Abstract
1. Sodium exchange was measured with 24Na in incubated guinea-pig cerebral-cortex slices maintained under adequate metabolic conditions with a steady content of fluid and ions resembling that of brain in vivo. 2. Evidence was obtained indicating that Na+ ions behaved in the inulin space as if they were extracellular, and that their entry into the non-inulin space of unstimulated tissue was about 10 times slower and could be separated, on the basis of complete exchangeability, into two components, a `fast' one, which reacted to electrical stimulation, and a `slow' one, exchanging at a rate of about 8μequiv./g./hr., which was not affected by stimulation. 3. The average rate of sodium turnover in unstimulated slices was 175–275μequiv./g./hr., whereas that for stimulated slices was approx. 4–6 times this, or 1050–1180μequiv./g./hr. The stimulated rate was equivalent to a turnover of 32% of the sodium in the non-inulin space/min., or 3mμequiv./g./impulse. 4. Response to the onset of stimulation appeared to be immediate, but after cessation of stimulation increased sodium movements persisted for several minutes before return to unstimulated values. 5. Calculations based on electrochemical gradients suggested that about one-quarter of the energy available from respiration was required for sodium and potassium transport at maximal rates in both unstimulated and stimulated cerebral-cortex slices.
Full text
PDF


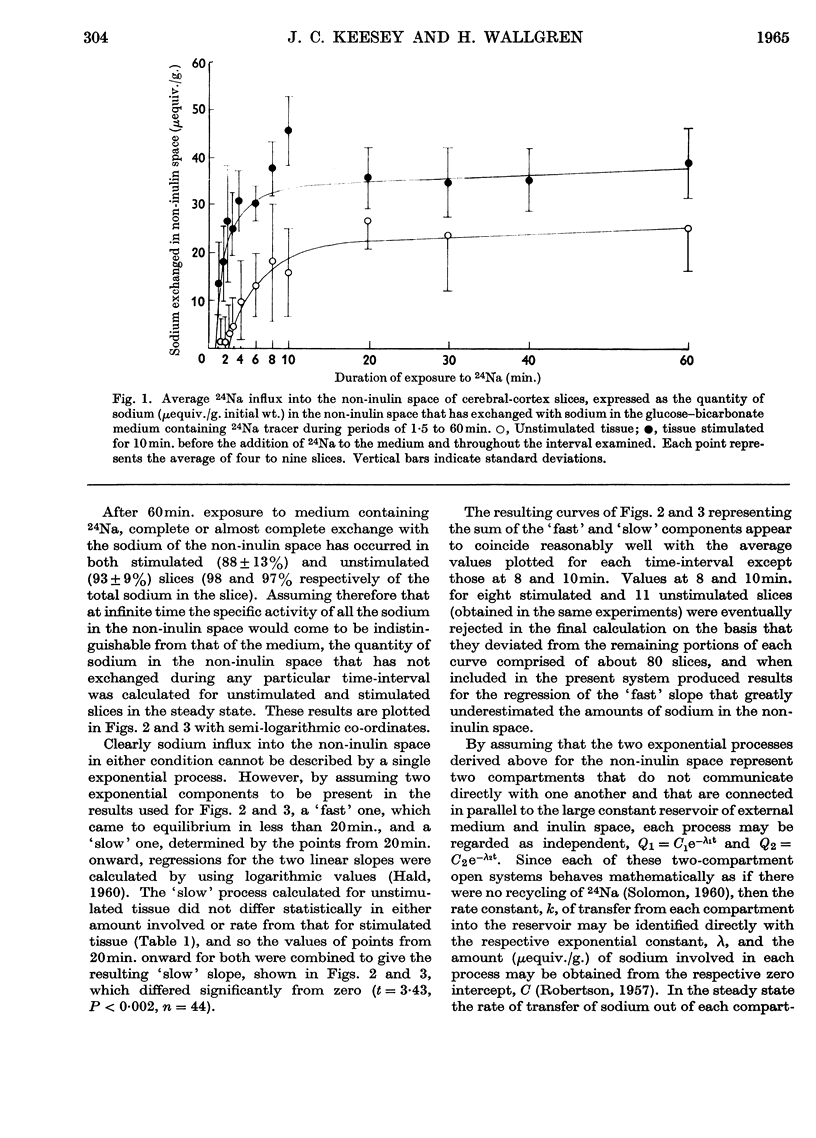
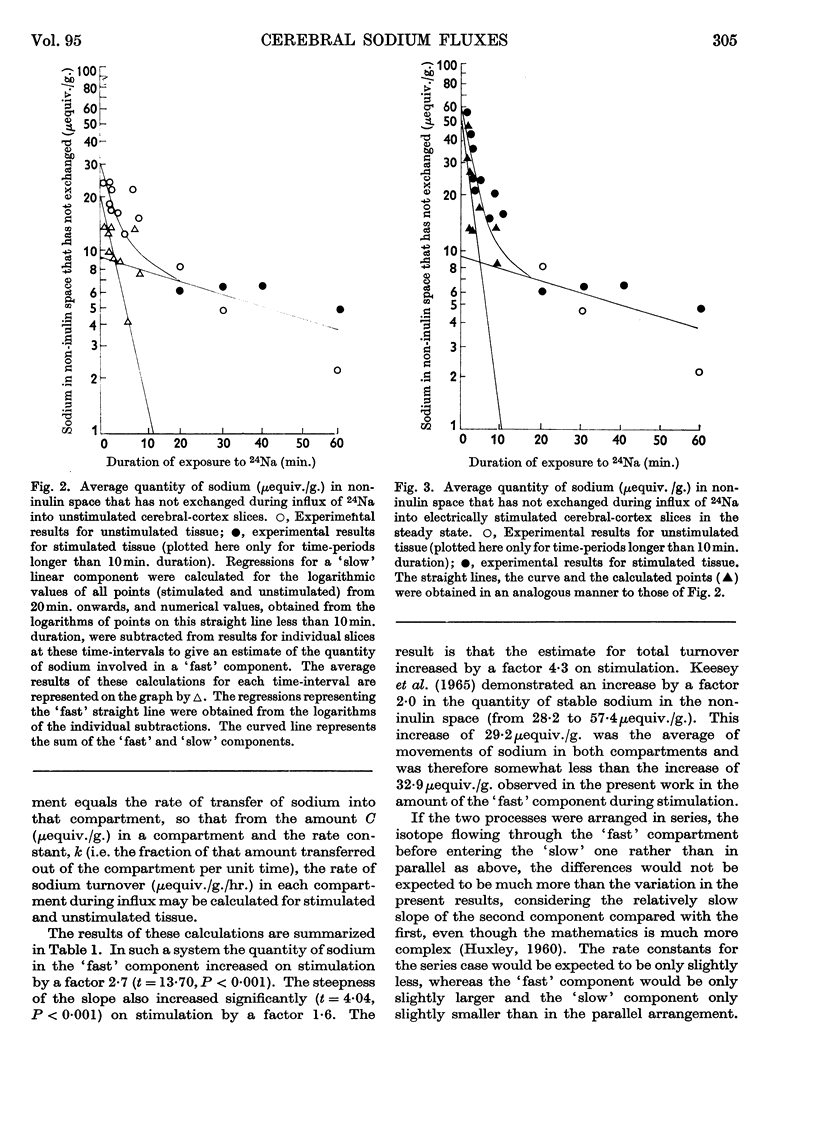
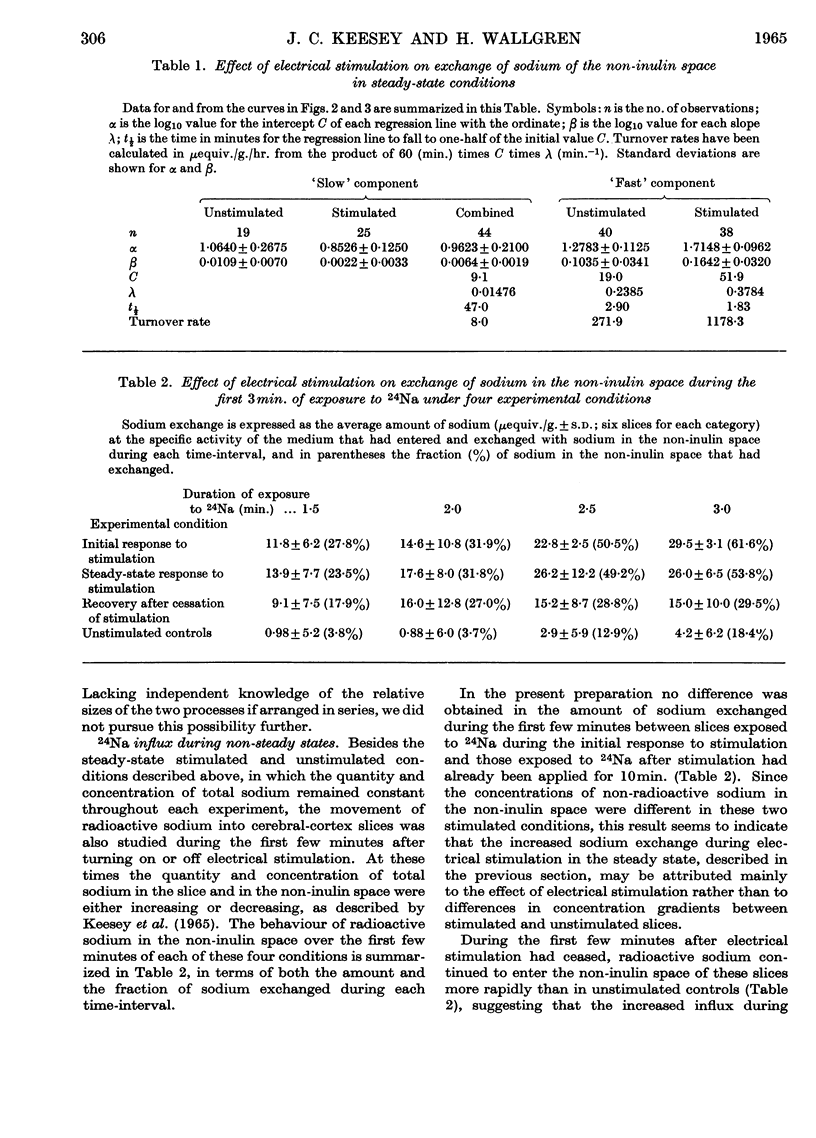
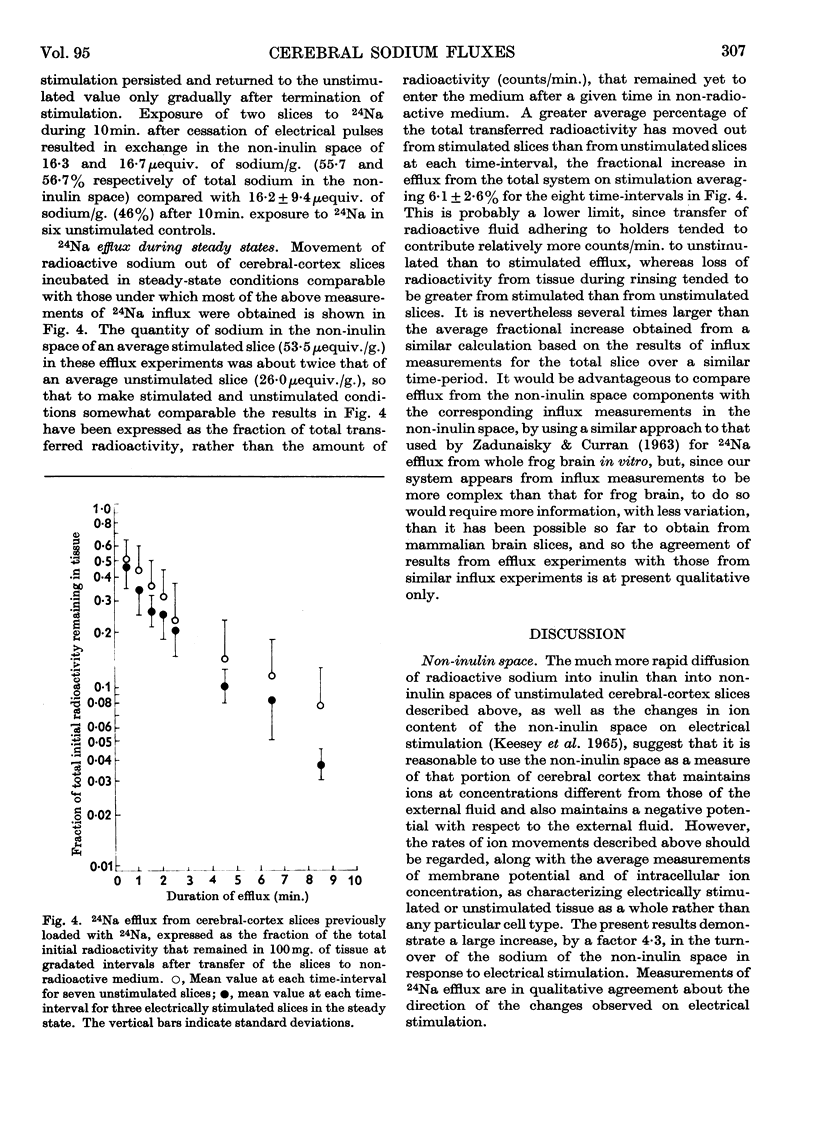



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AIRD R. B., GAROUTTE B. Diffusion from brain slices in vitro. J Cell Physiol. 1956 Oct;48(2):167–180. doi: 10.1002/jcp.1030480202. [DOI] [PubMed] [Google Scholar]
- BACHELARD H. S., CAMPBELL W. J., McILWAIN H. The sodium and other ions of mammalian cerebral tissues, maintained and electrically stimulated in vitro. Biochem J. 1962 Aug;84:225–232. doi: 10.1042/bj0840225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins J. T., McIlwain H. Electrical pulses and the potassium and other ions of isolated cerebral tissues. Biochem J. 1961 May;79(2):330–341. doi: 10.1042/bj0790330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILLMAN H. H., McILWAIN H. Membrane potentials in mammalian cerebral tissues in vitro: dependence on ionic environment. J Physiol. 1961 Jul;157:263–278. doi: 10.1113/jphysiol.1961.sp006720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L. Ionic movements and electrical activity in giant nerve fibres. Proc R Soc Lond B Biol Sci. 1958 Jan 1;148(930):1–37. doi: 10.1098/rspb.1958.0001. [DOI] [PubMed] [Google Scholar]
- KEESEY J. C., WALLGREN H., MCILWAIN H. THE SODIUM, POTASSIUM AND CHLORIDE OF CEREBRAL TISSUES: MAINTENANCE, CHANGE ON STIMULATION AND SUBSEQUENT RECOVERY. Biochem J. 1965 May;95:289–300. doi: 10.1042/bj0950289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KREBS H. A., EGGLESTON L. V., TERNER C. In vitro measurements of the turnover rate of potassium in brain and retina. Biochem J. 1951 May;48(5):530–537. doi: 10.1042/bj0480530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAPPIUS H. M., ROSENFELD M., JOHNSON D. M., ELLIOTT K. A. Effects of sodium-free media upon the metabolism and the potassium and water contents of brain slices. Can J Biochem Physiol. 1958 Feb;36(2):217–226. [PubMed] [Google Scholar]
- ROBERTSON J. S. Theory and use of tracers in determining transfer rates in biological systems. Physiol Rev. 1957 Apr;37(2):133–154. doi: 10.1152/physrev.1957.37.2.133. [DOI] [PubMed] [Google Scholar]
- SHANES A. M. Electrochemical aspects of physiological and pharmacological action in excitable cells. I. The resting cell and its alteration by extrinsic factors. Pharmacol Rev. 1958 Mar;10(1):59–164. [PubMed] [Google Scholar]
- VARON S., McILWAIN H. Fluid content and compartments in isolated cerebral tissues. J Neurochem. 1961 Dec;8:262–275. doi: 10.1111/j.1471-4159.1961.tb13552.x. [DOI] [PubMed] [Google Scholar]
- Zadunaisky J. A., Curran P. F. Sodium fluxes in isolated frog brain. Am J Physiol. 1963 Nov;205(5):949–956. doi: 10.1152/ajplegacy.1963.205.5.949. [DOI] [PubMed] [Google Scholar]


