Abstract
Testosterone is the classical male anabolic hormone, involved in sexual development, virilisation and regulation of body composition in adult men. Organic disease involving the hypothalamus, pituitary or testes may interfere with endogenous testosterone production. In such men, testosterone treatment effectively ameliorates symptoms and signs of androgen deficiency. However, non-gonadal factors including age, body mass index and medical comorbidities influence circulating testosterone, and older men have on average lower testosterone concentrations compared with younger men. In these men, testosterone treatment would be a pharmacological intervention requiring stringent justification via high-quality evidence from randomised controlled trials (RCTs). Recent RCTs show benefits of testosterone treatment to improve sexual function, anaemia and bone mineral density in older men, and to prevent or revert type 2 diabetes mellitus in men at high risk. Results from a large cardiovascular safety trial in men with or at risk of cardiovascular disease provide important reassurance as to cardiovascular and prostate safety of testosterone treatment. Key questions remain as to whether testosterone’s anabolic and other effects can be used safely to counter reductions in lean mass associated with incretin-based weight loss medications in men with obesity, and whether it might prevent disabilities including frailty, osteoporotic fractures and dementia in older men generally. This last question could be answered by a new testosterone RCT, targeting men in the 65–80 years age bracket, which would necessarily be large and of extended duration. A composite endpoint could be used which integrates potential benefits and risks, such as disability-free survival.
Key Points
| Men with hypothalamic, pituitary or testicular disease resulting in androgen deficiency warrant testosterone therapy in the absence of reversible causes or contraindications. |
| In men without organic hypogonadism, recent large trials show benefits of testosterone treatment to improve sexual function, anaemia and bone mineral density in older men and prevent or revert type 2 diabetes mellitus in men at high risk, and provide reassurance regarding cardiovascular and prostate safety of testosterone treatment. |
| Future randomised controlled trials would be necessary to test whether testosterone treatment could be used as an adjunct to incretin-based anti-obesity medications, and whether in older men it may enhance survival free of disabilities, including those arising from osteoporosis-related fractures and dementia. |
Introduction
Testosterone, the principal male anabolic hormone, has a central role in sexual development, and in adult men regulates libido, virilisation and body composition, increasing muscle mass and reducing fat [1]. Men with organic diseases of the hypothalamus, pituitary or testes which impair testosterone production manifest symptoms and signs of androgen deficiency, including changes in body composition, which are improved by testosterone treatment [2–5]. Older men without diseases of the hypothalamus, pituitary or testes tend to have lower circulating testosterone concentrations compared with younger men [6, 7]. For the purposes of this review, testosterone concentration refers to the total amount of testosterone in serum or plasma, including testosterone bound to sex hormone-binding globulin (SHBG) and other carrier proteins such as albumin, and any unbound fraction. Non-gonadal factors such as obesity and other medical comorbidities may influence functioning of the hypothalamic-pituitary-testicular (HPT) axis to lower testosterone concentrations [8–10]. In this respect, higher body mass index (BMI) is robustly associated with lower testosterone concentrations in adult men from young to old [10]. Previous studies have documented cross-sectional and longitudinal decreases in circulating testosterone concentrations in men, thought to occur from age 30 years onwards [8, 9, 11–14]. However, a recent individual participant data (IPD) meta-analysis of cohort studies including 25,149 community-dwelling men with circulating testosterone measured accurately using mass spectrometry showed that testosterone and luteinising hormone (LH) concentrations did not differ with age in men under the age of 70 years when sociodemographic factors including BMI were accounted for [10]. However, beyond the age of 70 years testosterone concentrations declined with age, whilst LH increased. Thus, biochemical evidence of impaired Leydig cell function appears relatively late in life, around the age of 70 years rather than 30 years.
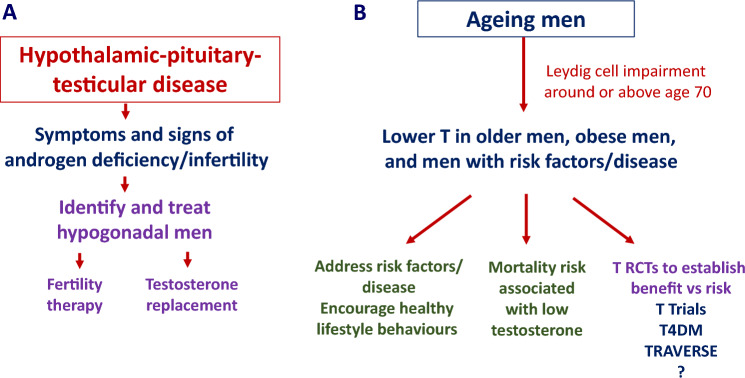
These findings highlight the importance of distinguishing between men with diseases of the hypothalamus, pituitary or testes resulting in androgen deficiency (organic hypogonadism) from men with an intact HPT axis with varying ages and medical comorbidities influencing testosterone concentrations (Fig. 1). The use of testosterone in adolescents and young adults, as well as in transgender medicine, is outside the scope of this article [15, 16]. This narrative review is based on targeted PubMed searches focussed on relevant publications in the last 10 years. The aims are to (i) illustrate the manner in which recent research findings have informed the diagnosis of androgen deficiency in men, (ii) discuss the implications of recent large randomised controlled trials (RCTs) of testosterone treatment in middle-aged to older men, and (iii) explore the potential roles of testosterone treatment as an anabolic adjunct to anti-obesity therapies and to prevent ill health in the expanding population of older men.
Fig. 1.
A Men with organic hypogonadism due to diseases affecting the hypothalamic-pituitary-testicular (HPT) axis may present with infertility or androgen deficiency, and warrant treatment. B Men with an intact HPT axis, who may be older, or have central adiposity/obesity and/or medical comorbidities, should be encouraged to engage in healthy lifestyle behaviours. T Trials, T4DM and TRAVERSE provide high-quality evidence as to benefits versus risks of testosterone treatment, with scope for a fourth, larger randomised controlled trial.
Management of Men with Organic Hypogonadism
Symptoms, Signs and Confirmatory Biochemical Testing
Men with diseases of the hypothalamus or pituitary gland which disrupt HPT axis function, or of the testes which impair testicular function, may present with reduced fertility, or with symptoms and signs of androgen deficiency. Some symptoms are non-specific, such as lethargy, fatigue, decreased energy and low mood. Others are organ specific, such as osteopenia, osteoporosis, reduced muscle mass and strength, increased fat mass and presence of gynaecomastia; even so, these can be present in eugonadal men [2]. Decreased libido is associated with low testosterone concentrations, whereas erectile dysfunction tends to have a multifactorial aetiology.
Where the clinical syndrome of androgen deficiency is present and organic disease of the hypothalamus, pituitary or testes is demonstrated, biochemical testing provides confirmation of low circulating testosterone and elevated LH in the presence of testicular disease. Where possible, fasting blood sampling should be done early morning, following a normal night’s sleep, with testosterone assayed using mass spectrometry [2]. When men wish to father children, considerations such as semen analysis and fertility treatment need to be addressed. For example, in men with hypogonadism due to pituitary disease, gonadotrophin treatment instead of testosterone may be employed to restore spermatogenesis. In the absence of reversible causes or contraindications, men with organic hypogonadism respond well to testosterone treatment with improvement in the symptoms and signs of androgen deficiency [2–5].
Testosterone Reference Ranges
Difficulties arise when men are categorised as being hypogonadal on the basis of having symptoms and signs consistent with androgen deficiency and a ‘low’ circulating testosterone on the basis of a semi-arbitrary cut-off, despite the absence of proven HPT axis disease [17]. These men are often older, have higher BMI or have medical comorbidities, in association with non-specific symptoms [8–10].
Defining ‘low’ testosterone is problematic because there are different reference ranges derived from different samples of healthy men. In 124 reproductively normal men aged 21–35 years, the testosterone reference range defined which was assayed using mass spectrometry and defined by the 2.5th and 97.5th percentiles (the middle 95% of testosterone values) was 10.4–30.1 nmol/L [6]. In 1185 non-obese men aged 19–39 years it was 9.2–31.8 nmol/L [18], and in very healthy men aged 70–89 years it was 6.4–25.7 nmol/L [7], both also assayed using mass spectrometry. Furthermore, when applying reference ranges to interpretation of results from individual men, the various socio-demographic, lifestyle and medical factors which influence testosterone concentrations need to be considered [10]. Therefore, it is difficult to justify applying a single testosterone cut-off value as a diagnostic test for men in general.
Issues with Calculating Free Testosterone
Testosterone concentrations are affected by concentrations of SHBG, its major carrier protein in the circulation. The utility of ‘free’ testosterone, namely the fraction not bound to SHBG or other carrier proteins, remains contentious [19, 20]. Equilibrium dialysis of serum under standardised conditions, followed by mass spectrometry assay of testosterone, indicates that 2–3% of serum total testosterone will cross the semi-permeable membrane to be quantitated in the dialysate [21]. Whether this fraction is present and stable in vivo, or represents at least in part testosterone bound to SHBG or other carrier proteins that dissociates in vitro and the relative biological activities of SHBG-bound versus unbound testosterone, remain unclear [19]. Equilibrium dialysis is labour intensive and not readily available.
Calculated free testosterone has been suggested as a surrogate for situations in which SHBG concentrations are abnormal [4, 5], but different calculations yield different results and clinically validated reference ranges are lacking [22, 23]. Calculations often incorporate assumptions regarding fixed dissociation constants for testosterone and SHBG, which may not be accurate [24]. A recent IPD meta-analysis found that calculating free testosterone using the Vermeulen equation produced a variable with a strong, linear, inverse association with age, demonstrating another major limitation of this approach [25]. These issues highlight the disadvantages of applying calculated free testosterone in the clinical setting.
Interpreting (Total) Testosterone in Relation to SHBG
In a large cross-sectional analysis of men from UK Biobank aged 40–69 years, (total) testosterone increased linearly with SHBG until the SHBG threshold of 60–80 nmol/L, after which there was a smaller increase in testosterone with higher SHBG concentrations [26]. Therefore, testosterone concentrations should be interpreted in the light of SHBG concentrations. Men can have a low testosterone in the presence of low SHBG without being hypogonadal, particularly if LH is normal [2]. If SHBG is normal, then non-gonadal factors associated with lower testosterone concentrations need to be considered, for example, age ≥ 70 years, higher BMI and medical comorbidities, for example, a history of diabetes or cancer [10].
The clinical diagnosis of hypogonadism can be confirmed by measurement of testosterone and SHBG, and hypothalamic/pituitary or testicular origins distinguished via measurement of LH as well as follicle stimulating hormone, which are elevated in men with testicular disease [2]. In men with organic hypogonadism due to diseases of the hypothalamus, pituitary or testes, testosterone replacement therapy can be considered to resolve the symptoms and signs of androgen deficiency [2–5].
Testosterone Intervention in Men with an Intact HPT Axis
Addressing Factors which Influence Testosterone Concentrations
Where HPT axis function has been affected by non-gonadal factors, resulting in a lower circulating testosterone concentration, the primary treatment should be directed at the underlying condition(s). In men with obesity, reducing excess weight subsequently increased testosterone concentrations [27]. Other comorbidities should be similarly addressed, for example, encouraging healthy lifestyle behaviours, addressing risk factors for disease and optimising the treatment of chronic conditions, extending to avoiding or minimising the use of opioid medications [2, 5].
In men with organic (or classical) hypogonadism presenting with androgen deficiency, testosterone treatment is indicated, and enrolling such men into a RCT would pose ethical issues. Here exogenous testosterone treatment replaces endogenous hormone whose production is impaired, and evidence of benefit derives from physiologic principles, open-label clinical trials of testosterone replacement and from experimental clinical studies (for review, see [28]). In the general population of men without organic diseases affecting the HPT axis, testosterone treatment would not be regarded as ‘replacement therapy’. Rather, it would be a pharmacological intervention where exogenous hormone is being given, which may suppress endogenous hormone production, and whose risks and benefits need to be defined on the basis of high-quality evidence from randomised controlled trials (RCTs) (Fig. 1) [29].
The Testosterone Trials
The Testosterone Trials (T Trials) were conducted in 788 men aged ≥ 65 years, with testosterone < 9.54 nmol/L, symptoms of reduced libido/sexual desire, difficulty walking or climbing stairs and/or reduced vitality [30]. The mean age of the study cohort was 72 years and BMI 31 kg/m2. In this study, 12 months of testosterone treatment (daily testosterone gel) improved sexual function, anaemia and bone mineral density, with some benefit on mood and depressive symptoms and walking distance [30, 31]. There was no significant effect on vitality, nor on overall cognitive functioning, but there was some evidence for improved executive functioning [30, 31]. These results indicated some, albeit limited, benefit of testosterone treatment in older men with symptoms and lower testosterone concentrations, who did not have known HPT axis disease. The findings from T Trials are complemented by results from two recent larger testosterone RCTs, Testosterone for the Prevention of Type 2 Diabetes Mellitus (T4DM) and Testosterone Replacement Therapy for Assessment of Long-term Vascular Events and Efficacy Response in Hypogonadal Men (TRAVERSE).
Testosterone for the Prevention of Type 2 Diabetes Mellitus
T4DM was designed from the outset as a trial to prevent type 2 diabetes mellitus in men at high risk [32]. For this reason, the T4DM study population comprised 1007 men, aged 50–74 years, waist circumference ≥ 95 cm, baseline testosterone ≤ 14 nmol/L and impaired glucose tolerance (IGT) or type 2 diabetes newly diagnosed on oral glucose tolerance testing (OGTT). The mean age was 60 years, BMI 35 kg/m2 and all men received a lifestyle intervention as background standard of care [33]. Men with known HPT axis disease were excluded.
The results from T4DM are summarised in Table 1; 2 years of testosterone treatment (intramuscular testosterone undecanoate, at baseline, 6 weeks then every 3 months) reduced the risk of type 2 diabetes (2-h glucose on OGTT ≥ 11.1 mmol/L) by 40%, beyond the effect of the lifestyle intervention [33]. Testosterone treatment reduced the average 2-h glucose values on OGTT by 0.75 mmol/L, and average fasting glucose values by 0.17 mmol/L. Of note, men receiving testosterone on top of the lifestyle intervention gained on average 0.4 kg muscle mass and lost 4.6 kg fat mass, whereas men on the lifestyle intervention and placebo lost 1.3 kg muscle mass and 1.9 kg fat [33]. Testosterone treatment also improved sexual function, bone microarchitecture and bone mineral density [33, 34]. Testosterone treatment improved all five International Index of Erectile Function-15 domain scores, with stronger effects on sexual desire and orgasmic function in older men, and sexual desire in men with higher depression scores [35]. There was a limited effect of testosterone treatment on health-related quality of life and psychosocial function, which improved with loss of excess weight [36]. Recovery of endogenous HPT axis function took 6–12 months after cessation of testosterone treatment [37]. Formal mediation analysis identified reduction in fat mass as a major contributor to the result [38]. In T4DM there was no signal for major cardiovascular adverse events, nor for prostate cancer. Whilst 22% of men in the testosterone group experienced a haematocrit ≥ 0.54 (compared with 1% in the placebo group), this frequently resolved on repeat (non-fasting) testing, and only 25 of 504 testosterone-treated men (5% of the testosterone group) were withdrawn from the study for this reason [33].
Table 1.
Characteristics of and major findings from two recent large testosterone randomised controlled trials, Testosterone for the Prevention of Type 2 Diabetes Mellitus (T4DM) and Testosterone Replacement Therapy for Assessment of Long-term Vascular Events and Efficacy Response in Hypogonadal Men (TRAVERSE)
| Attributes | T4DM [32–38] | TRAVERSE [39–42, 46–50] |
|---|---|---|
| Eligibility criteria | Age 50–74 years, waist circumference ≥ 95 cm, T ≤ 14 nmol/L, and impaired glucose tolerance (IGT) or new type 2 diabetes (T2D) diagnosed on oral glucose tolerance testing (OGTT) | Age 45–80 years, pre-existing cardiovascular disease or multiple risk factors, symptoms including decreased libido/sexual desire, decreased spontaneous erections, fatigue or decreased energy, low or depressed mood, loss of axillary or pubic hair or hot flashes, and T < 10.4 nmol/L |
| Baseline characteristics | N = 1007, mean age 60 years, BMI 35 kg/m2 | N = 5246, mean age 63 years, BMI 35 kg/m2 |
| Intervention | Intramuscular injections of testosterone undecanoate versus placebo for 2 years, all participants received a free lifestyle program (Weight Watchers) | Transdermal testosterone versus placebo for average duration of 22 months (average duration of follow-up 33 months) |
| Major findings | Testosterone treatment prevented or reverted T2D (on the basis of OGTT criteria). Testosterone treatment improved sexual function, bone microarchitecture and bone mineral density | There were similar rates of major adverse cardiovascular events, mortality and prostate cancer in both testosterone and placebo groups |
| Other results | On cessation of testosterone treatment, recovery of hypothalamic-pituitary-testicular axis function takes 6–12 months. Treatment effect partly mediated by changes in fat mass, abdominal fat, skeletal muscle mass, grip strength, SHBG and estradiol, but predominantly by changes in fat mass. Weight loss improved psychosocial function and quality of life more than testosterone treatment | There were higher rates of non-fatal arrhythmias and atrial fibrillation, and acute kidney injury in testosterone group, although overall risks were low. There were small numbers of pulmonary embolism events, but more occurred in testosterone group. Testosterone treatment improved anaemia, sexual function, mood and energy. More clinical fractures occurred in the testosterone group, but there were no significant differences in the small numbers of major osteoporotic, hip or clinical spine fractures between groups. Rates of conversion from pre-diabetes to diabetes (based on HbA1c criteria) were similar in both groups |
The TRAVERSE Study
By contrast, TRAVERSE was mandated by the Federal Drug Agency, and designed from the outset as a cardiovascular safety trial [39]. The trial recruited 5246 men, aged 45–80 years, with pre-existing cardiovascular disease or multiple risk factors, who had symptoms mostly consistent with but not specific for androgen deficiency, and who had a baseline testosterone < 10.4 nmol/L. The mean age was 63 years and BMI 35 kg/m2, and the average duration of treatment (daily transdermal testosterone gel) was 22 months with an average of 33 months follow-up, reflecting high discontinuation rates in both testosterone and placebo groups [40]. There was no difference in rates of major adverse cardiovascular events, overall mortality or prostate cancer between the groups [40, 41]. There were more atrial fibrillation, venous thromboembolic and acute kidney injury events in testosterone-treated men [40], but these differences were attenuated when men who experienced coronavirus disease 2019 (COVID-19) during the study were excluded [42].
Whilst previous meta-analyses of cardiovascular adverse events from smaller RCTs showed no significant differences with testosterone therapy [43–45], those studies were not powered for cardiovascular events as an outcome. Therefore, TRAVERSE, which was designed with major adverse cardiovascular events as the primary outcome of the trial, provides important reassurance as to the cardiovascular safety of testosterone therapy. These and other results from TRAVERSE are summarised in Table 1. As in T Trials and T4DM, testosterone treatment improved sexual function [46], and as in T Trials, testosterone improved anaemia [47]. Additionally, TRAVERSE found some evidence of improved mood [48]. However, there was no difference in rates of progression from pre-diabetes to diabetes (assessed largely on HbA1c criteria) [49]. Of note, men in the testosterone group experienced more fractures, albeit these tended to be smaller and more peripheral fractures, with the limited number of major osteoporotic fractures distributed similarly across groups [50].
Implications of T Trials, T4DM and TRAVERSE
Testosterone Improves Sexual Function
Major RCTs in older men, and in middle-aged to older men at high risk for type 2 diabetes or cardiovascular disease, demonstrated the effects of testosterone intervention to improve sexual function, typically defined using questionnaire responses [30, 33, 46]. A substantial proportion of men remain sexually active into their 70s and beyond, and many regard this as important [51]. The magnitude of benefit seen with testosterone treatment would need to be weighed against potential risks and the need for screening and safety monitoring, for instance, for haematocrit and prostate parameters. Raised haematocrit can usually be managed by reducing the testosterone dose (or frequency), but may rarely require venesection [3, 4].
Whilst T4DM and TRAVERSE both offer reassurance as to prostate safety, men with appreciable background risk should be screened for the presence of prostate cancer prior to starting therapy [3, 4]. On-treatment monitoring may result in more biochemical testing, and further investigation may uncover incidental disease [33, 40, 41]. Baseline evaluation of prostate cancer risk and further monitoring should aim to minimise unnecessary prostate biopsy whilst enabling detection of high-grade prostate cancers in men receiving testosterone therapy [52].
Broader consideration may need to extend to a societal debate, as to the acceptability of testosterone as a pharmacological intervention in men to improve sexual function. In the individual context, the availability and willingness of partners needs to be considered [51]. If erectile dysfunction is the primary problem, underlying issues should be addressed and therapeutic options such as phosphodiesterase type 5 inhibitors may be effective [53]. A recent Cochrane review found that in men with sexual dysfunction, testosterone therapy (up to 12 months) resulted in a small increase in erectile function scores, and little or no difference in prostate-related events or lower urinary tract symptoms [54].
Testosterone Reduces Risk of Type 2 Diabetes
The results from T4DM are unequivocal: testosterone treatment on a background of lifestyle intervention reduces risk of type 2 diabetes in men with IGT or newly diagnosed type 2 diabetes mellitus [33]. The magnitude of the effect is substantial. After 2 years, 87 of 413 men (21%) with available data in the placebo group and 55 of 443 men (12%) in the testosterone group had type 2 diabetes (relative risk 0.59; 95% confidence interval 0.43–0.80; p = 0.0007) [33]. The difference between the T4DM and TRAVERSE diabetes sub-study results may relate to intrinsic differences in trial design: in T4DM men received a background lifestyle intervention, and intramuscular testosterone undecanoate (rather than transdermal testosterone), administered by study nurses to assure adherence [33]. Additionally, T4DM used OGTT to assess outcomes, whereas TRAVERSE relied on HbA1c [33, 49].
A previous smaller open-label study had reported improvement in lean mass and marginally lower HbA1c in men treated with testosterone (fortnightly intramuscular testosterone cypionate) over 18 months [55]. However, HbA1c was similar in testosterone- and placebo-treated men in T4DM [33], and it may not be the optimal parameter to assess glycemia in the context of testosterone therapy, as testosterone affects erythropoiesis [56]. Therefore, testosterone treatment could be used in conjunction with lifestyle intervention to prevent or revert type 2 diabetes in men at high risk on the basis of the T4DM result. However, the optimal duration of treatment remains uncertain, and a background lifestyle intervention is necessary, as is close supervision and monitoring [33]. Importantly, exercise would probably be a better option.
The Testosterone and EXercise study (TEX), a 2 × 2 factorial randomised controlled 12-week trial of testosterone and exercise training combined, testosterone and usual activities, placebo and exercise, or placebo and usual activities, was conducted in a population of men similar to T4DM, except neither IGT nor newly diagnosed type 2 diabetes were required for inclusion and OGTTs were not performed [57]. Exercise improved cardiorespiratory fitness, reduced fat, increased muscle mass and improved endothelial function and ambulatory blood pressure [57–59]. Testosterone improved muscle mass, with some evidence of additive benefit of both exercise and testosterone for muscle mass and strength. However, it was clear that exercise training, when properly implemented, outperformed testosterone to produce prompt benefits [57–59].
Testosterone Improves Bone Mineral Density but Effects on Fracture Risk Remain Unclear
In a recent observational analysis of 205,973 men in the UK Biobank with 13.6 years follow-up, there were 11,088 incident cases of any fracture cases, 1680 of hip fracture and 1366 of forearm fracture [60]. In multivariable analyses adjusting for sociodemographic, lifestyle and medical factors, Fracture Risk Assessment Tool variables, vitamin D and SHBG, lower testosterone was associated with higher risks of any fracture, hip fracture and forearm fracture (hazard ratios for men in the lowest compared with highest quintile of testosterone values were 1.24 [95% confidence interval 1.16–1.33], 1.28 [1.08–1.51] and 1.51 [1.25–1.82], respectively) [60].
These observational data implicating lower testosterone with higher fracture risk are complemented by findings from T Trials, in which 12 months of testosterone treatment improved volumetric bone mineral density at spine and hip, and by the recent findings from T4DM [31, 34]. In T4DM, 2 years of testosterone treatment on a background of a lifestyle intervention increased cortical and total volumetric bone mineral density and increased cortical area and thickness at tibial and radial sites [34]. Testosterone increased areal bone mineral density at the hip and lumbar spine. These results suggest that testosterone treatment should reduce the risk of osteoporotic bone fractures. However, in TRAVERSE, men in the testosterone group treated on average for 22 months had a higher risk of fractures during the average of 33 months follow-up, compared with men receiving placebo [50]. It is important to note that there was no evaluation for osteoporosis or measurement of BMD at baseline, and most of these were fractures of smaller or peripheral bone, with smaller numbers of hip or major osteoporotic fractures which were similarly distributed between the two groups. Thus it is possible that the excess of smaller bone fractures in testosterone-treated men in TRAVERSE, some occurring within weeks of starting treatment, might be due to behavioural changes predisposing to trauma [61]. Clearly, a future large RCT with an extended duration of treatment would be needed to explore the outcome of either hip fracture or major osteoporotic fractures.
Exploring the Rationale for Future Testosterone Intervention Trials
Testosterone as an Adjunct to Anti-obesity Medications
Demographic shifts are producing ageing population structures in countries around the world, with increasing proportions of people transitioning from middle to older age groups. Superimposed on these trends is a global epidemic of overweight and obesity, predisposing to a range of cardiometabolic diseases including type 2 diabetes and cardiovascular disease [62]. However, we now have access to a class of anti-obesity medications based on pharmacological activation of the incretin system, which are highly effective at achieving weight loss. These agents include glucagon-like peptide-1 receptor agonists (GLP-1ra), combined agonists of both GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) receptors and triple agonists activating GLP-1, GIP and glucagon receptors, given as weekly subcutaneous injections. Semaglutide, a GLP-1ra, reduced body weight by 14.9% (versus 2.4% for placebo) over 68 weeks in adults with obesity or overweight with ≥ 1 weight-related condition [63]. Tirzepatide, a dual GLP-1 and GIP receptor agonist, resulted in 20.9% weight loss (versus 3.1% for placebo) after 72 weeks in middle-aged adults with obesity or overweight with one or more weight-related complications [64]. The triple GLP-1, GIP and glucagon receptor agonist retatrutide in adults with overweight/obesity resulted in weight loss of 24.2% of total body weight (versus 2.1% for placebo) with the highest dose administered after 48 weeks of treatment [65]. Adverse effects include nausea, diarrhoea, constipation and vomiting, and rebound weight gain occurs on cessation of therapy [66, 67].
Another consideration is that whilst a large amount of fat mass is lost, a substantial amount of lean mass, including muscle, is also lost [68, 69]. Overall, the benefits of losing excess fat, and concomitant improvements in cardiovascular risk factors, appears to predominate with a reduction in major adverse cardiovascular events reported for semaglutide [70]. Nevertheless, the longer-term effects of reduced muscle mass may be a predisposition to sarcopenia and frailty, with possible detrimental effects in older adults [71]. Testosterone is an anabolic agent which increases muscle mass and strength in younger and older men [72]. Other steroidal or non-steroidal agents which act on the androgen receptor have been considered for potential anabolic effects [73]. The role of testosterone or other androgen receptor ligands as an adjunct to incretin-based anti-obesity therapy has yet to be demonstrated in RCTs. However, exercise, particularly resistance exercise, may be effective and can be recommended readily along with attention to maintaining protein intake [68, 69].
Testosterone to Enhance Disability-Free Survival in Older Men
We know now that testosterone is lower in older men, with biochemical evidence of Leydig cell impairment appearing around and following age of 70 years [7, 10]. A recent meta-analysis including IPD from nine cohort studies with 24,109 men and 255,830 participant-years of follow-up, found a non-linear association of testosterone (measured using mass spectrometry) and mortality risk in adult men across all ages [74]. After adjusting for socio-demographic, lifestyle and medical factors, there was no association of testosterone with mortality risk for men with testosterone above a threshold of 7.4 nmol/L, but below this threshold mortality risk increased [74].
In men aged 70–89 years, lower testosterone concentrations have been reported to be associated with higher risks of incident stroke, dementia and fracture risk [75–78]. Recent analyses of UK Biobank data in men aged 40–69 years confirmed the association of lower testosterone with higher risks of incident fracture including hip fracture [60], and in men aged ≥ 50 years with higher risk of incident dementia [79]. These events, in addition to being associated with mortality risk, are also major contributors to disability and loss of independence in older men.
Retrospective case-control analyses of registries or prescription databases have associated testosterone treatment with lower mortality risk, including in men with type 2 diabetes (e.g. [80–83], for review, see [84]). However, causality remains unproven. In T Trials, there was no effect of testosterone treatment on cognition in older men, but that study was limited in sample size and duration of intervention [31]. In TRAVERSE, testosterone treatment in middle-aged to older men showed some improvement in mood [48], but the limited numbers of clinical fractures and small numbers of major osteoporotic and hip fractures make the fracture data difficult to interpret [50]. Therefore, a new testosterone RCT would need to be even larger, and of longer duration, most likely focussed on men in the transition from middle to older ages, for example, aged 65–80 years.
In TRAVERSE men treated with testosterone had a higher rate of atrial fibrillation (91 men in the testosterone group versus 63 in the placebo group, 3.5% versus 2.4%) [40]. In men who did not experience COVID-19 during the study, the overall rate and difference between groups was less (32 versus 26 men, 1.4% versus 1.1%) [42]. In an observational analysis of healthy older men, those with higher endogenous testosterone concentrations had a higher risk of developing atrial fibrillation (AF) [85]. By contrast, mechanistic studies have proposed possible beneficial effects of testosterone on the vasculature and heart (for review, see [86]), and a retrospective case-control study of men who had low testosterone concentrations found that those who normalised testosterone concentrations after testosterone therapy had a lower incidence of AF compared with men who received testosterone without normalisation or men who did not receive testosterone [87]. A new large testosterone RCT with randomisation and prospective ascertainment of incident AF could clarify this issue.
An overall outcome trial which integrated positive and potential adverse effects of treatment might be preferable. Therefore, a new RCT could be justified to test whether testosterone treatment in older men without pre-existing major medical comorbidities might enhance disability-free survival (Fig. 1, Table 2). Such an outcome, based on death or loss of independence resulting in admission to high-level residential care, could include pre-specified secondary outcomes such as dementia and hip fracture as contributors to the overall endpoint [29]. Other endpoints of interest may be changes in quality of life, mood and medication usage [88]. Furthermore, a larger RCT may allow for further analysis of quality-of-life outcomes, which could help inform men of their choices in consideration of testosterone treatment in older age.
Table 2.
Considerations in the rationale and design of a new, large testosterone randomised controlled trial
| Testosterone treatment | Unresolved issues | Design of new large testosterone trial |
|---|---|---|
|
Accepted clinical practice [2–5, 28] Men with organic (classical) hypogonadism should be considered for testosterone replacement therapy to resolve symptoms and signs of androgen deficiency. |
Observational data (causality unproven) [60, 74–79] In middle-aged to older men without organic (classical) hypogonadism, lower testosterone concentrations are associated with: • higher risk of any fracture or hip fracture • higher risk of incident stroke • higher risk of incident dementia • higher mortality risk (below a threshold of 7.4 nmol/L) |
Selection criteria • men aged 65–80 years, without major pre-existing medical comorbidities Primary outcome • disability-free survival (death or loss of independence resulting in admission to high-level residential care) Secondary endpoints • occurrence of hip fracture • diagnosis of dementia Other endpoints • quality of life • mood • medications usage Adjudicated safety endpoint • diagnosis of atrial fibrillation |
|
Evidence from T Trials, T4DM, TRAVERSE Testosterone intervention in men without organic (classical) hypogonadism with low-normal baseline testosterone concentrations*: • improves sexual function, anaemia and bone mineral density • prevents or reverts type 2 diabetes (in conjunction with lifestyle program) • reassurance regarding cardiovascular and prostate safety |
Findings from trials requiring further evaluation [40, 50] • association of testosterone therapy with incident atrial fibrillation • impact of testosterone therapy on risk of major osteoporotic or hip fracture |
*Note differences in cut-offs for baseline testosterone concentrations, and differences in other eligibility criteria for each trial (see main text)
Summary and Conclusions
Recent research findings have advanced understanding of changes in testosterone concentrations and the potential role of testosterone to influence health outcomes in male ageing. These new insights inform the clinical assessment of men with suspected androgen deficiency. Identification and appropriate treatment of men with organic disease of the hypothalamus, pituitary or testes remains a major priority for clinicians [2, 4, 5]. In men with an intact HPT axis, who may have lower testosterone concentrations in the setting of older age, central adiposity/obesity and/or medical comorbidities, optimising lifestyle behaviours and addressing treatable risk factors and disease are priorities [89]. However, treating specific underlying medical comorbidities might not improve testosterone concentrations in such men [90], and extensive discussion of specific disease conditions is beyond the scope of this review [91–93]. Under certain circumstances an individualised approach may be appropriate, with careful explanations of the risks versus benefits of specific approaches.
In the broader context of men with an intact HPT axis, recent large testosterone RCTs have shown effects of testosterone treatment to improve sexual function and bone density, and to prevent or revert type 2 diabetes in men at high risk, whilst also reassuring as to its cardiovascular and prostate safety. These provide a basis for discussion of benefits and risks of testosterone therapy with individual symptomatic men. However, whilst TRAVERSE was designed to address the key issue of cardiovascular safety [94], uncertainty remains over the risks of other vascular or cardiac events, such as AF, and fractures [40, 50]. Whether testosterone treatment modulates risks of major osteoporotic or hip fractures or occurrence of dementia and affects risks of disability and loss of independent living warrant further exploration [60, 77–79]. Therefore, whilst the findings from T Trials, T4DM and TRAVERSE may not alter the current clinical equipoise, they do provide a firm foundation for evaluating a new, very large testosterone RCT to test the hypothesis that testosterone treatment might enhance disability-free survival in older men.
Declarations
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Conflicts of Interest
B.B.Y. has received speaker honoraria and conference support from Bayer, Lilly and Besins Healthcare; research support from Lawley Pharmaceuticals, Lilly and Bayer; and participated in advisory roles for Lilly, Besins Healthcare, Ferring and Lawley Pharmaceuticals. J.J.M. is an NHMRC Leadership Fellow (IG 1173690). C.T. and C.M.D. have no relevant disclosures.
Availability of Data and Material
Not applicable.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Code Availability
Not applicable.
Author Contributions
B.B.Y. drafted the manuscript; C.T., C.M.D. and J.J.M. contributed important intellectual content; and all authors revised the manuscript in response to peer-review, and all authors approved the final version.
References
- 1.Handelsman DJ, et al. Androgen physiology, pharmacology, use and misuse. In: Feingold KR, Anawalt B, Blackman MR, et al., editors. Endotext. South Dartmouth: MDText.com, Inc.; 2020. p. 5. [Google Scholar]
- 2.Yeap BB, Grossmann M, McLachlan RI, et al. Endocrine Society of Australia position statement on male hypogonadism (part 1): assessment and indications for testosterone therapy. Med J Aust. 2016;205:173–8. [DOI] [PubMed] [Google Scholar]
- 3.Yeap BB, Grossmann M, McLachlan RI, et al. Endocrine Society of Australia position statement on male hypogonadism (part 2): treatment and therapeutic considerations. Med J Aust. 2016;205:228–31. [DOI] [PubMed] [Google Scholar]
- 4.Bhasin S, Brito JP, Cunningham GR, et al. Testosterone therapy in men with hypogonadism: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2018;103:1715–44. [DOI] [PubMed] [Google Scholar]
- 5.De Silva NL, Papanikolaou N, Grossmann M, et al. Male hypogonadism: pathogenesis, diagnosis, and management. Lancet Diabetes Endocrinol. 2024;12:761–74. [DOI] [PubMed] [Google Scholar]
- 6.Sikaris K, McLachlan RI, Kazlauskas R, de Kretser D, Holden CA, Handelsman DJ. Reproductive hormone reference intervals for healthy fertile young men: evaluation of automated platform assays. J Clin Endocrinol Metab. 2005;90:5928–36. [DOI] [PubMed] [Google Scholar]
- 7.Yeap BB, Alfonso H, Chubb SA, et al. Reference ranges and determinants of testosterone, dihydrotestosterone, and estradiol levels measured using liquid chromatography-tandem mass spectrometry in a population-based cohort of older men. J Clin Endocrinol Metab. 2012;97:4030–9. [DOI] [PubMed] [Google Scholar]
- 8.Shi Z, Araujo AB, Martin S, O’Loughlin P, Wittert GA. Longitudinal changes in testosterone over five years in community-dwelling men. J Clin Endocrinol Metab. 2013;98:3289–97. [DOI] [PubMed] [Google Scholar]
- 9.Camacho EM, Huhtaniemi IT, O’Neill TW, et al. Age-associated changes hypothalamic-pituitary-testicular function in middle-aged and older men modified by weight changes and lifestyle factors: longitudinal results from European Male Ageing Study. Eur J Endocrinol. 2013;168:445–55. [DOI] [PubMed] [Google Scholar]
- 10.Marriott RJ, Murray K, Adams RJ, et al. Factors associated with circulating sex hormones in men: individual participant data meta-analyses. Ann Intern Med. 2023;176:1221–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. J Clin Endocrinol Metab. 2001;86:724–31. [DOI] [PubMed] [Google Scholar]
- 12.Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts Male Aging Study. J Clin Endocrinol Metab. 2002;87:589–98. [DOI] [PubMed] [Google Scholar]
- 13.Handelsman DJ, Yeap BB, Flicker L, Martin S, Wittert GA, Ly LP. Age-specific population centiles for androgen status in men. Eur J Endocrinol. 2015;173:809–17. [DOI] [PubMed] [Google Scholar]
- 14.Yeap BB, Manning L, Chubb SAP, et al. Progressive impairment of testicular endocrine function in ageing men: testosterone and dihydrotestosterone decrease, and luteinizing hormone increases, in men transitioning from the 8th to 9th decades of life. Clin Endocrinol (Oxf). 2018;88:88–95. [DOI] [PubMed] [Google Scholar]
- 15.Cohen J, Nassau DE, Patel P, et al. Low testosterone in adolescents and young adults. Front Endocrinol. 2020;10:916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hodges-Simeon CR, Grail GPO, Albert G, et al. Testosterone therapy masculinizes speech and gender presentation in transgender men. Sci Rep. 2021;11:3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu FCW, Tajar A, Beynon JM, et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med. 2010;363:123–35. [DOI] [PubMed] [Google Scholar]
- 18.Travison TG, Vesper HW, Orwoll E, et al. Harmonized reference ranges for circulating testosterone levels in men of four cohort studies in the United States and Europe. J Clin Endocrinol Metab. 2017;102:1161–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Handelsman DJ. Free testosterone: pumping up the tires or ending the free ride? Endocr Rev. 2017;38:297–301. [DOI] [PubMed] [Google Scholar]
- 20.Nyamaah JA, Narinx N, Antonio L, Vanderschueren D. Use of calculated free testosterone in men: advantages and limitations. Curr Opin Endocrinol Diabetes Obes. 2024;31:230–5. [DOI] [PubMed] [Google Scholar]
- 21.Jasuja R, Pencina KM, Spencer DJ, et al. Reference intervals for free testosterone in adult men measured using a standardized equilibrium dialysis procedure. Andrology. 2023;11:125–33. [DOI] [PubMed] [Google Scholar]
- 22.Ly LP, Sartorius G, Hull L, et al. Accuracy of calculated free testosterone formulae in men. Clin Endocrinol (Oxf). 2010;73:382–8. [DOI] [PubMed] [Google Scholar]
- 23.Yeap BB, Wu FCW. Clinical practice update on testosterone therapy for male hypogonadism: contrasting perspectives to optimise care. Clin Endocrinol (Oxf). 2019;90:56–65. [DOI] [PubMed] [Google Scholar]
- 24.Goldman AL, Bhasin S, Wu FCW, Krishna M, Matsumoto AM, Jasuja R. A reappraisal of testosterone’s binding in circulation: physiological and clinical implications. Endocr Rev. 2017;38:302–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Narinx N, Marriott RJ, Murray K, et al. Sociodemographic, lifestyle and medical factors associated with calculated free testosterone concentrations in men: individual participant data meta-analyses. Eur J Endocrinol. 2024;191:523–34. [DOI] [PubMed] [Google Scholar]
- 26.Yeap BB, Marriott RJ, Antonio L, et al. Sociodemographic, lifestyle and medical influences on serum testosterone and sex hormone-binding globulin in men from UK Biobank. Clin Endocrinol (Oxf). 2021;94:290–302. [DOI] [PubMed] [Google Scholar]
- 27.Grossmann M. Low testosterone in men with type 2 diabetes: significance and treatment. J Clin Endocrinol Metab. 2011;96:2341–53. [DOI] [PubMed] [Google Scholar]
- 28.Grossmann M. Indications for testosterone therapy in men. Curr Opin Endocrinol Diabetes Obes. 2024;31:249–56. [DOI] [PubMed] [Google Scholar]
- 29.Yeap BB, Tran C, Douglass CM, McNeil JJ. Beyond T-Trials, T4DM and TRAVERSE: the next large testosterone randomized controlled trial. Curr Opin Endocrinol Diabetes Obes. 2024;31:222–9. [DOI] [PubMed] [Google Scholar]
- 30.Snyder PJ, Bhasin S, Cunningham GR, et al. Effects of testosterone treatment in older men. N Engl J Med. 2016;374:611–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Snyder PJ, Bhasin S, Cunningham GR, et al. Lessons from the testosterone trials. Endocr Rev. 2018;39:369–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wittert G, Atlantis E, Allan C, et al. Testosterone therapy to prevent type 2 diabetes mellitus in at-risk men (T4DM): design and implementation of a double-blind randomised controlled trial. Diabetes Obes Metab. 2019;21:772–80. [DOI] [PubMed] [Google Scholar]
- 33.Wittert G, Bracken K, Robledo KP, et al. Testosterone treatment to prevent or revert type 2 diabetes in men enrolled in a lifestyle program (T4DM): a randomised, double-blind, placebo-controlled, 2-year, phase 3b trial. Lancet Diabetes Endocrinol. 2021;9:32–45. [DOI] [PubMed] [Google Scholar]
- 34.Ng MTF, Hoermann R, Bracken K, et al. Effect of testosterone treatment on bone microarchitecture and bone mineral density in men: a two-year RCT. J Clin Endocrinol Metab. 2021;106:e3143–58. [DOI] [PubMed] [Google Scholar]
- 35.Wittert GA, Robledo KP, Handelsman DJ, et al. Testosterone treatment and sexual function in men: secondary analysis of the T4DM (Testosterone for Diabetes) trial. J Clin Endocrinol Metab. 2025. 10.1210/clinem/dgaf060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grossmann M, Robledo KP, Daniel M, et al. Testosterone treatment, weight loss and health-related quality of life and psychosocial function in men: a two-year RCT. J Endocrinol Metab. 2024;109:2019–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Handelsman DJ, Desai R, Conway AJ, et al. Recovery of male reproductive function after ceasing prolonged testosterone undecanoate injections. Eur J Endocrinol. 2022;186:307–18. [DOI] [PubMed] [Google Scholar]
- 38.Robledo KP, Marschner IC, Handelsman DJ, et al. Mediation analysis of the testosterone treatment effect to prevent type 2 diabetes in the T4DM trial. Eur J Endocrinol. 2023;188:613–20. [DOI] [PubMed] [Google Scholar]
- 39.Bhasin S, Lincoff AM, Basaria S, et al. Effects of long-term testosterone treatment on cardiovascular outcomes in men with hypogonadism: rationale and design of the TRAVERSE study. Am Heart J. 2022;245:41–50. [DOI] [PubMed] [Google Scholar]
- 40.Lincoff AM, Bhasin S, Flevaris P, et al. Cardiovascular safety of testosterone replacement therapy. N Engl J Med. 2023;389:107–17. [DOI] [PubMed] [Google Scholar]
- 41.Bhasin S, Travison TG, Pencina KM, et al. Prostate safety events during testosterone replacement therapy in men with hypogonadism: a randomized clinical trial. JAMA Netw Open. 2023;6: e2348692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pencina KM, Lincoff AM, Klein EA, et al. Testosterone replacement therapy and risk of COVID-19 and effect of COVID-19 on testosterone’s treatment effect. J Endocr Soc. 2025;9: bvaf002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corona G, Rastrelli G, Di Pasquale G, et al. Testosterone and cardiovascular risk: meta-analysis of interventional studies. J Sex Med. 2018;15:820–38. [DOI] [PubMed] [Google Scholar]
- 44.Diem SJ, Greer NL, MacDonald R, et al. Efficacy and safety of testosterone treatment in men: an evidence report for a clinical practice guideline by the American College of Physicians. Ann Intern Med. 2020;172:105–18. [DOI] [PubMed] [Google Scholar]
- 45.Hudson J, Cruickshank M, Quinton R, et al. Adverse cardiovascular events and mortality in men during testosterone treatment: an individual patient and aggregate data data meta-analysis. Lancet Healthy Longev. 2022;3:e381-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pencina KM, Travison TG, Cunningham GR, et al. Effect of testosterone replacement therapy on sexual function and hypogonadal symptoms in men with hypogonadism. J Clin Endocrinol Metab. 2024;109:569–80. [DOI] [PubMed] [Google Scholar]
- 47.Pencina KM, Travison TG, Artz AS, et al. Efficacy of testosterone replacement therapy in correcting anemia in men with hypogonadism: a randomized clinical trial. JAMA Netw Open. 2023;6: e2340030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bhasin S, Seidman S, Travison TG, et al. Depressive syndromes in men with hypogonadism in the TRAVERSE trial: response to testosterone-replacement therapy. J Clin Endocrinol Metab. 2024;109:1814–26. [DOI] [PubMed] [Google Scholar]
- 49.Bhasin S, Lincoff AM, Nissen SE, et al. Effect of testosterone on progression from prediabetes to diabetes in men with hypogonadism: a substudy of the TRAVERSE randomized clinical trial. JAMA Intern Med. 2024;184:353–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Snyder PJ, Bauer DC, Ellenberg SS, et al. Testosterone treatment and fractures in men with hypogonadism. N Engl J Med. 2024;390:203–11. [DOI] [PubMed] [Google Scholar]
- 51.Hyde Z, Flicker L, Hankey GJ, et al. Prevalence of sexual activity and associated factors in men aged 75–95 years: a cohort study. Ann Intern Med. 2010;153:693–702. [DOI] [PubMed] [Google Scholar]
- 52.Bhasin S, Thompson IM. Prostate risk and monitoring during testosterone replacement therapy. J Clin Endocrinol Metab. 2024;109:1975–83. [DOI] [PubMed] [Google Scholar]
- 53.Salonia A, Bettocchi C, Boeri L, et al. European Association of Urology guidelines on sexual and reproductive health – 2021 update: male sexual dysfunction. Eur Urol. 2021;80:333–57. [DOI] [PubMed] [Google Scholar]
- 54.Lee H, Hwang EC, Oh CK, et al. Testosterone replacement in men with sexual dysfunction (review). Cochrane Database Syst Rev. 2024;1: CD013071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deepika FNU, Ballato E, Colleluori G, et al. Baseline testosterone predicts body composition and metabolic response to testosterone therapy. Front Endocrinol. 2022;13: 915309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wittert GA, Grossmann M, Yeap BB, Handelsman DJ. Testosterone and type 2 diabetes prevention: translational lessons from the T4DM study. J Endocrinol. 2023;253: e220223. [DOI] [PubMed] [Google Scholar]
- 57.Chasland LC, Yeap BB, Maiorana AJ, et al. Testosterone and exercise: effects on fitness, body composition and strength in middle-to-older aged men with low-normal serum testosterone levels. Am J Physiol Heart Circ Physiol. 2021;320:H1985–98. [DOI] [PubMed] [Google Scholar]
- 58.Chasland LC, Naylor LH, Yeap BB, Maiorana AJ, Green DJ. Testosterone and exercise in middle-to-older aged men: combined and independent effects on vascular function. Hypertension. 2021;77:1095–105. [DOI] [PubMed] [Google Scholar]
- 59.Chasland LC, Green DJ, Schlaich MP, et al. Effects of testosterone, with and without exercise training, on ambulatory blood pressure in middle-aged and older men. Clin Endocrinol. 2021;95:176–86. [DOI] [PubMed] [Google Scholar]
- 60.Grahnemo L, Marriott RJ, Murray K, et al. Associations of serum testosterone and sex hormone-binding globulin with incident fractures in middle-aged to older men. J Clin Endocrinol Metab. 2024. 10.1210/clinem/dgae703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grossmann M, Anawalt BD. Breaking news—testosterone treatment and fractures in older men. N Engl J Med. 2024;390:267–8. [DOI] [PubMed] [Google Scholar]
- 62.NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet. 2016;387:1377–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wilding JPH, Batterham RL, Calanna S, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384:989–1002. [DOI] [PubMed] [Google Scholar]
- 64.Jastreboff AM, Aronne LJ, Ahmad NN, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387:205–16. [DOI] [PubMed] [Google Scholar]
- 65.Jastreboff AM, Kaplan LM, Frías JP, et al. Triple-hormone-receptor agonist retatrutide for obesity - a phase 2 trial. N Engl J Med. 2023;389:514–26. [DOI] [PubMed]
- 66.Wilding JPH, Batterham RL, Davies M, et al. Weight regain and cardiometabolic effects after withdrawal of semaglutide: the STEP 1 trial extension. Diabetes Obes Metab. 2022;24:1553–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aronne LJ, Sattar N, Horn DB, et al. Continued treatment with tirzepatide for maintenance of weight reduction in adults with obesity: the SURMOUNT-4 randomized clinical trial. JAMA. 2024;331:38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Conte C, Hall KD, Klein S. Is weight loss-induced muscle mass loss clinically relevant? JAMA. 2024;332:9–10. [DOI] [PubMed] [Google Scholar]
- 69.Locatelli JC, Costa JG, Haynes A, et al. Incretin-based weight loss pharmacotherapy: can resistance exercise optimize changes in body composition? Diabetes Care. 2024;47:1718–30. [DOI] [PubMed] [Google Scholar]
- 70.Lincoff AM, Brown-Frandsen K, Colhoun HM, et al. Semaglutide and cardiovascular outcomes in obesity without diabetes. N Engl J Med. 2023;389:2221–32. [DOI] [PubMed] [Google Scholar]
- 71.Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet. 2019;394:1365–75. [DOI] [PubMed] [Google Scholar]
- 72.Bhasin S, Woodhouse L, Casaburi R, et al. Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J Clin Endocrinol Metab. 2005;90:678–88. [DOI] [PubMed] [Google Scholar]
- 73.Bhasin S, Krishnan V, Storer TW, Steiner M, Dobs AS. Androgens and selective androgen receptor modulators to treat functional limitations associated with aging and chronic disease. J Gerontol A Biol Sci Med Sci. 2023;78(Suppl 1):25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yeap BB, Marriott RJ, Dwivedi G, et al. Associations of testosterone and related hormones with all-cause and cardiovascular mortality and incident cardiovascular disease in men: individual participant data meta-analyses. Ann Intern Med. 2024;177:768–81. [DOI] [PubMed] [Google Scholar]
- 75.Yeap BB, Hyde Z, Almeida OP, et al. Lower testosterone levels predict incident stroke and transient ischemic attack in older men. J Clin Endocrinol Metab. 2009;94:2353–9. [DOI] [PubMed] [Google Scholar]
- 76.Yeap BB, Alfonso H, Chubb SA, Hankey GJ, et al. In older men, higher plasma testosterone or dihydrotestosterone is an independent predictor for reduced incidence of stroke but not myocardial infarction. J Clin Endocrinol Metab. 2014;99:4565–73. [DOI] [PubMed] [Google Scholar]
- 77.Ford AH, Yeap BB, Flicker L, et al. Sex hormones and incident dementia in older men: the Health In Men Study. Psychoneuroendocrinology. 2018;98:139–47. [DOI] [PubMed] [Google Scholar]
- 78.Yeap BB, Alfonso H, Chubb SAP, et al. U-shaped association of plasma testosterone, and no association of plasma estradiol, with incidence of fractures in men. J Clin Endocrinol Metab. 2020;105: dgaa115. [DOI] [PubMed] [Google Scholar]
- 79.Marriott RJ, Murray K, Flicker L, et al. Lower serum testosterone concentrations are associated with a higher incidence of dementia in men: The UK Biobank prospective cohort study. Alzheimers Dement. 2022;18:1907–18. [DOI] [PubMed] [Google Scholar]
- 80.Muraleedharan V, Marsh H, Kapoor D, et al. Testosterone deficiency is associated with increased risk of mortality and testosterone replacement improves survival in men with type 2 diabetes. Eur J Endocrinol. 2013;169:725–33. [DOI] [PubMed] [Google Scholar]
- 81.Sharma R, Oni OA, Gupta K, et al. Normalization of testosterone level is associated with reduced incidence of myocardial infarction and mortality in men. Eur Heart J. 2015;36:2706–15. [DOI] [PubMed] [Google Scholar]
- 82.Wallis CJD, Lo K, Lee Y, et al. Survival and cardiovascular events in men treated with testosterone replacement therapy: an intention-to-treat observational cohort study. Lancet Diabetes Endocrinol. 2016;4:498–506. [DOI] [PubMed] [Google Scholar]
- 83.Oni OA, Dehkordi SHH, Jazayeri M-A, et al. Relation of testosterone normalization to mortality and myocardial infarction in men with previous myocardial infarction. Am J Cardiol. 2019;124:1171–8. [DOI] [PubMed] [Google Scholar]
- 84.Yeap BB, Dwivedi G, et al. Androgens and cardiovascular disease in men. In: Feingold KR, Anawalt B, Blackman MR, et al., editors. Endotext. South Dartmouth: MDText.com, Inc.; 2022. [PubMed] [Google Scholar]
- 85.Tran C, Yeap BB, Ball J, et al. Testosterone and the risk of incident atrial fibrillation in older men: further analysis of the ASPREE study. EClinicalMedicine. 2024;72: 102611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Barrientos G, Llanos P, Basualto-Alarcon C, et al. Androgen-regulated cardiac metabolism in aging men. Front Endocrinol. 2020;11:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sharma R, Oni OA, Gupta K, et al. Normalization of testosterone levels after testosterone replacement therapy is associated with decreased incidence of atrial fibrillation. J Am Heart Assoc. 2017;6: e004880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Indirli R, Lanzi V, Arosio M, et al. The association of hypogonadism with depression and its treatments. Front Endocrinol. 2023;14:1198437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Grossmann M, Matsumoto AM. A perspective on middle-aged and older men with functional hypogonadism: focus on holistic management. J Clin Endocrinol Metab. 2017;102:1067–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gosai JN, Charalampidis P, Nikolaidou T, et al. Revascularization with percutaneous coronary intervention does not affect androgen status in males with chronic stable angina. Andrology. 2016;4:486–91. [DOI] [PubMed] [Google Scholar]
- 91.Romejko K, Rymarz A, Sadownik H, et al. Testosterone deficiency as one of the major endocrine disorders in chronic kidney disease. Nutrients. 2022;14:3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Skiba R, Rymarz A, Matyjek A, et al. Testosterone replacement therapy in chronic kidney disease. Nutrients. 2022;14:3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Musicki B, Burnett AL. Testosterone deficiency in sickle cell disease: recognition and remediation. Front Endocrinol. 2022;13: 892184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Morgentaler A, Dhindsa S, Dobs AS, et al. Androgen Society position paper on cardiovascular risk with testosterone therapy. Mayo Clin Proc. 2024;99:1785–801. [DOI] [PubMed] [Google Scholar]