Abstract
Cilia are versatile, microtubule-based organelles that facilitate cellular signaling, motility, and environmental sensing in eukaryotic cells. These dynamic structures act as hubs for key developmental signaling pathways, while their assembly and disassembly are intricately regulated along cell cycle transitions. Recent findings show that factors regulating ciliogenesis and cilia dynamics often integrate their roles across other cellular processes, including cell cycle regulation, cytoskeletal organization, and intracellular trafficking, ensuring multilevel crosstalk of mechanisms controlling organogenesis. Disruptions in these shared regulators lead to broad defects associated with both ciliopathies and cancer. This review explores the crosstalk of regulatory mechanisms governing cilia assembly, disassembly, and maintenance during ciliary signaling and the cell cycle, along with the broader implications for development, tissue homeostasis, and disease.
Keywords: Cilia, Ciliary dynamics, Ciliary signaling, Cell cycle regulation, Tissue development, Ciliopathies, Cancer
Current model of primary cilia assembly and disassembly
Cilia are hair-like organelles composed of microtubules that extend from the cell surface of most eukaryotic cells. Cilia are probably the first ever observed cellular organelles, already described in the 17th century by a Dutch pioneer of microbiology and microscopy, Antonie van Leeuwenhoek [1]. Cilia can be motile or non-motile, with non-motile cilia referred to as primary cilia. The “primary” aspect of primary cilia relates to the time of their appearance, as primary cilium was noted to appear before the motile cilia in cells of rat lung epithelium [2]. Initially, primary cilia were thought to be vestigial, owing to their lack of motility. However, research over the past two decades has significantly twisted this perspective, revealing that primary cilia serve as crucial cellular antennas with essential roles in embryogenesis and tissue homeostasis [3, 4], [5]. Cilia significance has become even more evident with the discovery that a variety of human diseases, collectively termed ciliopathies, are linked to defects in ciliary structure and/or function [6, 7, 8]. In our review, we discuss primary cilia as dynamic organelles responding to various regulatory clues during their assembly, disassembly, and maintenance and, in turn, how the dynamic nature of primary cilia influences fundamental biological processes, including cell fate determination, tissue patterning, signal transduction, and cellular homeostasis.
Primary cilia and their structure
The primary cilium consists of the basal body, the transition zone (TZ), and the membrane-enclosed microtubule-based ciliary axoneme (Fig. 1A) [9]. The basal body originates from the older of the two centrioles of the centrosome, termed the mother centriole. Centrioles are barrel-like structures typically assembled according to radial nine-fold symmetry. A major part of their walls is made of nine microtubule triplets arranged in a circular pattern, which contributes to the structural integrity and overall stability of centrioles. The centriole’s most distal part transitions to a geometry reminiscent of an axoneme by containing microtubule doublets instead of triplets and being of smaller diameter than the proximal centriole part [10]. Only a fully matured mother centriole can serve as a basal body, having acquired two sets of appendages [11]. The structural integrity of the centriole distal part is critical for both distal (DAs) and subdistal (SDAs) appendages formation [12, 13].
Fig. 1.
Primary cilium structure and mechanism of intracellular ciliogenesis. A Primary cilium originates from the basal body (grey), modified centriole, which possesses two sets of appendages – distal (DAs) and subdistal (SDAs). The central structure is the membrane-enclosed microtubule-based ciliary axoneme (pink). The transition zone (TZ) acts as a diffusion barrier between the basal body and the ciliary axoneme. The ciliary membrane is rich in phosphoinositides (PIP), while phosphatidylinositol 4,5-bisphosphate (PIP2) levels are increased in the periciliary membrane at the cilium base; the phosphatidylinositol (3,4,5)-trisphosphate (PIP3) seems specifically enriched in the membrane surrounding the TZ. Active protein transport is facilitated by intraflagellar transport (IFT) machinery, which works in cooperation with the BBSome – cargo adapter complex. IFT is a bidirectional movement of complexes along the microtubules from the base of the cilium to its tip (anterograde transport) by kinesin motors and from the tip back to the ciliary base (retrograde transport) by dynein motors. B Intracellular ciliogenesis begins with the formation of the ciliary vesicle at the distal appendages, a process facilitated by EHD1/EHD3 and RAB34. TTBK2 phosphorylates CEP83 and other DAs components, enabling the ciliary vesicle to dock to the mother centriole. Ciliary vesicle membrane expansion is regulated by the RAB11/RABIN8/RAB8 cascade, driving the development and elongation of the ciliary membrane. Simultaneously, IFT mediates the growth of the axoneme, coordinating its assembly with the extension of the ciliary membrane. This image was created with BioRender.com
DAs are present as blades that attach to two adjacent microtubule triplets of the mother centriole wall, forming a structure resembling a nine-fold pinwheel [14]. The DAs blades consist of CEP83/CCDC41, CEP89/CCDC123, SCLT1, and CEP164, which are crucially involved with primary cilia assembly initiation [15, 16] and protein FBF1, involved in regulating ciliary gating [17, 18]. DAs are assembled in a hierarchical manner, with CEP83 being responsible for the recruitment of SCTL1 and CEP89. SCTL1 successively mediates FBF1 and CEP164 recruitment [15]. Mutations in DAs genes (demonstrated for CEP164, CEP83, and SCLT1) typically lead to nephronophthisis, a ciliopathy manifesting in kidneys [5, 6, 19]. Unlike DAs nine-fold symmetry arrangement, SDAs structure does not seem well conserved. Moreover, SDAs are not directly decisive in the regulation of cilium assembly or maintenance; instead, they act as anchorage points for centrosomal microtubules, hence connecting the basal body to the rest of the cell [20]. In turn, they have been implicated in the correct positioning of primary cilia [21], yet functional consequences are not clear. SDAs components include ODF2, Ninein, CEP170, CEP128, and others [20, 22]. Vertebrate cells typically contain only one fully matured mother centriole at a time, allowing for the formation of a single cilium [11]. However, multiciliated cells, including those found in the lung airway epithelium or kidneys, are an exception. During their differentiation, these cells generate up to hundreds of centrioles, which then serve as basal bodies [23].
The transition zone (TZ) delineates the transition between the basal body and the ciliary axoneme [24, 25]. Super-resolution studies have positioned the TZ approximately 100 nm above the DAs [26, 27, 28]. The TZ serves as a diffusion barrier for both membrane and soluble proteins to ensure the unique composition of the intra-ciliary milieu and, consequently, is essential for primary cilia function [25, 29]. Its arrangement and size may vary between different cell types, the common structural feature being the presence of Y-shaped linkers (termed Y-links) that crosslink axonemal microtubule doublets with the ciliary membrane [24, 26]. Many TZ proteins have already been identified [30, 31], most of which are linked to ciliopathies [8], which has then raised even more interest in studying the TZ. Nevertheless, the exact composition of Y-links remains elusive. TZ components cluster into several structurally and functionally distinct protein complexes [32]. One of them is the NPHP complex, which resides in the proximity of axonemal microtubules and consists of proteins such as NPHP1, NPHP4, and RPGRIP1L [25], [32]. Their mutations typically lead to nephronophthisis [8]. A second complex, termed MKS, comprises proteins located in or near the ciliary membrane (e.g., TCTN1-3, several TMEM proteins, AHI1, B9D1/2, etc.) [25], [32]. Corresponding mutations in MKS members are typically involved with Meckel and Joubert syndromes [5], [8]. Both complexes are engaged in several interactions, including mutual ones, interactions with CEP290 (a large protein commonly annotated as another MKS member), and proteins of the so-called Inversin compartment [25]. CEP290 and RPGRIP1L seem to act as the most upstream components in the hierarchy of individual TZ components recruitment [33].
The most common view of axonemal microtubule arrangement in primary cilia is the “9 + 0” configuration, where the central microtubule doublet, typical for the motile cilia with their “9 + 2” arrangement, is missing. While this simplistic model still holds credibility, it is apparent that the spectrum of different configurations is broader than originally anticipated, since the list of reported “exceptions from the rule” is rapidly enlarging. For instance, cilia found in olfactory neurons are immotile, albeit with “9 + 0” configuration [34]. Conversely, beta-cell “primary” cilia, with the typical “9 + 0” configuration, show movement in response to glucose stimulation, likely to facilitate insulin secretion [35]. Moreover, epithelial cell primary cilia seem to follow the “9 + 0” rule only in their proximal parts, while more distal regions of the axoneme exhibit a surprising level of heterogeneity and “disorganization”, with several individual microtubules often ending well before reaching the tip of the cilium [36], [37].
The ciliary membrane is continuous with the plasma membrane, the intermediate zone between them termed the periciliary membrane, which sometimes invaginates to form a ciliary pocket surrounding the proximal part of the axoneme [38], [39]. The ciliary membrane has a unique content of lipids and proteins, critical for correct ciliary functions [29], [40]. Phosphoinositides (PIPs) are perhaps the best example of lipids displaying a precise spatial organization within the cilium membrane. Specifically, the ciliary membrane is rich in PIP, while low in PIP2 and PIP3. Conversely, the plasma membrane has a high content of PIP2 and PIP3. PIP2 levels are increased in the periciliary membrane at the cilium base, while the PIP3 seems specifically enriched in the membrane surrounding the TZ [41]. This particular configuration is maintained by cilia-residing enzymes such as INPP5E, which converts the PIP2 into PIP inside the cilium to maintain the boundary [42], [43].
The soluble content of cilia (recently referred to as cilioplasm [44]) also evinces substantial differences from the composition of cytoplasm. Whereas soluble small molecules easily diffuse between the cilioplasm and cytosol, their concentrations can significantly differ between these two compartments. One such example is calcium, whose steady-state levels can be much higher in the cilioplasm than in the cytoplasm of the same cell [45]. Given that cilium volume is smaller than cell volume by several orders of magnitude, the effects of ciliary ions on cytosol can be safely considered negligible. Conversely, relatively subtle changes (by as little as “a few molecules”) can in fact mean dramatic differences in ion concentration in the context of cilia [46, 47]. For instance, it is estimated that a single molecule of cAMP translates into a 10 nM concentration increase of cAMP inside cilia [44]. This compartmentalization provides a conceptual base for primary cilia as sensitive and efficient signaling organelles [44, 47].
Primary cilia assembly regulation
Ciliogenesis activation is typical for postmitotic cells, as the cilium is accordingly disassembled prior to the entry of another round of mitosis [9]. Exit from the cell cycle and entry into quiescence is considered the most permissive condition for the assembly of a primary cilium [9]. Commensurate with that, perhaps the most commonly used protocol to facilitate ciliogenesis in mammalian cell culture is based on enriching the G1/G0 cell population via serum starvation [48]. Consequently, primary cilia were considered specific hallmarks of quiescent state [49]. Moreover, certain evidence suggests that primary cilia may even have a checkpoint function - guarding against cell cycle re-entry [50]. Nevertheless, based on data from live cell imaging and in vivo models, primary cilia apparently can be readily found not only in non-cycling but also in proliferating (yet non-mitotic) cells [51], [52]. Actually, vertebrate cells seem capable of assembling primary cilium throughout the cell cycle (except mitosis) [51]. Therefore, the timing of the onset of cilium assembly can be heterogeneous, and factors responsible for such asynchrony within a cell population are currently unknown. The earliest observed event in ciliogenesis, as documented in transmission electron microscopy (TEM) experiments, is the docking of the basal body DAs to the ciliary vesicle (CV) [2, 38] or directly to the apical cell membrane[53]. The former is a feature of the intracellular pathway of cilia assembly, where ciliogenesis already initiates in the cytoplasm, before the mother centriole attachment to the plasma membrane [54], [55]. The latter is common for the extracellular cilia assembly pathway of polarized cells, where ciliogenesis commences following mother centriole docking to the plasma membrane surface. This paper focuses on mechanisms of intracellular ciliogenesis, which is a more broadly used mechanism (Fig. 1B). Specific aspects of the extracellular cilium assembly pathway have previously been thoroughly addressed [56].
The large CV forms from the fusion of smaller vesicles, termed distal appendage vesicles (DAVs), attached to the DAs. Myosin-Va, implicated in the assembly and transport of DAVs, appears to be the earliest vesicle trafficking regulator recruited to the basal body [57]. Large CV formation depends on the coordinated action of two members of the EHD protein family, EHD1 and EHD3 [58]. Recruitment of EHD1/EDH3 to cilia base relies on the activity of small GTPase RAB34, localizing to ciliary sheath transiently forming during the assembly of primary cilia [59, 60]. When these proteins are depleted, DAVs can still dock, but the smaller vesicles fail to fuse, hence halting ciliogenesis [57], [58]. The CV membrane’s subsequent expansion, controlled by the RAB11/RABIN8/RAB8 cascade, leads to the formation and extension of the ciliary membrane, assembled in parallel with the growing axoneme. The small GTPase RAB11 is crucial for delivering vesicles containing RABIN8, a RAB8 guanine nucleotide exchange factor (GEF), to the mother centriole and for regulating RABIN8 activity. Locally enriched RABIN8 ensures the timely activation of small GTPase RAB8, promoting the extension of the ciliary membrane [61], [62], [63, 64]. Noteworthy, very few reports have documented an uncoupling of the growth of the primary cilium membrane and axoneme, suggesting tight cooperation of both processes by yet unclear mechanism [65].
The mother centriole-membrane interaction critically relies on intact DAs [15]. Meticulous TEM analyses showed that DAVs/CV docking is severely compromised when CEP164 [66, 67], CEP83/CCDC41 [68], CEP89/CEP123 [69], and to a lesser extent LRRC45 [70], are depleted. While the roles of SCLT1 and ANKRD26 in DAVs/CV docking have not been directly examined by TEM, SCLT1 is required for the recruitment of RAB34 [60, 71]. One open question concerns the mechanism regulating the DAs-CV interactions. It is plausible that individual DAs components and their domains directly mediate the interactions with CV regulators EHD1/3, Myosin-Va, or RAB34 to support efficient CV formation [71]. Certain aspects of such regulation are under the control of effector protein Tau Tubulin Kinase 2 (TTBK2) [72], recruited to DAs by interaction with CEP164 [73, 74]. The absence of TTBK2 prevents CV-mother centriole interactions, which seems to be partly mediated by its phosphorylation of CEP83 [75]. TTBK2 phosphorylates additional proteins involved in CV- mother centriole interactions (i.e. CEP164, CEP89, RABIN8) [73, 76], however, the functional relevance of this remains unclear.
Cilium growth occurs concurrently with the extension of the microtubule-based axoneme. However, the mechanism that triggers the outgrowth of microtubules from the mother centriole distal end is not entirely clear. A widely accepted hallmark of the onset of ciliogenesis in vertebrate cells is the removal of the centriole distal end proteins CEP97 and CP110 specifically from the mother centriole [77]. CEP97 and CP110 depend on each other for proper localization to the centriole distal end and form a complex that is commonly viewed as a “cap,” possibly sterically hindering the outgrowth of microtubules to form the axoneme [77, 78]. The removal of this “cap” depends on TTBK2 kinase activity [15, 72, 73], [79]. While it is not fully resolved how TTBK2 controls this process, one possibility is that TTBK2 directly acts on the CP110-CEP97 complex to induce its relocalization or destruction. Accordingly, TTBK2 has been shown to phosphorylate CEP97 [80]. Alternatively, TTBK2 might indirectly facilitate the removal of CP110-CEP97 by phosphorylating MPP9 [81].
Although CP110-CEP97 loss from the mother centriole serves as a reliable sign of ciliogenesis initiation in numerous studies, the function of these proteins appears more complex. For instance, depletion of CP110 or CEP97 was reported to cause artificially elongated centrioles [52, 78, 82] or facilitate primary cilia formation in certain cell types [81, 83]. Conversely, some evidence suggests these proteins play a positive role in cilia formation [84, 85, 86]. Therefore, the “cap to prevent microtubule growth” model seems overly simplistic for the emerging context-dependent role of CP110-CEP97.
Axoneme outgrowth is mediated by Intraflagellar Transport (IFT) proteins. IFT is a bidirectional movement of complexes along the axonemal doublet microtubules from the cilium base to its tip (anterograde transport) by kinesin motors (typically kinesin-II) and then from the tip back to the ciliary base (retrograde transport) by dynein 2 motors [87, 88]. The main IFT function is to deliver various cargo (e.g., ciliary components such as tubulin, receptors, etc.) into the cilia and back to promote cilium growth and maintenance. Cargo proteins can interact with IFT complexes directly (e.g. tubulin [89]) or via adaptor proteins [90]. Fully assembled IFT complexes comprise individual IFT proteins forming IFT-A and IFT-B subcomplexes, coupled with respective motor proteins [91]. Cryo-EM work in flagella of Chlamydomonas revealed that each microtubule doublet is used as a bidirectional double-track railway - anterograde IFT trains move along B-microtubules, and retrograde trains use A-microtubules for transport [92]. IFT complexes localize to two distinct pools at the ciliary base – one that overlaps with the DAs and the other within the TZ [93]. Their recruitment requires kinase activity of TTBK2 and intact DAs [71, 72], [73], however, exactly which DAs component(s) serve a physical docking platform for the interaction with IFTs is not clear. While IFTs are recruited to DAs even in nonciliated cells [73], their assembly into fully functional “trains“ seems to be completed within TZ, to be subsequently loaded inside the cilium [94, 95].
The transport of molecules inside and outside of cilium is critical for cilium growth, maintenance, and function. The current model posits that TZ together with DAs transformed into so-called transition fibers form a selective barrier between the cilium and the rest of a cell to control the ciliary entrance and exit [5, 20], [25]. Numerous studies have documented the existence of such a gating barrier at the ciliary base, yet the actual mechanism of sorting and transporting selected molecules across the barrier is not fully resolved. According to some evidence, the ciliary entry of various cargo is mediated by IFT activity. In this case, the cargo is simply dragged through the sorting barrier by the activity of IFT motors [5, 29]. In agreement with this model, anterograde IFT trains (likely cargo-loaded) are assembled at TZ, and inactivation of their motors impairs TZ localization of IFT trains and, successively, cilium elongation [5], [90], [95]. This mechanism likely ensures the ciliary entry of tubulin subunits, in cooperation with their free diffusion across the TZ [89, 96]. In addition, membrane-associated proteins and transmembrane receptors seem to enter the cilium independently of IFT motor activity. They rely instead on interactions with IFT-A subcomplex and adaptor protein TULP3 [97, 98]. TULP3 is a PIP2-binding protein, and its ciliary localization is restricted by the PIP2/PIP3 boundary, maintained by INPP5E (which itself is recruited to cilia in a TULP3-dependent manner) [97, 99].
The ciliary exit of many receptors is accordingly facilitated by the BBSome, an octameric cargo adaptor protein complex comprised of BBS1, BBS2, BBS4, BBS5, BBS7, BBS8, BBS9, and BBS18 subunits [64]. Mutations in any of the BBSome subunits lead to ciliopathy termed Barder-Biedl syndrome [100], albeit with various severity across tissues [101]. BBSome formation occurs in a step-wise fashion and is spatially governed by the BBS4 and BBS1 subunits [102, 103]. BBS4 localizes to pericentriolar satellites and recruits here the other subunits to form the pre-BBSome. BBS1 resides at the centrosome and facilitates pre-BBSome translocation and BBSome completion at the ciliary base. Moreover, BBS1 binds RABIN8 and thus serves as a bridge between RAB8/RAB11 dependent vesicular trafficking and the ciliary transport machinery [63, 64].
BBSome seems to be associated with IFT trains in many organisms. As Max Nachury stated, the BBSome may have been easily called IFT-C if not for historical reasons [98]. In fact, BBSome directly regulates IFT machinery movement in C.elegans [104]. Whether the analogous function is conserved in vertebrates is currently unclear. Nevertheless, the key function of BBSome, well documented across various model organisms, probably resides in regulating the ciliary exit of membrane receptors (e.g., SSTR3, GPR161, etc.) [98]. According to the current model, BBSome travels together with IFT particles in both anterograde and retrograde directions to retrieve ciliary receptors. Their ciliary exit, namely the passing through the TZ barrier, is subsequently facilitated by interactions with adaptor protein ARL6 (BBS3) [105].
DAs transformed into transition fibers are considered as another module regulating ciliary gating [40]. Such gating function is well expected, considering the intimate association of DAs and the membrane, implied by TEM or super-resolution microscopy studies [2, 17], [28, 38]. However, direct evidence of such function is lacking (and long-awaited), including the details on the interactions between individual DAs proteins and the membrane. One of the limitations is the critical role of several DAs proteins (namely CEP83, CEP164, and SCLT1) and TTBK2 in the initiation of the cilium assembly pathway (see earlier), hence the absence of any of these proteins halts ciliogenesis before any gating mechanism has a chance to step in. There is one exception though - FBF1. Deletion of FBF1 leads to moderate ciliogenesis defect [18, 71], allowing a careful analysis of primary cilia forming independently of FBF1. Indeed, lack of FBF1 in C. elegans or human cells causes defective cilia entry of IFT particles [18], together with reduced ciliary localization of SMO, PKD2, and SSTR3 receptors [17, 106]. The exact mechanism(s) that compromise the IFT/receptor recruitment here awaits to be identified.
Primary cilia disassembly regulation
It is well documented that primary cilium needs to be disassembled prior to the onset of mitosis, most likely to free the mother centriole for centrosome-mediated spindle organization and positioning [9], [107]. Serum-starved NIH3T3, hTERT-RPE1, or IMCD3 cells gradually lose cilia following serum addition [48, 49], [108], and asynchronous NIH3T3 (with ARL13B/Fucci sensor) were shown to disassemble primary cilia at G2/M [51]. An intriguing exception to this otherwise strictly followed rule is the Ptk1 cell line, in which primary cilium is able to persist even during early mitosis [109]. There are at least two modes for disassembling primary cilium. Consistent with the gradual shortening of flagella in Chlamydomonas before cell division [110], primary cilia of vertebrate cells can be slowly resorbed by axoneme depolymerization [108]. Moreover, the flagella of many protists can be lost in a process of rapid deciliation, which involves cutting off the ciliary axoneme by the microtubule-severing enzyme Katanin [111]. A similar mode of action also mediates primary cilia disassembly in vertebrate cells. Furthermore, both these modes of primary cilia removal can act separately as well as in combination [108]. While the identification of “switch factors”, controlling which mode of cilia assembly will be used requires additional work, it is becoming clear that the precise timing of cilia disassembly has significant physiological consequences. For instance, primary cilia of neuronal progenitors in developing chick neural tubes undergo timely remodeling of ciliary axoneme, which permits switching between specific branches of the Hedgehog (HH) signaling pathway crucial in neuronal patterning and differentiation [112] (see more on that in the HH signaling pathway chapter).
Gradual cilium disassembly requires destabilization and depolymerization of axonemal microtubules (Fig. 2). The upstream controlling mechanism involves mitotic kinase Aurora A (AURA), whose activity increases during the cell cycle, together with the size of the AURA pool at the base of primary cilia [113]. While the function of AURA upstream of cilia disassembly is well documented and conserved from Chlamydomonas to vertebrates [113, 114], the underlying mechanism is less clear. Available evidence from cell culture-based experiments suggests that a subset of histone deacetylates including HDAC6 or HDAC2, able to deacetylate tubulin, acts downstream of AURA to mediate the destabilization of axonemal microtubules [113, 115]. However, genetic ablation of HDAC6 or other HDACs does not lead to major ciliary phenotypes in vivo, indicating the involvement of compensatory actions and/or cell type specificity of the outlined mechanism [116, 117]. Still, considering both AURA and HDAC6 are enriched at the cilia base (with currently no evidence of their localization in the ciliary axoneme), one of the outstanding questions is how the signal from AURA/HDAC6 passes to the axonemal microtubules. A possible (though highly speculative) explanation may relate to the fact that HDAC6 prefers tubulin dimers over polymerized microtubules [118]. In such a scenario, HDAC6 would primarily act on tubulin subunits transported through the barrier at the cilia base.
Fig. 2.
Primary cilium dynamics during cell cycle. Primary cilia are disassembled before mitosis and reassembled after cell division during the early G1 phase. Cilium disassembly occurs through several potentially overlapping mechanisms, including gradual resorption mediated by AURA kinase and HDAC6, CDC42 and actin-dependent ectocytosis, and Katanin-dependent ciliary decapitation. Axoneme (light pink), basal body (grey) with distal appendages (DAs; pink). This image was created with BioRender.com
Disassembly of the primary cilium before mitosis is further controlled by several members of the kinesin 13 family (e.g., KIF2A, and KIF24). Unlike conventional kinesin motor proteins, kinesin 13 proteins do not “walk” along microtubules but possess the unique activity of ATP-dependent microtubule depolymerization to promote resorption of ciliary axoneme [119]. KIF2A is enriched at the base of primary cilia in the close vicinity of DAs/SDAs, and can also be found in the axoneme [79], [120]. The ability of kinesin 13 proteins to depolymerize microtubules is tightly controlled by upstream kinases. Phosphorylation by mitotic kinases such as PLK1 promotes its activity [120], while phosphorylation by TTBK2 counteracts KIF2A recruitment to the ciliary base and hampers its microtubule-depolymerizing activity [79, 121]. Similarly, the activity of axoneme-depolymerizing kinesin KIF24 is promoted by phosphorylation by NEK2 [122].
Another phenomenon, termed ectocytosis or cilia decapitation, has been recently linked to the regulation of primary cilia dynamics, eventually leading to their disassembly [123], [124] (Fig. 2). Here, events like the defective exit of ciliary receptors owing to impaired BBSome function [124], [125], or mitogen-induced INPP5E removal from primary cilia [123], trigger remodeling of ciliary actin and, accordingly, myosin-mediated abscission of the ciliary membrane and its release from the tip of cilia in a form of a small vesicle. Consequently, this may cause gradual resorption of the whole ciliary axoneme. Intriguingly, INPP5E removal from cilia and, successively, the shortening of ciliary axoneme requires AURA activity [123, 126], providing another means of how AURA activation contributes to primary cilia disassembly. Here, the shortening of primary cilia caused by BBSome deficiency can be reversed through both AURA and the intraciliary RHO GTPase CDC42 [125, 127], suggesting a close interplay between axoneme resorption and ciliary membrane turnover.
Crosstalk of cilia assembly and disassembly pathways with other regulatory mechanisms
The previous paragraphs have illustrated that primary cilia are highly dynamic organelles. In fact, as little as 5 min of halted IFT is enough to detect changes in primary cilia composition. Therefore, perhaps unexpected heterogeneity and asynchrony related to the initiation of their assembly and disassembly, respectively [51, 108], possibly reinforce a notion that having/not having a cilium is a result of counteracting interactions of pro-assembly and pro-resorption pathways. In this section, we plan to discuss some of these interactions thoroughly, including the relationship between cilia formation/resorption and cell cycle, the crosstalk between individual regulatory modules in cilium biology, along with the possible dual (non-ciliary) role of primary cilia regulators.
Cell cycle and primary cilia
As previously noted, primary cilia were originally viewed as hallmarks of quiescence, perhaps even having a checkpoint function. According to this model, primary cilium had to be resorbed before cell cycle re-entry and, conversely, a block of primary cilia disassembly led to cell cycle arrest [50]. While this model has gained some attention, especially when considering the possibility of halting tumor growth by inducing ciliogenesis in cancer cells, it remains controversial, with conflicting data in the literature. Actually, this raises a conundrum: How can organelle, resorbed prior to cell cycle re-entry, still convey proliferative signals (e.g. by HH ligands) important for G1-S transition? [9] Specifically, scaffolding protein trichoplein (TCHP) has been proposed to promote G1-S transition in ciliated cells by activating AURA and then dissembling cilia [128]. This model, however, has been challenged in a report indicating that TCHP regulates cell cycle progression independently of cilia status [129]. With the wide adaptation of live cell imaging microscopy, many reports have subsequently demonstrated the presence of primary cilia in cycling cells [9], [51, 130]. Still, considering the heterogeneity in the timing of primary cilia assembly/disassembly, the possibility of some feedback mechanism between primary cilia and cell cycle, eventually leading to cell cycle alterations between ciliated and non-ciliated cells cannot be completely dismissed.
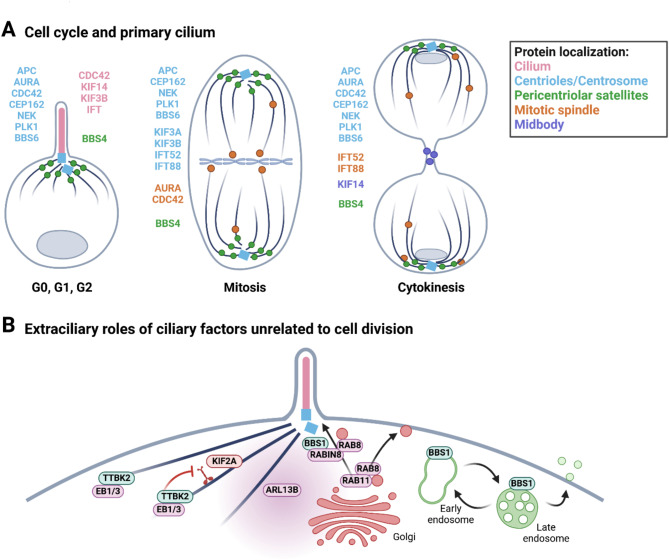
While direct evidence for the checkpoint role of primary cilia is currently lacking, the intimate relationship between primary cilia and the cell cycle is well documented (Fig. 3A). One aspect of this interconnection is the effect of cell cycle regulators on primary cilia dynamics. The key players here are proteins well known for their prominent roles in mitosis. They are typically associated with the centrosome/ciliary base throughout the cell cycle, starting with very low abundance at G1 and peaking their levels/activity in the G2 or M phase. These include, for instance, AURA (see above) and NEK kinases [131], as well as an anaphase-promoting factor (APC) [132]. Similarly to AURA, the activities of NEK kinases have been shown to promote cilia shortening/disassembly, while their depletion leads to ciliary axoneme elongation. These effects were observed in several models, including Chlamydomonas and Tetrahymena, as well as in mammalian cells, suggesting the role of NEKs is well conserved [131]. Mechanisms underlying the effects of NEK kinase activity include destabilization of microtubules [131] or, in the case of NEK2, disassembly of DAs [133]. The ciliary role of APC seems to be mediated by targeting NEK1 for degradation [132]. Interestingly, some mitotic regulators can act as ciliogenesis-promoting factors, e.g., by facilitating centriole maturation. One such example is PLK1, whose ectopic activation in interphase erases differences between mother and daughter centrioles (the daughter prematurely assembles DAs), and then, allows the formation of two primary cilia per cell [134]. Considering the orderly nature of the cell cycle, heterogeneity in the timing of primary cilia assembly/disassembly seen in several time-lapse experiments is somewhat unexpected. It implies that rather than providing an instructive signal coupled to a specific checkpoint, the cell cycle creates a permissive window for cilia assembly, fine-tuned by (potentially stochastic) fluctuations in the activities of key regulators.
Fig. 3.
Dual roles of cilia regulators in cell cycle and intracellular organization. A Established mitotic regulators such as AURA, NEKs, APC, CDC42, and PLK1 also play critical roles in regulating cilium disassembly. Common ciliary factors such as IFTs (e.g. IFT52 and IFT88), KIF3A/B, KIF14, CEP162, BBS4, and BBS6 also facilitate the formation of the mitotic spindle, chromosome alignment, and the progression of cytokinesis. B The factors involved in ciliogenesis and cilia function are vital to intracellular organization. TTBK2 binds to EB1/3 and promotes microtubule growth by suppressing depolymerizing activity of the KIF2A kinesin. ARL13B regulates the non-canonical Hedgehog (HH) pathway and is key in controlling actin organization. The RAB11/RABIN8/RAB8 cascade controls polarized vesicular trafficking to the plasma and ciliary membrane, where the latter is mediated by the BBS1-RABIN8 module. BBS1 is also involved in the endomembrane vesicular trafficking between the early and late endosomes. This image was created with BioRender.com
Perhaps the best-described functional connection between cilia and cell cycle is the extensive proliferation leading to cyst formation in kidneys – a hallmark feature of several ciliopathies[6]. In fact, kidney cyst formation, caused by mutation of cilia residing receptor polycystin 1/2 (PKD1/2) or DAs protein CEP164, can be mitigated by inhibition of cyclin-dependent kinases[19], [135]. Aberrant proliferation related to PKD1/2 mutations seems to be caused by deregulated Ca2+ signaling and cAMP production[136]. Another prominent driver of cell proliferation in kidneys is the Hedgehog (HH) signaling pathway. The causative relationship between cilia defects, deregulated HH signaling, and kidney cyst formation has been indicated for mutation of the IFT-A component, IFT139, that leads to bulbous cilia and an overactivated HH pathway[137], [138].
Another aspect of primary cilia–cell cycle connection is the role of several cilia components in cell cycle regulation, particularly during mitosis and cytokinesis. These effects appear to represent additional functions of these proteins, independent of primary cilia. Both mitosis and cytokinesis rely on the action of microtubules and associated proteins associated. Kinesins and other microtubule-binding proteins therefore represent a group where such cilia-unrelated function is anticipated[139]. KIF3A and KIF3B constitute the kinesin-II heterodimer, which, besides participating in the anterograde IFT in cilia, represents one of the most ubiquitously expressed kinesins, implicated in many forms of intracellular transport [140]. Furthermore, KIF3A/3B is associated with mitotic spindle in both Chlamydomonas and vertebrate cells, and expression of a mutated form of KIF3B leads to mitotic abnormalities and aneuploidy [141]. A different example is another microtubule-plus end motor, KIF14, originally identified as cytokinesis-regulating kinesin associated with the midbody [142, 143]. However, during interphase, KIF14 localizes to the axoneme of primary cilia to regulate cilia growth, IFT, and competence to convey HH pathway activation [144, 145]. The association between mitotic apparatus and cilia regulators extends to IFT and BBS proteins (see Table 1 for a detailed overview). This is perhaps the best documented for IFT88 (member of IFT-B complex). In dividing cells, IFT88 localizes to the mitotic spindle, where it takes part in a dynein1-driven complex to ensure the correct formation of astral microtubule arrays and spindle orientation. Consequently, IFT88 depletion leads to mitotic defects in cell culture cell lines, mice, and zebrafish [146]. Moreover, IFT88 has been implicated in the re-localization of AURB during cytokinesis [147], and, together with another IFT-B component, IFT52, in the clustering of supernumerary centrosomes in cancer cells [148]. Also, IFT proteins from both IFT-B and IFT-A subcomplex re-localize from basal bodies/cilia to cleavage furrow in dividing Chlamydomonas [149]. Interestingly, IFT-A and KIF3A have another way to affect the cell cycle independently of cilia – via regulation of the WNT/β-catenin pathway [150], [151]. Our WNT signaling chapter has further details. Other important primary cilia biology regulators with ties to proliferation control are BBS proteins. One of the initial BBS studies, which linked BBS4 to primary cilia and centriolar satellites, actually reported that BBS4 depletion leads to cell cycle arrest and prominent cytokinesis defects [152]. This proposed mechanism involves BBS4-PCM1 interaction in centriolar satellites and, accordingly, defective anchoring of microtubules in BBS4-depleted cells. Similarly, chaperonin BBS6 exhibits prominent localization to centrosome and centriolar satellites, and its depletion induces cytokinesis defects and the appearance of multinucleated cells [153]. One more example of a ciliary component with a cilia-unrelated role during cell division is CEP162, a protein with microtubule-binding ability residing at the centriole distal end to promote and restrict the TZ formation [154]. Additionally, CEP162 localizes to spindle microtubules and centrosomes in dividing cells, and its loss leads to abnormal mitosis and chromosome segregation defects [154], [155].
Table 1.
Cilia regulators with dual roles
| Protein(s) | Centrosome/cilia -related function | Extraciliary functions - cell cycle | Extraciliary functions- other |
|---|---|---|---|
| AURA | cilia disassembly [113, 114] | mitotic entry, spindle assembly, chromosome alignment [157] | |
| HDAC6 | cilia disassembly [113, 115] | microtubule deacetylation [116, 117] | |
| CDC42 | cilia shortening, actin-dependent ectocytosis [125] | chromosome alignment [157] | regulator of cell polarity and actin-based morphogenesis [158] |
| NEK kinases | cilia shortening/disassembly [131] |
destabilization of MTs [131] disassembly of DAs [133] |
|
| APC | stability of axonemal microtubules through targeting NEK1 [132] | anaphase promoting complex [157] | |
| PLK1 | centriole maturation [134] | mitosis progression [157] | |
|
KIF3A KIF3B |
anterograde IFT [140] |
intracellular transport [140] mutations in KIF3B - mitotic abnormalities and aneuploidy [141] |
intracellular transport [140] |
| KIF14 |
cilia growth and trafficking, HH activation [144] |
localization to midbody, cytokinesis progression [142, 143] | |
| IFT88 | ciliogenesis, IFT transport [87] |
spindle orientation, AURB positioning during cytokinesis [147] |
clustering of centrosomes in cancer cells [148] |
| BBS4 | BBSome-dependent trafficking [152] | cell cycle progression and cytokinesis [152] |
pre-BBSome assembly [102] exosome release [235] |
| BBS1 |
BBSome-dependent trafficking, ciliary gating by supporting TZ structure [102] |
endomembrane trafficking [162, 163] | |
| BBS6 | BBSome assembly [153] | progression of cytokinesis [153] |
CCT/TRiC-chaperonin complex [153] exosome release [235] |
| CEP162 | TZ formation [154] | chromosome segregation during mitosis [155] | |
| TTBK2 | primary cilia assembly [73–77, 80, 173, 174] | MTs dynamics and cell migration [121] | |
|
RAB11 RAB8 RABIN8 |
polarized trafficking of ciliary vesicles, outgrowth of ciliary membrane and ciliogenesis [61, 62, 64] | endosomal recycling, polarized transport of Golgi-derived vesicles [63, 159, 160] | |
| ARL13B | ciliary membrane composition; ciliary targeting of INPP5E and HH pathway components [165–167] | non-canonical HH signaling essential for the axon guidance [168, 169] |
Summarily, available evidence suggests that cells are efficiently re-purposing their regulatory elements, leading to a shared toolkit of regulators of cilia and cell cycle. Proteins commonly viewed as mitotic regulators (AURA, NEKs, APC) mediate the cilia disassembly to ensure cilia do not intervene with spindle function during cell division. Conversely, proteins involved in cilia biogenesis (IFT, BBS, KIF3A/3B, KIF14, etc.) often take over new tasks during mitosis or cytokinesis, as there is no cilium to care about at the moment. This opens an interesting possibility that some of the many pathologies found across different “ciliopathies” may in fact stem from non-ciliary functions of cilia regulators [156].
Extraciliary roles of ciliary factors
TTBK2 represents an example of a critical regulator of primary cilia assembly, with an additional role outside of cilia, not directly linked to cell division (Fig. 3B; Table 1). Outside of the basal body, TTBK2 interacts with microtubule plus-end tracking proteins (+ TIPs) such as EB1/3 to localize to cytoskeletal microtubules and regulate their dynamics and successive cell migration [121]. Interestingly, at least some activities of TTBK2, both inside and outside of primary cilia, rely on a common mechanism - phosphorylation and subsequent inactivation of microtubule-depolymerizing kinesin KIF2A [79], [121].
RAB11/RABIN8/RAB8 pathway activity is not restricted to primary cilia and is also involved in several aspects of vesicular trafficking in the cytosol, including endosomes recycling or transport of Golgi-derived vesicles to the cell membrane [63], [159], [160]. The BBS1 subunit of the BBSome interacts with RABIN8, linking the BBSome to the vesicular trafficking of ciliary proteins to the primary cilium base [64], [161]. BBS1 has also been detected on early and late endosomes in HEK293 cells [162], suggesting that BBSome is more broadly involved in the endomembrane system beyond its function in ciliary transport [163].
A surprising non-ciliary function has also been identified for ARL13B, which is perhaps the most commonly used marker to visualize primary cilia in microscopy-based experiments, and its mutations cause Joubert syndrome [48], [164]. ARL13B is a membrane-associated small GTPase of the ARF/ARL family, important for correct ciliary targeting and localization of INPP5E and several HH pathway components [165], [166], [167]. Recent work from the Caspary lab described ARL13BV358A mutant, which is unable to localize to primary cilia, and the cilia are shorter and show diminished INPP5E levels. Notably, ARL13BV358A still seems fully capable of rescuing major HH phenotypes related to ARL13b loss in mice [168], [169]. While ARL13BV358A is missing in primary cilia, it could be identified in axons and growth cones of commissural neurons, where it probably mediates non-canonical HH signaling relevant for axon guidance [169]. Considering the prominent role of ARL13B in disease and the well-supported model of HH pathway dependence on primary cilia in vertebrates, it would be very interesting to test the ability of ARL13BV358A to mediate HH signaling in a model with ablated ciliogenesis.
Interactions between individual modules of primary cilia
In the following section, we will discuss how individual cilia compartments affect each other to control the dynamics of the fully assembled organelle. We begin with the basal body, which is typically viewed as an inseparable part of cilium, and, via the DAs-associated activities previously noted, is critically involved in primary cilia assembly initiation (Figs. 1A and 4A). In the primary cilia of sensory neurons of the nematode C.elegans, the basal body is degraded following cilium assembly[170]. In fact, centrioles are lost in most cells in C. elegans, coinciding with the cessation of proliferation. Even though the basal body is lost, transition fiber-like structures still connect the proximal axoneme end with the membrane [170], indicating a function of this compartment exceeding ciliogenesis initiation. Indeed, emerging evidence from mice and cell culture cell lines has established DAs kinase TTBK2 capacity in primary cilia maintenance. TTBK2 activity ablation after cilia assembly effectuates their shortening, perturbed localization of IFT proteins and HH pathways components, and axonemal microtubules glutamylation reduction [171, 172]. Proposed mechanisms downstream from TTBK2 involve KIF2A inhibitory effects [79] and centriolar satellite remodeling [172].
Fig. 4.
Interactions between individual modules of primary cilia. A The basal body and pericentriolar satellites are crucial for the growth and maintenance of the ciliary axoneme. TTBK2 at the basal body promotes cilium growth by suppressing the depolymerizing kinesin KIF2A. In C. elegans, the basal body is degraded once the cilium is assembled. B Anterograde and retrograde IFT display functional crosstalk in cilium length control. Inhibition of the heteromeric KIF3A/3B kinesin II disrupts anterograde IFT and indirectly halts retrograde IFT, causing IFT particles to accumulate at the ciliary base. Inhibition of the dynein motor by ciliobrevin D or mutation in IFT-A complexes indirectly abrogates anterograde IFT. C Retrograde IFT is crucial for correct TZ establishment. Mutations in components of retrograde IFT lead to the mislocalization of TZ components inside the cilium, and the shortening of cilia. D Posttranslational modifications (PTMs) of axonemal tubulin are crucial for cilia function. Axoneme acetylation, glutamylation, and glycylation are involved in cilia beating and sperm motility, likely modulating the velocity of the anterograde IFT. PTMs can also compensate for each other; axoneme hyper-glycylation can compensate for axoneme deacetylation in zebrafish cilia, while axoneme hyper-glutamylation can compensate for axoneme deglycylation in murine photoreceptors. This image was created with BioRender.com
Dynamic interactions are also typical for the IFT. As noted earlier, disruption of IFT-dynein or IFT‐A complexes results in short, bulged primary cilia due to the accumulation of IFT proteins and their cargo. Conversely, mutations in IFT‐B components typically result in complete axoneme assembly failure [87]. Interestingly, while the general phenotypes of anterograde and retrograde defects, respectively, are well distinguishable, emerging evidence also suggests “crosstalk” between anterograde and retrograde transport (Fig. 4B). The putative sites for such interactions are the ciliary base and tip, respectively, where IFT trains rearrange to switch motors [87]. Acute inhibition of heteromeric KIF3A/3B kinesin II, for instance, completely halts both anterograde and retrograde transport in primary cilia in NIH3T3 cells within minutes [173]. However, dynein motor inhibition by ciliobrevin D halts anterograde IFT trains within 30 min of stalled retrograde transport [174]. Furthermore, CHE‐3, the C. elegans orthologue of the retrograde IFT‐dynein‐2 motor DYNC2H1 demonstrably affects both retrograde and anterograde transport [175]. Similar observations were reported in WDR60 and WDR34 dynein-2 mutants using mammalian cell lines [176]. One possible explanation for these phenotypes is the roadblock/traffic jam model, where stalled IFT trains impair “opposing” IFT train movement indirectly, by sterically limiting the “free road” available. Considering IFT-A and IFT-B trains show direction-specific usage of the two microtubules in a doublet to avoid collisions, at least in Chlamydomonas [92], reported reciprocal interactions between anterograde and retrograde transport machinery may indicate a more complex relationship. The modeling of IFT motor capacity to walk through crowded environments or pass obstacles warrants future investigation.
We have previously discussed the IFT trains being assembled at TZ and noted that IFT-A proteins participate in the import of membrane receptors through the TZ gate. Interestingly, IFT-TZ interactions seem to be even more complex with available evidence further implying IFT involvement in TZ assembly/maintenance regulation (Fig. 4C). The initial evidence of IFT involvement in TZ formation comes from Trypanosoma, where IFT mutations precipitate TZ shortening [177]. The requirement of functional IFT for correct TZ function is conserved in metazoan. Ablation of WDR60 or WDR34 in hTERT-RPE1 cells affects the expansion of RPGRIPL1 from the TZ inside the cilium and reduces the TMEM67 pool in the TZ [176]. Similarly, ectopic localization (expansion from the TZ into the proximal axoneme) of several TZ components is connected to the ablation of dynein motor CHE-3 in C. elegans [175]. Furthermore, such ectopic localization of several TZ components has also been established for several IFT-A mutants in C. elegans [170]. The functional consequence of IFT-TZ interactions is the prominent worsening of gating function in worms with ablated IFT and MKS over the single mutants [178]. One possible explanation of IFT effects on TZ is that TZ proteins begin their assembly in the most proximal region of the axoneme following ciliogenesis onset, yet a portion of these proteins can become mislocalized to distal ciliary regions and potentially create ectopic axoneme–membrane connections. The IFT retrograde machinery purpose may therefore be to assist in the retrieval of mis-localized TZ proteins [175], [178]. Interestingly, the antithesis of IFT-TZ interaction is that disrupting the TZ NPHP module restores the ciliary exit of “underpowered” retrograde trains in WDR-60 mutant, which otherwise accumulate inside the cilium [179]. The model of mutual regulation of IFT-TZ has support in association (though rare) of eye and kidney phenotypes, typical for TZ defects, with IFT mutations [8], [180].
New paradigms are emerging on possible mutual interactions of the ciliary axoneme and IFT motor proteins. Modulation of microtubule tracks in the ciliary axoneme, which fine-tunes the motor proteins walking on them, could be explained by the so-called “tubulin code”. The overall concept behind this term is that molecular patterns generated by combinations of tubulin isotypes and their posttranslational modifications (PTMs) such as acetylation, (poly)glutamylation, (poly)glycylation, detyrosination, etc., control the functions of microtubules [181], [182]. Almost all of the amino acid and length variation between different tubulin isotypes is confined to their unstructured C-terminal tails (CCTs). Consequently, the presence and absence of the CCT, respectively, and the type of its modification have substantial effects on the motility of several kinesin motors (including kinesin II) on purified microtubules in vitro [181]. Ciliary axonemes of both primary and motile cilia are rich in several tubulin PTMs [181], [184], which has been commonly associated (although not yet completely proved) with their considerably higher stability over the cytosolic microtubules. Thus, “guilt by association” evidence suggests that tubulin PTMs play a role in cilia biology (Fig. 4D). Indeed, several of these PTMs show non-uniform, pattern-like distribution in the axoneme [184]. The function of tubulin PTMs is reasonably well-documented for the motile cilia, where the glycylation and glutamylation are particularly enriched in the B microtubule of the doublet [184]. However, the role of tubulin modifications in primary cilia biology is less clear [181]. Polyglycylation of tubulin CCT is almost exclusively found in ciliary axonemes over cytosolic microtubules [181], and is essential for ciliogenesis, at least in protists. Tetrahymena strains with defective polyglycylation (where tubulin encoding genes were replaced with variants lacking corresponding glycylation sites) have profound defects in axoneme structure and exhibit impaired movement of flagella [182]. The situation seems to be different in mammals. Elimination of enzymes responsible for tubulin glycylation (TTLL3 and TTLL8) results in the absence of glycylated tubulin in cilia, albeit without a penetrant cilia-related phenotype (mice are viable and of normal morphology). Glycylation, therefore, does not seem to be critically involved in the assembly or maintenance of primary cilia. Instead, the defects in TTLL3/TTLL8 double knockout are restricted to the sperm flagella, inducing altered beating dynamics and then reduced fertility [185]. Similarly, aberrant (poly)glutamylation is linked to defective cilia beating in both Tetrahymena and mammals (where it often manifests in fertility and respiratory defects) [181], [186]. Significantly, axoneme glutamylation has been implicated in the regulation of the velocity of several ciliary motors and the release of cilia-derived extracellular vesicles in C.elegans [187]. Moreover, chemically-induced cilia de-glutamylation led to a slower anterograde movement of IFT88 containing trains in primary cilia of NIH3T3 cells [188]. If confirmed in other model systems, the glutamylation-IFT link could provide long-anticipated, yet currently lacking evidence of the functional impact of glutamylation/tubulin code on primary cilia biology. The purpose of acetylation in cilia is currently unclear, despite the fact that tubulin acetylation was the first described tubulin PTM [181]. Enzyme αTAT1 has been identified as the major regulator of tubulin acetylation, its ablation causing a loss of this PTM in mice, however excepting moderate fertility defects related to sperm motility, the mice are viable and develop normally [189]. Surprisingly, the cytosolic microtubules exhibited higher stability in the absence of αTAT1 [189]. We use this observation as a proxy to present another emerging phenomenon in tubulin PTMs regulation – compensatory mechanisms at the level of “writers”, “erasers”, or even between individual types of tubulin PTMs, which likely contribute to the lack of gross cilia phenotypes in various models. Loss of tubulin glycylation in mouse photoreceptors is accompanied by an increased glutamylation, perhaps due to competition of the corresponding enzymes for the same modification sites in the CTT [181]. Mutation of three enzymes responsible for tubulin deacetylation (SIRT2, HDAC6, and HDAC10) brings about the anticipated reduction of tubulin acetylation in cytosolic microtubules, yet concurrently decreased acetylation and increased glycylation of ciliary axonemes in zebrafish, and, furthermore, without a notable impact on ciliary function [117]. Clearly, resolving the nature of compensatory mechanisms is a crucial step in assessing the functional impact of tubulin PTMs on primary cilia biology. Since we have now discussed means in which the axoneme may affect walking motor proteins, it is worthwhile noting a rather hypothetical, yet very intriguing possibility that motor protein may communicate back to the cilium MT tracks. This new concept, currently demonstrated on prototypical cytosolic kinesins, kinesin 1 and 4, suggests that motors can not only induce conformational changes in tubulin owing to their walk, but the stepping may actually induce damage in the microtubule, which can be subsequently repaired by incorporation of free tubulins in the damage sites [190].
Substantively, three areas await further consideration: Whether this phenomenon additionally applies to ciliary kinesins, its potential impact for IFT, and whether the cilium developed efficient countermeasures to compensate/eliminate kinesin-induced changes in microtubule tracks.
Adaptive changes in primary cilium structure and composition to cell signaling pathways
Cilia are typically categorized into primary cilia (which are immotile and serve to sense and transduce signals), and motile cilia (which move extracellular fluids). However, increasing research reveals that both primary and motile cilia can modulate key developmental signaling pathways, such as HH, and WNT [191], [192]. Likewise, certain primary cilia can exhibit limited or specialized motility under specific conditions. As previously noted, pancreatic beta cells possess so-called hybrid cilia, which move to sense glucose levels and promote insulin production in the pancreas [35]. A temporally regulated change in primary cilia motility has been observed during the perinatal period of the choroid plexus development [193]. This transient motility shift was accompanied by changes in gene expression, suggesting a unique role in choroid plexus formation at this stage [193]. During tissue and organ development, cilia can change their identity and also undergo retraction and reassembly [127], [194], [195]. Moreover, emerging evidence suggests that cilia physically interact with neuronal axons via synapse-like connections [196]. These and other findings emphasize the need to view cilia as dynamic organelles with complex, context-specific adaptations in homeostasis, development, and regeneration. Recently, cilium’s involvement in developmental signaling pathway transduction was reviewed in great detail [3], [5]. At this juncture, we will explore how specific signals shape cilium composition and structure and accordingly, how cilia are associated with key signaling pathway regulations, crucial for cell differentiation and tissue morphogenesis.
Hedgehog signaling
Hedgehog (HH) signaling is a prototype of a cilia-dependent pathway decisive for growth and patterning of different tissues and organs during embryogenesis. Key molecular players comprise the transmembrane receptors PTCH, SMO, and GPR161, along with soluble factors including PKA, GLI proteins and the second messenger cAMP [197]. The pathway is induced via HH ligand binding to PTCH and its removal from cilia allowing SMO to enter. Recent studies indicate HH is transported in the neural tube to recipient cells with the help of cytonemes - specialized cellular actin-based extensions that facilitate targeted delivery [198]. SMO accumulation in cilia induces inhibitory receptor GPR161 export by β-arrestin-BBSome-IFT retrograde trains [105]. GPR161 removal decreases cAMP levels and leads to reduced PKA activity [199]. Consequently, GLI3FL (full-length) processing into its repressor form, GLI3R, is inhibited, permitting the transcription of HH-responsive genes. The cilium compartment provides spatial and temporal control over HH signaling, thereby differentiating the effects of ciliary versus non-ciliary cAMP and GPR161 activity [200], [201]. Changes in primary cilia composition induced by HH signaling are rapid and detectable within minutes upon HH pathway activation [202]. Several mechanisms have been proposed to mediate dynamic rearrangements of primary cilia in response to HH pathway activation. HH-mediated SMO activation promotes interactions of GPR161 and PTCH with β-arrestin, which results in the retrieval of both receptors from cilia by endocytosis [203], [204]. Acute HH pathway activation supports primary cilia shortening [125], [205], likely via activation of the ectocytosis/cilia decapitation feedback response [123], [124], [125](Fig. 5A). Certainly, the “heterogeneity/ plasticity” in the timing of the assembly/disassembly of primary cilia noted earlier likely mediates the robustness of HH pathway effects on cell proliferation. In this case, HH pathway activity in either G1-S of the previous cell cycle or the G1 phase of the cell cycle, when the decision is made, is sufficient to drive cell cycle entry [206]. This model is consistent with the remarkable finding that a decision “to divide” could have been made in virtually any phase of the previous cell cycle [207]. HH-induced signaling is thus propagated in a robust manner into the progeny to promote fate decisions within complex tissues.
Fig. 5.
Adaptive changes to cilia dynamics during cell signaling. A Activation of HH signaling triggers cilia shortening and promotes cell cycle entry, passing on this effect to the subsequent progeny (1). In fibro/adipogenic progenitors (FAPs), the length of cilia modulates muscle regeneration. Short or absent cilia on FAPs block adipogenesis and promote myogenesis, while long cilia favor adipogenesis (2). Similarly, HH signaling induces myoblast differentiation from muscle stem cells, where myoblasts lose cilia to form myofibrils (3). In BBSome deficiency, HH signaling triggers CDC42/actin-dependent ectocytosis of ciliary GPR161, leading to subsequent cilia shortening (4). After neural tube closure, HH-producing cells elongate cilia to attenuate their responsiveness to the HH pathway (5). During differentiation of neural progenitors, apical abscission of the ciliary axoneme from the basal body leads to a temporary cilia dysfunction. B The effects of WNT signaling on primary cilia dynamics are context-dependent. During tissue differentiation, WNT functions together with HH to either stimulate cilia disassembly in myoblasts or sustain cilia dependent signaling in bone marrow mesenchymal stem cells (BM MSCs) during osteogenesis (1). In MSCs, WNT and HH act inhibitory on adipo-commitment, leading to the emergence of adipocytes through IGF1R/FFAR4 cilia-dependent signaling and eventual cilia disassembly (1). In cell culture systems, activation of WNT with WNT3a ligand or pharmacological inhibition of WNT ligands secretion, does not alter number or length of cilia (2). C The effects of TGFβ signaling on cilia dynamics exhibit a context- and cell type-specific dependency. Activation of the TGFβ signaling pathway leads to the shortening of primary cilia in cultured chondrocytes (1), whereas it maintains cilia length in cilia of Xenopus left-right organizer (LRO) (2). D NOTCH signaling is primarily triggered by the fluid flow-induced alterations in cilia dynamics, which in turn promotes cilia function in diverse cellular systems, such as respiratory epithelium (1), zebrafish left-right organizer (LRO) (2) and endothelial cells in the myocardium (3). This image was created with BioRender.com
In muscles, HH signaling promotes the myogenic differentiation of the muscle stem cells [208], at the expense of fibrosis and inflammation [209]. Cilia of these stem cells maintain them in a quiescent state, preserving their ability to generate new myoblasts both during development and in response to stimuli, such as injury [195], [208], [210] (Fig. 5A). During aging, muscle stem cells lose their cilia, causing a decline in regenerative capacity [210]. The primary cilium of muscle stem cells transduces the HH pathway to ensure the proper timing of myoblast differentiation and proliferation [208]. Interestingly, defective cilia formation seems to drive these stem cells into premature proliferation, which can ultimately result in rhabdomyosarcoma [208]. Differentiated myoblasts lose their capacity to assemble primary cilia (hence to respond to HH pathway activation) during myofibrillar transition as they migrate and form myofibers [195]. Regenerative capacity of skeletal muscles is also dependent on fibro/adipogenic progenitors (FAPs) that dynamically modulate their ciliation during muscle regeneration. Preventing FAPs to ciliate blocks adipogenesis and promotes myogenesis following muscle injury [211]. Intriguingly, the mechanism involves the activation of HH target genes, due to the inability of FAPs with ablated primary cilia to generate GLI3R, which otherwise represses the HH target genes [211].
Furthermore, regulation of primary cilia length to fine-tune the responsiveness to HH pathway activation has been proposed to play a key role during the establishment of the dorsolateral axis in the forming neural tube (Fig. 5A). Here, primary cilia of the neural plate HH-responding cells (before the neural tube closure) are relatively short. Intriguingly, following the neural tube closure, HH-producing cells located in the floorplate elongate their cilia and concomitantly attenuate their responsiveness to HH pathway activation [212]. While the attenuation of the HH pathway in the floor plate depends on these elongating cilia, mechanisms driving their elongation and the HH pathway shutdown are still awaiting full determination [212]. One possibility is that the elongation is a consequence of the structural transformation of these cilia to become motile [213]. Cilia remodeling is also occurring during neurogenic divisions of progenitors in the neural tube, in a process termed apical abscission, which physically separates the axoneme and the basal body, hence rendering the cilium temporarily dysfunctional [214]. Remarkably, disrupting the cilium formation during or after its remodeling during the apical abscission impairs axonogenesis in newly born neurons, possibly via a non-canonical HH signaling pathway (not involving regulation of gene expression) [112].
In ciliopathy conditions, such as BBS, cells lack the retrograde adaptor BBSome for retrieval of GPR161 and additional receptors. Activation of the HH pathway in BBSome deficient cells also leads to cilia shortening [125], although the mechanism differs from that in wild-type conditions (Fig. 5A). HH induces hyperactivation here of intraciliary GTPase CDC42, which promotes actin-dependent ectocytosis of the ciliary content, including GPR161, and successively, shortens the ciliary axoneme [125]. Despite these alterations, HH signaling is not entirely suppressed in the BBS context [124], [215]. The GPR161 might likely be removed in ectosomes together with SMO in a bipartite complex [203] or additional HH modifiers, such as PKA [216]. In such cases, the ectocytosis-dependent decrease in cAMP and PKA activity might alleviate GLI3 phosphorylation and sustain low levels of HH signaling.
In Joubert syndrome, upon loss of INPP5E, cilia become shorter and HH signaling increases [123], [217]. INPP5E controls cilium dynamics via regulating ciliary PIP levels and TULP3-dependent import of receptors (e.g. GPR161) to cilia [42], [126]. AURA, as one of the key regulators of primary cilia disassembly, has been depicted to drive cilia shortening via depleting cilia of INPP5E [123]. It remains to be seen if AURA activity also mediates some of the reported effects of the HH pathway on primary cilia length. Overactive HH signaling and short cilia have been also identified in PINK1-deficient human and mouse models of familial Parkinson’s disease [218].
WNT signaling
Canonical (WNT/β-catenin) and non-canonical WNT (WNT/β-catenin-independent) signaling belong to the major developmental pathways with a somewhat controversial relationship to primary cilium. WNT/β-catenin signaling pathway, similar to the HH signaling pathway, is a potent driver of cell proliferation, with numerous implications for development and cancer [130], [219]. As the name suggests, its key element is the protein β-catenin, which is in response to WNT ligand stimulation translocated from the cytosol into the nucleus, where it, in cooperation with TCF/LEF proteins, drives gene expression [219]. Upon a WNT ligand binding to receptor Frizzled (FZD) and co-receptor LRP5/6, the signal is via activities of scaffolding protein DVL transduced to a complex of APC-AXIN-β-catenin, and kinases such as GSK3β and CK1. This causes the disassembly of this so-called degradation complex, β-catenin escapes GSK3β/CK1-mediated phosphorylation marking it for degradation, which then leads to the stabilization of β-catenin levels and import into the nucleus [219]. The non-canonical pathway is characterized by its β-catenin-independent status and typically involves branches such as WNT/PCP and WNT/Ca2+ [130, 220]. Discussion here is focused on the links between WNT/β-catenin signaling and primary cilia, as the connections between cilia and WNT/β-catenin- independent pathways (in particular the WNT/PCP) have been previously identified and thoroughly covered in several reviews [220], [221]. Several components of WNT signaling have been identified associated with basal body/centrosome (e.g. β-catenin, DVL, AXIN, APC) [130] and/or ciliary axoneme (e.g. LRP5/6, FZD) [222]. This evidence alone clearly argues for a link between primary cilia/centrosomes and the components of the WNT/β-catenin signaling pathway. However, functional consequences of such a link for cilia or WNT/β-catenin signaling are considerably less clear. Deregulation of cilia formation/function by acute depletion of some of its regulators (e.g. INVS, BBS1, BBS4, BBS6/MKS, etc.) [223], [224] or mutation of KIF3A in mouse fibroblast[225] leads to concomitant defects in WNT/β-catenin signaling. Conversely, mutation of ciliary regulators in vivo (e.g. IFT88, IFT172, INVS, DYNC2H1, and KIF3A) does not seem to cause major defects in WNT/β-catenin signaling, even though ciliogenesis and/or HH pathway are severely dysregulated [226], [227], [228]. One plausible explanation for these contradicting reports is the activation of compensatory mechanisms that lower the penetrance of the WNT-related phenotypes (in contrast to acute depletion/inhibition by siRNA or morpholino) in models with permanent gene deletion/mutation (e.g. owing to transcriptional adaptation following CRISPR/Cas9 gene editing [229]). Alternatively, some of these effects could be explained by the dual role of some of the cilia components previously discussed (Table 1). Interestingly, compelling evidence suggests that a key step of the WNT/β-catenin cascade, namely the translocation of β-catenin into the nucleus is controlled by IFT-A and kinesin II (KIF3A) proteins. Specifically, lack of IFT140 or KIF3A, though not IFT88, prevents nuclear accumulation of β-catenin following WNT ligand stimulation, independently of their role in primary cilia [150], [151]. Furthermore, reported effects (or their lack of) may reflect the high complexity of the cilia – WNT/ β-catenin signaling relationship (e.g., context-dependent effects, non-cell autonomous functions). The WNT/β-catenin signaling pathway is well established in the specification of motile cilia, via regulation of expression of transcription factor FOXJ, acting upstream of the induction of motile cilia formation [230], [231]. WNT signaling effects on primary cilia dynamics seem to be context-dependent (Fig. 5B). Analysis of cell lines such as hTERT-RPE1, HEK293, and NIH3T3 established that stimulation with WNT3a ligand, pharmacological inhibition of WNT ligands secretion, or genetic ablation of LRP5/6 receptors did not alter the number nor length of primary cilia [232], while ablation or knockdown of LRP5/6 impaired primary cilia formation in HEK293T cells [222]. The explanation may be the cell type-specific activation of a cilia-residing WNT signaling branch, independent of β-catenin and regulation of transcription, yet utilizing LRP5/6 and GSK3β, capable of modulating primary cilia dynamics [222]. The exact mechanism of how the ciliary WNT/GSK3β pathway contributes to cilia dynamics as well as the overall outcome of WNT signaling in ciliopathy conditions remains to be elucidated. In BBS, loss of BBSome stimulates premature cilia disassembly via ectocytosis/cilia decapitation [123], [124], [125]. Simultaneously, it disrupts WNT signaling and leads to altered differentiation of multiple tissues, including the retinal pigment epithelium (RPE) layer in the retina [127], [223], [233], [234]. It is not yet known whether the BBSome regulates the ciliary WNT/GSK pathway. Nevertheless, loss of the BBSome results in dysregulation of the cellular endomembrane system, causing increased exocytosis of the small secretory vesicles containing WNT regulators [235]. An intriguing possibility is that the BBSome may act to balance intraciliary and extraciliary WNT signaling, though this hypothesis needs to be thoroughly tested. It will now be crucial to explore how this WNT/GSK3β signaling route contributes to the overall WNT signaling outcome in ciliopathy models and examine its potential crosstalk with the HH pathway in relevant cases such as cancer and embryogenesis. In fact, considering that dysregulation of either the HH signaling pathway or signaling downstream of LRP5/6 induces neural tube closure defects [220], [236], a developing neural tube could represent a suitable model system to probe for HH pathway alternations in conditions with hampered WNT/(LRP5/6)/GSK3β signaling.
Another system offering room for HH-WNT interactions is mesenchymal cells (Fig. 5B). In mesenchymal cells of the soft palate, WNT/β-catenin signaling has been proposed to facilitate cilium disassembly and proliferation during myogenesis [237]. The WNT signaling is here likely accompanied by the HH pathway activation, which is essential for the proper myogenic differentiation as well [208, 210]. WNT and HH pathways are vital for driving the osteo-lineage commitment of mesenchymal stem cells (MSCs) during bone growth and upon mechanical loading [238, 239], [240]. Loss of IFT20 skews the osteo-/adipo-commitment of MSCs toward the adipogenic lineage [239], emphasizing the critical role of functional cilia in this process while still leaving open the possibility for IFT20 extraciliary functions. In contrast, the commitment of MSCs to adipogenic lineage is suppressed by WNT and HH pathways and driven by the IGF-1R and FFAR4 signaling [241], [242]. The primary cilium here is present only on committed pre-adipocytes to secure the onset of the adipogenic program and then disappears in mature adipocytes [242, 243], [244]. WNT signaling in MSCs also regulates the production of chemokines, such as CXCL12, being essential to maintaining hematopoiesis in bone marrow [245]. BBS patients and mouse models exhibit altered blood parameters, with a notable decrease in CXCL12 production observed in the bone marrow of BBS mouse models [246]. This indicates that ciliary factors, IFT20, BBS4, and BBS18 in MSCs regulate both the intrinsic and extrinsic functions of MSCs in bone tissue. However, it remains unclear whether BBSome deficiency influences the production of extracellular vesicles in MSCs, as seen in cell lines [235], and how this, consequently, impacts bone marrow homeostasis.
TGFβ signaling
The TGFβ signaling pathway regulates a wide range of cellular processes, including cell proliferation and differentiation. Upon stimulation with the TGFβ ligand, the cilia localized receptors TGFβIR and TGFβIIR become internalized via the clathrin-mediated endocytosis [247], [248]. Ciliary pocket (CiPo) is considered prominently decisive in TGFβ receptor retrieval, hence acting upstream of the phosphorylation of key effector proteins, SMAD2/3, to promote their transport into the nucleus to drive gene expression (reviewed in greater detail in [249]). Noteworthy, the involvement of CiPo in clathrin-mediated endocytosis of TGFβ receptors in vertebrates is pursuant to the function of the analogous region, the flagellar pocket, which serves as the sole site of endocytosis in protists [39]. A defect in TGFβ signaling has been reported following the ablation of IFT88 and CEP128 (a component of SDAs), causing defects in dorsoventral patterning in early embryogenesis [247], [248]. Surprisingly, depletion of CEP128 impairs ciliary TGFβ signaling while leaving primary cilia largely intact (in fact, its depletion facilitates cilia formation and promotes their length), leaving the contributing mechanism expected to hamper receptor trafficking at CiPo to be found.
Similar to the WNT signaling pathway, the effects of TGFβ signaling on cilia dynamics are context- and cell type-dependent (Fig. 5C). While activation of the TGFβ signaling pathway triggers shortening of primary cilia in cultured chondrocytes [250], cilia of Xenopus left-right organizer and other tissues reveal reduced length following the blocking of TGFβ pathway [251], and cilia remain largely intact in human mesenchymal stem cells in response to deregulated TGFβ signaling [252]. Underlying mechanisms involve the regulation of gene expression of key regulators of cilia assembly. Specifically, TGFβ signaling suppresses IFT88 expression in chondrocytic cells, resulting in a reduction in both the number and length of cilia [250]. Conversely, TGFβ signaling in Xenopus is required to sustain the expression of TZ components, and hence cilia maintenance [251].
Aberrant TGFβ signaling is linked to congenital heart diseases and skeletal anomalies, which are common features of ciliopathies and has been recently associated with Alstrom syndrome [223], [224], [225]. In mesenchymal stem cells, TGFβ signaling drives the differentiation of myogenic, chondrogenic, and adipogenic lineages [239], [253], [254]. Loss of IFT20 decreases TGFβ driven SMAD2/3 phosphorylation, impairs glucose metabolic homeostasis, and promotes adipolineage commitment of the bone marrow MSCs [239]. TGFβ signaling is critical for bone regeneration during fracture healing by regulating the expression of angiogenic and chondrogenic genes in chondrocytes and recruitment of the bone marrow MSCs to the site of injury [252], [253].
Decrease in primary cilia frequency or length upon loss of IFTs or by upregulation of cilia disassembly factors can be also linked to altered TGFβ-mediated extracellular matrix protein expression, which is a common prerequisite of fibrosis and cancer in many tissues, such as atrial fibrosis [254], lung pulmonary fibrosis [255], polycystic liver disease [256], liver fibrosis [257], cholangiocarcinoma [258], or colon cancer [259].
NOTCH signaling
NOTCH signaling is a developmental pathway controlling intercellular communication during tissue morphogenesis [260], its correct regulation relying on functional basal body/cilium, for instance, in developing skin [261] or in liver [262]. NOTCH signaling is transduced by the interaction of two cells, where one cell provides one of the DELTA-like (DLL1, DLL3, and DLL4) or JAGGED ligands (JAGGED1, JAGGED2) and the second one the receptors (NOTCH 1–4). Ligand–receptor interaction triggers γ-secretase-dependent cleavage of the NOTCH intracellular domain (NICD), which then translocates from the cytoplasm to the nucleus, where it promotes expression of key transcription factors Hes and Hey regulating the expression of downstream genes [263]. NOTCH pathway components, including the NOTCH receptor, were detected at the basal body and/or ciliary axoneme [163, 264]. The spatial organization of NOTCH signaling is regulated by the polarized trafficking module involving ARF4-RAB11 GTPases [264], [265]. Once at the membrane, the NOTCH receptor is continuously recycled through the endomembrane system back to the surface to maintain signaling capacity. Loss of BBS1, BBS4, or ALMS results in overactivation of the NOTCH pathway, likely owing to NOTCH retention in sorting endosomes [163].
Cilia-deficient keratinocytes in vitro and in mice embryonic epidermis have defects in NOTCH signaling [261]. Similarly, cKO of IFT88 leads to an increased proliferation yet decreased NOTCH signaling in corneal epithelial cells [266]. Furthermore, ablation of cilia-localized PKD2 in the liver induces increased NOTCH signaling and formation of cysts [262]. The primary cilium is also needed for hematogenic endothelial cell (HE) differentiation through the regulation of NOTCH signaling. HE cells give rise to hematopoietic stem and progenitor cells (HSPC). Reduced expression of HSPC markers, RUNX1 and CYMB, was observed in cilia-impaired zebrafish embryos due to reduced protein levels of NICD [267].
Studies examining the relationship between cilia dynamics and NOTCH signaling primarily focus on fluid flow-induced changes in cilia length and signal transduction, revealing an apparent interdependence between these two processes (Fig. 5D). Hyper-activation of the NOTCH pathway by overexpressing the NCID or DELTA-like ligand results in prolongation of cilia in the left-right organizer in zebrafish embryo. Here, the fluid flow-induced change in the cilium length triggered NOTCH signaling for transcriptional activation of laterality genes [268]. Similarly, in the endocardium, the mechanical shear stress signals via cilia activate the NOTCH pathway and regulate regeneration in zebrafish hearts [269]. During airway differentiation, NOTCH activity regulates levels of PROM1, a stem cell marker, to establish multiciliated cell diversity in the respiratory tract and modulate mucociliary clearance [270]. High NOTCH and PROM1 levels produce multiciliated cells with fast-beating cilia, whereas low levels result in cells with longer, low-beating cilia. It will be interesting to investigate whether increased mucociliary shear stress, induced e.g. by infections, increases cilia beating and further NOTCH activation similar to the shear stress in the endocardium or the fluid flow in the LRO [268], [269].
While not covered in this review, additional regulatory signaling circuits are involved in ciliogenesis control, including the tyrosine kinase signaling receptor, and HIPPO -YAP/TAZ pathway. Several excellent reviews concerning ciliogenesis control circuits may be referenced here [249], [271].
Managing cilia length and function: therapeutic strategies for converging mechanisms
Disruptions in ciliary dynamics are implicated in both ciliopathies and cancer progression, underscoring the therapeutic potential of targeting cilium-related processes. On one hand, strategies aimed at restoring cilium length and function show considerable promise in reducing cancer cell division and tumor growth [255], [258]. On the other hand, stabilization of the primary cilium helps cancer cells adopt a quiescent, stem-like state, which shields them from treatments based on kinase inhibitors [272]. In this part, we highlight small-molecule compounds that effectively modulate specific pathological mechanisms, including the regulation (both upregulation and downregulation) of the Hedgehog signaling pathway and the assembly and disassembly of primary cilia - processes closely associated with cancer development. Some of these inhibitors are already approved for specific conditions, and their use, either alone or in combination, holds the potential for addressing a broader range of cilia-associated disorders.
Targeting the HH signaling pathway
A key characteristic of cilium dysfunction is the disruption of signaling pathways, such as HH, which is commonly observed in conditions like Joubert syndrome, polycystic kidney disease, and Bardet-Biedl syndrome [5]. SMO inhibitors, such as cyclopamine or purmorphamine, have demonstrated potential in regulating excessive HH signaling caused by shortened or malformed cilia. This has been observed in conditions like Parkinson`s disease [218], in nephronophthisis associated with CEP290 mutations in Joubert syndrome [273], [274], and abnormal brain ventralization linked to dysfunctional INPP5E [217].
Increased HH signaling is also linked to the development of basal cell carcinoma and medulloblastoma [275], [276]. Here, the tumor growth can be driven by hyperactivated SMO in the presence of primary cilium and also by active GLI2, independent of the primary cilium. This indicates that the role of primary cilium is determined by the specific oncogene driving HH-triggered cancers, emphasizing the need for accurate diagnostics to select the most effective compounds or HH inhibitors to target pathogenic HH signaling [275], [276], [277]. In the case of brain tumors dependent on cilia, employment of TTBK1/2 inhibitors could represent a promising strategy to mitigate cilia-dependent HH upregulation [278], [279].
Activation of HH signaling plays a critical role in osteoarthritis [280] and can be suppressed by LiCl-mediated cilia prolongation in chondrocytes [281]. Similarly, LiCl treatment inhibits HH signaling in pancreatic ductal carcinoma cells leading to suppression of their tumorigenic phenotype [282]. Since LiCl is widely used for the treatment of people with bipolar disorders, it remains to be elucidated whether LiCl modulates the neuronal ciliary signaling pathway involving GPCRs, such as the dopamine receptor. Disrupted ciliary signaling, especially the HH pathway, could therefore serve as an ideal therapeutic target for ciliopathies, neurodegenerative disorders, and cancers driven by its dysfunction or exploitation.
Targeting the cilia disassembly factors HDAC6 and AURA
As previously noted, the mechanisms of the AURA/HDAC6 module in regulation cilia dynamics leave several questions unanswered. Importantly, however, strategies based on small molecules targeting those regulators seem to restore cilia homeostasis in several cilia dysfunction-related pathologies. In conditions like BBS and renal anomalies, including polycystic kidney disease and nephronophthisis, defects in cilia dynamics were mitigated using HDAC6 inhibitors [283], [284], such as tubacin [127], ACY-1215 [285] or tubastatin [286]. Similarly, in Rett syndrome, mutations in the MECP2 gene lead to microtubule instability, and tubacin treatment restored impaired HH signaling [287].
Another phenotype associated with ciliopathies like Bardet-Biedl, Alstrom, and MORM syndromes, is obesity [8]. Obese adipose-derived MSCs possess dysfunctional cilia with compromised HH signaling [288]. Notably, treatment with HDAC6 or AURA inhibitors, such as MLN8054, restored cilium length and expression of genes associated with osteogenic and adipogenic lineage commitment in these cells [244], [288]. This is in agreement with the view that primary cilia maintain stem cells in a quiescent state while preserving their capacity to differentiate, as noted earlier [208], [210], [239], [244].
HDAC6 inhibitors are also effective in cancer therapies, as many cancer cells rely on deregulated cilia dynamics for rapid cell division. In cholangiocytes, overexpression of HDAC6 drives increased cell proliferation, contributing to cystogenesis and tumor progression [258]. Thus, by stabilizing cilia and disrupting the timing of ciliary disassembly needed for mitotic entry, these inhibitors impair cancer cell proliferation. Furthermore, combining HDAC6 or AURA inhibitors with inhibitors of other pathways overactive in cancer cells, such as WNT or GFR (growth factor receptor) signaling [255], can more effectively halt cancer progression and potentially overcome resistance to single-pathway inhibitors.
Altogether, numerous studies underscore the importance of stabilizing cilia not only to address ciliopathies and cancer but also to preserve the regenerative potential of tissues. This highlights the therapeutic promise of HDAC6 and AURA inhibitors, which modulate ciliary dynamics and help restore normal cellular signaling in these conditions. An advantage of HDAC6 inhibitors is they are non-toxic to healthy tissues, and HDAC6-deficient mice do not exhibit any pathological phenotype under normal conditions [116]. However, mutations in HDAC6 lead to an increase in axoneme glycylation [117], suggesting that HDAC6 inhibitors may similarly activate compensatory mechanisms to preserve axoneme stability. HDAC6 is part of a large family, and certain HDAC6-selective inhibitors lack specificity, potentially causing off-target effects. Furthermore, HDACs are involved in various cellular processes, and off-target effects could lead to unexpected adverse phenotypes. Further research is therefore necessary to design more selective and effective HDAC6 inhibitors.
Therapeutical potential in compensatory mechanisms of ciliary modules
Understanding the mechanisms by which ciliary modules regulate cilia dynamics opens new avenues for developing new strategies to restore cilia function in ciliopathies. In BBS, cilia shortening is promoted by increased F-actin polymerization [125], [289] and elevated PIP2 within cilia [289]. Accumulation of PIP2 and F-actin in cilia phenocopies the loss of function INPP5E phenotype in Joubert syndrome [123]. Here, overexpressing INPP5E in olfactory neurons lacking BBSome restored cilia length and rescued odor detection and perception in BBS mice [289]. Another strategy to restore cilia length in BBS could involve suppressing the overactivated ciliary CDC42, thereby reducing excessive intraciliary actin polymerization and preventing ectocytosis to stabilize and elongate cilia [125]. One might potentially aim to target cargo import regulation [97], [144] to reduce the accumulation of GPCRs (SMO, GPR161, etc.) in cilia of BBSome-deficient cells, mitigating ectocytosis and overall cilia destabilization.
Challenges and future directions
Addressing shared mechanisms offers considerable potential for developing therapies applicable across multiple disorders, creating a framework for targeted and tailored treatment strategies. Combining inhibitors of ciliary disassembly, such as HDAC6 or AURA inhibitors, with approaches to modulate the activity of INPP5E, CDC42, or cargo import, could synergistically enhance cilia stabilization. Reducing actin polymerization might, for example, improve the effectiveness of disassembly inhibitors by mitigating another destabilizing force within cilia. By focusing on both structural (axonemal and F-actin dynamics) and functional (cargo trafficking and signaling) aspects of ciliary maintenance, these approaches offer a dual mechanism to restore cilia length and function, particularly in diseases where multiple pathways are disrupted.
Acknowledgements
We acknowledge BioRender.com for the creation of all figures included within this review.
Author contributions
LC and MH conceptualized and wrote the review. SS and OI designed and prepared the figures and table. All authors read and approved the final manuscript.
Funding
LC and MH acknowledge support from the Czech Science Foundation (grant 21–21612 S).
Data availability
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing Interests.
The authors declare that they have no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Lukáš Čajánek, Email: cajanek@med.muni.cz.
Martina Huranova, Email: martina.huranova@img.cas.cz.
References
- 1.Beales P, Jackson PK. Cilia - the prodigal organelle. Cilia. 2012;1:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sorokin SP. Reconstructions of centriole formation and ciliogenesis in mammalian lungs. J Cell Sci. 1968;3:207–30. [DOI] [PubMed] [Google Scholar]
- 3.Gopalakrishnan J, et al. Emerging principles of primary cilia dynamics in controlling tissue organization and function. EMBO J. 2023;42:e113891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bangs F, Anderson KV. Primary cilia and mammalian Hedgehog signaling. Cold Spring Harb Perspect Biol. 2017. 10.1101/cshperspect.a028175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mill P, Christensen ST, Pedersen LB. Primary cilia as dynamic and diverse signalling hubs in development and disease. Nat Rev Genet. 2023;24:421–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braun DA, Hildebrandt F, Ciliopathies. Cold Spring Harb Perspect Biol. 2017. 10.1101/cshperspect.a028191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitchison HM, Valente EM. Motile and non-motile cilia in human pathology: from function to phenotypes. J Pathol. 2017;241:294–309. [DOI] [PubMed] [Google Scholar]
- 8.Reiter JF, Leroux MR. Genes and molecular pathways underpinning ciliopathies. Nat Rev Mol Cell Biol. 2017. 10.1038/nrm.2017.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seeley ES, Nachury MV. The perennial organelle: assembly and disassembly of the primary cilium. J Cell Sci. 2010;123:511–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bornens M, Gonczy P. Centrosomes back in the limelight. Philos Trans R Soc Lond B Biol Sci. 2014. 10.1098/rstb.2013.0452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nigg EA, Holland AJ. Once and only once: mechanisms of centriole duplication and their deregulation in disease. Nat Rev Mol Cell Biol. 2018;19:297–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishikawa H, Kubo A, Tsukita S. Odf2-deficient mother centrioles lack distal/subdistal appendages and the ability to generate primary cilia. Nat Cell Biol. 2005;7:517–24. [DOI] [PubMed] [Google Scholar]
- 13.Balestra FR, Strnad P, Fluckiger I, Gonczy P. Discovering regulators of centriole biogenesis through siRNA-based functional genomics in human cells. Dev Cell. 2013;25:555–71. [DOI] [PubMed] [Google Scholar]
- 14.Bowler M, et al. High-resolution characterization of centriole distal appendage morphology and dynamics by correlative STORM and electron microscopy. Nat Commun. 2019;10:993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanos BE, et al. Centriole distal appendages promote membrane docking, leading to cilia initiation. Genes Dev. 2013;27:163–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang T-JB, Hsu JC-C, Yang TT. Single-molecule localization microscopy reveals the ultrastructural constitution of distal appendages in expanded mammalian centrioles. Nat Commun. 2023;14:1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang TT, et al. Super-resolution architecture of mammalian centriole distal appendages reveals distinct blade and matrix functional components. Nat Commun. 2018;9:2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei Q, et al. Transition fibre protein FBF1 is required for the ciliary entry of assembled intraflagellar transport complexes. Nat Commun. 2013;4:2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Airik R, Airik M, Schueler M, Bates CM, Hildebrandt F. Roscovitine blocks collecting duct cyst growth in Cep164-deficient kidneys. Kidney Int. 2019;96:320–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma D, Wang F, Teng J, Huang N, Chen J. Structure and function of distal and subdistal appendages of the mother centriole. J Cell Sci. 2023;136:jcs260560. [DOI] [PubMed] [Google Scholar]
- 21.Mazo G, Soplop N, Wang WJ, Uryu K, Tsou MB. Spatial control of primary ciliogenesis by subdistal appendages alters Sensation-Associated properties of cilia. Dev Cell. 2016. 10.1016/j.devcel.2016.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chong WM, et al. Super-resolution microscopy reveals coupling between mammalian centriole subdistal appendages and distal appendages. eLife. 2020;9:e53580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brooks ER, Wallingford JB. Multiciliated cells. Curr Biol CB. 2014;24:R973–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilula NB, Satir P. The ciliary necklace. A ciliary membrane specialization. J Cell Biol. 1972;53:494–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia-Gonzalo FR, Reiter JF. Open Sesame: how transition fibers and the transition zone control ciliary composition. Cold Spring Harb Perspect Biol. 2017;9:a028134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mercey O, Mukherjee S, Guichard P, Hamel V. The molecular architecture of the ciliary transition zones. Curr Opin Cell Biol. 2024;88:102361. [DOI] [PubMed] [Google Scholar]
- 27.Yang T. Superresolution pattern recognition reveals the architectural map of the ciliary transition zone. Sci Rep. 2015;5:14096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi X, et al. Super-resolution microscopy reveals that disruption of ciliary transition-zone architecture causes Joubert syndrome. Nat Cell Biol. 2017;19:1178–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nachury MV, Mick DU. Establishing and regulating the composition of cilia for signal transduction. Nat Rev Mol Cell Biol. 2019;20:389–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta GD, et al. A dynamic protein interaction landscape of the human centrosome-cilium interface. Cell. 2015;163:1484–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diener DR, Lupetti P, Rosenbaum JL. Proteomic analysis of isolated ciliary transition zones reveals the presence of ESCRT proteins. Curr Biol CB. 2015;25:379–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sang L, et al. Mapping the NPHP-JBTS-MKS protein network reveals ciliopathy disease genes and pathways. Cell. 2011;145:513–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiegering A, et al. Cell type-specific regulation of ciliary transition zone assembly in vertebrates. EMBO J. 2018;37:e97791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jenkins PM, McEwen DP, Martens JR. Olfactory cilia: linking sensory cilia function and human disease. Chem Senses. 2009;34:451–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cho JH, et al. Islet primary cilia motility controls insulin secretion. Sci Adv. 2022;8:eabq8486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun S, Fisher RL, Bowser SS, Pentecost BT, Sui H. Three-dimensional architecture of epithelial primary cilia. Proc. Natl. Acad. Sci. U. S. A. 2019;116:9370–9379. [DOI] [PMC free article] [PubMed]
- 37.Kiesel P, et al. The molecular structure of mammalian primary cilia revealed by cryo-electron tomography. Nat Struct Mol Biol. 2020;27:1115–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sorokin S. Centrioles and the formation of rudimentary cilia by fibroblasts and smooth muscle cells. J Cell Biol. 1962;15:363–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Molla-Herman A, et al. The ciliary pocket: an endocytic membrane domain at the base of primary and motile cilia. J Cell Sci. 2010;123:1785–95. [DOI] [PubMed] [Google Scholar]
- 40.Garcia G, Raleigh DR, Reiter JF. How the ciliary membrane is organized Inside-Out to communicate Outside-In. Curr Biol. 2018;28:R421–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Conduit SE, Davies EM, Fulcher AJ, Oorschot V, Mitchell CA. Superresolution microscopy reveals distinct phosphoinositide subdomains within the cilia transition zone. Front Cell Dev Biol. 2021;9:634649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garcia-Gonzalo FR, et al. Phosphoinositides regulate ciliary protein trafficking to modulate Hedgehog signaling. Dev Cell. 2015;34:400–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Conduit SE, Vanhaesebroeck B. Phosphoinositide lipids in primary cilia biology. Biochem J. 2020;477:3541–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hilgendorf KI, Myers BR, Reiter JF. Emerging mechanistic understanding of cilia function in cellular signalling. Nat Rev Mol Cell Biol. 2024;25:555–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Delling M, DeCaen PG, Doerner JF, Febvay S, Clapham DE. Primary cilia are specialized calcium signaling organelles. Nature. 2013;504:311–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paolocci E, Zaccolo M. Compartmentalised cAMP signalling in the primary cilium. Front Physiol. 2023. 10.3389/fphys.2023.1187134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nachury MV. How do cilia organize signalling cascades? Philos Trans R Soc B Biol Sci. 2014;369:20130465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Binó L, et al. A protocol for generation and live-cell imaging analysis of primary cilia reporter cell lines. STAR Protoc. 2022;3:101199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tucker RW, Pardee AB, Fujiwara K. Centriole ciliation is related to quiescence and DNA synthesis in 3T3 cells. Cell. 1979;17:527–35. [DOI] [PubMed] [Google Scholar]
- 50.Izawa I, Goto H, Kasahara K, Inagaki M. Current topics of functional links between primary cilia and cell cycle. Cilia. 2015. 10.1186/s13630-015-0021-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ford MJ, et al. A cell/cilia cycle biosensor for single-cell kinetics reveals persistence of cilia after G1/S transition is a general property in cells and mice. Dev Cell. 2018;47:509–e5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bangs FK, Schrode N, Hadjantonakis A-K, Anderson K. V. Lineage specificity of primary cilia in the mouse embryo. Nat Cell Biol. 2015;17:113–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sotelo JR. Trujillo-Cenóz, O. Electron microscope study on the development of ciliary components of the neural epithelium of the chick embryo. Z Für Zellforsch Mikrosk Anat. 1958;49:1–12. [DOI] [PubMed] [Google Scholar]
- 54.Kumar D, Reiter J. How the centriole builds its cilium: of mothers, daughters, and the acquisition of appendages. Curr Opin Struct Biol. 2021;66:41–8. [DOI] [PubMed] [Google Scholar]
- 55.Wang L, Dynlacht BD. The regulation of cilium assembly and disassembly in development and disease. Development. 2018. 10.1242/dev.151407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Labat-de-Hoz L, et al. A model for primary cilium biogenesis by polarized epithelial cells: role of the midbody remnant and associated specialized membranes. Front Cell Dev Biol. 2020;8:622918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu C-T, Chen H-Y, Tang TK. Myosin-Va is required for preciliary vesicle transportation to the mother centriole during ciliogenesis. Nat Cell Biol. 2018;20:175–85. [DOI] [PubMed] [Google Scholar]
- 58.Lu Q, et al. Early steps in primary cilium assembly require EHD1/EHD3-dependent ciliary vesicle formation. Nat Cell Biol. 2015. 10.1038/ncb310926612575 [Google Scholar]
- 59.Ganga AK, et al. Rab34 GTPase mediates ciliary membrane formation in the intracellular ciliogenesis pathway. Curr Biol. 2021;31:2895–e29057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stuck MW, Chong WM, Liao J-C, Pazour GJ. Rab34 is necessary for early stages of intracellular ciliogenesis. Curr Biol CB. 2021;31:2887–e28944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yoshimura S-I, Egerer J, Fuchs E, Haas AK, Barr FA. Functional dissection of Rab GTPases involved in primary cilium formation. J Cell Biol. 2007;178:363–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Westlake CJ, et al. Primary cilia membrane assembly is initiated by Rab11 and transport protein particle II (TRAPPII) complex-dependent trafficking of Rabin8 to the centrosome. Proc Natl Acad Sci U S A. 2011;108:2759–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Knödler A, et al. Coordination of Rab8 and Rab11 in primary ciliogenesis. Proc Natl Acad Sci U S A. 2010;107:6346–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nachury MV, et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129:1201–13. [DOI] [PubMed] [Google Scholar]
- 65.Shakya S, Westlake CJ. Recent advances in understanding assembly of the primary cilium membrane. Fac Rev. 2021;10:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schmidt KN, et al. Cep164 mediates vesicular docking to the mother centriole during early steps of ciliogenesis. J Cell Biol. 2012;199:1083–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Siller SS, et al. Conditional knockout mice for the distal appendage protein CEP164 reveal its essential roles in airway multiciliated cell differentiation. PLOS Genet. 2017;13:e1007128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Joo K, et al. CCDC41 is required for ciliary vesicle docking to the mother centriole. Proc Natl Acad Sci U S A. 2013;110:5987–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sillibourne JE, et al. Primary ciliogenesis requires the distal appendage component Cep123. Biol Open. 2013;2:535–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kurtulmus B, et al. LRRC45 contributes to early steps of axoneme extension. J Cell Sci. 2018;131:jcs223594. [DOI] [PubMed] [Google Scholar]
- 71.Kanie T, et al. A hierarchical pathway for assembly of the distal appendages that organize primary cilia. eLife. 2025;14:e85999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goetz SC, Liem KF Jr., Anderson K. V. The spinocerebellar ataxia-associated gene Tau tubulin kinase 2 controls the initiation of ciliogenesis. Cell. 2012;151:847–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cajanek L, Nigg EA. Cep164 triggers ciliogenesis by recruiting Tau tubulin kinase 2 to the mother centriole. Proc Natl Acad Sci U S A. 2014;111:E2841–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rosa E, Silva I, et al. Molecular mechanisms underlying the role of the centriolar CEP164-TTBK2 complex in ciliopathies. Struct Lond Engl 1993. 2022;30:114–e1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lo C-H, et al. Phosphorylation of CEP83 by TTBK2 is necessary for cilia initiation. J Cell Biol. 2019;218:3489–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bernatik O, et al. Phosphorylation of multiple proteins involved in ciliogenesis by Tau tubulin kinase 2. Mol Biol Cell. 2020;31:1032–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Spektor A, Tsang WY, Khoo D, Dynlacht BD. Cep97 and CP110 suppress a cilia assembly program. Cell. 2007;130:678–90. [DOI] [PubMed] [Google Scholar]
- 78.Kobayashi T, Tsang WY, Li J, Lane W, Dynlacht B. D. Centriolar Kinesin Kif24 interacts with CP110 to remodel microtubules and regulate ciliogenesis. Cell. 2011;145:914–25. [DOI] [PubMed] [Google Scholar]
- 79.Benk Vysloužil D, et al. Tau-tubulin kinase 2 restrains microtubule-depolymerizer KIF2A to support primary cilia growth. Cell Commun Signal. 2025;23:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Oda T, Chiba S, Nagai T, Mizuno K. Binding to Cep164, but not EB1, is essential for centriolar localization of TTBK2 and its function in ciliogenesis. Genes Cells. 2014;19:927–40. [DOI] [PubMed] [Google Scholar]
- 81.Huang N, et al. M-Phase phosphoprotein 9 regulates ciliogenesis by modulating CP110-CEP97 complex localization at the mother centriole. Nat Commun. 2018;9:4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schmidt TI, et al. Control of centriole length by CPAP and CP110. Curr Biol CB. 2009;19:1005–11. [DOI] [PubMed] [Google Scholar]
- 83.Prosser SL, Morrison CG. Centrin2 regulates CP110 removal in primary cilium formation. J Cell Biol. 2015;208:693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dobbelaere J, Schmidt Cernohorska M, Huranova M, Slade D, Dammermann A. Cep97 is required for centriole structural integrity and cilia formation in Drosophila. Curr Biol CB. 2020;30:3045–e30567. [DOI] [PubMed] [Google Scholar]
- 85.Yadav SP, et al. Centrosomal protein CP110 controls maturation of the mother centriole during cilia biogenesis. Dev Camb Engl. 2016;143:1491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Walentek P et al. Ciliary transcription factors and miRNAs precisely regulate Cp110 levels required for ciliary adhesions and ciliogenesis. eLife 2016;5:e17557. [DOI] [PMC free article] [PubMed]
- 87.Pedersen LB, Rosenbaum JL. Intraflagellar transport (IFT) role in ciliary assembly, resorption and signalling. Curr Top Dev Biol. 2008;85:23–61. [DOI] [PubMed]
- 88.Kozminski KG, Johnson KA, Forscher P, Rosenbaum JL. A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc Natl Acad Sci U S A. 1993;90:5519–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bhogaraju S, et al. Molecular basis of tubulin transport within the cilium by IFT74 and IFT81. Science. 2013;341:1009–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lechtreck K. Cargo adapters expand the transport range of intraflagellar transport. J Cell Sci. 2022;135:jcs260408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pigino G. Intraflagellar transport. Curr Biol CB. 2021;31:R530–6. [DOI] [PubMed] [Google Scholar]
- 92.Stepanek L, Pigino G. Microtubule doublets are double-track railways for intraflagellar transport trains. Science. 2016;352:721–4. [DOI] [PubMed] [Google Scholar]
- 93.Yang TT, et al. Superresolution pattern recognition reveals the architectural map of the ciliary transition zone. Sci Rep. 2015;5:14096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yang TT, Tran MNT, Chong WM, Huang C-E, Liao J-C. Single-particle tracking localization microscopy reveals nonaxonemal dynamics of intraflagellar transport proteins at the base of mammalian primary cilia. Mol Biol Cell. 2019;30:828–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.van den Hoek H, et al. In situ architecture of the ciliary base reveals the stepwise assembly of intraflagellar transport trains. Science. 2022;377:543–8. [DOI] [PubMed] [Google Scholar]
- 96.Van De Craft J, Harris JA, Kubo T, Witman GB, Lechtreck KF. Diffusion rather than intraflagellar transport likely provides most of the tubulin required for axonemal assembly in Chlamydomonas. J Cell Sci. 2020;133:jcs249805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mukhopadhyay S, et al. TULP3 bridges the IFT-A complex and membrane phosphoinositides to promote trafficking of G protein-coupled receptors into primary cilia. Genes Dev. 2010;24:2180–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nachury MV. The molecular machines that traffic signaling receptors into and out of cilia. Curr Opin Cell Biol. 2018;51:124–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Han S, et al. TULP3 is required for localization of membrane-associated proteins ARL13B and INPP5E to primary cilia. Biochem Biophys Res Commun. 2019;509:227–34. [DOI] [PubMed] [Google Scholar]
- 100.Ansley SJ, et al. Basal body dysfunction is a likely cause of pleiotropic Bardet-Biedl syndrome. Nature. 2003;425:628–33. [DOI] [PubMed] [Google Scholar]
- 101.Niederlova V, Modrak M, Tsyklauri O, Huranova M, Stepanek O. Meta-analysis of genotype-phenotype associations in Bardet-Biedl syndrome uncovers differences among causative genes. Hum Mutat. 2019;40:2068–87. [DOI] [PubMed] [Google Scholar]
- 102.Prasai A, et al. The BBSome assembly is spatially controlled by BBS1 and BBS4 in human cells. J Biol Chem. 2020;295:14279–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang Q, Yu D, Seo S, Stone EM, Sheffield VC. Intrinsic protein-Protein Interaction-mediated and Chaperonin-assisted sequential assembly of stable Bardet-Biedl syndrome protein complex, the BBSome. J Biol Chem. 2012;287:20625–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Blacque OE, et al. Loss of C. elegans BBS-7 and BBS-8 protein function results in cilia defects and compromised intraflagellar transport. Genes Dev. 2004;18:1630–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ye F, Nager AR, Nachury MV. BBSome trains remove activated GPCRs from cilia by enabling passage through the transition zone. J Cell Biol. 2018;217:1847–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yan H, et al. TALPID3 and ANKRD26 selectively orchestrate FBF1 localization and cilia gating. Nat Commun. 2020;11:2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nigg EA, Stearns T. The centrosome cycle: centriole biogenesis, duplication and inherent asymmetries. Nat Cell Biol. 2011;13:1154–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mirvis M, Siemers KA, Nelson WJ, Stearns TP. Primary cilium loss in mammalian cells occurs predominantly by whole-cilium shedding. PLOS Biol. 2019;17:e3000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rieder CL, Jensen CG, Jensen LCW. The resorption of primary cilia during mitosis in a vertebrate (PtK1) cell line. J Ultrastruct Res. 1979;68:173–85. [DOI] [PubMed] [Google Scholar]
- 110.Liang Y, Meng D, Zhu B, Pan J. Mechanism of ciliary disassembly. Cell Mol Life Sci. 2016;73:1787–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Quarmby LM. Cellular deflagellation. Int Rev Cytol. 2004;233:47–91. [DOI] [PubMed] [Google Scholar]
- 112.Toro-Tapia G, Das RM. Primary cilium remodeling mediates a cell signaling switch in differentiating neurons. Sci Adv. 2020;6:eabb0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pugacheva EN, Jablonski SA, Hartman TR, Henske EP, Golemis E. A. HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell. 2007;129:1351–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pan J, Wang Q, Snell WJ. An aurora kinase is essential for flagellar disassembly in Chlamydomonas. Dev Cell. 2004;6:445–51. [DOI] [PubMed] [Google Scholar]
- 115.Kobayashi T, et al. HDAC2 promotes loss of primary cilia in pancreatic ductal adenocarcinoma. EMBO Rep. 2017;18:334–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang Y, et al. Mice lacking histone deacetylase 6 have hyperacetylated tubulin but are viable and develop normally. Mol Cell Biol. 2008;28:1688–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Łysyganicz PK, et al. Loss of deacetylation enzymes Hdac6 and Sirt2 promotes acetylation of cytoplasmic tubulin, but suppresses axonemal acetylation in zebrafish cilia. Front Cell Dev Biol. 2021;9:676214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.McKenna ED, Sarbanes SL, Cummings SW, Roll-Mecak A. The tubulin code, from molecules to health and disease. Annu Rev Cell Dev Biol. 2023;39:331–61. [DOI] [PubMed] [Google Scholar]
- 119.Reilly ML, Benmerah A. Ciliary kinesins beyond IFT: cilium length, disassembly, cargo transport and signaling. Biol Cell. 2019. 10.1111/boc.201800074 [DOI] [PubMed] [Google Scholar]
- 120.Miyamoto T, et al. The Microtubule-Depolymerizing activity of a mitotic Kinesin protein KIF2A drives primary cilia disassembly coupled with cell proliferation. Cell Rep. 2015. 10.1016/j.celrep.2015.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Watanabe T, et al. TTBK2 with EB1/3 regulates microtubule dynamics in migrating cells through KIF2A phosphorylation. J Cell Biol. 2015;210:737–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kim S, Lee K, Choi J-H, Ringstad N, Dynlacht BD. Nek2 activation of Kif24 ensures cilium disassembly during the cell cycle. Nat Commun. 2015;6:8087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Phua SC, et al. Dynamic remodeling of membrane composition drives cell cycle through primary cilia excision. Cell. 2017;168:264–e27915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nager AR, et al. An actin network dispatches ciliary GPCRs into extracellular vesicles to modulate signaling. Cell. 2017;168:252–e26314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Prasai A, et al. BBSome-deficient cells activate intraciliary CDC42 to trigger actin-dependent ciliary ectocytosis. EMBO Rep. 2024. 10.1038/s44319-024-00326-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Stilling S, Kalliakoudas T, Benninghoven-Frey H, Inoue T, Falkenburger BH. PIP2 determines length and stability of primary cilia by balancing membrane turnovers. Commun Biol. 2022;5:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Patnaik SR, et al. Bardet-Biedl syndrome proteins regulate cilia disassembly during tissue maturation. Cell Mol Life Sci CMLS. 2019;76:757–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Inoko A, et al. Trichoplein and Aurora A block aberrant primary cilia assembly in proliferating cells. J Cell Biol. 2012;197:391–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Huang M, et al. Cell cycle arrest induced by trichoplein depletion is independent of cilia assembly. J Cell Physiol. 2022;237:2703–12. [DOI] [PubMed] [Google Scholar]
- 130.Bryja V, Cervenka I, Cajanek L. The connections of Wnt pathway components with cell cycle and centrosome: side effects or a hidden logic? Crit Rev Biochem Mol Biol. 2017;52:614–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fry AM, O’Regan L, Sabir SR, Bayliss R. Cell cycle regulation by the NEK family of protein kinases. J Cell Sci. 2012;125:4423–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang W, Wu T, Kirschner MW. The master cell cycle regulator APC-Cdc20 regulates ciliary length and disassembly of the primary cilium. eLife. 2014;3:e03083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Viol L et al. Nek2 kinase displaces distal appendages from the mother centriole prior to mitosis. J Cell Biol. 2020. 10.1083/jcb.201907136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kong D, et al. Centriole maturation requires regulated Plk1 activity during two consecutive cell cycles. J Cell Biol. 2014;206:855–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bukanov NO, Smith LA, Klinger KW, Ledbetter SR. Ibraghimov-Beskrovnaya, O. Long-lasting arrest of murine polycystic kidney disease with CDK inhibitor roscovitine. Nature. 2006;444:949–52. [DOI] [PubMed] [Google Scholar]
- 136.Chebib FT, Sussman CR, Wang X, Harris PC, Torres VE. Vasopressin and disruption of calcium signalling in polycystic kidney disease. Nat Rev Nephrol. 2015;11:451–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tran PV, et al. THM1 negatively modulates mouse Sonic Hedgehog signal transduction and affects retrograde intraflagellar transport in cilia. Nat Genet. 2008;40:403–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tran PV, et al. Downregulating Hedgehog signaling reduces renal cystogenic potential of mouse models. J Am Soc Nephrol JASN. 2014;25:2201–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ou G, Scholey JM. Motor Cooperation during mitosis and ciliogenesis. Annu Rev Cell Dev Biol. 2022;38:49–74. [DOI] [PubMed] [Google Scholar]
- 140.Hirokawa N, Noda Y, Tanaka Y, Niwa S. Kinesin superfamily motor proteins and intracellular transport. Nat Rev Mol Cell Biol. 2009;10:682–96. [DOI] [PubMed] [Google Scholar]
- 141.Haraguchi K, Hayashi T, Jimbo T, Yamamoto T, Akiyama T. Role of the Kinesin-2 family protein, KIF3, during mitosis. J Biol Chem. 2006;281:4094–9. [DOI] [PubMed] [Google Scholar]
- 142.Gruneberg U, et al. KIF14 and Citron kinase act together to promote efficient cytokinesis. J Cell Biol. 2006;172:363–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Carleton M, et al. RNA interference-mediated Silencing of mitotic Kinesin KIF14 disrupts cell cycle progression and induces cytokinesis failure. Mol Cell Biol. 2006;26:3853–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pejskova P et al. KIF14 controls ciliogenesis via regulation of Aurora A and is important for Hedgehog signaling. J Cell Biol. 2020. 10.1083/jcb.201904107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mikulenkova E et al. Kinesin-3 KIF14 Regulates Intraflagellar Transport Dynamics in Primary Cilia. 2025;644298 Preprint at 10.1101/2025.03.20.644298 (2025).
- 146.Delaval B, Bright A, Lawson ND, Doxsey S. The cilia protein IFT88 is required for spindle orientation in mitosis. Nat Cell Biol. 2011;13:461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Taulet N, et al. IFT proteins spatially control the geometry of cleavage furrow ingression and lumen positioning. Nat Commun. 2017;8:1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Vitre B, et al. IFT proteins interact with HSET to promote supernumerary centrosome clustering in mitosis. EMBO Rep. 2020;21:e49234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wood CR, et al. IFT proteins accumulate during cell division and localize to the cleavage furrow in Chlamydomonas. PLoS ONE. 2012;7:e30729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Vuong LT, et al. Kinesin-2 and IFT-A act as a complex promoting nuclear localization of β-catenin during Wnt signalling. Nat Commun. 2018;9:5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Balmer S, et al. Components of intraflagellar transport complex A function independently of the cilium to regulate canonical Wnt signaling in Drosophila. Dev Cell. 2015;34:705–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kim JC, et al. The Bardet-Biedl protein BBS4 targets cargo to the pericentriolar region and is required for microtubule anchoring and cell cycle progression. Nat Genet. 2004;36:462–70. [DOI] [PubMed] [Google Scholar]
- 153.Kim JC, et al. MKKS/BBS6, a divergent chaperonin-like protein linked to the obesity disorder Bardet-Biedl syndrome, is a novel centrosomal component required for cytokinesis. J Cell Sci. 2005;118:1007–20. [DOI] [PubMed] [Google Scholar]
- 154.Wang W-J, et al. CEP162 is an axoneme-recognition protein promoting ciliary transition zone assembly at the cilia base. Nat Cell Biol. 2013;15:591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Leon A, Omri B, Gely A, Klein C, Crisanti P. QN1/KIAA1009: a new essential protein for chromosome segregation and mitotic spindle assembly. Oncogene. 2006;25:1887–95. [DOI] [PubMed] [Google Scholar]
- 156.Lovera M, Lüders J. The ciliary impact of nonciliary gene mutations. Trends Cell Biol. 2021;31:876–87. [DOI] [PubMed] [Google Scholar]
- 157.Wieser S, Pines J. The biochemistry of mitosis. Cold Spring Harb Perspect Biol. 2015. 10.1101/cshperspect.a015776 [DOI] [PMC free article] [PubMed]
- 158.Pichaud F, Walther RF, Nunes de Almeida F. Regulation of Cdc42 and its effectors in epithelial morphogenesis. J Cell Sci. 2019. 10.1242/jcs.217869 [DOI] [PubMed]
- 159.Hehnly H, Chen C-T, Powers CM, Liu H-L, Doxsey S. The centrosome regulates the Rab11- dependent recycling endosome pathway at appendages of the mother centriole. Curr Biol. 2012;22:1944–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ang AL, et al. Recycling endosomes can serve as intermediates during transport from the golgi to the plasma membrane of MDCK cells. J Cell Biol. 2004;167:531–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jin H, et al. The conserved Bardet-Biedl syndrome proteins assemble a coat that traffics membrane proteins to cilia. Cell. 2010;141:1208–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Guo D-F, et al. The BBSome in POMC and AgRP neurons is necessary for body weight regulation and sorting of metabolic receptors. Diabetes. 2019;68:1591–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Leitch CC, Lodh S, Prieto-Echagüe V, Badano JL. Zaghloul, N. A. Basal body proteins regulate Notch signaling through endosomal trafficking. J Cell Sci. 2014;127:2407–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Cantagrel V, et al. Mutations in the cilia gene ARL13B lead to the classical form of Joubert syndrome. Am J Hum Genet. 2008;83:170–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Caspary T, Larkins CE, Anderson KV. The graded response to Sonic Hedgehog depends on cilia architecture. Dev Cell. 2007;12:767–78. [DOI] [PubMed] [Google Scholar]
- 166.Larkins CE, Aviles GDG, East MP, Kahn RA, Caspary T. Arl13b regulates ciliogenesis and the dynamic localization of Shh signaling proteins. Mol Biol Cell. 2011;22:4694–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Humbert MC, et al. ARL13B, PDE6D, and CEP164 form a functional network for INPP5E ciliary targeting. Proc Natl Acad Sci U S A. 2012;109:19691–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gigante ED, Taylor MR, Ivanova AA, Kahn RA, Caspary T. ARL13B regulates Sonic Hedgehog signaling from outside primary cilia. eLife. 2020. 10.7554/eLife.50434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ferent J, et al. The ciliary protein Arl13b functions outside of the primary cilium in Shh-mediated axon guidance. Cell Rep. 2019;29:3356–e33663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Serwas D, Su TY, Roessler M, Wang S, Dammermann A. Centrioles initiate cilia assembly but are dispensable for maturation and maintenance in C. elegans. J Cell Biol. 2017;216:1659–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bowie E, Norris R, Anderson KV, Goetz SC. Spinocerebellar ataxia type 11-associated alleles of Ttbk2 dominantly interfere with ciliogenesis and cilium stability. PLoS Genet. 2018;14:e1007844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Nguyen A, Goetz SC. TTBK2 controls cilium stability by regulating distinct modules of centrosomal proteins. Mol Biol Cell. 2023;34:ar8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Engelke MF, et al. Acute Inhibition of heterotrimeric Kinesin-2 function reveals mechanisms of intraflagellar transport in mammalian cilia. Curr Biol. 2019;29:1137–e11484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ye F, et al. Single molecule imaging reveals a major role for diffusion in the exploration of ciliary space by signaling receptors. eLife. 2013;2:e00654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Jensen VL, et al. Role for intraflagellar transport in building a functional transition zone. EMBO Rep. 2018;19:e45862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Vuolo L, Stevenson NL, Heesom KJ, Stephens DJ. Dynein-2 intermediate chains play crucial but distinct roles in primary cilia formation and function. eLife. 2018;7:e39655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Absalon S, et al. Intraflagellar transport and functional analysis of genes required for flagellum formation in trypanosomes. Mol Biol Cell. 2008;19:929–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Scheidel N, Blacque OE. Intraflagellar transport complex A genes differentially regulate cilium formation and transition zone gating. Curr Biol CB. 2018;28:3279–e32872. [DOI] [PubMed] [Google Scholar]
- 179.De-Castro ARG, et al. WDR60-mediated dynein-2 loading into cilia powers retrograde IFT and transition zone crossing. J Cell Biol. 2021;221:e202010178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bujakowska KM, et al. Mutations in IFT172 cause isolated retinal degeneration and Bardet–Biedl syndrome. Hum Mol Genet. 2015;24:230–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Janke C, Magiera MM. The tubulin code and its role in controlling microtubule properties and functions. Nat Rev Mol Cell Biol. 2020;21:307–26. [DOI] [PubMed] [Google Scholar]
- 182.Verhey KJ, Gaertig J. The tubulin code. Cell Cycle. 2007;6:2152–60. [DOI] [PubMed] [Google Scholar]
- 183.Sirajuddin M, Rice LM, Vale RD. Regulation of microtubule motors by tubulin isotypes and post-translational modifications. Nat Cell Biol. 2014;16:335–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Viar GA, Pigino G. Tubulin posttranslational modifications through the lens of new technologies. Curr Opin Cell Biol. 2024;88:102362. [DOI] [PubMed] [Google Scholar]
- 185.Gadadhar S, et al. Tubulin glycylation controls axonemal dynein activity, flagellar beat, and male fertility. Science. 2021;371:eabd4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Suryavanshi S, et al. Tubulin glutamylation regulates ciliary motility by altering inner dynein arm activity. Curr Biol CB. 2010;20:435–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.O’Hagan R, et al. Glutamylation regulates transport, specializes function, and sculpts the structure of cilia. Curr Biol. 2017;27:3430–e34416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hong S-R, et al. Spatiotemporal manipulation of ciliary glutamylation reveals its roles in intraciliary trafficking and Hedgehog signaling. Nat Commun. 2018;9:1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kalebic N, et al. αTAT1 is the major α-tubulin acetyltransferase in mice. Nat Commun. 2013;4:1962. [DOI] [PubMed] [Google Scholar]
- 190.Verhey KJ, Ohi R. Causes, costs and consequences of kinesin motors communicating through the microtubule lattice. J Cell Sci. 2023;136:jcs260735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Mao S, et al. Motile cilia of human airway epithelia contain Hedgehog signaling components that mediate noncanonical Hedgehog signaling. Proc Natl Acad Sci U S A. 2018;115:1370–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Seidl C, et al. Mucociliary Wnt signaling promotes cilia biogenesis and beating. Nat Commun. 2023;14:1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Nonami Y, Narita K, Nakamura H, Inoue T, Takeda S. Developmental changes in ciliary motility on choroid plexus epithelial cells during the perinatal period. Cytoskeleton. 2013;70:797–803. [DOI] [PubMed] [Google Scholar]
- 194.Ott CM, et al. Permanent Deconstruction of intracellular primary cilia in differentiating granule cell neurons. J Cell Biol. 2024;223:e202404038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Rozycki M, et al. The fate of the primary cilium during myofibroblast transition. Mol Biol Cell. 2014;25:643–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Müller A, et al. Structure, interaction and nervous connectivity of beta cell primary cilia. Nat Commun. 2024;15:9168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ingham PW. Hedgehog signaling. Curr Top Dev Biol. 2022;149:1–58. [DOI] [PubMed] [Google Scholar]
- 198.Hall ET, et al. Cytoneme signaling provides essential contributions to mammalian tissue patterning. Cell. 2024;187:276–e29323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Mukhopadhyay S, et al. The ciliary G-protein-coupled receptor Gpr161 negatively regulates the Sonic Hedgehog pathway via cAMP signaling. Cell. 2013;152:210–23. [DOI] [PubMed] [Google Scholar]
- 200.Truong ME, et al. Vertebrate cells differentially interpret ciliary and extraciliary cAMP. Cell. 2021;184:2911–e292618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hwang S-H, Somatilaka BN, White K, Mukhopadhyay S. Ciliary and extraciliary Gpr161 pools repress Hedgehog signaling in a tissue-specific manner. eLife. 2021. 10.7554/eLife.67121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.May EA, et al. Time-resolved proteomics profiling of the ciliary Hedgehog response. J Cell Biol. 2021;220:e202007207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Pal K, et al. Smoothened determines β-arrestin-mediated removal of the G protein-coupled receptor Gpr161 from the primary cilium. J Cell Biol. 2016;212:861–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Yue S, et al. Requirement of Smurf-mediated endocytosis of Patched1 in Sonic Hedgehog signal reception. eLife. 2014;3:e02555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ansari SS, et al. Sonic Hedgehog activates prostaglandin signaling to stabilize primary cilium length. J Cell Biol. 2024;223:e202306002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ho EK, Tsai AE, Stearns T. Transient primary cilia mediate robust Hedgehog Pathway-Dependent cell cycle control. Curr Biol CB. 2020;30:2829–e28355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Min M, Rong Y, Tian C, Spencer SL. Temporal integration of mitogen history in mother cells controls proliferation of daughter cells. Science. 2020;368:1261–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Fu W, Asp P, Canter B, Dynlacht B D. Primary cilia control Hedgehog signaling during muscle differentiation and are deregulated in rhabdomyosarcoma. Proc Natl Acad Sci U S A. 2014;111:9151–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Straface G, et al. Sonic Hedgehog regulates angiogenesis and myogenesis during post-natal skeletal muscle regeneration. J Cell Mol Med. 2009;13:2424–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Palla AR, et al. Primary cilia on muscle stem cells are critical to maintain regenerative capacity and are lost during aging. Nat Commun. 2022;13:1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kopinke D, Roberson EC, Reiter JF. Ciliary Hedgehog signaling restricts Injury-Induced adipogenesis. Cell. 2017;170:340–e35112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Cruz C, et al. Foxj1 regulates floor plate cilia architecture and modifies the response of cells to Sonic Hedgehog signalling. Dev Camb Engl. 2010;137:4271–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Hagenlocher C, Walentek P, Ller M, Thumberger C, T., Feistel K. Ciliogenesis and cerebrospinal fluid flow in the developing Xenopus brain are regulated by foxj1. Cilia 2013;2:12. [DOI] [PMC free article] [PubMed]
- 214.Das RM, Storey KG. Apical abscission alters cell polarity and dismantles the primary cilium during neurogenesis. Science. 2014;343:200–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Zhang Q, Seo S, Bugge K, Stone EM, Sheffield V. C. BBS proteins interact genetically with the IFT pathway to influence SHH-related phenotypes. Hum Mol Genet. 2012;21:1945–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Bachmann VA et al. Gpr161 anchoring of PKA consolidates GPCR and cAMP signaling. Proc. Natl. Acad. Sci. U. S. A. 2016;113:7786–7791. [DOI] [PMC free article] [PubMed]
- 217.Schembs L, et al. The ciliary gene INPP5E confers dorsal telencephalic identity to human cortical organoids by negatively regulating Sonic Hedgehog signaling. Cell Rep. 2022;39:110811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Schmidt S, et al. Primary cilia and SHH signaling impairments in human and mouse models of Parkinson’s disease. Nat Commun. 2022;13:4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Nusse R, Clevers H. Wnt/β-Catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169:985–99. [DOI] [PubMed] [Google Scholar]
- 220.Wallingford JB, Mitchell B. Strange as it May Seem: the many links between Wnt signaling, planar cell polarity, and cilia. Genes Dev. 2011;25:201–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.May-Simera HL, Kelley MW, Cilia. Wnt signaling, and the cytoskeleton. Cilia. 2012;1:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Zhang K, et al. Primary cilia are WNT-transducing organelles whose biogenesis is controlled by a WNT-PP1 axis. Dev Cell. 2023;58:139–e1548. [DOI] [PubMed] [Google Scholar]
- 223.Gerdes JM, et al. Disruption of the basal body compromises proteasomal function and perturbs intracellular Wnt response. Nat Genet. 2007;39:1350–60. [DOI] [PubMed] [Google Scholar]
- 224.Simons M, et al. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat Genet. 2005;37:537–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Corbit KC, et al. Kif3a constrains β-catenin-dependent Wnt signalling through dual ciliary and non-ciliary mechanisms. Nat Cell Biol. 2008;10:70–6. [DOI] [PubMed] [Google Scholar]
- 226.Huang P, Schier AF. Dampened Hedgehog signaling but normal Wnt signaling in zebrafish without cilia. Development. 2009;136:3089–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Ocbina PJR, Tuson M, Anderson KV. Primary cilia are not required for normal canonical Wnt signaling in the mouse embryo. PLoS ONE. 2009. 10.1371/journal.pone.0006839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Sugiyama N, Tsukiyama T, Yamaguchi TP, Yokoyama T. The canonical Wnt signaling pathway is not Involved in renal cyst development in the kidneys of Inv mutant mice. Kidney Int. 2011;79:957–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.El-Brolosy MA, et al. Genetic compensation triggered by mutant mRNA degradation. Nature. 2019;568:193–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Walentek P, Beyer T, Thumberger T, Schweickert A, Blum M. ATP4a is required for Wnt-dependent Foxj1 expression and leftward flow in Xenopus Left-Right development. Cell Rep. 2012;1:516–27. [DOI] [PubMed] [Google Scholar]
- 231.Yu X, Ng CP, Habacher H, Roy S. Foxj1 transcription factors are master regulators of the motile ciliogenic program. Nat Genet. 2008;40:1445–53. [DOI] [PubMed] [Google Scholar]
- 232.Bernatik O, Paclikova P, Kotrbova A, Bryja V, Cajanek L. Primary cilia formation does not rely on WNT/β-Catenin signaling. Front Cell Dev Biol. 2021;9:623753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Freke GM, et al. De-Suppression of mesenchymal cell identities and variable phenotypic outcomes associated with knockout of Bbs1. Cells. 2023;12:2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Horwitz A, Levi-Carmel N, Shnaider O, Birk R. BBS genes are involved in accelerated proliferation and early differentiation of BBS-related tissues. Differ Res Biol Divers. 2024;135:100745. [DOI] [PubMed] [Google Scholar]
- 235.Volz A-K, et al. Bardet-Biedl syndrome proteins modulate the release of bioactive extracellular vesicles. Nat Commun. 2021;12:5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Bryja V, et al. The extracellular domain of Lrp5/6 inhibits noncanonical Wnt signaling in vivo. Mol Biol Cell. 2009;20:924–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Janečková E, et al. Canonical Wnt signaling regulates soft palate development by mediating ciliary homeostasis. Development. 2023;150:dev201189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Holmen SL, et al. Essential role of β-Catenin in postnatal bone acquisition. J Biol Chem. 2005;280:21162–8. [DOI] [PubMed] [Google Scholar]
- 239.Li Y, Yang S, Liu Y, Qin L, Yang S. IFT20 governs mesenchymal stem cell fate through positively regulating TGF-β-Smad2/3-Glut1 signaling mediated glucose metabolism. Redox Biol. 2022;54:102373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Johnson GP, Fair S, Hoey DA. Primary cilium-mediated MSC mechanotransduction is dependent on Gpr161 regulation of Hedgehog signalling. Bone. 2021;145:115846. [DOI] [PubMed] [Google Scholar]
- 241.Hilgendorf KI, et al. Omega-3 fatty acids activate ciliary FFAR4 to control adipogenesis. Cell. 2019;179:1289–e130521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Zhu D, Shi S, Wang H, Liao K. Growth arrest induces primary-cilium formation and sensitizes IGF-1-receptor signaling during differentiation induction of 3T3-L1 preadipocytes. J Cell Sci. 2009;122:2760–8. [DOI] [PubMed] [Google Scholar]
- 243.Marion V, et al. Transient ciliogenesis involving Bardet-Biedl syndrome proteins is a fundamental characteristic of adipogenic differentiation. Proc Natl Acad Sci U S A. 2009;106:1820–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Ritter A, et al. Restoration of primary cilia in obese adipose-derived mesenchymal stem cells by inhibiting Aurora A or extracellular signal-regulated kinase. Stem Cell Res Ther. 2019;10:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Tamura M, Sato MM, Nashimoto M. Regulation of CXCL12 expression by canonical Wnt signaling in bone marrow stromal cells. Int J Biochem Cell Biol. 2011;43:760–7. [DOI] [PubMed] [Google Scholar]
- 246.Tsyklauri O, et al. Bardet-Biedl syndrome ciliopathy is linked to altered hematopoiesis and dysregulated self-tolerance. EMBO Rep. 2021;22:e50785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Clement CA, et al. TGF-β signaling is associated with endocytosis at the pocket region of the primary cilium. Cell Rep. 2013;3:1806–14. [DOI] [PubMed] [Google Scholar]
- 248.Mönnich M, et al. CEP128 localizes to the subdistal appendages of the mother centriole and regulates TGF-β/BMP signaling at the primary cilium. Cell Rep. 2018;22:2584–92. [DOI] [PubMed] [Google Scholar]
- 249.Christensen ST, Morthorst SK, Mogensen JB, Pedersen LB. Primary cilia and coordination of receptor tyrosine kinase (RTK) and transforming growth factor Β (TGF-β) signaling. Cold Spring Harb Perspect Biol. 2017;9:a028167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Kawasaki M, et al. TGF-β suppresses Ift88 expression in chondrocytic ATDC5 cells: TGF-β REGULATES IFT88. J Cell Physiol. 2015;230:2788–95. [DOI] [PubMed] [Google Scholar]
- 251.Tözser J, et al. TGF-β signaling regulates the differentiation of motile cilia. Cell Rep. 2015;11:1000–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Labour M-N, Riffault M, Christensen ST, Hoey DA. TGFβ1 - induced recruitment of human bone mesenchymal stem cells is mediated by the primary cilium in a SMAD3-dependent manner. Sci Rep. 2016;6:35542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Liu M, Alharbi M, Graves D, Yang S. IFT80 is required for fracture healing through controlling the regulation of TGF-β signaling in chondrocyte differentiation and function. J Bone Min Res Off J Am Soc Bone Min Res. 2020;35:571–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Kawasaki M, et al. Primary cilia suppress the fibrotic activity of atrial fibroblasts from patients with atrial fibrillation in vitro. Sci Rep. 2024;14:12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Zhang W, et al. Dual Inhibition of HDAC and tyrosine kinase signaling pathways with CUDC-907 attenuates TGFβ1 induced lung and tumor fibrosis. Cell Death Dis. 2020;11:765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Waddell SH, et al. A TGFβ-ECM-integrin signaling axis drives structural reconfiguration of the bile duct to promote polycystic liver disease. Sci Transl Med. 2023;15:eabq5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Hong R, et al. XIAP-mediated degradation of IFT88 disrupts HSC cilia to stimulate HSC activation and liver fibrosis. EMBO Rep. 2024;25:1055–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Gradilone SA, et al. HDAC6 Inhibition restores ciliary expression and decreases tumor growth. Cancer Res. 2013;73:2259–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Paul C, et al. Loss of primary cilia promotes inflammation and carcinogenesis. EMBO Rep. 2022;23:e55687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Zhou B, et al. Notch signaling pathway: architecture, disease, and therapeutics. Signal Transduct Target Ther. 2022;7:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Ezratty EJ, et al. A role for the primary cilium in Notch signaling and epidermal differentiation during skin development. Cell. 2011;145:1129–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Wang W, et al. IFT-A deficiency in juvenile mice impairs biliary development and exacerbates ADPKD liver disease. J Pathol. 2021;254:289–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Andersson ER, Sandberg R, Lendahl U. Notch signaling: simplicity in design, versatility in function. Dev Camb Engl. 2011;138:3593–612. [DOI] [PubMed] [Google Scholar]
- 264.Ezratty EJ, Pasolli HA, Fuchs EA. Presenilin-2-ARF4 trafficking axis modulates Notch signaling during epidermal differentiation. J Cell Biol. 2016;214:89–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Mazelova J, et al. Ciliary targeting motif VxPx directs assembly of a trafficking module through Arf4. EMBO J. 2009;28:183–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Grisanti L, Revenkova E, Gordon RE, Iomini C. Primary cilia maintain corneal epithelial homeostasis by regulation of the Notch signaling pathway. Dev Camb Engl. 2016;143:2160–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Liu Z, et al. Primary cilia regulate hematopoietic stem and progenitor cell specification through Notch signaling in zebrafish. Nat Commun. 2019;10:1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Lopes SS, et al. Notch signalling regulates left-right asymmetry through ciliary length control. Dev Camb Engl. 2010;137:3625–32. [DOI] [PubMed] [Google Scholar]
- 269.Li X, et al. Primary cilia mediate Klf2-dependant Notch activation in regenerating heart. Protein Cell. 2020;11:433–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Serra CFH, et al. Prominin 1 and Notch regulate ciliary length and dynamics in multiciliated cells of the airway epithelium. iScience. 2022;25:104751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Rausch V, Hansen CG. The Hippo pathway, YAP/TAZ, and the plasma membrane. Trends Cell Biol. 2020;30:32–48. [DOI] [PubMed] [Google Scholar]
- 272.Jenks AD, et al. Primary cilia mediate diverse kinase inhibitor resistance mechanisms in cancer. Cell Rep. 2018;23:3042–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Srivastava S, et al. A human patient-derived cellular model of Joubert syndrome reveals ciliary defects which can be rescued with targeted therapies. Hum Mol Genet. 2017;26:4657–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Hynes AM, et al. Murine Joubert syndrome reveals Hedgehog signaling defects as a potential therapeutic target for nephronophthisis. Proc Natl Acad Sci U S A. 2014;111:9893–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Wong SY, et al. Primary cilia can both mediate and suppress Hedgehog pathway–dependent tumorigenesis. Nat Med. 2009;15:1055–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Han Y-G, et al. Dual and opposing roles of primary cilia in medulloblastoma development. Nat Med. 2009;15:1062–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Wu VM, Chen SC, Arkin MR, Reiter JF. Small molecule inhibitors of Smoothened ciliary localization and ciliogenesis. Proc. Natl. Acad. Sci. 109, 13644–13649 (2012). [DOI] [PMC free article] [PubMed]
- 278.Halkina T, et al. Discovery of potent and brain-penetrant Tau tubulin kinase 1 (TTBK1) inhibitors that lower Tau phosphorylation in vivo. J Med Chem. 2021;64:6358–80. [DOI] [PubMed] [Google Scholar]
- 279.Bashore FM, et al. Modulation of Tau tubulin kinases (TTBK1 and TTBK2) impacts ciliogenesis. Sci Rep. 2023;13:6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Lin AC, et al. Modulating Hedgehog signaling can attenuate the severity of osteoarthritis. Nat Med. 2009;15:1421–5. [DOI] [PubMed] [Google Scholar]
- 281.Thompson CL, Wiles A, Poole CA, Knight MM. Lithium chloride modulates chondrocyte primary cilia and inhibits Hedgehog signaling. FASEB J Off Publ Fed Am Soc Exp Biol. 2016;30:716–26. [DOI] [PubMed] [Google Scholar]
- 282.Peng Z, et al. Lithium inhibits tumorigenic potential of PDA cells through targeting hedgehog-GLI signaling pathway. PLoS ONE. 2013;8:e61457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Cao Y, et al. Chemical modifier screen identifies HDAC inhibitors as suppressors of PKD models. Proc Natl Acad Sci U S A. 2009;106:21819–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Mergen M, et al. The nephronophthisis gene product NPHP2/Inversin interacts with Aurora A and interferes with HDAC6-mediated cilia disassembly. Nephrol Dial Transpl Off Publ Eur Dial Transpl Assoc - Eur Ren Assoc. 2013;28:2744–53. [DOI] [PubMed] [Google Scholar]
- 285.Yanda MK, Liu Q, Cebotaru V, Guggino WB, Cebotaru L. Histone deacetylase 6 inhibition reduces cysts by decreasing cAMP and Ca2 + in knock-out mouse models of polycystic kidney disease. J Biol Chem. 2017;292:17897–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Cebotaru L, et al. Inhibition of histone deacetylase 6 activity reduces cyst growth in polycystic kidney disease. Kidney Int. 2016;90:90–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Frasca A, et al. MECP2 mutations affect ciliogenesis: a novel perspective for Rett syndrome and related disorders. EMBO Mol Med. 2020;12:e10270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Ritter A, et al. Primary cilia are dysfunctional in obese adipose-derived mesenchymal stem cells. Stem Cell Rep. 2018;10:583–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Xie C, et al. Reversal of ciliary mechanisms of disassembly rescues olfactory dysfunction in ciliopathies. JCI Insight. 2022;7:e158736. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.