Abstract
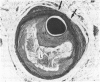
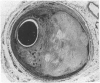
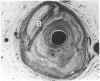
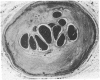
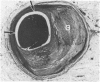
All segments of clinically significant stenosis in the coronary arteries of 54 men with stable angina were categorised according to the position of the plaques (eccentric or concentric) and the presence or absence of a pool of extracellular lipid. In the group as a whole, stenosis of greater than 50% by diameter was caused by concentric fibrous plaques in 48% of lesions, by concentric lipid plaques in 28%, by eccentric fibrous plaques in 12%, and by eccentric lipid plaques in 12%. In addition, 43 of the 54 patients had one or more stenoses with multiple channels (recanalisation). Eccentric plaques with an arc of normal vessel wall occupying more than 16% of the circumference of the residual lumen were considered to have a vasospastic potential and made up 15% of all lesions with stenosis of greater than 50% by diameter. Forty four per cent of plaques causing stenosis between 30% and 50% by diameter were eccentric and retained a considerable arc of normal media. These lesions were often in series with segments of higher grade stenosis that did not have an arc of normal media. The overall frequency of plaque types gave no indication of the proportions of different plaque types within an individual. In 15% of patients all the plaques causing greater than 50% diameter stenosis were fibrous and in 13% of patients all the plaques were of the lipid type. Most patients had mixtures of all plaque types in varying proportions. Plaques with a large pool of lipid were not found in 33% of patients whereas they formed greater than 90% of the plaques in 9% of patients. No segments of stenosis > 50% by diameter with a vasospastic potential were found in 44% of the patients but one or more such plaques was present in the the remaining 56%. Three patients (6%) each had five separate segments of stenosis with a vasospastic potential. The results indicate that even in a population of men with stable angina in whom diabetes is excluded the distribution of types of atheromatous lesions is very heterogenous.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brandt P. W., Partridge J. B., Wattie W. J. Coronary arteriography; method of presentation of the arteriogram report and a scoring system. Clin Radiol. 1977 Jul;28(4):361–365. doi: 10.1016/s0009-9260(77)80140-2. [DOI] [PubMed] [Google Scholar]
- Brown B. G., Bolson E. L., Dodge H. T. Dynamic mechanisms in human coronary stenosis. Circulation. 1984 Dec;70(6):917–922. doi: 10.1161/01.cir.70.6.917. [DOI] [PubMed] [Google Scholar]
- Brown B. G. Coronary vasospasm. Observations linking the clinical spectrum of ischemic heart disease to the dynamic pathology of coronary atherosclerosis. Arch Intern Med. 1981 May;141(6):716–722. doi: 10.1001/archinte.141.6.716. [DOI] [PubMed] [Google Scholar]
- Davies M. J., Thomas A. C. Plaque fissuring--the cause of acute myocardial infarction, sudden ischaemic death, and crescendo angina. Br Heart J. 1985 Apr;53(4):363–373. doi: 10.1136/hrt.53.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies M. J., Thomas A. Thrombosis and acute coronary-artery lesions in sudden cardiac ischemic death. N Engl J Med. 1984 May 3;310(18):1137–1140. doi: 10.1056/NEJM198405033101801. [DOI] [PubMed] [Google Scholar]
- Freudenberg H., Lichtlen P. R. Das normale Wandsegment bei Koronarstenosen--eine postmortale Studie. Z Kardiol. 1981 Dec;70(12):863–869. [PubMed] [Google Scholar]
- Gage J. E., Hess O. M., Murakami T., Ritter M., Grimm J., Krayenbuehl H. P. Vasoconstriction of stenotic coronary arteries during dynamic exercise in patients with classic angina pectoris: reversibility by nitroglycerin. Circulation. 1986 May;73(5):865–876. doi: 10.1161/01.cir.73.5.865. [DOI] [PubMed] [Google Scholar]
- Hort W., Moosdorf R., Kalbfleisch H., Köhler F., Milzner-Schwarz U., Frenzel H. Postmortale Untersuchungen über Lokalisation und Form der stärksten Stenosen in den Koronararterien und ihre Beziehung zu den Risikofaktoren. Z Kardiol. 1977 Jul;66(7):333–340. [PubMed] [Google Scholar]
- Lundberg B. Chemical composition and physical state of lipid deposits in atherosclerosis. Atherosclerosis. 1985 Jul;56(1):93–110. doi: 10.1016/0021-9150(85)90087-5. [DOI] [PubMed] [Google Scholar]
- Moise A., Théroux P., Taeymans Y., Waters D. D. Factors associated with progression of coronary artery disease in patients with normal or minimally narrowed coronary arteries. Am J Cardiol. 1985 Jul 1;56(1):30–34. doi: 10.1016/0002-9149(85)90561-2. [DOI] [PubMed] [Google Scholar]
- Quyyumi A. A., Al-Rufaie H. K., Olsen E. G., Fox K. M. Coronary anatomy in patients with various manifestations of three vessel coronary artery disease. Br Heart J. 1985 Oct;54(4):362–366. doi: 10.1136/hrt.54.4.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saner H. E., Gobel F. L., Salomonowitz E., Erlien D. A., Edwards J. E. The disease-free wall in coronary atherosclerosis: its relation to degree of obstruction. J Am Coll Cardiol. 1985 Nov;6(5):1096–1099. doi: 10.1016/s0735-1097(85)80314-4. [DOI] [PubMed] [Google Scholar]
- Thomas A. C., Davies M. J. Post-mortem investigation and quantification of coronary artery disease. Histopathology. 1985 Sep;9(9):959–976. doi: 10.1111/j.1365-2559.1985.tb02880.x. [DOI] [PubMed] [Google Scholar]
- Waller B. F. Coronary luminal shape and the arc of disease-free wall: morphologic observations and clinical relevance. J Am Coll Cardiol. 1985 Nov;6(5):1100–1101. doi: 10.1016/s0735-1097(85)80315-6. [DOI] [PubMed] [Google Scholar]