Abstract
Human T-cell leukemia virus type 1 (HTLV-1) but not HTLV-2 is associated with adult T-cell leukemia. We found that HTLV-2 Tax2 protein stimulated reporter gene expression regulated by the interleukin (IL)-2 promoter through the nuclear factor of activated T cells (NFAT) in a human T-cell line (Jurkat). However, the activity of HTLV-1 Tax1 was minimal in this system. T-cell lines immortalized by HTLV-2 but not HTLV-1 constitutively exhibited activated NFAT in the nucleus and constitutively expressed IL-2 mRNA. Cyclosporine A, an inhibitor of NFAT activation, abrogated the induction of IL-2 mRNA in HTLV-2-immortalized T-cell lines and concomitantly inhibited cell growth. This growth inhibition was rescued by the addition of IL-2 to the culture. Furthermore, anti-IL-2 receptor antibodies significantly reduced the proliferation of HTLV-2-infected T-cell lines but not that of HTLV-1-infected cells. Our results suggest that Tax2 activates an IL-2 autocrine loop mediated through NFAT that supports the growth of HTLV-2-infected cells under low-IL-2 conditions. This mechanism would be especially important in vivo, where this autocrine mechanism establishes a nonleukemogenic life-long HTLV-2 infection. The results also suggest that differences in long-term cytokine production between HTLV-1 and HTLV-2 infection are another factor for the differences in pathogenesis.
Adult T-cell leukemia is an aggressive form of leukemia characterized by monoclonal proliferation of T cells infected with human T-cell leukemia virus type 1 (HTLV-1) (15, 25, 30, 38). HTLV-1 is transmitted mainly from infected mothers to children through breast milk, however, the average age of adult T-cell leukemia patients is approximately 60 years (4), suggesting a long latency period. Furthermore, only a fraction (approximately 5%) of HTLV-1-infected individuals develop adult T-cell leukemia. Thus, HTLV-1-infection in and of itself is not sufficient for the development of adult T-cell leukemia, indicating that host factors have a critical role in the development of adult T-cell leukemia. Although not completely understood, there likely are at least two host factors involved in adult T-cell leukemia development: genetic and/or epigenetic changes in HTLV-1-infected T cells; and deterioration of host immune competence probably resulting from HTLV-1 infection itself (20, 25).
HTLV-2 is genetically and biologically similar to HTLV-1 (7, 34). Both HTLV-1 and HTLV-2 immortalize primary human T cells with similar efficiency in vitro (8, 27, 41), and like HTLV-1, HTLV-2 also establishes a life-long persistent infection in humans. However, unlike HTLV-1, HTLV-2 is not associated with the development of adult T-cell leukemia or other malignancies. Thus, it appears that HTLV-2 is unable to promote a certain step(s) in adult T-cell leukemia leukemogenesis. Which step(s) in adult T-cell leukemia development differs between HTLV-1 and HTLV-2 infections has not been defined, nor have the differences in mechanisms been elucidated.
HTLV-1 and HTLV-2 encode nine genes, including gag, pol, and env, but only a few viral genes are expressed in infected peripheral blood mononuclear cells (33). HTLV-1 tax1 and HTLV-2 tax2 are important regulatory genes, and accumulating evidence has shown that Tax1 and Tax2 expression plays a central role in immortalization of T cells and maintenance of persistent infections. For instance, mutant HTLV-1 and HTLV-2 viruses carrying inactive tax1 and tax2 genes, respectively, cannot immortalize primary human T cells (31, 32). The tax1 gene by itself has been shown to immortalize primary human T cells in the presence of interleukin-2 (IL-2) (2, 12). Tax1 has multiple functions in T cells, including the activation of cellular genes through transcription factors NF-κB, CREB/ATF, and AP-1 (10, 11, 17, 24, 42). Furthermore, Tax1 inactivates several tumor suppressor gene products, such as p53, MAD1, and p16INK4A (1, 19, 29, 35). Taken together, Tax1 and Tax2 should be key elements in the distinct pathogenic activities of these two viruses.
Tax1 and Tax2 share approximately 75% identity at the amino acid level and the two proteins exhibit indistinguishable activity with similar potency in vitro. Therefore, why Tax1 and Tax 2, sharing 75% amino acid identity and in vitro activity, are associated with distinct disease outcomes is still unclear.
Inducible and transient expression of cytokines, including IL-2, is crucial for antigen-induced differentiation and proliferation of T cells during immune responses. Such organized expression of cytokines in T cells is regulated by several transcription factors, including the nuclear factor of activated T cells (NFAT), AP-1, and NF-κB (40). In this study, we found that HTLV-2 Tax2 induces the expression of IL-2 through NFAT and that IL-2 promoted the proliferation of HTLV-2-infected T-cell lines. This result was specific to HTLV-2, since HTLV-1-infected T-cell lines did not produce IL-2. Thus, the results strongly suggest that aberrant activation of an IL-2 autocrine loop by Tax2 mediates life-long HTLV-2 infection. This autocrine immortalization mechanism allows establishment of a nonleukemogenic persistent infection, which is defective in promoting a certain step(s) of adult T-cell leukemia development. We will also discuss the long-term distinct gene expression through NFAT between HTLV-1 and HTLV-2 infection in terms of their distinct pathogeneses.
MATERIALS AND METHODS
Cells and culture conditions.
The human T-cell lines used in the present experiments have been characterized previously (18). Jurkat is an HTLV-1-negative human T-cell line. HUT102, SLB-1, and ILT-Koy are HTLV-1-positive human T-cell lines. Ton1, MoT, PBL01, and PBL2 are HTLV-2-positive human T-cell lines. Jurkat, HUT102, SLB-1, Ton1, and MoT were cultured in RPMI 1640 supplemented with 10% or 20% fetal bovine serum (FBS), 4 mM glutamine, penicillin (50 U/ml), and streptomycin (50 μg/ml) (RPMI/10% FBS). ILT-Koy, PBL01, and PBL2 are IL-2-dependent cells and therefore, they are cultured in RPMI/fetal bovine serum containing 1 nM of recombinant human IL-2.
Plasmids.
The tax1, tax2A, and tax2B genes and their chimeric genes were cloned into pHβPr-1-neo, which has a β-actin promoter for protein expression in mammalian cells and a neomycin resistance gene as a selection marker. All plasmids have been described previously (16). pNFAT-Luc is a luciferase expression plasmid regulated by three copies of the NFAT site (−286 to −249 of human IL-2) and the human IL-2 promoter (−64 to +47) (21, 28). pIL2Luc was constructed by insertion of the human IL-2 promoter (−541 to +57) into the pGL3-basic vector (4, 21). κB-Luc is a luciferase expression plasmid regulated by the κB element of the IL-2 receptor α-chain gene and the minimal HTLV-1 promoter. pGK/β-gal expresses β-galactosidase under the control of the phosphoglycerate kinase promoter and is used to normalize the transfection efficiency.
Transient transfection and luciferase assays.
Jurkat cells in RPMI 1640 medium without serum were seeded at 4 × 105 cells/well in a 24-well plate. The cells then were cotransfected with the Tax expression plasmid together with κB-Luc by using X-treamGENE Q2 (Roche Applied Science) or TransFectin (Bio-Rad Technologies) according to the manufacturer's instructions. Cell lysates were prepared from transfected cells, and the luciferase activity as well as β-galactosidase activity were determined using a luminometer (LUMAT LB9507, Berthold). The activity of luciferase was normalized to that of β-galactosidase. The assay was carried out three times to confirm reproducibility.
Western blotting.
Cell lysates (30 μg) were prepared from human T-cell lines using radioimmunoprecipitation assay (RIPA) buffer containing 25 mM Tris (pH 7.2), 150 mM NaCl, 1.0 mM EDTA, 1.0% NP-40, 0.5% deoxycholate, 0.1% sodium dodecyl sulfate, 1.0 mM phenylmethylsulfonyl fluoride, 20 μg/ml aprotinin, 1.0 mM Na3VO4, and 1.0 mM NaF, and they were size-separated by electrophoresis under reducing conditions in 8% polyacrylamide gel with sodium dodecyl sulfate. The proteins in the gel were electrotransferred onto a polyvinylidene difluoride membrane. The membrane was incubated with 5% skim milk for 1 h at room temperature to inhibit nonspecific binding and was further incubated with either anti-Tax1 mouse monoclonal antibody (Taxy-7) (37) or rabbit anti-Tax2B polyclonal serum (22). After being washed with TBS-T (20 mM Tris-HCl [pH 7.4], 150 mM NaCl, and 0.1% Tween 20), the membranes were incubated with either anti-mouse (for Tax1) or anti-rabbit (for Tax2B) immunoglobulin G conjugated with horseradish peroxidase (Bio-Rad Technologies). Protein bands in the membrane recognized by the antibodies were visualized using the ECL Western blotting detection system (Amersham Pharmacia Biotech). Yuetsu Tanaka (Ryukyu University) and W. W. Hall (University College Dublin) kindly provided the Taxy-7 antibody and the anti-Tax2B polyclonal serum, respectively.
RT-PCR.
Total RNA was isolated from human T-cell lines using ISOGEN (Nippon-gene), according to the manufacturer's instructions. Reverse transcription (RT)-PCR analysis for IL-2 mRNA was performed using 1 μg of total RNA and Ready-To-Go RT-PCR Beads (Amersham Pharmacia Biotech). Oligo(dT) was used to prime the reverse transcription step. For the RT step, the samples were incubated at 42°C for 25 min followed by 95°C for 5 min. The samples were stored at 4°C until use. Conditions for the first round of the PCR were 95°C for 2 min, followed by 30 cycles of 95°C for 1 min, 55°C for 1 min, and 72°C for 1 min, and finally one cycle of 72°C for 7 min. A 2-μl sample from the first round of PCR was added to a second PCR. The conditions for the second round of PCR were one cycle of 95°C for 2 min, followed by 20 cycles of 95°C for 1 min, 55°C for 1 min, and 72°C for 1 min, and one cycle of 72°C for 7 min.
The primers used for PCR were 5′-CATTGCACTAAGTCTTGCACTTGTCA-3′ and 5′-CGTTGATATTGCTGATTAAGTCCCTC-3′ for human IL-2. The β-actin-specific primers were purchased from Promega. The RT-PCR conditions for β-actin were 42°C for 25 min and 95°C for 5 min for RT, and one cycle of 95°C for 2 min, 20 cycles of 95°C for 1 min, 55°C for 1 min, and 72°C for 1 min, and finally one cycle of 72°C for 7 min for PCR. Thermal cycling was carried out using a GeneAmp PCR system 9700 (Applied Biosystems).
ELISA for quantitation of IL-2.
The amount of IL-2 protein in the culture supernatants of T-cell lines was measured by an enzyme-linked immunosorbent assay (ELISA) kit under instructions provided by the supplier (human IL-2 ELISA kit II; BD Biosciences Pharmingen).
Electrophoretic mobility shift assay.
To prepare nuclear extracts, human T-cell lines (5 × 106 to 10 × 106 cells) were washed with phosphate-buffered saline containing 1 mM Na3VO4 and 5 mM NaF and then treated with hypotonic buffer containing 0.2% NP-40, 20 mM HEPES (pH 7.9), 20 mM NaF, 1 mM Na3VO4, 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride, leupeptin (1 μg/ml), and aprotinin (1 μg/ml). Cell lysates were centrifuged, and the pellets then were treated with high-salt buffer supplemented with 20 mM HEPES, 420 mM NaCl, 20 mM NaF, 1 mM Na3VO4, 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride, leupeptin (1 μg/ml), aprotinin (1 μg/ml), and 20% glycerol at 4°C for 30 min. The lysates were mixed thoroughly by vortexing and incubated for 30 min at 4°C with rotation.
After centrifugation, the resultant supernatant was used as the nuclear extract in an electrophoretic mobility shift assay (EMSA). For EMSA, nuclear extract (15 μg) was preincubated with 1 μg of poly(dI:dC) in 20 μl of binding buffer containing 13 mM HEPES (pH 7.9), 65 mM NaCl, 0.15 mM EDTA, 8% glycerol, and 1 mM dithiothreitol for 15 min on ice. The 32P-labeled (5 × 104 cpm) double-stranded oligonucleotide probe was added to the reaction mixture and incubated for 15 min at room temperature. The complexes formed were separated from the unbound probe by electrophoresis in a 5% polyacrylamide gel containing 0.5x Tris-borate-EDTA and 2.5% glycerol. After separation, the gel was dried, and radioactivity in the dried gel was analyzed using a BAS5000 instrument (Fuji Film and Photo).
A 32P-labeled double-stranded synthetic oligonucleotide probe corresponding to the NFAT element (gatcGGAGGAAAAACTGTTTCATA) from the IL-2 gene was used as the NFAT site probe. To determine specificity, mutant NFAT (NFATm in the figure) and an unrelated oligonucleotide, gatcGGACCTTAAACTGTTTCATA and gatcGAAACTCAGCGCG, were used as competitors in EMSA. For the supershift assay, the nuclear extract was preincubated with anti-NFATp antibody (sc-7295; Santa Cruz Biotechnology) or control mouse immunoglobulin G2a (PP102; Chemicon) antibody for 30 min on ice before addition of the probe.
Cell growth inhibition by cyclosporine A or anti-IL-2R antibodies.
For the cyclosporine A assay, human T-cell lines (5 × 104/0.5 ml of RPMI/20% FBS with or without IL-2 in a 48-well plate) were cultured in the presence of 0.5 μM of cyclosporine A (Sigma). For the antibody assay, cells were cultured in the presence of 5 μg/ml of anti-IL-2 receptor (IL-2R) α-chain antibody (H31) and 5 μg/ml of anti-IL-2R β-chain antibody (TU27) (36). Cell morphology and viability were assessed using trypan blue staining.
RESULTS
Tax2 stimulates NFAT-dependent gene expression in a human T-cell line.
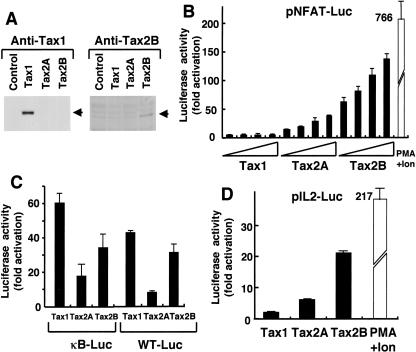
NFAT stimulates the expression of a number of cellular genes that are involved in T-cell activation and proliferation in response to antigen stimulation, such as IL-2, IL-3, IL-4, and Fas ligand (40). To gain insight into the distinct pathogenic activities of HTLV-1 and HTLV-2, we examined the effect of Tax1 and Tax2 protein expression on NFAT-dependent gene expression. For this purpose, plasmids encoding Tax1, Tax2B, or Tax2A were transiently transfected into Jurkat cells (human T-cell line) together with a luciferase reporter gene under the control of three copies of the NFAT binding site derived from the human IL-2 gene. Tax2B and Tax2A were derived from HTLV-2b and HTLV-2a subtypes, respectively. Forty-eight hours after transfection, the cells were harvested and luciferase activity was measured.
While Tax1 activated NFAT-dependent luciferase activity minimally (less than fivefold) relative to the control sample transfected with the pHβneo control plasmid, Tax2B prominently (more than 100-fold) induced luciferase activity (Fig. 1B). Similarly, Tax2A induced luciferase expression mediated by NFAT, and the activity of Tax2A was less than that of Tax2B but more than that of Tax1. Tax1, but not Tax2A or Tax2B, was detected in Jurkat cells by Western blot by anti-Tax1 antibody. On the other hand, Tax2B but not Tax2A or Tax1 was detected by anti-Tax2B antibody (Fig. 1A). Thus, Tax2 and Tax1 were expressed at a detectable level in Jurkat cells, although because the assay was not quantitative, it did not enable us to compare the expression levels between Tax1 and Tax2.
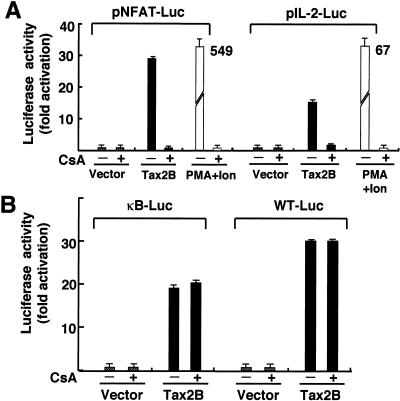
FIG. 1.
Tax2 stimulates IL-2 promoter activity in a T-cell line. (A) Jurkat cells (4 × 105 cells/0.5 ml) were transfected with either tax1, tax2A, or tax2B plasmid (2 μg). At 48 h after transfection, the cells were harvested, and the amount of Tax1 (left) or Tax2 (right) was measured by Western blot analysis. (B, C, and D) Jurkat cells were transfected with the indicated tax plasmid (0.01, 0.05, 0.1, and 0.5 μg) and 0.1 μg of pGK/β-gal together with 1 μg of either pNFAT-Luc (B), κB-Luc, WT-Luc (HTLV-1 21 bp) (C), or pIL-2-Luc (D). At 48 h after transfection, the cells were harvested, and the luciferase and β-galactosidase activities in the cell lysates were measured. As a positive control, Jurkat cells were treated with 20 ng/ml of PMA and 2 μM of ionomycin for 6 h before harvest. Luciferase activity was normalized to that of β-galactosidase. The fold activation represents the luciferase activity of cells transfected with the tax plasmid relative to that with the control plasmid. Error bars indicate standard deviations. Three independent experiments were carried out to confirm reproducibility.
Treatment of Jurkat cells with phorbol myristate acetate (PMA) and ionomycin, which mimics antigen stimulation, induced luciferase expression that was sixfold greater than that of Tax2B. Tax2 also stimulated luciferase expression regulated by NF-κB from the IL-2 receptor α-chain gene (κB-Luc) or by a Tax-inducible 21-bp sequence in the HTLV-1 long-terminal repeat (WT-Luc) in Jurkat cells (Fig. 1C). However, the levels of activation obtained with Tax2A and Tax2B were less than that with Tax1, indicating that the augmented activity of Tax2 relative to Tax1 is specifically mediated by NFAT.
Next, we examined whether Tax2 can stimulate an IL-2 promoter containing an NFAT site, as used above. Tax2A and Tax2B efficiently activated the expression of luciferase regulated by the IL-2 promoter, and as observed above, the activity was much greater than that of Tax1 (Fig. 1D). These results indicate that in a human T-cell line, Tax2 efficiently activated IL-2 gene expression through the NFAT site to a much greater level than Tax1.
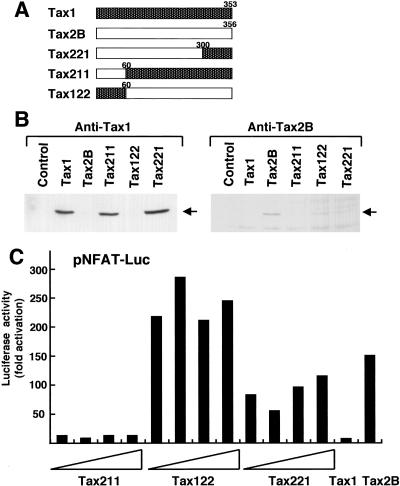
Identification of the region of Tax2 responsible for the augmented activation of NFAT.
To delineate the region of Tax2 responsible for its augmented NFAT activation relative to Tax1, we used chimeric tax constructs used previously (Fig. 2A). The result from the transient cotransfection study with pNFAT-Luc showed that the Tax122 and Tax221 proteins stimulated much greater luciferase expression mediated through NFAT than Tax1 and equivalent to Tax2B, whereas Tax211 was equivalent to Tax1 (Fig. 2C). These results indicated that the region between amino acids 60 and 300 of Tax2 contains the element required for NFAT activation that is distinct from Tax1. Western blot analysis showed that Tax211, Tax221, and Tax1 (detected with anti-Tax1 antibody), and Tax2B and Tax122 (detected with anti-Tax2B antibody) were expressed equivalently in Jurkat cells (Fig. 2B). Nevertheless, NFAT was activated by Tax221 significantly more than Tax1 and Tax211 were. Thus, Tax2B, Tax122, and Tax221 activate NFAT-dependent transcription more efficiently than Tax1.
FIG. 2.
Tax2 region is responsible for the activation of NFAT. (A) Structures of the chimeric genes and the position of the boundary between Tax1 and Tax2B are indicated. (B) Cell lysates were prepared from Jurkat cells transfected with the indicated plasmids (2 μg), and the amount of Tax in each lysate was measured by Western blot analysis using an anti-Tax1 antibody (left) or an anti-Tax2B antibody (right). Arrows indicate the Tax proteins recognized by the antibodies. (C) Jurkat cells were transfected with the indicated Tax expression plasmid (0.01 to 0.5 μg) together with the luciferase plasmid regulated by the NFAT element (pNFAT-Luc). Cell lysates were prepared from transfected cells, and luciferase activity was determined. Luciferase activity was normalized to that of β-galactosidase. The fold activation represents the luciferase activity of cells transfected with the tax plasmid relative to that with the control plasmid. Three independent experiments were carried out to confirm reproducibility.
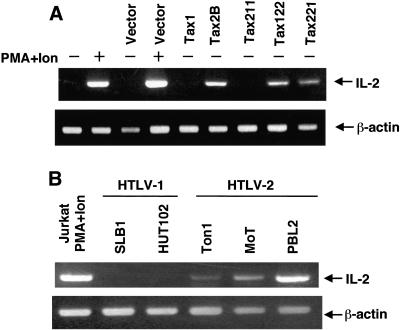
Tax2, but not Tax1, induces the expression of IL-2 in human T-cell lines.
The results from the transient transfection study above indicated that Tax2 stimulates the IL-2 promoter in the context of a plasmid transfection. In order to determine the biological relevance of this observation, we examined whether Tax2 can stimulate expression of the endogenous IL-2 gene. For this evaluation, plasmids encoding Tax2, Tax1, and their chimeric derivatives were transiently transfected into Jurkat cells. Forty-eight hours after transfection, total RNA was extracted from the cells by the Isogen method and reverse transcribed to obtain cDNA. IL-2 cDNA was amplified by PCR using primer sets corresponding to the human IL-2 gene.
The RT-PCR product specific to IL-2 was not detected in Jurkat cells transfected with the control plasmid, whereas it was detected in the cells transfected with the tax2B plasmid (Fig. 3A). Treatment of Jurkat cells with PMA and ionomycin induced expression of IL-2 mRNA that was greater than induction by Tax2. Consistent with the results from the luciferase assay, Tax1 and Tax211 did not induce the expression of IL-2 mRNA, whereas Tax122 and Tax221 did induce the expression (Fig. 3A), indicating that the activation of NFAT by Tax2 is responsible for the induction of endogenous IL-2 mRNA expression.
FIG. 3.
Tax2 induces the expression of IL-2 mRNA in T-cell lines. (A) Jurkat cells were transfected with the indicated plasmids. At 42 h after transfection, the cells were treated with PMA and ionomycin or medium alone for 6 h. Then the cells were harvested, and total RNA was extracted. From the isolated total RNA, the RT-PCR products corresponding to IL-2 and β-actin mRNA were amplified, and the products were size-separated on a 2% agarose gel stained with ethidium bromide. (B) Total RNA was prepared from the indicated HTLV-infected T-cell lines and Jurkat cells treated with PMA and ionomycin for 6 h. The RT-PCR products amplified from the isolated total RNA were size-separated on a 2% agarose gel stained with ethidium bromide. The experiments were repeated three times to confirm reproducibility.
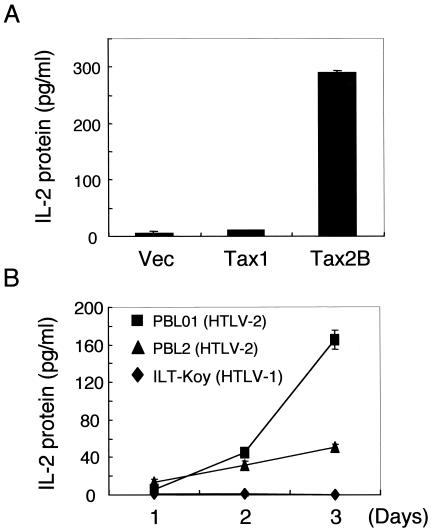
RT-PCR analysis also showed that three HTLV-2-infected T-cell lines (Ton1, MoT, and PBL2) but not two HTLV-1-infected cell lines (SLB1 and HUT102) constitutively expressed IL-2 mRNA (Fig. 3B). Among the three HTLV-2-positive cell lines, PBL2 cells expressed the highest level of IL-2 mRNA. Consistent with mRNA induction, an enzyme-linked immunosorbent assay for IL-2 protein showed that a transient transfection of the tax2 plasmid into Jurkat cells induced IL-2 protein expression, whereas transfection of tax1 did little (Fig. 4A). Moreover, two HTLV-2-infected T-cell lines but not an HTLV-1-infected one produced IL-2 in the absence of exogenous IL-2 in the culture supernatant, and the amounts increased till day 3 of culture (Fig. 4B). Thus, these results showed that Tax2, but not Tax1, can constitutively stimulate the expression of endogenous IL-2 protein in HTLV-2-infected T-cell lines.
FIG. 4.
Tax2 induces the expression of IL-2 protein in T-cell lines. (A) Jurkat cells were transfected with the indicated plasmids. At 48 h after transfection, the culture supernatant was collected, and the amount of IL-2 protein in the supernatant was measured by ELISA for IL-2. (B) The indicated HTLV-infected T-cell lines were cultured in the absence of IL-2. On the indicated days of culture, the culture supernatant was collected, and the amount of IL-2 protein in the supernatant was measured by ELISA for IL-2. The experiments were repeated twice to confirm reproducibility.
Activation of NFAT in HTLV-2-infected but not HTLV-1-infected T-cell lines.
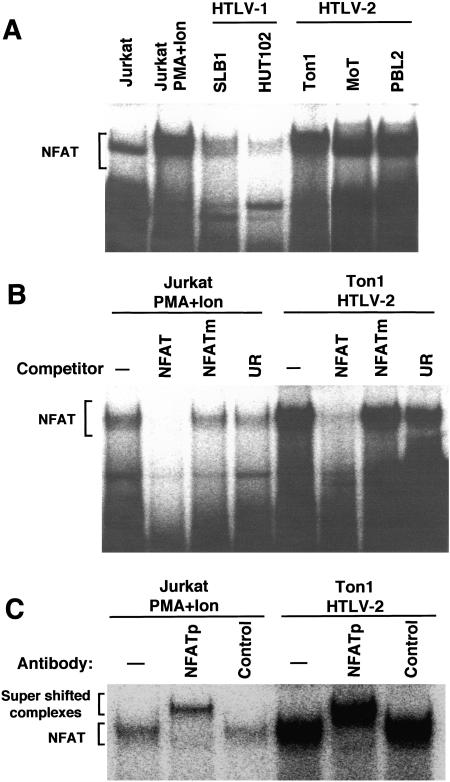
In resting T cells, NFAT is localized in the cytoplasm. Upon antigenic stimulation, NFATs translocate to the nucleus, bind NFAT response elements in cellular genes, and activate transcription. We next examined the amount of NFAT in the nucleus of various T-cell lines, by electrophoretic mobility shift assay (EMSA) using the NFAT binding site derived from the human IL-2 gene. Treatment of Jurkat cells with PMA and ionomycin induced a new complex that migrated slightly more slowly than the complex detected in untreated cells (Fig. 5A). The formation of this induced complex was selectively inhibited by an excess amount of homologous NFAT oligonucleotides, but not oligonucleotides containing mutations in the NFAT site or unrelated oligonucleotides (Fig. 5B).
FIG. 5.
HTLV-2-infected T-cell lines have constitutive NFAT activity. (A and B) Nuclear extracts prepared from the indicated T-cell lines and Jurkat cells treated with PMA and ionomycin were incubated with the NFAT site probe in the absence or presence of competitor. Binding to the NFAT site probe was measured by EMSA as described in Materials and Methods. The specific main complex with the NFAT element is indicated. Excess homologous NFAT oligonucleotides (NFAT), mutant NFAT oligonucleotides (NFATm), and unrelated oligonucleotides (UR) were used as competitors. (C) Nuclear extracts prepared from Ton1 cells or Jurkat cells treated with PMA and ionomycin were incubated with anti-NFATp antibody or the control antibody, followed by incubation with the NFAT site probe. The complex formed was analyzed by EMSA. The positions of the NFAT complex and the supershifted complex are indicated.
Three unstimulated HTLV-2-infected T-cell lines (Ton1, MoT, and PBL2) gave a complex with the same migration pattern as the induced pattern in Jurkat cells, and this complex was also inhibited specifically by NFAT oligonucleotides (Fig. 5A and 5B). NFAT is a family of transcription factors, including NFATp and NFATx (40). The supershift assay showed that the complex in an HTLV-2-infected T-cell line as well as in Jurkat cells treated with PMA and ionomycin contained NFATp protein, which is a dominant NFAT family member in activated T cells (Fig. 5C). Unlike HTLV-2 cell lines, two HTLV-1-infected T-cell lines had little NFAT activity localized to the nucleus. These results suggested that Tax2, but not Tax1, constitutively activated NFATp in HTLV-2-infected T-cell lines.
Cyclosporine A inhibits the activation of NFAT by Tax2.
Cyclosporine A is a well-characterized inhibitor of the NFAT activation pathway (9). Cyclosporine A forms a complex with cyclophilin, and this complex specifically inhibits the activity of calcineurin, a calmodulin-dependent phosphatase. Calcineurin is required to dephosphorylate NFAT prior to its translocation to the nucleus. We next examined whether cyclosporine A could inhibit the activation of NFAT by Tax2.
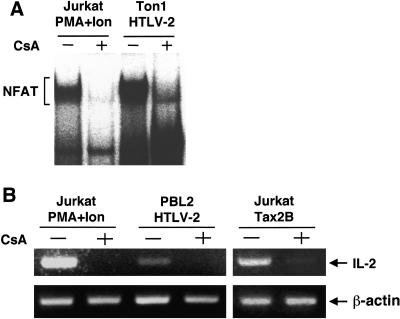
Cyclosporine A potently inhibited the Tax2 activation of luciferase expression from pNFAT-Luc as well as that from pIL-2-Luc in Jurkat cells (Fig. 6A). The inhibition by cyclosporine A was specific to the activation of NFAT, since the activation of NF-κB (κB-Luc) and viral CRE (WT-Luc) by Tax2 was unaffected by cyclosporine A (Fig. 6B). Consistent with these results, cyclosporine A also inhibited nuclear NFAT localization in HTLV-2-infected cells (Ton1) as well as in Jurkat cells treated with PMA and ionomycin (Fig. 7A). In addition, cyclosporine A inhibited the inducible expression of IL-2 mRNA in HTLV-2-infected cells (PBL2) as well as in Jurkat cells transfected with the tax2B plasmid and in Jurkat cells treated with PMA and ionomycin (Fig. 7B). These results indicated that the activation of NFAT by Tax2 was sensitive to cyclosporine A inhibition, suggesting that Tax2 activates NFAT at the level at or upstream of the cyclophilin/calcineurin point of the signaling pathway.
FIG. 6.
Cyclosporine A inhibits the activation of NFAT by Tax2. (A and B) Jurkat cells (4 × 105 cells) were transfected with the indicated plasmids. At 42 h after transfection, the cells were treated with PMA and ionomycin or medium alone for 6 h. In addition, the Jurkat cells were treated with 0.2 μM of cyclosporine A (CsA) 0.5 h before treatment with PMA and ionomycin or treated with 0.2 μM of cyclosporine A 6 h after transfection with the tax2 plasmid. Cell lysates were prepared and the luciferase and β-galactosidase activities were measured. Luciferase activity was normalized to that of β-galactosidase. The fold activation represents the luciferase activity of cells transfected with the tax2 plasmid relative to that with the control plasmid. Error bars indicate standard deviations. Three independent experiments were carried out to confirm reproducibility.
FIG. 7.
Cyclosporine A inhibits the induction of IL-2 mRNA by Tax2 in T-cell lines. (A) Jurkat cells were treated with 20 ng/ml of PMA and 2 μM of ionomycin for 6 h together with 1 μM of cyclosporine A (CsA) for 6.5 h. Ton1 cells were treated with cyclosporine A for 24 h. Nuclear extracts were prepared from the cells and incubated with the NFAT site probe. Binding to the NFAT site probe was measured by EMSA as described in Materials and Methods. The position of the specific main complex with the NFAT element is indicated. The position of the NFAT complex is indicated. (B) Jurkat cells were either transfected with the tax2B plasmid or treated with PMA and ionomycin. Then, Jurkat and Ton1 cells were treated with 1 μM of cyclosporine A (CsA) for 6.5 h (Jurkat) and 24 h (Ton1). Total RNA was isolated and the RT-PCR products corresponding to IL-2 and β-actin mRNAs were amplified. The products were size-separated on a 2% agarose gel stained with ethidium bromide. The experiments were repeated three times to confirm reproducibility.
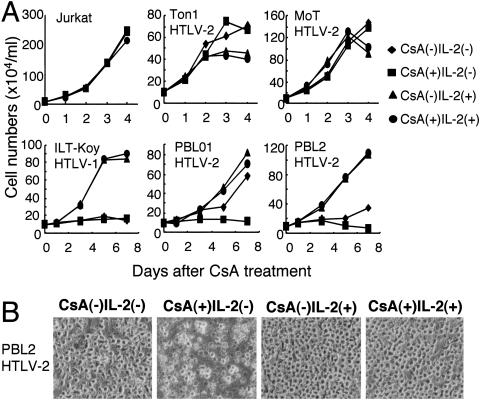
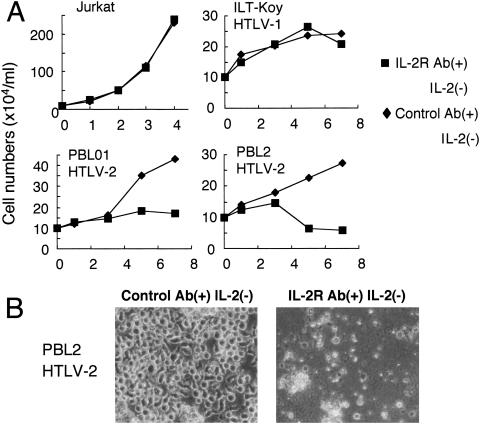
Then, we examined whether NFAT activation in HTLV-2-infected cells regulates their growth. To check this, we cultured HTLV-2-infected cells in the presence or absence of cyclosporine A. PBL2 and PBL01 are IL-2-dependent HTLV-2-immortalized T-cell lines. These two cell lines still grew in the absence of IL-2, but overall cell growth was reduced by culture with cyclosporine A for 3 to 7 days (Fig. 8). However, growth inhibition by cyclosporine A was abrogated by the addition of IL-2 to the culture. On the other hand, cyclosporine A negligibly affected the growth of HTLV-uninfected cells (Jurkat) and an IL-2-dependent HTLV-1-infected T-cell line (ILT-Koy), in either the absence or presence of IL-2. These results suggested that constitutive activation of NFAT supports the cell growth of IL-2-dependent HTLV-2-infected cells in the absence of IL-2.
FIG. 8.
Cyclosporine A inhibits growth of IL-2-dependent HTLV-2-infected cells. (A and B) The indicated T-cell lines were cultured in a combination of IL-2 and/or 0.5 μM of cyclosporine A (CsA) for 7 days. The number of viable cells (A) and the morphology of PBL2 cells at day 7 (B) were examined using a microscope. The experiments were repeated three times to confirm reproducibility.
Unlike in IL-2-dependent cells, cyclosporine A did not inhibit the growth of two IL-2-independent HTLV-2-infected T-cell lines (Ton1 and MoT) in the presence or absence of IL-2. MoT was derived from a hairy cell leukemia patient, while Ton1 was established by in vitro transformation with lethally irradiated HTLV-2-infected T cells. It should be noted that IL-2-independent immortalization by HTLV-2 in vitro occurs much less frequently than IL-2-dependent immortalization. Thus, these IL-2-independent T-cell lines are likely to have a genetic or epigenetic change(s), with such changes possibly conferring resistance to the inhibition of cell growth by cyclosporine A.
Finally, since NFAT regulates the induction of multiple cytokine genes, we examined whether IL-2 mediates the induced proliferation of HTLV-2-infected T-cell lines by Tax2 mediated through NFAT. For this purpose, two IL-2-dependent HTLV-2-infected T-cell lines, an IL-2-dependent HTLV-1-infected T-cell line, and IL-2-independent Jurkat cells were cultured in the absence of IL-2 and presence of two anti-IL-2 receptor antibodies (α-chain and β-chain), which can inhibit IL-2-dependent proliferation. Anti-IL-2 receptor antibodies reduced the growth only of the two HTLV-2-infected T-cell lines (Fig. 9). The effect of anti-IL-2 receptor antibodies was specific to HTLV-2-infected cell lines, since such cell growth inhibition was not observed for the HTLV-1-infected T-cell line or the Jurkat cell line. These results indicated that in HTLV-2-infected cells, induction of IL-2 production, mediated through NFAT activation, promotes proliferation.
FIG. 9.
Anti-IL-2R antibodies inhibit the growth of IL-2-dependent HTLV-2-infected cells. (A and B) The indicated T-cell lines were cultured in the absence of IL-2 together with anti-IL-2R antibodies (5 μg/ml of H31 and 5 μg/ml of TU27) or with 10 μg/ml of control antibody for 7 days. The number of viable cells (A) and the morphology of PBL2 cells at day 7 (B) were examined using a microscope. The experiments were repeated three times to confirm reproducibility.
DISCUSSION
Greene et al. reported that HTLV-2 Tax2 induces expression of IL-2 and IL-2R α-chain genes (13). However, whether induction of IL-2 gene expression plays a role in cell growth properties and phenotypes of HTLV-2-infected cells and how Tax2 activates IL-2 gene expression have not been characterized fully. In this study, we demonstrated that HTLV-2 Tax2 activated IL-2 gene expression, thereby promoting the proliferation of HTLV-2-infected T cells in the absence of IL-2. These results strongly suggest that an IL-2 autocrine loop is essential for maintaining the proliferation of HTLV-2-infected cells in low-IL-2 conditions in vitro as well as in vivo, thereby enabling establishment of life-long HTLV-2 infection. Unlike HTLV-2, HTLV-1 did not utilize the same IL-2 autocrine strategy for immortalization of T cells. These results suggest that immortalization of T cells by HTLV-2 through an IL-2 autocrine loop is a factor establishing a benign persistent infection which does not induce adult T-cell leukemia or related malignancies.
The anti-IL-2R β-chain antibody that we used here inhibits signaling by IL-15, which also promotes T-cell growth. Therefore, IL-15 may also be involved in the growth of HTLV-2-infected cells. Azimi et al. showed that HTLV-1 Tax1 induces the expression of IL-15 through NF-κB (5), but anti-IL-2R antibodies did not inhibit the growth or survival of HTLV-1-infected T cells (Fig. 9). Thus, it is unlikely that IL-15 is selectively involved in the growth of HTLV-2-infected cells but not HTLV-1-infected cells. Taken together, the experiments using anti-IL-2 receptor antibodies suggest that IL-2 produced from HTLV-2-infected T cells stimulates their growth.
IL-2 produced from HTLV-2-infected T-cell lines (PBL01 and PBL2) stimulated their growth but such IL-2 was not sufficient to support their continuous growth in vitro (Fig. 8 and 9). Thus, it is unclear whether IL-2 produced from HTLV-2-infected T-cell lines is sufficient for their continuous survival and cell growth in vivo. IL-2 induction by Tax2 was synergistically enhanced in Jurkat cells treated with PMA and ionomycin (data not shown), suggesting that antigen stimulation and Tax2 cooperatively induce the production of IL-2 in HTLV-2-infected T cells, which may fully support the survival and growth of HTLV-2-infected T cells in vivo. Further studies are, however, required to establish to what extent IL-2 contributes to the long-term survival and cell growth of HTLV-2-infected T cells in vivo.
There is no substantial evidence that growth of HTLV-1-infected cells is regulated by autocrine factors, including IL-2 (3, 39). Instead, several studies suggested that intracellular Tax1 directly attacks the host cell cycle machinery through a protein-protein interaction to promote cell growth. For instance, Tax1 interacts with several cell cycle regulators such as p16INK4A and CDK4, and the interactions are correlated with the cell cycle promotion induced by Tax1 (14, 35). HTLV-1-infected cells accumulate multiple genetic and epigenetic changes before adult T-cell leukemia development. Thus, the autocrine promotion of cell proliferation by HTLV-2 may result in less accumulation of genetic and/or epigenetic changes in infected cells.
In activated T cells, NFATp binds to the NFAT site on the IL-2 promoter as a complex with another transcription factor, AP-1 (6). AP-1 is a heterodimer or homodimer of Jun and Fos family members, and Tax1 activates AP-1-dependent transcription through inducing the expression of various AP-1 family members (11, 17). Stimulation via AP-1 does not, however, account for the differences in the interaction of Tax2 and Tax1 with the IL-2 NFAT site, since both Tax1 and Tax2 activate AP-1-dependent transcription, and their activities were equivalent (data not shown).
Experiments using chimeric Tax proteins indicated that the Tax2B amino acid region between 60 and 300 included a factor required for the augmented NFAT activity. Since this region in Tax2B and Tax2A differs by only one amino acid, the conserved amino acid variation(s) of Tax2B and Tax2A relative to Tax1 must account for the differences in NFAT activity. We are comprehensively delineating the domain of Tax2 responsible for this activity. As a next step, we will examine whether this Tax2-specific function is involved in the immortalization of primary T cells as well as persistent HTLV-2 infection in rabbit or rat model systems using a molecular clone of HTLV-2.
Several differences between Tax1 and Tax2 have been identified. For instance, Tax1 has greater transforming activity toward the Rat-1 fibroblast cell line (measured as colony formation in soft agar) than Tax2, and this difference is mediated by a PDZ domain binding motif (PBM) in Tax1, which is missing in Tax2. Since the PBM is localized at the C-terminal end of Tax1, PBM does not explain the reduced NFAT activation by Tax1 (16). Second, when the expression plasmids were transfected into a cell line, Tax2 localized in the cytoplasm while Tax1 localized in the nucleus (26). Thus, multiple differences between Tax1 and Tax2, including the activation of NFAT, are probably involved in the distinct pathogenesis of HTLV-1 and HTLV-2. Thus, further analysis is required to understand how the distinct pathogenesis of two related viruses is determined.
While NFAT, AP-1, and NF-κB are constitutively active in HTLV-2-infected cells, only AP-1 and NF-κB are constitutively activated in HTLV-1-infected cells. These three transcription factors are critical components of T-cell activation, differentiation, and proliferation during immune activation. These functions are carried out mainly through regulation of cytokine gene expression. Specifically, NFAT and AP-1 interact with each other to cooperatively regulate the expression of several cytokines, including IL-2, during an immune response (23). Thus, individuals infected with HTLV-1 and HTLV-2 would have distinct long-term cytokine profiles during persistent infection. Consequently, such distinct cytokine production may alter the immune status of HTLV infection, thereby being a factor responsible for the different modes of pathogenesis of HTLV-1 and HTLV-2.
In addition to adult T-cell leukemia, HTLV-1 is associated with several chronic inflammatory diseases such as HTLV-1-associated myelopathy/tropical spastic paraparesis and HTLV-1-associated uveitis. While HTLV-2 infection is not associated with development of adult T-cell leukemia, recent results suggest that HTLV-2 is associated with HTLV-1-associated myelopathy/tropical spastic paraparesis. Thus, cyclosporine A and anti-IL-2 receptor antibodies, especially cyclosporine A or related NFAT inhibitors, may be promising candidates for therapeutics for HTLV-2-associated diseases.
Acknowledgments
This work was supported in part by a Grant-in-Aid for Scientific Research on Priority Areas (C) and for Scientific Research (C) of Japan and grants from the National Institutes of Health (CA77556 and CA92009).
We thank William W. Hall and Mari Kannagi for the Tax2B plasmid, the antibody against Tax2B, and the HTLV-1-infected T-cell lines. We thank the Takeda pharmaceutical company for providing recombinant human IL-2. We also thank Sayoko Takizawa and Chika Yamamoto for excellent technical assistance and Kathleen Hayes for assistance in editing the manuscript.
REFERENCES
- 1.Akagi, T., H. Ono, N. Tsuchida, and K. Shimotohno. 1997. Aberrant expression and function of p53 in T cells immortalized by HTLV-I Tax1. FEBS Lett. 406:263-266. [DOI] [PubMed] [Google Scholar]
- 2.Akagi, T., and K. Shimotohno. 1993. Proliferative response of Tax1-transduced primary human T cells to anti-CD3 antibody stimulation by an interleukin-2-independent pathway. J. Virol. 67:1211-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arya, S. K., F. Wong-Staal, and R. C. Gallo. 1984. T-cell growth factor gene: lack of expression in human T-cell leukemia-lymphoma virus-infected cells. Science 223:1086-1087. [DOI] [PubMed] [Google Scholar]
- 4.Asada, H., N. Ishii, Y. Sasaki, K. Endo, H. Kasai, N. Tanaka, T. Takeshita, S. Tsuchiya, T. Konno, and K. Sugamura. 1999. Grf40, A novel Grb2 family member, is involved in T-cell signaling through interaction with SLP-76 and LAT. J. Exp. Med. 189:1383-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azimi, N., K. Brown, R. N. Bamford, Y. Tagaya, U. Siebenlist, and T. A. Waldmann. 1998. Human T-cell lymphotropic virus type I Tax protein trans-activates interleukin 15 gene transcription through an NF-kappaB site. Proc. Natl. Acad. Sci. USA 95:2452-2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boise, L. H., B. Petryniak, X. Mao, C. H. June, C. Y. Wang, T. Lindsten, R. Bravo, K. Kovary, J. M. Leiden, and C. B. Thompson. 1993. The NFAT-1 DNA binding complex in activated T cells contains Fra-1 and JunB. Mol. Cell. Biol. 13:1911-1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, I. S., J. McLaughlin, J. C. Gasson, S. C. Clark, and D. W. Golde. 1983. Molecular characterization of genome of a novel human T-cell leukaemia virus. Nature 305:502-505. [DOI] [PubMed] [Google Scholar]
- 8.Chen, I. S., S. G. Quan, and D. W. Golde. 1983. Human T-cell leukemia virus type II transforms normal human lymphocytes. Proc. Natl. Acad. Sci. USA 80:7006-7009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clipstone, N. A., and G. R. Crabtree. 1992. Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature 357:695-697. [DOI] [PubMed] [Google Scholar]
- 10.Cross, S. L., M. B. Feinberg, J. B. Wolf, N. J. Holbrook, F. Wong-Staal, and W. J. Leonard. 1987. Regulation of the human interleukin-2 receptor alpha chain promoter: activation of a nonfunctional promoter by the transactivator gene of HTLV-I. Cell 49:47-56. [DOI] [PubMed] [Google Scholar]
- 11.Fujii, M., T. Niki, T. Mori, T. Matsuda, M. Matsui, N. Nomura, and M. Seiki. 1991. HTLV-1 Tax induces expression of various immediate early serum responsive genes. Oncogene 6:1023-1029. [PubMed] [Google Scholar]
- 12.Grassmann, R., S. Berchtold, I. Radant, M. Alt, B. Fleckenstein, J. G. Sodroski, W. A. Haseltine, and U. Ramstedt. 1992. Role of human T-cell leukemia virus type 1 X region proteins in immortalization of primary human lymphocytes in culture. J. Virol. 66:4570-4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greene, W. C., W. J. Leonard, Y. Wano, P. B. Svetlik, N. J. Peffer, J. G. Sodroski, C. A. Rosen, W. C. Goh, and W. A. Haseltine. 1986. Trans-activator gene of HTLV-II induces IL-2 receptor and IL-2 cellular gene expression. Science 232:877-880. [DOI] [PubMed] [Google Scholar]
- 14.Haller, K., Y. Wu, E. Derow, I. Schmitt, K. T. Jeang, and R. Grassmann. 2002. Physical interaction of human T-cell leukemia virus type 1 Tax with cyclin-dependent kinase 4 stimulates the phosphorylation of retinoblastoma protein. Mol. Cell. Biol. 22:3327-3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hinuma, Y., K. Nagata, M. Hanaoka, M. Nakai, T. Matsumoto, K. I. Kinoshita, S. Shirakawa, and I. Miyoshi. 1981. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc. Natl. Acad. Sci. USA 78:6476-6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirata, A., M. Higuchi, A. Niinuma, M. Ohashi, M. Fukushi, M. Oie, M. Akiyama, Y. Tanaka, F. Gejyo, and M. Fujii. 2004. PDZ domain-binding motif of human T-cell leukemia virus type 1 Tax oncoprotein augments the transforming activity in a rat fibroblast cell line. Virology 318:327-336. [DOI] [PubMed] [Google Scholar]
- 17.Iwai, K., N. Mori, M. Oie, N. Yamamoto, and M. Fujii. 2001. Human T-cell leukemia virus type 1 tax protein activates transcription through AP-1 site by inducing DNA binding activity in T cells. Virology 279:38-46. [DOI] [PubMed] [Google Scholar]
- 18.Iwanaga, Y., Tsukahara.T., T. Ohashi, Y. Tanaka, M. Arai1, M. Nakamura, K. Ohtani, Y. Koya, M. Kannagi, N. Yamamoto, and M. Fujii. 1999. Human T-cell leukemia virus type 1 Tax protein abrogates interleukin 2 dependence in a mouse T-cell line. J. Virol. 73:1271-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin, D. Y., F. Spencer, and K. T. Jeang. 1998. Human T-cell leukemia virus type 1 oncoprotein Tax targets the human mitotic checkpoint protein MAD1. Cell 93:81-91. [DOI] [PubMed] [Google Scholar]
- 20.Katsuki, T., K. Katsuki, J. Imai, and Y. Hinuma. 1987. Immune suppression in healthy carriers of adult T-cell leukemia retrovirus (HTLV-I): impairment of T-cell control of Epstein-Barr virus-infected B-cells. Jpn. J. Cancer Res. 78:639-642. [PubMed] [Google Scholar]
- 21.Lee, H. J., N. Koyano-Nakagawa, Y. Naito, J. Nishida, N. Arai, K. Arai, and T. Yokota. 1993. cAMP activates the IL-5 promoter synergistically with phorbol ester through the signaling pathway involving protein kinase A in mouse thymoma line EL-4. J. Immunol. 151:6135-6142. [PubMed] [Google Scholar]
- 22.Lewis, M. J., P. Novoa, R. Ishak, M. Ishak, M. Salemi, A. M. Vandamme, M. H. Kaplan, and W. W. Hall. 2000. Isolation, cloning, and complete nucleotide sequence of a phenotypically distinct Brazilian isolate of human T-lymphotropic virus type II (HTLV-II). Virology 271:142-154. [DOI] [PubMed] [Google Scholar]
- 23.Macian, F., C. Lopez-Rodriguez, and A. Rao. 2001. Partners in transcription: NFAT and AP-1. Oncogene 20:2476-2489. [DOI] [PubMed] [Google Scholar]
- 24.Maruyama, M., H. Shibuya, H. Harada, M. Hatakeyama, M. Seiki, T. Fujita, J. Inoue, M. Yoshida, and T. Taniguchi. 1987. Evidence for aberrant activation of the interleukin-2 autocrine loop by HTLV-I-encoded p40x and T3/Ti complex triggering. Cell 48:343-350. [DOI] [PubMed] [Google Scholar]
- 25.Matsuoka, M. 2003. Human T-cell leukemia virus type I and adult T-cell leukemia. Oncogene 22:5131-5140. [DOI] [PubMed] [Google Scholar]
- 26.Meertens, L., S. Chevalier, R. Weil, A. Gessain, and R. Mahieux. 2004. A 10-amino acid domain within human T-cell leukemia virus type 1 and type 2 tax protein sequences is responsible for their divergent subcellular distribution. J. Biol. Chem. 279:43307-43320. [DOI] [PubMed] [Google Scholar]
- 27.Miyoshi, I., I. Kubonishi, S. Yoshimoto, T. Akagi, Y. Ohtsuki, Y. Shiraishi, K. Nagata, and Y. Hinuma. 1981. Type C virus particles in a cord T-cell line derived by co-cultivating normal human cord leukocytes and human leukaemic T cells. Nature 294:770-771. [DOI] [PubMed] [Google Scholar]
- 28.Northrop, J. P., K. S. Ullman, and G. R. Crabtree. 1993. Characterization of the nuclear and cytoplasmic components of the lymphoid-specific nuclear factor of activated T cells (NF-AT) complex. J. Biol. Chem. 268:2917-2923. [PubMed] [Google Scholar]
- 29.Pise-Masison, C. A., K. S. Choi, M. Radonovich, J. Dittmer, S. J. Kim, and J. N. Brady. 1998. Inhibition of p53 transactivation function by the human T-cell lymphotropic virus type 1 Tax protein. J. Virol. 72:1165-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poiesz, B. J., F. W. Ruscetti, A. F. Gazdar, P. A. Bunn, J. D. Minna, and R. C. Gallo. 1980. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA 77:7415-7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robek, M. D., and L. Ratner. 1999. Immortalization of CD4(+) and CD8(+) T lymphocytes by human T-cell leukemia virus type 1 Tax mutants expressed in a functional molecular clone. J. Virol. 73:4856-4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ross, T. M., S. M. Pettiford, and P. L. Green. 1996. The tax gene of human T-cell leukemia virus type 2 is essential for transformation of human T lymphocytes. J. Virol. 70:5194-5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seiki, M., S. Hattori, Y. Hirayama, and M. Yoshida. 1983. Hum. adult T-cell leukemia virus: Complete nucleotide sequence of the provirus genome integrated in leukemia cell DNA. Proc. Natl. Acad. Sci. USA 80:3618-3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sugamura, K., and Y. Hinuma. 1993. Human retroviruses: HTLV-I and HTLV-II, p. 399-435. In J. A. Levy (ed.), The Retroviridae, vol. 2. Plenum Press, New York, NY. [Google Scholar]
- 35.Suzuki, T., S. Kitao, H. Matsushime, and M. Yoshida. 1996. HTLV-1 Tax protein interacts with cyclin-dependent kinase inhibitor p16INK4A and counteracts its inhibitory activity towards CDK4. EMBO J. 15:1607-1614. [PMC free article] [PubMed] [Google Scholar]
- 36.Takeshita, T., Y. Goto, K. Tada, K. Nagata, H. Asao, and K. Sugamura. 1989. Monoclonal antibody defining a molecule possibly identical to the p75 subunit of interleukin 2 receptor. J. Exp. Med. 169:1323-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanaka, Y., A. Yoshida, H. Tozawa, H. Shida, H. Nyunoya, and K. Shimotohno. 1991. Production of a recombinant human T-cell leukemia virus type-I trans-activator (tax1) antigen and its utilization for generation of monoclonal antibodies against various epitopes on the tax1 antigen. Int. J. Cancer 48:623-630. [DOI] [PubMed] [Google Scholar]
- 38.Uchiyama, T., J. Yodoi, K. Sagawa, K. Takatsuki, and H. Uchino. 1977. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood 50:481-492. [PubMed] [Google Scholar]
- 39.Volkman, D. J., M. Popovic, R. C. Gallo, and A. S. Fauci. 1985. Human T-cell leukemia/lymphoma virus-infected antigen-specific T-cell clones: indiscriminant helper function and lymphokine production. J. Immunol. 134:4237-4243. [PubMed] [Google Scholar]
- 40.Winslow, M. M., J. R. Neilson, and G. R. Crabtree. 2003. Calcium signalling in lymphocytes. Curr. Opin. Immunol. 15:299-307. [DOI] [PubMed] [Google Scholar]
- 41.Yamamoto, N., M. Okada, Y. Koyanagi, M. Kannagi, and Y. Hinuma. 1982. Transformation of human leukocytes by cocultivation with an adult T-cell leukemia virus producer cell line. Science 217:737-739. [DOI] [PubMed] [Google Scholar]
- 42.Zhao, L. J., and C. Z. Giam. 1992. Human T-cell lymphotropic virus type I (HTLV-I) transcriptional activator, Tax, enhances CREB binding to HTLV-I 21-base-pair repeats by protein-protein interaction. Proc. Natl. Acad. Sci. USA 89:7070-7074. [DOI] [PMC free article] [PubMed] [Google Scholar]