Abstract
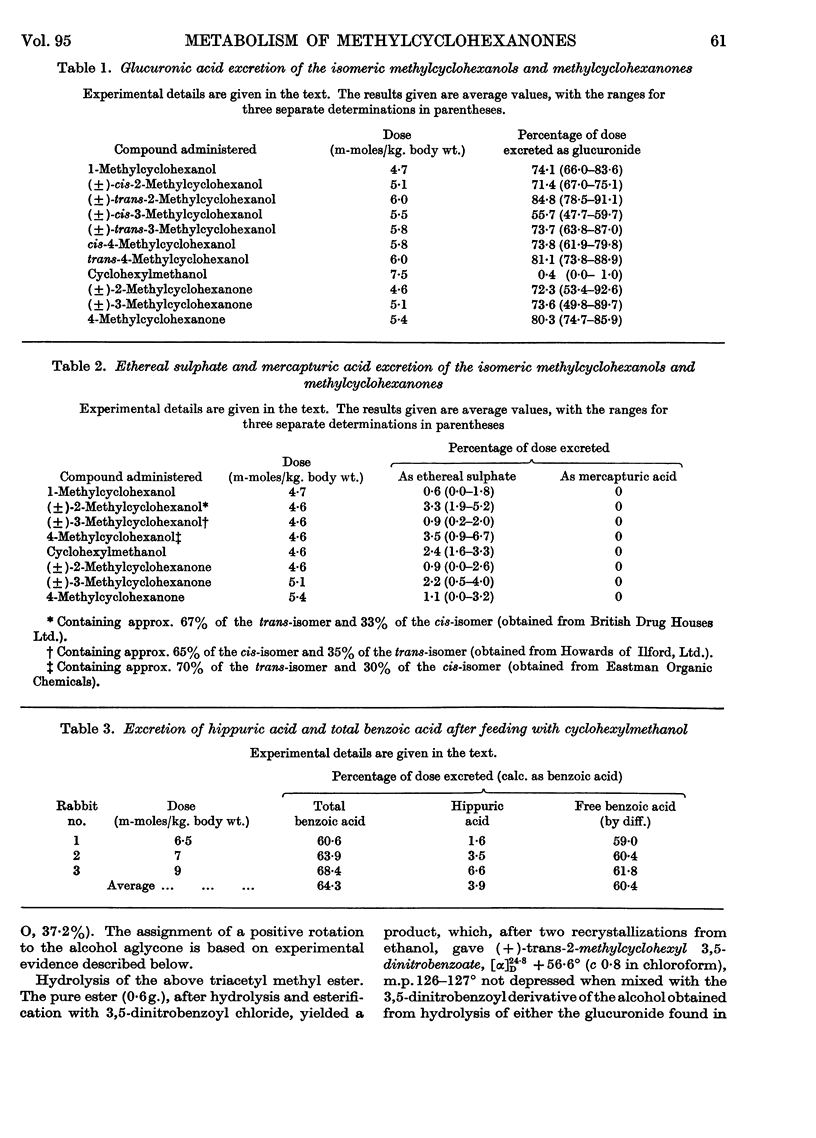
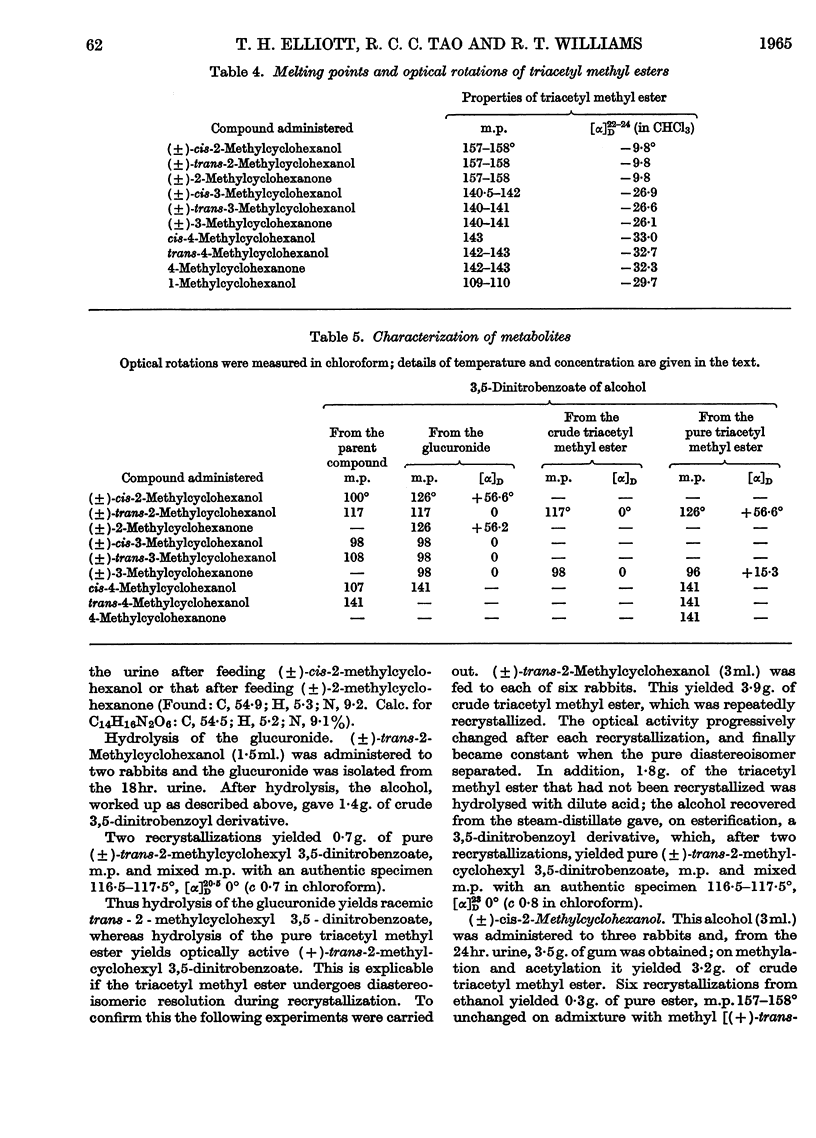
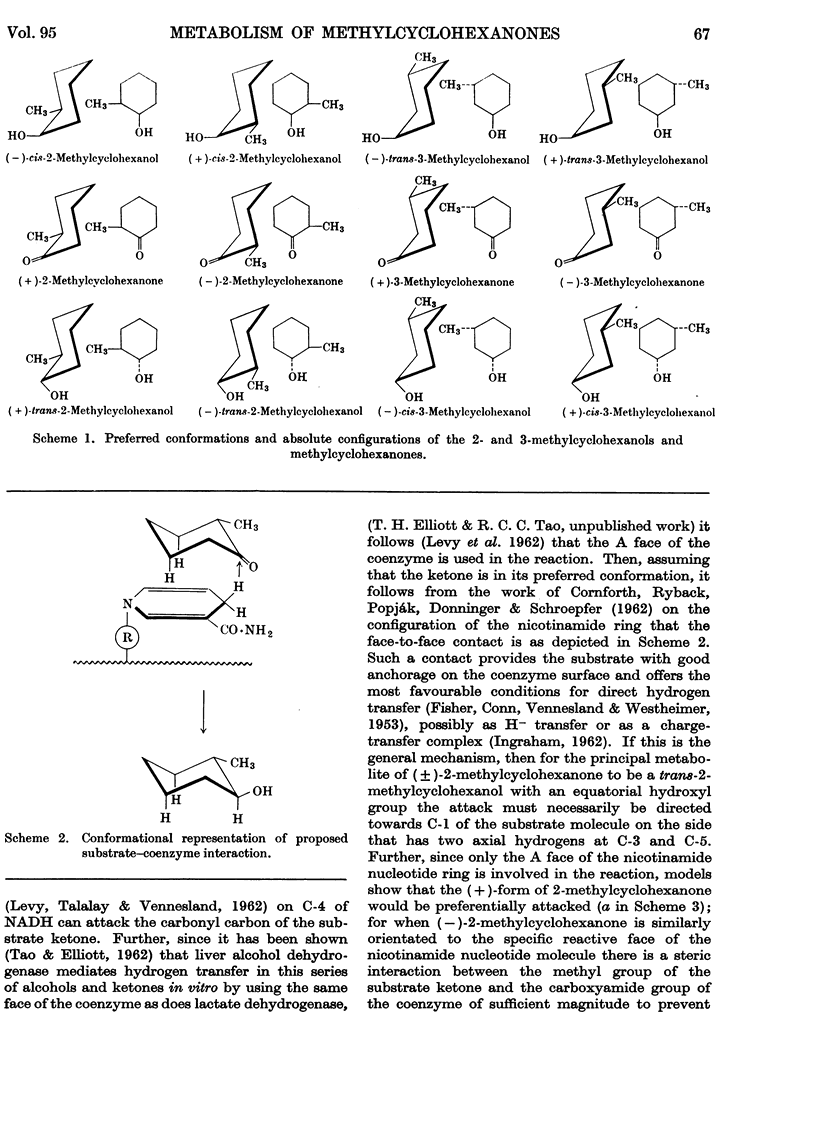
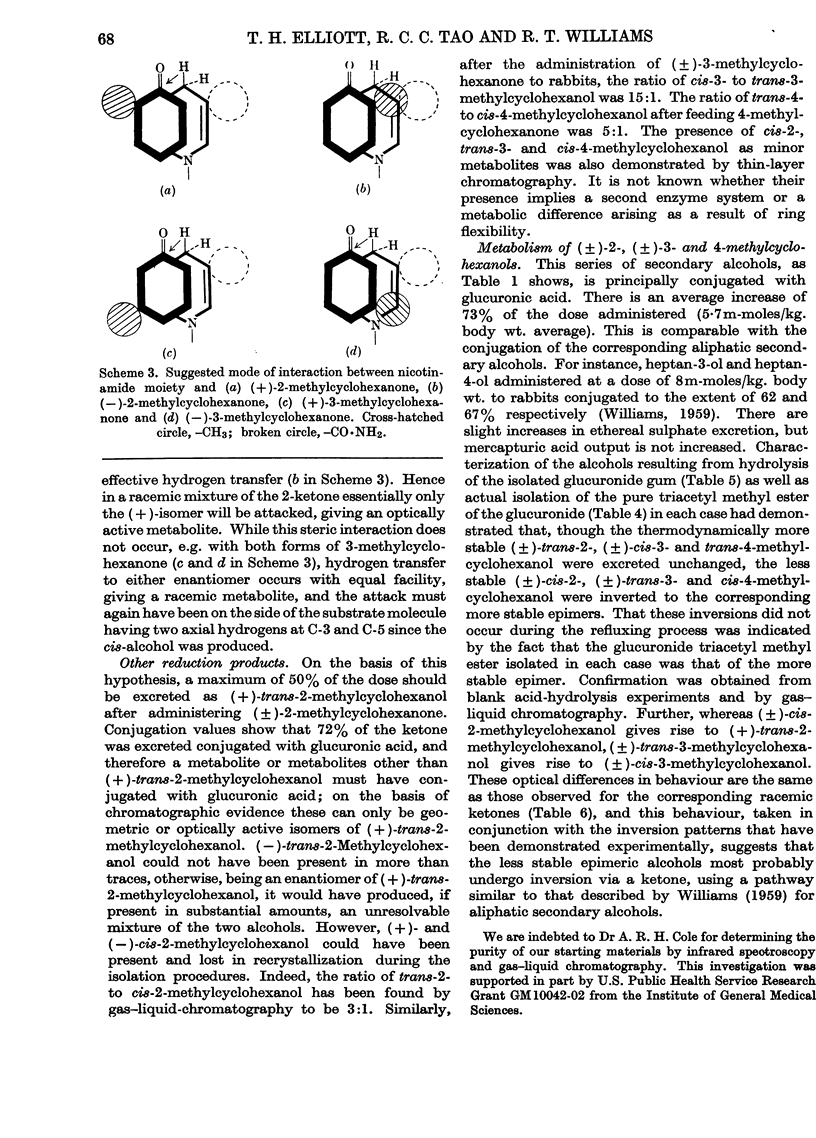
1. The seven isomeric optically inactive forms of methylcyclohexanol (i.e. 1-, and cis- and trans-2-, -3- and -4-) are excreted by rabbits mainly as glucuronides of the thermodynamically more stable forms of the alcohols. The eighth isomer, cyclohexylmethanol, however, undergoes aromatization in vivo, giving rise to benzoic acid and hippuric acid. The (±)-2-, (±-3- and 4-methylcyclohexanones are reduced in the rabbit and excreted mainly as the glucuronides of the thermodynamically more stable forms of the corresponding methylcyclohexanols. 2. Racemic cis- and trans-2-methylcyclohexanol and 2-methylcyclohexanone are all excreted as conjugated trans-2-methylcyclohexanol. However, when the (±)-cis-alcohol or the (±)-ketone is fed, (+)-trans-2-methylcyclohexanol is excreted, whereas when the (±)-trans-alcohol is fed it is excreted as the (±)-trans-alcohol. 3. Racemic cis- and trans-3-methylcyclohexanol and 3-methylcyclohexanone are all excreted as conjugated racemic cis-3-methylcyclohexanol. cis- and trans-4-Methylcyclohexanol and 4-methylcyclohexanone are all excreted as conjugated trans-4-methylcyclohexanol. 4. The metabolic differences between the various methylcyclohexanols are explicable in terms of their conformations and of Vennesland's (1958) hypothesis of the role of NADH in dehydrogenation reactions.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CORNFORTH J. W., RYBACK G. Stereochemistry of enzymic hydrogen transfer to pyridine nucleotides. Biochem Biophys Res Commun. 1962 Nov 27;9:371–375. doi: 10.1016/0006-291x(62)90018-9. [DOI] [PubMed] [Google Scholar]
- ELLIOTT T. H., PARKE D. V., WILLIAMS R. T. Studies in detoxication. 79. The metabolism of cyclo [14C] hexane and its derivatives. Biochem J. 1959 Jun;72(2):193–200. doi: 10.1042/bj0720193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLIOTT T. H., TAO R. C., WILLIAMS R. T. THE METABOLISM OF METHYLCYCLOHEXANE. Biochem J. 1965 Apr;95:70–76. doi: 10.1042/bj0950070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISHER H. F., CONN E. E., VENNESLAND B., WESTHEIMER F. H. The enzymatic transfer of hydrogen. I. The reaction catalyzed by alcohol dehydrogenase. J Biol Chem. 1953 Jun;202(2):687–697. [PubMed] [Google Scholar]
- Hanson S. W., Mills G. T., Williams R. T. A study of the determination of glucuronic acid by the naphthoresorcinol reaction, with the photoelectric absorptiometer. Biochem J. 1944;38(3):274–279. doi: 10.1042/bj0380274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAMIL I. A., SMITH J. N., WILLIAMS R. T. Studies in detoxication. 41. A study of the optical rotations of the amides and triacetyl methyl esters of some biosynthetic substituted phenylglucuronides. Biochem J. 1951 Dec;50(2):235–240. doi: 10.1042/bj0500235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN EYS J., SAN PIETRO A., KAPLAN N. O. [A mechanism for pyridine-nucleotide-dependent dependent dehydrogenases]. Science. 1958 Jun 20;127(3312):1443–1445. doi: 10.1126/science.127.3312.1443. [DOI] [PubMed] [Google Scholar]
- VENNESLAND B. Sterospecificity of hydrogen transfer in pyridine nucleotide dehydrogenase reactions. Fed Proc. 1958 Dec;17(4):1150–1157. [PubMed] [Google Scholar]


