Abstract
To study the impact of solar UV radiation (UVR) (280 to 400 nm) on the filamentous cyanobacterium Arthrospira (Spirulina) platensis, we examined the morphological changes and photosynthetic performance using an indoor-grown strain (which had not been exposed to sunlight for decades) and an outdoor-grown strain (which had been grown under sunlight for decades) while they were cultured with three solar radiation treatments: PAB (photosynthetically active radiation [PAR] plus UVR; 280 to 700 nm), PA (PAR plus UV-A; 320 to 700 nm), and P (PAR only; 400 to 700 nm). Solar UVR broke the spiral filaments of A. platensis exposed to full solar radiation in short-term low-cell-density cultures. This breakage was observed after 2 h for the indoor strain but after 4 to 6 h for the outdoor strain. Filament breakage also occurred in the cultures exposed to PAR alone; however, the extent of breakage was less than that observed for filaments exposed to full solar radiation. The spiral filaments broke and compressed when high-cell-density cultures were exposed to full solar radiation during long-term experiments. When UV-B was screened off, the filaments initially broke, but they elongated and became loosely arranged later (i.e., there were fewer spirals per unit of filament length). When UVR was filtered out, the spiral structure hardly broke or became looser. Photosynthetic O2 evolution in the presence of UVR was significantly suppressed in the indoor strain compared to the outdoor strain. UVR-induced inhibition increased with exposure time, and it was significantly lower in the outdoor strain. The concentration of UV-absorbing compounds was low in both strains, and there was no significant change in the amount regardless of the radiation treatment, suggesting that these compounds were not effectively used as protection against solar UVR. Self-shading, on the other hand, produced by compression of the spirals over adaptive time scales, seems to play an important role in protecting this species against deleterious UVR. Our findings suggest that the increase in UV-B irradiance due to ozone depletion not only might affect photosynthesis but also might alter the morphological development of filamentous cyanobacteria during acclimation or over adaptive time scales.
The depletion of the stratospheric ozone layer, mainly due to anthropogenically released pollutants such as chlorofluorocarbons, has resulted in an increase in the solar UV-B radiation (280 to 320 nm) that reaches the Earth's surface (10, 26). Ozone depletion is expected to increase and to spread over a broader range of altitudes and latitudes throughout most of the current century (49). UV-B radiation is potentially detrimental to all forms of life but is more detrimental to photosynthetic organisms, including cyanobacteria (21, 47, 48). The cyanobacteria are the largest and most widely distributed group of photosynthetic prokaryotes on the Earth, and they contribute markedly to global CO2 and N2 fixation (7, 15). It has been reported that UV-B not only impairs the motility and photoorientation of cyanobacteria (13) but also affects a number of physiological and biochemical processes, such as growth, survival, pigmentation, nitrogen metabolism enzymes (28, 43), CO2 uptake, and ribulose 1,5-bisphosphate carboxylase activity (27, 46). On the other hand, cyanobacteria are the oldest autotrophic inhabitants of the planet, and they may have been exposed to high UV radiation (UVR) levels during the early Precambrian era (12, 17). Therefore, they must have developed effective mechanisms to counteract detrimental effects of these highly energetic wavelengths (at present levels of UVR and possibly at higher levels that may occur in the future). Many species of cyanobacteria show wide variation in tolerance to UV-B and possess a variety of defense strategies, such as avoidance of brightly lit habitats (2, 8, 53), production of UV-absorbing compounds, such as mycosporine-like amino acids (MAAs) and scytonemin (19, 45), and active repair or de novo synthesis of DNA (41, 42). However, despite the large amount of literature on the effects of UVR on cyanobacteria, we are not aware of studies documenting the impact of solar UVR on their morphology.
Arthrospira (Spirulina) platensis is an economically well-known filamentous cyanobacterium that is commercially produced as a source of human health food (9), animal feed (31), and cosmetic colorants (11), and considerable efforts have been made to optimize the growth conditions of this organism for massive production (24, 40, 50, 52). The morphological features of Arthrospira species include characteristic regular helical coiling or spirals. The helicity has been used as a taxonomic criterion (30), as well as a way to select for high-quality strains (3). Environmental factors, such as light, temperature, and salinity, can affect the helical structure (22, 23, 25, 30); for example, the filaments change from straight to the typical helical shape when they are shifted to growth with a high intensity of visible light (16). However, we are not aware of any study documenting the effects of solar UVR on the morphology of Arthrospira.
In this work, we investigated the effects of solar radiation on the morphology and photosynthesis of A. platensis. The information obtained not only could provide a potential basis for optimizing the growth conditions for this species with increasing solar UV-B radiation but also could have special importance for understanding the overall impact of UVR on filamentous cyanobacteria.
MATERIALS AND METHODS
Organisms.
Two strains of A. (S.) platensis were used in our experiments. These strains were (i) strain 439, an indoor strain that was grown in the laboratory, had not been exposed to sunlight for decades, and was obtained from the Freshwater Algae Culture Collection of the Institute of Hydrobiology, Chinese Academy of Sciences, and (ii) strain D-0083, an outdoor strain that has been grown under sunlight for decades in the commercial production base and was obtained from Hainan DIC Microalgae Co. Ltd., Hainan, China. For these two strains we used three different culture concentrations, 330, 590, and 780 mg (dry weight) liter−1, in the experiments outlined below.
Solar radiation measurements and treatments.
Incident solar radiation (UV-B [280 to 315 nm], UV-A [315 to 400 nm], and PAR [400 to 700 nm]) was continuously monitored using a broadband filter radiometer (ELDONET; Real Time Computer Inc., Germany) (20) permanently installed on a roof at Shantou University (116.6°E, 23.3°N).
Subsamples of A. platensis were harvested during the exponential growth phase, resuspended in Zarrouk freshwater medium, and dispensed into 1-liter UV-transparent quartz tubes (inside diameter, 6.4 cm; length, 40 cm). The cultures were aerated (0.35 liter min−1) and maintained in a flowthrough water bath for temperature control at 18 to 20°C. The following three radiation treatments were used: (i) cells received full solar radiation (PAB treatment; 280 to 700 nm) in uncovered quartz tubes; (ii) cells received UV-A and PAR (PA treatment; 320 to 700 nm) in quartz tubes covered with Folex 320 (Montagefolie no. 10155099; Folex, Dreieich, Germany); and (iii) cells received only PAR (P treatment; 400 to 700 nm) in quartz tubes covered with Ultraphan film 395 (UV Opak; Digefra, Munich, Germany).
Morphological examination.
Morphological examinations were performed using a compound microscope (Zeiss Axioplan 2; Carl Zeiss, Germany). Digital images were recorded with a Zeiss Axicam camera and were analyzed with a Vision Analysis system (AxioVision 3.0). The length and helix pitch of 20 to 50 A. platensis filaments were measured in at least five fields. The helix pitch was measured by using the ichnography of the spiral-shaped trichome (i.e., the distance between two neighboring spirals).
To study morphological changes, cultures of A. platensis at initial concentrations of 330, 590, and 780 mg (dry weight) liter−1 were exposed to the PAB and P treatments for 6 h centered on local noon (from 10 a.m. to 4 p.m.) on 2 and 3 December 2003. Subsamples were taken every 2 h and examined under the microscope to determine morphological changes. Since there were no morphological changes in the two strains with the high biomass (780 mg [dry weight] liter−1) in 1 day, we conducted long-term experiments for 15 to 20 days to monitor potential morphological changes in the strains. In this long-term experiment, subsamples were taken daily at sunset for microscopic observation.
Assessment of photosynthetic inhibition.
Cultures of A. platensis at initial concentrations of 330 and 780 mg (dry weight) liter−1 were exposed to solar radiation by using the PAB and P treatments for 6 h (duplicate samples for each treatment). Oxygen evolution was measured at the beginning (i.e., time zero [t0]) and then every 2 h during the incubation period, which was centered on local noon (from 9 or 10 a.m. to 3 or 4 p.m.). Photosynthetic O2 evolution was determined with a five-channel oxymeter (Oxym 5; Real Time Computers Inc., Germany), which had five quartz chambers in a UV-transparent thermal jacket. Five Clark microelectrodes (model 5351; Yellow Spring Instruments Co.) were connected to the quartz chambers and interphased to a laptop computer; data were acquired every 2 s. Complete measurement of photosynthesis for the two radiation treatments took approximately 12 to 15 min, and the rate of O2 evolution was calculated from the slope of the curve of O2 concentration versus time. The relative photosynthetic inhibition due to UVR was calculated as follows: (PPAR − PUVR) · (PPAR)−1, where PPAR and PUVR are the photosynthetic O2 evolution rates in cells exposed to only PAR and to PAR plus UVR, respectively. In order to compare photosynthetic inhibition with different irradiances (i.e., on different days), the values described above were normalized to the mean PAR irradiance over the measurement period (i.e., 12 to 15 min).
Evaluation of UV-absorbing compounds and photosynthetic pigments.
Samples were taken from the cultures after 5 and 10 days of exposure and filtered onto Whatman GF/F glass fiber filters (25 mm). UV-absorbing compounds and photosynthetic pigments were extracted in 10 ml of absolute methanol overnight and centrifuged (5,000 × g for 5 min), and the extract was scanned at wavelengths of 250 to 750 nm using a scanning spectrophotometer (Shimadzu UV 2501-PC). The total concentration of UV-absorbing compounds was estimated from the peak at 334 nm as described by Dunlap et al. (14). The chlorophyll a (Chl a) concentration was calculated as described by Porra (36), and the concentration of carotenoids was determined using the equations of Parsons and Strickland (35).
Statistical analysis.
Analysis of variance and a t test were used to establish differences among the different radiation treatments and strains. A confidence level of 95% was used in all analyses.
RESULTS
Morphological changes.
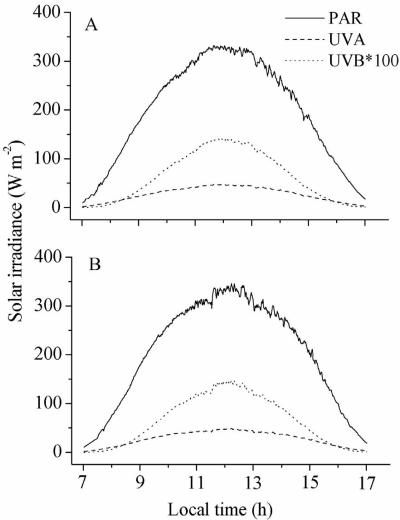
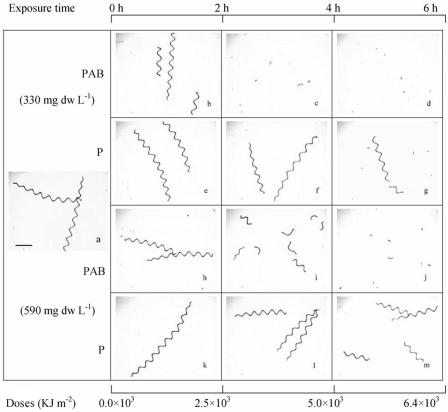
Morphological changes were examined for the indoor and outdoor strains on 2 December 2003 (Fig. 1A) and 3 December 2003 (Fig. 1B), respectively, when the maximum PAR irradiances were 329.2 and 341.1 W m−2, respectively. On these days, the maximum values for UV-A varied between 46.3 and 48.1 W m−2, whereas the maximum values for UV-B varied between 1.37 and 1.42 W m−2. The cells of indoor strain 439 were originally arranged in spring-shaped filaments that were ∼1.15 mm long with up to 12 spirals (Fig. 2a). Exposure to full solar radiation (i.e., PAB treatment) for 2 h led to breakage of the filaments in the low-biomass (330 mg [dry weight] liter−1) culture (Fig. 2b), whereas 4 to 6 h of exposure resulted in severe breakage of the spiral filaments into small pieces (Fig. 2c and d). In contrast, no significant changes in filament morphology were observed after 4 h of exposure to PAR (Fig. 2e and f), while breakage of filaments was induced by this treatment after 6 h of exposure (Fig. 2g) but to a lesser extent compared to the PAB treatment. In the culture with a biomass of 590 mg (dry weight) liter−1, the filaments did not break after 2 h of exposure to the PAB treatment (Fig. 2h), but breakage to form one or two helices was observed after 4 h of exposure (Fig. 2i); 6 h of exposure resulted in further breakage (Fig. 2j). As observed in the low-biomass culture, exposure to PAR for 2 to 4 h did not result in changes in filament morphology (Fig. 2k and l), and 6 h of exposure to PAR resulted in only slight breakage (Fig. 2m).
FIG. 1.
Solar irradiance curves for PAR, UV-A, and UV-B on 2 December 2003 (A) and 3 December 2003 (B), when morphological changes were evaluated (from 10 a.m. to 4 p.m.).
FIG. 2.
Spiral structure of A. (S.) platensis indoor-grown strain 439 exposed to different solar radiation treatments for 6 h centered on local noon. (a) Spiral structure at the beginning of the experiment (before exposure to solar radiation). Scale bar = 0.2 mm. (b to g) Spiral structure in low-biomass cultures (330 mg [dry weight] liter−1) exposed to full solar radiation (PAB) and only PAR (P) for 2, 4, and 6 h. (h to m) Spiral structure in high-biomass cultures (590 mg [dry weight] liter−1) exposed to the PAB and P treatments. dw, dry weight.
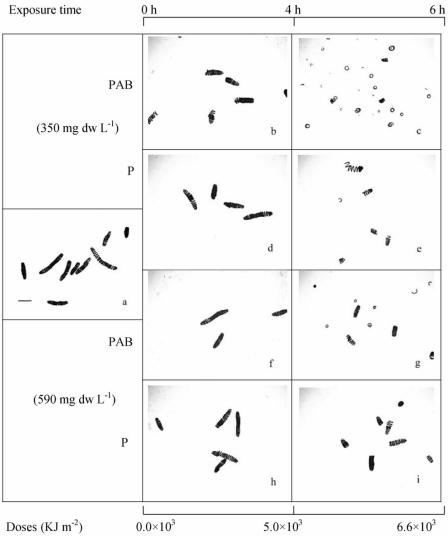
The cells of the outdoor-grown strain (D-0083) were arranged in a much tighter configuration; the filaments were ∼0.22 mm long with up to 19 spirals (Fig. 3a). Exposure to the PAB treatment for 4 h resulted in slight breakage of the filaments in the low-biomass (330 mg [dry weight] liter−1) culture (Fig. 3b). Severe breakage was observed after 6 h of exposure to full solar radiation (Fig. 3c), but small helices were still present. When cells were exposed to PAR for 4 h, the filaments did not break (Fig. 3d), but some breakage occurred after continuous exposure for 6 h (Fig. 3e). In contrast, no change in filament morphology was observed with the PAB treatment after 4 h of exposure in the culture containing 590 mg liter−1 (Fig. 3f); however, 6 h of exposure resulted in slight breakage of the filaments (Fig. 3g), but the extent of breakage was less than that in the low-biomass culture. Similarly, samples exposed to the P treatment did not show breakage of the filaments after 4 h of exposure (Fig. 3 h), but 6 h of exposure resulted in less breakage than the breakage observed with the PAB treatment or in the low-biomass culture (Fig. 3i).
FIG. 3.
Spiral structure of A. platensis outdoor-grown strain D-0083 exposed to different solar radiation treatments for 6 h centered on local noon. (a) Spiral structure at the beginning of the experiment (before exposure to solar radiation). Scale bar = 0.1 mm. (b to e) Spiral structure in low-biomass cultures (330 mg [dry weight] liter−1) exposed to full solar radiation (PAB) and only PAR (P) for 4 and 6 h. (f to i) Spiral structure in high-biomass cultures (590 mg [dry weight] liter−1) exposed to the PAB and P treatments. dw, dry weight.
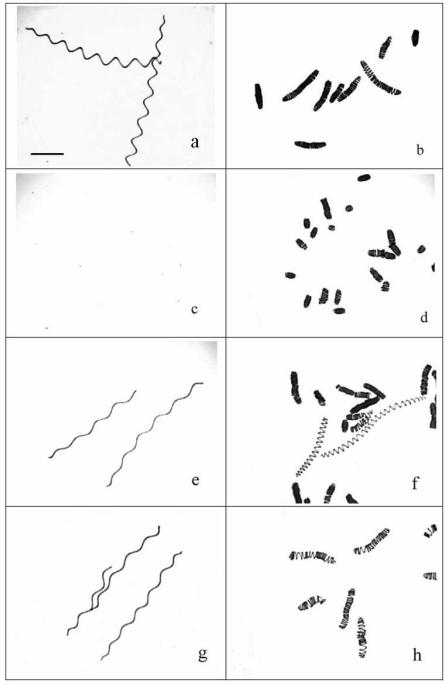
As mentioned above, no changes were observed in the short term (i.e., 6 h) for the two strains when the high-biomass cultures (i.e., 780 mg liter−1) were used. Thus, we investigated changes that could occur over longer time scales (i.e., weeks) when both strains of A. platensis were exposed to solar radiation using the three radiation treatments (i.e., PAB, PA, and P) (Fig. 4). Exposure to full solar radiation resulted in breakage of the spiral filaments within 5 and 10 days for the indoor and outdoor strains, respectively (Fig. 4c and d). Only cell debris was observed in the indoor strain culture after 5 days of exposure to PAB, and the culture became almost pellucid (Fig. 4c); on the other hand, shortened filaments of the outdoor strain had a tighter spiral structure (Fig. 4d). When UV-B was screened off, the spiral structure of A. platensis also broke during the initial period (1 to 2 days) (data not shown), but it sustained, elongated, and became loose later (Fig. 4e and f). When both UV-B and UV-A were filtered out, the spiral structure hardly broke, but it became loose (Fig. 4g and h).
FIG. 4.
Spiral structure of A. platensis indoor-grown strain 439 (a, c, e, and g) and outdoor-grown strain D-0083 (b, d, f, and h) exposed to different types of solar radiation in long-term cultures with a high level of biomass (780 mg [dry weight] liter−1). (a and b) Spiral structure at the beginning of the experiment (before exposure to solar radiation). Scale bar = 0.2 mm. (c and d) Death of cells of the indoor strain and breakage of the filaments of the outdoor strain exposed to the PAB treatment for 5 and 10 days, respectively. (e and f) Arrangement of the filament helix in the two strains exposed to the PA treatment for 15 days. (g and h) Loose helical structure when cells were exposed to only PAR for 10 and 20 days, respectively.
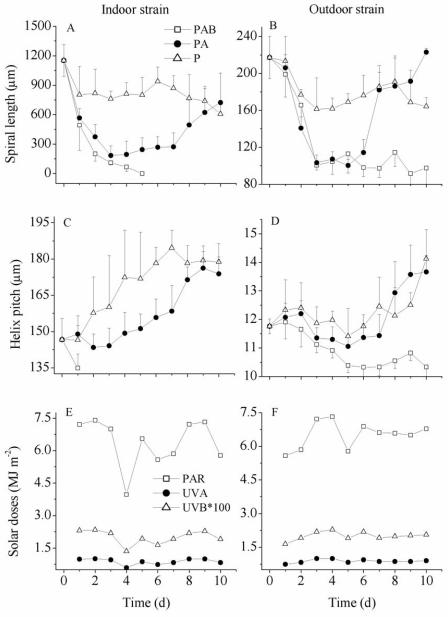
The trichome length (Fig. 5A and B) and the helical pitch (Fig. 5C and D) of A. platensis varied according to the radiation treatment imposed on the samples. In the indoor strain, the trichome length decreased significantly (P < 0.01) during the first 3 days of exposure to solar radiation and was 9.6% and 15.7% of the original mean value in the samples exposed to the PAB and PA treatments, respectively (Fig. 5A). The decrease in trichome length continued in the samples exposed to the PAB treatment until the length was almost zero on day 5. In the samples exposed to the PA treatment, the trichome length started to increase after day 3, but the difference was significant (P < 0.01) only after day 7. In the outdoor strain, however, the trichome length decreased to one-half of the initial value after 3 days of exposure to UVR (Fig. 5B). Continuous exposure to UVR did not result in a further decrease in the trichome length; exposure to UV-A, on the other hand, resulted in a significant (P < 0.01) increase in the trichome length after 6 days of exposure, and the length reached the original length on day 10. In samples exposed to only PAR there was an initial decrease in the trichome length after 1 and 2 days of exposure in the indoor and outdoor strains, respectively, but after this the trichome length did not vary significantly (P > 0.1). The helix pitch increased significantly (P < 0.01) with the PA and P treatments after 9 days of exposure to solar radiation in the indoor strain from the original value (146.6 ± 8.7 μm) to 176.2 ± 6.9 and 179.4 ± 6.1 μm, respectively (Fig. 5C), indicating that there was a decrease in the number of spirals per unit of filament length. A significant decrease (P < 0.01) in the helix pitch (about 8%), however, was observed in this strain with the PAB treatment after day 1 (Fig. 5C), indicating that the cell structure was compacted (i.e., there was an increased number of spirals per unit of filament length) in the presence of UVR. Breakage of trichomes into small pieces with the PAB treatment precluded measurement of the helix pitch after 1 day. Helix pitch in the outdoor strain also decreased significantly (P < 0.01) with the PAB treatment from the original value (11.8 ± 0.3 μm) to 10.3 ± 0.4 μm after day 5 (Fig. 5D), but it increased when UV-B or UVR was screened off. The increases, however, were significant only after day 9 (16%) and on day 10 (20%) (P < 0.01) with the PA and P treatments, respectively.
FIG. 5.
Structural changes in the spiral filaments of A. platensis indoor-grown strain 439 (A and C) and outdoor-grown strain D-0083 (B and D) as a function of time of exposure to solar radiation (E and F). (A and B) spiral length; (C and D) helix pitch; (E and F) daily solar doses during the exposure period. The vertical bars indicate the standard deviations around the means (n = 20 to 50).
Photosynthetic inhibition.
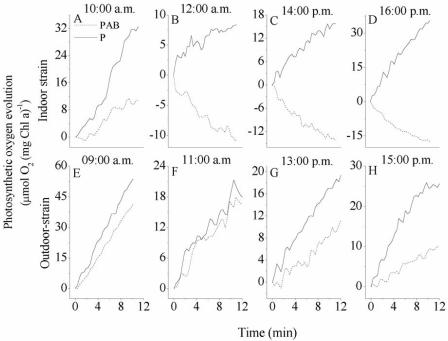
Together with the continuous breakage of the spiral structure of A. platensis exposed to full solar radiation during the short-term experiments (6 h) (Fig. 2 and 3), we observed significant UVR-induced reduction in photosynthetic O2 evolution (Fig. 6). Photosynthetic O2 evolution in the indoor strain was significantly suppressed (P < 0.01) in the presence of UVR at the beginning of the experiment (t0) (Fig. 6A) and after subsequent exposure to solar radiation (Fig. 6B, C, and D). Exposure to UVR for more than 2 h resulted in oxygen consumption (i.e., negative O2 evolution values) (Fig. 6B, C, and D). The outdoor strain also showed photoinhibitory effects when it was exposed to UVR, but to a lesser extent (Fig. 6E, F, G, and H). This strain displayed O2 production (i.e., photosynthesis) even at the highest UVR irradiance registered at noon (Fig. 6F and G). Photosynthetic O2 evolution was also suppressed in both strains when they were exposed only to PAR; however, the extent of suppression was much less than that observed with cells exposed to UVR plus PAR.
FIG. 6.
Photosynthetic oxygen evolution in A. platensis indoor-grown strain 439 (upper panels) and outdoor-grown strain D-0083 (lower panels) exposed to the PAB and P treatments for 6 h centered at local noon (from 10 a.m. to 4 p.m. or from 9 a.m. to 3 p.m.). Oxygen evolution was measured for 12 to 15 min every 2 h during the experiment. The initial biomass density for both strains was 780 mg (dry weight) liter−1.
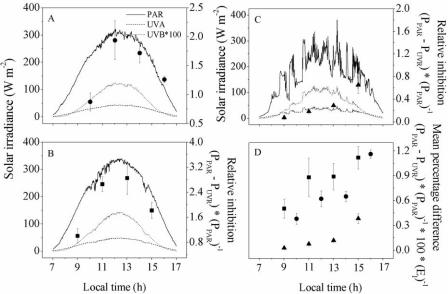
To better describe the inhibitory effects of UVR, we used the photosynthetic rate differences between cultures exposed to PAR plus UVR and cultures exposed to only PAR. Photosynthetic inhibition in the indoor strain reached a peak at noon and then decreased, following approximately the shape of the irradiance curve (Fig. 7A and B). On the other hand, in the outdoor strain, inhibition did not decrease in the afternoon (Fig. 7C). When we compared UVR-induced inhibition under different solar radiation conditions (i.e., different days) (Fig. 7D), we found that the inhibition increased with exposure time, and this was much more evident for the indoor strain (P < 0.01) and low biomass (P < 0.05) than for the outdoor strain and high biomass.
FIG. 7.
UVR-induced photosynthetic inhibition in A. platensis indoor-grown strain 439 and outdoor-grown strain D-0083. Cultures of A. platensis were exposed to the PAB and P treatments for 6 h centered at local noon. Each point represents the photosynthetic rate difference between cultures exposed to PAR plus UVR (PUVR) and cultures exposed to only PAR (PPAR). (A and B) Relative inhibition in the indoor strain cultured at densities of 780 and 330 mg (dry weight) liter−1, respectively. (C) Relative inhibition in the outdoor strain cultured at a density of 780 mg (dry weight) liter−1. (D) Percent inhibition normalized to the mean PAR irradiance (EI) received during measurements. Superimposed are the irradiance curves for PAR, UV-A, and UV-B during the experiment (9, 10, and 11 December 2003). The vertical bars indicate the standard deviations around the means (n = 4).
UV-absorbing compounds and pigments.
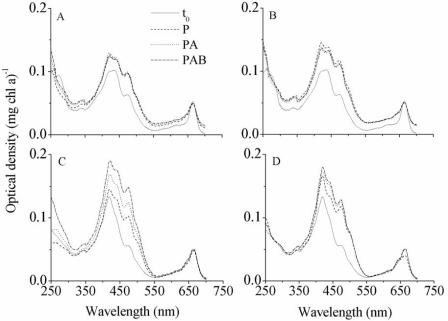
The spectral characteristics of the two strains of A. platensis subjected to the PAB, PA, and P treatments at t0 and after 5 and 10 days of exposure to solar radiation are shown in Fig. 8. There was a very small absorption peak of UV-absorbing compounds (absorbance between 310 and 360 nm) in these two strains (Fig. 8). Although the concentration of UV-absorbing compounds was very low in the indoor strain, it increased significantly during the PA and PAB treatments (P < 0.01) compared to the concentration at t0. In contrast, there was a much lower amount of UV-absorbing compounds in the outdoor strain, and no significant increase in UV-absorbing compounds was observed when this strain was exposed to UVR (Table 1). Similarly, the ratio of UV-absorbing compounds to Chl a increased in the indoor strain (P < 0.05) when cells were exposed to the PA and PAB treatments, but it remained constant (P > 0.05) in the outdoor strain (Table 1). There were no significant differences between radiation treatments with regard to the concentration of carotenoids for both strains (data not shown). However, a significant (P < 0.05) increase in the ratio of carotenoids to Chl a was observed with the PAB treatment for both strains compared to the ratio at t0.
FIG. 8.
Spectral characteristics of A. platensis indoor-grown strain 439 (A and B) and outdoor-grown strain D-0083 (C and D) exposed to PAB, PA, and P solar radiation. The ordinate is the optical density normalized to the concentration of Chl a. (A and C) Samples exposed for 5 days; (B and D) samples exposed for 10 days.
TABLE 1.
UV-absorbing compound contents and ratios of the UV-absorbing compounds and carotenoids to Chl a in A. platensis cells of indoor-grown strain 439 and outdoor-grown strain D-0083 exposed to PAB, PA, radiation for 10 days
| Radiation treatmenta | Concn of UV-absorbing compounds (μg g [dry wt]−1)
|
UV-absorbing compounds/Chl a (μg/mg)
|
Carotenoids/Chl a (μg/μg)
|
|||
|---|---|---|---|---|---|---|
| Strain 439 | Strain D-0083 | Strain 439 | Strain D-0083 | Strain 439 | Strain D-0083 | |
| Control | 0.43 ± 0.04b | 0.34 ± 0.02c | 0.10 ± 0.01 | 0.08 ± 0.01 | 0.32 ± 0.03 | 0.32 ± 0.01 |
| PA | 0.62 ± 0.01 | 0.32 ± 0.03 | 0.17 ± 0.01 | 0.11 ± 0.01 | 0.40 ± 0.02 | 0.43 ± 0.02 |
| PAB | 0.67 ± 0.01b | 0.33 ± 0.05c | 0.18 ± 0.01 | 0.11 ± 0.01 | 0.43 ± 0.01 | 0.49 ± 0.03 |
The control data are the data for time zero.
Significant at P < 0.01.
Not significant at P > 0.05.
DISCUSSION
Cyanobacteria date back to the Precambrian era, when UVR levels were much higher than they are today due to the lack of the ozone layer; thus, their light-harvesting proteins absorbed more than 99% of the incident UV-B radiation to shield the DNA molecule (29, 51). However, studies have shown that the DNA molecule is indeed degraded in cyanobacteria (6, 34) and that the physiology of these organisms responds negatively when they are exposed to elevated levels of UV-B (44). In particular, it has been shown that UV-B affects the photosynthetic electron transport and pigment-protein complexes of A. platensis (38). However, it is also now known that aquatic organisms have mechanisms to minimize and counteract the deleterious effects produced by highly energetic short wavelengths (1, 32).
Among the mechanisms that might play a significant role in protecting organisms against solar UVR is the production of photoprotective compounds, such as MAAs and scytonemin (1, 4, 18, 19). It has also been shown that cells with high concentrations of MAAs are more resistant to UVR than cells with small amounts of these compounds (18). In fact, MAA concentrations varying between 0.9 and 8.4 μg mg (dry weight)−1 have been measured in cyanobacterial isolates (18), and ratios of MAAs to Chl a in the range from 0.04 to 0.19 have been reported in cyanobacterial mats (37). In our study, we found that A. platensis contained a much lower concentration of UV-absorbing compounds (the highest value was 0.67 μg g [dry weight]−1 [Table 1]) and that the ratio of these compounds to Chl a was much lower than that in the cyanobacterial mats. Moreover, when the time period necessary to synthesize UV-absorbing compounds was assessed, it was found that in Nodularia spp. the levels were increased fivefold when the organisms were exposed to the full spectrum of solar radiation for 72 h (45). In contrast, in our study, an increase of 56% in the concentration of UV-absorbing compounds was observed in the indoor strain after 10 days of exposure to full solar radiation. This increased amount of UV-absorbing compounds might have played a small role in protection against UVR-induced damage, but it did not prevent the cells from being harmed, as seen in the breakage of filaments and photoinhibition (Fig. 2, 3, 6, and 7).
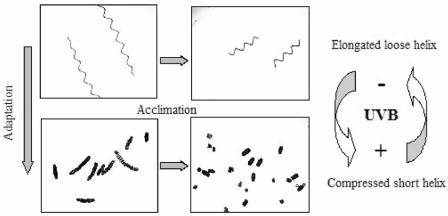
The low levels of UV-absorbing compounds found in the outdoor strain compared to those in the indoor strain (Table 1) or other related species indicate that the outdoor strain might have developed an alternative strategy for protection against harmful UVR levels. In fact, the effective self-shading produced by compressed spirals, seen in both strains when they were exposed to UV-B or produced in high-biomass cultures (Fig. 7), seems to be much more important for protecting the cells against high UVR levels. In particular, we propose that decreasing the helix pitch in the presence of UV-B (Fig. 5C and D) to obtain a more compact structure (i.e., minimum helix pitch) of spirals (Fig. 3a) is an effective protective mechanism, which results in self-shading and thus less photoinhibition (Fig. 6 and 7). Our results also suggest that there are different scales of adaptation and acclimation when cells receive full solar radiation (Fig. 9). Over short time scales (i.e., days) the trichome length decreases, resulting in breakage of filaments into small pieces. However, over long time scales (i.e., decades), adaptation to solar UVR can bring about changes in the spiral structure from a rather elongated helix to a very compressed helix. This compressed structure results in protection against high solar UV-B levels and provides an obvious advantage during growth in outdoor culture ponds or natural water. In addition, the increased ratio of carotenoids to Chl a with PAB treatment (Table 1) might indicate a protective function due to quenching of the highly reactive oxidants produced by UVR, as seen in other cyanobacteria (5).
FIG. 9.
Schematic representations of short- and long-term morphological changes in A. platensis as a function of exposure to UV-B.
Morphological changes in A. platensis, such as helix pitch, spiral width, or orientation, have been reported under laboratory conditions (22, 23, 25, 30, 33). It seems that laboratory conditions, without any impact of UVR, favor the development of straight forms (22, 23, 30). In addition, it was found that A. platensis strains with a tight helical structure could tolerate higher light intensity than strains with loose spirals (23) and that loose straight filaments could be transformed to tight coiled shapes when the cells were shifted to high-light conditions (16). Our study further demonstrates that A. platensis responds to solar UVR by changing its spiral structure. It appeared that such morphological changes from a loose helix to a tight helix, induced by UVR (as seen in our work) or by high levels of visible light (as determined by another researcher [16]), are associated with the protective strategy of this cyanobacterium to counteract solar UVR or high PAR levels by increasing self-shading.
Morphological characteristics of Arthrospira or Spirulina species are often related to product quality. In addition, the length of the spiral filament is very important for harvesting efficiency. Thus, breakage of filaments by solar UVR in outdoor cultures negatively influences the quality and yield of the harvested biomass. On the other hand, an increase in UV-B radiation due to ozone depletion, as forecast for the early 21st century, might alter the morphological development of cyanobacteria. Consequently, this effect on the shapes and sizes could ultimately affect higher trophic levels, as both metabolic rates (39) and grazing behavior in aquatic ecosystems highly depend on these parameters.
Acknowledgments
This work was supported by National Natural Science Foundation of China (project no. 90411018), by Hainan DIC Microalgae Co., and by Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) of Argentina (contribution no. 66 of Estación de Fotobiología Playa Unión).
We thank Zengling Ma for his assistance in measuring UV-absorbing compounds. This paper benefited from the comments of anonymous reviewers.
REFERENCES
- 1.Banaszak, A. T. 2003. Photoprotective physiological and biochemical response of aquatic organisms to UVR, p. 329-356. In E. W. Helbling and H. E. Zagarese (ed.), UV effects in aquatic organisms and ecosystems. The Royal Society of Chemistry, Cambridge, United Kingdom.
- 2.Bebout, B. M., and F. Garcia-Pichel. 1995. UV B-induced vertical migrations of cyanobacteria in a microbial mat. Appl. Environ. Microbiol. 61:4215-4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belay, A. 1997. Mass culture of Spirulina outdoors—the Earthrise farms experience, p. 131-158. In A. Vonshak (ed.), Spirulina platensis (Arthrospira): physiology, cell biology and biotechnology. Taylor & Francis Publishers, London, United Kingdom.
- 4.Brenowitz, S., and R. W. Castenholz. 1997. Long-term effects of UV and visible irradiance on natural populations of a scytonemin-containing cyanobacterium (Calothrix sp.). FEMS Microbiol. Ecol. 24:343-352. [Google Scholar]
- 5.Britton, G. 1995. Structure and properties of carotenoids in relation to function. FASEB J. 9:1551-1558. [PubMed] [Google Scholar]
- 6.Buma, A. G. J., P. Boelen, and W. H. Jeffrey. 2003. UVR-induced DNA damage in aquatic organisms, p. 291-327. In E. W. Helbling and H. E. Zagarese (ed.), UV effects in aquatic organisms and ecosystems. The Royal Society of Chemistry, Cambridge. United Kingdom.
- 7.Capone, D. J., J. P. Zehr, H. W. Paerl, B. Bergman, and E. J. Carpenter. 1997. Trichodesmium, a globally significant marine cyanobacterium. Science 276:1221-1229. [Google Scholar]
- 8.Castenholz, R. W. 1997. Multiple strategies for UV tolerance in cyanobacteria. Spectrum 10:10-16. [Google Scholar]
- 9.Ciferri, O. 1983. Spirulina, the edible microorganism. Microbiol. Rev. 47:551-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crutzen, P. J. 1992. Ultraviolet on the increase. Nature 356:104-105. [Google Scholar]
- 11.Dainippon Ink and Chemicals. 1985. Lina blue A (Natural blue colorant of Spirulina origin). Technical information. Dainippon Ink and Chemicals, Tokyo, Japan.
- 12.Dismukes, G. C., V. V. Klimov, S. V. Baranov, Y. N. Kozlov, J. DasGupta, and A. Tyryshkin. 2001. The origin of atmospheric oxygen on Earth: the innovation of oxygenic photosynthesis. Proc. Natl. Acad. Sci. USA 98:2170-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donkor, V., and D. P. Häder. 1991. Effects of solar and ultraviolet radiation on motility, photomovement and pigmentation in filamentous, gliding cyanobacterium. FEMS Microbiol. Ecol. 86:159-168. [Google Scholar]
- 14.Dunlap, W. C., G. A. Rae, E. W. Helbling, V. E. Villafañe, and O. Holm-Hansen. 1995. Ultraviolet-absorbing compounds in natural assemblages of Antarctic phytoplankton. Antarct. J. U. S. 30:323-326. [Google Scholar]
- 15.Ferreira, K. N., T. M. Iverson, K. Maghlaoui, J. Barber, and S. Iwata. 2004. Architecture of the photosynthetic oxygen-evolving center. Science 303:1831-1838. [DOI] [PubMed] [Google Scholar]
- 16.Fox, R. D. 1996. Spirulina. Production and potential. Edisud, Aixen-Provence, France.
- 17.Garcia-Pichel, F. 1998. Solar ultraviolet and the evolutionary history of cyanobacteria. Origins Life Evol. Biosph. 28:321-347. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Pichel, F., and R. W. Castenholz. 1993. Occurrence of UV-absorbing mycosporine-like compounds among cyanobacterial isolates and estimation of their screening capacity. Appl. Environ. Microbiol. 59:163-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia-Pichel, F., N. D. Sherry, and R. W. Castenholz. 1992. Evidence for an ultraviolet sunscreen role of the extracellular pigment scytonemin in the terrestrial cyanobacterium Chlorogloeopsis sp. Photochem. Photobiol. 56:17-23. [DOI] [PubMed] [Google Scholar]
- 20.Häder, D. P., M. Lebert, R. Marangoni, and G. Colombetti. 1999. ELDONET—European light dosimeter network hardware and software. J. Photochem. Photobiol. B Biol. 52:51-58. [DOI] [PubMed] [Google Scholar]
- 21.Häder, D. P. 2000. Effects of solar UV-B radiation on aquatic ecosystems. Adv. Space Res. 26:2029-2040. [DOI] [PubMed] [Google Scholar]
- 22.Jeeji Bai, N. 1985. Competitive exclusion or morphological transformation? A case study with Spirulina fusiformis. Arch. Hydrobiol. (Suppl. 71 Algol. Stud.) 38/39:191-199. [Google Scholar]
- 23.Jeeji Bai, N., and C. V. Seshadri. 1980. On coiling and uncoiling of trichomes in the genus Spirulina. Arch. Hydrobiol. (Suppl. 60 Algol. Stud.) 26:32-47. [Google Scholar]
- 24.Jensen, S., and G. Knutsen. 1993. Influence of light and temperature on photoinhibition of photosynthesis in Spirulina platensis. J. Appl. Phycol. 5:495-504. [Google Scholar]
- 25.Kebede, E. 1997. Response of Spirulina platensis (= Arthrospira fusiformis) from Lake Chitu, Ethiopia, to salinity stress from sodium salts. J. Appl. Phycol. 9:551-558. [Google Scholar]
- 26.Kerr, J., and C. McElroy. 1993. Evidence for large upward trends of ultraviolet-B radiation linked to ozone depletion. Science 262:1032-1034. [DOI] [PubMed] [Google Scholar]
- 27.Kumar, A., M. B. Tyagi, N. Singh, R. Tyagi, P. N. Jha, R. P. Sinha, and D. P. Häder. 2003. Role of white light in reversing UV-B-mediated effects in the N2-fixing cyanobacterium Anabaena BT2. J. Photochem. Photobiol. B Biol. 71:35-42. [DOI] [PubMed] [Google Scholar]
- 28.Kumar, A., P. P. Sinha, and D. P. Häder. 1996. Effect of UV-B on enzymes of nitrogen metabolism in the cyanobacterium Nostoc calcicola. J. Plant Physiol. 148:86-91. [Google Scholar]
- 29.Lao, K., and A. N. Glazer. 1996. Ultraviolet-B photodestruction of a light-harvesting complex. Proc. Natl. Acad. Sci. USA 93:5258-5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewin, R. A. 1980. Uncoiled variants of Spirulina platensis (Cyanophyceae: Oscillatoriaceae). Arch. Hydrobiol. (Suppl. 60 Algol. Stud.) 26:48-52. [Google Scholar]
- 31.Lu, J., G. Yoshizaki, K. Sakai, and T. Takeuchi. 2002. Acceptability of raw Spirulina platensis by larval tilapia Orechromis niloticus. Fish. Sci. (Tokyo) 68:51-58. [Google Scholar]
- 32.Malloy, K. D., M. A. Holman, D. Mitchell, and H. W. Detrich III. 1997. Solar UVB-induced DNA damage and photoenzymatic DNA repair in Antarctic zooplankton. Proc. Natl. Acad. Sci. USA 94:1258-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mühling, M., N. Harris, A. Belay, and B. A. Whitton. 2003. Reversal of helix orientation in the cyanobacterium Arthrospira. J. Phycol. 39:360-367. [Google Scholar]
- 34.O'Brien, P. A., and J. A. Houghton. 1982. Photoreactivation and excision repair of UV induced pyrimidine dimers in the unicellular cyanobacterium Gloeocapsa alpicola (Synechocystis PCC 6308). Photochem. Photobiol. 35:359-364. [DOI] [PubMed] [Google Scholar]
- 35.Parsons, T. R., and, J. D. H., Strickland. 1963. Discussion of spectrophotometric determination of marine plant pigments, with revised equation for ascertaining chlorophylls and carotenoids. J. Mar. Res. 21:155-163. [Google Scholar]
- 36.Porra, R. J. 2002. The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth. Res. 73:149-156. [DOI] [PubMed] [Google Scholar]
- 37.Quesada, A., W. F. Vincent, and D. R. S. Lean. 1999. Community and pigment structure of Arctic cyanobacterial assemblages: the occurrence and distribution of UV-absorbing compounds. FEMS Microbiol. Ecol. 28:315-323. [Google Scholar]
- 38.Rajagopal, S., S. D. S. Murthy, and P. Mohanty. 2000. Effect of ultraviolet-B radiation on intact cells of the cyanobacterium Spirulina platensis: characterization of the alterations in the thylakoid membranes. J. Photochem. Photobiol. B Biol. 54:61-66. [DOI] [PubMed] [Google Scholar]
- 39.Raven, J. A., and J. E. Kübler. 2002. New light on the scaling of metabolic rate with the size of algae. J. Phycol. 38:11-16. [Google Scholar]
- 40.Richmond, A., and J. U. Grobbelaar. 1986. Factors affecting the output rate of Spirulina platensis with reference to mass cultivation. Biomass 10:253-264. [Google Scholar]
- 41.Sass, L., C. Spetea, Z. Máté, F. Nagy, and I. Vass. 1997. Repair of UV-B-induced damage of photosystem II via de novo synthesis of the D1 and D2 reaction centre subunits in Synechocystis sp. PCC 6803. Photosynth. Res. 54:55-62. [Google Scholar]
- 42.Sinha, R. P., and D. P. Häder. 2002. UV-induced DNA damage and repair: a review. Photochem. Photobiol. Sci. 1:225-236. [DOI] [PubMed] [Google Scholar]
- 43.Sinha, R. P., H. D. Kumar, A. Kumar, and D. P. Häder. 1995. Effects of UV-B irradiation on growth, survival, pigmentation and nitrogen metabolism enzymes in cyanobacteria. Acta Protozool. 34:187-192. [Google Scholar]
- 44.Sinha, R. P., M. Klisch, A. Gröniger, and D.-P. Häder. 2001. Responses of aquatic algae and cyanobacteria to solar UV-B. Plant Ecol. 154:221-236. [Google Scholar]
- 45.Sinha, R. P., N. K. Ambasht, J. P. Sinha, M. Klisch, and D. P. Häder. 2003. UV-B-induced synthesis of mycosporine-like amino acids in three strains of Nodularia (cyanobacterium). J. Photochem. Photobiol. B Biol. 71:51-58. [DOI] [PubMed] [Google Scholar]
- 46.Sinha, R. P., N. Singh, A. Kumar, H. D. Kumar, and D. P. Häder. 1997. Impacts of ultraviolet-B irradiation on nitrogen-fixing cyanobacteria of rice paddy fields. J. Plant Physiol. 150:188-193. [Google Scholar]
- 47.Sinha, R. P., N. Singh, A. Kumar, H. D. Kumar, M. Häder, and D. P. Häder. 1996. Effects of UV irradiation on certain physiological and biochemical processes in cyanobacteria. J. Photochem. Photobiol. B Biol. 32:107-113. [Google Scholar]
- 48.Stapleton, A. E. 1992. Ultraviolet radiation and plants: burning questions. Plant Cell 4:1353-1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tabazadeh, A., M. L. Santee, M. Y. Danilin, H. C. Pumphrey, P. A. Newman, P. J. Hamill, and J. L. Mergenthaler. 2000. Quantifying denitrification and its effect on ozone recovery. Science 288:1407-1411. [DOI] [PubMed] [Google Scholar]
- 50.Torzillo, G., P. Bernardini, and J. Masojidek. 1998. On-line monitoring of chlorophyll fluorescence to assess the extent of photoinhibition of photosynthesis induced by high oxygen concentration and low temperature and its effect on the productivity of outdoor cultures of Spirulina platensis (cyanobacteria). J. Phycol. 34:504-510. [Google Scholar]
- 51.Van Baalen, C. 1968. The effects of ultraviolet irradiation on a coccoid blue-green alga: survival, photosynthesis, and photoreactivation. Plant Physiol. 43:1689-1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vonshak, A., and R. Guy. 1992. Photoadaptation, photoinhibition and productivity in the blue-green alga, Spirulina platensis grown outdoors. Plant Cell Environ. 15:613-616. [Google Scholar]
- 53.Xiong, F., J. Komenda, J. Kopecky, and L. Nedbal. 1997. Strategies of ultraviolet-B protection in microscopic algae. Physiol. Plant. 100:378-388. [Google Scholar]