Abstract
Background
While vaginitis is a leading cause of primary care visits among women with a gynecologic-related diagnosis, there are limited contemporary data on the healthcare burden. This study describes the real-world healthcare resource utilization (HCRU) of patients presenting with vaginitis symptoms in the United States (US) at symptom presentation and over long-term follow-up.
Methods
This retrospective study utilized IQVIA’s Longitudinal Prescription (LRx) and Medical Claims (Dx) databases to capture patients presenting with vaginitis symptoms from January 1, 2018 to September 30, 2022. The date of the first diagnosis code for vaginitis or related symptoms was considered the first clinical presentation (“index visit”). Healthcare visits, diagnostic testing, and treatments were assessed for patients at presentation (index date +2 days) and 12-month follow-up, stratified by pregnancy status at index. In a subset of patients with linkage to IQVIA Ambulatory EMR – US (AEMR), multivariable models were used to evaluate associations between insurance type, patient characteristics, diagnostic test(s) performed at presentation, and HCRU outcomes (subsequent vaginitis-related healthcare visits and ≥2 vaginitis treatment dates) over follow-up.
Results
A total of 18,745,351 people were documented with vaginitis symptoms or vaginitis in the study selection window, of which 4,000,615 patients met all selection criteria for analysis: 3,787,354 were not pregnant and 213,261 had evidence of pregnancy. About one-fourth (23.8%) of the non-pregnant cohort and half (47.6%) of the pregnant cohort had claims for at least 1 diagnostic test at symptom presentation, with traditional methods being most commonly used (44.1% and 36.4% for non-pregnant and pregnant patients, respectively), followed by direct probe (20.0% and 24.1%), and lastly nucleic acid amplification test (NAAT) panel (including bacterial vaginosis, vulvovaginal candidiasis, and trichomoniasis; 6.6% and 8.3%). Despite low diagnostic testing rates, 50.1% of the non-pregnant and 60.9% of the pregnant cohort received prescribed vaginitis treatment, most frequently metronidazole or fluconazole, and 28.8% of the non-pregnant and 30.9% of the pregnant cohort had subsequent vaginitis-related visits within 12 months. Among both the non-pregnant and pregnant cohorts, patients with Medicaid insurance had significantly higher odds of repeat healthcare visits and ≥2 treatment dates during follow-up relative to patients with commercial insurance.
Conclusion
This study demonstrated that vaginitis poses a high clinical burden in the US, possibly attributed to low diagnostic testing rates, use of tests with poor performance, and high rates of empiric treatment. There is an unmet need for rapid, accurate vaginitis diagnostic testing at the point-of-care to reduce empiric prescribing and improve diagnostic and treatment accuracy and efficiency.
Keywords: healthcare utilization, NAAT, vaginitis, vaginosis, infectious disease, diagnostics
AJOG MFM at a Glance.
Why was this study conducted?
This study investigated real-world diagnostic, healthcare resource utilization, and treatment patterns of people with vaginitis symptoms in the United States (US).
What are the key findings?
From 01/01/2018 to 09/30/2022, nearly 19 million people sought care for vaginitis symptoms, of which 4,000,615 were analyzed. We found underutilization of diagnostic tests overall, while 26% of non-pregnant and 22% of pregnant people received vaginitis treatment at their initial visit, suggesting high empiric prescribing rates. The analyzed cohort had >2 million vaginitis-related healthcare visits within 12 months after the initial visit.
What does this study add to what is already known?
This study highlights the clinical burden of vaginitis in the US and suggests inefficiencies and deficiencies in current diagnostic and treatment approaches. With misdiagnosis, mistreatment, and the challenge of antimicrobial resistance in vaginitis-associated organisms, there is a need for new point-of-care diagnostics to improve patient management.
Introduction
Vaginitis is a common reason for primary care visits among women with gynecologic-related diagnoses in the United States (US), frequently cited as accounting for >10 million office visits annually.1, 2, 3 The etiology determines appropriate treatment, with bacterial vaginosis (BV; accounting for 50% of cases), vulvovaginal candidiasis (VVC; 20−25% of cases), and trichomoniasis (TV; 15−20% of cases) being the most common causes.1,3, 4, 5, 6 Disease recurrence is common: > 50% of patients have symptomatic or confirmed BV within 6 months after treatment,7 10−20% of women have complicated VVC requiring special treatment considerations, including recurrent disease,8 and about 20% of patients with TV experience re-infection within 3 months of treatment.9 Recurrence may result from suboptimal management or re-infection, leading to additional outpatient healthcare resource utilization (HCRU) beyond the initial clinical presentation.1,4 Accurate and timely diagnosis is critical for resolving symptoms, especially for pregnant populations, as BV and TV have been associated with adverse neonatal outcomes, including preterm delivery and low birth weight.10,11
Various diagnostic methods are available to clinicians to inform patient management, including traditional methods (e.g., wet mount microscopy, culture, Amsel criteria, Gram stain and Nugent score) and advanced, molecular tests with or without amplification. Nucleic acid amplification testing (NAAT) has higher sensitivity and specificity than traditional methods and non-amplified direct molecular probe.2,12, 13, 14 The choice of diagnostic test may also have longer-term implications. A recent study found commercially-insured women receiving NAAT at their initial vaginitis encounter had significantly lower all-cause healthcare costs over 12 months follow-up vs. those clinically evaluated or receiving direct probe testing.3 However, real-world data on the disease-specific burden of diagnosing and treating vaginitis symptoms are limited. This study was undertaken to describe the diagnostic, HCRU, and treatment patterns of patients presenting with vaginitis symptoms using claims databases representing multiple insurance types, to provide a contemporary update on the clinical burden of vaginitis in the US.
Materials and methods
Study design
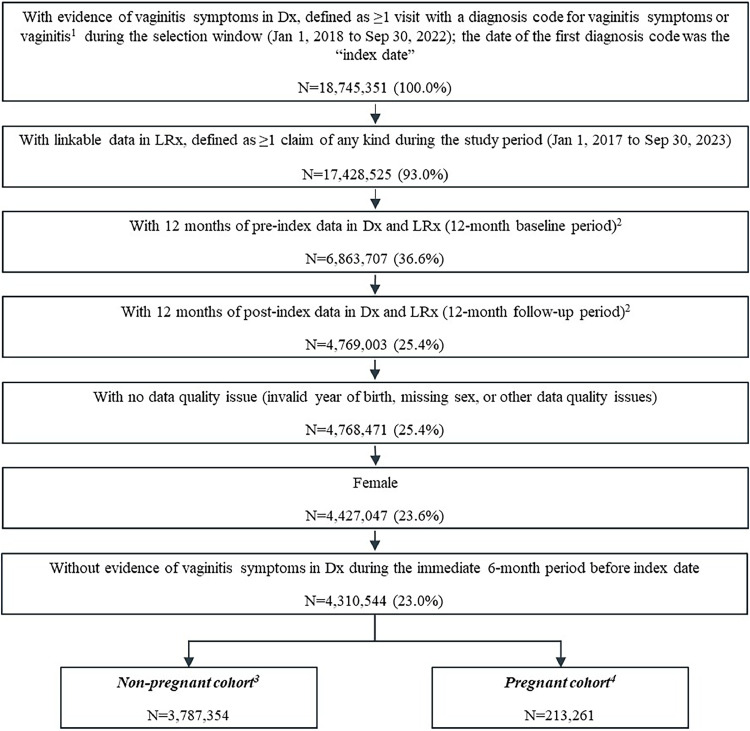
This retrospective cohort study leveraged IQVIA’s Longitudinal Prescription Claims (LRx) and Outpatient Medical Claims (Dx) databases from January 1, 2017 to September 30, 2023. LRx captures information on dispensed prescriptions sourced from retail, mail, long-term care, and specialty pharmacies, with 92% coverage of the retail channel, 72% coverage of standard mail service, and 76% of long-term care facilities in the US. Dx includes approximately 1 billion professional fee claims per year, representing about 75% of US physician activity. Patients with ≥1 International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) diagnosis code in Dx for vaginitis symptoms or vaginitis (Supplemental Table 1) were identified from January 1, 2018 to September 30, 2022 (selection window); the first diagnosis code date was the “index date” (Figure 1). All patients were required to be female, have 12 months of activity before (baseline period) and following (follow-up period) index in Dx and LRx to ensure stability of patient data, and have no vaginitis diagnoses within 6 months before index. Although vaginitis is considered an acute condition, 12 months follow-up was selected to assess real-world patient journeys within a timeframe consistent in which BV and VVC recurrence commonly occur.
Figure 1.
Patient selection
(A) Vaginitis symptoms were defined as ICD-10 L29.2, L29.3, N76.0-N76.3, N77.1, N89.8, N89.9, or N95.2 and vaginitis was defined as ICD-10 A51.0, A54.02, A56.02, A59.01, A60.04, B37.3X, or B96.89. (B) Patients were required to have consistent reporting of data from a medical provider in Dx and from the patient’s most frequently visited pharmacy in LRx each month during the baseline and follow-up periods. (C) The non-pregnant cohort excluded patients with any diagnosis codes for pregnancy in the baseline or follow-up period. (D) The pregnant cohort excluded patients not aged 12-54 at index date, or with evidence of delivery or abortive outcomes within 30 days prior to the index date.
Chen. Real-world clinical burden of patients presenting with vaginitis symptoms in the United States. AJOG Glob Rep 2025.
As vaginitis treatment may differ between non-pregnant and pregnant populations4 and pregnant patients may have more healthcare visits overall, patients were stratified into 2 cohorts: non-pregnant (without diagnosis code for pregnancy in baseline or follow-up) and pregnant (with ≥1 ICD-10 diagnosis code for pregnancy within index ± 30 days and age 12−54 years).
Study measures
Patient demographics and index visit characteristics were reported from the index date. Comorbidities and select risk factors for vaginitis (identified by ICD-10-CM codes) were reported from 12-month baseline and index. Outcomes included claimed diagnostic testing (from Current Procedural Terminology [CPT] codes), vaginitis-related healthcare visits (with ≥1 diagnosis code for vaginitis symptoms or vaginitis in any position), and vaginitis treatments (from National Drug Codes [NDC] for prescription fills or procedure codes for drug administrations; Supplemental Table 2) at symptom presentation (up to index + 2 days) and over 12-month follow-up (including index). Diagnostic tests of interest included traditional methods (vaginal pH, wet mount microscopy, whiff test, Gram stain, culture, and sialidase activity assay), direct probe, NAAT with 1-2 targets, and NAAT panel (i.e., with BV, VVC, and TV targets).
Analyses
The primary analysis was descriptive, with measures separately reported for the non-pregnant and pregnant cohorts. A secondary comparative analysis was conducted on the subset of patients with Medicaid or commercial insurance linkable to IQVIA Ambulatory EMR – US (AEMR; database description in Supplement) and with non-missing race/ethnicity. Since previous research suggests Medicaid-insured patients have a higher burden of BV than those with other insurance types,15 the secondary objective focused on Medicaid- and commercially-insured patients. Given differences in managing infectious and non-infectious vaginitis,4 and the high proportion of non-pregnant patients with evidence of atrophic vaginitis (Table 1), patients age ≥65 years were excluded to mitigate the impact of age-related atrophic vaginitis on outcomes. Logistic regression models evaluated the association between insurance type and the odds of repeat vaginitis-related healthcare visits and the odds of ≥2 vaginitis treatment claim dates during follow-up, adjusting for diagnostic test type at symptom presentation (any NAAT [panel or with 1−2 targets], direct probe, traditional methods, no claimed test), and key demographic and baseline characteristics). P-values <.05 were considered statistically significant. Analyses were conducted using SAS® 9.4 (SAS Institute Inc., Cary, NC).
Table 1.
Patient demographic and baseline clinical characteristics and characteristics of the index visit
| Measures | Non-pregnant cohort (N=3,787,354) |
Pregnant cohort (N=213,261) |
|---|---|---|
| Age at index date | ||
| Mean (SD) | 49.8 (21.0) | 28.4 (6.2) |
| Median (Q1, Q3) | 52 (34, 66) | 28 (24, 33) |
| Geographic region (n, %) | ||
| Northeast | 757,217 (20.0%) | 35,587 (16.7%) |
| Midwest | 812,253 (21.4%) | 49,515 (23.2%) |
| South | 1,592,773 (42.1%) | 90,871 (42.6%) |
| West | 625,057 (16.5%) | 37,287 (17.5%) |
| Other or missing | 54 (0.0%) | 1 (0.0%) |
| Payer type (n, %) | ||
| Commercial | 2,559,265 (67.6%) | 140,006 (65.7%) |
| Medicare (including Part D) | 760,097 (20.1%) | 2,360 (1.1%) |
| Medicaid1 | 467,950 (12.4%) | 70,893 (33.2%) |
| Cash payments | 42 (0.0%) | 2 (0.0%) |
| Index year (n, %) | ||
| 2018 | 1,201,479 (31.7%) | 65,080 (30.5%) |
| 2019 | 882,080 (23.3%) | 48,178 (22.6%) |
| 2020 | 639,635 (16.9%) | 38,273 (17.9%) |
| 2021 | 624,347 (16.5%) | 36,645 (17.2%) |
| 2022 | 439,813 (11.6%) | 25,085 (11.8%) |
| Baseline Charlson Comorbidity Index score | ||
| Mean (SD) | 0.9 (1.5) | 0.2 (0.6) |
| Median (Q1, Q3) | 0 (0, 1) | 0 (0, 0) |
| Baseline comorbidities (n, %)2 | ||
| Anxiety | 730,926 (19.3%) | 30,637 (14.4%) |
| Obesity | 654,768 (17.3%) | 37,629 (17.6%) |
| Depression | 559,840 (14.8%) | 21,923 (10.3%) |
| Chronic pulmonary disease | 553,839 (14.6%) | 15,917 (7.5%) |
| Diabetes, without chronic complications | 371,233 (9.8%) | 4,653 (2.2%) |
| Diabetes, with chronic complications | 217,031 (5.7%) | 722 (0.3%) |
| Renal disease | 190,616 (5.0%) | 544 (0.3%) |
| Baseline risk factors for vaginitis (n, %) | ||
| Infections with a predominantly sexual mode of transmission | 115,723 (3.1%) | 11,913 (5.6%) |
| Drug dependence | 68,040 (1.8%) | 4,666 (2.2%) |
| Nondependent abuse of drugs | 34,562 (0.9%) | 4,281 (2.0%) |
| Smoking | 58,967 (1.6%) | 2,656 (1.2%) |
| Provider specialty at the index visit (n, %) | ||
| Obstetrician/gynecologist | 1,323,622 (34.9%) | 98,965 (46.4%) |
| Primary care | 1,022,691 (27.0%) | 20,273 (9.5%) |
| ER/urgent care | 189,878 (5.0%) | 16,706 (7.8%) |
| Pediatrics | 182,665 (4.8%) | 643 (0.3%) |
| Fertility/reproductive medicine | 1,181 (0.0%) | 148 (0.1%) |
| Others3 | 708,433 (18.7%) | 38,180 (17.9%) |
| Unknown | 358,884 (9.5%) | 38,346 (18.0%) |
| Setting of care at the index visit (n, %) | ||
| Office visit | 2,719,849 (71.8%) | 114,553 (53.7%) |
| Independent laboratory4 | 284,021 (7.5%) | 36,820 (17.3%) |
| ER | 225,817 (6.0%) | 32,245 (15.1%) |
| Urgent care | 169,985 (4.5%) | 4,624 (2.2%) |
| Telehealth | 105,862 (2.8%) | 1,649 (0.8%) |
| Other | 279,282 (7.4%) | 23,319 (10.9%) |
| Select vaginitis diagnosis codes at the index visit (not mutually exclusive; n, %)5 | ||
| N76.0 (Acute vaginitis) | 1,051,876 (27.8%) | 74,468 (34.9%) |
| N89.8 (other specified noninflammatory disorders of vagina) | 921,311 (24.3%) | 91,052 (42.7%) |
| N95.2 (Postmenopausal atrophic vaginitis) | 763,593 (20.2%) | 223 (0.1%) |
| B96.89 (other specified bacterial agents as the cause of diseases classified elsewhere) | 563,146 (14.9%) | 22,084 (10.4%) |
| B37.3 (Candidiasis of vulva and vagina) | 547,570 (14.5%) | 33,687 (15.8%) |
| A59.01 (Trichomonal vulvovaginitis) | 22,655 (0.6%) | 4,763 (2.2%) |
ER, emergency room; SD, standard deviation; Q1, quartile 1; Q3, quartile 3.
Medicaid includes managed Medicaid.
Individual comorbidities from the Charlson Comorbidity Index and other comorbidities of interest were investigated. Comorbidities present in >5% of either cohort are presented.
Other specialties include non-specific specialties (e.g., nurse practitioner which was present in 9.7% of the pregnant cohort and 10.6% of the non-pregnant cohort) and specific specialties (e.g., clinical pathology, urology, cytopathology). There were no specific specialties present in >4% of either cohort.
Defined as a laboratory certified to perform diagnostic and/or clinical tests independent of an institution or a physician's office (e.g., LabCorp or Quest).
The top 5 vaginitis diagnosis codes in either cohort ae shown.
Chen. Real-world clinical burden of patients presenting with vaginitis symptoms in the United States. AJOG Glob Rep 2025.
Results
Participants
In total, 18,745,351 people sought care for vaginitis symptoms between January 1, 2018 to September 30, 2022. Of these, 4,000,615 met all selection criteria, including 3,787,354 non-pregnant and 213,261 pregnant patients (Figure 1).
Baseline characteristics
Table 1 presents characteristics of the non-pregnant and pregnant cohorts. The mean age (SD) of all non-pregnant patients was 49.8 (21.0) years, 67.6% were commercially-insured, and 42.1% were from the Southern US. The mean (SD) Charlson Comorbidity Index (CCI) score was low for the non-pregnant cohort, at 0.9 (1.5); the most common comorbidities were anxiety (19.3%), obesity (17.3%), and depression (14.8%). Vaginitis risk factors were each observed in <4% of the cohort. About one-third (34.9%) of the non-pregnant cohort presented to an obstetrician/gynecologist (Ob/Gyn) and 27.0% presented to primary care providers at index; most visits (71.8%) were in office settings.
The mean (SD) age of pregnant patients was 28.4 (6.2) years, 65.7% were commercially-insured, and 42.6% were in the Southern US. The pregnant cohort had a low baseline comorbidity burden (mean [SD] CCI, 0.2 [0.6]); obesity (17.6%), anxiety (14.4%), and depression (10.3%) were the most frequent comorbidities and risk factors for vaginitis were infrequently observed. Nearly half (46.4%) of the pregnant cohort visited an Ob/Gyn at index and 9.5% visited primary care providers; 53.7% of patients had their index visit in office settings.
Testing at symptom presentation
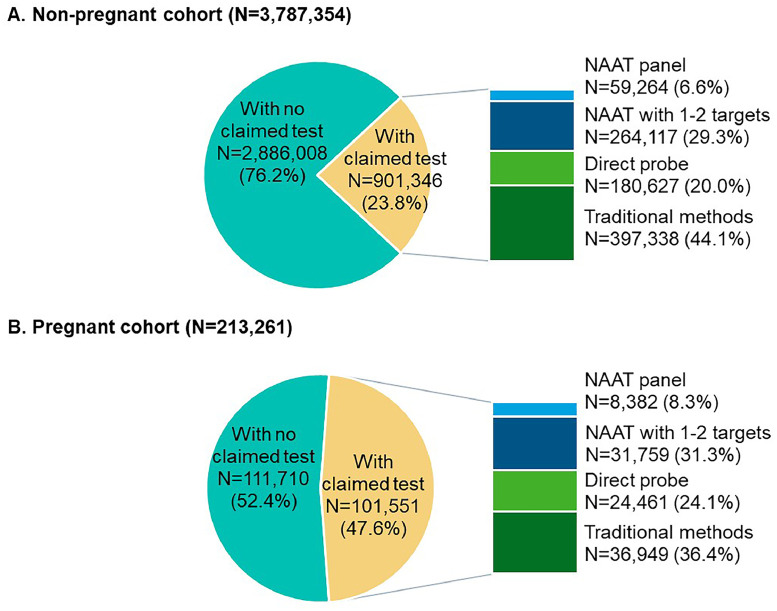
Figure 2 describes the distribution of vaginitis tests at symptom presentation. In total, 901,346 patients in the non-pregnant cohort (23.8%) had ≥1 vaginitis diagnostic test claim at symptom presentation and the remaining 2,886,008 patients (76.2%) did not have claimed tests. Among those with claimed tests, 44.1% received traditional methods, followed by NAAT with 1-2 targets (29.3%), direct probe (20.0%), and NAAT panel (6.6%) (Figure 2A). Claimed test types, stratified by BV (ICD-10 N76.0), VVC (ICD-10 B37.3), and TV (ICD-10 A59.01) diagnosis codes at index are shown in Figure 3A; NAAT panel remained infrequently used among these patients.
Figure 2.
Claimed vaginitis test types at symptom presentation in the non-pregnant (A) and pregnant (B) cohorts
A hierarchy was applied to test types at symptom presentation. Patients with multiple tests were first categorized under NAAT panel, followed by NAAT with 1−2 targets, direct probe, and traditional methods.
Chen. Real-world clinical burden of patients presenting with vaginitis symptoms in the United States. AJOG Glob Rep 2025.
Figure 3.
Test types at symptom presentation, stratified by BV, VVC, and TV diagnosis codes on the index date in the non- pregnant (A) and pregnant (B) cohorts
Chen. Real-world clinical burden of patients presenting with vaginitis symptoms in the United States. AJOG Glob Rep 2025.
There were 101,551 patients in the pregnant cohort (47.6%) with and 110,710 patients (52.4%) without claimed testing at symptom presentation. Of tested patients, more than one-third (36.4%) received traditional methods, followed by NAAT 1-2 targets (31.3%), direct probe (24.1%), and NAAT panel (8.3%) (Figure 2B). Among patients with BV, VVC, or TV diagnosis codes, NAAT panel remained the least commonly used test (Figure 3B).
Follow-up HCRU
After index, patients in the non-pregnant cohort had 1,918,647 additional vaginitis-related healthcare visits in total. Nearly one-third (28.8%) of non-pregnant patients had ≥1 vaginitis-related visit after index, including 4.6% (N=175,558) of patients with ≥3 visits (Figure 4). Although the vaginitis-related healthcare visits were identified by ICD-10 diagnostic codes, the medical claims database did not inform the specific reason for each visit (e.g., persistent symptoms, new symptoms, treatment follow-up); therefore, we were unable to assess the patient experience between the time of an index visit and subsequent vaginitis-related clinic visit. Furthermore, the non-pregnant cohort had 1,743,820 unique visits with claimed vaginitis tests during follow-up, including any tests at symptom presentation. Overall, 9.0% (N=22,724) of the non-pregnant cohort had ≥2 unique test dates during follow-up (Figure 5). Of non-pregnant patients without claimed tests at symptom presentation, 10.2% had claimed testing later in follow-up.
Figure 4.
Number of vaginitis-related visits after the index date and over the 12-month follow-up period in the non-pregnant and pregnant cohorts
Chen. Real-world clinical burden of patients presenting with vaginitis symptoms in the United States. AJOG Glob Rep 2025.
Figure 5.
Number of diagnostic test dates per patient over the 12-month follow-up period in the non-pregnant and pregnant cohorts
Chen. Real-world clinical burden of patients presenting with vaginitis symptoms in the United States. AJOG Glob Rep 2025.
During follow-up, the non-pregnant cohort had 4,688,934 vaginitis treatment claims. At index, 26.4% received vaginitis treatment and the proportion of patients with claimed treatments increased to 51.0% over the 12-month follow-up period; 9.6% of patients had ≥4 treatment claims. The most common treatments during follow-up were fluconazole (34.5%) and metronidazole (23.5%) (Table 2).
Table 2.
Vaginitis-related treatments over the 12-month follow-up period in the non-pregnant and pregnant cohorts
| Measures | Non-pregnant cohort (N=3,787,354) |
Pregnant cohort (N=213,261) |
|---|---|---|
| With ≥1 vaginitis-related treatment on the index date1(n, %) | 999,248 (26.4%) | 47,671 (22.4%) |
| With ≥1 vaginitis-related treatment during follow-up (n, %) | 1,931,055 (51.0%) | 129,833 (60.9%) |
| Vaginitis-related treatment claims per patient during follow-up (n, %) | ||
| 0 | 1,856,299 (49.0%) | 83,428 (39.1%) |
| 1 | 871,113 (23.0%) | 62,372 (29.2%) |
| 2 | 459,171 (12.1%) | 32,516 (15.2%) |
| 3 | 235,348 (6.2%) | 15,801 (7.4%) |
| 4+ | 365,423 (9.6%) | 19,144 (9.0%) |
| Specific vaginitis-related treatments during follow-up (n, %) | ||
| Fluconazole (any dose form) | 1,306,144 (34.5%) | 49,307 (23.1%) |
| Metronidazole (any dose form) | 888,877 (23.5%) | 78,927 (37.0%) |
| Clindamycin (any dose form) | 287,594 (7.6%) | 18,856 (8.8%) |
| Terconazole | 159,311 (4.2%) | 31,950 (15.0%) |
| Tinidazole | 28,766 (0.8%) | 901 (0.4%) |
| Clotrimazole | 26,238 (0.7%) | 7,082 (3.3%) |
| Miconazole | 19,921 (0.5%) | 6,792 (3.2%) |
| Secnidazole | 10,170 (0.3%) | 199 (0.1%) |
| Butoconazole | 3,456 (0.1%) | 512 (0.2%) |
| Ibrexafungerp | 748 (0.0%) | 14 (0.0%) |
| Tioconazole | 184 (0.0%) | 12 (0.0%) |
The index date is included in the 12-month follow-up period. The measures on the index date and the follow-up period are not mutually exclusive.
Chen. Real-world clinical burden of patients presenting with vaginitis symptoms in the United States. AJOG Glob Rep 2025.
In the pregnant cohort, 30.9% of patients had ≥1 subsequent vaginitis-related visit after index, totaling 105,171 subsequent visits (Figure 4). In addition, the cohort had 208,352 unique visit dates with claimed vaginitis tests during follow-up, with 23.9% of patients having ≥2 unique test dates (Figure 5). Of pregnant patients without claimed tests at initial symptom presentation, 21.5% had testing later in follow-up.
Most pregnant patients had ≥1 vaginitis-related treatment over follow-up (60.9%), totaling 281,240 treatment claims. About one-fourth of pregnant patients had treatment at index (22.4%). The most common treatments during follow-up were metronidazole (37.0%) and fluconazole (23.1%) (Table 2).
Secondary analysis
Between Medicaid- and commercially-insured patients linkable to AEMR, there were numeric differences in baseline comorbidities, vaginitis risk factors, and index visit characteristics (Supplemental Table 3). After adjusting for covariates listed in Table 3, Table 4, patients with Medicaid had significantly higher odds of repeat vaginitis-related visits during follow-up vs. commercially-insured patients (26−27% higher in the non-pregnant and pregnant cohorts). Additionally, patients with Medicaid had significantly higher odds of ≥2 vaginitis treatment dates (34% higher in non-pregnant and pregnant cohorts). Black patients (vs. White) and patients with vaginitis risk factors also had higher odds of both outcomes (repeat visits and ≥2 treatment dates).
Table 3.
Logistic regression models assessing the association between test type at index visit and the odds of repeat vaginitis visit and the odds of ≥2 vaginitis treatment claim dates during follow-up, among the non-pregnant cohort linked to AEMR (N=436,595)
| Outcome: With ≥1 vs. 0 subsequent vaginitis-related visits during follow-up |
Outcome: With ≥2 vaginitis treatment dates during follow-up |
|||
|---|---|---|---|---|
| Variables | Odds ratio (95% CI) | p-value | Odds ratio (95% CI) | p-value |
| Payer type (Medicaid vs. Commercial) | 1.26 (1.23, 1.28) | <.001 | 1.34 (1.30, 1.38) | <.001 |
| Test type at initial presentation (vs. no claimed test) | ||||
| NAAT panel or with 1−2 targets | 1.11 (1.08, 1.13) | <.001 | 1.09 (1.05, 1.14) | <.001 |
| Direct probe | 1.14 (1.10, 1.18) | <.001 | 1.15 (1.09, 1.21) | <.001 |
| Traditional methods | 1.21 (1.19, 1.24) | <.001 | 1.23 (1.19, 1.27) | <.001 |
| Age group (vs. 55+) | ||||
| 0−17 | 0.82 (0.80, 0.85) | <.001 | 0.65 (0.61, 0.68) | <.001 |
| 18−34 | 1.41 (1.38, 1.44) | <.001 | 1.49 (1.44, 1.53) | <.001 |
| 35−54 | 1.09 (1.07, 1.11) | <.001 | 1.08 (1.05, 1.11) | <.001 |
| Race/ethnicity (vs. White) | ||||
| Black | 1.36 (1.33, 1.38) | <.001 | 1.43 (1.39, 1.47) | <.001 |
| Hispanic | 1.04 (0.94, 1.15) | .43 | 1.16 (1.01, 1.34) | .03 |
| Other (Asian, Other, Two or more) | 1.11 (1.08, 1.15) | <.001 | 1.11 (1.06, 1.17) | <.001 |
| Geographic region (vs. South) | ||||
| Northeast | 1.07 (1.05, 1.09) | <.001 | 1.09 (1.05, 1.12) | <.001 |
| Midwest | 1.05 (1.03, 1.07) | <.001 | 1.09 (1.06, 1.12) | <.001 |
| West | 1.13 (1.11, 1.16) | <.001 | 1.20 (1.16, 1.24) | <.001 |
| Provider specialty for index visit (vs. primary care) | ||||
| Obstetrician/gynecologist | 0.97 (0.95, 0.99) | .003 | 0.96 (0.93, 0.99) | .006 |
| Emergency medicine1 | 0.88 (0.85, 0.92) | <.001 | 0.94 (0.89, 1.00) | .03 |
| All others | 1.04 (1.02, 1.06) | <.001 | 1.12 (1.09, 1.16) | <.001 |
| Risk factors for vaginitis (vs. none)2 | 1.29 (1.25, 1.33) | <.001 | 1.39 (1.33, 1.45) | <.001 |
Includes emergency room and urgent care.
Risk factors for vaginitis included the following categories of diagnosis codes from claims: infections with a predominantly sexual mode of transmission, drug dependence, and nondependent abuse of drugs.
Chen. Real-world clinical burden of patients presenting with vaginitis symptoms in the United States. AJOG Glob Rep 2025.
Table 4.
Logistic regression models assessing the association between test type at index visit and the odds of repeat vaginitis visit and the odds of ≥2 vaginitis treatment claim dates during follow-up, among the pregnant cohort linked to AEMR (N=25,112)
| Variables | Outcome: With ≥1 vs. 0 subsequent vaginitis-related visits during follow-up |
Outcome: With ≥2 vaginitis treatment dates during follow-up |
||
|---|---|---|---|---|
| Odds ratio (95% CI) | p-value | Odds ratio (95% CI) | p-value | |
| Payer type (Medicaid vs. Commercial) | 1.27 (1.20, 1.35) | <.001 | 1.34 (1.23, 1.46) | <.001 |
| Test type at initial presentation (vs. no claimed test) | ||||
| NAAT panel or with 1−2 targets | 1.18 (1.09, 1.28) | <.001 | 1.12 (1.00, 1.27) | .06 |
| Direct probe | 1.30 (1.18, 1.43) | <.001 | 1.39 (1.21, 1.58) | <.001 |
| Traditional methods | 1.08 (1.01, 1.16) | .03 | 1.02 (0.92, 1.14) | .68 |
| Age group (35+ vs. 12−34) | 0.77 (0.71, 0.82) | <.001 | 0.74 (0.66, 0.84) | <.001 |
| Race/ethnicity (vs. White) | ||||
| Black | 1.42 (1.33, 1.51) | <.001 | 1.50 (1.36, 1.65) | <.001 |
| Hispanic | 0.96 (0.73, 1.26) | .76 | 1.20 (0.82, 1.75) | .35 |
| Other (Asian, Other, Two or more) | 1.08 (0.98, 1.19) | .14 | 1.07 (0.92, 1.24) | .39 |
| Geographic region (vs. South) | ||||
| Northeast | 1.02 (0.94, 1.11) | .62 | 1.15 (1.01, 1.29) | .03 |
| Midwest | 1.03 (0.97, 1.10) | .35 | 1.02 (0.92, 1.13) | .72 |
| West | 0.95 (0.87, 1.05) | .31 | 0.96 (0.83, 1.12) | .62 |
| Provider specialty for index visit (vs. primary care) | ||||
| Obstetrician/gynecologist | 0.94 (0.86, 1.03) | .20 | 0.94 (0.82, 1.08) | .36 |
| Emergency medicine2 | 0.92 (0.81, 1.05) | .22 | 0.98 (0.81, 1.18) | .79 |
| All others | 0.94 (0.85, 1.03) | .17 | 0.95 (0.82, 1.09) | .44 |
| Risk factors for vaginitis (vs. none)2 | 1.33 (1.21, 1.46) | <.001 | 1.39 (1.23, 1.58) | <.001 |
1Includes emergency room and urgent care.
Risk factors for vaginitis included the following categories of diagnosis codes from claims: infections with a predominantly sexual mode of transmission, drug dependence, and nondependent abuse of drugs.
Chen. Real-world clinical burden of patients presenting with vaginitis symptoms in the United States. AJOG Glob Rep 2025.
Furthermore, patients who received diagnostic testing (vs. no testing) had significantly higher odds of both outcomes. Non-pregnant patients with NAAT, direct probe, and traditional methods had 11%, 14%, and 21% higher odds of repeat vaginitis-related visits and 9%, 15%, and 23% higher odds of ≥2 treatment dates during follow-up vs. patients without claimed tests, respectively (all P<.05). While odds ratios for both outcomes were highest for non-pregnant patients with traditional methods and lowest for patients with NAAT, pairwise comparisons between each test type were not conducted. Pregnant patients with NAAT, direct probe, and traditional methods had 18%, 30%, and 8% higher odds of repeat vaginitis-related visits (all P<.05) and 12%, 39%, and 2% higher odds of ≥2 treatment dates during follow-up (P<.05 for comparison with direct probe only) vs. patients without claimed tests, respectively.
Comment
Principal findings
This retrospective cohort study provides contemporary insights on the management of patients newly presenting with vaginitis symptoms in real-world settings, highlighting the significant clinical and resource utilization burden which vaginitis poses in the US. We analyzed 4,000,615 patients who sought care for vaginitis symptoms from 2018 to 2022, with 2,023,818 additional vaginitis-related visits, 1,952,172 diagnostic test dates, and 4,970,174 treatment claims over 12-month follow-up. This burden is likely underestimated, due to the approximately 14 million patients not meeting study selection criteria (e.g., due to claims database linkage, baseline or follow-up data requirements) and the unknown number who self-treated with over-the-counter medications, did not seek care, or received care/testing in settings not billed to insurance or captured in the databases (e.g., student health centers, public health or sexually transmitted infection [STI] clinics). Certain populations may also carry a disproportionate clinical burden. In secondary analyses, non-pregnant and pregnant patients with Medicaid (vs. commercially-insured), Black patients (vs. White), and patients with select risk factors (vs. no risk factors) had significantly higher odds of repeat vaginitis-related visits and additional treatment dates. These findings are consistent with prior research that shows vaginitis and associated complications disproportionately impact underserved and socioeconomically deprived populations, with the prevalence of BV, VVC, and TV highest among Black women compared to women of other races or ethnicities.16, 17, 18, 19
Results in the context of what is known
While commonly cited that vaginitis is responsible for >10 million office visits annually, recent statistics are unavailable.1, 2, 3 Our findings confirm at least 18.7 million people presented to the healthcare system with vaginitis symptoms or vaginitis diagnosis codes during the selection window. This study also suggests underutilization of NAATs, despite higher sensitivity than direct probe or traditional methods.2,12, 13, 14 This is particularly apparent among patients with suspected TV; only 19.4% of non-pregnant and 23.8% of pregnant patients with TV diagnoses were tested with NAAT. Given TV is associated with adverse birth outcomes, including preterm delivery, low birth weight, and augmented likelihood of HIV transmission,11,20 accurate testing and appropriate treatment are critical. Furthermore, most patients (76.2% of non-pregnant and 52.4% of pregnant cohorts) had no claimed diagnostic testing at symptom presentation, consistent with other claims-based studies.21,22 In contrast, a chart-based study reported 9% of patients were diagnosed empirically and point-of-care assessments were common (99% with visual evaluation of vaginal discharge and 56-66% with wet mount microscopy).23 While patients without claimed tests in the present study likely include those with unbilled testing, driven by a lack of CPT codes and underutilization of available CPT codes especially for low cost methodologies, and patients truly without testing, higher cost laboratory-based molecular methods like NAAT and direct probe are expected to be consistently billed. Among patients without billed testing at symptom presentation, many continued to seek vaginitis-related care (10.2% of the non-pregnant and 21.5% of the pregnant cohort without testing had claimed tests later in follow-up). Additionally, given 28.8% of the non-pregnant and 30.9% of the pregnant cohort had vaginitis-related visits after index and BV recurs in more than 50% of patients within 6 months,7 the frequency of follow-up visits and testing may indicate an excess HCRU burden due to unresolved or recurrent symptoms.
Clinical implications
In this large real-world cohort, most patients seeking vaginitis-related care did not have claimed testing at initial symptom presentation. Among patients with claimed testing, NAAT was underutilized, even when indicated for diagnosis of TV. Given the limitations of traditional test methods and direct probe, the 2024 update of the joint laboratory practice guidelines by the Infectious Diseases Society of America (IDSA) and American Society for Microbiology (ASM) recommend multiplex NAAT for vaginitis over traditional methods primarily due to higher sensitivity for BV, VVC, TV, and mixed infections, and over direct probe due to higher specificity for BV.24 Furthermore, approximately one-fourth of patients without claimed tests at symptom presentation received vaginitis-related treatment at index (results not presented), suggesting a high rate of empiric treatment, which is not recommended.25 With growing concerns of antimicrobial resistance among organisms associated with vaginitis and limited effective treatment options,26, 27, 28 these findings of potentially insufficient diagnostic testing and high empiric treatment rate suggest an unmet need for point-of-care molecular technologies and clinical strategies to improve diagnostic and prescribing efficiency and reduce vaginitis burden.
Research implications
Future studies evaluating the clinical utility of new point-of-care vaginitis NAAT panel are warranted, with the anticipated primary outcome of reducing empiric treatment. With point-of-care NAAT panels, accurate diagnosis and pathogen-directed treatment can be provided during a single healthcare visit. In the secondary analysis, there were numeric trends indicating patients with different test types at symptom presentation may have varying HCRU during follow-up. However, since all molecular tests described in this study were laboratory-based and expected to require a minimum 1-day turnaround, patients with highly accurate NAAT may have still required follow-up visits to obtain test results and may have been empirically treated in the meantime; thus, differences in repeat healthcare visits and number of treatment dates between patients with laboratory-based molecular tests (NAAT and direct probe) and traditional methods may not have been significant. While previous claims-based studies have suggested clinical utility of NAAT in reducing all-cause HCRU5 and costs,3,29 differences were small. The importance of timely treatment, despite being empiric, was reported in a cost-effectiveness analysis reporting immediate relief of symptoms (i.e., prescribing treatment based on pH while waiting for culture results) shortened symptom duration and reduced costs by reducing return office visits.30 However, the analysis was conducted in 2005 and did not include NAAT. Conditions that could have overlapping symptoms with vaginitis (e.g., urinary tract infection, STIs) should also be investigated, as they may impact testing and treatment patterns. For example, the reporting of chlamydia and gonorrhea testing patterns may elucidate the high number of patients without claimed tests, as testing for these STIs reportable to health departments may be prioritized over vaginitis.9
Strengths and limitations
The key strengths of this study lie in the study design. While previous claims database studies identified study populations with specific vaginitis diagnosis codes,3,5,6,21,29,31 the present study further included vaginitis symptoms for a more complete description of vaginitis burden. These studies also primarily leveraged claims databases with specific insurance types, while our study leveraged open claims databases capturing patients with commercial insurance, Medicare, Medicaid, and cash payments for greater generalizability. Lastly, other claims-based studies reported HCRU outcomes in exclusively non-pregnant cohorts or combined cohorts with predominantly non-pregnant patients.6,29,32 As pregnant patients are expected to have more frequent interactions with the healthcare system and management may differ, we reported the burden in pregnant patients separately.
There are also several limitations of the datasets. First, in open-source databases, there is a loss of visibility to healthcare activity outside of pharmacies and offices contributing to the databases. Second, claims data do not provide as much clinical detail as medical records; since they are primarily collected for billing and payment purposes, there is potential for under-coding of conditions (e.g., vaginitis risk factors). Finally, the study is limited to the clinical burden of vaginitis on the healthcare system through HCRU, which does not capture the significant humanistic and financial impact of persistent or recurrent vaginitis on patients, such as impact on sexual health, mental health and emotional well-being, productivity, social relationships, and quality of life.
Conclusions
This real-world study identified a large number of patients who sought care for vaginitis symptoms and confirms vaginitis poses a significant clinical burden in the US, although this burden is underestimated from claims data alone. Misdiagnosis and mistreatment of vaginitis could facilitate antimicrobial resistance, contribute to acquisition of STIs and forward transmission of TV, lead to adverse birth outcomes, and negatively affect women’s quality of life. Findings from this study suggest an unmet need for a rapid, accurate point-of-care diagnostic test to improve vaginitis diagnostic efficiency and increase informed prescribing, which may subsequently reduce the future burden of vaginitis.
CRediT authorship contribution statement
Justin Chen: Writing – review & editing, Visualization, Project administration, Methodology, Investigation, Conceptualization. Jenny Tse: Writing – review & editing, Visualization, Project administration, Methodology, Investigation, Conceptualization. Liucheng Shi: Software, Methodology, Investigation, Formal analysis, Data curation. Mindy M. Cheng: Writing – review & editing, Funding acquisition, Conceptualization. Rebecca Lillis: Writing – review & editing, Conceptualization. Aimee M. Near: Writing – review & editing, Supervision, Project administration, Methodology, Conceptualization.
Footnotes
Declaration of competing interest: Mindy M. Cheng is an employee of Cepheid, manufacturer of a multiplex vaginitis panel. Declarations of competing interest for Rebecca Lillis are described in Disclosures. Justin Chen and Jenny Tse were employed by IQVIA during the time the study was conducted. Liucheng Shi and Aimee Near are employed by IQVIA, which received funding from Cepheid to conduct this study and draft the manuscript.
Tweetable statement: Vaginitis is a common reason for clinic visits among women in the United States. This study provides a contemporary update on the significant clinical burden of managing vaginitis.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.xagr.2025.100504.
Appendix. Supplementary materials
References
- 1.Brown H., Drexler M. Improving the Diagnosis of Vulvovaginitis: Perspectives to Align Practice, Guidelines, and Awareness. Popul Health Manag. 2020;23:S3–s12. doi: 10.1089/pop.2020.0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Broache M., Cammarata C.L., Stonebraker E., Eckert K., Van Der Pol B., Taylor SN. Performance of a Vaginal Panel Assay Compared With the Clinical Diagnosis of Vaginitis. Obstet Gynecol. 2021;138:853–859. doi: 10.1097/AOG.0000000000004592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kong A.M., Jenkins D., Troeger K.A., Kim G., London RS. Diagnostic Testing of Vaginitis: Improving the Value of Care. Popul Health Manag. 2021;24:515–524. doi: 10.1089/pop.2021.0143. [DOI] [PubMed] [Google Scholar]
- 4.Paladine H.L., Desai UA. Vaginitis: Diagnosis and Treatment. Am Fam Physician. 2018;97:321–329. [PubMed] [Google Scholar]
- 5.Ackerman S.J., Knight T., Wahl P.M., Cartwright CP. Health care utilization and costs following amplified versus non-amplified molecular probe testing for symptomatic patients with suspected vulvovaginitis: a US commercial payer population. Clinicoecon Outcomes Res. 2019;11:179–189. doi: 10.2147/CEOR.S191831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watkins E., Chow C.M., Lingohr-Smith M., et al. Treatment patterns and economic burden of bacterial vaginosis among commercially insured women in the USA. J Comp Eff Res. 2024;13 doi: 10.57264/cer-2023-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vodstrcil L.A., Muzny C.A., Plummer E.L., Sobel J.D., Bradshaw CS. Bacterial vaginosis: drivers of recurrence and challenges and opportunities in partner treatment. BMC Med. 2021;19:194. doi: 10.1186/s12916-021-02077-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sobel J. Current treatment options for vulvovaginal candidiasis. Womens Health (Lond) 2005;1:253–261. doi: 10.2217/17455057.1.2.253. [DOI] [PubMed] [Google Scholar]
- 9.Workowski K.A., Bachmann L.H., Chan P.A., et al. Sexually Transmitted Infections Treatment Guidelines, 2021. MMWR Recomm Rep. 2021;70:1–187. doi: 10.15585/mmwr.rr7004a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Owens D.K., Davidson K.W., Krist A.H., et al. Screening for Bacterial Vaginosis in Pregnant Persons to Prevent Preterm Delivery: US Preventive Services Task Force Recommendation Statement. Jama. 2020;323:1286–1292. doi: 10.1001/jama.2020.2684. [DOI] [PubMed] [Google Scholar]
- 11.Van Gerwen O.T., MC Craig-Kuhn, Jones A.T., et al. Trichomoniasis and adverse birth outcomes: a systematic review and meta-analysis. Bjog. 2021;128:1907–1915. doi: 10.1111/1471-0528.16774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lowe N.K., Neal J.L., Ryan-Wenger N.A. Accuracy of the clinical diagnosis of vaginitis compared with a DNA probe laboratory standard. Obstet Gynecol. 2009;113:89–95. doi: 10.1097/AOG.0b013e3181909f63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cartwright C.P., Lembke B.D., Ramachandran K., et al. Comparison of nucleic acid amplification assays with BD affirm VPIII for diagnosis of vaginitis in symptomatic women. J Clin Microbiol. 2013;51:3694–3699. doi: 10.1128/JCM.01537-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Danby C.S., Althouse A.D., Hillier S.L., Wiesenfeld HC. Nucleic Acid Amplification Testing Compared With Cultures, Gram Stain, and Microscopy in the Diagnosis of Vaginitis. J Low Genit Tract Dis. 2021;25:76–80. doi: 10.1097/LGT.0000000000000576. [DOI] [PubMed] [Google Scholar]
- 15.Holzman C., Leventhal J.M., Qiu H., Jones N.M., Wang J. Factors linked to bacterial vaginosis in nonpregnant women. Am J Public Health. 2001;91:1664–1670. doi: 10.2105/ajph.91.10.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allsworth J.E., Peipert JF. Prevalence of bacterial vaginosis: 2001-2004 National Health and Nutrition Examination Survey data. Obstet Gynecol. 2007;109:114–120. doi: 10.1097/01.AOG.0000247627.84791.91. [DOI] [PubMed] [Google Scholar]
- 17.Bautista C.T., Wurapa E., Sateren W.B., Morris S., Hollingsworth B., Sanchez JL. Bacterial vaginosis: a synthesis of the literature on etiology, prevalence, risk factors, and relationship with chlamydia and gonorrhea infections. Mil Med Res. 2016;3:4. doi: 10.1186/s40779-016-0074-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flagg E.W., Meites E., Phillips C., Papp J., Torrone EA. Prevalence of Trichomonas vaginalis Among Civilian, Noninstitutionalized Male and Female Population Aged 14 to 59 Years: United States, 2013 to 2016. Sex Transm Dis. 2019;46:e93–e96. doi: 10.1097/OLQ.0000000000001013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foxman B., Barlow R., D'Arcy H., Gillespie B., Sobel J.D. Candida vaginitis: self-reported incidence and associated costs. Sex Transm Dis. 2000;27:230–235. doi: 10.1097/00007435-200004000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Masha S.C., Cools P., Sanders E.J., Vaneechoutte M., Crucitti T. Trichomonas vaginalis and HIV infection acquisition: a systematic review and meta-analysis. Sex Transm Infect. 2019;95:36–42. doi: 10.1136/sextrans-2018-053713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pinto C.N., Jung M., Wimmer M., et al. Differential Screening for Nonviral Sexually Transmitted Infections by Type of Vaginitis Testing. Sex Transm Dis. 2023;50:531–535. doi: 10.1097/OLQ.0000000000001820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benedict K., Singleton A.L., Jackson B.R., Molinari NAM. Survey of incidence, lifetime prevalence, and treatment of self-reported vulvovaginal candidiasis, United States. BMC Womens Health 2022. 2020;22:147. doi: 10.1186/s12905-022-01741-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nyirjesy P., Banker W.M., Bonus TM. Physician Awareness and Adherence to Clinical Practice Guidelines in the Diagnosis of Vaginitis Patients: A Retrospective Chart Review. Popul Health Manag. 2020;23:S13–s21. doi: 10.1089/pop.2020.0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller J.M., Binnicker M.J., Campbell S., et al. Guide to Utilization of the Microbiology Laboratory for Diagnosis of Infectious Diseases: 2024 Update by the Infectious Diseases Society of America (IDSA) and the American Society for Microbiology (ASM) Clin Infect Dis. 2024 doi: 10.1093/cid/ciae104. [DOI] [PubMed] [Google Scholar]
- 25.Marnach M.L., Wygant J.N., Casey PM. Evaluation and Management of Vaginitis. Mayo Clin Proc. 2022;97:347–358. doi: 10.1016/j.mayocp.2021.09.022. [DOI] [PubMed] [Google Scholar]
- 26.Muzny C.A., Sobel JD. The Role of Antimicrobial Resistance in Refractory and Recurrent Bacterial Vaginosis and Current Recommendations for Treatment. Antibiotics (Basel) 2022:11. doi: 10.3390/antibiotics11040500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sobel JD. Resistance to Fluconazole of Candida albicans in Vaginal Isolates: a 10-Year Study in a Clinical Referral Center. Antimicrob Agents Chemother. 2023;67 doi: 10.1128/aac.00181-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwebke J.R., Barrientes FJ. Prevalence of Trichomonas vaginalis isolates with resistance to metronidazole and tinidazole. Antimicrob Agents Chemother. 2006;50:4209–4210. doi: 10.1128/AAC.00814-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Troeger K.A., Thiel E.R., London RS. Optimizing Vaginitis Diagnosis to Reduce Health Care Costs in Nonpregnant Women Utilizing Molecular Diagnostics. Popul Health Manag. 2022;25:449–454. doi: 10.1089/pop.2022.0096. [DOI] [PubMed] [Google Scholar]
- 30.Carr P.L., Rothberg M.B., Friedman R.H., Felsenstein D., Pliskin JS. “Shotgun” versus sequential testing. Cost-effectiveness of diagnostic strategies for vaginitis. J Gen Intern Med. 2005;20:793–799. doi: 10.1111/j.1525-1497.2005.0188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watkins E., Chow C.M., Lingohr-Smith M., et al. Bacterial Vaginosis Treatment Patterns, Associated Complications, and Health Care Economic Burden of Women With Medicaid Coverage in the United States. Ann Pharmacother. 2024;58:480–493. doi: 10.1177/10600280231190701. [DOI] [PubMed] [Google Scholar]
- 32.Blumenfeld Y.J., Marić I., Stevenson D.K., Gibbs R.S., Shaw GM. Persistent Bacterial Vaginosis and Risk for Spontaneous Preterm Birth. Am J Perinatol. 2024;41 doi: 10.1055/s-0043-1770703. e2081-e88. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.