Abstract
Ferroptosis, the non-apoptotic, iron-dependent form of cell death is an unavoidable outcome and byproduct of cellular metabolism. Reactive oxygen species generation during metabolic activities transcends to Fe2+-induced lipid peroxidation, leading to ferroptosis. Cancer cells being highly metabolic are more prone to ferroptosis. However, their neoplastic nature enables them to bypass ferroptosis and become ferroptosis-resistant. The capability of cancer cells to reprogram its metabolic activities is one of its finest abilities to abort oxidative damage, and hence ferroptosis. Moreover, the reprogrammed metabolism of cancer cells, also associates with the radical trapping antioxidant systems to enhance the scavenging of ferroptosis and thereby tumor progression. Additionally, the TME, which is an inevitable part and regulator of carcinogenesis, presents an intricate cooperation with tumor metabolism to build an immuno-metabolic environment to regulate the sustenance of cell proliferation and survival. This review focuses on the current understanding of ferroptosis in carcinogenesis and its resistance acquired by cancer cells via several modulators including the radical trapping antioxidant systems, the reprogrammed metabolism, the TME, and intertwined role of cancer metabolism and tumor immunity. The reprogrammed metabolism section further comprehends the functional role of lipids, iron and glucose metabolism against ferroptosis defense separately. The affiliation of TME in ferroptosis regulation is further sectioned with reference to different immune cells present within the TME such as tumor-associated macrophages, tumor-infiltrating neutrophils, myeloid-derived suppressor cells, T-cells, natural killer cells, dendritic cells, and B-cells, modifying the TME in both pro and anti-tumorigenic manner. Subsequently, this review also discusses the convergence of immuno-metabolic environment in ferroptosis regulation, and eventually brings up research gaps in this context providing consequential and significant questions to explore for better understanding of the immuno-metabolic environment’s role in driving ferroptosis resistance for anti-cancer treatment progress.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12943-025-02337-3.
Keywords: Ferroptosis, Ferroptosis resistance, Metabolic reprogramming, Tumor microenvironment, Reprogrammed immune metabolic environment
Introduction
The cells in our body undergo periodic cell death to support tissue development and homeostasis. Cancer, however, is marked by uncontrolled cell division and growth, attaining a form of immortality by evading cell death mechanisms [1]. Cells can experience various forms of cell death depending on intra- or intercellular stress and environmental influences. These include apoptosis, necrosis, necroptosis, autophagy, pyroptosis, and ferroptosis [2]. Each type of cell death has implications for cancer development and anti-cancer therapy [3]. However, ferroptosis, a recently discovered non-apoptotic form of cell death, has major implications in carcinogenesis. On one side, ferroptosis has emerged as a novel strategy for reversing drug resistance in cancer [4]. On the other, cancer cells develop resistance to ferroptotic cell death. The ability to evade cell death, like ferroptosis, is one of the hallmarks of cancer, contributing to poor prognosis by promoting early relapse and drug resistance. This ferroptosis escape is regulated by various cellular processes including altered cell signaling pathways involving tumor-suppressors, pro-oncogenic factors, and pro-survival signaling [5]. In addition to these signaling pathways, metabolic and immune reprogramming significantly influences the progression and therapy resistance in aggressive cancers [6, 7]. However, these two traditional aspects of cancer biology namely tumor metabolism and tumor immunology not only regulate carcinogenesis separately but also unitedly affect cancer development and progression. They collectively form an immuno-metabolic environment, which affects carcinogenesis to a greater extent than they do separately. Furthermore, reprogramming of the immuno-metabolic environment enables the cancer cells to withstand the hostility at the tumor site, by protecting the cells from immunogenic regulations along with supplementing energy for rapid proliferation. For instance, high metabolic activity of cancer cells, particularly upregulated glycolysis results in large amount of lactate production thereby causing acidification of the tumor microenvironment (TME), which is referred to as TME acidosis [8]. As a consequence, normal cells die in the toxic TME, while the cancer cells survive and procure more space to proliferate, and parallelly facilitates the extracellular matrix (ECM) degradation, further enabling the cancer cells to evade and metastasise [9]. The TME acidosis also unifies with low oxygen levels (hypoxia), which upregulates and activates the hypoxia-inducible factor (HIF), a family of transcription factors, leading to activation of pro-cancerous metabolism and immune escape related genes [8, 10]. In addition, cancer associated fibroblasts (CAFs), a critical member of TME also contributes to the immuno-metabolic environment-induced cancer progression via the secretion of certain cytokines like IL- 6 [11]. Moreover, this reprogramed immuno-metabolic environment notably supports the escape of cancer cell death while promoting the death of non-cancerous, tumor killing cells. In this regard, the emergence of ferroptosis as a key vulnerability in cancer demands the essentiality of understanding its critical functioning, regulation, and resistance mechanism acquired by the cancer cells, not only by crucial traditional aspects of cancer biology, but also by the convergence of those aspects in the manner of immune-metabolic environment. Additionally, the association of tumor metabolism, tumor immunology, and mainly immune-metabolic environment with ferroptosis represents a potential therapeutic target for anti-cancer therapies to enhance anti-cancer immune response as well as to restore tumor suppressing cell signaling. This review therefore aims firstly to explore the mechanistic insights of cancer cell’s adaptations towards metabolic and immune responses to evade ferroptotic cell death, and then emphasizes on how the intrinsic metabolic changes and extrinsic immune interactions of cancer cells collectively confer ferroptosis resistance.
Ferroptosis
The term ferroptosis explains a morphologically, biochemically, and genetically distinct form of regulated cell death [12]. Morphologically, a cell dying from ferroptosis typically show distinct increase in the cellular membrane density. The plasma membrane as well as the membranes of cellular organelles especially mitochondrial membranes is ruptured due to increased density. Mitochondrial volume and cristate are also reduced as a consequence of ferroptosis [13]. In addition to this, ferroptosis has also been reported to induce endoplasmic reticulum (ER)-stress by activating increased pH and viscosity-mediated ER-stress signaling cascades [14]. ER-stress is further responsible for assisting ferroptosis through cation transport regulator 1 (CHAC1)-mediated glutathione (GSH) degradation [15]. Biochemically, ferroptosis is regarded as a non-apoptotic, iron-dependent form of cell death that is driven by the accumulation of lethal lipid peroxides [16]. Iron, which is an essential driver of metabolism, if not balanced correctly within the cell leads to reactive-oxygen species (ROS) generation through the Fenton reaction leading to ferroptosis induction in the cell [17]. In addition, the biochemical reactions of the cell that control the cellular metabolism are also the prime producers of free radicals or ROS [18]. The ROS once generated can attack the phospholipids (PLs) to produce phospholipid peroxides (PLOOH) and phospholipid hydroperoxyl radical (PLOO•). The presence of polyunsaturated fatty acids (PUFAs) in the membranes makes them vulnerable to ROS-mediated lipid peroxidation resulting in membrane damage [18]. Due to this, ferroptosis is regarded as a byproduct of cellular metabolism, which is being regulated by the cell for their survival.
Ferroptosis in cancer and anti-cancer therapy
The process of carcinogenesis is greatly affected by alterations in metabolic landscape of cancer cells. For cell proliferation, growth and survival, cancer cells mightily utilize both intra and inter cellular catabolic-anabolic pathways [6, 19, 20]. As a result of enhanced metabolic activity of the cancer cell they are immensely vulnerable to ROS-mediated cellular damage, which progresses to ferroptotic induction thereby fostering reduced cancer load. As a matter of fact, cancer research currently utilizes this self-destructive property of cancer cells to abrogate the disease through the induction of ferroptosis [4].
Ferroptosis as an anti-cancer therapy employs both the synergistic drug therapies as well as the nanoparticle-based therapies. Cisplatin treatment along with dihydroartemisinin, an antimalarial drug has been proven to reduce gastric cancer cell proliferation and invasion [21]. This drug synergy inhibits the glutathione peroxidase 4 (GPX4) activity and promote ferroptosis in these neoplastic cells, which suggest an effective perspective against cancer not only with cisplatin but with other chemotherapeutic drugs, even ferroptosis inducing drugs like sorafenib. Besides, the use of HDAC and PPAR inhibitors are quite popular for ovarian and breast cancer, however recent studies suggest an effectiveness of the HDAC and PPAR inhibitors against acute myeloid leukemia (AML) as well. The combinatorial treatment of HDAC inhibitor and PPAR agonist resulted in the reduced expression of leukemic stem cells in AML patients while providing no harm to the non-cancerous hematopoietic progenitor cells, and effectively induced ferroptosis in the leukemic cells, thereby supporting better survival rates of the patients [22]. Targeting cancer stem cells (CSCs) are one of the mandates against cancer eradication. CSCs are a predominant cause of early disease relapse, certain ferroptosis-inducing drugs like lapatinib, siramesine, sorafenib, and sulfasalazine are used to eliminate the population of CSCs and improve survival rates of cancer patients [23]. Additionally, doxorubicin, used to treat multiple cancer types like breast, ovarian, stomach etc. has the capacity to induce ferroptosis. Doxorubicin functions by intercalating into the DNA, breaks it down, and inhibits DNA repair leading to cell death. Furthermore, doxorubicin also has the ability to binds to mitochondrial DNA hampering mitochondrial functioning, which in turn surges the iron overload and results in mitochondrial ROS production-mediated lipid peroxidation and cell death [24]. Despite the fact that doxorubicin has the ability to induce ferroptosis in the cancer cells, doxorubicin also has adverse effects on non-cancerous cells. To overcome this, the application of nanomaterials has been employed to provide ferroptosis-based anti-tumor therapies, which has lesser side effects [25]. For instance, cisplatin’s efficacy to eliminate cancer cells is increased by iron-oxide carrier-mediated cisplatin release at the specific tumor site, without damaging the normal healthy cells [26]. In parallel, sorafenib’s ferroptosis inducing potential has been increased by implementing magnetic iron-oxide nanoparticles in hepatocellular carcinoma. These nanoparticles use magnetic hyperthermia to heat up the tumor site where the irons are released and Fenton reaction strengthens to generate ROS and thereby ferroptosis, both by sorafenib treatment and iron-oxides [27]. Apart from the operation of magnetic thermal energy, photothermal-based nanomaterials are also employed for tumor ablation. This technique engages glucose oxidase combined iron-oxide (Fe3O4) nanoparticles to target the tumor site. The glucose oxidase converts glucose to superoxides, used by Fe3+ ions to form even more ROS creating oxidative-stress and thereafter cellular ferroptosis of the tumor. In addition, this treatment considerably refines the anti-tumor immune response as well. Glucose oxidase-Fe3O4 complex-induced ferroptosis results in DAMP release within the tumor microenvironment (TME), which are taken up by the dendritic cells for helping T-cell maturation via antigen presentation, ultimately killing tumor cells and blocking tumor growth [28]. However, in spite of the promising advancement of anti-cancer therapies employing ferroptosis induction in the cancer cells, these neoplastic cells with high metabolic plasticity, overcome and counteract the oxidative damage-mediated ferroptotic cell death by certain protective mechanisms in the form of modified radical trapping antioxidant (RTA) systems, altered metabolic and anti-tumor immune response pathways, as well as reprogrammed immune-metabolic environment.
Tumor’s defense against ferroptosis
RTA system
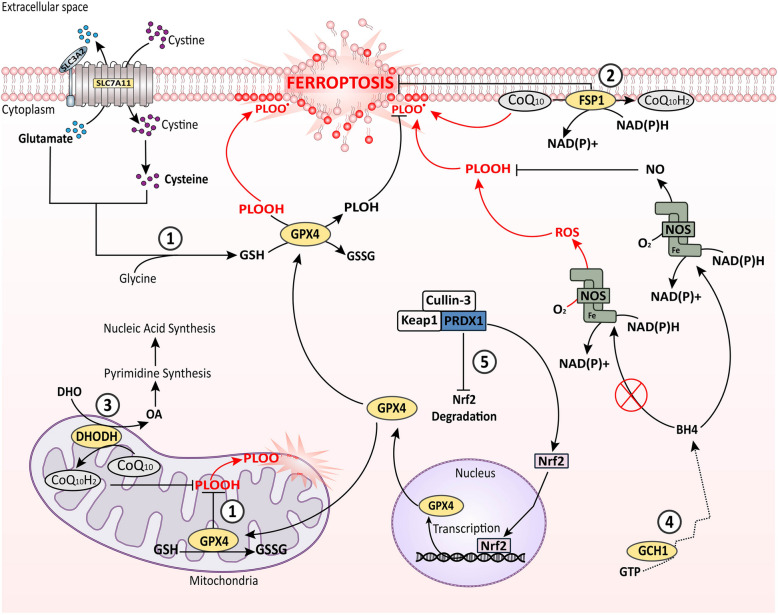
Oxidative damage is an intrinsic phenomenon in cellular physiology which essentially affects all the cell types. Every cell irrespective of its growth and proliferating capacity including normal, tumor, and non-tumor cells in the TME, as well as the tumor-associated cells, suffers from ROS-mediated oxidative damage, resulting in ferroptotic death. Hence, the tumor cell and those present in the TME forestall ferroptosis to persist with proliferation. On this account, RTA systems are the primary defense against ferroptosis and other forms of oxidative damages, which includes system Xc−-GPX4, ferroptosis suppressor protein 1(FSP1)-coenzyme Q10, dihydroorotate dehydrogenase (DHODH)-CoQ10, and GTP cyclohydrolase 1-tetrahydrobiopterin (GCH1-BH4) (Fig. 1) [29].
Fig. 1.
Outlook of ferroptosis with cellular RTA systems. ROS-mediated lipid-peroxidation is the prime cause of ferroptosis induction in the cell. Cells undergoing metabolic activity collaterally produce ROS, thereby inducing ferroptosis. To prevent this, cells employ several RTA systems; ① The GPX4 (cytoplasmic and mitochondrial) system, it utilizes GSH synthesised from cysteine to neutralize the ROS via a cycle of oxidation and reduction of GSH; ② The FSP1 (plasma membrane) system, and ③ the DHODH (mitochondrial) system, both utilizes CoQ10 to convert it into CoQ10H2 (non-toxic form), and subsequently reduce the ROS production. In addition, the FSP1 system uses NAD(P)H to neutralize ROS; ④ the GCH1/BH4 (cytoplasmic) system, in here GCH1 catalyzes the synthesis of the antioxidant BH4, which then binds to NOS and utilizes NAD(P)H to reduce O2 and produce NO thereby attenuating ROS generation; ⑤ PRDX1 system, it promotes the Nrf2 activity by inhibiting the Keap1-Cullin- 3 complex. PRDX1 bind to the Keap1-Cullin- 3, which is an inhibitor to Nrf2, thereby releasing and enabling Nrf2 entry into nucleus to activate of GPX4 gene transcription. All of these systems thereby effectively reduce PLOOH, and PLOO• generation, ultimately preventing ferroptosis. BH4: tetrahydrobiopterin, CoQ10: Coenzyme Q10, CoQ10H2: ubiquinol- 10, DHODH: dihydroorotate dehydrogenase, DHO: dihydroorotate, FSP1: ferroptosis suppressor protein 1, GCH1: GTP cyclohydrolase 1, GPX4: glutathione peroxidase 4, GSH: reduced glutathione, GSSG: oxidized glutathione, GTP: guanosine triphosphate, NADH: nicotinamide adenine dinucleotide (hydrogen), (reduced form), NAD(P)H: nicotinamide adenine dinucleotide phosphate, NO: nitric oxide, NOS: nitric oxide synthase, Nrf2: nuclear factor, OA: orate, PLOH: phospholipid alcohol, PLOOH: phospholipid peroxide, PLOO•: phospholipid hydroperoxyl radical, PRDX1: Peroxiredoxin- 1, ROS: reactive oxygen species, SLC3 A2: light chain subunit of System Xc− (cystine/glutamate antiporter), SLC7 A11: light chain subunit of System Xc− (cystine/glutamate antiporter)
The system Xc−GPX4 RTA system is the most well-known and major guardian of redox homeostasis maintenance that helps to neutralize the lipid peroxides within the cell [30]. On one end, the system Xc−, comprising of heavy chain SLC3 A2, and light chain SLC7 A11 subunit, functions as an antiporter of cystine/glutamate. For proper functioning of system Xc−, it requires fucosylation to be protected from degradation and destabilization. Receptor for activated C kinase 1 (RACK1) supplements the fucosylation of system Xc− by enhancing the expression of fructotransferase protein, FUT8, to stabilize and maintain its required cellular level within the cell [31]. Moreover, SLC7 A11 being a transmembrane protein and the core of system Xc−, its stability is also governed by structural proteins like caveolin- 1 (Cav- 1). Cav- 1 is a major structural and surface protein of lipid bilayers, which can both aid the structural stability of other transmembrane protein as well as mediate signal transduction [32]. Cav- 1 has been reported to interact with SLC7 A11 at cell membrane protecting it from proteasomal degradation, as well as protecting it at transcriptional level via AMPK/Nrf2 signaling [33]. Once system Xc− is stable, it takes up extracellular cystine in exchange for intracellular glutamate in a 1:1 ratio [30]. The internalized cystine is then reduced to cysteine, and then combined with glutamate and glycine to synthesize glutathione (GSH). The selenoprotein GPX4, on the other end, utilizes the GSH to reduce the esterified-oxidized fatty acids (FA) and cholesterol hyperoxides into respective non-toxic lipid alcohols to prevent ferroptotic death [34]. The dysfunctioning of system Xc−/GSH/GPX4 axis is a prime mediator of ferroptosis. Most cancer types have GPX4 overexpression to withstand ferroptosis [35–37]. Therefore, different classes of ferroptosis inducers like erastin, (Class I), RSL3 (Class II), FIN56, and CIL56 (Class III) are used to sensitize the cancer cells to ferroptotic death [38]. However, many cancers are hostile to agonists that inhibit the GPX4 system, which demonstrates the involvement of other cellular processes, mainly the antioxidant system in ferroptosis regulation.
Unlike GPX4, the FSP1 and DHODH systems, both of them prevent ferroptosis independent of GSH but in Coenzyme Q10 (CoQ10)-dependent manner [39]. Although both involve CoQ10, their cellular localization and functioning are different. FSP1, which was previously known as AIFM2 (Apoptosis-Inducing Factor Mitochondrial 2), is a plasma membrane protein but also found in the periphery of lipid droplets. At the plasma membrane, FSP1 reduces the redox-active CoQ10, also called ubiquinone- 10, to its non-toxic form CoQ10H2 (ubiquinol- 10), through its interactions with 6-hydroxyl-FAD, and NAD(P)H. Additionally, it also reduces Vitamin K to its hydroquinone (Vitamin K-H2) form by the same process. This reduced pool of CoQ10 and Vitamin K via FSP1 scavenges the lethal lipid peroxides thereby restricting ferroptosis in the cells [40]. On the other hand, DHODH, another flavin-dependent protein localized in the mitochondria, specifically in the outer face of inner mitochondrial membrane not only alters oxidative damage but simultaneously helps cancer cell proliferation. DHODH is a key enzyme in de novo pyrimidine nucleotide synthesis, where it catalyzes the formation of orotate from dihydroorotate (DHO), which are the initiators of pyrimidine production. During this process, DHODH utilizes CoQ10 as the electron acceptor, thereby reducing the potency of oxidative damage, and linking its function to with electron transport chain (ETC) and oxidative phosphorylation (OXPHOS) [41]. As a consequence, DHODH directly supports cancer progression by increasing pyrimidine yield needed for nucleic acid biosynthesis, enhancing the metabolic activity through OXHPHOS and conferring anti-ferroptosis phenotypes by decreasing the burden of redox-active molecules. Furthermore, it is also reported that DHODH and the mitochondrial GPX4 work together to suppress the propagation of lipid peroxidation at the mitochondrial membrane and reduce mitochondrial ferroptosis in cancer cells [41].
The fourth type of RTA system that participates in the defense against ferroptosis is the GCH1-BH4 system. GCH1 is a rate-limiting enzyme in the de novo biosynthesis of the anti-oxidant, BH4. In the presence of iron, BH4 acts as an essential cofactor of nitric oxide synthase (NOS) for the production of aromatic amino acids, neurotransmitters, and nitric oxide (NO) [42]. The binding of BH4 to NOS brings it to a coupled state, where it utilizes NAD(P)H as an electron donor to reduce O2 for the production of NO and L-citrulline as intermediate byproducts. The NO produced then results in the attenuation of ROS. However, the absence of BH4 or NOS dysfunction results in superoxide i.e., ROS generation due to uncoupling of electron transfer and O2 reduction from L-citrulline production [42]. Therefore, proper functioning of the GCH1/BH4 pathway effectively suppress ferroptosis by trapping ROS through NO production as well as by decreasing the CoQ10 and increasing CoQ10H2 concentration within the cells. Additionally, the GCH1-BH4 axis also causes lipid remodelling in cancer cells by selectively inhibiting the oxidation of specific PUFA-PLs like phosphatidylcholine shaving two PUFA tails, and it also works in parallel to GPX4 to counteract ferroptosis [43, 44]. Moreover, the ROS reducing activities of cancer cells are further associated with certain other peroxidases apart from the four-types discussed earlier. Peroxiredoxin- 1 (PRDX1), a highly functional RTA system, is overexpressed in cancer cells in order to bypass oxidative-stress-induced-damage [45–47]. PRDX1 exerts ferroptosis suppression by increasing Nrf2 activity to induce GPX4 functioning against ROS scavenging. Nrf2 is a crucial transcription factor that senses and protects cells from oxidative damage by regulating the expression of antioxidants. In contrast, Nrf2 is readily degraded by Keap1-Cullin- 3 complex-signaling at basal level. The Keap1-Cullin- 3 complex functions as the subunits of E3 ubiquitin ligase, and thus when Nrf2 is bound with Keap1-Cullin- 3 complex, is destined for ubiquitin-mediated proteasomal degradation. However, PRDX1 functions as a chaperone, and binds to the Cullin- 3-Keap1 complex to avoid the proteasomal degradation of Nrf2. This in turn enables Nrf2 to enter the nucleus and activate transcription of GPX4 gene to potentiate the ROS scavenging activity of the cell. [48]. Moreover, it in prostate cancer, it was reported that the oncogene holliday junction recognition protein favours the recycling of PRDX1, thereby providing anti-ferroptotic conditions [49].
In cellular physiology, however, the cell’s decision to undergo ferroptosis is dictated by the prevalence of one of the two cellular processes- PLOOH/PLOO• generation or RTA system over the other one. However, this decision in cancer cells is immensely affected by the metabolic plasticity of the cell and the TME, which comprises the tumor phenotypes regulating the immune cells.
Metabolic adaptation of cancer cells opposing ferroptosis
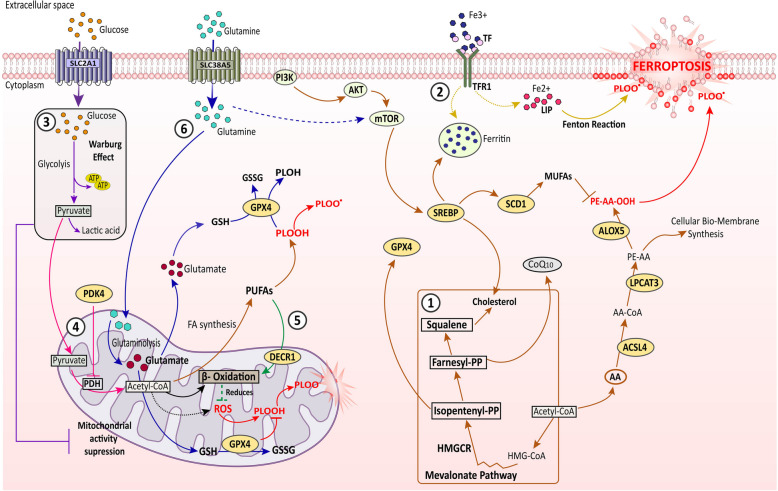
Metabolic reprogramming has long been regarded as a hallmark of cancer [50] since the discovery of “aerobic glycolysis” or Warburg effect in cancer cells, which explains upregulated anaerobic glycolysis producing large amounts of lactic acid from glucose in the presence of sufficient oxygen [51, 52]. Furthermore, altered lipid, amino acid as well as metal ion like iron metabolism proclaims much attention and ability to maintain cancer progression. These metabolic adaptations of cancer cells are linked with tumor metastasis, aggressiveness, and therapy resistance. Reprogrammed metabolism and metabolic signaling in cancer cells favor cell death escape not only by activating pro-survival processes but also by transforming the TME, thereby the anti-tumor immune response [53]. Tumor cell metabolism works with tumor immunity to develop cell death defense against apoptosis, autophagy and even ferroptosis [54]. Moreover, ferroptosis being an adjunct outcome of cellular metabolism, cancer cells have developed additional mechanisms to bypass the oxidative stress-induced-ferroptotic incidences to withstand the cell proliferation and disease progression (Fig. 2).
Fig. 2.
Involvement of cellular metabolism in ferroptosis regulation. ① the lipid metabolic pathways (brown arrows), this includes both ferroptosis inducing as well as ferroptosis suppressing conditions. On one end, PI3 K/Akt/mTOR mediated SREBP-SCD1 axis activation, promoting to MUFA generation, as well as the mevalonate pathway transduced cholesterol/CoQ10 axis functioning, both suppresses ferroptosis in cancer cells. On the other end, PUFA (AA) activation by ACSL4, esterification by LPCAT3, and thereby lipid peroxidation by ALOX5 promotes ferroptosis induction. ② the iron metabolism (yellow arrows), regulate ferroptosis via both pro-ferroptotic and anti-ferroptosis incidences. TF bound with circulating Fe3 + ions binds to TFR1 and is internalized, then either it contributes to LIP which promotes Fenton reaction mediated PLOOH and PLOO• generation, and ferroptosis, or can be stored as ferritin limiting redox active iron accumulation induced ferroptosis. ③ the glucose metabolism (purple arrows), which mainly exerts ferroptosis suppression. SLC2 A1 facilitated glucose uptake escalates Warburg effect, which reduces mitochondrial function-mediated ROS generation. ④ PDK4 action (pink arrows), it helps ferroptosis reduction in cancer cells by inhibiting PDH to catalyze pyruvate to acetyl-CoA. This acetyl-CoA would otherwise contribute to mitochondrial activity linked ROS generation, and PUFA synthesis. ⑤ DECR1 action (green arrows), it exerts anti-ferroptotic condition by accelerating β-oxidation of PUFAs to reduce ROS generation. ⑥ glutamate metabolism (blue arrows), SLC38 A5 assists the import of glutamine in the cell cytoplasm, which then enters into mitochondria, and undergo glutaminolysis to generate glutamate, which is then utilized by the GPX4 enzyme to reduce lipid peroxidation, and thereby reducing ferroptosis. Additionally, glutamate also activates mTOR signaling indirectly to further strengthen the lipid metabolic pathways for ferroptosis resistance. AA: arachidonic acid, AA-CoA: arachidonic acid-Coenzyme A, Acetyl-CoA: acetyl- Coenzyme A, ACSL4: Acyl-CoA synthetase long-chain family 4, ALOX5: arachidonate lipoxygenases 5, CoQ10: Coenzyme Q10, DECR1: dienoyl-CoA-reductase1, GPX4: glutathione peroxidase 4, GSH: reduced glutathione, GSSG: oxidized glutathione, HMGCR: HMG-CoA reductase, LIP: labile iron pool, LPCATs: lysophosphatidylcholine acyltransferase, MUFA: monounsaturated fatty acid, PLOH: phospholipid alcohol, PLOOH: phospholipid peroxide, PLOO•: phospholipid hydroperoxyl radical, PDH: pyruvate dehydrogenase, PDK4: pyruvate dehydrogenase kinase 4, PE-AA-OH: arachidonic acid lipid peroxide, PUFA: polyunsaturated fatty acid, SCD- 1: Stearoyl-CoA desaturase- 1, SLC2 A1: glucose transporter (GLUT1), SLC38 A5: amino acid transporter (glutamine) SREBP: sterol regulatory element-binding protein, TF: transferrin, TFR1: transferrin receptor 1. Direct lines: direct impact on the target molecule, Dotted lines: indirect impact on the target molecules, Black arrows: general cellular mechanisms irrespective of metabolic regulation of ferroptosis, Red arrows: ferroptosis commencing signal
Reprogrammed lipid metabolism
Amongst all metabolic pathways, altered lipid metabolism is an indispensable aspect of ferroptosis management in cancer cells. Since lipid peroxidation, specifically polyunsaturated fatty acid-phospholipid (PUFA-PL) peroxidation is the principal cause of ferroptosis, several lipid-metabolic enzymes like Acyl-CoA synthetase long-chain family (ACSLs), lysophosphatidylcholine acyltransferase (LPCATs) and lipoxygenases (LOXs) critically promotes ferroptosis [13]. ACSLs family member, ACSL4 is a biomarker of ferroptosis sensitivity, as it catalyzes the activation of PUFAs like arachidonic acid (AA) and eicosapentaenoic acid to form acyl-CoA-PUFA derivatives [55]. These ACSL4 enzyme products are further utilized by multiple LPCAT3 to esterify them into PUFA-PL like arachidonic acid containing phosphatidylethanolamines (AA-PE), and then they are incorporated into the bio-membranes of the cell membranes. The other family of enzymes, LOXs, especially the arachidonate lipoxygenases (ALOXs) uses the PUFA-PL as its substrate to mediate the formation of lipid radicals triggering ferroptosis [56]. However, the metabolic plasticity of cancer cells opposes the occurrence of ferroptosis by critically regulating the expression of several genes. ACSL4, which sensitizes cells to ferroptosis is frequently downregulated in various cancers including lung, and breast cancer [57, 58]. In contrast, ACSL3 drives cancer progression by activating monounsaturated fatty acids (MUFAs) like oleic acid (OA) and palmitoleic acid (PA) that displaces the PUFAs in the PL to reduce the sensitization of plasma and other cellular membrane to lipid oxidation [59]. Moreover, the expression of ALOX5 and ALOX12 is also reported to be downregulated in colorectal and bladder cancer helping the cells to escape ferroptosis [60, 61]. Notably, ALOX- 12 appears to be a vital player in p53-mediated ferroptosis in lymphoma. Cancer cells tend to overexpress SLC7 A11 and run GPX4 pathways for oxidative damage response. In p53-mediated ferroptotic tumor suppression, p53 enables ALOX12 activation through transcriptional suppression of SLC7 A11, which then enforces the accumulation of ROS [62]. However, the expression patterns of LPCATs vary with cancer types, and their role in tumorigenesis is also volatile in regard to pro and anti-oncogenic features [63].
Altered lipid metabolism being the central regulator of ferroptosis in cancer cells, the cellular activity and expression of certain master regulators of lipid metabolism itself influence ferroptotic phenotypes in cancer cells. One such regulator is the transcription factor, sterol regulatory element-binding proteins (SREBPs) [64]. The transcriptionally active form of SREBPs (nSREBPs) migrate to the nucleus and bind to sterol regulatory elements (SREs) in the promoter regions of its target gene like fatty acid synthase (FASN), acetyl-CoA carboxylase (ACC), ATP citrate lyase (ACLY), and stearoyl-CoA desaturase- 1 (SCD- 1), and initiate their transcription [65]. All these genes execute beneficial function in favour of cancer progression. FASN, a pro-carcinogenic lipid metabolism enzyme is overly expressed in various cancers. It is a key enzyme in the de novo lipid synthesis, in cancer it aids membrane biosynthesis-mediated cell proliferation through FA synthesis as well as cell metastasis and immune escape [66, 67]. Additionally, FASN has also been reported to promote MUFA and saturated FA levels in the membrane, thereby reducing lipid-peroxidation and ferroptosis [67, 68]. Recently, FASN has also been outlined to be critical player against sorafenib-induced ferroptosis in hepatocellular carcinoma cells [69]. In addition to FASN overexpression, upregulation of its transcription factor, SREBP is also been reported in cancer cells. SREBPs intensifies the sustained tumor growth through enhanced FA, cholesterol, and lipid droplets (LDs) biosynthesis [70]. The role of SREBP in persuading ferroptosis resistance is acquired by the activation of one of the major oncogenic cell signaling pathways; PI3 K/Akt/mTOR. Upon activation of this tumor-promoting pathway, it activates the SREBP1-mediated lipogenesis through SCD- 1. The SCD- 1 is an important enzyme in the biosynthesis of FAs. It catalyzes the formation of MUFAs like OA and PA from saturated FAs thereby repressing ferroptosis in cancer cells [71]. On the contrary, Akt-mediated suppression of SREBP2 maturation and activation poses ferroptosis resistance in colorectal cancer [72]. In these cells, B7H3, a transmembrane immunoregulatory protein, facilitates the Akt-mediated SREBP2 suppression and controls cholesterol metabolism, directing cell survival. Regardless of this finding, the association of SREBPs with iron metabolism presents an intertwined role of lipogenic regulators in iron-dependent ferroptotic cell death. SREBP2 has been reported to be involved directly in the transcription of transferrin to sequester intracellular iron pool to reduce ferroptosis in melanoma cells [73]. Furthermore, the SREBP-mediated ferroptosis resistance is associated with SLC38 A5-mediated glutamine uptake. SLC38 A5 is an amino-acid transporter that facilitates the import of amino acids like asparagine, glutamine, methionine, serine, and glycine but specifically transports glutamine in cancer cells. In the mitochondria, the glutamine is converted to glutamate via the glutaminolysis process, and glutamate is then exported to the cytoplasm to be utilized for GSH synthesis and GPX4 functioning. This SLC38 A5-mediated glutamine uptake activates the mTOR-SREBP1 axis to upregulate SCD1 expression and reduce ferroptosis in cancer cells, providing drug resistance [74]. Besides SREBPs, the synthesis of lipids is also regulated by lipin1 (LPIN1), which assists and promotes FA loading into the LDs in the cancer cells. LPIN1 is an important part of de novo lipogenesis which is responsible for the therapy resistance in colorectal cancer cells. It confers ferroptosis resistance in these cells by upregulating the LD formation and by facilitating the generation of diacylglycerols from phosphatidic acid via the ETS1-PTPN1-c-Src-CEBPβ pathway [75]. This association between LD and ferroptosis suppression in colorectal cancer cells present an able choice for reversing therapy resistance in the cells by targeting de novo lipogenesis. However, defeating ferroptosis is not always possible by the cancer cells. Some inherent properties of them increases the vulnerability towards ferroptosis. For instance, in chronic lymphocytic leukemia, the expression of lipid transporting protein apolipoprotein E (Apo E) is very high, which leads to ferroptosis induction in these cells [76]. Additionally, the copper binding property of Apo E increases sensitivity towards ferroptosis, because copper helps autophagic degradation of GPX4 reducing ROS scavenging capacities of these leukemia cells [76, 77]. In spite of this, aggressive subtype of chronic lymphocytic leukemia is insensitive towards Apo E, i.e., they have lower expression of Apo E protecting them from ferroptosis, surging carcinogenesis.
Cholesterol metabolism
Cholesterol, which is a crucial component of cell membrane also substantially affects ferroptosis apart from these core ferroptotic regulators like iron, glutamine, FAs, and glucose. Cholesterol is synthesized through the mevalonate cascade generating multiple sterol and non-sterol intermediates, favoring cancer progression and metastasis [78]. The vital intermediates like squalene and isoprenoid intermediates like isopentenyl pyrophosphate (IPP), geranyl-PP, and farnesyl-PP, generated during the synthesis of cholesterol are associated with tumor suppression due to their anti-ferroptotic function [79]. In lymphoma it was found that squalene protects the cells from oxidative ferroptotic cell death by altering the cellular lipid profile, acting as a metabolite of antioxidant properties [80]. Alternatively, IPP regulates the function of GPX4 antioxidants by promoting the maturation of selenocysteine t RNA (Sec-tRNA) [81]. Specific Sec-tRNA is then utilized in the translation of selenoprotein GPX4 to function as an RTA system [82, 83]. Additionally, IPP and subsequent farnesyl-PP also produce electron shuttling biomolecule CoQ10, which again participates in the RTA system of most cancers. Thus, inhibition of the mevalonate pathway through its rate-limiting enzyme HMGCR poses a potential ferroptosis inductive scenario in cancer cells.
Iron metabolism
The second form of metabolic pathway that represents the core of ferroptosis regulation system is iron metabolism [84]. Aberrant iron homeostasis is a characteristic feature of cancer cells, and this is regulated by various proteins that includes transferrin, transferrin receptor 1 (TFR1), and divalent metal transporter 1 (DMT1). Transferrin forms a complex with the circulating Fe3+ and binds to TFR1, then the whole complex is endocytosed. Within the endosome the Fe3+ is then converted to Fe2+, and then released into the labile iron pool (LIP) via divalent metal transporter 1 (DMT1) [85]. The LIP is the cytosolic pool of cheatable iron, comprising mainly of Fe2+ ions, which has a multifaceted function. On one hand, LIP functions as a source of iron storage and supplementation within the cells in the form of ferritin, and de novo biosynthesis of Fe-S clusters [86]. On the other hand, this LIP also drives Fenton reaction, where the redox active Fe2+ couples to superoxides like H2O2 to augment ROS generation, which eventually attacks PUFAs leading to ferroptosis [86]. Therefore, maintenance of iron homeostasis is critical for ferroptosis regulation. To balance the redox active iron pool, cancer cells upregulate the expression of ferritin as a protection against ROS generation, and thereby amplify cell proliferation and growth [87]. However, transferrin and TFR1 have a binate role in iron-dependent tumorigenesis. Import of iron by these two causes ferroptosis as well. Unsurprisingly, cancer overcomes this stress by upregulating RTA systems especially the GPX4 system [37, 88].
Glucose metabolism
The pioneer aspect of metabolic plasticity in cancer cells deals with metabolic reprogramming of glycolysis. The aerobic glycolysis in cancer cells serves as the core of energy production in the form of ATP and NADH [89]. It provides necessary metabolic intermediates for various biosynthetic processes to support tumorigenesis [90]. Eventually the altered glucose metabolism, i.e., Warburg effect, in the cancer cells prompts cell death escape including ferroptosis [91]. Moreover, the mitochondrial functioning in ferroptosis ties the link between the Warburg effect and ferroptosis. The mitochondrial enzyme DHODH associated with ETC Complex III converting CoQ10 into CoQ10H2, suppresses ferroptosis under the Warburg effect but in its absence generates ROS [92]. This phenomenon effectively attenuates the threat to lipid peroxidation-mediated ferroptotic death of cancer cells. Furthermore, the glucose- 6-phosphate produced during glycolysis, can also enter into the pentose phosphate pathway (PPP) leading to increased NAD(P)H generation. This NAD(P)H is further utilized by FSP1 enzyme to form CoQ10H2 and thereby restrict ferroptosis. Additionally, NAD(P)H indirectly supports the synthesis of GSH via donating hydrogen ions to cystine to generate cysteine, and eventually strengthening the GPX4 antioxidant machinery [93].
In parallel to glycolysis, glucose itself plays a critical role in ferroptosis regulation. Glucose starvation in the cells attenuates the prevalence of ferroptosis. Depleted glucose levels result in the activation of AMPK, which phosphorylates and inhibits the activity of ACC. In lipid metabolism ACC plays a crucial role in fatty acid biosynthesis, phosphorylation of ACC inactivates it, and halts lipid biosynthesis pathway, subsequently limiting the availability of PUFA for peroxidation reaction to that would otherwise trigger ferroptosis [94, 95]. The importance of glucose in ferroptosis regulation is further supported by the contribution of SLC2 A1-mediated glucose uptake in stimulating the SLC7 A11-inhibitor-induced ferroptosis in an ALOX5-dependent way [96]. Moreover, one of the end products of glycolysis, pyruvate, is either fermented to produce lactate or oxidized to form acetyl-CoA. The acetyl-CoA is further utilized in the citric acid cycle and subsequently FA and PUFA-oxidation meditated ferroptosis. However, pancreatic cancer cells have been reported to be exempted from this stress by pyruvate dehydrogenase kinase 4 (PDK4). PDK4 is a cytosolic enzyme, which efficiently phosphorylates and inhibits pyruvate dehydrogenase (PDH) activity. As a result, PDH is unable to oxidize pyruvate to form the acetyl-CoA, and thus, the entry of pyruvate in the mitochondria is restricted. Subsequently, the feasibility of ROS generation and ferroptosis induction is limited within the cancer cells, both by suppressed oxidation of pyruvate and reduced mitochondrial activity [97]. However, pyruvate oxidation to produce acetyl-CoA is also a vital phenomenon of cancer cells. Acetyl-CoA is a precursor molecule of FA synthesis which even though in the long run participates in ferroptosis induction through PUFA generation, is important for cell proliferation and membrane synthesis [98]. Beta-oxidation has been shown to suppress ferroptosis by utilizing acetyl-CoA to generate energy from PUFAs by the action of 2,4-dienoyl-CoA-reductase1 (DECR1) thereby limiting the availability of un-esterified PUFAs. In prostate cancer, the upregulated DECR1-mediated beta-oxidation of PUFAs not only protects the tumor from ferroptosis but also correlates with poor survival and treatment resistance [99, 100].
Tumor microenvironment in ferroptosis resistance
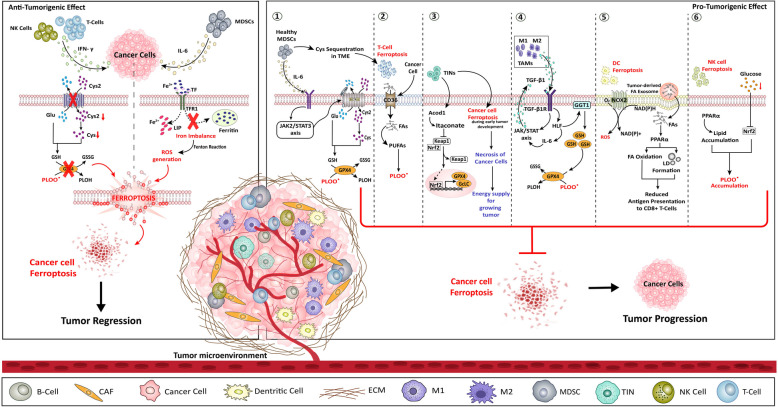
The immune surveillance of the cancer cells comprises of both immune and non-immune components that are present within the TME. This TME immensely regulates the tumorigenic phenotype of many cancers in pro-cancerous as well as in anti-cancerous manner [101]. The tumor niche that forms the TME consists of endothelial cells (EC), extracellular matrix (ECM), CAFs, tumor-associated macrophages (TAMs), tumor-infiltrated neutrophils (TINs), myeloid derived suppressor cells (MDSCs), regulatory T-cells (Treg), cytotoxic T-cells (Tc), natural killer cells (NKs), tumor-infiltrating lymphocyte B-cell, immune-inflammatory cells, etc. along with adipose cells, blood and lymphatic vascular networks [102]. Positive regulation of TME on tumorigenesis involves invasion and metastasis promotion, angiogenesis, tumor destruction evasion, enhanced pro-tumorigenic inflammation, and cell death resistance [103]. The association of TME with ferroptosis regulation encompasses all of the above factors (Fig. 3), which are also listed in Table 1.
Fig. 3.
Ferroptosis regulation by TME. The TME comprising of multiple immune cells regulate ferroptosis of cancer cells in both anti-tumorigenic as well as pro-tumorigenic. The anti-tumorigenic effect is induced only by T-Cells, NK cells, and MDSCs cells (left panel). Both T-cells and NK cells secretes IFN-γ, which thereby inhibits the system Xc−/GSH/GPX4 axis, thereby inducing ferroptosis in cancer cells. The MDSCs secretes IL- 6 and cause iron imbalance in the cancer cells, resulting in Fenton reaction-mediated ROS generation, lipid peroxidation and ultimately ferroptosis in cancer cells. Alternatively, the pro-tumorigenic regulation of ferroptosis is supported by MDSCs, T-Cells, TINs, TAMs, DCs, and NK cells (right panel). ① MDSCs secretes IL- 6 to activate JAK2/STAT3 pathway which then activates system Xc−/GSH/GPX4 axis to resist ferroptosis in cancer cells. Additionally, MDSCs sequesters Cys in the TME and make the T-Cells undergo ferroptosis. ② T-cells ferroptosis is moreover facilitated by cancer cell regulated CD36-mediated FA uptake in T-cells, which further favours PUFA generation leading to peroxidation of lipids. ③ TINs aids ferroptosis resistance in cancer cells by Acod1-mediated release of Keap1 inhibition on Nrf2 that promotes the transcriptional activation of the GPX4 and GcLc antioxidant gene to enhance ROS scavenging in the cells. In addition, TINs also induce ferroptosis of cancer cells at early stage and thereby necrosis of cancer cells to provide energy to proliferating cancer cells. ④ TAMs secrete TGF-β1, which activates HLF/GGT1 axis to enhance GSH production and reduce ferroptosis prevalence in cancer cells. The HLF activation in TINs further releases IL- 6 creating a positive feedback loop of TGF-β1 signaling via JAK/STAT axis. ⑤ DCs itself undergo ferroptosis via NOX2-mediated ROS generation, and also losses its function by the effect of tumor-derived exosome induced PPARα signaling, which reduces the antigen presenting capacity by LD accumulation and FA oxidation. ⑥ NK cells also themselves undergo ferroptosis within the TME, PPARα-mediated lipid accumulation and, the glucose deprived TME- Nrf2 inhibition collectively cause lipid peroxide accumulation, and thereby ferroptosis of NK cells. Acod1: aconitate decarboxylase 1, CAF: cancer associated fibroblast, Cys: cysteine, Cys2: Cystine, CD36: Clusters of differentiation 36 (fatty acid transporter), DC: dendritic cells, ECM: extracellular matrix, FA: fatty acid, GcLc: glutamate-cysteine ligase catalytic subunit, GGT1: gamma-glutamyl transferase 1Glu: glutamate, GPX4: glutathione peroxidase 4, GSH: glutathione, GSSG: oxidized glutathione, HLF: hepatic leukemia factor, IFN- γ: interferon-γ, IL- 6: interleukin- 6, JAK2: janus kinase 2, Keap1: kelch-like ECH-associated protein 1, LD: lipid droplet, LIP: labile iron pool, MDSCs: myeloid-derived suppressor cells, M1: macrophage type 1, M2: macrophage type 2, NADH: nicotinamide adenine dinucleotide (hydrogen), (reduced form), NAD(P)H: nicotinamide adenine dinucleotide phosphate NK: natural killer cells, NOX2: NADPH-oxidase Nrf2: nuclear factor erythroid 2-related factor, PLOH: phospholipid alcohol, PLOO•: phospholipid hydroperoxyl radical, PPARα: peroxisome proliferator-activated receptor alpha, PUFAs: polyunsaturated fatty acids, ROS: reactive oxygen species, TIN: tumor-infiltrating neutrophils TGF-β1: transforming growth factor-beta1, TGF-β1R: transforming growth factor-beta receptor type 1, STAT3: signal transducer and activator of transcription 3, SLC3 A2: light chain subunit of System Xc- (cystine/glutamate antiporter), SLC7 A11: light chain subunit of System Xc- (cystine/glutamate antiporter), Blue lipid bilayer: T-cell membrane, Yellow lipid bilayer: DC membrane, Black arrows: TME cell signaling, Red arrows: ferroptosis commencing signal
Table 1.
Summary table of immune cells regulating ferroptosis
| Cell type | Effect on ferroptosis in cancer cells | Key mediators | Reference(s) |
|---|---|---|---|
| TAMs | Promote ferroptosis resistance | TGF-β1 → HLF → GGT1 → GSH elevation | [104] |
| TINs | Promote ferroptosis resistance | Acod1→ Nrf2 → GPX4/GcLc → ROS scavenging | [105, 106] |
| MDSCs | Promote ferroptosis resistance | IL-6 → JAK2/STAT3 → System Xc- → GSH elevation | [107, 108] |
| T-Cells | Promote ferroptosis induction |
i. IFN- γ → System Xc-/GSH/GPX4 inhibition → ROS and LLPs accumulation ii. ACSL4 → PUFA generation → LLPs accumulation |
[109, 110] |
| NK Cells | Promote ferroptosis induction | IFN- γ → System Xc-/GSH/GPX4 inhibition → ROS and LLPs accumulation | [111] |
| DCs | Promote ferroptosis resistance | NOX2 → ROS accumulation → DC ferroptosis → antigen presentation reduced → anti-tumor immune escape | [112] |
TAMs
Among all the immune cells present within the TME, iron metabolism and ferroptosis are mostly studied in TAMs. TAMs secrete ferritin which promotes both, the cancer cell growth and metastasis, and hence their abundance is associated with poor prognosis of cancer [113, 114]. In murine model of breast carcinoma, Heme oxygenase (HO- 1) inhibition through Zinc-Protoporphyrin IX, not only reduced cancer growth, but also promoted a macrophage phenotype switching from M2 (pro-tumorigenic) to M1 (anti-tumorigenic) within the TAMs [115].
Under environmental stresses within the TME, K-Ras is released in the form of DAMPs during autophagy-dependent ferroptotic cell death of cancer cells. The released K-Ras are then taken up by the nearby macrophages which subsequently undergo STAT3-mediated FA oxidation and alter the polarization to M2 subtype, which not only promotes tumor progression but also correlates with reduced survival of cancer patients [116]. This phenomenon displays the duality of ferroptosis in cancer biology, and exclusively ascertains the fact that ferroptosis induction for tumor regression is not always beneficial for the host. However, this TAM polarization form M1 to M2, can be reversed by targeting lipid transport regulatory protein, apolipoprotein C1 (APOC1). Inhibition of APOC1 in TAM clusters of hepatocellular carcinomas resulted in increased iron accumulation and RTA system downregulation leading to increased M1 polarization via ferroptotic pathways, eventually reducing the tumor burden [117]. Iron level of TME can also alter M1/M2 polarization as enhanced iron level endorse the M1 polarization through increased ROS and p53 acetylation [118]. M1 sequester iron in ferritin within LIP, while M2 macrophages internalize, process and discharge iron at greater rates and have superior LIP [119]. Usually, M1 macrophages are more resistant to ferroptosis than M2 due to higher level of iNOS expression in M1 phenotype, despite the similar levels of GPX4 and ACSL4 in M1 and M2 subsets [120]. Enhanced iNOS expression resulted in higher yields of NO• and free radicals which interact with lipid peroxides and efficiently quench their assimilation into the membrane.
In breast cancer, especially in triple negative breast cancer (TNBC), TAM exhibits pro- tumorigenic role by averting ferroptosis. The communication between TNBC and the surrounding TAMs results in the secretion of TGF-β1 by TAMs. TGF-β1 binds to TGF- β1R on the TNBC cells, and subsequently activates hepatic leukemia factor (HLF). Active HLF then transactivates the production of IL- 6 as well as the ferroptosis suppressing protein, gamma-glutamyl transferase 1 (GGT1). IL- 6 produced by the TNBC cells promotes TGF-β1 secretion by TAMs through JAK-STAT, and GGT1 foster ferroptosis resistance in TNBC cells via enhanced intracellular GSH production. GGT1 is a plasma membrane bound protein that catalyses the breakdown of extracellular GSH to its component amino acids which are internalized by the cell to form intracellular GSH and thereby utilized by GPX4 for ferroptosis as well as oxidative burden suppression [104]. Additionally, the dual role of ferroptosis in both immunosuppression and stimulation in tumor immunity is exemplified by the type of cell undergoing ferroptosis.
TINs
The immunosuppressive tumor-infiltrating neutrophils (TINs) inflict a significant role in ferroptosis surveillance. TIN shields breast cancer cells against ferroptosis and promotes metastasis. In metastasizing breast cancer cells, TINs upregulate the activity of aconitate decarboxylase 1 (Acod1). Acod1 is primarily an enzyme found in mitochondria which catalyses the formation of itaconate from Cis-aconitate. This enzyme is emerged as a key regulator of immunometabolism during infection and inflammation. The itaconate thus formed, removes the Keap1 suppression on Nrf2 and activates it. The transcription factor Nrf2, then initiates the transcription of RTA system genes like GPX4 and glutamate-cysteine ligase catalytic subunit (GcLc) to bypass oxidative damage-mediated ferroptotic response. The presence of this TIN/Acod1/anti-ferroptosis axis assists the cells to survive in the hostile TME. In addition, this association also suggests a possible approach for synergistic anti-cancer therapy targeting TIN regulation through Acod1 inhibition [105, 106]. Interestingly, a pro-cancerous role of ferroptosis in cooperation with neutrophils has also been reported. Neutrophils in glioblastoma foster tumor growth by triggering necrosis [121]. Although necrosis is a form of cell death, but it supports tumor progression under insufficient blood supply or nutrient starvation [122]. During the development of tumors, early tissue damage recruits the neutrophils, which transfer certain myeloperoxidases containing granules in some of the growing tumor cells. In these tumor cells, myeloperoxidases induce lipid peroxidation and membrane disintegration but promotes overall tumor progression. It was reported that neutrophil-mediated ferroptosis of glioblastoma cells results in necrosis development during the advancement of tumor and that’s why ferroptosis has also recently been regarded as a form of regulated necrosis [121, 123].
MDSC
Myeloid-derived suppressor cells (MDSCs) are known for their immunosuppressive function on T-cells and natural killer (NK) cells. Abundance of MDSCs in tumors is usually linked with a poor prognosis, however cross-talk among TAMs and MDSCs within TME further heightens both of their suppressive functions [124, 125]. IL- 6 is markedly associated with MDSC accumulation; however, IL- 6 plays dual role within TME with respect to ferroptosis [107]. IL- 6 released from the myeloid cells can endorse ferroptosis resistance in cancer cells by enhancing the transcription of system Xc− via JAK2/STAT3 signaling [108]. Conversely, IL- 6 can also induce ferroptosis by disrupting the iron homeostasis [126]. This duality of IL- 6 is because of its capability to possess both cis and trans signaling. The anti-ferroptotic role of IL- 6 is associated with its cis-signaling pathway where it binds to cell surface receptor activating the downstream pathways. However, the pro-ferroptotic role is implicated by the trans-signaling pathways where IL- 6 binds to soluble transferrin, thereby compromising their homeostasis but enhancing iron-induced toxicity [127]. Moreover, IL- 6 secreted by CAFs, augments the production of MDSCs, which in turn strengthens ferroptosis resistance of cancer cells [128, 129].
In addition, ferroptosis of MDSCs play a crucial role in anti-cancer immunosuppression. During MDSC ferroptosis, it releases oxidized phospholipids which curbed T-cell expansion. MDSCs may also indirectly arbitrate inhibitory effects on T-cells through other ferroptosis-promoting mechanisms. It is reported that absence of a functional system Xc− within naive T-cells makes them extremely vulnerable as they are unable to import cysteine which is essential for their function and generation of GSH [130]. Hence, as MDSCs can import cysteine from TME but lack required machinery to export, they sequester the available cysteine and deprive T-cells [131]. Therefore, MDSCs render T-cells highly sensitive to ferroptosis by indirectly depleting their GSH production potential.
T-cell
CD8 + T-cell and CD4 + T helper (Th) cell subsets have different metabolic programs linked to their effector function and differentiation. Ferritin deprivation or CD71 blockade during T-cell activation, play pivotal role in activation and proliferation of CD8 + T-cells and CD4 + T-cells [132, 133]. Th1 cells are sensitive to low iron or iron withdrawn conditions. Additionally, both Th17 and T follicular helper (Tfh) cells depend on iron for their differentiation, while Th17 cells also require iron for IL- 17 production [134]. Among CD4 subsets, Treg contain less labile iron compared to Th1 cells, in spite of less CD71 expression [135]. Wu et al. showed that with low levels of free iron, Tregs can express higher levels of ferritin heavy chain (FTH) than conventional T-cells, prerequisite for maintenance of a stable intracellular iron homeostasis [136]. The high FTH expression also affects FOXP3 transcription by modulating ten-eleven translocation (TET) dioxygenase activity. TET dioxygenase demethylates the FOXP3 locus and thereby maintain high FOXP3 Treg levels. Therefore, targeting the iron regulation destabilizes Tregs in the TME and thus induce ferroptosis. Interestingly, iron promotes glycolytic activity in T-cells [137], and mTORC1 signaling [132], which suppresses ferroptosis. However, Feng et al. revealed that iron homeostasis in Tregs was regulated by MEK-ERK signaling which regulate CD71 expression and was independent of mTORC1 signaling [138].
Mostly, ferroptosis of immune cells causes immunosuppression whereas cancer cells undergoing ferroptosis promotes anti-tumor immunity [139]. On one end, secretion of interferon-γ (IFN- γ) by activated CD8 + T-cells within the TME kills the cancer cells through ferroptosis. The secreted INF-γ inhibits the system Xc−/GSH/GPX4 axis along with an increase in ACSL4 activity to expand the lipid-ROS burden [109, 110]. Opposingly, cancer cells undergoing ferroptosis release DAMPs like high mobility group box 1 (HMGB1), certain AA metabolites, and Prostaglandin E2 (PGE2) to survive and escape antitumor immune response [140]. Additionally, Bi-allelic deletion of GPX4 significantly reduced CD8 + T-cell numbers in the periphery while keeping CD4 + T-cells and Tregs intact, revealing differential need and sensitivity of ferroptosis within different T-cell subsets [141]. The CD8 + T-cell suppression in the TME is further associated with the overexpression of selenoprotein I (SELENOI) in ovarian cancer. SELENOI, a transmembrane protein, not only exerts anti-tumor immune response suppression but also reduces the expression of ferroptosis inducing ACSL4 and promotes Akt phosphorylation to support ovarian cancer cell proliferation and survival. Platin-based chemotherapeutic drug resistance in ovarian cancer is also linked to upregulated action of SELENOI. However, the core cellular pathways involved in SELENOI associated anti-ferroptosis features in ovarian and other cancer cells remains to be elucidated [142].
NK Cells
Natural Killer (NK) cells are innate lymphoid cells with highly cytotoxic and pro-inflammatory properties that play a central role through both direct killing of tumors and the release of pro-inflammatory cytokines that further stimulate anti-tumor immune responses [143]. Like T-Cells, NK cells also release IFN- γ to induce ferroptosis and kill cancer cells via system Xc-/GSH/GPX4 axis inhibition [111]. Moreover, recent reports reveal that ferroptosis itself may play a key role in NK cell dysfunction. NK cells become highly glycolytic in an mTOR dependent manner during their activation and increases CD71 expression for iron uptake [144]. The peroxisome proliferator-activated receptor (PPAR) pathway is dysregulated in NK cells, leading to lipid accumulation and worse anti-tumor responses [145]. Hence, an iron-starved, lipid-rich TME skews NK cell to a dysfunctional state that dampens the anti-tumor immunity. In addition, when NK cells are co-cultured with ovarian cancer patient ascites become dysfunctional with augmented expression of lipid peroxidation and ferroptosis genes. Conversely, activation of Nrf2 dependant anti-oxidation pathway rescued their function [146].
DC
Dendritic cells (DCs) are professional antigen presenting cells that migrate to tissues and process soluble and cell-associated antigens and presents to T-cells. Studies on ferroptosis in DCs are limited. Absence of GPX4 failed to alter total splenic DC population in mice [147]. Several evidences suggest that lipid oxidation and ferroptosis might be a critical regulator of DC functions. ROS production within DC by NADPH-oxidase (NOX) complex, NOX2 derive lipid peroxidation and membrane damage in endosomes may result in antigen leakage in cytosol hampering efficient antigen cross-presentation to CD8 + T-cells [112]. This situation may be reversed by α-tocopherol which acts as ROS scavenger. Tumor-derived exosomes carrying excessive FA to DCs resulted in decreased antigen presentation through the PPARα-mediated metabolic reprogramming associated to greater lipid droplet biogenesis and FA oxidation [148]. Tumor-derived factors upsurge lipid peroxidation in mouse DCs to trigger the transcription factor XBP1 which enhances lipid droplet production and diminish the antigen presentation [149].
B-cells
B-cell subsets capture a critical place within the TME, yet their role is indefinite and multifaceted [150]. Moreover, these B-cell lymphocytes may have differential sensitivities to ferroptosis, with GPX4 being required for B1 and marginal zone B-cell survival but extraneous in the development of B2 cells [151]. However, very little is known about B-cells in ferroptosis regulation of cancer cells, which requires more research.
Conclusively, in spite of this, the role of ROS, iron accumulation and thereby ferroptosis in tumorigenesis remains elusive. However, future research will decipher how different sources and mechanisms of lipid peroxidation in different immune cells depend upon iron and counter balance their functions within the TME.
Convergence of immuno-metabolic environment in ferroptosis regulation
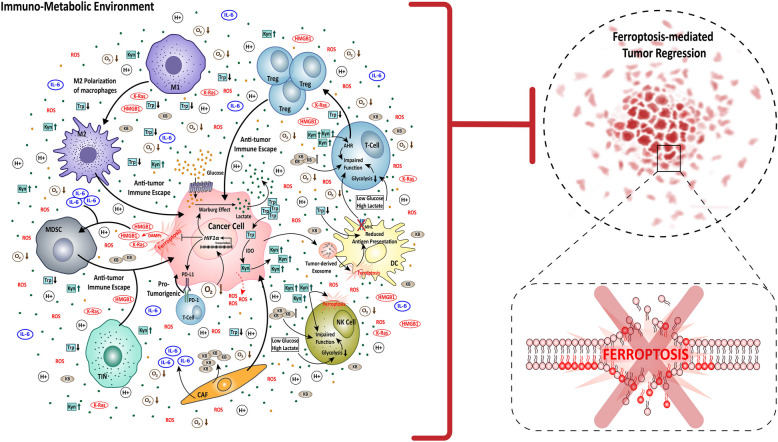
Metabolic reprogramming and immunogenic modification are the two core cellular systems that underlies the robustness of carcinogenesis. However, the convergence of these two core processes of cancer cells has greater impact on cancer development and progression. The integration as well as the cross-talk between tumor metabolism and immunogenic activity give rise to an immuno-metabolic environment at the tumor site within the TME. This immune-metabolic environment is often reprogrammed, and is governed by several factors such as nutrient and oxygen availability, vasculature system, and the heterocellular i.e., tumor and non-tumor cell metabolism and immune response. Consequently, the immuno-metabolic environment also reinforces ferroptosis regulation and resistance in the cancer cells (Fig. 4).
Fig. 4.
The network of Immuno-metabolic environment in ferroptosis resistance. The immuno-metabolic environment corresponds to the cross-talk between cancer cells, stromal cells like CAFs, and immune cells like T-cell, Tregs, DCs, M1 macrophages, M2 macrophages, MDSCs, and TINs. The cancer cells take up glucose and Trp from the TME, to produce lactate via Warburg effect and Kyn rich by Trp degradation, thereby making the TME acidic and Kyn-rich. Additionally, the CAFs release KBs in the TME, increasing the KB content high, and due to lower levels of oxygen, the TME is inherently hypoxic. All these four factors (high acid, Kyn and KB content with low oxygen) collectively promotes cancer progression. Parallelly, the cancer cells undergoing ferroptosis also favours pro-tumorigenic condition via the release of DAMPs like HMGB1 and K-Ras. All these factors together result in inhibition of ferroptosis-mediated tumor regression by promoting anti-tumor immune escape of the cancer cells. Mechanistically, the glucose deprived, lactate and Kyn rich condition impairs the function of T-cells, DC, NK, cells by generating Tregs, reducing antigen presenting capacity, inducing ferroptosis, respectively and additionally intensifies M2 polarization of macrophages for anti. The hypoxic condition activates HIF1α transcription, leading to HIF1α signaling upregulation, which thereby increases Warburg effect and PD-L1/PD- 1 signaling, and decreases ferroptosis in cancer cells. The DAMPs released by cancer cells as well as IL- 6 released by CAFs, activates MDSCs, thereby promoting anti-tumor immune escape. The TINs also promote cancer progression. Overall, the immuno-metabolic environment created by the cancer-non-cancer cell cross talk promotes cancer by inhibiting ferroptosis-mediated tumor regression and ferroptosis of cancers. AHR: aryl hydrocarbon receptor, CAF: cancer associated fibroblast, DAMPs: damage-associated molecular patterns, DC: dendritic cells, HIF1α: hypoxia-inducible factor 1-alpha, HMGB1: high mobility group box 1, IDO: indoleamine 2,3-dioxygenase, IL- 6: interleukin- 6 KB: ketone bodies, K-Ras: Kirsten rat sarcoma viral oncogene homolog, Kyn: kynurenine, MHC: Major histocompatibility complex, MDSC: myeloid-derived suppressor cells, M1: macrophage type 1, M2: macrophage type 2, NK: natural killer cells, ROS: reactive oxygen species, PD- 1: programmed cell death protein 1, PD-L1: programmed death-ligand 1, TIN: tumor infiltrating neutrophil, Treg: regulatory T-cells, Trp: tryptophan, Narrow arrows: signaling, Broad arrows: activation of the molecule/cell, Dotted arrows: indirect effect
The upregulated metabolic activity of cancer cells transforms the TME into a hypoxic acidic, and ketone body rich space. Hypoxia is an inherent property of tumor progression, while rapidly proliferating cancer cells enhances glucose utilization, which ultimately leads to lactate production-mediated acidification of the TME. These conditions are harmful to normal cells; however, cancer cells have evolved themselves to survive such conditions via several machineries. One of which is the activation of HIF proteins like HIF1α, and HIF2α. HIF1α is not only helpful for angiogenesis, cell proliferation, metastasis of cancer cells, but it has also been reported to facilitate ferroptosis resistance [152]. In solid tumors, HIF1α activates the transcription of SLC7 A11 gene, and eventually increases its activity in the cancer cells. SLC7 A1 is the transporter of glutamate, therefore high levels of glutamate inside the cell strengthens the functioning of classic anti-ferroptosis axis, the System Xc−/GSH/GPX4 system [152]. Besides, HIF1α also promotes ferroptosis resistance by escalating lactate production. HIF1α binds to glycolytic genes like GLUT1, HK1, and LDHA, upsurging Warburg effect and reducing ROS generation. Moreover, this lactate-rich TME demonstrates an immune-metabolic feedback loop regulating ferroptosis and immune escape. On end, cancer cells take up the lactate from TME via hydroxycarboxylic acid receptor 1 (HCAR1)/monocarboxylate transporter 1 (MCT1). Subsequently, SREBP1/SCD1 axis is activated to produce MUFAs instead of PUFAs by inhibiting AMPK signaling, leading to ferroptosis resistance in the cancer cells [153]. On the other end, the metabolic waste like lactic acid and hypoxic conditions impairs immune cells functioning, particularly T-Cell and NK cells. NK cells require glucose as their primary source of metabolic fuel, and glucose starvation in TME due to high metabolic demands of cancer cells, often leads to oxidative damage in NK cells impairing their anti-tumor functions [146]. Similarly in T-Cells, glucose deprivation results in suppressed glycolysis and mitochondrial function resulting in dysfunctional T-cell anti-tumor effect. All these factors ultimately foster immune escape of cancer cells [154]. However, in parallel to the impact of cancer cell metabolism on HIF1α activation in TME, the ferroptotic cancer cells releasing DAMPs in the form of HMGB1 also activates HIF1α signaling. Here, HIF1α leads to the transcriptional activation of CD274 (also known as PD-L1) which in turn causes immune suppression. CD274 is an inhibitory receptor of T-Cell, functioning as immune checkpoint, and thus due to increased expression of CD274 T-cell loses its anti-tumor effect, permitting tumor progression [111]. In addition, the PD- 1/PD-L1 axis is also influenced by receptor-tyrosine kinase known as TYRO3. The TYRO3 represses the anti–PD- 1/PD-L1 triggered ferroptosis of cancer cells by promoting Nrf2 expression and antioxidant functioning. Along with this TYRO3 also alters the M1/M2 macrophage ratio in TME, which ultimately establishes a pro-tumorigenic TME devoid of ferroptosis prevalence [155].
Moreover, apart from glucose, the immuno-metabolic environment also involves cholesterol and amino acid metabolism in the ferroptosis-mediated regulation of carcinogenesis. Cholesterol upregulates the expression of FA transporter protein CD36, thereby increasing FA-uptake in the CD8 + effector T-cells. Increased FA uptake then drives the peroxidation of lipids, and induces ferroptotic cell death of CD8 + T-cells, uplifting immune escape through T-cell depletion with the TME [156]. In regard to amino acid metabolism, tryptophan (Trp) metabolism, especially, Trp degradation pathway has been proven to be crucial for cancer cell proliferation, metastasis and immune escape [157]. Several enzymes such as indoleamine 2,3-dioxygenase (IDO), tryptophan 2,3-dioxygenase (TDO), and the intermediate byproduct Kynurenine (Kyn), involved in Trp degradation, play a major role in anti-tumor immune surveillance and tumor progression. Within the TME, cancer cells upregulate the IDO induced rapid degradation of Trp, and increases the amount of Kyn. This IDO/Kyn axis play dual role in supporting carcinogenesis. The Kyn effectively suppresses ferroptosis in cancer cells [158]. SLC7 A11, the core of cystine/glutamate antiporter overexpressed in cancer cells, imports Kyn in IDO1 non-expressing cancer cells. Later on, the imported Kyn is converted to its downstream derivatives like 3-hydroxyanthranilic acid (HAA) and 3-hydroxykynurenine (3HK), Nicotinamide adenine dinucleotide (NAD +), which then activates Nrf2 signaling to facilitate ROS scavenging and inhibit ferroptosis in the cancer cells [158]. Alternatively, L-Kyn alters the anti-cancer immunogenic response in the TME. It reduces the proliferation of CD8 + T-cells and promotes the generation of Tregs through aryl hydrocarbon receptor (AHR) signaling, leading to effector T-cell exhaustion within the TME. In addition, the IDO-L-Kyn-AHR axis also impairs NK cell and macrophage functioning, as well as engages PD- 1/PDL- 1-mediated cancer cell proliferative and survival pathways [159]. Moreover, L-Kyn has also been reported to act independent of AHR signaling and induce ferroptosis of NK cells intensifying immune escape of cancer cells [160]. Therefore, L-Kyn critically regulate carcinogenesis through a bidirectional ferroptosis associated feedback loop, and possess a potential target for anti-cancer therapies.
Despite the fact that cancer cells tend to rely on glucose creating an acidic environment at TME, the non-classical characteristic of TME is the enrichment of ketone bodies (KB). KB are produced during lipid metabolism. Even though cancer cells upregulates both glucose and lipid metabolism, KBs are produced by the stromal cells like CAFs [161]. The CAFs are reported to have dysfunctional mitochondria, which forces them to undergo ketogenesis to generate ATP. Eventually, CAFs secretes excess KBs with in the TME, which is not only toxic to normal cells but to cancer cells as well. These KBs within the TME has been reported to cause oxidative stress and ferroptosis [162]. Interestingly, a lipid bilayer structural protein, Cav- 1 has been reported to be associated with KB-mediated ferroptosis regulation in cancer cells [33]. The toxicity of KBs downregulates Cav- 1, which otherwise would provide anti-ferroptotic protection to the cancer cells through its interaction with SLC7 A11 and AMPK/Nrf2 signaling, enhancing the antioxidant machinery. Cav- 1 also has been reported to inhibit excess Fe2+ accumulation through FTH activity upregulation [163]. Moreover, the presence of Cav- 1 in lipid rafts, which are the key players in receptor mediated cell signaling, may also regulate immune cell types in the TME via TGF-β signaling, but limited information is available in this regard.
Furthermore, apart from tumor’s self-build immuno-metabolic environment within the TME, anti-cancer drug treatment also interestingly builds an ferroptosis escaping environment for the cancer cells. Drug treatment creates an even greater hypoxic condition within the TME to cause tumor reduction. However, the cross-talk between tumor cells, tumor-EC, and adipocytes attributes to the regeneration of tumor post drug discontinuation as well as during drug treatment. SCD1, the well-known ferroptosis regulator, and FABP4 has been proven to be typical for tumor regrowth. SCD1 present in cancer cells reduces ferroptosis via MUFA generation, while FABP4 activity of tumor-EC cells and adipocytes leads to LD production in hypoxic condition. As, a result PUFA generation is reduced and leading to reduced lethal lipid peroxides formation, which in turn resist ferroptosis and allows tumor relapse [164]. Moreover, a compelling role of ferroptosis in vasculature modification within the TME has also been reported. The high metabolic activity in TME facilitates pro-oxidative TME development, leading to the release of pro-angiogenic factor by both CAFs and cancer cells. This pro-oxidative TME is believed to be a result of ferroptosis which ultimately gives rise to EC hyperactivation and vessel-like structure formation mimicking angiogenesis [165].
Immuno-metabolic environment, a prospective intervention against ferroptosis-resistant cancer cells
The iron-dependent cell death, ferroptosis, being an aftermath of cell metabolism, provides a new perspective in cancer research. Today, multiple ferroptosis-induction-based anti-cancer treatments are being used to effectively reverse therapy resistance in several cancers, but cancer cell acquiring ferroptosis resistance significantly limits their therapeutic potential. In parallel, ferroptosis itself displays certain paradoxes in carcinogenesis, which interferes with ferroptosis-based cancer elimination. For instance, cancer cells undergoing ferroptosis releases certain types of DAMPs like K-Ras, HMGB1, and PGE2 that promotes the M2 polarization of macrophages, increases the expression of PD-L1 immune checkpoint and effector immune cells infiltration to effectively create an immunosuppressive TME, which in turn favours cancer progression. In addition, it is also reported that TINs induce ferroptosis based lipid peroxidation in glioblastoma cells which results in necrosis of tumor cells in their early development. This ferroptosis-associated necrosis thereby supports the development and progression of cancer, most certainly by nutrient supplementation at the onset of carcinogenesis. Therefore, all these findings inclusively suggests that it is not only that the cancer cells regulate ferroptosis, but the ferroptosis, an outcome of cell signaling in the form of cell death, can itself regulate the development and progression of cancer like a feedback mechanism. Moreover, the feedback loops further exist in immuno-metabolic environment of cancer cells within the TME. Glucose metabolism plays a major role in transforming the immuno-metabolic environment. The release of lactate in the TME due to Warburg effect, on one hand helps ferroptosis escape of cancer cells, and alternatively kills the population of T-cells and NK cells by ferroptosis to promote cancer progression. Alongside, cholesterol metabolism also transforms the immuno-metabolic environment in favour of cancer cells by inducing ferroptosis of cytotoxic T-cells while helping cancer cell proliferation. However, apart from these conventional metabolic regulators, the feedback loop of Trp, specifically its catabolic derivative L-Kyn, and the catalyzing enzyme IDO, holds immense scope in anti-cancer therapies, as it effectively impacts some crucial and most successful regulators of cancer. L-Kyn firstly, favours ferroptosis resistance in cancer cells by upregulating Nrf2, the master regulator of antioxidant machinery of cells. Secondly, it impairs both T-cell and NK cell anti-tumor response in the TME, and thirdly, it promotes PD- 1/PD-L1 mediated cancer progression. Therefore, Trp/IDO/L-Kyn axis is speculated to be a potent anti-cancer therapeutic approach, as it influences master regulators of cancer progression. Moreover, application of immune checkpoint inhibitors (CKIs) in association with Trp metabolism inhibitor, or the development of combinatorial therapies including CKIs, Trp metabolism modulators, as well as ferroptosis inducers, would also be favourable for effective eradication of cancer cells. Additionally, the implementation of CKIs like Anti-PD 1 (Nivolumab), along with glucose uptake inhibitors like Glutor, which is a selective inhibitor of GLUT 1/2/3, possess potential therapeutic values to reverse cancer progression. This Anti-PD 1/Glutor synergy will not only negatively affect the ferroptosis resistance abilities of cancer cells, but will also affect the TME in an anti-tumorigenic manner, suggesting a TME-targeted therapy for anti-cancer treatment. Furthermore, the paradox of ferroptosis, especially the release of DAMPs and their effect in the TME, considerably portrays ferroptosis as a form of immunogenic cell death (ICD). Conventionally, ICD refers to a type of regulated cell death triggered by immune responses, resulting in release of DAMPs by the dying cells, which then acts as antigens to further enhance the immune responses. Opposingly, ferroptosis as ICD of cancer cells associates with anti-tumor immunosuppression. This phenomenon limits the standalone application of ferroptosis inducing drugs to reduce cancer, but suggests a striking anti-tumor therapeutic approach by utilizing ferroptosis inducers in combination with DAMP-recognizing receptor inhibitors. This approach will allow targeted removal of cancer cells without the impairment of anti-tumor effector cells like T-cells and NK cells within the TME. Concurrently, synergistic therapeutic approaches involving immune cell type specific regulators along with a combination of ferroptosis inducer and metabolic drugs might also be advantageous against ferroptosis resistance of cancer cell following tumor regression. For instance, MDSCs targeting drugs, like the ones that inhibit the requirement of MDSCs at tumor site can be combined with glutamine metabolic drugs like glutaminase- 1 inhibitors to effectively reverse ferroptosis resistance, and immune escape, targeting reduced cancer burden. Parallelly, the utilization of FA transport inhibitors, like CD36 inhibitor (SMS121) combined with PPARα agonists like fenofibrate and clofibrate would strengthen ferroptosis inducing anti-cancer therapies, by effectively killing cancer cells while protecting beneficial immune cells like T-cells and DCs.
Moreover, the interdependence of immuno-metabolic environment surpasses the traditional metabolic cues translating the distinct involvement of structural protein like Cav- 1. This lipid raft membrane protein has been reported to have multifunctional role in signal transduction, but its association with immuno-metabolic environment to regulate ferroptosis in cancer cells and alter immune cell types in TME, presents novel approaches for anti-cancer treatment. Especially, the involvement of Cav- 1 with KBs points towards the development of TME-targeted anti-cancerous therapies. KBs or ketogenic diet has already been proven to be effective for the reduction of tumor burden. Accordingly, the administration of KBs combined with Cav- 1 inhibitors will possibly dominate cancer eradication by promoting anti-cancerous TME via suppressing ferroptosis and anti-tumor immune response escape as well as by inhibiting pro-oncogenic signal transduction in cancer cells.
Therefore, the immuno-metabolic ferroptosis resistance network in the cancer cells unfolds an unconventional and futuristic anti-cancerous therapeutic landscape, directing towards a multi-layered destruction policy against cancer.
Future perspective and conclusion
The emergence of cancer cell’s sensitivity towards ferroptosis although have led to an immense growth in anti-cancer therapies, the understanding of ferroptosis resistance in cancer cells yet have unexplored sections. For instance, cancer cells require high amount of cholesterol for cell proliferation, but this cholesterol rich condition results in oxidation of cholesterols leading to oxidative stress. Thus, it poses the question whether cholesterol oxidation has any effect on the ferroptosis prevalence in cancer cells, and if that so, how does these ever-proliferating cancer cells balance the oxidized and non-oxidized levels of cholesterol to resist ferroptosis. Simultaneously, the cholesterol-rich TME has been reported to be toxic for T-cells, but macrophages survive and undergo M2 polarization, therefore the mystery remains with the ability of macrophages to regulate cholesterol oxidative to survive in TME and thereby promote cancer progression. Additionally, the immuno-metabolic cross-talk between cancer cells and macrophages also put forwards the question whether they have any cooperative effect in neutralizing cholesterol oxidation in their favour but triggering T-cell toxicity. Furthermore, the functional role of lipid transporters in TAMs to cause iron accumulation, and thereby favouring M1 polarization needs more understanding of how a lipid transporter protein cancer regulate iron uptake or release in the cells. The question further transcends to whether any bioactive lipid molecule associates with iron to modulate iron transport and if so whether it can be utilized for directing iron accumulation in cancer cells to induce ferroptosis by targeting the immuno-metabolic environments. Moreover, having discussed the therapeutic approaches of anti-cancer drug treatment to eliminate cancer cells, it intrigues to explore whether the treatment of these drug alters or transforms the metabolic as well as the immuno-metabolic environment into ferroptosis resisting landscape to promote disease relapse. This area of anti-cancer drug induced alterations is less explored but crucial for understanding the plasticity of cancer cells and undoubtedly is a pristine domain of carcinogenesis to target for disease ablation.
In conclusion, ferroptosis has emerged as a new weapon in both targeting cancer cell’s therapy resistance as well as in cancer cell’s ability to resist anti-cancer therapies. Therefore, the deciding factor for pro-tumorigenic or anti-tumorigenic TME establishment is governed by dominance of one over the other, i.e., cancer cells over anti-tumor cells or vice versa. However, the flexibility of cancer cells in terms of metabolism, immune response and immuno-metabolic environment provides them an advantage to survive the hostile TME, while multiple challenges are encountered by anti-tumor cells to withstand the same TME. Overcoming the earlier discussed challenges will set the stage for efficient redemption of anti-cancer therapies, particularly ferroptosis based therapies to use cancer’s own weapon against itself.
Supplementary Information
Acknowledgements
We acknowledge the Department of Life Sciences, Presidency University for providing the infrastructural facility.
Authors’ contributions
S.B. wrote the major portion of the manuscript and made all the figures. S. Banerjee wrote a portion of the paper. V.S. critically suggested to improve the manuscript. S.M. conceptualized, reviewed, and corrected the manuscript..
Funding
The work is supported by the Departmental grant DBT-BUILDER: BT/INF/22/SP/45088/2022.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tuomela K, Ambrose AR, Davis DM. Escaping Death: How Cancer Cells and Infected Cells Resist Cell-Mediated Cytotoxicity. Front Immunol. 2022;13: 867098. 10.3389/fimmu.2022.867098/bibtex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang D, Kang R, Berghe TV, Vandenabeele P, Kroemer G. The molecular machinery of regulated cell death. Cell Res. 2019;29:347–64. 10.1038/S41422-019-0164-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peng F, Liao M, Qin R, Zhu S, Peng C, Fu L, Chen Y, Han B. Regulated cell death (RCD) in cancer: key pathways and targeted therapies. Signal Transduct Targeted Ther. 2022;7(17):1–66. 10.1038/s41392-022-01110-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang C, Liu X, Jin S, Chen Y, Guo R. Ferroptosis in cancer therapy: a novel approach to reversing drug resistance. Mol Cancer. 2022. 10.1186/S12943-022-01530-Y. [DOI] [PMC free article] [PubMed]
- 5.Sever R, Brugge JS. Signal Transduction in Cancer. Cold Spring Harb Perspect Med. 2015. 10.1101/cshperspect.a006098. [DOI] [PMC free article] [PubMed]
- 6.Faubert B, Solmonson A, DeBerardinis RJ. Metabolic reprogramming and cancer progression. Science. 2020;368. 10.1126/science.aaw5473. [DOI] [PMC free article] [PubMed]
- 7.Tsai CH, Chuang YM, Li X, Yu YR, Tzeng SF, Teoh ST, Lindblad KE, Di Matteo M, Cheng WC, Hsueh PC, Kao KC, Imrichova H, Duan L, Gallart-Ayala H, Hsiao PW, Mazzone M, Ivanesevic J, Liu X, de Visser KE, Lujambio A, Lunt SY, Kaech SM, Ho PC. Immunoediting instructs tumor metabolic reprogramming to support immune evasion. Cell Metab. 2023;35:118-133.e7. 10.1016/j.cmet.2022.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corbet C, Feron O. Tumour acidosis: from the passenger to the driver’s seat. Nat Rev Cancer. 2017;17:577–93. 10.1038/nrc.2017.77. [DOI] [PubMed] [Google Scholar]
- 9.Tafech A, Stéphanou A. On the Importance of Acidity in Cancer Cells and Therapy. Biology (Basel). 2024;13. 10.3390/biology13040225. [DOI] [PMC free article] [PubMed]
- 10.Chen Z, Han F, Du Y, Shi H, Zhou W. Hypoxic microenvironment in cancer: molecular mechanisms and therapeutic interventions. Signal Transduct Target Ther 2023;8. 10.1038/s41392-023-01332-8. [DOI] [PMC free article] [PubMed]
- 11.Wu X, Tao P, Zhou Q, Li J, Yu Z, Wang X, Li J, Li C, Yan M, Zhu Z, Liu B, Su L. IL-6 secreted by cancer-associated fibroblasts promotes epithelial-mesenchymal transition and metastasis of gastric cancer via JAK2/STAT3 signaling pathway. Oncotarget. 2017;8:20741–50. 10.18632/oncotarget.15119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B, Stockwell BR. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–72. 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen X, Comish PB, Tang D, Kang R. Characteristics and Biomarkers of Ferroptosis. Front Cell Dev Biol. 2021;9. 10.3389/fcell.2021.637162. [DOI] [PMC free article] [PubMed]
- 14.Zhao C, Yu D, He Z, Bao L, Feng L, Chen L, Liu Z, Hu X, Zhang N, Wang T, Fu Y. Endoplasmic reticulum stress-mediated autophagy activation is involved in cadmium-induced ferroptosis of renal tubular epithelial cells. Free Radic Biol Med. 2021;175:236–48. 10.1016/j.freeradbiomed.2021.09.008. [DOI] [PubMed] [Google Scholar]
- 15.Chu YM, Wang TX, Jia XF, Yang Y, Shi ZM, Cui GH, Huang QY, Ye H, Zhang XZ. Fuzheng Nizeng Decoction regulated ferroptosis and endoplasmic reticulum stress in the treatment of gastric precancerous lesions: A mechanistic study based on metabolomics coupled with transcriptomics. Front Pharmaco.l 2022;9. 10.3389/fphar.2022.1066244. [DOI] [PMC free article] [PubMed]
- 16.Li J, Cao F, Yin HL, Huang ZJ, Lin ZT, Mao N, Sun B, Wang G. Ferroptosis: past, present and future. Cell Death & Dis. 2020;11(2):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Formanowicz D, Radom M, Rybarczyk A, Formanowicz P. The role of Fenton reaction in ROS-induced toxicity underlying atherosclerosis - modeled and analyzed using a Petri net-based approach. Biosystems. 2018;165:71–87. 10.1016/j.biosystems.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 18.LJ Su, JH Zhang, H Gomez, R Murugan, X Hong, D Xu, F Jiang, ZY Peng. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxid Med Cell Longev. 2019. 10.1155/2019/5080843. [DOI] [PMC free article] [PubMed]
- 19.CA Lyssiotis, D Nagrath. Metabolic Reprogramming and Vulnerabilities in Cancer. Cancers (Basel). 2019;12. 10.3390/cancers12010090. [DOI] [PMC free article] [PubMed]
- 20.Lane AN, Higashi RM, Fan TWM. Metabolic reprogramming in tumors: Contributions of the tumor microenvironment. Genes Dis. 2020;7:185–98. 10.1016/j.gendis.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang H, Lu C, Zhou H, Zhao X, Huang C, Cheng Z, Liu G, You X. Synergistic effects of dihydroartemisinin and cisplatin on inducing ferroptosis in gastric cancer through GPX4 inhibition. Gastric Cancer. 2024. 10.1007/S10120-024-01574-7. [DOI] [PubMed] [Google Scholar]
- 22.Zhou H, Qin D, Xie C, Zhou J, Jia S, Zhou Z, Qiu Y, Xu B, Zha J. Combinations of HDAC Inhibitor and PPAR Agonist Induce Ferroptosis of Leukemic Stem Cell-like Cells in Acute Myeloid Leukemia. Clin Cancer Res. 2024;30:5430–44. 10.1158/1078-0432.ccr-24-0796. [DOI] [PubMed] [Google Scholar]
- 23.Elgendy SM, Alyammahi SK, Alhamad DW, Abdin SM, Omar HA. Ferroptosis: An emerging approach for targeting cancer stem cells and drug resistance. Crit Rev Oncol Hematol. 2020;155. 10.1016/j.critrevonc.2020.103095. [DOI] [PubMed]
- 24.Abe K, Ikeda M, Ide T, Tadokoro T, Miyamoto HD, Furusawa S, Tsutsui Y, Miyake R, Ishimaru K, Watanabe M, Matsushima S, Koumura T, Yamada KI, Imai H, Tsutsui H. Doxorubicin causes ferroptosis and cardiotoxicity by intercalating into mitochondrial DNA and disrupting Alas1-dependent heme synthesis. Sci Signal. 2022;15. 10.1126/scisignal.abn8017. [DOI] [PubMed]
- 25.Shen Z, Song J, Yung BC, Zhou Z, Wu A, Chen X. Emerging Strategies of Cancer Therapy Based on Ferroptosis. Adv Mater. 2018;30. 10.1002/ADMA.201704007. [DOI] [PMC free article] [PubMed]
- 26.Ma P, Xiao H, Yu C, Liu J, Cheng Z, Song H, Zhang X, Li C, Wang J, Gu Z, Lin J. Enhanced Cisplatin Chemotherapy by Iron Oxide Nanocarrier-Mediated Generation of Highly Toxic Reactive Oxygen Species. Nano Lett. 2017;17:928–37. 10.1021/acs.nanolett.6b04269. [DOI] [PubMed] [Google Scholar]
- 27.Tang Q, Wang Y, Yan B, Zhang J, Wang T, Fang Y, Ye Z, Zhang N, Zhang N, Wu Z, Fan H, Lyu Y, Liu X, Wu R. Intracellular Magnetic Hyperthermia Sensitizes Sorafenib to Orthotopic Hepatocellular Carcinoma Via Amplified Ferroptosis. ACS Nano. 2024;18. 10.1021/acsnano.4c09500. [DOI] [PubMed]
- 28.Li Y, Chen J, Xia Q, Shang J, He Y, Li Z, Chen Y, Gao F, Yu X, Yuan Z, Yin P. Photothermal Fe3O4 nanoparticles induced immunogenic ferroptosis for synergistic colorectal cancer therapy. J Nanobiotechnology. 2024;22. 10.1186/S12951-024-02909-3. [DOI] [PMC free article] [PubMed]
- 29.Žalytė E. Ferroptosis, Metabolic Rewiring, and Endometrial Cancer. International J Mol Sci 2024. 2023;25:75. 10.3390/ijms25010075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jyotsana N, Ta KT, DelGiorno KE. The Role of Cystine/Glutamate Antiporter SLC7A11/xCT in the Pathophysiology of Cancer. Front Oncol. 2022;12. 10.3389/fonc.2022.858462. [DOI] [PMC free article] [PubMed]
- 31.Yan A, Wu H, Jiang W. RACK1 inhibits ferroptosis of cervical cancer by enhancing SLC7A11 core-fucosylation. Glycoconj J. 2024;41. 10.1007/s10719-024-10167-6. [DOI] [PubMed]
- 32.Bhowmick S, Biswas T, Ahmed M, Roy D, Mondal S. Caveolin-1 and lipids: Association and their dualism in oncogenic regulation. Biochim Biophys Acta Rev Cancer. 2023;1878. 10.1007/s10719-024-10167-6. [DOI] [PubMed]
- 33.Liang X, Tian R, Li T, Wang H, Qin Y, Qian M, Fan J, Wang D, Cui H-Y, Jiang J. Integrative insights into the role of CAV1 in ketogenic diet and ferroptosis in pancreatic cancer. Cell Death Discov. 2025;11:139. 10.1038/s41420-025-02421-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weaver K, Skouta R. The Selenoprotein Glutathione Peroxidase 4: From Molecular Mechanisms to Novel Therapeutic Opportunities. Biomedicines. 2022;10. 10.3390/biomedicines10040891. [DOI] [PMC free article] [PubMed]
- 35.Kim JW, Min DW, Kim D, Kim J, Kim MJ, Lim H, Lee JY. GPX4 overexpressed non-small cell lung cancer cells are sensitive to RSL3-induced ferroptosis. Sci Rep. 2023;13. 10.1038/S41598-023-35978-9. [DOI] [PMC free article] [PubMed]
- 36.Wei S, Yu Z, Shi R, An L, Zhang Q, Zhang Q, Zhang T, Zhang J, Wang H. GPX4 suppresses ferroptosis to promote malignant progression of endometrial carcinoma via transcriptional activation by ELK1. BMC Cancer. 2022;22. 10.1186/s12885-022-09986-3. [DOI] [PMC free article] [PubMed]
- 37.Chen H, Peng F, Xu J, Wang G, Zhao Y. Increased expression of GPX4 promotes the tumorigenesis of thyroid cancer by inhibiting ferroptosis and predicts poor clinical outcomes. Aging. 2023;15:230–45. 10.18632/aging.204473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ghoochani A, Hsu EC, Aslan M, Rice MA, Nguyen HM, Brooks JD, Corey E, Paulmurugan R, Stoyanova T. Ferroptosis Inducers Are a Novel Therapeutic Approach for Advanced Prostate Cancer. Cancer Res. 2021;81:1583–94. 10.1158/0008-5472.can-20-3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee J, Roh JL. Unleashing Ferroptosis in Human Cancers: Targeting Ferroptosis Suppressor Protein 1 for Overcoming Therapy Resistance. Antioxidants (Basel). 2023;12. 10.3390/antiox12061218. [DOI] [PMC free article] [PubMed]
- 40.Zeng F, Chen X, Deng G. The anti-ferroptotic role of FSP1: current molecular mechanism and therapeutic approach. Molecular Biomedicine. 2022;3. 10.1186/s43556-022-00105-z. [DOI] [PMC free article] [PubMed]
- 41.Mao C, Liu X, Zhang Y, Lei G, Yan Y, Lee H, Koppula P, Wu S, Zhuang L, Fang B, Poyurovsky MV, Olszewski K, Gan B. DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature. 2021;593:586–90. 10.1038/S41586-021-03539-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gonçalves DA, Jasiulionis MG, de Melo FHM. The Role of the BH4 Cofactor in Nitric Oxide Synthase Activity and Cancer Progression: Two Sides of the Same Coin. Int J Mol Sci. 2021;22. 10.3390/ijms22179546. [DOI] [PMC free article] [PubMed]
- 43.Kraft VAN, Bezjian CT, Pfeiffer S, Ringelstetter L, Müller C, Zandkarimi F, Merl-Pham J, Bao X, Anastasov N, Kössl J, Brandner S, Daniels JD, Schmitt-Kopplin P, Hauck SM, Stockwell BR, Hadian K, Schick JA. GTP Cyclohydrolase 1/Tetrahydrobiopterin Counteract Ferroptosis through Lipid Remodeling. ACS Cent Sci. 2020;6:41–53. 10.1021/acscentsci.9b01063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soula M, Weber RA, Zilka O, Alwaseem H, La K, Yen F, Molina H, Garcia-Bermudez J, Pratt DA, Birsoy K. Metabolic determinants of cancer cell sensitivity to canonical ferroptosis inducers. Nat Chem Biol. 2020;16:1351–60. 10.1038/s41589-020-0613-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hao YY, Xiao WQ, Zhang HN, Yu NN, Park G, Han YH, Kwon T, Sun HN. Peroxiredoxin 1 modulates oxidative stress resistance and cell apoptosis through stemness in liver cancer under non-thermal plasma treatment. Biochem Biophys Res Commun. 2024;738: 150522. 10.1016/j.bbrc.2024.150522. [DOI] [PubMed] [Google Scholar]
- 46.Sun HN, Ma DY, Guo XY, Hao YY, Jin MH, Han YH, Jin X, Kwon T. Peroxiredoxin I and II as novel therapeutic molecular targets in cervical cancer treatment through regulation of endoplasmic reticulum stress induced by bleomycin. Cell Death Discovery. 2024;10:1–11. 10.1038/s41420-024-02039-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ding C, Fan X, Wu G. Peroxiredoxin 1 – an antioxidant enzyme in cancer. J Cell Mol Med. 2016;21:193. 10.1111/jcmm.12955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Song Y, Wang X, Sun Y, Yu N, Tian Y, Han J, Qu X, Yu X. PRDX1 inhibits ferroptosis by binding to Cullin-3 as a molecular chaperone in colorectal cancer. Int J Biol Sci. 2024;20:5070. 10.7150/ijbs.99804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lai W, Zhu W, Wu J, Huang J, Li X, Luo Y, Wang Y, Zeng H, Li M, Qiu X, Wen X. HJURP inhibits sensitivity to ferroptosis inducers in prostate cancer cells by enhancing the peroxidase activity of PRDX1. Redox Biol. 2024;77: 103392. 10.1016/j.redox.2024.103392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 51.Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J Gen Physiol. 1927;8:519–30. 10.1085/JGP.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Warburg O. On the origin of cancer cells. Science. 1956;123:309–14. 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 53.Farese RV, Currie E, Schulze A, Zechner R, Walther TC. Cellular Fatty Acid Metabolism and Cancer. Bone. 2014;23:1–7. 10.1016/j.cmet.2013.05.017.cellular. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mbah NE, Lyssiotis CA. Metabolic regulation of ferroptosis in the tumor microenvironment. J Biol Chem. 2022;298. 10.1016/j.jbc.2022.101617. [DOI] [PMC free article] [PubMed]
- 55.Yuan H, Li X, Zhang X, Kang R, Tang D. Identification of ACSL4 as a biomarker and contributor of ferroptosis. Biochem Biophys Res Commun. 2016;478:1338–43. 10.1016/j.bbrc.2016.08.124. [DOI] [PubMed] [Google Scholar]
- 56.Feng S, Tang D, Wang Y, Li X, Bao H, Tang C, Dong X, Li X, Yang Q, Yan Y, Yin Z, Shang T, Zheng K, Huang X, Wei Z, Wang K, Qi S. The mechanism of ferroptosis and its related diseases. Molecular Biomedicine. 2023;4. 10.1186/S43556-023-00142-2. [DOI] [PMC free article] [PubMed]
- 57.Zhang Y, Li S, Li F, Lv C, Kai Yang Q. High-fat diet impairs ferroptosis and promotes cancer invasiveness via downregulating tumor suppressor ACSL4 in lung adenocarcinoma. Biol Direct. 2021;16. 10.1186/S13062-021-00294-7. [DOI] [PMC free article] [PubMed]
- 58.Sha R, Xu Y, Yuan C, Sheng X, Wu Z, Peng J, Wang Y, Lin Y, Zhou L, Xu S, Zhang J, Yin W, Lu J. Predictive and prognostic impact of ferroptosis-related genes ACSL4 and GPX4 on breast cancer treated with neoadjuvant chemotherapy. E Bio Med. 2021;71. 10.1016/j.ebiom.2021.103560. [DOI] [PMC free article] [PubMed]
- 59.Yang Y, Zhu T, Wang X, Xiong F, Hu Z, Qiao X, Yuan X, Wang D. ACSL3 and ACSL4, Distinct Roles in Ferroptosis and Cancers. Cancers (Basel). 2022;14. 10.3390/cancers14235896. [DOI] [PMC free article] [PubMed]
- 60.Liu T, Xu X, Li J, Bai M, Zhu W, Liu Y, Liu S, Zhao Z, Li T, Jiang N, Bai Y, Jin Q, Zhang Y, Zheng Y, Zhou S, Zhan S, Sun Y, Liang G, Luo Y, Chen X, Guo H, Yang R. ALOX5 deficiency contributes to bladder cancer progression by mediating ferroptosis escape. Cell Death Dis. 2023;14. 10.1038/S41419-023-06333-7. [DOI] [PMC free article] [PubMed]
- 61.Weng S, Liu Z, Xu H, Ge X, Ren Y, Dang Q, Liu L, Zhang J, Luo P, Ren J, Han X. ALOX12: A Novel Insight in Bevacizumab Response, Immunotherapy Effect, and Prognosis of Colorectal Cancer. Front Immuno. 2022;13. 10.3389/fimmu.2022.910582. [DOI] [PMC free article] [PubMed]
- 62.Chu B, Kon N, Chen D, Li T, Liu T, Jiang L, Song S, Tavana O, Gu W. ALOX12 is required for p53-mediated tumour suppression through a distinct ferroptosis pathway. Nat Cell Biol. 2019;21:579–91. 10.1038/S41556-019-0305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lu Y, Liang H, Li X, Chen H, Yang C. Pan-cancer analysis identifies LPCATs family as a prognostic biomarker and validation of LPCAT4/WNT/β-catenin/c-JUN/ACSL3 in hepatocellular carcinoma. Aging. 2023;15:4699–713. 10.18632/AGING.204723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ma T, Du J, Zhang Y, Wang Y, Wang B, Zhang T. GPX4-independent ferroptosis-a new strategy in disease’s therapy. Cell Death Discov. 2022;8. 10.1038/S41420-022-01212-0. [DOI] [PMC free article] [PubMed]
- 65.Amemiya-Kudo M, Shimano H, Hasty AH, Yahagi N, Yoshikawa T, Matsuzaka T, Okazaki H, Tamura Y, Iizuka Y, Ohashi K, Osuga JI, Harada K, Gotoda T, Sato R, Kimura S, Ishibashi S, Yamada N. Transcriptional activities of nuclear SREBP-1a, -1c, and -2 to different target promoters of lipogenic and cholesterogenic genes. J Lipid Res. 2002;43:1220–35. 10.1194/jlr.m100417-jlr200. [PubMed] [Google Scholar]
- 66.Vanauberg D, Schulz C, Lefebvre T. Involvement of the pro-oncogenic enzyme fatty acid synthase in the hallmarks of cancer: a promising target in anti-cancer therapies. Oncogenesis. 2023;12. 10.1038/S41389-023-00460-8. [DOI] [PMC free article] [PubMed]
- 67.Varynskyi B, Schick JA. Hacking the Lipidome: New Ferroptosis Strategies in Cancer Therapy. Biomedicines. 2024;12. 10.3390/biomedicines12030541. [DOI] [PMC free article] [PubMed]
- 68.Rysman E, Brusselmans K, Scheys K, Timmermans L, Derua R, Munck S, Van Veldhoven PP, Waltregny D, Daniëls VW, Machiels J, Vanderhoydonc F, Smans K, Waelkens E, Verhoeven G, Swinnen JV. De novo lipogenesis protects cancer cells from free radicals and chemotherapeutics by promoting membrane lipid saturation. Cancer Res. 2010;70:8117–26. 10.1158/0008-5472.can-09-3871. [DOI] [PubMed] [Google Scholar]
- 69.Li Y, Yang W, Zheng Y, Dai W, Ji J, Wu L, Cheng Z, Zhang J, Li J, Xu X, Wu J, Yang M, Feng J, Guo C. Targeting fatty acid synthase modulates sensitivity of hepatocellular carcinoma to sorafenib via ferroptosis. J Exp Clin Cancer Res. 2023;42. 10.1186/S13046-022-02567-Z. [DOI] [PMC free article] [PubMed]
- 70.Zhao Q, Lin X, Wang G. Targeting SREBP-1-Mediated Lipogenesis as Potential Strategies for Cancer. Front Oncol. 2022;12. 10.3389/fonc.2022.952371. [DOI] [PMC free article] [PubMed]
- 71.Yi J, Zhu J, Wu J, Thompson CB, Jiang X. Oncogenic activation of PI3K-AKT-mTOR signaling suppresses ferroptosis via SREBP-mediated lipogenesis. Proc Natl Acad Sci U S A. 2020;117:31189–97. 10.1073/pnas.2017152117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jin H, Zhu M, Zhang D, Liu X, Guo Y, Xia L, Chen Y, Chen Y, Xu R, Liu C, Xi Q, Xia S, Shi T, Zhang G. B7H3 increases ferroptosis resistance by inhibiting cholesterol metabolism in colorectal cancer. Cancer Sci. 2023;114:4225–36. 10.1111/cas.15944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hong X, Roh W, Sullivan RJ, Wong KHK, Wittner BS, Guo H, Dubash TD, Sade-Feldman M, Wesley B, Horwitz E, Boland GM, Marvin DL, Bonesteel T, Lu C, Aguet F, Burr R, Freeman SS, Parida L, Calhoun K, Jewett MK, Nieman LT, Hacohen N, Näär AM, Ting DT, Toner M, Stott SL, Getz G, Maheswaran S, Haber DA. The Lipogenic Regulator SREBP2 Induces Transferrin in Circulating Melanoma Cells and Suppresses Ferroptosis. Cancer Discov. 2021;11:678–95. 10.1158/2159-8290.cd-19-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim MJ, Kim HS, Kang HW, Lee DE, Hong WC, Kim JH, Kim M, Cheong JH, Kim HJ, Park JS. SLC38A5 Modulates Ferroptosis to Overcome Gemcitabine Resistance in Pancreatic Cancer. Cells. 2023;12:2509. 10.3390/cells12202509/s1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jog E, Jainarayanan AK, La Ferlita A, Chakraborty A, Dalwai A, Yahya S, Shivashankar A, Choudhary BS, Chandramouli A, Kazi M, Jain D, Khapare N, Khan ABBK, Gera P, Patil P, Thorat R, Verma N, Sehgal L, Saklani A, Kamat SS, Dalal SN, Chaudhary N. Inhibiting de novo lipogenesis identifies a therapeutic vulnerability in therapy-resistant colorectal cancer. Redox Biol. 2024;79:103458. 10.1016/j.redox.2024.103458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nardi F, Del Prete R, Drago R, Di Rita A, Vallone FE, Ciofini S, Malchiodi M, Pezzella L, Tinti L, Cicaloni V, Salvini L, Licastro D, Pezacki AT, Chang CJ, Marotta G, Naldini A, Deaglio S, Vaisitti T, Gozzetti A, Bocchia M, Kabanova A. Apoliprotein E-mediated ferroptosis controls cellular proliferation in chronic lymphocytic leukemia. Leukemia. 2024;39:122. 10.1038/s41375-024-02442-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xue Q, Yan D, Chen X, Li X, Kang R, Klionsky DJ, Kroemer G, Chen X, Tang D, Liu J. Copper-dependent autophagic degradation of GPX4 drives ferroptosis. Autophagy. 2023;19:1982–96. 10.1080/15548627.2023.2165323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Guerra B, Recio C, Aranda-Tavío H, Guerra-Rodríguez M, García-Castellano JM, Fernández-Pérez L. The Mevalonate Pathway, a Metabolic Target in Cancer Therapy. Front Oncol. 2021;11. 10.3389/fonc.2021.626971. [DOI] [PMC free article] [PubMed]
- 79.Friedmann JP, Angeli D, Krysko V, Conrad M. Ferroptosis at the crossroads of cancer-acquired drug resistance and immune evasion. Nat Rev Cancer. 2019;19:405–14. [DOI] [PubMed] [Google Scholar]
- 80.Garcia-Bermudez J, Baudrier L, Bayraktar EC, Shen Y, La K, Guarecuco R, Yucel B, Fiore D, Tavora B, Freinkman E, Chan SH, Lewis C, Min W, Inghirami G, Sabatini DM, Birsoy K. Squalene accumulation in cholesterol auxotrophic lymphomas prevents oxidative cell death. Nature. 2019;567:118–22. 10.1038/S41586-019-0945-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Warner GJ, Berry MJ, Moustafa ME, Carlson BA, Hatfield DL, Faust JR. Inhibition of selenoprotein synthesis by selenocysteine tRNA[Ser]Sec lacking isopentenyladenosine. J Biol Chem. 2000;275:28110–9. 10.1074/jbc.m001280200. [DOI] [PubMed] [Google Scholar]
- 82.Miller BT, Ueta CB, Lau V, Jacomino KG, Wasserman LM, Kim BW. Statins and downstream inhibitors of the isoprenylation pathway increase type 2 iodothyronine deiodinase activity. Endocrinology. 2012;153:4039–48. 10.1210/en.2012-1117. [DOI] [PubMed] [Google Scholar]
- 83.Liang D, Minikes AM, Jiang X. Ferroptosis at the intersection of lipid metabolism and cellular signaling. Mol Cell. 2022;82:2215–27. 10.1016/j.molcel.2022.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen X, Yu C, Kang R, Tang D. Iron Metabolism in Ferroptosis. Front Cell Dev Biol. 2020;8. 10.3389/FCELL.2020.590226. [DOI] [PMC free article] [PubMed]
- 85.Qu L, He X, Tang Q, Fan X, Liu J, Lin A. Iron metabolism, ferroptosis, and lncRNA in cancer: knowns and unknowns. J Zhejiang Univ Sci B. 2022;23:844–62. 10.1631/jzus.b2200194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang S, Xin W, Anderson GJ, Li R, Gao L, Chen S, Zhao J, Liu S. Double-edge sword roles of iron in driving energy production versus instigating ferroptosis. Cell Death Dis. 2022;13. 10.1038/S41419-021-04490-1. [DOI] [PMC free article] [PubMed]
- 87.Brown RAM, Richardson KL, Kabir TD, Trinder D, Ganss R, Leedman PJ. Altered Iron Metabolism and Impact in Cancer Biology, Metastasis, and Immunology. Front Oncol. 2020;10. 10.3389/fonc.2020.00476. [DOI] [PMC free article] [PubMed]
- 88.Yang WS, Sriramaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji AF, Clish CB, Brown LM, Girotti AW, Cornish VW, Schreiber SL, Stockwell BR. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317–31. 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liberti MV, Locasale JW. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem Sci. 2016;41:211. 10.1016/j.tibs.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ediriweera MK, Jayasena S. The Role of Reprogrammed Glucose Metabolism in Cancer. Metabolites. 2023;13. 10.3390/metabo13030345. [DOI] [PMC free article] [PubMed]
- 91.Ždralević M, Vučetić M, Daher B, Marchiq I, Parks SK, Pouysségur J. Disrupting the “Warburg effect” re-routes cancer cells to OXPHOS offering a vulnerability point via ’ferroptosis’-induced cell death. Adv Biol Regul. 2018;68:55–63. 10.1016/j.jbior.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 92.Amos A, Amos A, Wu L, Xia H. The Warburg effect modulates DHODH role in ferroptosis: a review. Cell Commun Signal. 2023;21. 10.1186/S12964-022-01025-9. [DOI] [PMC free article] [PubMed]
- 93.Ye L, Wen X, Qin J, Zhang X, Wang Y, Wang Z, Zhou T, Di Y, He W. Metabolism-regulated ferroptosis in cancer progression and therapy. Cell Death Dis. 2024;15. 10.1038/S41419-024-06584-Y. [DOI] [PMC free article] [PubMed]
- 94.Lee H, Zandkarimi F, Zhang Y, Meena JK, Kim J, Zhuang L, Tyagi S, Ma L, Westbrook TF, Steinberg GR, Nakada D, Stockwell BR, Gan B. Energy-stress-mediated AMPK activation inhibits ferroptosis. Nat Cell Biol. 2020;22:225–34. 10.1038/S41556-020-0461-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bian X, Liu R, Meng Y, Xing D, Xu D, Lu Z. Lipid metabolism and cancer. J Exp Med. 2021;218. 10.1084/jem.20201606. [DOI] [PMC free article] [PubMed]
- 96.Liu J, Kang R, Tang D. Metabolic checkpoint of ferroptosis resistance. Mol Cell Oncol. 2021;8. 10.1080/23723556.2021.1901558. [DOI] [PMC free article] [PubMed]
- 97.Song X, Liu J, Kuang F, Chen X, Zeh HJ, Kang R, Kroemer G, Xie Y, Tang D. PDK4 dictates metabolic resistance to ferroptosis by suppressing pyruvate oxidation and fatty acid synthesis. Cell Rep. 2021;34. 10.1016/j.celrep.2021.108767. [DOI] [PubMed]
- 98.Hao Y, Yi Q, XiaoWu X, WeiBo C, GuangChen Z, XueMin C. Acetyl-CoA: An interplay between metabolism and epigenetics in cancer. Frontiers in Molecular Medicine. 2022;2:1044585. 10.3389/fmmed.2022.1044585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nassar ZD, Mah CY, Dehairs J, Burvenich IJG, Irani S, Centenera MM, Helm M, Shrestha RK, Moldovan M, Don AS, Holst J, Scott AM, Horvath LG, Lynn DJ, Selth LA, Hoy AJ, Swinnen JV, Butler LM. Human DECR1 is an androgen-repressed survival factor that regulates PUFA oxidation to protect prostate tumor cells from ferroptosis. Elife. 2020;9:1–34. 10.7554/elife.54166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Blomme A, Ford CA, Mui E, Patel R, Ntala C, Jamieson LE, Planque M, McGregor GH, Peixoto P, Hervouet E, Nixon C, Salji M, Gaughan L, Markert E, Repiscak P, Sumpton D, Blanco GR, Lilla S, Kamphorst JJ, Graham D, Faulds K, MacKay GM, Fendt SM, Zanivan S, Leung HY. 2,4-dienoyl-CoA reductase regulates lipid homeostasis in treatment-resistant prostate cancer. Nat Commun. 2020;11. 10.1038/s41467-020-16126-7. [DOI] [PMC free article] [PubMed]
- 101.Gonzalez H, Hagerling C, Werb Z. Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev. 2018;32:1267. 10.1101/gad.314617.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shen M, Kang Y. Complex interplay between tumor microenvironment and cancer therapy. Front Med. 2018;12:426–39. 10.1007/S11684-018-0663-7. [DOI] [PubMed] [Google Scholar]
- 103.Wang Q, Shao X, Zhang Y, Zhu M, Wang FXC, Mu J, Li J, Yao H, Chen K. Role of tumor microenvironment in cancer progression and therapeutic strategy. Cancer Med. 2023;12:11149–65. 10.1002/cam4.5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li H, Yang P, Wang JH, Zhang J, Ma Q, Jiang Y, Wu Y, Han T, Xiang D. HLF regulates ferroptosis, development and chemoresistance of triple-negative breast cancer by activating tumor cell-macrophage crosstalk. J Hematol Oncol. 2022;15. 10.1186/S13045-021-01223-X. [DOI] [PMC free article] [PubMed]
- 105.Zhao Y, Liu Z, Liu G, Zhang Y, Liu S, Gan D, Chang W, Peng X, Sung ES, Gilbert K, Zhu Y, Wang X, Zeng Z, Baldwin H, Ren G, Weaver J, Huron A, Mayberry T, Wang Q, Wang Y, Diaz-Rubio ME, Su X, Stack MS, Zhang S, Lu X, Sheldon RD, Li J, Zhang C, Wan J, Lu X. Neutrophils resist ferroptosis and promote breast cancer metastasis through aconitate decarboxylase 1. Cell Metab. 2023;35:1688-1703.e10. 10.1016/j.cmet.2023.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Luo L, Zhang Z, Weng Y, Zeng J. Ferroptosis-Related Gene GCLC Is a Novel Prognostic Molecular and Correlates with Immune Infiltrates in Lung Adenocarcinoma. Cells. 2022;11. 10.3390/cells11213371. [DOI] [PMC free article] [PubMed]
- 107.Weber R, Groth C, Lasser S, Arkhypov I, Petrova V, Altevogt P, Utikal J, Umansky V. IL-6 as a major regulator of MDSC activity and possible target for cancer immunotherapy. Cell Immunol. 2021;359. 10.1016/j.cellimm.2020.104254. [DOI] [PubMed]
- 108.Dai Y, Cui C, Jiao D, Zhu X. JAK/STAT signaling as a key regulator of ferroptosis: mechanisms and therapeutic potentials in cancer and diseases. Cancer Cell Int. 2025;25. 10.1186/S12935-025-03681-6. [DOI] [PMC free article] [PubMed]
- 109.Wang W, Green M, Choi JE, Gijón M, Kennedy PD, Johnson JK, Liao P, Lang X, Kryczek I, Sell A, Xia H, Zhou J, Li G, Li J, Li W, Wei S, Vatan L, Zhang H, Szeliga W, Gu W, Liu R, Lawrence TS, Lamb C, Tanno Y, Cieslik M, Stone E, Georgiou G, Chan TA, Chinnaiyan A, Zou W. CD8+ T cells regulate tumour ferroptosis during cancer immunotherapy. Nature. 2019;569:270–4. 10.1038/S41586-019-1170-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhou Q, Tao C, Yuan J, Pan F, Wang R. Ferroptosis, a subtle talk between immune system and cancer cells: To be or not to be? Biomed Pharmacother. 2023;165. 10.1016/j.biopha.2023.115251. [DOI] [PubMed]
- 111.Tang D, Kroemer G, Kang R. Ferroptosis in immunostimulation and immunosuppression. Immunol Rev. 2023. 10.1111/imr.13235. [DOI] [PubMed] [Google Scholar]
- 112.Dingjan I, Verboogen DRJ, Paardekooper LM, Revelo NH, Sittig SP, Visser LJ, Von Mollard GF, Henriet SSV, Figdor CG, Ter Beest M, Van Den Bogaart G. Lipid peroxidation causes endosomal antigen release for cross-presentation. Sci Rep. 2016;6. 10.1038/srep22064. [DOI] [PMC free article] [PubMed]
- 113.Dong D, Zhang G, Yang J, Zhao B, Wang S, Wang L, Zhang G, Shang P. The role of iron metabolism in cancer therapy focusing on tumor-associated macrophages. J Cell Physiol. 2019;234:8028–39. 10.1002/JCP.27569. [DOI] [PubMed] [Google Scholar]
- 114.Pinnix ZK, Miller LD, Wang W, D’Agostino R, Kute T, Willingham MC, Hatcher H, Tesfay L, Sui G, Di X, Torti SV, Torti FM. Ferroportin and iron regulation in breast cancer progression and prognosis. Sci Transl Med. 2010;2. 10.1126/scitranslmed.3001127. [DOI] [PMC free article] [PubMed]
- 115.Deng R, Wang SM, Yin T, Ye TH, Shen GB, Li L, Zhao JY, Sang YX, Duan XG, Wei YQ. Inhibition of tumor growth and alteration of associated macrophage cell type by an HO-1 inhibitor in breast carcinoma-bearing mice. Oncol Res. 2013;20:473–82. 10.3727/096504013x13715991125684. [DOI] [PubMed] [Google Scholar]
- 116.Dai E, Han L, Liu J, Xie Y, Kroemer G, Klionsky DJ, Zeh HJ, Kang R, Wang J, Tang D. Autophagy-dependent ferroptosis drives tumor-associated macrophage polarization via release and uptake of oncogenic KRAS protein. Autophagy. 2020;16:2069–83. 10.1080/15548627.2020.1714209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hao X, Zheng Z, Liu H, Zhang Y, Kang J, Kong X, Rong D, Sun G, Sun G, Liu L, Yu H, Tang W, Wang X. Inhibition of APOC1 promotes the transformation of M2 into M1 macrophages via the ferroptosis pathway and enhances anti-PD1 immunotherapy in hepatocellular carcinoma based on single-cell RNA sequencing. Redox Biol. 2022;56. 10.1016/j.redox.2022.102463. [DOI] [PMC free article] [PubMed]
- 118.Zhou Y, Que KT, Zhang Z, Yi ZJ, Zhao PX, You Y, Gong JP, Liu ZJ. Iron overloaded polarizes macrophage to proinflammation phenotype through ROS/acetyl-p53 pathway. Cancer Med. 2018;7:4012–22. 10.1002/cam4.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Corna G, Campana L, Pignatti E, Castiglioni A, Tagliafico E, Bosurgi L, Campanella A, Brunelli S, Manfredi AA, Apostoli P, Silvestri L, Camaschella C, Rovere-Querini P. Polarization dictates iron handling by inflammatory and alternatively activated macrophages. Haematologica. 2010;95:1814–22. 10.3324/haematol.2010.023879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.AA Kapralov, Q Yang, HH Dar, YY Tyurina, TS Anthonymuthu, R Kim, CM Croix, K Mikulska-Ruminska, B Liu, IH Shrivastava, VA Tyurin, HC Ting, YL Wu, Y Gao, GV Shurin, MA Artyukhova, LA Ponomareva, PS Timashev, RM Domingues, DA Stoyanovsky, JS Greenberger, RK Mallampalli, I Bahar, DI Gabrilovich, H Bayır, VE Kagan. Redox lipid reprogramming commands susceptibility of macrophages and microglia to ferroptotic death. Nat Chem Biol. 2020;16:278–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yee PP, Wei Y, Kim SY, Lu T, Chih SY, Lawson C, Tang M, Liu Z, Anderson B, Thamburaj K, Young MM, Aregawi DG, Glantz MJ, Zacharia BE, Specht CS, Wang HG, Li W. Neutrophil-induced ferroptosis promotes tumor necrosis in glioblastoma progression. Nat Commun. 2020;11. 10.1038/S41467-020-19193-Y. [DOI] [PMC free article] [PubMed]
- 122.Liu ZG, Jiao D. Necroptosis tumor necrosis and tumorigenesis. Cell Stress. 2020;4:1. 10.15698/cst2020.01.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Čepelak I, Dodig S, Dodig DČ. Ferroptosis: regulated cell death. Arh Hig Rada Toksikol. 2020;71:99–109. 10.2478/AIHT-2020-71-3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lasser SA, Ozbay Kurt FG, Arkhypov I, Utikal J, Umansky V. Myeloid-derived suppressor cells in cancer and cancer therapy. Nat Rev Clin Oncol. 2024;21:147–64. [DOI] [PubMed] [Google Scholar]
- 125.Ostrand-Rosenberg S, Sinha P, Beury DW, Clements VK. Cross-talk between myeloid-derived suppressor cells (MDSC), macrophages, and dendritic cells enhances tumor-induced immune suppression. Semin Cancer Biol. 2012;22:275–81. 10.1016/j.semcancer.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Han F, Li S, Yang Y, Bai Z. Interleukin-6 promotes ferroptosis in bronchial epithelial cells by inducing reactive oxygen species-dependent lipid peroxidation and disrupting iron homeostasis. Bioengineered. 2021;12:5279–88. 10.1080/21655979.2021.1964158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sterling JK, Kam TI, Guttha S, Park H, Baumann B, Mehrabani-Tabari AA, Schultz H, Anderson B, Alnemri A, Chou SC, Troncoso JC, Dawson VL, Dawson TM, Dunaief JL. Interleukin-6 triggers toxic neuronal iron sequestration in response to pathological α-synuclein. Cell Rep. 2022;38. 10.1016/j.celrep.2022.110358. [DOI] [PMC free article] [PubMed]
- 128.Zheng Z, Zheng X, Zhu Y, Yao Z, Zhao W, Zhu Y, Sun F, Mu X, Wang Y, He W, Liu Z, Wu K, Zheng J. IL-6 Promotes the Proliferation and Immunosuppressive Function of Myeloid-Derived Suppressor Cells via the MAPK Signaling Pathway in Bladder Cancer. Biomed Res Int. 2021. 10.1155/2021/5535578. [DOI] [PMC free article] [PubMed]
- 129.Sharma V, Sachdeva N, Gupta V, Nada R, Jacob J, Sahni D, Aggarwal A. IL-6 is associated with expansion of myeloid-derived suppressor cells and enhanced immunosuppression in pancreatic adenocarcinoma patients. Scand J Immunol. 2021;94. 10.1111/SJI.13107. [DOI] [PubMed]
- 130.Angelini G, Gardella S, Ardy M, Ciriolo MR, Filomeni G, Di Trapani G, Clarke F, Sitia R, Rubartelli A. Antigen-presenting dendritic cells provide the reducing extracellular microenvironment required for T lymphocyte activation. Proc Natl Acad Sci U S A. 2002;99:1491–6. 10.1073/pnas.022630299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Srivastava MK, Sinha P, Clements VK, Rodriguez P, Ostrand-Rosenberg S. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. 2010;70:68–77. 10.1158/0008-5472.CAN-09-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Frost JN, Tan TK, Abbas M, Wideman SK, Bonadonna M, Stoffel NU, Wray K, Kronsteiner B, Smits G, Campagna DR, Duarte TL, Lopes JM, Shah A, Armitage AE, Arezes J, Lim PJ, Preston AE, Ahern D, Teh M, Naylor C, Salio M, Gileadi U, Andrews SC, Dunachie SJ, Zimmermann MB, van der Klis FRM, Cerundolo V, Bannard O, Draper SJ, Townsend ARM, Galy B, Fleming MD, Lewis MC, Drakesmith H. Hepcidin-Mediated Hypoferremia Disrupts Immune Responses to Vaccination and Infection. Med (N Y). 2021;2:164-179.e12. 10.1016/j.medj.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Berg V, Modak M, Brell J, Puck A, Künig S, Jutz S, Steinberger P, Zlabinger GJ, Stöckl J. Iron Deprivation in Human T Cells Induces Nonproliferating Accessory Helper Cells. Immunohorizons. 2020;4:165–77. 10.4049/IMMUNOHORIZONS.2000003. [DOI] [PubMed] [Google Scholar]
- 134.Li L, Xia Y, Yuan S, Li F, Xie X, Luo Y, Yang XP, He R. Iron deprivation restrains the differentiation and pathogenicity of T helper 17 cell. J Leukoc Biol. 2021;110:1057–67. 10.1002/jlb.3ma0821-015r. [DOI] [PubMed] [Google Scholar]
- 135.Voss K, Sewell AE, Krystofiak ES, Gibson-Corley KN, Young AC, Basham JH, Sugiura A, Arner EN, Beavers WN, Kunkle DE, Dickson ME, Needle GA, Skaar EP, Rathmell WK, Ormseth MJ, Major AS, Rathmell JC. Elevated transferrin receptor impairs T cell metabolism and function in systemic lupus erythematosus. Sci Immunol. 2023;8. 10.1126/sciimmunol.abq0178. [DOI] [PMC free article] [PubMed]
- 136.Wu Q, Carlos AR, Braza F, Bergman ML, Kitoko JZ, Bastos-Amador P, Cuadrado E, Martins R, Oliveira BS, Martins VC, Scicluna BP, Landry JJM, Jung FE, Ademolue TW, Peitzsch M, Almeida-Santos J, Thompson J, Cardoso S, Ventura P, Slot M, Rontogianni S, Ribeiro V, Domingues VDS, Cabral IA, Weis S, Groth M, Ameneiro C, Fidalgo M, Wang F, Demengeot J, Amsen D, Soares MP. Ferritin heavy chain supports stability and function of the regulatory T cell lineage. EMBO J. 2024;43:1445–83. 10.1038/S44318-024-00064-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hidalgo V, Dy C, Fernandez Hidalgo O, Calvo FA. Simultaneous radiotherapy and cis-platinum for the treatment of brain metastases a pilot study. Am J Clin Oncol. 1987;10:205–9. 10.1097/00000421-198706000-00005. [DOI] [PubMed] [Google Scholar]
- 138.Feng P, Yang Q, Luo L, Sun Y, Lv W, Wan S, Guan Z, Xiao Z, Liu F, Li Z, Dong Z, Yang M. The kinase PDK1 regulates regulatory T cell survival via controlling redox homeostasis. Theranostics. 2021;11:9503–18. 10.7150/THNO.63992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gong C, Ji Q, Wu M, Tu Z, Lei K, Luo M, Liu J, Lin L, Li K, Li J, Huang K, Zhu X. Ferroptosis in tumor immunity and therapy. J Cell Mol Med. 2022;26:5565–79. 10.1111/jcmm.17529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wen Q, Liu J, Kang R, Zhou B, Tang D. The release and activity of HMGB1 in ferroptosis. Biochem Biophys Res Commun. 2019;510:278–83. 10.1016/j.bbrc.2019.01.090. [DOI] [PubMed] [Google Scholar]
- 141.Matsushita M, Freigang S, Schneider C, Conrad M, Bornkamm GW, Kopf M. T cell lipid peroxidation induces ferroptosis and prevents immunity to infection. J Exp Med. 2015;212:555–68. 10.1084/jem.20140857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Li J, Chen M, Huang D, Li Z, Chen Y, Huang J, Chen Y, Zhou Z, Yu Z. Inhibition of Selenoprotein I promotes ferroptosis and reverses resistance to platinum chemotherapy by impairing Akt phosphorylation in ovarian cancer. MedComm (Beijing). 2024;5: e70033. 10.1002/mco2.70033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wolf NK, Kissiov DU, Raulet DH. Roles of natural killer cells in immunity to cancer, and applications to immunotherapy. Nat Rev Immunol. 2023;23:90–105. 10.1038/S41577-022-00732-1. [DOI] [PubMed] [Google Scholar]
- 144.Donnelly RP, Loftus RM, Keating SE, Liou KT, Biron CA, Gardiner CM, Finlay DK. mTORC1-dependent metabolic reprogramming is a prerequisite for NK cell effector function. J Immunol. 2014;193:4477–84. 10.4049/JIMMUNOL.1401558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Michelet X, Dyck L, Hogan A, Loftus RM, Duquette D, Wei K, Beyaz S, Tavakkoli A, Foley C, Donnelly R, O’Farrelly C, Raverdeau M, Vernon A, Pettee W, O’Shea D, Nikolajczyk BS, Mills KHG, Brenner MB, Finlay D, Lynch L. Metabolic reprogramming of natural killer cells in obesity limits antitumor responses. Nat Immunol. 2018;19:1330–40. 10.1038/S41590-018-0251-7. [DOI] [PubMed] [Google Scholar]
- 146.Poznanski SM, Singh K, Ritchie TM, Aguiar JA, Fan IY, Portillo AL, Rojas EA, Vahedi F, El-Sayes A, Xing S, Butcher M, Lu Y, Doxey AC, Schertzer JD, Hirte HW, Ashkar AA. Metabolic flexibility determines human NK cell functional fate in the tumor microenvironment. Cell Metab. 2021;33:1205-1220.e5. 10.1016/j.cmet.2021.03.023. [DOI] [PubMed] [Google Scholar]
- 147.Piattini F, Matsushita M, Muri J, Bretscher P, Feng X, Freigang S, Dalli J, Schneider C, Kopf M. Differential sensitivity of inflammatory macrophages and alternatively activated macrophages to ferroptosis. Eur J Immunol. 2021;51:2417–29. 10.1002/eji.202049114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yin X, Zeng W, Wu B, Wang L, Wang Z, Tian H, Wang L, Jiang Y, Clay R, Wei X, Qin Y, Zhang F, Zhang C, Jin L, Liang W. PPARα Inhibition Overcomes Tumor-Derived Exosomal Lipid-Induced Dendritic Cell Dysfunction. Cell Rep. 2020;33. 10.1016/j.celrep.2020.108278. [DOI] [PMC free article] [PubMed]
- 149.Cubillos-Ruiz JR, Silberman PC, Rutkowski MR, Chopra S, Perales-Puchalt A, Song M, Zhang S, Bettigole SE, Gupta D, Holcomb K, Ellenson LH, Caputo T, Lee AH, Conejo-Garcia JR, Glimcher LH. ER Stress Sensor XBP1 Controls Anti-tumor Immunity by Disrupting Dendritic Cell Homeostasis. Cell. 2015;161:1527–38. 10.1016/j.cell.2015.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Laumont CM, Nelson BH. B cells in the tumor microenvironment: Multi-faceted organizers, regulators, and effectors of anti-tumor immunity. Cancer Cell. 2023;41:466–89. 10.1016/j.ccell.2023.02.017. [DOI] [PubMed] [Google Scholar]
- 151.Muri J, Thut H, Bornkamm GW, Kopf M. B1 and Marginal Zone B Cells but Not Follicular B2 Cells Require Gpx4 to Prevent Lipid Peroxidation and Ferroptosis. Cell Rep. 2019;29:2731-2744.e4. 10.1016/j.celrep.2019.10.070. [DOI] [PubMed] [Google Scholar]
- 152.Yang Z, Su W, Wei X, Qu S, Zhao D, Zhou J, Wang Y, Guan Q, Qin C, Xiang J, Zen K, Yao B. HIF-1α drives resistance to ferroptosis in solid tumors by promoting lactate production and activating SLC1A1. Cell Rep. 2023;42. 10.1016/j.celrep.2023.112945. [DOI] [PubMed]
- 153.Zhao Y, Li M, Yao X, Fei Y, Lin Z, Li Z, Cai K, Zhao Y, Luo Z. HCAR1/MCT1 Regulates Tumor Ferroptosis through the Lactate-Mediated AMPK-SCD1 Activity and Its Therapeutic Implications. Cell Rep. 2020;33. 10.1016/j.celrep.2020.108487. [DOI] [PubMed]
- 154.Scharping NE, Menk AV, Moreci RS, Whetstone RD, Dadey RE, Watkins SC, Ferris RL, Delgoffe GM. The Tumor Microenvironment Represses T Cell Mitochondrial Biogenesis to Drive Intratumoral T Cell Metabolic Insufficiency and Dysfunction. Immunity. 2016;45:374–88. 10.1016/j.immuni.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jiang Z, Lim SO, Yan M, Hsu JL, Yao J, Wei Y, Chang SS, Yamaguchi H, Lee HH, Ke B, Hsu JM, Chan LC, Hortobagyi GN, Yang L, Lin C, Yu D, Hung MC. TYRO3 induces anti-PD-1/PD-L1 therapy resistance by limiting innate immunity and tumoral ferroptosis. J Clin Invest. 2021;131. 10.1172/JCI139434. [DOI] [PMC free article] [PubMed]
- 156.Ma X, Xiao L, Liu L, Ye L, Su P, Bi E, Wang Q, Yang M, Qian J, Yi Q. CD36-mediated ferroptosis dampens intratumoral CD8+ T cell effector function and impairs their antitumor ability. Cell Metab. 2021;33:1001-1012.e5. 10.1016/j.cmet.2021.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Peyraud F, Guegan JP, Bodet D, Cousin S, Bessede A, Italiano A. Targeting Tryptophan Catabolism in Cancer Immunotherapy Era: Challenges and Perspectives. Front Immunol. 2022;13. 10.3389/fimmu.2022.807271. [DOI] [PMC free article] [PubMed]
- 158.Fiore A, Zeitler L, Russier M, Groß A, Hiller MK, Parker JL, Stier L, Köcher T, Newstead S, Murray PJ. Kynurenine importation by SLC7A11 propagates anti-ferroptotic signaling. Mol Cell. 2022;82:920-932.e7. 10.1016/j.molcel.2022.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.León-Letelier RA, Dou R, Vykoukal J, Sater AHA, Ostrin E, Hanash S, Fahrmann JF. The kynurenine pathway presents multi-faceted metabolic vulnerabilities in cancer. Front Oncol. 2023;13:1256769. 10.3389/fonc.2023.1256769/bibtex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cui JX, Xu XH, He T, Liu JJ, Xie TY, Tian W, Liu JY. L-kynurenine induces NK cell loss in gastric cancer microenvironment via promoting ferroptosis. J Exp Clin Cancer Res. 2023;42. 10.1186/S13046-023-02629-W. [DOI] [PMC free article] [PubMed]
- 161.Martinez-Outschoorn UE, Lin Z, Whitaker-Menezes D, Howell A, Sotgia F, Lisanti MP. Ketone body utilization drives tumor growth and metastasis. Cell Cycle. 2012;11:3964–71. 10.4161/CC.22137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ferrer M, Mourikis N, Davidson EE, Kleeman SO, Zaccaria M, Habel J, Rubino R, Flint TR, Connell CM, Lukey M, White EP, Coll AP, Venkitaraman AR, Janowitz T. Ketogenic diet promotes tumor ferroptosis but induces relative corticosterone deficiency that accelerates cachexia. BioRxiv. 2023. 10.1101/2023.02.17.528937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Deng GH, Wu CF, Li YJ, Shi H, Zhong WC, Hong MK, Li JJ, Zhao JM, Liu C, Qin MC, Zeng ZY, Zhang WM, Yung KKL, Lv ZP, Gao L. Caveolin-1 is critical for hepatic iron storage capacity in the development of nonalcoholic fatty liver disease. Mil Med Res. 2023;10. 10.1186/S40779-023-00487-3. [DOI] [PMC free article] [PubMed]
- 164.Luis G, Godfroid A, Nishiumi S, Cimino J, Blacher S, Maquoi E, Wery C, Collignon A, Longuespée R, Montero-Ruiz L, Dassoul I, Maloujahmoum N, Pottier C, Mazzucchelli G, Depauw E, Bellahcène A, Yoshida M, Noel A, Sounni NE. Tumor resistance to ferroptosis driven by Stearoyl-CoA Desaturase-1 (SCD1) in cancer cells and Fatty Acid Biding Protein-4 (FABP4) in tumor microenvironment promote tumor recurrence. Redox Biol. 2021;43. 10.1016/j.redox.2021.102006. [DOI] [PMC free article] [PubMed]
- 165.Lopes-Coelho F, Martins F, Hipólito A, Mendes C, Sequeira CO, Pires RF, Almeida AM, Bonifácio VDB, Pereira SA, J. Serp J. The Activation of Endothelial Cells Relies on a Ferroptosis-Like Mechanism: Novel Perspectives in Management of Angiogenesis and Cancer Therapy. Front Oncol. 2021;11. 10.3389/fonc.2021.656229. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.