Abstract
The heterogeneity of the immune response to Helicobacter pylori has always been noticed but has never been evaluated by obtaining a quantitative measure. For this purpose, sera were tested by enzyme-linked immunosorbent assay, and 207 positive serum specimens were subsequently tested by immunoblotting. The presence or absence of six specific bands was noted. The homology of the different profiles of bands was measured by calculating the Dice coefficient, and a dendrogram was constructed. Thirty-four profiles were found, with each profile containing from 1 to 43 serum specimens. At a level of 72% similarity, 115 of the serum specimens studied fell into eight profiles. At a level of 48% similarity, 186 of the serum specimens studied fell into 22 profiles. The difference in immunoblot profiles was probably linked to the host immune response, but infection with strains carrying different antigens cannot be ruled out.
Helicobacter pylori causes gastritis and plays a major role in the pathogenesis of peptic ulcer (16) and gastric cancer (13). Because of its uneven distribution on the gastric mucosa, some cases of H. pylori infection can be missed by culture of biopsy samples (3). Serological tests are useful because they circumvent this problem in that infected patients develop elevated serum immunoglobulin G (IgG) and possibly IgA antibodies as well as a local immune response (10, 24). Serological techniques provide a relatively noninvasive method for the diagnosis of H. pylori-associated diseases (12, 22) and are particularly well suited to seroepidemiological studies (14). A number of different techniques, i.e., hemagglutination, complement fixation, coagglutination, indirect immunofluorescence, and latex agglutination, have been used. Most studies, however, have used enzyme-linked immunosorbent assay (ELISA) and/or immunoblotting (Western blotting) (22).
The immune response to H. pylori in humans has been well studied qualitatively by using immunoblotting (1). This technique has not previously been used to compare individual patient responses quantitatively. In the present study, sera collected for an epidemiological study were analyzed by ELISA. The sera with results considered positive and in the “grey zone” were then analyzed by immunoblotting. The presence or absence of 6 of the 13 possible bands (2) revealed by immunoblotting were compared by the unweighted pair group method with averages (UPGMA) (20), and a dendrogram was constructed to illustrate the similarity between the immunoblot profiles of strains from different patients. The goal was to determine how many different profiles could be found and how they were related.
MATERIALS AND METHODS
Collection of sera.
The sera used for this study were collected for a cross-sectional epidemiological study of H. pylori infection in the Republic of San Marino (9). Subjects were selected from among the adults in the National Register after a random stratified sampling with proportional allocation by age, sex, and district. The 2,237 serum specimens collected were tested by ELISA, and all sera with results considered positive or in the grey zone were tested by immunoblotting. Of the 1,137 serum specimens which tested positive for antibodies to H. pylori by immunoblotting, 207 were chosen at random for study of the heterogeneity of the IgG response.
The ELISA was performed as described previously (18). The antigen used in this assay was a centrifuged sonicated extract of two strains identified as being from serogroups 1 and 3 by the schema of Lior (15). Antigen was used at 0.5 mg/ml. Sera were diluted 1:100 in phosphate-buffered saline containing Tween 20 and 5 mg of bovine serum albumin per ml and were tested in duplicate. A goat anti-human IgG peroxidase conjugate (Nordic Immunological Laboratories, Tilburg, The Netherlands) diluted 1:5,000 was used. The substrate, 2,2′-azino-di-(3-ethylbenzthiazolinsulfonate) (Boehringer Mannheim, Mannheim, Germany), was used at 220 mg/ml with 0.1% H2O2. Coloration was measured in a spectrophotometer at 405 nm. Optical densities above 0.400 were considered positive, and values between 0.350 and 0.400 were considered to be in the grey zone.
All sera considered to be positive or in the grey zone were then tested by a modification of an immunoblotting technique which has been previously described (4). For the immunoblotting, the antigen used was a whole-cell preparation of the same two strains used earlier for the ELISA. Antigen bands and a mixture of protein standards (Protein Molecular Weight Standards; range, 14,300 to 200,000; Bethesda Research Laboratories) were first separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and were then electrotransferred to nitrocellulose paper (Bio-Rad, Ivry Sur Seine, France) by using a Biolyon electrotransfer apparatus. Electrotransfer was carried out under semidry conditions in Trisma base (25 mM)-glycine (192 mM) containing methanol for 10 min at 12 V and for 1 h at 24 V. Proteins on nitrocellulose were immunoblotted in a Multiscreen device (Bio-Rad). After a blocking step of 1 h with 3% skim milk in Tris-buffered saline (TBS) with Tween 20 (0.05%), the immunoblots were washed three times for 5 min each time with TBS-Tween 20 and were then placed in contact with sera diluted 1:50 in TBS-Tween 20 with 1% skim milk for 2 h. After washing, goat anti-human IgG peroxidase conjugate diluted 1:500 was applied for 1 h. Blots were rewashed before adding the substrate. The substrate solution consisted of 1.6 mg of 4-chloro-1-naphthol per ml, 5 ml of cold methanol, and 15 μl of 30% H2O2 in 25 ml of TBS. Coloration of the substrate was carried out at room temperature in the dark for 30 min and was then stopped by washing with distilled H2O. Finally, the protein standards were restained with Poinceau S (Sigma, St. Quentin, Fallavier, France).
Among the 13 bands which can be present, 6 commonly noted bands which were considered to be major bands were studied. These particular bands were chosen because they are usually clear and unequivocal and can be measured with a certain degree of confidence. The additional bands potentially present can be more difficult to interpret. The molecular masses of the bands chosen were approximately 115,000, 93,000, 90,000, 58,000, 45,000, and 20,000 kDa. Their presence or absence was noted for 207 of the serum specimens tested.
Study of the similarities among profiles.
The distance between the different profiles (combinations of bands) was measured by calculating the Dice coefficient [(2 × the number of pairs of characteristics in common)/total number of characteristics in the two profiles](8). In this calculation only positive characteristics are taken into consideration.
The Dice coefficient was used in the UPGMA formula to assign levels of hierarchy in the similarity of profiles. The distance (d) between two clusters was measured as a function of the number of individuals in the cluster (M).
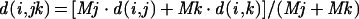 |
In this model, i is a given individual (profile or cluster of profiles) and jk is the cluster to which i is being compared, j being the last member added to the cluster and k being the initial or previous component (profile or cluster of profiles). By using the levels of hierarchy assigned by this model, a dendrogram was constructed to illustrate the relationship between the profiles.
RESULTS
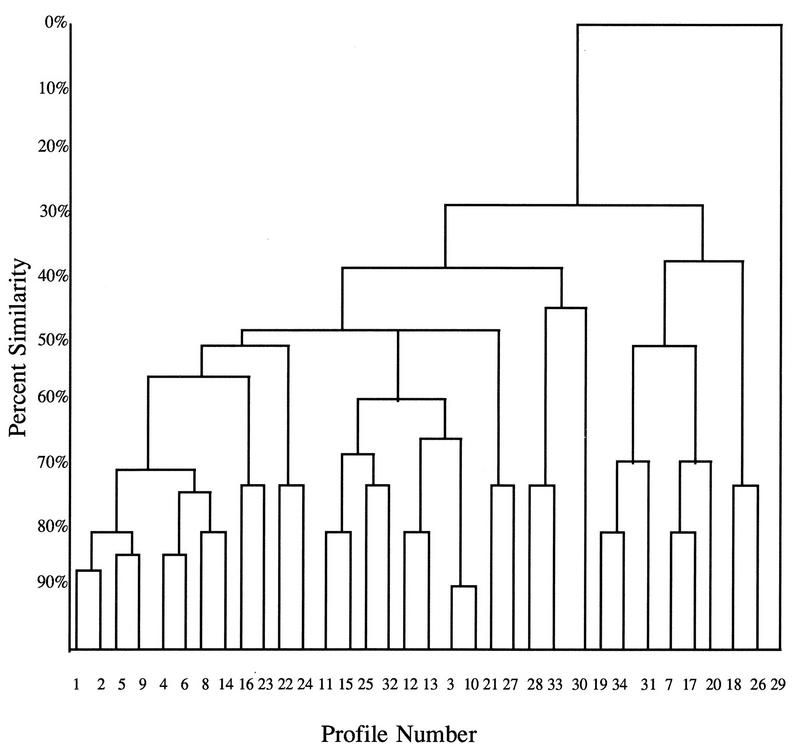
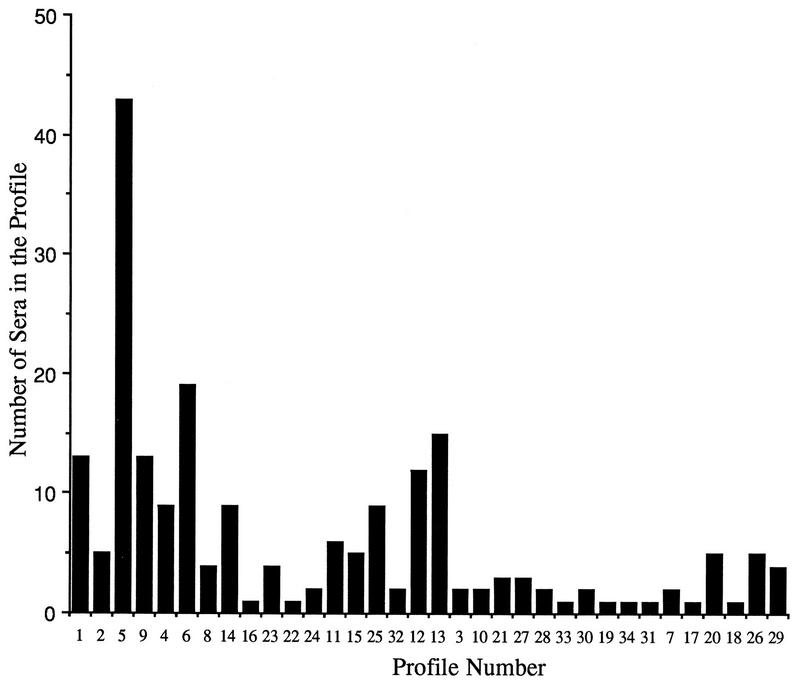
By using six bands, 34 profiles were found. The relationship between the different profiles is illustrated in Fig. 1. From 1 to 43 serum specimens were classified in each of these 34 profiles. The profile with the most serum specimens (43 serum specimens) was composed of five bands at 115,000, 93,000, 90,000, 58,000, and 45,000 kDa. The next most common profile contained 19 serum specimens and was composed of four bands at 115,000, 93,000, 90,000, and 58,000 kDa. Figure 2 illustrates the number of serum specimens in each profile. At a level of 72% similarity, 115 of the serum specimens studied fell into eight profiles. At a level of 48% similarity, 186 of the serum specimens studied fell into 22 profiles.
FIG. 1.
Dendrogram of 34 different profiles (combinations of six bands) revealed by immunoblotting of 207 serum specimens which had tested positive for H. pylori antibodies by ELISA.
FIG. 2.
Number of serum specimens in each profile.
Thirteen of the 34 profiles contained at least four of the bands studied. These 13 profiles grouped 130 serum specimens. Of these 130 serum specimens, 3 (2%) had ELISA results considered to be in the grey zone (optical densities of between 0.350 and 0.400). Of the remaining 77 serum specimens whose profiles contained three or fewer of the bands studied, 8 (10%) had ELISA results in the grey zone. Therefore, a total of 11 of the 207 serum specimens studied had ELISA results in the grey zone.
The profile (profile 1) with all six bands contained 13 serum specimens. Five profiles (profiles 2 to 5 and 34) contained five bands. As a group, they contained 59 serum specimens. The seven different profiles (profiles 6 to 12) with four bands grouped a total of 59 serum specimens. The 13 profiles with three bands were profiles 13 to 22 and 31 to 33. They grouped 46 serum specimens. Profiles with two bands (profiles 23 to 28) grouped 25 serum specimens. Two profiles had only one band, and these two (profiles 29 and 30) contained six serum specimens.
DISCUSSION
It is interesting that 17 of the 34 profiles found lacked a band at 115 kDa. These 17 profiles together contained 65 serum specimens (31%). Although in some cases differences in the molecular masses of the protein bands reported in the literature can be due to differences in the extraction procedures and gel electrophoresis techniques used, in the case of the 115-kDa band studied here, the differences observed by Faulde et al. (12) (130-kDa protein), Reiff et al. (19) (120-kDa protein), and von Wulffen (22) (110-kDa protein) are due to the structure of the gene coding for this protein (6). This gene has been found to contain sequences which can be repeated a variable number of times, affecting the length of the protein product. The results of this study are in agreement with those of previous studies (7, 12), which found that the prevalence of this antigen was 60 to 70% in patients with nonulcer dyspepsia. This antigen is of special importance because it is a marker for the cag pathogenicity island (5) which is more frequently found in strains isolated from patients with severe diseases (7, 23). In considering the frequency of antibody response to this protein, one must bear in mind that the sera for this study came from the general population and not a population of patients with gastric diseases.
Exactly where to place the cutoff point when interpreting ELISA data is a perennial problem. Some investigators even suggest that it should change as a function of the population studied (11). Immunoblotting can be used to verify questionable ELISA results, but it is not required to verify results after a certain level of positivity has been reached.
Human serum contains antibodies that react with a number of different H. pylori antigens (2). Several bands are detected by immunoblotting significantly more frequently with sera from H. pylori-positive patients than healthy controls. The total number of bands detected by individual sera is higher for H. pylori-positive patients than for healthy controls. The problem with immunoblotting, however, is that tertiary and quaternary protein structure can be affected by denaturation by sodium dodecyl sulfate and reduction of disulfide bonds during electrophoresis (21). This could lead to a loss of or a decrease in the antigenicity of the epitopes presented to the antibody.
In this study, the profiles obtained by immunoblotting were compared. The technique used to compare these profiles has been proposed for bacterial taxonomy. Although arbitrary, the results allow for a systematic comparison of the homology found between different profiles. The significance of the heterogeneity found is not addressed. For the immunoblotting analysis, all serum specimens were tested against the same strain and not the homologous strain (the strain isolated from the same patient). The differences in the profiles obtained by immunoblotting could be due to heterogeneity in the host immune response or to infection with strains with different antigens. Despite the genetic heterogeneity of H. pylori strains (17), most antigens are conserved. Although not proven, the diversity in immunoblotting profiles noted in this study was most likely due to the immune response of the host.
We used a collection of sera obtained from a well-designed epidemiological study in the general population in order to achieve insight into the extent of heterogeneity of the immune response. This provides the background and paves the way for the study of different groups of patients. The diversity of the immune responses observed with sera from asymptomatic H. pylori-infected subjects indicates that sera from patients with different clinical entities should also be studied in an attempt to find a specific profile which could be useful for diagnostic purposes.
REFERENCES
- 1.Andersen L P. The antibody response to Helicobacter pylori infection, and the value of serologic tests to detect H. pylori and for post-treatment monitoring. In: Goodwin C S, Worsley B W, editors. Helicobacter pylori: biology and clinical practice. Boca Raton, Fla: CRC Press, Inc.; 1993. pp. 285–305. [Google Scholar]
- 2.Andersen L P, Esperson F. Immoglobulin G antibodies to Helicobacter pylori in patients with dyspeptic symptoms investigated by the Western immunoblot technique. J Clin Microbiol. 1992;30:1743–1751. doi: 10.1128/jcm.30.7.1743-1751.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bayerdörfer E, Oertel H, Lehn N, Kasper G, Nannes G A, Sauerbruch T, Stolte M. Topographic association between active gastritis and Campylobacter pylori colonization. J Clin Pathol. 1989;42:834–839. doi: 10.1136/jcp.42.8.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burnette W N. Western blotting: electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein. Anal Biochem. 1981;56:480–488. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- 5.Censini S, Lange C, Xiang Z, Crabtree J E, Ghiara P, Borodovsky M, Rappioli R, Covacci A. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci USA. 1996;93:14548–14653. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Covacci A, Censini M, Bugnoli M, Petracca R, Burroni D, Macchia G, Massone A, Papini E, Xiang Z, Figura N, Rappuoli R. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc Natl Acad Sci USA. 1993;90:5791. doi: 10.1073/pnas.90.12.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crabtree J E, Taylor J D, Wyatt J I, Heatley R V, Shallcross T M, Tompins D S, Rathbone B J. Mucosal IgA recognition of Helicobacter pylori 120 kDa protein, peptic ulceration and gastric pathology. Lancet. 1991;338:332–335. doi: 10.1016/0140-6736(91)90477-7. [DOI] [PubMed] [Google Scholar]
- 8.Dice L R. Measures of the amount of ecological association between species. Ecology. 1991;109:273–278. [Google Scholar]
- 9.Gasbarrini G, Pretolani S, Bonvicini F, Gratto M R A, Tonelli E, Megraud F, Mayo K, Ghironzi G, Giulianelli G, Grassi M. A population based study of Helicobacter pylori infection in a European country: the San Marino study. Relations with gastrointestinal diseases. Gut. 1995;36:838–844. doi: 10.1136/gut.36.6.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodwin C S, Blincow E, Peterson G, Sanderson C, Cheng W, Marshall B, Warren J R, McCulloch R. Enzyme-linked immunosorbent assay for Campylobacter pyloridis: correlation with presence of C. pyloridis in the gastric mucosa. J Infect Dis. 1987;155:488–494. doi: 10.1093/infdis/155.3.488. [DOI] [PubMed] [Google Scholar]
- 11.Fauchère J-L. Evaluation of the anti-Helicobacter pylori serum antibody response. In: Lee A, Mégraud F, editors. Helicobacter pylori: Techniques for clinical diagnosis and basic research. London, United Kingdom: The W. B. Saunders Company Ltd.; 1996. p. 62. [Google Scholar]
- 12.Faulde M, Schröder J P, Sobe D. Serodiagnosis of Helicobacter pylori infections by detection of immunoglobulin G antibodies using an immunoblot technique and enzyme immunoassay. Eur J Clin Microbiol Infect Dis. 1992;11:589–594. doi: 10.1007/BF01961664. [DOI] [PubMed] [Google Scholar]
- 13.Forman, D. 1991. Helicobacter pylori infection and gastric carcinogenesis. Eur. J. Gastroenterol. Hepatol. 4(Suppl. 2):S31–S35.
- 14.Kosunen T U, Höök J, Rautelin H I, Myllyla G. Age-dependent increase of Campylobacter pylori antibodies in blood donors. Scand J Gastroenterol. 1989;24:110–114. doi: 10.3109/00365528909092247. [DOI] [PubMed] [Google Scholar]
- 15.Lior, H. 1991. Serological characterization of Helicobacter pylori—a provisional serotyping scheme. Ital. J. Gastroenterol. 23(Suppl. 2):42.
- 16.Mégraud F, Lamouliatte H. Helicobacter pylori and duodenal ulcer. Evidence suggesting causation. Dig Dis Sci. 1992;37:769–772. doi: 10.1007/BF01296437. [DOI] [PubMed] [Google Scholar]
- 17.Mégraud F. Helicobacter pylori species heterogenicity. In: Hunt R H, Tytgat G N T, editors. Basic mechanisms to clinical cure. Dordrecht, The Netherlands: Kluwer; 1994. pp. 28–40. [Google Scholar]
- 18.Rabbé M-P, Mégraud F. Titrage des anticorps anti-Campylobacter pylori par ELISA. Gastroenterol Clin Biol. 1988;12:587–588. [PubMed] [Google Scholar]
- 19.Reiff A, Jacobs E, Kist M. Seroepidemiological study of the immune response to Campylobacter pylori in potential risk groups. Eur J Clin Microbiol Infect Dis. 1989;8:592–596. doi: 10.1007/BF01968135. [DOI] [PubMed] [Google Scholar]
- 20.Sneath P H A, Sokal R R. Numerical taxonomy: the principles and practice of numerical classification. W. H. San Francisco, Calif: Freeman & Co.; 1973. [Google Scholar]
- 21.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.von Wulffen H. An assessment of serological tests for detection of Helicobacter pylori. Eur J Clin Microbiol Infect Dis. 1992;11:577–582. doi: 10.1007/BF01961662. [DOI] [PubMed] [Google Scholar]
- 23.Weel J F L, Vander Hulst R W M, Gerits Y, Roorda P, Feller M, Dankert J. The interrelationship between cytotoxin-associated gene A, vacuolating cytotoxin and Helicobacter-related diseases. J Infect Dis. 1996;173:1171–1175. doi: 10.1093/infdis/173.5.1171. [DOI] [PubMed] [Google Scholar]
- 24.Wyatt J I, Rathbone B J, Heatley R V. Local immune response to Campylobacter pyloridis in non-dyspepsia. J Clin Pathol. 1986;39:863–870. doi: 10.1136/jcp.39.8.863. [DOI] [PMC free article] [PubMed] [Google Scholar]