Abstract
Background
Lecanemab, a monoclonal antibody targeting amyloid beta, has recently been approved for treatment of early-stage Alzheimer’s disease (AD), demonstrating amyloid plaque reduction and slowing of cognitive decline in clinical trials. However, real-world data on its efficacy and safety remain limited. The Cognitive Neurology Unit at Tel Aviv Medical Center (TLVMC) established an infrastructure to facilitate advanced treatments for AD, utilising a multidisciplinary approach to patient screening, diagnosis, treatment initiation and follow up.
Methods
Lecanemab administration at the TLVMC commenced in November 2023. Patients with biomarker-confirmed early-stage AD were screened via a structured referral system, including neurological evaluations, MRI, lumbar puncture or Amyloid-PET, genetic testing, and multidisciplinary team (MDT) consensus discussions. Cognitive function was assessed using the Mini-Mental State Examination (MMSE) at baseline, six months, and twelve months. Safety monitoring included routine MRI scans for amyloid-related imaging abnormalities (ARIA).
Results
Between July 2023 and January 2025, 169 patients were screened and 86 initiated lecanemab treatment. By January 2025, 53 patients had reached the 6-month follow-up date. In the intention-to-treat (ITT) population, MMSE scores declined significantly over 6 months (F(1, 45.13) = 7.41, p =.009). Subgroup analysis revealed a significant decline in younger patients (n = 31; F(1, 24.67) = 8.06, p =.009), but not in older patients (n = 22; F(1, 19.25) = 0.67, p =.424). At 12 months, 31 patients had reached follow-up, with no significant change in MMSE scores observed (F(1, 17.18) = 2.49, p =.133). Age subgroup analysis was not performed at 12 months due to limited sample size. No significant correlations were found between baseline biomarkers and cognitive change. ARIA occurred in 18.6% of patients, mostly asymptomatic. One patient experienced symptomatic ARIA, required hospitalization with intravenous treatment, and discontinued therapy. A mixed-effects model showed no significant effect of ARIA on MMSE change (p =.264) and no interaction with time (p =.433). Infusion-related reactions occurred in 22.1%, all mild and transient. Treatment was discontinued in 19.8% of patients due to ARIA, financial barriers, comorbidities, or personal preference.
Conclusions
This real-world analysis demonstrates the feasibility and safety of Lecanemab administration for early-stage AD within a tertiary hospital setting. Establishing dedicated infrastructure enabled streamlined patient evaluations and treatment. The findings suggest a differential response across age groups, consistent with clinical trial data. Continued longitudinal follow-up is needed to assess long-term efficacy and safety.
Keywords: Alzheimer's disease, Anti-amyloid therapy, Lecanemab, Real-world data
Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder characterized by cognitive decline, behavioral changes, and ultimately, loss of independence. It is the most common cause of dementia, affecting millions worldwide and imposes a substantial burden on patients, caregivers, and healthcare systems [1]. Lecanemab, a monoclonal antibody targeting amyloid beta, was recently approved for the treatment of early-stage AD based on evidence demonstrating its ability to reduce amyloid plaque accumulation and slow cognitive decline [2]. This approval was a significant advancement in AD management, offering disease modifying therapy rather than symptomatic treatment alone. Real-world data on the efficacy and safety of Lecanemab outside of clinical trial settings is critically important, as it complements findings from clinical trials by reflecting the complexities and variability of routine clinical practice. Unlike controlled trial settings, real-world evidence captures a broader and more diverse patient population, including those with comorbidities, varying levels of disease severity, and different healthcare systems. Initial reports from other centers have recently begun to emerge [3–5], collectively expanding our understanding of Lecanemab’s safety, efficacy, and implementation challenges in everyday clinical settings. These contributions are essential for informing clinical decision-making, optimizing treatment strategies, and guiding policy and practice.
In 2021, following the FDA approval of Aducanumab for the treatment of early-stage AD [6], the Cognitive Neurology Unit at Tel Aviv Medical Center (TLVMC), a public tertiary hospital in central Israel, established the Center for Advanced Treatments in AD, an infrastructure to facilitate the administration of advanced therapies. This initiative stemmed from the recognition of the medical and operational complexities involved, as well as the view of the drug’s approval as a first step toward future advancements in the field.
To this end, a multidisciplinary (MDT) team comprising cognitive neurologists, neuroradiologists, nurses, and coordinators was assembled, and a collaborative model with the neurological day-admission unit was developed. Within the cognitive neurology unit, a patient referral screening system was implemented, prioritizing a fast-track pathway for individuals suspected of having early stages of AD. As part of this screening process, a requirement for an initial neurological evaluation and brain imaging prior to a patient’s first visit to the clinic was introduced. A dedicated clinical coordinator was recruited to help patients with private insurance reimbursements and to schedule treatments and scans.
All necessary diagnostic tests for treatment eligibility were made available within the hospital, including MRI, lumbar puncture (LP), AD biomarker testing, genetic testing for APOE status, and Amyloid-PET imaging. Specialized training was provided to the unit’s neurologists to offer genetic counseling before APOE testing as part of the pre-treatment evaluation, with almost all genetic testing conducted on-site. Neuroradiologists completed specific training for identification of amyloid-related imaging abnormalities (ARIA).
Prescription guidelines and restrictions in Israel are similar to those in the United States. There are no specific regulatory requirements concerning infrastructure or prescriber qualifications. The treatment is currently not covered by Israel’s public health insurance and must be paid for either out-of-pocket or through private insurance.
Patients were diagnosed according to clinical criteria with mild cognitive impairment (MCI) [7], mild and moderate dementia due to AD [1, 8]. The diagnosis was based on the clinical judgment of an experienced cognitive neurology specialist, taking into account the individual circumstances of each patient, reports of daily functioning provided by the patient and a knowledgeable informant, as well as various cognitive assessments, as determined appropriate by the clinician. Diagnosis of probable AD was confirmed by biomarker evidence of amyloid pathology [9, 10]. Assessment of treatment eligibility followed the American appropriate use recommendations published by Ramanan et al. [11], Cummings et al. [12] and Korean recommendations published by Park et al. [13], excluding patients receiving anticoagulation treatment. Additionally, it was decided not to administer treatment to ApoE ε4 homozygous individuals, due to the poorer safety profile observed in this population in the Clarity AD clinical trial [2], aligning with the later approval by regulatory authorities in the United Kingdom as well as in the European Union and the French appropriate use recommendations, published by Villain et al. [14].
Each candidate was discussed at a weekly MDT meeting, where treatment decisions were reached by consensus. Figure 1 illustrates the diagnostic workflow for patients undergoing evaluation for treatment eligibility.
Fig. 1.
Diagnostic workflow for patients undergoing evaluation for treatment eligibility
This report aims to evaluate the demographic characteristics, treatment response, and safety profile of early-stage AD patients treated with Lecanemab at the TLVMC. By analyzing follow-up outcomes, we seek to provide insights into the drug’s impact in a real-world clinical setting, contributing to the growing body of evidence on its use in routine practice.
Methods
Administration of Lecanemab to patients with early-stage AD commenced at the TLVMC in November 2023. Clinical assessments included demographic data, medical and neurological evaluations, cognitive testing, and genetic counseling with ApoE ε4 genotyping. Diagnosis was based on the clinical judgment of an experienced cognitive neurology specialist, incorporating the patient’s individual clinical context, reports of daily functioning from the patient and a knowledgeable informant, and a range of cognitive assessments, selected at the clinician’s discretion.
Eligible participants were diagnosed based on clinical criteria for MCI or mild dementia due to AD, confirmed by biomarker evidence of amyloid pathology [9, 10, 15].
The differentiation of dementia from MCI rested on the determination of whether or not there is significant interference in the ability to function at work or in usual daily activities. Mild dementia was determined for individuals who are able to function independently in many areas but are likely to require assistance with some activities to maximize independence and remain safe. They may still be able to drive, work and participate in their favorite activities. They may need more time to complete common daily tasks. Moderate dementia was diagnosed in individuals who already experience more problems with memory and language, are more likely to become confused, and find it harder to complete multi step tasks such as bathing and dressing. They may become incontinent at times, begin to have problems recognizing loved ones, and start showing personality and behavioral changes, including suspiciousness and agitation [1].
In our clinical practice, LP is generally recommended for the diagnosis of AD, as it provides comprehensive biomarker information. Amyloid PET imaging with Vizamyl™ (Flutemetamol F 18 Injection) is available at our institution and diagnostically useful; however, it remains significantly more costly and less accessible. All PET scans are interpreted by expert nuclear medicine physicians. Patients are informed of both diagnostic options and advised that a positive amyloid PET scan is sufficient to support diagnosis and guide treatment. In selected cases, when LP or PET results are borderline or inconclusive, the complementary method is recommended to achieve diagnostic clarity.
CSF biomarkers, including total ‘t-tau’, hyperphosphorylated tau (p-tau181), and amyloid beta 42 (Aβ42), were analyzed using the Euroimmun immunoassay (EUROIMMUN AG, Lübeck, Germany), based on enzyme-linked immunosorbent assay (ELISA) technology and standardized protocols. AD pathology was considered positive when p-tau181 levels exceeded 61 pg/ml, Aβ42 levels were below 570 pg/ml (borderline: 570–630 pg/ml), and t-tau levels were above 452 pg/ml (borderline: 290–452 pg/ml) [16]. Patients with borderline Aβ42 levels and clinical suspicion of AD were referred for confirmatory amyloid PET imaging using Vizamyl™ (Flutemetamol F 18 Injection) [17].
A contrast MRI, preferably on a 3T scanner and including FLAIR, SWI, and DWI sequences, was required to assess eligibility for Lecanemab therapy. If a prior MRI with these sequences had been done within the previous 12 months, it was accepted for approval.
Consistent with published recommendation, patients were excluded if they were homozygous for ApoE ε4 [14]; had any medical, neurological, or psychiatric condition that could contribute to cognitive impairment or suggest a non-AD diagnosis. Imaging exclusions included: >4 microhemorrhages (≤ 10 mm), any macrohemorrhage (> 10 mm), superficial siderosis, vasogenic edema, > 2 lacunar infarcts or territorial stroke, Fazekas 3 subcortical hyperintensities [18], amyloid beta-related angiitis (ABRA), cerebral amyloid angiopathy-related inflammation (CAA-ri), or other major intracranial pathologies. Clinical exclusions included recent stroke or TIA (within 12 months), any seizure history, severe mental illness or depression impairing informed consent or compliance, suicidal risk with biomarker disclosure, uncontrolled bleeding disorders, use of anticoagulants, immunologic diseases, or recent immunosuppressive therapy. Unstable medical conditions potentially interacting with Lecanemab were also exclusionary [12–14].
Lecanemab was administered intravenously every two weeks, without titration. Dosing was weight-based, with each patient receiving 10 mg/kg of body weight. Infusions were given in the neurological day-admission unit and lasted approximately one hour. To monitor for infusion-related reactions, patients were observed for two hours following the first and second infusions, with a follow-up phone call the next day. If no reactions occurred, the observation period for subsequent infusions was reduced to one hour. The first two infusions were scheduled in the morning, while subsequent infusions were typically given in the afternoon. In cases where an infusion was missed, the next dose was administered as soon as possible, with treatment continuing every two weeks thereafter.
Non-contrast MRIs were obtained after the 5th, 7th, and 14th infusions, as recommended in the prescribing information. Monitoring and dosing interruption for ARIA were implemented in accordance with the product information. In cases where ARIA was detected, treatment was temporarily suspended and follow-up imaging was performed as recommended. Resumption of therapy was guided by radiological resolution and clinical status, consistent with the protocol outlined in the prescribing information and published recommendations [12].
The Mini-Mental State Examination (MMSE) was used to assess cognitive decline at baseline, six and twelve months. Safety monitoring included regular clinical assessments and the recommended protocol of MRI scanning to detect ARIA, categorized as ARIA-edema (ARIA-E) and ARIA-hemorrhage (ARIA-H) [12, 19, 20]. Expedited neuroradiological reporting was carried out by expert neuroradiologists who completed specific training for identification of ARIA. Adverse events were recorded and classified according to severity and potential association with treatment.
This report includes data from all new patients who were referred to the cognitive neurology unit with suspected early-stage AD and were evaluated between July 2023 and January 2025, all patients who initiated treatment between November 2023 and January 2025, and all patients who had completed their six and twelve-month follow-up by end of January 2025. The patients who initiated treatment were grouped by baseline age into two categories: those 74 years and younger (“younger group”) and those 75 years and older (“older group”), in accordance with the subgroup analysis in the Clarity-AD clinical trial [2]. Due to the small sample size of patients 65 and younger, they were included in the younger group.
Written informed consent was obtained from all participants, who initiated treatment. Data from patients who did not commence treatment was collected under a retrospective data analysis IRB. The studies were approved by the institutional review board (IRB) of the TLVMC and conducted in accordance with the Declaration of Helsinki. The study adhered to international guidelines for clinical research, ensuring participant safety and data confidentiality.
All analyses were conducted by trained personnel with Statistical Package for the Social Sciences (SPSS) software (Version 29; SPSS, Inc., Chicago, IL, USA), and the alpha level was set at 0.05. Descriptive statistics were used to summarize baseline characteristics and biomarker data.
A linear mixed-effects model was used to assess changes in MMSE scores over time (at 6 and 12 months) in the intention-to-treat (ITT) population, with time as a fixed effect and subject ID as a repeated measure, using an unstructured covariance structure. The incidence of ARIA was reported as a percentage of treated patients.
Results
Patient group
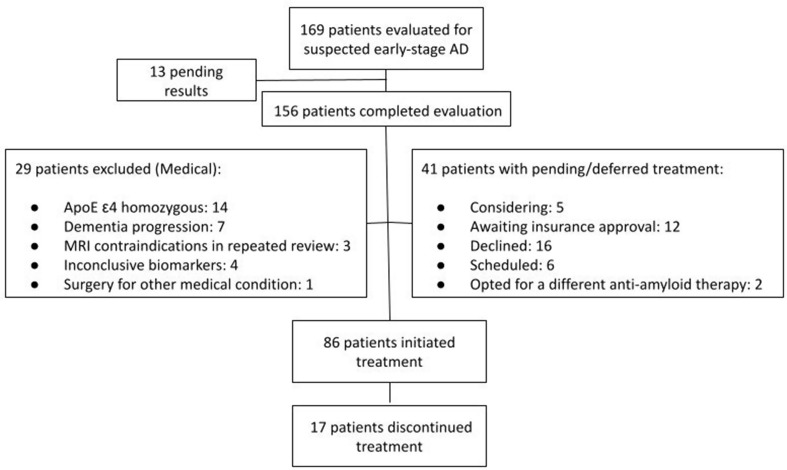
Between July 2023 and January 2025, a total of 169 patients with suspected early-stage AD had been evaluated, and 156 had completed the necessary clinical assessments and biomarker evaluations. In our cohort, 19 out of 156 patients (12.2%) underwent only an amyloid PET scan without LP. 70 patients did not commence treatment, of whom 29 were deemed unsuitable due to the following reasons: Fourteen (14/156, 8.97%) patients were ApoE ε4 homozygous, 7 patients progressed to moderate dementia during evaluation, 3 patients had baseline MRI findings that precluded treatment following repeat neuroradiological review, 4 patients had inconclusive biomarker results, and 1 patient required major surgery for an acute medical condition. At the time of writing, 5 patients are considering treatment, while 12 are in the process of securing insurance approval. Sixteen patients chose not to proceed with treatment, 2 patients opted for a different anti-amyloid therapy, and 6 patients are scheduled to begin treatment in the next month. Figure 2 illustrates the number of patients who underwent evaluation, initiated treatment, and withdrew from treatment.
Fig. 2.
Evaluations, treatment initiations and discontinuations
A total of 86 patients initiated treatment, of which 45 completed six month assessments and 18 completed twelve months assessments by January 2025. There were no significant differences in baseline characteristics between patients who have already completed six or twelve months of treatment and those who have not yet. Similarly, no differences were observed between the younger and older age groups, apart from age at onset. Table 1 summarizes baseline, 6 and 12-months characteristics.
Table 2.
Number of patients with ARIA according to type and severity*
| ARIA Type | Severity | Description | Number of Patients |
|---|---|---|---|
| ARIA-E | Mild | FLAIR hyperintensity confined to sulcus and/or cortex/subcortex white matter in one location < 5 cm | (n = 2) |
| Moderate | FLAIR hyperintensity 5 to 10 cm in single greatest dimension, or more than 1 site of involvement, each measuring < 10 cm | (n = 1) | |
| Severe | FLAIR hyperintensity > 10 cm with associated gyral swelling and sulcal effacement. One or more separate/independent sites of involvement may be noted | (n = 0) | |
| ARIA-H Microhemorrhage | Mild | ≤ 4 new incident microhemorrhages | (n = 10) |
| Moderate | 5 to 9 new incident microhemorrhages | (n = 2) | |
| Severe | 10 or more new incident microhemorrhages | (n = 0) | |
| ARIA-H Superficial Siderosis | Mild | 1 focal area of superficial siderosis | (n = 1) |
| Moderate | 2 focal areas of superficial siderosis | (n = 0) | |
| Severe | > 2 areas of superficial siderosis | (n = 0) |
* Table adapted from Cummings et al. 2023 [12]
Abbreviations: ARIA-E: Amyloid Related Imaging Abnormalities - Edema, ARIA-H: Amyloid Related Imaging Abnormalities – Hemorrhage.
Follow up data
By January 2025, 53 patients had completed or were expected to have completed their 6-month assessment. MMSE scores showed a significant decline over time (F(1, 45.13) = 7.41, p =.009). In subgroup analysis by age, a significant decline was observed in the younger group (n = 31; F(1, 24.67) = 8.06, p =.009), while no significant change was found in the older group (n = 22; F(1, 19.25) = 0.67, p =.424). Covariance estimates across all groups suggested greater variability at follow-up compared to baseline, with moderate within-subject consistency over time.
At 6 months, no significant difference in progression was observed between males and females (F(1, 52) = 0.21, p =.649).
By January 2025, 31 patients had completed or were expected to have completed their 12-month follow-up assessments. In the ITT population (n = 31), no significant change in MMSE scores was observed over the 12-month period (F(1, 17.18) = 2.49, p =.133). Covariance estimates indicated greater variability at 12 months compared to baseline, with moderate within-subject consistency. Due to the limited sample size at this time point, subgroup analyses by age and sex were not performed.
No significant correlations were observed between MMSE scores (at baseline, 6 months, or 12 months) and baseline CSF levels of t-tau, p-tau181, or Aβ42.
Safety
During this period, 16 out of 86 patients (18.6%) developed ARIA, as detailed in Table 2. All patients were asymptomatic, except for one who developed moderate ARIA-E with moderate ARIA-H (microhemorrhages) and presented with mild confusion. The patient was heterozygous for the APOE ε4 allele and had no baseline MRI risk factors for ARIA. She was admitted to the Neurology Department and received a five-day course of intravenous steroids. Symptoms resolved within approximately two weeks; however, the patient chose not to continue treatment.
Table 1.
Characteristics of the patients at baseline, 6 and 12 months
| All patients who initiated Tx (n = 86) | Patients ≤ 74 Years (Younger Group, n = 49) | Patients ≥ 75 Years (Older Group, n = 37) | Patients who completed 6-month follow-up (n = 45) * | Patients who completed 12-month follow-up (n = 18) * | |
|---|---|---|---|---|---|
| Age years (SD) | 71.99 (8.20) | 66.82 (6.97) | 78.84 (3.05) | 73.26 (7.27) | 73.06 (6.83) |
| Sex (n, % women) | 53 (62%) | 29 (59%) | 24 (65%) | 25 (54%) | 10 (56%) |
| Mean MMSE score (SD, min- max) | 23.96 (2.69) | 24.04 (2.78) | 23.86 (2.60) | 24.11 (2.59) | 24.55 (2.66) |
|
Clinical diagnosis (MCI (n, %), Mild AD (n, %) Moderate AD (n, %) |
MCI (62, 72%) Mild dementia (24, 28%) Moderate dementia (0, 0%) |
MCI (33, 67.3%) Mild dementia (16, 32.7%) Moderate dementia (0, 0%) |
MCI (29, 78.4%) Mild dementia (8, 21.6%) Moderate dementia (0, 0%) |
MCI (28, 62.3%) Mild dementia (16, 35.5%) Moderate dementia (1, 2.2%) |
MCI (9, 50%) Mild dementia (7, 38.9%) Moderate dementia (2, 11.1%) |
| Number of ApoEε4 carriers (n, %) | 46 (53%) | 26 (53%) | 20 (54%) | 25 (54%) | 12 (67%) |
| Mean baseline CSF t-tau (pg/ml) (SD)1 | 569.74 (279.27) | 603.37 (266.95) | 528.52 (292.75) | 575.81 (264.73) | 581.15 (330.82) |
| Mean baseline CSF p-tau181 (pg/ml) (SD) 2 | 154.17 (340.04) | 197.22 (461.05) | 104.18 (39.81) | 194.36 (461.42) | 119.53 (64.06) |
| Mean baseline CSF Amyloid beta 42 (pg/ml) (SD) 3 | 324.89 (159.75) | 298.13 (152.35) | 358.78 (164.99) | 332.39 (166.72) | 365.37 (210.39) |
Abbreviations: Tx: treatment; MMSE: Mini-Mental State Examination; MCI: Mild Cognitive Impairment; AD: Alzheimer’s Disease; CSF: cerebrospinal fluid
1 Normal levels below 452 pg/ml (borderline: 290–452 pg/ml)
2 normal levels below 61 pg/ml
3 Normal levels sbove 570 pg/ml (borderline: 570–630 pg/ml)
* without Intention To Treat (ITT) population
ARIA occurrence did not significantly influence the trajectory of cognitive change. In a linear mixed-effects model including ARIA as a covariate, neither the main effect of ARIA (F(1, 46.09) = 1.28, p =.264) nor the interaction between time and ARIA (F(1, 43.10) = 0.63, p =.433) reached statistical significance.
Two patients were diagnosed with new asymptomatic lacunar ischemic strokes during follow-up MRI, leading to treatment discontinuation. One of these patients resumed treatment a year later.
Nineteen patients (22.09%) developed infusion-related side effects, which were all mild and occurred only after the first and/or second dose. No hypersensitivity reactions occurred. No patients discontinued treatment as a result of these reactions.
A total of 17 out of 86 patients (19.76%) discontinued treatment at our center. Five patients discontinued due to ARIA, in accordance with published recommendations [12], as follows: One patient developed more than one area of superficial siderosis, 2 patients developed more than 10 microhemorrhages since treatment initiation, one patient experienced more than two episodes of ARIA, and finally the patient described above with symptomatic moderate ARIA-E and ARIA-H opted to discontinue. Two patients had their treatment temporarily suspended due to ARIA until follow-up MRI, one with mild ARIA-E and another with suspected superficial hemosiderosis. Anticoagulation was newly indicated for two patients, one due to newly diagnosed atrial fibrillation who is scheduled to restart treatment after a successful left atrial appendage closure procedure, and another due to newly diagnosed deep vein thrombosis which required lifetime anticoagulation and led to treatment discontinuation.
Other reasons for discontinuation included three patients who stopped treatment due to financial difficulties, four who chose to discontinue without specifying a reason, three patients discontinued due to other medical conditions (one with severe weight loss, one with suicidal thoughts, and one with asymptomatic ischemic stroke). One patient moved abroad and continued treatment elsewhere.
Discussion
Here we present real-world data on Lecanemab treatment for patients with early-stage AD from the Cognitive Neurology Unit at the TLVMC. The decision to administer advanced treatments for AD necessitated infrastructure adjustments including modifications to the referral system, and prioritization of patients suspected of having early-stage AD. This modification of the referral system likely explains the higher proportion of evaluated patients who initiated treatment, compared to both the clinical trial findings [2] and the initial real-world experience reported by Shields LBE et al. [3]. An expedited clinical evaluation, along with close collaboration with other in-hospital units, was also essential.
The establishment of the advanced treatment service for AD was made possible through substantial support from hospital management and the allocation of dedicated resources. Importantly, the revenue generated from the treatment program has covered the costs of additional staffing, allowing the service to operate without negatively impacting other clinical activities or departments.
Patients underwent accelerated evaluations enabling accurate identification of patients whose baseline clinical characteristics were consistent with biomarker-confirmed early-stage AD and who met eligibility criteria for treatment as per the label and published recommendations [12, 13]. Additionally, it was decided not to administer the treatment at this stage to individuals who are ApoE ε4 homozygous, aligning with the UK and EU regulatory decision, as well as the recently published French recommendation [14] due to the poorer safety profile observed in this population in the Clarity AD clinical trial [2].
The evaluation process involved, in most cases, three clinic visits, a baseline brain MRI, either an LP or an Amyloid PET scan, and genetic counseling and testing. LP was recommended; however, all patients were given the option to undergo an amyloid PET scan instead. Nonetheless, only 19/156 patients (12.2%) chose to undergo PET imaging alone. We believe this reflects patients’ good understanding of the diagnostic advantages of LP in establishing a highly probable diagnosis of AD. In addition, the cost likely played a significant role: amyloid PET scans are very expensive, not covered by public insurance, and only partially reimbursed by private insurance. In contrast, the LP procedure itself is fully covered by public insurance, and patients only pay out-of-pocket for the specific AD biomarker analysis, which is considerably less costly than a PET scan.
All MRIs were reviewed by a trained neuroradiologist, and each patient was presented and discussed in a weekly MDT meeting. Eligibility was primarily based on the overall clinical diagnosis of early-stage AD and the absence of exclusion criteria. While we report only the MMSE score at baseline, it is important to emphasize that the score alone was neither used to establish the diagnosis nor as an exclusion criterion. For example, patients with the logopenic variant of primary progressive aphasia often presented with lower MMSE scores due to language deficits, despite having well-preserved daily functioning. Such real-world diagnostic complexities were the reason for holding MDT discussions, during which each candidate was thoroughly reviewed. Importantly, The Israeli label follows the FDA’s and does not specify MMSE or any other specific cognitive score as an eligibility criterion, but rather refers broadly to the indication of early-stage AD.
Twenty-nine patients were excluded due to medical reasons, as detailed in Fig. 2. Notably, the length and complexity of the diagnostic work-up led to the exclusion of seven patients who progressed from mild to moderate dementia during the evaluation process, underscoring the urgent need for improvement in the speed and efficiency of assessment. This experience reflects the broader challenge of implementing early diagnosis and treatment for AD in real-world clinical settings [21–23]. As this is still the early phase of integrating disease-modifying therapies into practice, our findings highlight critical gaps in the current referral and assessment system. A more streamlined and time-sensitive diagnostic pathway is essential to ensure timely intervention, especially for patients at the borderline of eligibility. To improve the process, we suggest: prioritizing referrals for suspected early-stage Alzheimer’s with a fast-track triage system; implementing streamlined protocols with parallel scheduling of cognitive tests, biomarkers, and imaging; enhancing coordination between departments; using digital tools to monitor cognitive changes during evaluation; and educating referring clinicians on early signs and timely referral. Together, these efforts could reduce delays, prevent unnecessary exclusions, and improve access to treatment for patients who stand to benefit most.
16 out of 156 patients (10.2%) who completed the evaluation and were deemed eligible for treatment chose not to initiate therapy. We believe that the primary reasons for eligible patients declining treatment were financial considerations, as the therapy is not publicly funded and only partially covered by private insurance. Additionally, some patients expressed concerns regarding the treatment’s effectiveness and potential side effects, which may have influenced their decision.
Following six months of treatment patients treated with Lecanemab showed a significant decline in MMSE scores compared to baseline. Sub-group analysis revealed that following six months of treatment, older patients had no significant decline in MMSE scores, while younger patients experienced a greater and statistically significant decline in their MMSE scores. These findings, indicating a better response in older patients, are consistent with results from the clinical trial [2]. These differences are also consistent with previous findings showing that patients clinically diagnosed with AD at an older age tend to decline more slowly than those with a younger age at onset [24, 25].
In this group, no significant correlations were found between baseline, six and twelve month MMSE scores and CSF levels of t-tau, p-tau181, and Aβ42 at baseline, however this will need to be re-examined when further longitudinal data is available.
The rate of ARIA in this group (18.6%) was lower than that observed in the pivotal clinical trial [2], likely reflecting our exclusion of patients who were homozygous for ApoE ε4. This observation is consistent with the real-world findings of Shields LBE et al. [3], who reported that 4 out of 9 ApoE ε4 homozygous patients (44%) developed ARIA. Despite this precaution, ARIA still occurred in 16 out of 86 patients (18.6%). Five patients ultimately discontinued treatment due to ARIA-related findings, and one required hospitalization and treatment with intravenous steroids. These results highlight that even with risk-reduction strategies, ARIA remains a clinically relevant concern. They underscore the critical importance of rigorous safety monitoring through scheduled follow-up MRIs, timely interpretation of imaging results, and clear protocols for the management of ARIA when it arises. Continued vigilance and multidisciplinary coordination are essential to ensure early detection and appropriate response, especially as Lecanemab treatment expands in real-world settings.
Other side effects were mild infusion-related reactions, which occurred only after the first or second infusion, were consistent with the findings reported in the clinical trial [2].
Despite the potential for early treatment discontinuation due to ARIA or infusion-related reactions, we observed a steady increase in the number of patients initiating and continuing Lecanemab therapy over time. This trend likely reflects growing clinical experience, improved patient selection, and increased confidence among both clinicians and patients in the safety and management of the treatment.
While this report presents preliminary longitudinal data limited to 6- and 12-month follow-up, we recognize the importance of addressing treatment continuation beyond 18 months. In clinical practice, we actively monitor patients’ cognitive trajectories and assess for progression beyond the mild stage of dementia. In accordance with recent recommendations [14], we do not plan to continue Lecanemab treatment in patients who advance to moderate dementia or beyond, given the likely reduction in efficacy and increased treatment burden at this stage. These considerations are discussed with patients and their caregivers as part of routine follow-up and shared decision-making.
A key limitation of this report is its reliance on real-world clinical data, where routine assessments are often constrained by time and resource availability. In our clinic, the MMSE is the most frequently used cognitive measure. Although we recognize the value of more comprehensive neuropsychological testing, such evaluations are not routinely conducted in this setting and were therefore not available for the entire cohort. Additional limitations include the relatively small number of patients and, as with all real-world reports, the absence of a control group. Therefore, we cannot exclude the possibility that our cohort differed in other important ways from the clinical trial population [2].
Real-world data on Lecanemab treatment is increasingly recognized as essential, as it complements the results of randomized clinical trials by capturing the complexity and heterogeneity of routine clinical care [3]. Such data are invaluable for guiding clinical decisions, refining treatment protocols, and informing health policy. This real-world data analysis provides encouraging insights into the feasibility and safety of use of Lecanemab for early-stage AD in a tertiary hospital setting. Administration of advanced treatments for AD represents a significant shift in approach, requiring extensive infrastructure and manpower adaptations. Although challenging to implement, once the infrastructure is in place, it enables efficient patient workup and safe treatment for suitable patients, providing patients and their families access to these new disease modifying therapies.
Acknowledgements
We would like to thank all the patients and caregivers for agreeing to take part in this clinical data collection. We would also like to thank the TLVMC management for supporting the project of establishing the infrastructure and seeing this project as a priority for the hospital.
Abbreviations
- Aβ42
Amyloid Beta 42
- AD
Alzheimer’s Disease
- ARIA
Amyloid–Related Imaging Abnormalities
- ARIA-E
Amyloid–Related Imaging Abnormalities–Edema
- ARIA-H
Amyloid–Related Imaging Abnormalities–Hemorrhage
- ApoE
Apolipoprotein E
- CSF
Cerebrospinal Fluid
- ELISA
Enzyme–Linked Immunosorbent Assay
- FDA
U.S. Food and Drug Administration
- IRB
Institutional Review Board
- ITT
Intention to treat
- LP
Lumbar Puncture
- MDT
Multidisciplinary Team
- MCI
Mild Cognitive Impairment
- MMSE
Mini–Mental State Examination
- MRI
Magnetic Resonance Imaging
- PET
Positron Emission Tomography
- p-tau181
Hyperphosphorylated Tau 181
- SPSS
Statistical Package for the Social Sciences
- t-tau
Total Tau
- TLVMC
Tel Aviv Medical Center
Author contributions
NB, TS conceptualized and designed the study. NB, TS, TN, DS, NO, MHL, ABD, YA, AAA, AG, EA collected and analyzed the data. OA, EL reviewed all MRIs and provided information of ARIA. NB, TS interpreted the results and wrote the manuscript. All authors reviewed and approved the final manuscript.
Funding
Not applicable (this study was conducted as part of routine clinical work).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
This study was approved by the IRB committee of TLVMC, approval number 850 − 16. All participants provided written informed consent before inclusion in the study, in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable. This manuscript does not contain any individual person’s data in any form.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alzheimer’s Association. 2024 Alzheimer’s disease facts and figures. Alzheimers Dement. 2024;20(5):3708–3821. [DOI] [PMC free article] [PubMed]
- 2.van Dyck CH, Swanson CJ, Aisen P, Bateman RJ, Chen C, Gee M, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9–21. [DOI] [PubMed] [Google Scholar]
- 3.Shields LB, Hust H, Cooley SD, Cooper GE, Hart RN, Dennis BC, et al. Initial experience with lecanemab and lessons learned in 71 patients in a regional medical center. J Prev Alzheimers Dis. 2024;11(6):1549–62. [DOI] [PMC free article] [PubMed]
- 4.Pittock RR, Aakre JA, Castillo AM, Ramanan VK, Kremers WK, Jack CR, et al. Eligibility for Anti-Amyloid treatment in a Population-Based study of cognitive aging. Neurology. 2023;101(19):e1837–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parks AL, Thacker A, Dohan D, Gomez LA, Ritchie CS, Paladino J, et al. A qualitative study of people with Alzheimer’s disease in a memory clinic considering lecanemab treatment. J Alzheimers Dis. 2024. 10.1177/13872877251329519. [DOI] [PMC free article] [PubMed]
- 6.Budd Haeberlein S, Aisen PS, Barkhof F, Chalkias S, Chen T, Cohen S, et al. Two randomized phase 3 studies of aducanumab in early Alzheimer’s disease. J Prev Alzheimers Dis. 2022;9(2):197–210. [DOI] [PubMed] [Google Scholar]
- 7.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubois B, Villain N, Schneider L, Fox N, Campbell N, Galasko D, et al. Alzheimer disease as a Clinical-Biological Construct-An international working group recommendation. JAMA Neurol. 2024;81(12):1304–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jack CR, Andrews JS, Beach TG, Buracchio T, Dunn B, Graf A, et al. Revised criteria for diagnosis and staging of Alzheimer’s disease: Alzheimer’s association workgroup. Alzheimers Dement. 2024;20(8):5143–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramanan VK, Armstrong MJ, Choudhury P, Coerver KA, Hamilton RH, Klein BC, et al. Antiamyloid monoclonal antibody therapy for alzheimer disease: emerging issues in neurology. Neurology. 2023;101(19):842–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cummings J, Apostolova L, Rabinovici GD, Atri A, Aisen P, Greenberg S, et al. Lecanemab: appropriate use recommendations. J Prev Alzheimers Dis. 2023;10(3):362–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park KH, Kim GH, Kim C-H, Koh S-H, Moon SY, Park YH, et al. Lecanemab: appropriate use recommendations by Korean dementia association. Dement Neurocognitive Disord. 2024;23(4):165–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Villain N, Planche V, Lilamand M, Cordonnier C, Soto-Martin M, Mollion H, et al. Lecanemab for early Alzheimer’s disease: appropriate use recommendations from the French federation of memory clinics. J Prev Alzheimers Dis. 2025;12(4):100094. [DOI] [PubMed] [Google Scholar]
- 15.Dubois B, Villain N, Frisoni GB, Rabinovici GD, Sabbagh M, Cappa S, et al. Clinical diagnosis of Alzheimer’s disease: recommendations of the international working group. Lancet Neurol. 2021;20(6):484–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Römpler K, Arendt P, Brix B, Borchardt-Lohölter V, Schulz A, Busse M, et al. Evaluation of the EUROIMMUN automated chemiluminescence immunoassays for measurement of four core biomarkers for Alzheimer’s disease in cerebrospinal fluid. Practical Lab Med. 2024;41:e00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salloway S, Gamez JE, Singh U, Sadowsky CH, Villena T, Sabbagh MN, et al. Performance of [18F]flutemetamol amyloid imaging against the neuritic plaque component of CERAD and the current (2012) NIA-AA recommendations for the neuropathologic diagnosis of Alzheimer’s disease. Alzheimers Dement (Amst). 2017;9:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol. 1987;149(2):351–6. [DOI] [PubMed] [Google Scholar]
- 19.Honig LS, Sabbagh MN, van Dyck CH, Sperling RA, Hersch S, Matta A, et al. Updated safety results from phase 3 Lecanemab study in early Alzheimer’s disease. Alzheimers Res Ther. 2024;16(1):105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cogswell PM, Andrews TJ, Barakos JA, Barkhof F, Bash S, Benayoun MD, et al. Alzheimer’s disease Anti-Amyloid immunotherapies: imaging recommendations and practice considerations for ARIA monitoring. AJNR Am J Neuroradiol. 2024;46(1):24–32. [DOI] [PMC free article] [PubMed]
- 21.Bradshaw AC, Georges J. Anti-Amyloid therapies for alzheimer’s disease: an alzheimer Europe position paper and call to action. J Prev Alzheimers Dis. 2024;11(2):265–73. [DOI] [PubMed] [Google Scholar]
- 22.Lee J-H, Jia J, Ji Y, Kandiah N, Kim S, Mok V, et al. A framework for best practices and readiness in the advent of Anti-Amyloid therapy for early Alzheimer’s disease in Asia. J Alzheimers Dis. 2024;101(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenbloom MH, O’Donohue T, Zhou-Clark D, Mala D, Frazier A, Tarrant M, et al. A framework for the administration of Anti-amyloid monoclonal antibody treatments in Early-Stage Alzheimer’s disease. CNS Drugs. 2024;38(7):493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bernick C, Cummings J, Raman R, Sun X, Aisen P. Age and rate of cognitive decline in alzheimer disease: implications for clinical trials. Arch Neurol. 2012;69(7):901–5. [DOI] [PubMed] [Google Scholar]
- 25.Stanley K, Whitfield T, Kuchenbaecker K, Sanders O, Stevens T, Walker Z. Rate of cognitive decline in Alzheimer’s disease stratified by age. J Alzheimers Dis. 2019;69(4):1153–60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.