Abstract
Objectives
The effect of esketamine on perioperative neurocognitive dysfunction (PND) remains controversial. This systematic review and meta-analysis aimed to evaluate the impact of perioperative esketamine administration on PND.
Design
Systematic review and meta-analysis.
Data sources
PubMed, EMBASE, Web of Science and Cochrane Library were searched from their inception to 25 April 2024.
Eligibility criteria for selecting studies
We included the randomised controlled trials (RCTs) that compared single or continuous intravenous infusion of esketamine to saline among adult surgical patients without pre-existing neurocognitive disorders.
Data extraction and synthesis
Two reviewers independently extracted pertinent information from the included studies. Risk of bias was assessed using Cochrane’s risk of bias criteria. Risk ratios (RRs) and their corresponding 95% CIs were synthesised using a random-effects model. The overall evidence quality was appraised using the Grading of Recommendations Assessment, Development and Evaluation framework.
Results
10 RCTs were included in our meta-analysis, involving 854 surgical patients. Perioperative esketamine was associated with a reduced risk of postoperative delirium (POD) (relative risk (RR): 0.46, 95% CI: 0.30 to 0.71, p<0.001) and delayed neurocognitive recovery (dNCR) (RR: 0.41, 95% CI: 0.21 to 0.78, p<0.001). However, no statistically significant difference was found in the risk of postoperative neurocognitive disorder (post-NCD) at 3 months postsurgery between the esketamine and control groups (RR: 0.57, 95% CI: 0.19 to 1.73, p=0.40). Additionally, pain severity was reduced on postoperative day 1, with no difference in the risk of adverse events or length of hospital stay.
Conclusion
Perioperative esketamine reduces the risk of short-term PND, including POD and dNCR, without significantly affecting the incidence of adverse events or length of hospital stay. However, no significant differences were observed in the risk of post-NCD at 3 months following surgery. This systematic review and meta-analysis offers valuable data for PND research and clinical drug intervention strategies.
PROSPERO registration number
CRD42024538438.
Keywords: Systematic Review, Meta-Analysis, Adult anaesthesia
STRENGTHS AND LIMITATIONS OF THIS STUDY.
The study applied strict inclusion criteria, focusing only on studies reporting the standardised neuropsychological assessments.
The inclusion of only randomised controlled trials.
Various types of perioperative neurocognitive dysfunction, including postoperative delirium, delayed neurocognitive recovery and postoperative neurocognitive disorder, were included.
Most of the included studies were conducted in Chinese populations.
The limited number of studies and small sample sizes in some subgroup analyses.
Introduction
Neurocognitive disorder is a prevalent and well-documented complication following surgery and anaesthesia, particularly among the older adults. It is characterised by a discernible decline in memory, attention, orientation, information processing and social skills.1 The Perioperative Cognition Nomenclature Working Group has designated these clinical manifestations as perioperative neurocognitive dysfunction (PND), encompassing postoperative delirium (POD), delayed neurocognitive recovery (dNCR), postoperative neurocognitive disorder (post-NCD) and both mild and major cognitive impairments.2 The incidence of PND is acknowledged to vary based on the type of disorder, age, surgical procedure, anaesthesia type and additional factors. For example, a prior review synthesising various procedures and patient demographics reported POD incidence rates ranging from 13.2% to 41.7% and PND incidence rates from 8.9% to 46.1%.3 Moreover, PND is more prevalent among older patients, with incidence rates increasing with age.4 The rapid ageing of the general population, coupled with a higher number of surgeries performed on older patients, has thrust PND into the spotlight.5 Importantly, previous research indicates that PND can deteriorate quality of life, prolong hospital stays and escalate morbidity and mortality rates.6 7 Consequently, the identification and prevention of PND have become paramount concerns.8,10
Given its multifactorial aetiology,8 9 11 recent studies have highlighted the significant involvement of the N-methyl-D-aspartate (NMDA) receptor—an essential mediator of learning, memory and synaptic plasticity12 13—in the development of PND.14 15 Esketamine, the S-enantiomer of ketamine and an NMDA receptor antagonist, has been consistently confirmed in numerous studies for its antidepressant effects, particularly in alleviating postpartum depression.16 A network meta-analysis comparing different anaesthetic drugs in reducing the incidence of PND for older adults undergoing non-cardiac surgery revealed that there was no significant difference in the ketamine group compared with placebo.17 Contrastingly, preclinical studies in aged rats suggested that esketamine has an alleviating effect on neurocognitive impairment after surgery and anaesthesia.18 19 These findings, when considered alongside prior research highlighting differences in adverse reactions (eg, hallucinations and nightmares) and anxiolytic properties between the two agents,20 21 provide a compelling rationale to hypothesise divergent clinical efficacy in the prevention and management of PND. Consequently, ketamine and esketamine should not be conflated in evaluating their effects in prior studies, 22and independent analyses of esketamine are warranted.
However, data from previous clinical studies on the efficacy of esketamine in reducing the risk of PND are currently disparate. Given the potential of these agents to revolutionise perioperative care, synthesising the available evidence and providing guidance for clinical practice is imperative.
To address this clinical controversy and explore the potential impact of perioperative esketamine on the incidence of PND, we conducted a systematic review and meta-analysis of randomised controlled trials (RCTs) involving surgical patients, thereby providing a comprehensive and consolidated source of information for both clinical practitioners and researchers.
Materials and methods
The reporting of this meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines23 and adhered to a protocol registered with PROSPERO (CRD42024538438).
Search strategy
XS and LC independently executed a thorough search across various online databases, including PubMed, EMBASE, Web of Science and the Cochrane Library, with a focus on English-language articles containing the keywords “esketamine” and “PND.” The restriction to English-language articles was based on practical constraints, the predominance of high-quality research published in English and the need to ensure consistency and accuracy in data extraction and analysis. The literature search was comprehensive, covering all available records from their inception up to 25 April 2024. Any differences encountered were addressed through discussions with ST to achieve consensus. Specific search terms used for each database are provided in online supplemental table 1.
Study selection
The references obtained from the search were exported to Endnote V.2022 and assessed by XS and YZ independently. The initial screening involved evaluating titles and abstracts, followed by a thorough review of full texts for potentially relevant studies. Eligible studies were required to fulfil the “Population, Intervention, Comparator, Outcome, and Study design” (PICOS) criteria: (1) Population: adult surgical patients (≥18 years old) without pre-existing neurocognitive disorders; (2) Intervention: single or continuous intravenous infusion of esketamine; (3) Comparator: saline or no treatment; (4) Outcome: risk estimates or incidence of PND at defined postoperative time points (eg, 3, 7 or 30 days), measured by standardised neuropsychological assessments such as Mini-Mental State Examination (MMSE), International Study on Post-Operative Cognitive Dysfunction, Confusion Assessment Method (CAM) and Confusion Assessment Method for the Intensive Care Unit; (5) Study design: RCT. Studies not meeting these criteria, including those with inadequate data, animal studies or abstracts, were excluded. Disagreements regarding study eligibility were resolved through discussions involving ST.
Quality assessment
The risk of bias in the included RCTs was independently assessed by CL and SL using Cochrane’s risk of bias criteria,24 which covers aspects such as randomisation, allocation concealment, participant and personnel blinding, outcome assessment blinding, completeness of data, selective reporting and other biases. Each study was classified as having ‘low’, ‘high’ or ‘unclear’ risk. The overall evidence quality was appraised using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework.25,28 Discrepancies in quality assessments were reconciled through discussions with ST.
Data extraction
XS and ZW independently extracted pertinent information from the studies, resolving any discrepancies through discussions with JZ, XY and ST. Extracted data included the following: (1) study characteristics: first author’s name, publication year, country and sample size; (2) participant characteristics: age, gender proportion and type of surgeries; (3) esketamine administration route and dosage; (4) method and timing of neurocognitive assessments, along with types of PND (POD, dNCR or post-NCD); (5) number of PND cases and adverse events at least one postoperative time point. Furthermore, during the data extraction process, primary outcomes at long-term follow-up (postoperative 3 months) and secondary outcomes—including postoperative nausea and vomiting (PONV), cardiovascular adverse events, length of hospital stay and postoperative pain—were identified in several studies. These outcomes were subsequently analysed, where data sufficiency and clinical relevance permitted to provide a more comprehensive evaluation of esketamine’s effects.
Statistical analysis
Statistical analyses were performed using Stata software V.18.0. For dichotomous data, relevant information included the total number of patients in each group and the count of those developing PND. Risk ratios (RRs) and their corresponding 95% CIs were synthesised and mean differences (MD) or Standardized Mean Difference (SMD) (when different scales are used) with 95% CIs for continuous outcomes using a random-effects model due to expected clinical and methodological diversity. The Cochran Q statistic was employed to assess heterogeneity, quantified using the I² metric (with significant heterogeneity defined as I²>50% and p<0.05).29 For outcomes involving more than 10 studies, publication bias was evaluated through funnel plots and Egger’s tests.
Subgroup analyses were conducted to explore potential sources of heterogeneity and provide additional insights into the effects of esketamine. Specifically, subgroup analyses were performed based on the type of PND, timing of esketamine administration (before, during and after surgery) and patient age (older surgical patients vs non-older surgical patients). These subgroup analyses were conducted where data were available to assess whether the timing of esketamine administration or patient age influenced the risk of PND.
Patient and public involvement
None.
Results
Search results and study characteristics
The initial search identified 338 articles, of which 10 RCTs30,39were included in our systematic review and one RCT30 was excluded in our meta-analysis (figure 1). These studies included a total of 803 surgical patients, with a wide age range and diverse surgical procedures, such as cardiothoracic, abdominal, hip and knee surgeries. Most studies were conducted in Chinese populations, which may limit generalisability to other ethnic groups. Detailed study characteristics are presented in table 1 and online supplemental table 2. And the risk of bias assessment for the 10 RCTs is shown in online supplemental figure 1.
Figure 1. Flow diagram of study selection.
Table 1. Study characteristics.
| Study | Country | Age (years old) | N | Intervention(dose and route) | Surgery | Time | Scale | |
|---|---|---|---|---|---|---|---|---|
| Esketamine group | Control group | |||||||
| Helmar, 2016* | Austria | 62.2 (9.8), 58.4 (8.1)† | 61.0 (12.4) † | 57 | Low-dose group: 0.25 mg kg−1 intravenous+0.125 mg kg−1 h−1 ivgtt; minimal-dose group: a 0.9% saline intravenous+0.015 mg kg−1 h−1 ivgtt | Elective major open abdominal surgery | 48 hours | ICDSC |
| Xiaodan, 2022‡ | China | 57.14 (5.94)† | 56.46 (6.07), 58.81 (5.58)† | 110 | 0.3 mg kg−1 intravenous+0.2 mg kg−1 h−1 ivgtt | Video-assisted thoracoscopic surgery | 24 hours+3 months | MMSE |
| Juan, 2021 | China | 68.8 (3.6)† | 69.2 (5.4)† | 80 | 0.2 mg kg−1 intravenous | Unilateral total knee replacement | 24 hours+3 days | CAM |
| Chao, 2023 | China | 70.60 (7.63)† | 70.00 (6.25)† | 67 | 0.15 mg kg−1 intravenous | Gastrointestinal surgery | 7 days+3 months | ISPOCD |
| Jiamin, 2023 | China | 69.45 (4.35)† | 70.55 (4.24)† | 62 | 0.25 mg kg−1 intravenous+0.125 mg kg−1 h−1 ivgtt | Elective major abdominal surgery for gastrointestinal tumours | 3 days | CAM-ICU+MMSE |
| Zhaojun, 2024 | China | 69.20 (6.22)† | 71.47 (6.18)† | 87 | 0.25 mg kg−1 intravenous+0.1 mg kg−1 h−1 ivgtt | Laparoscopic gastrointestinal tumour surgery | 3 days | CAM |
| Jing, 2024 | China | NA | NA | 60 | 1 mg kg−1 diluted to 100 mL with normal saline for PCIA | Gastrointestinal surgery | 24 hours+3 days | CAM |
| Tianyuan, 2024* | China | 55.95 (9.08), 55.20 (9.23)† | 55.86 (10.60)† | 129 | Low-dose group: 0.2 mg kg−1 intravenous; high-dose group: 0.5 mg kg−1 intravenous | Elective non-cardiac thoracic surgery | 24 hours+3 days | MMSE |
| Xinglong, 2023 | China | 52.5 (44.0–57.0)§ | 51.5 (47.2–56.0)§ | 112 | 0.25 mg kg−1 intravenous | Cardiac valve surgery with cardiopulmonary bypass | 7 days | CAM or CAM-ICU |
| Tiantian, 2023 | China | 46.25 (6.77)† | 44.37 (9.74)† | 39 | 0.125 mg kg−1 intravenous | Elective laparoscopic gynaecological surgery | 7 days | CAM-ICU |
Presented as the study was designed for two groups using esketamine, but with two different doses.
Means (SD).
Presented as the study was designed for two groups without esketamine.
Median (IQR).
CAM, Confusion Assessment Method; CAM-ICU, Confusion Assessment Method for the Intensive Care Unit; ICDSC, Intensive Care Delirium Screening Checklist; ISPOCD, International Study on Postoperative Cognitive Dysfunction; MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment; NA, not reported; PCIA, patient-controlled intravenous analgesia.
Effects of perioperative esketamine on the incidence of perioperative neurocognitive dysfunction
Two points need particular attention. First, to obtain more robust results, two pooled analyses were conducted based on the grouping settings of the included studies. In the first pooled analysis, we included RCTs grouped into two groups. In the second, we included RCTs grouped into three groups and artificially divided them into experimental and control groups based on whether esketamine was used or not, combining them with the original two-group RCTs. Second, in some included studies, neurocognitive assessment results were reported at multiple time points. We combined results from different time points within 7 days postoperatively from the same study for analysis, applying the same method for results beyond 7 days.
Primary outcome
Within 7 days postoperatively
Nine RCTs were included in our analysis to investigate the effect of perioperative esketamine on the incidence of PND. The pooled results from seven RCTs with two groups indicated that perioperative esketamine was associated with a significantly lower risk of developing PND (RR: 0.44, 95% CI: 0.31 to 0.63, p<0.001, GRADE: high, figure 2A). Sensitivity analyses further supported these findings (online supplemental figure 2), with no significant heterogeneity (I² = 0.00%, p=0.98). The pooled results from six two-group RCTs and two three-group RCTs also showed a statistically significant reduction in the risk of developing PND with perioperative esketamine (RR: 0.51, 95% CI: 0.38 to 0.70, p<0.001, GRADE: high, figure 2B). Sensitivity analyses supported these findings as well (online supplemental figure 3), with no significant heterogeneity (I² = 0.00%, p=0.93).
Figure 2. (A) Forest plot showing the effect of perioperative esketamine on the perioperative neurocognitive dysfunction (PND) and subgroup analysis of postoperative delirium (POD) and delayed neurocognitive recovery (dNCR) within 7 days postoperatively. The relative risk (RR) was calculated by pooling six randomised controlled trials (RCTs) compared with two groups. (B) Forest plot showing the effect of perioperative esketamine on the PND and subgroup of POD and dNCR within 7 days postoperatively. The RR was calculated by pooling six RCTs compared with two groups and two RCTs compared with three groups.
Subgroup analysis by type of PND (POD and dNCR) further supported these findings (figure 2A,B). Removing two studies that failed to distinguish the older patients undergoing surgery, subgroup analysis based on older patients (≥65 or 60 years) showed that esketamine also reduced the risk of PND (onlinesupplemental figures 4 5). Subgroup analyses of esketamine use showed consistent results both before (for induction of anaesthesia) and during surgery (induction+maintenance of anaesthesia) (onlinesupplemental figures 6 7).
Regarding different time points within 7 days postoperatively, neurocognitive assessment results at 24, 48 and 72 hours after surgery were reported in three, one and seven articles, respectively. Perioperative esketamine was not associated with the risk of PND at 24 hours postoperatively (figure 3A); subgroup analyses of PND types, older surgical patients and esketamine use all showed the same results (onlinesupplemental figures 8 9). However, the small number of articles included in each subgroup limited the confidence of the results. At 48 hours postoperatively, Ma et al found no significant difference between groups with and without esketamine. Perioperative esketamine was associated with a reduced risk at 72 hours (figure 3B). Surprisingly, subgroup analyses showed inconsistent effects of esketamine on POD and dNCR within 72 hours after surgery, that is, it reduced the risk of POD (RR: 0.51, 95% CI: 0.38 to 0.70) but did not significantly change the risk of dNCR (RR: 0.63, 95% CI: 0.31 to 1.27). Subgroup analysis of older patients showed a reduced risk of PND (online supplemental figure 10). And subgroup analyses of esketamine use showed an inconsistent result between induction and induction+maintenance, but caution should be expressed as to whether only a small number of studies were included in each subgroup (online supplemental figure 11).
Figure 3. (A) Forest plot showing the effect of perioperative esketamine on the perioperative neurocognitive dysfunction (PND) and subgroup of postoperative delirium (POD) and delayed neurocognitive recovery (dNCR) at 24 hours postoperatively. (B) Forest plot showing the effect of perioperative esketamine on the PND and subgroup of POD and dNCR at 72 hours postoperatively. (C) Forest plot showing the effect of perioperative esketamine on the postoperative neurocognitive disorder (post-NCD) at 3 months postoperatively.
3-month postoperatively
Two RCTs reported the incidence of post-NCD at 3 months postoperatively. The pooled result indicated that perioperative esketamine did not significantly reduce the risk of developing PND (RR: 0.57, 95% CI: 0.19 to 1.73, p=0.40, GRADE: moderate), with no significant heterogeneity (I² = 0.00%, p=0.57). The pooled results of two articles are shown in figure 3C.
Effects of perioperative esketamine on the scores of neurocognitive assessments
Four articles reported neurocognitive scores assessed by Intensive Care Delirium Screening Checklist (ICDSC), Montreal Cognitive Assessment (MoCA) and MMSE. Due to differing scoring scales between assessment methods and the fact that all RCTs had three groups, we did not perform a pooled analysis of these scores. The results were inconsistent. Luo et al found that neither the 0.2 mg kg–1 nor 0.5 mg kg–1 dose of esketamine during induction improved CAM and MMSE scores at 1 and 3 days postsurgery compared with placebo. Chen et al found no improvement in MMSE scores at 1 day and 3 months postoperatively with a bolus of 0.3 mg kg–1 followed by an infusion of 0.2 mg kg–1 h–1 until 30 min before the end of the surgical procedure. Conversely, Helmar et al found that a 0.25 mg kg–1 intravenous bolus after induction followed by a 0.125 mg kg–1 h–1 continuous intravenous infusion improved ICDSC scores at 48 hours postsurgery. Zhao et al found that 0.5 mg kg–1 esketamine at induction and 2 or 4 µg kg–1 min–1 during maintenance improved MoCA scores 24 hours postsurgery compared with placebo.
Secondary outcomes
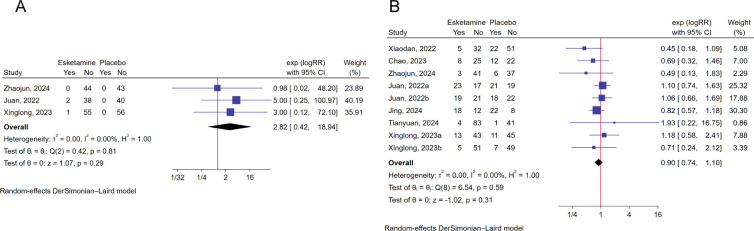
This meta-analysis found that perioperative esketamine was not associated with the risk of postoperative adverse events, including nightmare (RR: 2.82, 95% CI: 0.42 to 18.94, p=0.29, I²=0%, GRADE: high, figure 4A), PONV (RR: 0.90, 95% CI: 0.74 to 1.10, p=0.59, I²=0%, GRADE: high, figure 4B), postoperative cardiovascular adverse events (such as hypotension, hypertension, tachycardia and bradycardia) (RR: 1.03, 95% CI: 0.60 to 1.74, p=0.30, I2=59.13%, GRADE: low, figure 5A) and hallucination (RR: 1.65, 95% CI: 0.22 to 12.43, p=0.63, I2=0%, GRADE: moderate, figure 5B). Meanwhile, esketamine had no significant effect on length of hospital stay through pooling only two articles (SMD: −0.46, 95% CI: −0.93 to 0.02, p=0.06, I2=68.98%, GRADE: moderate, figure 5C).
Figure 4. (A) Forest plot showing the effect of perioperative esketamine on the postoperative nightmare. (B) Forest plot showing the effect of perioperative esketamine on the PONV.
Figure 5. (A) Forest plot showing the effect of perioperative esketamine on the postoperative cardiovascular adverse events and subgroup. Analysis of hypotension, hypertension, tachycardia and bradycardia. (B) Forest plot showing the effect of perioperative esketamine on hallucination. (C) Forest plot showing the effect of perioperative esketamine on length of hospital stay.
Regarding the postoperative pain, patients who received esketamine had lower pain severity on postoperative day 1 than those who did not, regardless of the method of pain assessment (SMD: −0.88, 95% CI: −1.33 to −0.44, p<0.001, I2=76.34%, GRADE: moderate, figure 6A). However, esketamine use could not relieve pain on the second and third days after surgery (SMD: −0.31, 95% CI: −0.88 to 0.27, p=0.30, I2=76.76%, GRADE: low; SMD: −0.28, 95% CI: −0.86 to 0.30, p=0.35, I2=67.75%, GRADE: low; figure 6B,C), through pooling two and three articles.
Figure 6. Forest plot showing the effect of perioperative esketamine on the postoperative pain and subgroup analysis of Visual Analogue Scale (VAS) and Numerical Rating Scale (NRS) scales. (A) At postoperative day 1. (B) At postoperative day 2. (C) At postoperative day 3.
The summary of evidence certainty
The level of certainty regarding the evidence for each outcome is presented in online supplemental table 3. The overall certainty of the evidence was considered high to low for the risk of PND within 7 days and 3 months postoperatively. For the secondary outcomes, including the effects on nightmares, PONV, postoperative cardiovascular adverse events, hallucination, length of hospital stay and pain, the overall certainty was considered high to low. And the primary factors for downgrading include inconsistency and imprecision.
Discussion
This meta-analysis revealed that perioperative esketamine use is associated with a reduced risk of POD and dNCR within 7 days after surgery. No significant differences were observed in the risk of PND at 3 months after surgery, based on the pooling of only two RCTs. No differences were observed in the risk of adverse events, including nightmares, PONV, cardiovascular adverse events and length of hospital stay between the groups. Evaluation of postoperative pain domains revealed that perioperative esketamine use reduced pain levels during the first 24 hours after surgery, whereas there was no positive effect during the 48 and 72 hours after surgery.
Several factors contribute to the novelty of this systematic review and meta-analysis: first, there are few previous clinical studies on esketamine, and second, most studies focus on its effects on negative emotions, depressive disorders and other mental health conditions. Recently, Zhou et al evaluated the effect of intraoperative subanesthetic doses of ketamine/esketamine versus no intervention in adults undergoing general anaesthesia on the incidence of PND.22 However, they found no statistically significant difference in the incidence of PND within 7 days when comparing the intraoperative subanesthetic dose of ketamine/esketamine with the control group, which contrasts with our findings. Only two of the nine RCTs in the previous meta-analysis examined esketamine, likely contributing to the discrepancy.
NMDA receptors, ionotropic glutamate receptors primarily located on neurons, consist of essential Glutamate Ionotropic Receptor NMDA Type Subunit 1 (GluN1) paired with GluN2 and/or GluN3.13 40 The relationship between NMDA receptors and PND is complex and not fully elucidated. Most studies have demonstrated that NMDA receptor function is bidirectional for PND15: physiological activation promotes neuron survival,41 while dysregulation through underactivation or overactivation may induce oxidative stress and cell death, contributing to cognitive deficits in various neuropsychiatric and neurodegenerative disorders.42 The results of this meta-analysis, showing a reduction in the risk of short-term neurocognitive impairment postsurgery with esketamine use, align with theoretical speculations and preclinical findings. Various perioperative stressors, such as local tissue hypoxia,43 pain14 and inflammation,11 44 can induce NMDA receptor overactivation, leading to PND. As an NMDA receptor antagonist, esketamine could theoretically mitigate PND by regulating this overactivation.15 Furthermore, animal studies have demonstrated that targeting NMDA receptor overactivation can improve neurocognitive impairment.45 46
In addition, esketamine may work by reducing neuroinflammation, which is a major cause of PND. Surgical trauma and anaesthesia may trigger inflammation throughout the body, which can lead to neuroinflammation and subsequent cognitive decline. Previous studies have shown that esketamine can inhibit the M1 polarisation of microglia, improve the Brain-derived Neurotrophic Factor-Tropomyosin Receptor Kinase B (BDNF-TrkB) signal dysfunction caused by surgical anaesthesia stimulation, and reverse postoperative behavioural abnormalities and cognitive decline in older rats.47
However, our results show that perioperative esketamine use has a differential effect on POD occurrence at 24, 48 and 72 hours after surgery. First, the results are weak due to the small number of studies included in the analysis, especially for dNCR. Second, the possible reasons for this result are as follows: previous studies have confirmed that there are many postoperative factors that can cause or aggravate POD, such as inflammation caused by surgery, pain, intraoperative anaesthesia drugs and postoperative analgesics.48,50 It can be observed that these risk factors gradually decrease with the extension of postoperative time or the gradual withdrawal of drugs. Previous observational studies have shown that the incidence of PND gradually decreases with the extension of postoperative time, which also supports this speculation.51,53 Therefore, esketamine may not be effective in the 24 or 48 hours after surgery when there are more risk factors and higher levels.
In addition, our analysis indicated that perioperative esketamine did not improve the post-NCD of 3 months postoperatively, a finding based on only two studies, warranting further investigation. Although this is a weak result, the risk of postoperative long-term neurocognitive performance is related to more factors compared with early onset, such as postoperative complication, home nursing, work, living habits after discharge and compliance with medical instructions, and esketamine use is only a point-in-time intervention, and it is theoretically hard to envisage how the choice of a single point can affect long-term outcomes.
This meta-analysis has several limitations. First, the wide age range of participants could influence outcomes, given varying neurocognitive levels across different age groups. Second, the diversity of surgical procedures, including cardiothoracic, abdominal, hip and knee surgery, introduces heterogeneity that might impact neurocognitive outcomes differently. Thirdly, most of these studies were conducted on the Chinese population, so these results need to be carefully generalised to all ethnic groups. Additionally, the dose of single injection and continuous infusion of esketamine differed between studies, which may have influenced the analysis results. Very few studies have investigated the clinical effects of different doses of esketamine, but there appears to be no difference in postoperative neurocognitive prevention between high and low doses of esketamine. However, the effect of esketamine dosage on the prevention of PND and the optimal dose need to be further explored.
Conclusion
Perioperative esketamine use is associated with a reduced risk of PND within 7 days after surgery, including POD and dNCR. However, no significant differences were observed in the risk of PND at 3 months after surgery based on the pooling of only two RCTs. And no differences were observed in the risk of adverse events, including nightmares, PONV, cardiovascular adverse events and length of hospital stay. This systematic review and meta-analysis provides reference data for PND research and clinical drug intervention strategies.
Supplementary material
Footnotes
Funding: This research was funded by the National Cancer Regional Medical Center Science and Education Foundation (No. BD2023008).
Prepub: Prepublication history and additional supplemental material for this paper are available online. To view these files, please visit the journal online (https://doi.org/10.1136/bmjopen-2024-095695).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Ethics approval: Not applicable.
Data availability free text: Not applicable.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1.Needham MJ, Webb CE, Bryden DC. Postoperative cognitive dysfunction and dementia: what we need to know and do. Br J Anaesth. 2017;119:i115–25. doi: 10.1093/bja/aex354. [DOI] [PubMed] [Google Scholar]
- 2.Evered L, Silbert B, Knopman DS, et al. Recommendations for the Nomenclature of Cognitive Change Associated with Anaesthesia and Surgery-2018. Anesthesiology. 2018;129:872–9. doi: 10.1097/ALN.0000000000002334. [DOI] [PubMed] [Google Scholar]
- 3.Fournier A, Krause R, Winterer G, et al. Biomarkers of postoperative delirium and cognitive dysfunction. Front Aging Neurosci. 2015;7:112. doi: 10.3389/fnagi.2015.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evered LA, Silbert BS. Postoperative Cognitive Dysfunction and Noncardiac Surgery. Anesth Analg. 2018;127:496–505. doi: 10.1213/ANE.0000000000003514. [DOI] [PubMed] [Google Scholar]
- 5.Dilmen OK, Meco BC, Evered LA, et al. Postoperative neurocognitive disorders: A clinical guide. J Clin Anesth. 2024;92:111320. doi: 10.1016/j.jclinane.2023.111320. [DOI] [PubMed] [Google Scholar]
- 6.Gleason LJ, Schmitt EM, Kosar CM, et al. Effect of Delirium and Other Major Complications on Outcomes After Elective Surgery in Older Adults. JAMA Surg. 2015;150:1134–40. doi: 10.1001/jamasurg.2015.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yan E, Veitch M, Saripella A, et al. Association between postoperative delirium and adverse outcomes in older surgical patients: A systematic review and meta-analysis. J Clin Anesth. 2023;90:111221. doi: 10.1016/j.jclinane.2023.111221. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y, Yang W, Xue J, et al. Neuroinflammation: The central enabler of postoperative cognitive dysfunction. Biomed Pharmacother. 2023;167:115582. doi: 10.1016/j.biopha.2023.115582. [DOI] [PubMed] [Google Scholar]
- 9.He L, Duan X, Li S, et al. Unveiling the role of astrocytes in postoperative cognitive dysfunction. Ageing Res Rev. 2024;95:102223. doi: 10.1016/j.arr.2024.102223. [DOI] [PubMed] [Google Scholar]
- 10.Singh NP, Makkar JK, Borle A, et al. Role of supplemental regional blocks on postoperative neurocognitive dysfunction after major non-cardiac surgeries: a systematic review and meta-analysis of randomized controlled trials. Reg Anesth Pain Med. 2024;49:49–58. doi: 10.1136/rapm-2022-104095. [DOI] [PubMed] [Google Scholar]
- 11.Terrando N, Eriksson LI, Ryu JK, et al. Resolving postoperative neuroinflammation and cognitive decline. Ann Neurol. 2011;70:986–95. doi: 10.1002/ana.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar A, Foster TC. Alteration in NMDA Receptor Mediated Glutamatergic Neurotransmission in the Hippocampus During Senescence. Neurochem Res. 2019;44:38–48. doi: 10.1007/s11064-018-2634-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakazawa K, Sapkota K. The origin of NMDA receptor hypofunction in schizophrenia. Pharmacol Ther. 2020;205:107426. doi: 10.1016/j.pharmthera.2019.107426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chi H, Kawano T, Tamura T, et al. Postoperative pain impairs subsequent performance on a spatial memory task via effects on N-methyl-D-aspartate receptor in aged rats. Life Sci. 2013;93:986–93. doi: 10.1016/j.lfs.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 15.Guan S, Li Y, Xin Y, et al. Deciphering the dual role of N-methyl-D-Aspartate receptor in postoperative cognitive dysfunction: A comprehensive review. Eur J Pharmacol. 2024;971:176520. doi: 10.1016/j.ejphar.2024.176520. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Liu H, Wei X. Effect of intraoperative and/or postoperative esketamine administration on preventing postpartum depression: A systematic review and meta-analysis. Psychiatry Res. 2024;335:115890. doi: 10.1016/j.psychres.2024.115890. [DOI] [PubMed] [Google Scholar]
- 17.Zeng K, Long J, Li Y, et al. Preventing postoperative cognitive dysfunction using anesthetic drugs in elderly patients undergoing noncardiac surgery: a systematic review and meta-analysis. Int J Surg. 2023;109:21–31. doi: 10.1097/JS9.0000000000000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, Wu Z-Y, Zheng W-C, et al. Esketamine alleviates postoperative cognitive decline via stimulator of interferon genes/ TANK-binding kinase 1 signaling pathway in aged rats. Brain Res Bull. 2022;187:169–80. doi: 10.1016/j.brainresbull.2022.07.004. [DOI] [PubMed] [Google Scholar]
- 19.Xu HJ, Li XP, Han LY. Role and mechanism of esketamine in improving postoperative cognitive dysfunction in aged mice through the TLR4/MyD88/p38 MAPK pathway. Kaohsiung J Med Sci. 2024;40:63–73. doi: 10.1002/kjm2.12778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J, Huang J, Yang S, et al. Pharmacokinetics and Safety of Esketamine in Chinese Patients Undergoing Painless Gastroscopy in Comparison with Ketamine: A Randomized, Open-Label Clinical Study. Drug Des Devel Ther. 2019;13:4135–44. doi: 10.2147/DDDT.S224553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bahji A, Vazquez GH, Zarate CA., Jr Comparative efficacy of racemic ketamine and esketamine for depression: A systematic review and meta-analysis. J Affect Disord. 2021;278:542–55. doi: 10.1016/j.jad.2020.09.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou N, Chen C, Liu Y, et al. Efficacy of intraoperative subanesthetic dose of ketamine/esketamine in preventing postoperative cognitive dysfunction: a systematic review and meta-analysis. Ther Adv Psychopharmacol. 2023;13:20451253231200261. doi: 10.1177/20451253231200261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brozek JL, Akl EA, Alonso-Coello P, et al. Grading quality of evidence and strength of recommendations in clinical practice guidelines. Part 1 of 3. An overview of the GRADE approach and grading quality of evidence about interventions. Allergy. 2009;64:669–77. doi: 10.1111/j.1398-9995.2009.01973.x. [DOI] [PubMed] [Google Scholar]
- 27.Brozek JL, Akl EA, Jaeschke R, et al. Grading quality of evidence and strength of recommendations in clinical practice guidelines: Part 2 of 3. The GRADE approach to grading quality of evidence about diagnostic tests and strategies. Allergy. 2009;64:1109–16. doi: 10.1111/j.1398-9995.2009.02083.x. [DOI] [PubMed] [Google Scholar]
- 28.Brożek JL, Akl EA, Compalati E, et al. Grading quality of evidence and strength of recommendations in clinical practice guidelines part 3 of 3. The GRADE approach to developing recommendations. Allergy. 2011;66:588–95. doi: 10.1111/j.1398-9995.2010.02530.x. [DOI] [PubMed] [Google Scholar]
- 29.Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:ED000142. doi: 10.1002/14651858.ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bornemann-Cimenti H, Wejbora M, Michaeli K, et al. The effects of minimal-dose versus low-dose S-ketamine on opioid consumption, hyperalgesia, and postoperative delirium: a triple-blinded, randomized, active- and placebo-controlled clinical trial. Minerva Anestesiol. 2016;82:1069–76. [PubMed] [Google Scholar]
- 31.Chen X, Liu Q, Fan L. Effects of thoracic paravertebral block combined with s-ketamine on postoperative pain and cognitive function after thoracoscopic surgery. Heliyon. 2022;8:e12231. doi: 10.1016/j.heliyon.2022.e12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han C, Ji H, Guo Y, et al. Effect of Subanesthetic Dose of Esketamine on Perioperative Neurocognitive Disorders in Elderly Undergoing Gastrointestinal Surgery: A Randomized Controlled Trial. Drug Des Devel Ther. 2023;17:863–73. doi: 10.2147/DDDT.S401161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jing Z, Han Y, Li Y, et al. Effect of subanesthetic dose of esketamine on postoperative pain in elderly patients undergoing laparoscopic gastrointestinal tumor Surgery:A prospective, double-blind, randomized controlled trial. Heliyon. 2024;10:e27593. doi: 10.1016/j.heliyon.2024.e27593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li J, Wang Z, Wang A, et al. Clinical effects of low-dose esketamine for anaesthesia induction in the elderly: A randomized controlled trial. J Clin Pharm Ther. 2022;47:759–66. doi: 10.1111/jcpt.13604. [DOI] [PubMed] [Google Scholar]
- 35.Liu J, Wang T, Song J, et al. Effect of esketamine on postoperative analgesia and postoperative delirium in elderly patients undergoing gastrointestinal surgery. BMC Anesthesiol. 2024;24:46. doi: 10.1186/s12871-024-02424-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luo T, Deng Z, Ren Q, et al. Effects of esketamine on postoperative negative emotions and early cognitive disorders in patients undergoing non-cardiac thoracic surgery: A randomized controlled trial. J Clin Anesth. 2024;95:111447. doi: 10.1016/j.jclinane.2024.111447. [DOI] [PubMed] [Google Scholar]
- 37.Xiong X, Shao Y, Chen D, et al. Effect of Esketamine on Postoperative Delirium in Patients Undergoing Cardiac Valve Replacement with Cardiopulmonary Bypass: A Randomized Controlled Trial. Anesth Analg. 2024;139:743–53. doi: 10.1213/ANE.0000000000006925. [DOI] [PubMed] [Google Scholar]
- 38.Ma J, Wang F, Wang J, et al. The Effect of Low-Dose Esketamine on Postoperative Neurocognitive Dysfunction in Elderly Patients Undergoing General Anesthesia for Gastrointestinal Tumors: A Randomized Controlled Trial. Drug Des Devel Ther. 2023;17:1945–57. doi: 10.2147/DDDT.S406568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu T, Zhang X, Li A, et al. Effects of intra-operative administration of subanesthetic s-ketamine on emergence from sevoflurane anesthesia: a randomized double-blind placebo-controlled study. BMC Anesthesiol. 2023;23:221. doi: 10.1186/s12871-023-02170-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hunt DL, Castillo PE. Synaptic plasticity of NMDA receptors: mechanisms and functional implications. Curr Opin Neurobiol. 2012;22:496–508. doi: 10.1016/j.conb.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Green MV, West AE. TRPing into excitotoxic neuronal death. Cell Calcium. 2021;93 doi: 10.1016/j.ceca.2020.102331. [DOI] [PubMed] [Google Scholar]
- 42.Le Douce J, Maugard M, Veran J, et al. Impairment of Glycolysis-Derived l-Serine Production in Astrocytes Contributes to Cognitive Deficits in Alzheimer’s Disease. Cell Metab. 2020;31:503–17. doi: 10.1016/j.cmet.2020.02.004. [DOI] [PubMed] [Google Scholar]
- 43.Li T, Luo Z, Liu Y, et al. Excessive Activation of NMDA Receptors Induced Neurodevelopmental Brain Damage and Cognitive Deficits in Rats Exposed to Intrauterine Hypoxia. Neurochem Res. 2018;43:566–80. doi: 10.1007/s11064-017-2451-1. [DOI] [PubMed] [Google Scholar]
- 44.Granger KT, Barnett JH. Postoperative cognitive dysfunction: an acute approach for the development of novel treatments for neuroinflammation. Drug Discov Today. 2021;26:1111–4. doi: 10.1016/j.drudis.2021.01.019. [DOI] [PubMed] [Google Scholar]
- 45.Chvojkova M, Kolar D, Kovacova K, et al. Pro-cognitive effects of dual tacrine derivatives acting as cholinesterase inhibitors and NMDA receptor antagonists. Biomed Pharmacother. 2024;176:116821. doi: 10.1016/j.biopha.2024.116821. [DOI] [PubMed] [Google Scholar]
- 46.Wang J, Akbari A, Chardahcherik M, et al. Ginger (Zingiber Officinale Roscoe) ameliorates ethanol-induced cognitive impairment by modulating NMDA and GABA-A receptors in rat hippocampus. Metab Brain Dis. 2024;39:67–76. doi: 10.1007/s11011-023-01301-8. [DOI] [PubMed] [Google Scholar]
- 47.Wen Y, Xu J, Shen J, et al. Esketamine Prevents Postoperative Emotional and Cognitive Dysfunction by Suppressing Microglial M1 Polarization and Regulating the BDNF-TrkB Pathway in Ageing Rats with Preoperative Sleep Disturbance. Mol Neurobiol. 2024;61:5680–98. doi: 10.1007/s12035-023-03860-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang S, Tao X-J, Ding S, et al. Associations between postoperative cognitive dysfunction, serum interleukin-6 and postoperative delirium among patients after coronary artery bypass grafting: A mediation analysis. Nurs Crit Care. 2024;29:1245–52. doi: 10.1111/nicc.13081. [DOI] [PubMed] [Google Scholar]
- 49.White N, Bazo-Alvarez JC, Koopmans M, et al. Understanding the association between pain and delirium in older hospital inpatients: systematic review and meta-analysis. Age Ageing. 2024;53:afae073. doi: 10.1093/ageing/afae073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scholz AFM, Oldroyd C, McCarthy K, et al. Systematic review and meta-analysis of risk factors for postoperative delirium among older patients undergoing gastrointestinal surgery. Br J Surg. 2016;103:e21–8. doi: 10.1002/bjs.10062. [DOI] [PubMed] [Google Scholar]
- 51.Liu P, Li Y, Wang X, et al. High serum interleukin-6 level is associated with increased risk of delirium in elderly patients after noncardiac surgery: a prospective cohort study. Chin Med J (Engl) 2013;126:3621–7. doi: 10.3760/cma.j.issn.0366-6999.20130211. [DOI] [PubMed] [Google Scholar]
- 52.Chang Y-L, Tsai Y-F, Lin P-J, et al. Prevalence and risk factors for postoperative delirium in a cardiovascular intensive care unit. Am J Crit Care. 2008;17:567–75. [PubMed] [Google Scholar]
- 53.Robinson TN, Raeburn CD, Tran ZV, et al. Postoperative delirium in the elderly: risk factors and outcomes. Ann Surg. 2009;249:173–8. doi: 10.1097/SLA.0b013e31818e4776. [DOI] [PubMed] [Google Scholar]