Abstract
Fibrosis-driven solid organ failure is a major world-wide health burden with few therapeutic options. Spiny mice (genus: Acomys) are terrestrial mammals that regenerate severe skin wounds without fibrotic scars to evade predators. Recent studies have shown that spiny mice also regenerate acute ischemic and traumatic injuries to kidney, heart, spinal cord, and skeletal muscle. A common feature of this evolved wound healing response is a lack of formation of fibrotic scar tissue that degrades organ function, inhibits regeneration, and leads to organ failure. Complex tissue regeneration is an extremely rare property among mammalian species. In this article, we discuss the evidence that Acomys represents an emerging model organism that offers a unique opportunity for the biomedical community to investigate and clinically translate molecular mechanisms of scarless wound healing and regeneration of organ function in a mammalian species.
Keywords: Acomys, Wound healing, Fibrosis, Evolution
Introduction
Many unique phenotypes in nature represent evolved adaptations to become either a better predator, a more elusive prey, or a more attractive mate (Endler 1986; Sterling and McCreless 2006; Conner and Corcoran 2012; Pembury Smith and Ruxton 2020). African spiny mice (genus: Acomys) are muroid rodent species native to arid and rocky habitats in Africa and the Middle East that evolved a number of unique adaptations be more elusive prey animals (Fig. 1). Their major predators are foxes, snakes, and raptors (Jones and Dayan 2000; Regula 2012). A deterrent against soft-mouthed predators is provided by the characteristic stiff spiny hairs that cover the dorsum of Acomys species, thus the common name “spiny mice” (Montandon et al. 2014; Jiang et al. 2019). Predatory raptors, however, are less likely to be deterred in this manner necessitating a different strategy to avoid predation. In this case, the property of skin shedding provides spiny mice with an effective avoidance capability (Seifert et al. 2012). The acquisition of this trait seems to have occurred along with a suite of phenotypes that permit long-term success of this strategy for species survival. These phenotypes include absence of infection in exposed subdermal tissues, lack of pain perception upon skin shedding, minimal bleeding, and failure to form scar tissue (Seifert et al. 2012; Maden and Brant 2019; Brewer et al. 2021). Scarless healing is accompanied by complete regeneration of skin including de novo hair follicles, sebaceous glands, cartilage, adipose tissue, nerves, and blood vessels in the correct architecture for restoration of structure and function of skin tissue (Seifert et al. 2012; Brant et al. 2015; Gawriluk et al. 2016; Matias Santos et al. 2016; Jiang et al. 2019; Maden and Brant 2019; Harn et al. 2021; Brewer et al., 2021).
Fig. 1.
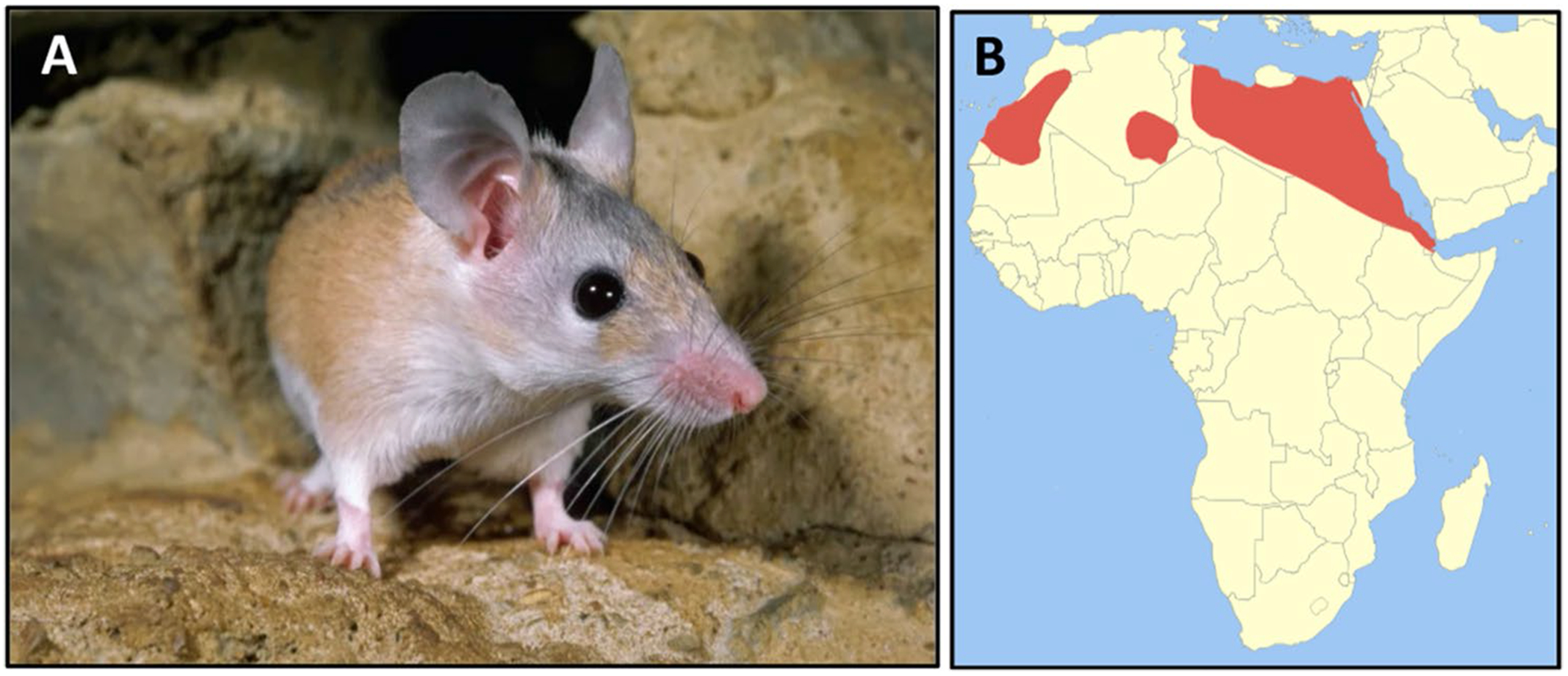
Acomys cahirinus—spiny mouse. A Adult spiny mice have narrow snouts, long whiskers, large ears, protruding black eyes, and spiny hairs along the dorsum. B These animals are adapted to hot arid climates and are found throughout northern Africa (red) and the Middle East (image licensed under the Creative Commons Attribution-Share Alike 3.0 Unported license-Wikimedia Commons)
It is this latter adaptation that has captured the attention of scientists worldwide (Maden and Varholick 2020; Gaire et al. 2021; Koopmans et al. 2021; Nogueira-Rodrigues et al. 2022; Okamura et al. 2022). Regeneration of complex tissue is extremely rare in adult mammals (Poss 2010; Maden 2018). Yet, most mammalian species, including humans, exhibit scarless regeneration in fetal or neonatal life (Colwell et al. 2003; Drenckhahn et al. 2008; Pratsinis et al. 2019; Porrello et al. 2011; Abrams et al. 2021). This may speak to the possibility that regeneration is a more broadly distributed property of mammalian genomes than is generally thought based on adult phenotypes of the comparatively few mammalian species in which it has been examined (Bely and Nyberg 2010; Andres-Mateos et al. 2012; Abrams et al. 2021; Murugan et al. 2022). If so, then the question becomes what are the mechanisms that repress this property in non-regenerating mammals and how can this potential be brought forward from its cryptic and repressed state (Bely and Sikes 2010; Cui et al. 2020; Williams et al. 2021; Abrams et al. 2021)? The fact that regeneration is both ancient (Fröbisch et al. 2014) and current, as the property in adults is now found scattered throughout the animal kingdom (Brockes et al. 2001), highlights its resiliency and suggests that strong selection pressures may be required to maintain expression in adult life. The cases of human fingertip regeneration (Illingworth 1974; Allan et al. 2006), as well as regeneration of surgical wounds in human fetal life (Colwell et al. 2003), suggest that this potential also resides in the human genome. Taken together, these findings argue that the information for organ regeneration may broadly exist in mammalian genomes but the expression of this information is efficiently silenced around the time of birth. Therefore, the possibility that spiny mice have avoided silencing this latent potential suggests that further study of this animal’s remarkable wound healing properties may reveal clues as to the silencing mechanism and its possible reversal in non-regenerating mammalian species.
Spiny mouse as an emerging model organism
Collectively, most investigators use only a small number of metazoan model organisms for their research (Goldstein and King 2016). These familiar models include mice, rats, zebrafish, Xenopus, C. elegans, and Drosophila. As an emerging model organism, spiny mice exhibit several unique phenotypes that are not found in traditional mammalian model organisms used for research (Gaire et al. 2021). In addition to the scarless wound healing trait introduced above, Bellofiore et al. reported that spiny mice are the first rodent species identified to exhibit menstruation (Bellofiore et al. 2017, 2021) thus offering a new genetic landscape for interrogation of the physiology and reproductive biology of mammalian menstruation. Acomys is also studied as an evolutionary model of sympatric speciation (the evolution of a new species from an existing species while both continue to inhabit the same territory and exhibit overlapping ranges) in its native desert habitat in Evolution Canyon, Israel (Hadid et al. 2014; Wang et al. 2022).
There is a growing, but still small, number of research colonies of Acomys species distributed throughout the world and in these colonies A. cahirinus (Egyptian spiny mouse) is most often found. As adults, these animals are about twice the size of laboratory mice of the C57BL/6 J strain (MusB6), the litters are smaller (~ 2–4 pups/litter), and gestation times longer (~ 40–50 days) than MusB6. Newborn spiny mice are covered with hair, eyes are open, ears unfurled, and within 2 or 3 days after birth are moving around actively exploring their environment (Keller and Burns 1989; Regula 2012; Haughton et al. 2016; Pinheiro et al. 2018). By the age of 2 months, spiny mice are sexually mature (Regula 2012; Haughton et al. 2016). Spiny mice develop stiff and sharp-tipped hairs covering their dorsum that are modified awl hairs and are thought to serve a protective function in deterring predators (Montandon et al. 2014). Adults are characterized by large ears, a narrow snout, long whiskers, and large protruding black eyes (Fig. 1). At least three species of Acomys have been reported to develop skin that tears easily at low tensile forces (Seifert et al. 2012; Harn et al. 2021). While the underlying changes in embryonic skin morphogenesis that produce anatomically “sheddable” skin in adults of these species are not known, it is likely that acquisition of this developmental phenotype was a critical step in the evolution of Acomys species as it allowed escape from predators via autotomy in their native habitats.
Scarless wound healing in an adult mammal
Vertebrate species that are capable of regeneration as adults will heal acute tissue injuries without fibrotic scars (Brockes and Kumar 2008; Chen and Poss 2017; Stockdale et al. 2018; Maden and Varholick 2020). The majority of those vertebrate species are not mammals and do not have a fully committed terrestrial life style. Thus, the bulk of the regeneration literature comes from studies that use axolotl (a regeneration-competent species of salamander), newts & other urodeles, Xenopus, and zebrafish. Neonatal mouse myocardium is capable of limited regeneration potential after ventricular apex resection, although this potential is restricted to the first few postnatal days (Porrello et al. 2011). As mice are altricial at birth (born in an underdeveloped state requiring care and feeding by parents), neonatal heart regeneration may be related to the general scarless wound healing phenotype observed in mammalian fetal life (Clancy et al. 2001; Colwell et al. 2003). Consistent with this, the same ventricular apex injuries heal with fibrotic scar by 7 days after birth and thereafter. Mouse digit tip regenerates after amputation in adults (Rinkevich et al. 2011; Lehoczky et al. 2011). Yet, it is only the extreme tip of the digit that regenerates limiting the overall relevance of this model for other tissues and organs. Current evidence suggests that spiny mice can regenerate a wide variety of tissues and organs as adults (Gaire et al. 2021; Okamura et al. 2022) and argues that Acomys should be added to the list of regenerators as a rare representative of the class Mammalia.
Skin
Given the damaging effects of tissue fibrosis on organ function (Wynn and Ramalingam 2012; Friedman et al. 2013; Humphreys 2018), the evolution of pathways for wound healing that minimize fibrosis and promote organ regeneration would seem advantageous (Fig. 2). The report by Siefert et al. showing that spiny mice can shed their dorsal skin to escape the grasp of predators and then fully regenerate the lost skin without fibrotic scarring suggests that Acomys species may have followed that evolutionary path (Seifert et al. 2012). These authors reported that the mean tensile strength of normal adult spiny mouse skin is at least 20-fold weaker than that of Swiss-Webster mice (Seifert et al. 2012). Moreover, skin shedding in spiny mice occurs by tearing without evidence of a pre-existing fracture plane, unlike the tail autotomy seen in geckos and other lizards (Baban et al. 2022; Ghatak 2022). This indicates that the composition of the extracellular matrix (ECM) of spiny mouse skin will be found to be considerably different than that of Swiss-Webster mice, as suggested by the opposing abundance ratios of collagens type I and type III in the two species and in the less dense and more porous arrangement of collagen fibers in spiny mouse skin (Seifert et al. 2012). Moreover, dermal fibroblasts isolated from dorsal skin of newborn Mus (CD1) and cultured on increasingly stiff matrices upregulated smooth muscle α-actin (Acta2) expression as a function of matrix stiffness, while newborn Acomys dermal fibroblasts do not upregulate Acta2 expression when cultured under the same conditions of matrix stiffness (Stewart et al. 2018).
Fig. 2.

Regeneration of ear hole injury in adult spiny mice. A Time course of ear hole closure and repigmentation in Mus (CD-1, top) and Acomys (spiny mouse, bottom). Note complete regeneration of ear hole injury in Acomys together with pigment and hair formation. B Histology (Safranin O stain). Left panel shows regeneration of normal ear architecture in vehicle-treated (mineral oil, MO) spiny mice with new cartilage (black dashed lines), adipocytes (asterisks), and hair follicles. (right panel) After treatment of Acomys with verteporfin (VP), an inhibitor of Yap-TEAD binding interactions (Brewer et al. 2021) between 3 and 5 weeks after ear punch injury, the resulting regenerated tissue is mispatterned and fibrotic. Significant reductions of new cartilage (dashed black lines) and adipocytes together with numerous ectopically located hair follicles appearing in the ventral region of de novo ear tissue at 5 weeks after injury (Brewer et al., 2021). Reprinted with permission from Developmental Cell
Tissue injury is typically accompanied by a localized innate immune response to promote wound healing (Eming et al. 2017; Moretti et al. 2022). A comprehensive analysis of the kinetics of leukocyte infiltration during ear hole regeneration in spiny mice was reported by Simkin et al. (2017). After determining that no significant differences in numbers of circulating monocytes, neutrophils, lymphocytes, and eosinophils preexisted in uninjured Acomys compared to Mus skin, the authors examined a time course of changes in leukocyte content in ear pinnae after injury. Over a 15 days time course, similar changes in CD11b-positive cells (neutrophils & macrophages) were found in Acomys and Mus ear tissues. Employing a pan-macrophage marker, IBA-1, no significant differences were found in the numbers of IBA-1-positive cells between the two species over the 15 days time course (Simkin et al. 2017). However, when lucigenin chemiluminescence was used to detect reactive oxygen species (ROS) production, much higher ROS levels were found in Acomys tissues at 1 days compared to Mus and this difference was maintained up to 5 days after injury (Simkin et al. 2017). Similar observations of sustained ROS production were reported for regeneration of zebrafish heart (Han et al. 2014) and Xenopus tadpole tail (Love et al. 2013) (reviewed in Helston and Amaya, 2021).
Macrophages are reported to be required for tissue regeneration in axolotl (Godwin et al. 2013), zebrafish (Lai et al. 2017), and neonatal mouse heart (Aurora et al. 2014). In a series of experiments to deplete phagocytes (mostly macrophages) using clodronate liposomes, Simkin et al. reported delays in blastema formation, cartilage histolysis, reepithelization, and closure of ear hole injuries in spiny mice (Simkin et al. 2017). These data provide evidence for a common cross-species requirement for macrophage function that is conserved in spiny mice. Precisely what mediators and macrophage subtypes are involved and what role they play in the cellular and molecular events underlying regeneration in spiny mice remain to be determined.
Although multiple cell types contribute to fibrotic matrix production, including perivascular cells (Passman et al. 2008; Kramann et al. 2015; Wu et al. 2016; Lu et al. 2020), endothelial cells (Yoshimatsu and Watabe 2022), and circulating fibrocytes (Reinhardt and Breuer 2021), a dominant role is played by the resident injury-activated myofibroblast (MF) (Hinz et al. 2012; van Putten et al. 2016; Henderson et al. 2020). Injury-induced MFs restore structural integrity of injured tissue but limit regeneration in most mammalian models (Darby et al. 1990; Tomasek et al. 2002; Hubert et al. 2021). In the skin, most MFs are derived from dermal fibroblasts (DFs) (Plikus et al. 2021; Talbott et al. 2022). DFs are now known to represent a number of distinct fibroblast subtypes that differ in embryonic origin, subtype-specific transcriptomes, anatomical position within skin tissue, and potentials for regeneration or scarring (Driskell et al. 2013; Rinkevich et al. 2015; Abbasi et al. 2020; Plikus et al. 2021; Talbott et al. 2022). Rinkevich et al. reported that DFs in the mouse arise from two independent origins in development that can be distinguished by whether or not their progenitor cells expressed engrailed-1 (EN1) (Rinkevich et al. 2015). The EN1-positive lineage produces DFs that are the source of most of the connective tissue in developing skin and as well as the fibrotic matrix that appears in adult skin after injury (Rinkevich et al. 2015; Mascharak et al. 2021). Moreover, tissue mechanics interact with lineage-specific DF properties to influence the balance between fibrosis and regeneration (Mascharak et al. 2021; Harn et al. 2021). Recent multi-omics and single cell ATAC-seq studies of developing and adult skin point to a significant degree of plasticity among fibroblast subtypes. Genetic pathways for DF plasticity may have provided the substrate for evolutionary selection or amplification of DF states that mediate skin regeneration in Acomys species (Seifert et al. 2012; Brant et al. 2016; Abbasi et al. 2020; Foster et al. 2021; Plikus et al. 2021; Thompson et al. 2022).
Mechanical forces and ECM-cytoskeleton-nuclear tension play major roles in morphogenesis and wound healing (Wells and Discher 2008; Humphrey et al. 2015; Cho et al. 2017). The Hippo-Yap signaling pathway has been linked to mechanosensing in a wide variety of tissues and organs (Dupont et al. 2011; Wu and Guan 2021). To address the role of Hippo-Yap signaling in scarless wound healing, Brewer et al. 2021examined Yap expression, localization, and function in repair of ear hole injuries in spiny mice (Brewer et al. 2021). No differences were found between Acomys and Mus dermal fibroblasts in Hippo pathway components or pathway activation upon cell–cell contact in vitro, a well-characterized activator of Hippo signaling (Gumbiner and Kim 2014; Brewer et al., 2021). Blocking Yap-TEAD interactions in vivo with verteporfin converted tissue regeneration to mispatterned repair and fibrosis in spiny mouse skin (Fig. 2). At the same time, however, studies in MusB6 skin showed that verteporfin treatment of excisional wounds in dorsal skin prevented fibrosis and promoted tissue regeneration (Mascharak et al. 2021). Brewer et al. suggested that there are several possible explanations for these opposing findings. One, is that the normal matrix stiffness of mouse skin is much greater than Acomys skin, as discussed above (Seifert et al. 2012). Matrix stiffness is a known activator of Yap-dependent fibrosis genes and thus inhibition of Yap signaling in the mouse would be predicted to be anti-fibrotic. Second, Acomys produce a regeneration blastema during the initial states of wound healing (Gawriluk et al. 2016) whereas Mus (C57BL/6, CD1) do not. Third, Hippo-Yap signaling is well-known to be highly context-dependent (Moya and Halder 2019). Therefore, in the course of evolution of scarless wound healing the genomic Yap-TEAD binding sites may have become “rewired” in Acomys and that a comparative analysis of Yap-TEAD ChIP-seq datasets in Acomys versus Mus dermal fibroblasts during wound healing would provide important clues for how to achieve scarless wound healing in other mammals. A similar “rewiring” of glycosylation biosynthetic pathways was proposed by Nogueira-Rodrigues et al., for regeneration of spinal cord injury in Acomys (Nogueira-Rodrigues et al. 2022).
Skeletal muscle
A single intramuscular injection of cardiotoxin leads to a rapid breakdown of skeletal muscle fibers that is then restored by activation, proliferation and differentiation of resident muscle stem cells, classically known as satellite cells (Garry et al. 2016). Prior to injury, Acomys and MusB6 were found to express similar levels of ECM proteins including collagen types I, IV, XII, XVII, fibronectin, laminin, and tenascin-C by immunofluorescence in tibialis anterior (TA) muscle (Maden et al. 2018). Collagen VI, however, was present in greater abundance in Acomys TA compared to Mus TA. Physiological stiffness of freshly isolated TA muscle was about twofold greater in Mus than in Acomys. Since cell migration, proliferation, and fibrosis are regulated by ECM stiffness, this difference in baseline muscle stiffness is noteworthy (Discher et al. 2005; Smith et al. 2017). Following a single injection of cardiotoxin, both spiny mice and C57BL/6 mice completely regenerated damaged TA muscle within 14 days with spiny mice regenerating muscle damage more rapidly than Mus (Maden et al. 2018). Regeneration occurred with similar changes in gene expression for myogenic transcription factors (myf5, myoD1, myoG) and TGFβ1, with higher levels of expression in Mus. Likewise, inflammation markers (including NF-kB) and ECM markers (collagen types I, III, & XII) were also increased in both species with much higher peak levels of expression in Mus. However, while cells expressing the macrophage M2 activation marker CD206 were present in equal numbers in both species at 4 days after cardiotoxin injury, cells expressing the M1 activation markers F4/80 or CD86 were present in abundance in damaged Mus muscle while they were undetectable in injured Acomys muscle (Maden et al. 2018).
Although repair of a single cardiotoxin injury was complete by 14 days in both species, striking differences between Mus and Acomys were observed after a series of five cardiotoxin injections each spaced 3 weeks apart. In this case, Mus muscle tissue regenerated very poorly after the fifth cardiotoxin injection with large numbers of adipocytes appearing among the few new muscle fibers that were formed (Maden et al. 2018). In contrast, skeletal muscle regeneration in spiny mice was robust with few, if any, adipocytes appearing in the regenerated muscle tissue. Thus, in addition to the previously described regeneration of panniculus carnosus muscle in skin (Seifert et al. 2012; Brant et al. 2016; Gawriluk et al. 2016; Matias Santos et al. 2016; Brewer et al. 2021), spiny mice are capable of complete regeneration of TA skeletal muscle even after repeated, closely-spaced necrotic injuries in vivo (Maden et al. 2018).
Kidney
Clinically significant end-stage kidney disease and loss of organ function is strongly linked to progressive tissue fibrosis (Duffield 2014; Hill et al. 2016; Humphreys 2018). Fibrosis is a reliable predictor of renal functional decline (Coresh et al. 2007), and chronic kidney disease (CKD) is among the strongest risk factors identified for cardiovascular disease (Go et al. 2004). There are few treatment options currently available for patients with end-stage kidney disease who often require long-term dialysis or kidney transplantation for survival (Chaudhry et al. 2022).
To test whether scarless wound healing observed in spiny mouse skin extended to wound healing in vital internal organs, such as the kidney, two aggressive models of acute kidney injury were employed (Okamura et al. 2021). Unilateral ureter obstruction (UUO) is a surgical procedure resulting in complete ligation of the ureter. This procedure leads to renal tubular expansion with activation of resident tubular epithelial cells. Activated epithelial cells signal to interstitial fibroblasts and perivascular cells, leading to rapid initiation and expansion of interstitial fibrosis (Kramann et al. 2015; Humphreys 2018) (Fig. 3). This sequence of events results in rapid loss of organ function and eventual organ failure (Moriyama et al. 1998; Li et al. 2013). A second form of kidney injury that is more commonly encountered clinically is an ischemia–reperfusion injury (IRI) produced by clamping the vascular pedicle for a given amount of time followed by release of the clamp for kidney reperfusion (Singbartl et al. 2000; Pennathur et al. 2015; Liu et al. 2017). IRI also produces rapidly-developing interstitial fibrosis in mice leading to loss of kidney function and organ failure (Okamura et al. 2021). Yet spiny mice, while exhibiting similar acute kidney damage as either inbred C57BL/6 or outbred CD1 mice, fail to progress to interstitial kidney fibrosis with no significant increases in total collagen content or Sirius Red-positive areas on kidney sections. By contrast, Mus kidneys exhibited nearly tenfold increases in total collagen content with corresponding increases in Sirius Red-positive interstitial tissues. Remarkably, injured spiny mouse kidneys completely regenerated normal renal function by 2 weeks after either type of injury (Okamura et al. 2021) (Fig. 3). Regeneration of kidney function was accompanied by rapid clearance of tubular casts, resolution of inflammation, and absence of fibrotic tissue formation. Transcriptomic analysis 2 days after obstructive injury suggested that the spiny mouse genome is poised to initiate a regenerative response at the time of the initial injury event (Okamura et al. 2021). For example, RNA seq analysis at 5 days after UUO injury revealed re-expression of a nephrogenic gene expression profile with rapid expansion of patches of cadherin-6-positive tubular epithelial cells in Acomys, but not in Mus, that correlated with regeneration of kidney function in spiny mice. Unlike ear hole injuries in skin, a regeneration blastema was not found in spiny mouse kidneys.
Fig. 3.

Regeneration of kidney function after acute renal injury in Acomys. (top left) Kidneys were injured either by permanent obstruction of the ureter (UUO, yellow arrow) or clamping of the renal artery for 40 min followed by release of the clamp allowing reperfusion of the ischemic kidney (IRI, blue arrow). (top right) Absence of fibrosis in obstructed kidneys by total collagen content measured as micrograms hydroxyproline per mg wet kidney weight in Acomys (red). By contrast, UUO produces a robust fibrotic response in MusB6 (blue) kidneys. (bottom left) Kidney function measured by blood urea nitrogen (BUN) levels 24 h after IRI injury. Note that both Acomys (Aco, red) and MusB6 (blue) exhibit similar levels of elevated BUN indicative of acute loss of kidney function. (bottom right) BUN levels at 2 weeks after IRI injury. Note further declines in kidney function in MusB6 (blue) progressing to organ failure. By contrast, BUN levels in Acomys (red) have returned to normal baseline values indicative of complete regeneration of organ function. Ns not significant, *p < 0.05, **p < 0.01, ***p < 0.001, **** p < 0.0001. (Okamura et al. 2021). Reprinted with permission from iScience. Figure created in BioRender
Heart
The response of spiny mouse hearts to myocardial ischemia (MI) following coronary ligation has been examined in three recently published studies (Qi et al. 2021; Koopmans et al. 2021; Peng et al. 2021). The results of the three studies are highly concordant with the common findings being: (1) spiny mouse hearts exhibit increased resistance to ischemic injury compared to Mus, (2) a robust angiogenic response after MI is observed in the infarct zone that persists over time in spiny mice, (3) a minimal infarct “scar” appears in spiny mouse hearts that is more porous and compliant than fibrotic scars in Mus hearts, and (4) as assessed by ultrasound imaging, spiny mouse hearts exhibit an improvement in cardiac function over time after MI while similarly injured Mus hearts exhibit a progressive decline in cardiac function over the same time period.
For example, Qi et al. showed that permanent ligation of the left main anterior descending coronary artery in adult spiny mice produced an acute drop in the ejection fraction by 1 day after MI to levels about 25% of uninjured controls (Qi et al. 2021). This reduction in ejection fraction was very similar to that seen in Mus hearts after MI for the same time period consistent with equivalent acute cardiac injuries sustained by both species. Over the next 4 weeks, spiny mice restored ejection fraction values to about 65% of uninjured controls while Mus hearts exhibited no improvement. By 13 weeks after MI, Mus hearts showed a 37% decrease in wall thickness of the left ventricular free wall while spiny mouse hearts exhibited no decrease in wall thickness. Qi et al. also reported that spiny mouse hearts had a smaller infarct zone with greatly reduced fibrotic scar area than did Mus hearts over the same time period (Qi et al. 2021). Using micro-CT analysis, Acomys hearts were found to have formed a much more substantial vascular network within the infarct zone with conduit-like arteries distributed throughout the fibrotic “scar” tissue indicative of high rates of blood flow. These vascular features in spiny mouse hearts departed sharply from the sparse distribution of small diameter vessels in the infarct zone of Mus hearts with large areas of scar tissue appearing avascular. Following daily injections of 5-bromo-2’-deoxyuridine (BrdU) starting at day 3 and continuing until day 14 after MI, Qi et al. reported that up to 20% of the BrdU-labeled cells in infarcted spiny mouse hearts were also labeled by sarcomeric α-actin and provisionally identified as proliferating cardiac myocytes (Qi et al. 2021).
Koopmans et al. similarly reported that the infarct “scar” tissue in spiny mouse hearts is substantially different from the familiar dense collagen-rich fibrotic scar that forms in similarly injured Mus hearts (Koopmans et al. 2021; Fu et al. 2018; Yokota et al. 2020). The collagen fibers in Acomys hearts are arranged in a highly porous “basket weave” pattern that has higher compliance and allows for greater ventricular conduction with minimal resistance to contraction than Mus scar tissue, features that are consistent with recovery of ejection fraction in spiny mouse hearts (Koopmans et al. 2021). By contrast, collagen fibers in Mus infarct scars are densely packed in parallel arrays that resist compression during ventricular contraction and lead to progressive loss of cardiac function as the scar tissue expands and matures over time (Fu et al. 2018; Yokota et al. 2020; Burke et al. 2021). Since neonatal mice are known to possess cardiac regeneration potential (Porrello et al. 2011), Koopmans et al. compared cardiac transcriptomes between adult spiny mouse hearts and neonatal Acomys and Mus hearts (Koopmans et al. 2021). Principal component analysis produced the intriguing result that adult spiny mouse hearts exhibit very little difference in overall gene expression when compared to neonatal spiny mouse cardiac transcriptomes suggesting that adult Acomys cardiac myocytes exist in an immature state. These results contrast with the comparison of neonatal versus adult Mus hearts where many differences in gene expression were found, corresponding to the maturation steps known to take place in the transition from newborn to adult mouse cardiac physiology via SRF-MRTF signaling (Guo et al. 2021; Miano et al. 2007). Taken together, these findings suggest that adult spiny mouse hearts maintain a neonatal pattern of gene expression with a minimal signature of postnatal maturation events.
Zebrafish hearts are known to regenerate apex resection injuries, and cardiac myocyte proliferation is a prominent component of the regenerative response (Kikuchi et al. 2010). Unlike adult mammalian hearts, zebrafish hearts maintain a predominant mononucleate cardiomyocyte population that serve as progenitors for the cells that proliferate after injury (Kikuchi and Poss 2012). Peng et al. reported a higher frequency of mononucleated cardiomyocytes in normal adult Acomys hearts (26%) compared to adult Mus hearts (6%) (Peng et al. 2021). Like the studies cited above, Peng et al. also found spiny mouse hearts are resistant to ischemic injury, develop increased vascular density in the infarct zone with proliferating endothelial cells and Acta2-positive mural cells, and produce a more porous and compliant “scar” tissue (Peng et al. 2021). Each of these features would contribute to an adult spiny mouse heart that is better able to preserve and restore cardiac function after MI.
Spinal cord
Injury to the spinal cord initiates a sequence of inflammatory responses involving microglial activation, influx of neutrophils and monocytes that migrate to the injury site, and formation of a glial/fibrotic scar that acts as a physical and chemical barrier to axon regeneration (Dias et al. 2021). Spiny mice exposed to spinal cord crush injury at the C3 level had reduced production of proinflammatory cytokines, increased expression of neurogenesis genes, absence of glial scar formation, rapid restoration of ability to void the bladder, restoration of weight-bearing and locomotion in hind limbs, and reduced TGFβ1 signaling compared to Mus with a similar injury (Streeter et al. 2020). The authors concluded that a blunted immune response and failure to produced fibrotic tissue may be characteristic, not only of spinal cord repair, but of wound healing in general for other spiny mouse tissues and organs (Streeter et al. 2020).
In a study of complete spinal cord transection at the T8 level, Nogueira-Rodrigues et al. reported that spiny mice form a “bridging matrix” across the lesion site whereas Mus produce a fibrotic scar (Nogueira-Rodrigues et al. 2022). While spiny mice develop numerous βIII-tubulin-positive axons, some fully myelinated, spanning the bridging matrix by 8 weeks after transection, Mus formed few, if any, such axons. Compound action potentials could be measured across the lesion site in 5 out of 10 injured spiny mice by 8 weeks while no signal conduction was detected in Mus at 8 weeks. As in skin, kidney, heart, and muscle, there were reduced levels of collagen fiber content by Masson’s Trichrome staining in the bridging matrix produced at the injury site in spiny mice while abundant densely-packed collagen fibers were found in Mus. These conclusions were supported by collagen type 1 immunostaining data spanning the injury site in the two species. RNA-seq analysis pointed to strong differences in acetylglucosaminyltransferase enzyme levels which included upregulation of keratan sulfate proteoglycan (KSPG) biosynthetic enzymes and downregulation of heparan sulfate proteoglycan (HSPG) biosynthetic enzymes in injured spiny mice spinal cords compared to Mus. In particular, β-1,3-N-acetylgalactosaminyltransferase 7 (β3gnt7) and carbohydrate sulfotransferase-15 (Chst15) were strongly increased in spiny mice compared to Mus (Nogueira-Rodrigues et al. 2022). β3gnt7 is a critical enzyme for KSPG production while Chst15 is required for chondroitin sulfate proteoglycan (CSPG) synthesis. When Chinese Hamster Ovary (CHO) cells overexpressing β3gnt7 were used as a substrate and Mus dorsal root ganglia or cortical neurons were plated on top of the CHO cells in vitro then greatly increased neurite and axon outgrowth was observed compared to vector only controls.
Taken together, these two studies show that spiny mice, unlike any other mammalian species tested, exhibit substantial regeneration of spinal cord injury and remarkable recovery of hind limb function after spinal cord transection (Streeter et al. 2020; Nogueira-Rodrigues et al. 2022; Wehner and Becker 2022). These data provide hope for the ~ 300,000 patients living with spinal cord injuries in the United States alone (Jain et al. 2015) that understanding spinal cord regeneration in Acomys may lead to more effective therapies in the near future.
Hibernating mammals
There is a curious parallel between wound healing in spiny mice and that reported for hibernating mammals. For example, Iaizzo et al. observed that hibernating black bears heal full thickness skin wounds with little or no infection, de novo formation of hair follicles, and an absence of fibrotic scars (Iaizzo et al. 2012). This is similar to the observations made of skeletal muscle injuries in hibernating 13-lined ground squirrels where healing occurs without fibrotic scars during hibernation (Andres-Mateos et al. 2012). The wound repair process is then completed with hair formation once the animals emerge naturally from hibernation (Andres-Mateos et al. 2012). These two examples come from cases of winter hibernation where core body temperatures are reduced compared to the awake state.
Yet a similar phenomenon also occurs in response to physiological stresses encountered in the native hot and dry environments that spiny mice normally inhabit. For example, a state of decreased metabolic activity, reduced heart and respiratory rate, and core body temperature called torpor is acquired by golden spiny mice (Acomys russatus) exposed to elevated temperatures and extended periods of food and water shortage commonly encountered in their native desert habitat (Grimpo et al. 2013; Mohr et al. 2020). In this case, a 50% food reduction led to a decrease in daily energy expenditure that was attributed to the metabolic effect of daily torpor (Grimpo et al. 2013). This physiologic state thus lowers metabolic activity in proportion to reduced food and water availability. Torpor therefore allows golden spiny mice to survive in their harsh native desert environment (Grimpo et al. 2014). Although these authors did not monitor wound healing during torpor, their findings raise the intriguing possibility that the unusual wound healing properties of adult spiny mice may have been achieved by gaining transcriptional access to scarless wound healing pathways without a requirement for physiological torpor or a hibernation-like metabolic state. Wound healing in hibernating mammals may therefore provide additional support for the idea that regeneration is a more broadly distributed property of mammalian genomes than is currently thought.
The spiny mouse genome
Wang et al. recently published a fully sequenced, assembled, and annotated Acomys cahirinus reference genome (Wang et al. 2022). These authors reported the spiny mouse genome has a total size of 2.27 Gb with 21,879 coding genes and 19 pseudochromosomes. They identified 586 distinct miRNAs, 1327 small nuclear RNAs, and ~ 878 Mb (39%) repetitive sequences (Wang et al. 2022). A phylogenetic tree analysis based on 6285 single-copy orthologous genes showed A. cahirinus was most closely related to A. dimidiatis followed by A. kempi, A. percivali and then A. russatus. Comparing the spiny mouse genome (A. cahirinus) to that of Mus musculus, 200 genes under positive selection in spiny mice were identified that were enriched in Gene Ontology (GO) terms related to embryonic morphogenesis and brain development (Wang et al. 2022). This intriguing finding is consistent with the response to injury studies reviewed above and is particularly relevant to the findings reported by Okamura et al. who identified re-expression of a set of nephrogenic progenitor genes in adult spiny mouse kidney 5 days after unilateral ureter obstruction injury (Okamura et al. 2021).
Sympatric speciation, first proposed by Darwin, is the evolution of a new species from an existing species while both continue to inhabit the same territory and exhibit overlapping ranges (Darwin 1859). Two populations of spiny mice appear to be to be undergoing sympatric speciation on the opposing slopes of Evolution Canyon, Israel (Hadid et al. 2014). These populations are under divergent selection as the European slope (ES) is exposed to morning sun, is cooler, and more densely forested whereas the opposite slope, the African slope (AS), is exposed to hot afternoon sun, is dryer, and is only sparsely forested (Li et al. 2016). To address the potential role of epigenetic regulation in relation to sympatric speciation in Evolution Canyon, Wang et al. carried out whole genome bisulfite sequencing and acquired DNA methylation patterns from their long-read DNA sequences (Oxford Nanopore Technologies, UK) of spiny mice from ES and AS. They report that an analysis of 28,636 differentially methylated regions revealed four functional enrichment categories related to sympatric speciation including DNA damage repair (Matsuoka et al. 2007), kidney system development, lipid storage and usage, and circadian rhythm (Wang et al. 2022).
Insights for mammalian organ regeneration
Progressive tissue fibrosis has proven to be an elusive therapeutic target. Ongoing mechanistic investigation continues to identify new methods and molecular players to unravel the pathogenesis of fibrosis that may provide effective targets for intervention (Doherty et al. 2010; Travers et al. 2022). Yet, a viable complementary direction is to look to nature for variant wound healing species that might be informative. For mammals, evolution of the genus Acomys has provided a potentially powerful resource for clues as to how to redirect mammalian wound healing from chronic inflammation and fibrosis to scarless wound repair and complex tissue regeneration. There are countless examples in nature where evolution by natural selection has produced innovative solutions to complex and difficult biological problems. For spiny mice, because the “solution” has evolved within the full complexity of a mammalian genome and mammalian physiology, we would anticipate fewer obstacles to clinical translation than many of the current approaches have encountered.
Summary and future directions
The studies reviewed above suggest that the spiny mouse (genus: Acomys) is an emerging model organism with exciting potential to identify mechanisms of complex tissue and organ regeneration that operate in the context of adult mammalian physiology (Fig. 4). Scarless wound healing with regeneration of mammalian organ function is a long-sought goal of the field of regenerative medicine. The remarkable wound healing properties that have evolved in the genus Acomys promise unique insights with potential clinical applications for mammalian tissue regeneration. Looking ahead, future studies will address (1) identification of the epigenetic controls for retention versus loss of fetal scarless wound healing phenotypes in Acomys versus Mus; (2) a more complete characterization of the composition and physical properties of the ECM in Acomys tissues and organs; (3) a more detailed investigation of the differences in immune responses to injury in Acomys versus Mus; and (4) an identification of cis-regulatory sequence changes that alter the levels, timing, or cell type specificity of the expression of key genes determining tissue responses to injury in spiny mice. These, and other studies, will provide additional key insights into the evolution of scarless wound healing in an adult mammal.
Fig. 4.

Acomys cahirinus (spiny mouse) (center top panel) is an emerging model organism to investigate and clinically translate molecular mechanisms of mammalian organ regeneration. Comparative studies have been reported of restored organ function after acute injury (from left to right) in kidney (Okamura et al. 2021); spinal cord (Nogueira-Rodrigues et al. 2022; Streeter et al. 2020), heart (Koopmans et al. 2021; Peng et al. 2021; Qi et al. 2021), skin (Brant et al. 2016; Brewer et al. 2021; Gawriluk et al. 2016; Harn et al. 2021; Matias et al. 2016; Jiang 2019; Seifert et al. 2012) and skeletal muscle (Maden et al. 2018). Some of the common findings across all studies of spiny mouse wound healing are indicated in the center boxed text. Figure created in BioRender
Funding
This work was supported by a Grant from the W.M. Keck Foundation; the US National Institutes of Health Grants 1R01DK114149, 5R01HL121877, and 5T32-DK007662, the Loie Power Robinson Stem Cell & Regenerative Medicine Fund, the Seattle Children’s Research Institute, and the Seattle Children’s Foundation. We thank our colleagues in the Center for Developmental Biology & Regenerative Medicine, Seattle Children’s Research Institute, and the Consortium for Fibrosis Research and Translation, University of Colorado, Anschutz Medical Campus.
Footnotes
Conflict of interest The authors declare that there are no financial or non-financial competing interests to disclose.
References
- Abbasi S, Sinha S, Labit E, Rosin NL, Yoon G, Rahmani W, Jaffer A, Sharma N, Hagner A, Shah P, Arora R, Yoon J, Islan A, Uchida A, Chang CK, Stratton JA, Scott RW, Rossi FMV, Underhill TM, Biernaskie J (2020) Distinct regulatory programs control the latent regenerative potential of dermal fibroblasts during wound healing. Cell Stem Cell 27:396–412. 10.1016/j.stem.2020.07.008 [DOI] [PubMed] [Google Scholar]
- Abrams MJ, Tan FH, Li Y, Basinger T, Heithe ML, Sarma A, Lee IT, Condiotte ZJ, Raffiee M, Dabiri JO, Gold DA, Goentoro L (2021) A conserved strategy for inducing appendage regeneration in moon jellyfish, Drosophila, and mice. eLife 10:e65092. 10.7554/eLife.65092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan CH, Fleckman P, Fernandes RJ, Hager B, James J, Wisecarver Z, Satterstrom FK, Gutierrez A, Norman A, Pirrone A, Underwood RA, Rubin BP, Zhang M, Ramay HR, Clark JM (2006) Tissue response and Msx1 expression after human fetal digit tip amputation in vitro. Wound Repair Regen 14:398–404. 10.1111/j.1743-6109.2006.00139.x [DOI] [PubMed] [Google Scholar]
- Andres-Mateos E, Mejias R, Soleimani A, Lin BM, Burks TN, Marx R, Lin B, Zellars RC, Zhang Y, Huso DL, Marr TG, Leinwand LA, Merriman DK, Cohn RD (2012) Impaired skeletal muscle regeneration in the absence of fibrosis during hibernation in 13-lined ground squirrels. PLoS ONE 7:e48884. 10.1371/journal.pone.0048884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurora AB, Porrello ER, Tan W, Mahmoud AI, Hill JA, Bassel-Duby R, Sadek HA, Olson EN (2014) Macrophages are required for neonatal heart regeneration. J Clin Invest 124:1382–1392. 10.1172/JCI72181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baban NS, Orozaliev A, Kirchhof S, Stubbs CJ, Song YA (2022) Biomimetic fracture model of lizard tail autotomy. Science 375:770–774. 10.1126/science.abh1614 [DOI] [PubMed] [Google Scholar]
- Bellofiore N, Ellery SJ, Mamrot J, Walker DW, Temple-Smith P, Dickinson H (2017) First evidence of a menstruating rodent: the spiny mouse (Acomys cahirinus). Am J Obstet Gynecol 216:4e01–40e11. 10.1016/j.ajog.2016.07.041 [DOI] [PubMed] [Google Scholar]
- Bellofiore N, George E, Vollenhoven B, Temple-Smith P (2021) Reproductive aging and menopause-like transition in the menstruating spiny mouse (Acomys cahirinus). Hum Reprod 36:3083–3094. 10.1093/humrep/deab215 [DOI] [PubMed] [Google Scholar]
- Bely AE, Nyberg KG (2010) Evolution of animal regeneration: reemergence of a field. Trends Ecol Evol 25:161–179. 10.1016/j.tree.2009.08.005 [DOI] [PubMed] [Google Scholar]
- Bely AE, Sikes JM (2010) Latent regeneration abilities persist following recent evolutionary loss in asexual annelids. Proc Natl Acad Sci USA 107:1464–1469. 10.1073/pnas.0907931107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brant JO, Lopez MC, Baker HV, Barbazuk WB, Maden M (2015) A comparative analysis of gene expression profiles during skin regeneration in mus and acomys. PLoS ONE 10:e0142931. 10.1371/journal.pone.0142931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brant JO, Yoon JH, Polvadore T, Barbazuk WB, Maden M (2016) Cellular events during scar-free skin regeneration in the spiny mouse. Wound Repair Regen 24:75–88. 10.1111/wrr.12385 [DOI] [PubMed] [Google Scholar]
- Brewer CM, Nelson BR, Wakenight P, Collins SJ, Okamura DM, Dong XR, Mahoney WM Jr, McKenna A, Shendure J, Timms A, Millen KJ, Majesky MW (2021) Adaptations in Hippo-Yap signaling and myofibroblast fate underlie scar-free ear appendage wound healing in spiny mice. Dev Cell 56:2722–2740. 10.1016/j.devcel.2021.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockes JP, Kumar A (2008) Comparative aspects of animal regeneration. Annu Rev Cell Dev Biol 24:525–549. 10.1146/annurev.cellbio.24.110707.175336 [DOI] [PubMed] [Google Scholar]
- Brockes JP, Kumar A, Velloso CP (2001) Regeneration as an evolutionary variable. J Anat 199:3–11. 10.1046/j.1469-7580.2001.19910003.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RM, Burgos Villar KN, Small EM (2021) Fibroblast contributions to ischemic cardiac remodeling. Cell Signal 77:109824. 10.1016/j.cellsig.2020.109824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry D, Chaudhry A, Peracha J, Sharif A (2022) Survival for waitlisted kidney failure patients receiving transplantation versus remaining on waiting list: systematic review and meta-analysis. BMJ 376:e068769. 10.1136/bmj-2021-068769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Poss KD (2017) Regeneration genetics. Annu Rev Genet 51:63–82. 10.1146/annurev-genet-120116-024554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S, Irianto J, Discher DE (2017) Mechanosensing by the nucleus: from pathways to scaling relationships. J Cell Biol 216:305–315. 10.1038/jcb.201610042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy B, Darlington RB, Finlay BL (2001) Translating developmental time across mammalian species. Neuroscience 105:7–17. 10.1016/s0306-4522(01)00171-3 [DOI] [PubMed] [Google Scholar]
- Colwell AS, Longaker MT, Lorenz HP (2003) Fetal wound healing. Front Biosci 8:s1240–s1248. 10.2741/1183 [DOI] [PubMed] [Google Scholar]
- Conner WE, Corcoran AJ (2012) Sound strategies: the 65-million-year-old battle between bats and insects. Annu Rev Entomol 57:21–39. 10.1146/annurev-ento-121510-133537 [DOI] [PubMed] [Google Scholar]
- Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, van Lente F, Levey AS (2007) Prevalence of chronic kidney disease in the United States. JAMA 298:2038–2047. 10.1001/jama.298.17.2038 [DOI] [PubMed] [Google Scholar]
- Cui M, Wang Z, Chen K, Shah AM, Tan W, Duan L, Sanchez-Ortiz E, Li H, Xu L, Liu N, Bassel-Duby R, Olson EN (2020) Dynamic transcriptional responses to injury of regenerative and non-regenerative cardiomyocytes by single-nucleus RNA sequencing. Dev Cell 53:102–116. 10.1016/j.devcel.2020.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby I, Skalli O, Gabbiani G (1990) Alpha-smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Invest 63:21–29 [PubMed] [Google Scholar]
- Darwin C (1859) On the origin of species by means of natural selection. John Murray, London [Google Scholar]
- Dias DO, Kalkitsas J, Kelahmetoglu Y, Estrada CP, Tatarishvili J, Doll S, JanssonL BS, Amiry-Moghaddam M, Ernst A, Huttner HA, Kokaia Z, Lindvall O, Brundin L, Frisén J, Göritz C (2021) Pericyte-derived fibrotic scarring is conserved across diverse central nervous system lesions. Nat Commun 12:5501. 10.1038/s41467-021-25585-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Discher DE, Janmey P, Wang YL (2005) Tissue cells feel and respond to the stiffness of their substrate. Science 310:1139–1143. 10.1126/science.1116995 [DOI] [PubMed] [Google Scholar]
- Doherty HE, Kim HS, Hiller S, Sulik KK, Maeda N (2010) A mouse strain where basal connective tissue growth factor gene expression can be switched from low to high. PLoS ONE 5:e12909. 10.1371/journal.pone.0012909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenckhahn JD, Schwarz QP, Gray S, Laskowski A, Kiriazis H, Ming Z, Harvey RP, Du XJ, Thorburn DR, Cox TC (2008) Compensatory growth of healthy cardiac cells in the presence of diseased cells restores tissue homeostasis during heart development. Dev Cell 15:521–533. 10.1016/j.devcel.2008.09.005 [DOI] [PubMed] [Google Scholar]
- Driskell RR, Lichtenberger BM, Hoste E, Kretzschmar K, Simons BD, Charalambous M, Ferron SR, Herault Y, Pavolic G, Ferguson-Smith AC, Watt FM (2013) Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature 504:277–281. 10.1038/nature12783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffield JS (2014) Cellular and molecular mechanisms in kidney fibrosis. J Clin Invest 124:2299–2306. 10.1172/JCI72267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, Elvassore N, Piccolo S (2011) Role of YAP/TZ in mechanotransduction. Nature 474:179–183. 10.1038/nature/10137 [DOI] [PubMed] [Google Scholar]
- Eming SA, Wynn TA, Martin P (2017) Inflammation and metabolism in tissue repair and regeneration. Science 356:1026–1030. 10.1126/science.aam7928 [DOI] [PubMed] [Google Scholar]
- Endler JA (1986) Defense against predators. In: Feder ME, Gauder GV (eds) Predatory-prey relationships. The University of Chicago Press, Chicago, pp 109–134 [Google Scholar]
- Foster DS, Januszyk M, Yost KE, Chinta MS, Gulati GS, Nguyen AT, Burcham AR, Salhotra A, Ransom RC, Henn D, Chen K, Mascharak S, Tolentino K, Titan AL, Jones RE, de Silva O, Leavitt WT, des MarshallJardins-Park CDHE, Hu MS, Wan DC, Wernig G, Wagh D, Coller J, Norton JA, Gurtner GC, Newman AM, Chang HY, Longaker MT (2021) Integrated spatial multipmics reveals fibroblast fate during tissue repair. Proc Natl Acad Sci USA 118:e2110025118. 10.1073/pnas.2110025118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman SL, Sheppard D, Duffield JS, Violette S (2013) Therapy for fibrotic diesases: nearing the starting line. Sci Transl Med 5:167sr1. 10.1126/scitranslmed.3004700 [DOI] [PubMed] [Google Scholar]
- Fröbisch NB, Bickelmann C, Witzmann F (2014) Early evolution of limb regeneration in tetrapods: evidence from a 300-million-year-old amphibian. Proc R Soc B 281:20141550. 10.1098/rspb.2014.1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, Khalil H, Kanisicak O, Boyer JG, Vagnozzi RJ, Baliken BD, Sargent MA, Prasad V, Valiente-Alandi I, Blaxall BC, Molkentin JD (2018) Specialized fibroblast differentiated states underlie scar formation in the infarcted mouse heart. J Clin Invest 128:2127–2143. 10.1172/JCI98215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaire J, Varholick JA, Rana S, Sunshine MD, Doré S, Barbaxuk WB, Fuller DD, Maden M, Simmons CS (2021) Spiny mouse (Acomys): an emerging research organism for regenerative medicine with applications beyond the skin. npiRegen Med 6:1. 10.1038/s41536-020-00111-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garry GA, Antony ML, Garry DJ (2016) Cardiotoxin induced injury and skeletal muscle regeneration. Methods Mol Biol 1460:61–71. 10.1007/978-1-4939-3810-0_6 [DOI] [PubMed] [Google Scholar]
- Gawriluk TR, Simkin J, Thompson KL, Biswas SK, Clare-Salzler Z, Kimani JM, Kiama SG, Smith JJ, Ezenwa VO, Seifert AW (2016) Comparative analysis of ear-hole closure identifies epimorphic regeneration as a discrete trait in mammals. Nat Commun 7:11164. 10.1038/ncomms11164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghatak A (2022) How does a lizard shed its tail? Science 375:721–722. 10.1126/science.abh1614 [DOI] [PubMed] [Google Scholar]
- Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY (2004) Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351:1296–1305. 10.1056/NEJMoa041031 [DOI] [PubMed] [Google Scholar]
- Godwin JW, Pinto AR, Rosenthal NA (2013) Macrophages are required for adult salamander limb regeneration. Proc Natl Acad Sci USA 110:9415–9420. 10.1073/pnas.1300290110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein B, King N (2016) The future of cell biology: emerging model organisms. Trends Cell Biol 26:818–824. 10.1016/j.tcb.2016.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimpo K, Legler K, Heldmaier G, Exner C (2013) That’s hot: golden spiny mice display torpor even at high ambient temperatures. J Comp Physiol B 183:567–581. 10.1007/s00360-012-0721-4 [DOI] [PubMed] [Google Scholar]
- Grimpo K, Kutschke M, Kastl A, Meyer CW, Heldmaier G, Exner C, Jastroch M (2014) Metabolic depression during warm torpor in the Golden spiny mouse (Acomys russatus) does not affect mitochondrial respiration and hydrogen peroxide release. Comp Biochem Physiol A Mol Integr Physiol 167:7–14. 10.1016/j.cbpa.2013.09.002 [DOI] [PubMed] [Google Scholar]
- Gumbiner BM, Kim NG (2014) The Hippo-YAP signaling pathway and contact inhibition of growth. J Cell Sci 127:709–717. 10.1242/jcs.140103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Cao Y, Jardin BD, Sethi I, Ma Q, Moghadaszadeh B, Troiano EC, Mazumdar N, Trembley MA, Small EM, Yuan GC, Beggs AH, Pu WT (2021) Sarcomeres regulate murine cardiomyocyte maturation through MRTF-SRF signaling. Proc Natl Acad Sci USA 118:e2008861118. 10.1073/pnas.2008861118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadid Y, Pavlicek T, Beiles A, Ianovici R, Raz S, Nevo E (2014) Sympatric incipient speciation of spiny mice Acomys at “Evolution Canyon”, Israel. Proc Natl Acad Sci USA 111:1043–1048. 10.1073/pnas.1322301111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han P, Zhou XH, Chang N, Xiao CL, Yan S, Ren H, Yang XZ, Zhang ML, Wu Q, Tang B, Diao JP, Zhu X, Zhang C, Li CY, Cheng H, Xiong JW (2014) Hydrogen peroxide primes heart regeneration with a derepression mechanism. Cell Res 24:1091–1107. 10.1038/cr.2014.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harn HIC, Wang SP, Lai YC, Van Handel B, Liang YC, Tsai S, Schiessl IM, Sarkar A, Xi H, Hughes M, Kaemmer S, Tang MJ, Peti-Peterdi J, Pyle AD, Woolley TE, Evseenko D, Jiang TX, Chuong CM (2021) Symmetry breaking of tissue mechanics in wound induced hair follicle regeneration of laboratory and spiny mice. Nat Commun 12:2595. 10.1038/s41467-021-22822-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughton CL, Gawriluk TR, Seifert AW (2016) The biology and husbandry of the African spiny mouse (Acomys cahirinus) and the research uses of a laboratory colony. J Am Assoc Lab Animal Sci 55:9–17 [PMC free article] [PubMed] [Google Scholar]
- Helston O, Amaya E (2021) Reactive oxygen species during heart regeneration in zebrafish: lessons for future clinical therapies. Wound Repair Regen 29:211–224. 10.1111/wrr.12892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson NC, Rieder F, Wynn TA (2020) Fibrosis: from mechanisms to medicines. Nature 587:555–566. 10.1038/s41586-020-2938-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill NR, Fatoba ST, Oke JL, Hirst JA, O’Callaghan CA, Lasserson DS, Hobbs FDR (2016) Global prevalence of chronic kidney disease—a systematic review and meta-analysis. PLoS ONE 11:e0158765. 10.1371/journal.pone.0158765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B, Phan SH, Thannikal VJ, Prunotto M, Desmoulière A, Varga J, De Weaver O, Mareel M, Gabbiani G (2012) Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am J Pathol 180:1340–1355. 10.1016/j.ajpath.2012.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert F, Payan SM, Pelce E, Bouchard L, Sturny R, Lenfant N, Mottola G, Collart F, Kelly RG, Rochais F (2021) FGF10 promotes cardiac repair through a dual cellular mechanism increasing cardiomyocyte renewal and inhibiting fibrosis. Cardiovasc Res. 10.1093/cvr/cvab340 [DOI] [PubMed] [Google Scholar]
- Humphrey JD, Schwartz MA, Tellides G, Milewicz DM (2015) Role of mechanotransduction in vascular biology: focus on thoracic aortic aneurysms and dissections. Circ Res 116:1448–1461. 10.1161/CIRCRESAHA.114.304936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys BD (2018) Mechanisms of renal fibrosis. Annu Rev Physiol 80:309–326. 10.1146/annurev-physiol-022516-034227 [DOI] [PubMed] [Google Scholar]
- Iaizzo PA, Laske TG, Harlow HJ, McClay CB, Garshelis DL (2012) Wound healing during hibernation by black bears (Ursus americanus) in the wild: elicitation of reduced scar formation. Integr Zool 7:48–60. 10.1111/j.1749-4877.2011.00280.x [DOI] [PubMed] [Google Scholar]
- Illingworth CM (1974) Trapped fingers and amputated finger tips in children. J Pediat Surg 9:853–858. 10.1016/s0022-3468(74)80220-4 [DOI] [PubMed] [Google Scholar]
- Jain NB, Ayers GD, Peterson EN, Harris MB, Morse L, O’Connor KC, Garshick E (2015) Traumatic spinal cord injury in the United States, 1933–2012. JAMA 313:2236–2243. 10.1001/jama.2015.6250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang TX, Harn HIC, Ou KL, Lei M, Chuong CM (2019) Comparative regenerative biology of spiny (Acomys cahirinus) and laboratory (Mus musculus) mouse skin. Exp Dermatol 28:442–449. 10.1111/exd.13899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M, Dayan T (2000) Foraging behavior and microhabitat use by spiny mice, Acomys cahirinus and A. russatus, in the presence of Blanford’s Fox (Vulpes cana) odor. J Chem Ecol 26:455–469. 10.1023/A:1005417707588 [DOI] [Google Scholar]
- Keller GL, Burns KA (1989) Husbandry and hematology of captive spiny mice (Acomys cahirinus). Lab Anim Sci 39:625–626 [PubMed] [Google Scholar]
- Kikuchi K, Poss KD (2012) Cardiac regenerative capacity and mechanisms. Annu Rev Cell Dev Biol 28:719–741. 10.1146/annurev-cellbio-101011-155739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi K, Holdway JE, Werdich AA, Anderson RM, Fang Y, Egnaczyk GF, Evans T, Macrae CA, Stainier DYR, Poss KD (2010) Primary contribution to zebrafish heart regeneration by gata4(+) cardiomyocytes. Nature 464:601–605. 10.1038/nature08804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopmans T, van Beijnum H, Roovers EF, Tomasso A, Malhotra D, Boeter J, Psathaki OE, Versteeg D, van Rooij E, Bartscherer K (2021) Ischemic tolerance and cardiac repair in the spiny mouse (Acomys). npiRegen Med 6:78. 10.1038/s41536-021-00188-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramann R, Schneider RK, DiRocco DP, Machado F, Flieg S, Bondzie PA, Henderson JM, Ebert BL, Humphreys BD (2015) Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell 16:51–66. 10.1016/j.stem.2014.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai SL, Marin-Juez R, Moura PL, Kuenne C, Lai JKH, Tsedeke AT, Guenther S, Looso M, Stainier DYR (2017) Reciprocal analyses in zebrafish and medaka reveal that harnessing the immune response promotes cardiac regeneration. eLife 6:25605. 10.7554/eLife.25606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehoczky JA, Robert B, Tabin CJ (2011) Mouse digit tip regeneration is mediated by fate-restricted progenitor cells. Proc Natl Acad Sci USA 108:20609–20614. 10.1073/pnas.1118017108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Mariappan N, Megyesi J, Shank B, Kannan K, Theus S, Price PM, Duffield JS, Portilla D (2013) Proximal tubule PPARα attenuates renal fibrosis and inflammation caused by unilateral ureteral obstruction. Am J Physiol Renal Physiol 305:F618–F627. 10.1152/ajprenal.00309.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Wang H, Cai Z, Wang L, Xu Q, Lövy M, Wang Z, Nevo E (2016) Sympatric speciation of spiny mice, Acomys, unfolded transcriptomically at Evolution Canyon, Israel. Proc Natl Acad Sci USA 113:8254–8259. 10.1073/pnas.1608743113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Kumar S, Dolzhenko E, Alvarado GF, Guo J, Lu C, Chen Y, Li M, Dessing MC, Parvez RK, Cippa PE, Krautzberger AM, Saribekyan G, Smith AD, McMahon AP (2017) Molecular characterization of the transition from acute to chronic kidney injury following ischemia/reperfusion. JCI Insight 2:e94716. https://doi.org/10.1172.jci.insight.94716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love NR, Chen Y, Ishibashi S, Kritsiligkou P, Lea R, Koh Y, Gallop JL, Dorey K, Amaya E (2013) Amputation-induced reactive oxygen species are required for successful Xenopus tadpole tail regeneration. Nat Cell Biol 15:222–228. 10.1038/ncb2659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S, Jolly AJ, Strand KA, Dubner AM, Mutryn MF, Moulton KS, Nemenoff RA, Majesky MW, Weiser-Evans MCM (2020) Smooth muscle-derived progenitor cell myofibroblast differentiation through KLF4 downregulation promotes arterial remodeling and fibrosis. JCI Insight 5(23):139445. 10.1172/jci.insight.139445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maden M (2018) The evolution of regeneration—where does that leave mammals? Int J Dev Biol 62:369–372. 10.1387/ijdb.180031mm [DOI] [PubMed] [Google Scholar]
- Maden M, Brant JO (2019) Insights into the regeneration of skin from Acomys, the spiny mouse. Exp Dermatol 28:436–441. 10.1111/exd.13847 [DOI] [PubMed] [Google Scholar]
- Maden M, Varholick JA (2020) Model systems for regeneration: the spiny mouse Acomys cahirinus. Development 147:167718. 10.1242/dev.167718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maden M, Brant JO, Rubiano A, Sandoval AGW, Simmons C, Mitchell R, Collin-Hooper H, Jacovson J, Omairi S, Patel K (2018) Perfect chronic skeletal muscle regeneration in adult spiny mice Acomys cahirinus. Sci Rep 8:8920. 10.1038/s41598-018-27178-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascharak S, des Jardins-Park HE, Davitt MF, Griffin M, Borrelli MR, Moore AL, Chen K, Buoto B, Chinta M, Foster DS, Shen AH, Januszyk M, Kwon SH, Wernig G, Wan DC, Lorenz HP, Gurtner GC, Longaker MT (2021) Preventing engrailed-1 activation in fibroblasts yields wound regeneration without scarring. Science 372:2374. 10.1126/science.aba2374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matias Santos D, Rita AM, Casanellas I, Brito Ova A, Araujo IM, Power D, Tiscornia G (2016) Ear wound regeneration in the African spiny mouse Acomys cahirinus. Regeneration 3:52–61. 10.1002/reg2.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka S, Ballif BA, Smogorzewska A, McDonald ER 3rd, Hurov KE, Luo J, Bakalarski CE, Zhao Z, Solimini N, Lerenthal Y, Shiloh Y, Gygi SP, Elledge SJ (2007) ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science 316:1160–1166. 10.1126/science,1140321. [DOI] [PubMed] [Google Scholar]
- Miano JM, Long X, Fujiwara K (2007) Serum response factor: master regulator of the actin cytoskeleton and contractile apparatus. Am J Physiol Cell Physiol 292:C70–C81. 10.1152/ajpcell.00386.2006 [DOI] [PubMed] [Google Scholar]
- Mohr SM, Bagriantsev SN, Bracheva EO (2020) Cellular, molecular, and physiological adaptations of hibernation: the solution to environmental challenges. Annu Rev Cell Dev Biol 36:315–338. 10.1146/annurev-cellbio-012820-095945 [DOI] [PubMed] [Google Scholar]
- Montandon SA, Tzika AC, Martins AF, Chopard B, Milinkovitch MC (2014) Two waves of anisotropic growth generate enlarged follicles in the spiny mouse. EvoDevo 5:33. 10.1186/2041-9139-5-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti L, Stalfort J, Barker TH, Abebayehu D (2022) The interplay of fibroblasts, the extracellular matrix, and inflammation in scar formation. J Biol Chem 298:101530. 10.1016/j.jbc.2021.101530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama T, Kawada N, Ando A, Yamauchi A, Horio M, Nagata K, Imai E, Hori M (1998) Up-regulation of HSP47 in the mouse kidney with unilateral ureteral obstruction. Kidney Int 54:110–119. 10.1046/j.1523-1755.1998.00964.x [DOI] [PubMed] [Google Scholar]
- Moya IM, Halder G (2019) Hippo-YAP/TAZ signaling in organ regeneration and regenerative medicine. Nat Rev Mol Cell Biol 20:211–226 [DOI] [PubMed] [Google Scholar]
- Murugan NJ, Vigran HJ, Miller KA, Golding A, Pham QL, Sperry MM, Rasumssen-Ivey C, Kane AW, Kaplan DL, Levin M (2022) Acute multidrug delivery via a wearable bioreactor facilitates long-term limb regeneration and functional recovery in adult Xenopus laevis. Sci Adv 8:eabj2164. 10.1126/sciadv.abj2164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira-Rodrigues J, Leite SC, Pinto-Costa R, Sousa SC, Luz LL, Sintra MA, Oliveira R, Monteiro AC, Pinheiro GG, Vitorino M, Silva JA, Simão S, Fernandes VE, Provaznik J, Benes V, Cruz CD, Safronov BV, Magalhães A, Reis CA, Vieira J, Vieira CP, Tiscórnia G, Araújo IM (2022) Rewired glycosylation activity promotes scarless regeneration and functional recovery in spiny mice after complete spinal cord resection. Dev Cell 57:1–11. 10.1016/j.devcel.2021.12.008 [DOI] [PubMed] [Google Scholar]
- Okamura DM, Brewer CM, Wakenight P, Bahrami N, Bernardi K, Tran A, Olson J, Shi X, Yeh SY, Piliponsky A, Collins SJ, Nguyen ED, Timms AE, MacDonald JW, Bammler TK, Nelson BN, Millen KJ, Beier DR, Majesky MW (2021) Spiny mice activate unique transcriptional programs after severe kidney injury regenerating organ function without fibrosis. iScience 24:103269. 10.1016/j.isci.2021.103269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura DM, Nguyen ED, Beier DR, Majesky MW (2022) Wound healing and regeneration in spiny mice (Acomys cahirinus). Curr Topics Dev Biol. 10.1016/bs.ctdb.2022.03.001 [DOI] [PubMed] [Google Scholar]
- Passman JN, Dong XR, Wu SP, Maguire CT, Hogan KA, Bautch VL, Majesky MW (2008) A sonic hedgehog signaling domain in the arterial adventitia supports resident Sca1+ smooth muscle progenitor cells. Proc Natl Acad Sci USA 105:9349–9354. 10.1073/pnas.0711382105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pembury Smith MQR, Ruxton GD (2020) Camouflage in predators. Biol Rev Camb Philos Soc 95:1325–1340. 10.1111/brv.12612. [DOI] [PubMed] [Google Scholar]
- Peng H, Shindo K, Donahue RR, Gao E, Ahern BM, Levitan BM, Tripathi H, Powell D, Noor A, Elmore GA, Satin J, Seifert AW, Abdel-Latif A (2021) Adult spiny mice (Acomys) exhibit endogenous cardiac recovery in response to myocardial infarction. npiRegen Med 6:74. 10.1038/s41536-021-00186-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennathur S, Pasichnyk K, Bahrami NM, Zeng L, Febbraio M, Yamaguchi I, Okamura DM (2015) The macrophage phagocytic receptor CD36 promotes fibrogenic pathways on removal of apoptotic cells during chronic kidney injury. Am J Pathol 185:2232–2245. 10.1016/j.ajpath.2015.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro G, Prata DF, Araújo IM, Tiscornia G (2018) The African spiny mouse [Acomys spp.] as an emerging model for development and regeneration. Lab Anim 52:565–576. 10.1177/0023677218769921 [DOI] [PubMed] [Google Scholar]
- Plikus MV, Wang X, Sinha S, Forte E, Thompson SM, Herzog EL, Driskell RR, Rosenthal N, Biernaskie J, Horsley V (2021) Fibroblasts: origins, definitions, and functions in health and disease. Cell 184:3852–3872. 10.1016/j.cell.2021.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrello ER, Mahmoud AI, Simpson E, Hill JA, Olson EN, Sadek HA (2011) Transient regenerative potential of the neonatal mouse heart. Science 331:1078–1080. 10.1126/science [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poss KD (2010) Advances in understanding tissue regenerative capacity and mechanisms in animals. Nat Rev Genet 11:710–722. 10.1038/nrg2879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratsinis H, Mavrogonatou E, Kletsas D (2019) Scarless wound healing: from development to senescence. Adv Drug Deliv Rev 146:325–343. 10.1016/j.addr.2018.04.011 [DOI] [PubMed] [Google Scholar]
- Qi Y, Dasa O, Maden M, Vohra R, Batra A, Walter G, Yarrow JF, Aranda JM Jr, Raizada MK, Pepine CJ (2021) Functional heart recovery in an adult mammal, the spiny mouse. Int J Cardiol 338:196–203. 10.1016/j.ijcard.2021.06.015 [DOI] [PubMed] [Google Scholar]
- Regula C (2012). “Acomys cahirinus” (On-line), Animal diversity web. Accessed February 23, 2022 at https://animaldiversity.org/accounts/Acomys_cahirinus/.
- Reinhardt JW, Breuer CK (2021) Fibrocytes: a critical review and practical guide. Front Immunol 12:784401. 10.3389/fimmu.2021.784401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinkevich Y, Lindau P, Ueno H, Longaker MT, Weissman IL (2011) Germ-layer and lineage-restricted stem/progenitors regenerate the mouse digit tip. Nature 476:409–413. 10.1038/nature10346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinkevich Y, Walmsley GG, Hu MS, Maan ZN, Newman AM, Drukker M, Januszyk M, Krampitz GW, Gurtner GC, Lorenz HP, Weissman IL, Longaker MT (2015) Identification and isolation of a dermal lineage with intrinsic fibrogenic potential. Science 348:aaa2151. 10.1126/science.aaa2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert AW, Kiama SG, Seifert MG, Goheen JR, Palmer TM, Maden M (2012) Skin shedding and tissue regeneration in African spiny mice (Acomys). Nature 489:561–565. 10.1038/nature11499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simkin J, Gawriluk TR, Gensel JC, Seifert AW (2017) Macrophages are necessary for epimorphic regeneration in African spiny mice. eLife 6:e24623. 10.7554/eLife.24623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singbartl K, Green SA, Ley K (2000) Blocking P-selectin protects from ischemia/reperfusion-induced acute renal failure. FASEB J 14:48–54. 10.1096/fasebj.14.1.48 [DOI] [PubMed] [Google Scholar]
- Smith L, Cho S, Discher DE (2017) Mechanosensing of matrix by stem cells: from matrix heterogeneity, contractility, and the nucleus in pore-migration to cardiogenesis and muscle stem cells in vivo. Semin Cell Dev Biol 71:84–98. 10.1016/j.semcdb.2017.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling EJ, McCreless EE (2006) Adaptations in the aye-aye: a review. In: Gould L, Sauther ML (eds) Lemurs: ecology and adaptation. Springer, New York, pp 159–184. 10.1007/978-0-387-34586-4_8 [DOI] [Google Scholar]
- Stewart DC, Serrano PN, Rubiano A, Yokosawa R, Sandler J, Mukhtar M, Brant JO, Maden M, Simmons CS (2018) Unique behavior of dermal cells from regenerative mammal, the African spiny mouse, in response to substrate stiffness. J Biomech 81:149–154. 10.1016/j.jbiomech.2018.10.005 [DOI] [PubMed] [Google Scholar]
- Stockdale WT, Lemieux ME, Killen AC, Zhao J, Hu Z, Riepsaame J, Hamilton N, Kudoh T, Riley RP, van Aerle R, Yamamoto Y, Mommersteeg MTM (2018) Heart regeneration in the Mexican cavefish. Cell Rep 25:1997–2007. 10.1016/j.celrep.2018.10.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeter KA, Sunshine MD, Brant JO, Sandoval AGW, Maden M, Fuller DD (2020) Molecular and histologic outcomes following spinal cord injury in spiny mice, Acomys cahirinus. J Comp Neurol 528:1535–1547. 10.1002/cne.24836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbott HE, Mascharak S, Griffin M, Wan DC, Longaker MT (2022) Wound healing, fibroblast heterogeneity, and fibrosis. Cell Stem Cell 29:1161–1180. 10.1016/j.stem.2022.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SM, Phan QM, Winuthayanon S, Driskell IM, Driskell RR (2022) Parallel single cell multi-omics analysis of neonatal skin reveals transitional fibroblast states that restricts differentiation into distinct fates. J Invest Dermatol. 10.1016/j/jid/2021.11.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA (2002) Myofibroblasts and mechano-regulation of connective tissue remodeling. Nat Rev Mol Cell Biol 3:349–363. 10.1038/nrm809 [DOI] [PubMed] [Google Scholar]
- Travers JG, Tharp CA, Rubino M, McKinsey TA (2022) Therapeutic targets for cardiac fibrosis: from old school to next-gen. J Clin Invest 132:e148554. 10.1172/JCI148554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Putten S, Shafieyan Y, Hinz B (2016) Mechanical control of cardiac myofibroblasts. J Mol Cell Cardiol 93:133–142. 10.1016/j.yjmcc.2015.11.025 [DOI] [PubMed] [Google Scholar]
- Wang Y, Qiao Z, Mao L, Li F, Liang X, An X, Zhang S, Liu X, Kuang Z, Wan N, Nevo E, Li K (2022) Sympatric speciation of the spiny mouse from evolution Canyon in Israel substantiated genomically and methylomically. Proc Natl Acad Sci USA 119:e2121822119. 10.1073/pnas.2121822119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehner D, Becker CG (2022) An exception to the rule? Regeneration of the injured spinal cord in the spiny mouse. Dev Cell 57:415–416. 10.1016/j.devcel.2022.02.002 [DOI] [PubMed] [Google Scholar]
- Wells RG, Discher DE (2008) Matrix elasticity, cytoskeletal tension, and TGF-beta: the insoluble and soluble meet. Sci Signal 1:13. 10.1126/stke.110pe13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MC, Patel JH, Kakebeen AD, Wills AE (2021) Nutrient availability contributes to a graded refractory period for regeneration in Xenopus tropicalis. Dev Biol 473:59–70. 10.1016/j.ydbio.2021.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Guan KL (2021) Hippo signaling in embryogenesis and development. Trends Biochem Sci 46:51–63. 10.1016/j.tibs.2020.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Montaniel KRC, Saleh MA, Xiao L, Chen W, Owens GK, Humphrey JD, Majesky MW, Paik DT, Hatzopoulos AK, Machur MS, Harrison DG (2016) Origin of matrix-producing cells that contribute to aortic fibrosis in hypertension. Hypertension 67:461–468. 10.1161/HYPERTENSIONAHA.115.06123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA, Ramalingam TR (2012) Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med 18:1028–1040. 10.1038/nm.2807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota T, McCourt J, Ma F, Ren S, Li S, Kim TH, Kurmangaliyev YZ, NasiriR AS, Nguyen T, Tan XHM, Zhou Y, Wu R, Rodriguez A, Cohn W, Wang Y, Whitelegge J, Ryazantsev S, Khademhosseini A, Teitell MA, Chiou PY, Birk DE, Rowat AC, Crosbie RH, Pellegrini M, Seldin M, Lusis AJ, Deb A (2020) Type V collagen in scar tissue regulates the size of scar after heart injury. Cell 182:545–562. 10.1016/j.cell.2020.06.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimatsu Y, Watabe T (2022) Emerging roles of inflammation-mediated endothelial-mesenchymal transition in health and disease. Inflamm Regen 42:9. 10.1186/s41232-021-00186-3 [DOI] [PMC free article] [PubMed] [Google Scholar]


