Abstract
Antibiotic release and transfer within environmental systems significantly impact ecological stability and human health, posing considerable safety risks. Consequently, accurate and efficient detection methods for antibiotics, particularly within complex environmental matrices, are essential. A growing body of research is focusing on antibiotic pollution in wastewater and other environmental settings. Nevertheless, pervasive matrix interferences in complex water matrices and the inherent limitations of standalone analytical approaches in sensitivity, detection range, and other performance metrics underscore the demand for more robust and versatile detection platforms. Here we show a comprehensive overview and critical assessment of antibiotic contaminants, examining their sources, environmental distribution, and current detection methods. We focus on emerging antibiotic detection technologies, comparing their performance and highlighting suitable application contexts. Furthermore, we discuss fundamental principles and historical advancements in antibiotic detection and analytical methodologies. Finally, we identify significant challenges for antibiotic detection in complex environments, suggest viable strategies for future improvements, and outline promising research directions. This review not only provides essential guidance for advancing environmental antibiotic monitoring but also sheds light on the development of a strategic framework for robust, integrated platforms enabling multiplexed antibiotic monitoring in challenging environmental water matrices.
Keywords: Antibiotics, Analytical methods, Detection, Environmental samples, Wastewater
Highlights
-
•
This review discusses recent advancements in antibiotic detection and their underlying analytical principles.
-
•
The strengths, limitations, and future potential of emerging antibiotic detection methods are critically assessed.
-
•
Major challenges of antibiotic detection in complex environments are identified, with strategies for future research.
1. Introduction
In recent years, antibiotics have become prominent due to exponential growth in their usage, which has established them as a significant category of emerging contaminants. Antibiotics constitute a unique class of substances primarily synthesized by microorganisms, including bacteria, fungi, and higher organisms, during their life activities. The main functions of antibiotics are to combat pathogens, inhibit bacteria, and disrupt the normal development of other living cells, including cancer cells. The primary mechanisms of action include inhibition of bacterial cell wall synthesis, enhancement of bacterial cell membrane permeability, disruption of bacterial protein synthesis, and inhibition of bacterial nucleic acid replication and transcription. Since their discovery and subsequent mass production, antibiotics have emerged as indispensable tools for preserving human health and the health of various animals and plants. The introduction of antibiotics has resulted in groundbreaking changes in fields such as medicine, biological cytology, livestock breeding, agricultural production, and aquaculture. However, with human society's advancement in production and daily life, the use of antibiotics has become widespread and extensive [[1], [2], [3], [4], [5]]. In a study conducted at the University of Oxford, researchers statistically analyzed antibiotic consumption in more than 200 countries over the past two decades [6]. The study revealed a steady global increase in antibiotic consumption, amounting to an approximately 46 % increase. This increase has resulted in large quantities of unconsumed and unmetabolized antibiotics entering surface water, soil, and groundwater through excretion by organisms. These antibiotics ultimately migrate continuously between water bodies and soil, leading to continuous accumulation. The significant presence of antibiotics in aquatic environments has the potential to disrupt the balance of microorganisms in ecosystems [7], induce antibiotic resistance in diverse bacteria [8], pose risks to human health [9], and ultimately contribute to a decline in biodiversity and ecosystem degradation [10,11]. Considering the increasingly severe public health situation, there is a growing and urgent need for efficient and convenient methods to detect antibiotics in water environments.
Traditionally, antibiotic detection relies on methods such as high-performance liquid chromatography (HPLC), mass spectrometry (MS), and the combined approach of liquid chromatography‒mass spectrometry (LC‒MS) [[12], [13], [14]]. Chromatography, in which a liquid is employed as a mobile phase, is the primary tool for detecting antibiotics in water. This method is grounded in the principles of traditional liquid chromatography, incorporates elements of gas chromatography theory, and utilizes equipment such as infusion pumps, separation columns, detectors, and computer control systems for analysis [15,16]. In contrast, MS is a technique that uses electric and magnetic fields to separate moving ions (e.g., charged atoms, molecules, or molecular fragments) based on their mass-to-charge ratios. MS can be used to measure the precise mass of these ions and to determine the composition of compounds. LC‒MS combines the separation capability of liquid chromatography with the measurement precision of mass spectrometry [[17], [18], [19], [20]]. This hybrid approach boasts greater analytical power and sensitivity than individual techniques; however, its enhanced capabilities require the use of more intricate technology and equipment, leading to increased detection costs. While these aforementioned methods are considered foundational in antibiotic detection, they are limited by their lengthy analysis times, intricate procedures, high costs, and complex sample preparation methods.
Considering the problems facing antibiotic detection, developing novel responsive detection technologies and integrated sensor devices is crucial. In recent years, numerous researchers have introduced various detection and analysis techniques [[21], [22], [23]]. Innovative techniques such as chromatography‒mass spectrometry, optical sensing, and electrochemical sensing have gained prominence in wastewater antibiotic detection [[24], [25], [26]]. In 2008, Xiao and colleagues at Peking University introduced a liquid chromatography‒electrospray tandem mass spectrometry method for analyzing quinolone antibiotics in wastewater. Shortly thereafter, researchers at Zhejiang University devised a liquid chromatography‒fluorescence method coupled with one-step solid-phase extraction to comprehensively detect multiple antibiotics in water [27]. In 2020, scholars at Donghua University proposed an impedance aptasensor based on TiO2-g-C3N4@AuNPs for the ultrasensitive detection of amoxicillin [28]. As shown in Fig. 1, these innovative approaches represent a crucial shift toward more efficient and convenient methodologies in the continuous quest for precise antibiotic detection. Moreover, developing integrated sensor devices enables more accurate detection and identification of antibiotics, providing more effective solutions for monitoring water environments.
Fig. 1.
Overview of methods used for antibiotic detection [29,30,95].
This review explores the distribution and sources of common antibiotics in diverse aquatic environments. It also offers an overview of the advancements in techniques for detecting and analyzing antibiotics while elucidating the principles underlying these methods. Furthermore, common antibiotic detection methods are comparatively analyzed, and the strengths of different approaches and their future potential are critically summarized. In the conclusion of this article, emerging trends in the detection of antibiotics in complex environments, including advancements in bioscience, optical sensing, and sensitive materials, are explored. Considering the importance of antibiotic detection in areas such as ecological environments and wastewater management, several potential new methods hold great promise for advancing antibiotic detection technologies. Moreover, the numerous challenges associated with the detection of antibiotics in complex environments are discussed, and potentially feasible strategies and directions for the future development of antibiotic detection are envisioned.
2. Antibiotics commonly found in wastewater
Significant amounts of antibiotics enter aquatic environments, primarily through the discharge of domestic sewage [31], medical wastewater [32,33], industrial wastewater [34,35], and wastewater from animal husbandry and aquaculture [[36], [37], [38]]. Among these sources, the supervision and treatment of domestic sewage, as well as medical and industrial wastewater, has been significantly improved by environmental agencies, primarily through establishing sewage treatment plants and medical waste management systems. Antibiotics from domestic sewage and medical wastewater are eventually discharged into wastewater treatment plants. Factories and enterprises must install appropriate sewage treatment equipment to remove pollutants from industrial wastewater, including antibiotic residues [39]. Numerous studies have demonstrated that adsorption and biodegradation, as established methods, are widely employed [40,41]. Furthermore, increased public awareness of antibiotic use in medical contexts has substantially reduced antibiotic residues in domestic sewage and medical wastewater [42]. Conversely, globally, over half of antibiotics are utilized in the production of food animals, with numerous livestock and aquaculture enterprises being the primary consumers of these drugs [43,44]. In large-scale breeding, antibiotics are used to prevent and treat animal infectious diseases and added to feed and drinking water as growth promoters [45]. Owing to their inherent characteristics, antibiotics are challenging to metabolize within organisms. As a result, substantial amounts of antibiotics are released into the environment with feces and other excreta [12]. Next, we analyze each type of wastewater individually.
2.1. Domestic sewage
In many countries, antibiotics are unregulated, which allows patients to purchase them independently and use them at home. The most commonly used antibiotics are penicillins and β-lactams [31]. Thus, antibiotics can be directly released into the environment by patients. Drugs that are not fully metabolized by the human body may also enter the sewage treatment system via urine or feces. In addition, some unused or expired drugs may be discarded, contributing to antibiotic contamination in aquatic environments [46]. As public awareness of medical and environmental issues has improved, the abuse of antibiotics has gradually decreased. However, the proper disposal of unused and expired drugs still requires significant attention from governments and international organizations, along with the enhancement of relevant laws and regulations and the implementation of appropriate measures [47].
2.2. Medical wastewater
Hospitals and medical activities play a crucial role in maintaining public health. Moreover, the threat posed to the ecosystem by medical wastewater must be considered. Medical wastewater is a primary source of antibiotics entering aquatic environments, soil, crops, and sewage treatment plants [[48], [49], [50]]. The characteristics of medical wastewater vary depending on factors such as geographic region, prescribed antibiotic type, type of medical institution, bed density, ward type, country, and seasonal variations [50]. Reports have indicated that antibiotics such as enrofloxacin, ciprofloxacin, ofloxacin, norfloxacin, sulfamethoxazole, trimethoprim, and metronidazole, as well as their metabolites, are present at high concentrations in hospital wastewater [49,51]. Among the various antibiotic transmission pathways, hospital wastewater collectors and sewage treatment plants play significant roles [52]. For several antibiotics, the removal efficiency of conventional treatment processes in sewage treatment plants is limited [53], necessitating the use of real-time online detection methods.
2.3. Industrial wastewater
Industrial wastewater is another significant source of antibiotics in aquatic environments. Notably, the concentration of antibiotics in wastewater from pharmaceutical factories is several orders of magnitude higher than that in domestic wastewater, reaching mg L−1 levels [54,55]. Reports have indicated that pharmaceutical wastewater from industrial production is a major contributor to elevated antibiotic residues in water environments in Europe [56] and Vietnam [57]. Similarly, in the Pudong New Area (Shanghai, China), industrial pharmaceutical wastewater is the primary source of antibiotic pollution in surface water [58].
2.4. Livestock breeding wastewater
Pig breeding is a common type of domestic livestock breeding. The antibiotics most widely used in the domestic pig breeding industry can be broadly categorized into four groups: sulfonamides, tetracyclines, macrolides, and chloramphenicol [59,60]. Table 1 shows the proportions of antibiotics used in livestock and meat production worldwide in recent years [61]. Domestic pig breeding is mainly individualized and free-range in vast areas of the world, including developing countries such as China, with a low degree of centralization. A previous study indicated that the majority of farmers and rural individuals lack accurate knowledge regarding the purpose of antibiotic use and the usage and dosage of specific antibiotics [[62], [63], [64]]. This disparity has led to many antibiotics in domestic pig manure. Approximately 30–90 % of antibiotics, depending on the type of antibiotic, enter water bodies, sediments, and soil through fecal matter [60,65,66].
Table 1.
| Penicillins (87.1 %) | Tetracyclines (87.1 %) | Macrolides (77.1 %) | Sulfonamides (70.0 %) | Quinolones (68.6 %) |
|---|---|---|---|---|
| Benzylpenicillin | Chlortetracycline | Tulathromycin | Sulfachlorpyridazine | Flumequine Miloxacin Nalidixic acid Oxolinic acid Ciprofloxacin Danofloxacin Difloxacin Enrofloxacin Marbofloxacin Norfloxacin Ofloxacin Orbifloxacin |
| Penethamate hydroxide | Doxycycline | Erythromycin | Sulfadiazine | |
| Penicillin procaine | Oxytetracycline | Josamycin | Sulfadimerazin | |
| Mecillinam | Tetracycline | Kitasamycin | Sulfadimethoxine | |
| Amoxicillin | Spiramycin | Sulfadimidine | ||
| Ampicillin | Tilmicosin | Sulfadoxine | ||
| Amoxicillin-clavulanic | Mirosamycin | Sulfafurazole | ||
| Ticarcillin | Terdecamycin | Sulfaguanidine | ||
| Tobicillin | Sulfamethazine | |||
| Aspoxicillin | Sulfadimethoxazole | |||
| Phenoxymethylpenicillin | Sulfamethoxine | |||
| Phenethicillin | Sulfamonomethoxine | |||
| Penicillin | Sulfaquinoxaline | |||
| Cloxacillin | Trimethoprim | |||
| Dicloxacillin | Sulfonamide | |||
| Nafcillin | Baquiloprim | |||
| Oxacillin | Trimethoprim |
2.5. Aquaculture wastewater
The Food and Agriculture Organization defines aquaculture as the cultivation of aquatic organisms, including fish, mollusks, crustaceans, and aquatic plants. The term “aquaculture industry” refers to a set of practices aimed at improving production efficiency. These practices may involve regular feeding, the use of medicines and fertilizers, and other methods to ensure yields. In aquaculture, antibiotics mainly treat common diseases and promote growth and development [67]. Common internationally approved antibiotics for aquaculture include fluoroquinolone, β-lactam, tetracycline, macrolide, and sulfonamide antibiotics. However, despite clear regulations on drug usage, a significant amount of misuse still occurs. Moreover, as aquatic drugs have low utilization rates, aquatic products can absorb only 20 %–30 % of these drugs. The remainder, a significant quantity, is discharged into surrounding waters or sediments, contributing to nonpoint source pollution [67]. High concentrations of antibiotics, including norfloxacin, ofloxacin, amoxicillin, erythromycin, tetracycline, and sulfamethoxazole, are commonly detected in aquaculture wastewater. These antibiotics have been detected in bodies of water across multiple countries (Table 2).
Table 2.
Antibiotic concentrations in typical water samples.
| Country | Category | Concentration (ng mL−1) | Reference |
|---|---|---|---|
| Mozambique | Sulfamethoxazole | 5.38 × 10−1 | [68] |
| Kenya, Nairobi River | Sulfamethoxazole | 3.89 × 10−1 | [69] |
| Croatia | Sulfamerazine | 1.10 × 10−1 | [56] |
| Vietnam, Mekong River | Sulfamethazine | 1.91 × 10−1 | [70] |
| China, Lake Poyang | Sulfadiazine | 5.62 × 10−2 | [71] |
| China, Huangpu River | Oxytetracycline | 4.87 × 10−2 | [72] |
| China, surface water in the Pearl River Delta | Doxycycline | 3.97 × 10−2 | [73] |
| China, water sources of Wuhan | Sulfadiazine | 5.36 × 10−2 | [74] |
| China, Yellow Sea coastal area | Sulfamethoxazole | 2.59 × 10−1 | [75] |
| Streptomycin | 3.41 × 10−2 | ||
| Tetracyclines | 2.34 × 10−1 | ||
| Sulfamethoxazole | 5.82 × 10−2 | ||
| Enrofloxacin | 4.99 × 10−2 | ||
| Sulfonamides | 1.44 × 10−2 | ||
| Ciprofloxacin | 2.11 × 10−2 | ||
| Enrofloxacin | 1.35 × 10−1 |
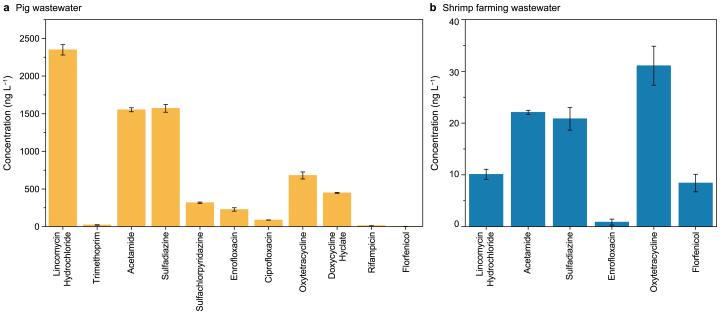
We conducted a field survey to assess the types and concentrations of antibiotics in pig and shrimp wastewater in Hainan Province, China. The concentrations of detected antibiotics were classified and quantified using the HPLC‒MS/MS method (Fig. 2). The results revealed that certain antibiotics in pig wastewater reached concentrations as high as μg L−1. The presence of high concentrations of various classes of antibiotics is of considerable concern. The unchecked use and release of antibiotics pose environmental risks and contribute to the development of antibiotic resistance in bacteria. This resistance complicates disease control and has led to the emergence of formidable resistant strains [76]. Therefore, regulating the use of antibiotics in the aquaculture industry is essential for both human health and environmental sustainability.
Fig. 2.
Types and concentrations of antibiotics in pig (a) and shrimp farming (b) wastewater in Hainan Province, China.
3. Detection of antibiotics in water environments
Antibiotic detection methods are commonly categorized into four groups: chromatography‒mass spectrometry methods [[77], [78], [79], [80], [81]], microbial‒immunoassay techniques [82,83], optical approaches, and electrochemical sensing techniques [[84], [85], [86], [87], [88]]. Previous research has described the principles and applications of HPLC, MS, and LC‒MS. Researchers have developed strategies to detect antibiotics using microorganisms in conjunction with immunoassays. For example, a 2008 study evaluated the growth dynamics of commercial Pseudomonas aeruginosa and soil-derived Pantoea agglomerans in antibiotic assays based on pH-dependent inhibition [89]. While microbial methods can save time, they may produce false positives or false negatives [83], leading to inaccurate results. Subsequently, new methods that combine the microbial detection of antibiotics with other methods have been proposed [90]. In one study, microorganisms were combined with high-performance liquid chromatography to analyze seven antibiotics, including sulfonamides and quinolones [91]. The antibiotic residue screening test (STAR) method and the 2,3,5-triphenyltetrazolium reduction (TTC) method were compared. The TTC method detected type 5 sulfonamide antibiotics more effectively at concentrations below 50 μg L−1 in dairy products than the STAR method. However, the STAR method effectively detects low concentrations of quinolone antibiotics, such as enrofloxacin and ciprofloxacin, in milk. In another study, luminescent-sensing bacteria were used to detect antibiotic concentrations in natural environments [92]. The well-known luciferase gene serves as a reporter gene, causing cells to glow without external light. The bioluminescence intensity is measurable, and cellular damage from antibiotics can trigger various bacterial repair systems. Despite its benefits, such as a short incubation time, this method faces challenges, such as intricate bacterial cultivation. In 2009, the Spanish Network Research Center for Biomaterials and Nanomedicine introduced an immunosensor capable of detecting sulfonamide antibiotics in milk [93]. Based on a microfluidic platform, this immunoassay detects biomolecule adsorption through changes in the waveguide evanescent field, achieving impressive detection limits in both buffer and milk. In the same year, the team introduced a groundbreaking optical biosensor designed around the wavelength principle that is aimed at facilitating the simultaneous screening of multiple analytes, including sulfonamide, quinolone, β-lactam, and tetracycline antibiotics in milk [94]. Using the evanescent wave phenomenon, this sensor effectively detected various antibiotic residues within milk samples through antigen‒antibody binding. The microbial‒immunoassay technique, known for its economical sample requirements, cost effectiveness, and widespread acceptance, possesses commendable attributes. However, certain limitations of this technique must be recognized, such as its intricate operation, reduced sensitivity, strict storage conditions, and limited selectivity.
In recent years, optical and electrochemical methods have been the primary techniques for detecting antibiotics [[95], [96], [97], [98], [99], [100]]. Optical methods have gained traction among scientists because of their straightforward visual results and ease of operation. In 2014, a breakthrough occurred when researchers introduced an enhanced fluorescence system for tetracycline detection that utilizes gold nanoclusters [101]. These nanoclusters act as fluorescence-enhancing agents, building a system around Eu3+–tetracycline complex supplemented by gold nanoclusters. This system can detect tetracycline within a linear range of 0.01–5 μM and boasts a detection limit of 4 nM and a signal-to-noise ratio of 3. Moreover, the practicality of this method in actual work (detection of tetracycline in human urine and milk samples) was verified. In addition to fluorescence detection, the colorimetric detection of antibiotics is intuitive. One notable example involves an alkali-etched manganese-based Prussian blue analog known for its superb oxidase-mimicking activity [102], pivotal in a tetracycline colorimetric sensing system. The process involves revealing the sites and surfaces of the analog via etching with NaOH, which notably increases its catalytic ability. Density functional theory is employed in the system to select the best molecularly imprinted polymer (MIP) layers for precise target identification. Its performance was evidenced by the consistent linear relationship with the absorbance at 652 nm for tetracycline concentrations ranging from 0.2 to 200.0 μM, with a detection threshold of 0.07 μM. Moreover, this system adeptly distinguishes specific targets from a variety of interferents. In another innovative project, engineers crafted a detection kit for the onsite identification of oxytetracycline in milk [103]. This ingenious kit simplifies the creation of lateral flow-based aptasensors by attaching tetracycline to a 7 kDa carrier protein and securing it onto a nitrocellulose membrane. Leveraging a 0.125 μM ligand‒gold conjugate, the system can visually identify tetracycline concentrations as low as 5 ng mL−1 in milk samples within only 10 min, comfortably meeting the standard threshold of 100 ng mL−1. Thus, this kit has emerged as a compelling solution for swift antibiotic detection and is especially crucial for ensuring food safety.
Electrochemistry has recently been used in sensing to detect specific antibiotics concentrations through different indicators, including impedance magnitude, redox peak intensity, and photocurrent response strength. For example, some innovators have crafted sensing probes using carbon quantum dots to amplify electrochemical signals [104]. When Hg ions and these sensing probes are introduced onto an electrode surface, they stimulate electron transfer, thereby increasing the generation of electrochemical signals. This tailored electrode shows excellent electrocatalytic activity toward Hg, with anode and cathode peaks at 0.53 and 0.42 V (vs. saturated calomel electrode [SCE]), respectively. The introduction of amoxicillin disrupts the sensing probe at the interface, suppressing electrochemical signal production. This probe exhibits an ideal linear response from 0 to 100 nM, with the detection threshold reduced to an impressive 15 nM. In a novel approach, researchers introduced a carbon-doped ZnO nanocomposite molecularly imprinted electrochemical sensor tailored for detecting oxytetracycline in milk [105]. When combined with acetylene black, this nanomaterial serves as a reinforcing conductive matrix, enhancing the efficiency of electron migration. Concurrently, based on electropolymerization, the MIP sensor represents a new standard, with a lower detection limit for oxytetracycline of 8.75 pM and a broad linear detection range spanning 0.01–1000.00 nM. Its exemplary selectivity and enduring stability are worth noting.
In conclusion, choosing and implementing techniques for detecting antibiotics in aquatic environments play a pivotal role in maintaining food safety, safeguarding environmental health, and facilitating accurate clinical diagnoses. While numerous methods can detect a variety of antibiotics, the unique chemical structure and properties of each antibiotic often necessitate specific detection approaches. In the following sections, we delve into the specialized detection techniques and nuances associated with several common antibiotics found in wastewater.
3.1. Detection of tetracycline antibiotics
In animal husbandry and aquaculture, tetracycline antibiotics are fundamental in antibacterial practices because they display potent efficacy against mycoplasma, chlamydia, spirochetes, and gram-negative and gram-positive bacteria. However, tetracycline resistance genes have been identified in pig farm wastewater, pig manure, and adjacent river systems, indicating substantial residual levels. This accumulation in aquatic environments has raised concerns regarding the environmental impact of these antibiotics [76,106]. The detection of tetracycline antibiotic residues has become imperative.
In recent years, there has been a surge in the development of tetracycline detection methods that leverage fluorescence-based strategies [85,[107], [108], [109], [110], [111], [112]]. Among these advancements, a dual-mode nanosensor that uses reduced carbon dots (r-CDs) to detect tetracycline has been introduced (Supplementary Material Fig. S1a) [113]. This innovative approach operates via a Förster resonance energy transfer mechanism, under which the presence of tetracycline suppresses the fluorescence emitted by r-CDs, enabling highly sensitive detection with a remarkable limit of detection (LOD) of 1.73 nM. Moreover, the color of the r-CDs exhibits an observable shift from colorless to red, providing an alternate detection method visible to the naked eye that achieves an LOD of 0.46 mM. These r-CDs demonstrate specificity and sensitivity in monitoring tetracycline within wastewater, underscoring their practical significance in diverse wastewater monitoring and treatment applications.
3.1.1. Semiconductor material fluorescence-based detection methods
Notably, a pioneering study in 2019 introduced a novel tetracycline fluorescence sensor leveraging molybdenum disulfide nanosheets (MoS2 NPs) [114]. The method involves a bottom-up hydrothermal process to synthesize molybdenum disulfide nanoparticles, which exhibit distinctive blue fluorescence at 430 nm. The fluorescence is significantly quenched by adding tetracycline owing to the combined internal filtering effect and electron transfer. This innovation led to the development of an MoS2 NP-based fluorescence sensor for tetracycline detection, which demonstrated practicality for examining tetracycline levels in milk powder, milk, and beef samples (with the ability to detect concentrations as low as 0.032 μM and a recovery rate between 88.46 % and 108.62 %).
In a separate breakthrough with exceptionally low detection limits, researchers introduced a zinc oxide tetrapod nanomaterial structure for the fluorescence immunodetection of tetracycline [115]. This novel material has a structure with needle-like branches, which vary in length from hundreds of nanometers to several micrometers, with an average thickness of tens of nanometers (Supplementary Material Fig. S1b). The ample surface area of this structure facilitates efficient bioreceptor immobilization and reactions. These ZnO nanotetrapods exhibit robust photoluminescence (PL) at room temperature, with small surface changes significantly impacting the PL. The PL signal of ZnO decreases with increasing tetracycline concentration, enabling precise measurements from 0.001 to 1.000 μg L−1 and an impressive decrease in the detection limit to 1.2 ng L−1.
3.1.2. Quantum dot fluorescence-based detection methods
With advancements in material technology, scientific experts have introduced the innovative concept of “quantum dots” as fluorescent probes. Owing to their quantum size, tungsten oxide quantum dots exhibit remarkable optical and electronic properties and have proven to be invaluable fluorescent probes for detecting tetracycline in animal products [116]. In this approach, tungsten disulfide (WS2) and hydrogen peroxide (H2O2) are used as the tungsten source and a sufficient oxidant (Supplementary Material Fig. S1c); additionally, a pot of ethanol is used, and templates, harsh conditions, or hazardous chemicals are not required. Blue luminescent tungsten oxide quantum dots were synthesized using a thermal method. These prepared quantum dots are significantly quenched by tetracycline owing to the collaborative effects of the internal filter effect, fluorescence resonance energy transfer, and photoinduced electron transfer. This method exhibits a linear relationship in detecting tetracycline at concentrations ranging from 0 to 50 μM, with a detection limit of 19 nM. The sensor monitors adulterated milk and milk powder samples, confirming its practical utility. In a separate endeavor, the researchers introduced a portable concept in which a smartphone was integrated with quantum dot fluorescence-based detection. A portable smart sensing platform was developed using silk fibroin–modified thiol-branched graphene oxide quantum dots (Supplementary Material Fig. S1d) [117]. This method efficiently enables the proportional fluorescence detection of tetracycline in real samples, demonstrating a linear range of 0–90 nM, with notably low detection limits across various samples, such as deionized water, chicken, fish, human serum, and honey (49.69, 47.76, 55.25, 47.90, and 45.78 nM, respectively). The enhanced luminescence properties of the material in liquid media are visible to the naked eye under 365 nm ultraviolet light, allowing the smartphone to detect color changes during sensing and translate them into readable Red-Green-Blue (RGB) data using 365 nm light-emitting diode. The portable sensor boasts a detection limit of 0.125 μM, facilitating swift onsite tetracycline detection.
3.1.3. Surface-enhanced Raman scattering-based detection methods
Surface-enhanced Raman scattering (SERS) is a pivotal tool in the detection and serves as an exceptionally sensitive optical identification methodology [[118], [119], [120]]. One pioneering study involved the creation of silver nanoparticle (AgNP) arrays through template-assisted electrochemical deposition of anodized aluminum oxide (AAO) (Supplementary Material Fig. S1e). In this study, scientists meticulously fine-tuned the periodic nanostructure to increase SERS activity by altering the nanopore diameter and silver NP sputtering time. Consequently, they managed to reduce the gap between silver NPs to less than 10 nm, effectively enabling the detection of tetracycline in milk at impressively low concentrations (1 × 10−9 M) [121]. This breakthrough highlights the potential of optimized nanostructures to push the boundaries of sensitive detection methodologies.
3.1.4. Electrochemistry-based detection methods
Electrochemical detection methods are gradually used in tetracycline trace detection [[122], [123], [124], [125]]. Researchers utilized the current values corresponding to the oxidation peak at −1.06 V and the reduction peak at 0.724 V of the electrochemically active metal‒organic frameworks Mo@MOF-808 and NH2-UiO-66 as response signals for detecting ultratrace levels of tetracycline. The analysis focused on monitoring changes in the intensities of the currents associated with these specific voltages, providing a sensitive and precise method for tetracycline detection [126]. This technique was utilized for the ultratrace detection of tetracycline (Supplementary Material Fig. S1f). Notably, this sensor demonstrates an extensive linear range for tetracycline (0.1–10000 nM) and an impressively low detection limit of 0.009792 nM. Furthermore, the sensor exhibits superior sensitivity, repeatability, and stability compared to single-signal sensors.
3.1.5. Scope and limitations of detection methods
For the detection of tetracycline, particularly in complex aquatic environments, the sample complexity must be addressed to identify the appropriate detection method. Fluorescence sensors offer high selectivity in detecting antibiotics in aquaculture wastewater, enabling the identification of specific antibiotics. However, fluorescence sensors are prone to photobleaching, which can degrade the sensor signal over time, compromising long-term stability and accuracy. Additionally, the complexity of aquaculture wastewater further impacts the performance of fluorescence sensors, making detection challenging and prone to error. SERS sensors face several challenges in practical applications, including their sensitivity, reproducibility, and range of applicability, particularly in complex aquatic environments, such as aquaculture wastewater. Electrochemical sensors encounter long-term stability issues in practical applications and can be influenced by environmental interference. Furthermore, owing to the widespread use of tetracyclines, there is a need for rapid, onsite, portable monitoring beyond the laboratory setting. This creates new challenges for detection methods. To overcome these limitations, emerging analytical strategies based on alternative mechanisms, such as electronic sensors, intelligent colorimetric sensors, and integrated electrochemical probes, are becoming essential for rapid analysis in diverse aquatic environments. These detection methods have become major focal points of research.
3.2. Detection of macrolide antibiotics
There is a lack of effective methods for detecting residues of azithromycin, a prominent macrolide antibiotic widely utilized in animal husbandry and aquaculture for respiratory antibacterial purposes. Innovative approaches predominantly rely on electrochemical principles [[127], [128], [129], [130]], with limited strategies reported.
3.2.1. Electrochemistry-based detection methods
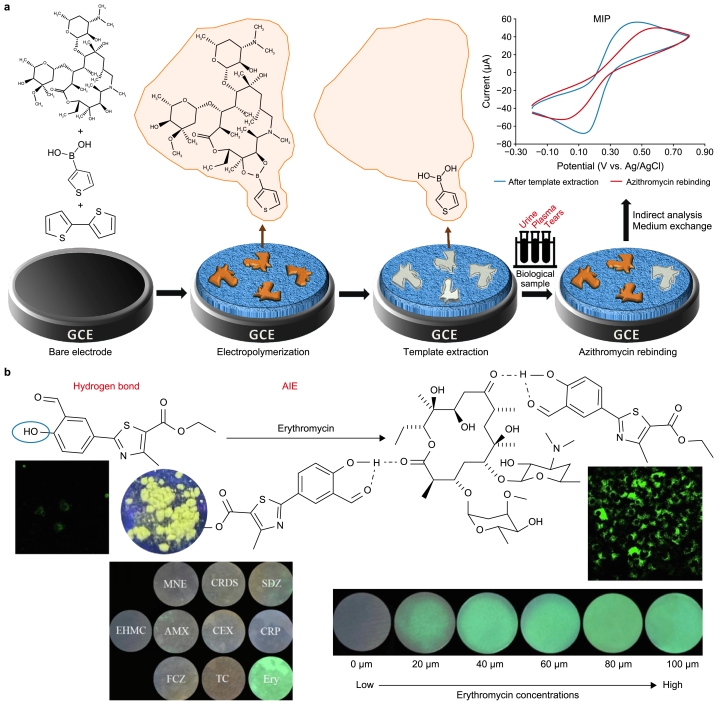
A typical work in 2020 proposed an electrochemical sensor for measuring azithromycin in various complex liquid environments (urine, tears, and plasma) [131]. This method hinges on using electrodeposition to craft molecularly imprinted polymers (MIPs) to form a bionic recognition layer approximately 75 nm thick on a glassy carbon electrode (Fig. 3a). The sensor exhibits a dynamic response range of 13.33–66.67 μM and boasts a remarkable detection limit of 0.85 nM for azithromycin. In addition to its impressive sensitivity, this sensor has demonstrated practical application viability in challenging environments, such as urine, tears, and plasma. Its advantages include a straightforward reusable design, an extended shelf life, and promising prospects for detecting macrolide antibiotics, specifically azithromycin.
Fig. 3.
Typical analysis and detection methods for azithromycin and erythromycin. a, Electrochemical method. MIP: molecularly imprinted polymer; GCE: glassy carbon electrode. Adapted from Ref. [131]. Copyright 2020, Elsevier. b, Fluorescence method. AIE: aggregation-induced emission. Adapted from Ref. [135]. Copyright 2023, Elsevier.
3.2.2. Fluorescence-based detection methods
In livestock and aquaculture fields, erythromycin is a common treatment for various bacterial infections. Unfortunately, this antibiotic frequently occurs in water environments [84,[132], [133], [134]]. A detection method was devised to address this issue using a fluorescent probe with aggregation-induced emission characteristics both in a solid state and in water/ether binary solvents [135]. This innovative approach allows for the identification of erythromycin in water. Moreover, a portable test strip developed based on this method enables precise and selective detection and determination of the concentration of erythromycin in diverse and complex settings, including various water sources and living cells (detectable down to a limit of 17.8 nM). Remarkably, the test paper exhibited robust green fluorescence only in the presence of erythromycin among the different antibiotic solutions (Fig. 3b). When various concentrations of erythromycin solutions were applied to the test paper, a distinct amplification of green fluorescence was visually apparent. This observation highlights the promising potential of this fluorescent test paper in evaluating erythromycin contamination.
3.2.3. Scope and limitations of detection methods
Detection methods for macrolide antibiotics with large rings have focused primarily on simple and cost-effective optical and electrochemical sensing strategies. This is mainly due to the high cost of chromatography‒mass spectrometry methods, which require complex equipment. These factors significantly limit the potential application of these methods for the portable and rapid detection of macrolide antibiotics. Therefore, developing new detection methods based on optical and electrochemical techniques holds great promise. Addressing practical needs and applying these methods in complex water environments remain challenging. Developing advanced materials with large surface areas and multiple reaction sites for electrochemical sensors can improve sensitivity and selectivity, but this remains challenging to achieve. Fluorescence sensors face several challenges in practical applications, such as reproducibility and application range. The reproducibility issue requires the introduction of internal standards and the preparation of highly uniform fluorescent materials. Additionally, expanding the application range of fluorescence sensors for the rapid in situ detection of macrolide antibiotics in aquaculture wastewater is crucial.
3.3. Detection of sulfonamide antibiotics
Detection methods for sulfonamide antibiotics have focused primarily on optical and electrochemical techniques [[136], [137], [138], [139], [140]].
3.3.1. Fluorescence-based quinolone antibiotic detection methods
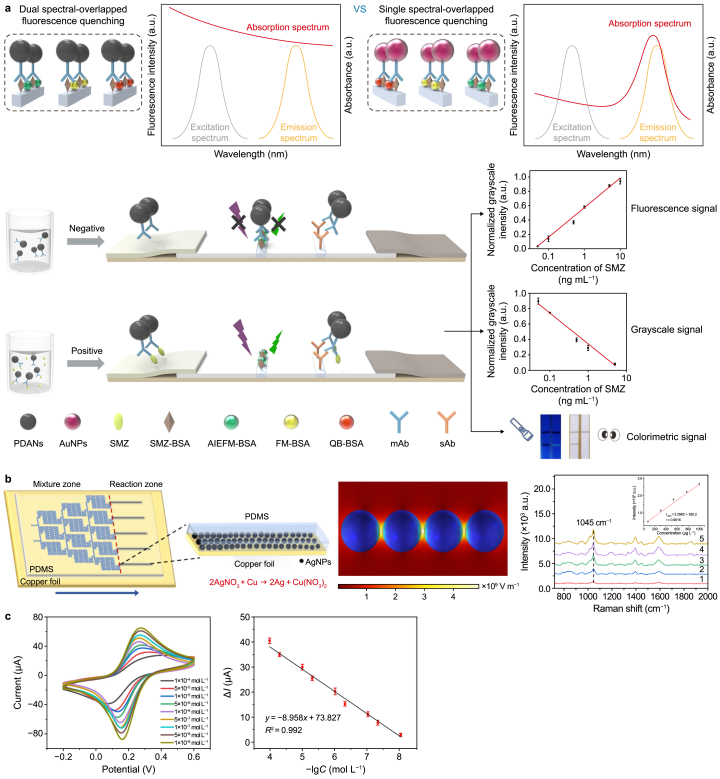
Fluorescence detection is a highly specific method commonly used to recognize antibiotics [[141], [142], [143]]. A 2022 study proposed a transverse-flow antibiotic assay for the sensitive detection of sulfamethoxazole based on dual-spectral overlapping fluorescence quenching of polydopamine nanospheres (PDANs) due to the internal filtering effect (Fig. 4a) [137]. The device consists of four components: an absorbent pad, a polyvinyl chloride pad, a sample pad, and a nitrocellulose membrane. Compared to traditional quenchers, PDANs can quench three types of fluorescent donors: aggregation-induced emission fluorescent microspheres, excitation and emission light of fluorescent microspheres, and quantum dot beads. This results in a significant increase in fluorescence intensity and a higher signal-to-noise ratio. The detection and visual detection limits of sulfamethoxazole in chicken meat obtained using this method are 0.043 and 0.5 ng mL−1, respectively. This method is also applicable for the onsite detection of aquaculture wastewater, demonstrating its practical application potential.
Fig. 4.
Typical analysis and detection methods for sulfonamide antibiotics. a, Fluorescence method. PDANs: polydopamine nanospheres; AuNPs: gold nanoparticles; SMZ: sulfamethazine; BSA: bovine serum albumin; AIEFM: aggregation-induced emission fluorescent microsphere; QB: quantum dot bead; FM: fluorescent microsphere; mAb: monoclonal antibody; sAb: secondary antibody. Adapted from Ref. [137]. Copyright 2022, Elsevier. b, Surface-enhanced Raman scattering method. PDMS: polydimethylsiloxane; AgNPs: silver nanoparticles. Adapted from Ref. [147]. Copyright 2024, Royal Society of Chemistry. c, Electrochemical method. DMSP: dimethyl sulfoxide; HAc: acetic acid; SDZ: sulfadiazine. Adapted from Ref. [151]. Copyright 2024, Springer.
In 2024, Zhang et al. developed a method for detecting sulfamethoxazole based on photoinduced electron transfer between imidazole ligands and copper ions [144]. Surface-modified upconversion nanoparticles bind to Cu2+ through electrostatic adsorption, leading to fluorescence quenching. The sensor exhibits excellent linearity over a wide concentration range (0.05–1000 ng mL−1), with a detection limit of 0.04 ng mL−1. It is highly selective and is unaffected by common substances in aquatic media. These findings indicate that the sensor has great potential for detecting residual sulfamethoxazole in aquatic products.
3.3.2. Surface-enhanced Raman scattering-based quinolone antibiotic detection methods
The SERS technique is a sensitive detection method frequently used to recognize sulfonamide antibiotics [[145], [146], [147], [148]]. Sun et al. designed a multifunctional structure containing gold nanoparticles embedded within a zirconium-based metal‒organic framework [146]. Methods such as analyte enrichment, Raman signal enhancement, and internal standard calibration demonstrated excellent SERS-sensing performance for sulfonamide antibiotics (sulfamethoxazole, sulfamethoxazole, sulfamethoxazole, and sulfaquinoxaline). The extensive π-bond conjugation system of Zr-MOF significantly enhances the concentration of sulfonamide antibiotics through π–π interactions. The embedded antibiotics are vertically adsorbed on the Au surface through coordination interactions between Au and two oxygen atoms or between Au and the terminal amino group. The above four antibiotics were detected in real honey samples at concentrations ranging from 2.8 to 5.2 ng g−1 within 10 min.
In the same year, Huang et al. designed a copper/polydimethylsiloxane microfluidic chip (Fig. 4b) [147]. SERS substrates were synthesized through displacement reactions between silver and copper in microfluidic channels, forming a microfluidic SERS nanoparticle (Ag NPs)/Cu chip. Combined with a concentration gradient microchannel network design, this chip can generate a standard curve for a sample with a single injection on a single chip. The linear range for sulfamethoxazole analysis is 75–1000 mg L−1, with a detection limit of 28.4 μg L−1. Sulfonamide pyrimidine was detected in aquatic products, with recovery rates ranging from 82.7 % to 110 %. The entire testing process for sulfamethoxazole detection can be completed within 20 min.
3.3.3. Electrochemistry-based quinolone antibiotic detection methods
Recently, many detection methods based on electrochemical methods have been proposed [[149], [150], [151], [152], [153]].
One notable study stands out because of the wide linear range (1 × 10−8‒1 × 10−4 mol L−1) and low detection limit (1.30 × 10−9 mol L−1). The researchers developed a novel molecularly imprinted electrochemical sensor for the specific detection of sulfadiazine in aquaculture feed (Fig. 4c) [151]. Niobium carbide (Nb2CTx), a typical two-dimensional layered nanomaterial, has good conductivity and a unique structure. It was assembled with one-dimensional silver nanowires (AgNWs) to form quasi-3D composite nanomaterials (Nb2CTx/AgNWs) (Supplementary Material Fig. S2). As a spacer material, AgNWs prevent aggregation of Nb2CTx and the collapse of the Nb2CTx layer. Simultaneously, the synergistic effect between the nanomaterials creates a rapid electron transfer channel. The Nb2CTx/AgNW composite enhances the electrical signals. This approach has been successfully applied to detect sulfamethoxazole in aquaculture feed.
3.3.4. Scope and limitations of detection methods
In sulfonamide antibiotic detection, emerging strategies have focused primarily on fluorescence, SERS, and electrochemical methods. This is primarily due to the complexity of chromatography‒mass spectrometry methods and the need for expensive equipment. These factors limit the applicability of these methods for portable and rapid sulfonamide antibiotic detection. Emerging detection strategies require developing new materials and interdisciplinary collaboration among fields such as biology, chemistry, and environmental monitoring. Furthermore, these methods should not be confined to laboratory settings but should be adaptable to real-world environments, which present unique challenges for practical implementation. Overall, intelligent visual colorimetric sensing and portable SERS detection devices will likely become promising key approaches for sulfonamide antibiotic detection. These methods are expected to enable the in situ detection of sulfonamide antibiotics in aquaculture wastewater.
3.4. Detection of quinolone antibiotics
Quinolone antibiotics are commonly used in the breeding industry; thus, their effective detection is crucial. Typical quinolone antibiotics include ofloxacin, ciprofloxacin, and norfloxacin. Detection methods for ofloxacin focus predominantly on optical and electrochemical techniques [[154], [155], [156], [157], [158]].
3.4.1. Fluorescence-based quinolone antibiotic detection methods
In 2022, researchers proposed a multianalyte fluorescent probe based on biocompatible chitosan-capped CdS (CTS-CdS) quantum dots to detect environmentally harmful organic contaminants [154]. A straightforward water chemistry method was employed for the probe to prepare the CTS-CdS quantum dots. The probe demonstrates unique fluorescence properties with high selectivity for various contaminants in wastewater. Specifically, it shows a lower detection limit (0.006 μM) for ofloxacin and exhibits good stability for the trace detection of organic contaminants in real samples.
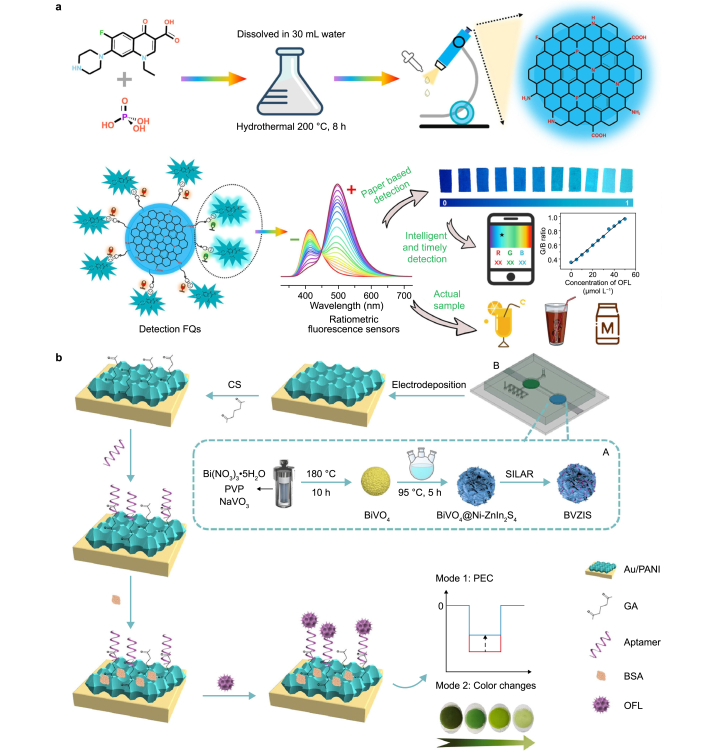
In 2023, a groundbreaking study introduced a novel ratiometric fluorescence detection approach for ofloxacin and its l-isomer levofloxacin (LEV) using tridoped graphene quantum dots (T-GQDs) [159]. This innovative method harnessed norfloxacin and phosphoric acid as doping sources to synthesize T-GQDs in a single step. An intelligent RGB‒time color analysis method was also devised in this study based on fluorescent test paper, enabling the real-time detection of OFL/LEV (Fig. 5a). The fundamental concept revolves around the electrostatic bond between T-GQDs and OFL/LEV, which induces an intermolecular electron transfer effect. This transfer reduces fluorescence intensity as electrons move from the T-GQDs to OFL/LEV. The sensor achieves impressive detection limits of 46 and 67 nM for the two antibiotics. Moreover, it can detect these substances in complex water environments, such as juice, carbonated drinks, and milk. In the same year, a rapid and energy-efficient two-step synthesis method was developed to produce self-passivated fluorescent water-soluble CDs (wsCDs) from sustainable microcrystalline cellulose materials [156]. The aqueous solution of wsCD exhibits blue emission under ultraviolet light, with a fluorescence quantum yield of approximately 6 %. Additionally, wsCDs were employed for the specific detection of ofloxacin, achieving a detection limit of approximately 0.025 ppm. Antibacterial experiments with wsCDs indicated no toxic effects at a concentration of 1 mg mL−1, supporting their biocompatibility.
Fig. 5.
Typical analysis and detection methods for ofloxacin. a, Fluorescence/colorimetric method. Adapted from Ref. [159]. Copyright 2023, Elsevier. b, Electrochemical method. CS: chitosan; BVZIS: BiVO4@Ni-ZnIn2S4/Bi2S3; SILAR: successive ionic layer adsorption and reaction; PVP: polyvinyl pyrrolidone; GA: glutaraldehyde; BSA: bovine serum albumin; OFL: ofloxacin. Adapted from Ref. [160]. Copyright 2023, American Chemical Society.
3.4.2. Electrochemistry-based quinolone antibiotic detection methods
Along a different line of research, scientists have introduced an electrochemical analysis method for detecting ofloxacin [160]. This method involves a dual-mode microfluidic analysis device combining photoelectrochemical sensing and electrochromic visual analysis to detect ofloxacin. A stable electron supply is provided in this strategy by designing a dual direct z-scheme BiVO4@Ni-ZnIn2S4/Bi2S3 heterojunction as a photoanode (Fig. 5b). The dual z-scheme structure of the composite expedites electron migration. A polyaniline-modified electrochromic material, Au/PANI, was subsequently electrodeposited on the photocathode to affix the aptamer and enable visual reading. The Au/PANI material directly accepts electrons from the photoanode, causing a rapid decrease in polyaniline and a visible color change from blue to green. This method shows remarkable OFL electrochemical and colorimetric (RGB-green) detection limits of 18 and 30 fg mL−1, respectively, enabling the sensitive detection of OFL in milk and river water.
3.4.3. Quantum dot fluorescence-based quinolone antibiotic detection methods
In recent years, there has been a surge in proposals of various innovative methods for detecting ciprofloxacin, marking a significant advancement in sensing technologies [[161], [162], [163], [164], [165], [166], [167], [168]].
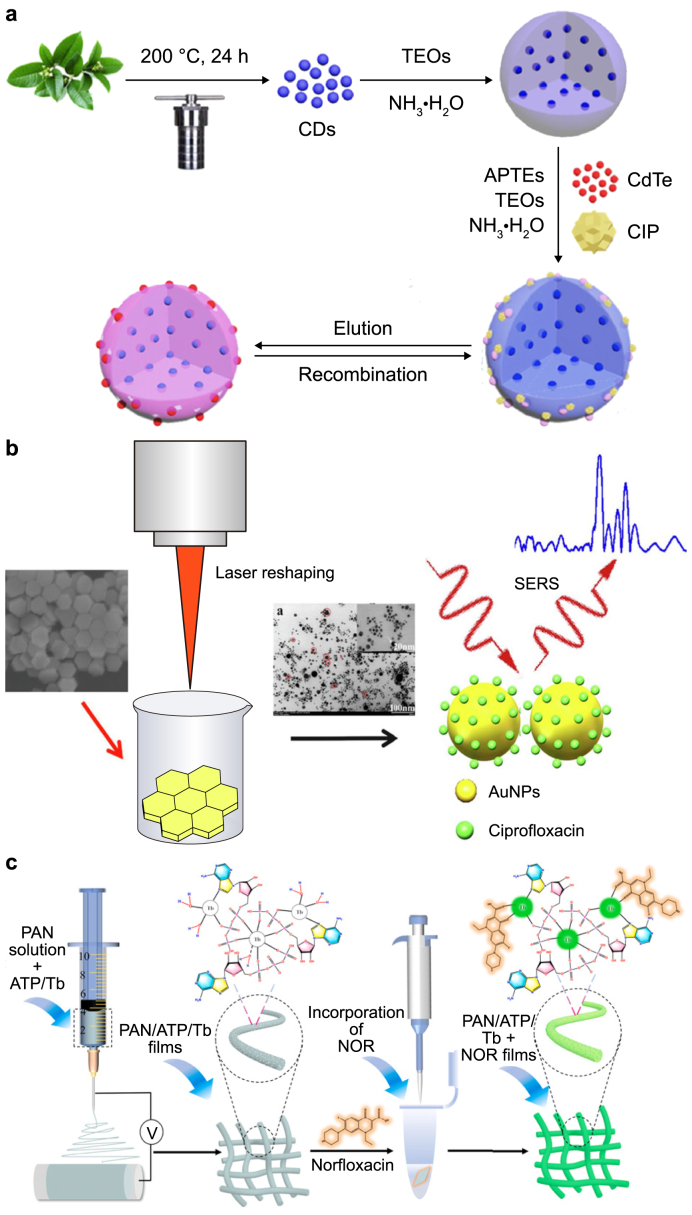
Researchers have presented a pioneering approach to ciprofloxacin sensing by developing a multifunctional composite material composed of carbon dots and CdTe quantum dots [167]. This innovative composite material was created using osmanthus leaves as a carbon source and polyethylenimine as a nitrogen source to generate highly fluorescent CDs through a hydrothermal method (Fig. 6a). The resulting blue CDs were combined with red CdTe quantum dots to construct MIPs@CdTe/CDs@SiO2. The selectivity for and sensitivity to ciprofloxacin were evaluated using TiO2/CDs/CdTe quantum dots as photocatalysts for ciprofloxacin degradation. The strategy exhibits a remarkable detection limit for ciprofloxacin of 0.0127 nM and demonstrates a linear testing range of 0–60 nM. It was successfully applied to monitor ciprofloxacin levels in human urine.
Fig. 6.
Typical analysis and detection methods for ciprofloxacin and norfloxacin. a, Fluorescence method. CDs: carbon dots; TEOs: tetraethoxysilane; APTEs: 3-aminopropyltriethoxysilane; CIP: ciprofloxacin. Adapted from Ref. [167]. Copyright 2019, Elsevier. b, Surface-enhanced Raman scattering (SERS) method. AuNPs: gold nanoparticle. Adapted from Ref. [169]. Copyright 2022, Elsevier. c, Fluorescence method. PAN: polyacrylonitrile; ATP: adenosine triphosphate; NOR: norfloxacin. Adapted from Ref. [179]. Copyright 2023, Elsevier.
3.4.4. Surface-enhanced Raman scattering-based quinolone antibiotic detection methods
SERS is frequently employed as a prominent optical detection method for quinolone antibiotic detection. Researchers manipulated femtosecond and nanosecond lasers to shape hexagonal gold sheets into particles of various sizes and forms and then utilized them for the label-free SERS detection of ciprofloxacin (Fig. 6b) [169]. By reshaping gold nanoparticles into spherical–hexagonal structures with a diameter of 120 nm and spherical–triangular combinations with a diameter of 6 nm via laser action, this method achieves a remarkable LOD of 7.5 × 10−8 M for ciprofloxacin in milk. It leverages the ultrafast laser reshaping of gold nanoparticles, paving the way for the straightforward and portable detection of contaminants.
Semiconductors have increasingly been used to prepare SERS substrates [[170], [171], [172]]. In 2023, Jiang et al. successfully synthesized multifunctional materials using a layer-by-layer method. Fe3O4@mTiO2@Ag core‒shell nanoparticles enabled the in situ detection of quinolone antibiotics in aquaculture wastewater from water plants [173]. The results revealed that the minimum detectable concentrations of the investigated antibiotics were 1 × 10−9 M (ciprofloxacin, enrofloxacin, and norfloxacin) and 1 × 10−8 M (difloxacin hydrochloride), and the detection performance was enhanced by Fe3O4@mTiO2@Ag NPs. Furthermore, a strong quantitative relationship was observed between the antibiotic concentration and the SERS peak intensity within a specific detection range. The spiked assay results for actual aquaculture water samples revealed that the recoveries for six antibiotics ranged from 82.9 % to 113.5 %, with relative standard deviations ranging from 1.71 % to 7.24 %. This approach provides a multifunctional solution for the low-concentration detection and effective degradation of antibiotics in aquaculture water.
3.4.5. Method for detecting quinolone antibiotics based on SERS microfluidic channels
The combination of microfluidic and SERS detection strategies is becoming increasingly popular [[174], [175], [176], [177]]. In a distinct approach, researchers integrated SERS substrates onto the surfaces of optical fiber cores within microfluidic channels to detect ciprofloxacin and norfloxacin residues in water [178]. This innovative strategy entails using self-assembled Ag particles as the SERS substrate and constructing a microfluidic sensing platform through hollow-core optical fibers (Supplementary Material Fig. S3). The experimental results revealed LODs for ciprofloxacin (0.1 nM) and norfloxacin (0.01 nM) substantially below the maximum residue limit specified by the European Union (3.01 × 10−7 M). This solution offers a novel approach for the label-free microfluidic detection of antibiotic residues within optical fibers and holds promise for applications in the realm of environmental antibiotic water pollution detection.
3.4.6. Electrospinning-based quinolone antibiotic detection methods
A different approach was developed using electrospinning technology to combine polyacrylonitrile fibers with adenosine triphosphate (ATP)-rare earth metal Tb3+ complexes (ATP/Tb) for the onsite detection of norfloxacin in a thin-film sensor (Fig. 6c) [179]. The main operational mechanism involves the gradual enhancement of the bright green fluorescence produced by the system in the presence of norfloxacin. This sensor exhibits a strong linear response to norfloxacin within a wide concentration range of 0.04–30 μM, with a detection limit of 16 nM. When coupled with a smartphone color recognition application, this thin-film sensing strategy facilitates onsite norfloxacin testing. The development of this strategy, coupled with intelligent portable identification, holds considerable significance for rapid detection in water environments.
3.4.7. Scope and limitations of detection methods
Quinolone antibiotic detection strategies focus predominantly on electrochemical and fluorescence methods. The success of these strategies relies heavily on integrating novel materials and necessitates interdisciplinary collaboration across the fields of biology, chemistry, and environmental monitoring, creating new challenges for the research community. The main limitations of electrochemical sensors are their poor repeatability, stability, and portability. Fluorescence sensors are prone to signal degradation over time due to photobleaching and face challenges in preparing complex materials. Moreover, these methods are highly susceptible to the complex composition of aquaculture wastewater. Additionally, these methods should be extended beyond laboratory settings and applied to real-world environments, which presents unique challenges for practical implementation. Overall, intelligent visual colorimetric sensing, electrochemical trace detection, and similar strategies hold promise as crucial approaches for feasible quinolone antibiotic detection. However, these methods must be capable of in situ detection in real-world environments.
3.5. Detection of β-lactam antibiotics
β-lactam antibiotics, predominantly penicillin and its derivatives, such as amoxicillin, are extensively used in animal husbandry with notable irregularities. Consequently, the precise detection of β-lactam antibiotics is highly important [[180], [181], [182], [183], [184], [185]].
3.5.1. Surface-enhanced Raman scattering-based β-lactam antibiotic detection methods
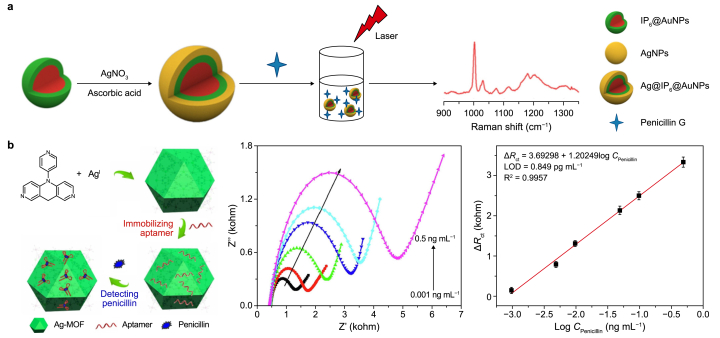
A conventional approach involves the creation of a SERS substrate known as Ag@IP6@AuNPs (Fig. 7a) [184]. This substrate was achieved by enveloping gold nanoparticles protected by inositol hexaphosphate (IP6) within a silver shell. The efficacy of this substrate is evident, as it was successfully used to identify trace amounts of penicillin residues in milk, demonstrating a linear quantitative detection range between 10−5 and 10−11 M, with a detection limit of 10−3 nM. Meeting the sensitivity requirements stipulated by the European Union's maximum residue limit for penicillin in dairy products (1.2 × 10−8 M), this detection system holds substantial promise for practical application.
Fig. 7.
Typical analysis and detection methods for β-lactams and erythromycin. a, Surface-enhanced Raman scattering method. Adapted from Ref. [184]. Copyright 2021, Elsevier. b, Electrochemical method. MOF: metal–organic framework; LOD: Limit of detection. Adapted from Ref. [188]. Copyright 2020, Elsevier.
3.5.2. Electrochemistry-based β-lactam antibiotic detection methods
Electrochemical sensing strategies also play a pivotal role in penicillin detection [185,186]. For example, a carbazole-based porous organic polymer (POP) is synthesized via the Friedel−Crafts coupling reaction between 2,4,6-tri(9H-carbazol-9-yl)-1,3,5-triazine and 2,4,6-tris(bromomethyl)mesitylene. The synthesized POP can immobilize aptamers to create an electrochemical aptasensor that detects penicillin with exceptional sensitivity, high selectivity, excellent reproducibility, and remarkable stability. Furthermore, the POP-based electrochemical aptasensor can quantitatively detect ultratrace levels of penicillin (0.001 ng mL−1) in real-world samples [187].
In another typical work, researchers engineered a biosensor based on Ag(I)-MOF materials by assembling Ag(I) salts of tripyridin-4-ylamine with distinct anions (SiF62− versus CH3SO3−) (Fig. 7b) [188]. This biosensor exhibits exceptional selectivity for penicillin under interference from other antibiotics and boasts an impressive minimum detection limit of 0.849 pg mL−1, effectively monitoring penicillin content in milk samples.
3.5.3. Fluorescence-based β-lactam antibiotic detection methods
The current phase of innovative amoxicillin detection strategies predominantly emphasizes [26,[189], [190], [191]] using electrochemical and fluorescence colorimetric methods. A notable example of amoxicillin detection via an electrochemical approach has previously been highlighted [87,104]. In addition to the aforementioned electrochemical detection method, in a different study, fluorescent apparatus employing specially synthesized green safety materials, i.e., red carbon dots (RCDs) and blue carbon dots (BCDs), was engineered (Supplementary Material Fig. S4). This nanoprobe was designed for amoxicillin detection [192]. Fundamentally, the premise of this research lies in the occurrence of a reaction that induces hydrogen bonding in the presence of amoxicillin. This reaction notably amplifies the blue fluorescence intensity of the BCDs, resulting in a significant color change from red to blue. Furthermore, for practical applications, the tailored fluorescent handheld probe, when integrated with smart devices, enables the direct and quantitative detection of amoxicillin in real samples. This device boasts a fast detection time and a notably low detection limit (2.39 nM). The efficacy of this approach suggests promise for application in the precise trace detection of amoxicillin.
3.5.4. Scope and limitations of detection methods
Currently, most β-lactam antibiotic detection methods are confined to laboratory settings, and their practical deployment is limited by the complex preparation required for sensing materials. Additionally, the sensors show limited adaptability to various environmental factors, such as pH, temperature, and humidity. Therefore, a key challenge moving forward is to enable the application of β-lactam antibiotic sensors in real-world environments. For example, fluorescence combined with smart devices, such as smartphones, as discussed in Section 3.5.3, represents an effective onsite wastewater detection strategy. However, issues related to the stability and rapid analysis of optical metrology and software remain, requiring advanced software support and adding to operational challenges.
4. Challenges and potential solutions
Table 3 provides a comprehensive overview of the detection strategies utilized for various prominent antibiotics and their corresponding detection performance metrics. These methods serve as valuable reference points in the dynamic landscape of antibiotic testing. In the future, efforts should focus on streamlining sample processing, improving the portability of analytical equipment, reducing usage costs, and ensuring the clarity and intuitiveness of test results. These coordinated efforts will significantly contribute to advancing the effectiveness and accessibility of antibiotic testing methodologies.
Table 3.
Detection performance of various representative antibiotic detection strategies.
| Antibiotic | Detection strategy | Examination range | Detection limit | Reference |
|---|---|---|---|---|
| Tetracycline | Fluorescence | 0–160 μM | 1.73 nM | [113] |
| Ofloxacin | Colorimetric | 0–90 nM | 4.78 × 10−1 nM | [117] |
| Ciprofloxacin | SERS | 0–104 nM | 1 nM | [119] |
| Norfloxacin | Electrochemistry | 0–30 μM | 9.792 × 10−3 nM | [126] |
| Azithromycin | Fluorescence | 0.1–105 pg mL−1 | 46 nM | [159] |
| Erythromycin | Electrochemistry | 0–60 nM | 18 fg mL−1 | [160] |
| Penicillin | Fluorescence | 0.1–106 nM | 0.0127 nM | [167] |
| Amoxicillin | SERS | 0.04–30 μM | 0.1 nM | [178] |
| Fluorescence | 0.01–106 nM | 16 nM | [166] | |
| SERS | 13.33‒6 × 104 μM | 0.01 nM | [178] | |
| Electrochemistry | 0–100 μM | 0.85 nM | [131] | |
| Fluorescence | 10−5‒10−11 M | 17.8 nM | [135] | |
| SERS | 1.43–429.12 μM | 10−3 nM | [184] | |
| Electrochemistry | 0–6 μM | 0.849 pg mL−1 | [188] | |
| Fluorescence | 0–100 nM | 825 nM | [26] | |
| Colorimetric | 2.39 nM | [192] | ||
| Electrochemistry | 15 nM | [104] |
5. Summary and future prospects
In recent decades, as the quality of industrial and agricultural production has improved, there has been a growing emphasis on material needs for a better quality of life and for protecting the environment, which are crucial for survival. This emphasis has led to a significant increase in demand for the detection of residual antibiotics in the environment since they are emerging contaminants in various types of wastewater. Many detection and analysis strategies have been reported to effectively detect concentrations of emerging antibiotic contaminants in the natural environment. Generally, gas chromatography‒mass spectrometry, a traditional method for antibiotic detection, possesses characteristics such as high precision, sensitivity, and good selectivity. However, this method has several disadvantages, including expensive instrumentation, lengthy pretreatment times, and the need for specialized operators, which greatly limits its practical application. Consequently, numerous emerging detection methods have been introduced, such as microbiological analysis, optical methods, and electrochemical sensing. This paper explored the primary sources and types of residual antibiotics in wastewater from livestock and aquaculture, briefly reviewed various detection methods and their principles, outlined several emerging approaches for detecting typical antibiotics, and summarized the detection performance of each method, highlighting their excellent detection limits.
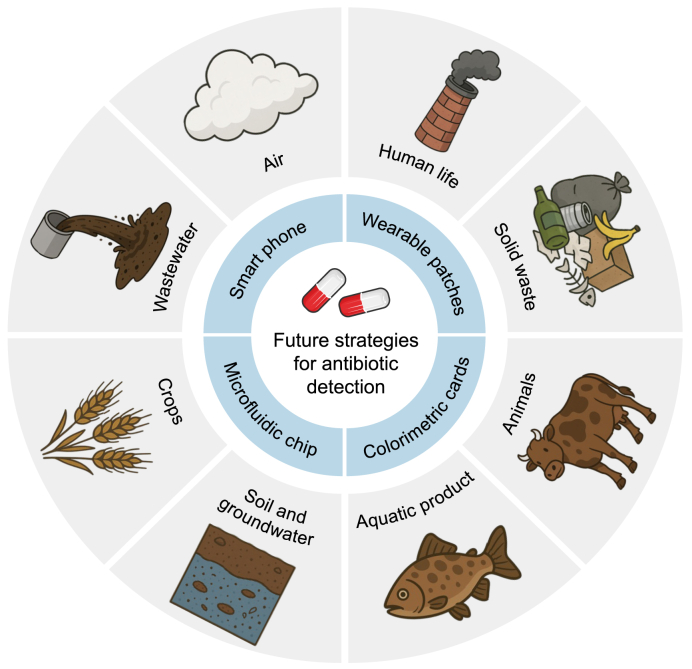
To meet the market demand for efficient and cost-effective strategies for detecting antibiotics in complex water environments, detection methods and sensor structures should be tailored to specific needs and detection conditions. Despite numerous reported methods for detecting antibiotics in complex environments, these strategies still face limitations and shortcomings in widespread application. Significant progress has been made in microbial methods for antibiotic detection. Microbial sensing strategies offer high specificity for detecting trace levels of antibiotics. However, practical applications pose challenges adapting to sensing platforms and selecting appropriate biomolecules. Ensuring compatibility between materials and biomolecules and establishing stable and effective connections between biomolecules and the target substance are crucial considerations. These issues are key challenges for the future of microbial detection strategies. Compared to traditional analytical approaches, optical antibiotic detection methods (e.g., fluorescence, colorimetric, and SERS) offer speed, intuitiveness, and relatively low detection limits. Moreover, when integrated with intelligent platforms, these methods demonstrate the advantage of rapid onsite monitoring. However, the preparation of fluorescent probes, SERS substrates, and various new materials, as well as the potential introduction of new pollution sources into aquatic environments through these probes, poses practical challenges that must be addressed. Electrochemical sensing methods offer ultralow detection limits and high sensitivity, making them promising for trace detection applications. However, these methods encounter similar challenges regarding probe preparation, the outdoor stability of electrochemical workstations, and portability. In the future (Fig. 8), integrating sensors with the Internet of Things (IoT) or artificial intelligence technologies will enable remote data acquisition, online analysis, and cloud database upload across a broader range of fields. This integration can facilitate sampling and testing at remote locations while efficiently organizing data. For example, combining electrochemical and optical sensors with IoT technology can continuously monitor multiple points in aquaculture wastewater, providing rapid responses to discharging pollutants such as antibiotics. Additionally, the miniaturization and portability of devices, such as portable Raman sensing units, visualized fluorescent test strips, and smartphone image recognition systems, can support rapid onsite detection, ideal for daily inspections with lower precision requirements. These devices, in turn, leverage the specificity of advanced environmentally sensitive materials, offering accurate compositional analyses under various water conditions.
Fig. 8.
Potential strategies for detecting antibiotics in various complex environments.
In summary, detecting antibiotics in complex environments via emerging methods involves innovations in material preparation, platform integration, and practical device applications. To address the challenges various detection strategies face, future efforts should prioritize developing stable, highly accurate, portable, cost-effective, and environmentally friendly sensors, thus enabling in situ detection in complex aquatic environments. In the future, portable in situ detection utilizing smartphones and wearable chips is expected to have a wide range of applications. With advancements in device fabrication technology and updates in integration strategies, we believe that antibiotic detection methods based on new materials and platforms will revolutionize achievements in biology, the environment, health care, and energy.
CRediT authorship contribution statement
Xinyu Chang: Writing – review & editing, Writing – original draft, Visualization, Validation, Software, Resources, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Junchi Cui: Methodology, Formal analysis, Conceptualization. Guihua Wang: Investigation, Conceptualization, Resources. Shujuan Meng: Software, Resources, Formal analysis, Methodology. Lingling Chen: Supervision, Conceptualization, Funding acquisition, Project administration, Writing – review & editing. Meng Zhang: Project administration, Funding acquisition, Conceptualization, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
National Natural Science Foundation of China (52122008, U24A20188, 52370003). Guangdong Basic and Applied Basic Research Foundation (2023B1515020035).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ese.2025.100572.
Contributor Information
Lingling Chen, Email: chenlingling@sztu.edu.cn.
Meng Zhang, Email: mengzhang10@buaa.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Martinez J.L. Antibiotics and antibiotic resistance genes in natural environments. Science. 2008;321:365–367. doi: 10.1126/science.1159483. [DOI] [PubMed] [Google Scholar]
- 2.Jorgensen P.S., Aktipis A., Brown Z., Carroll S.P., Downes S., Dunn R.R., et al. Carroll, Antibiotic and pesticide susceptibility and the Anthropocene operating space. Nat. Sustain. 2018;1:632–641. [Google Scholar]
- 3.Fisher M.C., Hawkins N.J., Sanglard D., Gurr S.J. Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science. 2018;360:739–742. doi: 10.1126/science.aap7999. [DOI] [PubMed] [Google Scholar]
- 4.Parnanen K.M.M., Narciso-da-Rocha C., Kneis D., Berendonk T.U., Cacace D., Do T.T., et al. Antibiotic resistance in European wastewater treatment plants mirrors the pattern of clinical antibiotic resistance prevalence. Sci. Adv. 2019;5 doi: 10.1126/sciadv.aau9124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peng Z., Hu Z.Z., Li Z.G., Zhang X.X., Jia C.Y., Li T.Z., et al. Antimicrobial resistance and population genomics of multidrug-resistant Escherichia coli in pig farms in mainland China. Nat. Commun. 2022;13 doi: 10.1038/s41467-022-28750-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Browne A.J., Chipeta M.G., Haines-Woodhouse G., Kumaran E.P.A., Hamadani B.H.K., Zaraa S., et al. Global antibiotic consumption and usage in humans, 2000-18: a spatial modelling study. Lancet Planet. Heath. 2021;5:E893–E904. doi: 10.1016/S2542-5196(21)00280-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouki C., Venieri D., Diamadopoulos E. Detection and fate of antibiotic resistant bacteria in wastewater treatment plants: a review. Ecotoxicol. Environ. Saf. 2013;91:1–9. doi: 10.1016/j.ecoenv.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 8.Chen H.Y., Jing L.J., Teng Y.G., Wang J.S. Multimedia fate modeling and risk assessment of antibiotics in a water-scarce megacity. J. Hazard. Mater. 2018;348:75–83. doi: 10.1016/j.jhazmat.2018.01.033. [DOI] [PubMed] [Google Scholar]
- 9.Anjali R., Shanthakumar S. Insights on the current status of occurrence and removal of antibiotics in wastewater by advanced oxidation processes. J. Environ. Manag. 2019;246:51–62. doi: 10.1016/j.jenvman.2019.05.090. [DOI] [PubMed] [Google Scholar]
- 10.Wang J.L., Chu L.B., Wojnarovits L., Takacs E. Occurrence and fate of antibiotics, antibiotic resistant genes (ARGs) and antibiotic resistant bacteria (ARB) in municipal wastewater treatment plant: an overview. Sci. Total Environ. 2020;744 doi: 10.1016/j.scitotenv.2020.140997. [DOI] [PubMed] [Google Scholar]
- 11.Wang J.L., Zhuan R. Degradation of antibiotics by advanced oxidation processes: an overview. Sci. Total Environ. 2020;701 doi: 10.1016/j.scitotenv.2019.135023. [DOI] [PubMed] [Google Scholar]
- 12.Wang J.L., Wang S.Z. Removal of pharmaceuticals and personal care products (PPCPs) from wastewater: a review. J. Environ. Manag. 2016;182:620–640. doi: 10.1016/j.jenvman.2016.07.049. [DOI] [PubMed] [Google Scholar]
- 13.Lan L.Y., Yao Y., Ping J.F., Ying Y.B. Recent advances in nanomaterial-based biosensors for antibiotics detection. Biosens. Bioelectron. 2017;91:504–514. doi: 10.1016/j.bios.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Zhang M., Shen J.L., Zhong Y.C., Ding T., Dissanayake P.D., Yang Y., et al. Sorption of pharmaceuticals and personal care products (PPCPs) from water and wastewater by carbonaceous materials: a review. Environ. Sci. Ecotechnol. 2022;52:727–766. [Google Scholar]
- 15.Ternes T., Bonerz M., Schmidt T. Determination of neutral pharmaceuticals in wastewater and rivers by liquid chromatography-electrospray tandem mass spectrometry. J. Chromatogr. A. 2001;938:175–185. doi: 10.1016/s0021-9673(01)01205-5. [DOI] [PubMed] [Google Scholar]
- 16.Noedler K., Licha T., Bester K., Sauter M. Development of a multi-residue analytical method, based on liquid chromatography-tandem mass spectrometry, for the simultaneous determination of 46 micro-contaminants in aqueous samples. J. Chromatogr. A. 2010;1217:6511–6521. doi: 10.1016/j.chroma.2010.08.048. [DOI] [PubMed] [Google Scholar]
- 17.Conley J.M., Symes S.J., Kindelberger S.A., Richards S.A. Rapid liquid chromatography-tandem mass spectrometry method for the determination of a broad mixture of pharmaceuticals in surface water. J. Chromatogr. A. 2008;185:206–215. doi: 10.1016/j.chroma.2008.01.064. [DOI] [PubMed] [Google Scholar]
- 18.Li B., Zhang T., Xu Z.Y., Fang H.H.P. Rapid analysis of 21 antibiotics of multiple classes in municipal wastewater using ultra performance liquid chromatography-tandem mass spectrometry. Anal. Chim. Acta. 2009;645:64–72. doi: 10.1016/j.aca.2009.04.042. [DOI] [PubMed] [Google Scholar]
- 19.Loos R., Carvalho R., Antonio D.C., Cornero S., Locoro G., Tavazzi S., et al. EU-wide monitoring survey on emerging polar organic contaminants in wastewater treatment plant effluents. Water Res. 2013;47:6475–6487. doi: 10.1016/j.watres.2013.08.024. [DOI] [PubMed] [Google Scholar]
- 20.Liu P.X., Zhang H.M., Feng Y.J., Yang F.L., Zhang J.P. Removal of trace antibiotics from wastewater: a systematic study of nanofiltration combined with ozone-based advanced oxidation processes. Chem. Eng. J. 2014;240:211–220. [Google Scholar]
- 21.Sousa J.C.G., Ribeiro A.R., Barbosa M.O., Pereira M.F.R., Silva A.M.T. A review on environmental monitoring of water organic pollutants identified by EU guidelines. J. Hazard. Mater. 2018;344:146–162. doi: 10.1016/j.jhazmat.2017.09.058. [DOI] [PubMed] [Google Scholar]
- 22.Liu C.B., Li B.L., Liu M., Mao S. Demand, status, and prospect of antibiotics detection in the environment. Sensor. Actuat. B-Chem. 2022;369 [Google Scholar]
- 23.Zhou Y.Y., Wang J.L. Detection and removal technologies for ammonium and antibiotics in agricultural wastewater: recent advances and prospective. Chemosphere. 2023;334 doi: 10.1016/j.chemosphere.2023.139027. [DOI] [PubMed] [Google Scholar]
- 24.Tong C.L., Zhuo X.J., Guo Y. Occurrence and risk assessment of four typical fluoroquinolone antibiotics in raw and treated sewage and in receiving waters in Hangzhou, China. J. Agric. Food Chem. 2011;59:7303–7309. doi: 10.1021/jf2013937. [DOI] [PubMed] [Google Scholar]
- 25.Castro G., Rodriguez I., Ramil M., Cela R. Selective determination of sartan drugs in environmental water samples by mixed-mode solid-phase extraction and liquid chromatography tandem mass spectrometry. Chemosphere. 2019;224:562–571. doi: 10.1016/j.chemosphere.2019.02.137. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X., Ren Y.K., Ji Z., Fan J. Sensitive detection of amoxicillin in aqueous solution with novel fluorescent probes containing boron-doped carbon quantum dots. J. Mol. Liq. 2020;311 [Google Scholar]
- 27.Xiao Y., Chang H., Jia A., Hu J.Y. Trace analysis of quinolone and fluoroquinolone antibiotics from wastewaters by liquid chromatography-electrospray tandem mass spectrometry. J. Chromatogr. A. 2008;1214:100–108. doi: 10.1016/j.chroma.2008.10.090. [DOI] [PubMed] [Google Scholar]
- 28.Song J.L., Huang M.H., Jiang N., Zheng S.Y., Mu T.W., Meng L.J., et al. Ultrasensitive detection of amoxicillin by TiO2-g-C3N4@AuNPs impedimetric aptasensor: fabrication, optimization, and mechanism. J. Hazard. Mater. 2020;391 doi: 10.1016/j.jhazmat.2020.122024. [DOI] [PubMed] [Google Scholar]
- 29.Hou H., Bai X.J., Xing C.Y., Gu N.Y., Zhang B.L., Tang J.L. Aptamer-based cantilever array sensors for oxytetracycline detection. Anal. Chem. 2013;85:2010–2014. doi: 10.1021/ac3037574. [DOI] [PubMed] [Google Scholar]
- 30.Gajda A., Jablonski A., Bladek T., Posyniak A. Oral fluid as a biological material for antemortem detection of oxytetracycline in pigs by liquid chromatography-tandem mass spectrometry. J. Agric. Food Chem. 2017;65:494–500. doi: 10.1021/acs.jafc.6b05205. [DOI] [PubMed] [Google Scholar]
- 31.Lyu J., Yang L.S., Zhang L., Ye B.X., Wang L. Antibiotics in soil and water in China-a systematic review and source analysis. Environ. Pollut. 2020;266 doi: 10.1016/j.envpol.2020.115147. [DOI] [PubMed] [Google Scholar]
- 32.Sellier A., Khaska S., Salle C.L.L. Assessment of the occurrence of 455 pharmaceutical compounds in sludge according to their physical and chemical properties: a review. J. Hazard. Mater. 2022;426 doi: 10.1016/j.jhazmat.2021.128104. [DOI] [PubMed] [Google Scholar]
- 33.Gadipelly C., Perez-Gonzalez A., Yadav G.D., Ortiz I., Ibanez R., Rathod V.K., Marathe K.V. Pharmaceutical industry wastewater: review of the technologies for water treatment and reuse. Ind. Eng. Chem. Res. 2014;53:11571–11592. [Google Scholar]
- 34.Li F.F., Chen L.J., Chen W.D., Bao Y.Y., Zheng Y.H., Huang B., et al. Antibiotics in coastal water and sediments of the East China Sea: distribution, ecological risk assessment and indicators screening. Mar. Pollut. Bull. 2020;151 doi: 10.1016/j.marpolbul.2019.110810. [DOI] [PubMed] [Google Scholar]
- 35.Oharisi O.O.L., Ncube S., Nyoni H., Madikizela M.L., Olowoyo O.J., Maseko B.R. Occurrence and prevalence of antibiotics in wastewater treatment plants and effluent receiving rivers in South Africa using UHPLC-MS determination. J. Environ. Manag. 2023;345 doi: 10.1016/j.jenvman.2023.118621. [DOI] [PubMed] [Google Scholar]
- 36.Ahmad N.N.R., Ang W.L., Teow Y.H., Mohammad A.W., Hilal N. Nanofiltration membrane processes for water recycling, reuse and product recovery within various industries: a review. J. Water Process Eng. 2020;45 [Google Scholar]
- 37.Kuemmerer K. Antibiotics in the aquatic environment-A review-Part I. Chemosphere. 2009;75:417–434. doi: 10.1016/j.chemosphere.2008.11.086. [DOI] [PubMed] [Google Scholar]
- 38.Singer A.C., Shaw H., Rhodes V., Hart A. Review of antimicrobial resistance in the environment and its relevance to environmental regulators. Front. Microbiol. 2016;7:1728. doi: 10.3389/fmicb.2016.01728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bao Y.Y., Li F.F., Chen L.J., Mu Q.L., Huang B., Wen D.H. Fate of antibiotics in engineered wastewater systems and receiving water environment: a case study on the coast of Hangzhou Bay, China. Sci. Total Environ. 2021;769 doi: 10.1016/j.scitotenv.2020.144642. [DOI] [PubMed] [Google Scholar]
- 40.Luo Y.L., Guo W.S., Ngo H.H., Nghiem L.D., Hai F.I., Zhang J., et al. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014;473:619–641. doi: 10.1016/j.scitotenv.2013.12.065. [DOI] [PubMed] [Google Scholar]
- 41.Wang L., Qiang Z.M., Li Y.G., Ben W.W. An insight into the removal of fluoroquinolones in activated sludge process: sorption and biodegradation characteristics. J. Environ. Sci. 2017;56:263–271. doi: 10.1016/j.jes.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 42.Oberoi A.S., Jia Y.Y., Zhang H.Q., Khanal S.K., Lu H. Insights into the fate and removal of antibiotics in engineered biological treatment systems: a critical review. Environ. Sci. Technol. 2019;53:7234–7264. doi: 10.1021/acs.est.9b01131. [DOI] [PubMed] [Google Scholar]
- 43.Klein E.Y., Van Boeckel T.P., Martinez E.M., Pant S., Gandra S., Levin S.A., et al. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA. 2018;115:E3463–E3470. doi: 10.1073/pnas.1717295115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu C.M., Wang Y.C., Shi X.M., Wang S., Ren H.W., Shen Z.Q., et al. Rapid rise of the ESBL and mcr-1 genes in Escherichia coli of chicken origin in China. Emerg. Microb. Infect. 2018;7:1–10. doi: 10.1038/s41426-018-0033-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He X.T., Deng M.C., Wang Q., Yang Y.T., Yang Y.F., Nie X.P. Residues and health risk assessment of quinolones and sulfonamides in cultured fish from Pearl River Delta, China. Aquaculture. 2016;458:38–46. [Google Scholar]
- 46.Gezahegn T., Tegegne B., Zewge F., Chandravanshi B.S. Salting-out assisted liquid–liquid extraction for the determination of ciprofloxacin residues in water samples by high performance liquid chromatography–diode array detector. BMC Biochem. 2019;13:28. doi: 10.1186/s13065-019-0543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zou M.Y., Tian W.J., Zhao J., Chu M.L., Song T.T. Quinolone antibiotics in sewage treatment plants with activated sludge treatment processes: a review on source, concentration and removal. Process Saf. Environ. 2022;160:116–129. [Google Scholar]
- 48.Santos L.H.M.L.M., Gros M., Rodriguez-Mozaz S., Delerue-Matos C., Pena A., Barcelo D., Montenegro M.C.B.S.M. Contribution of hospital effluents to the load of pharmaceuticals in urban wastewaters: identification of ecologically relevant pharmaceuticals. Sci. Total Environ. 2013;461:302–316. doi: 10.1016/j.scitotenv.2013.04.077. [DOI] [PubMed] [Google Scholar]
- 49.Thomas K.V., Dye C., Schlabach M., Langford K.H. Source to sink tracking of selected human pharmaceuticals from two Oslo city hospitals and a wastewater treatment works. J. Environ. Monit. 2007;9:1410–1418. doi: 10.1039/b709745j. [DOI] [PubMed] [Google Scholar]
- 50.Verlicchi P., Galletti A., Petrovic M., BarcelÓ D. Hospital effluents as a source of emerging pollutants: an overview of micropollutants and sustainable treatment options. J. Hydrol. 2010;389:416–428. [Google Scholar]
- 51.Mutiyar P.K., Mittal A.K. Occurrences and fate of selected human antibiotics in influents and effluents of sewage treatment plant and effluent-receiving river Yamuna in Delhi (India) Environ. Monit. Assess. 2014;186:541–557. doi: 10.1007/s10661-013-3398-6. [DOI] [PubMed] [Google Scholar]
- 52.Varela A.R., Nunes O.C., Manaia C.M. Quinolone resistant Aeromonas spp. as carriers and potential tracers of acquired antibiotic resistance in hospital and municipal wastewater. Sci. Total Environ. 2016;542:665–671. doi: 10.1016/j.scitotenv.2015.10.124. [DOI] [PubMed] [Google Scholar]
- 53.Verlicchi P., Al Aukidy M., Zambello E. What have we learned from worldwide experiences on the management and treatment of hospital effluent?—an overview and a discussion on perspectives. Sci. Total Environ. 2015;514:467–491. doi: 10.1016/j.scitotenv.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cardoso O., Porcher J.-M., Sanchez W. Factory-discharged pharmaceuticals could be a relevant source of aquatic environment contamination: review of evidence and need for knowledge. Chemosphere. 2014;115:20–30. doi: 10.1016/j.chemosphere.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 55.Wang K., Zhuang T., Su Z.X., Chi M.H., Wang H.C. Antibiotic residues in wastewaters from sewage treatment plants and pharmaceutical industries: occurrence, removal and environmental impacts. Sci. Total Environ. 2021;788 doi: 10.1016/j.scitotenv.2021.147811. [DOI] [PubMed] [Google Scholar]
- 56.Bielen A., Simatovic A., Kosic-Vuksic J., Senta I., Ahel M., Babic S., et al. Negative environmental impacts of antibiotic-contaminated effluents from pharmaceutical industries. Water Res. 2017;126:79–87. doi: 10.1016/j.watres.2017.09.019. [DOI] [PubMed] [Google Scholar]
- 57.Thai P.K., Ky L.X., Binh V.N., Nhung P.H., Nhan P.T., et al. Occurrence of antibiotic residues and antibiotic-resistant bacteria in effluents of pharmaceutical manufacturers and other sources around Hanoi. Vietnam. Sci. Total Environ. 2018;645:393–400. doi: 10.1016/j.scitotenv.2018.07.126. [DOI] [PubMed] [Google Scholar]
- 58.Pan C., Bao Y., Xu B., Xu B.T. Seasonal variation of antibiotics in surface water of Pudong new area of Shanghai, China and the occurrence in typical wastewater sources. Chemosphere. 2020;239 doi: 10.1016/j.chemosphere.2019.124816. [DOI] [PubMed] [Google Scholar]
- 59.Zhang Q.Q., Ying G.G., Pan C.G., Liu Y.S., Zhao J.L. Comprehensive evaluation of antibiotics emission and fate in the river basins of China: source analysis, multimedia modeling, and linkage to bacterial resistance. Environ. Sci. Technol. 2015;49:6772–6782. doi: 10.1021/acs.est.5b00729. [DOI] [PubMed] [Google Scholar]
- 60.Zhang R.M., Liu X., Wang S.L., Fang L.X., Sun J., Liu Y.H., Liao X.P. Distribution patterns of antibiotic resistance genes and their bacterial hosts in pig farm wastewater treatment systems and soil fertilized with pig manure. Sci. Total Environ. 2021;758 doi: 10.1016/j.scitotenv.2020.143654. [DOI] [PubMed] [Google Scholar]
- 61.Robles-Jimenez L.E., Aranda-Aguirre E., Castelan-Ortega O.A., Shettino-Bermudez B.S., Ortiz-Salinas R., Miranda M., et al. Worldwide traceability of antibiotic residues from livestock in wastewater and soil: a systematic review. Animals. 2022;12:60. doi: 10.3390/ani12010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Proceedings of the 75th General Session . OIE list of antimicrobials of veterinary importance; 2007. World Organization for Animal Health (OIE) [Google Scholar]
- 63.World Health Organization . Licence: CC BY-NC-SA 3.0 IGO; World Health Organization; Geneva, Switzerland: 2017. WHO Guidelines on Use of Medically Important Antimicrobials in Food-Producing Animals. [Google Scholar]
- 64.Dyar O.J., Yin J., Ding L.L., Wikander K., Zhang T.Y., Sun C.T., et al. Antibiotic use in people and pigs: a One Health survey of rural residents' knowledge, attitudes and practices in Shandong province, China. J. Antimicrob. Chemother. 2018;73:2893–2899. doi: 10.1093/jac/dky240. [DOI] [PubMed] [Google Scholar]
- 65.Sarmah A.K., Meyer M.T., Boxall A.B.A. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere. 2006;65:725–759. doi: 10.1016/j.chemosphere.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 66.Jia S.Y., Zhang X.X., Miao Y., Zhao Y.T., Ye L., Li B., Zhang T. Fate of antibiotic resistance genes and their associations with bacterial community in livestock breeding wastewater and its receiving river water. Water Res. 2017;124:259–268. doi: 10.1016/j.watres.2017.07.061. [DOI] [PubMed] [Google Scholar]
- 67.Xu Z.A., Li T., Bi J., Wang C. Spatiotemporal heterogeneity of antibiotic pollution and ecological risk assessment in Taihu Lake Basin, China. Sci. Total Environ. 2018;643:14–20. doi: 10.1016/j.scitotenv.2018.06.175. [DOI] [PubMed] [Google Scholar]
- 68.Segura P.A., Takada H., Correa J.A., El Saadi K., Koike T., Onwona-Agyeman S., et al. Global occurrence of anti-infectives in contaminated surface waters: impact of income inequality between countries. Environ. Int. 2015;80:89–97. doi: 10.1016/j.envint.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 69.Madikizela L.M., Tavengwa N.T., Chimuka L. Status of pharmaceuticals in African water bodies: occurrence, removal and analytical methods. J. Environ. Manag. 2017;193:211–220. doi: 10.1016/j.jenvman.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 70.Managaki S., Murata A., Takada H., Tuyen B.C., Chiem N.H. Distribution of macrolides, sulfonamides, and trimethoprim in tropical waters: ubiquitous occurrence of veterinary antibiotics in the Mekong Delta. Environ. Sci. Technol. 2007;41:8004–8010. doi: 10.1021/es0709021. [DOI] [PubMed] [Google Scholar]
- 71.Ding H.J., Wu Y.X., Zhang W.H., Zhong J.Y., Lou Q., Yang P., Fang Y.Y. Occurrence, distribution, and risk assessment of antibiotics in the surface water of Poyang Lake, the largest freshwater lake in China. Chemosphere. 2017;184:137–147. doi: 10.1016/j.chemosphere.2017.05.148. [DOI] [PubMed] [Google Scholar]
- 72.Chen K., Zhou J.L. Occurrence and behavior of antibiotics in water and sediments from the Huangpu River, Shanghai, China. Chemosphere. 2014;95:604–612. doi: 10.1016/j.chemosphere.2013.09.119. [DOI] [PubMed] [Google Scholar]
- 73.Pan M., Wong C.K.C., Chu L.M. Distribution of antibiotics in wastewater-irrigated soils and their accumulation in vegetable crops in the pearl river delta, Southern China. J. Agric. Food Chem. 2014;62:11062–11069. doi: 10.1021/jf503850v. [DOI] [PubMed] [Google Scholar]
- 74.Wang J., Yu Y., Jiang J.Y., Li B.L., Xie W.M., Li G.Z. Study on the distribution characteristics and risk assessment of antibiotics and resistance genes in water sources of Wuhan. Toxics. 2024;12:507. doi: 10.3390/toxics12070507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Han Q.F., Zhao S., Zhang X.R., Wang X.L., Song C., Wang S.G. Distribution, combined pollution and risk assessment of antibiotics in typical marine aquaculture farms surrounding the Yellow Sea, North China. Environ. Int. 2020;138 doi: 10.1016/j.envint.2020.105551. [DOI] [PubMed] [Google Scholar]
- 76.Yang Y.W., Liv Z.X., Xing S.C., Liao X.D. The correlation between antibiotic resistance gene abundance and microbial community resistance in pig farm wastewater and surrounding rivers. Ecotoxicol. Environ. Saf. 2019;182 doi: 10.1016/j.ecoenv.2019.109452. [DOI] [PubMed] [Google Scholar]
- 77.Gros M., Rodriguez-Mozaz S., Barcelo D. Rapid analysis of multiclass antibiotic residues and some of their metabolites in hospital, urban wastewater and river water by ultra-high-performance liquid chromatography coupled to quadrupole-linear ion trap tandem mass spectrometry. J. Chromatogr. A. 2013;1292:173–188. doi: 10.1016/j.chroma.2012.12.072. [DOI] [PubMed] [Google Scholar]
- 78.Moudgil P., Bedi J.S., Aulakh R.S., Gill J.P.S., Kumar A. Validation of HPLC multi-residue method for determination of fluoroquinolones, tetracycline, Sulphonamides and chloramphenicol residues in bovine milk. Food Anal. Methods. 2019;12:338–346. [Google Scholar]
- 79.Vuran B., Ulusoy H.I., Sarp G., Yilmaz E., Morgul U., Kabir A., et al. Determination of chloramphenicol and tetracycline residues in milk samples by means of nanofiber coated magnetic particles prior to high-performance liquid chromatography-diode array detection. Talanta. 2021;230 doi: 10.1016/j.talanta.2021.122307. [DOI] [PubMed] [Google Scholar]
- 80.Moema D., Makwakwa T.A., Gebreyohannes B.E., Dube S., Nindi M.M. Hollow fiber liquid phase microextraction of fluoroquinolones in chicken livers followed by high pressure liquid chromatography: greenness assessment using National Environmental Methods Index Label (NEMI), green analytical procedure index (GAPI), Analytical GREEnness metric (AGREE), and Eco Scale. J. Food Compos. Anal. 2023;117 [Google Scholar]
- 81.Bohm D.A., Stachel C.S., Gowik P. Validation of a multi-residue method for the determination of several antibiotic groups in honey by LC-MS/MS. Anal. Bioanal. Chem. 2012;403:2943–2953. doi: 10.1007/s00216-012-5868-z. [DOI] [PubMed] [Google Scholar]
- 82.Sierra D., Contreras A., Sanchez A., Luengo C., Corrales J.C., Morales C.T., et al. Detection limits of non-β-lactam antibiotics in goat's milk by microbiological residues screening tests. J. Dairy Sci. 2009;92:4200–4206. doi: 10.3168/jds.2009-2101. [DOI] [PubMed] [Google Scholar]
- 83.Wang X., Gong J.H., Yuan B.L., Chen Z.F., Jiang J.C. Sensitive and multiplexed detection of antibiotics using a suspension array platform based on silica-agarose hybrid microbeads. J. Hazard. Mater. 2019;373:115–121. doi: 10.1016/j.jhazmat.2019.03.081. [DOI] [PubMed] [Google Scholar]
- 84.Shrivastav A.M., Usha S.P., Gupta B.D. Highly sensitive and selective erythromycin nanosensor employing fiber optic SPR/ERY imprinted nanostructure: application in milk and honey. Biosens. Bioelectron. 2017;90:516–524. doi: 10.1016/j.bios.2016.10.041. [DOI] [PubMed] [Google Scholar]
- 85.Guo M., Wang R., Jin Z.C., Zhang X.Y., Jokerst J.V., Sun Y.T., Sun L.P. Hyperbranched molecularly imprinted photoactive polymers and its detection of tetracycline antibiotics. ACS Appl. Polym. Mater. 2022;4:1234–1242. [Google Scholar]
- 86.Yang Y.Y., Liu X.H., Meng S., Mao S., Tao W.Q., Li Z. Molecularly imprinted polymers-isolated AuNP-enhanced CdTe QD fluorescence sensor for selective and sensitive oxytetracycline detection in real water samples. J. Hazard. Mater. 2023;458 doi: 10.1016/j.jhazmat.2023.131941. [DOI] [PubMed] [Google Scholar]
- 87.Anh N.T., Dinh N.X., Tuan H.V., Doan M.Q., Anh N.H., Khi N.T., et al. Eco-friendly copper nanomaterials-based dual-mode optical nanosensors for ultrasensitive trace determination of amoxicillin antibiotics residue in tap water samples. Mater. Res. Bull. 2022;147 [Google Scholar]
- 88.Zhang Z., Huang L.L., Sheng S.C., Jiang C.Y., Wang Y.P. MIL-125(Ti)-derived COOH functionalized TiO2 grafted molecularly imprinted polymers for photoelectrochemical sensing of ofloxacin. Sensor. Actuat. B-Chem. 2021;343 [Google Scholar]
- 89.Tappe W.G., Zarfl C., Kummer S., Burauel P., Vereecken H., Groeneweg J. Growth-inhibitory effects of sulfonamides at different pH: dissimilar susceptibility patterns of a soil bacterium and a test bacterium used for antibiotic assays. Chemosphere. 2008;72:836–843. doi: 10.1016/j.chemosphere.2008.02.041. [DOI] [PubMed] [Google Scholar]
- 90.Ajibola A.S., Tisler S., Zwiener C. Simultaneous determination of multiclass antibiotics in sewage sludge based on QuEChERS extraction and liquid chromatography-tandem mass spectrometry. Anal. Methods. 2020;12:576–586. [Google Scholar]
- 91.Chung H.H., Lee J.B., Chung Y.H., Lee K.G. Analysis of sulfonamide and quinolone antibiotic residues in Korean milk using microbial assays and high performance liquid chromatography. Food Chem. 2009;113:297–301. [Google Scholar]
- 92.Eltzov E., Ben-Yosef D.Z., Kushmaro A., Marks R. Detection of sub-inhibitory antibiotic concentrations via luminescent sensing bacteria and prediction of their mode of action. Sensor. Actuat. B-Chem. 2008;129:685–692. [Google Scholar]
- 93.Adrian J., Pasche S., Diserens J.M., Sanchez-Baeza F., Gao H., Marco M.P., Voirin G. Waveguide interrogated optical immunosensor (WIOS) for detection of sulfonamide antibiotics in milk. Biosens. Bioelectron. 2009;24:3340–3346. doi: 10.1016/j.bios.2009.04.036. [DOI] [PubMed] [Google Scholar]
- 94.Adrian J., Pasche S., Pinacho D.G., Font H., Diserens J.M., Sanchez-Baeza F., et al. Wavelength-interrogated optical biosensor for multi-analyte screening of sulfonamide, fluoroquinolone, β-lactam and tetracycline antibiotics in milk. Trac-Trend. Anal. Chem. 2009;28:769–777. [Google Scholar]
- 95.Chang K.W., Cheng H.W., Shiue J., Wang J.K., Wang Y.L., Huang N.T. Antibiotic susceptibility test with surface-enhanced Raman scattering in a microfluidic system. Anal. Chem. 2019;91:10988–10995. doi: 10.1021/acs.analchem.9b01027. [DOI] [PubMed] [Google Scholar]
- 96.Huang H.K., Cheng H.W., Liao C.C., Lin S.J., Chen Y.Z., Wang J.K., Wang Y.L., Huang N.T. Bacteria encapsulation and rapid antibiotic susceptibility test using a microfluidic microwell device integrating surface-enhanced Raman scattering. Lab Chip. 2020;20:2520–2528. doi: 10.1039/d0lc00425a. [DOI] [PubMed] [Google Scholar]
- 97.Chinnappan R., Eissa S., Alotaibi A., Siddiqua A., Alsager O.A., Zourob M. In vitro selection of DNA aptamers and their integration in a competitive voltammetric biosensor for azlocillin determination in waste water. Anal. Chim. Acta. 2020;1101:149–156. doi: 10.1016/j.aca.2019.12.023. [DOI] [PubMed] [Google Scholar]
- 98.Chen K.H., Lee S.H., Kok L.C., Ishdorj T.O., Chang H.Y., Tseng F.G. A 3D-ACEK/SERS system for highly efficient and selectable electrokinetic bacteria concentration/detection/antibiotic-susceptibility-test on whole blood. Biosens. Bioelectron. 2022;197 doi: 10.1016/j.bios.2021.113740. [DOI] [PubMed] [Google Scholar]
- 99.Huang Y.H., Wei H., Santiago P.J., Thrift W.J., Ragan R., Jiang S.Y. Sensing antibiotics in wastewater using surface-enhanced Raman scattering. Environ. Sci. Technol. 2023;57:4880–4891. doi: 10.1021/acs.est.3c00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nehru R., Gnanakrishnan S., Chen C.W., Dong C.D. Nanoscale Mn3O4 electrocatalyst as improved electrode materials for electrochemical sensing of chloramphenicol drug. ACS Appl. Nano Mater. 2023;6:1235–1249. [Google Scholar]
- 101.Yang X.M., Zhu S.S., Dou Y., Zhuo Y., Luo Y.W., Feng Y.J. Novel and remarkable enhanced-fluorescence system based on gold nanoclusters for detection of tetracycline. Talanta. 2014;122:36–42. doi: 10.1016/j.talanta.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 102.Liu B.X., Zhu H.J., Liu J.J., Wang M.Z., Pan J.M., Feng R.L., et al. Alkali-etched imprinted Mn-based prussian blue Analogues with superior oxidase-mimetic activity and precise recognition for tetracycline colorimetric sensing. ACS Appl. Mater. Interfaces. 2023;15:24736–24746. doi: 10.1021/acsami.3c02207. [DOI] [PubMed] [Google Scholar]
- 103.Birader K., Kumar P., Tammineni Y., Barla J.A., Reddy S., Suman P. Colorimetric aptasensor for on-site detection of oxytetracycline antibiotic in milk. Food Chem. 2021;356 doi: 10.1016/j.foodchem.2021.129659. [DOI] [PubMed] [Google Scholar]
- 104.Chacko A.R., Narayanan R., Korah B.K., Mathew S., Abraham T., Mathew B. Solution-based carbon quantum dots as electrochemical "on-off" switch in aqueous medium: engineering of a sustainable green sensor and its computational investigation. Microchem. J. 2023;191 [Google Scholar]
- 105.Zhang H.F., Pan Q.H., Cai W.Y., Shi X.W., Yang D.P., Lin H.T., Qiu E.H. C-doped ZnO nanocomposites molecularly imprinted photoelectrochemical sensor for ultrasensitive and selective detection of oxytetracycline in milk. Food Chem. 2023;426 doi: 10.1016/j.foodchem.2023.136535. [DOI] [PubMed] [Google Scholar]
- 106.Gupta S.K., Shin H., Han D., Hur H.G., Unno T. Metagenomic analysis reveals the prevalence and persistence of antibiotic- and heavy metal-resistance genes in wastewater treatment plant. J. Microbiol. 2018;56:408–415. doi: 10.1007/s12275-018-8195-z. [DOI] [PubMed] [Google Scholar]
- 107.Han L., Fan Y.Z., Qing M., Liu S.G., Yang Y.Z., Li N.B., Luo H.Q. Smartphones and test paper-assisted ratiometric fluorescent sensors for semi-quantitative and visual assay of tetracycline based on the target-induced synergistic effect of antenna effect and inner filter effect. ACS Appl. Mater. Interfaces. 2020;12:47099–47107. doi: 10.1021/acsami.0c15482. [DOI] [PubMed] [Google Scholar]
- 108.Yu J.H., Liu H., Wang Y., Li J.H., Wu D.Z., Wang X.D. Fluorescent sensing system based on molecularly imprinted phase-change microcapsules and carbon quantum dots for high-efficient detection of tetracycline. J. Colloid Interface Sci. 2021;599:332–350. doi: 10.1016/j.jcis.2021.04.094. [DOI] [PubMed] [Google Scholar]
- 109.Zhuang Q., Zhang C., Zhuang H.Y., Deng H.Y., Lin X.P., Li Y., Chen H., Xie A.M., Dong W. Heteroatom-free conjugated tetraphenylethylene polymers for selectively fluorescent detection of tetracycline. Anal. Chim. Acta. 2022;1190 doi: 10.1016/j.aca.2021.339236. [DOI] [PubMed] [Google Scholar]
- 110.Sun P.Y., Yang D.Y., Li J., Zhang Y.D. Aggregation-induced emission of 4-formyl-3-hydroxybenzoic acid for the ratiometric fluorescence detection of tetracycline antibiotics. Dyes Pigments. 2022;197 [Google Scholar]
- 111.Kang S.R., Liu X.J., Wang Z.X., Wu Y., Dou M.Y., Yang H., et al. Functionalized 2D defect g-C3N4 for artificial photosynthesis of H2O2 and synchronizing tetracycline fluorescence detection and degradation. Environ. Res. 2023;232 doi: 10.1016/j.envres.2023.116345. [DOI] [PubMed] [Google Scholar]
- 112.Che H.C., Nie Y.L., Tian X.K., Li Y. New method for morphological identification and simultaneous quantification of multiple tetracyclines by a white fluorescent probe. J. Hazard. Mater. 2023;441 doi: 10.1016/j.jhazmat.2022.129956. [DOI] [PubMed] [Google Scholar]
- 113.Fu Q., Long C.C., Qin L.F., Jiang Z.X., Qing T.P., Zhang P., Feng B. Fluorescent and colorimetric dual-mode detection of tetracycline in wastewater based on heteroatoms-doped reduced state carbon dots. Environ. Pollut. 2021;283 doi: 10.1016/j.envpol.2021.117109. [DOI] [PubMed] [Google Scholar]
- 114.Jia P., Bu T., Sun X.Y., Liu Y.N., Liu J.H., Wang Q.Z., Shui Y.H., Guo S.W., Wang L. A sensitive and selective approach for detection of tetracyclines using fluorescent molybdenum disulfide nanoplates. Food Chem. 2019;297 doi: 10.1016/j.foodchem.2019.124969. [DOI] [PubMed] [Google Scholar]
- 115.Bras M., Zanoni J., Falcao B.P., Leitao J.P., Costa F.M., Monteiro T., et al. Label-free nanoscale ZnO tetrapod-based transducers for tetracycline detection. ACS Appl. Nano Mater. 2022;5:1232–1243. [Google Scholar]
- 116.Xin W., Li L.W., Hong J., Hui Z.S., Wang Q.Z., Sun X.Y., Li W. Highly selective and sensitive fluorescence detection of tetracyclines based on novel tungsten oxide quantum dots. Food Chem. 2022;374 doi: 10.1016/j.foodchem.2021.131774. [DOI] [PubMed] [Google Scholar]
- 117.Goswami K.J., Sen Sarma N. "Click" reaction-mediated silk fibroin-functionalized thiol-branched graphene oxide quantum dots for smart sensing of tetracycline. ACS Omega. 2023;8:21914–21928. doi: 10.1021/acsomega.3c01753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lin S.J., Chao P.H., Cheng H.W., Wang J.K., Wang Y.L., Han Y.Y., Huang N.T. An antibiotic concentration gradient microfluidic device integrating surface-enhanced Raman spectroscopy for multiplex antimicrobial susceptibility testing. Lab Chip. 2022;22:1805–1814. doi: 10.1039/d2lc00012a. [DOI] [PubMed] [Google Scholar]
- 119.Jin S., Park E., Guo S., Park Y., Park J., Yoo H.S., Park J.H., Chen L., Jung Y.M. Process monitoring of photocatalytic degradation of 2,4-dinitrotoluene by Au-decorated Fe3O4@TiO2 nanoparticles: surface-enhanced Raman scattering method. Spectrochim. Acta. 2022;275 doi: 10.1016/j.saa.2022.121155. [DOI] [PubMed] [Google Scholar]
- 120.Kumar E.A., Kokulnathan T., Wang T.J., Kumar K.M.A., Chang Y.H. Coaxially electrospun TiO2@Au core-shell nanofibers for ultrasensitive and reusable SERS detection of furazolidone. J. Environ. Chem. Eng. 2023;11 [Google Scholar]
- 121.Muhammad M., Yan B., Yao G.H., Chao K.L., Zhu C.H., Huang Q. Surface-enhanced Raman spectroscopy for trace detection of tetracycline and dicyandiamide in milk using transparent substrate of Ag nanoparticle arrays. ACS Appl. Nano Mater. 2020;3:7066–7075. [Google Scholar]
- 122.Lin J.Y., Qian J.C., Wang Y.P., Yang Y., Zhang Y.X., Chen J.P., Chen X.W., Chen Z.G. Quantum dots@porous carbon platform for the electrochemical sensing of oxytetracycline. Microchem. J. 2021;167 [Google Scholar]
- 123.Peng B., Zhang Z.L., Tang L., Ouyang X.L., Zhu X., Chen L., et al. Self-powered photoelectrochemical aptasensor for oxytetracycline cathodic detection based on a dual Z-scheme WO3/g-C3N4/MnO2 photoanode. Anal. Chem. 2021;93:9129–9138. doi: 10.1021/acs.analchem.1c00929. [DOI] [PubMed] [Google Scholar]
- 124.Dang X.M., Shi Z.J., Sun Z.J., Li Y., Hu X., Zhao H.M. Ultrasensitive sandwich-type photoelectrochemcial oxytetracycline sensing platform based on MnIn2S4/WO3 (Yb, Tm) functionalized rGO film. J. Electroanal. Chem. 2022;915 [Google Scholar]
- 125.Ling L.Y., Yuan C., Xu Q.Y., Li T.H., Zhu M.S., Zhai C.Y. Directional charge separation on 2D/2D BiVO4/MXene for the enhanced photoelectrochemical detection of oxytetracycline antibiotic in water. Surf. Interfaces. 2023;36 [Google Scholar]
- 126.Liang N.N., Hu X.T., Zhang X.A., Li W.T., Guo Z., Huang X.W., et al. Ratiometric sensing for ultratrace tetracycline using electrochemically active metal-organic frameworks as response signals. J. Agric. Food Chem. 2023;71:7584–7592. doi: 10.1021/acs.jafc.3c00846. [DOI] [PubMed] [Google Scholar]
- 127.Guo X.Q., Liu Y., Dong W.J., Hu Q., Li Y., Shuang S.M., et al. Azithromycin detection in cells and tablets by N,S co-doped carbon quantum dots. Spectrochim. Acta. 2021;252 doi: 10.1016/j.saa.2021.119506. [DOI] [PubMed] [Google Scholar]
- 128.Cho K.Y., Jung C.H., Oh W.C. Novel synthesis of Ca2+-doped MgAl2O3-G-SiO2 mesoporous nanospheres toward sensing effects for selective electrochemical performance of azithromycin. Biotechnol. Bioproc. Eng. 2022;27:831–842. [Google Scholar]
- 129.Mahnashi H.M., Mahmoud A.M., Alkahtani A.S., El-Wekil M.M. Simultaneous electrochemical detection of azithromycin and hydroxychloroquine based on VS2 QDs embedded N, S @graphene aerogel/cCNTs 3D nanostructure. Microchem. J. 2021;163 doi: 10.1016/j.microc.2021.105925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sopaj F., Loshaj F., Contini A., Mehmeti E., Veseli A. Preparation of an efficient and selective voltammetric sensor based on screen printed carbon ink electrode modified with TiO2 nanoparticles for Azithromycin quantification. Results Chem. 2023;6 [Google Scholar]
- 131.Stoian I.A., Iacob B.C., Dudas C.L., Barbu-Tudoran L., Bogdan D., Marian I.O., et al. Biomimetic electrochemical sensor for the highly selective detection of azithromycin in biological samples. Biosens. Bioelectron. 2020;155 doi: 10.1016/j.bios.2020.112098. [DOI] [PubMed] [Google Scholar]
- 132.Li T.H., Li X.F., Liu H., Deng Z.W., Zhang Y.S., Zhang Z.M., et al. Preparation and characterization of molecularly imprinted polymers based on β-cyclodextrin-stabilized Pickering emulsion polymerization for selective recognition of erythromycin from river water and milk. J. Separ. Sci. 2020;43:3683–3690. doi: 10.1002/jssc.201901255. [DOI] [PubMed] [Google Scholar]
- 133.Du Y.P., Liu D., Wang M., Guo F.K., Lin J.S. Preparation of DNA aptamer and development of lateral flow aptasensor combining recombinase polymerase amplification for detection of erythromycin. Biosens. Bioelectron. 2021;181 doi: 10.1016/j.bios.2021.113157. [DOI] [PubMed] [Google Scholar]
- 134.Jain P., Jagtap S., Chauhan M., Motghare R.V. Electrocatalytic behaviour of self-assembled Cu-chitosan/f-MWCNT on glassy carbon electrode for detection of erythromycin in various samples. Sens. Biosens. Res. 2023;41 [Google Scholar]
- 135.Li N.N., Xu X.Y., Qiu P., Li Y.X., Yu X.S., Gao Y.E., et al. A novel AIE material for sensing of Erythromycin in pure water by hydrogen bond in portable test strips and cellular imaging applications. Spectrochim. Acta. 2023;303 doi: 10.1016/j.saa.2023.123272. [DOI] [PubMed] [Google Scholar]
- 136.Sgobbi L.F., Razzino Claudia A., Machado Sergio A.S. A disposable electrochemical sensor for simultaneous detection of sulfamethoxazole and trimethoprim antibiotics in urine based on multiwalled nanotubes decorated with Prussian blue nanocubes modified screen-printed electrode. Electrochim. Acta. 2016;191:1010–1017. [Google Scholar]
- 137.Wang Z.X., Xing K.Y., Ding N.S., Wang S.H., Zhang G.G., Lai W.H. Lateral flow immunoassay based on dual spectral-overlapped fluorescence quenching of polydopamine nanospheres for sensitive detection of sulfamethazine. J. Hazard. Mater. 2022;423 doi: 10.1016/j.jhazmat.2021.127204. [DOI] [PubMed] [Google Scholar]
- 138.Nehru R., Chen C.W., Dong C.D. Sonochemically synthesized rod-like bismuth phosphate and carbon black hybrid electrocatalyst for electrochemical monitoring of hazardous sulfamethazine. J. Environ. Chem. Eng. 2023;11 [Google Scholar]
- 139.Lalmalsawmi J., Tiwari D., Lee S.M., Kim D.J., Kim Hyunook. Efficient electrochemical sensor for trace detection of sulfamethazine in spring water: use of novel nanocomposite material coated with Ag or Au nanoparticles. Microchem. J. 2022;179 [Google Scholar]
- 140.Senthil Kumar P., Padmalaya G., Elavarasan N., Sreeja B.S. A selective analysis of sulfamethoxazole–Trimethoprim in tablet formulations using graphene oxide-zinc oxide quantum dots based nanocomposite modified glassy carbon electrode. Chemosphere. 2023;332 doi: 10.1016/j.chemosphere.2023.138814. [DOI] [PubMed] [Google Scholar]
- 141.Jabbar H.S. Paper-based analytical device for sensitive colorimetric determination of sulfonamides in pharmaceutical samples. Spectrochim. Acat A. 2024;304 doi: 10.1016/j.saa.2023.123336. [DOI] [PubMed] [Google Scholar]
- 142.Long Y.Q., Liu J.J., Wu Y.J., Fu L., Bai L.J. A novel “on-off-on” electrochemiluminescence aptasensor based on resonance energy transfer as signal amplification strategy for ultrasensitive detection of sulfadiazine in different samples. Food Chem. 2024;456 doi: 10.1016/j.foodchem.2024.140025. [DOI] [PubMed] [Google Scholar]
- 143.Yang W.M., Liu C.H., Zhang B.L., Wu W.Z., Cao Y., Huang W.H., Xu W.Z. Construction of a nitrogen-doped carbon quantum dot fluorescent molecularly imprinted sensor for ultra-sensitive detection of sulfadiazine in pork samples. Food Anal. Methods. 2024;17:1689–1701. [Google Scholar]
- 144.Zhang S., Li S.H., Li D., Wu J.Z., Jiao T.H., Wei J., et al. Chen Quansheng. Sulfadiazine detection in aquatic products using upconversion nanosensor based on photo-induced electron transfer with imidazole ligands and copper ions. Food Chem. 2024;456 doi: 10.1016/j.foodchem.2024.139992. [DOI] [PubMed] [Google Scholar]
- 145.Ji K.X., Liu P., Wu C.Y., Li Q., Ge Y., Wen Y.Q., et al. A deep learning strategy for discrimination and detection of multi-sulfonamides residues in aquatic environments using gold nanoparticles-decorated violet phosphorene SERS substrates. Sensor Actuat. B-Chem. 2023;386 [Google Scholar]
- 146.Sun Y.B., Dong Y.F., Wang P.L., Cheng J. Zr-MOF-based substrate with inherent internal standards facilitates reliable SERS analysis of sulfonamide antibiotics. Sensor Actuat. B-Chem. 2025;423 [Google Scholar]
- 147.Huang J.Y., Xia L., Xiao X.H., Li G.K. A recyclable PDMS microfluidic surface-enhanced Raman scattering Cu/AgNP chip for the analysis of sulfadiazine in aquatic products. New J. Chem. 2024;48:11457–11464. [Google Scholar]
- 148.Lu Y.S., Li C.W., Wang Y., Wang Z.Y., Liu C., Fan H.T., Sun T. A SERS responsive DGT sensing device for on-site determination of organic contaminants underwater. ACS Sens. 2023;8:3762–3771. doi: 10.1021/acssensors.3c01169. [DOI] [PubMed] [Google Scholar]
- 149.Wei X.B., Zhang Z.R., Zhang L.F., Xu X.H. Synthesis of molecularly imprinted polymers/NiCo2O4 nanoneedle arrays on 3D graphene electrode for determination of sulfadimidine residue in food. J. Mater. Sci. 2019;54:2066–2078. [Google Scholar]
- 150.Javanshiri-Ghasemabadi J., Sadeghi S. Facile fabrication of an electrochemical sensor for the determination of two sulfonamide antibiotics in milk, honey and water samples using the effective modification of carbon paste electrode with graphitic carbon nitride and manganese oxide nanostructures. J. Food Compos. Anal. 2023;120 [Google Scholar]
- 151.Wang Y.F., He J.W., Wu J., Hao W., Cai L., Wang H.Y., Fang G.Z., Wang S. A novel molecularly imprinted electrochemical sensor based on quasi-three-dimensional nanomaterials Nb2CTx/AgNWs for specific detection of sulfadiazine. Microchim. Acta. 2024;191:720. doi: 10.1007/s00604-024-06805-3. [DOI] [PubMed] [Google Scholar]
- 152.Shanlee S.S.R., Ruspika S., Chen S.M., Balaji R., Liao Y.C., Chandrasekar N. Multi-walled carbon nanotube wrapped rare earth tungstate nanosheet composites for selective and rapid detection of sulfadiazine. Chem. Eng. J. 2024;492 [Google Scholar]
- 153.Chopra S., Balkhandia M., Khatak M., Sagar N., Agrawal V.V. Molecularly imprinted electrochemical sensor based on APTES-functionalized indium tin oxide electrode for the determination of sulfadiazine. Microchim. Acta. 2025;192:6. doi: 10.1007/s00604-024-06866-4. [DOI] [PubMed] [Google Scholar]
- 154.Kaur J., Komal A., Sheoran A., Nidhi V., Kumar V., Tikoo K., et al. Development of perceptive multi-analyte sensing platform based on fluorescent CdS QDs for selective and sensitive assay of virulent contaminants in aqueous medium. J. Environ. Chem. Eng. 2022;10 [Google Scholar]
- 155.Taherizadeh M., Jahani S., Moradalizadeh M., Foroughi M.M. Synthesis of a dual-functional terbium doped copper oxide nanoflowers for high-efficiently electrochemical sensing of ofloxacin, pefloxacin and gatifloxacin. Talanta. 2023;255 doi: 10.1016/j.talanta.2022.124216. [DOI] [PubMed] [Google Scholar]
- 156.Aggarwal R., Garg A.K., Kumar V., Jonwal H., Sethi S., Gadiyaram S., et al. Cellulose-derived carbon dots for inner filter effect-based selective sensing of ofloxacin antibiotics. ACS Appl. Nano Mater. 2023;6:6518–6527. [Google Scholar]
- 157.Qi X.J., Tao S.Y. MWCNT modified Ni-Fe LDH/BiVO4 heterojunction: boosted visible-light-driven photoelectrochemical aptasensor for ofloxacin detection. RSC Adv. 2022;12:24269–24277. doi: 10.1039/d2ra03981h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Liu Z.P., Jin M.L., Lu H., Yao J.Y., Wang X., Zhou G.F., Shui L.L. Molecularly imprinted polymer decorated 3D-framework of functionalized multi-walled carbon nanotubes for ultrasensitive electrochemical sensing of Norfloxacin in pharmaceutical formulations and rat plasma. Sensor. Actuat. B-Chem. 2019;288:363–372. [Google Scholar]
- 159.Wang C.X., Qin F.F., Tang S.Y., Li X.M., Li T.T., Guo G.Q., et al. Construction of graphene quantum dots ratiometric fluorescent probe by intermolecular electron transfer effect for intelligent and real-time visual detection of ofloxacin and its L-isomer in daily drink. Food Chem. 2023;411 doi: 10.1016/j.foodchem.2023.135514. [DOI] [PubMed] [Google Scholar]
- 160.Wu T.T., Du Y., Gao Z.F., Xu K., Dai L., Liu L., et al. Dual direct Z-scheme heterojunction with stable electron supply to a Au/PANI photocathode for ultrasensitive photoelectrochemical and electrochromic visualization detection of ofloxacin in a microfluidic sensing platform. Anal. Chem. 2023;95:1627–1634. doi: 10.1021/acs.analchem.2c04740. [DOI] [PubMed] [Google Scholar]
- 161.Heidarian S.M.T., Sani A.T., Danesh M., Ramezani M., Alibolandi M., Gerayelou G., et al. A novel electrochemical approach for the ultrasensitive detection of fluoroquinolones based on a double-labelled aptamer to surpass complementary strands of aptamer lying flat. Sensor. Actuat. B-Chem. 2021;334 [Google Scholar]
- 162.Xiong Y.Q., Zhang D., Ye C.Z., Wang Y., Deng X.R., Deng D.W., et al. Ultra-sensitive detection of ciprofloxacin hydrochloride in milk by molecularly imprinted electrochemical sensor based on S-CoFe-MOFs/AuNPs. J. Food Compos. Anal. 2023;122 [Google Scholar]
- 163.Liu J., Wang B., Huang H.C., Jian D., Lu Y.A., Shan Y.K., et al. Quantitative ciprofloxacin on-site rapid detections using quantum dot microsphere based immunochromatographic test strips. Food Chem. 2021;335 doi: 10.1016/j.foodchem.2020.127596. [DOI] [PubMed] [Google Scholar]
- 164.Liu C., Mueller-Botticher L., Liu C., Popp J., Fischer D., Cialla-May D. Raman-based detection of ciprofloxacin and its degradation in pharmaceutical formulations. Talanta. 2022;250 doi: 10.1016/j.talanta.2022.123719. [DOI] [PubMed] [Google Scholar]
- 165.Sharma K., Kaur M., Tewatia P., Kumar V., Paulik C., Yoshitake H., et al. Ultra-sensitive detection and scavenging of arsenic ions and ciprofloxacin using 3D multipurpose hemicellulose based aerogel: adsorption mechanism and RSM optimization. Bioresour. Technol. 2023;389 doi: 10.1016/j.biortech.2023.129825. [DOI] [PubMed] [Google Scholar]
- 166.Wang W.J., Zhang L., Dong W.H., Wei K.Y., Li J., Sun J.A., et al. A colorimetric aptasensor fabricated with group-specific split aptamers and complex nanozyme for enrofloxacin and ciprofloxacin determination. J. Hazard. Mater. 2023;458 doi: 10.1016/j.jhazmat.2023.131995. [DOI] [PubMed] [Google Scholar]
- 167.Liu X.Q., Wang T., Lu Y., Wang W.J., Zhou Z.P., Yan Y.S. Constructing carbon dots and CdTe quantum dots multi-functional composites for ultrasensitive sensing and rapid degrading ciprofloxacin. Sensor. Actuat. B-Chem. 2019;289:242–251. [Google Scholar]
- 168.Zokhtareh R., Rahimnejad M., Najafpour-Darzi G., Karimi-Maleh H. A new approach to electrochemical sensing of a wildly used antibiotic, ciprofloxacin. Measurement. 2023;215 [Google Scholar]
- 169.Zhang A., Feng J.J., Yan J.C., Hu M.Y., Zhang L., Zeng H.P. Laser reshaping of gold nanoparticles for highly sensitive SERS detection of ciprofloxacin. Appl. Surf. Sci. 2022;583 [Google Scholar]
- 170.Xu J.W., Shi X.M., Yi M.Y., Chi Y.Z., Guo S., Mao Z., et al. Improved charge-transfer enhancement by codoping Mg/Zn into ZrO2 for quinolone antibiotic drug determination. Appl. Surf. Sci. 2023;639 [Google Scholar]
- 171.Xu J.W., Shi X.M., Yi M.Y., Chi Y.Z., Mao Z., Yang B., Jung Y.M. Lithium-doped ZrO2 nanoparticles for SERS-based norfloxacin drug detection. Spectrochim. Acta. 2025;326 doi: 10.1016/j.saa.2024.125239. [DOI] [PubMed] [Google Scholar]
- 172.Yu C.Y., Chung C.K. Facile nanofabrication and simultaneous color-and-Raman hybrid mechanism of economic SERS substrate with tunable structure color for high enhancement factor. Appl. Surf. Sci. 2025;681 [Google Scholar]
- 173.Jiang Y., Wang X.C., Zhao G., Shi Y.Y., Wu Y. In-situ SERS detection of quinolone antibiotic residues in aquaculture water by multifunctional Fe3O4@mTiO2@Ag nanoparticles. Spectrochim. Acta. 2023;302 doi: 10.1016/j.saa.2023.123056. [DOI] [PubMed] [Google Scholar]
- 174.Gao D.H., Yang X.H., Teng P.P., Liu Z.H., Yang J., Kong D.P., et al. Optofluidic in-fiber integrated surface-enhanced Raman spectroscopy detection based on a hollow optical fiber with a suspended core. Opt. Lett. 2019;44:5173–5176. doi: 10.1364/OL.44.005173. [DOI] [PubMed] [Google Scholar]
- 175.Jin Y., Dou M.H., Zhuo S.Q., Li Q.J., Wang F.Y., Li J.L. Advances in microfluidic analysis of residual antibiotics in food. Food Control. 2022;136 [Google Scholar]
- 176.Li H.Y., Chu R., Cao J.Y., Zhou F., Guo K.K., Zhang Q.M., et al. Sensitive and reproducible on-chip SERS detection by side-polished fiber probes integrated with microfluidic chips. Measurement. 2023;218 [Google Scholar]
- 177.Yao F.Q., Wang J.M., Zhang W., Wang Z.T., Li Y.F., Sun H., Chen Q., Liang P. A microfluidic platform for minute-scale synthesizing Au@Ag nanocubes. Mater. Today Chem. 2023;34 [Google Scholar]
- 178.Teng P.P., Gao D.H., Yang X.H., Luo M., Kong D.P., Gao S., et al. In situ SERS detection of quinolone antibiotic residues in a water environment based on optofluidic in-fiber integrated Ag nanoparticles. Appl. Opt. 2021;60:6659–6664. doi: 10.1364/AO.426611. [DOI] [PubMed] [Google Scholar]
- 179.Wang J.Y., Feng Y.T., Zhao X.Y., Tian Y.H., Duan Y.X. Electrospun nanofiber-based indicatorpaper sensing platform for fluorescence and visualization detection of norfloxacin. Biosens. Bioelectron. 2023;238 doi: 10.1016/j.bios.2023.115562. [DOI] [PubMed] [Google Scholar]
- 180.Abdullah S.M., Rachid S. On column binding a real-time biosensor for β-lactam antibiotics quantification. Molecules. 2020;25:1248. doi: 10.3390/molecules25051248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Nag P., Sadani K., Mohapatra S., Mukherji S., Mukherji S. Evanescent wave optical fiber sensors using enzymatic hydrolysis on nanostructured polyaniline for detection of β-lactam antibiotics in food and environment. Anal. Chem. 2021;93:2299–2308. doi: 10.1021/acs.analchem.0c04169. [DOI] [PubMed] [Google Scholar]
- 182.Xie S.T., He R., Zhu Q.F., Shui L.L., Yang R.Z., Shen S.T., et al. Label-free optical sensor based on liquid crystal sessile droplet array for penicillin G determination. Colloids Surf. 2022;644 [Google Scholar]
- 183.Liao G.Q., Liu H., Wang X.D. More accurate and more efficient: penicillinase-immobilized phase-change microcapsules for detection and removal of penicillins under microenvironmental thermal management. J. Environ. Chem. Eng. 2023;11 [Google Scholar]
- 184.Wang T.S., Wang H., Zhu A.N., Wu Y.P., Guo X.Y., Wen Y., Yang H.F. Preparation of gold core and silver shell substrate with inositol hexaphosphate inner gap for Raman detection of trace Penicillin G. Sensor. Actuat. B-Chem. 2021;346 [Google Scholar]
- 185.Vafaye S.E., Rahman A., Safaeian S., Adabi M. An electrochemical aptasensor based on electrospun carbon nanofiber mat and gold nanoparticles for the sensitive detection of Penicillin in milk. J. Food Meas. Char. 2021;15:876–882. [Google Scholar]
- 186.Xue Y.Q., Yang X.T., Sun X.L., Han Z.Y., Sun J.C., He H.M. Reversible structural transformation of CuI-TbIII heterometallic MOFs with highly efficient detection capability toward penicillin. Inorg. Chem. 2021;60:11081–11089. doi: 10.1021/acs.inorgchem.1c00952. [DOI] [PubMed] [Google Scholar]
- 187.Yuan R.R., He H.M. Construction of an electrochemical aptasensor based on a carbazole-bearing porous organic polymer for rapid and ultrasensitive detection of penicillin. Appl. Surf. Sci. 2021;563 [Google Scholar]
- 188.He H.M., Wang S.Q., Han Z.Y., Tian X.H., Zhang W.W., Li C.P., Du M. Construction of electrochemical aptasensors with Ag(I) metal−organic frameworks toward high-efficient detection of ultra-trace penicillin. Appl. Surf. Sci. 2020;531 [Google Scholar]
- 189.Wang L., Zhou G., Guan X.L., Zhao L. Rapid preparation of surface-enhanced Raman substrate in microfluidic channel for trace detection of amoxicillin. Spectrochim. Acat A. 2020;235 doi: 10.1016/j.saa.2020.118262. [DOI] [PubMed] [Google Scholar]
- 190.Lowdon J.W., Dilien H., Van Grinsven B., Eersels K., Cleij T.J. Colorimetric sensing of amoxicillin facilitated by molecularly imprinted polymers. Polymers. 2021;13:2221. doi: 10.3390/polym13132221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lu H., Huang Y., Cui H.Y., Li L., Ding Y.P. A molecularly imprinted electrochemical aptasensor based on zinc oxide and co-deposited gold nanoparticles/reduced graphene oxide composite for detection of amoxicillin. Microchim. Acta. 2022;189:421. doi: 10.1007/s00604-022-05497-x. [DOI] [PubMed] [Google Scholar]
- 192.Li L.F., Yang L., Lin D., Xu S.H., Mei C.S., Yu S.M., Jiang C.L. Hydrogen-bond induced enhanced emission ratiometric fluorescent handy needle for visualization assay of amoxicillin by smartphone sensing platform. J. Hazard. Mater. 2022;444 doi: 10.1016/j.jhazmat.2022.130403. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.