Abstract
Exposure to certain metal and metalloid species, such as arsenic, cadmium, chromium, and nickel, has been associated with an increased risk of cancer in humans. The biological effects of these metals are thought to result from induction of reactive oxygen species (ROS) and inhibition of DNA repair enzymes, although alterations in signal transduction pathways may also be involved in tumor development. To better understand metal toxicity and its connection to ROS, we have compared the effects of arsenite and hydrogen peroxide in wild-type and mutant strains of the fission yeast Schizosaccharomyces pombe. An atf1Δ pap1Δ strain, which is defective in two transcription factors that control stress responses, is extremely sensitive to hydrogen peroxide but not to arsenite. A strain that lacks the transcription factor Zip1 has the opposite relationship. Spc1 (Sty1) mitogen-activated protein kinase (MAPK), a homologue of mammalian p38 MAPK, and the upstream MAPK kinase (MAPKK) Wis1 are essential for survival of both arsenite and hydrogen peroxide. Inactivation of two MAPKK kinases, Win1 and Wis4, almost completely eliminates Spc1 activation by arsenite, yet these cells survive arsenite treatment. The two-component phosphorelay protein Mcs4, which acts upstream of Win1 and Wis4 and is required for Spc1 activation in response to oxidative stress, is not required for Spc1 activation in response to arsenite. We conclude that the toxic effects of arsenic are not strongly connected to oxidative stress and that although Spc1 is activated by arsenic exposure, the basal activity of Spc1 is largely sufficient for the survival of arsenic.
Arsenic and cadmium, along with other metals and metalloids, are a natural part of the environment and yet present serious health risks when they are found in high concentrations. Arsenic is a common contaminant at toxic waste sites, and high levels of arsenic occur naturally in soil, rocks, and water in some areas of the world. Epidemiological studies have established that exposure to certain metals and metalloids is associated with an increased risk of cancer, and in particular there is strong evidence that chromium, nickel, beryllium, cadmium, and arsenic are human carcinogens (8). Arsenic is especially worrying in countries such as Bangladesh, Argentina, and India, where it is found in high concentrations in groundwater. In these places there is clear evidence of an association between the intake of arsenic and increased risk of skin, lung, and bladder cancers (22).
Arsenic exists in inorganic and organic forms and in different oxidation states (−3, 0, +3, +5). With regard to environmental exposure, the main concern is trivalent and pentavalent oxidation states (arsenite and arsenate, respectively) (14). Arsenite can react with sulfur-containing compounds, such as glutathione (GSH) or cysteine, and arsenate can substitute for phosphorus in several biochemical reactions. Trivalent organic arsenicals are potent inhibitors of GSH reductase and thioredoxin reductase (16, 35). Glutathione is found in millimolar concentrations in mammalian cells and is considered a good marker of the redox state of the cell. Therefore, inhibition of GSH reductase by arsenic can substantially increase cellular oxidation levels.
Several reports have described the capacity of arsenite to damage DNA. Arsenite inhibits base excision repair and nucleotide excision repair mechanisms, leading to accumulation of oxidative DNA damage after arsenite treatment (1, 10-12, 26). Furthermore, poly(ADP-ribosyl)ation of nuclear proteins is one of the immediate cellular responses to DNA damage induced by ionizing radiation, alkylating agents, and oxidants (11). The reaction is catalyzed predominantly by PARP-1. PARP-1 is inhibited by arsenite at nanomolar concentrations; this protein has two zinc finger motifs that could be targeted by arsenite, by substitution of the zinc ion and modification of the activity of the protein (41).
However, arsenic is not a significant mutagen in mammalian cells. Hence, it has been considered a tumor promoter or copromoter. In some systems, solar UV radiation can increase its carcinogenic capacity by cotreatment with arsenic (23). In some population studies, an association between arsenic intake from drinking water and smoking has been shown to have synergistic effects in cancer development (4, 38).
There is an extensive body of evidence indicating that arsenite can induce transformation through activation of signal transduction pathways involved in cell proliferation, repression of antiproliferative pathways, and even inhibition of cell cycle checkpoints (22). Signaling components affected by metals include growth factor receptors, G proteins, mitogen-activated protein kinases (MAPKs), and nuclear transcription factors (8). Among the latter, AP-1 and NF-κB activities have been shown to be targeted by arsenite (13).
Studies of the mechanisms of stress response, signal transduction, and cell cycle control in the fission yeast Schizosaccharomyces pombe have provided an important framework for investigating analogous mechanisms in higher eukaryotes. A well-studied stress-activated MAPK pathway in this organism, analogous to the p38 pathway in mammals, has as its central component the MAPK homologue Spc1, also known as Sty1 and Phh1 (15, 17, 30). The Spc1 pathway is activated in response to many types of agents that cause cytotoxic stress, such as UV radiation, heat, hyperosmolarity, and hydrogen peroxide (5, 6, 17, 30, 31, 40). In most cases Spc1 is essential for survival of cells exposed to these forms of stress. Two transcription factors, Atf1 and Pap1, homologues of human ATF-2 and c-Jun, respectively, are necessary for the response to different types of stress (5, 31, 36, 37, 40). Another transcription factor, Zip1, has been shown to be specifically involved in the response to cadmium (9).
Yeast model systems present a good opportunity to investigate the relationship between toxic metals and the effects of reactive oxygen species (ROS). Here we build on earlier studies to compare the biological effects of arsenite and hydrogen peroxide in wild-type and mutant strains of fission yeast. From these studies we conclude that in fission yeast these two stress agents elicit distinct biological responses and are detected by different primary sensors.
MATERIALS AND METHODS
Yeast strains, media, and general methods.
Cell growth conditions and media were as described previously (18). All strains, unless otherwise indicated, were h−ura4-D18 leu1-32. Their genotypes were as follows: PR109, wild type; KS1497, atf1::ura4+ (31); KS1366, spc1::ura4+ (30); KS1779, atf1-HA6His(ura4+) (31); KS1376, spc1+-HA6His(ura4+) (30); KS1484, pap1::ura4+ (lab stock); KS1535, pap1::ura4+ atf1::ura4+ (lab stock); KS1559, mcs4-13 spc1+-HA6His(ura4+) (33); GD1682, wis1::ura4+ spc1+-HA6His(ura4+) (lab stock); KS2086, wis1(AA)-12myc(ura4+) spc1+-HA6His(ura4+) (32); KS1667, wis4::ura4+ spc1+-HA6His(ura4+) (33); KS2148, win1-1 spc1+-HA6His(ura4+) his7-366 (lab stock); KS2189, win1-1 wis4::his7+ spc1+-HA6His(ura4+) his7-366 (lab stock); KS2209, win1-1 wis4::his7+ pyp1::leu2+ spc1+-HA6His(ura4+) his7-366 (lab stock); MR3566, win1-1 wis4::his7+ pyp2::kanMX6 spc1+-HA6His(ura4+) his7-366 (this work); VB1700, mcs4(D412N) spc1+-HA6His(ura4+) his7-366 ade6-M210 (2); CLP52-1, zip1− (sup9) ura4+ leu1+ (9). For plate survival assays, serial dilutions of yeast culture were plated in media containing sodium arsenite (10 μM to 10 mM).
Protein methods.
Detection of phosphorylated Spc1 by using an anti-phosphorylated p38 (Thr-180/Tyr-182) MAPK antibody (New England Biolabs) and Ni affinity purification were performed as described previously (7). The hemagglutinin (HA) epitope was detected using mouse monoclonal antibodies (12CA5).
RESULTS
Sensitivity of fission yeast mutants to sodium arsenite.
If metal compounds such as arsenic cause toxicity by stimulating the intracellular production of ROS, then the cellular mechanisms that are required to survive exposure to toxic metals should overlap with those that protect cells from oxidative stress caused by hydrogen peroxide. This hypothesis was investigated by analyzing the growth of various mutant strains in the presence of arsenite or hydrogen peroxide. We first analyzed spc1Δ, atf1Δ, pap1Δ, and zip1Δ cells. As noted above, hydrogen peroxide exposure leads to activation of the MAPK Spc1 (p38 homologue) (6), triggering a transcriptional response that is largely mediated by a heterodimeric transcription factor that consists of Atf1 (ATF-2 homologue) and Pcr1 (21). The transcription factor Pap1 (AP-1) is also involved in the response, especially at low concentrations of hydrogen peroxide (21, 37, 39). Zip1 is a transcription factor that is essential for the response to cadmium in fission yeast (9).
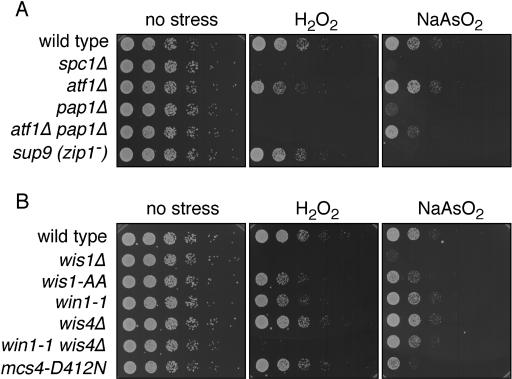
As shown before (37), and in the cell dilution plating assays shown in Fig. 1A, elimination of the MAPK Spc1 causes sensitivity to sodium arsenite (0.3 mM) and hydrogen peroxide (0.1 mM). Previous studies have shown that Pap1 is important for survival when cells are exposed to a low concentration of hydrogen peroxide, whereas Atf1-Pcr1 is required to survive exposure to higher concentrations of hydrogen peroxide (21). The atf1Δ cells were modestly sensitive to this low concentration of hydrogen peroxide, but they did not appear to be at all sensitive to the sodium arsenite. These findings indicated that activation of the Atf1-Pcr1 transcription factor is not the primary mechanism by which Spc1 promotes resistance to sodium arsenite. Consistent with the evidence that Pap1 is especially important for survival of low concentrations of hydrogen peroxide, we found that pap1Δ cells were sensitive to 0.1 mM hydrogen peroxide. Unexpectedly, the pap1Δ cells were also quite sensitive to 0.1 mM arsenite.
FIG. 1.
Viability of fission yeast mutants in response to sodium arsenite and hydrogen peroxide. (A) Serial dilutions (1/5) of wild-type, spc1Δ, atf1Δ, pap1Δ, atf1Δ pap1Δ, and zip1− (sup9) cultures were plated on YES, YES plus 0.1 mM hydrogen peroxide, or YES plus 0.3 mM sodium arsenite plates. Pictures were taken after 2 days of growth at 30°C. (B) Serial dilutions (1/5) of wild-type, wis1Δ, wis1(AA), win1-1, wis4Δ, win1-1 wis4Δ, and mcs4(D412N) cultures were plated on YES, YES plus 0.1 mM hydrogen peroxide, or YES plus 0.3 mM sodium arsenite plates. Pictures were taken after 2 days of growth at 30°C.
The observation that pap1Δ cells were sensitive to 0.1 mM arsenite was consistent with the possibility that exposure to 0.1 mM arsenite might lead to a modest elevation in ROS, perhaps to a level that is roughly equivalent to that caused by lower concentrations of hydrogen peroxide. If this were the case, we would expect that atf1Δ and pap1Δ mutations might display additive or synergistic interactions in the survival of arsenite exposure. To address this possibility, we created an atf1Δ pap1Δ double-mutant strain and tested its survival of hydrogen peroxide and arsenite exposure. As expected, the atf1Δ pap1Δ strain was unable to survive 0.1 mM hydrogen peroxide. However, this strain was significantly more resistant to 0.1 mM arsenite than the pap1Δ strain. The atf1Δ pap1Δ strain was not equivalent to the wild type or the atf1Δ strain, but its survival was reproducibly better than that of the pap1Δ strain.
We tested the sensitivity of zip1Δ cells to arsenite and found that they were very sensitive to arsenite but not at all sensitive to hydrogen peroxide (Fig. 1A). From these results we conclude that the function of Zip1 is specific for survival of metals or metallic compounds and that Zip1 is unlikely to be necessary for survival of ROS that might be generated as a consequence of exposure to these metals.
Requirement of Spc1 activators in the response to arsenite.
As shown above, the MAPK Spc1 is vitally important for survival of arsenite, as well as other types of stress such as hyperosmotic growth media, cold, heat shock, and UV irradiation (5, 6, 17, 30, 31, 40). In response to stress, Spc1 is phosphorylated by the MAPK kinase (MAPKK) Wis1 at tyrosine and threonine residues in a conserved TGY motif. This phosphorylation activates Spc1, resulting in the phosphorylation of its substrates, such as Atf1, Srk1, and Cmk2 (25, 31, 34). Wis1 is phosphorylated and activated by two MAPKK kinases (MAPKKKs), Win1 and Wis4 (also known as Wak1 and Wik1) (24, 28, 29, 32, 33). In response to hydrogen peroxide but not arsenite, Mcs4, a phosphorelay component, is necessary for the activation of the MAPKKKs (2, 28, 33). We decided to study which elements of this pathway are required for the response to arsenite in S. pombe, using mutants in the different components of the pathway.
The wis1Δ deletion mutant showed high sensitivity to 0.1 mM hydrogen peroxide and 0.1 mM arsenite, consistent with its essential role in the activation of Spc1 (Fig. 1B). A wis1(AA) strain, which expresses a form of Wis1 that cannot be phosphorylated and activated by the upstream MAPKKKs, showed a weaker sensitivity to hydrogen peroxide and arsenite. We conclude that Wis1(AA) protein has a basal activity that is sufficient for resistance to arsenite. The win1-1 mutation, which inactivates the Win1 MAPKKK, was also weakly sensitive to hydrogen peroxide but was not sensitive to arsenite. The wis4Δ null mutation, which deletes the gene that encodes the MAPKKK Wis4, had no effect on cell survival in the presence of hydrogen peroxide or arsenite. However, there was a striking difference when a win1-1 wis4Δ double mutant was plated on medium containing 0.1 mM hydrogen peroxide or 0.1 mM arsenite. This strain was extremely sensitive to hydrogen peroxide but not to arsenite. This result further supports the notion that the toxic effects of arsenite cannot be attributed to generation of ROS.
Interestingly, the mutant in Mcs4, mcs4(D412N), showed increased sensitivity to arsenite but not to hydrogen peroxide. This pattern was opposite that seen in the win1-1 wis4Δ double mutant, and again these results point out the differences in signaling between hydrogen peroxide and arsenite stresses.
Participation of Mcs4 in the response to arsenite.
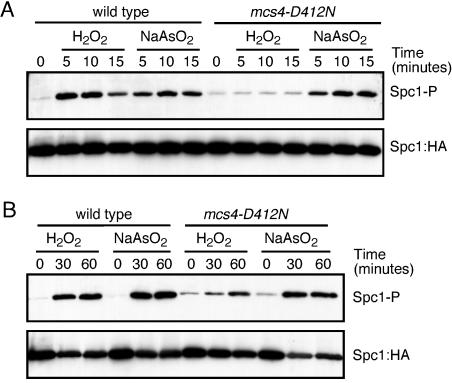
Mcs4 is an activator of the Spc1 pathway, working upstream of the MAPKKKs Wis4 and Win1 in response to hydrogen peroxide. We analyzed the effect of the mcs4(D412N) mutation on the activation of Spc1 in response to arsenite. Mcs4 makes a major contribution to Spc1 activation in response to hydrogen peroxide treatment (2, 19). However, the signal triggered by arsenite treatment is less dependent on (or independent of) Mcs4 activity (2). We compared the phosphorylation of Spc1 in the wild type or the mcs4(D412N) mutant after treatment with hydrogen peroxide or arsenite. Mutations in D412 of Mcs4 abolish the activity of the protein by removing the aspartate residue involved in phosphotransfer. Under our conditions, 1 mM hydrogen peroxide and 0.5 mM sodium arsenite produced very similar phosphorylation of Spc1 in wild-type cells (Fig. 2A). When the same experiment was performed with mcs4(D412N) mutants, we observed a complete abolishment of Spc1 phosphorylation after hydrogen peroxide treatment but no effect of Spc1 phosphorylation after sodium arsenite treatment (Fig. 2A). This result further confirms the differences between hydrogen peroxide and arsenite signaling. With longer times of stress treatment, however, the phosphorylation of Spc1 after hydrogen peroxide treatment almost reached the wild-type level (Fig. 2B). Therefore, Mcs4 is involved in the optimal activation of Spc1 after hydrogen peroxide exposure.
FIG. 2.
Function of Mcs4 in hydrogen peroxide versus arsenite stress. (A) Six-histidine-tagged Spc1::HA was purified from wild-type or mcs4(D412N) cells treated with 0.5 mM sodium arsenite or 1 mM hydrogen peroxide for 5 to 15 min. Phosphorylated Spc1 (Spc1-P) was detected using anti-phosphorylated p38. Spc1::HA was used as a loading control. (B) Phosphorylated Spc1 was detected from extracts obtained from wild-type or mcs4(D412N) cells treated with 0.5 mM sodium arsenite or 1 mM hydrogen peroxide for up to 60 min. Spc1::HA was used as a loading control.
Roles of the MAPKK Wis1 and the MAPKKKs Wis4 and Win1 in the response to arsenite stress.
Wis1 is the only MAPKK phosphorylating Spc1 under all the stress conditions described to date (17, 30). In response to arsenite, wis1Δ mutants do not show phosphorylation of Spc1, indicating that Wis1 is the only MAPKK involved in Spc1 activation after arsenite treatment (Fig. 3).
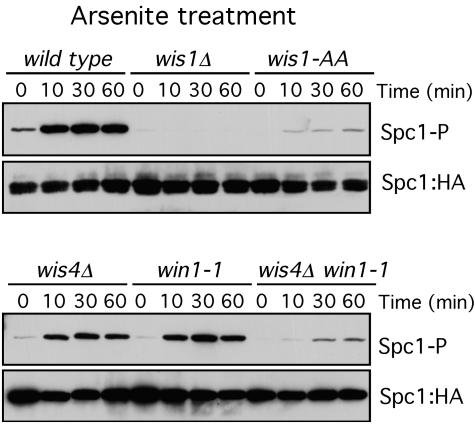
FIG. 3.
Roles of Wis1, Win1, and Wis4 in the transmission of the arsenite signal. Spc1 phosphorylation (Spc1-P) was monitored in the wild type and in wis1Δ, wis1(AA), wis4Δ, win1-1, and wis4Δ win1-1 mutants. Cells were treated with 0.5 mM sodium arsenite. Spc1::HA was used as a loading control.
As described before, Wis1 mutants in which the MAPKKK phosphorylation sites have been replaced by alanine [wis1(AA)] are unable to transmit most of the stress signal to Spc1 (27, 32). In response to arsenite, wis1(AA) mutants show a decreased level of Spc1 phosphorylation, but phosphorylation is still present and induced after arsenite treatment, indicating that arsenite can activate Spc1 by a mechanism independent of Wis1 phosphorylation (Fig. 3).
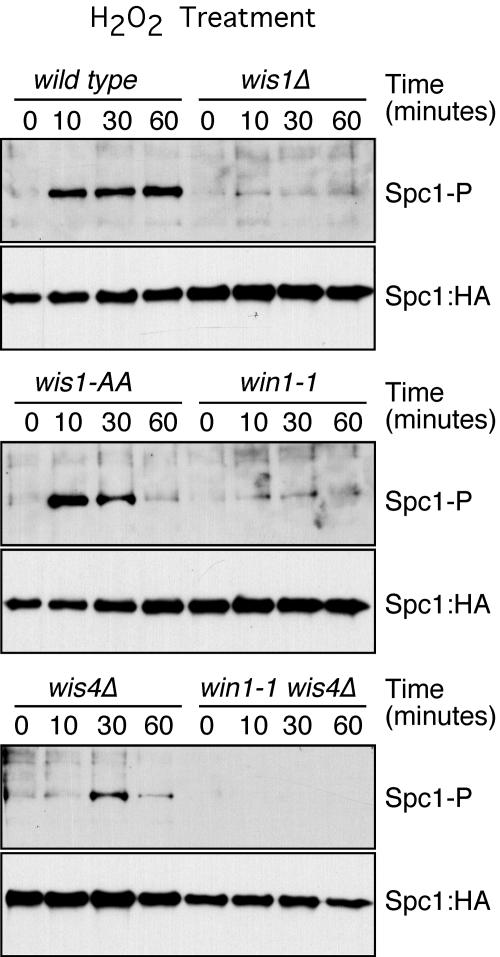
To confirm this result, we analyzed the phosphorylation of Spc1 in the MAPKKK-null wis4Δ and win1-1 mutants. The level of phosphorylation of Spc1 after arsenite treatment was reduced in the wis4Δ and win1-1 mutants. In the wis4Δ win1-1 double mutant, the phosphorylation of Spc1 after arsenite treatment was reduced to the levels observed in wis1(AA) mutants, confirming that MAPKKK phosphorylation of Wis1 is the principal step in Spc1 activation (Fig. 3) but there is another way by which arsenite activates Spc1 phosphorylation independently of Wis1 phosphorylation. In contrast, the activation of Spc1 in response to hydrogen peroxide was totally dependent on the coordinated activity of Win1 and Wis4. In the absence of both kinases, the phosphorylation of Spc1 in response to hydrogen peroxide was below the level of detection (Fig. 4). These results are consistent with those of the survival assays (Fig. 1B) and underscore the differences between arsenite and hydrogen peroxide signaling. Although wis1(AA) and wis4Δ win1-1 mutants were severely defective at activating Spc1 in response to arsenite (Fig. 3), these mutants were only modestly defective at survival of arsenite exposure (Fig. 1). The weak activation of Spc1 in these mutants is apparently sufficient to provide a large degree of protection from the toxic effects of arsenite.
FIG. 4.
Roles of Wis1, Win1, and Wis4 in the transmission of the hydrogen peroxide signal. Spc1 phosphorylation (Spc1-P) was monitored in the wild type and in wis1Δ, wis1(AA), win1-1, wis4Δ, and wis4Δ win1-1 mutants. Cells were treated with 1 mM hydrogen peroxide. Spc1::HA was used as a loading control.
Participation of the phosphatase Pyp1 in regulation of Spc1 during arsenite stress.
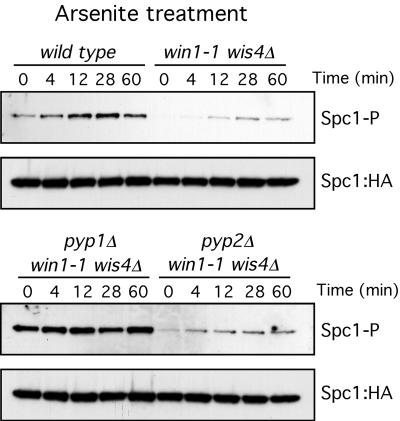
The results described above demonstrate that, in response to arsenite, a minor part of Spc1 phosphorylation is independent of Wis1 phosphorylation (Fig. 3). We reasoned that another way of increasing the level of phosphorylation of Spc1, independently of Wis1 phosphorylation, is to inhibit the activity of the phosphatases that eliminate those phosphate groups. It has been reported that, in mammalian cells, JNK phosphatase activity is inhibited by arsenite (3). In fission yeast, changes in the association of Pyp1 with Spc1 have been linked to activation of Spc1 by heat stress (20). We therefore decided to analyze whether the Wis1-independent activation of Spc1 was due to inhibition of the phosphatase Pyp1 or Pyp2. We compared the phosphorylation of Spc1 after arsenite treatment in mutants that lack MAPKKK activity. In this way, any downstream activity would be more easily detected. In this mutant background, we eliminated Pyp1 and Pyp2 to test if they were responsible for the increased activation of Spc1 in win1-1 wis4Δ or wis1(AA) backgrounds. As shown in Fig. 5, Spc1 phosphorylation increased after arsenite treatment in wis4Δ win1-1 pyp2Δ mutants but remained constant and not induced in wis4Δ win1-1 pyp1Δ mutants, indicating that Pyp1 activity is necessary to increase the phosphorylation levels of Spc1 after arsenite treatment.
FIG. 5.
Pyp1 participates in arsenite signaling. Spc1 phosphorylation (Spc1-P) was monitored in the wild type and in wis4Δ win1-1, wis4Δ win1-1 pyp1Δ, and wis4Δ win1-1 pyp2Δ mutants. Cells were treated with 0.5 mM sodium arsenite. Spc1::HA was used as a loading control.
DISCUSSION
In this report, several elements of the response to arsenic have been analyzed and compared to those involved in the response to hydrogen peroxide. The overall conclusion from these studies is that the responses to arsenic and hydrogen peroxide are quite different, with distinct requirements for signaling and survival. These studies indicate that ROS generation is unlikely to account for most of the toxic effects of arsenic, even though some proteins such as Spc1 are vital for survival of both arsenic and ROS. The transcription factors Atf1, Pap1, and Zip1 have different importance for cell survival depending on the stress encountered. The activation of the MAPK Spc1 in response to arsenite is independent of Mcs4 (2; also our results) but mostly dependent on the activity of the MAPKKKs Wis4 and Win1. Interestingly, a wis4Δ win1-1 strain is profoundly defective in Spc1 activation in response to arsenic exposure, yet the mutant cells are only moderately defective in survival of arsenic exposure. Finally, it appears that negative regulation of the tyrosine phosphatase Pyp1 is responsible for some of the activation of Spc1 phosphorylation in response to arsenite.
Transcription factors required for arsenite response.
The transcription factors Atf1, Pap1, and Zip1 have distinct and largely nonoverlapping roles in the survival of hydrogen peroxide and arsenic. As shown in Fig. 1A, atf1Δ mutants are not sensitive to sodium arsenite and are only mildly sensitive to a low concentration of hydrogen peroxide, indicating that Atf1 is not an essential factor in the response to arsenite. On the other hand, Pap1 seems to be very important for both hydrogen peroxide and sodium arsenite response. An absence of Pap1 activity leads to very low viability of S. pombe cells under both types of stress.
Interestingly, the atf1Δ pap1Δ double mutant displays an unexpected behavior upon exposure to hydrogen peroxide and sodium arsenite. When exposed to hydrogen peroxide, atf1Δ pap1Δ cells have very low viability, consistent with the sensitivities of the single mutants. However, when exposed to sodium arsenite, the atf1Δ pap1Δ cells are less sensitive than pap1Δ cells, indicating that Atf1 could function as a negative factor in the response to arsenite. The atf1Δ pap1Δ strain was not equivalent to the wild type or the atf1Δ strain, but its survival was reproducibly better than that of the pap1Δ strain. This result recalls a previous study in which we found that atf1Δ significantly suppresses the UV light sensitivity of spc1Δ mutants (5). In this situation it appeared that elimination of Atf1 increased the basal expression of Atf1- and Spc1-regulated genes in the spc1Δ mutant background. A similar phenomenon might explain why the atf1Δ mutation suppresses the arsenic sensitivity of pap1Δ cells. In future studies it may be interesting to investigate this possibility and also to examine whether the atf1Δ mutation suppresses the arsenic sensitivity of spc1Δ mutants.
We also tested whether zip1Δ cells were sensitive to hydrogen peroxide and sodium arsenite. In contrast to atf1Δ pap1Δ cells, zip1Δ cells were very sensitive to sodium arsenite but not to hydrogen peroxide. Taken together, these results indicate that on the one hand, hydrogen peroxide and sodium arsenite have very different effects on fission yeast physiology, even though some of their effects seem to be similar (e.g., pap1Δ mutant sensitivities). On the other hand, it seems that the transcriptional program put in place to respond to different types of stress requires several transcription factors, such as Atf1, Pap1, and Zip1, with a different degree of importance and functional requirement for each type of stress.
Pathway of activation of Spc1 in response to arsenite exposure.
Spc1 is activated in response to sodium arsenite (2; also this work), and its activity is essential for the response (37; also this work). Spc1 is phosphorylated and activated by the MAPK kinase Wis1 (Fig. 3). Most of the activation of Spc1 is mediated by the joint activity of the two MAPKKKs of the pathway, Wis4 and Win1. Mutants in both kinases show great reductions in the level of Spc1 phosphorylation in response to arsenite (Fig. 3). Mutations in the Wis1 sites phosphorylated by Wis4 and Win1 have identical effects on Spc1 activation (Fig. 3), confirming that the transmission of most of the arsenite signal occurs through activation of the MAPKKKs Wis4 and Win1.
Interestingly, previous reports have shown that Mcs4 phosphorylation is not necessary for the phosphorylation of Spc1 in response to arsenite (2). Using an Mcs4 allele unable to perform its phosphorelay function [mcs4(D412N)], we saw a complete abolishment of hydrogen peroxide signaling, measured by the level of Spc1 phosphorylation at early time points (Fig. 2A). At longer times of hydrogen peroxide exposure, Spc1 becomes phosphorylated even in the mcs4(D412N) mutant, perhaps explaining the survival of this mutant on hydrogen peroxide plates (Fig. 1B and 2B). We have observed that mcs4(D412N) cells appear to be more sensitive to arsenite than win1-1 wis4Δ double-mutant cells (Fig. 1). Yet Spc1 phosphorylation is dramatically reduced in the win1-1 wis4Δ double mutant (Fig. 3) but only moderately affected in mcs4(D412N) cells (Fig. 2). These observations suggest that Mcs4 might have a role in the arsenite response that is independent of Spc1 activation. The absence of this function, perhaps combined with the moderate defect in Spc1 activation in mcs4(D412N) cells, might account for the increased sensitivity of these cells to arsenite.
To assess which other points of the pathway were targeted by arsenite signaling independently of the MAPKKKs, we used the wis4Δ win1-1 pyp1Δ and wis4Δ win1-1 pyp2Δ triple mutants. Dephosphorylation and inactivation of Spc1 are carried out mainly by the dual phosphatase Pyp1 (17, 30). If arsenite inhibits Pyp1, we would expect that in response to arsenite, the levels of Spc1 phosphorylation would not change in the wis4Δ win1-1 pyp1Δ triple mutant. Otherwise, if the signaling is independent of Pyp1 or if Pyp1 is not the only target, we would expect changes in Spc1 phosphorylation after arsenite treatment in the wis4Δ win1-1 pyp1Δ mutant. As shown in Fig. 5, Spc1 phosphorylation did not increase in the wis4Δ win1-1 pyp1Δ triple mutant, indicating that Pyp1 is targeted by arsenite. These data are reminiscent of a study of the heat shock response in fission yeast in which it was found that negative regulation of Pyp1 is responsible, at least in part, for the activation of Spc1 (20). That study found that heat stress, but not osmotic stress, led to a decrease in the association of Pyp1 with Spc1 in vivo (20). In fact, in that study, the investigators also observed that arsenite impairs the interaction of Pyp1 with Spc1 (K. Shiozaki, personal communication). The findings suggest that heat shock and arsenite stress have a underlying commonality that leads to negative regulation of Pyp1.
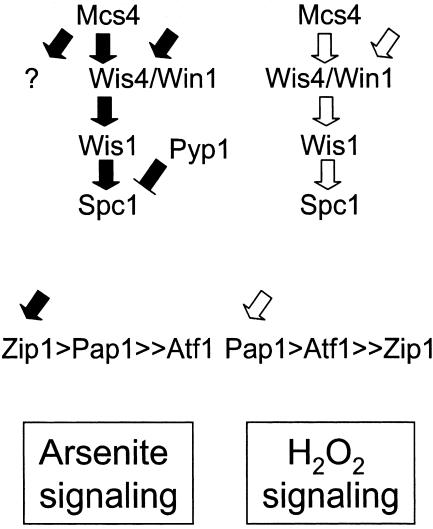
Taking all these results together, we can propose a model in which Spc1 is activated in response to arsenite by a mechanism largely dependent on Wis4 and Win1, with some signaling mediated through a mechanism downstream of the MAPKKKs that possibly involves the inactivation of Pyp1 (Fig. 6). The upstream activators of Wis4 and Win1 in response to arsenite are not known yet. Our current studies are focused on identifying these factors
FIG. 6.
Model for arsenite signaling versus hydrogen peroxide in Schizosaccharomyces pombe. See the text for a more detailed description.
Acknowledgments
We thank Jonathan Millar and Takashi Toda for providing strains. We thank the Scripps Cell Cycle Groups and members of the Russell laboratory for support and encouragement. Thanks to Victoria Martin and the anonymous reviewers for critical reading of the manuscript.
The project described here was supported by grant ES10337 from the National Institute of Environmental Health Sciences, NIH (to P.R.).
The contents of this report are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NIH.
REFERENCES
- 1.Bau, D. T., T. S. Wang, C. H. Chung, A. S. Wang, and K. Y. Jan. 2002. Oxidative DNA adducts and DNA-protein cross-links are the major DNA lesions induced by arsenite. Environ. Health Perspect. 110:753-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buck, V., J. Quinn, T. Soto Pino, H. Martin, J. Saldanha, K. Makino, B. A. Morgan, and J. B. Millar. 2001. Peroxide sensors for the fission yeast stress-activated mitogen-activated protein kinase pathway. Mol. Biol. Cell 12:407-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cavigelli, M., W. W. Li, A. Lin, B. Su, K. Yoshioka, and M. Karin. 1996. The tumor promoter arsenite stimulates AP-1 activity by inhibiting a JNK phosphatase. EMBO J. 15:6269-6279. [PMC free article] [PubMed] [Google Scholar]
- 4.Chiou, H. Y., Y. M. Hsueh, K. F. Liaw, S. F. Horng, M. H. Chiang, Y. S. Pu, J. S. Lin, C. H. Huang, and C. J. Chen. 1995. Incidence of internal cancers and ingested inorganic arsenic: a seven-year follow-up study in Taiwan. Cancer Res. 55:1296-1300. [PubMed] [Google Scholar]
- 5.Degols, G., and P. Russell. 1997. Discrete roles of the Spc1 kinase and the Atf1 transcription factor in the UV response of Schizosaccharomyces pombe. Mol. Cell. Biol. 17:3356-3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Degols, G., K. Shiozaki, and P. Russell. 1996. Activation and regulation of the Spc1 stress-activated protein kinase in Schizosaccharomyces pombe. Mol. Cell. Biol. 16:2870-2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaits, F., G. Degols, K. Shiozaki, and P. Russell. 1998. Phosphorylation and association with the transcription factor Atf1 regulate localization of Spc1/Sty1 stress-activated kinase in fission yeast. Genes Dev. 12:1464-1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris, G. K., and X. Shi. 2003. Signaling by carcinogenic metals and metal-induced reactive oxygen species. Mutat. Res. 533:183-200. [DOI] [PubMed] [Google Scholar]
- 9.Harrison, C., S. Katayama, S. Dhut, D. Chen, N. Jones, J. Bahler, and T. Toda. 2005. SCF(Pof1)-ubiquitin and its target Zip1 transcription factor mediate cadmium response in fission yeast. EMBO J. 24:599-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartwig, A., M. Asmuss, I. Ehleben, U. Herzer, D. Kostelac, A. Pelzer, T. Schwerdtle, and A. Burkle. 2002. Interference by toxic metal ions with DNA repair processes and cell cycle control: molecular mechanisms. Environ. Health Perspect. 110:797-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hartwig, A., H. Blessing, T. Schwerdtle, and I. Walter. 2003. Modulation of DNA repair processes by arsenic and selenium compounds. Toxicology 193:161-169. [DOI] [PubMed] [Google Scholar]
- 12.Hartwig, A., and T. Schwerdtle. 2002. Interactions by carcinogenic metal compounds with DNA repair processes: toxicological implications. Toxicol. Lett. 127:47-54. [DOI] [PubMed] [Google Scholar]
- 13.Hu, Y., X. Jin, and E. T. Snow. 2002. Effect of arsenic on transcription factor AP-1 and NF-κB DNA binding activity and related gene expression. Toxicol. Lett. 133:33-45. [DOI] [PubMed] [Google Scholar]
- 14.Hughes, M. F. 2002. Arsenic toxicity and potential mechanisms of action. Toxicol. Lett. 133:1-16. [DOI] [PubMed] [Google Scholar]
- 15.Kato, T., Jr., K. Okazaki, H. Murakami, S. Stettler, P. A. Fantes, and H. Okayama. 1996. Stress signal, mediated by a Hog1-like MAP kinase, controls sexual development in fission yeast. FEBS Lett. 378:207-212. [DOI] [PubMed] [Google Scholar]
- 16.Lin, S., W. R. Cullen, and D. J. Thomas. 1999. Methylarsenicals and arsinothiols are potent inhibitors of mouse liver thioredoxin reductase. Chem. Res. Toxicol. 12:924-930. [DOI] [PubMed] [Google Scholar]
- 17.Millar, J. B., V. Buck, and M. G. Wilkinson. 1995. Pyp1 and Pyp2 PTPases dephosphorylate an osmosensing MAP kinase controlling cell size at division in fission yeast. Genes Dev. 9:2117-2130. [DOI] [PubMed] [Google Scholar]
- 18.Moreno, S., A. Klar, and P. Nurse. 1991. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 194:795-823. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen, A. N., A. Lee, W. Place, and K. Shiozaki. 2000. Multistep phosphorelay proteins transmit oxidative stress signals to the fission yeast stress-activated protein kinase. Mol. Biol. Cell 11:1169-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen, A. N., and K. Shiozaki. 1999. Heat-shock-induced activation of stress MAP kinase is regulated by threonine- and tyrosine-specific phosphatases. Genes Dev. 13:1653-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quinn, J., V. J. Findlay, K. Dawson, J. B. Millar, N. Jones, B. A. Morgan, and W. M. Toone. 2002. Distinct regulatory proteins control the graded transcriptional response to increasing H2O2 levels in fission yeast Schizosaccharomyces pombe. Mol. Biol. Cell 13:805-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rossman, T. G. 2003. Mechanism of arsenic carcinogenesis: an integrated approach. Mutat. Res. 533:37-65. [DOI] [PubMed] [Google Scholar]
- 23.Rossman, T. G., A. N. Uddin, F. J. Burns, and M. C. Bosland. 2001. Arsenite is a cocarcinogen with solar ultraviolet radiation for mouse skin: an animal model for arsenic carcinogenesis. Toxicol. Appl. Pharmacol. 176:64-71. [DOI] [PubMed] [Google Scholar]
- 24.Samejima, I., S. Mackie, and P. A. Fantes. 1997. Multiple modes of activation of the stress-responsive MAP kinase pathway in fission yeast. EMBO J. 16:6162-6170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanchez-Piris, M., F. Posas, V. Alemany, I. Winge, E. Hidalgo, O. Bachs, and R. Aligue. 2002. The serine/threonine kinase Cmk2 is required for oxidative stress response in fission yeast. J. Biol. Chem. 277:17722-17727. [DOI] [PubMed] [Google Scholar]
- 26.Schwerdtle, T., I. Walter, I. Mackiw, and A. Hartwig. 2003. Induction of oxidative DNA damage by arsenite and its trivalent and pentavalent methylated metabolites in cultured human cells and isolated DNA. Carcinogenesis 24:967-974. [DOI] [PubMed] [Google Scholar]
- 27.Shieh, J. C., H. Martin, and J. B. Millar. 1998. Evidence for a novel MAPKKK-independent pathway controlling the stress activated Sty1/Spc1 MAP kinase in fission yeast. J. Cell Sci. 111:2799-2807. [DOI] [PubMed] [Google Scholar]
- 28.Shieh, J. C., M. G. Wilkinson, V. Buck, B. A. Morgan, K. Makino, and J. B. Millar. 1997. The Mcs4 response regulator coordinately controls the stress-activated Wak1-Wis1-Sty1 MAP kinase pathway and fission yeast cell cycle. Genes Dev. 11:1008-1022. [DOI] [PubMed] [Google Scholar]
- 29.Shieh, J. C., M. G. Wilkinson, and J. B. Millar. 1998. The Win1 mitotic regulator is a component of the fission yeast stress-activated Sty1 MAPK pathway. Mol. Biol. Cell 9:311-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shiozaki, K., and P. Russell. 1995. Cell-cycle control linked to extracellular environment by MAP kinase pathway in fission yeast. Nature 378:739-743. [DOI] [PubMed] [Google Scholar]
- 31.Shiozaki, K., and P. Russell. 1996. Conjugation, meiosis, and the osmotic stress response are regulated by Spc1 kinase through Atf1 transcription factor in fission yeast. Genes Dev. 10:2276-2288. [DOI] [PubMed] [Google Scholar]
- 32.Shiozaki, K., M. Shiozaki, and P. Russell. 1998. Heat stress activates fission yeast Spc1/StyI MAPK by a MEKK-independent mechanism. Mol. Biol. Cell 9:1339-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shiozaki, K., M. Shiozaki, and P. Russell. 1997. Mcs4 mitotic catastrophe suppressor regulates the fission yeast cell cycle through the Wik1-Wis1-Spc1 kinase cascade. Mol. Biol. Cell 8:409-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith, D. A., W. M. Toone, D. Chen Murchie, J. Bahler, N. Jones, B. A. Morgan, and J. Quinn. 2002. The Srk1 protein kinase is a target for the Sty1 stress-activated MAPK in fission yeast. J. Biol. Chem. 277:33411-33421. [DOI] [PubMed] [Google Scholar]
- 35.Styblo, M., S. V. Serves, W. R. Cullen, and D. J. Thomas. 1997. Comparative inhibition of yeast glutathione reductase by arsenicals and arsenothiols. Chem. Res. Toxicol. 10:27-33. [DOI] [PubMed] [Google Scholar]
- 36.Toda, T., M. Shimanuki, and M. Yanagida. 1991. Fission yeast genes that confer resistance to staurosporine encode an AP-1-like transcription factor and a protein kinase related to the mammalian ERK1/MAP2 and budding yeast FUS3 and KSS1 kinases. Genes Dev. 5:60-73. [DOI] [PubMed] [Google Scholar]
- 37.Toone, W. M., S. Kuge, M. Samuels, B. A. Morgan, T. Toda, and N. Jones. 1998. Regulation of the fission yeast transcription factor Pap1 by oxidative stress: requirement for the nuclear export factor Crm1 (Exportin) and the stress-activated MAP kinase Sty1/Spc1. Genes Dev. 12:1453-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsuda, T., A. Babazono, E. Yamamoto, N. Kurumatani, Y. Mino, T. Ogawa, Y. Kishi, and H. Aoyama. 1995. Ingested arsenic and internal cancer: a historical cohort study followed for 33 years. Am. J. Epidemiol. 141:198-209. [DOI] [PubMed] [Google Scholar]
- 39.Vivancos, A. P., E. A. Castillo, N. Jones, J. Ayte, and E. Hidalgo. 2004. Activation of the redox sensor Pap1 by hydrogen peroxide requires modulation of the intracellular oxidant concentration. Mol. Microbiol. 52:1427-1435. [DOI] [PubMed] [Google Scholar]
- 40.Wilkinson, M. G., M. Samuels, T. Takeda, W. M. Toone, J. C. Shieh, T. Toda, J. B. Millar, and N. Jones. 1996. The Atf1 transcription factor is a target for the Sty1 stress-activated MAP kinase pathway in fission yeast. Genes Dev. 10:2289-2301. [DOI] [PubMed] [Google Scholar]
- 41.Yager, J. W., and J. K. Wiencke. 1997. Inhibition of poly(ADP-ribose) polymerase by arsenite. Mutat. Res. 386:345-351. [DOI] [PubMed] [Google Scholar]