ABSTRACT
Metabolic Dysfunction-Associated Steatotic Liver Disease(MASLD) is increasing in prevalence worldwide and has become the greatest potential risk for cirrhosis and hepatocellular liver cancer. Currently, the role of gut microbiota in the development of MASLD has become a research hotspot. The development of MASLD can affect the homeostasis of gut microbiota, and significant changes in the composition or abundance of gut microbiota and its metabolite abnormalities can influence disease progression. The regulation of gut microbiota is an important strategy and novel target for the treatment of MASLD with good prospects. In this paper, we summarize the role of gut microbiota and its metabolites in the pathogenesis of MASLD, and describe the potential preventive and therapeutic efficacy of gut microbiota as a noninvasive marker to regulate the pathogenesis of MASLD based on the “gut-hepatic axis”, which will provide new therapeutic ideas for the clinic.
KEYWORDS: Metabolic dysfunction-associated steatotic liver disease, gut-liver axis, gut microbiota, probiotics, metabolites
Metabolic dysfunction-associated steatotic liver disease (MASLD) is a chronic progressive liver disease primarily characterized by overnutrition and insulin resistance in genetically predisposed individuals. MASLD may progress to metabolic dysfunction-associated steatohepatitis (MASH), further inducing liver fibrosis and cirrhosis. The prognosis of MASLD is mainly related to cardiovascular disease. The combination of progressive fibrosis significantly increases the incidence of hepatocellular carcinoma (HCC). The global prevalence of MASLD is as high as 37.8%, with considerable regional variations. This disease is highly prevalent in overweight and obese populations, although it also affects some non-obese or lean MASLD patients. Over the past two decades, the prevalence of MASLD in Chinese adults has been approximately 29.6%, with a higher incidence in males (34.8%) compared with females (23.5%).1 The disease is also gradually replacing viral hepatitis and is poised to rank first among chronic liver diseases in China. Hence, MASLD has emerged as a critical public concern worldwide, underscoring the urgent demand for enhanced screening and prevention strategies. The clinical management of MASLD faces serious challenges due to the lack of effective diagnostic and therapeutic tools, thus imposing a heavy burden on healthcare systems. Currently, the crosstalk between the liver and gut has been recognized by an increasing number of scholars.2–4 In MASLD, significant changes in the composition and metabolites of gut microbiota suggest that therapeutic strategies targeting the gut microbiota may contribute to the decelerated progression of steatohepatitis and fibrosis. Therefore, the potential clinical value of gut microbiota profiles as noninvasive markers of metabolism-associated steatohepatitis is analyzed in this paper based on the influence of gut microbiota and its metabolites in the pathogenesis of MASLD. Furthermore, the potential preventive and therapeutic interventions related to gut microbiota for the treatment of MASLD are also explored from the perspectives of probiotics, prebiotics, synbiotics, fecal microbiota transplantation (FMT), and emerging therapies.
Gut microbiota and the “Gut-liver axis”
The gut microbiota, one of the most complex micro ecosystems in the human body, is involved in maintaining intestinal homeostasis through a variety of mechanisms, including metabolites and immune regulation. The composition and function of intestinal microorganisms are not only directly related to intestinal diseases, but also closely related to the pathogenesis of MASLD through the gut-liver axis, which is a bidirectional interaction pathway.
Physiological profile and role of gut microbiota
Gut microbiota refers to microorganisms in the gastrointestinal system that pass through the screening of saliva, gastric acid, digestive enzymes, and various digestive organs.5 Broadly speaking, gut microbiota consists of diverse compositions, including not only intestinal bacteria but also fungi, viruses, and archaea. The total number of bacteria in the normal intestine can reach 10–100 trillion, mainly located in the distal small intestine and colon.6 Gut microbiota is predominantly composed of specialized anaerobes. The core groups include the Gram-positive Firmicutes and the Gram-negative Bacteroidetes, and the other groups mainly comprise Aspergillus and Actinobacterium.7 Gut microbiota is in a dynamic state, and its composition is influenced by various factors, including diet, drug use, and circadian rhythms. There are significant inter- and intra-individual variations in the composition of the gut microbiota over time.8 Gut microbiota is involved in a variety of physiological processes in the body and is vital in nutrient absorption, substance metabolism, and immunomodulation. Over the past few years, scholars have acquired more knowledge about the characteristics of gut microbiota and have reported that changes in the ecology and composition of gut microbiota can affect host health. At present, it has been validated that the occurrence of various diseases is related to the state of intestinal microbiota, such as metabolic syndrome,9 atherosclerosis,10 Parkinson’s disease,11 cognitive dysfunction,12 asthma, and chronic obstructive pulmonary disease (COPD).13 Meanwhile, increasing attention has been paid to the crosstalk between the liver and gut.14 Therefore, exploring the physiological role of gut microbiota is of significance for identifying the occurrence and development of MASLD.
Physiological structure and characteristics of the “gut-liver axis”
The term “gut-liver axis” was first proposed more than 100 years ago to identify alterations in the composition of the gut microbiota in patients with chronic liver disease. Through an investigation into the impaired intestinal motility, intestinal permeability, and bacterial translocation in patients with chronic liver disease, scholars have gradually unraveled the role of gut microbiota and metabolites in the development of MASLD.15 During embryonic development, both the liver and the intestine originate from the ventral anterior endoderm, and they are intrinsically linked. The intestine and liver have close bidirectional communication through the bile ducts, portal vein, and somatic circulation. Hepatocytes can synthesize and secrete bile, which contains abundant IgA, bicarbonate, antimicrobial peptides, and bile acids, and they can communicate directly with the intestine through the biliary system.16 Nutrients absorbed from the intestine and the metabolites of gut microbiota, including carbohydrates, lipids, amino acids, secondary bile acids, ethanol, and short-chain fatty acids, flow into the liver through the portal vein. The liver absorbs nutrients, processes the metabolites of gut microbiota, and then supplies hepatic metabolites or inflammatory mediators to the intestine via arterial blood in the systemic circulation. These findings demonstrate that the gut and liver are closely linked at the physiological level, and the gut microbiota and liver are influenced by each other in terms of their functions.
Relationship between gut microbiota and metabolic dysfunction-associated steatotic liver disease
The development and progression of MASLD are associated with changes in the gut microbiota. This is primarily evidenced by an imbalance in the composition of the gut microbiota in patients with MASLD, with alterations in the abundance of specific gut microbiota potentially impacting the risk of developing MASLD. Furthermore, the compositional characteristics of the gut microbiota undergo alterations during the course of MASLD. Consequently, these observations imply that the gut microbiota exerts a significant influence on the development of MASLD and that its dynamics may serve as a crucial diagnostic tool and a vital indicator of the progression of the disease.
Alterations in gut microbiota and the development of metabolic dysfunction-associated steatotic liver disease
At present, the pathogenesis of MASLD has not been fully clarified, and some scholars have proposed the “multiple strikes” hypothesis, namely that insulin resistance, lipid metabolism disorders, nutritional factors, gut microbiota dysbiosis, genetic determinants, and epigenetic factors jointly affect the development of MASLD (Figure 1).17 Unhealthy dietary habits and fewer physical activities are associated with the progression of MASLD.18 Host genetics can also influence the composition and function of gut microbiota. More specifically, MASLD is characterized by familial aggregation,19 and there are more significant similarities in the gut microbiota between identical twins than between dizygotic twins.20 The patatin-like phospholipase domain-containing 3 (PNPLA3), transmembrane 6 superfamily member 2 (TM6SF2), membrane-bound O-acyltransferase-containing structural domain 7 (MBOAT7) and transmembrane 6 superfamily member 2 (TM6SF2) genes are strongly related to the onset of MASLD.21–24
Figure 1.

MASLD progression. The development of MASLD is associated with insulin resistance, genetic susceptibility, gut microbiota dysbiosis, and other factors. The composition and function of the gut microbiota affect MASLD through the “gut-liver axis”. The spectrum of diseases includes metabolic dysfunction-related fatty liver, liver fibrosis, cirrhosis, and hepatocellular carcinoma. Abbreviations: HCC: hepatocellular carcinoma.
Created in https://BioRender.com.
Patients with MASLD may have small intestinal bacterial overgrowth (SIBO). SIBO is defined as an increased number or abnormal type of bacteria in the gut that may induce gastrointestinal symptoms. In a meta-analysis of 10 studies, the prevalence of SIBO was higher in the MASLD group than in the control group (OR = 3.82, 95% CI: 1.93–7.59).25 Another meta-analysis including 18 studies showed that the incidence of SIBO was 35.0% (95% CI: 24.4%-47.2%) and 41.1% (95% CI: 21.9%-63.4%) in patients with MASLD and MASH, respectively.26
Patients with MASLD may exhibit significant change in the abundance and composition of gut microbiota. Compared with healthy people, the abundance of the families Enterococcus, Bacteroides, and Muribaculaceae is lower in patients with MASLD, while the abundance of the genera Acidobacteria and Escherichia coli is higher in these patients.27 Boursier et al. found that in MASH patients with fibrosis at or above stage 2, the abundance of the genera Bacteroides, Bifidobacterium, and Prevotella increased significantly, while the abundance of Prevotella decreased.28 Through an analysis based on 16s RNA gene amplicon sequencing, Li et al. found that the families Ruminococcaceae and Veillonellaceae were associated with the severity of fibrosis in non-obese subjects with MASLD.29 Jeffery et al. conducted a study based on metagenomic sequencing and found that the abundance of the phyla Bacteroidetes, Verrucomicrobia, and Firmicutes was higher in MASH children with mild fibrosis or without fibrosis. Additionally, they also revealed that the abundance of the phyla Bacteroidetes and Proteobacteria was higher in MASH children with moderate to severe fibrosis.30 These studies suggest that as the disease progresses, patients with MASLD have a higher abundance of potentially pathogenic microbiota (such as Enterobacteriaceae and Enterococcaceae) but a lower abundance of commensal microbiota in the gut. In addition, Demir et al. performed sequencing for the fungal internal transcribed spacer region 2 in the feces of 78 patients with MASLD, and they demonstrated that non-obese patients with MASLD at advanced stages had a specific fungal composition and an enhanced systemic immune response to Candida albicans.31 Greta et al. revealed that the fungi Scopulariopsis, Kluyveromyces, Malassezia restricta, and Scopulariopsis corpus could be used to discriminate alcohol-related liver disease from MASLD.32 Although the alterations in the abundance or composition of intestinal t microbiota in patients with MASLD have been corroborated in many studies, it remains unclear whether gut dysbiosis exacerbates MASLD or whether these alterations are caused by this disease.
Exploration of the causal relationship between gut microbiota and MASLD
With the continuous development of the gut-liver axis doctrine, more and more evidence suggests that gut microbiota play an important role in the development of MASLD. However, the development of MASLD is associated with many factors such as obesity, diabetes mellitus, hyperlipidemia, insulin resistance, and genetic susceptibility. Most of the current studies on the correlation between gut microbiota and MASLD are not only affected by the above confounding factors, but also are mostly case-control studies, which cannot effectively elucidate the causal relationship between the two.
However, two-sample Mendelian randomization (MR) can effectively avoid the confounding factors and reverse causality in observational studies, as well as the problems of representativeness and feasibility in case-control studies.33 Recently, many scholars have adopted MR to assess the causal relationship between gut microbiota and MASLD at multiple biological levels, such as phylum, class, order, family, genus, and species, etc. Long et al. found that by MR, Enterobacteriales, Enterobacteriaceae, and Trichosporonaceae UCG-004 and Prevotella9 increase the increased risk of MASLD. Dorea and Veillonella can increase the risk of MASH, while Oscillospira and Ruminococcaceae UCG-013 reduce the risk of MASH.34 Ouyang et al. found a negative correlation between the abundance of Ruminococcus2 in the gastrointestinal tract and the incidence of MASLD in a two-sample Mendelian randomization study. This protective effect seems likely to be mediated by increased levels of cysteine-glutathione as well as 3-indoleglyoxylic acid.35 Because 3-indoleglyoxylic acid secreted by rumenococci 2 may help reduce the incidence of MASLD through its ability to protect the integrity of the intestinal wall and inhibit the production of inflammatory factors.36 However, this result contradicts the findings of a study by Boursier et al. that showed an elevated abundance of rumenococci in the gut microbiota of MASLD patients.28,37 It is possible that because of the remarkable diversity of the genus Rumococcus, different subtypes of Rumococcus have different effects on the host organism, leading to conflicting conclusions.38 In addition, phylum Tenericutes, class Deltaproteobacteria and class Mollicutes were significantly associated with the risk of MASLD.39 Lactobacillaceae, Christensenellaceae, and Intestinibacter were negatively associated with MASLD. While OCoriobacteriia, Actinomycetales, Ruminococcaceae_UCG005 were positively associated with MASLD.40
These studies explain the causal relationship between gut microbiota and MASLD, and changes in the abundance of specific gut flora may influence the risk of developing MASLD. Although MR is relatively reliable for inferring a causal relationship between gut microbiota and MASLD, it needs to be further validated by randomized controlled trials to improve the credibility and robustness of the results obtained from two-sample Mendelian randomization studies. In conclusion, MR is useful for screening the gut microbiota associated with MASLD, provides new ideas for the prevention and treatment of MASLD, and contributes to a deeper knowledge and understanding of the gut-liver axis.
Progression of metabolic dysfunction-associated steatotic liver disease detected by the characterization of gut microbiota
There are significant alternations in the abundance and composition of gut microbiota in patients with MASLD. This disease at different stages may also be associated with different characteristics of the constitution of gut microbiota. These characteristics can be employed to assess the progression of MASLD. Loomba et al. examined the characteristics of gut microbiota in MASLD patients with advanced fibrosis by a prospective cohort study. The metagenomic sequencing of fecal microbiota was performed in 86 patients with biopsy-proven MASLD. Among these patients, 72 patients had mild to moderate fibrosis (i.e., stages 0–2), and 14 patients had advanced fibrosis (i.e., stages 3–4). They also constructed a randomized forest model incorporating 37 bacterial traits, age, body mass index, and other characteristics that differed between the early and advanced phenotypes of fibrosis. The area under the receiver operating characteristic (ROC) curve (AUC) of this model was 0.936, indicating its robustness and statistically significant diagnostic accuracy for detecting advanced fibrosis in MASLD.30 Caussy et al. also examined gut microbiota characteristics in patients with MASLD at the cirrhotic stage in the twin and family cohorts in a prospective cohort study and sequenced 16sRNA profiles of gut microbiota. They designed a random forest model incorporating 27 bacterial profiles, age, body mass index, and other characteristics. This model was verified in a validation cohort comprising the relatives of MASLD patients at the stage of cirrhosis (AUC = 0.87), demonstrating robust diagnostic accuracy (AUC = 0.92).41
In addition to bacteria, enteroviruses are also significantly altered in the late fibrotic stage of MASLD. The characteristics of enteroviruses have been explored in inflammatory bowel disease,42 colorectal cancer,43 and type I diabetes.44 Lang et al. developed a clinical data model based on fecal viral diversity by the reverse transcription and metagenomic sequencing of fecal viral nucleic acids. The AUC of this model for MASLD activity scores and advanced fibrosis was 0.95 and 0.88, respectively. This model exhibited higher accuracy for identifying patients with severe MASLD and fibrosis compared with other models that were constructed based only on clinical or bacterial data. Overall, this model provides a better option for identifying the features of gut microbiota in patients with severe MASLD and fibrosis.45
These studies suggest that gut microbiota characterization can provide a noninvasive method for detecting advanced fibrosis in MASLD. Although these models using the microbiome as a marker to diagnose fibrosis in MASLD have been established, there has been no further cross-regional validation to assess clinical relevance.
Notably, Howell Leung et al. recently demonstrated, for the first time in a prospective cohort, that the composition and function of the gut microbiota of patients differed from that of healthy subjects even before the ultrasound diagnosis of MASLD, and constructed a microbiota prediction model that was able to predict the risk of NAFLD onset over the next 4 years in healthy subjects with or without MASLD. By analyzing the macrogenomic data for bacterial diversity, although the study found no significant differences between the case and control groups in terms of overall bacterial composition, the two groups did differ significantly compared to patients who had already been diagnosed with MASLD at baseline. The initial colony model used 14 colony composition, function, and metabolite profiles, categorized according to MASLD status after 4.6 years, with an area under the ROC curve of 0.72. When age, BMI, HDL, and fasting insulin were added, the area under the ROC curve for the final model was up to 0.80. The final model’s efficacy remained stable in cross-regional external validation, with an area under the ROC curve of 0.72. The area under the ROC curve ranged from 0.72 to 0.78. Comparison of the colony prediction model with three previously reported clinical prediction models for MASLD resulted in superior results to the clinical prediction models, and all three clinical prediction models showed a significant improvement in predictive efficacy when incorporating colony-related features.46 This independent cohort study identifies microbiome features in the early stages of hepatic steatosis and constructs a risk prediction model for the development of MASLD, which provides a new direction for exploring microbiome-based MASLD prevention and intervention.
Although these studies confirmed the validity of the gut microbiota characterization models at different stages of MASLD progression, yet it is challenging to apply these models in clinical trials. This is because the baseline gut microbiota is determined by many factors, including age, gender, geographic location, and lifestyle factors. This therefore means that validated tests must be developed to ensure ease of diagnosis and reproducibility.
Influencing mechanisms of gut microbiota in the progression of metabolic dysfunction-associated steatotic liver disease
The gut and liver are connected by a complex network of anatomical structures, including the bile ducts, portal vein, and the body’s circulation system. This network facilitates bidirectional communication between the two organs. The liver communicates with the intestine by releasing bile acids and biologically active mediators into the biliary tract and somatic circulation. In the intestine, gut microbiota metabolites are transferred to the liver through the portal vein. During this process, the intestinal barrier plays a crucial role in absorbing essential nutrients and preventing invasion by intestinal luminal microbiota. When the intestinal barrier is compromised, the gut microbiota and its metabolites, such as pathogen-associated molecular patterns and endogenous ethanol, can influence the progression of MASLD through a variety of mechanisms (Figure 2).
Figure 2.
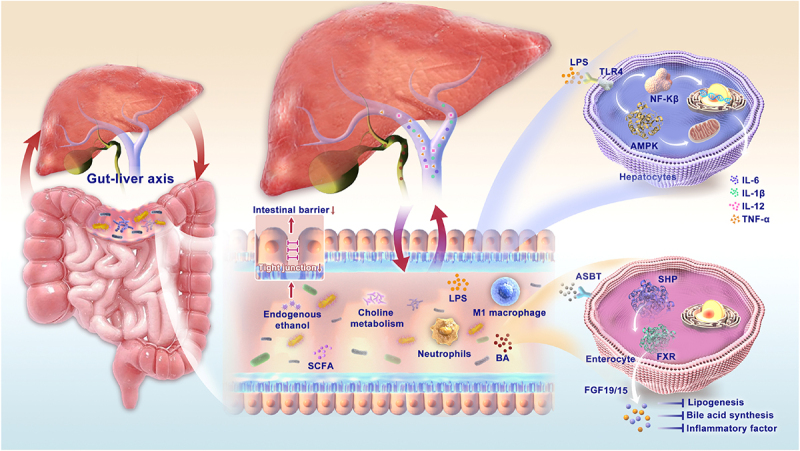
The mechanism of the gut-liver axis: the gut and liver are connected by a complex network of anatomical structures, including the bile ducts, portal vein, and the body’s circulation system. This network facilitates bidirectional communication between the two organs. The liver communicates with the intestine by releasing bile acids and biologically active mediators into the biliary tract and somatic circulation. In the intestine, gut microbiota metabolites are transferred to the liver through the portal vein. The gut microbiota and their metabolites, such as pathogen-associated molecular patterns and endogenous ethanol, SCFA, and BA, can influence the progression of MASLD through a variety of mechanisms. Abbreviations: SCFA: short-chain fatty acids; BA: bile acids; LPS: lipopolysaccharide; AMPK: AMP-activated protein kinase; NF-κB: nuclear factor-κB; IL: interleukin; TNF: tumor necrosis factor; ASBT: apical sodium-bile acid transporter; SHP: small heterodimer partner; FXR: farnesoid X receptor; FGF: fibroblast growth factor.
Increased intestinal barrier permeability exacerbates the progression of metabolic dysfunction-associated steatotic liver disease
The intestinal barrier consists of multiple defense lines (Figure 3). The first defense line is the mucus layer. Specifically, the mucus layer consists of water, lipids, mucins, and electrolytes, which isolates bacteria and other antigens from direct exposure to the intestinal epithelium.47 In the ileocytes of mice after the induction by a high-fat diet (HFD), the expression of CFTR was down-regulated, and mucus synthesis and secretion were blocked, resulting in a thinner and less dense mucus layer.48 The thinning and rupture of the mucus layer is an early feature of MASLD.49 The second defense line is the intestinal epithelial barrier. Specifically, it is mainly composed of epithelial cells with tight junctions (TJs) that restrict the passage of live bacteria but allow the passage of small molecules (such as short-chain fatty acids) through the paracellular gap.50 Three intercellular junction complexes including TJs, desmosomes and adherens junctions are characteristic of the epithelial layer. TJs consist of transmembrane proteins and related proteins involved in intramembrane linkages and vesicle trafficking. TJ transmembrane proteins consist of the claudins, TJ-associated proteins, and junctional adhesion molecules (JAMs). In addition, intracellular scaffold proteins such as zonula occludens (ZO)-1, ZO-2, and ZO-3, perform regulatory functions by connecting the TJ to the actin cytoskeleton via transmembrane proteins. The capacity of the intestinal epithelial barrier is related to the expression of TJ proteins.51 It has been reported that the expression of such TJ proteins as occludin and ZO-1 is down-regulated in patients with MASLD.52 Mice with the disruption of the gene encoding junctional adhesion molecule A (JAM-A) had defective intestinal epithelial permeability, and more severe steatohepatitis was detected in these HFD-induced mice.53 As revealed in a meta-analysis, patients with MASLD had increased intestinal permeability, which was proved by the oral disaccharide test and detection of serum zonulin levels.54 The third defense line is the immune defense system. Specifically, it is mainly composed of various immune cells and secretory IgA. Secretory IgA is mainly secreted by plasma cells in the inner layer of the gastrointestinal mucosa, which can protect the intestinal mucosa from pathogen adhesion and regulate the composition of gut microbiota.55 Some studies have shown that the serum level of secretory IgA is significantly higher in individuals with severe MASH than in those with mild MASH, which may be correlated with fibrosis in the advanced stages of this disease.56 Patients with MASLD have a decreased level of FOXP3-expressing regulatory T cells but an increased level of Th1 and CD8+ T cells within the intestinal lamina propria. Trypsin-like enzymes from mast cells are responsible for ZO-1 cleavage, thus down-regulating the JAM-A expression and increasing the permeability of the gut epithelial barrier.57
Figure 3.

Intestinal mechanical barrier: 1. Mucus layer is an external mechanical barrier composed of mucus. 2. Epithelial layer. 3. Tight junctions consist of proteins controlling paracellular pathways, adherens junctions, bridges, and gap junctions. 4. Intestinal vascular barrier. Abbreviations: LPS: lipopolysaccharide; IgA: immunoglobulin A.
Created in https://BioRender.com.
Moreover, there is an intestinal vascular barrier consisting of vascular endothelial cells, pericytes, and intestinal neuroglia, which prevents the transfer of pathogens from the lamina propria to the portal circulation.58 In addition to the down-regulated expression of ZO-1 in the intestinal epithelial barrier, the expression of plasma membrane vesicle-associated protein-1 was up-regulated significantly in HFD-induced mice, which may correlate with the disrupted integrity of the gut vascular barrier.59 In mice model of MASLD, a dextran fluorescent probe tracer was injected intravenously into the HFD-fed mice to cross the intestinal vascular barrier and reach the lamina propria of the ileum, suggesting a significant increase in the permeability of the intestinal vascular barrier.60 Further studies revealed that this process was induced by the disruption of the intestinal vascular barrier through interference with the WNT/β-catenin signaling pathway.61 These results suggest that the intestinal barrier contains multiple anatomical and functional structure layers, ranging from the intact mucus layer, the intestinal epithelial barrier, and the intestinal vascular barrier, to the intestinal immune system, which together maintain the integrity of the intestinal barrier. When the intestinal barrier is impaired, gut microbiota and its metabolites can be transferred to the liver via the portal vein, leading to liver injury and inflammation or aggravating existing liver disease.
Relationship between gut microbiota metabolites and metabolic dysfunction-associated steatotic liver disease
Pathogen-associated molecular patterns contribute to the progression of metabolic dysfunction-associated steatotic liver disease
The disruption of the intestinal barrier in the early stage of MASLD makes pathogen-associated molecular patterns (PAMPs) more susceptible to translocation. PAMPs are some molecular structures in pathogens, including Gram-negative bacteria-associated lipopolysaccharide (LPS), Gram-positive bacteria-associated lipoproteins and peptidoglycans, yeast glycans in yeast, and DNA from viruses and bacteria.62 PAMPs can be recognized by pattern recognition receptors (PRRs) on immune cells in the gut lamina propria and liver, thus triggering an immune response. PRRs include Toll-like receptors (TLRs), purinergic P2X7 receptors, inflammatory vesicle family thermoproteins, and GMP-AMP synthases.63 Among them, TLRs play a key role (Figure 4). The serum level of LPS is notably higher in MASLD patients than in healthy people, and its level is correlated with the stage of MASLD, which can be regarded as a key factor for exacerbating MASLD.64 LPS stimulates the recruitment of TLR4 through the myeloid differentiation primary response gene 88 (MyD88) pathway or MyD88-independent signaling, which activates the classical nuclear factor-κB (NF-κB) and mitogen-activated protein kinase (MAPK) signaling pathways. This further leads to the expression of pro-inflammatory cytokines, including tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), interleukin-8 (IL-8), interleukin-12 (IL-12), and chemokines (including interferon-γ and monocyte chemoattractant protein-1 [MCP-1]), thereby promoting inflammation.65 This process involves multiple intracellular cascades, including the stress response and promyelocytic leukemia protein kinase, c-Jun NH 2 -terminal kinase (JNK), and P38 mitogen-activated protein kinase, which trigger the transcription of proinflammatory genes and promote the hepatic migration of neutrophils and monocytes, thus promoting the low-grade systemic inflammation and liver injury induced by oxidative stress mediators (namely nitrogen- and oxygen-responsive substances).66 TLR4/NF-κB signaling not only aggravates the hepatic cellular inflammatory response and steatosis but also up-regulates the expression of the Notch ligand Jagged1 (JAG1) and induces liver fibrosis.67 Mitochondrial DNA released from hepatocytes injured by lipid overload induces the up-regulated expression of macrophage IL-33 via TLR 9 and enhances the lipopolysaccharide-induced production of inflammatory cytokines.68 In MASLD mice, PAMPs induced the activation and polarization of hepatic macrophages to M1-type macrophages via the TLR2-NF-kB/NLRP3-Caspase-1 pathway and activated the mTOR-S6K1-SREBP-1/PPAR-α signaling pathway, thus leading to a shift in lipid metabolism from triglyceride oxidation to triglyceride synthesis.69 In the liver, the activation of TLR4 by gut-derived LPS activates NK-κB, which promotes the synthesis of cytokines and pro-inflammatory factors, including the chemokines CC-chemokine ligand 2 (CCL2) and IL-8, which attract neutrophils and macrophages to the liver, respectively.14
Figure 4.

Types of TLRs. TLR1, 2, 4, 5, and 6 conjugate to molecules associated with bacterial membranes. TLR4 is one of the most studied TLRs and conjugates to LPS from the cell wall of gram-negative bacteria. TLR3, 7, 8, and 9 recognise viral, bacterial or endogenous nucleic acid. Abbreviations: TLR: toll-like receptor; NF-κB: nuclear factor- κB; MyD88: myeloid differentiation factor 88; IFR: interferon regulatory factor; PAMP: pathogen-associated molecular pattern.
Created in https://BioRender.com.
Notably, the activation of the TLR4-Nf-κB pathway stimulates the production of pro-IL-β. Interleukin-1β (IL-1β) amplifies the synthesis of proinflammatory cytokines through the autocrine mechanism, which makes hepatocytes more prone to apoptosis, thus promoting hepatic fat accumulating and accelerating hepatic fibrosis.70 In addition, the NOD-like receptor protein 3 (NLRP3) can stimulate immunity by forming inflammatory vesicles with apoptosis-associated speck-like protein containing a CARD (ASC, an apoptosis-associated speck-like CARD-domain protein), thus activating procaspase 1.71 Inflammatory vesicle dysfunction may lead to an excessive hepatic inflammatory response, liver fibrosis, and cell death. In addition, patients with MASH have been found to have an elevated plasma level of mitochondrial DNA, which can act as a potent TLR-9 activator. It was also revealed that TLR-9-deficient mice were protective against HFD-induced hepatosteatosis and inflammation, highlighting the significance of the TLR-9 pathway in regulating inflammation in MASH.72 The above results corroborate the acting mechanism of PAMPs in the pathogenesis of MASLD.
Endogenous ethanol promotes the progression of metabolic dysfunction-associated steatotic liver disease
Under normal conditions, the concentration of blood ethanol increases in humans after the ingestion of nonalcoholic foods, which have been confirmed to be continuously fermented by gut microbiota to produce ethanol. Under normal conditions, endogenous ethanol is rapidly and completely removed from the portal bloodstream, which is typically achieved by hepatic ethanol dehydrogenase, the microsomal ethanol oxidation system, and catalase.73 Patients with MASLD have significantly higher plasma endogenous ethanol concentrations produced by gut microbiota compared with healthy individuals.74 The high alcohol-producing Klebsiella pneumoniae in gut microbiota has been confirmed to cause a fatty liver via the 2,3-butanediol fermentation pathway.75 Some researchers have identified other ethanol-producing microbiota in the gut, such as Limosilactobacillus fermentum, Enterocloster bolteae, Mediterraneibacter gnavus, Streptococcus mutans, Pichia kudriavzevii, Candida albicans and Candida glabrata Yeasts.76,77
Endogenous ethanol can disrupt the integrity of the intestinal barrier through several mechanisms. Specifically, acetaldehyde, a metabolite of ethanol, can down-regulate the expression of TJ proteins (such as ZO-1 and claudin-1) in IECs through a protein phosphatase 2A-dependent mechanism,78 thereby impairing the structural integrity of intestinal TJs. Ethanol can significantly elevate the level of apoptosis-related marker proteins, such as intestinal cytochrome P450 2E1 (CYP2E1), inducible nitric oxide synthase (iNOS), and nitrulin, proving that ethanol can disrupt the intestinal barrier.79 Ethanol may also impair the mucosal repair capacity of the intestinal barrier through β-catenin signaling dysregulation, which inhibits the proliferation of intestinal stem cells,80 further exacerbating the damage of the gastrointestinal barrier. The ethanol-induced increase in gastrointestinal permeability promotes the movement of more microbe-associated molecular patterns (MAMPs) and triggers an immune response that may lead to liver inflammation.81
Endogenous ethanol can not only increase intestinal permeability but also affect lipid metabolism in the liver and hepatocyte inflammation. Although endogenous ethanol exacerbates fat accumulation in hepatocytes, it is not directly involved in fatty acid synthesis. Ethanol can ultimately increase fatty acid synthesis in the liver through the activation of transcription factors for fatty acid synthesis such as sterol regulatory element binding protein-1c (SREBP-1c).82 In the metabolism process of ethanol to acetaldehyde, NAD+ is consumed, which increases the ratio of NADH to NAD+ and disrupts fatty acid oxidation, leading to hepatic steatosis.73 The metabolism of ethanol to acetaldehyde can affect the expression of SREBP1c and peroxisome proliferator-activated receptor alpha (PPAR-α) by regulating their transcription, modulating the immune response activation, endoplasmic reticulum stress-related variants in adenosine and lipocalin levels, and reducing the activity of the regulatory protein ATAT3, thus contributing to fatty acid accumulation.83 Ethanol can stimulate the activation of PPAR-α, which results in the down-regulated expression of various genes related to free fatty acid transport and oxidation.70 Besides, ethanol can also induce oxidative stress by disrupting the mitochondrial respiratory chain of hepatocytes, thus leading to the production of reactive oxygen species (ROS).84 In addition, acetaldehyde exacerbates oxidative stress induced by ethanol consumption by binding to glutathione (GSH), an important antioxidant in the liver, thereby leading to a decrease in the hepatic antioxidant capacity.70 Ultimately, ROS generated after exposure to ethanol can interact with proteins to alter their structure and function. Further, these ROS can even directly combine with and destroy DNA, causing impaired cellular function and increasing the risk of hepatic carcinoma.85
Overall, the mechanisms by which endogenous ethanol promotes the development of MASLD are similar to those by which exogenous ethanol causes MASLD. Long-term exposure to endogenous ethanol can cause liver injury. However, there are no treatment guidelines for the excessive production of endogenous ethanol, and most clinical evidence is summarized from case reports. Therefore, it is essential to further investigate the genetic and metabolic characteristics of these ethanol-producing bacteria.
Effects of short-chain fatty acids on metabolic dysfunction-associated steatotic liver disease
A short-chain fatty acid (SCFA) is an organic fatty acid synthesized by the gut microbiota through the fermentation of indigestible carbohydrates and soluble dietary fiber, mainly including acetate, propionate, butyrate, and valerate.86 High levels of SCFAs are produced in the intestine mainly by the genera Clostridium, Bacillus, and Butyricum.87 Intestinal SCFAs can enter the liver through the hepatic portal vein, accounting for about 5%-10% of the energy supply.88 SCFAs can delay the development of MASLD by improving the intestinal barrier function, regulating the appetite, and exerting anti-inflammatory effects.
SCFAs can directly enhance the intestinal barrier function via G protein-coupled receptors (GPCRs), including GPR41 and GPR43, which affect a range of cells, including columnar cells, epithelial stem cells, and enteroendocrine cells. For instance, GPCRs can regulate epithelial cell turnover and maintain epithelial barrier integrity.89 SCFAs can stimulate prostaglandin production by subepithelial myofibroblasts and up-regulate the expression of mucin.90 Butyrate can up-regulate the expression of TJ proteins and increase their abundance by activating the synthesis of Akt/mTOR-mediated proteins, including claudin 3 and claudin 4 in epithelial cells.91 It has been demonstrated that SCFAs can reverse the decreased transepithelial resistance, increased paracellular permeability, and disrupted morphology of TJ proteins observed upon lipopolysaccharide (LPS) stimulation by inhibiting autophagy and acting as a histone deacetylase (HDAC) inhibitor to suppress NLRP3 inflammatory vesicles.92 In addition to maintaining the integrity of the intestinal barrier through the formation of TJs, SCFAs can also support the renewal of IECs. Butyrate can inhibit the proliferation of intestinal stem cells and progenitor cells and delay wound repair through the transcription factor fork-head box O3 (FOXO3).93 The butyrate-dependent inhibition of histone deacetylase 8 (HDAC8) can prevent IECs from death during intestinal inflammation by suppressing the expression of hexokinase 2 and subsequently inhibiting mitochondrial respiration.94 Butyrate increases the oxygen consumption of IECs, thereby stabilizing the expression of hypoxia-inducible factors (HIFs) to promote intestinal barrier repair.95
SCFAs can promote the expression of PPAR-1α, activate the AMP-activated protein kinase (AMPK) signaling pathway, promote fatty acid oxidation, and inhibit lipogenesis.96 Besides, SCFAs can also inhibit the synthesis of hepatic fatty acids by down-regulating the expression of genes related to hepatic adipogenesis, primarily by regulating the expression of sterol regulatory element-binding protein-1c (SREBP-1c).97 Additionally, SCFAs stimulate the secretion of glucagon-like peptide-1 (GLP-1) and peptide YY (PYY) via free fatty acid receptor 2 (FFAR2), which promotes satiety and regulates insulin sensitivity through the activation of endocrine receptors in the hypothalamus and the nucleus tractus solitarius.98 Charlotte et al. found that SCFAs were not dependent on FFAR2/FFAR3 but primarily acted as energy sources for rat colonocytes, subsequently triggering the secretion of GLP-1.99 Nicola et al. further showed that SCFAs can influence food intake by modulating orexin/hypothalamic orexin neuron inhibition mediated by orexigenic neuron activities.100 Moreover, SCFAs can alter gene transcription via HDACs, an enzyme that can catalyze the clearance of acetyl groups from lysine residues on histones, thereby reducing gene transcription. Butyrate may exert an anti-inflammatory effect by inhibiting HDACs. Active butyrate has an anti-inflammatory effect on hepatic Kupffer cells. The supplementation of butyrate to rats in vivo enhanced the ability of blast cells in the liver to produce immunosuppressive prostaglandin E2 (PFE2), namely that the supplementation of butyrate to the blast cells in the liver in vitro increased the production of PGE2 and inhibited the secretion of tumor necrosis factors (TNFs).89 SCFAs are associated with the development of MASLD, and butyrate plays a particularly important role in this process. Therefore, targeting the metabolism of SCFAs exhibits the potential to prevent MASLD.
Bile acids alleviate metabolic dysfunction-associated steatotic liver disease
Bile acids (BAs) can be classified into primary and secondary bile acids. Specifically, primary bile acids are synthesized by hepatocytes and stored in the gallbladder, while secondary bile acids are products of bacterial metabolism. The cholesterol biosynthesis of bile acids is initiated by cholesterol 7a-hydroxylase (CYP7A1) in the classical pathway in hepatocytes or sterol 27-hydroxylase (CYP27A1) in the alternative pathway in extrahepatic tissues. When food is consumed, bile acids are released into the duodenum, where they are metabolized by gut microbiota in the gastrointestinal tract. Approximately 95% of the bile acids are reabsorbed before reaching the end of the ileum and circulating through the gut-liver axis. The remaining 5% of bile acids enter the colon and contribute to the production of deoxycholic acid (DCA) through dehydroxylation and reabsorption.
Bile acids can act as emulsifiers to promote fat absorption, and they are also closely related to various metabolic regulation patterns in the body. The serum level of total bile acids is elevated in adult patients with MASH.101 It has been stated that the level of total bile acids is elevated in diet-induced MASH mice, which is closely related to the severity of fibrosis.102 Bile acids regulate bile acid metabolism, energy metabolism, lipid metabolism, glucose metabolism, and immune cell function through the activation of nuclear or membrane receptors, such as farnesoid X receptors (FXRs), vitamin D receptors, pregnane X receptors, and G protein-coupled receptors5. Additionally, bile acids can maintain intestinal epithelial integrity through the expression of ZO-1 and claudin-1 stimulated by FXRs, and they can regulate mucin production to maintain the integrity of the gastrointestinal vascular barrier.103
Bile acids can affect glucose and lipid metabolism through the FXR signaling pathway. Besides, bile acids can inhibit gluconeogenesis through FXR-dependent and non-FXR-dependent mechanisms. In addition, they can also indirectly regulate glucose homeostasis through the FXR-dependent induction of intestinal fibroblast growth factor 15 (FGF15) (FGF19 in humans),104 down-regulates the expression of SREBP-1c, reduces adipogenesis, and increases fatty acid β-oxidation.105 Moreover, bile acids can promote the synthesis and release of insulin through the activation of GLP-1 by the G-protein-coupled bile acid receptor (Takeda G-protein-coupled receptor 5 [TGR5]). Furthermore, they can also protect pancreatic β-cells by inhibiting apoptosis and stimulating β-cell proliferation, thus reducing insulin resistance.106
Bile acids also play an essential role in immunomodulation through the FXR signaling pathway. The activation of FXRs inhibits NF-κB and reduces hepatic inflammation. Bile acid signaling through FXRs and TGR5 affects TLR-dependent pathways and NRLP3-dependent inflammatory vesicle activation, thereby modulating immune effects.107,108 The results show that FXRs have a critical role in the progression of MASLD. FXR agonists can lower the level of triglycerides through multiple mechanisms, including the inhibition of atypical nuclear receptor small heterodimers by SREBP-1c and the FXR-dependent induction of PPAR-α.109
Gut microbiota can convert primary bile acids into secondary bile acids, and bile acids can also influence the composition of gut microbiota. The diets with a high level of bile acids significantly inhibit the phyla Anabaena and Actinobacteria.110 Nie et al. found that 3-succinylcholic acid promoted the growth of Akkermansia muciniphila in the gut.111 Thus, the interaction between bile acids and gut microbiota is bidirectional, and its effects on MASLD are highly complex. Bile acids are the end products of cholesterol metabolism and play a key role in regulating gut microbiota. Bile acids facilitate the digestion and absorption of dietary fats and fat-soluble vitamins, thus regulating host metabolism and immunity. Therefore, targeting bile acid metabolism and signaling is expected to become a novel therapeutic option for MASLD.
Impaired choline metabolism affects metabolic dysfunction-associated steatotic liver disease
Choline engaged in the synthesis of phospholipids, betaine, acetylcholine, and trimethylamine (TMA). Due to its importance for liver function, brain development, nerve function, and muscle movement, choline is considered an indispensable nutrient for the human body. Rodents fed on a choline-deficient diet have been used to mimic nonalcoholic steatohepatitis in humans. Choline can be processed into phosphatidylcholine, which contributes to the excretion of very low-density proteins from the liver and prevents hepatic steatosis. A choline-deficient diet can reduce the diversity of gut microbiota by altering the representative strains in the gut microbiota.112 Besides, this diet may also disrupt the integrity of the intestinal epithelial barrier and exacerbate MASLD by down-regulating the expression of TJ proteins such as ZO-1.113 Juliette et al. found that a choline-deficient diet down-regulated the expression of the WNT/β-catenin signaling pathway, further up-regulating the expression of plasmalemma vesicle associated protein-1 (PV-1) and inducing a disruption to the intestinal vascular barrier.61
It has been found that gut microbiota, including Anaerococcus hydrogenalis, Clostridium asparagiforme, Clostridium hathewayi, Clostridium sporogenes, Escherichia fergusonii, Proteus penneri, Providencia rettgeri, and Edwardsiella tarda, can convert choline to TMA.114,115 TMA is absorbed by the intestinal epithelium and then transported through the portal vein to the liver, where it is oxidized to trimethylamine N-oxide (TMAO).116 As revealed in some clinical studies, the level of TMAO in the blood is associated with the progress of MASLD, and a high level of TMAO may increase the risk of MASLD in adults.117
TMAO induces lipid accumulation, inflammatory infiltration, liver injury, and liver fibrosis in zebrafish, while activating the protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK) signaling pathway for endoplasmic reticulum stress in the liver of zebrafish.118 Nian et al. found that TMAO disrupted the structure and function of the intestinal barrier at different levels, which further activated the TLR 4/MyD 88/NF-κB pathway and inhibited the WNT/β-catenin pathway. TMAO can also induce endothelial dysfunction via the capillarization of liver sinusoidal endothelial cells (LSECs) and simultaneously regulate macrophage polarization.119 In addition to damaging the intestinal epithelium, Miyata et al. further found that TMAO ingestion ameliorated liver injury in FXR-deficient mice by altering the metabolism of bile acids and cholesterol in an FXR-independent manner.120 However, Zhao et al. found that in high-fat and high-cholesterol diet-induced MASH rats, TMAO intervention inhibited intestinal cholesterol uptake by down-regulating the cholesterol influx-associated protein (Niemann-Pick C1-like 1 protein [NPC1L1]), while up-regulating cholesterol efflux-associated proteins (ATP binding cassette subfamily G member 5/8 [ABCG 5/8]), which attenuated cholesterol-induced endoplasmic reticulum stress and cell death in the liver.121 Based on these mechanisms, a high serum level of TMAO may aggravate MASLD. However, whether the serum level of TMAO can be used as a biomarker for MASLD still needs further exploration.
Effects of other gut microbiota metabolites on metabolic dysfunction-associated steatotic liver disease
Tryptophan is an essential amino acid that can be converted to a variety of bioactive substances in the human body. The addition of tryptophan to foods reduces the severity of MASLD by reducing fat accumulation and relieving the disruption of bioelectrical properties, and it can also prevent a decrease in the synthesis of adenosine triphosphate (ATP) in mitochondria.122 Approximately 5% of tryptophan is converted to indole metabolites by gut microbiota, which may alleviate MASLD. In HFD-induced hepatic steatosis mice, Indole-3-acetic acid (IAA) reduced the infiltration of macrophages in liver tissues, decreased the level of chemotactic protein-1 and TNF-α in monocytes, and down-regulated the expression of genes involved in adipogenesis.123 Zhao et al. found that IAA induced the expression of TJ proteins such as ZO-1 and occludin, which enhanced the intestinal barrier, reduced endotoxin leakage, inhibited NF-κB signaling, decreased the level of such pro-inflammatory factors as TNF-α and IL-1β, and inhibited the expression of fibrogenic proteins and collagenous proteins, thereby attenuating hepatic steatosis.124 Zhu et al. confirmed that indole supplementation significantly reduced the severity of hepatic steatosis, inflammation, and hepatic fibrosis in MASLD mice. Some cellular experiments revealed that indole supplementation inhibited the fibrotic activation of hepatic stellate cells (HSCs) as well as intestinal pro-inflammatory responses, thereby attenuating MASH.125
Other gut microbiota metabolites such as Trimethyl-5-aminovaleric acid (TMAVA) have also been implicated in the pathogenesis of MASLD. Zhao et al. found that the plasma level of TMAVA was elevated in patients with hepatic steatosis, and TMAVA supplementation in HFD-fed mice exacerbated MASLD by inhibiting γ-butyl betaine hydroxylase and reducing carnitine synthesis and fatty acid oxidation.126 The metabolites of gut microbiota generally affect gastrointestinal barrier integrity and affect hepatic steatosis, inflammatory responses, and oxidative stress by influencing various signaling pathways. A more thorough insight into the acting mechanisms of these metabolites may contribute to identifying the pathogenic targets of MASLD, thus promoting the development of drugs with MASLD-specific targets.
Progress in the application of intestinal probiotics in the treatment of metabolic dysfunction-associated steatotic liver disease
The pathophysiological mechanisms of MASLD are extremely complex, involving multiple pathogenic pathways that have not yet been fully elucidated. To date, there is only one potent drug (namely, Resmetirom) approved by the Food and Drug Administration (FDA) for the clinical treatment of MASLD.127 However, it is only indicated for patients with stage F2 and F3 fibrotic MASH who have not progressed to cirrhosis. This also suggests that existing MASH therapeutics cannot meet the clinical need. Clinical practice guidelines from the European and American Societies of Hepatology recommend a low-calorie diet combined with exercise to achieve a degree of weight loss in overweight or obese patients with MASLD, thus improving biochemical liver function and alleviating histological hepatic steatosis. However, individuals who have had the disease still face a recurrence rate of up to 49%.128 Therefore, there is still an urgent demand for novel drugs. Currently, there is widespread interest in the treatment of MASLD by modulating the constitutive species or metabolites of gut microbiota, including antibiotics, probiotics, prebiotics, synbiotics, and gut microbiota transplants). Although antibiotics can remove or prevent specific bacterial infections or colonization in the gut to maintain the composition of gut microbiota, the use of antibiotics inevitably leads to the emergence of resistance in individuals, which limits their application. Engineered bacteria and phage therapies are emerging therapeutic strategies that may have shown precision in targeting gut microbiota.
Probiotics
Probiotics are safe and live bacteria that can promote host health. The effectiveness of probiotics for MASLD treatment has been verified in a growing number of animal experiments (Table 1). In MASLD, Lactobacillus and Bifidobacterium are the most common conventional probiotics. Conventional probiotics can fulfill functions in the treatment of MASLD through several mechanisms, including regulating the composition or abundance of gut microbiota; influencing the metabolism of bile acids, which in turn affects hepatic lipid and glucose homeostasis; maintaining the integrity of the gastrointestinal barrier; down-regulating the expression of pro-inflammatory factors as well as the production of SCFAs; reducing oxidative stress. For example, dietary complex probiotics promote ileal FXR signaling and up-regulate the hepatic FXR/SHP pathway to negatively regulate the synthesis of bile acid. Meanwhile, modulating FXR signaling can stimulate PPAR-α-mediated lipid oxidation and inhibit SREBP1c/PPAR-γ-mediated lipid synthesis.139 Acetate produced by the fermentation using probiotics in the intestinal lumen can regulate insulin sensitivity via hepatic FFAR2, thereby preventing the progression of MASLD.140 The probiotic Bacillus monomorphus could promote glucosamine production through the synthesis of 3-succinylated cholic acid (3-sucCA), which in turn stimulated the growth of the probiotic bacterium Ackermannia mucinophilus, thus remodeling gut microbiota and decelerating the progression of MASH in mice.111
Table 1.
Preclinical studies on the effect of different probiotics on MAFLD.
| Probiotics | Animal model | Experimental Methods | Mechanism of action |
|---|---|---|---|
| Bacteroides thetaiotaomicron (B. theta)129 | HFD-fed male mice | B. theta tube-feeding was administered continuously 3 times a week for 12 weeks. | 1) Regulation of gut microbiota. 2) Enhances folate synthates and regulates folate metabolism. 3) Regulation of unsaturated fatty acids. |
| Lactococcus lactis KF140 (LL-KF140)130 | CML and HFD-fed mice | LL-KF140 was administered for 8 weeks. | 1) Reduced adipogenic gene expression. 2) Significantly reduced poor glucose tolerance and insulin resistance. |
| Bacteroides ovatus131 | HFHC-fed male C57BL/6 J mice | Bacteroides ovatus tube feeding method for 8 weeks. | 1) Reduced adipogenic gene expression. 2) Regulates gut microbiota. 3) Suppression of the inflammatory response. 4) Increase SCFA levels. |
| Lactobacillus rhamnosus GG132 | HFHF-fed male Wistar rats | GG administered by oral force-feeding for 6 weeks | 1) Reduced adipogenic gene expression. |
| Lactobacillus oris133 | C57BL–6 ob/ob mice | Oral Lactobacillus oris for 5 weeks | 1) Regulates fatty acid synthesis. 2) Promotes the conversion of cholesterol to bile acids. |
| Bifidobacterium134 | WD fed C57BL/6 J mice | Bifidobacterium feeding for 8 weeks | 1) Regulation of gut microbiota and their metabolites. 2) Strengthening the intestinal barrier. 3) Regulation of lipid metabolism and modulation of inflammatory responses. |
| Lactobacillus plantarum ATCC14917135 | HFD-fed male Sprague Dawley rats | Lactobacillus plantarum ATCC14917 intervention 8 weeks | 1) Regulation of oxidative stress. 2) Relieves liver inflammation. 3) Regulates gut microbiota. |
| Cholesterol lowering (CL) probiotics136 | HFD-fed male FXR knockout mice | Gavage with probiotics for 16 weeks | 1) Suppression of liver inflammation. 2) Restore the balance of gut microbiota. 3) Correct bile acid composition and content. |
| Bifidobacterium animalis subsp. lactis SF137 | HFD-fed C57BL/6N mice | Probiotics given by gavage for 12 weeks | 1) Regulation of lipid metabolism and reduction of insulin resistance. 2) Reduces oxidative stress and inflammatory responses. 3) Restoring the intestinal barrier. 4) Regulation of gut microbiota abundance. |
| Mutaflor(®) Probiotics138 | HFHSD-fed male Wistar rats | Mutaflor(®) probiotics were administered by tube feeding for 12 weeks. | 1) Reduces inflammatory response. 2) Reduces liver fibrosis. |
Abbreviation: HFD: high-fat diet; CML: Nε-carboxymethyl-lysine; HFHCD: high-fat and high-cholesterol diet; HFHFD: high-fat high-fructose diet; WD: western diet; HFHSD: high-fat high sucrose diet.
It is noteworthy that Akkermansia muciniphila has become a research hotspot for the probiotic treatment of MASLD in recent years. Akkermansia muciniphila is a Gram-negative anaerobic bacterium that colonizes the mucus layer of the intestine and alleviates MASLD through three ways. Firstly, extracellular vesicles produced by the metabolism of Akkermansia muciniphila can be used as a delivery vehicle to improve the depth and thickness of mucosal crypts induced by HFDs and thus improve intestinal permeability. This further up-regulates the expression of TJ-related proteins, including ZO-1, occludin, and claudin-1, thus improving the expression of MASLD in obese mice and humans.141,142 Secondly, Akkermansia muciniphila can regulate the hepatic immune microenvironment through the TLR2-γδT17 cell axis by secreting cytokines such as IL-17A and IL-22 as well as regulating the conversion of macrophages into anti-inflammatory macrophages, which in turn reduces hepatic inflammation.143 Thirdly, Akkermansia muciniphila can lower blood glucose and inhibit gastric emptying by stimulating GLP-1 secretion.144 The above findings suggest that probiotics may delay the progression of MASLD by regulating hepatic lipid metabolism, improving insulin resistance, inhibiting hepatic inflammatory responses, influencing the composition of gut microbiota, or improving the permeability of the gut microbiota barrier. In addition, it has been validated in clinical trials that probiotics can mitigate MASLD (Table 2), which is in line with the results of animal experiments. Overall, probiotics are effective in the treatment of MASLD. However, there are currently no strains or probiotic combinations to achieve clinical therapeutic effects. Therefore, additional studies are needed through the standardization of strains, probiotic doses, and treatment duration to assess their long-term efficacy and clarify their precise acting mechanisms.
Table 2.
Clinical treatment with probiotics.
| Probiotics | Number of patients | Duration of treatment | Effect |
|---|---|---|---|
| Probiotic mix Lactobacillus acidophilus + Lactobacillus rhamnosus + Lactobacillus paracasei + Bifidobacterium lactis145 | 44 | 24 weeks |
|
| Multi-strain probiotics (MCP®BCMC®strains) containing Lactobacillus acidophilus, Lactobacillus casei subspecies, Lactobacillus lactis, Bifidobacterium bifidum, Bifidobacterium infantis and Bifidobacterium longum146 | 40 | 6 months |
|
| VSL#3 [A highly concentrated multi-strain probiotic blend containing one strain of Streptococcus thermophilus BT01, three strains of Bifidobacterium, and four strains of Lactobacilli]147 | 60 | 3 months |
|
| Multi-strain probiotics of six different Lactobacillus and Bifidobacterium species (MCP®BCMC®strains: Lactobacillus acidophilus, Lactobacillus casei subspecies, Lactobacillus lactis, Bifidobacterium bifidum, Bifidobacterium infantis and Bifidobacterium longum).148 | 39 | 6 months |
|
| Lactobacillus Lb149 | 50 | 3 months |
|
| Lactobacillus acidophilus and Bifidobacterium lactis150 | 48 | 6 months |
|
| Lactobacillus acidophilus Lactobacillus acidophilus Bifidobacterium breve, B. longum, L. delbrueckii susp. bulgaricus, L. helveticus, L. plantarum, L. rhamnosus, L. casei, Lactococcus lactis susp. lactis, Streptococcus thermophiles151 | 48 | 12 weeks |
|
Abbreviation: LDL-C: low-density lipoprotein-cholesterol; HDL-C: high-density lipoprotein-cholesterol; TG: triglycerides; AST: aspartate aminotransferase; ALT: alanine aminotransferase; BMI: body mass index.
World Health Organization(WHO) suggests that probiotics are beneficial to their intended hosts if provided in sufficient quantities.152 The dose range of probiotics for the treatment of MASLD in existing clinical research trials varies widely, usually 5 × 106/1010 CFU/day.153 There are few studies that have systematically explored the linear relationship between probiotic dose and the effect of treatment of MASLD. However, probiotics have been explored in the dose effect of antibiotic-associated diarrhea and other diseases. Studies on fecal recovery and antibiotic-associated diarrhea (AAD) have shown a dose-response relationship. Early observations by Van Niel et al. are consistent with their earlier observations, where they observed a dose-response to the inclusion of four probiotic products in the study in an AAD analysis, with a dose of less than 1010 CFU being ineffective.154 Similarly, Johnston et al. observed that higher doses (more than 5 × 109 CFU) were more effective than lower doses (less than 5 × 109 CFU) based on 15 studies and 10 different probiotic products.155 These studies suggest that higher doses of probiotics are most effective in treating antibiotic-associated diarrhea. In addition, probiotics provide beneficial effects in the treatment of necrotizing enterocolitis. However, studies have shown no clear correlation between dose and risk ratio of necrotizing small bowel colitis.156 In other diseases, no clear dose effect has been observed.157 However, the absence of evidence of a dose effect does not mean that no dose effect exists. A meta-analysis on meta-analysis showed that leptin levels in patients with MASLD decreased more than other clinical indicators in response to probiotic and synbiotic supplementation. Although the degree of leptin decline did not show significant differences in terms of intervention form (probiotic/synbiotic type), total bacterial dose, length of follow-up, or quality of randomized controlled trials (RCTs), the following trend was observed: the decline in leptin levels was more pronounced when probiotics/synbiotics were supplemented in the form of dietary supplements, at a low daily dose, and for a duration of ≥12 weeks.158 Currently, there is a lack of standardized dosage recommendations for probiotics in the treatment of MASLD, and more studies are needed to compare the effects of different dosages due to the wide variation in populations, regimens, and endpoints.
This is because the mechanism by which probiotics regulate MASLD is complex and involves the regulation of multiple metabolites. Acetate produced by Bifidobacterium pseudolongum inhibits hepatocellular carcinoma associated with nonalcoholic fatty liver disease.159 Lactobacillus rhamnosus GG depletes intestinal fatty acids and defends against the initial stages of MASLD development earlier than in vitro changes in intestinal permeability or inflammation.160 A randomized controlled trial showed that a multi-strain combination probiotic was superior to a single strain in improving liver enzymes, hepatic steatosis, and fibrosis scores.161 Yang et al. analyzed a total of 35 RCTs involving 2212 patients with MASLD and showed that Lactobacillus, Bifidobacterium, and Streptococcus may be the most effective probiotic combinations for the treatment of liver enzymes, lipid profile, and inflammatory factors.162
Prebiotics and synbiotics
Prebiotics are substrates that are selectively utilized by host microorganisms and confer health benefits on hosts.163 Prebiotics are mainly composed of polysaccharides (such as inulin, cellulose, pectin, and resistant starch), oligosaccharides (such as oligofructose, oligogalactose, oligoisomaltose, and lactofructose), and non-carbohydrates (such as flavonoids). A growing number of experiments have demonstrated the significant efficacy of prebiotics in the treatment of MASLD (Table 3). Prebiotics can reduce triglyceride accumulation by stimulating fatty acid oxidation via PPAR-α and reduce cholesterol accumulation by inhibiting SREBP-2-dependent cholesterol synthesis.164
Table 3.
Preclinical trials of prebiotics and synbiotics.
| Prebiotics/Synbiotics | Animal model | Breeding method | Effect |
|---|---|---|---|
| Prebiotics | |||
| Melissa Officinalis Extract (MFE)164 | 5-week-old male C57BL/6/J mice | MFE was given daily by oral gavage for 4 weeks. |
|
| Sarsaparilla Polysaccharide (SCP)165 | Feeding 8-week-old male C57BL/6/J mice | SCP by gavage daily for 12 weeks. |
|
| Sea buckthorn polysaccharide (SP)166 | Male Wistar rats | SP solution was given by oral gavage for 4 weeks. |
|
| Yellow Tea Polysaccharide (YTP)167 | HFD-fed 4-week-old male C57 BL/6 J mice | Gavage YTP for 12 weeks |
|
| Inulin168 | HSD-fed mice |
|
|
| NUTRIOSE® Soluble Fibre169 | IGN-deficient mice fed a high-fat diet |
|
|
| Auricularia auriculae polysaccharide (Auricularia auriculae polysaccharide)170 | HFHCD combined with carbon tetrachloride induced in 6–8 week C57BL/6 male mice | Gavage for 8 weeks. |
|
| Low molecular weight polysaccharide RPP-2 from Pueraria lobata root171 | HFD-fed C57BL/6J mice | Gavage for 12 weeks |
|
| IOEC Extract172 | Fructose-fed C57BL/6J mice | Gavage for 12 weeks |
|
| Oligofructose and cassis polyphenol extracts173 | HFD-fed Zucker rats | Add oligofructose and cassis polyphenol extract to your diet. |
|
| Artemisia argyi polysaccharide174 | Intraperitoneal injection of lipopolysaccharide-induced intestinal inflammation in mice | 21 days by gavage |
|
| Mulberry leaf aqueous extract175 | HFD-fed ICR mice | Gavage for 6 weeks |
|
| Anionic polysaccharide GG176 | HFD-fed C57BL/6J mice | Gavage for 12 weeks |
|
| Synbiotics | |||
| Bifidobacteria, Streptococcus thermophilus and Inulin (HS)177 | HFD-fed male mice | Gavage for 8 weeks |
|
| Contains piperine, fulvic acid, silymarin herbs and a probiotic blend (F3)178 | HFD-fed C57BL/6 J male mice | Gavage for 1 month |
|
| Synbiotic composition of Lactobacillus acidophilus, Bifidobacterium infantis and konjac glucomannan oligosaccharides179 | HFD-fed C57 BL/6 J male mice | Gavage for 12 weeks |
|
| Mu l tistrain probiotic mixture and prebiotic inulin fibre180 | HSHFD-fed rats | Gavage for 12 weeks |
|
| Prebiotic oat beta-glucan (OAT) and the probiotic Lactobacillus rhamnosus GG181 | HFD-fed C57BL/6N mice | Gavage for 17 weeks |
|
Abbreviation: HFD: high-fat diet; HSD: high sucrose diet; HFHCD: high-fat and high-cholesterol diet; HFHFD: high-fat high-fructose diet; HSHFD: high-sucrose high-fat diet.
Sea buckthorn polysaccharides can also attenuate hepatic lipid accumulation by regulating the Nrf-2/HO-1 signaling pathway.166 Silymarin is a flavonolignan extracted from the fruit of the Chinese medicine silymarin and has been confirmed to be beneficial to the treatment of liver disease.182 In a mouse model of MASLD, silymarin as a prebiotic inhibited NF-κB signaling by enhancing FXR transcriptional activities, thus ameliorating insulin resistance and decreasing inflammation levels.183 In clinical trials, silymarin reduced liver fibrosis in adult patients with biopsy-proven MASH.184 Resveratrol can prevent HFD-induced insulin resistance and hepatic steatosis by targeting the miR-34a/SIRT1 axis.185 Several clinical trials have revealed that prebiotic supplementation in patients with MASLD improves blood lipids (Table 4). However, a recent clinical trial based on 19 subjects showed no significant changes in the hepatic fat content and the level of metabolic and inflammatory mediators in liver function tests in MASLD patients supplemented with inulin.189 The undefined effectiveness of prebiotics due to different treatment courses and the heterogeneity in the patient populations necessitates large-scale and long-term trials.
Table 4.
Clinical trials of prebiotics and synbiotics.
| Prebiotics and synbiotics | Clusters | Span | Effect |
|---|---|---|---|
| Oligofructose186 | 33 | 9 months |
|
| Amazonian berry camu-camu187 | 30 | 12 weeks |
|
| Inulin and metronidazole188 | 60 | 12 weeks |
|
| ITFs Inulin-based fructans189 | 19 | 12 weeks |
|
| 64 × 109 CFU of Lactobacillus and Bifidobacterium and 6.4 g of inulin in the synbiotic190 | 84 | 12 weeks |
|
| Bifidobacterium lactis, Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus salivarius, and Lactococcus lactis, 5 probiotics, and inulin from agave inulin191 | 86 | 7 weeks |
|
| Spores of Bacillus coagulans (GBI-30) plus inulin192 | 53 | Lasts 12 weeks |
|
| Oligofructose and Bifidobacterium lactis subspecies193 | 104 | 10–14 months |
|
| L. fermentum strains and acacia gum194 | 180 | 3 months |
|
Abbreviation: NAS: nonalcoholic fatty liver activity score; AST: aspartate aminotransferase; ALT: alanine aminotransferase; TNF: tumor Necrosis Factor; NF- κB: nuclear factor- κB.
The Global Prebiotic Association (GPA) defines the prebiotic effect as “a health or performance benefit due to a change in the composition and/or activity of the microbiota as a direct or indirect result of the utilization of specific and well-defined compounds or ingredients by microorganisms.195 Traditional medicinal extracts have been used in China for thousands of years to prevent and treat diseases, and the vast majority of them are mainly taken orally. Many of their active ingredients enter the gastrointestinal tract and are metabolized by intestinal flora before being absorbed and exerting pharmacological effects, such as polysaccharides and flavonoids. Traditional medicinal extracts have an impact on MASLD through the prebiotic effect, such as powdered kudzu polysaccharide,196 maitake polysaccharide,197 flavonoid-rich mulberry leaf,175 and so on. They maintain gut microbiota homeostasis by affecting the composition of the gut microbiota. In addition to altering the composition of gut microbiota, medicinal polysaccharides can be fermented into SCFAs by gut microbes.174,198 Yang et al. found that Puerarin effectively ameliorated metabolic disorders and dysbiosis of the gut microbiota in obese mice, mainly through FXR-dependent pathways.199 In recent years, more and more scholars have studied the effects of traditional medicinal food extracts on MASLD through the gut microbiota, and have achieved good results in animal experiments. However, it is not clear what mechanism plays a dominant role, and the complexity of the composition of traditional medicinal food extracts is an important challenge for their clinical use.
There are indeed fewer studies that systematically explore the linear relationship between prebiotic dose and the effectiveness of treatment of MASLD. While prebiotics are generally considered safe, the results based on the limited studies suggest that prebiotic dosage also affects its safety. For example, low (2.5–10 g/day) and high (40–50 g/day) doses of prebiotics can cause flatulence and osmotic diarrhea, respectively. A dose of 2.5-10 g of prebiotics per day is needed to perform its beneficial functions on human health.200 Factors such as gut microbiota composition and baseline metabolic status may influence the dose-response of prebiotics and need to be combined with individualized therapy. The reason for this may be that prebiotics can be fermented by gut microbiota to produce gases such as hydrogen, carbon dioxide, or methane, which are rapidly broken down by gut microbiota in the intestines to produce gas, leading to abdominal distension. In addition, prebiotics are indigestible, and their entrance into the intestinal tract increases the permeability of the intestinal lumen, causing water to enter the intestinal lumen, which may lead to diarrhea when used in high doses.201 Therefore, in the development of prebiotic products, it is necessary to evaluate the gas production of prebiotics and the health effects of different doses, to determine the effective dose and the maximum tolerable dose. In a dose-effect study of prebiotics, it was found that the daily intake of fructo-oligosaccharides needed to reach more than 10 g to produce the effect of bifidobacteria in a healthy population, while the daily intake of inulin only needed to be 2.5–5.0 g to produce the same effect.202 Another intervention study of galacto-oligosaccharides (β-GOS), an oligogalactose mixture, in elderly subjects found that β-GOS increased the abundance of bifidobacteria in the intestinal tract, as well as increased the blood levels of the anti-inflammatory cytokine IL-10 and significantly decreased the expression of the inflammatory factor IL-1β, compared to placebo.203 These findings are often difficult to obtain through in vitro simulated fermentation experiments. Therefore, for the evaluation of the efficacy of prebiotics, it is recommended to use in vitro screening combined with in vivo validation to clarify the effective dose range of prebiotics.
Synbiotics are a combination of probiotics and prebiotics. The probiotic and inulin mixture may delay the progression of MASH through genes related to the Hippo signaling pathway, such as YAP1, LATS1, and NF2, as well as the epigenetic regulator miR-1205-lncRNA SRD 5A 3-AS 1.180 A meta-analysis involving 634 patients with MASLD showed that synbiotic supplementation improved liver function, regulated lipid metabolism, and reduced hepatic fibrosis in patients with MASLD, but these effects should be confirmed by further studies.204 However, synbiotics have not been compared with probiotics or prebiotics alone in terms of their efficacy in the treatment of MASLD. Furthermore, it is necessary to identify the synbiotic combinations that are most effective in the treatment of MASLD.
Fecal microbiota transplantation
Fecal microbiota transplantation (FMT) is the transplantation of functional gut microbiota from healthy human faces into the intestines of patients to reestablish a new gut microbiota for the treatment of intestinal and extra-intestinal diseases. This therapy has been successfully used in the treatment of patients with refractory and recurrent Clostridium difficile (C. difficile) infection.205 It is also more commonly used in the treatment of inflammatory bowel disease and irritable bowel syndrome. In a MASH mouse model induced by HFDs, it was proved that FMT could correct intestinal dysbiosis, increase the intestinal butyrate concentration and the intestinal TJ protein level, and reduce the intrahepatic lipid accumulation and serum proinflammatory cytokine levels to improve liver inflammation.206 Recently, washed microbiota transplantation (WMT), a modified gut microbiota transplantation method, was validated to up-regulate the expression of hepatic homing C-X-C motif chemokine receptor 6 (CXCR6) in group 3 innate lymphoid cells (ILC3s), which promoted the migration of ILC3s to the liver of MASLD mice via the CXCL16/CXCR6 axis. Besides, it was found that the severity of this disease was negatively correlated with the proportion of ILC3s in the liver, suggesting that WMT and targeting hepatic homing of ILC3s may be a novel approach for the treatment of MASLD.207 Daniela et al. found an increased abundance of wild fungi and the potentially probiotic bacterium Wechsler’s Braunschweig in homozygous FMT compared with autologous FMT, as well as the presence of an altered level of intestinal-derived phenylacetylcarnitine and phenylacetylglutamine. In addition, there were extensive changes in hepatic DNA methylation profiles, particularly the altered methylation status of threonyl-tRNA synthetase 1 and zinc finger protein 57. These studies suggest that FMT may induce changes in the metabolic pathways from gut microbiota to the liver.208
In a randomized clinical study based on 21 subjects with hepatic steatosis, a trend toward improvement in hepatic necroinflammatory scores was found in patients after FMT. In the homozygous FMT group, the expression of a number of hepatic genes associated with inflammation and lipid metabolism was significantly altered. However, their liver function biochemistry did not improve significantly.209 A recent randomized clinical trial based on 75 patients with MASLD demonstrated a significant reduction in hepatic fat in patients after FMT, despite that there was no significant difference in their lipid levels. Notably, FMT can exert a greater effect on the gut microbiota structure in lean MASLD patients than in obese MASLD patients.210 The results indicated a tendency toward enhancement in necroinflammatory scores, gut microbiota composition, as well as plasma metabolites and hepatic gene expression involved in inflammation and lipid metabolism.211 While these findings are encouraging, further research is necessary to formally assess the efficacy of FMT in reversing histologic alterations in the liver and slowing the progression of MASLD. Because the composition of gut microbiota changes with age, genetics, and dietary habits of individuals. Therefore, healthy individuals who may provide fecal microbiota should be selected based on more stringent and standardized criteria, patients with the same stage of MASLD should also be enrolled in the treatment of MASLD using FMT in clinical trials. Furthermore, MASLD should be treated based on more rational administration approaches and doses, in a trial based on a larger sample size.
Engineered bacteria and phage therapy
Engineered bacteria are new types of bacteria that have been modified by genetic engineering techniques to obtain superior traits. As mentioned earlier, it is difficult to control the effects of probiotics in clinical trials due to the diversity of probiotics and their non-targeted properties. Among the probiotics, Lactobacillus and Bifidobacterium have favorable safety and stability. They are often used as precursors for engineered bacteria. Currently, some experiments using engineered bacteria as targeted therapies have been conducted, mainly for metabolic diseases. Interleukin (IL) acts as a cytokine that regulates lectins to mediate intestinal immune responses against pathogens. In some studies, the engineering of Lactococcus lactis has been performed to express IL-10 and IL-36 in the intestines to improve the intestinal environment in mice, thereby reducing hepatic inflammation in mice.212,213 In addition to delivering cytokines, engineered bacteria can also be employed to deliver beneficial peptides. GLP-1 can regulate glucose metabolic homeostasis. Ma et al.converted Lactobacillus gattii to a GLP-secreting engineered bacterium, which significantly increased insulin levels and glucose tolerance in diabetic rats.214 They also found that the engineered bacteria attenuated steatotic liver disease in obese mice and increased the a-diversity of gut microbiota, but these bacteria did not significantly alter the relative abundance of the Firmicutes and Bacteroidetes.215 Engineered bacteria can also replenish molecules that are deficient in the gut. N-acylethanolamides (NAEs) are molecules that can provide a feeling of satiety. Chen et al. compensated for the reduction by developing an E. coli-engineered bacterium that synthesized NAEs, resulting in a reduction in weight gain and fat accumulation in HFD-fed mice compared with control mice.216 By binding fructose dehydrogenase and mannitol dehydrogenase to glucose transporter proteins encoded by Aeromonas sp., Somabhai et al. found that mice showed an increase in SCFAs and the potent antioxidant compound PQQ, which significantly suppressed the elevated level of hepatic triglycerides and cholesterol.217 These studies suggest that engineered bacteria can exert biological effects in the gut by synthesizing specific bioactive molecules. The downstream effects of the changes include lipid metabolism regulation, antimicrobial effects, and immunomodulation. However, foreign genes of interest are specifically inserted into carrier bacteria are still very experimental. Before promoting engineered bacterial therapies, key issues such as their biosafety, therapeutic efficacy, and efficient delivery must be addressed to ensure the controllability of the bacterial vectors in vivo, low toxicity to the host, and precision of drug release, which are specific challenges encountered in current research.
Phages are prokaryotic viruses that infect and kill bacteria. As one of the components of gut microbiota, viruses have the possibility of influencing the development of MASLD. In noninvasive predictive models, changes in gut viruses combined with clinical data designed to assess the severity of liver fibrosis can be employed to assess the severity of liver fibrosis to a larger extent. Lin et al. blocked endogenous ethanol production by the phage-targeted removal of high alcohol-producing Klebsiella pneumoniae and mitigated hepatic steatosis and inflammation in mice. Notably, the therapy does not induce significant changes in gut microbiota.218 Ichikawa et al. reduced the abundance of Klebsiella pneumoniae and attenuated hepatic inflammation in mice susceptible to hepatobiliary injury by lysogenic phage mixtures.219 To date, however, there has been no direct pathogenic relationship between the gut microbiota in the development of MASLD, except for the highly alcoholic Klebsiella pneumoniae, which has been clearly recognized as the causative bacterium, which has limited the application of phages in MASLD. However, phage therapy targeting the gut microbiota in liver disease has not yet been studied outside of mouse models, which requires further evaluation of not only efficacy but also clinical safety in humans. Now no clinical trials are available since the phage therapy for MASLD treatment is still in its infancy. Although phages are highly specific as a natural antimicrobial agent and can effectively eliminate target bacteria without affecting other gut bacteria. This therapy reduces the risk of antibiotic resistance compared with conventional antibiotic therapies. However, before promoting engineered bacterial therapies, key issues such as their biosafety, therapeutic efficacy, and efficient delivery must be addressed to ensure the controllability of the bacterial vectors in vivo, low toxicity to the host, and precision of drug release, which are specific challenges encountered in current research.
Summary and outlook
As the most common chronic liver disease, MASLD has become a serious public concern worldwide. However, there are still considerable challenges in the clinical treatment of MASLD due to the unclear pathogenesis of MASLD. Based on the influence of gut microbiota and its metabolites in the pathogenesis of MASLD, this paper illustrates that gut microbiota profiles can be used as noninvasive markers of metabolism-associated steatohepatitis. Furthermore, the potential preventive and therapeutic interventions related to gut microbiota for the treatment of MASLD are also explored from probiotics, prebiotics, synbiotics, FMT, and emerging therapies.
The diagnostic and prognostic assessment of MASLD can be achieved based on gut microbiota-related biomarkers. However, it is difficult to achieve the transition from experimental modeling to clinical application. This may be explained that MASLD is a heterogeneous disease with a phenotype that results from the interaction of multiple factors, including gender, age, genetic background, medication history, and lifestyle. The stratification by classifying patients into high- or low-risk categories based on the severity of MASLD before treatment can identify patients who may achieve favorable outcomes by targeted gut microbiota therapies. In addition, the gut microbiota or the concentration of gut microbiota metabolites of patients is typically examined based on their fecal samples, which limits the understanding of the entire gastric microbiota and its metabolites. Therefore, it is necessary to develop more comprehensive and convenient techniques to measure microbiota and their metabolite concentrations in vivo.
Given the epidemiological trend of MASLD, it is necessary to develop a prevention and treatment protocol for MASLD with a focus on the targeting of gut microbiota. Despite many challenges in the field, investigating the mechanisms by which targeting the gut microbiota contributes to the pathogenesis of MASLD may eliminate the limitations of the current treatment of MASLD based on the “gut-liver axis”, ultimately benefiting more patients with MASLD.
Funding Statement
This work was supported by Grant [JCYJ20240813120109013] from the Shenzhen Natural Science Foundation Project, Grants [82102076] from the National Scientific Foundation Committee of China; Supported by Shenzhen Key Medical Discipline Construction Fund [No. SZXK051]; Supported by Sanming Project of Medicine in Shenzhen [No. SZSM202111011]; National Key Research and Development Program of China [No. 2023YFC3402605].
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Zhou J, Zhou F, Wang W, Zhang X-J, Ji Y-X, Zhang P, She Z-G, Zhu L, Cai J, Li H.. Epidemiological features of NAFLD from 1999 to 2018 in China. Hepatol (Baltim, Md). 2020;71(5):1851–34. doi: 10.1002/hep.31150. [DOI] [PubMed] [Google Scholar]
- 2.Benedé-Ubieto R, Cubero FJ, Nevzorova YA. Breaking the barriers: the role of gut homeostasis in metabolic-associated steatotic liver disease (MASLD). Gut Microbes. 2024;16(1):2331460. doi: 10.1080/19490976.2024.2331460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koning M, Herrema H, Nieuwdorp M, Meijnikman AS. Targeting nonalcoholic fatty liver disease via gut microbiome-centered therapies. Gut Microbes. 2023;15(1):2226922. doi: 10.1080/19490976.2023.2226922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruuskanen MO, Åberg F, Männistö V, Havulinna AS, Méric G, Liu Y, Loomba R, Vázquez-Baeza Y, Tripathi A, Valsta LM, et al. Links between gut microbiome composition and fatty liver disease in a large population sample. Gut Microbes. 2021;13(1):1–22. doi: 10.1080/19490976.2021.1888673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Vos WM, Tilg H, Van Hul M, Cani PD. Gut microbiome and health: mechanistic insights. Gut. 2022;71(5):1020–1032. doi: 10.1136/gutjnl-2021-326789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilbert JA, Blaser MJ, Caporaso JG, Jansson JK, Lynch SV, Knight R. Current understanding of the human microbiome. Nat Med. 2018;24(4):392–400. doi: 10.1038/nm.4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dieterich W, Schink M, Zopf Y. Microbiota in the gastrointestinal tract. Medical sciences (Basel, Switzerland. Med Sci. 2018;6(4):116. doi: 10.3390/medsci6040116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Falony G, Joossens M, Vieira-Silva S, Wang J, Darzi Y, Faust K, Kurilshikov A, Bonder MJ, Valles-Colomer M, Vandeputte D, et al. Population-level analysis of gut microbiome variation. Science (New York, NY). 2016;352(6285):560–564. doi: 10.1126/science.aad3503. [DOI] [PubMed] [Google Scholar]
- 9.Schoeler M, Caesar R. Dietary lipids, gut microbiota and lipid metabolism. Rev Endocr Metab Disord. 2019;20(4):461–472. doi: 10.1007/s11154-019-09512-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jonsson AL, Bäckhed F. Role of gut microbiota in atherosclerosis. Nat Rev Cardiol. 2017;14(2):79–87. doi: 10.1038/nrcardio.2016.183. [DOI] [PubMed] [Google Scholar]
- 11.Sun MF, Shen YQ. Dysbiosis of gut microbiota and microbial metabolites in Parkinson’s disease. Ageing Res Reviews. 2018;45:53–61. doi: 10.1016/j.arr.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Agirman G, Yu KB, Hsiao EY. Signaling inflammation across the gut-brain axis. Science (New York, NY). 2021;374(6571):1087–1092. doi: 10.1126/science.abi6087. [DOI] [PubMed] [Google Scholar]
- 13.Budden KF, Gellatly SL, Wood DLA, Cooper MA, Morrison M, Hugenholtz P, Hansbro P, et al. Emerging pathogenic links between microbiota and the gut-lung axis. Nat Rev Microbiol. 2017;15:55–63. [DOI] [PubMed] [Google Scholar]
- 14.Hsu CL, Schnabl B. The gut–liver axis and gut microbiota in health and liver disease. Nat Rev Microbiol. 2023;21(11):719–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bouin M, Vincent C, Bouhier K, Debruyne D, Fatome A, Piquet M-A, Verwaerde J-C, Dao T. Increased oro-cecal transit time in grade I or II hepatic encephalopathy. Gastroentérologie Clinique et Biologique. 2004;28(12):1240–1244. doi: 10.1016/S0399-8320(04)95217-7. [DOI] [PubMed] [Google Scholar]
- 16.Stärkel P, Schnabl B. Bidirectional communication between liver and gut during alcoholic liver disease. Semin Liver Dis. 2016;36(4):331–339. doi: 10.1055/s-0036-1593882. [DOI] [PubMed] [Google Scholar]
- 17.Loomba R, Friedman SL, Shulman GI. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell. 2021;184(10):2537–2564. doi: 10.1016/j.cell.2021.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ouyang X, Cirillo P, Sautin Y, McCall S, Bruchette JL, Diehl AM, Johnson RJ, Abdelmalek MF. Fructose consumption as a risk factor for non-alcoholic fatty liver disease. J Hepatol. 2008;48(6):993–999. doi: 10.1016/j.jhep.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loomba R, Hwang S, O’Donnell CJ, Ellison RC, Vasan RS, D’Agostino RB, Liang TJ, Fox CS. Parental obesity and offspring serum alanine and aspartate aminotransferase levels: the Framingham heart study. Gastroenterology. 2008;134(4):953–959. doi: 10.1053/j.gastro.2008.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodrich JK, Waters J, Poole A, Sutter J, Koren O, Blekhman R, Beaumont M, Van Treuren W, Knight R, Bell J, et al. Human genetics shape the gut microbiome. Cell. 2014;159(4):789–799. doi: 10.1016/j.cell.2014.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chalasani N, Guo X, Loomba R, Goodarzi MO, Haritunians T, Kwon S, Cui J, Taylor KD, Wilson L, Cummings OW, et al. Genome-wide association study identifies variants associated with histologic features of nonalcoholic fatty liver disease. Gastroenterology. 2010;139(5):1567–1576.e6. doi: 10.1053/j.gastro.2010.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Falleti E, Fabris C, Cmet S, Cussigh A, Bitetto D, Fontanini E, Fornasiere E, Bignulin S, Fumolo E, Bignulin E, et al. PNPLA3 rs738409C/G polymorphism in cirrhosis: relationship with the aetiology of liver disease and hepatocellular carcinoma occurrence. Liver Int. 2011;31(8):1137–1143. doi: 10.1111/j.1478-3231.2011.02534.x. [DOI] [PubMed] [Google Scholar]
- 23.Kozlitina J, Smagris E, Stender S, Nordestgaard BG, Zhou HH, Tybjærg-Hansen A, Vogt TF, Hobbs HH, Cohen JC. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2014;46(4):352–356. doi: 10.1038/ng.2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sookoian S, Rotman Y, Valenti L. Genetics of metabolic dysfunction-associated Steatotic liver disease: the state of the art update. Clin Gastroenterol Hepatol. 2024;22(11):2177–2187.e2173. doi: 10.1016/j.cgh.2024.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wijarnpreecha K, Lou S, Watthanasuntorn K, Kroner PT, Cheungpasitporn W, Lukens FJ, Pungpapong S, Keaveny AP, Ungprasert P. Small intestinal bacterial overgrowth and nonalcoholic fatty liver disease: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2020;32(5):601–608. doi: 10.1097/MEG.0000000000001541. [DOI] [PubMed] [Google Scholar]
- 26.Gudan A, Jamioł-Milc D, Hawryłkowicz V, Skonieczna-Żydecka K, Stachowska E. The prevalence of small intestinal bacterial overgrowth in patients with non-alcoholic liver diseases: NAFLD, NASH, fibrosis, cirrhosis—A systematic review, meta-analysis and meta-regression. Nutrients. 2022;14(24):5261. doi: 10.3390/nu14245261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ha S, Wong VW, Zhang X, Yu J. Interplay between gut microbiome, host genetic and epigenetic modifications in MASLD and MASLD-related hepatocellular carcinoma. Gut. 2024;74:141–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boursier J, Mueller O, Barret M, Machado M, Fizanne L, Araujo‐Perez F, Guy CD, Seed PC, Rawls JF, David LA, et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology (Baltimore, Md. 2016;63(3):764–775. doi: 10.1002/hep.28356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee G, You HJ, Bajaj JS, Joo SK, Yu J, Park S, Kang H, Park JH, Kim JH, Lee DH, et al. Distinct signatures of gut microbiome and metabolites associated with significant fibrosis in non-obese NAFLD. Nat Commun. 2020;11(1):4982. doi: 10.1038/s41467-020-18754-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwimmer JB, Johnson JS, Angeles JE, Behling C, Belt PH, Borecki I, Bross C, Durelle J, Goyal NP, Hamilton G, et al. Microbiome signatures associated with steatohepatitis and moderate to severe fibrosis in children with nonalcoholic fatty liver disease. Gastroenterology. 2019;157(4):1109–1122. doi: 10.1053/j.gastro.2019.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Demir M, Lang S, Hartmann P, Duan Y, Martin A, Miyamoto Y, Bondareva M, Zhang X, Wang Y, Kasper P, et al. The fecal mycobiome in non-alcoholic fatty liver disease. J Hepatol. 2022;76(4):788–799. doi: 10.1016/j.jhep.2021.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Viebahn G, Hartmann P, Lang S, Demir M, Zhang X, Fouts DE, Stärkel P, Schnabl B. Fungal signature differentiates alcohol-associated liver disease from nonalcoholic fatty liver disease. Gut Microbes. 2024;16(1):2307586. doi: 10.1080/19490976.2024.2307586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, Laurin C, Burgess S, Bowden J, Langdon R, et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife. 2018;7. doi: 10.7554/eLife.34408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhai Q, Wu H, Zheng S, Zhong T, Du C, Yuan J, Peng J, Cai C, Li J. Association between gut microbiota and NAFLD/NASH: a bidirectional two-sample Mendelian randomization study. Front Cell Infect Microbiol. 2023;13:1294826. doi: 10.3389/fcimb.2023.1294826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ouyang C, Liu P, Liu Y, Lan J, Liu Q. Metabolites mediate the causal associations between gut microbiota and NAFLD: a Mendelian randomization study. BMC Gastroenterol. 2024;24(1):244. doi: 10.1186/s12876-024-03277-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bansal T, Alaniz RC, Wood TK, Jayaraman A. The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation. Proc Natl Acad Sci USA. 2010;107(1):228–233. doi: 10.1073/pnas.0906112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shimada Y, Kinoshita M, Harada K, Mizutani M, Masahata K, Kayama H, Takeda K. Commensal bacteria-dependent indole production enhances epithelial barrier function in the colon. PLOS ONE. 2013;8(11):e80604. doi: 10.1371/journal.pone.0080604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sartor RB. Key questions to guide a better understanding of host–commensal microbiota interactions in intestinal inflammation. Mucosal Immunol. 2011;4(2):127–132. doi: 10.1038/mi.2010.87. [DOI] [PubMed] [Google Scholar]
- 39.Dai X, Jiang K, Ma X, Hu H, Mo X, Huang K, Jiang Q, Chen Y, Liu C. Mendelian randomization suggests a causal relationship between gut microbiota and nonalcoholic fatty liver disease in humans. Medicine (Baltimore). 2024;103(12):e37478. doi: 10.1097/MD.0000000000037478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Y, Liang X, Lyu Y, Wang K, Han L, Wang Y, Sun J, Chi C. Association between the gut microbiota and nonalcoholic fatty liver disease: a two-sample Mendelian randomization study. Dig Liver Dis. 2023;55(11):1464–1471. doi: 10.1016/j.dld.2023.07.014. [DOI] [PubMed] [Google Scholar]
- 41.Caussy C, Tripathi A, Humphrey G, Bassirian S, Singh S, Faulkner C, Bettencourt R, Rizo E, Richards L, Xu ZZ, et al. A gut microbiome signature for cirrhosis due to nonalcoholic fatty liver disease. Nat Commun. 2019;10(1):1406. doi: 10.1038/s41467-019-09455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clooney AG, Sutton TDS, Shkoporov AN, Holohan RK, Daly KM, O’Regan O, Ryan FJ, Draper LA, Plevy SE, Ross RP, et al. Whole-Virome analysis Sheds Light on viral dark matter in inflammatory bowel disease. Cell Host & Microbe. 2019;26(6):764–778.e765. doi: 10.1016/j.chom.2019.10.009. [DOI] [PubMed] [Google Scholar]
- 43.Nakatsu G, Zhou H, Wu WKK, Wong SH, Coker OO, Dai Z, Li X, Szeto C-H, Sugimura N, Lam TYT, et al. Alterations in enteric virome are associated with colorectal cancer and survival outcomes. Gastroenterology. 2018;155(2):529–541.e525. doi: 10.1053/j.gastro.2018.04.018. [DOI] [PubMed] [Google Scholar]
- 44.Vehik K, Lynch KF, Wong MC, Tian X, Ross MC, Gibbs RA, Ajami NJ, Petrosino JF, Rewers M, Toppari J, et al. Prospective virome analyses in young children at increased genetic risk for type 1 diabetes. Nat Med. 2019;25(12):1865–1872. doi: 10.1038/s41591-019-0667-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lang S, Demir M, Martin A, Jiang L, Zhang X, Duan Y, Gao B, Wisplinghoff H, Kasper P, Roderburg C, et al. Intestinal virome signature associated with severity of nonalcoholic fatty liver disease. Gastroenterology. 2020;159(5):1839–1852. doi: 10.1053/j.gastro.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leung H, Long X, Ni Y, Qian L, Nychas E, Siliceo SL, Pohl D, Hanhineva K, Liu Y, Xu A, et al. Risk assessment with gut microbiome and metabolite markers in NAFLD development. Sci Transl Med. 2022;14(648):eabk0855. doi: 10.1126/scitranslmed.abk0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johansson ME, Sjövall H, Hansson GC. The gastrointestinal mucus system in health and disease. Nat Rev Gastroenterol Hepatol. 2013;10(6):352–361. doi: 10.1038/nrgastro.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rohr MW, Narasimhulu CA, Rudeski-Rohr TA, Parthasarathy S. Negative effects of a high-fat diet on intestinal permeability: a review. Adv Nutr (Bethesda, Md). 2020;11(1):77–91. doi: 10.1093/advances/nmz061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang B, Li J, Fu J, Shao L, Yang L, Shi J. Interaction between mucus layer and gut microbiota in non-alcoholic fatty liver disease: soil and seeds. Chin Med J. 2023;136(12):1390–1400. doi: 10.1097/CM9.0000000000002711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gudan A, Kozłowska-Petriczko K, Wunsch E, Bodnarczuk T, Stachowska E. Small intestinal bacterial overgrowth and non-alcoholic fatty liver disease: what do we know in 2023? Nutrients. 2023;15(6):1323. doi: 10.3390/nu15061323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pabst O, Hornef MW, Schaap FG, Cerovic V, Clavel T, Bruns T. Gut–liver axis: barriers and functional circuits. Nat Rev Gastroenterol Hepatol. 2023;20(7):447–461. doi: 10.1038/s41575-023-00771-6. [DOI] [PubMed] [Google Scholar]
- 52.Chakaroun RM, Massier L, Kovacs P. Gut microbiome, intestinal permeability, and tissue bacteria in metabolic disease: perpetrators or bystanders? Nutrients. 2020;12(4):1082. doi: 10.3390/nu12041082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rahman K, Desai C, Iyer SS, Thorn NE, Kumar P, Liu Y, Smith T, Neish AS, Li H, Tan S, et al. Loss of junctional adhesion molecule a promotes severe steatohepatitis in mice on a Diet High in saturated Fat, fructose, and cholesterol. Gastroenterology. 2016;151(4):733–746.e712. doi: 10.1053/j.gastro.2016.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Munck TJI, Xu P, Verwijs HJA, Masclee AAM, Jonkers D, Verbeek J, Koek GH. Intestinal permeability in human nonalcoholic fatty liver disease: a systematic review and meta-analysis. Liver Int. 2020;40(12):2906–2916. doi: 10.1111/liv.14696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pietrzak B, Tomela K, Olejnik-Schmidt A, Mackiewicz A, Schmidt M. Secretory IgA in intestinal mucosal secretions as an adaptive barrier against microbial cells. Int J Mol Sci. 2020;21(23):9254. doi: 10.3390/ijms21239254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McPherson S, Henderson E, Burt AD, Day CP, Anstee QM. Serum immunoglobulin levels predict fibrosis in patients with non-alcoholic fatty liver disease. J Hepatol. 2014;60(5):1055–1062. doi: 10.1016/j.jhep.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 57.Wilcz-Villega EM, McClean S, O’Sullivan MA. Mast cell tryptase reduces junctional adhesion molecule-A (JAM-A) expression in intestinal epithelial cells: implications for the mechanisms of barrier dysfunction in irritable bowel syndrome. Am J Gastroenterol. 2013;108(7):1140–1151. doi: 10.1038/ajg.2013.92. [DOI] [PubMed] [Google Scholar]
- 58.Spadoni I, Fornasa G, Rescigno M. Organ-specific protection mediated by cooperation between vascular and epithelial barriers. Nat Rev Immunol. 2017;17(12):761–773. doi: 10.1038/nri.2017.100. [DOI] [PubMed] [Google Scholar]
- 59.Spadoni I, Zagato E, Bertocchi A, Paolinelli R, Hot E, Di Sabatino A, Caprioli F, Bottiglieri L, Oldani A, Viale G, et al. A gut-vascular barrier controls the systemic dissemination of bacteria. Science (New York, NY). 2015;350(6262):830–834. doi: 10.1126/science.aad0135. [DOI] [PubMed] [Google Scholar]
- 60.Cheng C, Tan J, Qian W, Zhang L, Hou X. Gut inflammation exacerbates hepatic injury in the high-fat diet induced NAFLD mouse: attention to the gut-vascular barrier dysfunction. Life Sci. 2018;209:157–166. doi: 10.1016/j.lfs.2018.08.017. [DOI] [PubMed] [Google Scholar]
- 61.Mouries J, Brescia P, Silvestri A, Spadoni I, Sorribas M, Wiest R, Mileti E, Galbiati M, Invernizzi P, Adorini L, et al. Microbiota-driven gut vascular barrier disruption is a prerequisite for non-alcoholic steatohepatitis development. J Hepatol. 2019;71(6):1216–1228. doi: 10.1016/j.jhep.2019.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Y, Song E, Bai B, Vanhoutte PM. Toll-like receptors mediating vascular malfunction: lessons from receptor subtypes. Pharmacology & Therapeutics. 2016;158:91–100. doi: 10.1016/j.pharmthera.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 63.Shaker ME. The contribution of sterile inflammation to the fatty liver disease and the potential therapies. Biomed & Pharmacother = Biomedecine & Pharmacotherapie. 2022;148:112789. doi: 10.1016/j.biopha.2022.112789. [DOI] [PubMed] [Google Scholar]
- 64.Imajo K, Fujita K, Yoneda M, Nozaki Y, Ogawa Y, Shinohara Y, Kato S, Mawatari H, Shibata W, Kitani H, et al. Hyperresponsivity to low-dose endotoxin during progression to nonalcoholic steatohepatitis is regulated by leptin-mediated signaling. Cell Metab. 2012;16(1):44–54. doi: 10.1016/j.cmet.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 65.Chen L, Zhang L, Hua H, Liu L, Mao Y, Wang R. Interactions between toll-like receptors signaling pathway and gut microbiota in host homeostasis. Immun Inflam & Dis. 2024;12(7):e1356. doi: 10.1002/iid3.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mencin A, Kluwe J, Schwabe RF. Toll-like receptors as targets in chronic liver diseases. Gut. 2009;58(5):704–720. doi: 10.1136/gut.2008.156307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yu J, Zhu C, Wang X, Kim K, Bartolome A, Dongiovanni P, Yates KP, Valenti L, Carrer M, Sadowski T, et al. Hepatocyte TLR4 triggers inter-hepatocyte Jagged1/Notch signaling to determine NASH-induced fibrosis. Sci Transl Med. 2021;13(599). doi: 10.1126/scitranslmed.abe1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gao Y, Wang Y, Liu H, Liu Z, Zhao J. Mitochondrial DNA from hepatocytes induces upregulation of interleukin-33 expression of macrophages in nonalcoholic steatohepatitis. Dig Liver Dis. 2020;52(6):637–643. doi: 10.1016/j.dld.2020.03.021. [DOI] [PubMed] [Google Scholar]
- 69.Zhang Y, Jiang W, Xu J, Wu N, Wang Y, Lin T, Liu Y, Liu Y. E. coli NF73-1 isolated from NASH patients aggravates NAFLD in mice by translocating into the liver and stimulating M1 polarization. Front Cell Infect Microbiol. 2020;10:535940. doi: 10.3389/fcimb.2020.535940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wahli W, Michalik L. PPARs at the crossroads of lipid signaling and inflammation. Trends Endocrinol Metab: TEM. 2012;23(7):351–363. doi: 10.1016/j.tem.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 71.Yang G, Lee HE, Lee JY. A pharmacological inhibitor of NLRP3 inflammasome prevents non-alcoholic fatty liver disease in a mouse model induced by high fat diet. Sci Rep. 2016;6(1):24399. doi: 10.1038/srep24399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Garcia-Martinez I, Santoro N, Chen Y, Hoque R, Ouyang X, Caprio S, Shlomchik MJ, Coffman RL, Candia A, Mehal WZ. Hepatocyte mitochondrial DNA drives nonalcoholic steatohepatitis by activation of TLR9. J Clin Invest. 2016;126(3):859–864. doi: 10.1172/JCI83885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Meijnikman AS, Nieuwdorp M, Schnabl B. Endogenous ethanol production in health and disease. Nat Rev Gastroenterol Hepatol. 2024;21(8):556–571. doi: 10.1038/s41575-024-00937-w. [DOI] [PubMed] [Google Scholar]
- 74.Meijnikman AS, Davids M, Herrema H, Aydin O, Tremaroli V, Rios-Morales M, Levels H, Bruin S, de Brauw M, Verheij J, et al. Microbiome-derived ethanol in nonalcoholic fatty liver disease. Nat Med. 2022;28(10):2100–2106. doi: 10.1038/s41591-022-02016-6. [DOI] [PubMed] [Google Scholar]
- 75.Yuan J, Chen C, Cui J, Lu J, Yan C, Wei X, Zhao X, Li N, Li S, Xue G, et al. Fatty liver disease caused by High-Alcohol-Producing Klebsiella pneumoniae. Cell Metab. 2019;30(6):1172. doi: 10.1016/j.cmet.2019.11.006. [DOI] [PubMed] [Google Scholar]
- 76.Mbaye B, Magdy Wasfy R, Borentain P, Tidjani Alou M, Mottola G, Bossi V, Caputo A, Gerolami R, Million M. Increased fecal ethanol and enriched ethanol-producing gut bacteria Limosilactobacillus fermentum, enterocloster bolteae, mediterraneibacter gnavus and streptococcus mutans in nonalcoholic steatohepatitis. Front Cell Infect Microbiol. 2023;13:1279354. doi: 10.3389/fcimb.2023.1279354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mbaye B, Borentain P, Magdy Wasfy R, Alou MT, Armstrong N, Mottola G, Meddeb L, Ranque S, Gérolami R, Million M, et al. Endogenous ethanol and triglyceride production by gut Pichia kudriavzevii, Candida albicans and Candida glabrata yeasts in non-alcoholic steatohepatitis. Cells. 2022;11(21):3390. doi: 10.3390/cells11213390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dunagan M, Chaudhry K, Samak G, Rao RK. Acetaldehyde disrupts tight junctions in caco-2 cell monolayers by a protein phosphatase 2A-dependent mechanism. Am J Physiol Gastrointestinal Liver Physiol. 2012;303(12):G1356–G1364. doi: 10.1152/ajpgi.00526.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cho YE, Yu LR, Abdelmegeed MA, Yoo SH, Song BJ. Apoptosis of enterocytes and nitration of junctional complex proteins promote alcohol-induced gut leakiness and liver injury. J Hepatol. 2018;69(1):142–153. doi: 10.1016/j.jhep.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pierce SF. The critical care nurse: an ethicist by trade. Crit Care Nurs Q. 1989;12(3):75–78. doi: 10.1097/00002727-198912000-00009. [DOI] [PubMed] [Google Scholar]
- 81.Díaz LA, Arab JP, Louvet A, Bataller R, Arrese M. The intersection between alcohol-related liver disease and nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2023;20(12):764–783. doi: 10.1038/s41575-023-00822-y. [DOI] [PubMed] [Google Scholar]
- 82.Zhang D, Tong X, Nelson BB, Jin E, Sit J, Charney N, Yang M, Omary MB, Yin L. The hepatic BMAL1/AKT/lipogenesis axis protects against alcoholic liver disease in mice via promoting PPARα pathway. Hepatol (Baltim, Md). 2018;68(3):883–896. doi: 10.1002/hep.29878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Seitz HK, Bataller R, Cortez-Pinto H, Gao B, Gual A, Lackner C, Mathurin P, Mueller S, Szabo G, Tsukamoto H. Alcoholic liver disease. Nat Rev Dis Primers. 2018;4(1):16. doi: 10.1038/s41572-018-0014-7. [DOI] [PubMed] [Google Scholar]
- 84.Fromenty B, Roden M. Mitochondrial alterations in fatty liver diseases. J Hepatol. 2023;78(2):415–429. doi: 10.1016/j.jhep.2022.09.020. [DOI] [PubMed] [Google Scholar]
- 85.Seitz HK, Stickel F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat Rev Cancer. 2007;7(8):599–612. doi: 10.1038/nrc2191. [DOI] [PubMed] [Google Scholar]
- 86.Qu S, Yu Z, Zhou Y, Wang S, Jia M, Chen T, Zhang X. Gut microbiota modulates neurotransmitter and gut-brain signaling. Microbiol Res. 2024;287:127858. doi: 10.1016/j.micres.2024.127858. [DOI] [PubMed] [Google Scholar]
- 87.Munukka E, Ahtiainen JP, Puigbó P, Jalkanen S, Pahkala K, Keskitalo A, Kujala UM, Pietilä S, Hollmén M, Elo L, et al. Six-week endurance exercise alters gut metagenome that is not reflected in systemic metabolism in over-weight women. Front Microbiol. 2018;9:2323. doi: 10.3389/fmicb.2018.02323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM, Czaja MJ. Autophagy regulates lipid metabolism. Nature. 2009;458(7242):1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mann ER, Lam YK, Uhlig HH. Short-chain fatty acids: linking diet, the microbiome and immunity. Nat Rev Immunol. 2024;24(8):577–595. doi: 10.1038/s41577-024-01014-8. [DOI] [PubMed] [Google Scholar]
- 90.Willemsen LE, Koetsier MA, van Deventer SJ, van Tol EA. Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E(1) and E(2) production by intestinal myofibroblasts. Gut. 2003;52(10):1442–1447. doi: 10.1136/gut.52.10.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yan H, Ajuwon KM. Butyrate modifies intestinal barrier function in IPEC-J2 cells through a selective upregulation of tight junction proteins and activation of the akt signaling pathway. PLOS ONE. 2017;12(6):e0179586. doi: 10.1371/journal.pone.0179586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Feng Y, Wang Y, Wang P, Huang Y, Wang F. Short-chain fatty acids manifest stimulative and protective effects on intestinal barrier function through the inhibition of NLRP3 inflammasome and autophagy. Cell Physiol Biochem. 2018;49(1):190–205. doi: 10.1159/000492853. [DOI] [PubMed] [Google Scholar]
- 93.Kaiko GE, Ryu SH, Koues OI, Collins PL, Solnica-Krezel L, Pearce EJ, Pearce EL, Oltz EM, Stappenbeck TS. The colonic crypt protects stem cells from microbiota-derived metabolites. Cell. 2016;165(7):1708–1720. doi: 10.1016/j.cell.2016.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hinrichsen F, Hamm J, Westermann M, Schröder L, Shima K, Mishra N, Walker A, Sommer N, Klischies K, Prasse D, et al. Microbial regulation of hexokinase 2 links mitochondrial metabolism and cell death in colitis. Cell Metab. 2021;33(12):2355–2366.e2358. doi: 10.1016/j.cmet.2021.11.004. [DOI] [PubMed] [Google Scholar]
- 95.Fachi JL, Felipe JDS, Pral LP, da Silva BK, Corrêa RO, de Andrade MCP, da Fonseca DM, Basso PJ, Câmara NOS, de Sales e Souza ÉL, et al. Butyrate protects mice from clostridium difficile-induced colitis through an HIF-1-Dependent mechanism. Cell Rep. 2019;27(3):750–761.e757. doi: 10.1016/j.celrep.2019.03.054. [DOI] [PubMed] [Google Scholar]
- 96.Rai RP, Liu Y, Iyer SS, Liu S, Gupta B, Desai C, Kumar P, Smith T, Singhi AD, Nusrat A, et al. Blocking integrin α4β7-mediated CD4 T cell recruitment to the intestine and liver protects mice from western diet-induced non-alcoholic steatohepatitis. J Hepatol. 2020;73(5):1013–1022. doi: 10.1016/j.jhep.2020.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sun C, Qiu C, Zhang Y, Yan M, Tan J, He J, Yang D, Wang D, Wu L. Lactiplantibacillus plantarum NKK20 alleviates high-fat-diet-induced nonalcoholic fatty liver disease in mice through regulating bile acid anabolism. Molecules (Basel, Switzerland. Oxycedrus Needles Berries Mol. 2023;28(10):4042. doi: 10.3390/molecules28104042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Psichas A, Sleeth ML, Murphy KG, Brooks L, Bewick GA, Hanyaloglu AC, Ghatei MA, Bloom SR, Frost G. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int J Obes (2005). 2015;39(3):424–429. doi: 10.1038/ijo.2014.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Christiansen CB, Gabe MBN, Svendsen B, Dragsted LO, Rosenkilde MM, Holst JJ. The impact of short-chain fatty acids on GLP-1 and PYY secretion from the isolated perfused rat colon. Am J Physiol Gastrointestinal Liver Physiol. 2018;315(1):G53–g65. doi: 10.1152/ajpgi.00346.2017. [DOI] [PubMed] [Google Scholar]
- 100.Forte N, Marfella B, Nicois A, Palomba L, Paris D, Motta A, Pina Mollica M, Di Marzo V, Cristino L. The short-chain fatty acid acetate modulates orexin/hypocretin neurons: a novel mechanism in gut-brain axis regulation of energy homeostasis and feeding. Biochem Pharmacol. 2024;226:116383. doi: 10.1016/j.bcp.2024.116383. [DOI] [PubMed] [Google Scholar]
- 101.Nimer N, Choucair I, Wang Z, Nemet I, Li L, Gukasyan J, Weeks TL, Alkhouri N, Zein N, Tang WHW, et al. Bile acids profile, histopathological indices and genetic variants for non-alcoholic fatty liver disease progression. Metab: Clin And Exp. 2021;116:154457. doi: 10.1016/j.metabol.2020.154457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Suga T, Yamaguchi H, Ogura J, Shoji S, Maekawa M, Mano N. Altered bile acid composition and disposition in a mouse model of non-alcoholic steatohepatitis. Toxicol Appl Pharm. 2019;379:114664. doi: 10.1016/j.taap.2019.114664. [DOI] [PubMed] [Google Scholar]
- 103.Sorribas M, Jakob MO, Yilmaz B, Li H, Stutz D, Noser Y, de Gottardi A, Moghadamrad S, Hassan M, Albillos A, et al. FXR modulates the gut-vascular barrier by regulating the entry sites for bacterial translocation in experimental cirrhosis. J Hepatol. 2019;71(6):1126–1140. doi: 10.1016/j.jhep.2019.06.017. [DOI] [PubMed] [Google Scholar]
- 104.Jahn D, Rau M, Hermanns HM, Geier A. Mechanisms of enterohepatic fibroblast growth factor 15/19 signaling in health and disease. Cytokine & Growth Factor Rev. 2015;26(6):625–635. doi: 10.1016/j.cytogfr.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 105.Wieland A, Frank DN, Harnke B, Bambha K. Systematic review: microbial dysbiosis and nonalcoholic fatty liver disease. Alimentary Pharmacol & Ther. 2015;42(9):1051–1063. doi: 10.1111/apt.13376. [DOI] [PubMed] [Google Scholar]
- 106.Zhang CH, Zhou B-G, Sheng J-Q, Chen Y, Cao Y-Q, Chen C. Molecular mechanisms of hepatic insulin resistance in nonalcoholic fatty liver disease and potential treatment strategies. Pharmacol Res. 2020;159:104984. doi: 10.1016/j.phrs.2020.104984. [DOI] [PubMed] [Google Scholar]
- 107.Renga B, Mencarelli A, Cipriani S, D’Amore C, Carino A, Bruno A, Francisci D, Zampella A, Distrutti E, Fiorucci S, et al. The bile acid sensor FXR is required for immune-regulatory activities of TLR-9 in intestinal inflammation. PLOS ONE. 2013;8(1):e54472. doi: 10.1371/journal.pone.0054472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Guo C, Xie S, Chi Z, Zhang J, Liu Y, Zhang L, Zheng M, Zhang X, Xia D, Ke Y, et al. Bile acids control inflammation and metabolic disorder through inhibition of NLRP3 inflammasome. Immunity. 2016;45(4):802–816. doi: 10.1016/j.immuni.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 109.Fuchs CD, Traussnigg SA, Trauner M. Nuclear receptor modulation for the treatment of nonalcoholic fatty liver disease. Semin Liver Dis. 2016;36(1):69–86. doi: 10.1055/s-0036-1571296. [DOI] [PubMed] [Google Scholar]
- 110.Collins SL, Stine JG, Bisanz JE, Okafor CD, Patterson AD. Bile acids and the gut microbiota: metabolic interactions and impacts on disease. Nat Rev Microbiol. 2023;21(4):236–247. doi: 10.1038/s41579-022-00805-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nie Q, Luo X, Wang K, Ding Y, Jia S, Zhao Q, Li M, Zhang J, Zhuo Y, Lin J, et al. Gut symbionts alleviate MASH through a secondary bile acid biosynthetic pathway. Cell. 2024;187(11):2717–2734.e2733. doi: 10.1016/j.cell.2024.03.034. [DOI] [PubMed] [Google Scholar]
- 112.Schneider KM, Mohs A, Kilic K, Candels LS, Elfers C, Bennek E, Schneider LB, Heymann F, Gassler N, Penders J, et al. Intestinal microbiota protects against MCD diet-induced steatohepatitis. Int J Mol Sci. 2019;20(2):308. doi: 10.3390/ijms20020308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sawada Y, Kawaratani H, Kubo T, Fujinaga Y, Furukawa M, Saikawa S, Sato S, Seki K, Takaya H, Okura Y, et al. Combining probiotics and an angiotensin-II type 1 receptor blocker has beneficial effects on hepatic fibrogenesis in a rat model of non-alcoholic steatohepatitis. Hepatol Res. 2019;49(3):284–295. doi: 10.1111/hepr.13281. [DOI] [PubMed] [Google Scholar]
- 114.Romano KA, Vivas EI, Amador-Noguez D, Rey FE. Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-oxide. mBio. 2015;6(2):e02481. doi: 10.1128/mBio.02481-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chhibber-Goel J, Gaur A, Singhal V, Parakh N, Bhargava B, Sharma A. The complex metabolism of trimethylamine in humans: endogenous and exogenous sources. Expert reviews in molecular medicine. Expert Rev Mol Med. 2016;18:e8. doi: 10.1017/erm.2016.6. [DOI] [PubMed] [Google Scholar]
- 116.Bennett BJ, Vallim T, Wang Z, Shih D, Meng Y, Gregory J, Allayee H, Lee R, Graham M, Crooke R, et al. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 2013;17(1):49–60. doi: 10.1016/j.cmet.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.León-Mimila P, Villamil-Ramírez H, Li XS, Shih DM, Hui ST, Ocampo-Medina E, López-Contreras B, Morán-Ramos S, Olivares-Arevalo M, Grandini-Rosales P, et al. Trimethylamine N-oxide levels are associated with NASH in obese subjects with type 2 diabetes. Diabetes Metab. 2021;47(2):101183. doi: 10.1016/j.diabet.2020.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yang B, Tang G, Wang M, Ni Y, Tong J, Hu C, Zhou M, Jiao K, Li Z. Trimethylamine N-oxide induces non-alcoholic fatty liver disease by activating the PERK. Toxicol Lett. 2024;400:93–103. doi: 10.1016/j.toxlet.2024.08.009. [DOI] [PubMed] [Google Scholar]
- 119.Nian F, Chen Y, Xia Q, Zhu C, Wu L, Lu X. Gut microbiota metabolite trimethylamine N-oxide promoted NAFLD progression by exacerbating intestinal barrier disruption and intrahepatic cellular imbalance. Int Immunopharmacol. 2024;142:113173. doi: 10.1016/j.intimp.2024.113173. [DOI] [PubMed] [Google Scholar]
- 120.Miyata M, Takeda K, Nagira S, Sugiura Y. Trimethylamine N-oxide ameliorates hepatic damage including reduction of hepatic bile acids and cholesterol in Fxr-null mice. Int J Food Sci Nutr. 2024;75(4):385–395. doi: 10.1080/09637486.2024.2346765. [DOI] [PubMed] [Google Scholar]
- 121.Zhao ZH, Xin F-Z, Zhou D, Xue Y-Q, Liu X-L, Yang R-X, Pan Q, Fan J-G. Trimethylamine N-oxide attenuates high-fat high-cholesterol diet-induced steatohepatitis by reducing hepatic cholesterol overload in rats. World J Gastroenterol. 2019;25(20):2450–2462. doi: 10.3748/wjg.v25.i20.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yanko R, Levashov M, Chaka OG, Nosar V, Khasabov S, Khasabova I. Tryptophan prevents the development of non-alcoholic fatty liver disease. Diabetes, Metab Syndrome Obesity. 2023;16:4195–4204. doi: 10.2147/DMSO.S444278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ji Y, Gao Y, Chen H, Yin Y, Zhang W. Indole-3-Acetic Acid Alleviates Nonalcoholic Fatty Liver Disease in Mice via Attenuation of Hepatic Lipogenesis, and Oxidative and Inflammatory Stress. Nutrients. 2019;11(9):2062. doi: 10.3390/nu11092062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhao ZH, Xin F-Z, Xue Y, Hu Z, Han Y, Ma F, Zhou D, Liu X-L, Cui A, Liu Z, et al. Indole-3-propionic acid inhibits gut dysbiosis and endotoxin leakage to attenuate steatohepatitis in rats. Exp Mol Med. 2019;51(9):1–14. doi: 10.1038/s12276-019-0304-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhu B, Li H, Lu B, Guo X, Wu C, Wang F, Li Q, Xie L, Glaser S, Francis H, et al. Corrigendum to “Indole supplementation ameliorates MCD-induced NASH in mice” [J Nutrit Biochem 2022;107:109041]. J nutritional Biochem. 2024;133:109705. doi: 10.1016/j.jnutbio.2024.109705. [DOI] [PubMed] [Google Scholar]
- 126.Zhao M, Zhao L, Xiong X, He Y, Huang W, Liu Z, Ji L, Pan B, Guo X, Wang L, et al. TMAVA, a Metabolite of Intestinal Microbes, Is Increased in Plasma From Patients With Liver Steatosis, Inhibits γ-Butyrobetaine Hydroxylase, and Exacerbates Fatty Liver in Mice. Gastroenterology. 2020;158(8):2266–2281.e2227. doi: 10.1053/j.gastro.2020.02.033. [DOI] [PubMed] [Google Scholar]
- 127.Ledford H. First US drug approved for a liver disease surging around the world. Nature. 2024; doi: 10.1038/d41586-024-00747-9. [DOI] [PubMed] [Google Scholar]
- 128.Nakanishi N, Hashimoto Y, Okamura T, Ohbora A, Kojima T, Hamaguchi M, Fukui M. A weight regain of 1.5 kg or more and lack of exercise are associated with nonalcoholic fatty liver disease recurrence in men. Sci Rep. 2021;11(1):19992. doi: 10.1038/s41598-021-99036-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li H, Wang X-K, Tang M, Lei L, Li J-R, Sun H, Jiang J, Dong B, Li H-Y, Jiang J-D, et al. Bacteroides thetaiotaomicron ameliorates mouse hepatic steatosis through regulating gut microbial composition, gut-liver folate and unsaturated fatty acids metabolism. Gut Microbes. 2024;16(1):2304159. doi: 10.1080/19490976.2024.2304159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lee HB, Park M, Lee S-Y, Ha SK, Kim Y, Lee K-W, Park H-Y. Lactococcus lactis KF140 Ameliorates Nonalcoholic Fatty Liver Disease Induced by N ε -Carboxymethyl-Lysine and High-Fat Diet. Mol nutr Food Res. 2024;68(16):e2400260. doi: 10.1002/mnfr.202400260. [DOI] [PubMed] [Google Scholar]
- 131.Sun C, Xiong X, Liu M, Liang Q, Zhao Q, Wei G, Shi J, Li X. Bacteroides ovatus alleviates high-fat and high-cholesterol -induced nonalcoholic fatty liver disease via gut-liver axis. Biomed & Pharmacother = Biomedecine & pharmacotherapie. 2024;178:117156. doi: 10.1016/j.biopha.2024.117156. [DOI] [PubMed] [Google Scholar]
- 132.Arellano-García LI, Milton-Laskibar I, Martínez JA, Arán-González M, Portillo MP. Comparative effects of viable Lactobacillus rhamnosus GG and its heat-inactivated paraprobiotic in the prevention of high-fat high-fructose diet-induced non-alcoholic fatty liver disease in rats. BioFactors (Oxford, Engl). 2024;51(1). doi: 10.1002/biof.2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhao Y, Li C, Luan Z, Chen J, Wang C, Jing Y, Qi S, Zhao Z, Zhang H, Wu J, et al. Lactobacillus oris improves non-alcoholic fatty liver in mice and inhibits endogenous cholesterol biosynthesis. Sci Rep. 2023;13(1):12946. doi: 10.1038/s41598-023-38530-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yoon SJ, Yu JS, Min BH, Gupta H, Won S-M, Park HJ, Han SH, Kim B-Y, Kim KH, Kim BK, et al. Bifidobacterium-derived short-chain fatty acids and indole compounds attenuate nonalcoholic fatty liver disease by modulating gut-liver axis. Front Microbiol. 2023;14:1129904. doi: 10.3389/fmicb.2023.1129904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wen X, Liu H, Luo X, Lui L, Fan J, Xing Y, Wang J, Qiao X, Li N, Wang G. Supplementation of Lactobacillus plantarum ATCC14917 mitigates non-alcoholic fatty liver disease in high-fat-diet-fed rats. Front Microbiol. 2023;14:1146672. doi: 10.3389/fmicb.2023.1146672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yang M, Wang H, Bukhari I, Zhao Y, Huang H, Yu Y, Sun X, Mi Y, Mei L, Zheng P. Effects of cholesterol-lowering probiotics on non-alcoholic fatty liver disease in FXR gene knockout mice. Front Nutr. 2023;10:1121203. doi: 10.3389/fnut.2023.1121203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lv H, Tao F, Peng L, Chen S, Ren Z, Chen J, Yu B, Wei H, Wan C. In Vitro Probiotic Properties of Bifidobacterium animalis subsp. lactis SF and Its Alleviating Effect on Non-Alcoholic Fatty Liver Disease. Nutrients. 2023;15(6):1355. doi: 10.3390/nu15061355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hany NM, Eissa S, Basyouni M, Hasanin AH, Aboul-Ela YM, Elmagd NMA, Montasser IF, Ali MA, Skipp PJ, Matboli M. Modulation of hepatic stellate cells by Mutaflor® probiotic in non-alcoholic fatty liver disease management. J Transl Med. 2022;20(1):342. doi: 10.1186/s12967-022-03543-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yang D, Wei X, Zhang B, Zhu R, Hu H, Fan X, Du H, Chen X, Zhang Z, Zhao M, et al. Probiotics protect against hepatic steatosis in tris (2-chloroethyl) phosphate-induced metabolic disorder of mice via FXR signaling. Food Chem Toxicol : Int J published Br Ind Biol Res Assoc. 2022;169:113440. doi: 10.1016/j.fct.2022.113440. [DOI] [PubMed] [Google Scholar]
- 140.Aoki R, Onuki M, Hattori K, Ito M, Yamada T, Kamikado K, Kim Y-G, Nakamoto N, Kimura I, Clarke JM, et al. Commensal microbe-derived acetate suppresses NAFLD/NASH development via hepatic FFAR2 signalling in mice. Microbiome. 2021;9(1):188. doi: 10.1186/s40168-021-01125-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Depommier C, Everard A, Druart C, Plovier H, Van Hul M, Vieira-Silva S, Falony G, Raes J, Maiter D, Delzenne NM, et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat Med. 2019;25(7):1096–1103. doi: 10.1038/s41591-019-0495-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Plovier H, Everard A, Druart C, Depommier C, Van Hul M, Geurts L, Chilloux J, Ottman N, Duparc T, Lichtenstein L, et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med. 2017;23(1):107–113. doi: 10.1038/nm.4236. [DOI] [PubMed] [Google Scholar]
- 143.Han Y, Ling Q, Wu L, Wang X, Wang Z, Chen J, Zheng Z, Zhou Z, Jia L, Li L, et al. Akkermansia muciniphila inhibits nonalcoholic steatohepatitis by orchestrating TLR2-activated γδT17 cell and macrophage polarization. Gut Microbes. 2023;15(1):2221485. doi: 10.1080/19490976.2023.2221485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Niu H, Zhou M, Zogona D, Xing Z, Wu T, Chen R, Cui D, Liang F, Xu X. Akkermansia muciniphila: a potential candidate for ameliorating metabolic diseases. Front Immunol. 2024;15:1370658. doi: 10.3389/fimmu.2024.1370658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Silva-Sperb AS, Moraes HA, Barcelos STA, de Moura BC, Longo L, Michalczuk MT, Cerski CTS, Uribe-Cruz C, da Silveira TR, Álvares-da-Silva MR, et al. Probiotic supplementation for 24 weeks in patients with non-alcoholic steatohepatitis: the PROBILIVER randomized clinical trial. Front Nutr. 2024;11:1362694. doi: 10.3389/fnut.2024.1362694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ayob N, Muhammad Nawawi KN, Mohamad nor MH, Raja Ali RA, Ahmad HF, Oon SF, Mohd Mokhtar N. The Effects of Probiotics on Small Intestinal Microbiota Composition, Inflammatory Cytokines and Intestinal Permeability in Patients with Non-Alcoholic Fatty Liver Disease. Biomedicines. 2023;11(2):640. doi: 10.3390/biomedicines11020640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Derosa G, Guasti L, D’Angelo A, Martinotti C, Valentino MC, Di Matteo S, Bruno GM, Maresca AM, Gaudio GV, Maffioli P. Probiotic Therapy With VSL#3® in Patients With NAFLD: A Randomized Clinical Trial. Front Nutr. 2022;9:846873. doi: 10.3389/fnut.2022.846873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mohamad nor MH, Ayob N, Mokhtar NM, Raja Ali RA, Tan GC, Wong Z, Shafiee NH, Wong YP, Mustangin M, Nawawi KNM. The effect of probiotics (MCP® BCMC® Strains) on hepatic steatosis, small intestinal mucosal immune function, and intestinal barrier in patients with non-alcoholic fatty liver disease. Nutrients. 2021;13(9):3192. doi: 10.3390/nu13093192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Abd El Hamid AA, Mohamed AE, Mohamed MS, Amin GEED, Elessawy HAA, Allam MF. The effect of probiotic supplementation on non-alcoholic fatty liver disease (NAFLD) fibrosis score in patients attending a tertiary hospital clinic in Cairo, Egypt. BMC Gastroenterol. 2024;24(1):354. doi: 10.1186/s12876-024-03424-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Escouto GS, Port GZ, Tovo CV, Fernandes SA, Peres A, Dorneles GP, Houde VP, Varin TV, Pilon G, Marette A, et al. Probiotic supplementation, hepatic fibrosis, and the microbiota profile in patients with nonalcoholic steatohepatitis: a randomized controlled trial. J Drug Issues Nutr. 2023;153(7):1984–1993. doi: 10.1016/j.tjnut.2023.05.019. [DOI] [PubMed] [Google Scholar]
- 151.Crommen S, Rheinwalt KP, Plamper A, Simon M-C, Rösler D, Fimmers R, Egert S, Metzner C. A Specifically Tailored Multistrain Probiotic and Micronutrient Mixture Affects Nonalcoholic Fatty Liver Disease—Related Markers in Patients with Obesity after Mini Gastric Bypass Surgery. J Drug Issues Nutr. 2022;152(2):408–418. doi: 10.1093/jn/nxab392. [DOI] [PubMed] [Google Scholar]
- 152.Cohen PA. Probiotic Safety—No Guarantees. JAMA Intern Med. 2018;178(12):1577–1578. doi: 10.1001/jamainternmed.2018.5403. [DOI] [PubMed] [Google Scholar]
- 153.Rong L, Ch’ng D, Jia P, Tsoi KKF, Wong SH, Sung JJY. Use of probiotics, prebiotics, and synbiotics in non-alcoholic fatty liver disease: A systematic review and meta-analysis. J Gastroen Hepatol. 2023;38(10):1682–1694. doi: 10.1111/jgh.16256. [DOI] [PubMed] [Google Scholar]
- 154.Van Niel CW, Feudtner C, Garrison MM, Christakis DA. Lactobacillus therapy for acute infectious diarrhea in children: a meta-analysis. Pediatrics. 2002;109(4):678–684. doi: 10.1542/peds.109.4.678. [DOI] [PubMed] [Google Scholar]
- 155.Guo Q, Goldenberg JZ, Humphrey C, El Dib R, Johnston BC. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst Rev. 2019;4:Cd004827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Aceti A, Gori D, Barone G, Callegari ML, Di Mauro A, Fantini MP, Indrio F, Maggio L, Meneghin F, Morelli L, et al. Probiotics for prevention of necrotizing enterocolitis in preterm infants: systematic review and meta-analysis. Ital J Pediatr. 2015;41(1):89. doi: 10.1186/s13052-015-0199-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ouwehand AC. A review of dose-responses of probiotics in human studies. Benef Microbes. 2017;8(2):143–151. doi: 10.3920/BM2016.0140. [DOI] [PubMed] [Google Scholar]
- 158.Noormohammadi M, Ghorbani Z, Löber U, Mahdavi-Roshan M, Bartolomaeus TUP, Kazemi A, Shoaibinobarian N, Forslund SK. The effect of probiotic and synbiotic supplementation on appetite-regulating hormones and desire to eat: A systematic review and meta-analysis of clinical trials. Pharmacol Res. 2023;187:106614. doi: 10.1016/j.phrs.2022.106614. [DOI] [PubMed] [Google Scholar]
- 159.Song Q, Zhang X, Liu W, Wei H, Liang W, Zhou Y, Ding Y, Ji F, Ho-Kwan Cheung A, Wong N, et al. Bifidobacterium pseudolongum-generated acetate suppresses non-alcoholic fatty liver disease-associated hepatocellular carcinoma. J Hepatol. 2023;79(6):1352–1365. doi: 10.1016/j.jhep.2023.07.005. [DOI] [PubMed] [Google Scholar]
- 160.Jang HR, Kim UK, Jang K, Song YS, Cha J-Y, Yi H, Youn J. A protective mechanism of probiotic Lactobacillus against hepatic steatosis via reducing host intestinal fatty acid absorption. Exp Mol Med. 2019;51(12):1–14. doi: 10.1038/s12276-019-0352-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wang W, Shi LP, Shi L, Xu L. Efficacy of probiotics on the treatment of non-alcoholic fatty liver disease. Zhonghua Nei Ke Za Zhi. 2018;57(2):101–106. doi: 10.3760/cma.j.issn.0578-1426.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 162.Yang Y, Yang L, Wu J, Hu J, Wan M, Bie J, Li J, Pan D, Sun G, Yang C. Optimal probiotic combinations for treating nonalcoholic fatty liver disease: A systematic review and network meta-analysis. Clin Nutr (Edinburgh, Scotland). 2024;43(6):1224–1239. doi: 10.1016/j.clnu.2024.04.004. [DOI] [PubMed] [Google Scholar]
- 163.Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, Scott K, Stanton C, Swanson KS, Cani PD, et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14(8):491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- 164.Zhao W, Guo M, Feng J, Gu Z, Zhao J, Zhang H, Wang G, Chen W. Myristica fragrans Extract Regulates Gut Microbes and Metabolites to Attenuate Hepatic Inflammation and Lipid Metabolism Disorders via the AhR–FAS and NF-κB Signaling Pathways in Mice with Non-Alcoholic Fatty Liver Disease. Nutrients. 2022;14(9):1699. doi: 10.3390/nu14091699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhang W, Yu L, Yang Q, Zhang J, Wang W, Hu X, Li J, Zheng G. Smilax China L. polysaccharide prevents HFD induced-NAFLD by regulating hepatic fat metabolism and gut microbiota. Phytomed : Int J Phytother Phytopharmacol. 2024;127:155478. doi: 10.1016/j.phymed.2024.155478. [DOI] [PubMed] [Google Scholar]
- 166.Yan Y, Yuan H, Yang F, Na H, Yu X, Liu J, Wang Y. Seabuckthorn polysaccharides mitigate hepatic steatosis by modulating the Nrf-2/HO-1 pathway and gut microbiota. AMB Express. 2024;14(1):100. doi: 10.1186/s13568-024-01756-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Huang Y, Chen H, Chen J, Wu Q, Zhang W, Li D, Lu Y, Chen Y. Yellow tea polysaccharides protect against non-alcoholic fatty liver disease via regulation of gut microbiota and bile acid metabolism in mice. Phytomed : Int J Phytother Phytopharmacol. 2024;133:155919. doi: 10.1016/j.phymed.2024.155919. [DOI] [PubMed] [Google Scholar]
- 168.Yang Z, Su H, Lv Y, Tao H, Jiang Y, Ni Z, Peng L, Chen X. Inulin intervention attenuates hepatic steatosis in rats via modulating gut microbiota and maintaining intestinal barrier function. Food Res Int. 2023;163:112309. doi: 10.1016/j.foodres.2022.112309. [DOI] [PubMed] [Google Scholar]
- 169.Vily-Petit J, Soty M, Silva M, Micoud M, Bron C, Guérin-Deremaux L, Mithieux G. Improvement of energy metabolism associated with NUTRIOSE® soluble fiber, a dietary ingredient exhibiting prebiotic properties, requires intestinal gluconeogenesis. Food Res Int. 2023;167:112723. doi: 10.1016/j.foodres.2023.112723. [DOI] [PubMed] [Google Scholar]
- 170.Shu Y, Huang Y, Dong W, Fan X, Sun Y, Chen G, Zeng X, Ye H. The polysaccharides from Auricularia auricula alleviate non-alcoholic fatty liver disease via modulating gut microbiota and bile acids metabolism. Int J Biol Macromolecules. 2023;246:125662. doi: 10.1016/j.ijbiomac.2023.125662. [DOI] [PubMed] [Google Scholar]
- 171.Li Q, Liu W, Zhang H, Chen C, Liu R, Hou H, Luo Q, Yu Q, Ouyang H, Feng Y, et al. α-D-1, 3-glucan from Radix Puerariae thomsonii improves NAFLD by regulating the intestinal flora and metabolites. Carbohyd Polym. 2023;299:120197. doi: 10.1016/j.carbpol.2022.120197. [DOI] [PubMed] [Google Scholar]
- 172.Im ST, Mun H, Park S, Kang H, Kim WC, Heo S-J, Lee S-H. Ishige okamurae Celluclast extract ameliorates non-alcoholic fatty liver in high-fructose diet-fed mice by modulation of lipid metabolism and gut microbiota composition. Food Chem Toxicol : an Int J published Br Ind Biol Res Assoc. 2023;177:113864. doi: 10.1016/j.fct.2023.113864. [DOI] [PubMed] [Google Scholar]
- 173.Fotschki B, Sójka M, Kosmala M, Juśkiewicz J. Prebiotics Together with Raspberry Polyphenolic Extract Mitigate the Development of Nonalcoholic Fatty Liver Diseases in Zucker Rats. Nutrients. 2023;15(14):3115. doi: 10.3390/nu15143115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ning E-J, Sun C-W, Wang X-F, Chen L, Li F-F, Zhang L-X, Wang L-P, Ma Y-N, Zhu J, Li X, et al. Artemisia argyi polysaccharide alleviates intestinal inflammation and intestinal flora dysbiosis in lipopolysaccharide-treated mice. Food & Med Homol. 2024;1(1):9420008. doi: 10.26599/FMH.2024.9420008. [DOI] [Google Scholar]
- 175.Liu Y-F, Ling N, Zhang B, Chen C, Mo X-N, Cai J-Y, Tan X-D, Yu Q-M. Flavonoid-Rich mulberry leaf extract modulate lipid metabolism, antioxidant capacity, and gut microbiota in high-fat diet-induced obesity: potential roles of FGF21 and SOCS2. Food & Med Homol. 2024;1(2):9420016. doi: 10.26599/FMH.2024.9420016. [DOI] [Google Scholar]
- 176.Do MH, Lee HHL, Lee J-E, Park M, Oh M-J, Lee H-B, Park J-H, Jhun H, Kim JH, Kang C-H, et al. Gellan gum prevents non-alcoholic fatty liver disease by modulating the gut microbiota and metabolites. Food Chem. 2023;400:134038. doi: 10.1016/j.foodchem.2022.134038. [DOI] [PubMed] [Google Scholar]
- 177.Zhang C, Fang T, Shi L, Wang Y, Deng X, Wang J, Zhou Y. The synbiotic combination of probiotics and inulin improves NAFLD though modulating gut microbiota. J nutritional Biochem. 2024;125:109546. doi: 10.1016/j.jnutbio.2023.109546. [DOI] [PubMed] [Google Scholar]
- 178.Ralli T, Ahmad S, Saifi Z, Alhalmi A, Aeri V, Aqil M, Kohli K. Exploring the therapeutic potential of silymarin-based herbal remedy (prebiotic) and probiotic blend in a mouse model of NAFLD: Insights into gut microbiota modulation and liver health. Heliyon. 2024;10(12):e33505. doi: 10.1016/j.heliyon.2024.e33505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kang Y, Ren P, Shen X, Kuang X, Yang X, Liu H, Yan H, Yang H, Kang X, Ding Z, et al. A newly synbiotic combination alleviates obesity by modulating the gut microbiota–fat axis and inhibiting the hepatic TLR4/NF-κB Signaling Pathway. Mol nutr Food Res. 2023;67(24):e2300141. doi: 10.1002/mnfr.202300141. [DOI] [PubMed] [Google Scholar]
- 180.Gadallah SH, Eissa S, Ghanem HM, Ahmed EK, Hasanin AH, El Mahdy MM, Matboli M. Probiotic-prebiotic-synbiotic modulation of (YAP1, LATS1 and NF2 mRNAs/miR-1205/lncRNA SRD5A3-AS1) panel in NASH animal model. Biomed & Pharmacother = Biomedecine & pharmacotherapie. 2021;140:111781. doi: 10.1016/j.biopha.2021.111781. [DOI] [PubMed] [Google Scholar]
- 181.Yau YF, El‐Nezami H, Galano J-M, Kundi ZM, Durand T, Lee JCY. Lactobacillus rhamnosus GG and oat beta-glucan regulated fatty acid profiles along the gut-liver-brain axis of mice fed with high fat diet and demonstrated antioxidant and anti-inflammatory potentials. Mol nutr Food Res. 2020;64(18):e2000566. doi: 10.1002/mnfr.202000566. [DOI] [PubMed] [Google Scholar]
- 182.Abenavoli L, Izzo AA, Milić N, Cicala C, Santini A, Capasso R. Milk thistle (Silybum marianum): A concise overview on its chemistry, pharmacological, and nutraceutical uses in liver diseases. Phytotherapy Res : PTR. 2018;32(11):2202–2213. doi: 10.1002/ptr.6171. [DOI] [PubMed] [Google Scholar]
- 183.Gu M, Zhao P, Huang J, Zhao Y, Wang Y, Li Y, Li Y, Fan S, Ma Y-M, Tong Q, et al. Silymarin Ameliorates Metabolic Dysfunction Associated with Diet-Induced Obesity via Activation of Farnesyl X Receptor. Front Pharmacol. 2016;7:345. doi: 10.3389/fphar.2016.00345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wah Kheong C, Nik Mustapha NR, Mahadeva S. A Randomized Trial of Silymarin for the Treatment of Nonalcoholic Steatohepatitis. Clin Gastroenterol Hepatol. 2017;15(12):1940–1949.e1948. doi: 10.1016/j.cgh.2017.04.016. [DOI] [PubMed] [Google Scholar]
- 185.BinMowyna MN, AlFaris NA, Al-Sanea EA, AlTamimi JZ, Aldayel TS. Resveratrol attenuates against high-fat-diet-promoted non-alcoholic fatty liver disease in rats mainly by targeting the miR-34a/SIRT1 axis. Arch Physiol Biochem. 2024;130(3):300–315. doi: 10.1080/13813455.2022.2046106. [DOI] [PubMed] [Google Scholar]
- 186.Bomhof MR, Parnell JA, Ramay HR, Crotty P, Rioux KP, Probert CS, Jayakumar S, Raman M, Reimer RA. Histological improvement of non-alcoholic steatohepatitis with a prebiotic: a pilot clinical trial. Eur J Nutr. 2019;58(4):1735–1745. doi: 10.1007/s00394-018-1721-2. [DOI] [PubMed] [Google Scholar]
- 187.Agrinier AL, Morissette A, Daoust L, Gignac T, Marois J, Varin TV, Pilon G, Larose É, Gagnon C, Desjardins Y, et al. Camu-camu decreases hepatic steatosis and liver injury markers in overweight, hypertriglyceridemic individuals: A randomized crossover trial. Cell Reports Med. 2024;5(8):101682. doi: 10.1016/j.xcrm.2024.101682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Chong CYL, Orr D, Plank LD, Vatanen T, O’Sullivan JM, Murphy R. Randomised Double-Blind Placebo-Controlled Trial of Inulin with Metronidazole in Non-Alcoholic Fatty Liver Disease (NAFLD). Nutrients. 2020;12(4):937. doi: 10.3390/nu12040937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Reshef N, Gophna U, Reshef L, Konikoff F, Gabay G, Zornitzki T, Knobler H, Maor Y. Prebiotic Treatment in Patients with Nonalcoholic Fatty Liver Disease (NAFLD)—A Randomized Pilot Trial. Nutrients. 2024;16(11):1571. doi: 10.3390/nu16111571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mitrović M, Dobrosavljević A, Odanović O, Knežević-Ivanovski T, Kralj Đ, Erceg S, Perućica A, Svorcan P, Stanković-Popović V. The effects of synbiotics on the liver steatosis, inflammation, and gut microbiome of metabolic dysfunction-associated liver disease patients-randomized trial. Rom J Intern Med = Revue Roum de medecine interne. 2024;62(2):184–193. doi: 10.2478/rjim-2024-0004. [DOI] [PubMed] [Google Scholar]
- 191.Mantri A, Köhlmoos A, Schelski DS, Seel W, Stoffel-Wagner B, Krawitz P, Stehle P, Holst JJ, Weber B, Koban L, et al. Impact of Synbiotic Intake on Liver Metabolism in Metabolically Healthy Participants and Its Potential Preventive Effect on Metabolic-Dysfunction-Associated Fatty Liver Disease (MAFLD): A Randomized, Placebo-Controlled, Double-Blinded Clinical Trial. Nutrients. 2024;16(9):1300. doi: 10.3390/nu16091300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Abhari K, Saadati S, Yari Z, Hosseini H, Hedayati M, Abhari S, Alavian SM, Hekmatdoost A. The effects of Bacillus coagulans supplementation in patients with non-alcoholic fatty liver disease: A randomized, placebo-controlled, clinical trial. Clin Nutr ESPEN. 2020;39:53–60. doi: 10.1016/j.clnesp.2020.06.020. [DOI] [PubMed] [Google Scholar]
- 193.Scorletti E, Afolabi PR, Miles EA, Smith DE, Almehmadi A, Alshathry A, Childs CE, Del Fabbro S, Bilson J, Moyses HE, et al. Synbiotics alter fecal microbiomes, but not liver fat or fibrosis, in a randomized trial of patients with nonalcoholic fatty liver disease. Gastroenterology. 2020;158(6):1597–1610.e1597. doi: 10.1053/j.gastro.2020.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Laue C, Papazova E, Pannenbeckers A, Schrezenmeir J. Effect of a probiotic and a synbiotic on body fat mass, body weight and traits of metabolic syndrome in individuals with abdominal overweight: a human, double-blind, randomised, controlled clinical study. Nutrients. 2023;15(13):3039. doi: 10.3390/nu15133039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Deehan EC, Al Antwan S, Witwer RS, Guerra P, John T, Monheit L. Revisiting the concepts of prebiotic and prebiotic effect in light of scientific and regulatory progress—a consensus paper from the global prebiotic association. Adv nutr. 2024;15(12):100329. doi: 10.1016/j.advnut.2024.100329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Li Q, Liu W, Feng Y, Hou H, Zhang Z, Yu Q, Zhou Y, Luo Q, Luo Y, Ouyang H, et al. Radix Puerariae thomsonii polysaccharide (RPP) improves inflammation and lipid peroxidation in alcohol and high-fat diet mice by regulating gut microbiota. Int J Biol Macromolecules. 2022;209:858–870. doi: 10.1016/j.ijbiomac.2022.04.067. [DOI] [PubMed] [Google Scholar]
- 197.Wang X, Shi L, Wang X, Feng Y, Wang Y. MDG-1, an Ophiopogon polysaccharide, restrains process of non-alcoholic fatty liver disease via modulating the gut-liver axis. Int J Biol Macromolecules. 2019;141:1013–1021. doi: 10.1016/j.ijbiomac.2019.09.007. [DOI] [PubMed] [Google Scholar]
- 198.Gao LL, Ma J-M, Fan Y-N, Zhang Y-N, Ge R, Tao X-J, Zhang M-W, Gao Q-H, Yang J-J. Lycium barbarum polysaccharide combined with aerobic exercise ameliorated nonalcoholic fatty liver disease through restoring gut microbiota, intestinal barrier and inhibiting hepatic inflammation. Int J Biol Macromolecules. 2021;183:1379–1392. doi: 10.1016/j.ijbiomac.2021.05.066. [DOI] [PubMed] [Google Scholar]
- 199.Yang CW, Liu H-M, Chang Z-Y, Liu G-H, Chang H-H, Huang P-Y, Lee T-Y. Puerarin Modulates Hepatic Farnesoid X Receptor and Gut Microbiota in High-Fat Diet-Induced Obese Mice. Int J Mol Sci. 2024;25(10):5274. doi: 10.3390/ijms25105274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Davani-Davari D, Negahdaripour M, Karimzadeh I, Seifan M, Mohkam M, Masoumi S, Berenjian A, Ghasemi Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods (Basel, Switz). 2019;8(3):92. doi: 10.3390/foods8030092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Cunningham M, Azcarate-Peril MA, Barnard A, Benoit V, Grimaldi R, Guyonnet D, Holscher HD, Hunter K, Manurung S, Obis D, et al. Shaping the Future of Probiotics and Prebiotics. Trends Microbiol. 2021;29(8):667–685. doi: 10.1016/j.tim.2021.01.003. [DOI] [PubMed] [Google Scholar]
- 202.Guarino MPL, Altomare A, Emerenziani S, Di Rosa C, Ribolsi M, Balestrieri P, Iovino P, Rocchi G, Cicala M. Mechanisms of Action of Prebiotics and Their Effects on Gastro-Intestinal Disorders in Adults. Nutrients. 2020;12(4):1037. doi: 10.3390/nu12041037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Vulevic J, Juric A, Walton GE, Claus SP, Tzortzis G, Toward RE, Gibson GR. Influence of galacto-oligosaccharide mixture (B-GOS) on gut microbiota, immune parameters and metabonomics in elderly persons. Br J Nutr. 2015;114(4):586–595. doi: 10.1017/S0007114515001889. [DOI] [PubMed] [Google Scholar]
- 204.Cai J, Dong J, Chen D, Ye H. The effect of synbiotics in patients with NAFLD: a systematic review and meta-analysis. Therap Adv Gastroenterol. 2023;16:17562848231174299. doi: 10.1177/17562848231174299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, Visser CE, Kuijper EJ, Bartelsman JFWM, Tijssen JGP, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368(5):407–415. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 206.Shou D, Luo Q, Tang W, Cao C, Huang H, Chen H, Zhou Y. Hepatobiliary and pancreatic: Multi-donor fecal microbiota transplantation attenuated high-fat diet-induced hepatic steatosis in mice by remodeling the gut microbiota. J Gastroen Hepatol. 2023;38(12):2195–2205. doi: 10.1111/jgh.16359. [DOI] [PubMed] [Google Scholar]
- 207.Zhong HJ, Zhuang Y-P, Xie X, Song J-Y, Wang S-Q, Wu L, Zhan Y-Q, Wu Q, He X-X. Washed microbiota transplantation promotes homing of group 3 innate lymphoid cells to the liver via the CXCL16/CXCR6 axis: a potential treatment for metabolic-associated fatty liver disease. Gut Microbes. 2024;16(1):2372881. doi: 10.1080/19490976.2024.2372881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Stols-Gonçalves D, Mak AL, Madsen MS, van der Vossen EWJ, Bruinstroop E, Henneman P, Mol F, Scheithauer TPM, Smits L, Witjes J, et al. Faecal Microbiota transplantation affects liver DNA methylation in Non-alcoholic fatty liver disease: a multi-omics approach. Gut Microbes. 2023;15(1):2223330. doi: 10.1080/19490976.2023.2223330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Witjes JJ, Smits LP, Pekmez CT, Prodan A, Meijnikman AS, Troelstra MA, Bouter KEC, Herrema H, Levin E, Holleboom AG, et al. Donor Fecal Microbiota Transplantation Alters Gut Microbiota and Metabolites in Obese Individuals With Steatohepatitis. Hepatol Commun. 2020;4(11):1578–1590. doi: 10.1002/hep4.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Xue L, Deng Z, Luo W, He X, Chen Y. Effect of Fecal Microbiota Transplantation on Non-Alcoholic Fatty Liver Disease: A Randomized Clinical Trial. Front Cell Infect Microbiol. 2022;12:759306. doi: 10.3389/fcimb.2022.759306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Vrieze A, Van Nood E, Holleman F, Salojärvi J, Kootte RS, Bartelsman JFWM, Dallinga–Thie GM, Ackermans MT, Serlie MJ, Oozeer R, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143(4):913–916.e917. doi: 10.1053/j.gastro.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 212.Steidler L, Neirynck S, Huyghebaert N, Snoeck V, Vermeire A, Goddeeris B, Cox E, Remon JP, Remaut E. Biological containment of genetically modified Lactococcus lactis for intestinal delivery of human interleukin 10. Nat Biotechnol. 2003;21(7):785–789. doi: 10.1038/nbt840. [DOI] [PubMed] [Google Scholar]
- 213.Yoda M, Takase S, Suzuki K, Murakami A, Namai F, Sato T, Fujii T, Tochio T, Shimosato T. Development of engineered IL-36γ-hypersecreting Lactococcus lactis to improve the intestinal environment. World J Microbiol Biotechnol. 2024;40(11):363. doi: 10.1007/s11274-024-04157-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ma J, Li C, Wang J, Gu J. Genetically engineered Escherichia coli Nissle 1917 secreting GLP-1 analog exhibits potential antiobesity effect in high-fat diet-induced obesity mice. Obesity (Silver Spring, Md). 2020;28(2):315–322. doi: 10.1002/oby.22700. [DOI] [PubMed] [Google Scholar]
- 215.Ma J, Wang J, Xu L, Liu Y, Gu J. The beneficial effects of genetically engineered Escherichia coli Nissle 1917 in obese C57BL/6J mice. Int J Obes (2005). 2022;46(5):1002–1008. doi: 10.1038/s41366-022-01073-8. [DOI] [PubMed] [Google Scholar]
- 216.Chen Z, Guo L, Zhang Y, Walzem RL, Pendergast JS, Printz RL, Morris LC, Matafonova E, Stien X, Kang L, et al. Incorporation of therapeutically modified bacteria into gut microbiota inhibits obesity. J Clin Invest. 2014;124(8):3391–3406. doi: 10.1172/JCI72517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Somabhai CA, Raghuvanshi R, Nareshkumar G. Genetically Engineered Escherichia coli Nissle 1917 Synbiotics Reduce Metabolic Effects Induced by Chronic Consumption of Dietary Fructose. PLOS ONE. 2016;11(10):e0164860. doi: 10.1371/journal.pone.0164860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Gan L, Feng Y, Du B, Fu H, Tian Z, Xue G, Yan C, Cui X, Zhang R, Cui J, et al. Bacteriophage targeting microbiota alleviates non-alcoholic fatty liver disease induced by high alcohol-producing Klebsiella pneumoniae. Nat Commun. 2023;14(1):3215. doi: 10.1038/s41467-023-39028-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Ichikawa M, Nakamoto N, Kredo-Russo S, Weinstock E, Weiner IN, Khabra E, Ben-Ishai N, Inbar D, Kowalsman N, Mordoch R, et al. Bacteriophage therapy against pathological Klebsiella pneumoniae ameliorates the course of primary sclerosing cholangitis. Nat Commun. 2023;14(1):3261. doi: 10.1038/s41467-023-39029-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


