Abstract
Cardiovascular diseases are paramount cause of morbidity in aging population and aging disrupts normal circadian rhythm cycle. Circadian rhythms, regulated by the suprachiasmatic nucleus in the brain, profoundly influence cardiovascular health through intricate neurobiological mechanisms. These rhythms regulate gene expression in cardiomyocytes, modulate autonomic nervous system (ANS) activity, and synchronize cardiovascular functions with environmental cues, ultimately impacting heart rate, blood pressure, and susceptibility to cardiac events. The intricate relationship between circadian rhythms and cardiovascular health emphasizes the critical role of brain-heart communication in physiological processes.
This review explores the neurobiology of circadian clock in cardiovascular disease, exploring how peripheral clocks in cardiovascular tissues influence organ physiology and how their disruption contributes to pathogenesis. The examination of neurobiological pathways linking circadian clock to cardiovascular disease, including ANS function, neuroendocrine signaling, and inflammatory responses, highlights the interplay between brain and heart. By probing environmental and lifestyle factors that modulate the circadian clock, as well as sex-specific variations in circadian rhythms, the review provides a comprehensive understanding of how these factors impact cardiovascular health. The discussion of emerging concepts, such as exosome-mediated intracellular communication in circadian physiology, offers new insights into the molecular mechanisms underlying brain-heart interactions. Furthermore, the exploration of diagnostic potential and therapeutic strategies, particularly chronotherapy, emphasizes the importance of targeting the circadian clock for disease prevention and treatment in cardiovascular medicine. This comprehensive assessment not only advances our understanding about circadian clock's role in cardiovascular health but also paves the way for innovative approaches in theranostic, ultimately improving patient outcomes.
Keywords: Neuroscience, Ageing disease, Circadian rhythm, Cardiovascular system, Brain-heart communication, Extracellular vesicles, Chronotherapy
Highlights
-
•
Suprachiasmatic nucleus (SCN) regulates heart rhythms via autonomic pathways and brain-heart connections.
-
•
Circadian disruption increases risks of arrhythmias, infarction, and heart failure through maladaptive remodeling.
-
•
Neuroendocrine-inflammatory crosstalk links circadian misalignment to cardiovascular dysfunction and disease progression.
-
•
Chronotherapy aligns treatments with circadian biology to optimize outcomes in cardiovascular medicine.
1. Introduction
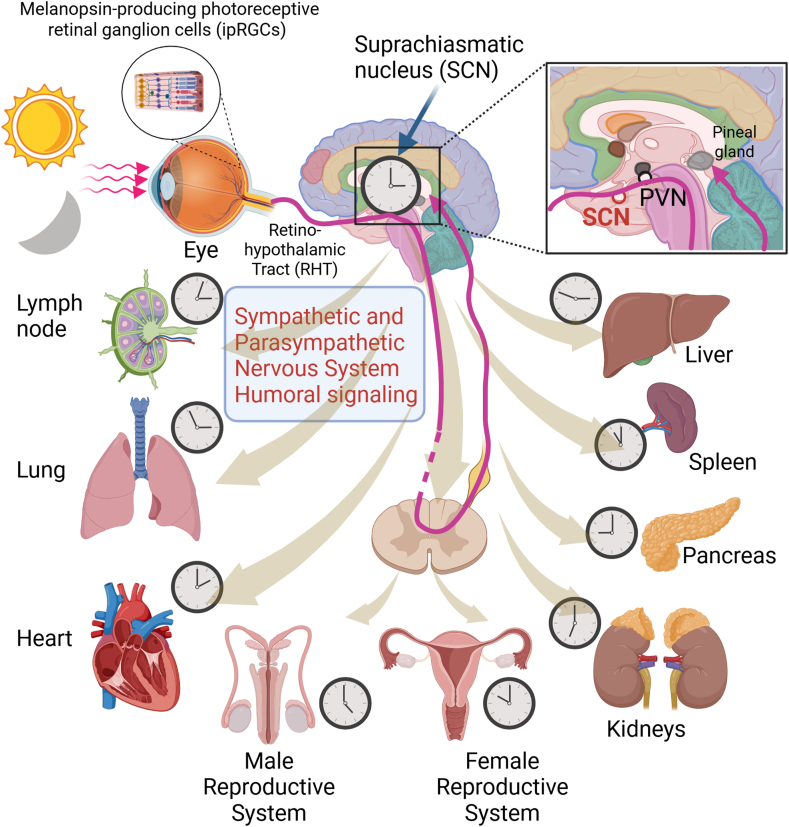
The circadian clock is an evolutionarily conserved biological system that coordinates physiological and behavioral processes in a 24-h rhythm, enabling organisms to anticipate and adapt to daily environmental changes such as light-dark cycles (Bass and Lazar, 2016; Takahashi, 2017). At its core, the circadian rhythm is governed by a network of molecular clocks, within the suprachiasmatic nucleus (SCN) in the hypothalamus serving as the central pacemaker that synchronizes peripheral clocks located in virtually all tissues, including the heart, blood vessels, and kidneys (Koronowski and Sassone-Corsi, 2021; Hastings et al., 2018; Mohawk et al., 2012). The circadian rhythm, orchestrated by the SCN, synchronizes body-wide clocks through neural and hormonal pathways. Light signals, detected by specialized retinal cells, travel via the retino-hypothalamic tract to the SCN, initiating a cascade through the paraventricular nucleus (PVN), brainstem, and spinal cord to the pineal gland (as shown in Fig. 1). The molecular machinery of the circadian clock involves a set of core clock genes, including CLOCK, BMAL1, PER, and CRY, which operate through transcriptional-translational feedback loops (TTFLs) to regulate the expression of clock-controlled genes (CCGs) that influence diverse physiological processes (Pilorz et al., 2018; Bass and Lazar, 2016). This intricate system ensures temporal coordination of cardiovascular functions, such as blood pressure, heart rate, and endothelial function, which exhibit robust diurnal variations (Martino and Sole, 2009; Monfredi and Lakatta, 2019; Atkinson et al., 2010; Curtis et al., 2007). For instance, blood pressure typically dips during sleep and rises in the early morning, a pattern regulated by the interplay between the SCN and peripheral clocks in the cardiovascular system (CVS) (Douma and Gumz, 2018; Smolensky et al., 2017; Faraci and Scheer, 2024; Hermida et al., 2011). Disruption of these rhythms, as seen in shift workers or individuals with sleep disorders, has been epidemiologically linked to an increased risk of cardiovascular diseases (CVD), including hypertension, myocardial infarction (MI), and atherosclerosis (Wong et al., 2023; Vyas et al., 2012). Studies have shown that circadian misalignment can lead to dysregulation of the autonomic nervous system (ANS), impaired glucose metabolism, and increased systemic inflammation, all of which contribute to CVD pathogenesis (Chellappa et al., 2019; Young et al., 2024; Morris et al., 2016; Scheer et al., 2009). Evidently, the circadian rhythm is a crucial conduit between brain and heart.
Fig. 1.
Composition of body-wide clocks via circadian rhythm. The circadian rhythm coordinates body-wide clocks, optimizing physiology for environmental changes. Light signals, detected by specialized retinal cells, reach the suprachiasmatic nucleus (SCN) via the retino-hypothalamic tract (RHT). The SCN then synchronizes peripheral clocks through neural and hormonal pathways. Feeding and exercise also influence circadian rhythms, which regulate various physiological processes including metabolism and immune function. The zoom-in image of SCN shows the neuroanatomical pathway depicting that the light activates retinal neurons, triggering a signal cascade through the RHT to SCN, PVN, brainstem, and spinal cord. This pathway ultimately reaches the pineal gland via the superior cervical ganglion.
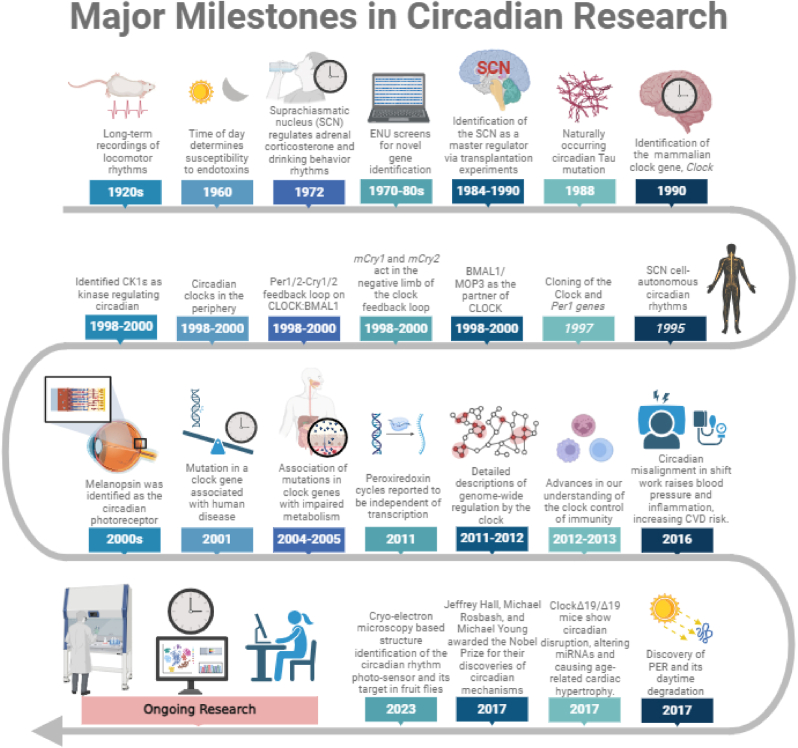
For the past several decades, a plethora of milestones and scientific breakthroughs have been achieved as illustrated on Fig. 2. It highlights crucial discoveries that advanced our understanding of biological clocks, from early observations to recent molecular insights into circadian rhythms. The purpose of this review is to explore the neurobiological mechanisms underlying the connection between the circadian clock and cardiovascular disease, with a focus on how disruptions in central and peripheral clocks contribute to CVD development. By elucidating these mechanisms, we aim to highlight potential therapeutic targets, such as pharmacological modulation of clock genes or chronotherapy; timed administration of medications to align with circadian rhythms, to improve cardiovascular outcomes (Gumz et al., 2023; Festus et al., 2024; Smolensky et al., 2010). Understanding the interplay between circadian biology and cardiovascular health not only provides insights into disease mechanisms but also opens new avenues for personalized medicine, where circadian rhythms can be leveraged to optimize prevention and treatment strategies for CVD (Takeda and Maemura, 2011).
Fig. 2.
Major advancements in circadian research. A schematic representation charting pivotal discoveries and milestones in the field of circadian rhythm studies, spanning over a century of scientific progress and breakthroughs in understanding biological clock mechanisms.
2. The circadian clock: molecular and neurobiological mechanisms
In the brain, the SCN consists of two key regions: the ventrolateral subdivision, which receives direct retinal input, and the dorsomedial subdivision, which primarily receives input from hypothalamic nuclei (Fig. 1) (Mieda, 2020). It regulates various physiological processes through a complex network of neurons and glial cells (Mieda, 2020; Mistlberger, 2005; Patton and Hastings, 2018). In addition, the SCN coordinates the body's circadian rhythms by synchronizing peripheral clocks and systemic rhythms through neuronal and hormonal signals (Astiz et al., 2019; Myung et al., 2018). At the molecular level, the circadian clock mechanism operates via TTFLs (Abe et al., 2015; Richards and Gumz, 2013). The core components of this machinery are the clock proteins CLOCK and BMAL1, which heterodimerize and bind to E-box elements in target gene promoters, driving the positive transcription arm of the TTFLs (Richards and Gumz, 2013; Orozco-Solis and Aguilar-Arnal, 2020). These target genes include Period (Per) and Cryptochrome (Cry), whose protein products form a repressor complex that inhibits CLOCK:BMAL1 activity, constituting the negative feedback arm of the loop (Richards and Gumz, 2013; Orozco-Solis and Aguilar-Arnal, 2020). Additional regulatory loops involve nuclear receptors such as REV-ERB and ROR, which modulate Bmal1 transcription (Orozco-Solis and Aguilar-Arnal, 2020; Duez and Staels, 2008; Kojetin and Burris, 2014). Fig. 3 depicts the major events in the molecular clock of mammalian.
Fig. 3.
Events in a mammalian molecular clock. The mammalian circadian clock operates through intricate transcriptional-translational feedback loops, cycling approximately every 24 h. At its core, CLOCK or NPAS2 partners with BMAL1, forming heterodimers that bind to E-box elements in gene promoters. This activates transcription of clock-controlled genes, including Per and Cry. As PER and CRY proteins accumulate, they form complexes that inhibit their own transcription, completing the negative feedback loop. An auxiliary loop involves ROR and REV-ERB proteins, which regulate Bmal1 expression. Additionally, the clock controls NAD + biosynthesis through NAMPT, influencing SIRT1 activity and further modulating clock gene expression. This complex system allows for tissue-specific variations in circadian gene expression patterns.
Post-translational modifications, particularly phosphorylation of PER and CRY proteins by casein kinases, play a crucial role in regulating the oscillatory period length (Orozco-Solis and Aguilar-Arnal, 2020; Brenna and Albrecht, 2020; Hirano et al., 2016). Epigenetic mechanisms significantly contribute to circadian regulation, with the coordinated action of the molecular clock and epigenetic remodelers underlying circadian chromatin transitions (Orozco-Solis and Aguilar-Arnal, 2020; Zhu and Belden, 2020; Papazyan et al., 2016; Bellet and Sassone-Corsi, 2010). For instance, rhythmic histone acetylation is achieved through the recruitment of histone acetyltransferases p300 and CREB-binding protein (CBP) by CLOCK:BMAL1 (Doi et al., 2006; Curtis et al., 2004). The NAD + dependent histone deacetylase SIRT1 also plays a vital role in circadian epigenetic regulation, with its activity oscillating due to rhythmic biosynthesis of its cofactor NAD+ (Orozco-Solis and Aguilar-Arnal, 2020; Rehan et al., 2014; Nakahata et al., 2009). This intricate molecular machinery within the SCN, coupled with its ability to receive light inputs from photosensitive retinal ganglion cells, allows it to entrain to the environment and coordinate the subordinate cellular clocks throughout the body (Rizvi and Majumdar, 2024; Kofuji et al., 2016). The influence of SCN extends to various physiological processes, including sleep-wake cycles, appetite, autonomic functions, and neuroendocrine regulation, emphasizing its fundamental role in maintaining temporal organization in mammals (Mieda, 2020; Mistlberger, 2005). Apparently, the SCN synchronizes circadian rhythms via neuronal and hormonal signals, with CLOCK:BMAL1-driven TTFLs and epigenetic modifications converging mechanistically, though the precise integration of chromatin and metabolic cues remains a debate.
3. Peripheral clocks in cardiovascular tissues
Peripheral clocks in cardiovascular tissues play a crucial role in regulating circadian rhythms of cardiovascular function, working in concert with the central clock in the SCN. (Meloni et al., 2013; Csoma and Bikov, 2023; Bass and Lazar, 2016b). In the heart, cardiomyocytes exhibit rhythmic expression of core clock genes and CCGs, with up to 10 % of the cardiac transcriptome showing circadian oscillations (Lecacheur et al., 2024). These oscillations regulate critical cardiac functions, such as the expression of ion channels like Kv1.5, Kv4.2, and Scn5a, which are involved in cardiac electrophysiology (Richards and Gumz, 2013; Diekman and Wei, 2021). The peripheral clock in blood vessels also demonstrates circadian rhythmicity, with smooth muscle BMAL1 participating in blood pressure regulation (Douma and Gumz, 2018; Crnko et al., 2018; Xie et al., 2015). Beesley et al. conducted an intriguing study to explore how circadian rhythms affect heart function in mice cardiomyocytes. They found the circadian clock gene expression of PER2 in cardiomyocytes is cell-autonomous and can be amplified by β-adrenergic signaling suggesting how circadian rhythms are regulated in cardiomyocytes and their potential implications for cardiac function (Beesley et al., 2016).
Endothelial cells, crucial for vascular homeostasis, exhibit circadian control over coagulation factors like plasminogen activator inhibitor-1 (PAI-1) and thrombomodulin, as well as cell cycle regulators CCNA1 and CDK1 (Mieda, 2020; Lecacheur et al., 2024). The interaction between central and peripheral clocks is complex, with the SCN playing a central role in the synchronization of peripheral clocks through neural and humoral signals (Barclay et al., 2012; Ramkisoensing and Meijer, 2015). However, peripheral clocks can also be affected by local zeitgebers such as food intake and physical activity, allowing for tissue-specific temporal organization (Koronowski and Sassone-Corsi, 2021), (Mieda, 2020), (Hower et al., 2018). This intricate network of central and peripheral clocks enables the CVS to anticipate and adapt to daily environmental changes. Fig. 4 depicts the mechanism underlying circadian regulation of cardiac function including cardiac contractility, electrophysiology, and metabolism. The SCN affects peripheral clocks via the SNS, while hormones like T3 and neurotransmitters such as VIP fine-tune cardiac rhythms. Voltage-gated calcium channels (VGCC) play a crucial role in translating circadian signals into functional cardiac outputs (Lecacheur et al., 2024; Xu et al., 2021).
Fig. 4.
Circadian regulation of cardiac function. The cardiomyocyte core molecular clock, entrained by external cues, operates autonomously. BMAL1 and CLOCK heterodimerize, initiating expression of REV-ERB, PER1-3, and CRY1-2, which suppress BMAL1 and CLOCK, creating rhythmic oscillations. These transcription factors modulate output genes affecting cardiac contractility, electrophysiology, metabolism, and repair. The diagram illustrates key components and pathways, including AKT, GSK-3β, PIK3R1, SCN, SNS, T3, VGCC, and VIP, demonstrating the complex interplay between the circadian clock and cardiac function.
Disruption of this synchronization, either between the central clock and peripheral clocks or among different peripheral clocks, can lead to cardiovascular dysfunction (Young and Bray, 2007). For instance, cardiomyocyte-specific deletion of the Bmal1 gene results in a slower heart rate and increased susceptibility to arrhythmias (Richards and Gumz, 2013; Lefta et al., 2012; Schroder et al., 2013). Moreover, desynchronization between internal clocks and the external environment, as seen in shift work or irregular social schedules, has been associated with an increased risk of cardiovascular diseases (Young et al., 2024; Mieda, 2020). Understanding the molecular mechanisms underlying these peripheral clocks and their interaction with the central clock is crucial for developing novel therapeutic approaches to manage cardiovascular disorders and optimize treatment timing, a concept known as chronotherapy (Festus et al., 2024; Mieda, 2020; Richards and Gumz, 2013; Lee et al., 2021). Evidently, peripheral cardiovascular clocks, coordinated by the SCN, regulate rhythmic gene expression and cardiac function. Most studies agree that BMAL1 and CLOCK proteins are key to how heart clocks work, but it's still debated how much local signals versus the brain's clock control these rhythms.
4. Circadian rhythm disruption and cardiovascular disease
4.1. Mechanism of circadian disruption causing cardiovascular diseases
Circadian disruption significantly impacts cardiovascular health through multiple interconnected mechanisms (as listed in Table 1). Dysregulation of the ANS activity is a key factor, as evidenced by altered heart rate variability and blood pressure patterns in individuals experiencing circadian misalignment (Young et al., 2024; Mieda, 2020; Richards and Gumz, 2013). This disruption can lead to prolonged QTc intervals (the time from the start of the Q wave to the end of the T wave on an electrocardiogram (ECG), measuring ventricular depolarization and repolarization) and increased susceptibility to arrhythmias, particularly in shift workers (Mieda, 2020; Meloni et al., 2013). Impaired metabolic and hormonal rhythms also play a crucial role in associating circadian disruption to CVD. Cortisol and melatonin, two hormones with strong circadian patterns, are particularly affected. Disrupted cortisol rhythms can lead to increased inflammation and metabolic dysfunction, while altered melatonin secretion due to light exposure at night can impact sleep quality and cardiovascular function (Myung et al., 2018; Hower et al., 2018; Ansu Baidoo and Knutson, 2023). Oxidative stress and inflammation are exacerbated by circadian disruption, contributing significantly to CVD risk. Studies have shown that circadian rhythm disruption impairs tissue homeostasis and exacerbates chronic inflammation (Orozco-Solis and Aguilar-Arnal, 2020; Csoma and Bikov, 2023). Moreover, mice lacking core clock genes exhibit increased reactive oxygen species (ROS) levels in various tissues, suggesting a direct link between circadian rhythms and oxidative stress regulation (Hower et al., 2018; Fanjul-Moles and López-Riquelme, 2016). The interplay between these mechanisms creates a complex network of physiological changes that increase CVD risk. For instance, disrupted sleep patterns and exposure to artificial light, especially blue light, can alter melatonin secretion, leading to decreased heart rate variability and increased cardiovascular stress (Myung et al., 2018). Additionally, the circadian clock regulates immune cell infiltration during cardiac events, with disruption leading to altered immune responses that may exacerbate cardiovascular damage (Myung et al., 2018; Aziz et al., 2021). Apparently, insight into these mechanisms is crucial for developing targeted interventions and chronotherapeutic approaches to mitigate the cardiovascular risks associated with circadian disruption (Smolensky et al., 2016; Ruan et al., 2021).
Table 1.
Effect of circadian dysregulation on cardiovascular function.
| Clock Gene Manipulation | Cardiovascular Effects | Ref. |
|---|---|---|
| Bmal1 knockout | Arrhythmic behaviour, loss of circadian rhythms in peripheral tissues, reduced lifespan | Haque et al. (2019) |
| Per2 mutation | Impaired endothelium-dependent relaxation, decreased NO production | Viswambharan et al. (2007) |
| SCN-specific Bmal1 knockout | Loss of circadian behavioral rhythms, but peripheral clocks remain rhythmic | Haque et al. (2019) |
| Astrocyte-specific Bmal1 knockout | Delayed activity onset, slower entrainment to new light-dark cycles | Haque et al. (2019) |
| Ventral forebrain Bmal1 knockout | Altered timing of circadian behavioral patterns | Haque et al. (2019) |
| Per2 knockdown in cardiomyocytes | Increased cell death and mitochondrial dysfunction under stress | Bhaskara et al. (2024) |
| VSMC Bmal1 knockout | Aggravated atherosclerotic lesions; increased VMSC migration, monocyte transmigration, ROS levels and VSMC apoptosis | Lin et al. (2022) |
| Postnatal cardiomyocyte Bmal1 knockout | Increased cardiac hypertrophy, augmented fibrosis, promoted pressure overload-induced cardiac remodeling | Liang et al. (2022) |
| PER1 knockout | Desynchrony of circadian rhythm, increased salt-sensitive hypertension | Zietara et al. (2022) |
| PER1/PER2 knockout | Increased cardiac hypertrophy, increased cardiomyocyte proliferation | Tampakakis et al. (2021) |
| Cry1/Cry2 knockout | Impaired blood reperfusion, reduced capillary density, impaired angiogenesis | Tsuzuki et al. (2021) |
| Cardiomyocyte REV-ERBα/β knockout | Severe cardiac dysfunction, ventricular dilation, pressure overload | Li et al. (2022) |
Circadian dysregulation is intricately correlated to various cardiovascular conditions, highlighting the critical role of the body's internal clock in maintaining cardiovascular health. Table 1 lists the various effects of circadian dysregulation on cardiovascular function via manipulation of clock genes (Crnko et al., 2019a). Hypertension and non-dipping blood pressure patterns are closely associated with circadian rhythm disruptions, with studies showing that the normal nocturnal dip in blood pressure is absent in individuals with circadian misalignment (Mieda, 2020). MI exhibits a striking circadian pattern, with a peak incidence in the morning hours, particularly between 6 a.m. and noon (Richards and Gumz, 2013; Boari et al., 2007). This timing coincides with the morning surge in sympathetic activity and increased platelet aggregability, demonstrating the influence of circadian rhythms on cardiovascular events (Orozco-Solis and Aguilar-Arnal, 2020; Thosar et al., 2018; Guo and Stein, 2003). Arrhythmias and heart failure also display circadian variations, with ventricular arrhythmias showing a bimodal distribution peaking in the morning and afternoon (Young and Bray, 2007; El et al., 2023; Miyake et al., 2017). The circadian clock regulates cardiac ion channel expression, including SCN5A and KChIP2, which affects cardiac electrophysiology and susceptibility to arrhythmias (Black et al., 2019). In heart failure, disrupted circadian rhythms contribute to altered heart rate variability and worsened outcomes (Orozco-Solis and Aguilar-Arnal, 2020). Atherosclerosis progression is influenced by circadian rhythms, with disrupted or misaligned circadian patterns promoting endothelial dysfunction, a key factor in atherogenesis (Zhang et al., 2020). Circadian clock genes regulate endothelial nitric oxide production, and their disruption leads to impaired vascular function (Myung et al., 2018). Furthermore, the circadian system modulates inflammatory processes and lipid metabolism, both crucial in atherosclerosis development (Mieda, 2020). These findings underscore the importance of maintaining proper circadian rhythms for cardiovascular health and suggest that targeting the circadian system could be a novel therapeutic approach for cardiovascular diseases (Festus et al., 2024; Mieda, 2020; Kiss et al., 2024).
4.2. Epigenetics of circadian disruption in CVDs
Epigenetic mechanisms play a crucial role in regulating gene expression and cellular function in the heart (Ordovás and Smith, 2010; Shi et al., 2022; Thej et al., 2024; Thej and Kishore, 2024). Additionally, circadian rhythms, which regulate biological processes over a 24-h cycle, substantially impact cardiac physiology (Young, 2023). Understanding how these two regulatory mechanisms interact could result in discovering novel therapeutic targets for treating cardiovascular diseases. The interaction of epigenetics and circadian regulation may be crucial for maintaining cardiovascular homeostasis (Škrlec, 2021) Epigenetic modifications modulate the expression and function of circadian clock genes in the heart, affecting metabolic, electrophysiological, and structural properties of cardiac tissue (Takeda and Maemura, 2015).
DNA methylation dynamically regulates circadian gene expression in cardiomyocytes, influencing metabolic and electrophysiological pathways (Joska et al., 2014). It is plausible that the hypermethylation of BMAL1 and PER2 could result in dysfunctional cardiomyocytes, leading to impaired circadian rhythmicity and metabolic imbalance (Li et al., 2020). Disruption of circadian gene expressions due to DNA methylation changes can lead to a reprogramming of these metabolic pathways (Joska et al., 2014). For example, altered DNA methylation patterns have been associated with a shift towards glycolytic metabolism and suppression of oxidative phosphorylation, complemented by significant downregulation of CLOCK, indicative of a fetal-like metabolic state observed in heart failure (Pepin et al., 2019). Protein O-GlcNAcylation, a metabolism-linked post-translational modification (PTM), exhibits circadian rhythmicity in the heart, regulating clock proteins, metabolic enzymes, and myocardial function (Durgan et al., 2011). It acts as a metabolic sensor, influencing cardiac contractility, energy metabolism, and stress responses, with protective roles in ischemia but dysregulation in heart failure. Targeting circadian O-GlcNAcylation could offer novel therapeutic strategies for metabolic and ischemic heart diseases (Lal et al., 2024).
Histone acetylation and methylation patterns play a crucial role in controlling circadian gene oscillations in various tissues including cardiac (Doi et al., 2006; Feng and Lazar, 2012). CLOCK itself possesses histone acetyltransferase activity, acetylating histones at circadian gene promoters (Aguilar-Arnal and Sassone-Corsi, 2015). Loss of BMAL1 disrupts histone modification patterns, leading to maladaptive transcriptional programs that promote heart failure (Arrieta et al., 2024). Additionally, microRNAs could regulate post-transcriptional circadian gene networks in the heart (Škrlec, 2023). The knockout of miR-125b led to an accumulation of mitochondrial DNA while simultaneously reducing ATP production, suggesting a disruption in mitochondrial function (Chen et al., 2021). Apparently, the epigenetic modifications, including DNA methylation, histone changes, and O-GlcNAcylation, regulate cardiac circadian genes, influencing metabolism and function. These regulatory RNAs offer potential therapeutic avenues for restoring circadian rhythm integrity in CVD.
4.3. Impact of environmental and lifestyle factors on circadian clock leading to CVD
Environmental and lifestyle factors significantly impact the circadian clock, potentially leading to CVD. Shift work, sleep deprivation, and artificial light exposure are major disruptors of circadian rhythms. Night shift workers experience elevated blood pressure, increased inflammatory markers, and higher CVD risk. Chronic exposure to artificial light at night suppresses melatonin production and reduces day-night variability in blood pressure and heart rate, increasing cardiovascular risk (Hower et al., 2018; Molcan et al., 2024). A study of 60,000 people found that those with the highest light pollution exposure were 23 % more likely to be hospitalized for coronary heart disease and 29 % more likely to die from it (Orozco-Solis and Aguilar-Arnal, 2020; Jemielita et al., 2025).
Diet and timing of food intake also play crucial roles in circadian regulation. High-calorie diets and obesity disrupt circadian patterns, leading to reprogramming of the cardiac circadian transcriptome and increased expression of adverse cardiac remodeling markers (28)(74) (Grosjean et al., 2023). Irregular eating patterns can misalign peripheral clocks with the central clock, contributing to metabolic disorders and CVD risk. The interplay between these factors creates a complex network of physiological changes that increase CVD risk. For instance, shift workers often experience disrupted sleep patterns, altered meal timing, and increased exposure to artificial light, all of which contribute to circadian misalignment and subsequent cardiovascular complications (Mieda, 2020). Environmental and lifestyle factors, such as shift work and night shifts—prevalent since the Industrial Revolution—disrupt circadian rhythms, increasing CVD risk. Unhealthy habits like fast food, smoking, and alcohol further raise the likelihood of hypertension, heart failure, and coronary artery disease (Chellappa et al., 2019; Takeda and Maemura, 2011; Skogstad et al., 2019; Lecacheur et al., 2024). The rise of remote work, driven by COVID-19 and digital technologies, has blurred work-life boundaries. Prolonged nighttime exposure to blue light from screens disrupts circadian rhythms, impairing sleep, blood pressure, and glucose regulation (Gooley et al., 2011). The effects of artificial light at night on the cardiovascular system are primarily mediated through the ANS. Study shows both diurnal and nocturnal animals exhibit weakened circadian coordination affecting involuntary physiological functions, including heart rate and blood pressure (Molcan et al., 2024).
Growing evidence links CCGs to cardiac physiology. Tsai et al. showed that in cardiomyocyte clock mutant mice, the circadian clock suppresses hormone-sensitive lipase via AMPK, disrupting triglyceride metabolism and causing cardiac steatosis. (Tsai et al., 2010). These findings highlight the importance of the circadian clock in maintaining cardiac metabolic homeostasis and suggest that disruptions to this clock can lead to adverse cardiac outcomes. Apparently, understanding these mechanisms is crucial for developing targeted interventions and chronotherapeutic approaches to mitigate the cardiovascular risks associated with modern lifestyles and environmental factors (Crenguța Nicolae et al., 2022). While evidence links environmental and lifestyle factors to circadian disruption and increased CVD risk, methodological limitations persist. Many studies have small sample sizes, lack longitudinal follow-up, and often rely on animal models, which require further follow-up studies to generalize findings and fully understand species-specific effects or long-term cardiovascular outcomes.
4.4. Sex-specific circadian rhythm variations in cardiovascular health and disease
Sex-specific differences in circadian rhythms profoundly affect cardiovascular function. Females typically have higher resting and active heart rates, faster repolarization, and greater high-frequency heart rate variability, indicating parasympathetic dominance, while males show higher low-frequency variability, reflecting sympathetic regulation during active periods (Škrlec et al., 2021; Walton et al., 2022). Additionally, women tend to have higher nocturnal systolic and diastolic blood pressure compared to men (Škrlec et al., 2021). At the molecular level, sex-specific differences in circadian transcriptomes have been identified. Female hearts express a greater number of rhythmically expressed genes (REGs) than male hearts, with distinct temporal patterns. The core circadian gene Bmal1 plays a critical role in mediating these differences. Cardiomyocyte-specific knockout of Bmal1 diminishes sex-specific variations in the cardiac transcriptome and reduces differentially expressed genes between male and female hearts by approximately eightfold (Zhang et al., 2024, 2025). This highlights the importance of Bmal1 in regulating sex-specific gene expression in the heart. Sex hormones further modulate these differences. Estrogens are protective against CVDs in premenopausal women by influencing circadian clock gene expression. However, this protection diminishes post-menopause as estrogen levels decline and testosterone levels rise, increasing the risk of hypertension and coronary artery disease (Škrlec et al., 2021; Walton et al., 2022). Moreover, gonadal hormones directly or indirectly affect the SCN, the central circadian pacemaker, contributing to sex-specific rhythmicity (Walton et al., 2022). These sex-specific circadian variations have clinical implications for CVD management. Chronotherapy—timing treatments according to circadian rhythms—may benefit both sexes but require careful consideration of these differences. For instance, women may be more sensitive to circadian disruptions associated with sleep disorders or shift work, potentially exacerbating cardiovascular risks (Škrlec et al., 2021; Walton et al., 2022). Apparently, sex-specific differences in circadian regulation of the CVS arise from molecular, hormonal, and physiological factors. Fig. 5A–B depict cardiovascular circadian rhythms exhibit sex-specific variations. Females show earlier chronotypes and more rhythmically expressed genes in the heart. Bmal1 regulates sex-specific cardiac gene expression. Estrogen modulates clock genes, influencing sex-specific circadian rhythms.
Fig. 5.
Sex-specific circadian rhythm variations in cardiovascular system in health and diseases. (A) A 24-h timeline illustrating chronotypes, with arrows indicating later chronotypes for males and earlier for females. (B) The core circadian clock is regulated by a set of genes, including Bmal1, Clock, Cry, and Per. These genes form a feedback loop that drives the circadian rhythm. Recent studies have shown that these genes are not only essential for maintaining circadian homeostasis but also play a role in sex-specific gene expression in the heart. For instance, (a) Bmal1 has been identified as a key factor in conferring sex-specific differences in cardiac gene expression. Hearts from female mice exhibit more rhythmically expressed genes (REGs) compared to male hearts, and the loss of Bmal1 in cardiomyocytes diminishes these sex-specific differences. (b) Estrogen receptors are present in various tissues, including the heart, and have been shown to regulate the expression of clock genes. For example, estrogen has been found to modulate the expression of Per2 and Bmal1 in the heart, suggesting a mechanism by which estrogen influences circadian rhythms in a sex-specific manner. (c) The circadian clock regulates blood pressure, with disruptions in circadian rhythms leading to increased blood pressure variability and hypertension. Studies have shown that males are more susceptible to the adverse effects of circadian disruption on blood pressure regulation, while females will develop more severe atherosclerosis.
Sexual dimorphism in cardiovascular diseases has long been established. For example, males typically have a higher risk of experiencing a cardiovascular event compared to females (Pana et al., 2023). Cardiovascular risk factors like hypertension, diabetes, and hypercholesterolemia are more prevalent in females, raising their risk of major events. Preclinical studies show males, unlike females, develop heart failure and cardiac fibrosis earlier (Nguyen et al., 2024; Douglas et al., 1998). These differences have been associated with sex chromosomes as well as sex hormones and their receptors (Rabinovich-Nikitin et al., 2023). Recently, studies have begun to link the disruption of circadian rhythms and the development of cardiovascular events. The basic mechanisms of the circadian rhythm include influencing temperature, hormone release, digestion, sleep, and mood. This includes cardiovascular functions, such as blood pressure, heart rate, endothelial dysfunction, and thrombus formation (Guo and Stein, 2003; Martino et al., 2008; Škrlec et al., 2021).
The localization of sex steroid receptors to the suprachiasmatic nucleus, where the circadian rhythm is located, as well as sex differences in the expression of androgen and estrogen receptors, may indicate the direct and differential role of gonadal hormones on circadian rhythm (Adan and Natale, 2002). This is further suggested by a postmortem brain study in which circadian rhythm genes PER2, PER3, and ARNT1 peaked significantly earlier in the dorsolateral prefrontal cortex of females than males (Adan and Natale, 2002). Another study points to sex differences within single nucleotide polymorphisms (SNPs). In 10 SNPs from CLOCK, BMAL1, PER, and CRY associated with cardiovascular risk factors in earlier research (Englund et al., 2009; Škrlec et al., 2018; Garaulet and Madrid, 2009; Leu et al., 2015), namely that variations in the circadian genes differ between females and males with MI.
Beyond the basic mechanisms, sex differences in how the circadian rhythm contributes to a 24-h daily gene expression program termed clock output have been reported in mouse liver, as well as human studies looking at genotype-tissue expression (Talamanca et al., 2023; Astafev et al., 2024; Anderson et al., 2023). These studies suggest that males and females may experience different consequences from circadian rhythms. It is thought to be that females may be more sensitive to circadian rhythms (Duffy et al., 2011; Boivin et al., 2016). Females are more likely to have higher blood pressure at night than males, as well as higher heart rates and an increased risk ratio of 1.41–1.64 for experiencing insomnia (Boivin et al., 2016). It was also suggested that females not only showed significant sex differences in the diurnal and circadian variation of sleep and alertness, but a significantly shorter stage 1 sleep and non-REM sleep compared to males (Boivin et al., 2016). Overall, females may face higher risk of circadian disruptions impacting cardiovascular events, but the underlying mechanisms and influence of lifestyle factors on these sex-specific differences require further investigation.
5. Neurobiological pathways connecting the circadian clock to CVD
5.1. Autonomic nervous system
The SCN exerts control over sympathetic and parasympathetic activity via multi-synaptic neural connections to the heart, as demonstrated by retrograde tracing studies showing a " Suprachiasmatic Nucleus-Paraventricular nucleus of the hypothalamus-Superior cervical ganglion-Heart (SCN-PVN-SCG-Heart)" sympathetic axis (Richards and Gumz, 2013). This pathway allows the circadian clock to modulate cardiovascular parameters such as heart rate and blood pressure, which exhibit distinct diurnal patterns. Sympathetic activity peaks around midday, while cardiac vagal activity reaches its maximum in the morning, around 8 a.m. (Hower et al., 2018). These circadian variations in autonomic tone significantly impact cardiovascular function, with blood pressure typically higher in the morning and lower in the evening (Myung et al., 2018; Panza et al., 1991; Portaluppi et al., 2012). The influence of SCN extends to other cardiovascular processes, including endothelial cell function, platelet aggregation, and thrombus formation, all of which follow 24-h cycles (Myung et al., 2018; Mergenthaler et al., 2024; Rana et al., 2020). Disruption of these circadian rhythms, such as through shift work or irregular schedules, can lead to autonomic imbalance and increased risk of cardiovascular events (Young and Bray, 2007; Portaluppi et al., 2012). Studies have shown that SCN lesions abolish circadian patterns in multiple physiological outputs, including heart rate, while pharmacological sympathectomy selectively reduces the heart rate circadian rhythmicity (Warren et al., 1994). This underscores the key role of sympathetic nervous system in circadian regulation of cardiovascular function, highlighting the need for chronotherapeutic strategies to reduce cardiovascular risks linked to circadian disruption (Richards and Gumz, 2013; Young and Bray, 2007).
5.2. Neuroendocrine pathways
Neuroendocrine pathways play a crucial role in circadian regulation, with melatonin and cortisol serving as key hormones in this process. Melatonin, produced by the pineal gland, rises at night and peaks between 2 a.m. and 4 a.m., signaling the onset of sleep and regulating the sleep-wake cycle (28)(82). Cortisol, on the other hand, follows an opposing rhythm, peaking in the early morning around 8 a.m. and gradually decreasing throughout the day (Mieda, 2020; Orozco-Solis and Aguilar-Arnal, 2020; Stalder et al., 2025). This cortisol awakening response helps initiate wakefulness and prepares the body for daily activities (Young and Bray, 2007; Clow et al., 2010). The interaction between the hypothalamic-pituitary-adrenal (HPA) axis and the circadian clock is complex and bidirectional. SCN influences the HPA axis through multi-synaptic neural connections, regulating the rhythmic secretion of corticotropin-releasing hormone (CRH) from the PVN of the hypothalamus (Hower et al., 2018; Ding et al., 2018). This, in turn, drives the pulsatile release of adrenocorticotropic hormone (ACTH) from the pituitary, ultimately controlling cortisol production in the adrenal glands (Myung et al., 2018; Lightman et al., 2020). Conversely, glucocorticoids can influence peripheral clocks throughout the body, acting as systemic synchronizers (Richards and Gumz, 2013; Spencer et al., 2018). The HPA axis and circadian system are tightly intertwined, with disruptions in one often affecting the other. For instance, chronic stress or circadian misalignment can lead to dysregulation of both systems, potentially contributing to various health issues, including cardiovascular diseases (Mieda, 2020; Young and Bray, 2007). This emphasizes the importance of neuroendocrine pathways and their interactions for developing targeted interventions to maintain circadian health and stress resilience (Kinlein and Karatsoreos, 2020).
5.3. Inflammatory and immune responses
The circadian clock exerts significant control over immune cell activity and cytokine production, playing a crucial role in inflammatory responses and CVD. Circadian rhythms regulate the expression of key immune-related genes, including Toll-like receptors and cytokines, through core clock proteins such as BMAL1, REV-ERBα, and CLOCK (Mieda, 2020). This regulation results in diurnal variations of immune cell functions, with lymphocyte counts oscillating daily in humans (Myung et al., 2018; Beam et al., 2020; Druzd et al., 2017; Haus and Smolensky, 1999). The clock's influence extends to proinflammatory cytokine secretion, with TNF-α production in macrophages varying based on the time of endotoxin challenge (Myung et al., 2018; Chen et al., 2020). Disruption of circadian rhythms, as seen in shift work, can lead to elevated systemic inflammatory markers and increased CVD risk (Myung et al., 2018). Inflammation, in turn, can affect clock function, with endotoxin treatment rapidly decreasing levels of clock proteins (Myung et al., 2018; Curtis et al., 2014). This bidirectional relationship between circadian rhythms and inflammation contributes to CVD pathogenesis, as evidenced by the circadian pattern of cardiovascular events like MI peaking in the early morning (Hower et al., 2018; Takeda and Maemura, 2016). Apparently, these interactions are crucial for developing chronotherapeutic approaches to mitigate inflammation-related cardiovascular risks (Huang et al., 2023).
5.4. Role of exosomal intracellular communication in circadian rhythm-based CVD
Exosomes, small extracellular vesicles ranging from 30 to 120 nm in diameter, serve as critical mediators of intercellular communication. They transport various macromolecules, including proteins, lipids, DNA, mRNA, and non-coding RNA, to recipient cells, thereby influencing cellular functions (Chen et al., 2022). Recent studies have shown that circadian rhythms can modulate the content and function of circulating exosomes, suggesting a potential mechanism by which circadian disruption may contribute to CVD pathogenesis (Chen et al., 2022; Khalyfa et al., 2020). Khalyfa et al. found that circadian misalignment from simulated night shifts alters the miRNA content of circulating exosomes, which then suppress clock gene expression in multiple metabolically active cell types, broadly disrupting peripheral circadian regulation (Khalyfa et al., 2020). Furthermore, the study showed that exosomes from the night shift condition could phase-shift the expression of BMAL1 in human adipocytes by approximately 6.2 h compared to exosomes from the day shift condition. This finding suggests that exosomes can communicate circadian misalignment to peripheral tissues, potentially disrupting metabolic homeostasis and contributing to insulin resistance (Khalyfa et al., 2020). The role of specific miRNAs in this process has also been elucidated. For instance, hsa-mir-3614-5p was identified as a functionally relevant mediator of night shift-induced circadian misalignment, with downstream effects on cellular metabolic function (Khalyfa et al., 2020). These findings suggest exosomal miRNAs could serve as biomarkers and therapeutic targets for circadian-related CVD. Yeung et al. also showed circadian protein cargo in small EVs peaks at specific times, impacting cardiovascular health and disease progression (Yeung et al., 2022). Apparently, exosomal signaling is a key link between circadian disruption and cardiovascular disease, as altered miRNA and protein cargo can broadly impact peripheral clock gene expression and metabolic function.
6. Diagnostic implications of circadian rhythm dysfunction in CVDs
Circadian rhythm dysfunction plays a crucial diagnostic role in cardiovascular disease, as it regulates blood pressure, heart rate, and vascular function. Disruption-often from shift work or sleep disorders-raises CVD risk, increasing hypertension, arrhythmia, and myocardial infarction by disturbing normal blood pressure rhythms (Rabinovich-Nikitin et al., 2019; Chellappa et al., 2019; Morris et al., 2016). At the molecular level, circadian dysfunction affects endothelial function by disrupting nitric oxide production and promoting inflammation. Experimental evidence indicates that mutations in core clock genes such as Bmal1 and Clock lead to endothelial dysfunction, reduced glucose metabolism, and accelerated cardiomyopathy (Rabinovich-Nikitin et al., 2019; Thosar et al., 2018; Chellappa et al., 2019). Additionally, circadian disruption alters the rhythmic expression of CCGs involved in cardiac metabolism and repair, exacerbating CVD progression (Young, 2023; Buurma et al., 2019). Clinically, circadian patterns influence the timing of adverse cardiovascular events. Morning hours are associated with a higher incidence of MI and stroke due to increased sympathetic activity and platelet aggregation. This temporal pattern underscores the importance of chronotherapy; timing medical interventions to align with circadian rhythms. For example, afternoon surgeries have been shown to reduce perioperative cardiac events compared to morning procedures (Chellappa et al., 2019; Morris et al., 2016). Apparently, diagnosing circadian rhythm dysfunction provides critical insights into CVD risk stratification and management. Table 2 presents a range of novel biomarkers linked to circadian disruption in cardiovascular disease, highlighting emerging molecular and physiological indicators that may improve risk assessment and guide personalized interventions. Incorporating chronobiological assessments into clinical practice could improve outcomes by enabling personalized therapeutic strategies aligned with patients' circadian profiles. Additionally, a large multicenter study investigated whether cardiac troponin I (cTnI) blood concentrations, unlike troponin T, exhibit a diurnal rhythm and if this variation affects acute myocardial infarction (AMI) diagnosis. Using four different (high sensitivity) cTnI assays in over 2600 emergency patients, researchers found no significant differences in cTnI levels or diagnostic accuracy between morning and evening presentations. All assays showed consistently high accuracy for AMI detection regardless of sampling time. These findings suggest that, unlike some cardiac biomarkers, cTnI is not influenced by circadian rhythms, supporting its reliability for AMI diagnosis at any time of day (Wildi et al., 2018). Importantly, considering individual variability is crucial for diagnosis and treatment, even among patients of similar sex, age, or ethnicity. Standard blood pressure targets may overlook important vascular anomalies, while personalized, time-specific monitoring can better identify risk. Diagnosing based on circadian amplitude and acrophase, rather than average values alone, may enable tailored drug timing, improving management and prevention of vascular issues (Halberg et al., 2013; Crnko et al., 2019b).
Table 2.
Emerging biomarkers associated with circadian dysregulation in CVD.
| Biomarker | Mechanism of Action | Status | Ref. |
|---|---|---|---|
| Plasminogen Activator Inhibitor-1 (PAI-1) | Circadian-regulated protein involved in fibrinolysis; elevated levels during circadian misalignment promote thrombosis. | Identified as circadian regulated, under investigation. | McHill et al. (2024) |
| Tissue Factor Pathway Inhibitor (TFPI) | Reduced during circadian misalignment, contributing to hypercoagulable states and increased cardiovascular risk. | Under investigation. | McHill et al. (2024) |
| Coagulation Factor VII | Exhibits 24-h rhythmicity; altered levels during circadian misalignment may increase coagulation risk. | Under investigation. | McHill et al. (2024) |
| BloodCCD | Measures circadian clock disruption through RNA-sequencing of 42 clock-regulated genes in blood samples. | Clinical trials for circadian dysfunction detection. | Altman et al. (2022) |
| miRNAs (e.g., QSOX1, LIPCAR, MICRA) | Circadian variations in miRNA expression reflect inflammatory and metabolic changes linked to cardiovascular disease. | Investigational biomarkers for CAD and heart failure. | Vanhaverbeke et al. (2022) |
| Nicotinamide Phosphoribosyl transferase (NAMPT) | Regulates NAD + biosynthesis; circadian oscillations influence energy metabolism and cardiovascular homeostasis. | Preclinical studies. | Acosta-Rodríguez et al. (2021) |
| TimeTeller (Wearable Device Output) | Non-invasive monitoring of circadian rhythm outputs like body temperature and heart rate for personalized diagnostics. | Emerging tool; under evaluation. | Dose et al. (2023) |
| ARNTL, CRY2, BHLHE41, NPAS2 | Circadian rhythm-related hub genes that serve as biomarkers for diagnosis and treatment of heart failure | Under investigation. | Sun et al. (2023) |
| C-Reactive Protein, IL-6 | Pro-inflammatory markers linked to increased oxidative stress and disrupted circadian rhythms, upregulated in shift workers | Under investigation. | Wong et al. (2023) |
| Plasminogen activator inhibitor-1, Angiopoietin-2, Insulin-like growth factor binding protein-4, Follistatin-related protein-3, and Endoplasmic reticulum resident protein-29 | Circadian regulated proteins associated decreased tissue factor pathway inhibitor and increased tissue factor within the coagulation pathway during circadian misalignment; promote hypercoagulative state. | Under investigation. | McHill et al. (2024) |
7. Therapeutic implications and chronotherapy
7.1. Targeting the circadian clock for CVD prevention and treatment
Targeting the circadian clock for CVD prevention and treatment has emerged as a promising approach. Pharmacological modulation of clock genes and pathways offers potential therapeutic benefits. Small molecules targeting clock proteins such as RORs, REV-ERBs, and CRY1/2 have shown promise in preclinical studies (Mieda, 2020). For instance, the REV-ERB agonist SR9009 demonstrated beneficial effects on cardiac remodeling after pressure overload (Mieda, 2020; Reitz et al., 2019). Inhibiting REV-ERBs using SR8278 has also shown potential for cardio-protection in cardiac injury (Mieda, 2020; Huang et al., 2022). However, further characterization of these clock-targeting molecules is needed to determine their precise mechanisms of action. Lifestyle interventions targeting circadian rhythms have also shown promise for CVD prevention. Time-restricted eating (TRE), which extends nighttime fasting duration to more than 12 h, has been associated with improvements in key cardiovascular health indicators such as reduced body weight, blood pressure, and inflammation (Richards and Gumz, 2013). However, results have not always been consistent, and more research is needed to establish the direct association between nighttime fasting duration and CVD risk (Richards and Gumz, 2013; St-Onge et al., 2017). Timed exercise, particularly moderate aerobic morning exercise, has been shown to help reduce cardiovascular risk factors such as high blood pressure (Mieda, 2020; Cornelissen et al., 2009). Light therapy, which helps entrain the central circadian clock, may also have potential benefits for cardiovascular health. Additionally, adopting earlier daily eating patterns, such as having earlier first and last meals of the day, has been associated with a lower risk of cardiovascular outcomes (Richards and Gumz, 2013). These findings suggest that both pharmacological and lifestyle interventions targeting the circadian clock may offer novel approaches for CVD prevention and treatment.
7.2. Chronotherapy in cardiovascular medicine
Chronotherapy in cardiovascular medicine harnesses circadian rhythms to enhance treatment outcomes and minimize side effects. Notably, the Hygia study found that bedtime dosing of antihypertensives reduced cardiovascular events by 45 % compared to morning dosing in hypertensive patients (Hermida et al., 2020). This approach aligns with the body's natural circadian blood pressure variations, potentially offering superior protection against cardiovascular events. Statin administration timing also impacts efficacy, with evening dosing generally recommended due to the circadian rhythm of cholesterol synthesis. Personalized approaches based on circadian phenotypes emerge as a promising strategy in cardiovascular chronotherapy. Table 3 provides an overview of both experimental and clinically approved drugs, illustrating their mechanisms of action and potential roles in modulating circadian rhythms for CVD management. Individual variations in circadian rhythms, influenced by factors such as chronotype, diet, and exercise, necessitate tailored treatment schedules (Hower et al., 2018). ZeitZeiger, a method for predicting circadian time from gene expression, has shown potential in personalizing chronotherapy by accurately estimating an individual's internal circadian time (Richards and Gumz, 2013). This approach could help identify “chronofit” patients who would benefit most from personalized chronotherapy (Myung et al., 2018). However, implementing personalized chronotherapy in clinical practice requires further validation through prospective studies (Orozco-Solis and Aguilar-Arnal, 2020; Young and Bray, 2007).
Table 3.
Experimental and clinical drugs acting on circadian pathway.
| Drug | Mechanism of Action | Status | Ref. |
|---|---|---|---|
| Tasimelteon (Hetlioz) | Melatonin receptor agonist (MT1 and MT2); entrains the circadian clock in totally blind individuals with non-24-h sleep-wake disorder. | FDA-approved for non-24-h sleep-wake disorder. | Tsuey Tse (2014) |
| Melatonin | Agonist for melatonin receptors (MT1 and MT2); synchronizes circadian rhythms and regulates sleep-wake cycles. | Widely available; used off-label for circadian rhythm disorders. | (Tsuey Tse, 2014), (Ruan et al., 2021), (Tsuey Tse, 2014) |
| Modafinil/Armodafinil | Promotes wakefulness by modulating dopamine transporters; used for shift work sleep disorder. | FDA-approved for shift work disorder. | Servid (2021) |
| Nobiletin | ROR agonist; enhances circadian clock amplitude and metabolic protection in preclinical studies. | Under investigation in preclinical studies. | Ruan et al. (2021) |
| KL001 | Stabilizes CRY; lengthens the circadian period and reduces amplitude. | Experimental; preclinical development. | Ruan et al. (2021) |
| CK1 Inhibitors | Stabilize PER proteins by inhibiting casein kinase 1 (CK1); modulate circadian period length. | Experimental; preclinical development. | Ruan et al. (2021) |
| REV-ERB Agonists | Target nuclear receptor REV-ERB to regulate circadian transcription and metabolism. | Under investigation in preclinical studies. | Sulli et al. (2018) |
| Ramelteon | Produces significant phase advance of human melatonin rhythm, facilitates resynchronization; melatonin agonist, decreases fibrosis severity | Under investigation in clinical studies. | (Richardson et al., 2008), (Aydin et al., 2023) |
| Agomelatine | Acts via non-selective MT1/MT2 melatonin receptor agonist, prevents oxidative stress, induces anti-inflammatory effects | Under investigation in clinical studies. | Promsan et al. (2022) |
| Tasimelteon | Melatonin receptor agonist with higher affinity for MT2 receptors, retrains circadian clock | FDA-approved treatment | Pavkovic and Kothare (2022) |
8. Conclusion
Circadian rhythms are fundamental to cardiovascular health, governed by complex neurobiological mechanisms. The suprachiasmatic nucleus (SCN) in the hypothalamus acts as the body's central circadian pacemaker, regulating cardiovascular function through the autonomic nervous system and neuroendocrine pathways. This leads to daily fluctuations in blood pressure, heart rate, and endothelial function. Disruptions to these rhythms—such as those caused by shift work or irregular routines—are linked to a higher risk of CVD. Studies show that maintaining consistent sleep schedules and adopting earlier mealtimes can help lower this risk, emphasizing the role of circadian health in prevention. The field of circadian medicine is rapidly evolving, with research focused on understanding the mechanisms connecting circadian disruption to CVD and developing targeted interventions. Chronotherapy, such as taking antihypertensive medications at bedtime, has demonstrated significant benefits in reducing cardiovascular events. Personalized approaches based on individual circadian profiles are emerging, and integrating circadian principles into treatment and lifestyle—like optimizing meal timing and sleep—offers promising, cost-effective strategies for CVD prevention and improved outcomes. Despite a huge leap in the cardiac circadian rhythm research, it is crucial to consider various conflicting results based on species differences (human versus mice study) as mentioned in Table 4.
Table 4.
Conflicting findings in human versus animals in the context of circadian studies.
| Conflicting Finding | Human Studies | Animal Studies | Ref. |
|---|---|---|---|
| Sex-specific CVD risk from circadian disruption | Daylight saving time shifts increase MI risk, with higher risk in women in spring and men in fall; confounding factors like sleep and chronotype not controlled. | Rodent studies show greater circadian-dependent MI mortality in males; controlled genetic/environmental manipulations reveal direct sex-specific effects. | (Young, 2023; Rabinovich-Nikitin et al., 2019) |
| Mechanisms of circadian influence | Mechanisms linking circadian disruption to CVD remain cryptic, with multiple confounders (sleep deprivation, hormonal changes, inflammation). | Genetic ablation of clock genes (e.g., CLOCK/BMAL1) in mice leads to cardiomyopathy, arrhythmias, and altered metabolism, directly linking clock disruption to pathology. | (Young, 2023), (Eckle et al., 2024), (Rabinovich-Nikitin et al., 2019) |
| Role of central vs. peripheral clocks | Human studies often cannot distinguish effects of central (brain) vs. peripheral (heart) clocks due to complexity of environmental cues. | Animal models can isolate central and peripheral clock contributions; disruption in specific tissues (e.g., cardiomyocytes) demonstrates distinct roles in cardiac rhythms and disease. | (Young, 2023; Rabinovich-Nikitin et al., 2019; Hayter et al., 2021) |
| Impact of environmental circadian misalignment | Shift work, daylight saving, and Intensive Care Unit (ICU) environments linked to increased CVD risk, but with variable and sometimes contradictory findings due to uncontrolled variables. | Controlled circadian misalignment (e.g., jet lag simulation) in animals consistently increases CVD risk and inflammatory responses, independent of sleep loss. | (Rabinovich-Nikitin et al., 2019), (Morris et al., 2016) |
| Chronotherapy and biomarker rhythms | Human biomarker studies show circadian variation, but clinical translation for optimized therapy timing remains inconsistent. | Animal studies demonstrate robust circadian regulation of cardiac gene/protein expression and response to therapy, supporting chronotherapy strategies. | (Young, 2023; Rabinovich-Nikitin et al., 2019; Scheer et al., 2010) |
9. Future perspectives
Future directions in circadian medicine for CVD risk prediction and management are promising. The development of circadian-based biomarkers for CVD risk prediction is an emerging area of research. Recent studies have identified transcriptomic biomarkers that can predict CVD with up to 96 % accuracy, highlighting the potential of using circadian-regulated genes as diagnostic tools (Richards and Gumz, 2013). Additionally, the integration of multiple biomarkers from different pathophysiological pathways has shown improved prognostic accuracy compared to single biomarkers (Mieda, 2020). Advances in circadian medicine and precision health are focusing on understanding how circadian misalignment affects CVD risk biomarkers. Short-term circadian misalignment has been found to influence many putative biomarkers of CVD risk, suggesting the need for further research into long-term effects. The incorporation of circadian rhythms into risk assessment models may enhance the prediction of incident CVD, as demonstrated by studies showing that increments in plasma cardiac biomarkers robustly predict increased hazard of incident CVD (Hower et al., 2018). Furthermore, the development of machine learning (ML) models for CVD risk assessment using circadian-based biomarkers is a promising avenue for personalized medicine (Zhou et al., 2024). ML models have been widely utilized for various biomedical as well as pharmaceutical purposes including classification or prediction-based disease stratification, and identification of originating parent cells of exosomes (Thakur et al., 2020a, 2020b). The development of ML models for CVD risk assessment using circadian-based biomarkers holds significant promise for personalized medicine. Recent studies demonstrate that ML models integrating proteomic and circadian data outperform traditional risk scores in predicting CVD events, offering improved accuracy and individualized risk profiles. These models enable nuanced analysis of temporal biomarker variations, enhancing early detection and prevention strategies (Zhou et al., 2024; Climente-González et al., 2025; Ambale-Venkatesh et al., 2017). Neural network-based machine learning (NNML) models, including both classical and quantum-enhanced approaches, offer powerful tools for the early detection of CVDs by analyzing complex molecular and genomic datasets from both healthy individuals and patients. These models can identify subtle patterns and biomarkers associated with disease onset and progression, enabling more accurate risk stratification. Looking ahead, integrating ML algorithms with Internet of Things (IoT) technologies (facilitate connection of devices with sensors and software to exchange data autonomously) could facilitate the development of real-time, wearable diagnostic devices. Such integration would enable continuous monitoring and personalized prognosis, ultimately enhancing the early diagnosis, prevention, and management of CVDs in clinical settings. In addition, biosensors could be developed for rapid detection of various CVD-specific EVs, which could be potentially utilized for minimally invasive liquid biopsy of CVD.
As our comprehension of the circadian system's role in cardiovascular health deepens, there is growing potential for circadian medicine to revolutionize CVD prevention and treatment strategies, leading to more precise and effective interventions (Mieda, 2020). However, implementing circadian-based interventions for CVD faces several challenges. First, translating findings from animal models to humans is complex due to differences in circadian biology between species and a lack of definitive biomarkers for circadian disruption in humans (Eckle et al., 2024). Clinical studies are limited, with most interventions tested only in small cohorts or under controlled conditions, which may not reflect real-world variability (Morris et al., 2016). Additionally, optimal timing for drug administration or lifestyle interventions remains unclear and may need to be individualized. Key research priorities include large-scale, longitudinal human studies, development of reliable circadian biomarkers, and personalized chronotherapy protocols to maximize efficacy and minimize adverse effects (Young, 2023; Lecacheur et al., 2024).
CRediT authorship contribution statement
Abhimanyu Thakur: Writing – original draft, Visualization, Validation, Project administration, Formal analysis, Data curation, Conceptualization. Raj Kishore: Writing – review & editing, Supervision, Funding acquisition.
Funding
This work was supported, in part, by National Institutes of Health grants, HL134608, HL147841 and HL169405.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
We appreciate the scientific discussion and assistance from Christopher Wittmann, Maddy Cohen, Charan Thej, Vandana Mallaredy, Elena McMullan, Hajime Kubo, Darukeshwara Joladarashi, and Zhongjian Cheng. All the figures were generated using Biorender.com.
References
- Abe J., Hiyama T.B., Mukaiyama A., Son S., Mori T., Saito S., Osako M., Wolanin J., Yamashita E., Kondo T., Akiyama S. Atomic-scale origins of slowness in the cyanobacterial circadian clock. Science. 2015;349(6245):312–316. doi: 10.1126/science.1261040. [DOI] [PubMed] [Google Scholar]
- Acosta-Rodríguez V.A., Rijo-Ferreira F., Green C.B., Takahashi J.S. Importance of circadian timing for aging and longevity. Nat. Commun. 2021;12(1):2862. doi: 10.1038/s41467-021-22922-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adan A., Natale V. Gender differences in morningness–eveningness preference. Chronobiol. Int. 2002;19(4):709–720. doi: 10.1081/cbi-120005390. [DOI] [PubMed] [Google Scholar]
- Aguilar-Arnal L., Sassone-Corsi P. Chromatin landscape and circadian dynamics: spatial and temporal organization of clock transcription. Proc. Natl. Acad. Sci. 2015;112(22):6863–6870. doi: 10.1073/pnas.1411264111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman B.J., Bautista J., Culakova E., Morris K.M., DeRollo R.E., Outland E., Kleckner A., Kleckner I.R., Gilmore N.J., Esparaz B.T., Kuzma C.S., Woude AC Vander, Lin P.-J., Hughey J.J., Mustian K.M. Abstract 3215: BloodCCD is a novel biomarker to detect circadian rhythm disruption in cancer survivors with insomnia. Cancer Res. 2022;82(12_Suppl. ment) 3215–3215. [Google Scholar]
- Ambale-Venkatesh B., Yang X., Wu C.O., Liu K., Hundley W.G., McClelland R., Gomes A.S., Folsom A.R., Shea S., Guallar E., Bluemke D.A., Lima J.A.C. Cardiovascular event prediction by machine learning. Circ. Res. 2017;121(9):1092–1101. doi: 10.1161/CIRCRESAHA.117.311312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S.T., Meng H., Brooks T.G., Tang S.Y., Lordan R., Sengupta A., Nayak S., Mřela A., Sarantopoulou D., Lahens N.F., Weljie A., Grant G.R., Bushman F.D., FitzGerald G.A. Sexual dimorphism in the response to chronic circadian misalignment on a high-fat diet. Sci. Transl. Med. 2023;15(696) doi: 10.1126/scitranslmed.abo2022. [DOI] [PubMed] [Google Scholar]
- Ansu Baidoo V., Knutson K.L. Associations between circadian disruption and cardiometabolic disease risk: a review. Obesity (Silver Spring, Md.) 2023;31(3):615–624. doi: 10.1002/oby.23666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrieta A., Chapski D.J., Reese A., Kimball T.H., Song K., Rosa-Garrido M., Vondriska T.M. Circadian control of histone turnover during cardiac development and growth. J. Biol. Chem. 2024;300(7) doi: 10.1016/j.jbc.2024.107434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astafev A.A., Mezhnina V., Poe A., Jiang P., Kondratov R.V. Sexual dimorphism of circadian liver transcriptome. iScience. 2024;27(4) doi: 10.1016/j.isci.2024.109483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astiz M., Heyde I., Oster H. Mechanisms of communication in the mammalian circadian timing system. Int. J. Mol. Sci. 2019;20(2):343. doi: 10.3390/ijms20020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson G., Jones H., Ainslie P.N. Circadian variation in the circulatory responses to exercise: relevance to the morning peaks in strokes and cardiac events. Eur. J. Appl. Physiol. 2010;108(1):15–29. doi: 10.1007/s00421-009-1243-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydin P., Aksakalli-Magden Z.B., Civelek M.S., Karabulut-Uzuncakmak S., Mokhtare B., Ozkaraca M., Alper F., Halici Z. The melatonin agonist ramelteon attenuates bleomycin-induced lung fibrosis by suppressing the NLRP3/TGF-Β1/HMGB1 signaling pathway. Adv. Med. Sci. 2023;68(2):322–331. doi: 10.1016/j.advms.2023.09.004. [DOI] [PubMed] [Google Scholar]
- Aziz I.S., McMahon A.M., Friedman D., Rabinovich-Nikitin I., Kirshenbaum L.A., Martino T.A. Circadian influence on inflammatory response during cardiovascular disease. Curr. Opin. Pharmacol. 2021;57:60–70. doi: 10.1016/j.coph.2020.11.007. [DOI] [PubMed] [Google Scholar]
- Barclay J.L., Tsang A.H., Oster H. 2012. Interaction of Central and Peripheral Clocks in Physiological Regulation; pp. 163–181. [DOI] [PubMed] [Google Scholar]
- Bass J., Lazar M.A. Circadian time signatures of fitness and disease. Science. 2016;354(6315):994–999. doi: 10.1126/science.aah4965. [DOI] [PubMed] [Google Scholar]
- Beam C.A., Wasserfall C., Woodwyk A., Akers M., Rauch H., Blok T., Mason P., Vos D., Perry D., Brusko T., Peakman M., Atkinson M. Synchronization of the normal human peripheral immune system: a comprehensive circadian systems immunology analysis. Sci. Rep. 2020;10(1):672. doi: 10.1038/s41598-019-56951-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beesley S., Noguchi T., Welsh D.K. Cardiomyocyte circadian oscillations are cell-autonomous, amplified by β-adrenergic signaling, and synchronized in cardiac ventricle tissue. PLoS One. 2016;11(7) doi: 10.1371/journal.pone.0159618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellet M.M., Sassone-Corsi P. Mammalian circadian clock and metabolism – the epigenetic link. J. Cell Sci. 2010;123(22):3837–3848. doi: 10.1242/jcs.051649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskara M., Anjorin O., Yoniles A., Liu J., Wang M. Importance of Per2 in cardiac mitochondrial protection during stress. Sci. Rep. 2024;14(1):1290. doi: 10.1038/s41598-024-51799-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black N., D'Souza A., Wang Y., Piggins H., Dobrzynski H., Morris G., Boyett M.R. Circadian rhythm of cardiac electrophysiology, arrhythmogenesis, and the underlying mechanisms. Heart Rhythm. 2019;16(2):298–307. doi: 10.1016/j.hrthm.2018.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boari B., Salmi R., Gallerani M., Malagoni A.M., Manfredini F., Manfredini R. Acute myocardial infarction: circadian, weekly, and seasonal patterns of occurrence. Biol. Rhythm Res. 2007;38(3):155–167. [Google Scholar]
- Boivin D.B., Shechter A., Boudreau P., Begum E.A., Ng Ying-Kin N.M.K. Diurnal and circadian variation of sleep and alertness in men vs. naturally cycling women. Proc. Natl. Acad. Sci. 2016;113(39):10980–10985. doi: 10.1073/pnas.1524484113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenna A., Albrecht U. Phosphorylation and circadian molecular timing. Front. Physiol. 2020;11 doi: 10.3389/fphys.2020.612510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buurma M., van Diemen J.J.K., Thijs A., Numans M.E., Bonten T.N. Circadian rhythm of cardiovascular disease: the potential of chronotherapy with aspirin. Front. Cardiovasc. Med. 2019;6 doi: 10.3389/fcvm.2019.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellappa S.L., Vujovic N., Williams J.S., Scheer F.A.J.L. Impact of circadian disruption on cardiovascular function and disease. Trends Endocrinol. Metabol. 2019;30(10):767–779. doi: 10.1016/j.tem.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Fuller K.K., Dunlap J.C., Loros J.J. A pro- and anti-inflammatory Axis modulates the macrophage circadian clock. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.00867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.-Y., Lee D.S., Choong O.K., Chang S.-K., Hsu T., Nicholson M.W., Liu L.-W., Lin P.-J., Ruan S.-C., Lin S.-W., Hu C.-Y., Hsieh P.C.H. Cardiac-specific microRNA-125b deficiency induces perinatal death and cardiac hypertrophy. Sci. Rep. 2021;11(1):2377. doi: 10.1038/s41598-021-81700-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Huang Q., Wang Z., Zhuang X., Lin S., Shi Q. Therapeutic potential of exosomes/miRNAs in polycystic ovary syndrome induced by the alteration of circadian rhythms. Front. Endocrinol. 2022;13 doi: 10.3389/fendo.2022.918805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Climente-González H., Oh M., Chajewska U., Hosseini R., Mukherjee S., Gan W., Traylor M., Hu S., Fatemifar G., Ghouse J., Del Villar P.P., Vernet E., Koelling N., Du L., Abraham R., et al. Interpretable machine learning leverages proteomics to improve cardiovascular disease risk prediction and biomarker identification. Commun. Med. 2025;5(1):170. doi: 10.1038/s43856-025-00872-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clow A., Hucklebridge F., Thorn L. 2010. The Cortisol Awakening Response in Context; pp. 153–175. [DOI] [PubMed] [Google Scholar]
- Cornelissen V.A., Arnout J., Holvoet P., Fagard R.H. Influence of exercise at lower and higher intensity on blood pressure and cardiovascular risk factors at older age. J. Hypertens. 2009;27(4):753–762. doi: 10.1097/HJH.0b013e328322cf60. [DOI] [PubMed] [Google Scholar]
- Crenguța Nicolae A., Dumitrescu I.-B., Cristina Diaconu C., Elena Ritivoiu M., Adella Sirbu C., Manuela Drăgoi C. Circadian Rhythm - New Insights into Physiological and Pathological Implications. IntechOpen; 2022. Chronotherapy advances in the management of chronic neurological and cardiovascular diseases: complex interactions of circadian rhythm environmental inputs, nutrition and drug administration and their impact on human health. [Google Scholar]
- Crnko S., Cour M., Van Laake L.W., Lecour S. Vasculature on the clock: circadian rhythm and vascular dysfunction. Vasc. Pharmacol. 2018;108:1–7. doi: 10.1016/j.vph.2018.05.003. [DOI] [PubMed] [Google Scholar]
- Crnko S., Du Pré B.C., Sluijter J.P.G., Van Laake L.W. Circadian rhythms and the molecular clock in cardiovascular biology and disease. Nat. Rev. Cardiol. 2019;16(7):437–447. doi: 10.1038/s41569-019-0167-4. [DOI] [PubMed] [Google Scholar]
- Crnko S., Du Pré B.C., Sluijter J.P.G., Van Laake L.W. Circadian rhythms and the molecular clock in cardiovascular biology and disease. Nat. Rev. Cardiol. 2019;16(7):437–447. doi: 10.1038/s41569-019-0167-4. [DOI] [PubMed] [Google Scholar]
- Csoma B., Bikov A. The role of the circadian rhythm in dyslipidaemia and vascular inflammation leading to atherosclerosis. Int. J. Mol. Sci. 2023;24(18) doi: 10.3390/ijms241814145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis A.M., Seo S., Westgate E.J., Rudic R.D., Smyth E.M., Chakravarti D., FitzGerald G.A., McNamara P. Histone acetyltransferase-dependent chromatin remodeling and the vascular clock. J. Biol. Chem. 2004;279(8):7091–7097. doi: 10.1074/jbc.M311973200. [DOI] [PubMed] [Google Scholar]
- Curtis A.M., Cheng Y., Kapoor S., Reilly D., Price T.S., FitzGerald G.A. Circadian variation of blood pressure and the vascular response to asynchronous stress. Proc. Natl. Acad. Sci. 2007;104(9):3450–3455. doi: 10.1073/pnas.0611680104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis A.M., Bellet M.M., Sassone-Corsi P., O'Neill L.A.J. Circadian clock proteins and immunity. Immunity (Camb., Mass.) 2014;40(2):178–186. doi: 10.1016/j.immuni.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Diekman C.O., Wei N. Circadian rhythms of early afterdepolarizations and ventricular arrhythmias in a cardiomyocyte model. Biophys. J. 2021;120(2):319–333. doi: 10.1016/j.bpj.2020.11.2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding G., Gong Y., Eckel-Mahan K.L., Sun Z. 2018. Central Circadian Clock Regulates Energy Metabolism; pp. 79–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi M., Hirayama J., Sassone-Corsi P. Circadian regulator CLOCK is a histone acetyltransferase. Cell. 2006;125(3):497–508. doi: 10.1016/j.cell.2006.03.033. [DOI] [PubMed] [Google Scholar]
- Dose B., Yalçin M., Dries S.P.M., Relógio A. TimeTeller for timing health: the potential of circadian medicine to improve performance, prevent disease and optimize treatment. Frontiers in Digital Health. 2023;5 doi: 10.3389/fdgth.2023.1157654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas P.S., Katz S.E., Weinberg E.O., Chen M.H., Bishop S.P., Lorell B.H. Hypertrophic remodeling: gender differences in the early response to left ventricular pressure overload. J. Am. Coll. Cardiol. 1998;32(4):1118–1125. doi: 10.1016/s0735-1097(98)00347-7. [DOI] [PubMed] [Google Scholar]
- Douma L.G., Gumz M.L. Circadian clock-mediated regulation of blood pressure. Free Radic. Biol. Med. 2018;119:108–114. doi: 10.1016/j.freeradbiomed.2017.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druzd D., Matveeva O., Ince L., Harrison U., He W., Schmal C., Herzel H., Tsang A.H., Kawakami N., Leliavski A., Uhl O., Yao L., Sander L.E., Chen C.-S., Kraus K., et al. Lymphocyte circadian clocks control lymph node trafficking and adaptive immune responses. Immunity (Camb., Mass.) 2017;46(1):120–132. doi: 10.1016/j.immuni.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duez H., Staels B. The nuclear receptors Rev-erbs and RORs integrate circadian rhythms and metabolism. Diabetes Vasc. Dis. Res. 2008;5(2):82–88. doi: 10.3132/dvdr.2008.0014. [DOI] [PubMed] [Google Scholar]
- Duffy J.F., Cain S.W., Chang A.-M., Phillips A.J.K., Münch M.Y., Gronfier C., Wyatt J.K., Dijk D.-J., Wright K.P., Czeisler C.A. Sex difference in the near-24-hour intrinsic period of the human circadian timing system. Proc. Natl. Acad. Sci. 2011;108(Suppl. ment_3):15602–15608. doi: 10.1073/pnas.1010666108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durgan D.J., Pat B.M., Laczy B., Bradley J.A., Tsai J.-Y., Grenett M.H., Ratcliffe W.F., Brewer R.A., Nagendran J., Villegas-Montoya C., Zou C., Zou L., Johnson R.L., Dyck J.R.B., Bray M.S., et al. O-GlcNAcylation, novel post-translational modification linking myocardial metabolism and cardiomyocyte circadian clock. J. Biol. Chem. 2011;286(52):44606–44619. doi: 10.1074/jbc.M111.278903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckle T., Bertazzo J., Khatua T.N., Fatemi Tabatabaei S.R., Moori Bakhtiari N., Walker L.A., Martino T.A. Circadian influences on myocardial ischemia-reperfusion injury and heart failure. Circ. Res. 2024;134(6):675–694. doi: 10.1161/CIRCRESAHA.123.323522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Jamal N., Lordan R., Teegarden S.L., Grosser T., FitzGerald G. The circadian biology of heart failure. Circ. Res. 2023;132(2):223–237. doi: 10.1161/CIRCRESAHA.122.321369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund A., Kovanen L., Saarikoski S.T., Haukka J., Reunanen A., Aromaa A., Lönnqvist J., Partonen T. NPAS2 and PER2 are linked to risk factors of the metabolic syndrome. J. Circadian Rhythms. 2009;7(0):5. doi: 10.1186/1740-3391-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanjul-Moles M.L., López-Riquelme G.O. Relationship between oxidative stress, circadian rhythms, and AMD. Oxid. Med. Cell. Longev. 2016;2016(1) doi: 10.1155/2016/7420637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraci F.M., Scheer F.A.J.L. Hypertension: causes and consequences of circadian rhythms in blood pressure. Circ. Res. 2024;134(6):810–832. doi: 10.1161/CIRCRESAHA.124.323515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng D., Lazar M.A. Clocks, metabolism, and the epigenome. Mol. Cell. 2012;47(2):158–167. doi: 10.1016/j.molcel.2012.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festus I.D., Spilberg J., Young M.E., Cain S., Khoshnevis S., Smolensky M.H., Zaheer F., Descalzi G., Martino T.A. Pioneering new frontiers in circadian medicine chronotherapies for cardiovascular health. Trends Endocrinol. Metabol. 2024;35(7):607–623. doi: 10.1016/j.tem.2024.02.011. [DOI] [PubMed] [Google Scholar]
- Garaulet M., Madrid J.A. Chronobiology, genetics and metabolic syndrome. Curr. Opin. Lipidol. 2009;20(2):127–134. doi: 10.1097/MOL.0b013e3283292399. [DOI] [PubMed] [Google Scholar]
- Gooley J.J., Chamberlain K., Smith K.A., Khalsa S.B.S., Rajaratnam S.M.W., Van Reen E., Zeitzer J.M., Czeisler C.A., Lockley S.W. Exposure to room light before bedtime suppresses melatonin onset and shortens melatonin duration in humans. J. Clin. Endocrinol. Metabol. 2011;96(3):E463–E472. doi: 10.1210/jc.2010-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosjean E., Simonneaux V., Challet E. Reciprocal interactions between circadian clocks, food intake, and energy metabolism. Biology (Basel) 2023;12(4):539. doi: 10.3390/biology12040539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumz M.L., Shimbo D., Abdalla M., Balijepalli R.C., Benedict C., Chen Y., Earnest D.J., Gamble K.L., Garrison S.R., Gong M.C., Hogenesch J.B., Hong Y., Ivy J.R., Joe B., Laposky A.D., et al. Toward precision medicine: circadian rhythm of blood pressure and chronotherapy for hypertension - 2021 NHLBI workshop report. Hypertension. 2023;80(3):503–522. doi: 10.1161/HYPERTENSIONAHA.122.19372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y i-F, Stein P.K. Circadian rhythm in the cardiovascular system: chronocardiology. Am. Heart J. 2003;145(5):779–786. doi: 10.1016/S0002-8703(02)94797-6. [DOI] [PubMed] [Google Scholar]
- Halberg F., Powell D., Otsuka K., Watanabe Y., Beaty L.A., Rosch P., Czaplicki J., Hillman D., Schwartzkopff O., Cornelissen G. Diagnosing vascular variability anomalies, not only MESOR-hypertension. Am. J. Physiol. Heart Circ. Physiol. 2013;305(3):H279–H294. doi: 10.1152/ajpheart.00212.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque S.N., Booreddy S.R., Welsh D.K. Effects of BMAL1 manipulation on the brain's master circadian clock and behavior. Yale J. Biol. Med. 2019;92(2):251–258. [PMC free article] [PubMed] [Google Scholar]
- Hastings M.H., Maywood E.S., Brancaccio M. Generation of circadian rhythms in the suprachiasmatic nucleus. Nat. Rev. Neurosci. 2018;19(8):453–469. doi: 10.1038/s41583-018-0026-z. [DOI] [PubMed] [Google Scholar]
- Haus E., Smolensky M.H. Biologic rhythms in the immune system. Chronobiol. Int. 1999;16(5):581–622. doi: 10.3109/07420529908998730. [DOI] [PubMed] [Google Scholar]
- Hayter E.A., Wehrens S.M.T., Van Dongen H.P.A., Stangherlin A., Gaddameedhi S., Crooks E., Barron N.J., Venetucci L.A., O'Neill J.S., Brown T.M., Skene D.J., Trafford A.W., Bechtold D.A. Author Correction: distinct circadian mechanisms govern cardiac rhythms and susceptibility to arrhythmia. Nat. Commun. 2021;12(1):7284. doi: 10.1038/s41467-021-27498-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermida R.C., Ayala D.E., Fernandez J.R., Portaluppi F., Fabbian F., Smolensky M.H. Circadian rhythms in blood pressure regulation and optimization of hypertension treatment with ACE inhibitor and ARB medications. Am. J. Hypertens. 2011;24(4):383–391. doi: 10.1038/ajh.2010.217. [DOI] [PubMed] [Google Scholar]
- Hermida R.C., Crespo J.J., Domínguez-Sardiña M., Otero A., Moyá A., Ríos M.T., Sineiro E., Castiñeira M.C., Callejas P.A., Pousa L., Salgado J.L., Durán C., Sánchez J.J., Fernández J.R., Mojón A., et al. Bedtime hypertension treatment improves cardiovascular risk reduction: the Hygia Chronotherapy Trial. Eur. Heart J. 2020;41(48):4565–4576. doi: 10.1093/eurheartj/ehz754. [DOI] [PubMed] [Google Scholar]
- Hirano A., Fu Y.-H., Ptáček L.J. The intricate dance of post-translational modifications in the rhythm of life. Nat. Struct. Mol. Biol. 2016;23(12):1053–1060. doi: 10.1038/nsmb.3326. [DOI] [PubMed] [Google Scholar]
- Hower I.M., Harper S.A., Buford T.W. Circadian rhythms, exercise, and cardiovascular health. J. Circadian Rhythms. 2018;16(1) doi: 10.5334/jcr.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q., Tian L., Zhao X., Lei S., Zhao B., Qiu Z., Xia Z.-Y. Rev-erbs agonist SR9009 alleviates ischemia-reperfusion injury by heightening endogenous cardioprotection at onset of type-2 diabetes in rats: down-regulating ferritinophagy/ferroptosis signaling. Biomed. Pharmacother. 2022;154 doi: 10.1016/j.biopha.2022.113595. [DOI] [PubMed] [Google Scholar]
- Huang H., Mehta A., Kalmanovich J., Anand A., Bejarano M.C., Garg T., Khan N., Tonpouwo G.K., Shkodina A.D., Bardhan M. Immunological and inflammatory effects of infectious diseases in circadian rhythm disruption and future therapeutic directions. Mol. Biol. Rep. 2023;50(4):3739–3753. doi: 10.1007/s11033-023-08276-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemielita P., Lip G.Y.H., Kurasz A., Święczkowski M., Krepsztul-Jemielita A., Dobrzycki S., Kuźma Ł. Noise and light exposure and cardiovascular outcomes: a review of evidence, potential mechanisms and implications. Trends Cardiovasc. Med. 2025;35(1):62–69. doi: 10.1016/j.tcm.2024.07.001. [DOI] [PubMed] [Google Scholar]
- Joska T.M., Zaman R., Belden W.J. Regulated DNA methylation and the circadian clock: implications in cancer. Biology (Basel) 2014;3(3):560–577. doi: 10.3390/biology3030560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalyfa A., Gaddameedhi S., Crooks E., Zhang C., Li Y., Qiao Z., Trzepizur W., Kay S.A., Andrade J., Satterfield B.C., Hansen D.A., Kheirandish-Gozal L., Van Dongen H.P.A., Gozal D. Circulating exosomal miRNAs signal circadian misalignment to peripheral metabolic tissues. Int. J. Mol. Sci. 2020;21(17):6396. doi: 10.3390/ijms21176396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinlein S.A., Karatsoreos I.N. The hypothalamic-pituitary-adrenal axis as a substrate for stress resilience: interactions with the circadian clock. Front. Neuroendocrinol. 2020;56 doi: 10.1016/j.yfrne.2019.100819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss M.G., Cohen O., McAlpine C.S., Swirski F.K. Influence of sleep on physiological systems in atherosclerosis. Nature Cardiovascular Research. 2024;3(11):1284–1300. doi: 10.1038/s44161-024-00560-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofuji P., Mure L.S., Massman L.J., Purrier N., Panda S., Engeland W.C. Intrinsically photosensitive retinal ganglion cells (ipRGCs) are necessary for light entrainment of peripheral clocks. PLoS One. 2016;11(12) doi: 10.1371/journal.pone.0168651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojetin D.J., Burris T.P. REV-ERB and ROR nuclear receptors as drug targets. Nat. Rev. Drug Discov. 2014;13(3):197–216. doi: 10.1038/nrd4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koronowski K.B., Sassone-Corsi P. Communicating clocks shape circadian homeostasis. Science. 2021;371(6530) doi: 10.1126/science.abd0951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal H., Verma S.K., Wang Y., Xie M., Young M.E. Circadian rhythms in cardiovascular metabolism. Circ. Res. 2024;134(6):635–658. doi: 10.1161/CIRCRESAHA.123.323520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecacheur M., Ammerlaan D.J.M., Dierickx P. Circadian rhythms in cardiovascular (dys)function: approaches for future therapeutics. npj Cardiovascular Health. 2024;1(1):21. [Google Scholar]
- Lee Y., Field J.M., Sehgal A. Circadian rhythms, disease and chronotherapy. J. Biol. Rhythm. 2021;36(6):503–531. doi: 10.1177/07487304211044301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefta M., Campbell K.S., Feng H.-Z., Jin J.-P., Esser K.A. Development of dilated cardiomyopathy in Bmal1 -deficient mice. Am. J. Physiol. Heart Circ. Physiol. 2012;303(4):H475–H485. doi: 10.1152/ajpheart.00238.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leu H.-B., Chung C.-M., Lin S.-J., Chiang K.-M., Yang H.-C., Ho H.-Y., Ting C.-T., Lin T.-H., Sheu S.-H., Tsai W.-C., Chen J.-H., Yin W.-H., Chiu T.-Y., Chen C.-I., Fann C.S., et al. Association of circadian genes with diurnal blood pressure changes and non-dipper essential hypertension: a genetic association with young-onset hypertension. Hypertens. Res. 2015;38(2):155–162. doi: 10.1038/hr.2014.152. [DOI] [PubMed] [Google Scholar]
- Li Y., Shan Y., Kilaru G.K., Berto S., Wang G.-Z., Cox K.H., Yoo S.-H., Yang S., Konopka G., Takahashi J.S. Epigenetic inheritance of circadian period in clonal cells. eLife. 2020;9 doi: 10.7554/eLife.54186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Song S., Tien C., Qi L., Graves A., Nasiotis E., Burris T.P., Zhao Y., Sun Z., Zhang L. SR9009 improves heart function after pressure overload independent of cardiac REV-ERB. Front. Cardiovasc. Med. 2022;9 doi: 10.3389/fcvm.2022.952114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Q., Xu H., Liu M., Qian L., Yan J., Yang G., Chen L. Postnatal deletion of Bmal1 in cardiomyocyte promotes pressure overload induced cardiac remodeling in mice. J. Am. Heart Assoc. 2022;11(13) doi: 10.1161/JAHA.121.025021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightman S.L., Birnie M.T., Conway-Campbell B.L. Dynamics of ACTH and cortisol secretion and implications for disease. Endocr. Rev. 2020;41(3) doi: 10.1210/endrev/bnaa002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C., Xu L., Tang X., Li X., Lu C., Cheng Q., Jiang J., Shen Y., Yan D., Qian R., Fu W., Guo D. Clock gene Bmal1 disruption in vascular smooth muscle cells worsens carotid atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 2022;42(5):565–579. doi: 10.1161/ATVBAHA.121.316480. [DOI] [PubMed] [Google Scholar]
- Martino T.A., Sole M.J. Molecular time. Circ. Res. 2009;105(11):1047–1061. doi: 10.1161/CIRCRESAHA.109.206201. [DOI] [PubMed] [Google Scholar]
- Martino T.A., Oudit G.Y., Herzenberg A.M., Tata N., Koletar M.M., Kabir G.M., Belsham D.D., Backx P.H., Ralph M.R., Sole M.J. Circadian rhythm disorganization produces profound cardiovascular and renal disease in hamsters. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;294(5):R1675–R1683. doi: 10.1152/ajpregu.00829.2007. [DOI] [PubMed] [Google Scholar]
- McHill A.W., Melanson E.L., Wright K.P., Depner C.M. Circadian misalignment disrupts biomarkers of cardiovascular disease risk and promotes a hypercoagulable state. Eur. J. Neurosci. 2024;60(7):5450–5466. doi: 10.1111/ejn.16468. [DOI] [PubMed] [Google Scholar]
- Meloni M., Setzu D., Del Rio A., Campagna M., Cocco P. QTc interval and electrocardiographic changes by type of shift work. Am. J. Ind. Med. 2013;56(10):1174–1179. doi: 10.1002/ajim.22207. [DOI] [PubMed] [Google Scholar]
- Mergenthaler P., Balami J.S., Neuhaus A.A., Mottahedin A., Albers G.W., Rothwell P.M., Saver J.L., Young M.E., Buchan A.M. Stroke in the time of circadian medicine. Circ. Res. 2024;134(6):770–790. doi: 10.1161/CIRCRESAHA.124.323508. [DOI] [PubMed] [Google Scholar]
- Mieda M. The central circadian clock of the suprachiasmatic nucleus as an ensemble of multiple oscillatory neurons. Neurosci. Res. 2020;156:24–31. doi: 10.1016/j.neures.2019.08.003. [DOI] [PubMed] [Google Scholar]
- Mistlberger R.E. Circadian regulation of sleep in mammals: role of the suprachiasmatic nucleus. Brain Res. Rev. 2005;49(3):429–454. doi: 10.1016/j.brainresrev.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Miyake C.Y., Asaki S.Y., Webster G., Czosek R.J., Atallah J., Avasarala K., Rao S.O., Thomas P.E., Kim J.J., Valdes S.O., de la Uz C., Wang Y., Wehrens X.H.T., Abrams D. Circadian variation of ventricular arrhythmias in catecholaminergic polymorphic ventricular tachycardia. JACC (J. Am. Coll. Cardiol.): Clinical Electrophysiology. 2017;3(11):1308–1317. doi: 10.1016/j.jacep.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohawk J.A., Green C.B., Takahashi J.S. Central and peripheral circadian clocks in mammals. Annu. Rev. Neurosci. 2012;35(1):445–462. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molcan L., Babarikova K., Cvikova D., Kincelova N., Kubincova L., Mauer Sutovska H. Artificial light at night suppresses the day-night cardiovascular variability: evidence from humans and rats. Pflueg. Arch. Eur. J. Physiol. 2024;476(3):295–306. doi: 10.1007/s00424-023-02901-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monfredi O., Lakatta E.G. Complexities in cardiovascular rhythmicity: perspectives on circadian normality, ageing and disease. Cardiovasc. Res. 2019;115(11):1576–1595. doi: 10.1093/cvr/cvz112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris C.J., Purvis T.E., Hu K., Scheer F.A.J.L. Circadian misalignment increases cardiovascular disease risk factors in humans. Proc. Natl. Acad. Sci. 2016;113(10) doi: 10.1073/pnas.1516953113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myung J., Schmal C., Hong S., Tsukizawa Y., Rose P., Zhang Y., Holtzman M.J., De Schutter E., Herzel H., Bordyugov G., Takumi T. The choroid plexus is an important circadian clock component. Nat. Commun. 2018;9(1):1062. doi: 10.1038/s41467-018-03507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahata Y., Sahar S., Astarita G., Kaluzova M., Sassone-Corsi P. Circadian control of the NAD + salvage pathway by CLOCK-SIRT1. Science. 2009;324(5927):654–657. doi: 10.1126/science.1170803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen A.H., Hurwitz M., Sullivan S.A., Saad A., Kennedy J.L.W., Sharma G. Update on sex specific risk factors in cardiovascular disease. Front. Cardiovasc. Med. 2024;11 doi: 10.3389/fcvm.2024.1352675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordovás J.M., Smith C.E. Epigenetics and cardiovascular disease. Nat. Rev. Cardiol. 2010;7(9):510–519. doi: 10.1038/nrcardio.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco-Solis R., Aguilar-Arnal L. Circadian regulation of immunity through epigenetic mechanisms. Front. Cell. Infect. Microbiol. 2020;10 doi: 10.3389/fcimb.2020.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pana T.A., Mamas M.A., Wareham N.J., Khaw K.T., Dawson D.K., Myint P.K. Sex specific lifetime risk of incident cardiovascular events. Results from a large British population-based cohort study. Eur. Heart J. 2023;44(Suppl. ment_2) [Google Scholar]
- Panza J.A., Epstein S.E., Quyyumi A.A. Circadian variation in vascular tone and its relation to α-sympathetic vasoconstrictor activity. N. Engl. J. Med. 1991;325(14):986–990. doi: 10.1056/NEJM199110033251402. [DOI] [PubMed] [Google Scholar]
- Papazyan R., Zhang Y., Lazar M.A. Genetic and epigenomic mechanisms of mammalian circadian transcription. Nat. Struct. Mol. Biol. 2016;23(12):1045–1052. doi: 10.1038/nsmb.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton A.P., Hastings M.H. The suprachiasmatic nucleus. Curr. Biol. 2018;28(15):R816–R822. doi: 10.1016/j.cub.2018.06.052. [DOI] [PubMed] [Google Scholar]
- Pavkovic I.M., Kothare S.V. Pharmacologic approaches to insomnia and other sleep disorders in children. Curr. Treat. Options Neurol. 2022;24(4):129–153. [Google Scholar]
- Pepin M.E., Drakos S., Ha C.-M., Tristani-Firouzi M., Selzman C.H., Fang J.C., Wende A.R., Wever-Pinzon O. DNA methylation reprograms cardiac metabolic gene expression in end-stage human heart failure. Am. J. Physiol. Heart Circ. Physiol. 2019;317(4):H674–H684. doi: 10.1152/ajpheart.00016.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilorz V., Helfrich-Förster C., Oster H. The role of the circadian clock system in physiology. Pflueg. Arch. Eur. J. Physiol. 2018;470(2):227–239. doi: 10.1007/s00424-017-2103-y. [DOI] [PubMed] [Google Scholar]
- Portaluppi F., Tiseo R., Smolensky M.H., Hermida R.C., Ayala D.E., Fabbian F. Circadian rhythms and cardiovascular health. Sleep Med. Rev. 2012;16(2):151–166. doi: 10.1016/j.smrv.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Promsan S., Thongnak L., Pengrattanachot N., Phengpol N., Sutthasupha P., Lungkaphin A. Agomelatine, a structural analog of melatonin, improves kidney dysfunction through regulating the AMPK/mTOR signaling pathway to promote autophagy in obese rats. Food Chem. Toxicol. 2022;165 doi: 10.1016/j.fct.2022.113190. [DOI] [PubMed] [Google Scholar]
- Rabinovich-Nikitin I., Lieberman B., Martino T.A., Kirshenbaum L.A. Circadian-regulated cell death in cardiovascular diseases. Circulation (New York, N. Y.) 2019;139(7):965–980. doi: 10.1161/CIRCULATIONAHA.118.036550. [DOI] [PubMed] [Google Scholar]
- Rabinovich-Nikitin I., Crandall M., Kirshenbaum L.A. Circadian regulation of genetic and hormonal risk factors of cardiovascular disease in women. Can. J. Physiol. Pharmacol. 2023;101(1):1–7. doi: 10.1139/cjpp-2022-0222. [DOI] [PubMed] [Google Scholar]
- Ramkisoensing A., Meijer J.H. Synchronization of biological clock neurons by light and peripheral feedback systems promotes circadian rhythms and health. Front. Neurol. 2015;6 doi: 10.3389/fneur.2015.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana S., Prabhu S.D., Young M.E. Chronobiological influence over cardiovascular function. Circ. Res. 2020;126(2):258–279. doi: 10.1161/CIRCRESAHA.119.313349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehan L., Laszki-Szcząchor K., Sobieszczańska M., Polak-Jonkisz D. SIRT1 and NAD as regulators of ageing. Life Sci. 2014;105(1–2):1–6. doi: 10.1016/j.lfs.2014.03.015. [DOI] [PubMed] [Google Scholar]
- Reitz C.J., Alibhai F.J., Khatua T.N., Rasouli M., Bridle B.W., Burris T.P., Martino T.A. SR9009 administered for one day after myocardial ischemia-reperfusion prevents heart failure in mice by targeting the cardiac inflammasome. Commun. Biol. 2019;2(1):353. doi: 10.1038/s42003-019-0595-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards J., Gumz M.L. Mechanism of the circadian clock in physiology. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013;304(12):R1053–R1064. doi: 10.1152/ajpregu.00066.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson G.S., Zee P.C., Wang-Weigand S., Rodriguez L., Peng X. Circadian phase-shifting effects of repeated ramelteon administration in healthy adults. J. Clin. Sleep Med. : JCSM : official publication of the American Academy of Sleep Medicine. 2008;4(5):456–461. [PMC free article] [PubMed] [Google Scholar]
- Rizvi S.I., Majumdar G. Circadian Clock and Aging. Springer Nature Singapore; Singapore: 2024. Mechanisms of circadian oscillations; pp. 31–71. [Google Scholar]
- Ruan W., Yuan X., Eltzschig H.K. Circadian rhythm as a therapeutic target. Nat. Rev. Drug Discov. 2021;20(4):287–307. doi: 10.1038/s41573-020-00109-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer F.A.J.L., Hilton M.F., Mantzoros C.S., Shea S.A. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl. Acad. Sci. 2009;106(11):4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer F.A.J.L., Hu K., Evoniuk H., Kelly E.E., Malhotra A., Hilton M.F., Shea S.A. Impact of the human circadian system, exercise, and their interaction on cardiovascular function. Proc. Natl. Acad. Sci. 2010;107(47):20541–20546. doi: 10.1073/pnas.1006749107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder E.A., Lefta M., Zhang X., Bartos D.C., Feng H.-Z., Zhao Y., Patwardhan A., Jin J.-P., Esser K.A., Delisle B.P. The cardiomyocyte molecular clock, regulation of Scn5a , and arrhythmia susceptibility. Am. J. Physiol. Cell Physiol. 2013;304(10):C954–C965. doi: 10.1152/ajpcell.00383.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servid S. 2021. Drug Class Review: Circadian Rhythm Sleep Disorders. [Google Scholar]
- Shi Y., Zhang H., Huang S., Yin L., Wang F., Luo P., Huang H. Epigenetic regulation in cardiovascular disease: mechanisms and advances in clinical trials. Signal Transduct. Targeted Ther. 2022;7(1):200. doi: 10.1038/s41392-022-01055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skogstad M., Mamen A., Lunde L.-K., Ulvestad B., Matre D., Aass H.C.D., Øvstebø R., Nielsen P., Samuelsen K.N., Skare Ø., Sirnes P.A. Shift work including night work and long working hours in industrial plants increases the risk of atherosclerosis. Int. J. Environ. Res. Publ. Health. 2019;16(3):521. doi: 10.3390/ijerph16030521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Škrlec I. Cardiac Diseases - Novel Aspects of Cardiac Risk, Cardiorenal Pathology and Cardiac Interventions. IntechOpen; 2021. Epigenetics of circadian rhythm disruption in cardiovascular diseases. [Google Scholar]
- Škrlec I. 2023. Circadian System microRNAs – Role in the Development of Cardiovascular Diseases; pp. 225–267. [DOI] [PubMed] [Google Scholar]
- Škrlec I., Milic J., Heffer M., Peterlin B., Wagner J. Genetic variations in circadian rhythm genes and susceptibility for myocardial infarction. Genet. Mol. Biol. 2018;41(2):403–409. doi: 10.1590/1678-4685-GMB-2017-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Škrlec I., Talapko J., Juzbašić M., Steiner R. Sex differences in circadian clock genes and myocardial infarction susceptibility. Journal of cardiovascular development and disease. 2021;8(5) doi: 10.3390/jcdd8050053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolensky M.H., Hermida R.C., Ayala D.E., Tiseo R., Portaluppi F. Administration–time-dependent effects of blood pressure-lowering medications: basis for the chronotherapy of hypertension. Blood Pres. Monit. 2010;15(4):173–180. doi: 10.1097/MBP.0b013e32833c7308. [DOI] [PubMed] [Google Scholar]
- Smolensky M.H., Hermida R.C., Reinberg A., Sackett-Lundeen L., Portaluppi F. Circadian disruption: new clinical perspective of disease pathology and basis for chronotherapeutic intervention. Chronobiol. Int. 2016;33(8):1101–1119. doi: 10.1080/07420528.2016.1184678. [DOI] [PubMed] [Google Scholar]
- Smolensky M.H., Hermida R.C., Portaluppi F. Circadian mechanisms of 24-hour blood pressure regulation and patterning. Sleep Med. Rev. 2017;33:4–16. doi: 10.1016/j.smrv.2016.02.003. [DOI] [PubMed] [Google Scholar]
- Spencer R.L., Chun L.E., Hartsock M.J., Woodruff E.R. Glucocorticoid hormones are both a major circadian signal and major stress signal: how this shared signal contributes to a dynamic relationship between the circadian and stress systems. Front. Neuroendocrinol. 2018;49:52–71. doi: 10.1016/j.yfrne.2017.12.005. [DOI] [PubMed] [Google Scholar]
- St-Onge M.-P., Ard J., Baskin M.L., Chiuve S.E., Johnson H.M., Kris-Etherton P., Varady K. Meal timing and frequency: implications for cardiovascular disease prevention: a scientific statement from the American heart association. Circulation (New York, N. Y.) 2017;135(9) doi: 10.1161/CIR.0000000000000476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalder T., Oster H., Abelson J.L., Huthsteiner K., Klucken T., Clow A. The cortisol awakening response: regulation and functional significance. Endocr. Rev. 2025;46(1):43–59. doi: 10.1210/endrev/bnae024. [DOI] [PubMed] [Google Scholar]
- Sulli G., Manoogian E.N.C., Taub P.R., Panda S. Training the circadian clock, clocking the drugs, and drugging the clock to prevent, manage, and treat chronic diseases. Trends Pharmacol. Sci. 2018;39(9):812–827. doi: 10.1016/j.tips.2018.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q., Zhao J., Liu L., Wang X., Gu X. Identification of the potential biomarkers associated with circadian rhythms in heart failure. PeerJ. 2023;11 doi: 10.7717/peerj.14734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi J.S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 2017;18(3):164–179. doi: 10.1038/nrg.2016.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda N., Maemura K. Circadian clock and cardiovascular disease. J. Cardiol. 2011;57(3):249–256. doi: 10.1016/j.jjcc.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Takeda N., Maemura K. The role of clock genes and circadian rhythm in the development of cardiovascular diseases. Cell. Mol. Life Sci. : CMLS. 2015;72(17):3225–3234. doi: 10.1007/s00018-015-1923-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda N., Maemura K. Circadian clock and the onset of cardiovascular events. Hypertens. Res. 2016;39(6):383–390. doi: 10.1038/hr.2016.9. [DOI] [PubMed] [Google Scholar]
- Talamanca L., Gobet C., Naef F. Sex-dimorphic and age-dependent organization of 24-hour gene expression rhythms in humans. Science. 2023;379(6631):478–483. doi: 10.1126/science.add0846. [DOI] [PubMed] [Google Scholar]
- Tampakakis E., Gangrade H., Glavaris S., Htet M., Murphy S., Lin B.L., Liu T., Saberi A., Miyamoto M., Kowalski W., Mukouyama Y.-S., Lee G., Minichiello L., Kwon C. Heart neurons use clock genes to control myocyte proliferation. Sci. Adv. 2021;7(49) doi: 10.1126/sciadv.abh4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur A., Mishra A.P., Panda B., Sweta K., Majhi B. Detection of disease-specific parent cells via distinct population of nano-vesicles by machine learning. Curr. Pharm. Des. 2020;26(32):3985–3996. doi: 10.2174/1381612826666200422091753. [DOI] [PubMed] [Google Scholar]
- Thakur A., Mishra A.P., Panda B., Rodríguez D.C.S., Gaurav I., Majhi B. Application of artificial intelligence in pharmaceutical and biomedical studies. Curr. Pharm. Des. 2020;26(29):3569–3578. doi: 10.2174/1381612826666200515131245. [DOI] [PubMed] [Google Scholar]
- Thej C., Kishore R. Epigenetic regulation of sex dimorphism in cardiovascular health. Can. J. Physiol. Pharmacol. 2024;102(9):498–510. doi: 10.1139/cjpp-2023-0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thej C., Roy R., Cheng Z., Garikipati V.N.S., Truongcao M.M., Joladarashi D., Mallaredy V., Cimini M., Gonzalez C., Magadum A., Ghosh J., Benedict C., Koch W.J., Kishore R. Epigenetic mechanisms regulate sex differences in cardiac reparative functions of bone marrow progenitor cells. NPJ Regen. Med. 2024;9(1):17. doi: 10.1038/s41536-024-00362-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thosar S.S., Butler M.P., Shea S.A. Role of the circadian system in cardiovascular disease. J. Clin. Investig. 2018;128(6):2157–2167. doi: 10.1172/JCI80590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai J.-Y., Kienesberger P.C., Pulinilkunnil T., Sailors M.H., Durgan D.J., Villegas-Montoya C., Jahoor A., Gonzalez R., Garvey M.E., Boland B., Blasier Z., McElfresh T.A., Nannegari V., Chow C.-W., Heird W.C., et al. Direct regulation of myocardial triglyceride metabolism by the cardiomyocyte circadian clock. J. Biol. Chem. 2010;285(5):2918–2929. doi: 10.1074/jbc.M109.077800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuey Tse M. Circadian rhythm drug approved. Nat. Biotechnol. 2014;32(4) 303–303. [Google Scholar]
- Tsuzuki K., Shimizu Y., Suzuki J., Pu Z., Yamaguchi S., Fujikawa Y., Kato K., Ohashi K., Takefuji M., Bando Y.K., Ouchi N., Calvert J.W., Shibata R., Murohara T. Adverse effect of circadian rhythm disorder on reparative angiogenesis in hind limb ischemia. J. Am. Heart Assoc. 2021;10(16) doi: 10.1161/JAHA.121.020896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhaverbeke M., Attard R., Bartekova M., Ben-Aicha S., Brandenburger T., de Gonzalo-Calvo D., Emanueli C., Farrugia R., Grillari J., Hackl M., Kalocayova B., Martelli F., Scholz M., Wettinger S.B., Devaux Y. Peripheral blood RNA biomarkers for cardiovascular disease from bench to bedside: a position paper from the EU-CardioRNA COST action CA17129. Cardiovasc. Res. 2022;118(16):3183–3197. doi: 10.1093/cvr/cvab327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswambharan H., Carvas J.M., Antic V., Marecic A., Jud C., Zaugg C.E., Ming X.-F., Montani J.-P., Albrecht U., Yang Z. Mutation of the circadian clock gene Per2 alters vascular endothelial function. Circulation (New York, N. Y.) 2007;115(16):2188–2195. doi: 10.1161/CIRCULATIONAHA.106.653303. [DOI] [PubMed] [Google Scholar]
- Vyas M.V., Garg A.X., Iansavichus A.V., Costella J., Donner A., Laugsand L.E., Janszky I., Mrkobrada M., Parraga G., Hackam D.G. Shift work and vascular events: systematic review and meta-analysis. BMJ. 2012;345(jul26 1) doi: 10.1136/bmj.e4800. e4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton J.C., Bumgarner J.R., Nelson R.J. Sex differences in circadian rhythms. Cold Spring Harbor Perspect. Biol. 2022;14(7) doi: 10.1101/cshperspect.a039107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren W.S., Champney T.H., Cassone V.M. The suprachiasmatic nucleus controls the circadian rhythm of heart rate via the sympathetic nervous system. Physiol. Behav. 1994;55(6):1091–1099. doi: 10.1016/0031-9384(94)90392-1. [DOI] [PubMed] [Google Scholar]
- Wildi K., Singeisen H., Twerenbold R., Badertscher P., Wussler D., Klinkenberg L.J.J., Meex S.J.R., Nestelberger T., Boeddinghaus J., Miró Ò., Martin-Sanchez F.J., Morawiec B., Muzyk P., Parenica J., Keller D.I., et al. Circadian rhythm of cardiac troponin I and its clinical impact on the diagnostic accuracy for acute myocardial infarction. Int. J. Cardiol. 2018;270:14–20. doi: 10.1016/j.ijcard.2018.05.136. [DOI] [PubMed] [Google Scholar]
- Wong R., Crane A., Sheth J., Mayrovitz H.N. Shift work as a cardiovascular disease risk factor: a narrative review. Cureus. 2023 doi: 10.7759/cureus.41186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z., Su W., Liu S., Zhao G., Esser K., Schroder E.A., Lefta M., Stauss H.M., Guo Z., Gong M.C. Smooth-muscle BMAL1 participates in blood pressure circadian rhythm regulation. J. Clin. Investig. 2015;125(1):324–336. doi: 10.1172/JCI76881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W., Jain M.K., Zhang L. Molecular link between circadian clocks and cardiac function: a network of core clock, slave clock, and effectors. Curr. Opin. Pharmacol. 2021;57:28–40. doi: 10.1016/j.coph.2020.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung C.-Y.C., Dondelinger F., Schoof E.M., Georg B., Lu Y., Zheng Z., Zhang J., Hannibal J., Fahrenkrug J., Kjaer M. Circadian regulation of protein cargo in extracellular vesicles. Sci. Adv. 2022;8(14) doi: 10.1126/sciadv.abc9061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young M.E. The cardiac circadian clock. JACC (J. Am. Coll. Cardiol.): Basic to Translational Science. 2023;8(12):1613–1628. doi: 10.1016/j.jacbts.2023.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young M.E., Bray M.S. Potential role for peripheral circadian clock dyssynchrony in the pathogenesis of cardiovascular dysfunction. Sleep Med. 2007;8(6):656–667. doi: 10.1016/j.sleep.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young M.J., Heanue S., Kanki M., Moneghetti K.J. Circadian disruption and its impact on the cardiovascular system. Trends in Endocrinology & Metabolism. 2024 doi: 10.1016/j.tem.2024.11.010. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Yu B., Wang X., Luo C., Zhou T., Zheng X., Ding J. Circadian rhythm and atherosclerosis. Exp. Ther. Med. 2020;20(5) doi: 10.3892/etm.2020.9224. (Review) 1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Procopio S.B., Ding H., Semel M.G., Schroder E.A., Seward T.S., Du P., Wu K., Johnson S.R., Prabhat A., Schneider D.J., Stumpf I.G., Rozmus E.R., Huo Z., Delisle B.P., et al. 2024. New Role for Cardiomyocyte Bmal1 in the Regulation of Sex-specific Heart Transcriptomes. [Google Scholar]
- Zhang X., Procopio S.B., Ding H., Semel M.G., Schroder E.A., Viggars M.R., Seward T.S., Du P., Wu K., Johnson S.R., Prabhat A., Schneider D.J., Stumpf I.G., Rozmus E.R., Huo Z., et al. The core circadian clock factor, Bmal1 , transduces sex-specific differences in both rhythmic and nonrhythmic gene expression in the mouse heart. Function. 2025;6(1) doi: 10.1093/function/zqae053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Sun X., Zhao H., Xie F., Li B., Zhang J. Biomarker identification and risk assessment of cardiovascular disease based on untargeted metabolomics and machine learning. Sci. Rep. 2024;14(1) doi: 10.1038/s41598-024-77352-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q., Belden W.J. Molecular regulation of circadian chromatin. J. Mol. Biol. 2020;432(12):3466–3482. doi: 10.1016/j.jmb.2020.01.009. [DOI] [PubMed] [Google Scholar]
- Zietara A., Spires D.R., Juffre A., Costello H.M., Crislip G.R., Douma L.G., Levchenko V., Dissanayake L.V., Klemens C.A., Nikolaienko O., Geurts A.M., Gumz M.L., Staruschenko A. Knockout of the circadian clock protein PER1 (Period1) exacerbates hypertension and increases kidney injury in dahl salt-sensitive rats. Hypertension. 2022;79(11):2519–2529. doi: 10.1161/HYPERTENSIONAHA.122.19316. [DOI] [PMC free article] [PubMed] [Google Scholar]