Abstract
The protein kinase C (PKC) pathway is involved in the maintenance of cell shape and cell integrity in Saccharomyces cerevisiae. Here, we show that this pathway mediates tolerance to low pH and that the Bck1 and Slt2 proteins belonging to the mitogen-activated protein kinase cascade are essential for cell survival at low pH. The PKC pathway is activated during acidification of the extracellular environment, and this activation depends mainly on the Mid2p cell wall sensor. Rgd1p, which encodes a Rho GTPase-activating protein for the small G proteins Rho3p and Rho4p, also plays a role in low-pH response. The rgd1Δ strain is sensitive to low pH, and Rgd1p activates the PKC pathway in an acidic environment. Inactivation of both genes in the double mutant rgd1Δ mid2Δ strain renders yeast cells unable to survive at low pH as in bck1Δ and slt2Δ strains. Our data provide evidence for the existence of two distinct ways, one involving Mid2p and the other involving Rgd1p, with both converging to the cell integrity pathway to mediate low-pH tolerance in Saccharomyces cerevisiae. Nevertheless, even if Rgd1p acts on the PKC pathway, it seems that its mediating action on low-pH tolerance is not limited to this pathway. As the Mid2p amount plays a role in rgd1Δ sensitivity to low pH, Mid2p seems to act more like a molecular rheostat, controlling the level of PKC pathway activity and thus allowing phenotypical expression of RGD1 inactivation.
The fungal cell wall is an essential cellular boundary that controls many transport processes, cellular metabolism, and all communications with the extracellular environment. Because of its mechanical strength, it allows cells to withstand turgor pressure and consequently prevents cell lysis (7, 32). In the yeast Saccharomyces cerevisiae, a mitogen-activated protein kinase (MAPK) cascade is essential for the sensing of cell integrity under a variety of environmental conditions and morphological events and also in transducing signals to adapt cell wall biosynthesis (19). This cascade is activated by the protein kinase Pkc1p and constitutes the protein kinase C (PKC) cell integrity pathway (21). Cell wall alterations are detected by plasma membrane sensors of the WSC family (Wsc1p to Wsc4p) and by Mid2p and its homologue, Mtl1p (17, 18, 31, 38, 61). Among the WSC family members, Wsc1p/Slg1p/Hcs77p plays the major role in maintaining cell wall integrity (17, 61). Wsc1p is required for the viability of yeast cells during vegetative growth under various stress conditions, including heat stress, oxidative stress, hypoosmotic stress, ethanol, DNA-damaging drugs, and caspofungin (11, 51, 65). Mid2p is required during pheromone-induced cell morphogenesis but also for resistance to cell wall perturbation (13, 50). It has been reported that Wsc1p and Mid2p partially fulfill overlapping functions (9, 31, 50). Upon activation, they stimulate the exchange activity of the guanine nucleotide exchange factor Rom2p and thereby activate the small GTPase Rho1p (48). Among other functions, Rho1p is thought to stabilize and localize Pkc1p in the growth area, where the kinases Pkh1p and Pkh2p phosphorylate Pkc1p (24, 54). The main target of activated Pkc1p is the MAPK cascade module consisting of Bck1p MAPK kinase kinase (35); Mkk1p and Mkk2p (25), two redundant MAPK kinases; and the MAPK Slt2p/Mpk1p (34, 41). Upon activation by Mkk1/2p, Slt2p phosphorylates the transcription factors Rlm1p (63) and SBF, the latter being composed of Swi4 and Swi6 proteins (39). Most of the genes regulated by activation of the PKC pathway are known to encode integral cell wall proteins (CWPs) or proteins involved in cell wall biogenesis (26).
We previously reported the existence of functional interactions between the PKC pathway and the Rho GTPase-activating protein (RhoGAP) Rgd1p (9, 10). Rgd1p is a GTPase-activating protein acting on the two small yeast GTPases, Rho3p and Rho4p (14). These Rho proteins are involved in the regulation of exocytosis, the polarization of actin cytoskeleton (1, 53), and cytokinesis (23, 28, 45). Loss of Rgd1p greatly enhances the phenotype due to deletion of WSC1, and the small-budded cells carrying both mutations die because of defects in cell wall structure and lysis upon bud growth (31). Moreover, inactivation of MID2 exacerbates the phenotype of an rgd1Δ mutant and leads to an increase in the amount of dead cells observed at late exponential phase in liquid minimal medium (9). In addition, several suppressors of the lethality associated with the rgd1Δ mutation were isolated. Among them, we identified the genes encoding the two sensors Mid2p and Mtl1p, the two partially redundant GTPases Rho1p and Rho2p, and the MAP kinases Mkk1p and Slt2p (10). The dominant active BCK1-20 allele (35) was also found to be a strong suppressor of rgd1Δ (10).
The rgd1Δ mutation leads to sensitivity to low pH. Indeed, the rgd1Δ strain dies to approximately 15% at late exponential phase when minimal medium acidity increases or dies to 50% 6 hours following an acidic shock (15). These new data, considering the genetic links between rgd1Δ and the PKC pathway, led us to study the effect of low-pH stress on the PKC pathway and on its upstream and downstream elements. The PKC pathway was activated under low-pH conditions, and the cell wall sensor Mid2p was identified as decisive in the activation of this pathway. We also show that Bck1p and Slt2p components of the MAPK cascade are essential in an acidic environment. In addition, we have examined RGD1 involvement in the PKC pathway at low pH. We report a decrease of the activity of the PKC pathway at low pH in an rgd1Δ strain. Moreover, we show that the lack of Mid2p function exacerbates the rgd1Δ lethality in an acidic environment and that additional copies of MID2 suppress this lethality. Mid2p seems to act like a molecular rheostat for RGD1 inactivation, probably by changing PKC pathway activity. Our results are consistent with the existence of two ways, one involving Mid2p and the other involving Rgd1p, both converging to the cell integrity pathway to transduce signal at low pH; the simultaneous interruption of both ways renders yeast cells unable to survive under low-pH stress. Nevertheless, in contrast to what was observed with bck1Δ or slt2Δ strains, osmotic support does not eliminate the rgd1Δ and rgd1Δ mid2Δ lethality provoked by acidic shock, suggesting that the mediating action of Rgd1p on low-pH tolerance is not limited to the PKC pathway.
MATERIALS AND METHODS
Strains and growth conditions.
The Saccharomyces cerevisiae strains used in the present study were mainly obtained from the EUROSCARF knockout collection (http://www.uni-frankfurt.de/fb15/mikro/euroscarf/) and are isogenic derivatives of BY4742 (Table 1). Unless otherwise indicated, yeast strains were grown routinely at 30°C either in YPD (1% Bacto yeast extract, 2% Bacto peptone, 2% glucose) or in synthetic minimal YNB (0.67% yeast nitrogen base without amino acids [Difco], 2% glucose) medium supplemented with bases and amino acids when necessary. The buffered YNB medium was prepared by adding 1% succinic acid and 0.6% NaOH, and the final pH was 5.5 after autoclaving. The percentage of cell mortality was determined after methylene blue staining by counting the blue cells with an optical microscope as previously reported (10).
TABLE 1.
List of yeast strains used in this study
| Strain | Relevant genotype | Source or reference |
|---|---|---|
| X2180-1A | MATaSUC2 mal mel gal2 CUP1 | Y.G.S.C.a |
| LBG3-1B | MATaSUC2 mal mel gal2 CUP1 ura3-52 his3-11,15 rgd1::HIS3 | 9 |
| rgd1Δ mid2Δ | MATaura3-52 leu2 trp1Δ63 his3-11,15 rgd1::HIS3 mid2::TRP1 | 10 |
| BY4742 | MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 | EUROSCARF |
| cch1Δ | MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 cch1::KanMX4 | EUROSCARF |
| fks1Δ | MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 fks1::KanMX4 | EUROSCARF |
| fks2Δ | MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 fks2::KanMX4 | EUROSCARF |
| hog1Δ | MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 hog1::KanMX4 | EUROSCARF |
| mid1Δ | MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 mid1::KanMX4 | EUROSCARF |
| mid2Δ | MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 mid2::KanMX4 | EUROSCARF |
| pst1Δ | MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 pst1::KanMX4 | EUROSCARF |
| rgd1Δ | MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 rgd1::KanMX4 | EUROSCARF |
| rlm1Δ | MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 rlm1::KanMX4 | EUROSCARF |
| rom1Δ | MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 rom1::KanMX4 | EUROSCARF |
| rom2Δ | MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 rom2::KanMX4 | EUROSCARF |
| slt2Δ | MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 slt2::KanMX4 | EUROSCARF |
| swi4Δ | MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 swi4::KanMX4 | EUROSCARF |
| swi6Δ | MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 swi6::KanMX4 | EUROSCARF |
| wsc1Δ | MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 wsc1::KanMX4 | EUROSCARF |
| bck1Δ | MATaura3 trp1 his3Δ rme1 HMLa bck1::HIS3MX6 | M. Hall |
| pkc1Δ | MATaade2 trp1 leu2 ura3 his3 pkc1::URA3 | M. Hall |
| BCK1-20 | MATaura3-52 leu2-3,112 trp1-1 his4 can1r BCK1-20 | D. Levin |
| YOC784 | MATaade2 his3 leu2 lys2 trp1 ura3 rho1::HIS3 ade3::[RHO1 LEU2] | 22 |
| YOC772 | MATaade2 his3 leu2 lys2 trp1 ura3 rho1::HIS3 ade3::[rho1-2 LEU2] | 22 |
| YOC773 | MATaade2 his3 leu2 lys2 trp1 ura3 rho1::HIS3 ade3::[rho1-3 LEU2] | 22 |
| YOC774 | MATaade2 his3 leu2 lys2 trp1 ura3 rho1::HIS3 ade3::[rho1-4 LEU2] | 22 |
| YOC776 | MATaade2 his3 leu2 lys2 trp1 ura3 rho1::HIS3 ade3::[rho1-5 LEU2] | 22 |
Y.G.S.C., Yeast Genetic Stock Center (University of California, Berkeley).
Plasmids.
The multicopy plasmid YEp357R (44) was used to follow gene expression: it contains the lacZ coding sequence without its ATG and its promoter sequence. The region containing the promoter and the beginning of the coding sequence (−700 to +45 bp) of PST1 was amplified by PCR using oligonucleotides containing the XbaI and EcoRI cleavage sites, respectively. DNA amplification was performed from genomic yeast DNA using the Pwo DNA polymerase (Roche Molecular Biochemicals). The PCR fragment was cloned in the XbaI and EcoRI restriction sites of YEp357R as described previously by Sambrook et al. (57). In the resulting plasmid, YEp357R-PST1, the promoter of PST1 and the beginning of the coding sequence are fused in frame with the lacZ sequence encoding the β-galactosidase. This construction was verified by sequencing. Cloning and transformation were carried out in Escherichia coli XL1-Blue (Stratagene). For control experiments, we used the YEp357R-ADE1 plasmid (15) carrying the ADE1 promoter (−500 to +45 bp from ATG) fused to the lacZ coding sequence. Yeasts were transformed by the method described previously by Gietz et al. (16).
Stress procedures.
Yeast cells were grown overnight in YNB medium supplemented with metabolites if necessary until an optical density at 600 nm (OD600) of 0.5 was reached, and the culture was split into two parts. One culture was kept under standard growth conditions as a control, and the other was submitted to stress. In the case of heat shock, the cells were grown overnight at 21°C, and then half of the cells were quickly shifted to a temperature of 39°C. For low-pH stress, the cells were grown at 30°C during the whole experiment, and hydrochloric acid was added until the pH of the liquid medium reached a final pH of 3.5 or 2.8 (15).
β-Galactosidase liquid assay.
Cells containing the YEp357R-PST1 or the YEp357R-ADE1 plasmid were grown in selective medium. For each assay, a volume of culture medium corresponding to 3 × 108 cells was harvested. After 1 minute of centrifugation at 10,000 × g, cell pellets were stored at −20°C until transcriptional activity measurement. Cells were resuspended with 100 μl of 0.1 M phosphate buffer (Na2HPO4/NaH2PO4, pH 7.0) before disruption by mechanical shaking in the presence of glass beads (Mini-Beadbeater; Biospec Products). After centrifugation for 1 minute at 10,000 × g, the protein concentration in the supernatant was determined by a Bradford assay, and 10 μg of total protein was incubated at 30°C in 1 ml of 0.1 M phosphate buffer containing 0.8 mg of ONPG (o-nitrophenyl-β-d-galactopyranoside). After a 20-min incubation, the reaction was stopped by the addition of 500 μl of 1 M Na2CO3. β-Galactosidase activity was measured with a spectrophotometer at 420 nm. Results are presented in nanomoles per minute per milligram of protein, with 4.5 × 10−3 OD420 units representing 1 nmol of ONPG cleaved (5). We verified that with 10 μg of protein extract, ortho-nitrophenol production by β-galactosidase was linear during 30 min. For each condition, three independent measurements were determined, and variation was always <15%. Figures present the results obtained from one representative clone.
Preparation of yeast extracts and immunoblotting.
Yeast cells were harvested by centrifugation, and proteins were extracted according to the NaOH-trichloroacetic acid method (52). Protein extract corresponding to 107 cells was separated by sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis and electrotransferred onto a nitrocellulose membrane for Western blotting. Phosphorylated Slt2p was detected by using an anti-phospho-p44/42 MAP kinase (Thr202/Tyr204) antibody (Cell Signaling, Beverley, Mass.) at a 1:2,000 dilution to detect dually phosphorylated Slt2p as described previously (41). Total Slt2p was detected with anti-GST-Slt2p antibodies (a generous gift from H. Martin and M. Molina, Madrid, Spain) at a 1:1,000 dilution. Protein-antibody complexes were visualized by enhanced chemiluminescence with the Lumi-LightPLUS (Roche) system. To estimate the Slt2p activation level, the signal intensities for each time were determined using the Scion Image program (Scion Corporation, Md.), and the ratio of phospho-Slt2p to total Slt2p was determined.
RESULTS
PST1-lacZ expression is enhanced at low pH in an Rlm1p-dependent manner.
Loss of the RhoGAP encoding RGD1 function results in cell mortality in YNB medium at late exponential phase, and this lethality is caused by the natural acidification of minimal medium during growth (15). The pH of synthetic medium was determined to be around 5.7 at the beginning of culture, and this value, which was found to decrease concomitantly with biomass increase, reached 2.6 at stationary phase. We demonstrated that rgd1Δ lethality appears when pH is below 3.2/3.1 (15). This lethality was shown to be suppressed by RHO1 and RHO2 encoding two small GTPases, MKK1, encoding one of the MAP kinase kinases in the PKC pathway, and MTL1, a MID2 homolog (10). These data, which indicate functional links between RGD1 and the cell integrity pathway, led us to investigate the effect of low pH upon the PKC pathway. To address this point, we developed a reporter system to evaluate its activity conveniently. Activation of the Rlm1p transcription factor by the Slt2p kinase increases the transcription of genes encoding proteins mainly involved in cell wall biogenesis and maintenance (26). Among these genes, PST1 codes for a glycosyl-phosphatidylinositol (GPI) protein secreted by protoplasts regenerating their cell walls (47). The PST1 gene, which has a promoter region containing three Rlm1p binding sites [CTA(A/T)4TAG], was chosen in order to follow the activity of the PKC pathway. Its expression is strongly activated in a yeast strain carrying the constitutively active MKK1S386P, and this activation disappears in an rlm1Δ strain (26). Under the experimental conditions described previously (26), the major part of the transcriptional activity of PST1 and thus the PKC pathway was controlled by the Rlm1p transcription factor. We fused 700 bp of the PST1 promoter and the first 45 bp of the coding sequence in frame with the lacZ reporter gene in the YEp357R vector. To validate our reporter system, as the PKC pathway was shown to be activated after heat shock (27), we tested the activation of PST1-lacZ expression in a wild-type strain cultivated at 21°C or after heat shock (39°C) during 4 h. Whereas no PST1 transcriptional activation was observed at 21°C, the PST1 transcription was greatly increased, fourfold after heat shock, as expected for a reporter controlled by the PKC pathway.
Effect of low pH on PST1-driven lacZ expression was then determined in wild-type and rlm1Δ strains carrying YEp357R-PST1 (Fig. 1). We first carried out three types of low-pH shock on the wild-type strain: one shock lowering the pH of standard minimal medium from 3.5 to 2.8 and two shocks from the medium buffered at pH 5.5, i.e., from 5.5 to 2.8 and from 5.5 to 3.5. The last condition has already been used to show the nonimplication of the PKC pathway in the parietal modifications due to low pH (29). The follow-up of PST1 promoter activity was carried out by measuring β-galactosidase activity 4 hours after the shock (Fig. 1A). In the wild-type background, PST1 expression was activated by three kinds of low-pH shock. At pH 5.5 or 3.5 without shock, the values of PST1 expression were comparable. The level of activation did not depend on the pH difference (ΔpH) but seemed to be more in relation with the strength (the low pH value) of the acid shock. Indeed, the lower the final pH, the stronger the activation of the PST1 transcription. The activation triggered by low-pH stress was found to be specific to the PST1 promoter and not the consequence of a general increase of transcription in cells stressed by low pH, as determined by the follow-up of ADE1-lacZ expression (15) and also shown by a global transcriptional study at low pH (6). To perform the experiment in the rlm1Δ background (Fig. 1A), we used only the two types of acid shock which gave the strongest PST1 promoter activation in the wild-type strain (i.e., from pH 5.5 to 2.8 and from pH 3.5 to 2.8). In the rlm1Δ strain without shock, we observed that the level of PST1 expression was slightly dependent on pH medium. When pH medium was buffered to 5.5, the level of PST1 transcription was very low in an rlm1Δ strain. If the medium was naturally acidified during growth up to pH 3.5, the rlm1Δ strain was able to slightly increase the basal level of PST1 transcription in an Rlm1p-independent manner. In an rlm1Δ strain, transcription of the PST1-lacZ reporter was not activated under any acidic stress conditions, showing that activation of PST1 transcription under conditions of low-pH stress was entirely dependent on the Rlm1p transcription factor. These data suggest that low-pH stress activates the PKC pathway.
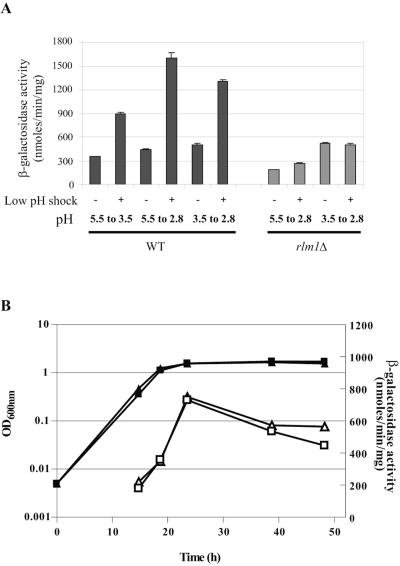
FIG. 1.
Effect of low pH on expression of the reporter gene PST1-lacZ in wild-type (WT) and rlm1Δ strains. (A) Cells transformed with YEp357R-PST1 were grown at 30°C to an OD600 of 0.5 in YNB either in standard minimal medium or in medium buffered at pH 5.5. For each experiment, the culture was divided into two parts. The first culture was kept under standard conditions as an unshocked control. The pH of the second culture was decreased from 5.5 to 3.5 or 2.8 for buffered YNB medium and 3.5 to 2.8 for standard YNB medium. β-Galactosidase activity was measured 4 hours after the low-pH stress as described in Materials and Methods. Results were from three independent assays, and the error bar indicates mean error. (B) Cells transformed with YEp357R-PST1 were grown at 30°C in minimal medium, and β-galactosidase activity was measured during growth. WT (triangles), rlm1Δ (squares).
To further explore the role of Rlm1p during the natural acidification of culture medium, both wild-type and rlm1Δ strains were cultivated in minimal medium, and PST1-driven lacZ expression was followed during growth (Fig. 1B). The β-galactosidase activity progressively increased during the exponential growth phase and then diminished after entry into the stationary phase. No difference was observed between the strains, indicating that in the absence of acidic shock, the basal PST1-lacZ expression was not dependent of Rlm1p. The variation of β-galactosidase activity might be in relation with the general activity and physiological adaptation of yeast cells during growth.
The RhoGAP Rgd1p is required for PST1 activation under conditions of low-pH stress.
Previous analysis revealed a lower PST1 RNA level in rgd1Δ cells cultivated in minimal medium when cells entered into the stationary phase (9). To study the effect of RGD1 inactivation on Rlm1p activation at low pH and then on the PKC pathway, we examined the PST1 expression in wild-type and rgd1Δ strains. For comparison with the previous data, the experiments were done in the X2180 background. First, we looked at the transcriptional activity of PST1-lacZ in both strains during growth in minimal medium (Fig. 2A). Consistent with Northern blot analysis (9), when cells reached the stationary phase and during this growth phase, PST1 transcription was less induced in rgd1Δ than in the wild type. The genetic background of BY4742 and X2180 strains might explain the variation of β-galactosidase activity in wild-type cells at stationary phase. To explore the role of pH on variation in PST1 transcription observed during growth, the wild-type and rgd1Δ strains were submitted to acid shock (Fig. 2B). In wild-type cells, lowering the pH greatly increased β-galactosidase activity. This increase was lower in the rgd1Δ background, and a lower β-galactosidase activity was also observed before the low-pH stress. The difference of β-galactosidase activity could not be explained by rgd1Δ mortality, as 1 hour after the shock, the mortality rate was still low. Moreover, the enzyme activity measured from the ADE1-lacZ reporter was similar in shocked rgd1Δ and wild-type cells, showing that the diminution of PST1 expression in rgd1Δ was not due to a general decrease of transcriptional activity in acidic conditions. These results, in agreement with previous data showing functional links between RGD1 and the PKC pathway (10), suggest that the cell integrity pathway is required under acidic growth conditions.
FIG. 2.
Effect of rgd1Δ mutation on expression of the reporter gene PST1-lacZ during growth and after low-pH shock. The wild-type (square) and rgd1Δ (triangle) strains in the X2180 background were transformed with YEp357R-PST1. (A) Expression of PST1-lacZ (open symbols) in both strains was determined by measuring β-galactosidase during growth (closed symbols) in minimal medium. (B) Cells were grown in minimal medium up to an OD600 of 0.5, and the pH was brought from 3.5 to 2.8. PST1-lacZ expression was measured for 6 hours after the acid shock.
The Mid2p cell surface sensor is also required for PST1 activation under low-pH stress conditions.
Several proteins have been involved in PKC pathway activation under stress conditions. Wsc1p (11, 65) and Mid2p (59) are among the most cited stress sensors in yeast. These proteins possess a plasma membrane domain with a small cytoplasmic extremity and a long extracellular extremity rich in serine and threonine which is highly O mannosylated (48). We investigated whether PST1 activation after low-pH stress was dependent on the Wsc1p and Mid2p sensors. To perform this study, we followed the β-galactosidase activity in wsc1Δ and mid2Δ strains transformed with the PST1-lacZ reporter construction (Fig. 3A). Before the shock, we observed that the level of PST1 expression in exponentially growing wsc1Δ cells (OD600 of 0.5; pH of medium, 3.5) was similar to that of the wild type but was decreased in a mid2Δ strain. These results suggest that under our experimental conditions, the basal activity of the PKC pathway depends on the presence of MID2 but not of WSC1. The low-pH shock was carried out by decreasing the pH from 3.5 to 2.8. As expected, the PST1-driven lacZ expression was enhanced in the wild-type strain after low-pH stress. In the mid2Δ strain, PST1 expression after low-pH stress was much weaker than that in the wild type. MID2 thus seems decisive for PST1 activation after low-pH stress. In the wsc1Δ strain, activation of PST1 expression was as fast as that in the wild-type strain during the first hours after the shock, but the β-galactosidase activity reached the plateau more quickly, and this plateau was lower than that in the wild type (Fig. 3A). There are two possible explanations for this result: (i) WSC1 could be responsible for part of the PKC pathway activation, and particularly in a later growth phase after low-pH shock, or (ii) the reduction of plateau level could be the consequence of a general decrease in transcriptional activity, as the wsc1Δ strain showed a mortality rate near 25% 1 hour after the shock. To discriminate between the two hypotheses, ADE1-lacZ expression was followed in the wsc1Δ mutant as well as in the wild type under the same experimental conditions after an acidic shock. Whereas the β-galactosidase activity was identical in wild-type cells, whether shocked or not, in the wsc1Δ strain, the ADE1 transcriptional activity was decreased by low-pH stress in the same way as was observed for PST1-lacZ (Fig. 3B). Thus, we can exclude that the decrease of PST1 transcription triggered by low-pH stress in wsc1Δ was completely specific to this gene. As expected for mid2Δ, the ADE1-lacZ expression was similar to that in the wild-type strain (Fig. 3B). These results were more consistent with a general lowering of the gene expression level caused by the appearance of wsc1Δ cell lethality following the low-pH stress. In contrast to MID2, WSC1 does not seem to specifically act in the activation of PST1 expression at low pH.
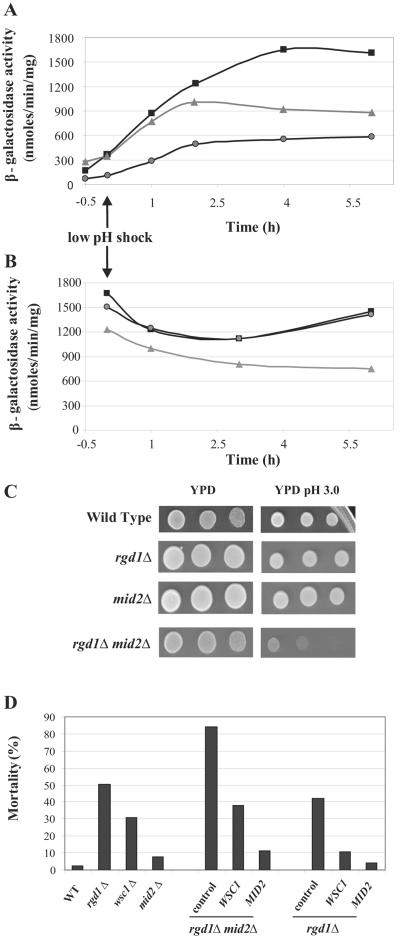
FIG. 3.
(A) Effect of WSC1 and MID2 inactivation on expression of the PST1-lacZ reporter gene during low-pH stress. Cells transformed with YEp357R-PST1 were grown in YNB medium to an OD600 of 0.5. The pH was decreased from 3.5 to 2.8 at time zero (indicated by an arrow). β-Galactosidase activity was followed during 6 hours after the shock in wild-type (▪), wsc1Δ (▴), and mid2Δ (•) strains. (B) Effect of WSC1 and MID2 inactivation on expression of the ADE1-lacZ reporter gene during low-pH stress. Cells were transformed with the YEp357R-ADE1 plasmid. The experimental conditions and symbols are the sameas described above (A). (C) Effect of RGD1 and MID2 inactivation on low-pH tolerance. Cells of wild-type, rgd1Δ, mid2Δ, and rgd1Δ mid2Δ strains and 10-fold serial dilutions thereof were spotted onto YPD and YPD medium adjusted at pH 3.0 by adding hydrochloric acid and incubated for 3 days at 30°C. (D) Suppression of rgd1Δ and rgd1Δ mid2Δ sensitivity to low pH by WSC1 and MID2 overexpression. The viability of indicated mutants as well as the viability of the rgd1Δ and rgd1Δ mid2Δ strains transformed with YEP352 WSC1-3HA, YEP352 MID2-3HA, or control vector were determined after low-pH stress. Cells were grown in YNB medium to an OD600 of about 0.5, and the pH was decreased from 3.6 to 2.8. Viability was measured after 6 h. Data concerning the effect of WSC1 and MID2 overexpression were averages from results obtained from three independent transformants. WT, wild type.
Synthetic lethality between RGD1 and MID2 depends on low pH.
Inactivation of the RGD1 gene results in a 15% cell mortality at the entry into stationary phase in minimal medium (9). Moreover, when MID2 is inactivated, strong enhancement of rgd1Δ mortality (∼60%) at the same growth phase is observed (10). Thus, RGD1 and MID2 can be considered conditional colethal genes because lethality of the double mutant appears only in particular growth conditions, i.e., at the entry of stationary phase and in minimal medium.
Considering the involvement of rgd1Δ in low-pH response (15), we examined the sensitivity of rgd1Δ, mid2Δ, and rgd1Δ mid2Δ mutants to acid growth conditions to find out the origin of the rgd1Δ mid2Δ colethality. Cells were diluted and spotted onto plates of rich YPD medium brought or not brought to pH 3.0 (Fig. 3C). Whereas all the strains grew similarly on regular YPD medium, the rgd1Δ mid2Δ strain grew slowly on YPD medium at pH 3.0. The rgd1Δ, mid2Δ, and wild-type strains presented apparent similar growth on solid YPD medium at pH 3.0. However, the data obtained from the same strains grown in liquid minimal medium and submitted to low-pH shock (Fig. 3D) showed the previously reported rgd1Δ sensitivity (15) as well as the hypersensitivity of the rgd1Δ mid2Δ double mutant to low pH. No mortality was detected by growing the double mutant in buffered medium at pH 5.5, which contrasts with our observations in standard minimal medium at late exponential phase when the medium pH was decreased (data not shown). Taken together, these results indicate that like rgd1Δ, the rgd1Δ mid2Δ colethality was caused by natural medium acidification during growth. In addition, these data suggest that RGD1 and MID2, which both give a stronger growth defect when inactivated, act in two distinct ways in low-pH tolerance in Saccharomyces cerevisiae. It is noteworthy that RGD1 is also synthetically lethal with WSC1; however, in this case, cell mortality does not depend on the pH of culture medium (9, 50).
MID2 and WSC1 suppress the rgd1Δ lethality at low pH.
Taking the results in PST1 transcriptional activity into account, we wondered whether Mid2p and Rgd1p were regulators of the PKC pathway during low-pH stress. The Wsc1p cell wall sensor, although involved in activation of the PKC pathway after various stresses (heat stress, oxidative stress, ethanol stress, and DNA damage [65]), did not show a direct involvement in the low-pH stress response. To further investigate the functional links between the Mid2p and Wsc1p sensors and the RhoGAP Rgd1p, we examined the effect of WSC1 and MID2 overexpression on low-pH sensitivity of rgd1Δ and rgd1Δ mid2Δ strains. We first scored for viability of strains inactivated for these genes in response to low-pH stress in liquid minimal medium (Fig. 3D). The mutant strains inactivated for WSC1, MID2, and RGD1 were subjected to low-pH shock, and mortality was measured 6 h after the shock. The mid2Δ strain, although it had a lower activity of the PKC pathway, presented a cell survival rate comparable with that of the wild type. The rgd1Δ and wsc1Δ strains died to about 50% and 30%, respectively. The rgd1Δ mid2Δ strain was particularly sensitive to shock (85% ± 5% of mortality) and consequently was unable to properly respond to low pH (Fig. 3D). We transformed rgd1Δ and rgd1Δ mid2Δ strains with the YEp352 multicopy vector carrying the WSC1 or MID2 gene under its own promoter (48). We found that WSC1 or MID2 overexpression suppressed the mortality of the rgd1Δ mid2Δ strain (Fig. 3D). However, although both WSC1 and MID2 play on PKC pathway activity, MID2 had the strongest suppressor effect. Indeed, while WSC1 overexpression was able to suppress the viability defect of the double mutant down to the rgd1Δ mortality level, the MID2 overexpression provoked a greater decrease in mortality, down to 11% (±1%). A comparable result was observed in an rgd1Δ strain: MID2 suppressed the rgd1Δ strain lethality more strongly than WSC1, suggesting that MID2 had a more important role in low-pH response. Taken together, these results suggest that MID2 and WSC1 overexpression can compensate for the PKC pathway defect linked to loss of Rgd1p function, and the two sensors might suppress the rgd1Δ mutation by increasing PKC pathway activity. These results provided further evidence for the existence of two pathways, one involving Mid2p and possibly Wsc1p and the other involving Rgd1p, both acting on the cell integrity pathway to mediate low-pH tolerance in Saccharomyces cerevisiae.
Low-pH stress activates the PKC signaling pathway through Slt2p phosphorylation.
We next investigated whether an increase in Rlm1p-dependent expression of the PST1-lacZ gene at low pH was linked to PKC pathway activation. Activation of the PKC pathway leads to phosphorylation of Slt2p on Thr190/Tyr192 residues (34). Therefore, we tested whether low-pH stress increased the amount of dually phosphorylated Slt2p. The stress was performed from cells growing in minimal medium by shifting the pH from 3.7 to 2.8. To analyze Slt2p phosphorylation, we used a phospho-specific antibody raised against dually phosphorylated p44/42 MAP kinase and a specific anti-Slt2p as described in Materials and Methods. Low-pH stress triggered a non-immediate but lasting Slt2p phosphorylation (Fig. 4); induction of Slt2p phosphorylation started 20 min after the acid shock and was still observed at least 3 hours after lowering the pH of the medium (not shown). Consistent with the previous result, Slt2p phosphorylation was also observed from wild-type cells after 2 hours of growth without shock; this activation might be linked to natural acidification of minimal medium (15). In addition, we noticed that the amount of Slt2p increased after low-pH stress and to a lesser extent after 1 hour without stress. This increase is consistent with the rise in the level of SLT2 mRNA reported at low pH (56) and with the feedback effect of PKC pathway activation on SLT2 expression (26). The ratio of phospho-Slt2p to total Slt2p was calculated; it indicates an increase of the amount of the phosphorylated form compared to total Slt2p 20 minutes after the low-pH shock, contrary to what was observed in the absence of shock. Taken together, these data indicate that low-pH stress activates the PKC pathway, which leads to phosphorylation of Slt2p accompanied by the synthesis of Slt2p in a later phase and then activation of Rlm1p-dependent transcription of target genes like PST1.
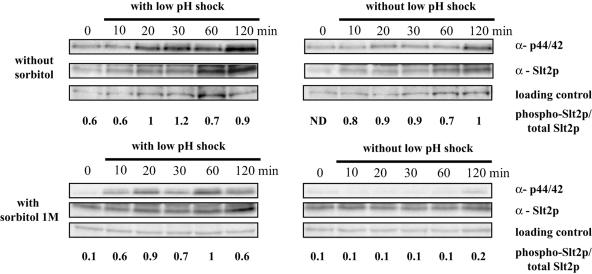
FIG. 4.
Immunoblot analysis of total and phosphorylated Slt2p after low-pH shock or not. Exponentially growing wild-type cells (OD600 of 0.4) were exposed to low-pH stress by shifting the pH from 3.7 to 2.8 in the presence or absence of sorbitol (1 M). Control experiments were carried out from cells grown under standard conditions. Aliquots were collected for various times, starting immediately after hydrochloric acid addition (0 min). Cell extracts were subjected to immunoblotting with anti-Slt2p and phospho-p44/42 MAP kinase antibodies as described in Materials and Methods. A nonspecific band revealed by anti-Slt2p antibody was used as a loading control. The ratio of phospho-Slt2p to total Slt2p was determined for each time by measuring the signal intensity with the Scion Image program. ND, not determined.
The same study was carried out with wild-type cells grown in minimal medium with 1 M sorbitol (Fig. 4). As expected (8), a very weak signal corresponding to Slt2p dual phosphorylation was detected under standard growth conditions, except at 120 min. When cells were shocked, the phosphorylation of Slt2p was still detected, even 10 min after the shock, showing that the addition of the osmotic stabilizer did not significantly affect Slt2p activation resulting from low pH. The slight increase in signal intensity observed at 120 min for unshocked cells was consistent with the absence of effect of sorbitol. This result indicates that the stimulus that regulates the induction of the PKC1-MAPK pathway provoked by low pH was not associated with cell wall changes which can be stabilized by osmotic pressure.
Components of the PKC signaling pathway are required for low-pH stress tolerance.
The aforementioned results suggest that the PKC pathway is necessary for low-pH stress tolerance. To gain further insight into the involvement of the PKC pathway, we wanted to know whether PKC pathway mutants were sensitive to medium acidity. We examined the viability of various PKC pathway mutants, as well as mutants altered for upstream and downstream components of this pathway, on solid YPD medium whose pH was adjusted to 3.0 (Fig. 5A). We tested the inactivated strains for ROM2, encoding the exchange factor of Rho1p, and for its homologue, ROM1. We also tested the strains inactivated for the MAPK kinase kinase BCK1 or for the MAP kinase SLT2 but not for MKK1 or MKK2 because of the partial redundancy between these two genes (41). Growth of the rlm1Δ strain was also examined on these media. The strains inactivated for the ROM1 and ROM2 genes, upstream from PKC1, and for the RLM1 gene downstream from the MAP kinase cascade did not present growth difference at pH 3.0. On the other hand, the strains inactivated for the BCK1 and SLT2 genes, encoding components of the MAPK cascade, were sensitive to low pH. To check if this sensitivity to low-pH stress was only linked to the lack of the activity of the PKC pathway and not caused by its upregulation, we tested the behavior of a strain containing the overactivated BCK1-20 allele (35). This strain did not show any particular sensitivity to low-pH stress, indicating that only interruption of the PKC pathway was detrimental for cell survival at low pH. In the literature, it has been reported that low external pH induces HOG1-dependent changes in the organization of the yeast cell wall and that modification does not depend on Slt2p (29). We examined whether a HOG1-inactivated strain was sensitive to low pH. On YPD medium at pH 3.0, the hog1Δ strain did not show any growth defect. This result indicates that even if the HOG pathway is involved in the low-pH stress response, Hog1p is not an essential component at low pH, in contrast to components of the PKC pathway.
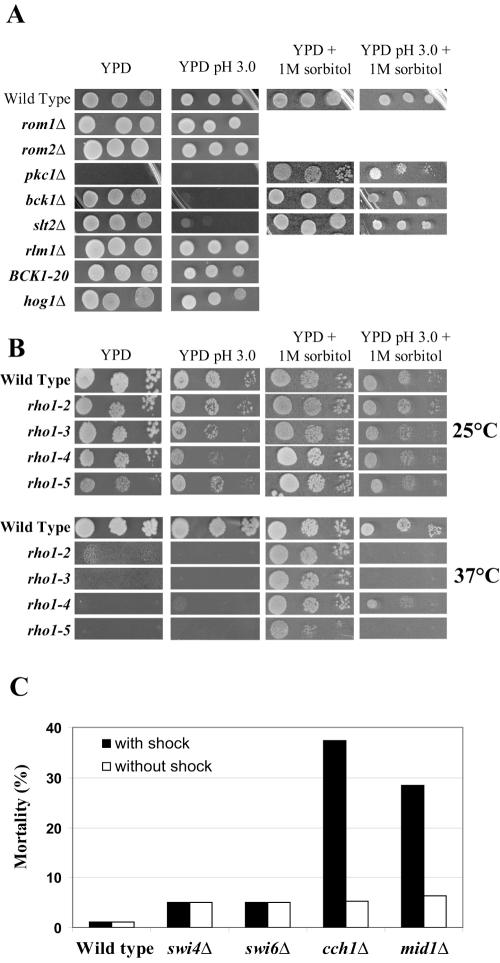
FIG. 5.
Study of the sensitivity of different mutant strains to low pH. (A) Effect of inactivation of genes encoding proteins of the PKC pathway and upstream and downstream components, of BCK1 overactivation (BCK1-20 allele), as well as of hog1Δ on low-pH tolerance. Cells of the indicated strains and 10-fold serial dilutions thereof were spotted onto plates of YPD medium, YPD medium at pH 3.0, YPD medium plus 1 M sorbitol, and YPD medium at pH 3.0 plus 1 M sorbitol and incubated for 3 days at 30°C. (B) Effect of temperature-sensitive rho1 mutations on growth at low pH. Cells were spotted onto YPD medium as indicated above (A). The plates were incubated at 25°C or 37°C for 2.5 days. (C) Study of sensitivity of swi4Δ, swi6Δ, cch1Δ, and mid1Δ mutants to low pH. Cells were grown in YNB medium to an OD600 of about 0.5, and the pH was decreased from 3.6 to 2.8. Cell viability was measured 6 h after the shock or not.
The PKC pathway is involved in several functions: maintenance of cell wall integrity, actin cytoskeleton polarization (22, 42), and regulation of ribosome synthesis (46). We investigated whether the growth defect observed during low-pH stress could be compensated by an osmotic stabilizer. bck1Δ and slt2Δ mutants, which have an impaired maintenance of cell wall integrity and which were sensitive to the acidity of the medium, saw their growth restored in the presence of 1 M sorbitol (Fig. 5A). Sorbitol increases the osmotic pressure outside the cell and avoids lysis of yeast cells with a defective cell wall (36, 60). One consequence of the low-pH stress could be to accentuate the weakening of the cell wall in the bck1Δ and slt2Δ mutants, thus explaining the compensating effect of sorbitol. Thus, these data provide evidence that the MAPK cascade module is essential for low-pH tolerance through cell wall maintenance.
The essential PKC1 and RHO1 genes were also tested for sensitivity to low pH. Growth of a pkc1Δ strain is restored in the presence of an osmotic stabilizer (Fig. 5A), so we tested this strain in the presence of 1 M sorbitol. Like bck1Δ and slt2Δ, no growth difference was observed for the pkc1Δ strain on YPD medium with 1 M sorbitol at normal pH (5.8) or at low pH (Fig. 5A). For RHO1, several thermosensitive mutations were identified and characterized (49); on one hand, rho1-2 and rho1-5 lack the ability to activate the Pkc1p-Slt2p pathway, and on the other hand, rho1-3 and rho1-4 present severe defects in glucan synthase activation (56). These strains were grown on solid YPD medium adjusted to pH 3.0 at 25°C and 37°C (Fig. 5B). At 25°C, the thermosensitive strains did not present growth defects at low pH. At 37°C, as expected, sorbitol compensated for the effects of all thermosensitive mutations in standard YPD medium. When the pH was lowered to 3.0, only rho1-4 grew on YPD medium with sorbitol, contrary to rho1-2, rho1-3, and rho1-5 mutants. The different response of rho1-4 and rho1-3 strains reported as belonging to the same mutant class made interpretation of results difficult, but nevertheless, all the results obtained from the thermosensitive mutants suggested that the Rho1p GTPase was also involved in pH tolerance. Taken together, the data indicated the involvement of the MAP kinase cascade starting from Rho1p up to the Slt2p kinase; the Rlm1p transcription factor, although necessary for PST1 activation in acid medium, did not seem to be essential for low-pH tolerance.
Growth test on solid YPD medium at pH 3 was not sensitive enough to reveal weak or intermediary sensitivity to low pH. Therefore, the lethality of strains inactivated for ROM2, ROM1, RLM1, and also PST1, one Rlm1p target, was analyzed in liquid minimal medium after the pH was shifted from 3.5 to 2.8. Only rom2Δ showed a slight lethality (about 10%) after 6 h under acidic growth conditions. In addition, so as to clarify the effect of high-temperature-sensitive mutations in the RHO1 gene, the fks1Δ and fks2Δ strains deficient for the β1-3 glucan synthase were also tested. Rho1p binds and activates Fks1p and Fks2p, two closely related catalytic subunits of β1-3 glucan synthase (49). The acidic shock was without effect on fksΔ strains, indicating that the pH effect does not involve the glucan synthase activity, and thus, Rho1p might act in acidic conditions by signaling to Pkc1p. We also tested the effect of sorbitol on the rgd1Δ and rgd1Δ mid2Δ strains as well as bck1Δ and slt2Δ grown in liquid minimal medium. We found again that the osmotic stabilizer prevented mortality of bck1Δ and slt2Δ mutants at pH 2.8, but concerning rgd1Δ and rgd1Δ mid2Δ, the presence of sorbitol was without effect on lethality at low pH. These data seem to indicate that the action of Rgd1p is not restricted to the PKC pathway to mediate low-pH tolerance.
As the acidic shock did not change rlm1Δ viability although the PKC-MAPK cascade was essential under these conditions, we decided to test inactivated strains for other Slt2p-dependent targets. Activated Slt2 kinase phosphorylates the Swi6p transcription factor; Swi6p binds to Swi4p, and both proteins make the SBF involved in cell cycle regulation (39). Slt2p directly or indirectly stimulates the Cch1-Mid1 Ca2+ channel under conditions of endoplasmic reticulum stress (2). Cch1p and Mid1p are both required for a high-affinity Ca2+ influx system and for growth in low-calcium environments (37). We then examined cell viability of strains deficient for these different components at pH 2.8 (Fig. 5C). Lowering the pH only led to mortality of cch1Δ and mid1Δ mutants with 37% and 28% of dead cells, respectively, 4 hours after the shock. This last observation suggested that the calcium channel is involved in cell viability at low pH as a result of PKC pathway activation.
DISCUSSION
Extracellular pH is a key environmental signal that influences the growth and physiology of yeast (12). During yeast growth, extracellular medium is acidified due to the activity of plasma membrane H+-ATPase and to the extrusion of acidic metabolites from cells (33). This low pH induces various adaptations in cell wall composition and structure. For example, at acidic pH, the cell wall chitin level is lower than that in cells growing at a higher pH, which might be attributed to an increase in chitinase levels at acidic pH values (4). The cells also become highly resistant to the recombinant β1,3-glucanase Quantazyme and use the alkali-sensitive linkage between CWPs and β1,3-glucan more extensively, resulting in a more efficient incorporation of Pir-CWPs and an increase in double-linked GPI-CWPs (29). Interestingly, the increase in Quantazyme resistance at low pH is dependent on the Hog1p MAP kinase pathway but not on Slt2p. However, the HOG-dependent changes in the cell wall are not essential because the hog1Δ strain does not show any growth defects under low-pH conditions (29).
In this work, we demonstrate the involvement of the PKC cell integrity pathway in response to low-pH stress in Saccharomyces cerevisiae. The PKC signal transduction pathway is essential for sensing cell integrity under a variety of environmental conditions or morphogenetic events (21). Phosphorylation of Slt2p has been shown to occur in response to high temperature, exposure to exogenous mating pheromone, or hypoosmotic shock (3, 40, 64). Signaling is activated persistently in response to growth at elevated temperatures (27), whereas hypoosmotic shock induces a rapid but transient activation of signaling (8). Agents that cause cell wall stress, such calcofluor white and Congo red, also activate Slt2p phosphorylation, but this occurs after a long time (13, 51). Our results show that low-pH stress also results in Slt2p activation followed by Slt2p synthesis; however, the MAP kinase dual phosphorylation caused by acidic shock occurred later compared to the kinetics of Slt2 activation after hypoosmotic or heat shocks. The Slt2p response suggests that activation of the cell integrity pathway provoked by low pH could correspond to an adaptive process more than an immediate response driven by a MAP kinase pathway in response to an external stimulus. We question whether constant exposure to low pH causes increasingly greater structural changes to the cell wall and/or plasma membrane which in turn activate the PKC pathway. The addition of sorbitol to medium did not abolish Slt2p activation, and induction is even faster with the appearance of Slt2p phosphorylation 10 minutes after the shock. This result, which shows that MAPK pathway activation cannot be prevented by providing osmotic support, suggests that this activation is not the response to a general mechanism of cell wall adaptation. The acidification of medium could act through changes inside the yeast cell. Such a hypothesis has been put forward to explain Slt2p activation when yeast cells are stressed using an oxidative agent like hydrogen peroxide (62). Hydrogen peroxide exercises its action at the intracellular level, mainly by affecting a cellular function that is closely related to the polarization of the actin cytoskeleton (62). In our case, one possibility was a drastic decrease in the intracellular pH following the acidic shock. Signaling does not seem to involve variation of the intracellular pH, as its estimation in wild-type cells before and after the acidic shock revealed very close pH values (15).
Using a microarray approach, Causton et al. (6) have shown that the transcription of genes encoding proteins acting upstream of the PKC pathway (i.e., MID2, SLG1, ROM2, and RHO1) and in the PKC pathway itself (i.e., BCK1 and MKK1/2) is not activated after a low-pH shock from 6.0 to 4.0 in rich YPD medium. Transcription of SLT2 is activated slightly, approximately twice as much, while that of PST1, one of the outputs of Slt2 activation, is activated up to fivefold. In this study, we described how the expression of the PST1 gene is enhanced after various low-pH stresses, and it is particularly high when the ΔpH is important. In addition, we show that the whole activation of PST1 transcription during low-pH shock is controlled by the Rlm1p transcription factor. The enhancement of PKC pathway activity as well as the Rlm1p-dependent PST1 activation at low pH suggest that expression of other genes controlled by Rlm1p is also increased under these conditions. A previous genome-wide survey of genes whose expression was altered in response to Slt2p activation indicated that about 20 genes were upregulated (26). Among Rlm1p-regulated genes (26), global transcript analysis (6) revealed that in addition to PST1, transcription of most of the genes coding for GPI proteins (SED1, CWP1, ECM19, and YLR194c) is activated in response to low-pH stress. This is also the case with PIR2, encoding an O-glycosylated cell wall protein of the PIR family proteins (30). Thus, the Rlm1p-dependent transcriptional activation at low pH seems to be specific to cell wall-related genes. We can imagine that variation of expression of these genes might act in cell wall maintenance and adaptation to acidic growth conditions. The PKC pathway would then cooperate with the HOG pathway for low-pH response in yeast cells; the PKC pathway activates transcription of some GPI- and PIR-CWPs (6, 26), and the HOG pathway allows a better anchoring of these proteins to the cell wall structure (29). However, as the rlm1Δ and hog1Δ strains normally grow at a low pH, the variation in gene expression controlled by Rlm1p and Hog1p, respectively, is not essential for yeast viability under acidic growth conditions.
Some mechano-sensors have been described as being activators of the PKC pathway. Among them, Wsc1p is known to respond to various stresses (heat stress, oxidative stress, hypoosmotic stress, ethanol, DNA-damaging drugs, and caspofungin [11, 51, 65]) and seems to play a role in the overall mechanism needed to protect cells from damage and unfavorable environments. The cell wall sensor Mid2p responds more particularly to cell wall perturbations (13, 50). We show in this work that low-pH activation of the PKC pathway is greatly reduced in the mid2Δ strain. However, although the mid2Δ mutation strongly affects the PST1-driven lacZ expression, the mid2Δ strain exhibits a cell viability similar to that of the wild-type strain after a low-pH shock or during natural medium acidification. Thus, the low activation of the PKC pathway observed in a mid2Δ strain is still sufficient for cell survival under low-pH conditions. On the contrary, the wsc1Δ strain died in acid medium. However, the decrease of PST1 transcription beginning 2 hours after the shock does not seem specifically linked to pH response but is more related to the loss of wsc1Δ cell viability under these conditions. The wsc1Δ cell mortality exhibited at low pH must be linked to cell wall weakness, as inactivation of WSC1 results in cell wall impairment (59) and in mortality, which can be prevented by osmotic support (9). Thus, whereas Wsc1p does not directly participate in low-pH signaling but has a more general action in that stress response, Mid2p plays a major role in signal transduction to low pH in Saccharomyces cerevisiae.
In previous work (9, 10), we reported that MID2 genetically interacts with RGD1. MID2 deletion exacerbates the rgd1Δ strain mortality observed in stationary phase, whereas its overexpression suppresses the rgd1Δ growth defect. In fact, the RGD1 gene, which encodes the GTPase-activating protein for Rho3p and Rho4p, is also involved in the low-pH stress response. Indeed, RGD1 transcription is activated during medium acidification (i.e., during growth in liquid minimal medium or after a low-pH shock), and RGD1 inactivation leads to cell mortality in these conditions, indicating that RGD1 plays a key role in the low-pH stress response (15). We have already shown that an increase in the PKC pathway activity in an rgd1Δ strain suppresses lethality at late exponential phase (10). We also demonstrate here that the RhoGAP Rgd1p is required to increase PST1 expression at low pH. Thus, Rgd1p, which seems to be involved in PKC pathway activation, appears to be a major regulator involved in yeast survival following low-pH stress. We show that the synthetic lethality of the rgd1Δ mid2Δ strain is also specific to acidic environments. On solid medium at pH 3.0, neither the mid2Δ strain nor the rgd1Δ strain, which nevertheless displays sensitivity to low pH in liquid medium, presents a growth defect. The existence of rgd1Δ mid2Δ colethality indicates that RGD1 and MID2 are part of a common response to low-pH stress. MID2 overexpression suppresses the rgd1Δ mid2Δ mortality during low-pH stress more efficiently than WSC1. Similar results were observed in an rgd1Δ strain, indicating that MID2 was able to specifically compensate for the defect related to low-pH response. Thus, although the growth of the mid2Δ strain was not affected by acidic conditions, the Mid2p amount in yeast cells plays a role in allowing or not allowing the appearance of rgd1Δ sensitivity to low pH. Mid2p seems to act like a molecular rheostat for RGD1 inactivation, probably by changing PKC pathway activity and, by way of consequence, the yeast's physiological background. Taken together, these data are consistent with the existence of two ways, one involving Mid2p and the other involving Rgd1p, with both converging in the cell integrity pathway, to participate in low-pH tolerance in Saccharomyces cerevisiae. If the functional link between the Mid2p sensor and the PKC pathway is well established (48), the connection between Rgd1p and this pathway has yet to be discovered. However, considering the absence of sensitivity of the mid2Δ strain to low pH, rgd1Δ cell lethality cannot be explained by the decrease of the PKC pathway alone.
Our work establishes that the function of the Bck1p and Slt2p proteins belonging to the MAPK cascade of the PKC pathway as well as the Rho1 GTPase are required for cell survival in acidic environments, whereas the upstream and downstream components are dispensable. These results suggest the existence of a specific function associated with the MAPK cascade which is essential for the low-pH survival response. In addition, we found that slt2Δ and bck1Δ mortality was prevented by adding sorbitol. Hence, it seems that the lethality induced by low-pH stress and rescued by increasing the extracellular osmotic pressure is linked here to cell wall impairment (19). Our results show that the PKC cell integrity pathway is activated in response to low-pH stress and that cell wall adaptation to this stress requires the presence of a functional PKC-MAPK cascade. This seems to indicate that there is a function of the PKC-MAPK module which is essential for low-pH tolerance but different from the activation of the PKC pathway. Contrary to bck1Δ or slt2Δ strains, osmotic support does not eliminate the rgd1Δ and rgd1Δ mid2Δ lethality provoked by acidic shock, providing evidence for the existence of cellular mechanisms acting differently through the PKC-MAPK cascade and the RhoGAP Rgd1p to mediate low-pH tolerance. This last Rgd1p effect would not be compensable by sorbitol. We have shown that the Cch1 and Mid1 proteins, two effectors of Slt2p and components for the calcium channel, are involved in cell viability at low pH. Intracellular biological changes in rgd1Δ, like intracellular ionic variation or pH decrease (15), could explain the sensitivity of mutants to low pH. A link between the lack of Rgd1p and pH sensitivity might be considered through the defective regulation of Rho3 and Rho4 GTPases. A loss of Rgd1p function leads to an accumulation of active forms of Rho3p and Rho4p (55), and consistent with the above-described hypothesis, overexpressing the constitutively active form of Rho3 leads to sensitivity to low pH as for rgd1Δ (unpublished results).
Taken together, our data support a model for yeast response to acidic stress (Fig. 6). During low-pH stress, the HOG pathway is activated, resulting in the activation of Msn2p and Msn4p transcription factors (29, 43, 58). When activated, these two factors would enhance the expression of various genes, one of which is RGD1 (15). These genes would then positively regulate the PKC pathway. This model is consistent with the results of Hahn and Thiele (20), which indicates that SLT2 transcription is enhanced in a HOG1-dependent manner but also in an RLM1-dependent manner and which suggests that the HOG pathway is able to activate the PKC pathway. At the same time, low-pH stress would be able to activate the PKC pathway, thanks to Mid2p sensor. Upstream of Pkc1p, there would be at least two ways to activate the MAPK pathway: the first one involving Mid2p and the second one involving Rgd1p. Each of these would lead to Pkc1p and thus activation of the cell integrity pathway. Indeed, a protein partner of Rgd1p which could be the functional link between Rgd1p and Pkc1p was identified. A study of the relationship between this protein and Rgd1p has been undertaken and will provide the key to how Rgd1p is connected to the PKC pathway. The simultaneous blocks of these two ways upstream of Pkc1p (e.g., in the rgd1Δ mid2Δ strain) or the interruption of the PKC pathway downstream of Pkc1p (e.g., in bck1Δ or slt2Δ strains) then makes yeast cells unable to survive low-pH stress. Further study of the effect of low pH would contribute to a better understanding of the response to this key environmental signal in Saccharomyces cerevisiae.
FIG. 6.
Model of PKC pathway activation in response to low-pH stress (see Discussion). The speculative functional links are indicated with dotted lines.
Acknowledgments
We thank A. Claveres and M. F. Peypouquet for technical assistance and O. Roumanie for critical reading. English usage was checked by Joanna Pageze. We are grateful to M. Hall for providing bck1::HIS3MX6 (PA109-1c) and the isogenic wild-type strain (Jk9-3da) as well as the different high-temperature-sensitive rho1 mutants and to D. Levin for providing strain BCK1-20 (DL245) and YEp352-Wsc1HA and YEp352-Mid2HA plasmids. The anti-GST-Slt2p antibodies were a kind gift from H. Martin and M. Molina.
This work was supported by grants from the University Victor Segalen Bordeaux 2, CNRS, and Regional Council of Aquitaine. S.C. and X.G. are recipients of a MENRT fellowship.
REFERENCES
- 1.Adamo, J. E., G. Rossi, and P. Brennwald. 1999. The Rho GTPase Rho3 has a direct role in exocytosis that is distinct from its role in actin polarity. Mol. Biol. Cell 10:4121-4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonilla, M., and K. W. Cunningham. 2003. Mitogen-activated protein kinase stimulation of Ca(2+) signaling is required for survival of endoplasmic reticulum stress in yeast. Mol. Biol. Cell 14:4296-4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buehrer, B. M., and B. Errede. 1997. Coordination of the mating and cell integrity mitogen-activated protein kinase pathways in Saccharomyces cerevisiae. Mol. Cell. Biol. 17:6517-6525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cabib, E., A. Sburlati, B. Bowers, and S. J. Silverman. 1989. Chitin synthase 1, an auxiliary enzyme for chitin synthesis in Saccharomyces cerevisiae. J. Cell Biol. 108:1665-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casadaban, M. J., A. Martinez-Arias, S. K. Shapira, and J. Chou. 1983. Beta-galactosidase gene fusions for analyzing gene expression in Escherichia coli and yeast. Methods Enzymol. 100:293-308. [DOI] [PubMed] [Google Scholar]
- 6.Causton, H. C., B. Ren, S. S. Koh, C. T. Harbison, E. Kanin, E. G. Jennings, T. I. Lee, H. L. True, E. S. Lander, and R. A. Young. 2001. Remodeling of yeast genome expression in response to environmental changes. Mol. Biol. Cell 12:323-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cid, V. J., A. Duran, F. del Rey, M. P. Snyder, C. Nombela, and M. Sanchez. 1995. Molecular basis of cell integrity and morphogenesis in Saccharomyces cerevisiae. Microbiol. Rev. 59:345-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davenport, K. R., M. Sohaskey, Y. Kamada, D. E. Levin, and M. C. Gustin. 1995. A second osmosensing signal transduction pathway in yeast. Hypotonic shock activates the PKC1 protein kinase-regulated cell integrity pathway. J. Biol. Chem. 270:30157-30161. [DOI] [PubMed] [Google Scholar]
- 9.de Bettignies, G., C. Barthe, C. Morel, M. F. Peypouquet, F. Doignon, and M. Crouzet. 1999. RGD1 genetically interacts with MID2 and SLG1, encoding two putative sensors for cell integrity signalling in Saccharomyces cerevisiae. Yeast 15:1719-1731. [DOI] [PubMed] [Google Scholar]
- 10.de Bettignies, G., D. Thoraval, C. Morel, M. F. Peypouquet, and M. Crouzet. 2001. Overactivation of the protein kinase C-signaling pathway suppresses the defects of cells lacking the Rho3/Rho4-GAP Rgd1p in Saccharomyces cerevisiae. Genetics 159:1435-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delley, P. A., and M. N. Hall. 1999. Cell wall stress depolarizes cell growth via hyperactivation of RHO1. J. Cell Biol. 147:163-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denison, S. H. 2000. pH regulation of gene expression in fungi. Fungal Genet. Biol. 29:61-71. [DOI] [PubMed] [Google Scholar]
- 13.de Nobel, H., C. Ruiz, H. Martin, W. Morris, S. Brul, M. Molina, and F. M. Klis. 2000. Cell wall perturbation in yeast results in dual phosphorylation of the Slt2/Mpk1 MAP kinase and in an Slt2-mediated increase in FKS2-lacZ expression, glucanase resistance and thermotolerance. Microbiology 146:2121-2132. [DOI] [PubMed] [Google Scholar]
- 14.Doignon, F., C. Weinachter, O. Roumanie, and M. Crouzet. 1999. The yeast Rgd1p is a GTPase activating protein of the Rho3 and Rho4 proteins. FEBS Lett. 459:458-462. [DOI] [PubMed] [Google Scholar]
- 15.Gatti, X., G. de Bettignies, S. Claret, F. Doignon, M. Crouzet, and D. Thoraval. 2005. RGD1, encoding a RhoGAP involved in low-pH survival, is an Msn2p/Msn4p regulated gene in Saccharomyces cerevisiae. Gene 351:159-169. [DOI] [PubMed] [Google Scholar]
- 16.Gietz, R. D., R. H. Schiestl, A. R. Willems, and R. A. Woods. 1995. Studies on the transformation of intact yeast cells by the LiAc/SS-DNA/PEG procedure. Yeast 11:355-360. [DOI] [PubMed] [Google Scholar]
- 17.Gray, J. V., J. P. Ogas, Y. Kamada, M. Stone, D. E. Levin, and I. Herskowitz. 1997. A role for the Pkc1 MAP kinase pathway of Saccharomyces cerevisiae in bud emergence and identification of a putative upstream regulator. EMBO J. 16:4924-4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green, R., G. Lesage, A. M. Sdicu, P. Menard, and H. Bussey. 2003. A synthetic analysis of the Saccharomyces cerevisiae stress sensor Mid2p, and identification of a Mid2p-interacting protein, Zeo1p, that modulates the PKC1-MPK1 cell integrity pathway. Microbiology 149:2487-2499. [DOI] [PubMed] [Google Scholar]
- 19.Gustin, M. C., J. Albertyn, M. Alexander, and K. Davenport. 1998. MAP kinase pathways in the yeast Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 62:1264-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hahn, J. S., and D. J. Thiele. 2002. Regulation of the Saccharomyces cerevisiae Slt2 kinase pathway by the stress-inducible Sdp1 dual specificity phosphatase. J. Biol. Chem. 277:21278-21284. [DOI] [PubMed] [Google Scholar]
- 21.Heinisch, J. J., A. Lorberg, H. P. Schmitz, and J. J. Jacoby. 1999. The protein kinase C-mediated MAP kinase pathway involved in the maintenance of cellular integrity in Saccharomyces cerevisiae. Mol. Microbiol. 32:671-680. [DOI] [PubMed] [Google Scholar]
- 22.Helliwell, S. B., A. Schmidt, Y. Ohya, and M. N. Hall. 1998. The Rho1 effector Pkc1, but not Bni1, mediates signalling from Tor2 to the actin cytoskeleton. Curr. Biol. 8:1211-1214. [DOI] [PubMed] [Google Scholar]
- 23.Imamura, H., K. Tanaka, T. Hihara, M. Umikawa, T. Kamei, K. Takahashi, T. Sasaki, and Y. Takai. 1997. Bni1p and Bnr1p: downstream targets of the Rho family small G-proteins which interact with profilin and regulate actin cytoskeleton in Saccharomyces cerevisiae. EMBO J. 16:2745-2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inagaki, M., T. Schmelzle, K. Yamaguchi, K. Irie, M. N. Hall, and K. Matsumoto. 1999. PDK1 homologs activate the Pkc1-mitogen-activated protein kinase pathway in yeast. Mol. Cell. Biol. 19:8344-8352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Irie, K., M. Takase, K. S. Lee, D. E. Levin, H. Araki, K. Matsumoto, and Y. Oshima. 1993. MKK1 and MKK2, which encode Saccharomyces cerevisiae mitogen-activated protein kinase-kinase homologs, function in the pathway mediated by protein kinase C. Mol. Cell. Biol. 13:3076-3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jung, U. S., and D. E. Levin. 1999. Genome-wide analysis of gene expression regulated by the yeast cell wall integrity signalling pathway. Mol. Microbiol. 34:1049-1057. [DOI] [PubMed] [Google Scholar]
- 27.Kamada, Y., U. S. Jung, J. Piotrowski, and D. E. Levin. 1995. The protein kinase C-activated MAP kinase pathway of Saccharomyces cerevisiae mediates a novel aspect of the heat shock response. Genes Dev. 9:1559-1571. [DOI] [PubMed] [Google Scholar]
- 28.Kamei, T., K. Tanaka, T. Hihara, M. Umikawa, H. Imamura, M. Kikyo, K. Ozaki, and Y. Takai. 1998. Interaction of Bnr1p with a novel Src homology 3 domain-containing Hof1p. Implication in cytokinesis in Saccharomyces cerevisiae. J. Biol. Chem. 273:28341-28345. [DOI] [PubMed] [Google Scholar]
- 29.Kapteyn, J. C., B. ter Riet, E. Vink, S. Blad, H. De Nobel, H. Van Den Ende, and F. M. Klis. 2001. Low external pH induces HOG1-dependent changes in the organization of the Saccharomyces cerevisiae cell wall. Mol. Microbiol. 39:469-479. [DOI] [PubMed] [Google Scholar]
- 30.Kapteyn, J. C., P. Van Egmond, E. Sievi, H. Van Den Ende, M. Makarow, and F. M. Klis. 1999. The contribution of the O-glycosylated protein Pir2p/Hsp150 to the construction of the yeast cell wall in wild-type cells and beta 1,6-glucan-deficient mutants. Mol. Microbiol. 31:1835-1844. [DOI] [PubMed] [Google Scholar]
- 31.Ketela, T., R. Green, and H. Bussey. 1999. Saccharomyces cerevisiae mid2p is a potential cell wall stress sensor and upstream activator of the PKC1-MPK1 cell integrity pathway. J. Bacteriol. 181:3330-3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klis, F. M., P. Mol, K. Hellingwerf, and S. Brul. 2002. Dynamics of cell wall structure in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 26:239-256. [DOI] [PubMed] [Google Scholar]
- 33.Kotyk, A., G. Lapathitis, and S. Krenkova. 1999. Glucose- and K(+)-induced acidification in different yeast species. Folia Microbiol. (Prague) 44:295-298. [DOI] [PubMed] [Google Scholar]
- 34.Lee, K. S., K. Irie, Y. Gotoh, Y. Watanabe, H. Araki, E. Nishida, K. Matsumoto, and D. E. Levin. 1993. A yeast mitogen-activated protein kinase homolog (Mpk1p) mediates signalling by protein kinase C. Mol. Cell. Biol. 13:3067-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee, K. S., and D. E. Levin. 1992. Dominant mutations in a gene encoding a putative protein kinase (BCK1) bypass the requirement for a Saccharomyces cerevisiae protein kinase C homolog. Mol. Cell. Biol. 12:172-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levin, D. E., and E. Bartlett-Heubusch. 1992. Mutants in the S. cerevisiae PKC1 gene display a cell cycle-specific osmotic stability defect. J. Cell Biol. 116:1221-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Locke, E. G., M. Bonilla, L. Liang, Y. Takita, and K. W. Cunningham. 2000. A homolog of voltage-gated Ca2+ channels stimulated by depletion of secretory Ca2+ in yeast. Mol. Cell. Biol. 20:6686-6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lussier, M., A. M. White, J. Sheraton, T. di Paolo, J. Treadwell, S. B. Southard, C. I. Horenstein, J. Chen-Weiner, A. F. Ram, J. C. Kapteyn, T. W. Roemer, D. H. Vo, D. C. Bondoc, J. Hall, W. W. Zhong, A. M. Sdicu, J. Davies, F. M. Klis, P. W. Robbins, and H. Bussey. 1997. Large scale identification of genes involved in cell surface biosynthesis and architecture in Saccharomyces cerevisiae. Genetics 147:435-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Madden, K., Y. J. Sheu, K. Baetz, B. Andrews, and M. Snyder. 1997. SBF cell cycle regulator as a target of the yeast PKC-MAP kinase pathway. Science 275:1781-1784. [DOI] [PubMed] [Google Scholar]
- 40.Martin, H., J. Arroyo, M. Sanchez, M. Molina, and C. Nombela. 1993. Activity of the yeast MAP kinase homologue Slt2 is critically required for cell integrity at 37 degrees C. Mol. Gen. Genet. 241:177-184. [DOI] [PubMed] [Google Scholar]
- 41.Martin, H., J. M. Rodriguez-Pachon, C. Ruiz, C. Nombela, and M. Molina. 2000. Regulatory mechanisms for modulation of signaling through the cell integrity Slt2-mediated pathway in Saccharomyces cerevisiae. J. Biol. Chem. 275:1511-1519. [DOI] [PubMed] [Google Scholar]
- 42.Mazzoni, C., P. Zarov, A. Rambourg, and C. Mann. 1993. The SLT2 (MPK1) MAP kinase homolog is involved in polarized cell growth in Saccharomyces cerevisiae. J. Cell Biol. 123:1821-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moskvina, E., E. M. Imre, and H. Ruis. 1999. Stress factors acting at the level of the plasma membrane induce transcription via the stress response element (STRE) of the yeast Saccharomyces cerevisiae. Mol. Microbiol. 32:1263-1272. [DOI] [PubMed] [Google Scholar]
- 44.Myers, A. M., A. Tzagoloff, D. M. Kinney, and C. J. Lusty. 1986. Yeast shuttle and integrative vectors with multiple cloning sites suitable for construction of lacZ fusions. Gene 45:299-310. [DOI] [PubMed] [Google Scholar]
- 45.Nakano, K., T. Mutoh, R. Arai, and I. Mabuchi. 2003. The small GTPase Rho4 is involved in controlling cell morphology and septation in fission yeast. Genes Cells 8:357-370. [DOI] [PubMed] [Google Scholar]
- 46.Nierras, C. R., and J. R. Warner. 1999. Protein kinase C enables the regulatory circuit that connects membrane synthesis to ribosome synthesis in Saccharomyces cerevisiae. J. Biol. Chem. 274:13235-13241. [DOI] [PubMed] [Google Scholar]
- 47.Pardo, M., L. Monteoliva, J. Pla, M. Sanchez, C. Gil, and C. Nombela. 1999. Two-dimensional analysis of proteins secreted by Saccharomyces cerevisiae regenerating protoplasts: a novel approach to study the cell wall. Yeast 15:459-472. [DOI] [PubMed] [Google Scholar]
- 48.Philip, B., and D. E. Levin. 2001. Wsc1 and Mid2 are cell surface sensors for cell wall integrity signaling that act through Rom2, a guanine nucleotide exchange factor for Rho1. Mol. Cell. Biol. 21:271-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qadota, H., C. P. Python, S. B. Inoue, M. Arisawa, Y. Anraku, Y. Zheng, T. Watanabe, D. E. Levin, and Y. Ohya. 1996. Identification of yeast Rho1p GTPase as a regulatory subunit of 1,3-beta-glucan synthase. Science 272:279-281. [DOI] [PubMed] [Google Scholar]
- 50.Rajavel, M., B. Philip, B. M. Buehrer, B. Errede, and D. E. Levin. 1999. Mid2 is a putative sensor for cell integrity signaling in Saccharomyces cerevisiae. Mol. Cell. Biol. 19:3969-3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reinoso-Martin, C., C. Schuller, M. Schuetzer-Muehlbauer, and K. Kuchler. 2003. The yeast protein kinase C cell integrity pathway mediates tolerance to the antifungal drug caspofungin through activation of Slt2p mitogen-activated protein kinase signaling. Eukaryot. Cell 2:1200-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Riezman, H., T. Hase, A. P. van Loon, L. A. Grivell, K. Suda, and G. Schatz. 1983. Import of proteins into mitochondria: a 70 kilodalton outer membrane protein with a large carboxy-terminal deletion is still transported to the outer membrane. EMBO J. 2:2161-2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Robinson, N. G., L. Guo, J. Imai, E. A. Toh, Y. Matsui, and F. Tamanoi. 1999. Rho3 of Saccharomyces cerevisiae, which regulates the actin cytoskeleton and exocytosis, is a GTPase which interacts with Myo2 and Exo70. Mol. Cell. Biol. 19:3580-3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roelants, F. M., P. D. Torrance, N. Bezman, and J. Thorner. 2002. Pkh1 and pkh2 differentially phosphorylate and activate ypk1 and ykr2 and define protein kinase modules required for maintenance of cell wall integrity. Mol. Biol. Cell 13:3005-3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roumanie, O., M. F. Peypouquet, M. Bonneu, D. Thoraval, F. Doignon, and M. Crouzet. 2000. Evidence for the genetic interaction between the actin-binding protein Vrp1 and the RhoGAP Rgd1 mediated through Rho3p and Rho4p in Saccharomyces cerevisiae. Mol. Microbiol. 36:1403-1414. [DOI] [PubMed] [Google Scholar]
- 56.Saka, A., M. Abe, H. Okano, M. Minemura, H. Qadota, T. Utsugi, A. Mino, K. Tanaka, Y. Takai, and Y. Ohya. 2001. Complementing yeast rho1 mutation groups with distinct functional defects. J. Biol. Chem. 276:46165-46171. [DOI] [PubMed] [Google Scholar]
- 57.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 58.Schuller, C., J. L. Brewster, M. R. Alexander, M. C. Gustin, and H. Ruis. 1994. The HOG pathway controls osmotic regulation of transcription via the stress response element (STRE) of the Saccharomyces cerevisiae CTT1 gene. EMBO J. 13:4382-4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sekiya-Kawasaki, M., M. Abe, A. Saka, D. Watanabe, K. Kono, M. Minemura-Asakawa, S. Ishihara, T. Watanabe, and Y. Ohya. 2002. Dissection of upstream regulatory components of the Rho1p effector, 1,3-beta-glucan synthase, in Saccharomyces cerevisiae. Genetics 162:663-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Torres, L., H. Martin, M. I. Garcia-Saez, J. Arroyo, M. Molina, M. Sanchez, and C. Nombela. 1991. A protein kinase gene complements the lytic phenotype of Saccharomyces cerevisiae lyt2 mutants. Mol. Microbiol. 5:2845-2854. [DOI] [PubMed] [Google Scholar]
- 61.Verna, J., A. Lodder, K. Lee, A. Vagts, and R. Ballester. 1997. A family of genes required for maintenance of cell wall integrity and for the stress response in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 94:13804-13809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vilella, F., E. Herrero, J. Torres, and M. A. de la Torre-Ruiz. 2005. Pkc1 and the upstream elements of the cell integrity pathway in Saccharomyces cerevisiae, Rom2 and Mtl1, are required for cellular responses to oxidative stress. J. Biol. Chem. 280:9149-9159. [DOI] [PubMed] [Google Scholar]
- 63.Watanabe, Y., G. Takaesu, M. Hagiwara, K. Irie, and K. Matsumoto. 1997. Characterization of a serum response factor-like protein in Saccharomyces cerevisiae, Rlm1, which has transcriptional activity regulated by the Mpk1 (Slt2) mitogen-activated protein kinase pathway. Mol. Cell. Biol. 17:2615-2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zarzov, P., C. Mazzoni, and C. Mann. 1996. The SLT2 (MPK1) MAP kinase is activated during periods of polarized cell growth in yeast. EMBO J. 15:83-91. [PMC free article] [PubMed] [Google Scholar]
- 65.Zu, T., J. Verna, and R. Ballester. 2001. Mutations in WSC genes for putative stress receptors result in sensitivity to multiple stress conditions and impairment of Rlm1-dependent gene expression in Saccharomyces cerevisiae. Mol. Genet. Genomics 266:142-155. [DOI] [PubMed] [Google Scholar]