Abstract
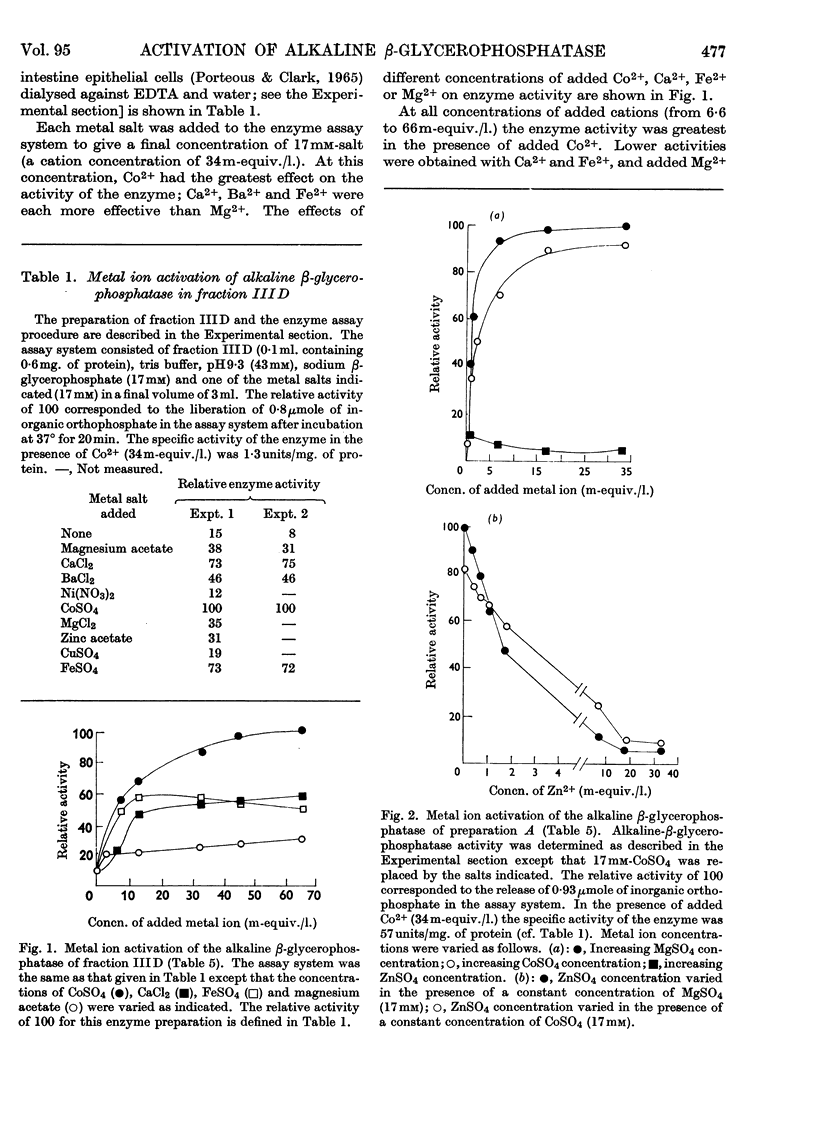
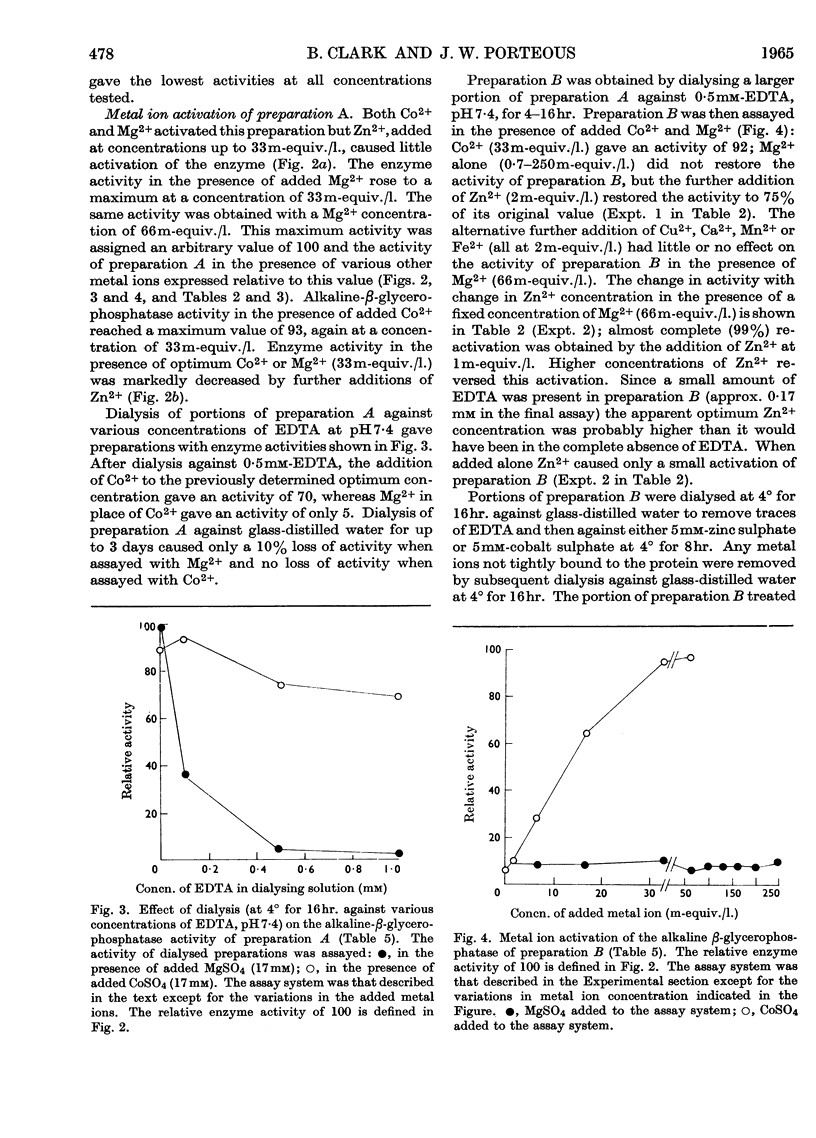
1. A fraction of intestinal epithelial cells from rabbit small intestine that contained nuclei and microvillus membranes served as a source of alkaline-β-glycerophosphatase activity. 2. The greater part of the enzyme activity could be released from the subcellular particles by disintegration of the latter, followed by centrifugation at 40000g and butanol extraction of the resulting sediment. 3. Further purification of the enzyme was achieved by diethylaminoethylcellulose chromatography and by gel filtration. 4. Dialysis of the purified enzyme preparations against EDTA gave an essentially inactive enzyme. High activity could be restored by adding Zn2++Mg2+, Zn2++Co2+, Mg2++Co2+ or Co2+ alone to these inactive preparations. Neither Zn2+ nor Mg2+ added singly to the assay system restored more than a small part of the enzyme activity. 5. The optimum Zn2+ concentration was about 0·2–1m-equiv./l., whereas Mg2+ and Co2+ had optimum concentrations about 30–60m-equiv./l. 6. If added in excess of the optimum concentration, Zn2+ strongly inhibited the enzyme under all conditions tested. 7. In the presence of an optimum concentration of Co2+ (33m-equiv./l.) in tris buffer at the optimum pH (8·8 at 37°), Km for the β-glycerophosphatase was 0·3mm.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALVAREZ E. F., LORA-TAMAYO M. Purification of kidney alkaline phosphatase. Biochem J. 1958 Jun;69(2):312–320. doi: 10.1042/bj0690312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abul-Fadl M. A., King E. J. Purification of alkaline phosphatase by tryptic digestion. Biochem J. 1949;44(4):434–435. [PMC free article] [PubMed] [Google Scholar]
- COOPER R. THE BIOSYNTHESIS OF COPROPORPHYRINOGEN, MAGNESIUM PROTOPORPHYRIN MONOMETHYL ESTER AND BACTERIOCHLOROPHYLL BY RHODOPSEUDOMONAS CAPSULATA. Biochem J. 1963 Oct;89:100–108. doi: 10.1042/bj0890100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENGSTROM L. Studies on calf-intestinal alkaline phosphatase. I. Chromatographic purification, microheterogeneity and some other properties of the purified enzyme. Biochim Biophys Acta. 1961 Sep 2;52:36–48. doi: 10.1016/0006-3002(61)90901-5. [DOI] [PubMed] [Google Scholar]
- GAREN A., LEVINTHAL C. A fine-structure genetic and chemical study of the enzyme alkaline phosphatase of E. coli. I. Purification and characterization of alkaline phosphatase. Biochim Biophys Acta. 1960 Mar 11;38:470–483. doi: 10.1016/0006-3002(60)91282-8. [DOI] [PubMed] [Google Scholar]
- GOMORI G. The complex nature of alkaline phosphatase. Biochim Biophys Acta. 1952 Feb;8(2):162–172. doi: 10.1016/0006-3002(52)90026-7. [DOI] [PubMed] [Google Scholar]
- GROSSBERG A. L., HARRIS E. H., SCHLAMOWITZ M. Enrichment and separation of alkaline phosphatase activities of human tissues by chromatography on cellulose ion-exchange adsorbents. Arch Biochem Biophys. 1961 May;93:267–277. doi: 10.1016/0003-9861(61)90261-2. [DOI] [PubMed] [Google Scholar]
- HOFSTEE B. H. Alkaline phosphatase. I. Mechanism of action of Zn, Mg, glycine, versene and hydrogen ions. Arch Biochem Biophys. 1955 Dec;59(2):352–365. doi: 10.1016/0003-9861(55)90502-6. [DOI] [PubMed] [Google Scholar]
- King E. J. The colorimetric determination of phosphorus. Biochem J. 1932;26(2):292–297. doi: 10.1042/bj0260292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINTHAL C., SIGNER E. R., FETHEROLF K. Reactivation and hybridization of reduced alkaline phosphatase. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1230–1237. doi: 10.1073/pnas.48.7.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATHIES J. C. Preparation and properties of highly purified alkaline phosphatase from swine kidneys. J Biol Chem. 1958 Nov;233(5):1121–1127. [PubMed] [Google Scholar]
- MORTON R. K. Some properties of alkaline phosphatase of cow's milk and calf intestinal mucosa. Biochem J. 1955 Aug;60(4):573–582. doi: 10.1042/bj0600573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOSS D. W., CAMPBELL D. M., ANAGNOSTOU-KAKARAS E., KING E. J. Characterization of tissue alkaline phosphatases and their partial purification by starch-gel electrophoresis. Biochem J. 1961 Nov;81:441–447. doi: 10.1042/bj0810441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOSS D. W., KING E. J. Properties of alkaline-phosphatase fractions separated by starch-gel electrophoresis. Biochem J. 1962 Jul;84:192–195. doi: 10.1042/bj0840192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PLOCKE D. J., LEVINTHAL C., VALLEE B. L. Alkaline phosphatase of Escherichia coli: a zinc metalloenzyme. Biochemistry. 1962 May 25;1:373–378. doi: 10.1021/bi00909a001. [DOI] [PubMed] [Google Scholar]
- PLOCKE D. J., VALLEE B. L. Interaction of alkaline phosphatase of E. coli with metal ions and chelating agents. Biochemistry. 1962 Nov;1:1039–1043. doi: 10.1021/bi00912a014. [DOI] [PubMed] [Google Scholar]
- REITHEL F. J. THE DISSOCIATION AND ASSOCIATION OF PROTEIN STRUCTURES. Adv Protein Chem. 1963;18:123–226. doi: 10.1016/s0065-3233(08)60269-7. [DOI] [PubMed] [Google Scholar]
- ROCHE J., THOAI N. V. Phosphatase alkaline. Adv Enzymol Relat Subj Biochem. 1950;10:83–122. [PubMed] [Google Scholar]
- TRUBOWITZ S., FELDMAN D., MORGENSTERN S. W., HUNT V. M. The isolation, purification and some properties of the alkaline phosphatase of human leucocytes. Biochem J. 1961 Aug;80:369–374. doi: 10.1042/bj0800369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VALLEE B. L. Zinc and metalloenzymes. Adv Protein Chem. 1955;10:317–384. doi: 10.1016/s0065-3233(08)60108-4. [DOI] [PubMed] [Google Scholar]


