Abstract
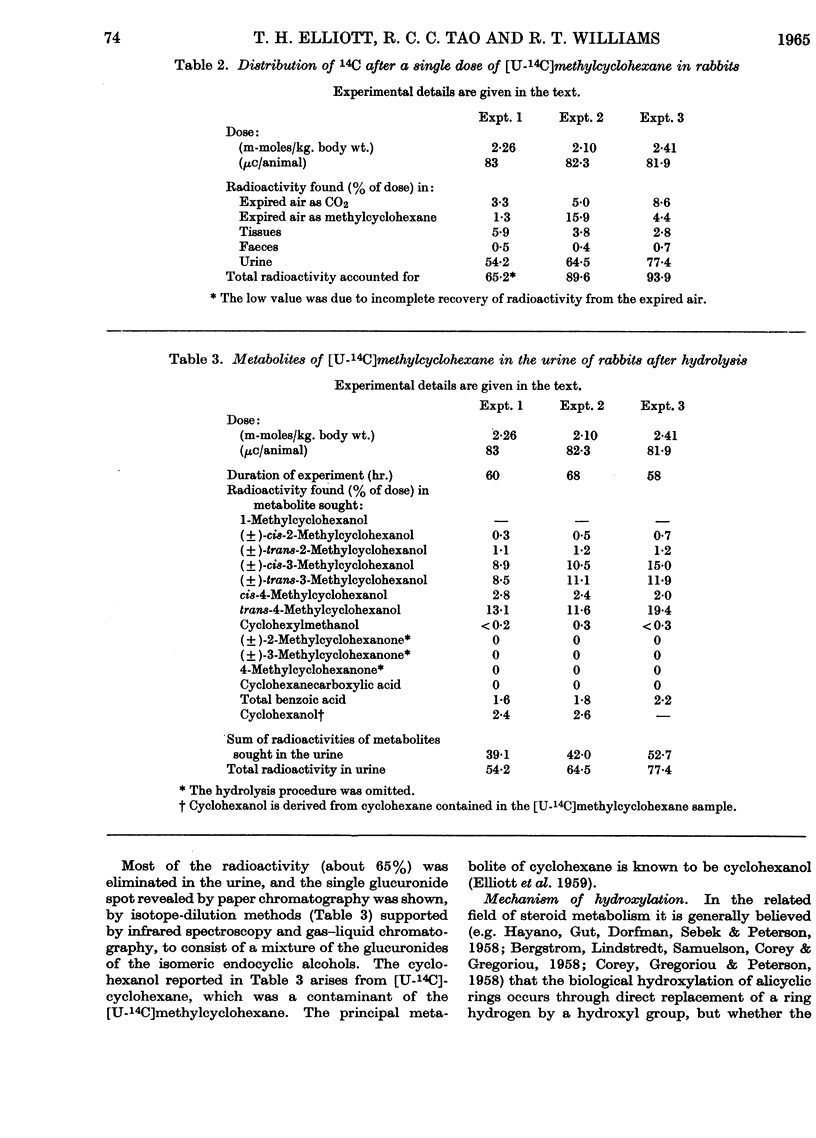
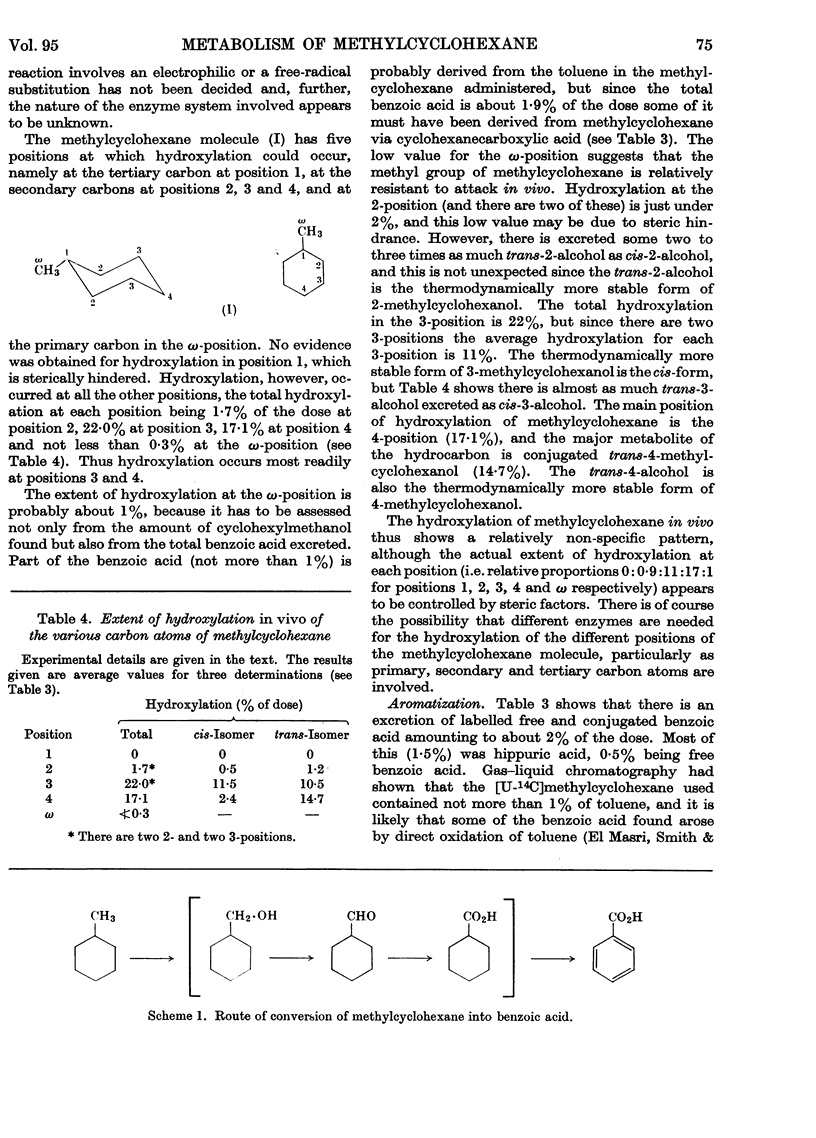
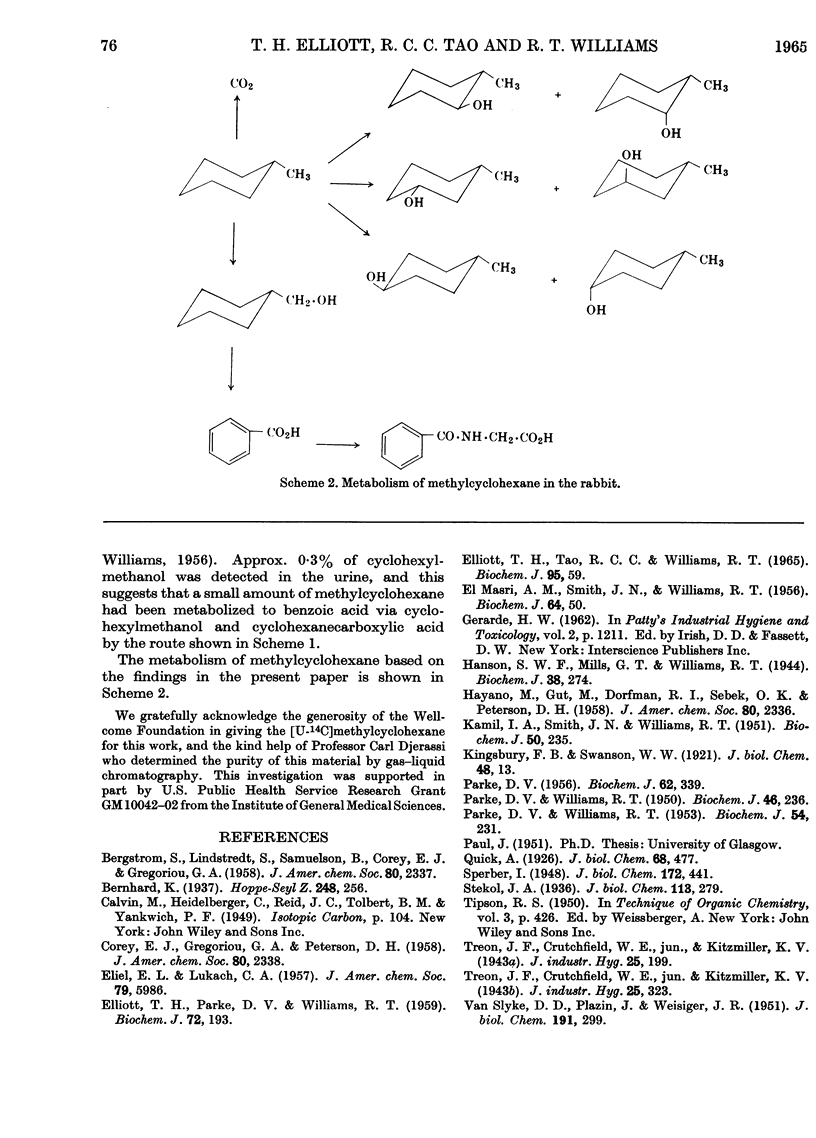
1. When [U-14C]methylcyclohexane is fed to rabbits (dose 2–2·5m-moles/kg. body wt.), 65% of the radioactivity is excreted in the urine as metabolites, 0·5% appears in the faeces and about 15% in the expired air, some 4–5% remaining in the body in about 60hr. after dosing. The 15% of the dose appearing in the expired air consists of unchanged methylcyclohexane (10%) and 14CO2 (5%). The low output of 14CO2 shows that reactions leading to complete oxidation of methylcyclohexane are of minor importance. 2. The main metabolite found in the urine was the glucuronide of trans-4-methylcyclohexanol which was isolated. Seven methylcyclohexanols were found in the urine as conjugated glucuronides. The amounts of these were determined by isotope dilution to be as follows: cis-2-, 0·6%; trans-2-, 1·2%; cis-3-, 11·5%; trans-3-, 10·5%; cis-4-, 2·4%; trans-4-methylcyclohexanol, 14·7%, cyclohexylmethanol, 0·3%. No 1-methylcyclohexanol was found. There was evidence also that a small amount (approx. 1%) of the hydrocarbon aromatized to benzoic acid, probably via cyclohexylmethanol and cyclohexane-carboxylic acid. 3. The pattern of hydroxylation and the various amounts of the isomers found suggest that the hydroxylation in vivo of methylcyclohexane is dependent on steric factors in the molecule, hydroxylation occurring to the greatest extent at the carbon atom furthest away from the methyl group.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ELLIOTT T. H., PARKE D. V., WILLIAMS R. T. Studies in detoxication. 79. The metabolism of cyclo [14C] hexane and its derivatives. Biochem J. 1959 Jun;72(2):193–200. doi: 10.1042/bj0720193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLIOTT T. H., TAO R. C., WILLIAMS R. T. STEREOCHEMICAL ASPECTS OF THE METABOLISM OF THE ISOMERIC METHYLCYCLOHEXANOLS AND METHYLCYCLOHEXANONES. Biochem J. 1965 Apr;95:59–69. doi: 10.1042/bj0950059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson S. W., Mills G. T., Williams R. T. A study of the determination of glucuronic acid by the naphthoresorcinol reaction, with the photoelectric absorptiometer. Biochem J. 1944;38(3):274–279. doi: 10.1042/bj0380274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAMIL I. A., SMITH J. N., WILLIAMS R. T. Studies in detoxication. 41. A study of the optical rotations of the amides and triacetyl methyl esters of some biosynthetic substituted phenylglucuronides. Biochem J. 1951 Dec;50(2):235–240. doi: 10.1042/bj0500235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARKE D. V. Studies in detoxication. 68. The metabolism of [14C]nitrobenzene in the rabbit and guinea pig. Biochem J. 1956 Feb;62(2):339–346. doi: 10.1042/bj0620339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARKE D. V., WILLIAMS R. T. Studies in detoxication. XLIX. The metabolism of benzene containing (14C1) benzene. Biochem J. 1953 May;54(2):231–238. doi: 10.1042/bj0540231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parke D. V., Williams R. T. Studies in detoxication. 30. The metabolism of benzene. (a) The determination of benzene. (b) The elimination of unchanged benzene by rabbits. Biochem J. 1950 Feb;46(2):236–243. doi: 10.1042/bj0460236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN SLYKE D. D., PLAZIN J., WEISIGER J. R. Reagents for the Van Slyke-Folch wet carbon combustion. J Biol Chem. 1951 Jul;191(1):299–304. [PubMed] [Google Scholar]


