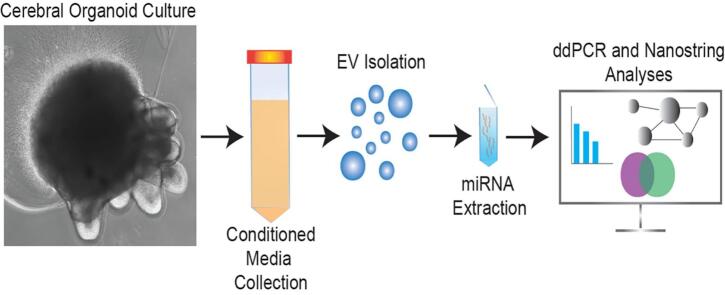
Graphical abstract
Keywords: Cerebral organoids, microRNA, Extracellular vesicles, Neurotoxicity, New approach methodologies, Neurodegenerative disease
Highlights
-
•
Extracellular vesicles (EVs) were isolated from cerebral organoids and were characterized via several methods.
-
•
Cerebral-organoid-derived EVs contained RNA in sufficient quantities for robust analysis using droplet-digital PCR and NanoString.
-
•
NanoString was used to characterize miRNA content of the cerebral-organoid-derived EVs.
-
•
Cerebral organoids were found to released EVs containing miRNA targeting several pathways involved in neural development and neurodegenerative disease.
-
•
This study highlights the potential use of cerebral organoids for studying brain-related EVs.
Abstract
Environmental toxicants can contribute to the development of several neurodegenerative diseases. However, the mechanisms behind this pathology are still incompletely understood. Prompt diagnosis of impending neurodegeneration is crucial for early interventions to prevent cognitive decline. Towards this end, accurate biomarkers for early neurodegenerative processes and exposure risk are needed. Extracellular vesicles (EVs) are lipid particles released by cells which contain many bioactive molecules including miRNAs. EVs may serve both as a route of propagating neurotoxic phenotypes and as a source of biomarkers for neurological disease. However, the exact mechanisms though which EVs could spread the deleterious effects of toxicants and the full spectrum of their usage as biomarkers remain unclear. Organoid models have several advantages, including potential for use in high-throughput toxicant testing and applications in personalized medicine and disease models. However, few studies have examined EV release in brain organoids to determine if the EVs could contain useful biomarkers. We employed several technologies to characterize EVs released by human cerebral organoids and their associated miRNAs. We identified that cerebral organoids consistently release EV-associated miRNA in quantities sufficient for robust analysis with NanoString. Further, pathway analyses revealed that terms related to neurodegenerative disease and nervous system signaling are associated with the recovered miRNAs. Together, these data suggest that cerebral organoids have utility as a tool for the discovery of EV-associated miRNAs involved in neurodegenerative disease and neurotoxicity.
1. Introduction
Accumulating evidence shows that exposure to environmental toxicants such as metals and pesticides can contribute to the development of neurodegenerative diseases including Alzheimer’s Disease (AD), dementia, and Parkinson’s Disease (PD) (Chin-Chan et al., 2015). Although there has been increased interest towards understanding the role of toxicants such as pesticides in the development of AD and neurodegenerative diseases, the underlying mechanisms remain unclear (Li et al., 2021). Other environmental toxicants can also adversely affect the nervous system. For example, it has become increasingly recognized that inhaled particulate matter can affect the brain, contributing to the development of neurodegenerative disease and impacting cognition (Kritikos et al., 2020). Exposure to heavy metals like lead in childhood disrupts brain development and cognition, although the minimum detrimental dose and duration of exposure are still undetermined (Heidari et al., 2021). Further, mixtures of metals may exert a synergistic effect, resulting in greater neurotoxicity than exposure to a single toxicant, highlighting the need for additional biomarkers of toxicity and exposure severity (Martin et al., 2021). Early diagnosis of neurotoxicity and neurodegenerative disease is critical for implementing treatments to prevent cognitive decline. However, effective risk biomarkers are lacking (Couch, 2023).
Extracellular vesicles (EVs) are lipid-bound particles released from cells that contain bioactive molecules including proteins and miRNA (Yáñez-Mó et al., 2015). EVs may have utility as biomarkers of neurodegeneration. Proteins that may be predictive of neurodegenerative diseases, specifically AD, PD, and Huntington’s, have been identified in EVs isolated from urine samples (Wang et al., 2019). Blood-derived EVs have also been found to differ between persons with AD and healthy individuals, containing miRNAs that may be indicative of disease progression (Aharon et al., 2020). EVs may also be predictive of neurological disorders. The concentrations of brain-derived plasma EVs have been observed to differ in patients with cognitive impairments (Visconte et al., 2023). For example, individuals with Autism Spectrum Disorder were found to have increased levels of EV-associated protein and mitochondrial DNA as compared to control persons (Tsilioni and Theoharides, 2018). EVs may also be indicative of neurotoxicity. For example, methylmercury exposure in rat models triggered neurological deficits accompanied by a decrease in abundance of small EVs circulating in plasma (Arrifano et al., 2021). EVs from human astrocytes that had been exposed to ketamine were toxic to neuronal cells in culture, while EVs from untreated astrocytes did not have this property (Penning et al., 2021). EVs may represent a source of informative molecules such as miRNAs. However, more high-throughput, reproducible techniques are still needed to fully translate EVs into reliable biomarkers of neurotoxicity and neurological disease (Couch, 2023).
High-throughput testing of potential toxicants can be easily conducted in human organoids, which have more commonalities with tissues in the body than 2D cell culture models. Additionally, patient-derived organoids can be used to create models of neurological disease (Kim et al., 2020). Several studies have isolated EVs from organoids including pancreatic (Buenafe et al., 2022, Zeold et al., 2021), retinal (Arthur et al., 2023, Zhou et al., 2021), intestinal (Szvicsek et al., 2019), salivary (Chansaenroj et al., 2022), and colon carcinoma (Tauro et al., 2013), but there is still a paucity of research examining EV release from organoids, particularly those of the brain. Forebrain organoids have been reported to release EVs capable of being labeled with iron oxide nanoparticles (Liu et al., 2022). In this prior study, a comprehensive analysis of EV-associated miRNA was not undertaken, but six miRNAs were detected with RT-qPCR (miR-10, miR-19a, miR-133b, miR-21, miR-22, miR-221). In addition, small EVs were previously isolated from iPSC-derived cerebral organoid models and were observed to promote the differentiation of dopaminergic neurons (Ji et al., 2023). Expression patterns of miRNAs have been successfully studied in EV preparations isolated from patient-derived forebrain organoid disease models (Bahram Sangani et al., 2024). However, EVs released by cerebral organoids and their associated miRNA remain otherwise minimally studied. Cerebral organoids are an excellent in vitro model to study aspects of the human brain. Although no in vitro model can yet fully mimic the full complexity of organs in vivo, cerebral organoids contain multiple differentiated cell types and structures found in the human brain, in a genetically human background. Cerebral organoids have been documented to contain neurons, neural progenitors, and neuroepithelia organized into rosette structures surrounding fluid-filled cavities. Also, discrete regions of cells expressing markers found in forebrain/hindbrain tissues, dorsal cortex, hippocampal and prefrontal tissues, ventral cortex, choroid plexus, and retinal tissue have been identified in cerebral organoids (Lancaster et al., 2013).
Here, we isolated and characterized EVs from mature cerebral organoids. We performed a comprehensive analysis of associated miRNAs using NanoString and identified several targeted pathways associated with neurodegeneration and nervous system signaling. We conclude that cerebral organoids release EVs consistently in quantities sufficient for comprehensive miRNA analysis. Cerebral organoids could thus be used as a tool for the identification of novel biomarkers and miRNA signaling pathways during cerebral development and in response to drug treatments, toxic exposures, or disease models.
2. Materials and methods
2.1. Human embryonic stem cell culture
H9 human embryonic stem cells (hESCs) were cultured in mTeSR + medium (Thermo Fisher) 37 °C and 5 % CO2 on plates pre-coated with Matrigel (Corning) for 30 min at 37 °C in DMEM/F:12 (Thermo Fisher) at a concentration of 1.2 µl/ml/cm2. At approximately 80 % confluence, plates of cells were passaged using 0.5 mM EDTA (Gibco) and seeded at a ratio of approximately 1:5–1:10 onto new plates. Cultures were passaged no more than 5 times and monitored for any morphological changes indicative of differentiation. Cultures with differentiating colonies were discarded.
2.2. Cerebral organoid generation
H9 ESCs were used to generate cerebral organoids using the StemDiff Cerebral Organoid Kit (StemCell Technologies) according to the manufacturer’s protocol as previously described (Silver et al., 2024). Briefly, 1×TrypLE/0.5 mM EDTA/PBS (Gibco) was used to trypsinize cells. EB Formation Medium (StemDiff Cerebral Differentiation Kit; StemCell Technologies) supplemented with 10 µM of Y27632 was used to resuspend the cell pellet, and cells were seeded at a density of 2 × 104 cells/mL, 50 µL per well in a 384 round bottom ultra-low attachment plate (Corning). The plate was centrifuged at 200g × 5 min to coalesce the cells. This was defined as day 0 of growth. Media was replaced with EB Formation Medium on growth days 1 and 3. Media was replaced with induction Medium (StemDiff Cerebral Differentiation Kit; StemCell Technologies) on growth day 5. On growth day 7, the tissues were individually embedded in Matrigel (Corning) and transferred to a 24-well ultra-low attachment plate (Thermo Fisher) in 500 µl of Expansion Medium (StemDiff Cerebral Differentiation Kit; StemCell Technologies), 4 tissues/well, and incubated at 37 °C on an orbital shaker for 72 h. On growth day 10, the media was changed to 1 ml of Maturation Medium (StemDiff Cerebral Differentiation Kit; StemCell Technologies), and the media changed every 3–4 days until maturity (day 41). Conditioned media was collected from three biological replicates (separate batches) between growth days 41 and 49 for EV isolation. On growth day 58, 10 µM GW 4869 (Tocris) was added to half of the organoids in the batch, and DMSO (1:1000) was added to the other half as a control (one plate each). After 24 h incubation, conditioned media (20–25 mL) was collected from each condition for EV isolation.
2.3. Isolation of extracellular vesicles
The following protocol was adapted from previous studies (Ngalame et al., 2018, Purushothaman, 2019). Conditioned media was collected from each batch of cerebral organoids (totaling approximately 45 ml media from about 200 organoids) and centrifuged at 2,000g for 10 min at 4 °C. The supernatant was further centrifuged at 10,000g for 30 min at 4 °C and filtered through a 0.2 µm vacuum-driven filter. To increase EV yield, Total Exosome Isolation Reagent (from cell culture media) (Thermo Fisher Scientific) was added to the media at a ratio of 1:2, mixed well, and stored overnight at 4 °C. The media and reagent solution were then centrifuged at 100,000g for 70 min at 4 °C using a Beckman L8-80 M ultracentrifuge equipped with a SW 32 Ti swinging bucket rotor. The supernatant was removed, and the pellet resuspended in approximately 10 mL 1×PBS which had previously been filtered (0.22 µm). The suspension was centrifuged again at 100,000g for 70 min at 4 °C, and all except for approximately 300–400 µL PBS removed. The EV pellet was resuspended in the remaining PBS and stored at −80°c until RNA or protein extraction.
2.4. Electron microscopy imaging of extracellular vesicles
Samples were fixed after being pelleted by combining 25 µl of sample with 25 µl of 8 % paraformaldehyde/PBS for at least 10 min, for a final concentration of 4 % paraformaldehyde. Samples were stored at 4 °C until time of imaging. 10 µl of the sample suspension was then pipetted onto a 300-mesh formvar carbon coated grid for 2 min at room temperature. The grid was then stained with 1 % uranyl acetate for 1 min and allowed to air dry at room temperature. Digital images were captured with a Gatan Orius SC1000 side mount camera attached to an FEI T12 transmission electron microscope operating at an accelerating voltage of 80 kV.
2.5. RNA extraction and western blotting
Half of the total EV yield from each replicate (−25 µL used for electron microscopy analysis) was used to extract RNA using the Maxwell automated nucleic acid extraction system with the Maxwell® RSC miRNA Tissue Kit (AS1460, Promega) according to the manufacturer’s protocol. Fragment analysis was performed on a 5200 Fragment Analyzer using the Agilent DNF-470 Small RNA Kit (DNF-470-55, Agilent).
The remaining half of the EV sample yield was used for western blotting. Proteins were extracted by combining sample 1:1 with RIPA buffer (Thermo Fisher) supplemented with 25× Complete Protease Inhibitor (Thermo Fisher), incubating for 20 min at 4 °C and homogenizing by vortex. Protein samples were stored at −80 °C until time of use. 13 µL protein solution was combined with 5 µL NuPage Reaction Buffer and 1 µL Reducing Agent (Thermo Fisher) and incubated for 10 min at 70 °C. Samples were loaded onto a NuPAGE™ 4 to 12 %, Bis-Tris gel (Thermo Fisher), and run for 35 min at 200 V in NuPage MES Run Buffer (Thermo Fisher) plus NuPage Antioxidant (Thermo Fisher). An iBlot™ Gel Transfer Device (Thermo Fisher) was used to transfer the proteins onto nitrocellulose membranes. Ponceau S solution (Thermo Fisher) was used to confirm protein transfer to the membranes. Protein-containing membranes were blocked for 15 min in EveryBlot Blocking Reagent (Bio-Rad). The membranes were then incubated overnight at 4 °C in the following primary antibody solutions diluted 1:1000 in Bio-Rad EveryBlot Blocking Reagent: rabbit anti-VDAC (Abcam), rabbit anti-CD63 (Abcam), rabbit anti-TSG101 (Abcam). Following primary antibody incubation, membranes were washed 3 × 5 min in TBST (1× tris-buffered saline (TBS; Bio-Rad) plus 0.1 % tween-20 (Sigma)). Membranes were then incubated for 45 min at room temperature with secondary antibody anti-rabbit-HRP (Novus) diluted 1:5000 in EveryBlot Blocking Reagent (Bio-Rad). Membranes were washed 3 × 15 min in TBST. HRP was visualized using SuperSignal™ West Pico Plus Chemiluminescent Substrate (Thermo Fisher).
2.6. Droplet-digital PCR analysis of RNA
RNA copy numbers were evaluated using droplet-digital PCR (ddPCR; six-probe system). The 1-Step RT-ddPCR Advanced Kit for Probes (Bio-Rad) was used following the manufacturer’s protocol for 25 µL reaction volumes with 2 µL of RNA per reaction and 1 µL each of the following probes: ND1 (Cy5.5, 12005573, Bio-Rad), MT-CO2 (HEX, 10031255, Bio-Rad), and GAPDH (ROX, 12017425, Bio-Rad). Droplets were generated using the Automated Droplet Generator (Bio-Rad, CA, USA). PCR was performed with an annealing temperature of 60 °C for 1 min × 40 cycles. Droplets were read using the QX600 Droplet Reader (Bio-Rad, CA, USA). Thresholding was applied equally across all samples for each probe.
2.7. miRNA detection and analysis
miRNA expression was examined with the NanoString© platform (https://www.nanostring.com) utilizing the Human miRNA codeset (Ns_H_Mir_v3B) that measures 798 endogenous miRNAs and 6 housekeeping (HK) mRNAs. 50 ng of each total RNA sample was prepared as per the manufacturer’s instructions. Only samples with an RNA concentration >20 ng/µL were of sufficient concentration to use in NanoString analysis. A total of n = 6 replicates taken between growth days 41–49 were of sufficient concentration to include in the final NanoString analysis. RNA expression was quantified on the nCounter Digital AnalyzerTM and raw and adjusted counts were generated with nSolver (v4.0)TM software. 23 of 24 samples passed nSolver’s initial QA/QC checks. In sum, all data were adjusted utilizing the manufacturer’s positive and negative experimental control probes. For organoid samples, data were normalized with 3 highly correlated HK genes. For the EV samples, data were normalized to the ligation controls. Compiled raw and data adjusted with positive/negative controls and the HK genes/ligation controls were exported as.csv files. nSolver normalized data were then imported into Partek_v7.0 and log2 transformed for further QA/QC. Probes below the limit of detectivity were excluded.
2.8. EV-associated miRNA gene and pathway analysis
DIANA-miRPath v4.0 (Tastsoglou et al., 2023) was used with TarBase v8.0 to perform a KEGG analysis analyzing the gene union of miRNA found in EVs. The top 20 enriched terms are shown. Ingenuity pathway analysis (IPA) was used to further analyze pathways and functions associated with genes encoded by mRNA targeted by the CO-EV-associated miRNA. Targets of miRNA were first determined using the miRNA Target Filter analysis tool in IPA using Ingenuity Expert Findings, miRecords, and TarBase as sources. The species was specified as human, and miRNA confidence was limited to “experimentally observed”. The resulting list of mRNAs was used to perform a further IPA Core Expression Analysis using all databases in the IPA directory with species specified as human. For all analyses, a p-value cutoff of p < 0.05 was used (–log(p–value) > 1.3).
3. Results
3.1. Cerebral organoids release small extracellular vesicles
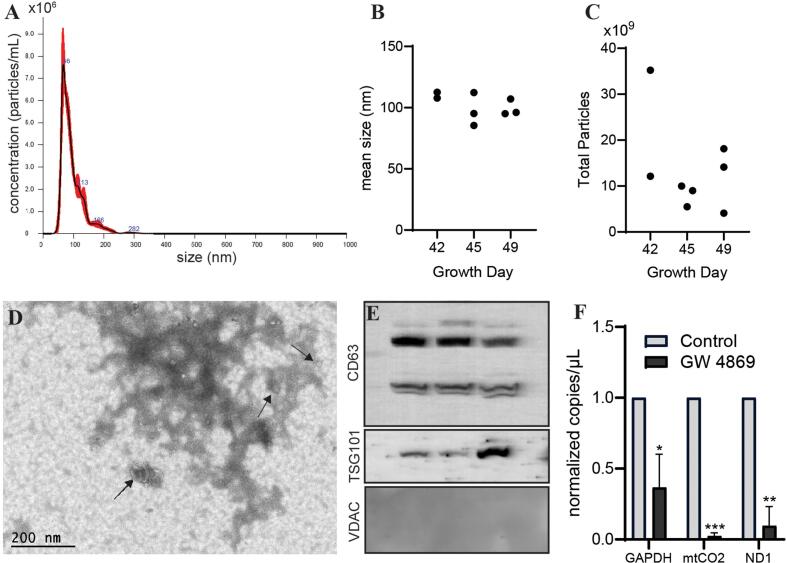
Cerebral organoids were generated from human embryonic stem cells (hESCs) using a commercially available kit and successful differentiation was verified as previously described (Silver et al., 2024). Conditioned medium was collected from mature cerebral organoids (>growth day 40), and extracellular particles were isolated using a modified ultracentrifugation protocol combined with an EV precipitation reagent to maximize EV yield (see Materials and Methods). Several methodologies were used to confirm that the particles possessed characteristics of small EVs. Nanoparticle Tracking Analysis (NTA) was used to assess size and concentration of the particles. NTA revealed that nearly all particles were under 200 nm in diameter (Fig. 1A). Further, particles isolated from conditioned media taken from a few different time points post-maturation of the organoids (growth days 42–49) maintained a similar mean size of approximately 100 nm (Fig. 1B). A total particle count of at least 2 × 109 was obtained from all time points (Fig. 1C). Electron microscopy analysis revealed particles of around 100 nm in diameter with a circular cup-like structure, as well as smaller circular particles ranging from 20-50 nm (Fig. 1D). Protein analysis identified the presence of CD63, a membrane protein commonly enriched in EVs, and TSG101, a primarily cytoplasmic protein reported to be enriched in small EVs (<200 nm) and exosomes (Kowal et al., 2016). The mitochondrial protein VDAC was not detected (Fig. 1E). To further confirm that our isolated particles were indeed small EVs, we evaluated RNA cargo from EVs released by cerebral organoids treated with or without GW 4869, an inhibitor of exosome release (Trajkovic et al., 2008). Droplet-digital PCR (ddPCR) was employed to quantify copy number of GAPDH and two mitochondrial markers (ND1, mtCO2) in the EV-associated RNA, transcripts which have previously been observed to be highly represented in EVs (Luo et al., 2022). Normalized copy number of all three markers was significantly decreased as compared to control (Fig. 1F). This observation implies that release of at least some of the recovered EVs may be impacted by the exosome inhibitor GW 4869. Together, these data suggest that cerebral organoids release particles with characteristics of small EVs.
Fig. 1.
Cerebral organoids release particles with properties of small extracellular vesicles. (A) Representative nanoparticle tracking analysis histogram showing the relative size distribution of particles isolated from mature cerebral organoids. Mean size (B) and total particle count recovered per batch of (approximately 200) organoids each (C) compared in particles extracted from cerebral organoids at three time points post-maturation. Shown are individual data points from separate biological replicates. (D) Tunneling Electron Microscopy (TEM) image of particles isolated from mature cerebral organoids. Scale bar represents 200 nm. (E) Western Blot performed using protein extracted from particles derived from cerebral organoids on growth day 45. Three biological replicates are shown, using antibodies against the EV markers CD63 and TSG101, and the mitochondrial protein VDAC. (F) Droplet-digital PCR showing abundance of RNA transcripts corresponding to GAPDH, mtCO2, or ND1 in EVs isolated from cerebral organoids treated with or without GW 4869. Graph shows average concentrations + S.D. of n = 3 biological replicates. Samples were compared using a one-sample t-test comparing to a hypothetical value of 1.0. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
3.2. Cerebral-organoid-derived EVs contain miRNAs which may target pathways associated with neurological disease
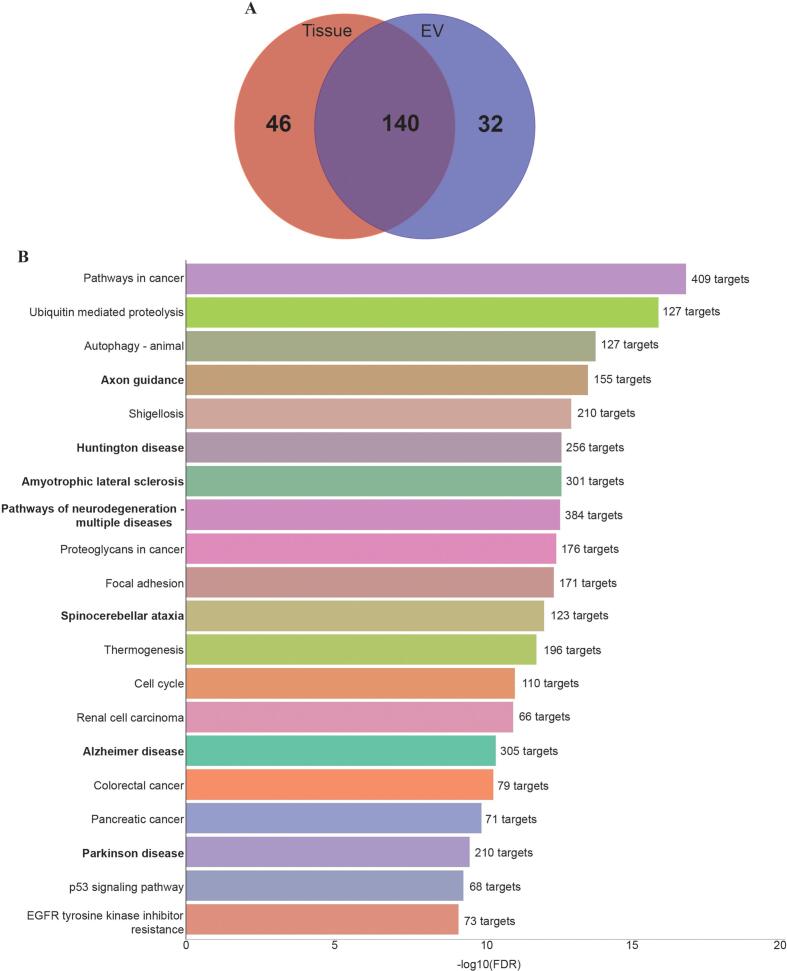
RNA was obtained from the cerebral-organoid-derived EVs (CO-EVs) in concentrations ranging from approximately 10–65 ng/µL (Supplementary Fig. 1A). Fragment analysis showed that the isolated RNA consisted of primarily short (10–40 nt) sequences (Supplementary Fig. 1B). The EV-associated RNA was further examined with the NanoString© platform using a Human miRNA codeset that measures 798 miRNAs. RNA derived from EVs was compared to RNA extracted from mature cerebral organoid tissues. A total of 172 miRNAs were identified in the EV-associated samples. Of these, 32 were unique to the EV samples and were not detected in the tissues (Fig. 2A). To characterize the EV-associated miRNA, a KEGG analysis of miRNA gene targets was performed using the DIANA-miRPath tools (Tastsoglou et al., 2023). Seven of the top 20 enriched terms were associated with neurological disease, including Axon Guidance, Huntington Disease, Amyotrophic Lateral Sclerosis, Pathways of Neurodegeneration, Spinocerebellar Ataxia, Alzheimer’s Disease, and Parkinson’s Disease (Fig. 2B).
Fig. 2.
Cerebral-Organoid-Derived EVs contain miRNAs which may target genes associated with neurological disease. (A) Venn diagram illustrating the numbers of miRNAs identified in cerebral organoid tissue versus CO-EVs using NanoString. (B) Top 20 enriched terms identified through a KEGG analysis using the DIANA-miRPath tool.
3.3. CO-EV-associated miRNAs may target genes associated with development, disease, and toxicity
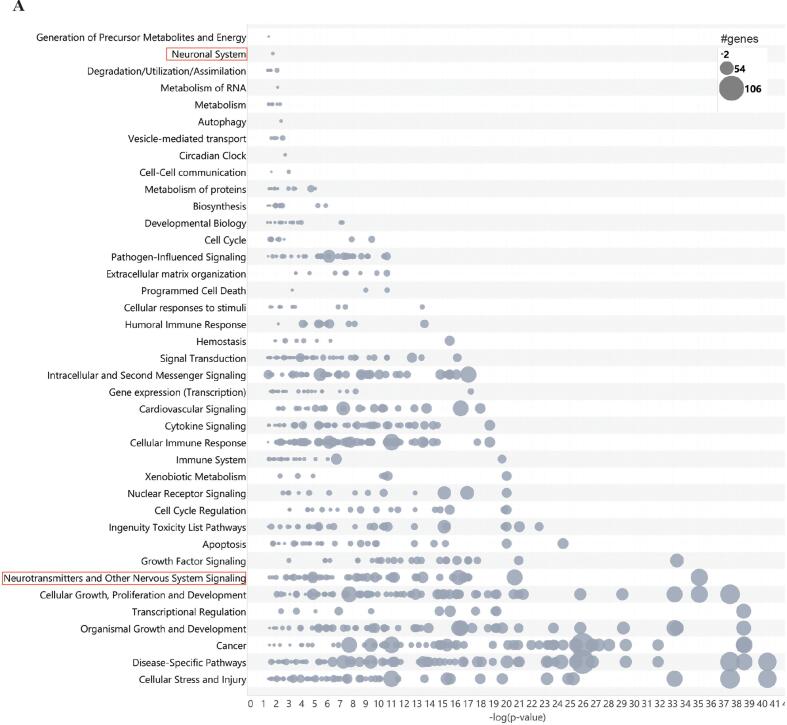
To further understand target pathways of CO-EV-associated miRNA, Ingenuity Pathway Analysis (IPA) was used to obtain a list of genes targeted by our recovered miRNAs. We then performed a core analysis using all available IPA databases to assess pathways associated with these target genes. This analysis revealed 862 different mRNAs targeted by our recovered CO-EV-associated miRNAs. Fig. 3A shows pathway categories associated with the targeted molecules, ranked by p-value and number of genes per pathway contained within each category. Notably, the top 10 most highly represented categories included those associated with development, toxicity, and neurological signaling, including Cellular Stress and Injury, Organismal Growth and Development, Neurotransmitters and Other Nervous System Signaling, Apoptosis, and Ingenuity Toxicity List Pathways.
Fig. 3.
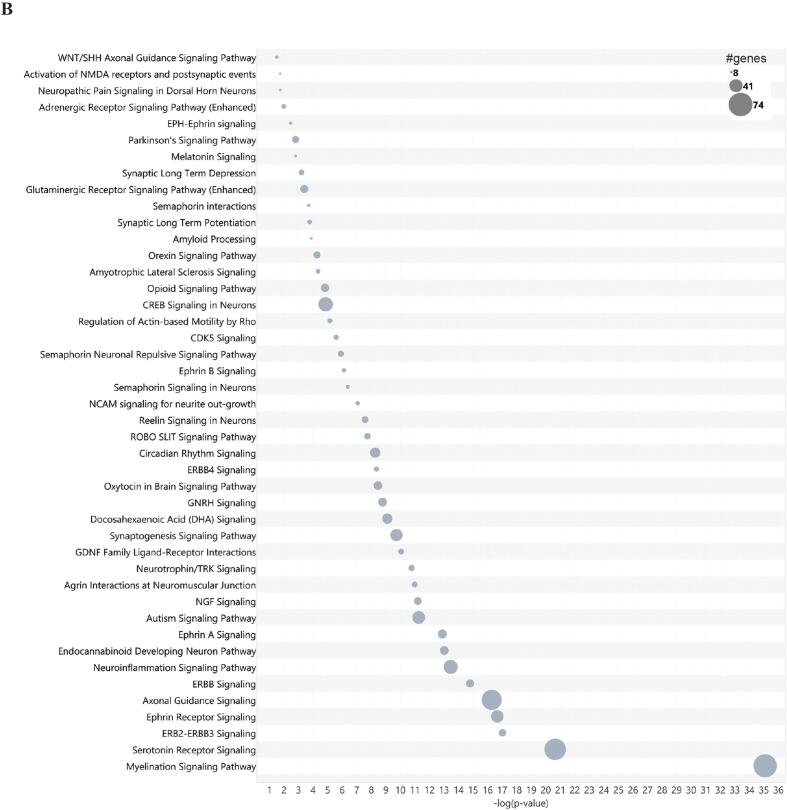
CO-EV-associated miRNA targets genes involved in pathways of cellular stress, development, and nervous system signaling. (A) Bubble plot showing the results of IPA core pathway analysis ranked by p-value and number of genes belonging to each pathway within the category. −log(p-value) is shown on the x-axis and pathway category is shown on the y-axis. A −log(p-value) cutoff of 1.3 was used for all categories. Red boxes indicate categories analyzed further in panel B. (B) Bubble plot showing specific pathways contained within the categories of Neurotransmitters and Other Nervous System Signaling and Neuronal System, ranked by p-value and number of genes within the pathway. −log(p-value) is shown on the x-axis and pathway name is shown on the y-axis. A −log(p-value) cutoff of 1.3 was used for all pathways. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.4. Genes involved in nervous system pathways and neurodegenerative disease are potentially targeted by CO-EV associated miRNAs
Since we were particularly interested in the capacity of cerebral organoids to serve as tools for the study of EV-associated biomarkers of neurotoxicity and neurodegeneration, we wished to further elucidate specific pathways contained within categories related to neuronal and nervous system signaling. Fig. 3B shows specific pathways contained within the categories Neurotransmitters and Other Nervous System Signaling and Neuronal System ranked by p-value and number of genes within each pathway. Myelination Signaling was the most highly represented pathway. Axonal Guidance was among the top five most highly represented pathways, consistent with previous findings using the DIANA-miRPath analysis tool. Neuroinflammation Signaling was also highly represented. Autism Signaling was among the ten most highly ranked pathways. Other pathways associated with neurodevelopment such as Synaptogenesis and Endocannabinoid Developing Neuron Pathway were also highly represented. Pathways which may have association with mechanisms of toxicity such as Opioid Signaling and disease terms that had also been identified in the prior KEGG analysis (Amyotrophic Lateral Sclerosis, Parkinson’s Signaling Pathway) were also present.
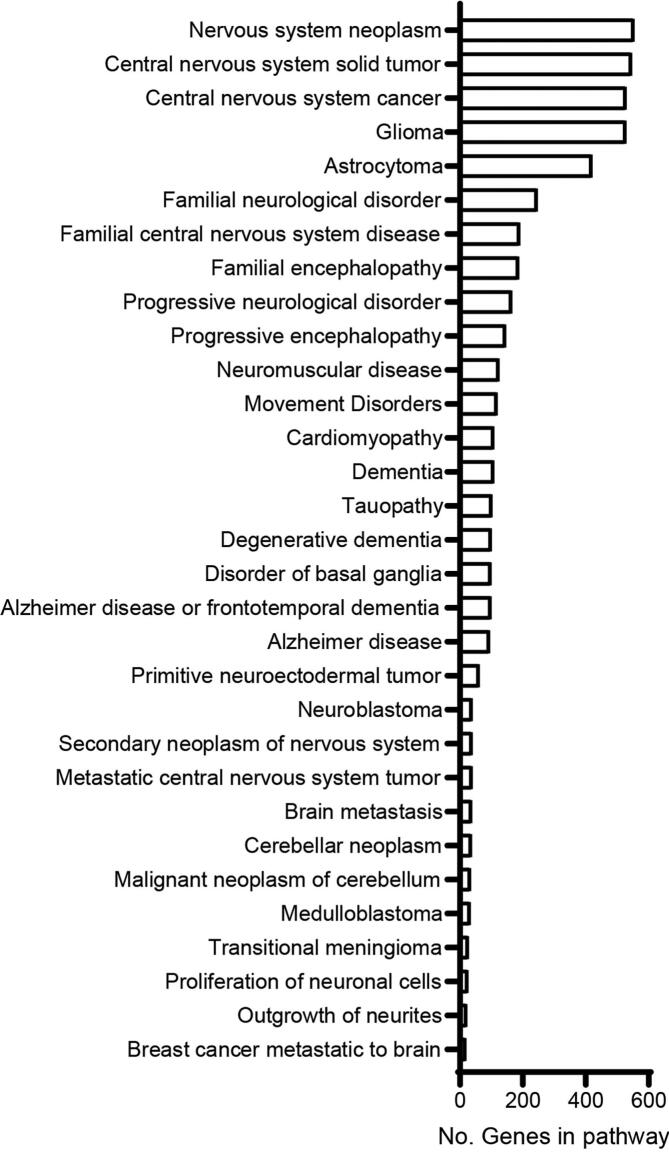
To delve more deeply into pathways of neurological disease targeted by miRNA contained within CO-EVs, we examined genes categorized based on related diseases and functions using IPA core analysis. These pathways were filtered to look specifically at those associated with neurological disease and nervous system function (Fig. 4). The largest number of genes were associated with neoplasms of the nervous system and brain. Many genes were also related to neurological and movement disorders. Several highly represented terms involved pathways of Dementia and AD.
Fig. 4.
Neurological diseases and functions associated with CO-EV-derived miRNA gene targets. Plot shows the number of genes targeted by CO-EV-associated miRNAs within the categories of neurological disease and nervous system function with a p-value < 0.05 as determined by IPA core analysis of diseases and functions. Number of genes is shown on the x-axis and associated disease or function term on the y-axis.
4. Discussion
In this study, we used a modified ultracentrifugation method to isolate small EVs from cerebral organoids. We observed that EVs ranging approximately from 30-150 nm in diameter were consistently recoverable from mature cerebral organoids. Further, we determined that the CO-EVs contained miRNAs in quantities sufficient for robust analysis using NanoString. KEGG analysis of the CO-EV-associated miRNA identified terms associated with neurodegenerative diseases including AD and PD. Further, IPA core analysis of genes targeted by the CO-EV-associated miRNA revealed numerous neurological pathways such as Myelination Signaling, Serotonin Receptor Signaling, Axonal Guidance, and Neuroinflammation. Notably, IPA analysis also revealed that many of these targeted genes were involved in pathways associated with Autism. This observation complements our previous findings that cerebral organoids release cell-free DNA sequences mapping to genes associated with the disease ontology terms Autism Spectrum Disorder and Intellectual Disability (Silver et al., 2024).
Our observation that CO-EVs contain miRNA in sufficient quantities for robust analysis underscores the potential of cerebral organoids as a tool for biomarker study. EVs have also been proposed to participate in spreading neurotoxic effects by transporting proteins and miRNA cargo between cells and tissues (Nicholson et al., 2022). For instance, EVs derived from myoblasts cultured from muscle biopsies of patients with Amyotrophic Lateral Sclerosis were more numerous than EVs derived from healthy individuals and induced neurotoxicity when applied to neurons in culture (Le Gall et al., 2022). EVs of neuronal or astrocytic lineage from the plasma taken from patients with AD also induced toxicity in cultured neurons (Nogueras-Ortiz et al., 2020). EV-encapsulated miR-21 specifically has been found to cause neurotoxicity (Yelamanchili et al., 2015). However, the exact mechanisms through which EVs mediate neurotoxicity and the full range of miRNAs involved in this process still require further research. CO-EVs may prove a useful tool for understanding EV-induced neurotoxicity.
Our finding that CO-EV-associated miRNAs are associated with AD, Amyotrophic Lateral Sclerosis, Huntington Disease, and pathways of neurodegeneration suggests that cerebral organoids release EVs that contain miRNAs relevant to pathways of neurological disease. Our observation that target genes were associated with pathways connected to Neuroinflammation, Amyloid Processing, PD, and Autism provides further evidence that EVs from cerebral organoids carry miRNAs relevant to neurological disease pathways. However, our analysis also revealed a number of very broad terms as well, including pathways related to the cell cycle and pathways associated with various cancers. An intriguing area of future study would be designing organoid models to examine EVs in the context of brain cancers. Towards this end, investigating the impact of extracellular matrix composition on EV-miRNA makeup would also be a valuable area of future work. Further research is required to dissect the full spectrum of pathologies that CO-EVs could be used to study.
Absence of VDAC in EV preparations implies that mitochondrial fragments are not present in our EV fractions. However, mitochondrial RNA transcripts were observed. An investigation of the differential sorting mechanisms of RNA and protein into EVs released from cerebral organoids is beyond the scope of this study, but would be a fascinating area of future work. Our observation that GW 4869 decreased concentrations of EV-associated RNA from both genomic (GAPDH) and mitochondrial (mtCO2, ND1) origin suggests that at least part of the EV population we recovered from the cerebral organoids may have originated from an endocytic pathway (Kim et al., 2022). However, additional experimentation including particle counts and protein analyses are needed to fully understand the impact of pharmacological inhibitors such as GW 4869 on EVs released by cerebral organoids. Although our data strongly suggest that cerebral organoids release particles with characteristics of small EVs, further research is needed to ascertain the exact origin and classification of EVs released by cerebral organoids, and to establish their mechanisms of release. Notably, we detected a subset of miRNAs which were present in the EV preparations but not in associated tissues. Although this observation appears counterintuitive at first glance, previous reports have documented that packaging of RNA into EVs can alter the transcriptional profile of their parent tissues (O’Grady et al., 2022). Another possibility is biogenesis or processing of miRNAs inside of EVs post-release (Melo et al., 2014). Further study into such mechanisms would be a valuable area of future research. Additionally, whether other types of cerebral organoids, such as neurological disease models or patient-derived organoids, release EV-encapsulated miRNA in quantities sufficient for comprehensive analysis requires further investigation. Although our organoid models are more complex than 2D cell cultures and contain multiple differentiated cell types, they do not contain some important cell types such as microglia which are present in vivo. Future advancements in brain organoid culture will be valuable towards the study of EVs in vitro. In addition, cerebral organoids often exhibit heterogeneity across batches. The analysis of our recovered EVs gives a general picture of representative miRNA content, on average, throughout batches of cerebral organoids. However, future efforts could also include more individualized screening of organoids, to assess differences in EV content between individual organoids. A technical challenge to be overcome for such efforts, however, is recovery of very low concentrations of miRNA from small EV quantities.
An intriguing prospect would be if cerebral organoids treated with known or potential neurotoxicants could be used to identify EV-associated miRNAs that are indicative of exposure severity or impending neurodegeneration. Future research in this area would be of value towards discovering novel EV-related biomarkers of neurotoxicity. Previously, we identified that cerebral organoids release cell-free DNA corresponding to genes associated with neurodevelopment and neurological disorders such as Autism Spectrum Disorder (Silver et al., 2024). Together, these studies suggest that cerebral organoids may be a promising new approach methodology for the identification of circulating biomarkers and bioactive molecules.
In summary, here we have characterized EV-associated miRNAs derived from cerebral organoids generated from hESCs. We determined that CO-EVs contain miRNA in quantities sufficient for analysis using NanoString. Further, pathway analysis of the miRNAs and their targeted genes identified several terms related to neurodegenerative diseases and nervous system signaling. Future efforts include examining EV release in cerebral organoids treated with neurotoxicants and organoid models of neurological disease. Another future avenue of research includes investigating mechanisms through which EVs propagate neurotoxic phenotypes. Together, our data suggest that CO-EVs have potential as a tool to identify novel early risk biomarkers and to elucidate mechanisms of EV-propagated neurotoxicity.
CRediT authorship contribution statement
Brian B. Silver: Conceptualization, Methodology, Investigation, Visualization, Writing – original draft. Rick Fannin: Methodology, Formal analysis. Kevin Gerrish: Resources, Supervision, Writing – review & editing. Erik J. Tokar: Supervision, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by NIEHS grants ES102546 to KG and ES103378-01 to ET.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We wish to acknowledge the NIEHS Genomics Core and especially Wesley Gladwell for assistance with droplet-digital PCR and NanoString services. We also wish to thank the Electron Microscopy Support Team at NIEHS for preparation of electron microscopy images.
Footnotes
This article is part of a special issue entitled: ‘Stem Cells’ published in Current Research in Toxicology.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crtox.2025.100229.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Extracellular vesicles derived from mature cerebral organoids contain measurable quantities of miRNA. (A) Total concentrations of RNA extracted from CO-EVs. Shown are individual data points taken from three biological replicates at three time points post-maturation. (B) Representative fragment analysis of RNA extracted from a sample of CO-EVs.
References
- Aharon A., Spector P., Ahmad R.S., Horrany N., Sabbach A., Brenner B., Aharon-Peretz J. Extracellular vesicles of Alzheimer's disease patients as a biomarker for disease progression. Mol. Neurobiol. 2020;57(10):4156–4169. doi: 10.1007/s12035-020-02013-1. [DOI] [PubMed] [Google Scholar]
- Arrifano G.D., Augusto-Oliveira M., Sealey-Bright M., Zainal J., Imbiriba L., Fernandes L.M.P., Maia C.S.F., Anthony D., Crespo-Lopez M.E. Contributing to understand the crosstalk between brain and periphery in methylmercury intoxication: neurotoxicity and extracellular vesicles. Int. J. Mol. Sci. 2021;22:19. doi: 10.3390/ijms221910855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur P., Kandoi S., Sun L., Kalvala A., Kutlehria S., Bhattacharya S., Kulkarni T., Nimma R., Li Y., Lamba D.A., Singh M. Biophysical, molecular and proteomic profiling of human retinal organoid-derived exosomes. Pharm. Res. 2023;40(4):801–816. doi: 10.1007/s11095-022-03350-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahram Sangani N., Koetsier J., Gomes A.R., Diogo M.M., Fernandes T.G., Bouwman F.G., Mariman E., Ghazvini M., Gribnau J., Curfs L.M. Involvement of extracellular vesicle microRNA clusters in developing healthy and Rett syndrome brain organoids. Cell. Mol. Life Sci. 2024;81(1):1–16. doi: 10.1007/s00018-024-05409-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buenafe A.C., Dorrell C., Reddy A.P., Klimek J., Marks D.L. Proteomic analysis distinguishes extracellular vesicles produced by cancerous versus healthy pancreatic organoids. Sci. Rep. 2022;12(1):3556. doi: 10.1038/s41598-022-07451-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chansaenroj A., Adine C., Charoenlappanit S., Roytrakul S., Sariya L., Osathanon T., Rungarunlert S., Urkasemsin G., Chaisuparat R., Yodmuang S., Souza G.R., Ferreira J.N. Magnetic bioassembly platforms towards the generation of extracellular vesicles from human salivary gland functional organoids for epithelial repair. Bioact. Mater. 2022;18:151–163. doi: 10.1016/j.bioactmat.2022.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin-Chan M., Navarro-Yepes J., Quintanilla-Vega B. Environmental pollutants as risk factors for neurodegenerative disorders: Alzheimer and Parkinson diseases. Front. Cell Neurosci. 2015;9 doi: 10.3389/fncel.2015.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couch Y. Challenges associated with using extracellular vesicles as biomarkers in neurodegenerative disease. Expert Rev. Mol. Diagn. 2023;23(12):1091–1105. doi: 10.1080/14737159.2023.2277373. [DOI] [PubMed] [Google Scholar]
- Heidari S., Mostafaei S., Razazian N., Rajati M., Saeedi A., Rajati F. Correlation between lead exposure and cognitive function in 12-year-old children: a systematic review and meta-analysis. Environ. Sci. Pollut. Res. 2021;28(32):43064–43073. doi: 10.1007/s11356-021-14712-w. [DOI] [PubMed] [Google Scholar]
- Ji X., Zhou S., Wang N., Wang J., Wu Y., Duan Y., Ni P., Zhang J., Yu S. Cerebral-organoid-derived exosomes alleviate oxidative stress and promote LMX1A-dependent dopaminergic differentiation. Int. J. Mol. Sci. 2023;24(13) doi: 10.3390/ijms241311048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Koo B.K., Knoblich J.A. Human organoids: model systems for human biology and medicine. Nat. Rev. Mol. Cell Biol. 2020;21(10):571–584. doi: 10.1038/s41580-020-0259-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.H., Lee C.H., Baek M.C. Dissecting exosome inhibitors: therapeutic insights into small-molecule chemicals against cancer. Exp. Mol. Med. 2022;54(11):1833–1843. doi: 10.1038/s12276-022-00898-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowal J., Arras G., Colombo M., Jouve M., Morath J.P., Primdal-Bengtson B., Dingli F., Loew D., Tkach M., Thery C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. PNAS. 2016;113(8):E968–E977. doi: 10.1073/pnas.1521230113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritikos M., Gandy S.E., Meliker J.R., Luft B.J., Clouston S.A.P. Acute versus chronic exposures to inhaled particulate matter and neurocognitive dysfunction: pathways to Alzheimer's disease or a related dementia. J. Alzheimers Dis. 2020;78(3):871–886. doi: 10.3233/JAD-200679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster M.A., Renner M., Martin C.-A., Wenzel D., Bicknell L.S., Hurles M.E., Homfray T., Penninger J.M., Jackson A.P., Knoblich J.A. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501(7467):373–379. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gall L., Duddy W.J., Martinat C., Mariot V., Connolly O., Milla V., Anakor E., Ouandaogo Z.G., Millecamps S., Lainé J., Vijayakumar U.G., Knoblach S., Raoul C., Lucas O., Loeffler J.P., Bede P., Behin A., Blasco H., Bruneteau G., Amador M.D., Devos D., Henriques A., Hesters A., Lacomblez L., Laforet P., Langlet T., Leblanc P., Le Forestier N., Maisonobe T., Meininger V., Robelin L., Salachas F., Stojkovic T., Querin G., Dumonceaux J., Browne G.B., De Aguilar J.L.G., Duguez S., Pradat P.F. Muscle cells of sporadic amyotrophic lateral sclerosis patients secrete neurotoxic vesicles. J. Cachexia Sarcopeni. 2022;13(2):1385–1402. doi: 10.1002/jcsm.12945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.N., Fang R.Y., Liu Z.H., Jiang L.P., Zhang J.D., Li H.H., Liu C.Y., Li F. The association between toxic pesticide environmental exposure and Alzheimer's disease: a scientometric and visualization analysis. Chemosphere. 2021;263 doi: 10.1016/j.chemosphere.2020.128238. [DOI] [PubMed] [Google Scholar]
- Liu C., Helsper S., Marzano M., Chen X., Muok L., Esmonde C., Zeng C., Sun L., Grant S.C., Li Y. Human forebrain organoid-derived extracellular vesicle labeling with iron oxides for in vitro magnetic resonance imaging. Biomedicines. 2022;10(12) doi: 10.3390/biomedicines10123060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo T., Chen S.Y., Qiu Z.X., Miao Y.R., Ding Y., Pan X.Y., Li Y., Lei Q., Guo A.Y. Transcriptomic features in a single extracellular vesicle via single‐cell RNA sequencing. Small Methods. 2022;6(11) doi: 10.1002/smtd.202200881. [DOI] [PubMed] [Google Scholar]
- Martin K.V., Sucharew H., Dietrich K.N., Parsons P.J., Palmer C.D., Wright R., Amarasiriwardena C., Smith D.R., Haynes E.N. Co-exposure to manganese and lead and pediatric neurocognition in East Liverpool, Ohio. Environ. Res. 2021;202 doi: 10.1016/j.envres.2021.111644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo S.A., Sugimoto H., O’Connell J.T., Kato N., Villanueva A., Vidal A., Qiu L., Vitkin E., Perelman L.T., Melo C.A. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell. 2014;26(5):707–721. doi: 10.1016/j.ccell.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngalame N.N.O., Luz A.L., Makia N., Tokar E.J. Arsenic alters exosome quantity and cargo to mediate stem cell recruitment into a cancer stem cell-like phenotype. Toxicol. Sci. 2018;165(1):40–49. doi: 10.1093/toxsci/kfy176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson S., Baccarelli A., Prada D. Role of brain extracellular vesicles in air pollution-related cognitive impairment and neurodegeneration. Environ. Res. 2022;204(Pt C) doi: 10.1016/j.envres.2021.112316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueras-Ortiz C.J., Mahairaki V., Delgado-Peraza F., Das D., Avgerinos K., Eren E., Hentschel M., Goetzl E.J., Mattson M.P., Kapogiannis D. Astrocyte- and neuron-derived extracellular vesicles from Alzheimer's disease patients effect complement-mediated neurotoxicity. Cells. 2020;9(7) doi: 10.3390/cells9071618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Grady T., Njock M.-S., Lion M., Bruyr J., Mariavelle E., Galvan B., Boeckx A., Struman I., Dequiedt F. Sorting and packaging of RNA into extracellular vesicles shape intracellular transcript levels. BMC Biol. 2022;20(1):72. doi: 10.1186/s12915-022-01277-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penning D.H., Cazacu S., Jevtovic-Todorovic V., Kalkanis S., Lewis M.C., Brodie C. Neuron-glia crosstalk plays a major role in the neurotoxic effects of ketamine via extracellular vesicles. Anesth. Analg. 2021;132(5s_Suppl):558–559. doi: 10.3389/fcell.2021.691648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purushothaman A. Exosomes from cell culture-conditioned medium: isolation by ultracentrifugation and characterization. Methods Mol. Biol. 2019;1952:233–244. doi: 10.1007/978-1-4939-9133-4_19. [DOI] [PubMed] [Google Scholar]
- Silver B.B., Brooks A., Gerrish K., Tokar E.J. Isolation and characterization of cell-free DNA from cerebral organoids. Int. J. Mol. Sci. 2024;25(10) doi: 10.3390/ijms25105522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szvicsek Z., Oszvald A., Szabo L., Sandor G.O., Kelemen A., Soos A.A., Paloczi K., Harsanyi L., Tolgyes T., Dede K., Bursics A., Buzas E.I., Zeold A., Wiener Z. Extracellular vesicle release from intestinal organoids is modulated by Apc mutation and other colorectal cancer progression factors. Cell Mol. Life Sci. 2019;76(12):2463–2476. doi: 10.1007/s00018-019-03052-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tastsoglou S., Skoufos G., Miliotis M., Karagkouni D., Koutsoukos I., Karavangeli A., Kardaras F.S., Hatzigeorgiou A.G. DIANA-miRPath v4.0: expanding target-based miRNA functional analysis in cell-type and tissue contexts. Nucl. Acids Res. 2023;51(W1):W154–W159. doi: 10.1093/nar/gkad431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauro B.J., Greening D.W., Mathias R.A., Mathivanan S., Ji H., Simpson R.J. Two distinct populations of exosomes are released from LIM1863 colon carcinoma cell-derived organoids. Mol. Cell. Proteomics. 2013;12(3):587–598. doi: 10.1074/mcp.M112.021303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trajkovic K., Hsu C., Chiantia S., Rajendran L., Wenzel D., Wieland F., Schwille P., Brügger B., Simons M. Ceramide triggers budding of exosome vesicles into multivesicular Endosomes. Science. 2008;319(5867):1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- Tsilioni I., Theoharides T.C. Extracellular vesicles are increased in the serum of children with autism spectrum disorder, contain mitochondrial DNA, and stimulate human microglia to secrete IL-1β. J. Neuroinflamm. 2018;15 doi: 10.1186/s12974-018-1275-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visconte C., Golia M.T., Fenoglio C., Serpente M., Gabrielli M., Arcaro M., Sorrentino F., Busnelli M., Arighi A., Fumagalli G., Rotondo E., Rossi P., Arosio B., Scarpini E., Verderio C., Galimberti D. Plasma microglial-derived extracellular vesicles are increased in frail patients with Mild Cognitive Impairment and exert a neurotoxic effect. Geroscience. 2023;45(3):1557–1571. doi: 10.1007/s11357-023-00746-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Kojima K., Mobley J.A., West A.B. Proteomic analysis of urinary extracellular vesicles reveal biomarkers for neurologic disease. EBioMedicine. 2019;45:351–361. doi: 10.1016/j.ebiom.2019.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yáñez-Mó M., Siljander P.R.M., Andreu Z., Zavec A.B., Borràs F.E., Buzas E.I., Buzas K., Casal E., Cappello F., Carvalho J., Colás E., Cordeiro-da Silva A., Fais S., Falcon-Perez J.M., Ghobrial I.M., Giebel B., Gimona M., Graner M., Gursel I., Gursel M., Heegaard N.H.H., Hendrix A., Kierulf P., Kokubun K., Kosanovic M., Kralj-Iglic V., Krämer-Albers E.M., Laitinen S., Lässer C., Lener T., Ligeti E., Line A., Lipps G., Llorente A., Lötvall J., Mancek-Keber M., Marcilla A., Mittelbrunn M., Nazarenko I., Nolte-t' Hoen E.N.M., Nyman T.A., O'Driscoll L., Olivan M., Oliveira C., Pállinger É., del Portillo H.A., Reventós J., Rigau M., Rohde E., Sammar M., Sánchez-Madrid F., Santarém N., Schallmoser K., Ostenfeld M.S., Stoorvogel W., Stukelj R., Van der Grein S.G., Vasconcelos M.H., Wauben M.H.M., De Wever O. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles. 2015;4 doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelamanchili S.V., Lamberty B.G., Rennard D.A., Morsey B.M., Hochfelder C.G., Meays B.M., Levy E., Fox H.S. MiR-21 in extracellular vesicles leads to neurotoxicity via TLR7 signaling in SIV neurological disease. PLoS Pathog. 2015;11(7) doi: 10.1371/journal.ppat.1005032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeold A., Sandor G.O., Kiss A., Soos A.A., Tolgyes T., Bursics A., Szucs A., Harsanyi L., Kittel A., Gezsi A., Buzas E.I., Wiener Z. Shared extracellular vesicle miRNA profiles of matched ductal pancreatic adenocarcinoma organoids and blood plasma samples show the power of organoid technology. Cell Mol. Life Sci. 2021;78(6):3005–3020. doi: 10.1007/s00018-020-03703-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Flores-Bellver M., Pan J., Benito-Martin A., Shi C., Onwumere O., Mighty J., Qian J., Zhong X., Hogue T., Amponsah-Antwi B., Einbond L., Gharbaran R., Wu H., Chen B.J., Zheng Z., Tchaikovskaya T., Zhang X., Peinado H., Canto-Soler M.V., Redenti S. Human retinal organoids release extracellular vesicles that regulate gene expression in target human retinal progenitor cells. Sci. Rep. 2021;11(1):21128. doi: 10.1038/s41598-021-00542-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Extracellular vesicles derived from mature cerebral organoids contain measurable quantities of miRNA. (A) Total concentrations of RNA extracted from CO-EVs. Shown are individual data points taken from three biological replicates at three time points post-maturation. (B) Representative fragment analysis of RNA extracted from a sample of CO-EVs.