumovirus-associated community-acquired pneumonia in adults. J Clin Virol. 2019;117:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hijano DR, Maron G, Hayden RT. Respiratory viral infections in patients with cancer or undergoing hematopoietic cell transplant. Front Microbiol. 2018;9:3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Samuel S, Nanjappa S, Cooper CD, Greene JN. Human metapneumovirus infection in immunocompromised patients. Cancer Control. 2016;23:442–5. [DOI] [PubMed] [Google Scholar]
- 79.Yim KC, Mousa JJ, Blanco JC, Kim S, Boukhvalova MS. Human metapneumovirus (hMPV) infection and MPV467 treatment in immunocompromised cotton rats Sigmodon hispidus. Viruses. 2023;15:476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bhasin A, Nguyen DC, Briggs BJ, Nam HH. The burden of RSV, hMPV, and PIV amongst hospitalized adults in the united States from 2016 to 2019. J Hosp Med 2024. [DOI] [PubMed]
- 81.Zhao H, Feng Q, Feng Z, Zhu Y, Ai J, Xu B, Deng L, Sun Y, Li C, Jin R, et al. Clinical characteristics and molecular epidemiology of human metapneumovirus in children with acute lower respiratory tract infections in China, 2017 to 2019: A multicentre prospective observational study. Virol Sin. 2022;37:874–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rodriguez PE, Frutos MC, Adamo MP, Cuffini C, Cámara JA, Paglini MG, Moreno L, Cámara A. Human metapneumovirus: epidemiology and genotype diversity in children and adult patients with respiratory infection in Córdoba, Argentina. PLoS ONE. 2021;15:e0244093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Panda S, Mohakud NK, Pena L, Kumar S. Human metapneumovirus: review of an important respiratory pathogen. Int J Infect Dis. 2014;25:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Matsuda S, Nakamura M, Hirano E, Kiyota N, Omura T, Suzuki Y, Noda M, Kimura H. Characteristics of human metapneumovirus infection prevailing in hospital wards housing patients with severe disabilities. Jpn J Infect Dis. 2013;66:195–200. [DOI] [PubMed] [Google Scholar]
- 85.Haas LE, de Rijk NX, Thijsen SF. Human metapneumovirus infections on the ICU: a report of three cases. Ann Intensive Care. 2012;2:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Miyakawa R, Zhang H, Brooks WA, Prosperi C, Baggett HC, Feikin DR, Hammitt LL, Howie SRC, Kotloff KL, Levine OS, et al. Epidemiology of human metapneumovirus among children with severe or very severe pneumonia in high pneumonia burden settings: the pneumonia etiology research for child health (PERCH) study experience. Clin Microbiol Infect. 2025;31:441–50. [DOI] [PubMed] [Google Scholar]
- 87.Heikkinen T, Osterback R, Peltola V, Jartti T, Vainionpää R. Human metapneumovirus infections in children. Emerg Infect Dis. 2008;14:101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Esposito S, Daleno C, Prunotto G, Scala A, Tagliabue C, Borzani I, Fossali E, Pelucchi C, Principi N. Impact of viral infections in children with community-acquired pneumonia: results of a study of 17 respiratory viruses. Influenza Other Respir Viruses. 2013;7:18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shakya M, Chu HY, Englund JA, Briggs-Hagen M, Carone M, Kuntz JL, Lockwood T, Midgley CM, Schmidt MA, Starita L, et al. Epidemiology of symptomatic human metapneumovirus infection in the CASCADIA Community-Based Cohort - Oregon and Washington, 2022–2024. MMWR Morb Mortal Wkly Rep. 2025;74:188–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ji W, Chen Y, Han S, Dai B, Li K, Li S, Li Z, Chen S, Zhang Y, Zhang X, et al. Clinical and epidemiological characteristics of 96 pediatric human metapneumovirus infections in Henan, China after COVID-19 pandemic: a retrospective analysis. Virol J. 2024;21:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jain S, Williams DJ, Arnold SR, Ampofo K, Bramley AM, Reed C, Stockmann C, Anderson EJ, Grijalva CG, Self WH, et al. Community-acquired pneumonia requiring hospitalization among U.S. Children. N Engl J Med. 2015;372:835–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Debur MC, Vidal LR, Stroparo E, Nogueira MB, Almeida SM, Takahashi GA, Rotta I, Pereira LA, Silveira CS, Delfraro A, et al. Impact of human metapneumovirus infection on in and outpatients for the years 2006–2008 in Southern Brazil. Mem Inst Oswaldo Cruz. 2010;105:1010–8. [DOI] [PubMed] [Google Scholar]
- 93.Hacker K, Kuan G, Vydiswaran N, Chowell-Puente G, Patel M, Sanchez N, Lopez R, Ojeda S, Lopez B, Mousa J, et al. Pediatric burden and seasonality of human metapneumovirus over 5 years in Managua, Nicaragua. Influenza Other Respir Viruses. 2022;16:1112–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li J, Song CL, Wang T, Ye YL, Du JR, Li SH, Zhu JM. Etiological and epidemiological characteristics of severe acute respiratory infection caused by multiple viruses and Mycoplasma pneumoniae in adult patients in Jinshan, Shanghai: A pilot hospital-based surveillance study. PLoS ONE. 2021;16:e0248750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li J, Wang Z, Gonzalez R, Xiao Y, Zhou H, Zhang J, Paranhos-Baccala G, Vernet G, Jin Q, Wang J, Hung T. Prevalence of human metapneumovirus in adults with acute respiratory tract infection in Beijing, China. J Infect. 2012;64:96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ng DC, Liew CH, Tan KK, Awang EHB, Nazri F, Maran AKT, Mohan V, Ramachandran D, Chok M, Teh CH, et al. Clinical comparison of HMPV and RSV infections in hospitalised Malaysian children: A propensity score matched study. Clin Respir J. 2024;18:e13747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Walsh EE, Peterson DR, Falsey AR. Human metapneumovirus infections in adults: another piece of the puzzle. Arch Intern Med. 2008;168:2489–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Debiaggi M, Canducci F, Ceresola ER, Clementi M. The role of infections and coinfections with newly identified and emerging respiratory viruses in children. Virol J. 2012;9:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Semple MG, Cowell A, Dove W, Greensill J, McNamara PS, Halfhide C, Shears P, Smyth RL, Hart CA. Dual infection of infants by human metapneumovirus and human respiratory syncytial virus is strongly associated with severe bronchiolitis. J Infect Dis. 2005;191:382–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Esposito S, Mastrolia MV. Metapneumovirus infections and respiratory complications. Semin Respir Crit Care Med. 2016;37:512–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Papenburg J, Hamelin M, Ouhoummane N, Carbonneau J, Ouakki M, Raymond F, Robitaille L, Corbeil J, Caouette G, Frenette L, De Serres G, Boivin G. Comparison of risk factors for human metapneumovirus and respiratory syncytial virus disease severity in young children. J Infect Dis. 2012;206:178–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nunes MC, Cutland CL, Klugman KP, Madhi SA. Pneumococcal conjugate vaccine protection against Coronavirus-Associated pneumonia hospitalization in children living with and without HIV. mBio. 2021;12. 10.1128/mbio.02347-02320 [DOI] [PMC free article] [PubMed]
- 103.Hahn A, Wang W, Jaggi P, Dvorchik I, Ramilo O, Koranyi K, Mejias A. Human metapneumovirus infections are associated with severe morbidity in hospitalized children of all ages. Epidemiol Infect. 2013;141:2213–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Williams JV, Edwards KM, Weinberg GA, Griffin MR, Hall CB, Zhu Y, Szilagyi PG, Wang CK, Yang C-F, Silva D, et al. Population-Based incidence of human metapneumovirus infection among hospitalized children. J Infect Dis. 2010;201:1890–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sánchez Fernández I, Rebollo Polo M, Muñoz-Almagro C, Monfort Carretero L, Fernández Ureña S. Rueda Muñoz A, colomé Roura R, Pérez dueñas B: human metapneumovirus in the cerebrospinal fluid of a patient with acute encephalitis. Arch Neurol. 2012;69:649–52. [DOI] [PubMed] [Google Scholar]
- 106.Tomatis Souverbielle C, Sánchez PJ, Erdem G. Metapneumovirus infections are uncommon in infants younger than 60 days of age admitted for Sepsis evaluation. Pediatr Infect Dis J. 2023;42:e52–3. [DOI] [PubMed] [Google Scholar]
- 107.Shinkai M, Ota S, Ishikawa N, Tanimoto T, Suzuki H, Abe S, Vandendijck Y, Nakayama Y, Murata Y. Burden of respiratory syncytial virus, human metapneumovirus and influenza virus infections in Japanese adults in the hospitalized acute respiratory tract infection study. Respir Investig. 2024;62:717–25. [DOI] [PubMed] [Google Scholar]
- 108.Philippot Q, Rammaert B, Dauriat G, Daubin C, Schlemmer F, Costantini A, Tandjaoui-Lambiotte Y, Neuville M, Desrochettes E, Ferré A, et al. Human metapneumovirus infection is associated with a substantial morbidity and mortality burden in adult inpatients. Heliyon. 2024;10:e33231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Khan A, Khanna V, Majumdar K. Demographics, clinical presentation and outcome of metapneumovirus infection in adults: A case series analysis at Scarborough general hospital, united Kingdom. Cureus. 2024;16:e73292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Falsey AR, Walsh EE, House S, Vandenijck Y, Ren X, Keim S, Kang D, Peeters P, Witek J, Ispas G. Risk factors and medical resource utilization of respiratory syncytial virus, human metapneumovirus, and Influenza-Related hospitalizations in Adults-A global study during the 2017–2019 epidemic seasons (Hospitalized acute respiratory tract infection [HARTI] study). Open Forum Infect Dis. 2021;8:ofab491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Loubet P, Mathieu P, Lenzi N, Galtier F, Lainé F, Lesieur Z, Vanhems P, Duval X, Postil D, Amour S et al. Characteristics of human metapneumovirus infection in adults hospitalized for community-acquired influenza-like illness in France, 2012–2018: a retrospective observational study. Clin Microbiol Infect 2021, 27:127.e121-127.e126. [DOI] [PMC free article] [PubMed]
- 112.Foulongne V, Lechiche C, Reynes J, Segondy M. [Human metapneumovirus pneumonia in an adult patient hospitalized for suspected severe acute respiratory syndrome (SARS)]. Presse Med. 2004;33:1006–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kan-o K, Ramirez R, MacDonald MI, Rolph M, Rudd PA, Spann KM, Mahalingam S, Bardin PG, Thomas BJ. Human metapneumovirus infection in chronic obstructive pulmonary disease: impact of glucocorticosteroids and interferon. J Infect Dis. 2017;215:1536–45. [DOI] [PubMed] [Google Scholar]
- 114.Furuta T, Hasegawa S, Mizutani M, Iwai T, Ohbuchi N, Kawano S, Tashiro N, Uchida M, Hasegawa M, Motoyama M, et al. Burden of human metapneumovirus and respiratory syncytial virus infections in asthmatic children. Pediatr Infect Dis J. 2018;37:1107–11. [DOI] [PubMed] [Google Scholar]
- 115.Egli A, Bucher C, Dumoulin A, Stern M, Buser A, Bubendorf L, Gregor M, Servida P, Sommer G, Bremerich J, et al. Human metapneumovirus infection after allogeneic hematopoietic stem cell transplantation. Infection. 2012;40:677–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sumino KC, Agapov E, Pierce RA, Trulock EP, Pfeifer JD, Ritter JH, Gaudreault-Keener M, Storch GA, Holtzman MJ. Detection of severe human metapneumovirus infection by real-time polymerase chain reaction and histopathological assessment. J Infect Dis. 2005;192:1052–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Groome MJ, Moyes J, Cohen C, Walaza S, Tempia S, Pretorius M, Hellferscee O, Chhagan M, Haffejee S, Dawood H, et al. Human metapneumovirus-associated severe acute respiratory illness hospitalisation in HIV-infected and HIV-uninfected South African children and adults. J Clin Virol. 2015;69:125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kapandji N, Darmon M, Valade S, Salmona M, Legoff J, Zafrani L, Azoulay E, Lemiale V. Clinical significance of human metapneumovirus detection in critically ill adults with lower respiratory tract infections. Ann Intensive Care. 2023;13:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.El Chaer F, Shah DP, Kmeid J, Ariza-Heredia EJ, Hosing CM, Mulanovich VE, Chemaly RF. Burden of human metapneumovirus infections in patients with cancer: risk factors and outcomes. Cancer. 2017;123:2329–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Piñana JL, Tridello G, Xhaard A, Wendel L, Montoro J, Vazquez L, Heras I, Ljungman P, Mikulska M, Salmenniemi U, et al. Upper and/or lower respiratory tract infection caused by human metapneumovirus after allogeneic hematopoietic stem cell transplantation. J Infect Dis. 2024;229:83–94. [DOI] [PubMed] [Google Scholar]
- 121.Debiaggi M, Canducci F, Sampaolo M, Marinozzi MC, Parea M, Terulla C, Colombo AA, Alessandrino EP, Bragotti LZ, Arghittu M, et al. Persistent symptomless human metapneumovirus infection in hematopoietic stem cell transplant recipients. J Infect Dis. 2006;194:474–8. [DOI] [PubMed] [Google Scholar]
- 122.Permpalung N, Sait AS, Bazemore K, Avery RK, Mathew J, Shah PD. Human metapneumovirus and parainfluenza virus infections in lung transplant recipients: the effects on lung allograft and clinical outcomes. Transplantation. 2021;105:2625–31. [DOI] [PubMed] [Google Scholar]
- 123.Fathima S, Lee BE, May-Hadford J, Mukhi S, Drews SJ. Use of an innovative web-based laboratory surveillance platform to analyze mixed infections between human metapneumovirus (hMPV) and other respiratory viruses Circulating in Alberta (AB), Canada (2009–2012). Viruses. 2012;4:2754–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wan Q, Li Y-w, Cheng Y, Hu H. The impact of bacterial Co-Infection on hospitalized children with human rhinovirus and human metapneumovirus infections: A retrospective analytical Cross-Sectional study. Jundishapur J Microbiol 2023;16.
- 125.Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986;140:543–6. [DOI] [PubMed] [Google Scholar]
- 126.De Paulis M, Gilio AE, Ferraro AA, Ferronato AE, do Sacramento PR, Botosso VF, Oliveira DB, Marinheiro JC, Hársi CM, Durigon EL, Vieira SE. Severity of viral coinfection in hospitalized infants with respiratory syncytial virus infection. J Pediatr (Rio J). 2011;87:307–13. [DOI] [PubMed] [Google Scholar]
- 127.Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O’Brien KL, Roca A, Wright PF, Bruce N. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375:1545–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang X, Li Y, Deloria-Knoll M, Madhi SA, Cohen C, Ali A, Basnet S, Bassat Q, Brooks WA, Chittaganpitch M. Global burden of acute lower respiratory infection associated with human metapneumovirus in children under 5 years in 2018: a systematic review and modelling study. Lancet Global Health. 2021;9:e33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Steinberg R, Marty V, Korten I, Aebi C, Latzin P, Agyeman PK. Epidemiology and clinical characteristics of human metapneumovirus infections in hospitalized children in two consecutive postpandemic years. Pediatr Infect Dis J. 2024;43:e141–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Otomaru H, Nguyen HAT, Vo HM, Toizumi M, Le MN, Mizuta K, Moriuchi H, Bui MX, Dang DA, Yoshida L-M. A decade of human metapneumovirus in hospitalized children with acute respiratory infection: molecular epidemiology in central Vietnam, 2007–2017. Sci Rep. 2023;13:15757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Costa-Filho RC, Saddy F, Costa JLF, Tavares LR, Castro Faria Neto HC. The silent threat of human metapneumovirus: clinical challenges and diagnostic insights from a severe pneumonia case. Microorganisms. 2025;13:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ghattas C, Mossad SB. Fatal human metapneumovirus and influenza B virus coinfection in an allogeneic hematopoietic stem cell transplant recipient. Transpl Infect Dis. 2012;14:E41–43. [DOI] [PubMed] [Google Scholar]
- 133.Ibrahim S, Scott M, Bixler D, Pedersen R, Tripp J, Carter K, Erdman D, Schneider E, Britton C. Outbreaks of human metapneumovirus in two skilled nursing facilities—West Virginia and Idaho, 2011–2012. Morb Mortal Wkly Rep. 2013;62:909. [PMC free article] [PubMed] [Google Scholar]
- 134.Edwards KM, Zhu Y, Griffin MR, Weinberg GA, Hall CB, Szilagyi PG, Staat MA, Iwane M, Prill MM, Williams JV. Burden of human metapneumovirus infection in young children. N Engl J Med. 2013;368:633–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Venkataraman A. Assessing the Impact of Comorbidities on Respiratory Virus Prevalence Across Age Groups in Washington State. 2024.
- 136.Shehu MT, Pascual A, Kapinos P, El Khoury MY. Mortality and morbidity of human metapneumovirus infection in the Pre-COVID-19 era: the value of the Charlson comorbidity index on outcome prediction. Cureus 2024;16. [DOI] [PMC free article] [PubMed]
- 137.Carter P, Lagan J, Fortune C, Bhatt DL, Vestbo J, Niven R, Chaudhuri N, Schelbert EB, Potluri R, Miller CA. Association of cardiovascular disease with respiratory disease. J Am Coll Cardiol. 2019;73:2166–77. [DOI] [PubMed] [Google Scholar]
- 138.Bhat TA, Panzica L, Kalathil SG, Thanavala Y. Immune dysfunction in patients with chronic obstructive pulmonary disease. Annals Am Thorac Soc. 2015;12:S169–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gurgone D, McShane L, McSharry C, Guzik TJ, Maffia P. Cytokines at the interplay between asthma and atherosclerosis? Front Pharmacol. 2020;11:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Branche AR, Falsey AR. Parainfluenza virus infection. Seminars in respiratory and critical care medicine. Thieme Medical; 2016;538–54. [DOI] [PMC free article] [PubMed]
- 141.Lynch JP, Fishbein M, Echavarria M. Adenovirus. Seminars in respiratory and critical care medicine. © Thieme Medical; 2011;494–511.
- 142.Singhal T. A review of coronavirus disease-2019 (COVID-19). Indian J Pediatr. 2020;87:281–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Feng Y, He T, Zhang B, Yuan H, Zhou Y. Epidemiology and diagnosis technologies of human metapneumovirus in China: a mini review. Virol J. 2024;21:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Azar MM, Landry ML. Detection of influenza A and B viruses and respiratory syncytial virus by use of clinical laboratory improvement amendments of 1988 (CLIA)-waived point-of-care assays: a paradigm shift to molecular tests. J Clin Microbiol. 2018;56. 10.1128/jcm. 00367– 00318. [DOI] [PMC free article] [PubMed]
- 145.Parker KG, Gandra S, Matushek S, Beavis KG, Tesic V, Charnot-Katsikas A. Comparison of 3 nucleic acid amplification tests and a rapid antigen test with culture for the detection of group A Streptococci from throat swabs. J Appl Lab Med. 2019;4:164–9. [DOI] [PubMed] [Google Scholar]
- 146.García-Arroyo L, Prim N, Martí N, Roig MC, Navarro F, Rabella N. Benefits and drawbacks of molecular techniques for diagnosis of viral respiratory infections. Experience with two multiplex PCR assays. J Med Virol. 2016;88:45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cobo F. Application of molecular diagnostic techniques for viral testing. Open Virol J. 2012;6:104–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bachman J. Reverse-transcription PCR (RT-PCR). Methods Enzymol. 2013;530:67–74. [DOI] [PubMed] [Google Scholar]
- 149.Vemula SV, Zhao J, Liu J, Wang X, Biswas S, Hewlett I. Current approaches for diagnosis of influenza virus infections in humans. Viruses. 2016;8:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Trombetta VK, Chan YL, Bankowski MJ. Are rapid influenza antigen tests still clinically useful in today’s molecular diagnostics world? Hawai’i J Med Public Health. 2018;77:226. [PMC free article] [PubMed] [Google Scholar]
- 151.BinSaeed AA, Siddiqui AR, Mandil AM, Torchyan AA, Tayel SA, Shaikh SA, Habib HA, Al-Khattaf AS. The role of rapid testing and clinical decision in the diagnosis of human influenza A H1N1 infection. Saudi Med J. 2014;35:277–84. [PubMed] [Google Scholar]
- 152.Bhandary R, Boloor R. Detection of respiratory syncytial virus using direct fluorescent antibody assay in paediatric patients with acute respiratory tract infection. J Clin Diagn Research: JCDR. 2016;10:DC10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rochelet M, Solanas S, Grossiord C, Maréchal P, Résa C, Vienney F, Barranger C, Joannes M. A thin layer-based amperometric enzyme immunoassay for the rapid and sensitive diagnosis of respiratory syncytial virus infections. Talanta. 2012;100:139–44. [DOI] [PubMed] [Google Scholar]
- 154.Todd AK, Costa AM, Waller G, Daley AJ, Barr IG, Deng YM. Rapid detection of human respiratory syncytial virus A and B by duplex real-time RT-PCR. J Virol Methods. 2021;294:114171. [DOI] [PubMed] [Google Scholar]
- 155.Shirato K, Yano T, Senba S, Akachi S, Kobayashi T, Nishinaka T, Notomi T, Matsuyama S. Detection of middle East respiratory syndrome coronavirus using reverse transcription loop-mediated isothermal amplification (RT-LAMP). Virol J. 2014;11:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lu X, Whitaker B, Sakthivel SKK, Kamili S, Rose LE, Lowe L, Mohareb E, Elassal EM, Al-sanouri T, Haddadin A. Real-time reverse transcription-PCR assay panel for middle East respiratory syndrome coronavirus. J Clin Microbiol. 2014;52:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bhadra S, Jiang YS, Kumar MR, Johnson RF, Hensley LE, Ellington AD. Real-time sequence-validated loop-mediated isothermal amplification assays for detection of middle East respiratory syndrome coronavirus (MERS-CoV). PLoS ONE. 2015;10:e0123126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wang N, Luo C, Liu H, Yang X, Hu B, Zhang W, Li B, Zhu Y, Zhu G, Shen X. Characterization of a new member of alphacoronavirus with unique genomic features in Rhinolophus bats. Viruses. 2019;11:379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Biserni GB, Scarpini S, Dondi A, Biagi C, Pierantoni L, Masetti R, Sureshkumar S, Rocca A, Lanari M. Potential diagnostic and prognostic biomarkers for adenovirus respiratory infection in children and young adults. Viruses 2021;13. [DOI] [PMC free article] [PubMed]
- 160.Al-Siyabi T, Binkhamis K, Wilcox M, Wong S, Pabbaraju K, Tellier R, Hatchette TF, LeBlanc JJ. A cost effective real-time PCR for the detection of adenovirus from viral swabs. Virol J. 2013;10:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Do DH, Laus S, Leber A, Marcon MJ, Jordan JA, Martin JM, Wadowsky RM. A one-step, real-time PCR assay for rapid detection of rhinovirus. J Mol Diagn. 2010;12:102–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sikazwe CT, Chidlow GR, Imrie A, Smith DW. Reliable quantification of rhinovirus species C using real-time PCR. J Virol Methods. 2016;235:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Indumathi CP, Gunanasekaran P, Kaveri K, Arunagiri K, Mohana S, Sheriff AK, SureshBabu BV, Padmapriya P, Senthilraja R, Fathima G. Isolation & molecular characterization of human parainfluenza virus in Chennai, India. Indian J Med Res. 2015;142:583–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Choudhary ML, Anand SP, Sonawane NS, Chadha MS. Development of real-time RT-PCR for detection of human metapneumovirus and genetic analysis of Circulating strains (2009–2011) in Pune, India. Arch Virol. 2014;159:217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.González LA, Vázquez Y, Mora JE, Palavecino CE, Bertrand P, Ferrés M, Contreras AM, Beckhaus AA, Riedel CA, Bueno SM. Evaluation of monoclonal antibodies that detect conserved proteins from respiratory syncytial virus, metapneumovirus and adenovirus in human samples. J Virol Methods. 2018;254:51–64. [DOI] [PubMed] [Google Scholar]
- 166.Munharo S, Nayupe S, Mbulaje P, Patel P, Banda C, Gacutno KJA, Lin X, Shawa IT, Lucero-Prisno DE III. Challenges of COVID-19 testing in low-middle income countries (LMICs): the case of Malawi. J Lab Precision Med 2020;5.
- 167.Kumar M, Pahuja S, Khare P, Kumar A. Current challenges and future perspectives of diagnosis of hepatitis B virus. Diagnostics (Basel) 2023;13. [DOI] [PMC free article] [PubMed]
- 168.Umubyeyi Nyaruhirira A, Scholten JN, Gidado M, Suarez PG. Coronavirus disease 2019 diagnosis in Low- and Middle-Income countries: the big new bully disrupting TB and HIV diagnostic services. J Mol Diagn. 2022;24:289–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gemeda ST, Soboksa NE, Tefera YM, Desta AF, Gari SR. PCR-based detection of pathogens in improved water sources: a scoping review protocol of the evidence in low-income and middle-income countries. BMJ Open. 2022;12:e057154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Fofana DB, Coulibaly TA, Maiga M, Nguyen T, Gozlan J, Diarra Z, Koné A, Cissoko Y, Maiga AI, Hawkins CA, et al. A multiplexed real-time PCR assay for simultaneous quantification of human immunodeficiency virus and hepatitis B virus for low‐and‐middle‐ income countries. J Virol Methods. 2024;330:115026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kilmarx PH, Simbi R. Progress and challenges in scaling up laboratory monitoring of HIV treatment. PLoS Med. 2016;13:e1002089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chamas C, Barbeitas MM, Correa M, Kameda K, de Oliveira ACD, Villarinho L. Innovation in diagnostics: addressing gaps in low- and middle-income countries. Bull World Health Organ. 2022;100:467–a467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sarna M, Le H, Taye BW, Glass K, Levy A, Richmond P, Moore HC. Clinical outcomes and severity of laboratory-confirmed RSV compared with influenza, parainfluenza and human metapneumovirus in Australian children attending secondary care. BMJ Open Respir Res 2024;11. [DOI] [PMC free article] [PubMed]
- 174.de Zwart A, Riezebos-Brilman A, Lunter G, Vonk J, Glanville AR, Gottlieb J, Permpalung N, Kerstjens H, Alffenaar JW, Verschuuren E. Respiratory syncytial virus, human metapneumovirus, and parainfluenza virus infections in lung transplant recipients: A systematic review of outcomes and treatment strategies. Clin Infect Dis. 2022;74:2252–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Principi N, Esposito S. Paediatric human metapneumovirus infection: epidemiology, prevention and therapy. J Clin Virol. 2014;59:141–7. [DOI] [PubMed] [Google Scholar]
- 176.Kumar P, Srivastava M. Prophylactic and therapeutic approaches for human metapneumovirus. Virusdisease. 2018;29:434–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Guo L, Li L, Liu L, Zhang T, Sun M. Neutralising antibodies against human metapneumovirus. Lancet Microbe. 2023;4:e732–44. [DOI] [PubMed] [Google Scholar]
- 178.Miller RJ, Mousa JJ. Structural basis for respiratory syncytial virus and human metapneumovirus neutralization. Curr Opin Virol. 2023;61:101337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Decool H, Gonnin L, Gutsche I, Sizun C, Eléouët JF, Galloux M. Interactions between the nucleoprotein and the phosphoprotein of pneumoviruses: structural insight for rational design of antivirals. Viruses 2021;13. [DOI] [PMC free article] [PubMed]
- 180.Troncoso-Bravo T, Ramírez MA, Loaiza RA, Román-Cárdenas C, Papazisis G, Garrido D, González PA, Bueno SM, Kalergis AM. Advancement in the development of mRNA-based vaccines for respiratory viruses. Immunology. 2024;173:481–96. [DOI] [PubMed] [Google Scholar]
- 181.Shi HJ, Kim NY, Eom SA, Kim-Jeon MD, Oh SS, Moon BS, Kwon MJ, Eom JS. Effects of Non-Pharmacological interventions on respiratory viruses other than SARS-CoV-2: analysis of laboratory surveillance and literature review from 2018 to 2021. J Korean Med Sci. 2022;37:e172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Odusanya OO, Odugbemi BA, Odugbemi TO, Ajisegiri WS. COVID-19: A review of the effectiveness of non-pharmacological interventions. Nigerian Postgrad Med J. 2020;27:261–7. [DOI] [PubMed] [Google Scholar]
- 183.Oh J, Lee J-K, Schwarz D, Ratcliffe HL, Markuns JF, Hirschhorn LR. National response to COVID-19 in the Republic of Korea and lessons learned for other countries. Health Syst Reform. 2020;6:e1753464. [DOI] [PubMed] [Google Scholar]
- 184.Lévy C, Aerts L, Hamelin M-È, Granier C, Szécsi J, Lavillette D, Boivin G, Cosset F-L. Virus-like particle vaccine induces cross-protection against human metapneumovirus infections in mice. Vaccine. 2013;31:2778–85. [DOI] [PubMed] [Google Scholar]
- 185.Lukacs NW, Malinczak CA. Harnessing cellular immunity for vaccination against respiratory viruses. Vaccines (Basel) 2020;8. [DOI] [PMC free article] [PubMed]
- 186.Zhang X, Knoth C, Pham A, Lima A, Dominguez R, Ibarra-Flores I, Lopez JC, Uy D, Silbert S, Patel A, et al. The clinical performance of the biocode respiratory pathogen panel for the detection of viruses and Bacteria from nasopharyngeal swabs. Microbiol Spectr. 2023;11:e0404422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Leber AL, Lisby JG, Hansen G, Relich RF, Schneider UV, Granato P, Young S, Pareja J, Hannet I. Multicenter evaluation of the QIAstat-Dx respiratory panel for detection of viruses and Bacteria in nasopharyngeal swab specimens. J Clin Microbiol 2020;58. [DOI] [PMC free article] [PubMed]
- 188.Parčina M, Schneider UV, Visseaux B, Jozić R, Hannet I, Lisby JG. Multicenter evaluation of the QIAstat respiratory Panel-A new rapid highly multiplexed PCR based assay for diagnosis of acute respiratory tract infections. PLoS ONE. 2020;15:e0230183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Anand M, Nimmala P. Seasonal incidence of respiratory viral infections in Telangana, India: utility of a multiplex PCR assay to Bridge the knowledge gap. Trop Med Int Health. 2020;25:1503–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.