Abstract
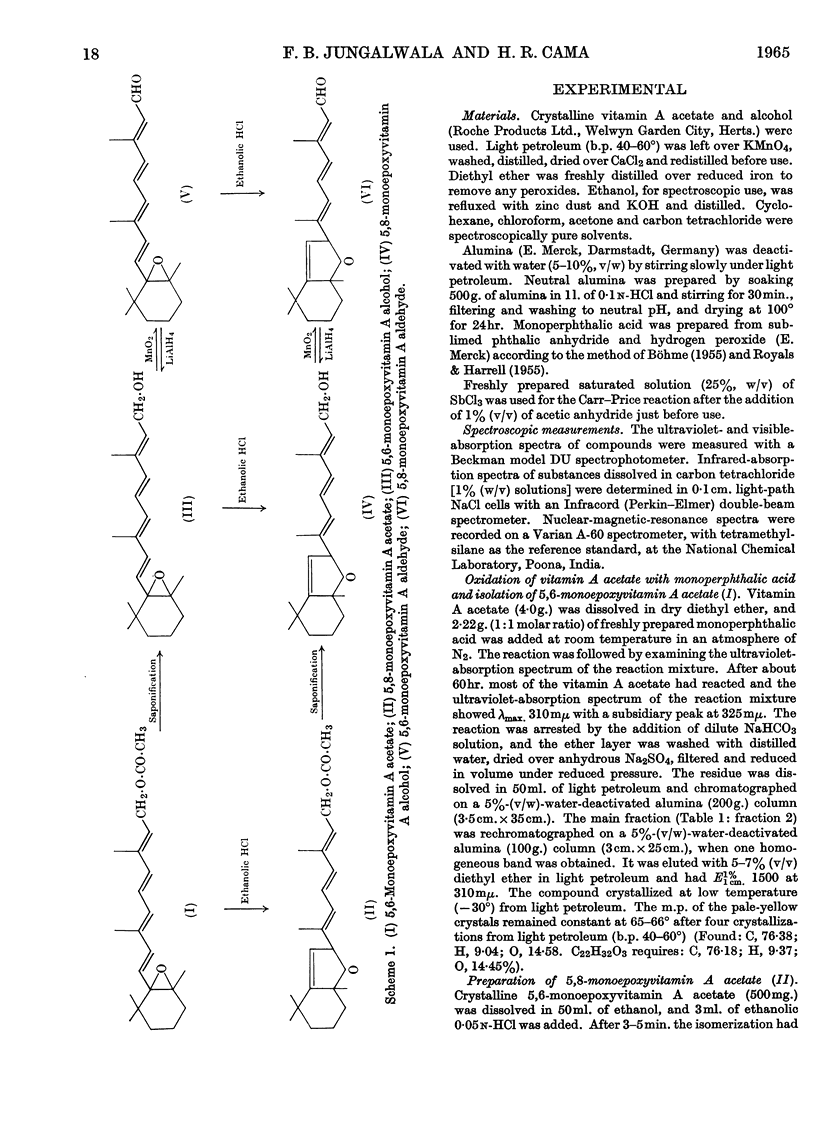
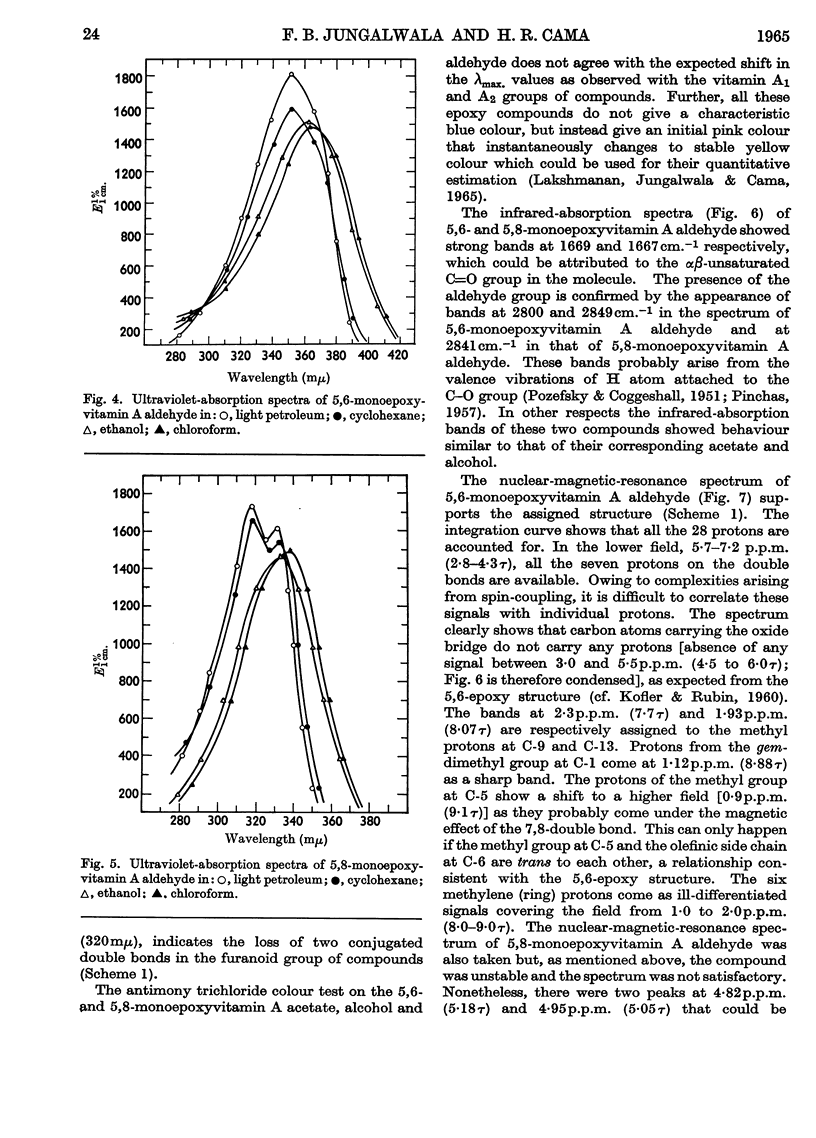
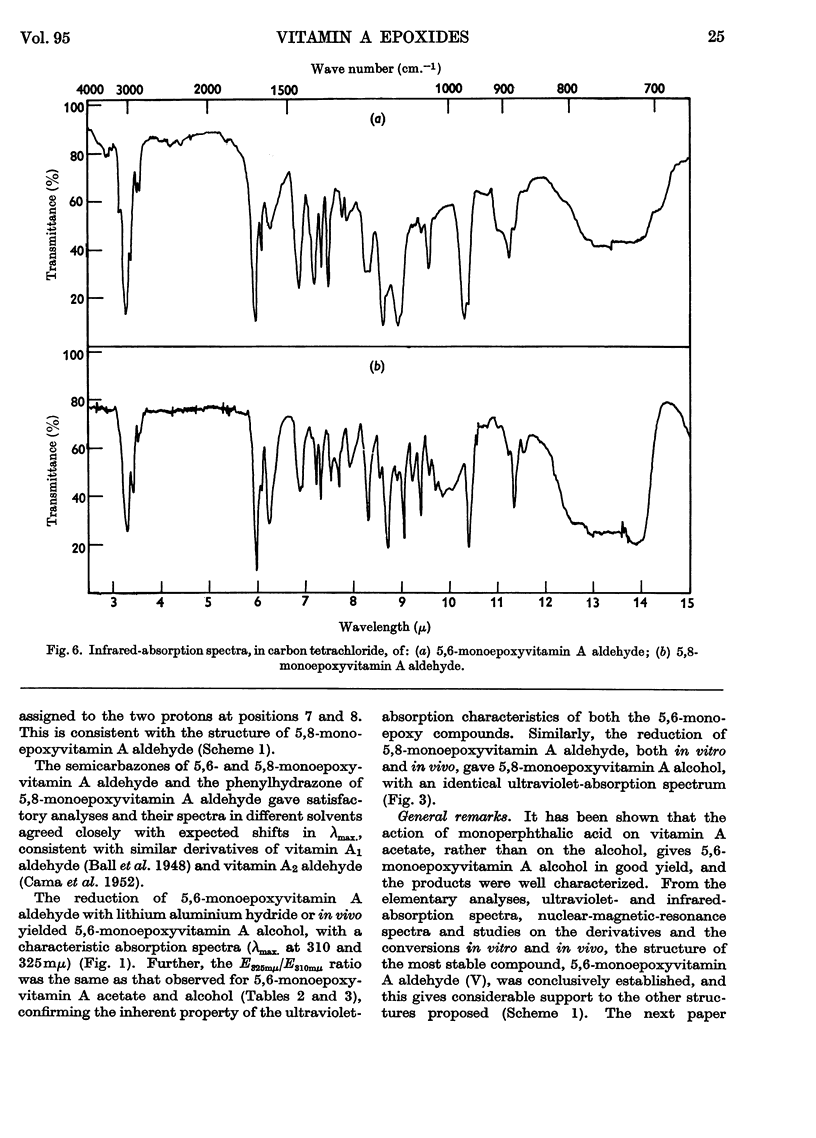
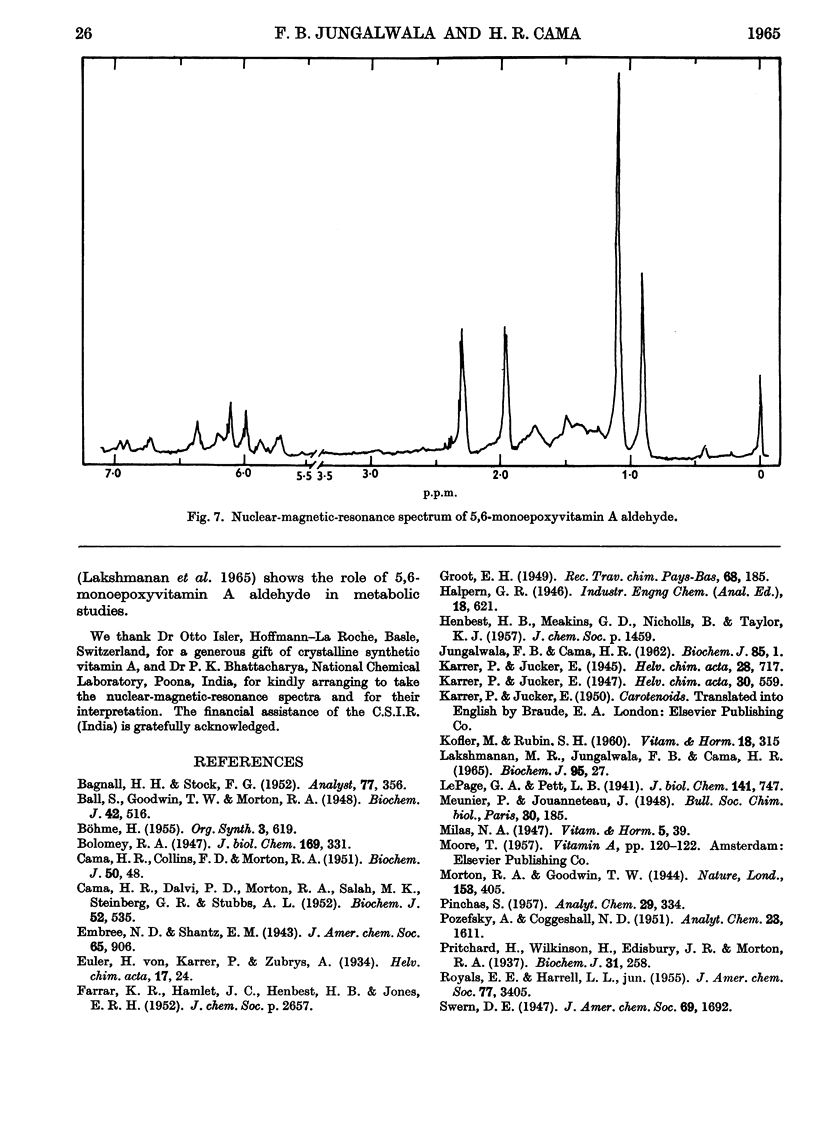
1. Oxidation of vitamin A acetate with monoperphthalic acid gave 5,6-monoepoxyvitamin A acetate, C22H32O3, obtained as pale-yellow crystals, m.p. 65–66°. 2. Saponification of 5,6-monoepoxyvitamin A acetate yielded 5,6-monoepoxyvitamin A alcohol, which was readily oxidized with manganese dioxide to 5,6-monoepoxyvitamin A aldehyde, obtained as yellow crystals, m.p. 101–102°. It was the most stable of all the epoxy compounds studied. 3. Treatment of the 5,6-epoxy compounds with ethanolic hydrochloric acid gave the corresponding 5,8-epoxy (furanoid) compounds. 5,8-Monoepoxyvitamin A aldehyde was obtained as crystals, m.p. 104–105°, but was very unstable. 4. Crystalline semicarbazones and phenylhydrazones with constant melting points and characteristic spectra were prepared from 5,6- and 5,8-monoepoxyvitamin A aldehyde. 5. Reduction of 5,6- and 5,8-monoepoxyvitamin A aldehyde with lithium aluminium hydride gave the corresponding 5,6- and 5,8-monoepoxyvitamin A alcohol. 6. 5,6- and 5,8-Monoepoxyvitamin A aldehyde were fed to vitamin A-deficient rats, and the compounds obtained from the livers of rats were indistinguishable from the reduction products obtained with lithium aluminium hydride. 7. The structures of the epoxy compounds were confirmed by their chromatographic behaviour, elemental analyses, ultraviolet-, visible- and infrared-absorption spectra and nuclear-magnetic-resonance spectra.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball S., Goodwin T. W., Morton R. A. Studies on vitamin A: 5. The preparation of retinene(1)-vitamin A aldehyde. Biochem J. 1948;42(4):516–523. doi: 10.1042/bj0420516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMA H. R., COLLINS F. D., MORTON R. A. Studies in vitamin A. XVII. Spectroscopic properties of all-trans-vitamin A and vitamin A acetate; analysis of liver oils. Biochem J. 1951 Nov;50(1):48–60. doi: 10.1042/bj0500048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMA H. R., DALVI P. D., MORTON R. A., SALAH M. K., STEINBERG G. R., STUBBS A. L. Studies in vitamin A. XIX. Preparation and properties of retinene2. Biochem J. 1952 Dec;52(4):535–540. doi: 10.1042/bj0520535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JUNGALWALA F. B., CAMA H. R. Carotenoids in Delonix regia (Gul Mohr) flower. Biochem J. 1962 Oct;85:1–8. doi: 10.1042/bj0850001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOFLER M., RUBIN S. H. Physicochemical assay of vitamin A and related compounds. Vitam Horm. 1960;18:315–339. doi: 10.1016/s0083-6729(08)60867-5. [DOI] [PubMed] [Google Scholar]
- LAKSHMANAN M. R., JUNGALWALA F. B., CAMA H. R. METABOLISM AND BIOLOGICAL POTENCY OF 5,6-MONOEPOXYVITAMIN A ALDEHYDE IN THE RAT. Biochem J. 1965 Apr;95:27–34. doi: 10.1042/bj0950027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard H., Wilkinson H. A discrepancy between biological assays and other methods of determining vitamin A. II. Biochem J. 1937 Feb;31(2):258–265. doi: 10.1042/bj0310258. [DOI] [PMC free article] [PubMed] [Google Scholar]


