Abstract
Tumor metastasis is the leading cause of high mortality in most cancers, and numerous studies have demonstrated that the malignant crosstalk of multiple components in the tumor microenvironment (TME) together promotes tumor metastasis. Cancer-associated fibroblasts (CAFs) are the major stromal cells and crosstalk centers in the TME of various kinds of tumors, such as breast cancer, pancreatic cancer, and prostate cancer. Recently, the CAF-induced pro-tumor metastatic TME has gained wide attention, being considered as one of the effective targets for tumor therapy. With in-depth research, CAFs have been found to promote tumor metastasis through multiple mechanisms, such as inducing epithelial–mesenchymal transition in tumor cells, remodeling the extracellular matrix, protecting circulating tumor cells, and facilitating the formation of a pre-metastatic niche. To enhance the anti-tumor metastasis effect, therapeutic strategies designed by combining nano-drug delivery systems with CAF modulation are undoubtedly a desirable choice, as evidenced by the research over the past decades. Herein, we introduce the physiological properties of CAFs, detail the possible mechanisms whereby CAFs promote tumor metastasis, categorize CAFs-based nano-drug delivery strategies according to their anti-metastasis functions and discuss the current challenges, possible solutions, as well as the future directions in order to provide a theoretical basis and reference for the utilization of CAFs-based nano-drug delivery strategies to promote tumor metastasis therapy.
Key words: Tumor metastasis, Cancer-associated fibroblasts, Nano-drug delivery system, Epithelial–mesenchymal transition, Extracellular matrix, Pre-metastatic niche, Circulating tumor cells, Tumor microenvironment
Graphical abstract
Nano-drug delivery systems are designed to deplete cancer-associated fibroblasts (CAFs), inhibit CAF activation or normalize CAFs, block the effects of CAFs, and turn CAFs into “friends” to exert anti-tumor metastasis effects.
1. Introduction
Tumor metastasis, the leading cause of high mortality in most cancers, is a tremendous challenge that urgently needs to be overcome in cancer therapy1, 2, 3. Crucially, a large number of studies have shown that tumor metastasis is not an autonomous process of tumor cells but rather a result of the combined effects of various components in the tumor microenvironment (TME)4. The TME can be regarded as a complex and highly structured ecosystem, mainly composed of cellular components (tumor cells, multiple immune cells, fibroblasts, etc.) and non-cellular components (cytokines, proteins, extracellular matrix, etc.)5,6. The crosstalk between tumor cells and multiple components in the TME changes dynamically throughout tumor progression, which is the primary contributor to tumor growth, invasion, and metastasis7. Therefore, identifying different targets and exploring strategies to regulate TME is currently a hot research direction in anti-tumor metastasis.
Cancer-associated fibroblasts (CAFs) are the most abundant stromal cells in the TME of desmoplastic tumors8. For example, CAFs account for 50%–70% of the total cells in the breast cancer TME9, whereas in pancreatic cancer they can even reach 90% of the tumor mass10, often acting as a crosstalk center in the TME11, 12, 13. Recently, a study stated that CAFs could promote lung cancer metastasis through mitophagy and mtDNA transfer14, indicating that CAFs are closely associated with tumor metastasis and are now a hot research topic. Specifically, CAFs can be activated from various precursor cells, such as tissue-resident fibroblasts, quiescent stellate cells, epithelial cells, etc., via different signaling pathways15,16. It is well established that CAFs can inhibit the function of immune cells in the TME and modulate the extracellular matrix (ECM) by secreting a variety of cytokines or metabolites, thus facilitating the formation of a “tumor hospitable” TME, which provides an immunosuppressive environment and hinders drug permeation for tumor cell survival4,17. Afterward, the crosstalk between CAFs and tumor cells will exert pro-metastatic effects by inducing epithelial–mesenchymal transition (EMT) of tumor cells, remodeling ECM, protecting circulating tumor cells (CTCs), and facilitating the formation of a pre-metastatic niche18, 19, 20. Interestingly, the effects of CAFs on tumor metastasis are mostly focused on hematogenous metastasis at this stage21, 22, 23, 24. Therefore, the term “tumor metastasis” in this article refers to hematogenous metastasis. Moreover, tumor tissues tend to generate richer blood vessels to meet the more intense proliferation needs of tumor cells, with this also offering more opportunities for CAFs to promote hematogenous metastasis of tumor cells25. In this context, CAFs have become a major target for anti-tumor metastasis based on TME modulation.
Nano-drug delivery systems (NDDS) are an emerging product of utilizing nanotechnology in drug delivery to improve drug efficacy and reduce side effects26. Anti-tumor strategies with the assistance of NDDS can be used in the diagnosis, treatment, and prevention of tumors, which have received increasing attention27,28. Over the past 30 years, various NDDS such as nanoparticles, liposomes, micelles, and nanoemulsions have been developed for tumor treatment based on different therapeutic desires and drug properties, bringing new light to anti-tumor metastasis and showing remarkable progress29, 30, 31. By optimizing their physicochemical properties, NDDS can improve the solubility, membrane permeability, and pharmacokinetic behavior of loaded drugs32. In addition, specific nano-sized drug delivery systems can be passively accumulated at the tumor site due to the enhanced permeability and retention (EPR) effect in tumor tissues33. On the other hand, with the incorporation of functional molecules, the drug delivery efficacy can be further enhanced, such as by achieving targeted delivery, slow or sustained release, and improving the deep penetration of drugs at the tumor site34. However, merely inhibiting tumor cells is not sufficient to achieve the desired anti-tumor metastasis effect due to the presence of CAFs-enriched TME35. Thus, recent studies have shifted their attention to the suppression of CAFs. Most of the CAFs-based NDDS studies at this stage are comprehensive treatment strategies, which usually exhibit better anti-tumor metastasis effects36. This is mainly due to the comprehensive treatment strategy that can inhibit the direct promoting effect of CAFs on tumor metastasis37. In addition, it can also enhance the effects of chemotherapeutic agents on tumor cells in combination therapy by inhibiting the CAFs-mediated unfriendly TME, ultimately reducing the risk of tumor metastasis and hindering the malignant progression of tumors38,39.
This review focuses on the specific mechanisms by which CAFs promote tumor metastasis and the current CAF-based nano-drug delivery strategies. Different from most of the current reviews concerning the effects of anti-CAF nanomedicine on tumor growth, we paid more attention to “tumor metastasis” and summarized the latest research cases. More importantly, we have screened the classic studies of anti-CAF NDDS against tumor metastasis. We elaborate on the role of CAFs in the key stages of tumor cell metastasis and, for the first time, summarize the detailed functions and anti-tumor metastasis efficiency of CAFs-based NDDS in terms of suppressing CAF activation, depleting CAFs, normalizing CAFs, modulating CAF effects, and turning CAFs into “friends”. We have attempted to summarize the progress of CAFs-based NDDS in the field of anti-tumor metastasis from a novel perspective in detail. In addition, we propose the challenges and possible solutions in the current studies of CAFs-based NDDS, as well as the promising research directions in the future, aiming to provide a reference for the research of CAFs-based NDDS for anti-tumor metastasis.
2. Cancer-associated fibroblasts (CAFs)
2.1. Definition and features of CAFs
Defining CAFs first requires a clear understanding of fibroblasts. The history of fibroblasts can be traced back to 1858 when the German pathologist Rudolf Virchow first described fibroblasts as a distinct cell type called “spindle cells of connective tissue”. Later, Ernst Ziegler first used the term “fibroblast” to identify this type of cell that produces new connective tissue after healing in 189540. Nowadays, fibroblasts are referred to canonically as cells that create and maintain an anatomically diverse array of ECM-rich connective tissues to support a broad range of essential organ functions41. In such conditions, CAFs can be defined as spindle-shaped fibroblasts located in or near tumor tissues that lack lineage markers for epithelial cells, endothelial cells, and hematopoietic cells, which functionally contribute to the promotion of tumor malignant behavior42. For these features, CAFs are distinguished from normal fibroblasts by both their appearance and their biological properties. In terms of appearance, CAFs are usually larger in morphology, with an indented nucleus and a cell cytoplasm with more branching structures43,44. As for biological properties, CAFs have greater proliferation, migration, and secretion abilities. Compared with normal fibroblasts, CAFs are more metabolically active and can secrete more ECM factors, such as envelope growth factor, secreted protein acidic and rich in cysteine (SPARC), and tenascin, etc. In addition, CAFs produce more collagen, with a rigid and constricted pattern of collagen deposition45.
2.2. Origins and biomarkers of CAFs
CAFs originate from various cells through the activation of diverse signaling pathways (Fig. 1). There is now a great deal of evidence confirming the existence of a variety of cellular precursors of CAFs46. For example, tissue-resident fibroblasts and stellate cells (e.g., pancreatic and hepatic stellate cells) can be recruited and activated into CAFs by cytokines such as TGF-β47, 48, 49, platelet-derived growth factor (PDGF)15,50, fibroblast growth factor 2 (FGF-2)51,52, stromal-derived factor 1 (SDF-1/CXCL12)53, and reactive oxygen species (ROS)54. The mesenchymal stem cells (MSCs) are also one of the origins of CAFs; cytokines such as TGF-β, the chemokine ligand family (SDF-1/CXCL12, CXCL16, CCL2, CCL5), and other cytokines are involved in the transformation of MSCs to CAFs16,55. Adipocytes can also be transformed into CAFs with high expression of fibroblast-specific protein-1 (FSP-1) under the stimulation of TGF-β156,57. With further studies, more potential sources of CAFs were identified, such as epithelial and endothelial cells that can be activated and differentiated into CAFs through the processes of epithelial–mesenchymal transition (EMT) and endothelial–mesenchymal transition (EndMT), respectively58,59. Pericytes and smooth muscle cells can be transformed into CAFs in response to TGF-β1 and WNT3A60, and monocytes can be transformed into CAFs through monocyte myoblast trans-differentiation (MMT) induced by ROS through the p38 neutrophil-activated protein kinase signaling pathway61.
Figure 1.
The possible origins of CAFs in the TME.
Clarifying the biomarkers of CAFs can help to distinguish CAFs from other stromal cells in TME, which is more conducive to the subsequent precision therapy against CAFs. Previous studies have shown that fibroblast activation protein (FAP)62, α-smooth muscle actin (α-SMA)63, fibroblast-specific protein (FSP-1/S100A4)64, platelet growth factor receptor α/β (PDGFRα/β)45,65, TENASCIN-C66, PERIOSTIN67, and chondroitin sulfate proteoglycan (NG2)68 can be considered as a set of biomarkers to define CAFs. Although these biomarkers are not specific to CAFs, a combination of them can help identify CAFs. In addition, some mesenchymal markers such as VIMENTIN, FIBRONECTIN, type I collagen, prolyl 4-hydroxylase, and fibroblast surface proteins can also be used as markers to aid in the identification of CAFs45,69,70. A recent study found that the membrane receptor protein ITGA5 could serve as a novel biomarker for CAFs, and its expression showed good agreement with the classical CAF markers FAP and α-SMA71. CAFs with different biomarkers may have specific biological functions; for example, α-SMA and FAP-positive CAFs can produce and regulate ECM, promote cell-ECM adhesion, exert immunosuppressive effects, and increase the alignment and stiffness of ECM, which is closely associated with the invasion and metastasis of tumor cells72,73. Further, the fibroblast-specific protein S100A4 exists relatively specifically on CAFs, and the S100A4-positively expressed CAFs can perform a unique tumor-protective role in immune surveillance by producing collagen17,74. With increasing research, CAVEOLIN-1 and G protein-coupled receptor 77 (GPR 77) have been found to act as biomarkers for a subgroup of pro-tumorigenic CAFs, which play a key role in promoting the malignant progression of tumor cells75,76. There are commonly used biomarkers of CAFs and their characteristics, as shown in Table 1 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107.
Table 1.
Commonly used biomarkers of CAFs and their characteristics.
| Expression site | Biomarker | Cross-expressed cell | Cancer with CAFs that express the marker | Biological function | Ref. |
|---|---|---|---|---|---|
| Cell membrane | FAP |
|
Breast cancer, lung cancer, colorectal, renal cancer, and ovarian cancer, etc. | Promoting tumor metastasis, immune evasion, and ECM remolding | 77, 78, 79 |
| PDGFRα/β |
|
Breast cancer, colorectal cancer, and cervical cancer, etc. | Promoting tumor metastasis, M2 macrophage polarization, angiogenesis | 80,81 | |
| CAVEOLIN-1 |
|
Pancreatic cancer, breast cancer, and gastric cancer, etc. | Promoting tumor invasion | 82,83 | |
| CD10 |
|
Breast cancer, colorectal cancer, and gastric cancer, etc. | Promoting tumor cell stemness | 84, 85, 86 | |
| GPR77 |
|
Breast cancer, gastric cancer, and ovarian cancer, etc. | Promoting tumor progression | 87,88 | |
| ITGA5 |
|
Breast cancer, colorectal cancer, and glioblastoma, etc. | Promoting tumor metastasis, tumor cell stemness, and angiogenesis | 89,90 | |
| NG2 |
|
Breast cancer, melanoma, squamous cell cancer, neuroblastoma, mesothelioma | Promoting tumor cell migration | 91, 92, 93 | |
| Cytoplasmic | α-SMA |
|
Pancreatic cancer, breast cancer, lung cancer, liver cancer, colon cancer, and renal cancer, etc. | Promoting tumor cell proliferation, ECM remodeling, and tumor cell stemness | 94,95 |
| VIMENTIN |
|
PDAC, breast cancer, prostate cancer, lung cancer, gastric cancer, etc. | Promoting tumor cell growth, invasion, and migration | 96, 97, 98 | |
| S100A4/FSP-1 |
|
Breast cancer, lung cancer, gastric cancer, endometrioid adenocarcinoma, etc. | Promoting tumor metastasis, immune evasion | 99 | |
| DESMIN |
|
Colorectal cancer, and gastric cancer, etc. | Promoting tumor cell growth, invasion, and migration | 100,101 | |
| ECM protein | TENASCIN-C |
|
Pancreatic cancer, breast cancer, prostate cancer, and cervical cancer, etc. | Promoting tumor metastasis and angiogenesis | 102, 103, 104 |
| PERIOSTIN |
|
Breast cancer, lung cancer, prostate cancer, and esophageal squamous cell cancer, etc. | Promoting tumor cell stemness, invasion and metastasis | 105, 106, 107 |
‒, not applicable. CAFs, cancer-associated fibroblasts; FAP, fibroblast activation protein; CD, cluster of differentiation; ECM, extracellular matrix; PDGFRα/β, platelet growth factor receptor α/β; GPR77, G protein-coupled receptor 77; ITGA5, integrin alpha5; NG2, chondroitin sulfate proteoglycan; α-SMA, α-smooth muscle actin; PDAC, pancreatic ductal adenocarcinoma; EMT, epithelial–mesenchymal transition; FSP-1, fibroblast-specific protein-1.
3. Mechanisms of CAFs in promoting tumor metastasis
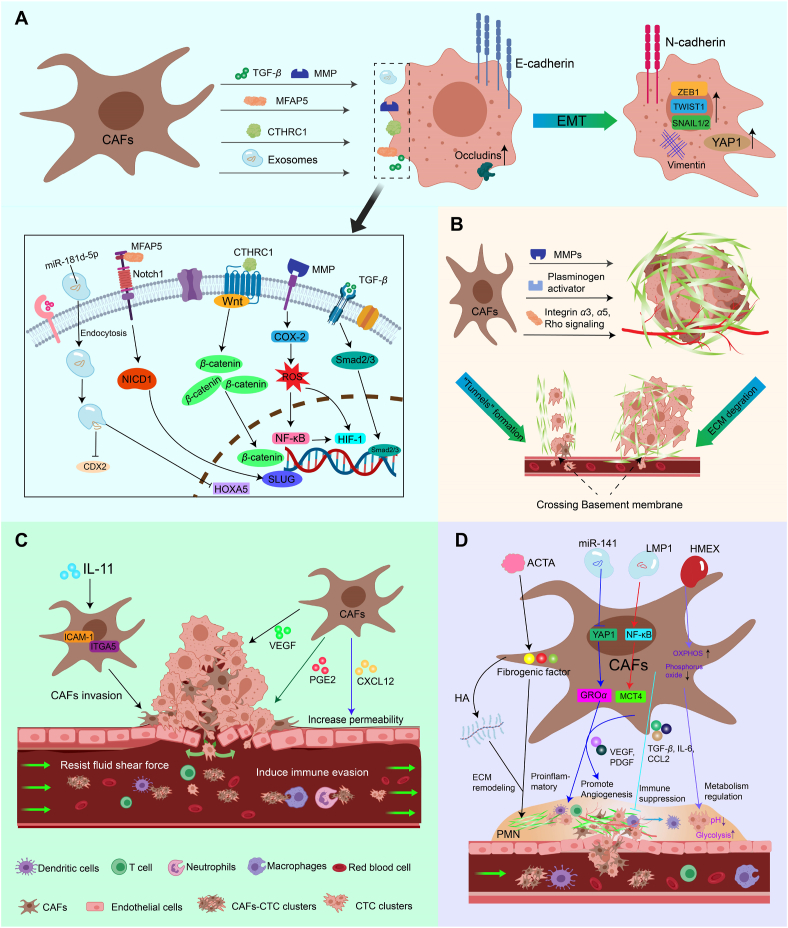
A great deal of evidence demonstrates that CAFs are present in different tumor types and behave as the primary stromal cells in the TME to construct a favorable environment for tumor development, which ultimately promotes the malignant progression of tumors to evolve into metastatic cancers through interactions with tumor cells108,109. According to the steps for tumor cells undergoing metastasis, CAFs were found to promote tumor cell invasion and metastasis by inducing EMT of tumor cells, regulating ECM, protecting CTCs that entered the blood circulation, and forming pre-metastatic ecological niches (Fig. 2).
Figure 2.
The specific mechanisms by which CAFs promote tumor metastasis. (A) Induction of EMT in tumor cells. CAFs can promote EMT in tumor cells by secreting TGF-β, MMP, MFAP5, CTHRC1 and exosomes containing miR-181d-5p. (B) Remodeling of ECM. CAFs promote tumor cell crossing the basement membrane via “tunnel” formation and ECM degradation, which in turn promotes tumor distant metastasis (C) Protection of CTCs. CAFs acquire an invasive phenotype and increased vascular permeability enables CAFs to better form CAFs-CTC clusters with tumor cells, thereby protecting CTCs from fluid shear forces and the immune system in the blood circulation (D) Facilitating the formation of PMN. CAFs facilitate PMN formation via ECM remodeling, pro-inflammatory effects, pro-angiogenesis, immune suppression and metabolism regulation.
3.1. Inducing EMT of tumor cells
EMT, the process by which tumor cells acquire a migratory and invasive phenotype in their primary location, is the mechanism by which tumor cells initiate invasion, and it provides epithelial cells with stem cell-like properties such as self-renewal, which in turn accelerates the metastatic process of tumor cells110. CAFs have been confirmed to be critical for the EMT process in desmoplastic tumors such as breast cancer, pancreatic cancer, prostate cancer, and lung cancer (Fig. 2A). A recent investigation of breast cancer patients revealed that the high CAFs patient group was significantly enriched in EMT-related signaling pathways111. Generally, tumor cells undergoing the EMT process will lose epithelial markers such as E-CADHERIN and gain the mesenchymal marker N-CADHERIN, and Matsumura et al.112 suggested that CAFs induce the formation of breast tumor cell clusters in a hybrid epithelial/mesenchymal state (E-CADHERIN Lo ZEB1 Hi), which leads to collective invasion113.
Previous studies have found that CAFs can induce EMT in tumor cells by secreting a variety of cytokines, including TGF-β18, epidermal growth factor (EGF), PDGF, hepatocyte growth factor (HGF)114, and SDF-1/CXCL12115. In addition, Giannoni et al.116 noted that CAF-secreted MMPs induce EMT in prostate cancer cells via the cyclooxygenase-2 (COX-2)/nuclear factor-κB (NF-κB)/hypoxia-inducible factor 1 (HIF-1) signaling pathway. A recent study found that collagen triple helix repeat protein 1 (CTHRC1), secreted by CAFs isolated from breast cancer tissues, can activate the WNT/β-catenin signaling pathway, in turn promoting EMT and the invasiveness of tumor cells117. According to Chen et al.118, microfibril-associated protein 5 (MFAP5) secreted by CAFs activated NOTCH1 signaling, which upregulated the expression of intracellular activation structural domain (NICD1) and the transcription factor SLUG, thereby facilitating the EMT of tumor cells. Moreover, CAFs were proven to promote the expression of YES-associated protein 1 (YAP1), VIMENTIN, and CD44 in tumor spheroids, and high expression of YAP1 was associated with a more severe EMT status and stromal index119. Further, CAFs can secrete exosomes that are loaded with proteins, lipids, and RNAs to affect TME and play an important role in tumor invasion and metastasis120. For example, it has been found that miR-181d-5p-containing exosomes released by CAFs downregulate CDX2 and HOXA5, and the downregulation of HOXA5 decreases the levels of E-CADHERIN and VIMENTIN and increases the levels of N-CADHERIN, SLUG, SNAIL1, TWIST1, ZEB1, and ZEB2, which accelerates the EMT process in MCF-7 cells121. It has also been noted that exosomal miR-625–3p from CAFs may promote migration, invasion, and EMT of CRC cells by inhibiting the CELF2/WWOX pathway122. In addition, CAFs activated from breast adipose tissue-derived mesenchymal stromal/stem cells could highly upregulate the gene expression of EMT transcriptional factors, including ZEB1, TWIST1, SNAIL1, and SNAIL2, with an associated increase in VIMENTIN as well as a decrease in CDH1 (coding for the calcium ion-dependent cell adhesion molecule E-CADHERIN)123,124.
3.2. Remodeling the ECM
The ECM of tumors is a highly dynamic, three-dimensional supramolecular entity composed of a range of molecules, including collagen, elastin, proteoglycans, laminin, and glycosaminoglycans125,126. As a central part of the TME, the ECM not only provides support and protection for tumor cells but also contributes to tumor cell metastasis by regulating cell motility and mediating intercellular signaling127. Usually, the ECM is classified into two major types: the interstitial and pericellular matrices. Basement membrane (BM) is a type of pericellular matrix found between epithelial cells and connective tissue128. In the early stages of tumor progression, the BM separates the tumor cells from the surrounding stromal cells, forming a physical barrier that prevents tumor cells from spreading129,130. As the tumor further develops into the metastatic stage, tumor cells break through the BM to enter the mesenchyme and invade the surrounding tissues; this process is accompanied by ECM remodeling131. It was found that the invasion of tumor cells through the BM involves both protein hydrolysis-dependent and protein hydrolysis-independent routes, and CAFs have a critical effect on both of them (Fig. 2B). For the invasion of tumor cells in a protein hydrolysis-dependent pathway, it has been extensively demonstrated that CAFs secrete broad-spectrum matrix metalloproteinase (MMP) such as MMP-1, -2, -3, -9, -11, -13, -14, -19, and fibrinogen activator that can directly degrade the ECM, and the higher MMP secretion contributes to the disruption of the BM and facilitates the breakthrough of tumor cells132,133. For the pathway that is not dependent on protein hydrolysis, CAFs exert mechanical force on the ECM through integrin α3, α5, and Rho signaling-mediated myosin-ECM interactions, thereby aligning collagen fibers from a random mesh-like appearance to a parallel pattern45,134, providing a more convenient “tunnel” for tumor cells to traverse the ECM. Similarly, Cannone et al.135 also noted that tumor cells connect with CAFs through the BM layer, utilizing their contractile properties to exert physical forces on the BM and enlarge the existing pores to complete invasion in a protein hydrolysis-independent manner. In addition, Papanicolaou et al.136 used temporal proteomic analyses of cellular tumor exfoliation and discovered that XII-type collagen secreted by CAFs alters the structure of I-type collagen, thereby creating an invasive microenvironment that supports tumor metastasis, which provides new insights into the mechanisms by which CAFs remodel the ECM. Another study has shown that full-length collagen VI from the ECM of obese mammary glands is a novel driver of TNBC cell invasion, and the mechanism was identified to promote TNBC cell invasion through NG2-EGFR crosstalk and MAPK signaling pathways137.
3.3. Protecting circulating tumor cells
Circulating tumor cells (CTCs) generally refer to these tumor cells derived from primary or metastatic tumors that acquire the ability to detach from the BM and invade the blood vessels138. It was found that CAFs play an important role in this process (Fig. 2C). Mitsui et al.139 stated that the release of prostaglandin E2 (PGE2) by CAFs may be essential for promoting tumor cell migration into the blood vessels. In addition, CAFs can promote angiogenesis through secreting VEGF, which provides more opportunities for invasive tumor cells to infiltrate blood vessels and develop hematogenous metastasis140. Moreover, chemokines such as CXCL12 secreted by CAFs could increase the vascular permeability of local microvascular analogs through paracrine signaling and promote vascular endothelial infiltration of breast cancer cells141,142.
CTCs may participate in blood circulation in two forms, either by circulating alone or in clusters with other cells, and clustered CTCs have a higher metastatic potential than individual CTCs143,144. Importantly, the direct physical interactions between CAFs and tumor cells may mediate the formation of heterotypic clusters of CAFs and CTCs (CAFs-CTC clusters), so that CAFs can travel with tumor cells in the blood circulation145, 146, 147, 148. Besides, CAFs acquire an invasive phenotype that also contributes to their accompanying CTCs entering the circulation, and it has been pointed out that IL11 can modulate ICAM-1 and ITGA5 signaling pathways to promote the migratory and invasive features of CAFs, which consequently facilitates the formation of metastatic CAFs-CTC clusters149. Subsequent findings confirm that CAFs-CTC clusters have a higher in vivo metastatic potential than homologous CTC clusters. The formation of this heterogeneous cluster may be related to the adhesion and stemness marker CD44 in a subpopulation of CTCs and CAFs150.
After entering the circulation, CTCs tend to be exposed to more fluid shear stress than the primary site, and CAFs that accompany CTCs into the blood circulation will act as bodyguards for CTCs to enhance their survival in the blood stream144,151. Specifically, CAFs in the CTCs-CAFs clusters have been proven to induce shear resistance in tumor cells via strong cellular adhesions and soluble factors (CCL2, CCL7, and CXCL5), which promote stable cell aggregates and can activate signaling pathways involved in cell survival and immune evasion, respectively148,152, 153, 154, 155. In summary, the protective effect of CAFs on CTCs in the blood circulation effectively promotes tumor cell metastasis, and the identification of CAFs-CTC clusters has become a powerful indicator for monitoring cancer progression in clinical practice156,157.
3.4. Facilitating the formation of pre-metastatic niche
The metastasis of tumor cells to distant tissues or organs and their colonization at the target site to start a new round of unrestrained growth can be understood as the process of “seeding in the soil”. The “seeds” usually refer to the CTCs that invade the blood vessels after crossing the ECM, while the “soil” is the microenvironment formed by the primary tumor through the release of exosomes, cytokines, and other signaling molecules to remotely recruit immune cells and stromal cells to alter the ECM of the distant organs, making it suitable for the colonization and growth of CTCs. Such a “hospitable” microenvironment is also known as the pre-metastatic niche (PMN)158, 159, 160. PMN facilitates the formation of metastatic foci, which is a critical stage in the malignant progression of cancer. It is well established that CAFs are important stromal components of PMN, which can assist in the development of PMN that is suitable for tumor growth through remodeling the ECM at the metastatic site, regulating metabolism, and immunosuppression (Fig. 2D), thereby promoting the metastatic process of tumor cells20,161.
Initially, tumor cells could activate normal fibroblasts in the host organ to form new CAFs by deriving a series of soluble mediators and extracellular vesicles (EVs) carrying tumor information162. For example, tumor cells could secrete Hsa-miR-141-3p (miR-141)-containing exosomes that target YAP1, a critical effector of the Hippo pathway, and reduce the intranuclear YAPA/TAZ ratio, which may reprogram stromal fibroblasts into pro-inflammatory CAFs and enhance the expression of growth-regulated oncogene α (GROα) in CAFs163,164. Tumor cell-derived Evs rich in integrin beta-like 1 (ITGBL1) from rectal cancer (CRC) can activate fibroblasts through NF-κB signaling, ultimately promoting PMN formation and rectal cancer metastasis165. In addition, Wu et al.166 found that epstein-barr virus (EBV)-encoded latent membrane protein 1 (LMP1) could also activate the NF-κB–P65 signaling pathway through extracellular vesicular transport and upregulate the expression of MCT4 proteins in CAFs, which promoted PMN formation and tumor proliferation.
Thereafter, the CAFs activated from the host organ and the CAFs accompanying CTCs will promote PMN formation by remodeling the ECM at the metastatic site, such as secreting large amounts of collagen to increase the stiffness of the ECM167,168. Notably, the increased ECM stiffness promotes tumor cells to secrete exosomes carrying tumor-promoting molecules, which in turn increases the risk of cancer metastasis169. A recent study indicated that activin A, a member of the TXGF-β superfamily, upregulates the expression of pro-fibrotic factors in CAFs, leading to collagen deposition in the pre-metastatic ecological niche of the lungs and facilitating the process of lung metastasis in breast cancer170. In addition, hyaluronic acid (HA) with a high molecular weight that ranges from 500 to 6000 kDa secreted by CAFs is an essential component of the ECM in PMN, and the secretion of HA is often accompanied by an increase in the levels of type I collagen as well as α-SMA, an EMT marker, which also contributes to the formation of PMN and promotes cancer metastasis171.
In addition, CAFs can promote angiogenesis in the PMN through the secretion of VEGF, PDGF, etc. to meet the initial growth requirements of newly colonized tumor cells140,172. On the other hand, Shu et al.173 found that CAFs exposed to exosomes from human melanoma increased oxidative phosphorylation of aerobic sugar degradation, leading to extracellular acidification, which in turn promoted glycolysis in tumor cells and facilitated PMN formation through metabolic regulation. Additionally, the colorectal cancer cell-derived exosome HSPC111 has been observed to alter the lipid metabolism of CAFs by phosphorylating ATP-citrate lyase, favoring PMN formation and CRC liver metastasis174. Another important fact is that CAFs can promote the formation of PMN by mediating the inflammatory microenvironment. Shani et al.175 reported that up-regulation of IL33 in CAFs from breast cancer patients with spontaneous lung metastasis altered the immune microenvironment of the metastatic PMN towards type 2 inflammation and facilitated lung metastasis of breast cancer, showing us a novel type of interaction axis between CAFs and immune cells. In addition, numerous studies have demonstrated that CAFs can recruit immunosuppressive cells such as myeloid-derived suppressor cells (MDSC) into the PMN by secreting cytokines like TGF-β176, IL-6 and IL-8177, CCL2178, and complement factors179 to reduce T-cell infiltration and prevent the killing of tumor cells by the immune system180. Further, a similar study revealed that CD38+ CAFs can inhibit M1 macrophage activation and T cell proliferation, thereby fostering the formation of immunosuppressive PMN181.
3.5. Other functions of CAFs in fostering tumor metastasis
In addition to the aforementioned role of CAFs in promoting tumor cell metastasis, several studies have reported that CAFs may facilitate the malignant behavior of tumors via other mechanisms. Hypoxia occurs in most growing solid tumors, and a recent study showed that breast cancer-associated fibroblasts BrC4f undergo mesenchymal-epithelial transformation induced by “pulsed hypoxia” to become more aggressive epithelioid tumor cells, which ultimately accelerates the metastasis of breast cancer182. CAVEOLIN-1 is a scaffolding protein and a major component of the caveolae/lipid rafts. Huang et al.183 showed that CAFs lacking CAVEOLIN-1 expression enhanced the secretion of TGF-β1, which activated the TGF-β1/SMAD signaling pathway in the tumor cells, thereby promoting cancer metastasis. Interestingly, a previous study mentioned that CAFs may promote tumor cell expansion, invasion, and metastasis through mechanical stress, which simply manifested as CAFs exerting pressure on the invasive front of the tumor due to their movement across from the tumor periphery to the core, encouraging the invasive growth of tumor cells in the forked finger model184. Further, it is well known that the interaction between cancer cells and surrounding fibroblasts significantly influences cancer growth, metabolism, metastasis, and progression, based on which it has been noted that the secretion of hydrogen peroxide by tumor cells to the TME induces neighboring stromal cells, mainly CAFs, to undergo aerobic glycolysis and produce large amounts of energy-rich “fuels” (e.g., pyruvate, ketone bodies, fatty acids, and lactic acid), which serve as the “nutrients” for tumor cells, thus promoting their proliferation and metastasis185.
4. Strategies of nanomedicine to modulate CAFs for anti-metastasis therapy
With the deepening research on nanotechnology, suppressing the pro-tumor functions of CAFs by constructing NDDS such as nanoparticles, liposomes, micelles, and nanoemulsions loaded with active ingredients has become a hot topic in the field of anti-tumor metastasis (Fig. 3). NDDS has been confirmed to improve the solubility and stability of drugs, alter the in vivo pharmacokinetic behavior of drugs, enhance the targeting of drugs, and thus strengthen the therapeutic efficacy and reduce the systemic toxicity of drugs186,187. In recent decades, CAF modulation strategies based on NDDS have shown great potential in mitigating the risk of tumor metastasis38,188. As discussed earlier, CAFs are critical in all steps of tumor cell metastasis, and therefore, developing a series of anti-CAF strategies with the assistance of NDDS may provide promising therapy for tumor metastasis. Recent studies related to CAFs-based NDDS include depleting CAFs (Fig. 3A), suppressing activation or normalizing CAFs (Fig. 3B), modulating CAFs effects on tumor cells and ECM (Fig. 3C), and even turning CAFs into “friends” (Fig. 3D).
Figure 3.
Strategies of CAFs-based NDDS against tumor metastasis. (A) Depleting CAFs. NDDS can directly deplete CAFs by passive or active targeting and in turn reduce the promote effect of CAFs on tumor metastasis. (B) Suppressing CAFs activation or normalizing CAFs. NDDS can suppress the activation of CAFs or normalize CAFs via reprogramming, thereby inhibiting the pro-metastatic function of CAFs. (C) Modulating the effects of CAFs on tumor cells and the ECM. NDDS can inhibit the secretion of pro-tumor cytokines by CAFs to suppress the malignant behavior of tumor cells or inhibit the collagen secretion of CAFs to reduce the ECM stiffness, ultimately exerting anti-tumor metastatic effects. (D) Turning CAFs into “friends”. NDDS can be designed to reprogram CAFs to act as drug delivery tools or to secrete anti-tumor molecules to activate immunity and induce apoptosis of tumor cells, manifesting as turning CAFs into “friends”.
4.1. Depletion of CAFs
Depletion of CAFs is the most direct way to block the effect chain of CAFs, which is a hot research topic in inhibiting the function of CAFs. The depletion of CAFs can directly silence all the downstream pro-tumorigenic effects regulated by CAFs, thus reducing the risk of tumor metastasis. To deplete CAFs, NDDS are often designed to enable the loaded active drug to target as many CAFs as possible and then promote CAF depletion through direct killing effects. The NDDS targeting CAF depletion is mainly classified into two types: passive targeting and active targeting. Specifically, passive targeting is usually based on the TME and the physiological properties of CAFs, while active targeting-mediated CAF depletion is usually realized through ligand-receptor-based targeting.
4.1.1. Depletion of CAFs mediated by passive targeting
Passive targeted depletion of CAFs is primarily achieved based on the physiological configuration and properties of the TME enriched with CAFs. It is well recognized that nanoparticles of appropriate size can penetrate the tumor blood vessels via the EPR effect to be passively enriched in the tumor tissue189, 190, 191. Since CAFs tend to localize near the BM of tumor blood vessels in most tumors, drug-loaded nanoparticles come into contact with CAFs earlier compared to the tumor cells after crossing the tumor blood vessels38, and this property can be exploited to simplify the design of the nano-formulation, which is one of the advantages of passively targeted CAF depletion. On the other hand, prolonging the circulation time of the NDDS is also a means to enhance their passive targeting via the EPR effect190,192. In previous studies, polymeric nanoparticles of a conjugate of PEGylated carboxymethylcellulose and docetaxel (Cellax-DTX) were fabricated with a particle size of 120 nm, which exhibited a distinctive long-circulation behavior. In a metastatic pancreatic cancer model, more than 90% of Cellax-DTX nanoparticles were able to aggregate in CAFs through the enhanced EPR effect, leading to long-term depletion of CAF cell populations and significantly reducing the metastatic risk of pancreatic cancer193,194. On the other hand, tumor cells utilize glycolysis rather than oxidative phosphorylation as the primary means of energy release, which leads to an increase in lactate loading in the extracellular environment and thus makes an acidic TME195. Therefore, Li et al.196 synthesized doxorubicin (DOX)-loaded dendrimers and co-loaded them with R848 (a toll-like 7/8 receptor agonist) and losartan (LOS) into pH-responsive liposomes. The liposomes were enriched in tumors and then disassembled in the acidic TME to release the loaded drug, where LOS could effectively antagonize the angiotensin type 1 receptor and inhibit the proliferation of CAFs. In combination with chemotherapeutic agents, liposomes can induce immunogenic cell death (ICD) of tumor cells, recruit and promote the maturation of dendritic cells (DCs), which in turn improves antigen presentation and activates T-cell responses, and inhibit lung metastasis of breast cancer by depleting CAFs. In another study, acid-responsive nanoparticles were constructed using random copolymers of hexyl methacrylate (HMA), dimethyl aminoethyl methacrylate (DMAEMA), and methacrylic acid (MAA). The results demonstrated that this system was able to disrupt the cell membranes of tumor cells and CAFs, exhibiting undifferentiated cytotoxicity. In the in vivo study, the nanoparticles inhibited the expression of ECM components by depleting the CAFs and rendered the originally dense pancreatic tumor tissues more permeable, which effectively inhibited the distant metastasis of pancreatic cancer cells in the lung and liver197.
4.1.2. Depletion of CAFs mediated by active targeting
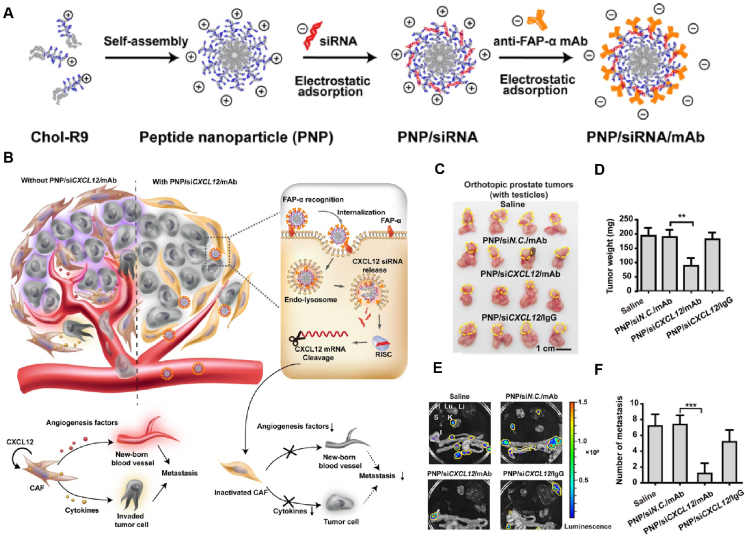
FAP is highly upregulated on CAFs, therefore, FAP-targeted NDDS is a classical targeting strategy for actively targeting CAFs and a popular research direction for depleting CAFs198. It has been reported that FAP-α monoclonal antibodies (anti-FAP-α mAb) modified on cell-penetrating peptide nanoparticles loaded with CXCL12-siRNA were able to significantly enhance the targeting of CAFs and specifically down-regulate the expression of CXCL12 in CAFs, which effectively inhibited the metastasis of prostate tumors by depleting CAFs199,200. The results showed that the metastasis inhibition rate was as high as 86% compared with the control group (Fig. 4200). In addition, FAP-mediated targeting of CAFs can also be combined with vaccine delivery. In a study, two computer-predicted immunodominant FAP-specific epitope peptides obtained by ex vivo antitumor efficacy screening were modified on lipid nanoparticles loaded with the Toll-like receptor 9 agonist CpG to obtain a nano vaccine, and immunization can be achieved by depletion of FAP-overexpressed CAFs, which induced antigen-specific CD4+ T-cell responses in addition to CD8+ T-cell responses. HE staining of lung tissues revealed that the nano-vaccine substantially reduced lung metastasis from subcutaneously implanted primary T-lymphoma cells compared with untreated controls201. Apart from the FAP, CAFs have also been reported to highly express N-CADHERIN protein on the cell surface. Shen et al.202 prepared poly (lactic-co-glycolic) acid nanoemulsions capable of targeting CAFs based on N-CADHERIN aptamers (NC3S) for the delivery of DOX and HGF-targeted siRNA, which was highly specific for CAFs and was effective in inducing their apoptosis, thus reducing ECM deposition caused by collagen secretion from CAFs. Interestingly, the delivered siRNA could prevent the propagation of CAFs through the autocrine closure of HGF. Therefore, the invasion and metastasis of colorectal cancer cells were significantly inhibited under these synergistic effects. Besides, a study designed telmisartan (TEL)-grafted glycolipid micelles loaded with DOX, in which TEL has a high affinity to the angiotensin II type I receptor overexpressed on CAFs, thus facilitating the targeted depletion via inducing CAF apoptosis. In contrast, Tel-CSOSA/DOX eliminates the “finger-like” ECM caused by the apoptosis of CAFs, which contributes to a more uniform and deeper penetration after drug delivery. Notably, the elimination of CAFs inhibited the secretion of MMP-9, which ultimately suppressed the invasion and metastasis of breast cancer cells203. More detailed information on the studies of NDDS is available for CAFs targeting depletion is summarized in Table 2193,196,197,200, 201, 202, 203.
Figure 4.
Representative cases of NDDS against tumor metastasis by depleting CAFs. (A) Construction of the PNP/siRNA/mAb nanosystem through supramolecular assembly. (B) Proposed mechanism of PNP/siCXCL12/mAb-mediated metastasis inhibition and CPP-mediated transfection of CXCL12 siRNA in CAFs. (C) Images of prostate tumors with testicles. Yellow dashed lines represent the locations of the primary tumor. (D)Weight of isolated tumors (without prostate and testicles) in each group. (E) Ex vivo bioluminescence images of metastasis foci on major organs on Day 14. H, heart; Lu, lung; Li, liver; S, spleen; K, kidney; I, intestines. Yellow dashed lines enclosed the locations of the metastatic tumors. (F) Numbers of tumor metastases in main organs observed by bioluminescence imaging. Reprinted with permission from Ref. 200. Copyright © 2019 American Chemical Society.
Table 2.
NDDS for CAFs targeting depletion.
| Targeting mechanism | Loaded drug | Delivery system and typical feature | Tumor metastasis model | Mechanism of anti-metastasis | Effectiveness of anti-metastasis | Ref. |
|---|---|---|---|---|---|---|
| EPR effect | Cellax-DTX | Nanoparticles; materials and drugs coupled by chemical bonds | Total primary human pancreatic xenografts tumor metastasis in peritoneal tissues | Inhibit the ECM remodeling of CAFs | 60% metastasis reduction compared to the control group | 193 |
| R848, LOS, DOX | Liposomes; pH-responsive | Breast cancer lung metastasis | Inhibit the ECM remodeling of CAFs | 90% metastasis reduction compared to the control group | 196 | |
| HMA, DMAEMA, MAA | Nanoparticles; pH-sensitive Long-circulating |
Pancreatic cancer metastasis in lung and liver | Inhibit the ECM remodeling of CAFs | – | 197 | |
| Affinity of anti-FAP-α mAb and FAP-α | CXCL12-siRNA | Nanoparticles; self-assembly | Prostate tumors metastasis in major organs | Reduce EMT induced by CAFs | 86% metastasis reduction compared to the control group | 200 |
| Affinity of FAP-specific epitope peptides and FAP-α | CpG | Nanoparticles; peptides with dominant epitopes on the surface | T-lymphoma lung metastasis | Inhibit the ECM remodeling of CAFs | 73% metastasis reduction compared to the control group | 201 |
| Affinity of N-cadherin aptamers and N-cadherin | DOX, HGF-siRNA | Nanoemulsions; surface aptamer modification | Colorectal cancer lung metastasis | Inhibit the ECM remodeling of CAFs | 90% metastasis reduction compared to the control group | 202 |
| Affinity of TEL and angiotensin II type I receptor | TEL, DOX | Micelles; self-assembly Sequential targeting |
Breast cancer metastasis | Inhibit the ECM remodeling of CAFs | – | 203 |
‒, not applicable. DTX, docetaxel; ECM, extracellular matrix; CAFs, cancer-associated fibroblasts; EPR, enhanced permeability and retention; R848, Resiquimod; LOS, losartan; DOX, doxorubicin; HMA, hexyl methacrylate; DMAEMA, dimethyl aminoethyl methacrylate; MAA, methacrylic acid; FAP, fibroblast activation protein; CXCL12, C–X–C motif chemokine ligand 12; EMT, epithelial–mesenchymal transition; HGF, hepatocyte growth factor; TEL, telmisartan.
4.2. Suppress activation or normalization of CAFs
It is noteworthy that the direct depletion of CAFs may promote the proliferation of cancer stem cells and thus increase the invasiveness and metastasis of tumors204,205. Therefore, other than the depletion of CAFs, an NDDS can be designed to suppress CAF activation or normalize CAFs, which can simply and efficiently reduce their promotion effect on tumor metastasis.
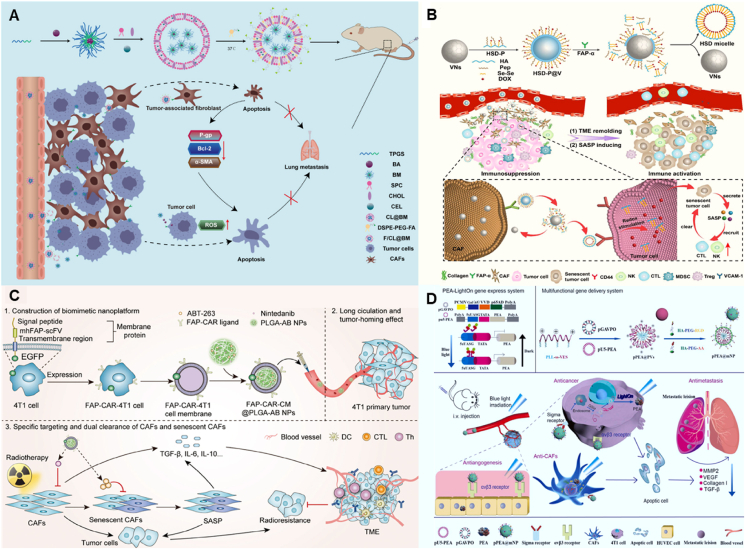
During the process of CAF activation, TGF-β is the most common cytokine that activates other cells into CAFs, and it is involved in the transformation of nearly all cells into CAFs. Moreover, TGF-β is also a key cytokine in maintaining the phenotype of CAFs, and suppression of TGF-β in the TME could reverse CAFs to a quiescent state206,207. Therefore, nano-drug delivery strategies that inhibit CAF activation or normalize CAFs by modulating TGF-β and, in turn, reduce tumor metastasis have gained extensive research. In this regard, Wu et al.208 prepared hyaluronic acid-modified pH-sensitive liposomes co-loaded with capsaicin (CAP) and TEL (GTHLs), which enable targeted delivery through the affinity between hyaluronic acid and CD44 receptor that is highly expressed on CAFs. Thereafter, CAP could obviously inhibit the activation of CAFs from hepatic stellate cells by blocking the TGF-β/SMAD signaling pathway, with a significantly smaller α-SMA-positive area, thereby disrupting the “CAFs-ECM” axis and inhibiting the metastasis of liver cancer in the lung. Meanwhile, by detecting the expression levels of EMT-related proteins after the treatment, it was found that the expressions of VIMENTIN and E-CADHERIN were significantly reduced and elevated, respectively, indicating that the liposomes could effectively reverse EMT induced by CAFs. Similarly, a study conjugated human relaxin-2 (RLX) with superparamagnetic iron oxide nanoparticles (PLX-SPION) to exert a targeting effect on pancreatic stellate cells via the binding of RLX to the extracellular structural domain of relaxin family peptide receptor 1. The results showed that PLX-SPION inhibited the TGF-β-induced differentiation of pancreatic stellate cells into CAFs, thereby inhibiting the invasion and migration of tumor cells via suppressing the pSMAD2 signaling pathway209. In addition, Xu et al.210 developed a novel aminoethyl anisamide (AEAA)-modified and puerarin (PUE)-loaded nanoemulsion for targeting purposes by binding to the sigma receptor expressed by CAFs, and it significantly reduces the highly expressed α-SMA phenotype of CAFs in breast cancer by about 6-fold compared to the PBS group. RT-PCR results showed that TGF-β was more than 10-fold reduced after nanoemulsion treatment, ultimately improving the chemotherapeutic efficacy of paclitaxel (PTX) and suppressing breast cancer metastasis in the lung to some extent. Moreover, a study designed silybin (SIL)-loaded biomimetic nanoparticles coated with anisamide (AA)-modified red blood cell membrane, which could significantly reduce the expression of TGF-β and suppress the α-SMA + CAFs in tumor tissues. The mechanism study found that the nanoparticles decreased breast cancer cell metastasis by affecting the TGF-β/Twist/EMT pathway211. A recent study developed self-assembling nanoparticles (FPC@S) loaded with a photosensitizer (protoporphyrin IX, PR4) and an antifibrotic drug (SIS3), which enabled targeted delivery by modifying an amphiphilic chimeric peptide, Fmoc-K(PpIX)-CREKA (FPC), to bind specifically to FN that is highly expressed in CAFs. It was found that SIS3 restored the low α-SMA and FN phenotypes of CAFs by inhibiting the phosphorylation of SMAD3 in the TGF-β pathway rather than killing CAFs. In addition, by combining with photodynamic therapy, the natural physical barrier formed by the dense ECM in fibrotic breast cancer is eliminated, thus allowing drugs and immune cells to penetrate deeper into the tumor for better efficacy, while FPC@S can be used in combination with immune checkpoint blockade therapy to effectively suppress primary, distant and metastatic tumors (Fig. 5212).
Figure 5.
Representative cases of NDDS against tumor metastasis by normalizing CAFs. (A) The formation of FPC@S through the self-assembly of FN targeting chimeric peptide (Fmoc-K(PpIX)-CREKA) and anti-fibrotic drug (SIS3). (B) By remodeling ECM and reprogramming CAFs, FPC@S broke the physical barrier of fibrotic BRCA to facilitate drug penetration, immune cell infiltration, and improve hypoxia, thereby enhancing photodynamic immunotherapy. (C) Images of scratch healing experiment on fibrotic BRCA after treatment with different drugs for 24 h. In which, #1−#6 meant blank, SIS3, FPC, FPC@S, FPC + Light, and FPC@S + Light treatment. (D) H&E-stained images of mouse lung sections after various treatments. Black arrows pointed to metastatic tumor lesions, and dashed boxes showed magnified views of selected metastatic tumor lesions. In which, #1−#6 meant blank, αPD-L1, FPC@S, FPC@S + αPD-L1, FPC@S + Light, and FPC@S + αPD-L1 + Light treatment. Reprinted with permission from Ref. 212. Copyright © 2024 American Chemical Society.
In addition to TGF-β, there are many other mechanisms involved in the activation and phenotype maintenance of CAFs. For example, CAFs are highly dependent on the cystine transporter SLC7A11 for cystine uptake and glutathione synthesis. Therefore, Sharbeen et al.213 developed SLC7A11-small interfering RNA (siRNA)-Star 3 nanoparticles via gene editing technology and improved their passive targeting ability by modification with PEG, which reduced SLC7A11 protein levels in pancreatic tumors in situ, inhibited the proliferation of SLC7A11high CAFs, and finally decreased the incidence of metastasis in PDAC214. The endothelin-1 (ET-1) axis consists of ET-1 ligands and their receptors, which are involved in various physiological and pathological processes such as cell proliferation, migration, and invasion215. Son et al.216 prepared macitentan (MAC)-loaded polymeric nanoparticles with passive targeting capability, which inhibited breast cancer lung metastasis by blocking the ET-1 axis and inhibiting the activation of CAFs, resulting in a decrease in fibrosis markers such as α-smooth muscle actin (αSMA), fibronectin, and collagen. In addition, Wang et al.217 developed a nano-hydrogel for the controlled release of a potent CAF suppressor (NOX4 inhibitor, NOX4i) and liposomal DOX (L-Dox). The results indicated that NOX4i effectively countered CAF activation, improved the effect of L-Dox on tumor cells, and finally inhibited lung metastasis of osteosarcoma. More detailed information on the studies of NDDS for inhibiting CAF activation or normalizing CAFs is summarized in Table 3208, 209, 210, 211, 212, 213,216,217.
Table 3.
NDDS for inhibiting the CAFs activation or normalizing CAFs.
| Targeting mechanism | Involved signaling pathway | Loaded drug | Delivery system and typical feature | Tumor metastasis model | Mechanism of anti-metastasis | Effectiveness of anti-metastasis | Ref. |
|---|---|---|---|---|---|---|---|
| Affinity of HA and CD44 | TGF-β/SMAD | CAP, TEL | Liposomes; pH-sensitive | Liver cancer lung metastasis | Inhibit the ECM remodeling of CAFs and reduce EMT induced by CAFs | 86% metastasis reduction compared to the control group | 208 |
| Affinity of RLX and RXFP1 | RLX | Nanoparticles; magnetic responsive | Pancreatic cancer metastasis | Inhibit the ECM remodeling of CAFs | Cell migration was reduced by about 50% after treatment compared to the control group | 209 | |
| Affinity of AEAA and sigma | PUE | Nanoemulsion; long-term stability | Breast cancer lung metastasis | Inhibit the ECM remodeling of CAFs | – | 210 | |
| Affinity of AA and sigma | SIL | Nanoparticles; biomimetic | Breast cancer metastasis | Inhibit the ECM remodeling of CAFs and reduce EMT induced by CAFs | – | 211 | |
| Affinity of FPC and FN | SIS3, PR4 | Nanoparticles; self-assembly, acid responsive | Breast cancer lung metastasis | Inhibit the ECM remodeling of CAFs and reduce EMT induced by CAFs | 86% metastasis reduction compared to the control group | 212 | |
| EPR effect | SLC7A11 | SLC7A11-siRNA | Nanoparticles; self-assembly | PDAC metastasis in liver, lung, spleen, and lymph node | Inhibit the ECM remodeling of CAFs | 50% metastasis reduction compared to the control group | 213 |
| EPR effect | ET-1 axis | MAC | Nanoparticles; highly stable | Breast cancer lung metastasis | Inhibit the ECM remodeling of CAFs | – | 216 |
| In situ administration | NOX4 | NOX4i | Sequential drug release | Osteosarcoma lung metastasis | Inhibit the ECM remodeling of CAFs | – | 217 |
‒, not applicable. HA, hualuronic acid; CD, cluster of differentiation; CAP, capsaicin; TEL, telmisartan; ECM, extracellular matrix; CAFs, cancer-associated fibroblasts; EMT, epithelial–mesenchymal transition; RLX, relaxin-2; RXFP1, relaxin family peptide receptor type 1; AEAA, aminoethyl anisamide; TGF-β, transforming growth factor-beta; PUE, puerarin; AA, anisamide; SIL, silybin; FPC, Fmoc-K(PpIX)-CREKA; SIS3, Sugar insensitive 3; PR4, protoporphyrin IX; EPR, enhanced permeability and retention; SLC7A11, solute carrier family 7 member 11 Gene; PDAC, pancreatic ductal adenocarcinoma; ET-1, endothelin-1; MAC, macitentan; Nox4, NADPH oxidase 4; Nox4i, NADPH oxidase 4 inhibitor.
4.3. Modulating the effect of CAFs on tumor cells and the ECM
As described above, tumor cells and ECM have been reported as the main targets regulated by CAFs; therefore, for CAFs that are already exerting pro-tumorigenic effects, blocking their effects on tumor cells and ECM is also a common nano-drug delivery strategy targeting CAFs. More detailed information on the studies of NDDS for modulating the effects of CAFs on tumor cells and the ECM is summarized in Table 4 218,219,221, 222, 223,227,228,230,231,233,234.
Table 4.
NDDS for modulating the effects of CAFs on tumor cells and the ECM.
| Targeting mechanism | Effect on CAFs | Loaded drug | Delivery system and typical feature | Tumor metastasis model | Mechanism of anti-metastasis | Effectiveness of anti-metastasis | Ref. |
|---|---|---|---|---|---|---|---|
| EPR effect | Reduce the secretion of CXCL12 and IL-6 | RES | Nanoparticles; prepared by single emulsion solvent evaporation method | Oral cancer metastasis in kidney, liver, brain, neck lymph nodes and lung | Inhibit the ECM remodeling of CAFs and reduce EMT induced by CAFs | 75% metastasis reduction compared to the control group | 218 |
| Affinity of AEAA and sigma | Reduce the secretion of TGF-β, CXCL12, and IL-6 | MIT, CEL | Nanoparticles; TME-responsive (pH and GSH) |
Melanoma metastasis in liver and lung | Inhibit the ECM remodeling of CAFs | – | 219 |
| Affinity of MMP-2 and TME, spatial targeting of CAFs. | Reduce the secretion of Wnt16 | GA, DGL-GEM | Nanoparticles; size-switchable, MMP-2-sensitive | Breast cancer metastasis in major organs | Inhibit the ECM remodeling of CAFs | – | 221 |
| Affinity of MMP-2 and TME | QUE, PTX | Liposomes; MMP-sensitive, sequential delivery, dual-targeting, hybrid micelle-in-liposome |
Breast cancer lung metastasis | Inhibit the ECM remodeling of CAFs | – | 222 | |
| Homologous targeting | Reduce the secretion of lactate | PFK15, PTX | Nanoparticles; dual-targeting, Homologous targeting, biomimetic |
Breast cancer metastasis | Inhibit the ECM remodeling of CAFs | – | 223 |
| Intratumoral administration | Inhibit the synthesis and secretion of collagen | LOS,DOX | Hydrogel, liposomes; self-assembly | Breast cancer lung metastasis | Inhibit the ECM remodeling of CAFs and reduce EMT induced by CAFs | 80% metastasis reduction compared to the control group | 227 |
| Intratumoral administration | LOS, TEL | Fiber fragments; sequential drug release | Breast cancer lung metastasis | Inhibit the ECM remodeling of CAFs | 90% metastasis reduction compared to the control group | 228 | |
| Affinity of AEAA and sigma | LOS, DOX, ferric ions | Carbon dots; CAFs-responsive; sequential and spatiotemporal release |
Breast cancer lung metastasis | Inhibit the ECM remodeling of CAFs | 100% metastasis reduction compared to the control group | 230 | |
| Specific drug release with esterase-responsive properties | TPL | Micelles; esterase-responsive property | Gastric cancer metastasis in the peritoneal cavity | Inhibit the ECM remodeling of CAFs | 90% metastasis reduction compared to the control group | 231 | |
| Specific drug release with CTSB-responsive | DAS, epirubicin | Nanoparticles; sequential delivery, cathepsin B-responsive, pH-responsive |
Breast cancer lung metastasis | Inhibit the ECM remodeling of CAFs | – | 233 | |
| Homologous targeting | Inhibit the secretion of MMP-14 | ART | Nanoparticles; homologous targeting, biomimetic | Breast cancer metastasis | Inhibit the ECM remodeling of CAFs | – | 234 |
‒, not applicable. CAFs, cancer-associated fibroblasts; ECM, extracellular matrix; EPR, enhanced permeability and retention; CXCL12, C-X-C motif chemokine ligand 12; IL-6, interleukin- 6; RES, resveratrol; EMT, epithelial–mesenchymal transition; AEAA, aminoethyl anisamide; TGF-β, transforming growth factor-beta; MIT, mitoxantrone; CEL, celastrol; TME, tumor microenvironment; GSH,Glutathione; MMP-2, matrix metallopeptidase 2; Wnt16, wnt family member 16; GA, glycyrrhetinic acid; DGL-GEM, dendrigraft poly-l-lysine-gemcitabin; QUE, quercetin; PTX, paclitaxel; LOS, losartan; DOX, doxorubicin; TEL, telmisartan; TPL, triptolide; CTSB, cathepsin B; DAS, Dasatinib; ART, artesunate.
4.3.1. Blocking the effects of CAFs on tumor cells
As one of the highly abundant components of TME, CAFs can promote the malignant process of tumor cells by secreting a series of cytokines. For example, a study prepared resveratrol-loaded polymer nanoparticles (RES-NPs) and found that they reduced the conversion of quiescent HFB cells to CAFs-like phenotype (high expression of FAP, α-SMA, and VIMENTIN) and inhibited the secretion of CXCL12 and IL-6 by CAFs. The treatment of RES-NPs cuts off the regulatory effects of CAFs on tumor cells by secreting cytokines, reducing the growth and proliferation of cancer stem cells, and ultimately decreasing the metastasis risk of oral cancer218. Similarly, Liu et al.219 prepared AA-modified nanoparticles loaded with mitoxantrone (MIT) and celastrol (CEL). The results showed that the nanoparticles were able to reduce the secretion of TGF-β, CXCL12, and IL-6 by CAFs, effectively eliminate the immunosuppressive microenvironment, empower immunogenic cell death, and restore the tumor antigen recognition, trigger the overall anti-tumor immunity, and thus significantly inhibit the metastasis of melanoma in the liver and lung. In addition, WNT16 has been confirmed to be secreted by CAFs and leads to drug resistance, which promotes tumor cell malignant behavior220. A study has prepared MMP-2 responsive nanoparticles loaded with gemcitabine dendritic polylysine-conjugated small nanoparticles (DGL-GEM) and 18β-glycyrrhetinic acid (GA). The nanoparticles would release DGL-GEM in response to the MMP-2, and due to the small particle size, they can penetrate deeply and kill the tumors, while the larger GA-loaded nanoparticles accumulate around the tumor vasculature and are absorbed by the CAFs, where GA can regulate the secretion of WNT16 in the CAFs and inhibit breast cancer metastasis in major organs221. Besides, Duan et al.222 constructed quercetin (QUE) and PTX co-loaded liposomes modified with the neovascular-targeting peptide NGR. The nanoparticles released QUE upon interaction with MMP-2 that is highly expressed in TME and were uptaken by CAFs, which then down-regulated the WNT16 expression of CAFs, resulting in significant inhibition of chemoresistance and breast cancer lung metastasis. It has been reported that glycolytic metabolites produced by CAF, such as lactate, pyruvate, and ketone bodies, enter the tricarboxylic acid cycle of neighboring cancer cells to rapidly and efficiently produce adenosine triphosphate (ATP), which enables cancer cells to grow and proliferate rapidly in a nutrient-poor TME. Therefore, Zang et al.223 prepared hybridized membranes of CAFs and breast cancer cells, which were modified on the surface of solid lipid nanoparticles loaded with PTX and the glycolysis inhibitor PFK15. Modification of the fibroblast membrane confers the nanoparticles with homologous targeting ability. The results showed that PFK15 can prevent the glycolysis of CAFs and block the metabolic support of CAFs to tumor cells. In addition, lactate contributes to the histone lactylation in macrophages that regulates gene transcription, which is related to tumor growth and metastasis. Therefore, the decrease in lactate caused by this nanoparticle could potentially reduce breast cancer metastasis173.
4.3.2. Blocking the regulation of CAFs on ECM stiffness and degradation
Numerous studies have shown that both stiffness and degradation of the ECM can promote tumor cell invasion and metastasis224, 225, 226. On the one hand, collagen secreted by CAFs is an important contributor to the increased stiffness of the ECM. Therefore, a study prepared an injectable peptide hydrogel loaded with LOS, which was injected intratumorally and could be retained in the tumor for 9 days, thereby increasing its uptake by CAFs, and found that this system significantly inhibited the secretion of collagen by CAFs and blocked the pro-ECM communication network of CAFs, ultimately enhancing the chemotherapeutic effect of DOX-loaded PEGylated liposomes, which suppressed the growth and lung metastasis of breast cancer227. In addition, Chen et al.228 electrospun a mixture of LOS and TEL into fiber fragments, and the sustained release of LOS and TEL more strongly inhibited collagen synthesis by CAFs, down-regulated hypoxia-inducible factor α (HIF-α) expression, and reduced breast cancer metastasis in the lung. Carbon dots, a new class of fluorescent carbon materials with diameters of less than 10 nm, have emerged as potential materials for biosensing, drug delivery, and bioimaging due to their excellent optical properties, high biocompatibility, and low toxicity229. Hou et al.230 developed AEAA-modified CAFs responsive to honeycomb carbon dots (CDs), where DOX and immunotherapeutic enhancers (ferric ions) were immobilized on the surface of the CDs and LOS loaded within the mesopores. The drug-loaded nanocomposites aggregated in the tumor tissue and decomposed into individual CDs, releasing LOS that could alleviate the hypoxic environment caused by CAFs and reduce the expression of collagen, at which time individual drug-loaded CDs and iron ions could effectively penetrate into the tumor depth and enhance ICD, as well as promote T-cells, NK-cells infiltration and down-regulation of immune-cells recruitment (Treg and MDSC), and increase the pro-inflammatory cytokine secretion (IFN-γ, IL-6, and TNF-α), ultimately preventing lung metastasis of breast cancer (Fig. 6230). In addition, a study encapsulated the precursor of triptolide (TPL), triptolide-naphthalene sulfonamide (TPL-nsa), in amphiphilic SN38 polymeric micelles based on the principle of precursor design and found that TPL could significantly reduce the expression of collagen, FAP, and α-smooth muscle actin (α-SMA) in tumors. Meanwhile, due to the high esterase concentration in tumor tissues, PSN38 polymer containing phenolic ester structure with esterase-responsive properties could achieve tumor-specific drug release231,232. In another study, Zhang et al.233 prepared dasatinib (DAS, CAF modifier)-loaded dendritic poly (oligo (ethylene glycol) methyl ether methacrylate) (POEGMA)-based nanomedicines with cathepsin B (CTSB) responsiveness (P-DAS), which can reverse the phenotype of CAFs to reduce collagen by downregulating collagen anabolism and energy metabolism, thereby reducing the ECM deposition. The in vivo study revealed that this nanomedicine, combined with epirubicin, effectively inhibited breast cancer lung metastasis. Besides, as mentioned earlier, MMP secreted by CAFs leads to degradation of the ECM, providing tumor cells with “tunnels” for metastasis. For example, MMP-14, a member of the MMP family, promotes tumor invasion by degrading collagen. Zheng et al.234 prepared TNBC cell membrane-modified poly (lactic acid)-glycolic acid (PLGA) biomimetic nanoparticles loaded with artesunate (ART) to enhance the accumulation of ARS in different mouse tumor models through homologous targeting and found that the nanoparticles could inhibit the secretion of MMP-14 by CAFs, which may ultimately reduce distant metastasis of breast cancer.
Figure 6.
Representative case of NDDS against tumor metastasis by inhibiting the remodeling effect of CAFs on the ECM. (A) Schematic illustration for the preparation of drugs-loaded nanoassemblies. (B) The transformation and enhanced antitumor immunity mechanism of Pep-APCDs@Fe/DOX-LOS. (C) Images and H&E-stained sections of lungs collected from mice after various treatments. Tumor metastasis sites are pointed out by arrows. (D) Number of metastatic lung nodules. Reprinted with permission from Ref. 230. Copyright © 2023 Wiley.
4.4. Turning CAFs into “friends”
In addition to the above strategies, many studies have also been conducted to modulate CAFs to become our “friends” and perform anti-tumor functions. Specifically, the turn-foe-into-friend strategy mainly focuses on utilizing the characteristics of CAF distribution and function in tumor tissues to further facilitate anti-metastasis nano-drug delivery.
In a recent study, Yuan et al.235 prepared an AEAA-modified lipid-polymer hybrid drug delivery system (PI/JGC/L-A) that could create a CAF “barrel” with JQ1, stimulating the secretion of gemcitabine (GEM)-loaded exosomes from the CAF “barrel” to the deep tumor site, and leveraging the CAF “barrel” to secrete IL-12, realizing effective drug delivery and inhibiting PDAC metastasis. In addition, a study has prepared lipid-coated protamine DNA complex nanoparticles loaded with a plasmid encoding a secretable TNF-associated factor, sTRAIL, in which the loaded plasmid was delivered to CAFs by taking advantage of the feature of off-target distribution of anticancer nanoparticles to CAFs. After three doses in a mouse xenograft model of bladder cancer, it could make approximately 70% of CAFs turn into sTRAIL-producing tools, and sTRAIL secreted by CAFs can induce apoptosis of tumor cells, thereby inhibiting liver and lung metastasis of pancreatic cancer236. Inspired by the finding that CAFs have similar processing abilities to professional antigen-presenting cells (APCs), turning CAFs into “friends” can also be combined with immunotherapy. Geng et al.237 proposed a strategy to transform CAFs into APCs through in situ engineering to reactivate immune activation. The temperature-controlled NDDS is composed of molten eutectic hybrids, fusion plasmids, and chitosan modified by AEAA. The heat shock protein 70-initiated plasmid activated the expression of CD86 on CAFs through non-destructive photothermal activation, at which CAFs could be regarded as APCs, thereby presenting MHC I–Ag and inducing the activation and proliferation of antigen-specific CD8+ T cells. In addition, engineered CAFs can also secrete PD-L1 capture proteins for ICB therapy. The results of in vivo experiments showed that turning CAFs into “friends” could lead to a tumor suppression rate of approximately 85% in highly fibrotic breast cancer and effectively inhibit the tumor lung metastasis (Fig. 7237). As mentioned earlier, tumor growth and metastasis are highly dependent on the pericellular structure, and CAFs, as the main cell type for collagen production in the tumor stroma, can prevent tumor cell escape through collagen-mediated encapsulation. In this regard, Liu et al.238 developed gadolinium fullerenol-containing nanoparticles. Under acidic conditions, the reduced size of the nanoparticles would allow for better penetration into tumor hypoxic tissues. At the same time, the nanoparticles could activate the TNFR2/p38 MAPK signaling pathway by promoting the binding activity of tumor necrosis factor α (TNF-α) and its receptor, which in turn enhanced the expression of cellular collagen from CAFs and stimulated the formation of dense fibrous cages to inhibit the metastasis of tumor cells in the form of capture rather than toxicity, which provided a new idea for the development of antimetastatic therapy based on CAFs.
Figure 7.
Representative cases of NDDS against tumor metastasis by turning CAFs to “friends”. Schematic illustration of “turn foes to friends” strategy for anti-tumor immunotherapy. (A) Process of preparation of TNP@CS-A/pDNA complexes. (B) Illustration of non-destructive photoactivatable CAF engineering for reversing immune tolerance microenvironment to amplify immunotherapy. (C) Photographs and H&E staining of lung metastatic nodules. (D) Quantitative analysis of lung metastatic nodules. Light irradiation (+) was conducted with a 660 nm laser at the power density of 2 W/cm2 for 30 min. Reprinted with permission from Ref. 237. Copyright © 2023 Published by Elsevier.
5. Challenges and outlook
Although nano-drug delivery strategies designed with CAFs as targets have shown great potential in anti-tumor metastasis, their clinical translation is not as optimistic as the positive results obtained in the laboratory. It is found that no CAFs-based NDDS has entered the clinical trial stage so far, which indicates that the relevant research still faces non-negligible challenges. This section summarizes the primary challenges faced by CAF-based NDDS and the possible solutions.
5.1. Qualitative, quantitative, and modeling studies of CAFs
Accurate identification, localization, and clarification of the function and amount of CAFs are conducive to a clearer understanding of CAFs, which is the basis for designing CAF-based nano-drug delivery strategies. However, the heterogeneity of tumors, intercellular crosstalk, and their complex evolutionary processes bring a lot of challenges for CAF-related research.
As mentioned earlier, CAFs have a wide range of origins, leading to phenotypic heterogeneity and functional heterogeneity, which poses a great challenge for researchers to accurately categorize newly discovered CAFs239,240. Currently, the function of CAFs can be used to qualify CAFs, classifying them into the appropriate categories. In fact, function should be linked to cell surface biomarkers in future studies to facilitate further analysis of CAFs when functional tests are not applicable, which requires that researchers pay more attention to the expression of CAF biomarkers80. If necessary, multiple biomarkers and their abundance differences can be jointly applied to clarify the possible attribution of CAFs. In addition, factors such as immune functions, lineage history, and metabolic status should be taken into consideration in classifying CAFs, which can comprehensively reflect the characteristics of CAFs in order to accurately incorporate them into the CAF subtypes that can be currently identified by cellular transcriptome sequencing techniques, such as myofibroblasts, inflammatory CAFs, and antigen-presenting CAFs in pancreatic cancer241. In addition, there are many deficiencies in quantitative studies of CAFs in tumor tissues, and it is also a challenge to clarify the amount and proportion of CAFs in tumor tissues after identifying the possible properties and functions of CAFs. Currently, the quantitative status of CAFs is often determined using CAF surface markers (e.g., α-SMA) in conjunction with antibody recognition; however, high-quality antibodies targeting CAF subtype markers are very scarce, and the optimization process remains challenging. In recent studies, it has been pointed out that cytometry by time of flight (CyTOF) flow cytometry can utilize mass spectrometry principles for high-dimensional single-cell analysis of cells. Compared with traditional flow cytometry using fluorescent dyes for cell labeling, CyTOF based on metal isotope labeling and mass spectrometry has a higher resolution and a larger analytical range242. This method can simultaneously detect dozens of different protein markers at the same time, thus exhibiting great potential in the quantitative detection of CAFs13,243.
On the other hand, the construction of in vitro and in vivo CAFs research models that reflect the real TME is another challenge at present since the function continuity is questionable as the culture time of CAFs is prolonged, and a detailed characterization of the functional changes during the long-term culture of CAFs is therefore needed. Encouragingly, several studies have demonstrated that the original phenotype of CAFs is better maintained under 3D culture conditions, and combining this with the ever-increasing ability to manipulate progenitor cells will allow experiments to be performed with human cells that more closely resemble the TME42. In addition, combining techniques from other disciplines can further enhance the potential of the in vitro CAF models. A recent study developed a novel high-throughput screening platform in which a matrix produced by CAFs is generated in 384-well plates, imaged with an automated confocal microscope to clarify what changes have occurred in the ECM, and analyzed with a customized MATLAB script to screen for drugs and possible action targets that can effectively alter the ECM. This platform enables assay miniaturization, which helps to simplify experimental operations, save time and money, and ultimately enable high-throughput screening applications244. Although a lot of CAF biological models have been designed and constructed in vitro, it has been repeatedly shown that CAFs in culture are not able to fully recapitulate the functional properties of CAFs in vivo, so it is particularly essential to build suitable in vivo CAFs models245. For the exploration of CAFs in vivo, a commonly used method is to inject a mixture of CAFs and tumor cells, allow them to grow naturally until tumor formation, and then measure the functions of CAFs via the assay described above. However, such a modeling approach skips the early stage of tumor cell development, and the final models will include a mixture of injected CAFs and fibroblasts from the host mouse, which will affect the subsequent determination of CAF functions. Cre-lox manipulation is an important tool widely used in mouse experiments for selective expression or knockdown of genes of interest in a cell-specific manner246. Transgenic manipulation of CAFs by the Cre-lox system can address these issues, but the complexity of the technology still leads to many challenges, such as the lack of a Cre driver line specific for CAFs, which may play a counterproductive role in different tumor types. FLP is an enzyme that catalyzes recombination between two short FRT DNA sequences and is often used to excise genomic fragments flanking FRT sites to activate or knock down gene expression. Combining Cre-lox and FLP-FRT techniques seems to bring new horizons for manipulating tumors and fibroblasts, allowing the measurement of various indices related to CAF function once the tumor has formed, but this method requires significant resources and time, which may be an obstacle for many researchers42,247. In future studies, fate mapping to assess CAFs and immune cell changes through tumor progression, digital/multiple spatial analysis of tissue proteins and RNA to assess spatial changes in the TME, spatial transcriptomics, and digital pathology can be considered. In addition, the use of techniques such as 3D tissue culture to explore the characteristics of CAFs at different experimental stages (primary, early isolation, and long-term) as well as different clinical stages (pathological grading and staging), furthermore, penetrating microscopy can provide real-time visualization of in vivo cell-to-cell interactions, which can also be helpful in the study of the longitudinal progression of CAFs45,109.
5.2. The mechanism of CAFs-based NDDS against tumor metastasis should be further elucidated
Although the CAFs-based NDDS shows great potential for anti-metastasis therapy, no relevant NDDS has been clinically translated, which indicates that there are still potential problems in the research process. The first problem of the CAFs-based NDDS is “whether it works”, which is primarily focused on the anti-tumor strategy of CAFs depletion. As mentioned earlier, many studies have explained the mechanism by which the antitumor effect is produced as the targeted depletion of CAFs. Indeed, it remains controversial whether depletion of CAFs would achieve the desired effect. For example, the study by Ozdemir et al.248 noted that in the spontaneous PDAC mouse model of α-SMA-thymidine kinase transgenic mice, depletion of α-SMA+ myofibroblasts enhanced hypoxic and infiltration of immune-suppressing CD3+Foxp3+ Treg cells in tumors, induced tumor EMT and stem cell features, which ultimately accelerated the metastatic progression of the tumor248. In addition, it has also been pointed out that killing CAFs with toxic drugs leads to the production of WNT16, resulting in drug resistance in adjacent tumor cells and affecting subsequent tumor therapy210. This suggests that more attention should be paid to future studies based on CAF depletion, as well as verifying whether the depletion of CAFs produces similar effects to those described above, to clarify the effectiveness of the constructed NDDS. Further, it is recommended that the normalization of CAFs mentioned above be considered as a starting point, thereby avoiding the adverse effects that may arise from CAF depletion.
On the other hand, most studies conclude that CAFs-based NDDS have an anti-tumor metastasis effect based only on their overall effect, and the influence of regulating CAFs alone on tumor metastasis is always ignored. A number of related studies often lacked the group of regulated CAFs alone in their experimental design, thus affecting further research on the detailed mechanism of tumor metastasis inhibition by regulating CAFs individually. In addition, the deposition of ECM due to collagen secretion by CAFs is often interpreted as a barrier to drug penetration. However, collagen XII and collagen VI have been proved to have a direct effect on tumor metastasis136,137, which is often ignored in most current studies. This would affect the judgment of the direct role of CAFs in tumor metastasis to a certain extent, therefore it is necessary to pay more attention in subsequent studies.
Although the mechanism of CAFs in promoting tumor metastasis is well studied, there is still a lack of in-depth research on how these CAFs-based NDDS exert anti-tumor metastasis efficacy based on CAF regulation. For the specific aspects of tumor metastasis inhibited by CAFs-based NDDS, we found that these aspects were basically distributed only in ECM remodeling and EMT induction, while there were almost no studies about the effects of anti-CAFs NDDS on protecting CTC and facilitating the formation of PMN. Most of the conclusions are based on the simple detection of metastatic foci characteristics or indirectly reflecting the metastasis of tumor cells by determining the tumor growth volume and plotting survival curves, which do not accurately visualize the specific mechanisms of anti-tumor metastasis of these CAFs-based NDDS. Meanwhile, there is a lack of quantitative indexes for the evaluation of metastatic effects, which makes it inconvenient to compare the anti-tumor metastatic effects among different nano-drug delivery strategies. In the evaluation of antitumor drug efficacy, the research content should be refined to analyze the antitumor metastasis mechanism of NDDS as comprehensively as possible, which is more beneficial to further optimization and application. For example, a multilayer paper-supported co-culture system developed by Lin et al.249 consists of a tumor layer infiltrated with breast cancer cells, a layer infiltrated with lung cells, and multiple invasive layers in between, offering the possibility of studying breast cancer lung metastasis both temporally and spatially. Clarifying the mechanism of “how it works” related to CAFs will help determine future research directions.
5.3. Difficulties in precise targeting and long-term efficacy of CAFs-based NDDS
Another major challenge in the study of CAFs-based NDDS against tumor metastasis is to achieve precise drug delivery to CAFs, of which the main reason is the heterogeneity of CAFs and the lack of specific biomarkers. As mentioned earlier, part of the NDDS has inhibited tumor metastasis by affecting the function of CAFs through targeted drug delivery. However, most of these targeting strategies do not differentiate subtypes of CAFs, and the targets chosen are not unique to CAFs, such as the commonly used target FAP, which, in addition to being highly expressed in CAFs, is also expressed in some tumor cells such as sarcomas, mesotheliomas, and esophagus250. In addition, the sigma receptor, which is highly expressed by CAFs, has also been reported to be expressed on tumor cells as well251. Although these markers are expressed at lower levels in other cells than CAFs, they still affect the efficiency of drug delivery from NDDS to CAFs to some extent. Moreover, the CAFs-targeted NDDS may have possible adverse effects on normal cells that express the common CAF biomarkers. This suggests that in future studies, we should avoid selecting markers with high cross-expression levels as the target of NDDS in CAFs, which can further improve the precise drug delivery of CAFs-based NDDS and ultimately achieve the desirable anti-tumor metastasis effect. In this regard, studies have been conducted to overcome possible biomarker expression differences on the cell surface by extracting CAF membranes and incorporating them into NDDS to construct biomimetic homologous targeting-based NDDS. Notably, the biomimetic CAFs-based NDDS poses higher safety requirements. In the study of cell membrane-coated nanoparticle-mediated CAF targeting, the ratio of the membrane to nucleus is usually variable, which is more inclined to enhance the targeting effect and ignores the discussion of its safety252. Therefore, it is necessary to focus on safety while utilizing biomaterials to enhance the targeting efficiency of CAF-based NDDS.
It is well known that the long-term use of chemotherapeutic agents is prone to cause drug resistance and reduce drug effects253. At present, a large number of CAFs-based NDDS are designed to modulate CAFs and, at the same time, attenuate drug resistance, ultimately achieving excellent anti-metastasis effects254. However, the vast majority of CAFs-based NDDS studies have neglected to examine the long-term effectiveness and drug resistance of the formulations, which is another of the limitations of the research in this field currently. Considering the advantages of natural products, the development of natural products as therapeutic agents, along with the design of more precise targeted drug delivery strategies, is expected to avoid potential drug resistance and enhance the long-term effectiveness of CAFs-based NDDS, which can be a direction for further exploration.
5.4. Combination therapy with CAFs-based NDDS against tumor metastasis
In order to improve the antitumor efficacy, more and more attention has been paid to combination therapies, which are expected to achieve better therapeutic effects by combining CAFs-based NDDS with chemotherapy, immunotherapy, radiotherapy, and photothermal therapy (Fig. 8)255. For example, Li et al.256 co-loaded tretinoin with small-sized micelles containing betulinic acid in liposomes (CL@BM) by thin-film hydration method, and further introduced folic acid as the targeting fraction (F/CL@BM) to achieve better targeting effect. The results demonstrated that by sequential delivery of two drugs, specific removal of CAFs and increased penetration of chemotherapeutic agents could effectively inhibit tumor growth and metastasis in TNBC, providing a new strategy for the chemotherapeutic treatment of TNBC (Fig. 8A256). In addition, a report on the combination of CAFs-based NDDS and immunotherapy claimed that a CAFs-triggered structurally transformable nanoassembly (HSD-P@V) could deliver valsartan (a CAFs regulator) and DOX directionally to specific targets. Anti-tumor therapy was accomplished by recruiting effector immune cells to remove senescent cells. The results showed that the nanoassemblies significantly inhibited tumor growth and lung metastasis, and prolonged the survival of hormonal mice. This work provides a promising paradigm for delivering multisite nanodrugs for cancer immunotherapy (Fig. 8B 257). Radiation resistance in breast cancer leads to residual or recurrent lesions and is an important reason for radiotherapy failure258. It has been found that CAFs can directly activate the stem cell properties of tumor cells via paracrine exosomes, thus affecting the sensitivity of tumor cells to radiation therapy259. Therefore, Jian et al.260 triggered systemic anti-tumor immunity by constructing an engineered cancer cell mimicry nanoplatforms for dual-targeted clearance of CAFs, which resulted in improved radiation resistance and the most total reduction of breast cancer metastasis (Fig. 8C260). In addition, Cheng et al.261 developed a dual-targeted nano-drug delivery system for CAFs and breast cancer cells to deliver the LightOn gene expression system, which spatiotemporally and spatially controllably expresses virulent Pseudomonas aeruginosa exotoxin A under blue-light irradiation, ultimately exhibiting potent antitumor, CAFs inhibition, and anti-metastasis effect (Fig. 8D261).
Figure 8.
Typical cases of CAFs-based NDDS in combination with other therapeutic approaches for reducing tumor metastasis. (A) Assembly and mechanism of folate-coated micelles-in-liposomes loaded with BA and CEL (F/CL@BM) to accumulate at tumor sites and induce apoptosis in, respectively, tumor-associated fibroblasts (TAFs) and tumor cells. Reprinted with permission from Ref. 256. Copyright © 2024 Dove Medical Press Ltd. (B) Schematic illustration for the preparation and transformation of HSD-P@V and anti-tumor immune mechanism of HSD-P@V. Reprinted with permission from Ref. 257. Copyright © 2024 Shenyang Pharmaceutical University. Published by Elsevier. (C) Schematic Illustration of FAP-CAR-CM@PLGA-AB NPs construction and dual-clearance of CAFs and SC CAFs to improve radiation resistance. Reprinted with permission from Ref. 260. Copyright © 2024 Wiley. (D) Dual-targeting, high-affinity nano delivery system for light controlled Pseudomonas exotoxin A gene treatment of early breast cancer and lung metastasis. Reprinted with permission from Ref. 261. Copyright © 2023 Published by Elsevier.
Although various CAFs-based NDDS for anti-tumor metastasis therapy have been widely studied and exhibited good anti-metastasis effects in animal experiments, their clinical applications have not been reported so far. This is probably caused by the fact that nano-formulations with complex structures are relatively less favorable for industrial production, thereby limiting their transition from the “Bench” to the “Bedside”262. According to the previous summary of the tumor metastasis inhibition efficiency of each delivery system, the best antitumor metastasis effect reported as we know was achieved by the carbon dots-based formulation with an inhibition rate of 100%230. However, liposomes, nanoemulsions, and nanoparticles based on CAFs also exhibited excellent anti-tumor metastatic effects (inhibition rate of 86%–90%). Moreover, considering the large proportion of currently marketed nano-formulations, liposomes may have a more desirable cost and production ease. Therefore, liposomes may be the most promising dosage for future CAFs-based NDDS development263. With constant exploration, simpler and more potent CAFs-based NDDSs will be designed and investigated in the future, which can also be used in combination with other therapeutic approaches to suppress tumor metastasis in clinical.
6. Conclusions
As the crosstalk center of stromal cells in the TME, CAFs exert a critical role in tumor metastasis, and nano-drug delivery strategies designed based on CAF regulation demonstrate great potential in suppressing tumor metastasis. This review summarizes the mechanisms by which CAFs promote tumor metastasis, mainly via inducing EMT in tumor cells, remodeling the ECM, protecting CTCs, and facilitating PMN formation. In addition, this article, for the first time, summarizes the anti-metastasis CAFs-based nano-drug delivery strategies in terms of their functions, such as depleting CAFs, suppressing the activation or normalizing CAFs, modulating CAF effects on tumor cells and the ECM, and turning CAFs into “friends”. These insights can help to further understand CAFs and provide a reference for the design of subsequent NDDS. Despite the promising research progress of these strategies at the laboratory level, there has been no clinical translation of the formulations so far, which suggests that there are still some challenges. The qualitative and quantitative issues derived from the heterogeneity of CAFs and the effectiveness as well as safety of the NDDS based on CAFs are the challenges that need to be overcome urgently. In the future, it is essential to focus on the linkage between the functions of CAFs and their biomarkers so as to clarify the attribution and properties of CAFs, which is the basis for the subsequent research. For the research of CAFs-based nano-drug delivery strategies, an in vitro evaluation model that can simulate the actual growth of tumors should be developed, and emphasis should be placed on the cross-fertilization of multidisciplinary specialties, such as pharmaceutics, materials science, and medical image science, to combine the aforementioned devices and approaches to determine “whether” and “how” CAFs-based nano-drug delivery strategies reduced tumor metastasis. At the same time, safety should be taken as an essential evaluation index, thus decreasing the obstacles in the clinical translation of these drug delivery systems. Additionally, CAFs-based nano-drug delivery strategies might be combined with immunotherapy, photodynamic therapy, and radiation therapy to achieve more efficient anti-tumor metastasis effects in the future. It is believed that with the improvement of basic theories and the breakthrough of critical experimental techniques, CAFs-based nano-drug delivery strategies will achieve a more long-term development in anti-tumor metastasis.
Author contributions
Linghui Zou: Writing – original draft, Investigation, Conceptualization. Peng Xian: Writing – original draft, Investigation. Qing Pu: Investigation. Yangjie Song: Investigation. Shuting Ni: Writing – review & editing, Supervision. Lei Chen: Investigation. Kaili Hu: Writing – review & editing, Validation, Supervision, Resources, Funding acquisition.
Conflicts of interest
The authors have no conflicts of interest to declare.
Acknowledgments
The financial support for this work was from the National Natural Science Foundation of China (Grant No. 82274281, 81773909), “Shuguang Program” supported by Shanghai Education Development Foundation and Shanghai Municipal Education Commission (22SG41,China), Shanghai Talent Development Funds (Grant No.201665, China), and the combination of the medical care and health project of the Shanghai University of Traditional Chinese Medicine (YYKC‒2021‒01‒008, China).
Footnotes
Peer review under the responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Shuting Ni, Email: stella_ni2021@163.com.
Lei Chen, Email: chenlei2114@shutcm.edu.cn.
Kaili Hu, Email: kaili-hu@163.com.
References
- 1.Bergers G., Fendt S.M. The metabolism of cancer cells during metastasis. Nat Rev Cancer. 2021;21:162–180. doi: 10.1038/s41568-020-00320-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang W., Wang F., Hu C., Zhou Y., Gao H., Hu J. The progress and perspective of nanoparticle-enabled tumor metastasis treatment. Acta Pharm Sin B. 2020;10:2037–2053. doi: 10.1016/j.apsb.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park M., Kim D., Ko S., Kim A., Mo K., Yoon H. Breast cancer metastasis: mechanisms and therapeutic implications. Int J Mol Sci. 2022;23:6806. doi: 10.3390/ijms23126806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Visser K.E., Joyce J.A. The evolving tumor microenvironment: from cancer initiation to metastatic outgrowth. Cancer Cell. 2023;41:374–403. doi: 10.1016/j.ccell.2023.02.016. [DOI] [PubMed] [Google Scholar]
- 5.Baghban R., Roshangar L., Jahanban-Esfahlan R., Seidi K., Ebrahimi-Kalan A., Jaymand M., et al. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun Signal. 2020;18:59. doi: 10.1186/s12964-020-0530-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naser R., Fakhoury I., El-Fouani A., Abi-Habib R., El-Sibai M. Role of the tumor microenvironment in cancer hallmarks and targeted therapy. Int J Oncol. 2023;62:23. doi: 10.3892/ijo.2022.5471. [DOI] [PubMed] [Google Scholar]
- 7.Malla R.R., Kiran P. Tumor microenvironment pathways: cross regulation in breast cancer metastasis. Genes Dis. 2022;9:310–324. doi: 10.1016/j.gendis.2020.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen R., Huang L., Hu K. Natural products remodel cancer-associated fibroblasts in desmoplastic tumors. Acta Pharm Sin B. 2020;10:2140–2155. doi: 10.1016/j.apsb.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xin L., Gao J.F., Zheng Z.L., Chen Y.Y., Lv S.X., Zhao Z.K., et al. Fibroblast activation protein-α as a target in the bench-to-bedside diagnosis and treatment of tumors: a narrative review. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.648187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao M.K., Zhuang A.B., Fang Y. Cancer-associated fibroblast-derived exosomal miRNA-320a promotes macrophage M2 polarization in vitro by regulating PTEN/PI3Kγ signaling in pancreatic cancer. J Oncol. 2022;2022 doi: 10.1155/2022/9514697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarkar M., Nguyen T., Gundre E., Ogunlusi O., El-Sobky M., Giri B., et al. Cancer-associated fibroblasts: the chief architect in the tumor microenvironment. Front Cell Dev Biol. 2023;11 doi: 10.3389/fcell.2023.1089068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y.B., Gan G.F., Wang B.C., Wu J.L., Cao Y., Zhu D., et al. Cancer-associated fibroblasts promote irradiated cancer cell recovery through autophagy. EBioMedicine. 2017;17:45–56. doi: 10.1016/j.ebiom.2017.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lavie D., Ben-Shmuel A., Erez N., Scherz-Shouval R. Cancer-associated fibroblasts in the single-cell era. Nat Cancer. 2022;3:793–807. doi: 10.1038/s43018-022-00411-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou Z., Qu C.H., Zhou P.J., Zhou Q., Li D., Wu X., et al. Extracellular vesicles activated cancer-associated fibroblasts promote lung cancer metastasis through mitophagy and mtDNA transfer. J Exp Clin Cancer Res. 2024;43:158. doi: 10.1186/s13046-024-03077-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X.M., Song E.W. Turning foes to friends: targeting cancer-associated fibroblasts. Nat Rev Drug Discov. 2019;18:99–115. doi: 10.1038/s41573-018-0004-1. [DOI] [PubMed] [Google Scholar]
- 16.Mao X.Q., Xu J., Wang W., Liang C., Hua J., Liu J., et al. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives. Mol Cancer. 2021;20:131. doi: 10.1186/s12943-021-01428-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu Y.F., Li X.Y., Wang L., Hong X.W., Yang J. Metabolic reprogramming and crosstalk of cancer-related fibroblasts and immune cells in the tumor microenvironment. Front Endocrinol. 2022;13 doi: 10.3389/fendo.2022.988295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang M.L., Fu M.R., Wang J., Xia C.H., Zhang H., Xiong Y.Q., et al. TGF-β1-activated cancer-associated fibroblasts promote breast cancer invasion, metastasis and epithelial-mesenchymal transition by autophagy or overexpression of FAP-α. Biochem Pharmacol. 2021;188 doi: 10.1016/j.bcp.2021.114527. [DOI] [PubMed] [Google Scholar]
- 19.Li X., Yong T.Y., Wei Z.H., Bie N., Zhang X.Q., Zhan G.T., et al. Reversing insufficient photothermal therapy-induced tumor relapse and metastasis by regulating cancer-associated fibroblasts. Nat Commun. 2022;13:2794. doi: 10.1038/s41467-022-30306-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dong G.Z., Chen P., Xu Y.T., Liu T.Y., Yin R. Cancer-associated fibroblasts: key criminals of tumor pre-metastatic niche. Cancer Lett. 2023;566 doi: 10.1016/j.canlet.2023.216234. [DOI] [PubMed] [Google Scholar]
- 21.Verginadis I.I., Avgousti H., Monslow J., Skoufos G., Chinga F., Kim K., et al. A stromal Integrated Stress Response activates perivascular cancer-associated fibroblasts to drive angiogenesis and tumour progression. Nat Cell Biol. 2022;24:940–953. doi: 10.1038/s41556-022-00918-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang X.Y., Zhang M., Sun H., Wang X., Wang X., Sheng W.Q., et al. The role of transcription factors in the crosstalk between cancer-associated fibroblasts and tumor cells. J Adv Res. 2025;67:121–132. doi: 10.1016/j.jare.2024.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsu W.-H., LaBella K.A., Lin Y.Y., Xu P., Lee R., Hsieh C.-E., et al. Oncogenic KRAS drives lipofibrogenesis to promote angiogenesis and colon cancer progression. Cancer Discov. 2023;13:2652–2673. doi: 10.1158/2159-8290.CD-22-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu B.L., Zhang B.F., Qi J.L., Zhou H.Y., Tan L.C., Huang J.L., et al. Targeting MFGE8 secreted by cancer-associated fibroblasts blocks angiogenesis and metastasis in esophageal squamous cell carcinoma. Proc Natl Acad Sci U S A. 2023;120 doi: 10.1073/pnas.2307914120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang S., Chen C., Qiu Y., Xu C., Yao J. Paying attention to tumor blood vessels: cancer phototherapy assisted with nano delivery strategies. Biomaterials. 2021;268 doi: 10.1016/j.biomaterials.2020.120562. [DOI] [PubMed] [Google Scholar]
- 26.Sahu T., Ratre Y.K., Chauhan S., Bhaskar L., Nair M.P., Verma H.K. Nanotechnology based drug delivery system: current strategies and emerging therapeutic potential for medical science. J Drug Deliv Sci Technol. 2021;63 [Google Scholar]
- 27.Liu R., Luo C., Pang Z.Q., Zhang J.M., Ruan S.B., Wu M.Y., et al. Advances of nanoparticles as drug delivery systems for disease diagnosis and treatment. Chin Chem Lett. 2023;34 [Google Scholar]
- 28.Huda S., Alam M.A., Sharma P.K. Smart nanocarriers-based drug delivery for cancer therapy: an innovative and developing strategy. J Drug Deliv Sci Technol. 2020;60 [Google Scholar]
- 29.Tang L., Li J., Zhao Q.Q., Pan T., Zhong H., Wang W. Advanced and innovative nano-systems for anticancer targeted drug delivery. Pharmaceutics. 2021;13:1151. doi: 10.3390/pharmaceutics13081151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sánchez-López E., Guerra M., Dias-Ferreira J., Lopez-Machado A., Ettcheto M., Cano A., et al. Current applications of nanoemulsions in cancer therapeutics. Nanomaterials. 2019;9:821. doi: 10.3390/nano9060821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Handa M., Beg S., Shukla R., Barkat M.A., Choudhry H., Singh K.K. Recent advances in lipid-engineered multifunctional nanophytomedicines for cancer targeting. J Control Release. 2021;340:48–59. doi: 10.1016/j.jconrel.2021.10.025. [DOI] [PubMed] [Google Scholar]
- 32.Wang J.J., Ni Q.K., Wang Y.F., Zhang Y.X., He H.Y., Gao D.W., et al. Nanoscale drug delivery systems for controllable drug behaviors by multi-stage barrier penetration. J Control Release. 2021;331:282–295. doi: 10.1016/j.jconrel.2020.08.045. [DOI] [PubMed] [Google Scholar]
- 33.Kashkooli F.M., Soltani M., Souri M. Controlled anti-cancer drug release through advanced nano-drug delivery systems: static and dynamic targeting strategies. J Control Release. 2020;327:316–349. doi: 10.1016/j.jconrel.2020.08.012. [DOI] [PubMed] [Google Scholar]
- 34.Zhou Y., Chen X.C., Cao J., Gao H.L. Overcoming the biological barriers in the tumor microenvironment for improving drug delivery and efficacy. J Mater Chem B. 2020;8:6765–6781. doi: 10.1039/d0tb00649a. [DOI] [PubMed] [Google Scholar]
- 35.Junttila M.R., De Sauvage F.J. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature. 2013;501:346–354. doi: 10.1038/nature12626. [DOI] [PubMed] [Google Scholar]
- 36.Gu X.Y., Gao Y.Z., Wang P., Wang L.X., Peng H.B., He Y.Z., et al. Nano-delivery systems focused on tumor microenvironment regulation and biomimetic strategies for treatment of breast cancer metastasis. J Control Release. 2021;333:374–390. doi: 10.1016/j.jconrel.2021.03.039. [DOI] [PubMed] [Google Scholar]
- 37.Qu J.H., Yan Z.L., Lei D.F., Zhong T.N., Fang C.Z., Wen Z.H., et al. Effect of bioactive black phosphorus nanomaterials on cancer-associated fibroblast heterogeneity in pancreatic cancer. ACS Nano. 2024;18:19354–19368. doi: 10.1021/acsnano.4c06147. [DOI] [PubMed] [Google Scholar]
- 38.Guo J., Zeng H.T., Chen Y. Emerging nano-drug delivery systems targeting cancer-associated fibroblasts for improved antitumor effect and tumor drug penetration. Mol Pharma. 2020;17:1028–1048. doi: 10.1021/acs.molpharmaceut.0c00014. [DOI] [PubMed] [Google Scholar]
- 39.Tang L., Mei Y.J., Shen Y., He S., Xiao Q.Q., Yin Y., et al. Nanoparticle-mediated targeted drug delivery to remodel tumor microenvironment for cancer therapy. Int J Nanomedicine. 2021;16:5811–5829. doi: 10.2147/IJN.S321416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang X., Sinha S., Forte E., Thompson S., Herzog E., Driskell R., et al. Fibroblasts: origins, definitions, and functions in health and disease. Cell. 2021;184:3852–3872. doi: 10.1016/j.cell.2021.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Plikus M.V., Wang X.J., Sinha S., Forte E., Thompson S.M., Herzog E.L., et al. Fibroblasts: origins, definitions, and functions in health and disease. Cell. 2021;184:3852–3872. doi: 10.1016/j.cell.2021.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sahai E., Astsaturov I., Cukierman E., DeNardo D.G., Egeblad M., Evans R.M., et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Cancer. 2020;20:174–186. doi: 10.1038/s41568-019-0238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fozzatti L., Cheng S.Y. Tumor cells and cancer-associated fibroblasts: a synergistic crosstalk to promote thyroid cancer. Endocrinol Metab. 2020;35:673–680. doi: 10.3803/EnM.2020.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu T.Y., Han C.C., Wang S.W., Fang P.Q., Ma Z.F., Xu L., et al. Cancer-associated fibroblasts: an emerging target of anti-cancer immunotherapy. J Hematol Oncol. 2019;12:86. doi: 10.1186/s13045-019-0770-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Glabman R.A., Choyke P.L., Sato N. Cancer-associated fibroblasts: tumorigenicity and targeting for cancer therapy. Cancers. 2022;14:3906. doi: 10.3390/cancers14163906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamamoto Y., Kasashima H., Fukui Y., Tsujio G., Yashiro M., Maeda K. The heterogeneity of cancer-associated fibroblast subpopulations: their origins, biomarkers, and roles in the tumor microenvironment. Cancer Sci. 2023;114:16–24. doi: 10.1111/cas.15609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Caja L., Dituri F., Mancarella S., Caballero-Diaz D., Moustakas A., Giannelli G., et al. TGF-β and the tissue microenvironment: relevance in fibrosis and cancer. Int J Mol Sci. 2018;19:1294. doi: 10.3390/ijms19051294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jena B.C., Sarkar S., Rout L., Mandal M. The transformation of cancer-associated fibroblasts: current perspectives on the role of TGF-β in CAF mediated tumor progression and therapeutic resistance. Cancer Lett. 2021;520:222–232. doi: 10.1016/j.canlet.2021.08.002. [DOI] [PubMed] [Google Scholar]
- 49.Ghahremanifard P., Chanda A., Bonni S., Bose P. TGF-β mediated immune evasion in cancer—spotlight on cancer-associated fibroblasts. Cancers. 2020;12:3650. doi: 10.3390/cancers12123650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoshida G.J. Regulation of heterogeneous cancer-associated fibroblasts: the molecular pathology of activated signaling pathways. J Exp Clin Cancer Res. 2020;39:112. doi: 10.1186/s13046-020-01611-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Awaji M., Futakuchi M., Heavican T., Iqbal J., Singh R.K. Cancer-associated fibroblasts enhance survival and progression of the aggressive pancreatic tumor via FGF-2 and CXCL8. Cancer Microenviron. 2019;12:37–46. doi: 10.1007/s12307-019-00223-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Q., Wang Y., Liu F.S. Cancer-associated fibroblasts: versatile mediators in remodeling the tumor microenvironment. Cell Signal. 2022;103 doi: 10.1016/j.cellsig.2022.110567. [DOI] [PubMed] [Google Scholar]
- 53.Galland S., Stamenkovic I. Mesenchymal stromal cells in cancer: a review of their immunomodulatory functions and dual effects on tumor progression. J Pathol. 2020;250:555–572. doi: 10.1002/path.5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tommelein J., Verset L., Boterberg T., Demetter P., Bracke M., De Wever O. Cancer-associated fibroblasts connect metastasis-promoting communication in colorectal cancer. Front Oncol. 2015;5:63. doi: 10.3389/fonc.2015.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li X.B., Chen M.F., Lu W.J., Tang J., Deng L.J., Wen Q., et al. Targeting FAPα-expressing tumor-associated mesenchymal stromal cells inhibits triple-negative breast cancer pulmonary metastasis. Cancer Lett. 2021;503:32–42. doi: 10.1016/j.canlet.2021.01.013. [DOI] [PubMed] [Google Scholar]
- 56.Jotzu C., Alt E., Welte G., Li J., Hennessy B.T., Devarajan E., et al. Adipose tissue derived stem cells differentiate into carcinoma-associated fibroblast-like cells under the influence of tumor derived factors. Cell Oncol. 2011;34:55–67. doi: 10.1007/s13402-011-0012-1. [DOI] [PubMed] [Google Scholar]
- 57.Hamabe-Horiike T., Harada Si, Yoshida K., Kinoshita J., Yamaguchi T., Fushida S. Adipocytes contribute to tumor progression and invasion of peritoneal metastasis by interacting with gastric cancer cells as cancer associated fibroblasts. Cancer Rep. 2023;6 doi: 10.1002/cnr2.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fiori M.E., Di Franco S., Villanova L., Bianca P., Stassi G., De Maria R. Cancer-associated fibroblasts as abettors of tumor progression at the crossroads of EMT and therapy resistance. Mol Cancer. 2019;18:70. doi: 10.1186/s12943-019-0994-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ma J., Sanchez-Duffhues G., Goumans M.-J., Ten Dijke P. TGF-β-induced endothelial to mesenchymal transition in disease and tissue engineering. Front Cell Dev Biol. 2020;8:260. doi: 10.3389/fcell.2020.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fang Z.L., Meng Q.C., Xu J., Wang W., Zhang B., Liu J., et al. Signaling pathways in cancer-associated fibroblasts: recent advances and future perspectives. Cancer Commun. 2023;43:3–41. doi: 10.1002/cac2.12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang X., He C.B., Hua X., Kan A., Mao Y.Z., Sun S.X., et al. Oxidative stress induces monocyte-to-myofibroblast transdifferentiation through p38 in pancreatic ductal adenocarcinoma. Clin Transl Med. 2020;10 doi: 10.1002/ctm2.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Greimelmaier K., Klopp N., Mairinger E., Wessolly M., Borchert S., Steinborn J., et al. Fibroblast activation protein-α expression in fibroblasts is common in the tumor microenvironment of colorectal cancer and may serve as a therapeutic target. Pathol Oncol Res. 2023;29 doi: 10.3389/pore.2023.1611163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nielsen S.H., Willumsen N., Leeming D.J., Daniels S.J., Brix S., Karsdal M.A., et al. Serological assessment of activated fibroblasts by alpha-smooth muscle actin (α-SMA): a noninvasive biomarker of activated fibroblasts in lung disorders. Transl Oncol. 2019;12:368–374. doi: 10.1016/j.tranon.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Im S.B., Cho J.M., Kim H.B., Shin D.-H., Kwon M.S., Lee I.Y., et al. FSP-1 expression in cancer cells is relevant to long-term oncological outcomes in nonmetastatic colorectal cancer. Korean J Clin Oncol. 2022;18:66–77. doi: 10.14216/kjco.22009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Appiah-Kubi K., Wang Y., Qian H., Wu M., Yao X.Y., Wu Y., et al. Platelet-derived growth factor receptor/platelet-derived growth factor (PDGFR/PDGF) system is a prognostic and treatment response biomarker with multifarious therapeutic targets in cancers. Tumor Biol. 2016;37:10053–10066. doi: 10.1007/s13277-016-5069-z. [DOI] [PubMed] [Google Scholar]
- 66.Ni W.D., Yang Z.T., Cui C.A., Cui Y., Fang L.Y., Xuan Y.H. TENASCIN-C is a potential cancer-associated fibroblasts marker and predicts poor prognosis in prostate cancer. Biochem Biophys Res Commun. 2017;486:607–612. doi: 10.1016/j.bbrc.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 67.Ratajczak-Wielgomas K., Grzegrzolka J., Piotrowska A., Gomulkiewicz A., Witkiewicz W., Dziegiel P. PERIOSTIN expression in cancer-associated fibroblasts of invasive ductal breast carcinoma. Oncol Rep. 2016;36:2745–2754. doi: 10.3892/or.2016.5095. [DOI] [PubMed] [Google Scholar]
- 68.Jung Y.Y., Lee Y.K., Koo J.S. Expression of cancer-associated fibroblast-related proteins in adipose stroma of breast cancer. Tumor Biol. 2015;36:8685–8695. doi: 10.1007/s13277-015-3594-9. [DOI] [PubMed] [Google Scholar]
- 69.Nurmik M., Ullmann P., Rodriguez F., Haan S., Letellier E. In search of definitions: cancer-associated fibroblasts and their markers. Int J Cancer. 2020;146:895–905. doi: 10.1002/ijc.32193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang L., Xu A.M., Liu S., Liu W., Li T.J. Cancer-associated fibroblasts in digestive tumors. World J Gastroenterol. 2014;20:17804–17818. doi: 10.3748/wjg.v20.i47.17804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lu T., Oomens L., Terstappen L., Prakash J. In vivo detection of circulating cancer-associated fibroblasts in breast tumor mouse xenograft: impact of tumor stroma and chemotherapy. Cancers. 2023;15:1127. doi: 10.3390/cancers15041127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xue M.Y., Tong Y.S., Xiong Y.Z., Yu C.H. Role of cancer-associated fibroblasts in the progression, therapeutic resistance and targeted therapy of oesophageal squamous cell carcinoma. Front Oncol. 2023;13 doi: 10.3389/fonc.2023.1257266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mukaida N., Sasaki S. Fibroblasts, an inconspicuous but essential player in colon cancer development and progression. World J Gastroenterol. 2016;22:5301–5316. doi: 10.3748/wjg.v22.i23.5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jiang H., Hegde S., DeNardo D.G. Tumor-associated fibrosis as a regulator of tumor immunity and response to immunotherapy. Cancer Immunol Immunother. 2017;66:1037–1048. doi: 10.1007/s00262-017-2003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Han C.C., Liu T.Y., Yin R. Biomarkers for cancer-associated fibroblasts. Biomarker Res. 2020;8:64. doi: 10.1186/s40364-020-00245-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shen X.J., Zhang H., Tang G.S., Wang X.D., Zheng R., Wang Y., et al. CAVEOLIN-1 is a modulator of fibroblast activation and a potential biomarker for gastric cancer. Int J Biol Sci. 2015;11:370–379. doi: 10.7150/ijbs.10666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kalaei Z., Manafi-Farid R., Rashidi B., Kiani F.K., Zarei A., Fathi M., et al. The Prognostic and therapeutic value and clinical implications of fibroblast activation protein-α as a novel biomarker in colorectal cancer. Cell Commun Signal. 2023;21:139. doi: 10.1186/s12964-023-01151-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhao Y., Liu Y.P., Jia Y.L., Wang X.X., He J.K., Zhen S.M., et al. Fibroblast activation protein in the tumor microenvironment predicts outcomes of PD-1 blockade therapy in advanced non-small cell lung cancer. J Cancer Res Clin Oncol. 2023;149:3469–3483. doi: 10.1007/s00432-022-04250-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Solano-Iturri J.D., Errarte P., Etxezarraga M.C., Echevarria E., Angulo J., López J.I., et al. Altered tissue and plasma levels of fibroblast activation protein-α (FAP) in renal tumours. Cancers. 2020;12:3393. doi: 10.3390/cancers12113393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhao Z., Li T.M., Yuan Y., Zhu Y.M. What is new in cancer-associated fibroblast biomarkers? Cell Commun Signal. 2023;21:96. doi: 10.1186/s12964-023-01125-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Akiyama T., Yasuda T., Uchihara T., Yasuda-Yoshihara N., Tan B.J.Y., Yonemura A., et al. Stromal reprogramming through dual PDGFRα/β blockade boosts the efficacy of anti–PD-1 immunotherapy in fibrotic tumors. Cancer Res. 2023;83:753–770. doi: 10.1158/0008-5472.CAN-22-1890. [DOI] [PubMed] [Google Scholar]
- 82.Wang S.W., Xu K.L., Ruan S.Q., Zhao L.L., Chen L.R. Overexpression of CAVEOLIN-1 in cancer-associated fibroblasts predicts good outcome in breast cancer. Breast Care. 2012;7:477–483. doi: 10.1159/000345464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yamao T., Yamashita Y.I., Yamamura K., Nakao Y., Tsukamoto M., Nakagawa S., et al. Cellular senescence, represented by expression of CAVEOLIN-1, in cancer-associated fibroblasts promotes tumor invasion in pancreatic cancer. Ann Surg Oncol. 2019;26:1552–1559. doi: 10.1245/s10434-019-07266-2. [DOI] [PubMed] [Google Scholar]
- 84.Yu S.B., Lu Y.W., Su A., Chen J.N., Li J., Zhou B.X., et al. A CD10-OGP membrane peptolytic signaling axis in fibroblasts regulates lipid metabolism of cancer stem cells via SCD1. Adv Sci. 2021;8 doi: 10.1002/advs.202101848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhao Z.H., Zhu Ym. FAP, CD10, and GPR77-labeled CAFs cause neoadjuvant chemotherapy resistance by inducing EMT and CSC in gastric cancer. BMC cancer. 2023;23:507. doi: 10.1186/s12885-023-11011-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Khanh D.T., Mekata E., Mukaisho Ki, Sugihara H., Shimizu T., Shiomi H., et al. Transmembrane mucin MUC 1 overexpression and its association with CD 10+ myeloid cells, transforming growth factor-β1 expression, and tumor budding grade in colorectal cancer. Cancer Sci. 2013;104:958–964. doi: 10.1111/cas.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Su S.C., Chen J.N., Yao H.R., Liu J., Yu S.B., Lao L.Y., et al. CD10+ GPR77+ cancer-associated fibroblasts promote cancer formation and chemoresistance by sustaining cancer stemness. Cell. 2018;172:841–856. doi: 10.1016/j.cell.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 88.Zhang M., Chen Z.X., Wang Y., Zhao H.B., Du Y. The role of cancer-associated fibroblasts in ovarian cancer. Cancers. 2022;14:2637. doi: 10.3390/cancers14112637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang L.Y., Guo Q., Guan G.F., Cheng W., Cheng P., Wu A.H. Integrin beta 5 is a prognostic biomarker and potential therapeutic target in glioblastoma. Front Oncol. 2019;9:904. doi: 10.3389/fonc.2019.00904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lu L., Gao Y.H., Huang D.F., Liu H., Yin D.Z., Li M., et al. Targeting integrin α5 in fibroblasts potentiates colorectal cancer response to PD-L1 blockade by affecting extracellular-matrix deposition. J Immunother Cancer. 2023;11 doi: 10.1136/jitc-2023-007447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Park S.Y., Kim H.M., Koo J.S. Differential expression of cancer-associated fibroblast-related proteins according to molecular subtype and stromal histology in breast cancer. Breast Cancer Res Treat. 2015;149:727–741. doi: 10.1007/s10549-015-3291-9. [DOI] [PubMed] [Google Scholar]
- 92.Chen K., Yong J., Zauner R., Wally V., Whitelock J., Sajinovic M., et al. Chondroitin sulfate proteoglycan 4 as a marker for aggressive squamous cell carcinoma. Cancers. 2022;14:5564. doi: 10.3390/cancers14225564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mellai M., Casalone C., Corona C., Crociara P., Favole A., Cassoni P., et al. Chondroitin sulphate proteoglycans in the tumour microenvironment. Adv Exp Med Biol. 2020;1272:73–92. doi: 10.1007/978-3-030-48457-6_5. [DOI] [PubMed] [Google Scholar]
- 94.Errarte P., Larrinaga G., López J.I. The role of cancer-associated fibroblasts in renal cell carcinoma. An example of tumor modulation through tumor/non-tumor cell interactions. J Adv Res. 2020;21:103–108. doi: 10.1016/j.jare.2019.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li Q.L., Li M.H., Zheng K.X., Tang S., Ma S.L. Expression pattern analysis and drug differential sensitivity of cancer-associated fibroblasts in triple-negative breast cancer. Transl Oncol. 2021;14 doi: 10.1016/j.tranon.2020.100891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fang T.Y., Zhang L., Yin X., Wang Y.F., Zhang X.H., Bian X.L., et al. The prognostic marker elastin correlates with epithelial-mesenchymal transition and VIMENTIN-positive fibroblasts in gastric cancer. J Pathol Clin Res. 2023;9:56–72. doi: 10.1002/cjp2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nomura S. Identification, friend or foe: VIMENTIN and α-smooth muscle actin in cancer-associated fibroblasts. Ann Surg Oncol. 2019;26:4191–4192. doi: 10.1245/s10434-019-07894-8. [DOI] [PubMed] [Google Scholar]
- 98.Maehira H., Miyake T., Iida H., Tokuda A., Mori H., Yasukawa D., et al. VIMENTIN expression in tumor microenvironment predicts survival in pancreatic ductal adenocarcinoma: heterogeneity in fibroblast population. Ann Surg Oncol. 2019;26:4791–4804. doi: 10.1245/s10434-019-07891-x. [DOI] [PubMed] [Google Scholar]
- 99.Hu D.D., Li Z.Q., Zheng B., Lin X.X., Pan Y.H., Gong P.R., et al. Cancer-associated fibroblasts in breast cancer: challenges and opportunities. Cancer Commun. 2022;42:401–434. doi: 10.1002/cac2.12291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sugai T., Uesugi N., Kitada Y., Yamada N., Osakabe M., Eizuka M., et al. Analysis of the expression of cancer-associated fibroblast-and EMT-related proteins in submucosal invasive colorectal cancer. J Cancer. 2018;9:2702–2712. doi: 10.7150/jca.25646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ma Y.C., Zhu J., Chen S.W., Li T.Y., Ma J., Guo S.H., et al. Activated gastric cancer-associated fibroblasts contribute to the malignant phenotype and 5-FU resistance via paracrine action in gastric cancer. Cancer Cell Int. 2018;18:104. doi: 10.1186/s12935-018-0599-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yang Z.T., Ni W.D., Cui C.N., Fang L.Y., Xuan Y.H. Tenascin C is a prognostic determinant and potential cancer-associated fibroblasts marker for breast ductal carcinoma. Exp Mol Pathol. 2017;102:262–267. doi: 10.1016/j.yexmp.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 103.Yang Z.T., Ni W.D., Liu X.X., Xuan Y.H., Jin Y. TENASCIN-C is a diagnostic marker for cervical cancer and a potential marker for cancer-associated fibroblasts in cervical carcinoma. Int J Clin Exp Pathol. 2017;10:139–147. [Google Scholar]
- 104.Furuhashi S., Morita Y., Matsumoto A., Ida S., Muraki R., Kitajima R., et al. Tenascin C in pancreatic cancer-associated fibroblasts enhances epithelial mesenchymal transition and is associated with resistance to immune checkpoint inhibitor. Am J Cancer Res. 2023;13:5641–5655. [PMC free article] [PubMed] [Google Scholar]
- 105.Doldi V., Lecchi M., Ljevar S., Colecchia M., Campi E., Centonze G., et al. Potential of the stromal matricellular protein PERIOSTIN as a biomarker to improve risk assessment in prostate cancer. Int J Mol Sci. 2022;23:7987. doi: 10.3390/ijms23147987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Takatsu F., Suzawa K., Tomida S., Thu Y.M., Sakaguchi M., Toji T., et al. PERIOSTIN secreted by cancer-associated fibroblasts promotes cancer progression and drug resistance in non-small cell lung cancer. J Mol Med. 2023;101:1603–1614. doi: 10.1007/s00109-023-02384-7. [DOI] [PubMed] [Google Scholar]
- 107.Ishibashi Y., Mochizuki S., Horiuchi K., Tsujimoto H., Kouzu K., Kishi Y., et al. PERIOSTIN derived from cancer-associated fibroblasts promotes esophageal squamous cell carcinoma progression via ADAM17 activation. Biochim Biophys Acta Mol Basis Dis. 2023;1869 doi: 10.1016/j.bbadis.2023.166669. [DOI] [PubMed] [Google Scholar]
- 108.Liao Z.H., Tan Z.W., Zhu P.C., Tan N.S. Cancer-associated fibroblasts in tumor microenvironment–Accomplices in tumor malignancy. Cell Immunol. 2019;343 doi: 10.1016/j.cellimm.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 109.Ping Q.R., Yan R.P., Cheng X., Wang W.J., Zhong Y.M., Hou Z.L., et al. Cancer-associated fibroblasts: overview, progress, challenges, and directions. Cancer Gene Ther. 2021;28:984–999. doi: 10.1038/s41417-021-00318-4. [DOI] [PubMed] [Google Scholar]
- 110.Mani S.A., Guo W.J., Liao M.J., Eaton E.N., Ayyanan A., Zhou A.Y., et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xu A., Xu X.N., Luo Z., Huang X., Gong R.Q., Fu D.Y. Identification of prognostic cancer-associated fibroblast markers in luminal breast cancer using weighted gene co-expression network analysis. Front Oncol. 2023;13 doi: 10.3389/fonc.2023.1191660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Matsumura Y., Ito Y., Mezawa Y., Sulidan K., Daigo Y., Hiraga T., et al. Stromal fibroblasts induce metastatic tumor cell clusters via epithelial–mesenchymal plasticity. Life Sci Alliance. 2019;2 doi: 10.26508/lsa.201900425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Giannoni E., Parri M., Chiarugi P. EMT and oxidative stress: a bidirectional interplay affecting tumor malignancy. Antioxid Redox Signal. 2012;16:1248–1263. doi: 10.1089/ars.2011.4280. [DOI] [PubMed] [Google Scholar]
- 114.Qiao A.X., Gu F., Guo X.J., Zhang X.M., Fu L. Breast cancer-associated fibroblasts: their roles in tumor initiation, progression and clinical applications. Front Med. 2016;10:33–40. doi: 10.1007/s11684-016-0431-5. [DOI] [PubMed] [Google Scholar]
- 115.Wang Y.Y., Lan W., Xu M.X., Song J., Mao J., Li C.Y., et al. Cancer-associated fibroblast-derived SDF-1 induces epithelial-mesenchymal transition of lung adenocarcinoma via CXCR4/β-catenin/PPARδ signalling. Cell Death Dis. 2021;12:214. doi: 10.1038/s41419-021-03509-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Giannoni E., Bianchini F., Calorini L., Chiarugi P. Cancer associated fibroblasts exploit reactive oxygen species through a proinflammatory signature leading to epithelial mesenchymal transition and stemness. Antioxid Redox Signal. 2011;14:2361–2371. doi: 10.1089/ars.2010.3727. [DOI] [PubMed] [Google Scholar]
- 117.Li H.X., Liu W., Zhang X.Y., Wang Y.F. Cancer-associated fibroblast-secreted collagen triple helix repeat containing-1 promotes breast cancer cell migration, invasiveness and epithelial-mesenchymal transition by activating the WNT/β-catenin pathway. Oncol Lett. 2021;22:814. doi: 10.3892/ol.2021.13075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chen Z., Yan X., Li K., Ling Y., Kang H. Stromal fibroblast-derived MFAP5 promotes the invasion and migration of breast cancer cells via Notch1/slug signaling. Clin Transl Oncol. 2020;22:522–531. doi: 10.1007/s12094-019-02156-1. [DOI] [PubMed] [Google Scholar]
- 119.Es H.A., Cox T.R., Sarafraz-Yazdi E., Thiery J.P., Warkiani M.E. Pirfenidone reduces epithelial-mesenchymal transition and spheroid formation in breast carcinoma through targeting cancer-associated fibroblasts (CAFs) Cancers. 2021;13:5118. doi: 10.3390/cancers13205118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gou Z.X., Li J.N., Liu J.M., Yang N. The hidden messengers: cancer associated fibroblasts-derived exosomal miRNAs as key regulators of cancer malignancy. Front Cell Dev Biol. 2024;12 doi: 10.3389/fcell.2024.1378302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang H.B., Wei H., Wang J.S., Li L., Chen A.Y., Li Z.G. MicroRNA-181d-5p-containing exosomes derived from CAFs promote EMT by regulating CDX2/HOXA5 in breast cancer. Mol Ther Nucleic Acids. 2020;19:654–667. doi: 10.1016/j.omtn.2019.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang Y., Yin C.J., Wei C., Xia S., Qiao Z., Zhang X.W., et al. Exosomal miR-625-3p secreted by cancer-associated fibroblasts in colorectal cancer promotes EMT and chemotherapeutic resistance by blocking the CELF2/WWOX pathway. Pharmacol Res. 2022;186 doi: 10.1016/j.phrs.2022.106534. [DOI] [PubMed] [Google Scholar]
- 123.Ritter A., Kreis N.N., Roth S., Friemel A., Safdar B.K., Hoock S.C., et al. Cancer-educated mammary adipose tissue-derived stromal/stem cells in obesity and breast cancer: spatial regulation and function. J Exp Clin Cancer Res. 2023;42:35. doi: 10.1186/s13046-022-02592-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ritter A., Kreis N.N., Hoock S.C., Solbach C., Louwen F., Yuan J.P. Adipose tissue-derived mesenchymal stromal/stem cells, obesity and the tumor microenvironment of breast cancer. Cancers. 2022;14:3908. doi: 10.3390/cancers14163908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lepucki A., Orlińska K., Mielczarek-Palacz A., Kabut J., Olczyk P., Komosińska-Vassev K. The role of extracellular matrix proteins in breast cancer. J Clin Med. 2022;11:1250. doi: 10.3390/jcm11051250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cox T.R. The matrix in cancer. Nat Rev Cancer. 2021;21:217–238. doi: 10.1038/s41568-020-00329-7. [DOI] [PubMed] [Google Scholar]
- 127.Henke E., Nandigama R., Ergün S. Extracellular matrix in the tumor microenvironment and its impact on cancer therapy. Front Mol Biosci. 2020;6:160. doi: 10.3389/fmolb.2019.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Theocharis A.D., Skandalis S.S., Gialeli C., Karamanos N.K. Extracellular matrix structure. Adv Drug Deliv Rev. 2016;97:4–27. doi: 10.1016/j.addr.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 129.Sala M., Ros M., Saltel F. A complex and evolutive character: two face aspects of ECM in tumor progression. Front Oncol. 2020;10:1620. doi: 10.3389/fonc.2020.01620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sakhneny L., Epshtein A., Landsman L. Pericytes contribute to the islet basement membranes to promote beta-cell gene expression. Sci Rep. 2021;11:2378. doi: 10.1038/s41598-021-81774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chang J., Chaudhuri O. Beyond proteases: basement membrane mechanics and cancer invasion. J Cell Biol. 2019;218:2456–2469. doi: 10.1083/jcb.201903066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kessenbrock K., Plaks V., Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nii T., Kuwahara T., Makino K., Tabata Y. A co-culture system of three-dimensional tumor-associated macrophages and three-dimensional cancer-associated fibroblasts combined with biomolecule release for cancer cell migration. Tissue Eng A. 2020;26:1272–1282. doi: 10.1089/ten.TEA.2020.0095. [DOI] [PubMed] [Google Scholar]
- 134.Dumont N., Liu B., DeFilippis R.A., Chang H., Rabban J.T., Karnezis A.N., et al. Breast fibroblasts modulate early dissemination, tumorigenesis, and metastasis through alteration of extracellular matrix characteristics. Neoplasia. 2013;15:249–262. doi: 10.1593/neo.121950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cannone S., Greco M.R., Carvalho T.M.A., Guizouarn H., Soriani O., Di Molfetta D., et al. Cancer associated fibroblast (CAF) regulation of PDAC parenchymal (CPC) and CSC phenotypes is modulated by ECM composition. Cancers. 2022;14:3737. doi: 10.3390/cancers14153737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Papanicolaou M., Parker A.L., Yam M., Filipe E.C., Wu S.Z., Chitty J.L., et al. Temporal profiling of the breast tumour microenvironment reveals collagen XII as a driver of metastasis. Nat Commun. 2022;13:4587. doi: 10.1038/s41467-022-32255-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wishart A.L., Conner S.J., Guarin J.R., Fatherree J.P., Peng Y., McGinn R.A., et al. Decellularized extracellular matrix scaffolds identify full-length collagen VI as a driver of breast cancer cell invasion in obesity and metastasis. Sci Adv. 2020;6 doi: 10.1126/sciadv.abc3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Li X.Y., Dong M., Zang X.Y., Li M.Y., Zhou J.Y., Ma J.J., et al. The emerging role of circulating tumor cells in cancer management. Am J Transl Res. 2020;12:332–342. [PMC free article] [PubMed] [Google Scholar]
- 139.Mitsui Y., Tomonobu N., Watanabe M., Kinoshita R., Sumardika I.W., Youyi C., et al. Upregulation of mobility in pancreatic cancer cells by secreted S100A11 through activation of surrounding fibroblasts. Oncol Res. 2019;27:945–956. doi: 10.3727/096504019X15555408784978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mei S.S., Chen X., Wang K., Chen Y.X. Tumor microenvironment in ovarian cancer peritoneal metastasis. Cancer Cell Intl. 2023;23:11. doi: 10.1186/s12935-023-02854-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Holter J.C., Chang C.W., Avendano A., Garg A.A., Verma A.K., Charan M., et al. Fibroblast-derived CXCL12 increases vascular permeability in a 3-D microfluidic model independent of extracellular matrix contractility. Front Bioeng Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.888431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Choi Y.P., Lee J.H., Gao M.Q., Kim B.G., Kang S., Kim S.H., et al. Cancer-associated fibroblast promote transmigration through endothelial brain cells in three-dimensional in vitro models. Int J Cancer. 2014;135:2024–2033. doi: 10.1002/ijc.28848. [DOI] [PubMed] [Google Scholar]
- 143.Lozar T., Gersak K., Cemazar M., Kuhar C.G., Jesenko T. The biology and clinical potential of circulating tumor cells. Radiol Oncol. 2019;53:131–147. doi: 10.2478/raon-2019-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chen Q., Zou J.Y., He Y., Pan Y.H., Yang G.J., Zhao H., et al. A narrative review of circulating tumor cells clusters: a key morphology of cancer cells in circulation promote hematogenous metastasis. Front Oncol. 2022;12 doi: 10.3389/fonc.2022.944487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Aramini B., Masciale V., Arienti C., Dominici M., Stella F., Martinelli G., et al. Cancer stem cells (CSCs), circulating tumor cells (CTCs) and their interplay with cancer associated fibroblasts (CAFs): a new world of targets and treatments. Cancers. 2022;14:2048. doi: 10.3390/cancers14102408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ortiz-Otero N., Marshall J.R., Lash B., King M.R. Chemotherapy-induced release of circulating-tumor cells into the bloodstream in collective migration units with cancer-associated fibroblasts in metastatic cancer patients. BMC Cancer. 2020;20:873. doi: 10.1186/s12885-020-07376-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Luo H.J., Tu G., Liu Z.M., Liu M.R. Cancer-associated fibroblasts: a multifaceted driver of breast cancer progression. Cancer Lett. 2015;361:155–163. doi: 10.1016/j.canlet.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 148.Hurtado P., Martínez-Pena I., Piñeiro R. Dangerous liaisons: circulating tumor cells (CTCs) and cancer-associated fibroblasts (CAFs) Cancers. 2020;12:2861. doi: 10.3390/cancers12102861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cirillo F., Pellegrino M., Talia M., Perrotta I.D., Rigiracciolo D.C., Spinelli A., et al. Estrogen receptor variant ERα46 and insulin receptor drive in primary breast cancer cells growth effects and interleukin 11 induction prompting the motility of cancer-associated fibroblasts. Clin Transl Med. 2021;11 doi: 10.1002/ctm2.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sharma U., Medina-Saenz K., Miller P.C., Troness B., Spartz A., Sandoval-Leon A., et al. Heterotypic clustering of circulating tumor cells and circulating cancer-associated fibroblasts facilitates breast cancer metastasis. Breast Cancer Res Treat. 2021;189:63–80. doi: 10.1007/s10549-021-06299-0. [DOI] [PubMed] [Google Scholar]
- 151.Rajput S., Sharma P.K., Malviya R. Fluid mechanics in circulating tumour cells: role in metastasis and treatment strategies. Med Drug Discov. 2023 [Google Scholar]
- 152.Labernadie A., Kato T., Brugués A., Serra-Picamal X., Derzsi S., Arwert E., et al. A mechanically active heterotypic E-CADHERIN/N-CADHERIN adhesion enables fibroblasts to drive cancer cell invasion. Nat Cell Biol. 2017;19:224–237. doi: 10.1038/ncb3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sun Y.F., Wu L., Liu S.P., Jiang M.M., Hu B., Zhou K.Q., et al. Dissecting spatial heterogeneity and the immune-evasion mechanism of CTCs by single-cell RNA-seq in hepatocellular carcinoma. Nat Commun. 2021;12:4091. doi: 10.1038/s41467-021-24386-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ortiz-Otero N., Clinch A.B., Hope J., Wang W., Reinhart-King C.A., King M.R. Cancer associated fibroblasts confer shear resistance to circulating tumor cells during prostate cancer metastatic progression. Oncotarget. 2020;11:1037–1050. doi: 10.18632/oncotarget.27510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Duda D.G., Duyverman A.M.M.J., Kohno M., Snuderl M., Steller E.J.A., Fukumura D., et al. Malignant cells facilitate lung metastasis by bringing their own soil. Proc Natl Acad Sci U S A. 2010;107:21677–21682. doi: 10.1073/pnas.1016234107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Muchlińska A., Wenta R., Ścińska W., Markiewicz A., Suchodolska G., Senkus E., et al. Improved characterization of circulating tumor cells and cancer-associated fibroblasts in one-tube assay in breast cancer patients using imaging flow cytometry. Cancers. 2023;15:4169. doi: 10.3390/cancers15164169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ortiz-Otero N., Marshall J.R., Glenn A., Matloubieh J., Joseph J., Sahasrabudhe D.M., et al. TRAIL-coated leukocytes to kill circulating tumor cells in the flowing blood from prostate cancer patients. BMC Cancer. 2021;21:898. doi: 10.1186/s12885-021-08589-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yang L., Li T., Shi H., Zhou Z., Huang Z., Lei X. The cellular and molecular components involved in pre-metastatic niche formation in colorectal cancer liver metastasis. Expert Rev Gastroenterol Hepatol. 2021;15:389–399. doi: 10.1080/17474124.2021.1848543. [DOI] [PubMed] [Google Scholar]
- 159.Peinado H., Zhang H., Matei I.R., Costa-Silva B., Hoshino A., Rodrigues G., et al. Pre-metastatic niches: organ-specific homes for metastases. Nat Rev Cancer. 2017;17:302–317. doi: 10.1038/nrc.2017.6. [DOI] [PubMed] [Google Scholar]
- 160.Geissler M., Jia W.Y., Kiraz E.N., Kulacz I., Liu X., Rombach A., et al. The brain pre-metastatic niche: biological and technical advancements. Int J Mol Sci. 2023;24 doi: 10.3390/ijms241210055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Han Q., Tan S.R., Gong L.Q., Li G.Q., Wu Q.L., Chen L., et al. Omental cancer-associated fibroblast-derived exosomes with low microRNA-29c-3p promote ovarian cancer peritoneal metastasis. Cancer Sci. 2023;114:1929–1942. doi: 10.1111/cas.15726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mezawa Y., Orimo A. The roles of tumor-and metastasis-promoting carcinoma-associated fibroblasts in human carcinomas. Cell Tissue Res. 2016;365:675–689. doi: 10.1007/s00441-016-2471-1. [DOI] [PubMed] [Google Scholar]
- 163.Sheta M., Taha E.A., Lu Y., Eguchi T. Extracellular vesicles: new classification and tumor immunosuppression. Biology. 2023;12:110. doi: 10.3390/biology12010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mo Y.L., Leung L.L., Mak C.S.L., Wang X., Chan W.S., Hui L.M.N., et al. Tumor-secreted exosomal miR-141 activates tumor-stroma interactions and controls premetastatic niche formation in ovarian cancer metastasis. Mol Cancer. 2023;22:4. doi: 10.1186/s12943-022-01703-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Li R.X., Zhou J., Wu X.N., Li H.Z., Pu Y.Z., Liu N.N., et al. Jianpi Jiedu Recipe inhibits colorectal cancer liver metastasis via regulating ITGBL1-rich extracellular vesicles mediated activation of cancer-associated fibroblasts. Phytomedicine. 2022;100 doi: 10.1016/j.phymed.2022.154082. [DOI] [PubMed] [Google Scholar]
- 166.Wu X., Zhou Z., Xu S., Liao C., Chen X.L., Li B., et al. Extracellular vesicle packaged LMP1-activated fibroblasts promote tumor progression via autophagy and stroma-tumor metabolism coupling. Cancer Lett. 2020;478:93–106. doi: 10.1016/j.canlet.2020.03.004. [DOI] [PubMed] [Google Scholar]
- 167.Liu M.M., Yang J., Xu B.S., Zhang X. Tumor metastasis: mechanistic insights and therapeutic interventions. MedComm. 2021;2:587–617. doi: 10.1002/mco2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhao Z.H., Li T.M., Sun L.P., Yuan Y., Zhu Y.M. Potential mechanisms of cancer-associated fibroblasts in therapeutic resistance. Biomed Pharmacother. 2023;166 doi: 10.1016/j.biopha.2023.115425. [DOI] [PubMed] [Google Scholar]
- 169.Wu B., Liu D.A., Guan L., Myint P.K., Chin L., Dang H., et al. Stiff matrix induces exosome secretion to promote tumour growth. Nat Cell Biol. 2023;25:415–424. doi: 10.1038/s41556-023-01092-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Cohen N., Mundhe D., Deasy S.K., Adler O., Ershaid N., Shami T., et al. Breast cancer-secreted factors promote lung metastasis by signaling systemically to induce a fibrotic pre-metastatic niche. Cancer Res. 2023;83:3354–3367. doi: 10.1158/0008-5472.CAN-22-3707. [DOI] [PubMed] [Google Scholar]
- 171.Kokoretsis D., Maniaki E.K., Kyriakopoulou K., Koutsakis C., Piperigkou Z., Karamanos N.K. Hyaluronan as "Agent Smith" in cancer extracellular matrix pathobiology: regulatory roles in immune response, cancer progression and targeting. IUBMB Life. 2022;74:943–954. doi: 10.1002/iub.2608. [DOI] [PubMed] [Google Scholar]
- 172.Li X., Jiang E.H., Zhao H., Chen Y., Xu Y.M., Feng C.Y., et al. Glycometabolic reprogramming-mediated proangiogenic phenotype enhancement of cancer-associated fibroblasts in oral squamous cell carcinoma: role of PGC-1α/PFKFB3 axis. Br J Cancer. 2022;127:449–461. doi: 10.1038/s41416-022-01818-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Shu S.L., Yang Y.C., Allen C.L., Maguire O., Minderman H., Sen A., et al. Metabolic reprogramming of stromal fibroblasts by melanoma exosome microRNA favours a pre-metastatic microenvironment. Sci Rep. 2018;8 doi: 10.1038/s41598-018-31323-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zhang C., Wang X.Y., Zhang P., He T.C., Han J.H., Zhang R., et al. Cancer-derived exosomal HSPC111 promotes colorectal cancer liver metastasis by reprogramming lipid metabolism in cancer-associated fibroblasts. Cell Death Dis. 2022;13:57. doi: 10.1038/s41419-022-04506-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Shani O., Vorobyov T., Monteran L., Lavie D., Cohen N., Raz Y., et al. Fibroblast-derived IL33 facilitates breast cancer metastasis by modifying the immune microenvironment and driving type 2 immunity. Cancer Res. 2020;80:5317–5329. doi: 10.1158/0008-5472.CAN-20-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zhong B.P., Cheng B., Huang X., Xiao Q.M., Niu Z.T., Chen Y.F., et al. Colorectal cancer-associated fibroblasts promote metastasis by up-regulating LRG1 through stromal IL-6/STAT3 signaling. Cell Death Dis. 2021;13:16. doi: 10.1038/s41419-021-04461-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Dong Q., Liu X., Cheng K., Sheng J.H., Kong J., Liu T.J. Pre-metastatic niche formation in different organs induced by tumor extracellular vesicles. Front Cell Dev Biol. 2021;9 doi: 10.3389/fcell.2021.733627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Servais C., Erez N. From sentinel cells to inflammatory culprits: cancer-associated fibroblasts in tumour-related inflammation. J Pathol. 2013;229:198–207. doi: 10.1002/path.4103. [DOI] [PubMed] [Google Scholar]
- 179.Monteran L., Ershaid N., Doron H., Zait Y., Scharff Y., Ben-Yosef S., et al. Chemotherapy-induced complement signaling modulates immunosuppression and metastatic relapse in breast cancer. Nat Commun. 2022;13:5797. doi: 10.1038/s41467-022-33598-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zhang J., Gu C.Y., Song Q.Q., Zhu M.Q., Xu Y.Q., Xiao M.B., et al. Identifying cancer-associated fibroblasts as emerging targets for hepatocellular carcinoma. Cell Biosci. 2020;10:127. doi: 10.1186/s13578-020-00488-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Walterskirchen N., Mueller C., Ramos C., Zeindl S., Stang S., Herzog D., et al. Metastatic colorectal carcinoma-associated fibroblasts have immunosuppressive properties related to increased IGFBP2 expression. Cancer Lett. 2022;540 doi: 10.1016/j.canlet.2022.215737. [DOI] [PubMed] [Google Scholar]
- 182.Nushtaeva A., Ermakov M., Abdurakhmanova M., Troitskaya O., Belovezhets T., Varlamov M., et al. “Pulsed hypoxia” gradually reprograms breast cancer fibroblasts into pro-tumorigenic cells via mesenchymal–epithelial transition. Int J Mol Sci. 2023;24:2494. doi: 10.3390/ijms24032494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Huang Q.Y., Wu L.Y., Wang Y., Kong X., Xiao X.H., Huang Q.Y., et al. Caveolin-1-deficient fibroblasts promote migration, invasion, and stemness via activating the TGF-β/SMAD signaling pathway in breast cancer cells. Acta Biochim Biophys Sin. 2022;54:1587–1598. doi: 10.3724/abbs.2022150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Karagiannis G.S., Poutahidis T., Erdman S.E., Kirsch R., Riddell R.H., Diamandis E.P. Cancer-associated fibroblasts drive the progression of metastasis through both paracrine and mechanical pressure on cancer tissue. Mol Cancer Res. 2012;10:1403–1418. doi: 10.1158/1541-7786.MCR-12-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Liang L., Li W.T., Li X., Jin X., Liao Q.J., Li Y.L., et al. 'Reverse Warburg effect' of cancer-associated fibroblasts. Int J Oncol. 2022;60:67. doi: 10.3892/ijo.2022.5357. (Review) [DOI] [PubMed] [Google Scholar]
- 186.Hari S.K., Gauba A., Shrivastava N., Tripathi R.M., Jain S.K., Pandey A.K. Polymeric micelles and cancer therapy: an ingenious multimodal tumor-targeted drug delivery system. Drug Deliv Transl Res. 2023;13:135–163. doi: 10.1007/s13346-022-01197-4. [DOI] [PubMed] [Google Scholar]
- 187.Babadi D., Dadashzadeh S., Osouli M., Abbasian Z., Daryabari M.S., Sadrai S., et al. Biopharmaceutical and pharmacokinetic aspects of nanocarrier-mediated oral delivery of poorly soluble drugs. J Drug Deliv Sci Technol. 2021;62 [Google Scholar]
- 188.Tanaka H.Y., Nakazawa T., Enomoto A., Masamune A., Kano M.R. Therapeutic strategies to overcome fibrotic barriers to nanomedicine in the pancreatic tumor microenvironment. Cancers. 2023;15:724. doi: 10.3390/cancers15030724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Greish K. Enhanced permeability and retention effect for selective targeting of anticancer nanomedicine: are we there yet? Drug Discov Today Technol. 2012;9:e71–e174. doi: 10.1016/j.ddtec.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 190.Subhan M.A., Yalamarty S.S.K., Filipczak N., Parveen F., Torchilin V.P. Recent advances in tumor targeting via EPR effect for cancer treatment. J Pers Med. 2021;11:571. doi: 10.3390/jpm11060571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Fang J., Islam W., Maeda H. Exploiting the dynamics of the EPR effect and strategies to improve the therapeutic effects of nanomedicines by using EPR effect enhancers. Adv Drug Deliv Rev. 2020;157:142–160. doi: 10.1016/j.addr.2020.06.005. [DOI] [PubMed] [Google Scholar]
- 192.Torchilin V.P. Passive and active drug targeting: drug delivery to tumors as an example. Handb Exp Pharmacol. 2010;197:3–53. doi: 10.1007/978-3-642-00477-3_1. [DOI] [PubMed] [Google Scholar]
- 193.Ernsting M.J., Hoang B., Lohse I., Undzys E., Cao P., Do T., et al. Targeting of metastasis-promoting tumor-associated fibroblasts and modulation of pancreatic tumor-associated stroma with a carboxymethylcellulose-docetaxel nanoparticle. J Control Release. 2015;206:122–130. doi: 10.1016/j.jconrel.2015.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Murakami M., Ernsting M.J., Undzys E., Holwell N., Foltz W.D., Li S.D. Docetaxel conjugate nanoparticles that target α-smooth muscle actin-expressing stromal cells suppress breast cancer metastasis. Cancer Res. 2013;73:4862–4871. doi: 10.1158/0008-5472.CAN-13-0062. [DOI] [PubMed] [Google Scholar]
- 195.Peng J.H., Cui Y.Y., Xu S.P., Wu X.W., Huang Y., Zhou W.B., et al. Altered glycolysis results in drug-resistant in clinical tumor therapy. Oncol Lett. 2021;21:369. doi: 10.3892/ol.2021.12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Li X.Y., Luo Y., Huang Z.J., Wang Y., Wu J., Zhou S.B. Multifunctional liposomes remodeling tumor immune microenvironment for tumor chemoimmunotherapy. Small Methods. 2023;7 doi: 10.1002/smtd.202201327. [DOI] [PubMed] [Google Scholar]
- 197.Fan F., Jin L.J., Yang L.H. pH-Sensitive nanoparticles composed solely of membrane-disruptive macromolecules for treating pancreatic cancer. ACS Appl Mater Inter. 2021;13:12824–12835. doi: 10.1021/acsami.0c16576. [DOI] [PubMed] [Google Scholar]
- 198.Tansi F.L., Rüger R., Böhm C., Steiniger F., Kontermann R.E., Teichgraeber U.K., et al. Activatable bispecific liposomes bearing fibroblast activation protein directed single chain fragment/Trastuzumab deliver encapsulated cargo into the nuclei of tumor cells and the tumor microenvironment simultaneously. Acta Biomater. 2017;54:281–293. doi: 10.1016/j.actbio.2017.03.033. [DOI] [PubMed] [Google Scholar]
- 199.Ji T.J., Ding Y.P., Zhao Y., Wang J., Qin H., Liu X.M., et al. Peptide assembly integration of fibroblast-targeting and cell-penetration features for enhanced antitumor drug delivery. Adv Mater. 2015;27:1865–1873. doi: 10.1002/adma.201404715. [DOI] [PubMed] [Google Scholar]
- 200.Lang J.Y., Zhao X., Qi Y.Q., Zhang Y.L., Han X.X., Ding Y.P., et al. Reshaping Prostate tumor microenvironment to suppress metastasis via cancer-associated fibroblast inactivation with peptide-assembly-based nanosystem. ACS Nano. 2019;13:12357–12371. doi: 10.1021/acsnano.9b04857. [DOI] [PubMed] [Google Scholar]
- 201.Shin H., Kim Y., Jon S. Nanovaccine displaying immunodominant T cell epitopes of fibroblast activation protein is effective against desmoplastic tumors. ACS Nano. 2023;17:10337–10352. doi: 10.1021/acsnano.3c00764. [DOI] [PubMed] [Google Scholar]
- 202.Shen W.Q., Yao P.A., Li W.J., Gu C.J., Gao T., Cao Y., et al. Cancer-associated fibroblast-targeted nanodrugs reshape colorectal tumor microenvironments to suppress tumor proliferation, metastasis and improve drug penetration. J Mater Chem B. 2023;11:1871–1880. doi: 10.1039/d2tb02253b. [DOI] [PubMed] [Google Scholar]
- 203.Zhu Y., Wen L.J., Shao S.H., Tan Y.N., Meng T.T., Yang X.Q., et al. Inhibition of tumor-promoting stroma to enforce subsequently targeting AT(1)R on tumor cells by pathological inspired micelles. Biomaterials. 2018;161:33–46. doi: 10.1016/j.biomaterials.2018.01.023. [DOI] [PubMed] [Google Scholar]
- 204.Chan T.S., Shaked Y., Tsai K.K. Targeting the interplay between cancer fibroblasts, mesenchymal stem cells, and cancer stem cells in desmoplastic cancers. Front Oncol. 2019;9:688. doi: 10.3389/fonc.2019.00688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Guo D.X., Ji X.Y., Xie H., Ma J., Xu C.C., Zhou Y.F., et al. Targeted reprogramming of vitamin B(3) metabolism as a nanotherapeutic strategy towards chemoresistant cancers. Adv Mater. 2023;35 doi: 10.1002/adma.202301257. [DOI] [PubMed] [Google Scholar]
- 206.Czekay R.-P., Cheon D.-J., Samarakoon R., Kutz S.M., Higgins P.J. Cancer-associated fibroblasts: mechanisms of tumor progression and novel therapeutic targets. Cancers. 2022;14:1231. doi: 10.3390/cancers14051231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Shi X.K., Young C.D., Zhou H.M., Wang X.J. Transforming growth factor-β signaling in fibrotic diseases and cancer-associated fibroblasts. Biomolecules. 2020;10:1666. doi: 10.3390/biom10121666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Wu J.L., Wang F.Q., Dong J.P., Zhang S.Q., Li N., Zhao H.F., et al. Therapeutic response of multifunctional lipid and micelle formulation in hepatocellular carcinoma. ACS Appl Mater Inter. 2022;14:45110–45123. doi: 10.1021/acsami.2c10446. [DOI] [PubMed] [Google Scholar]
- 209.Mardhian D.F., Storm G., Bansal R., Prakash J. Nano-targeted relaxin impairs fibrosis and tumor growth in pancreatic cancer and improves the efficacy of gemcitabine in vivo. J Control Release. 2018;290:1–10. doi: 10.1016/j.jconrel.2018.09.031. [DOI] [PubMed] [Google Scholar]
- 210.Xu H., Hu M.Y., Liu M.R., An S., Guan K.Y., Wang M.L., et al. Nano-puerarin regulates tumor microenvironment and facilitates chemo- and immunotherapy in murine triple negative breast cancer model. Biomaterials. 2020;235 doi: 10.1016/j.biomaterials.2020.119769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Wu Y.F., Chen R.J., Ni S.T., Hu K.L. Biomimetic “nano-spears” for CAFs-targeting: splintered three “shields” with enhanced cisplatin anti-TNBC efficiency. J Control Release. 2024;370:556–569. doi: 10.1016/j.jconrel.2024.04.053. [DOI] [PubMed] [Google Scholar]
- 212.Qiu Z.W., Zhong Y.T., Lu Z.M., Yan N., Kong R.J., Huang J.Q., et al. Breaking physical barrier of fibrotic breast cancer for photodynamic immunotherapy by remodeling tumor extracellular matrix and reprogramming cancer-associated fibroblasts. ACS Nano. 2024;18:9713–9735. doi: 10.1021/acsnano.4c01499. [DOI] [PubMed] [Google Scholar]
- 213.Sharbeen G., McCarroll J.A., Akerman A., Kopecky C., Youkhana J., Kokkinos J., et al. Cancer-associated fibroblasts in pancreatic ductal adenocarcinoma determine response to SLC7A11 inhibition. Cancer Res. 2021;81:3461–3479. doi: 10.1158/0008-5472.CAN-20-2496. [DOI] [PubMed] [Google Scholar]
- 214.Teo J., McCarroll J.A., Boyer C., Youkhana J., Sagnella S.M., Duong H.T.T., et al. A rationally optimized nanoparticle system for the delivery of RNA interference therapeutics into pancreatic tumors in vivo. Biomacromolecules. 2016;17:2337–2351. doi: 10.1021/acs.biomac.6b00185. [DOI] [PubMed] [Google Scholar]
- 215.Liu W., Zhang Q.Q., Zhang Y., Sun L.L., Xiao H., Luo B. Epstein-barr virus regulates endothelin-1 expression through the ERK/FOXO1 pathway in EBV-associated gastric cancer. Microbiol Spectr. 2023;11 doi: 10.1128/spectrum.00898-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Son S., Shin J.M., Shin S., Kim C.H., Lee J.A., Ko H., et al. Repurposing macitentan with nanoparticle modulates tumor microenvironment to potentiate immune checkpoint blockade. Biomaterials. 2021;276 doi: 10.1016/j.biomaterials.2021.121058. [DOI] [PubMed] [Google Scholar]
- 217.Wang H., Chen Y., Wei R., Zhang J.L., Zhu J.H., Wang W.B., et al. Synergistic chemoimmunotherapy augmentation via sequential nanocomposite hydrogel-mediated reprogramming of cancer-associated fibroblasts in osteosarcoma. Adv Mater. 2024;36 doi: 10.1002/adma.202309591. [DOI] [PubMed] [Google Scholar]
- 218.Pradhan R., Paul S., Das B., Sinha S., Dash S.R., Mandal M., et al. Resveratrol nanoparticle attenuates metastasis and angiogenesis by deregulating inflammatory cytokines through inhibition of CAFs in oral cancer by CXCL-12/IL-6-dependent pathway. J Nutr Biochem. 2023;113 doi: 10.1016/j.jnutbio.2022.109257. [DOI] [PubMed] [Google Scholar]
- 219.Liu Q., Chen F.Q., Hou L., Shen L.M., Zhang X.Q., Wang D.G., et al. Nanocarrier-mediated chemo-immunotherapy arrested cancer progression and induced tumor dormancy in desmoplastic melanoma. ACS Nano. 2018;12:7812–7825. doi: 10.1021/acsnano.8b01890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Xiang L., Song Z.D., Rong G.H. Taxotere-induced WNT16 expression in carcinoma-associated fibroblasts might associate with progression and chemoresistance of breast cancer. Ann Clin Lab Sci. 2020;50:205–212. [PubMed] [Google Scholar]
- 221.Cun X.L., Chen J.T., Li M.M., He X., Tang X., Guo R., et al. Tumor-associated fibroblast-targeted regulation and deep tumor delivery of chemotherapeutic drugs with a multifunctional size-switchable nanoparticle. ACS Appl Mater Inter. 2019;11:39545–39559. doi: 10.1021/acsami.9b13957. [DOI] [PubMed] [Google Scholar]
- 222.Duan H.X., Liu C., Hou Y., Liu Y.H., Zhang Z.A., Zhao H.M., et al. Sequential delivery of quercetin and paclitaxel for the fibrotic tumor microenvironment remodeling and chemotherapy potentiation via a dual-targeting hybrid micelle-in-liposome system. ACS Appl Mater Inter. 2022;14:10102–10116. doi: 10.1021/acsami.1c23166. [DOI] [PubMed] [Google Scholar]
- 223.Zang S.Y., Huang K.X., Li J.X., Ren K.B., Li T., He X., et al. Metabolic reprogramming by dual-targeting biomimetic nanoparticles for enhanced tumor chemo-immunotherapy. Acta Biomater. 2022;148:181–193. doi: 10.1016/j.actbio.2022.05.045. [DOI] [PubMed] [Google Scholar]
- 224.Najafi M., Farhood B., Mortezaee K. Extracellular matrix (ECM) stiffness and degradation as cancer drivers. J Cell Biochem. 2019;120:2782–2790. doi: 10.1002/jcb.27681. [DOI] [PubMed] [Google Scholar]
- 225.Walker C., Mojares E., del Río Hernández A. Role of extracellular matrix in development and cancer progression. Int J Mol Sci. 2018;19:3028. doi: 10.3390/ijms19103028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Fang M., Yuan J.P., Peng C.W., Li Y. Collagen as a double-edged sword in tumor progression. Tumor Biol. 2014;35:2871–2882. doi: 10.1007/s13277-013-1511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Hu C.H., Liu X.Y., Ran W., Meng J., Zhai Y.H., Zhang P.C., et al. Regulating cancer associated fibroblasts with losartan-loaded injectable peptide hydrogel to potentiate chemotherapy in inhibiting growth and lung metastasis of triple negative breast cancer. Biomaterials. 2017;144:60–72. doi: 10.1016/j.biomaterials.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 228.Chen Z.J., Liu W.P., Wang X., Liu Y., Li X.H. Sequential drug release to modulate collagen synthesis and promote micelle penetration in tumors. ACS Biomater Sci Eng. 2019;5:1343–1353. doi: 10.1021/acsbiomaterials.8b01600. [DOI] [PubMed] [Google Scholar]
- 229.Tuerhong M., Xu Y., Yin X.B. Review on carbon dots and their applications. Chin J Anal Chem. 2017;45:139–150. [Google Scholar]
- 230.Hou L., Chen D.D., Wang R., Wang R.T., Zhang H.J., Zhang Z.Z., et al. Transformable honeycomb-like nanoassemblies of carbon dots for regulated multisite delivery and enhanced antitumor chemoimmunotherapy. Angew Chem Int Ed Engl. 2021;60:6581–6592. doi: 10.1002/anie.202014397. [DOI] [PubMed] [Google Scholar]
- 231.Zheng S., Wang J.F., Ding N., Chen W.W., Chen H.D., Xue M., et al. Prodrug polymeric micelles integrating cancer-associated fibroblasts deactivation and synergistic chemotherapy for gastric cancer. J Nanobiotechnology. 2021;19:381. doi: 10.1186/s12951-021-01127-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Pan J.X., Lai Y., Zhang S.N., Zhang H.J., Shan Y.M., Huang L.J., et al. Self-adaptive nanoregulator to mitigate dynamic immune evasion of pancreatic cancer. Adv Mater. 2023;35 doi: 10.1002/adma.202305798. [DOI] [PubMed] [Google Scholar]
- 233.Zhang Y.X., Fang Z.X., Pan D.Y., Li Y.K., Zhou J., Chen H.Y., et al. Dendritic polymer-based nanomedicines remodel the tumor stroma: improve drug penetration and enhance antitumor immune response. Adv Mater. 2024;36 doi: 10.1002/adma.202401304. [DOI] [PubMed] [Google Scholar]
- 234.Zheng D.D., Zhou J., Qian L., Liu X.J., Chang C., Tang S., et al. Biomimetic nanoparticles drive the mechanism understanding of shear-wave elasticity stiffness in triple negative breast cancers to predict clinical treatment. Bioact Mater. 2023;22:567–587. doi: 10.1016/j.bioactmat.2022.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Yuan S.J., Mu W.W., Liu S.J., Liu M.C., Xia Z.X., Liang S., et al. Transforming cancer-associated fibroblast barrier into drug depots to boost chemo-immunotherapy in “shooting fish in a barrel” pattern. ACS Nano. 2023;17:13611–13626. doi: 10.1021/acsnano.3c02272. [DOI] [PubMed] [Google Scholar]
- 236.Miao L., Liu Q., Lin C.M., Luo C., Wang Y.H., Liu L.N., et al. Targeting tumor-associated fibroblasts for therapeutic delivery in desmoplastic tumors. Cancer Res. 2017;77:719–731. doi: 10.1158/0008-5472.CAN-16-0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Geng S.Z., Xiang T.T., Zhang Y.Y., Guo P.K., Zhang H.L., Zhang Z.Z., et al. Safe engineering of cancer-associated fibroblasts enhances checkpoint blockade immunotherapy. J Control Release. 2023;356:272–287. doi: 10.1016/j.jconrel.2023.02.041. [DOI] [PubMed] [Google Scholar]
- 238.Liu J., Kang S.G., Wang P., Wang Y., Lv X.N., Liu Y., et al. Molecular mechanism of Gd@C(82)(OH)(22) increasing collagen expression: implication for encaging tumor. Biomaterials. 2018;152:24–36. doi: 10.1016/j.biomaterials.2017.10.027. [DOI] [PubMed] [Google Scholar]
- 239.Ishii G., Ochiai A., Neri S. Phenotypic and functional heterogeneity of cancer-associated fibroblast within the tumor microenvironment. Adv Drug Deliv Rev. 2016;99:186–196. doi: 10.1016/j.addr.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 240.Chen Y., McAndrews K.M., Kalluri R. Clinical and therapeutic relevance of cancer-associated fibroblasts. Nat Rev Clin Oncol. 2021;18:792–804. doi: 10.1038/s41571-021-00546-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Geng X.L., Chen H.Z., Zhao L., Hu J.S., Yang W.B., Li G.Q., et al. Cancer-associated fibroblast (CAF) heterogeneity and targeting therapy of CAFs in pancreatic cancer. Front Cell Dev Biol. 2021;9 doi: 10.3389/fcell.2021.655152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Tracey L.J., An Y., Justice M.J. CyTOF: an emerging technology for single-cell proteomics in the mouse. Curr Protoc. 2021;1:e118. doi: 10.1002/cpz1.118. [DOI] [PubMed] [Google Scholar]
- 243.Cords L., Tietscher S., Anzeneder T., Langwieder C., Rees M., de Souza N., et al. Cancer-associated fibroblast classification in single-cell and spatial proteomics data. Nat Commun. 2023;14:4294. doi: 10.1038/s41467-023-39762-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Jones C.E., Sharick J.T., Sizemore S.T., Cukierman E., Strohecker A.M., Leight J.L. A miniaturized screening platform to identify novel regulators of extracellular matrix alignment. Cancer Res Commun. 2022;2:1471–1486. doi: 10.1158/2767-9764.CRC-22-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Poon S., Ailles L.E. Modeling the role of cancer-associated fibroblasts in tumor cell invasion. Cancers. 2022;14:962. doi: 10.3390/cancers14040962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Song A.J., Palmiter R.D. Detecting and avoiding problems when using the Cre–lox system. Trends Genet. 2018;34:333–340. doi: 10.1016/j.tig.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Hubbard E.J.A. FLP/FRT and Cre/lox recombination technology in C. elegans. Methods. 2014;68:417–424. doi: 10.1016/j.ymeth.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Ozdemir B.C., Pentcheva-Hoang T., Carstens J.L., Zheng X., Wu C.C., Simpson T.R., et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25:719–734. doi: 10.1016/j.ccr.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Lin D.G., Chen X., Lin Z., Lin J.Q., Liu Y., Liu D.Y. Paper-supported co-culture system for dynamic investigations of the lung-tropic migration of breast cancer cells. Biomed Mater. 2021;16 doi: 10.1088/1748-605X/abc28c. [DOI] [PubMed] [Google Scholar]
- 250.Zboralski D., Hoehne A., Bredenbeck A., Schumann A., Nguyen M., Schneider E., et al. Preclinical evaluation of FAP-2286 for fibroblast activation protein targeted radionuclide imaging and therapy. Eur J Nucl Med Mol Imaging. 2022;49:3651–3667. doi: 10.1007/s00259-022-05842-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Pergolizzi J., Varrassi G., Coleman M., Breve F., Christo D.K., Christo P.J., et al. The sigma enigma: a narrative review of sigma receptors. Cureus. 2023;15 doi: 10.7759/cureus.35756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Ghosh S., Girigoswami K., Girigoswami A. Membrane-encapsulated camouflaged nanomedicines in drug delivery. Nanomedicine. 2019;14:2067–2082. doi: 10.2217/nnm-2019-0155. [DOI] [PubMed] [Google Scholar]
- 253.Sun L., Sun J.H., Li C.Y., Wu K.Y., Gu Z.Y., Guo L., et al. STAT3-specific nanocarrier for shRNA/drug dual delivery and tumor synergistic therapy. Bioact Mater. 2024;41:137–157. doi: 10.1016/j.bioactmat.2024.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Mollah F., Varamini P. Overcoming therapy resistance and relapse in TNBC: emerging technologies to target breast cancer-associated fibroblasts. Biomedicines. 2021;9:1921. doi: 10.3390/biomedicines9121921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Guo L., Zhang Y.P., Al-Jamal K.T. Recent progress in nanotechnology-based drug carriers for celastrol delivery. Biomater Sci. 2021;9:6355–6380. doi: 10.1039/d1bm00639h. [DOI] [PubMed] [Google Scholar]
- 256.Li C.H., Wang Z., Zhang Y.F., Zhu Y.Q., Xu M.C., Lei H., et al. Efficient sequential co-delivery nanosystem for inhibition of tumor and tumor-associated fibroblast-induced resistance and metastasis. Int J Nanomedicine. 2024;19:1749–1766. doi: 10.2147/IJN.S427783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Liang C.L., Zhang G., Guo L.L., Ding X.Y., Yang H., Zhang H.L., et al. Spatiotemporal transformable nano-assembly for on-demand drug delivery to enhance anti-tumor immunotherapy. Asian J Pharm Sci. 2024;19 doi: 10.1016/j.ajps.2024.100888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Pajic M., Froio D., Daly S., Doculara L., Millar E., Graham P.H., et al. miR-139-5p modulates radiotherapy resistance in breast cancer by repressing multiple gene networks of DNA repair and ROS defense. Cancer Res. 2018;78:501–515. doi: 10.1158/0008-5472.CAN-16-3105. [DOI] [PubMed] [Google Scholar]
- 259.Vermeulen L., De Sousa E.M.F., van der Heijden M., Cameron K., de Jong J.H., Borovski T., et al. WNT activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol. 2010;12:468–476. doi: 10.1038/ncb2048. [DOI] [PubMed] [Google Scholar]
- 260.Jian C., Wu T.T., Wang L.T., Gao C., Fu Z.W., Zhang Q., et al. Biomimetic nanoplatform for dual-targeted clearance of activated and senescent cancer-associated fibroblasts to improve radiation resistance in breast cancer. Small. 2024;20 doi: 10.1002/smll.202309279. [DOI] [PubMed] [Google Scholar]
- 261.Cheng Y., Zou J.F., He M.Y., Hou X.Y., Wang H.T., Xu J.J., et al. Spatiotemporally controlled Pseudomonas exotoxin transgene system combined with multifunctional nanoparticles for breast cancer antimetastatic therapy. J Control Release. 2024;367:167–183. doi: 10.1016/j.jconrel.2023.08.011. [DOI] [PubMed] [Google Scholar]
- 262.Havel H., Finch G., Strode P., Wolfgang M., Zale S., Bobe I., et al. Nanomedicines: from bench to bedside and beyond. AAPS J. 2016;18:1373–1378. doi: 10.1208/s12248-016-9961-7. [DOI] [PubMed] [Google Scholar]
- 263.Tenchov R., Bird R., Curtze A.E., Zhou Q.Q. Lipid nanoparticles—from liposomes to mRNA vaccine delivery, a landscape of research diversity and advancement. ACS Nano. 2021;15:16982–17015. doi: 10.1021/acsnano.1c04996. [DOI] [PubMed] [Google Scholar]