Abstract
SHP-2 is a positive component of many receptor tyrosine kinase signaling pathways. The related protein-tyrosine phosphatase (PTP) SHP-1 usually acts as a negative regulator. The precise domains utilized by SHP-2 to transmit positive signals in vivo and the basis for specificity between SHP-1 and SHP-2 are not clear. In Xenopus, SHP-2 is required for mesoderm induction and completion of gastrulation. We investigated the effects of SHP-2 mutants and SHP-2/SHP-1 chimeras on basic fibroblast growth factor-induced mesoderm induction. Both SH2 domains and the PTP domain are required for normal SHP-2 function in this pathway. The N-terminal SH2 domain is absolutely required, whereas the C-terminal SH2 contributes to wild-type function. The C-terminal tyrosyl phosphorylation sites and proline-rich region are dispensable, arguing against adapter models of SHP-2 function. Although the SH2 domains contribute to SHP-2 specificity, studies of SHP chimeras reveal that substantial specificity resides in the PTP domain. Thus, PTP domains exhibit biologically relevant specificity in vivo, and noncatalytic and catalytic domains of PTPs contribute to specificity in a combinatorial fashion.
Growth factor-initiated signal transduction is required in multiple developmental pathways. Mice with naturally occurring mutations in receptor tyrosine kinases (RTKs), such as c-kit (W) (reviewed in reference 65) and the epidermal growth factor receptor (EGFR) (42), have gross phenotypic abnormalities, as well as signaling defects in specific cell populations. The EGFR homolog let-23 is essential for vulval development in Caenorhabditis elegans (5), whereas multiple RTKs are required for normal Drosophila development (reviewed in references 18, 19, 59, 60, and 68). In Xenopus, overexpression of a dominant negative form of fibroblast growth factor (FGF) receptor 2 (FGFR2) results in severe gastrulation defects and abnormal mesoderm formation (3, 4). Likewise, mice lacking FGFR1 die prior to or during gastrulation (16, 86), demonstrating that FGF signaling pathways are critical for early development in vertebrates.
Biochemical and genetic studies have identified several downstream components of RTK signaling cascades. Following growth factor binding, tyrosyl phosphorylation sites on the activated receptor recruit secondary signaling molecules containing SH2 domains, which couple RTK activation to cytoskeletal and nuclear events (reviewed in reference 13). SH2-containing secondary signaling proteins include enzymes, such as phospholipase Cγ (PLCγ) and SHP-2, and adapters, which contain other modules such as SH3, PTB, and PH domains. One central pathway in signaling from multiple RTKs is the Ras/Raf/MEK/mitogen-activated protein (MAP) kinase (MAPK) cascade (reviewed in references 44 and 67). This pathway involves the SH2/SH3-containing adapter, Grb2, which is bound via its SH3 domains to the Ras exchange protein, Sos. The Grb2 SH2 domains either bind directly to activated RTKs or, in some pathways, bind to adapters such as IRS-1, FRS-2 (35), and/or Shc (for a review, see reference 46). Genetic evidence for the role of Grb2 was provided by studies of its Drosophila homolog, drk (53), and its C. elegans homolog, sem-5 (12). The importance of the MAPK pathway in RTK-driven developmental pathways also has been demonstrated in invertebrates (reviewed in reference 83). However, the roles of most other secondary signaling molecules have not been validated by genetic analyses. There is virtually no information regarding the functions of specific subdomains in vivo.
SH2-containing protein-tyrosine phosphatases (SHPs) are key components of RTK signaling pathways and play important developmental roles (reviewed in references 48 and 78). SHPs share the same overall architecture, with two SH2 domains at their N termini, a protein-tyrosine phosphatase (PTP) domain, and a C terminus containing sites for tyrosyl and seryl/threonyl phosphorylation and, in some cases, a proline-rich domain (for reviews, see references 22 and 47). Two SHPs exist in vertebrates. SHP-1 is expressed primarily in hematopoietic and epithelial cells, where it acts predominantly as a negative regulator of RTK and cytokine receptor signaling pathways (for reviews, see references 10, 48, and 79). This is illustrated most vividly by the phenotype of mice with defective SHP-1 genes (motheaten and motheaten viable mice [reviewed in reference 10;]]). The ubiquitously expressed SHP-2, despite a high degree of sequence similarity to SHP-1 (approximately 60% overall identity), appears to have distinct functions. Experiments with tissue culture cells using dominant negative mutants (1, 8, 52, 87, 88) or antibody microinjection approaches (8, 28, 64, 85) established SHP-2 as a required positive component in several RTK pathways, acting upstream of MAPK. The Drosophila homolog of SHP-2, corkscrew (csw), is required in multiple developmental pathways (2, 57, 58). In Xenopus, SHP-2 is required for basic (bFGF)-induced MAPK activation, mesodermal marker induction, and completion of gastrulation; these functions cannot be subserved by SHP-1 (74). Most likely, SHP-2 has a similar role in early development of all vertebrates, since mice homozygous for targeted disruptions of SHP-2 die by embryonic day 10.5 (6, 66) and exhibit gastrulation defects and impaired bFGF and platelet-derived growth factor signaling (66).
Why the two vertebrate SHPs have distinct biological functions has remained unclear. They appear to participate in several of the same pathways; in some cases, they even bind to the same receptors (e.g., c-Kit, IL3-Rβ, and EpoR) (reviewed in references 48 and 79). Their SH2 domains seem to recognize similar phosphotyrosyl peptides (see Discussion). Both also are enzymatically activated in vitro upon binding of phosphotyrosyl peptides to their respective SH2 domains (14, 15, 31, 38, 54, 56, 61, 63, 73, 80).
The molecular details by which SHP-2 participates in RTK signaling in vivo also have remained unclear. SHP-2 binds, via its SH2 domains, to phosphotyrosine motifs in several activated RTKs and adapters or accessory molecules, including IRS-1 (36), Gab1 (30), Dos (29), and SHPS1/SIRP1α (25, 33). Nevertheless, the importance of the SH2 domains in signaling in vivo has not been demonstrated explicitly. In response to stimulation by some growth factors, SHP-2 is phosphorylated on one or both of the tyrosyl residues in its C-terminal tail, Y542 and Y580 (9, 82). These sites can bind to Grb2 (9, 40, 75, 76, 84), which led to the suggestion that SHP-2 might act as an adapter, coupling RTKs to the Ras pathway (9, 40, 47). However, the biological significance of this interaction has not been demonstrated. Recently, tyrosyl-phosphorylated SHP-2 also has been found to bind to the SH2 domain of the inositol monophosphatase SHIP in cytokine-stimulated hematopoietic cells (41); the function of this complex and whether it occurs in nonhematopoietic cells remain unclear. Between the two C-terminal tyrosines is a proline-rich region, which potentially could bind SH3 and/or WW domain-containing proteins. SHP-1 lacks this proline-rich region, raising the possibility that it could contribute to SHP signaling and/or specificity. However, no proteins have been shown to bind to the SHP-2 proline-rich region, nor has a function for this domain been demonstrated. Experiments using PTP-inactive mutants of SHP-2 strongly suggest that the PTP domain is required for normal signaling (see above), but whether the PTP domain also contributes significantly to SHP specificity has remained undetermined.
We chose to address these unresolved questions by using bFGF-induced mesoderm induction as an experimental model. Signals from vegetal pole cells of Xenopus embryos direct the presumptive ectodermal cells of the animal cap to adopt a mesodermal cell fate (50). Mesoderm induction can be evoked, to various degrees, by purified growth factors, including bFGF, serving as the basis for the animal cap assay (reviewed in reference 34). Animal cap explants cultured in low-salt media undergo ectodermal differentiation. Addition of bFGF results in dramatic elongation of the explants, MAPK activation, and induction of mesodermal markers.
It has been shown previously that expression of an SHP-2 mutant lacking 31 amino acids of the PTP domain (ΔP) (see Fig. 1A) in animal caps blocks normal bFGF responses, demonstrating that SHP-2 and, in particular, its PTP domain are required downstream of the Xenopus FGFR (XFGFR) (74). We now have determined which other domains of SHP-2 are necessary for transmission of the XFGFR signal. Both SH2 domains of SHP-2 are required, although the N-terminal SH2 domain (N-SH2) is more critical than the C-terminal SH2 domain (C-SH2). The C-terminal tyrosines and the proline-rich region are dispensable for rescue of the dominant negative effects of ΔP, suggesting that the adapter model is not the major mechanism for bFGF signal transmission. Finally, by analyzing the effects of SHP-2/SHP-1 chimeras, we have found that the PTP domain of SHP-2 accounts for much of the specificity between SHP-1 and SHP-2 in bFGF-induced mesoderm induction. Our results establish an absolute requirement for the SH2 and PTP domains of SHP-2 in this pathway, provide the first demonstration that biological specificity resides within the phosphatase domain of a PTP in vivo, and suggest that PTP specificity is determined by combinatorial mechanisms.
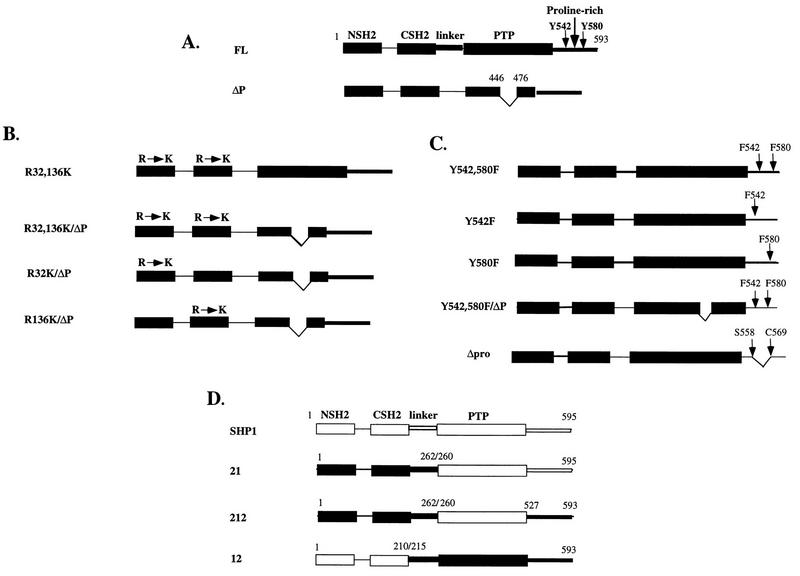
FIG. 1.
Microinjection constructs: schematic representations of human SHP-2 and chimeric cDNA clones showing functional domains that might participate in bFGF signaling, including the two SH2 domains, the SH2-PTP linker, the PTP domain, the C-terminal tyrosine phosphorylation sites, and the proline-rich region. Amino acid numbers corresponding to human SHP-2 (24) are indicated above the diagram. (A) Full-length SHP-2 and ΔP, a mutant with a 31-amino-acid deletion in the PTP domain, which acts as a dominant negative mutant. (B) SH2 domain mutants with point mutations in the essential βb5 arginine of both SH2 domains (R32,138K) or individual N-SH2 (R32K) or C-SH2 (R138K) domains in the context of WT SHP-2 or ΔP, as indicated. (C) C-terminal tail mutants with tyrosine (Y)-to-phenylalanine (F) mutations at position 542 and/or 580 in the context of WT SHP-2 or ΔP, as indicated. Δpro, 10-amino-acid deletion of the proline-rich region between the two tyrosines. (D) Chimeras between SHP-2 and SHP-1. SHP-1 domains (white boxes) and SHP-2 domains (black boxes) are indicated. Shown are SHP-1; the 21 chimera, containing the SH2 domains and linker region of SHP-2 fused to the PTP and C-terminal tail of SHP-1; the 212 chimera, containing the SH2 domains and linker of SHP-2 fused to the PTP domain of SHP-1 and the C-terminal tail of SHP-2; and the 12 chimera, containing the SH2 domains of SHP-1 fused to the linker, PTP domain, and C-terminal tail of SHP-2.
MATERIALS AND METHODS
Mutant construction.
Constructs containing R→K mutations in the essential arginines within the FLVRES sequences of N-SH2 (R32), C-SH2 (R138), and both SH2 domains (R32,138) of human SHP-2, cloned into pET vectors, were described previously (73). An upstream EcoRI site was introduced into the 5′ ends of the pET constructs by PCR using the oligonucleotides 5′-CTGACTGAATTCATGACATCGCGGAG-3′ and 5′-ATCTCTGGTCTCAGCTAA-3′, with pET FLVRES mutants as templates. The 0.7-kb products were digested with EcoRI and BglII and cloned via three-way ligations with either a BglII-EcoRI 3′ fragment of wild-type (WT) SHP-2 or a BglII-EcoRI 3′ fragment of ΔP into pXT7SR1 vectors (17) or pSP64RI (74), each linearized with EcoRI. pSP64RI-R32K, pXT7SRI-R138K, and pXT7SRI-R32,138K contain point mutations of the essential arginines in the FLVRES sequence in the context of the full-length protein. pXT7SRI-R32K/ΔP, pSP64RI-R138K/ΔP, and pXT7SRI-R32,138K/ΔP contain point mutations of the essential arginines in the FLVRES sequence in the context of ΔP; this deletion includes the essential VHCSAG motif (74).
Single and double Y→F mutants of the human SHP-2 C-terminal tyrosines have been described previously (74). Δpro was generated by overlap extension PCR, as follows. The oligonucleotide 5′-GGAGATCAGAGCTGTGCAGAAATGAGAG-3′ and the standard T3 primer were used in a PCR with human SHP-2 cDNA (24) as a template to generate a 0.3-kb fragment representing the 3′ portion of the construct, and the oligonucleotides 5′-CGGAATTCAACATGACATCGCGGAG-3′ (Ek1s) and 5′-CCTCTAGTCTCGACACGTCTTTACTCTC-3′ were used to generate the 1.7-kb 5′ portion of the construct. These products were purified and used as templates in a second round of PCR with Ek1s and T3 to generate the full-length 2.0-kb product. This 2.0-kb fragment was blunt-end cloned into pBluescriptKS (BSKS) (Stratagene). A 0.6-kb PstI-EcoRI fragment was isolated from the BSKS clone and subcloned via three-way ligation with a 1.4-kb EcoRI-PstI fragment of WT SHP-2 into EcoRI-linearized pSP64RI. The resultant protein contains a 10-amino-acid deletion (amino acids P559 to P568) of the sequence PLPPCTPTPP.
The 21 chimera contains the SH2 domains and linker region of SHP-2 and the PTP domain and the C terminus of SHP-1 (see Fig. 1D). It was constructed by overlap extension PCR, as follows. The oligonucleotides Ek1S and 5′-CCCTTCCAGACGCTGGTGGAGAAGTTTGCACTCCTGTTGTTG-3′ (italics and boldface indicate SHP-1 and SHP-2 sequence, respectively, in all oligonucleotides) were used in a PCR to generate a 0.83-kb fragment, with human SHP-2 cDNA as a template. The oligonucleotides 5′-CAACAACAGGAGTGCAAACTTCTCCACCAGCGTCTGGAAGGG-3′ and 5′-CTTCTTGAATTCGGCATGGCCACCTGAG-3′ (A8) were used to generate a 1.2-kb fragment, with human SHP-1 (62) as a template. The 0.83- and 1.2-kb products were purified and used as templates in a second round of PCR in conjunction with Ek1s and A8 to generate the full-length 2.0-kb 21 fragment. The PCR product was blunt-end cloned into BSKS and then subcloned as an EcoRI fragment into pSP64RI linearized with EcoRI. The resulting protein contains amino acids 1 to 262 of SHP-2 fused to amino acids 260 to 595 of SHP-1.
The 212 chimera contains the SH2 domains, the linker and C terminus of SHP-2, and the PTP domain of SHP-1. It was generated by overlap extension PCR, as follows. The oligonucleotides 5′-CTTCAATCCTGCGCTGAGTGGTTTCAATGAACTG-3′ and Ek1s were used to generate a 1.6-kb fragment with chimera 21 used as a template. The oligonucleotides 5′-CAGTTCATTGAAACCACTCAGCGCAGGATTGAAG-3′ and T3 were used to generate a 0.3-kb fragment with human SHP-2 cDNA used as a template. The 1.6- and 0.3-kb products were purified and used as templates in a second-round reaction with Ek1s and T3 to generate the full-length 1.9-kb clone. The 1.9-kb fragment was blunt-end cloned into BSKS, and then chimera 212 was subcloned into pSP64RI as an EcoRI fragment. The resulting protein contains amino acids 1 to 262 of SHP-2, amino acids 260 to 519 of SHP-1, and amino acids 527 to 593 of SHP-2 (see Fig. 1D).
The 12 chimera contains the SH2 domains of SHP-1 and the linker, PTP, and C terminus of SHP-2 (see Fig. 1D). It was constructed by overlap extension PCR, as follows. The oligonucleotides 5′-CCGGAATTCCCTCTGGGGAAGC-3′ (A1) and 5′-CGAGTCGTGTTAAGGGGCTGCCGCAGGTAGACAAAG-3′ were used to generate a 0.65-kb fragment, with human SHP-1 cDNA as a template. The oligonucleotides 5′-CTTTGTCTACCTGCGGCAGCCCCTTAACACGACTCG-3′ and T3 were used to generate a 1.25-kb fragment, with human SHP-2 cDNA as a template. The 0.65- and 1.25-kb products were purified and used as templates in a second-round reaction with A1 and T3 to generate the full-length 1.9-kb product of chimera 12. The 1.9-kb full-length product was blunt-end cloned into BSKS, and then chimera 12 was subcloned into pSP64RI as an EcoRI fragment. The resulting protein contains amino acids 1 to 210 of SHP-1 fused to amino acids 215 to 593 of SHP-2 (see Fig. 1D).
All regions of constructs that were generated by PCR were confirmed by automated sequencing (Applied Biotechnology) to ensure that there were no PCR-generated mutations. Further details regarding the generation of these constructs are available from A. O’Reilly upon request.
Plasmid constructs and in vitro transcription.
For in vitro transcription, constructs were subcloned into the pSP64RI (74) or pXT7SRI (17) vectors, which contain a polylinker flanked by Xenopus β-globin 5′ and 3′ untranslated sequences. In vitro transcription of linearized plasmids was carried out by using SP6 polymerase for constructs in pSP64RI and T7 polymerase for constructs in pXT7SRI and a MEGAscript kit (Ambion).
Embryo manipulations.
Fertilization and embryo culture in 0.1× MMR were performed as described previously (49). Embryos were transferred to 0.5× MMR–3% Ficoll (Pharmacia) and injected with 10 nl of RNA in the animal pole of both cells of two-cell-stage embryos. The concentration of ΔP mRNA injected was determined by preliminary experiments with each batch of RNA in which the minimal level required to block bFGF-dependent elongation was determined. The RNA concentrations for all other mutants were determined by the amount required to produce protein levels comparable to those of ΔP. Animal pole explants were excised at stage 7.5 to 8.5 and analyzed as described previously (74).
RNA and protein analysis.
RNA extraction and Northern analysis of late mesodermal markers were performed as described elsewhere (32). For MAPK shift assays, animals caps were isolated at stage 7.5 to 8 and dissociated in calcium-free, magnesium-free normal amphibian media (27). Dissociated cells were collected and stimulated for 5 min at 25°C with 100 ng of Xenopus bFGF per ml and then pelleted for 10 s at 14,000 rpm. The pellets were lysed immediately in Nonidet P-40 (NP-40) lysis buffer (1% NP-40, 150 mM NaCl, 50 mM Tris [pH 7.4]) containing protease inhibitors (10 μg of leupeptin per ml, 1 μg of aprotinin per ml, 1 μg of pepstatin A per ml, 1 μg of antipain per ml, and 20 μg of phenylmethylsulfonyl fluoride per ml) and phosphatase inhibitors (1 mM sodium vanadate) and incubated for 10 min on ice. The lysates were clarified for 10 min at 14,000 rpm at 4°C, electrophoresed, and transferred onto Immobilon P membranes (Millipore). Immunoblots were probed with rabbit polyclonal anti-Xenopus MAPK C-terminal antibodies (a generous gift of James Maller). Levels of SHP-2 and chimeric proteins containing the SHP-2 N terminus were quantified by probing total NP-40 lysates from animal caps at stage 8 or 9 with mouse monoclonal antibodies against the N-SH2 of SHP-2 (Transduction Laboratories). For experiments comparing expression of chimera 12 with that of SHP-2, polyclonal antibodies against the C terminus of SHP-2 (Santa Cruz Biotechnology, Inc.) were used instead.
Glutathione S-transferase (GST) fusion proteins and PTP assays.
SHP-2, SHP-1, and chimeras 21, 212, and 12 were subcloned into the EcoRI site of pGEX4T (Pharmacia). The constructs were transformed into DH5α. Log-phase bacterial cultures (100 ml) were induced with 0.1 μM isopropyl-β-d-thiogalactopyranoside (IPTG) (Gibco-BRL) for 4 h at 37°C. Bacterial pellets were lysed in 1× STE (10 mM Tris-Cl [pH 7.5], 10 mM NaCl, 1 mM EDTA) containing 100 μg of lysozyme per ml for 10 min on ice and then pelleted at 4°C and 12,500 rpm for 15 min. Triton X-100 was added to a final concentration of 1.9%. The lysates were incubated with 250 μl of a 50% slurry of glutathione agarose (Sigma) for 1 h. The beads were washed four times in ice-cold phosphate-buffered saline, and proteins were eluted with 15 mM glutathione in 50 mM Tris–150 mM NaCl–5 mM dithiothreitol at pH 7.4 and then dialyzed for 4 h in 50 mM HEPES–150 mM NaCl. Protein concentrations were determined by Coomassie staining and bicinchoninic acid assay (Pierce).
PTP assays were performed in 30 mM sodium acetate–150 mM NaCl–5 mM dithiothreitol (pH 5.5) with 20 mM para-nitrophenyl phosphate as the substrate. All assays were carried out in the linear range of the product-time curve. SHP-1 and chimera 212 assays were performed with 0.05 μg of protein for 3 and 5 min, respectively. SHP-2 and chimera 12 assays were done with 0.50 μg of protein for 20 and 10 min, respectively. Absorbances were measured at 410 nm. Specific activities are represented as units per milligram ± standard error of the mean of triplicate determinations, where 1 U is 1 μmol/min.
RESULTS
To determine which domains of SHP-2 are required for signaling downstream of the XFGFR, we generated a series of mutants with alterations in the SHP-2 SH2 domains and the C-terminal tail (Fig. 1B and C). For subsequent studies to assess which domains contribute to specificity in XFGFR signal transduction, we generated several SHP-2/SHP-1 chimeras (Fig. 1D). RNA was prepared from each of these constructs by in vitro transcription (see Materials and Methods) and injected into both animal poles of two-cell embryos. Animal caps from injected and control embryos were monitored for their ability to elongate, activate MAPK, and induce expression of the late mesodermal marker, muscle actin, in response to bFGF stimulation. In one series of experiments, we examined whether mutations in various SHP-2 domains, when introduced into the ΔP mutant, retained the ability to block these bFGF-stimulated events (Fig. 1B and C). Mutants (in the context of ΔP) that fail to block bFGF signaling indicate a requirement for that domain to mediate the ΔP block and, by inference, a requirement for that domain to transmit the SHP-2 signal. Due to the nature of dominant negative experiments, proteins that block signaling must be expressed at levels substantially higher than that of endogenous SHP-2, creating the possibility that subtle effects of functional-domain mutations on the ability of ΔP to block signaling might not be detected. For this reason, we also utilized a complementary, more sensitive approach in which the functional-domain mutants were introduced into WT SHP-2 (Fig. 1B and C) and then were assessed for their ability, when coexpressed with ΔP, to rescue the ΔP-induced block of FGF signaling. For these experiments, we used the minimal amount of ΔP required to block animal cap elongation (as determined by preliminary titration experiments). Low levels of the mutant proteins were coexpressed with this minimal amount of ΔP. The abilities of mutant and WT SHP-2 to rescue were compared. Again, we interpreted mutant SHP-2 proteins that failed to rescue the ΔP-induced block as indicating a requirement for that domain of SHP-2 in bFGF signaling.
The SH2 domains of SHP-2 are required for bFGF-induced signaling in animal caps.
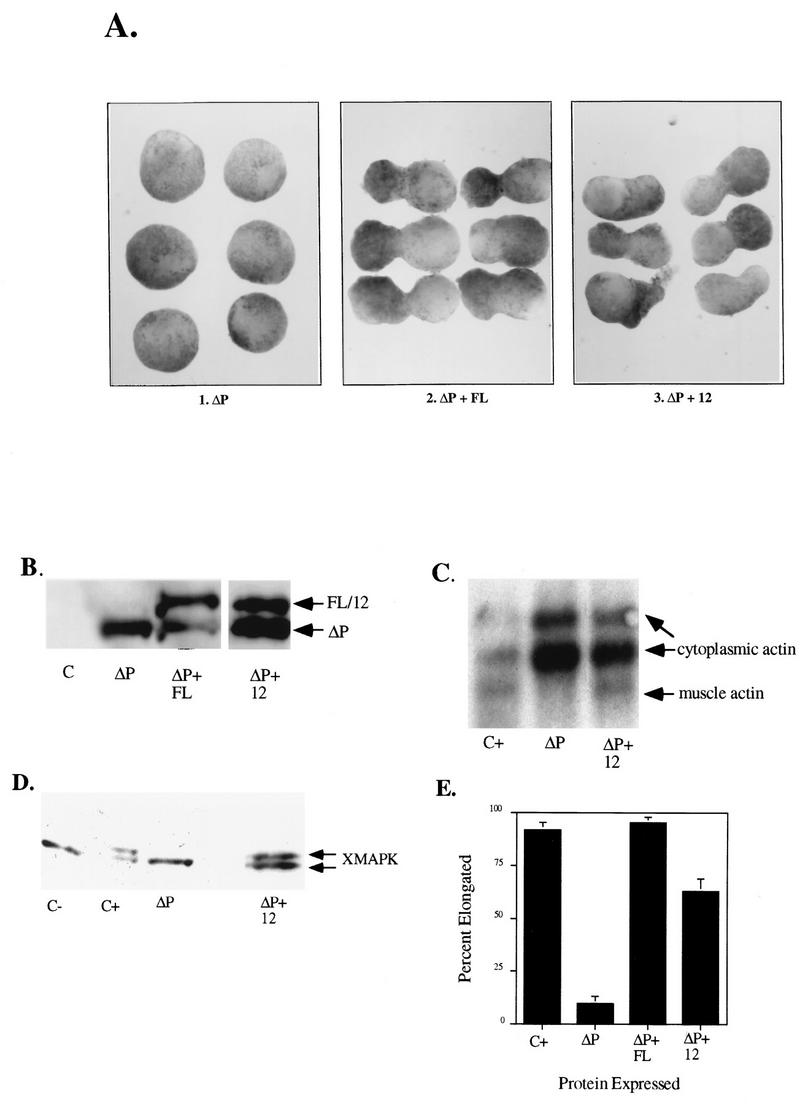
To assess the role of the SH2 domains of SHP-2, we generated mutants in which the essential FLVRES motifs of N-SH2, C-SH2, or both SH2s (N+C−SH2) were converted to FLVKES in the context of WT SHP-2 or ΔP (Fig. 1B). Previous studies revealed that such mutants display markedly reduced (approximately 50-fold), although not absent, binding to phosphotyrosyl peptides in vitro (73). Normally, animal caps undergo dramatic elongation in response to stimulation with bFGF (Fig. 2A, C+) which is accompanied by induction of mesodermal markers, including muscle actin (Fig. 3C), and activation of MAPK (Fig. 3D). As was shown previously (74), ΔP blocks elongation (Fig. 2A and others), the activation-dependent shift of MAPK (Fig. 2C and others), and muscle actin mRNA induction (Fig. 3C and others), most likely by competing with endogenous, full-length SHP-2 for its target(s). Mutation of the essential arginines of both SH2 domains (R32,138K/ΔP) abolished the ability of ΔP to act as a dominant negative mutant in the bFGF pathway: animal caps from embryos injected with R32,138K/ΔP RNA elongated at all doses tested (Fig. 2A). The failure to block was not due to an inability of the mutant protein to be expressed stably, since levels of R32,138K/ΔP protein ranging from equal to the minimal amount of ΔP required to block elongation (Fig. 2B) to up to 10-fold-higher levels (data not shown) failed to block elongation. R32,138K/ΔP also failed to block activation of MAPK (Fig. 2C). This result demonstrates that at least one functional SH2 domain is required for the dominant negative effect of ΔP on bFGF signaling and supports a model in which ΔP acts as a dominant negative mutant by binding, via its SH2 domain(s), to one or more endogenous phosphotyrosyl proteins, thus preventing proper localization and/or activation of endogenous SHP-2.
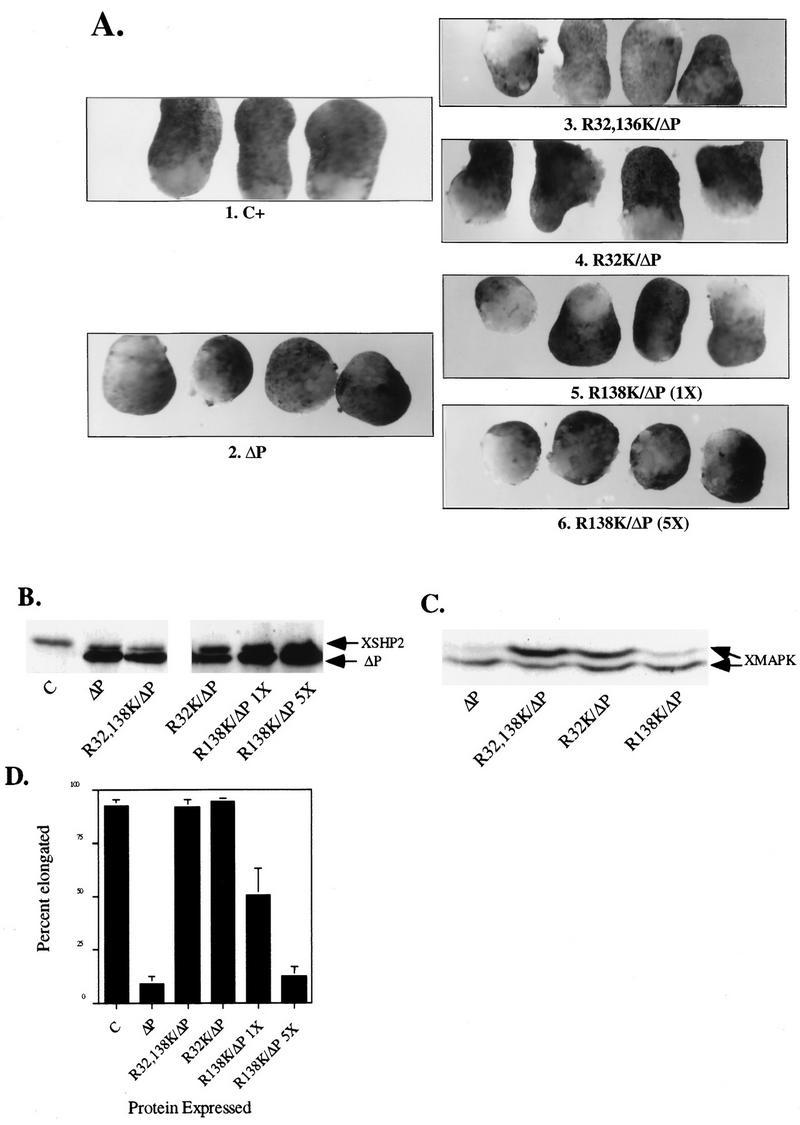
FIG. 2.
SH2 domains are required for ΔP to act as a dominant negative mutant. Representative experimental results show animal cap elongation, protein levels, and MAPK activation following injection of the indicated SHP-2 mutants. (A) bFGF-stimulated animal caps at stage 10.5. The injected mRNA is indicated beneath each panel. C+, uninjected animal caps stimulated for 2 h with bFGF (100 ng/ml). These caps are elongated. In contrast, ΔP-injected caps (ΔP) show no elongation and are scored as blocked. 1× and 5×, concentrations used (see Materials and Methods). (B) Immunoblot analysis of injected animal caps at stage 8. Total lysates of animal caps were probed with anti-PTP1D/SHP-2 monoclonal antibodies (Transduction Laboratories). ΔP proteins are indicated (lower band). This antibody cross-reacts with endogenous Xenopus SHP-2 (XSHP2), which serves as a loading control. The injected mRNA is indicated beneath each lane. (C) MAPK activation in animal caps stimulated with bFGF (100 ng/ml) at 25°C for 5 min. Total lysates of animal caps were probed with anti-Xenopus MAPK (anti-XMAPK antibodies) (see Materials and Methods). The injected mRNA is indicated beneath each lane. The amount of R138/ΔP injected here is equivalent to 5× in panel A. (D) Quantitation of animal cap elongation. Mean percentages of elongated caps are shown. Error bars show the standard error of the mean for each injected mRNA. Injected mRNA is indicated beneath each lane. Percentages are based on a minimum of 45 total animal caps in three or more separate experiments.
FIG. 3.
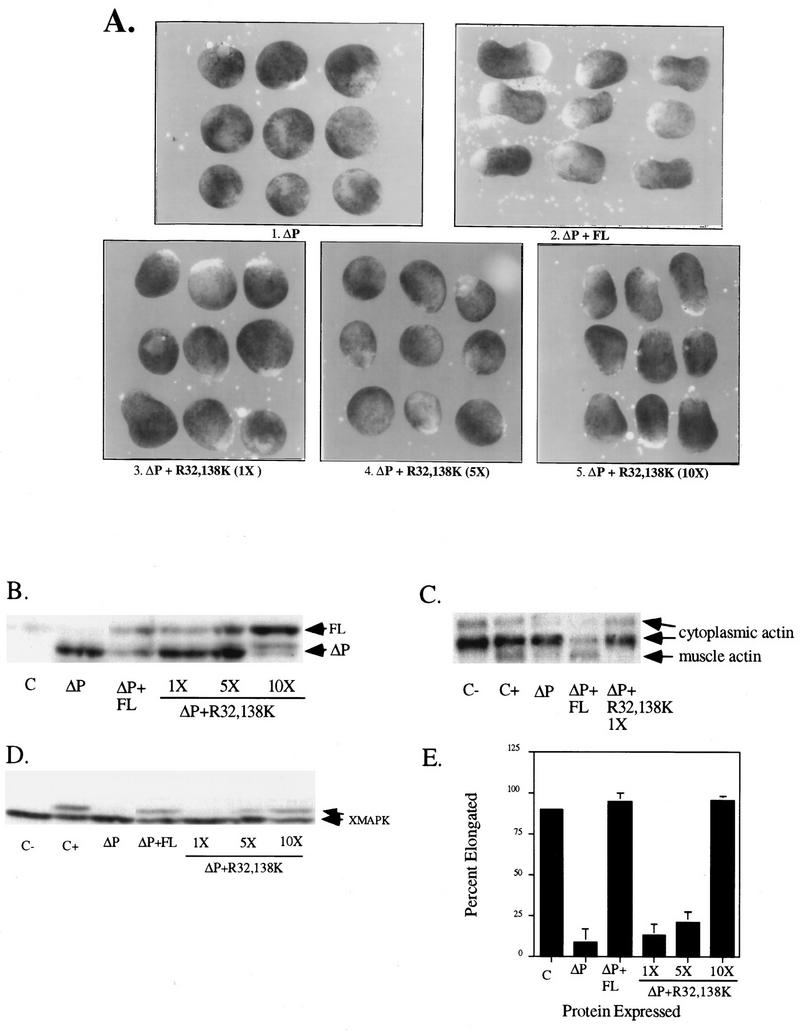
Intact SH2 domains are required for SHP-2 to rescue the ΔP-induced block of bFGF signaling. Representative experimental results show elongation, protein levels, muscle actin induction, and MAPK activation in response to bFGF stimulation of animal caps from embryos injected with the indicated RNAs. (A) bFGF-stimulated animal caps at stage 10.5 were analyzed as for Fig. 2A. Shown are caps from embryos injected with ΔP alone (ΔP), with ΔP plus WT SHP-2 (ΔP + FL), or with ΔP plus the indicated increasing amounts of the R32,138K mutant (1×, 5×, and 10×). (B) Immunoblot analysis of the injected animal caps from panel A. (C) Northern blot analysis of induction of the mesoderm-specific marker muscle actin. Caps collected at stage 21 were analyzed for expression of muscle actin (lowest band). The two upper bands represent cytoplasmic actin, which cross-reacts with the probe and acts as a loading control for the experiment. The injected mRNA is indicated beneath each lane. (D) MAPK activation in animal caps injected with the indicated constructs, monitored as described for Fig. 2D. XMAPK, Xenopus MAPK. (E) Quantitation of animal cap elongation, as described for Fig. 2D. The injected mRNA is indicated beneath each lane. Percentages are based on a minimum of 30 total animal caps in three separate experiments.
To confirm these results and to specify which SH2 domain, if either, is more important for SHP-2 signaling, we injected RNAs from the single SH2 domain mutants (in the context of ΔP). Animal caps expressing R32K/ΔP (the N-SH2 mutant) also elongated in response to bFGF at all doses tested (Fig. 2A and B and data not shown), indicating that the N-SH2 domain is required for the dominant negative effects of ΔP in bFGF signaling. When R138K/ΔP (the C-SH2 mutant) was expressed at levels comparable to that of ΔP, elongation was partially blocked, although clearly to a lesser extent than upon ΔP expression (Fig. 2A). At 5- to 10-fold-higher protein levels (Fig. 2B), R138K/ΔP efficiently blocked animal cap elongation (Fig. 2A) and MAPK activation (Fig. 2C). These data suggest that C-SH2 contributes to SHP-2 signaling since at higher levels of expression, the C-SH2 mutant (in the context of ΔP) can effectively block elongation and MAPK activation. However, C-SH2 appears to be less critical than N-SH2 in this pathway.
We also investigated the roles of the SH2 domains by using a rescue assay. Previously, it was shown that complete rescue of the ΔP block can be obtained upon coexpression of WT SHP-2 at levels barely detectable above that of endogenous SHP-2 (reference 74 and Fig. 3B). Comparable amounts of R32,138K protein (in the context of WT SHP-2) failed to rescue the ΔP block of elongation (Fig. 3A and B), muscle actin expression (Fig. 3C), or MAPK activation (Fig. 3D). Much higher levels of R32,138K did rescue the ΔP-induced block (Fig. 3A, B, and D); these results are quantified in Fig. 3E. However, injection of amounts of R32,138K RNA sufficient to rescue the elongation block resulted in inhibition of ΔP expression to levels below the minimum required for observation of a block (Fig. 3B), presumably because high levels of RNA saturate the translational machinery. Conceivably, however, at very high levels, the R32,138K protein may be able to find its correct target, either because the FLVRES motif mutation we employed decreases but does not eliminate SHP-2 SH2 domain binding capacity or because the requirement for SH2-directed targeting is obviated in cells expressing such high levels of R32,138K protein. In contrast to the effects of mutation of both SH2 domains, and similar to our earlier findings (see above), the C-SH2 domain (R138K) mutant remains capable of rescuing the ΔP-induced block. Titration experiments reveal that the dose of R138K required to rescue elongation is similar to that of WT-SHP-2, indicating again that N-SH2 is sufficient for bFGF signaling (data not shown).
The C-terminal tyrosines and proline-rich domain are dispensable for rescue of the ΔP block of bFGF signaling.
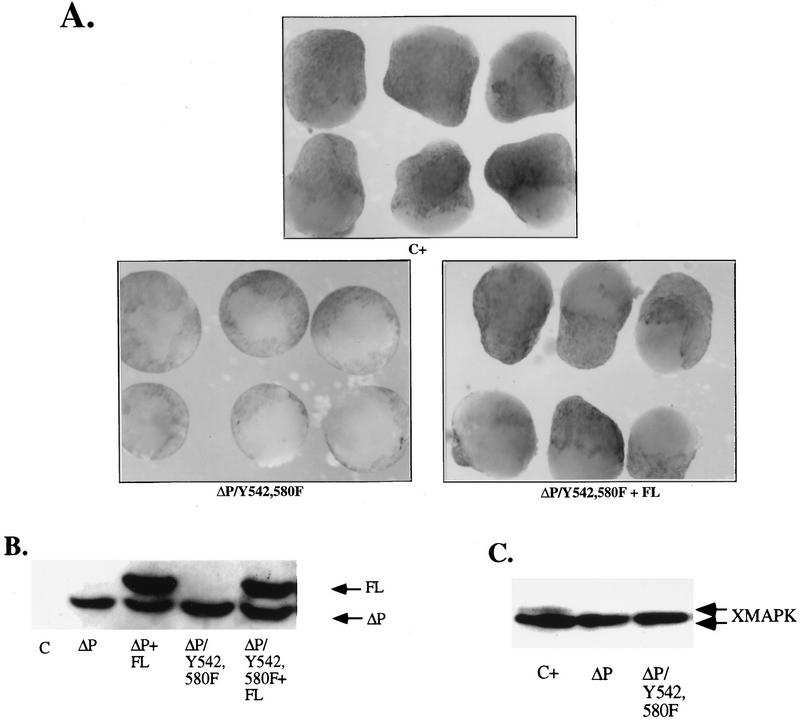
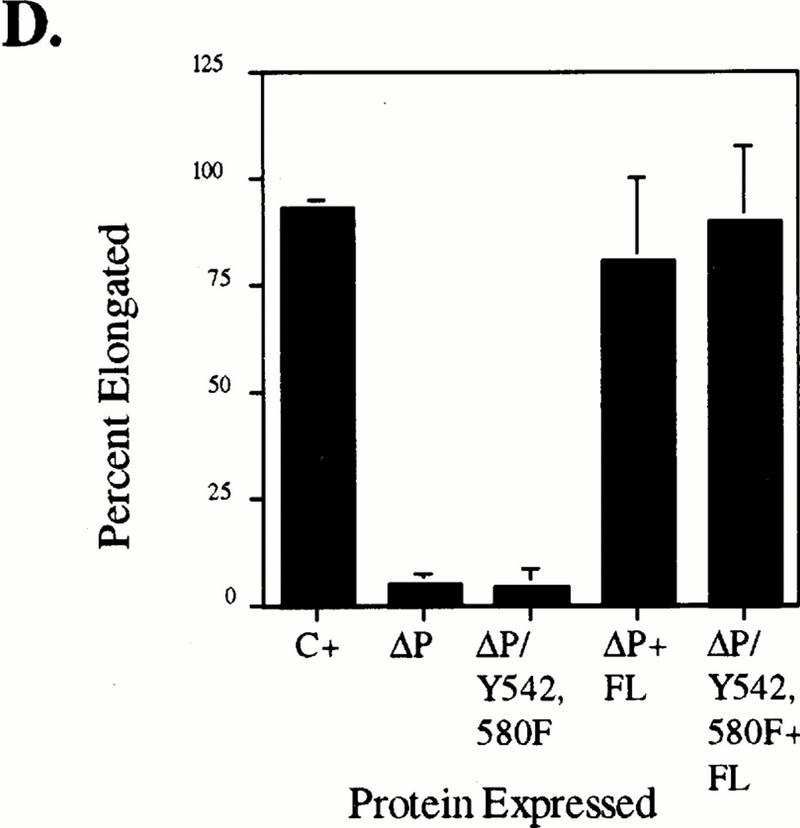
We next examined the role of domains within the C-terminal tail of SHP-2. Phosphorylated Y542 and Y580 both can bind Grb2, which led to the proposal that by binding Grb2, SHP-2 links RTKs to Ras activation (see the introduction). Tyrosyl phosphorylation of WT SHP-2 in response to bFGF treatment of animal caps is weak and inconsistent, although catalytically inactive forms of SHP-2, such as ΔP, are constitutively and strongly phosphorylated (data not shown). This suggests that SHP-2 probably is phosphorylated in this system but then rapidly autodephosphorylates. We were concerned that instead of sending a positive signal via Grb2, the increased tyrosyl phosphorylation of the SHP-2 C terminus in catalytically impaired mutants such as ΔP might actually sequester Grb2. If this were the case, the block in bFGF signaling caused by mutants such as ΔP might be artifactual, rather than reflecting a specific role for the SHP-2 catalytic domain in bFGF signaling; this concern has been raised also by others (71). To rule out this possibility, we generated a triple mutant (Y542,580F/ΔP) containing the ΔP deletion and both Y542 and Y580 converted to phenylalanine (Fig. 1C). Animal caps expressing levels of the triple-mutant protein equal to the level of ΔP required to block bFGF signaling (Fig. 4B) failed to elongate (Fig. 4A) or activate MAPK (Fig. 4C) in response to bFGF stimulation. The effects of this mutant were rescued by small amounts of WT SHP-2 (Fig. 4A), demonstrating that the triple-mutant blocks mesoderm induction by a mechanism similar to that of ΔP. In addition to confirming that the PTP domain itself is required for bFGF signaling, this result demonstrates that the C-terminal tyrosines are not required for the dominant negative effects of the ΔP mutant.
FIG. 4.
Tyrosine-to-phenylalanine mutations do not affect the ability of ΔP to block bFGF signaling. Representative experimental results show animal cap elongation, protein levels, and MAPK activation. (A) bFGF-stimulated animal caps at stage 10.5, prepared from embryos injected with the indicated mRNA. (B) Immunoblot analysis, carried out as described for Fig. 2B. Positions of ΔP proteins and WT full-length SHP-2 (FL) are indicated. In this exposure, endogenous Xenopus SHP-2 levels are too low to be detected. The injected mRNA is indicated beneath each lane. (C) MAPK activation in animal caps assayed as described for Fig. 2C. The injected mRNA is indicated beneath each lane. XMAPK, Xenopus MAPK. (D) Quantitation of animal cap elongation, as for Fig. 2D. The injected mRNA is indicated beneath each lane. Percentages are based on a minimum of 45 total animal caps in three or more separate experiments.
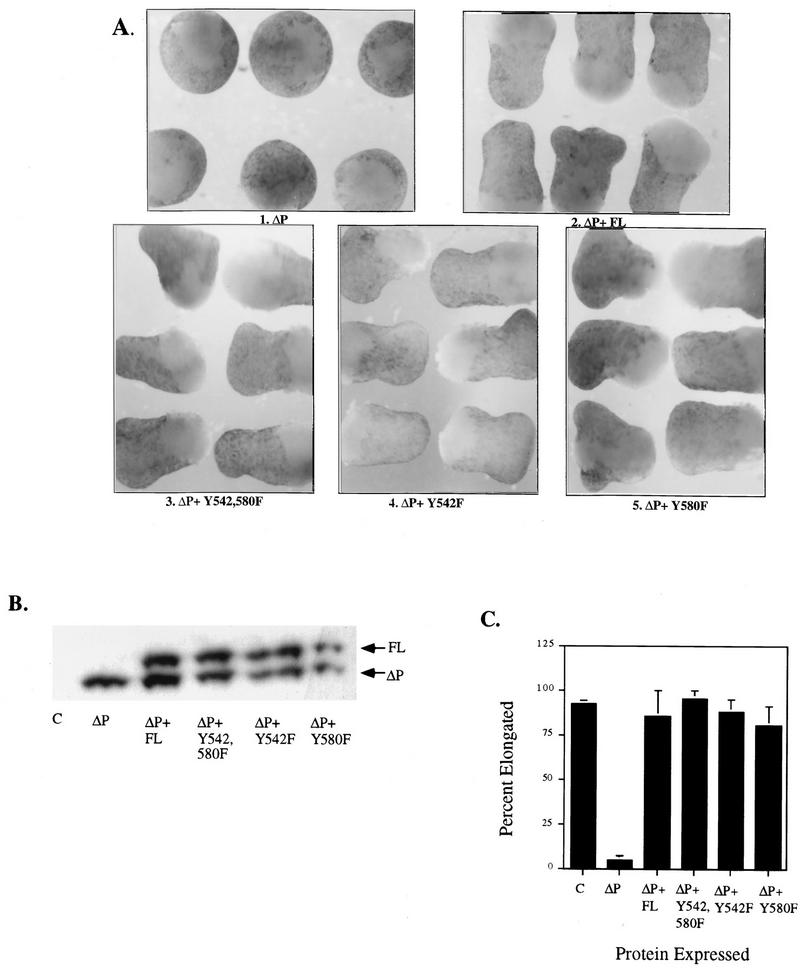
Additionally, animal caps overexpressing tyrosine-to-phenylalanine point mutations in the context of WT SHP-2 elongated normally (data not shown). Moreover, when expressed at levels comparable to that of WT SHP-2, Y542,580F, Y542F, or Y580F (Fig. 1C) rescued the ΔP-induced block of elongation (Fig. 5A and B), indicating that these sites are dispensable for efficient rescue of ΔP in bFGF signaling.
FIG. 5.
Mutation of tyrosines to phenylalanines does not affect the ability of full-length SHP-2 to rescue the ΔP block. (A) bFGF-stimulated animal caps at stage 10.5 from embryos injected with the indicated mRNA. Shown are caps injected with ΔP alone (ΔP), ΔP plus WT full-length SHP-2 (ΔP + FL), and ΔP plus the indicated single or double tyrosyl phosphorylation site mutants. (B) Immunoblot analysis of SHP-2 expression, as described for Fig. 2B. (C) Quantitation of animal cap elongation, as for Fig. 2D. Percentages are based on a minimum of 70 total animal caps in three or more separate experiments.
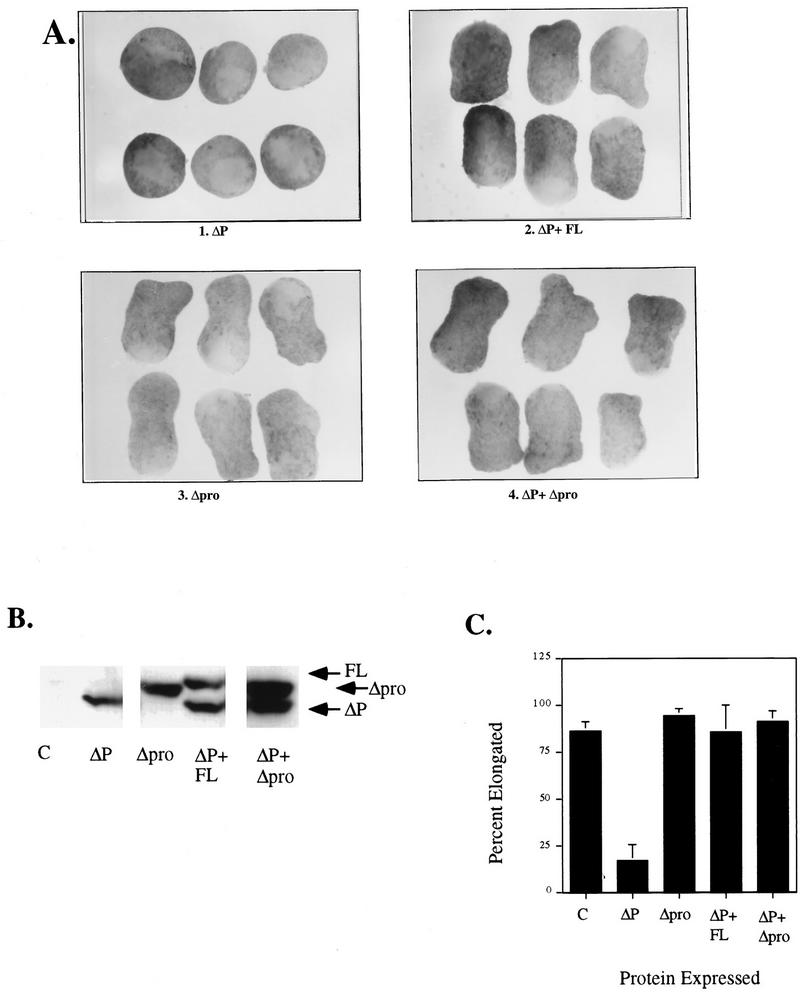
To assess the requirement for the proline-rich region in the C-terminal tail, we expressed an SHP-2 mutant with a 10-amino-acid deletion spanning this region (Δpro). This deletion eliminates the six prolines in the C-terminal tail (Fig. 1) and thus should prevent interaction with potential SH3 or WW domain-containing binding partners. Expression of Δpro failed to block animal cap elongation (Fig. 6A) or MAPK activation at all doses tested (data not shown), indicating that this region is not essential for bFGF signaling. More importantly, Δpro was as potent as WT SHP-2 for rescuing the ΔP-induced block (Fig. 6A and B and data not shown), providing strong evidence that the proline-rich region is not essential for SHP-2 signaling in the bFGF pathway.
FIG. 6.
Deletion of the C-terminal prolines has no effect on the ability of SHP-2 to rescue the ΔP block. Representative experimental results show animal cap elongation and protein levels. (A) bFGF-stimulated animal caps from embryos injected with the indicated mRNAs. (B) Immunoblot analysis, as described for Fig. 2B. (C) Quantitation of animal cap elongation, as for Fig. 2D. Percentages are based on a minimum of 37 total animal caps in two or more separate experiments.
Determinants of SHP specificity map to the PTP domain.
The above results (combined with our previous studies) show that the SH2 domains of SHP-2, and N-SH2 in particular, as well as the PTP domain are required for bFGF-stimulated animal cap elongation, MAPK activation and mesodermal marker induction. SHP-1 cannot substitute for SHP-2 in this system (reference 74 and data not shown), so we next addressed the question of which domain(s) is responsible for this difference. All known nontransmembrane PTPs contain noncatalytic domains capable of directing interactions with other proteins and/or lipids. Since isolated PTP catalytic domains generally display relatively low levels of substrate specificity, it has been suggested that targeting PTP catalytic domains to the correct location in the cell is a (the) major determinant of PTP specificity (45).
We decided to address this question by monitoring the effects of expression of a series of SHP-2/SHP-1 chimeric proteins. These constructs were designed to maintain the structural integrity of the individual SHP domains (Fig. 1D). Extensive structural information for N-SH2 (39) and N+C−SH2 (20) of SHP-2 allowed us to predict where the SH2 domains end and the SH2-PTP linker for both SHP-2 and SHP-1 begins and to design constructs based on these predictions. All constructs contained the linker region of SHP-2. Likewise, the PTP domains of SHP-2 and SHP-1 were modelled on the known crystal structure of PTP1B (7) in order to locate likely essential structural elements in the primary sequence. Fusions of the SH2 domains and the linker region to the presumptive α1 helix of the PTP domain of each PTP, as predicted from the PTP1B structure, were made. Similarly, C-terminal structural components were located by comparison with PTP1B, and the predicted α6 helix of each PTP domain was fused to the appropriate C-terminal tail.
Each of the chimeras, along with WT SHP-1 and SHP-2, was expressed in bacteria as GST fusion proteins and purified by affinity chromatography on glutathione agarose beads. The 212 and 12 chimeric proteins, as well as GST–SHP-1 and GST–SHP-2, displayed enzymatic activity against the artificial substrate para-nitrophenyl phosphate (with mean specific activities [± standard errors of the means] of 6.604 ± 0.591, 0.8726 ± 0.0555, 4.254 ± 0.586, and 0.2208 ± 0.0248 U/mg, respectively, where 1 U is 1 μmol/min). These data support the idea that the structural integrity of each PTP domain was maintained in the fusion proteins and, by inference, in the eukaryotic expression constructs. As has been observed previously, SHP-2 has a lower specific activity than SHP-1 (55, 72). Interestingly, the specific activities of the 212 and 12 chimeric proteins tended to correlate with the origin of the PTP domain.
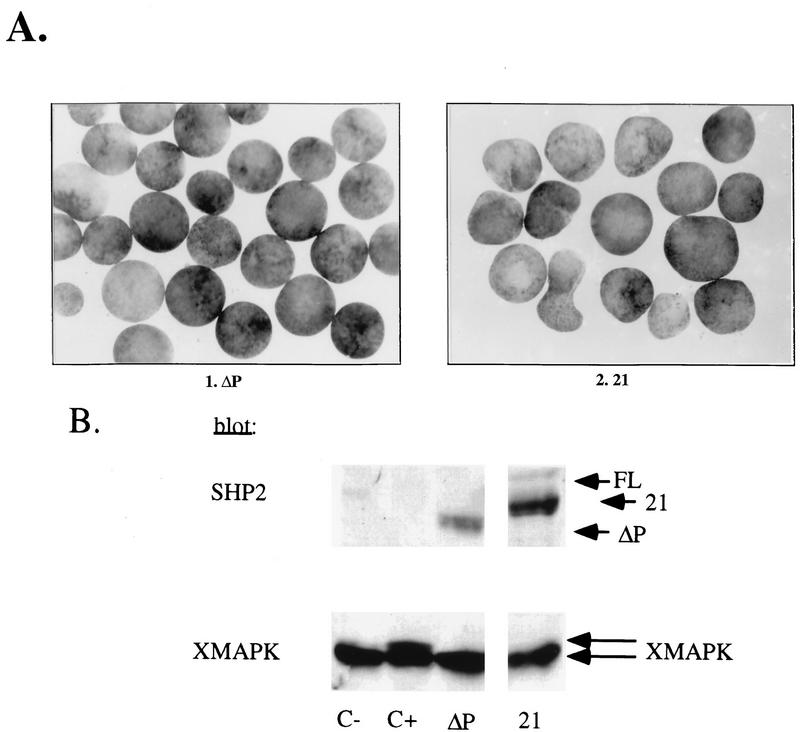
Injection of RNA encoding the 21 chimera, which contains the SH2 domains and linker region of SHP-2 (amino acids 1 to 262), fused to the PTP domain and C-terminal tail of SHP-1 (amino acids 260 to 595) resulted in a dominant negative phenotype indistinguishable from that produced by ΔP (Fig. 7A and B). This chimeric molecule should be able to bind to the same phosphotyrosyl proteins as does endogenous SHP-2, but nevertheless it failed to transmit an appropriate signal. Therefore, specific regions in the PTP domain and/or C terminus of SHP-2 must be required for transmission of positive signals from the XFGFR.
FIG. 7.
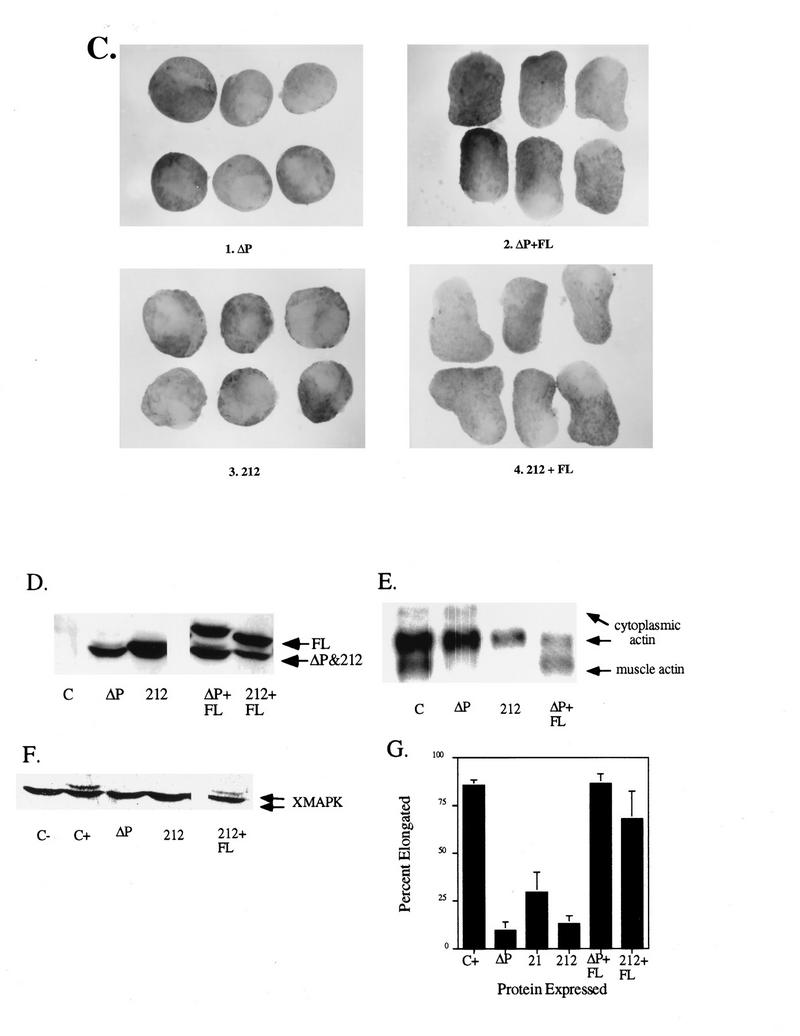
Chimeras containing the PTP domain of SHP-1 act as dominant negative mutants in the animal cap assay. Representative experimental results show animal cap elongation, protein levels, muscle actin induction, and MAPK activation. (A) bFGF-stimulated animal caps at stage 10.5 from embryos injected with either ΔP or the 21 chimera. Note that the two constructs block elongation equivalently. (B) Immunoblot analysis as described for Fig. 2B and MAPK activation in animal caps, assessed as for Fig. 2C. The injected mRNA is indicated beneath each lane. XMAPK, Xenopus MAPK. (C) bFGF-stimulated animal caps at stage 10.5. Shown are caps from embryos injected with ΔP alone (ΔP), ΔP plus WT full-length SHP-2 (ΔP + FL), the 212 chimera alone (212), and the 212 chimera plus WT full-length SHP-2 (212 + FL). (D) Immunoblot analysis as in Fig. 2B. (E) Northern blot analysis of muscle actin mRNA induction, carried out as for Fig. 3C. No muscle actin band is seen in either the ΔP or the 212 lane, even upon longer exposure. (F) MAPK activation in animal caps, as in Fig. 2C. The injected mRNA is indicated beneath each lane. (G) Quantitation of animal cap elongation, as in Fig. 2D. Percentages are based on a minimum of 45 total animal caps in three or more separate experiments.
To further delineate the determinants of specificity, we generated the 212 chimera, which contains the SH2 domains and linker region of SHP-2 (amino acids 1 to 262), the PTP domain of SHP-1 from the presumptive α1 to α6 helices (amino acids 260 to 519), and the entire C-terminal tail of SHP-2 (amino acids 527 to 593). This chimera also exhibited a dominant negative phenotype, as assessed by blocking of elongation (Fig. 7C and G) and muscle actin induction (Fig. 7E). These results demonstrate clearly that targeting of the PTP domain of SHP-1 to normal SHP-2 binding sites is not sufficient for biologically relevant bFGF signaling. MAPK activation in response to bFGF also was blocked by expression of the 212 chimera (Fig. 7D), demonstrating that the PTP domain of SHP-2 is absolutely required for receptor-proximal events, as well as later biological effects. Importantly, the dominant negative phenotypes produced by the 21 or the 212 chimera were rescued efficiently by small amounts of WT SHP-2 (Fig. 7C to G). These rescue experiments establish that the inhibitory effects of these chimeras on bFGF-induced signaling are due to their ability to interfere specifically with the function of endogenous SHP-2, instead of being a gratuitous consequence of, for example, a deregulated PTP.
To verify that biological specificity resides within the PTP domain, we tested the effects of the converse chimera, 12, containing the SH2 domains of SHP-1 (amino acids 1 to 210) and the linker, PTP domain, and C-terminal tail of SHP-2 (amino acids 215 to 593). Animal caps expressing the 12 chimera alone elongated normally (data not shown). This result might be explained if the 12 chimera failed to target to the correct phosphotyrosyl protein(s) in animal pole cells (i.e., if the 12 chimera acted as a null mutant). Alternatively, since overexpression of WT SHP-2 has little effect on bFGF-induced events in animal caps, chimera 12 might be behaving like WT SHP-2 in this assay. To distinguish between these possibilities, we used the rescue assay. The 12 chimera was coexpressed with the minimal amount of ΔP required to block bFGF-induced signaling. Remarkably, the 12 chimera partially rescued the ΔP-induced block of elongation (Fig. 8A), muscle actin induction (Fig. 8C), and MAPK activation (Fig. 8D). In the experiment whose results are shown in Fig. 8, a relatively small amount of the 12 chimera was expressed (compared with the level of expression of ΔP). In other experiments, in which the 12 chimera was expressed at levels substantially higher than the level of WT SHP-2 needed to effect full rescue, rescue by the chimera remained incomplete (data not shown).
FIG. 8.
The ΔP block is partially rescued by coexpression of a chimera containing the PTP domain of SHP-2. Representative experimental results show animal cap elongation, protein levels, muscle actin induction, and MAPK activation. (A) bFGF-stimulated animal caps at stage 10.5 from embryos injected with the indicated mRNAs. Note that ΔP is fully rescued by coexpression of WT full-length SHP-2 (ΔP + FL) and partially rescued upon coexpression of the 12 chimera (ΔP + 12). (B) Immunoblot analysis carried out as for Fig. 2B, except that an antibody directed against the C terminus of SHP-2 (Santa Cruz Biotechnology) was used to allow comparison of the levels of full-length SHP-2, ΔP, and the 12 chimera protein, which share the C terminus of SHP-2 (see Materials and Methods for details). (C) Northern blot analysis of induction of muscle actin mRNA, as for Fig. 3C. (D) MAPK activation in animal caps injected with the indicated constructs, analyzed as for Fig. 2C. XMAPK, Xenopus MAPK. (E) Quantitation of animal cap elongation, as in Fig. 2D. Percentages are based on a minimum of 88 total animal caps in three or more separate experiments.
Two separate conclusions can be reached from these experiments. The SH2 domains of SHP-1 probably do not bind to SHP-2 binding partners with an affinity equal to that of the SH2 domains of SHP-2, since rescue with the 12 chimera was only partial; thus, some specificity resides in the SH2 domains of SHP-2. Equally important, however, to restore proper signaling in vivo, the correct PTP domain (i.e., from SHP-2) must be present in the chimeric protein, implying that substantial biological specificity resides within the PTP domains of the two closely related SHPs. Moreover, the region responsible for specificity maps to amino acids 263 to 527 of SHP-2.
DISCUSSION
Using mesoderm induction in animal cap explants as a model system, we have elucidated the structural requirements for SHP-2 function in bFGF signaling. We found that both SH2 domains are important for normal signaling, although N-SH2 plays the more critical role. Surprisingly, we can find no role for the C-terminal tyrosines in this system, arguing strongly that the Grb2 adapter model for positive signaling by SHP-2 (9, 40, 47) does not apply to the bFGF pathway. Likewise, the proline-rich domain appears to be dispensable for bFGF signaling. The PTP domain is essential and, presumably via its ability to dephosphorylate only certain phosphotyrosyl proteins, is a, if not the, major determinant of biological specificity in vivo. The SH2 domains also appear to contribute to SHP specificity. Our results identify for the first time key structural determinants of biological specificity for a member of the PTP family.
Previous work suggests that the SH2 domains of SHPs may serve at least two types of function. First, several groups have shown that SHP-1 and SHP-2 are targeted to various plasma membrane proteins upon stimulation with growth factors or cytokines. In some cases, the SHPs bind directly to RTKs themselves (21, 37, 81); in others, SHPs may bind to transmembrane proteins, such as SHPS-1/SIRP family members (25, 33, 51, 52, 89), or to adapter or accessory molecules, such as IRS-1 (36), Gab1 (30), and FRS-2 (35). SH2 domain-directed binding serves to relocate SHPs to new intracellular locations, where they may gain access to potential substrates. Additionally, however, in vitro studies using recombinant SHPs and known phosphotyrosyl peptide ligands for their SH2 domains have suggested that SH2 domain engagement increases catalytic activity (14, 15, 31, 38, 54, 56, 61, 63, 73, 80). Basally, SHP-2 and SHP-1 appear to exist in a closed conformation wherein the SH2 domains bind to and inhibit the PTP domain; SH2 engagement relieves inhibition, presumably by promoting conversion to an open conformation. For SHP-1, N-SH2 alone can repress basal activity; C-SH2 cannot subserve this function (54). However, both SH2 domains can bind to phosphotyrosyl peptide targets. We cannot determine from our data which of these functions accounts for the critical requirement for the SHP-2 SH2 domains for proper bFGF-induced signaling. However, if, as in SHP-1, only N-SH2 of SHP-2 participates in basal repression, whereas both SH2 domains can direct binding to the appropriate intracellular target(s), the absolute requirement for N-SH2, as well as the contribution of C-SH2, may be explained (Fig. 2 and 3).
Homozygous deletion of 64 amino acids in the N-SH2 domain of murine SHP-2 results in mice with severe developmental abnormalities and impaired bFGF signaling (66). Although these results are consistent with a requirement for N-SH2 in bFGF signaling, another explanation is possible. The deletion mutant retains the essential FLVRES motif of N-SH2, but this mutant most likely is nonfunctional for either appropriate targeting or basal repression. Therefore, it is unclear whether the phenotype of these mice results solely from loss of normal SHP-2 function (hypomorphism) or a combination of hypomorphism and abnormal function (neomorphism) resulting from the presence of an activated PTP in an abnormal cellular location. Our data indicate that the SH2 domains of SHP-2 are indeed essential contributors to SHP-2 function in bFGF signaling in vivo.
The SH2 domains of SHP-2 also appear to account for some of the specificity between SHP-1 and SHP-2. The 12 chimera rescues the ΔP-induced block only partially (Fig. 8). If the SH2 domains of SHP-1 and SHP-2 were interchangeable, this chimera would be equivalent to WT SHP-2 in the rescue assay. Most likely, the SH2 domains of SHP-1 have an affinity which is lower than those of SHP-2 (but not absent) for the target of the ΔP SH2 domains. When expressed at high enough levels, the SH2 domains of the 12 chimera can cross over and bind what are normally SHP-2 binding targets. Consistent with the idea of some specificity resident within the SH2 domains, WT SHP-1 does not act as a dominant negative mutant for bFGF-induced signaling in animal caps (53a). Again, if the SH2 domains of the two SHPs were fully interchangeable, the results of our experiments with the 21 and 212 chimeras would predict that targeting of an incorrect PTP domain (i.e., SHP-1) to the normal location of SHP-2 would lead to a block in signaling.
Further study is needed to clarify the molecular basis by which the SH2 domains of the two SHPs confer specificity. Optimal binding sequences, determined by peptide library screening, for the N-SH2 domains of both SHPs have been determined (references 11, 69, and 70 and Table 1). The similarity between these consensus sequences renders it easy to see why, particularly at high concentrations, the two SHPs may be able to bind to the same intracellular target (see above). The precise determinants within phosphotyrosyl peptide ligands for the SHPs that permit discrimination between them remain unclear. Comparison of peptide sequences of binding targets for SHP-2 shows a general trend in which no basic residues are present between amino acids −2 and +5 (with position 0 being pY), an observation that is compatible with the crystal structures of the SHP-2 SH2 domains (20, 39). In contrast, many SHP-1 binding sites that have been mapped have basic residues within the region from −2 to +5 (Table 1). However, some proposed binding sites for SHP-2 (e.g., CTLA-4 [43]) deviate from these simple consensus sequences. It is possible that sequences distant from the phosphotyrosyl peptide ligand within proteins that bind to the SH2 domains of SHP-2 may influence binding; such influences would not be reflected in attempts to define a linear consensus sequence. Similarly, additional structural determinants within the SH2 domains may participate in recognition of specific peptides. Determination of the crystal structure of the SH2 domains of SHP-1 bound to an appropriate ligand and comparison with the available structures of SHP-2 should provide key insights into the resolution of these questions.
TABLE 1.
Binding sequences for the N-SH2 domains of SHP-2 and SHP-1a
| Peptide | Sequence |
|---|---|
| −−−pY+++++ | |
| 321pY12345 | |
| SHP-2 | |
| IRS-1 1172 | SLNpYIDLDLVK |
| PDGFR 1009 | SVLpYTAVQPNE |
| EpoR 425 | SFEpYTILDPSS |
| PECAM-1 663 | DVQpYTEVQV |
| SHP-1 | |
| EpoR 429/431 | HLKpYLVVSD |
| hKIR 303 | EVTpYSMVRF |
| mFcgRIIBI 309 | TITpYSLLKH |
| mCD22 863 | DVDpYVTLKH |
| mCD22 783 | TVSpYAILRFP |
In general, SHP-2 binding sites fit the consensus xxpYI/V-nonbasic-V/I/L/P-nonbasic-hydrophobic. The consensus sequence for SHP-1 SH2 domains has been recently determined as I/V xpYxxL. Note the prevalence of acidic and nonbasic residues within −2 to +5 in SHP-2 binding sequences, especially at +2 and +4 (boldface), and the presence of basic residues within −2 to +5 for SHP-1 binding sequences (italic). Although these consensus sequences account for most phosphotyrosyl peptides known to bind SHP-2 or SHP-1, there are exceptions (not shown). It is possible that sequences in these proteins distal to the tyrosyl phosphorylation site may modify binding (for details, see Discussion).
The C terminus of SHP-2 has structural motifs that could play important roles in signal transduction, including two tyrosyl phosphorylation sites, a proline-rich stretch, and potential serine phosphorylation sites. The proline-rich region and at least one of the two C-terminal tyrosines are conserved from Drosophila Csw to vertebrate SHP-2 (24, 58, 74), suggesting that this region contributes to signaling in at least some pathways. Studies of SHP-2 function in mammalian cells and in Xenopus have relied heavily on catalytically inactive mutants of SHP-2, which exhibit abnormally high levels of tyrosyl phosphorylation in response to some growth factors, including insulin and Xenopus bFGF (53a, 71). Since tyrosyl-phosphorylated SHP-2 can recruit Grb2 via SH2 domain interactions, we were concerned that aberrantly phosphorylated SHP-2 might sequester Grb2 away from its normal role in promoting Ras activation. Aberrant phosphorylation might also promote excessive SHP-2/SHIP association, with unclear functional consequences.
Our finding that the triple mutant ΔP/Y542,580F acts as a potent dominant negative mutant (Fig. 4) argues against the possibility that the dominant negative effects of ΔP (and other catalytically inactive mutants) might be the result of inappropriately sustained tyrosyl phosphorylation and subsequent Grb2 binding. Furthermore, our results argue that the C-terminal tyrosines of SHP-2 are not absolutely required for positive signaling in the bFGF pathway, since tyrosine-to-phenylalanine mutants rescue the dominant negative effects of ΔP in a manner indistinguishable from that of WT SHP-2 (Fig. 5). Deletion of the proline-rich region also has no effect on the ability of SHP-2 to rescue the ΔP-induced block (Fig. 6). We attempted to study the signaling properties of an SHP-2 deletion mutant completely lacking its C terminus but were unable to detect accumulation of the mutant protein in Xenopus embryos. Therefore, we cannot exclude the possibility that other motifs within the C terminus (e.g., seryl phosphorylation sites) play some role in the positive signaling function of SHP-2 or that this region plays a modulatory role in bFGF-induced mesoderm induction that is undetectable in our assays.
Our results clearly establish that the PTP domain, together with the SH2 domains of SHP-2, is primarily responsible for its positive signaling functions. Since SHP-1 cannot replace SHP-2 in early Xenopus development (74) (Fig. 7 and data not shown), we questioned which regions of the two PTPs are critical for specificity in vivo by studying a series of SHP-2/SHP-1 chimeras. Both the 21 and the 212 chimeras act as potent dominant negative mutants, providing strong evidence that in vivo specificity resides within the PTP domain: even when targeted to the correct location in the cell by the SH2 domains of SHP, the PTP domain of SHP-1 cannot substitute for the PTP domain of SHP-2. Most likely, residues within the PTP domains determine substrate specificity. However, we cannot exclude the possibility that specificity is dependent solely upon the magnitude of the catalytic activity resident within the PTP domains of the two SHPs. Since proteins containing the PTP domain of SHP-1 have 30-fold-higher activity against artificial substrates than those containing the PTP domain of SHP-2, it is possible that the dominant negative effects are a result of too much PTP activity. Determination of residues responsible for substrate binding and for setting the level of activity for each PTP should resolve this issue.
Compared to the amount of ΔP required, somewhat larger amounts of chimeric protein are needed to block animal cap elongation (Fig. 7 and data not shown). The N-SH2 domains of both SHPs are known to bind to and inhibit the PTP domain (see above). The endpoints of the deleted region within ΔP fall within important secondary structural elements, as predicted from the crystal structure of PTP-1B (7), so the PTP domain of ΔP probably is unfolded and thus unable to bind to the N-SH2. Therefore, ΔP likely exists basally in an open conformation. Conversely, the chimeras are likely to be basally repressed through binding of their N-SH2 domains to the PTP domain. Energy would be required to open this closed conformation. Accordingly, larger amounts of the chimera proteins may be required to exert the same degree of inhibition of bFGF signaling.
Perhaps the most compelling evidence that the PTP domain determines specificity in vivo is provided by the ability of the 12 chimera to partially rescue the ΔP-induced block. Although the 21 and 212 chimeras retain PTP activity in vitro, it remained formally possible that other parts of these molecules were improperly folded, leading to an artifactual block of signaling activity. However, the positive finding that the 12 chimera can to a large extent rescue the effects of a dominant negative SHP-2 mutant clearly shows that the PTP domain of SHP-2 provides critical information for proper signaling in vivo.
Our results are consistent with several recent studies using tissue culture systems. Using substrate-trapping approaches, p130Cas was identified as a likely substrate of PTP-PEST, arguing for PTP domain specificity for this nontransmembrane PTP as well (26). Chimeras similar to those used here were used in transient-transfection experiments with 293 cells (77). The researchers of that study concluded that the PTP domain of SHP-1 confers specificity for dephosphorylation of the EGFR. The biological significance of these observations is unclear, since the EGFR has not been identified as a bona fide substrate for SHP-1 in vivo. Moreover, the enzymatic activities of these chimeras were not reported, nor was it clear that the chimeras were expressed at comparable levels following transient transfection. Although these studies, together with our results, clearly indicate that the PTP domain can confer considerable specificity for several nontransmembrane PTPs, for others targeting seems to play a more dominant role. For example, although full-length PTP-1B appears to have highly restricted substrate specificity in transient-transfection studies (23), truncated PTP-1B lacking its C-terminal targeting sequence is relatively nonselective (26).
Most likely, all nontransmembrane PTPs use a combination of specificity conferred by targeting sequences and intrinsic PTP domain specificity to select the correct substrate(s) in vivo. By combining specificities resident in noncatalytic and catalytic domains, rapid evolution of a large number of selective PTPs may have been facilitated. For the SHPs, combinatorial specificity probably is of particular importance, since in several signaling pathways SHP-2 and SHP-1 appear to be targeted to similar, if not identical, sites, yet have drastically different downstream effects. The existence of specificity within the PTP domains of the SHPs would allow each molecule to select different targets from the same local milieu, potentially explaining the distinct effects of SHP-2 and SHP-1. Moreover, it may be possible to exploit the specificity resident within PTP catalytic domains to develop highly selective PTP inhibitors. Future studies should be directed to understanding the structural details underlying the specificities of the PTP domains of the SHPs.
ACKNOWLEDGMENTS
We thank S. Sokol and K. Itoh (Beth Israel-Deaconess Medical Center, Boston, Mass.) for use of their microinjection facilities, technical assistance, and Xenopus bFGF; J. Maller (University of Colorado School of Medicine, Denver) for Xenopus MAPK antibodies; and M. Hagel (Beth Israel-Deaconess Medical Center) for BL21 competent cells. We also thank D. Barford (Oxford University) and J. Timms (Beth Israel-Deaconess Medical Center) for useful insights and discussions regarding PTP structure.
This work was supported by NIH grant R01 CA49152 (to B.G.N.).
REFERENCES
- 1.Ali S, Chen Z, Lebrun J-J, Vogel W, Kharitonenkov A, Kelly P A, Ullrich A. PTP1D is a positive regulator of the prolactin signal leading to β-casein promoter activation. EMBO J. 1996;15:135–142. [PMC free article] [PubMed] [Google Scholar]
- 2.Allard J D, Chang H C, Herbst R, McNeill H, Simon M A. The SH2-containing tyrosine phosphatase corkscrew is required during signaling by sevenless, Ras1 and Raf. Development. 1996;122:1137–1146. doi: 10.1242/dev.122.4.1137. [DOI] [PubMed] [Google Scholar]
- 3.Amaya E, Musci T J, Kirschner M W. Expression of a dominant negative mutant of the FGF receptor disrupts mesoderm formation in Xenopus embryos. Cell. 1991;66:257–260. doi: 10.1016/0092-8674(91)90616-7. [DOI] [PubMed] [Google Scholar]
- 4.Amaya E, Stein P A, Musci T J, Kirschner M W. FGF signalling in the early specification of mesoderm in Xenopus. Development. 1993;118:477–487. doi: 10.1242/dev.118.2.477. [DOI] [PubMed] [Google Scholar]
- 5.Aroian R V, Koga M, Mendel J E, Ohshima Y, Sternberg P W. The let-23 gene necessary for Caenorhabditis elegans vulval induction encodes a tyrosine kinase of the EGF receptor subfamily. Nature. 1990;348:693–699. doi: 10.1038/348693a0. [DOI] [PubMed] [Google Scholar]
- 6.Arrandale J M, Gore-Willse A, Rocks S, Ren J M, Zhu J, Davis A, Livingston J N, Rabin D U. Insulin signaling in mice expressing reduced levels of Syp. J Biol Chem. 1996;271:21353–21358. doi: 10.1074/jbc.271.35.21353. [DOI] [PubMed] [Google Scholar]
- 7.Barford D, Flint A J, Tonks N K. Crystal structure of human protein tyrosine phosphatase 1B. Science. 1994;263:1397–1404. [PubMed] [Google Scholar]
- 8.Bennett A M, Hausdorff S F, O’Reilly A M, Freeman R M, Jr, Neel B G. Multiple requirements for SH-PTP2 in epidermal growth factor-mediated cell cycle progression. Mol Cell Biol. 1996;16:1189–1202. doi: 10.1128/mcb.16.3.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bennett A M, Tang T L, Sugimoto S, Walsh C T, Neel B G. Binding of tyrosyl phosphorylated SH2-containing tyrosyl phosphatase SHPTP2 to Grb2/Sos1 couples platelet-derived growth factor receptor β to the Ras signaling pathways. Proc Natl Acad Sci USA. 1994;91:7335–7339. doi: 10.1073/pnas.91.15.7335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bignon J S, Siminovitch K A. Identification of PTP1C mutation as a genetic defect in motheaten and viable motheaten mice: a step toward defining the roles of protein tyrosine phosphatases in the regulation of hemopoietic cell differentiation and function. Clin Immunol Immunopathol. 1994;73:168–179. doi: 10.1006/clin.1994.1185. [DOI] [PubMed] [Google Scholar]
- 11.Burshtyn D N, Yang W, Yi T, Long E O. A novel phosphotyrosine motif with a critical amino acid at position −2 for the SH2 domain-mediated activation of the tyrosine phosphatase SHP-1. J Biol Chem. 1997;272:13066–13072. doi: 10.1074/jbc.272.20.13066. [DOI] [PubMed] [Google Scholar]
- 12.Clark S G, Stern M J, Horvitz H R. C. elegans cell-signaling gene sem-5 encodes a protein with SH2 and SH3 domains. Nature. 1992;356:340–344. doi: 10.1038/356340a0. [DOI] [PubMed] [Google Scholar]
- 13.Cohen G B, Ren R, Baltimore D. Modular binding domains in signal transduction proteins. Cell. 1995;80:237–248. doi: 10.1016/0092-8674(95)90406-9. [DOI] [PubMed] [Google Scholar]
- 14.Dechert U, Adam M, Harder K W, Clark-Lewis I, Jirik F. Characterization of protein tyrosine phosphatase SH-PTP2. Study of phosphopeptide substrates and possible regulatory role of SH2 domains. J Biol Chem. 1994;269:5602–5611. [PubMed] [Google Scholar]
- 15.Dechert U, Affolter M, Harder K W, Matthews J, Owen P, Clark-Lewis I, Thomas M L, Aebersold R, Jirik F R. Comparison of the specificity of bacterially expressed cytoplasmid protein-tyrosine phosphatases SHP and SH-PTP2 towards synthetic phosphopeptide substrates. Eur J Biochem. 1995;231:673–681. doi: 10.1111/j.1432-1033.1995.0673d.x. [DOI] [PubMed] [Google Scholar]
- 16.Deng C-X, Wynshaw-Boris A, Shen M M, Daugherty C, Ornitz D M, Leder P. Murine FGFR-1 is required for early postimplantation growth and axial organization. Genes Dev. 1994;8:3045–3057. doi: 10.1101/gad.8.24.3045. [DOI] [PubMed] [Google Scholar]
- 17.Dominguez I, Itoh K, Sokol S. Role of glycogen synthase kinase 3 beta as a negative regulator of dorsoventral axis formation in Xenopus embryos. Proc Natl Acad Sci USA. 1995;92:8498–8502. doi: 10.1073/pnas.92.18.8498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duffy J B, Perrimon N. Recent advances in understanding signal transduction pathways in worms and flies. Curr Opin Cell Biol. 1996;8:231–238. doi: 10.1016/s0955-0674(96)80070-6. [DOI] [PubMed] [Google Scholar]
- 19.Duffy J B, Perrimon N. The torso pathway in Drosophila: lessons on receptor tyrosine kinase signaling and pattern formation. Dev Biol. 1994;166:380–395. doi: 10.1006/dbio.1994.1324. [DOI] [PubMed] [Google Scholar]
- 20.Eck M J, Pluskey S, Trub T, Harrison S C, Shoelson S E. Spatial constraints on the recognition of phosphoproteins by the tandem SH2 domains of the phosphatase SH-PTP2. Nature. 1996;379:277–280. doi: 10.1038/379277a0. [DOI] [PubMed] [Google Scholar]
- 21.Feng G-S, Hui C-C, Pawson T. SH2-containing phosphotyrosine phosphatase as a target of protein-tyrosine kinases. Science. 1993;259:1607–1611. doi: 10.1126/science.8096088. [DOI] [PubMed] [Google Scholar]
- 22.Feng G S, Pawson T. Phosphotyrosine phosphatases with SH2 domains: regulators of signal transduction. Trends Genet. 1994;10:54–58. doi: 10.1016/0168-9525(94)90149-x. [DOI] [PubMed] [Google Scholar]
- 23.Flint A J, Tiganis T, Barford D, Tonks N K. Development of “substrate trapping” mutants to identify physiological substrates of protein-tyrosine phosphatases. Proc Natl Acad Sci USA. 1997;94:1680–1685. doi: 10.1073/pnas.94.5.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freeman R M, Jr, Plutzky J, Neel B G. Identification of a human src-homology 2 (SH2) containing tyrosine phosphatase: a putative homolog of Drosophila corkscrew. Proc Natl Acad Sci USA. 1992;89:11239–11243. doi: 10.1073/pnas.89.23.11239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fujioka Y, Matozaki T, Noguchi T, Iwamatsu A, Yamao T, Takahashi N, Tsuda M, Takada T, Kasuga M. A novel membrane glycoprotein, SHPS-1, that binds the SH2-domain-containing protein tyrosine phosphatase SHP-2 in response to mitogens and cell adhesion. Mol Cell Biol. 1996;16:6887–6899. doi: 10.1128/mcb.16.12.6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garton A J, Flint A J, Tonks N K. Identification of p130cas as a substrate for the cytosolic protein tyrosine phosphatase PTP-PEST. Mol Cell Biol. 1996;16:6408–6418. doi: 10.1128/mcb.16.11.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Green J B A, Smith J C. Graded changes in dose of a Xenopus activin A homologue elicit stepwise transitions in embryonic cell fate. Nature. 1990;347:391–394. doi: 10.1038/347391a0. [DOI] [PubMed] [Google Scholar]
- 28.Hausdorff S F, Bennett A M, Neel B G, Birnbaum M J. Different signaling roles of SHPTP2 in insulin-induced GLUT1 expression and GLUT4 translocation. J Biol Chem. 1995;270:12965–12968. doi: 10.1074/jbc.270.22.12965. [DOI] [PubMed] [Google Scholar]
- 29.Herbst R, Carroll P M, Allard J D, Schilling J, Raabe T, Simon M A. Daughter of Sevenless is a substrate of the phosphotyrosine phosphatase corkscrew and functions during Sevenless signaling. Cell. 1996;85:899–909. doi: 10.1016/s0092-8674(00)81273-8. [DOI] [PubMed] [Google Scholar]
- 30.Holgado-Madruga M, Emlet D R, Moscatello D K, Godwin A K, Wong A J. A Grb2-associated docking protein in EGF- and insulin-receptor signalling. Nature. 1996;379:560–564. doi: 10.1038/379560a0. [DOI] [PubMed] [Google Scholar]
- 31.Huyer G, Li Z M, Adam M, Huckle W R, Ramachandran C. Direct determination of the sequence recognition requirements of the SH2 domains of SH-PTP2. Biochemistry. 1995;34:1040–1049. doi: 10.1021/bi00003a039. [DOI] [PubMed] [Google Scholar]
- 32.Itoh K, Sokol S. Heparan sulfate proteoglycans are required for mesoderm formation in Xenopus embryos. Development. 1994;121:2703–2711. doi: 10.1242/dev.120.9.2703. [DOI] [PubMed] [Google Scholar]
- 33.Kharitonenkov A, Chen Z, Sures I, Wang H, Schilling J, Ullrich A. A family of proteins that inhibit signaling though tyrosine kinase receptors. Nature. 1997;386:181–186. doi: 10.1038/386181a0. [DOI] [PubMed] [Google Scholar]
- 34.Klein P S, Melton D A. Hormonal regulation of embryogenesis: the formation of mesoderm in Xenopus laevis. Endocrine Rev. 1994;15:326–341. doi: 10.1210/edrv-15-3-326. [DOI] [PubMed] [Google Scholar]
- 35.Kouhara H, Hadari Y, Spivak-Kroizman T, Schilling J, Bar-Sagi D, Lax I, Schlessinger J. A lipid-anchored Grb2-binding protein that links FGF-receptor activation to the Ras/MAPK signaling pathway. Cell. 1997;89:693–702. doi: 10.1016/s0092-8674(00)80252-4. [DOI] [PubMed] [Google Scholar]
- 36.Kuhne M R, Pawson T, Lienhard G E, Feng G-S. The insulin receptor substrate 1 associates with the SH2-containing phosphotyrosine phosphatase Syp. J Biol Chem. 1993;268:11479–11481. [PubMed] [Google Scholar]
- 37.Lechleider R J, Freeman R M, Neel B G. Tyrosyl phosphorylation and growth factor receptor association of the human corkscrew homologue, SH-PTP2. J Biol Chem. 1993;268:13434–13438. [PubMed] [Google Scholar]
- 38.Lechleider R J, Sugimoto S, Bennett A M, Kashishian A S, Cooper J A, Shoelson S E, Walsh C T, Neel B G. Activation of the SH2-containing phosphotyrosine phosphatase SH-PTP2 by its binding site 1009, on the human platelet-derived growth factor b. J Biol Chem. 1993;268:21478–21481. [PubMed] [Google Scholar]
- 39.Lee C-H, Kominos D, Jacques S, Margolis B, Schlessinger J, Shoelson S E, Kuriyan J. Crystal structures of peptide complexes of the N-terminal SH2 domain of the Syp tyrosine phosphatase. Structure. 1994;15:423–438. doi: 10.1016/s0969-2126(00)00044-7. [DOI] [PubMed] [Google Scholar]
- 40.Li W, Nishimura R, Kashishian A, Batzer A G, Kim W J H, Cooper J A, Schlessinger J. A new function for a phosphotyrosine phosphatase: linking GRB2-Sos to a receptor tyrosine kinase. Mol Cell Biol. 1994;14:509–517. doi: 10.1128/mcb.14.1.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu L, Damen J E, Ware M D, Krystal G. Interleukin-3 induces the association of the inositol 5-phosphatase SHIP with SHP-2. J Biol Chem. 1997;272:10998–11001. doi: 10.1074/jbc.272.17.10998. [DOI] [PubMed] [Google Scholar]
- 42.Luettke N C, Phillips H K, Qiu T H, Copeland N G, Earp H S, Jenkins N A, Lee D C. The mouse waved-2 phenotype results from a point mutation in the EGF receptor tyrosine kinase. Genes Dev. 1994;8:399–413. doi: 10.1101/gad.8.4.399. [DOI] [PubMed] [Google Scholar]
- 43.Marengere L E M, Waterhouse P, Duncan G S, Mittrucker H-W, Feng G-S, Mak T W. Regulation of T cell receptor signaling by tyrosine phosphatase SYP association with CTLA-4. Science. 1996;272:1170–1173. doi: 10.1126/science.272.5265.1170. [DOI] [PubMed] [Google Scholar]
- 44.Marshall C J. MAP kinase kinase kinase, MAP kinase kinase, and MAP kinase. Curr Opin Genet Dev. 1994;4:82–89. doi: 10.1016/0959-437x(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 45.Mauro L J, Dixon J E. “Zip codes” direct intracellular protein tyrosine phosphatases to the correct cellular “address.”. Trends Biochem Sci. 1994;19:151–155. doi: 10.1016/0968-0004(94)90274-7. [DOI] [PubMed] [Google Scholar]
- 46.McCormick F. Activators and effectors of ras p21 proteins. Curr Opin Genet Dev. 1994;4:71–76. doi: 10.1016/0959-437x(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 47.Neel B G. Structure and function of SH2-domain containing tyrosine phosphatases. Semin Cell Biol. 1993;4:419–432. doi: 10.1006/scel.1993.1050. [DOI] [PubMed] [Google Scholar]
- 48.Neel B G, Tonks N K. Protein tyrosine phosphatases in signal transduction. Curr Opin Cell Biol. 1997;9:193–204. doi: 10.1016/s0955-0674(97)80063-4. [DOI] [PubMed] [Google Scholar]
- 49.Newport K, Kirschner M. A major developmental transition in early Xenopus embryos. I. Characterization and timing of cellular changes at the midblastula stage. Cell. 1982;30:675–686. doi: 10.1016/0092-8674(82)90272-0. [DOI] [PubMed] [Google Scholar]
- 50.Nieuwkoop P D. The formation of mesoderm in urodelean amphibians. I. Induction by the endoderm. Wilhelm Roux’ Arch. 1969;162:341–373. doi: 10.1007/BF00578701. [DOI] [PubMed] [Google Scholar]
- 51.Noguchi T, Matozaki T, Fujioka Y, Yamao T, Tsuda M, Takada T, Kasuga M. Characterization of a 115-kDa protein that binds to SH-PTP2, a protein-tyrosine phosphatase with Src homology 2 domains, in Chinese hamster ovary cells. J Biol Chem. 1996;271:27652–27658. doi: 10.1074/jbc.271.44.27652. [DOI] [PubMed] [Google Scholar]
- 52.Noguchi T, Matozaki T, Horita K, Fujioka Y, Kasuga M. Role of SH-PTP2, a protein-tyrosine phosphatase with Src homology 2 domains, in insulin-stimulated Ras activation. Mol Cell Biol. 1994;14:6674–6682. doi: 10.1128/mcb.14.10.6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Olivier J P, Raabe T, Henkemeyer M, Dickson B, Mbamalu G, Margolis B, Schlessinger J, Hafen E, Pawson T. A Drosophila SH2-SH3 adaptor protein implicated in coupling of the sevenless tyrosine kinase to an activator of Ras guanine nucleotide exchange, Sos. Cell. 1993;73:179–191. doi: 10.1016/0092-8674(93)90170-u. [DOI] [PubMed] [Google Scholar]
- 53a.O’Reilly, A. M., and B. G. Neel. Unpublished observations.
- 54.Pei D, Lorenz U, Klingmuller U, Neel B G, Walsh C T. Intramolecular regulation of protein tyrosine phosphatase SH-PTP1: a new function for Src homology 2 domains. Biochemistry. 1994;33:15483–15493. doi: 10.1021/bi00255a030. [DOI] [PubMed] [Google Scholar]
- 55.Pei D, Neel B G, Walsh C T. Overexpression, purification, and characterization of Src homology 2-containing protein tyrosine phosphatase. Proc Natl Acad Sci USA. 1993;90:1092–1096. doi: 10.1073/pnas.90.3.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pei D, Wang J, Walsh C T. Differential functions of the two Src homology 2 domains in protein tyrosine phosphatase SH-PTP1. Proc Natl Acad Sci USA. 1996;93:1141–1145. doi: 10.1073/pnas.93.3.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perkins L A, Johnson M R, Melnick M B, Perrimon N. The non-receptor protein tyrosine phosphatase Corkscrew functions in multiple receptor tyrosine kinase pathways in Drosophila. Dev Biol. 1996;180:63–81. doi: 10.1006/dbio.1996.0285. [DOI] [PubMed] [Google Scholar]
- 58.Perkins L A, Larsen I, Perrimon N. The Drosophila corkscrew gene encodes a putative protein tyrosine phosphatase that functions to transduce the terminal signal from the receptor tyrosine kinase torso. Cell. 1992;70:225–236. doi: 10.1016/0092-8674(92)90098-w. [DOI] [PubMed] [Google Scholar]
- 59.Perrimon N. Signalling pathways initiated by receptor tyrosine kinases in Drosophila. Curr Opin Cell Biol. 1994;6:260–266. doi: 10.1016/0955-0674(94)90145-7. [DOI] [PubMed] [Google Scholar]
- 60.Perrimon N, Perkins L A. There must be 50 ways to rule the signal: the case of the Drosophila EGF receptor. Cell. 1997;89:13–16. doi: 10.1016/s0092-8674(00)80177-4. [DOI] [PubMed] [Google Scholar]
- 61.Pluskey S, Wandless T J, Walsh C T, Shoelson S E. Potent stimulation of SH-PTP2 phosphatase activity by simultaneous occupancy of both SH2 domains. J Biol Chem. 1995;270:2897–2900. doi: 10.1074/jbc.270.7.2897. [DOI] [PubMed] [Google Scholar]
- 62.Plutzky J, Neel B G, Rosenberg R D. Isolation of a novel SRC homology 2 (SH2) containing tyrosine phosphatase. Proc Natl Acad Sci USA. 1992;89:1123–1127. doi: 10.1073/pnas.89.3.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pregel M J, Shen S-H, Storer A C. Regulation of protein tyrosine phosphatase 1C: opposing effects of the two src homology 2 domains. Protein Eng. 1995;8:1309–1316. doi: 10.1093/protein/8.12.1309. [DOI] [PubMed] [Google Scholar]
- 64.Roche S, McGlade J, Jones M, Gish G D, Pawson T, Courtneidge S A. Requirement of phospholipase C gamma, the tyrosine phosphatase Syp and the adaptor proteins Shc and Nck for PDGF-induced DNA synthesis: evidence for the existence of Ras-dependent and Ras-independent pathways. EMBO J. 1996;15:4940–4948. [PMC free article] [PubMed] [Google Scholar]
- 65.Rottapel R, Reedijik M, Williams D E, Lyman S D, Anderson D M, Pawson T, Bernstein A. The Steel/W transduction pathway: kit autophosphorylation and its association with a unique subset of cytoplasmic signalling proteins is induced by the steel factor. Mol Cell Biol. 1991;11:3043–3051. doi: 10.1128/mcb.11.6.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saxton T M, Henkemeyer M, Gasca S, Shen R, Rossi D J, Shalaby F, Feng G-S, Pawson T. Abnormal mesoderm patterning in mouse embryos mutant for the SH2 tyrosine phosphatase SHP-2. EMBO J. 1997;16:2352–2364. doi: 10.1093/emboj/16.9.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Seger R, Krebs E G. The MAPK signaling cascade. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- 68.Simon M A. Signal transduction during the development of the Drosophila R7 photoreceptor. Dev Biol. 1994;166:431–442. doi: 10.1006/dbio.1994.1327. [DOI] [PubMed] [Google Scholar]
- 69.Songyang Z, Blechner S, Hoagland N, Hoekstra M F, Piwnica-Worms H, Cantley L C. Use of an oriented peptide library to determine the optimal substrates of protein kinases. Curr Biol. 1994;4:973–982. doi: 10.1016/s0960-9822(00)00221-9. [DOI] [PubMed] [Google Scholar]
- 70.Songyang Z, Shoelson S E, Chaudhuri M, Gish G, Pawson T, Haser W G, King F, Roberts T, Ratnofsky S, Lechleider R J, Neel B G, Birge R B, Fajardo J E, Chou M M, Hanafusa H, Schaffhausen B, Cantley L C. SH2 domains recognize specific phosphopeptide sequences. Cell. 1993;72:767–778. doi: 10.1016/0092-8674(93)90404-e. [DOI] [PubMed] [Google Scholar]
- 71.Stein-Gerlach M, Kharitonenkov A, Vogel W, Ali S, Ullrich A. Protein-tyrosine phosphatase 1D modulates its own state of tyrosine phosphorylation. J Biol Chem. 1995;270:24635–24637. doi: 10.1074/jbc.270.42.24635. [DOI] [PubMed] [Google Scholar]
- 72.Sugimoto S, Lechleider R J, Shoelson S E, Neel B G, Walsh C T. Expression, purification, and characterization of SH2-containing protein tyrosine phosphatase, SH-PTP2. J Biol Chem. 1993;268:22771–22776. [PubMed] [Google Scholar]
- 73.Sugimoto S, Wandless T J, Shoelson S E, Neel B G, Walsh C T. Activation of the SH2-containing protein tyrosine phosphatase, SH-PTP2, by phosphotyrosine containing peptides derived from insulin receptor substrate-1. J Biol Chem. 1993;268:2733–2736. [PubMed] [Google Scholar]
- 74.Tang T L, Freeman R M, O’Reilly A M, Neel B G, Sokol S Y. The SH2-containing protein tyrosine phosphatase SH-PTP2 is required upstream of MAP kinase for early Xenopus development. Cell. 1995;80:473–483. doi: 10.1016/0092-8674(95)90498-0. [DOI] [PubMed] [Google Scholar]
- 75.Tauchi T, Feng G-S, Marshall M S, Shen R, Mantel C, Pawson T, Broxmeyer H E. The ubiquitously expressed Syp phosphatase interacts with c-kit and Grb2 in hematopoietic cells. J Biol Chem. 1994;269:25206–25211. [PubMed] [Google Scholar]
- 76.Tauchi T, Feng G S, Shen R, Hoatlin M, Bagby G C, Jr, Kabat D, Lu L, Broxmeyer H E. Involvement of SH2-containing phosphotyrosine phosphatase Syp in erythropoietin receptor signal transduction pathways. J Biol Chem. 1995;270:5631–5635. doi: 10.1074/jbc.270.10.5631. [DOI] [PubMed] [Google Scholar]
- 77.Tenev T, Keilhack H, Tomic S, Stoyanov B, Stein-Gerlach M, Lammers R, Krivtsov A V, Ullrich A, Bohmer F D. Both SH2 domains are involved in interaction of SHP-1 with the epidermal growth factor receptor but cannot confer receptor-directed activity to SHP-1/SHP-2 chimera. J Biol Chem. 1997;272:5966–5973. doi: 10.1074/jbc.272.9.5966. [DOI] [PubMed] [Google Scholar]
- 78.Tonks N K. Protein tyrosine phosphatases and the control of cellular signaling responses. Adv Pharmacol. 1996;36:91–119. doi: 10.1016/s1054-3589(08)60578-5. [DOI] [PubMed] [Google Scholar]
- 79.Tonks N K, Neel B G. From form to function: signaling by protein tyrosine phosphatases. Cell. 1996;87:365–368. doi: 10.1016/s0092-8674(00)81357-4. [DOI] [PubMed] [Google Scholar]
- 80.Townley R, Shen S-H, Banville D, Ramachandran C. Inhibition of the activity of protein tyrosine phosphatase 1C by its SH2 domains. Biochemistry. 1993;32:13414–13418. doi: 10.1021/bi00212a006. [DOI] [PubMed] [Google Scholar]
- 81.Vogel W, Lammers R, Huang J, Ullrich A. Activation of phosphotyrosine phosphatase by tyrosine phosphorylation. Science. 1993;259:1611–1614. doi: 10.1126/science.7681217. [DOI] [PubMed] [Google Scholar]
- 82.Vogel W, Ullrich A. Multiple in vivo phosphorylated tyrosine phosphatase SHP-2 engages binding to Grb2 via Tyrosine 584. Cell Growth Differ. 1996;7:1589–1597. [PubMed] [Google Scholar]
- 83.Waskiewicz A J, Cooper J A. Mitogen and stress-response pathways—MAP kinase cascades and phosphatase regulation in mammals and yeast. Curr Opin Cell Biol. 1995;7:798–805. doi: 10.1016/0955-0674(95)80063-8. [DOI] [PubMed] [Google Scholar]
- 84.Welham M J, Dechert U, Leslie K B, Jirik F, Schrader J W. Interleukin (IL)-3 and granulocyte/macrophage colony-stimulating factor, but not IL-4, induce tyrosine phosphorylation, activation, and association of SHPTP2 with Grb2 and phosphatidylinositol 3′-kinase. J Biol Chem. 1994;269:23764–23768. [PubMed] [Google Scholar]
- 85.Xiao S, Rose D W, Sasaoka T, Maegawa H, Burke T R J, Roller P P, Shoelson S E, Olefsky J M. Syp (SH-PTP2) is a positive mediator of growth factor-stimulated mitogenic signal transduction. J Biol Chem. 1994;269:21244–21248. [PubMed] [Google Scholar]
- 86.Yamaguchi T P, Harpal K, Henkemeyer M, Rossant J. Fgfr-1 is required for embryonic growth and mesodermal patterning during mouse gastrulation. Genes Dev. 1994;8:3032–3044. doi: 10.1101/gad.8.24.3032. [DOI] [PubMed] [Google Scholar]
- 87.Yamauchi K, Milarski K L, Saltiel A R, Pessin J E. Protein-tyrosine-phosphatase SHPTP2 is a required positive effector for insulin downstream signaling. Proc Natl Acad Sci USA. 1995;92:664–668. doi: 10.1073/pnas.92.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yamauchi K, Pessin J E. Epidermal growth factor-induced association of the SHPTP2 protein tyrosine phosphatase with a 115-kDa phosphotyrosine protein. J Biol Chem. 1995;270:14871–14874. doi: 10.1074/jbc.270.25.14871. [DOI] [PubMed] [Google Scholar]
- 89.Yamauchi K, Ribon V, Saltiel A R, Pessin J E. Identification of the major SHPTP2-binding protein that is tyrosine-phosphorylated in response to insulin. J Biol Chem. 1995;270:17716–17722. doi: 10.1074/jbc.270.30.17716. [DOI] [PubMed] [Google Scholar]